- 1Department of Medical Oncology, Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Oncology, The Third People’s Hospital of ChengduChengdu, China
- 3Department of Medical Oncology, Sichuan Cancer HospitalChengdu, China
- 4Department of Head and Neck Oncology, West China Hospital, Sichuan University, Chengdu, China
Purpose: The aim of the study was to evaluate the cost-effectiveness of PEGylated recombinant human granulocyte–stimulating factor (PEG-rhG-CSF) as a means of achieving primary and secondary prophylaxis against chemotherapy-induced neutropenia cancer cases.
Methods: Individuals who underwent PEG-rhG-CSF therapeutics were monitored for 12 months, together with thorough examination of individual medical records for extracting medical care costs. Both prophylaxis-based therapeutic options (primary/secondary) were scrutinized for cost-effectiveness, using a decision-making analysis model which derived the perspective of Chinese payers. One-way and probabilistic sensitivity analyses were used to assess the robustness of the model.
Results: In summary, 130 clinical cases treated using PEG-rhG-CSF prophylaxis were included in this study: 51 within the primary prophylaxis (PP) group and 79 within the secondary prophylaxis (SP) group. Compared with SP, PP-based PEG-rhG-CSF successfully contributed to a 14.3% reduction in febrile neutropenia. In general, PP was estimated to reduce costs by $4,701.81 in comparison to SP, with a gain of 0.02 quality-adjusted life years (QALYs). Equivalent results were found in differing febrile neutropenia (FN) risk subgroups. Sensitivity analyses found the model outputs to be most affected for the average time of hospitalization and for the cost of FN.
Conclusion: From the perspective of Chinese payers, PP with PEG-rhG-CSF should be considered cost-effective compared to SP strategies in patients who received chemotherapy regimens with a middle- to high-risk of FN.
Introduction
Chemotherapy-induced febrile neutropenia (FN) is a main repercussion derived from myelosuppression stemming from chemotherapeutic measures (Aarts et al., 2013). FN can lead to lowering dose limits of chemotherapy drugs, delayed chemotherapy, and severe infections. Such repercussions typically increase treatment costs, together with reducing the efficacy of chemotherapy and quality of survival, affecting patient prognosis and possibly even leading to death (Gisselbrecht et al., 1997; Tirelli et al., 1998). Therefore, primary/secondary prophylaxis measures employing granulocyte colony–stimulating factor (G-CSF) were recommended by national and international guidelines as a measure for reducing FN development within the most vulnerable patients incurring elevated risks of FN (Aapro et al., 2010; Langford and Chrisp, 2010; Smith et al., 2015). FN risk assessments are based upon multiple patient parameters, including demographic data, and primary disease parameters (such as tumor progression status), together with therapeutic parameters such as the degree of aggressiveness in chemotherapy approaches on the patient. Consequently, prophylaxis protocols that rely solely on the type and intensity of chemotherapy regimens can result in exposing patients to a greater number of FN events.
Currently, the effective and widely used drug for treating chemotherapy-induced febrile granulocytopenia is recombinant human granulocyte colony–stimulating factor (rhG-CSF) (Kuderer et al., 2007; Wang et al., 2015). However, rhG-CSF usually requires multiple doses to achieve improvement, leading to exacerbated drug-related adverse effects, which undoubtedly prolongs the treatment cycle and increases the physical and psychological stress in patients (Kuwabara et al., 1996; Bhana, 2007). PEGylated recombinant human granulocyte–stimulating factor (PEG-rhG-CSF) is a chemically modified version of rhG-CSF using monomethoxy polyethylene glycol, which has the characteristic of relieving neutrophil deficiency with a single dose (Holmes et al., 2002; Green et al., 2003; Kubo et al., 2016; Cerchione et al., 2017). Based on its convenience, stability and efficacy, long-acting PEGylated rhG-CSF is recommended by relevant treatment guidelines and widely used in clinical practice (Aapro et al., 2010; The society of chemothera, 2019; Network.linical Pra, 2020).
