- Hematology Unit, Businco Hospital, Department of Medical Sciences and Public Health, University of Cagliari, Cagliari, Italy
Background: Off-target effects in chronic myeloid leukemia (CML) patients treated with tyrosine kinase inhibitors (TKIs) are associated with cardiovascular toxicity. Hypertension represents an important cardiovascular complication and, if not appropriately managed, can contribute to developing thrombotic events. Third-generation TKI ponatinib is associated with hypertension development, and its use is more restricted than in the past. Few data are reported for second-generation TKI, nilotinib, dasatinib, and bosutinib. The aim of this article was to evaluate with a systematic review and meta-analysis the real incidence of hypertension in CML patients treated with second- or third-generation TKI.
Methods: The PubMed database, Web of Science, Scopus, and ClinicalTrials.gov were systematically searched for studies published between January 1, 2000, and January 30, 2021; the following terms were entered in the database queries: Cardiovascular, Chronic Myeloid Leukemia, CML, Tyrosine kinases inhibitor, TKI, and Hypertension. The study was carried out according to the Preferred Reporting Items for Systematic and Meta-Analyses (PRISMA) statement.
Results: A pooled analysis of hypertension incidence was 10% for all new-generation TKI, with an even higher prevalence with ponatinib (17%). The comparison with the first-generation imatinib confirmed that nilotinib was associated with a significantly increased risk of hypertension (RR 2; 95% CI; 1.39-2.88, I2=0%, z=3.73, p=0.0002). The greatest risk was found with ponatinib (RR 9.21; 95% CI; 2.86-29.66, z=3.72, p=0.0002).
Conclusion: Hypertension is a common cardiovascular complication in CML patients treated with second- or third-generation TKI.
Introduction
Chronic myeloid leukemia (CML) is a hematological disease characterized by the uncontrolled proliferation of hematopoietic stem cells due to a characteristic genetic anomaly causing the synthesis of the abnormal protein Bcl-Abl1 (Faderl et al., 1999). Tyrosine kinase inhibitors (TKIs) specifically targeting the Bcl-Abl1 protein have been developed, resulting in a dramatic change in the prognosis of the disease (Hochhaus et al., 2017). Nowadays, several molecules have emerged, together with imatinib, in the treatment of CML (Cortes et al., 2016a; Hochhaus et al., 2016a; Cortes et al., 2018a; Cortes et al., 2018b). Second- and third-generation TKI can provide faster molecular responses but are considered less safe than first-generation drugs. Although all second-generation TKIs can be used as first-line treatments, evidence-based guidelines recommend taking into account target profundity of molecular response and TKI safety profiles for the final treatment decision (Fachi et al., 2018; Haguet et al., 2020). The use of a second-generation TKI over imatinib is particularly recommended for patients with moderate- or high-risk Sokal scores. Second-generation TKIs are also recommended for younger patients because of the higher probability of treatment-free remission with these TKIs (Deininger et al., 2020; Hochhaus et al., 2020). Due to the growing number of long-surviving patients who undergo TKI treatment for many years, the problem of long-term toxicities has emerged (Steegmann et al., 2016). Cardiovascular (CV) toxicity has a potentially important impact on long-term morbidity and mortality in these patients. Particularly, nilotinib and third-generation TKI ponatinib are more frequently associated with the onset of cardiovascular events, especially thrombotic events (Aghel et al., 2017). Hypertension represents, per se, a comorbidity that can increase the CV risk of patients (Piepoli et al., 2016). Exacerbation of hypertension and an increase of new events were reported, especially with the use of ponatinib since its pivotal trials (Cortes et al., 2013; Lipton et al., 2016). Since then, many studies have highlighted its cardiotoxic profile and the possible mechanism (Valent et al., 2017). Currently, limited use of ponatinib in those patients who already have cardiovascular comorbidities has been recommended (Deininger et al., 2020). In contrast, second-generation TKIs, such as dasatinib and bosutinib, seem to be safer (Medeiros et al., 2018).
The aim of this systematic review and meta-analysis has been to evaluate the real incidence of hypertension, considering also the real-life data in CML patients treated with new-generation TKI (NGTKI).
Materials and Methods
Search Strategy