Increasingly, cost-effectiveness analysis is used to compare the costs and health outcomes of different interventions to provide a basis for policy decisions. Moreover, long-acting PEGylated rhG-CSF has been highlighted in previous pharmacoeconomic studies as being cost-effective (Lyman et al., 2009; Sebban et al., 2012; Perrier et al., 2013). Consequently, further research on the cost-effective benefits of population-based primary or secondary prophylaxis with PEG-rhG-CSF is essential to optimize medical resource allocations for chemotherapy-induced FN. Although several investigations have explored the cost/medical benefit of PEGylated rhG-CSF for primary versus secondary prophylaxis for oncology patients in multiple countries (Ramsey et al., 2009; Fust et al., 2014; Hill et al., 2014; Fust et al., 2017), most of these studies were based on published clinical trials and were limited to specific oncology patients, leading to a paucity of data in the real world. Furthermore, to our knowledge, no relevant studies have explicitly evaluated the cost-effectiveness of PEG-rhG-CSF primary versus secondary prophylaxis in Chinese cancer patients. Due to regional and health insurance plan limitations, available cost-effectiveness data from Western countries may not be generalizable to Asian populations.
The investigation described later analyzed the cost-effectiveness of PEG-rhG-CSF as primary and secondary prophylactic measures against FN from the perspective of Chinese payers. This was achieved by the use of the hospital information system (HIS) database of several hospitals in southwestern China from 2015 to 2019, with the intention of evaluating the economics of PEG-rhG-CSF and providing real-world evidence for rational and economical clinical deployment of this drug.
Materials and Methods
Data Collection
This study was accepted by the Biomedical Ethics Committee of West China Hospital of Sichuan University, without the requirement for informed, written consent by patients. In this analysis, medical records from three hospitals in southwest China, from 2015 to 2019, were retrospectively collected to assess the effectiveness, together with costs, for PEG-rhG-CSF therapies in oncology patients. The criteria for including patients into this study were as follows: a) adult age (18–75 years); b) malignancy diagnosed by pathological histology or cytology; c) receiving chemotherapy with medium- to high-risk FN regimen; and d) having received PEG-rhG-CSF as primary or secondary prophylaxis during chemotherapy. Exclusion criteria were as follows: a) bone marrow metastasis and b) no data on absolute neutrophil count after granulocyte-stimulating factor prophylaxis or treatment.
Model Construction
Both prophylaxis-based therapeutic options (primary/secondary prophylaxis) were scrutinized for cost-effectiveness, using a decision-making analysis model derived from Chinese payer’s perspective. For the purpose of this study, primary prophylaxis (PP) was defined as the PEG-rhG-CSF therapy commencing at the start (1st) of the chemotherapeutic cycle (or in a previous cycle without a neutropenic event). Secondary prophylaxis (SP) was defined for PEG-rhG-CSF therapy that was commenced following a neutropenic event immediately preceding the chemotherapeutic cycle. Clinical effectiveness was measured by neutropenic events, including neutropenia without fever or infection and FN (defined as neutropenia combined with fever and/or infection). A log of neutropenic incidents was recorded for every patient during each chemotherapy cycle. The model creation and analysis were carried out using TreeAge software (TreeAge Pro® statistical package 2020 R1, Williamstown, Massachusetts, United States), SPSS software 26 (IBM, Armonk, New York, United States), and Microsoft Excel 2019 (Microsoft, Redmond, Washington, United States). Each model chemotherapy cycle constituted 4 weeks (1 month) across a 12-month time horizon, matching this study’s follow-up time. The primary outcome for this investigation was the incremental cost-effectiveness ratio (ICER; a ratio-based the difference of cost and effectiveness variations between PP and SP therapeutics). Consequently, the ICER value was evaluated against the willingness to pay (WTP; highest price a patient is comfortable to pay for an individual QALY). Based on the WHO research and guidelines, the WTP for this specific study was set at three-fold the individual Chinese citizen’s GDP (USD 30,313.52-2019) (Molassiotis et al., 2002; Eichler et al., 2004; Murray et al., 2000; O (2002). The world hea, 2002).
Cost and Utilities
The cost and cost-effectiveness were conducted from the Chinese payer system perspective but were limited to direct medical cost only. Such costs include G-CSF, PEG-rhG-CSF, and antibiotic therapies, laboratory tests and hospitalization costs. Direct medical cost evaluation was collected from centralized procurement data in the Chinese market, Hospital Information System, and published the literature (Table 1). Since multiple tumor types and various treatment options were included in the study, the cost for chemotherapy drugs was not taken into consideration. All costs were recorded in USD (February 15, 2021 exchange rate, 6.9851 RMB = 1 US dollar) (Sigsgaard et al., 2001).
Sensitivity Analysis
A series of deterministic sensitivity analyses were conducted to explore whether primary PEG-rhG-CSF prophylaxis is cost-effective when the cost, utility values, and other parameters of PEG-rhG-CSF vary over a broader range. The variables in the deterministic sensitivity analysis varied within confidence intervals or ±30%. Based on the distribution characteristics of each parameter, a γ distribution was used for the cost parameter, and a β distribution was used for the health utility values. Then, we performed 1,000 Monte Carlo simulations for probabilistic sensitivity analysis. The univariate sensitivity analysis results were expressed as tornado plots, and the probabilistic sensitivity analysis results were expressed as cost–benefit acceptability curves. We also analyzed the possibility of primary PEG-rhG-CSF prophylaxis being cost-effective in the subgroups with different FN risks.
Results
Patient Datasets
One hundred thirty (130) patients fulfilling the inclusion and exclusion criteria for our study, of which 51 participants underwent PEG-rhG-CSF PP, while 79 participants underwent PEG-rhG-CSF SP. In the PP group, a total of 68.6% of patients received chemotherapeutic cycles with an FN risk being >20%, while in the SP group, this percentage was 45.6%. Among PP patients, 21.6% had metastatic cancer, compared with 21.5% of patients with metastatic cancer in the SP group. Baseline characteristics of the PEG-rhG-CSF–treated patients are presented in Table 2.
Economic Evaluation
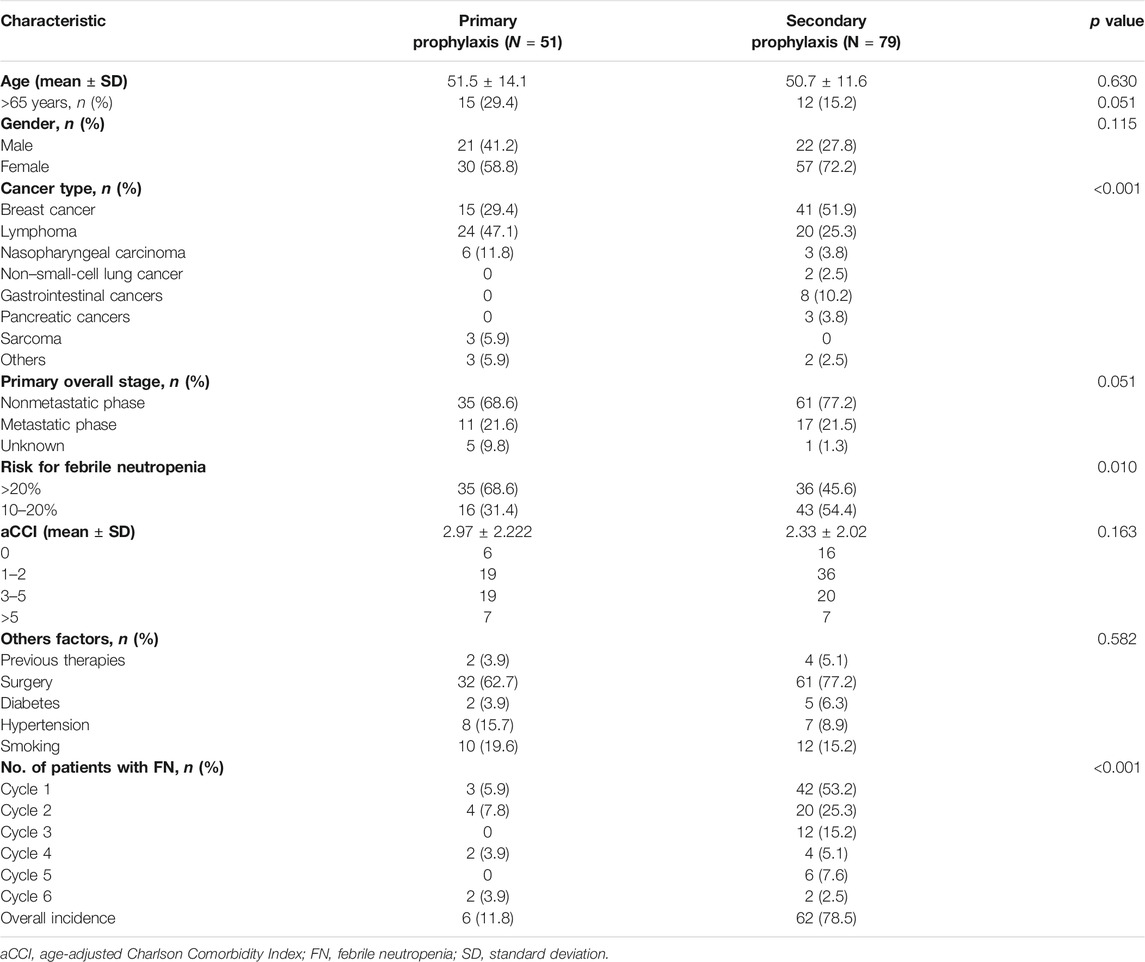
In the PP arm, 6 of 51 patients (11.8%) suffered a minimum of one FN event, while in the SP arm, FN afflicted 62 out of 79 patients (78.5%). From Chinese payers’ perspective, the average cost of primary PEG-rhG-CSF prophylaxis is $11,611.62, with an average gain of 0.73 QALYs per patient over a 1-year time horizon. The average cost of secondary PEG-rhG-CSF prophylaxis was $16,313.43, with an average gain of 0.71 QALYs (Figure 1). For all patients in this study, PEG-rhG-CSF as primary prophylaxis dominated all comparators on FN events avoided and QALYs gained. Consensus results were also observed in the subgroup analysis of patients who received chemotherapy regimens with a risk of FN greater than 20% and patients who received chemotherapy regimens with an FN risk of 10–20%(Table 3).
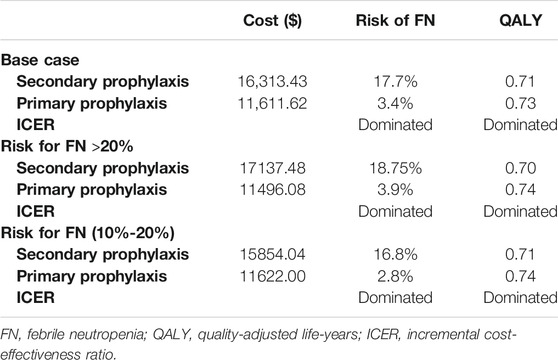
TABLE 3. Base case and subgroups cost-effectiveness results for PEG-rhG-CSF as primary versus secondary prophylaxis.
Sensitivity Analysis
All one-way sensitivity investigation results are represented in Figure 2. One-way sensitivity analysis highlighted that this model’s reliability was more vulnerable to the mean hospitalization time frame in both study groups, together with the costs associated with FN clinical management, since both parameters have the greatest influence on ICER values. Notwithstanding, ICER was still identified to have a reduced value compared to WTP when all variable parameters shifted in value within the set range. Probabilistic sensitivity analysis consistently showed dominated results by PP, in comparison to SP (Figure 3).
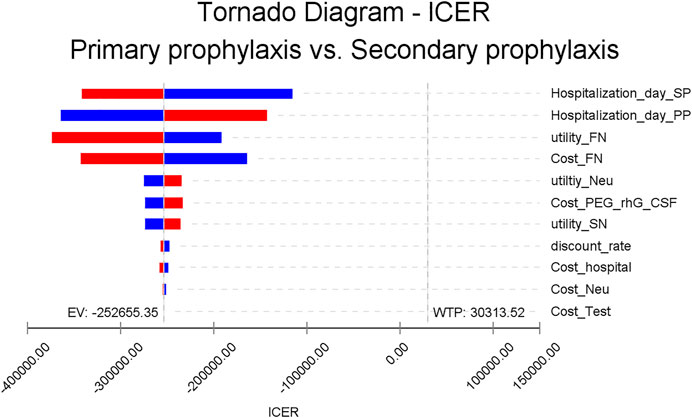
FIGURE 2. One-way sensitivity analysis. This diagram shows incremental cost-effectiveness ratio (ICER) of PP vs SP for different model input parameters. PEG-rhG-CSF, PEGylated recombinant human granulocyte–stimulating factor; FN, febrile neutropenia; SN, severe neutropenia; PP, primary prophylaxis; SP, secondary prophylaxis; Neu, neutropenia.
Discussion
In this study, Markov models were employed for assessing the cost-efficiency for PEG-rhG-CSF by retrospectively collecting and analyzing data on the effectiveness and cost of using this drug as PP and SP within three large hospitals. Compared with SP of PEG-rhG-CSF, this study revealed that PP of PEG-rhG-CSF not only increased the QLAY by 0.02 for patients with malignancies receiving intermediate- to high-risk FN chemotherapy, but there was also a reduction in FN events by 14.3%, together with a reduction in costs by $4,701.81. The analyzed model robustness was most vulnerable to the mean hospitalization time frame endured by both study groups, reflecting the impact of hospitalization costs on outcomes. One-way sensitivity was not used to analyze FN incidence since it was fixed in the model as a transition probability.
This study found that PP therapy incurred lower costs than SP therapy, having a lower FN incidence rate and a higher level of QALY. In the subgroup analysis, the median cost per QALY gained for PP patients was $15,535.24 and $15,705.41 in the FN high-and medium-risk groups, which was $8,946.87 and $6,624.22 lower than for secondary prevention patients, respectively. Such results were in conformity with reports from other nations focusing on this research niche (Hill et al., 2014). In Belgium, a study of PP and SP, including long-acting PEGylated rhG-CSF, found PP to be a cost-effective therapy for both breast cancer and non-Hodgkin’s lymphoma at a €30,000/QALY threshold (Fust et al., 2017). Similar conclusions regarding the cost-effectiveness of PP with long-acting PEGylated rhG-CSF versus SP have been reported in other studies in the United Kingdom, Singapore, and the United States (Gruschkus et al., 2011; Fust et al., 2014; Wang et al., 2016). Here, we expanded on past analyses by including different tumor types and obtained the incidence of FN for each cycle of the patients from multiple centers. We also analyzed the cost-effectiveness of PEG in FN intermediate- and high-risk chemotherapy regimens separately and therefore was pertinent for more complex clinical practice decision-making.
Overall, this investigation concludes that primary prophylaxis using G-CSF is a dominant strategy, when compared with secondary G-CSF-based prophylaxis for such oncology cases. Compared with PEG-rhG-CSF for secondary prophylaxis, albeit a more frequent use of PEG-rhG-CSF for primary prophylaxis results in a relatively higher cost, primary prophylaxis still proved to have enhanced cost-effectiveness potential against FN, also when FN-associated treatment costs are taken into account. However, unlike SP, PP does not fall within Chinese medical insurance provider coverage plans, leaving no reimbursement options for patients undergoing PP. Consequently, the findings from this study could provide empirical data to re-evaluate the reimbursement scheme.
As with all observational studies based on hospital information system, this study does carry limitations. In the first instance, these study data were based on past medical records, leading to several baseline variations (patient demographics, chemotherapy regimens, cancer type, the risk for febrile neutropenia, etc.) between the PP and SP groups. For the difference in the incidence of FN with chemotherapy regimens in the PP and SP groups, patients receiving moderate- and high-risk chemotherapy regimens in the two groups were analyzed separately and the findings were consistent with the overall population. In addition, regarding the age-adjusted Charlson comorbidity index and other factors that may be associated with the incidence of FN, as mentioned in the NCCN guidelines (16), we found no significant differences between the PP and SP groups. Overall, the cost of PEG⁃rhG⁃CSF is relatively expensive for patients in China, and only patients receiving secondary prevention are considered for reimbursement by medical insurance. We hope that more data will be available for further research following changes in health insurance policies, lower drug prices, or the inclusion of more institutions. Second, adverse events associated with PEG⁃rhG⁃CSF treatment were not considered in the model since PEG⁃rhG⁃CSF has relatively few adverse effects. Furthermore, the assessment of healthcare resource utilization, based on expert opinion, introduced a high degree of uncertainty into the model analysis. However, in the sensitivity analysis, all essential parameters were examined, and the model was stable when the parameters were varied. The probabilistic sensitivity analysis provided further evidence that PP is most likely a cost-effective option.
To our knowledge, this is the first real-world cost-effective study on PEG-rhG-CSF prophylaxis, from a Chinese payer’s perspective. In summary, for patients with tumors receiving chemotherapy regimens carrying >10% risk of incurring FN, primary prophylaxis with PEG-rhG-CSF reduces FN-related incidences, increases the likelihood of receiving a full dose, a full course of chemotherapy, and additional QALY benefits. Therefore, PEG-rhG-CSF is ideal for primary prophylaxis in terms of cost-effectiveness and has a reference value for adjusting health insurance reimbursement policies.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by the Biomedical Ethics Committee of West China Hospital of Sichuan University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
QW coordinated and performed data analyses, reported study results, and drafted the manuscript. JZ, ZL, JZ, and JC assisted with the analyses. QL and YL contributed to the interpretation of the results and reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYJC18010).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aapro, M. S., Bohlius, J., Cameron, D. A., Dal Lago, L., Donnelly, J. P., Kearney, N., et al. (2010). 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur. J. Cancer 47 (1), 8–32. doi:10.1016/j.ejca.2010.10.013
Aarts, M. J., Grutters, J. P., Peters, F. P., Mandigers, C. M., Dercksen, M. W., Stouthard, J. M., et al. (2013). Cost effectiveness of primary pegfilgrastim prophylaxis in patients with breast cancer at risk of febrile neutropenia. J. Clin. Oncol. 31 (34), 4283–4289. doi:10.1200/jco.2012.48.3644
Bhana, N. (2007). Granulocyte colony-stimulating factors in the management of chemotherapy-induced neutropenia: evidence based review. Curr. Opin. Oncol. 19 (4), 328–335. doi:10.1097/01.cco.0000275309.58868.11
Cerchione, C., De Renzo, A., Di Perna, M., Della Pepa, R., Pugliese, N., Catalano, L., et al. (2017). Pegfilgrastim in primary prophylaxis of febrile neutropenia following frontline bendamustine plus rituximab treatment in patients with indolent non-hodgkin lymphoma: a single center, real-life experience. Support Care Cancer 25 (3), 839–845. doi:10.1007/s00520-016-3468-8
Eichler, H. G., Kong, S. X., Gerth, W. C., Mavros, P., and Jönsson, B. (2004). Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health 7 (5), 518–528. doi:10.1111/j.1524-4733.2004.75003.x
Fust, K., Li, X., Maschio, M., Barron, R., Weinstein, M. C., Parthan, A., et al. (2014). Cost-effectiveness of prophylaxis treatment strategies for febrile neutropenia in patients with recurrent ovarian cancer. Gynecol. Oncol. 133 (3), 446–453. doi:10.1016/j.ygyno.2014.03.014
Fust, K., Li, X., Maschio, M., Villa, G., Parthan, A., Barron, R., et al. (2017). Cost-Effectiveness Analysis of Prophylaxis Treatment Strategies to Reduce the Incidence of Febrile Neutropenia in Patients with Early-Stage Breast Cancer or Non-hodgkin Lymphoma. PharmacoEconomics 35 (4), 425–438. doi:10.1007/s40273-016-0474-0
Gisselbrecht, C., Haioun, C., Lepage, E., Bastion, Y., Tilly, H., Bosly, A., et al. (1997). Placebo-controlled phase III study of lenograstim (glycosylated recombinant human granulocyte colony-stimulating factor) in aggressive non-Hodgkin's lymphoma: factors influencing chemotherapy administration. Groupe d'Etude des Lymphomes de l'Adulte. Leuk. Lymphoma 25 (3-4), 289–300. doi:10.3109/10428199709114168
Gold, H. T., Hall, M. J., Blinder, V., and Schackman, B. R. (2009). Cost effectiveness of pharmacogenetic testing for uridine diphosphate glucuronosyltransferase 1A1 before irinotecan administration for metastatic colorectal cancer. Cancer 115 (17), 3858–3867. doi:10.1002/cncr.24428
Green, M. D., Koelbl, H., Baselga, J., Galid, A., Guillem, V., Gascon, P., et al. (2003). A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann. Oncol. 14 (1), 29–35. doi:10.1093/annonc/mdg019
Gruschkus, S. K., Lairson, D., Dunn, J. K., Risser, J., and Du, X. L. (2011). Cost-effectiveness of white blood cell growth factor use among a large nationwide cohort of elderly non-hodgkin's lymphoma patients treated with chemotherapy. Value Health 14 (2), 253–262. doi:10.1016/j.jval.2010.09.010
Hill, G., Barron, R., Fust, K., Skornicki, M. E., Taylor, D. C., Weinstein, M. C., et al. (2014). Primary vs secondary prophylaxis with pegfilgrastim for the reduction of febrile neutropenia risk in patients receiving chemotherapy for non-hodgkin's lymphoma: cost-effectiveness analyses. J. Med. Econ. 17 (1), 32–42. doi:10.3111/13696998.2013.844160
Holmes, F. A., O'Shaughnessy, J. A., Vukelja, S., Jones, S. E., Shogan, J., Savin, M., et al. (2002). Blinded, randomized, multicenter study to evaluate single administration pegfilgrastim once per cycle versus daily filgrastim as an adjunct to chemotherapy in patients with high-risk stage II or stage III/IV breast cancer. J. Clin. Oncol. 20 (3), 727–731. doi:10.1200/jco.2002.20.3.727
Kubo, K., Miyazaki, Y., Murayama, T., Shimazaki, R., Usui, N., Urabe, A., et al. (2016). A randomized, double-blind trial of pegfilgrastim versus filgrastim for the management of neutropenia during CHASE(R) chemotherapy for malignant lymphoma. Br. J. Haematol. 174 (4), 563–570. doi:10.1111/bjh.14088
Kuderer, N. M., Dale, D. C., Crawford, J., and Lyman, G. H. (2007). Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J. Clin. Oncol. 25 (21), 3158–3167. doi:10.1200/jco.2006.08.8823
Kuwabara, T., Kobayashi, S., and Sugiyama, Y. (1996). Pharmacokinetics and pharmacodynamics of a recombinant human granulocyte colony-stimulating factor. Drug Metab. Rev. 28 (4), 625–658. doi:10.3109/03602539608994020
Langford, P., and Chrisp, P. (2010). Fosaprepitant and aprepitant: an update of the evidence for their place in the prevention of chemotherapy-induced nausea and vomiting. Core Evid. 5, 77–90. doi:10.2147/ce.s6012
Lyman, G. H., Lalla, A., Barron, R. L., and Dubois, R. W. (2009). Cost-effectiveness of pegfilgrastim versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the United States. Clin. Ther. 31 (5), 1092–1104. doi:10.1016/j.clinthera.2009.05.003
Molassiotis, A., Yam, B. M., Yung, H., Chan, F. Y., and Mok, T. S. (2002). Pretreatment factors predicting the development of postchemotherapy nausea and vomiting in Chinese breast cancer patients. Support Care Cancer 10 (2), 139–145. doi:10.1007/s00520-001-0321-4
Murray, C. J., Evans, D. B., Acharya, A., and Baltussen, R. M. (2000). Development of WHO guidelines on generalized cost-effectiveness analysis. Health Econ. 9 (3), 235–251. doi:10.1002/(sici)1099-1050(200004)9:3<235::aid-hec502>3.0.co;2-o
Nafees, B., Stafford, M., Gavriel, S., Bhalla, S., and Watkins, J. (2008). Health state utilities for non small cell lung cancer. Health Qual. Life Outcomes 6, 84. doi:10.1186/1477-7525-6-84
Network. NCC Clinical Practice Guidelines in Oncology. Hematopoietic Growth Factors. (NCCN). Version 2. 2020[cited 2021 February 15] https://www.nccn.org/professionals/physician_gls/pdf/growthfactors.pdf.
Pater, J., Slamet, L., Zee, B., Osoba, D., Warr, D., and Rusthoven, J. (1994). Inconsistency of prognostic factors for post-chemotherapy nausea and vomiting. Support Care Cancer 2 (3), 161–166. doi:10.1007/BF00417474
Perrier, L., Lefranc, A., Pérol, D., Quittet, P., Schmidt-Tanguy, A., Siani, C., et al. (2013). Cost effectiveness of pegfilgrastim versus filgrastim after high-dose chemotherapy and autologous stem cell transplantation in patients with lymphoma and myeloma: an economic evaluation of the PALM Trial. Appl. Health Econ. Health Pol. 11 (2), 129–138. doi:10.1007/s40258-013-0011-7
Ramsey, S. D., Liu, Z., Boer, R., Sullivan, S. D., Malin, J., Doan, Q. V., et al. (2009). Cost-effectiveness of primary versus secondary prophylaxis with pegfilgrastim in women with early-stage breast cancer receiving chemotherapy. Value Health 12 (2), 217–225. doi:10.1111/j.1524-4733.2008.00434.x
Rojas, C., Raje, M., Tsukamoto, T., and Slusher, B. S. (2014). Molecular mechanisms of 5-HT(3) and NK(1) receptor antagonists in prevention of emesis. Eur. J. Pharmacol. 722, 26–37. doi:10.1016/j.ejphar.2013.08.049
Salgia, R., Mambetsariev, I., Hewelt, B., Achuthan, S., Li, H., Poroyko, V., et al. (2018). Modeling small cell lung cancer (SCLC) biology through deterministic and stochastic mathematical models. Oncotarget 9 (40), 26226–26242. doi:10.18632/oncotarget.25360
Sebban, C., Lefranc, A., Perrier, L., Moreau, P., Espinouse, D., Schmidt, A., et al. (2012). A randomised phase II study of the efficacy, safety and cost-effectiveness of pegfilgrastim and filgrastim after autologous stem cell transplant for lymphoma and myeloma (PALM study). Eur. J. Cancer 48 (5), 713–720. doi:10.1016/j.ejca.2011.12.016
Sigsgaard, T., Herrstedt, J., Handberg, J., Kjaer, M., and Dombernowsky, P. (2001). Ondansetron plus metopimazine compared with ondansetron plus metopimazine plus prednisolone as antiemetic prophylaxis in patients receiving multiple cycles of moderately emetogenic chemotherapy. J. Clin. Oncol. 19 (7), 2091–2097. doi:10.1200/jco.2001.19.7.2091
Smith, T. J., Bohlke, K., Lyman, G. H., Carson, K. R., Crawford, J., Cross, S. J., et al. (2015). Recommendations for the Use of WBC Growth Factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 33 (28), 3199–3212. doi:10.1200/jco.2015.62.3488
The society of chemotherapy CA-CA (2019). Consensus on the clinical diagnosis, treatment, and prevention of chemotherapy-induced neutropenia in China (2019 edition)[J]. CHINESE JOURNAL CLINICAL ONCOLOGY 46 (17), 876. doi:10.3969/j.issn.1000-8179.2019.17.913
Tirelli, U., Errante, D., Van Glabbeke, M., Teodorovic, I., Kluin-Nelemans, J. C., Thomas, J., et al. (1998). CHOP is the standard regimen in patients > or = 70 years of age with intermediate-grade and high-grade non-hodgkin's lymphoma: results of a randomized study of the European Organization for Research and Treatment of Cancer Lymphoma Cooperative Study Group. J. Clin. Oncol. 16 (1), 27–34. doi:10.1200/jco.1998.16.1.27
Wang, L., Baser, O., Kutikova, L., Page, J. H., and Barron, R. (2015). The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer 23 (11), 3131–3140. doi:10.1007/s00520-015-2686-9
Wang, X. J., Tang, T., Farid, M., Quek, R., Tao, M., Lim, S. T., et al. (2016). Routine Primary Prophylaxis for Febrile Neutropenia with Biosimilar Granulocyte Colony-Stimulating Factor (Nivestim) or Pegfilgrastim Is Cost Effective in Non-hodgkin Lymphoma Patients undergoing Curative-Intent R-CHOP Chemotherapy. PloS one 11 (2), e0148901. doi:10.1371/journal.pone.0148901
Weycker, D., Malin, J., Edelsberg, J., Glass, A., Gokhale, M., and Oster, G. (2008). Cost of neutropenic complications of chemotherapy. Ann. Oncol. 19 (3), 454–460. doi:10.1093/annonc/mdm525
WHO (2002). The world health report 2002: reducing risks, promoting healthy life. Geneva, Switzerland: World Health Organization.
Keywords: real-world, cost-effectiveness, PEGylated recombinant human granulocyte-stimulating factor, febrile neutropenia, prophylaxis
Citation: Wu Q, Li Q, Zhang J, Luo Z, Zhou J, Chen J and Luo Y (2021) Comparison of Primary and Secondary Prophylaxis Using PEGylated Recombinant Human Granulocyte–Stimulating Factor as a Cost-Effective Measure in Malignant Neoplasms: A Multicenter Retrospective Study. Front. Pharmacol. 12:690874. doi: 10.3389/fphar.2021.690874
Received: 07 April 2021; Accepted: 09 August 2021;
Published: 29 October 2021.
Edited by:
Domenico Criscuolo, Italian Society of Pharmaceutical Medicine, ItalyReviewed by:
Pere Gascon, University of Barcelona, SpainClaudio Cerchione, Istituto Scientifico Romagnolo per lo Studio e il Trattamento dei Tumori (IRCCS), Italy
Lan Huang, BeyondSpring Pharmaceuticals, Inc., United States
Copyright © 2021 Wu, Li, Zhang, Luo, Zhou, Chen and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Luo, luyo2021@163.com
 Qiuji Wu
Qiuji Wu Qiu Li
Qiu Li Jun Zhang2
Jun Zhang2 Jin Zhou
Jin Zhou Yong Luo
Yong Luo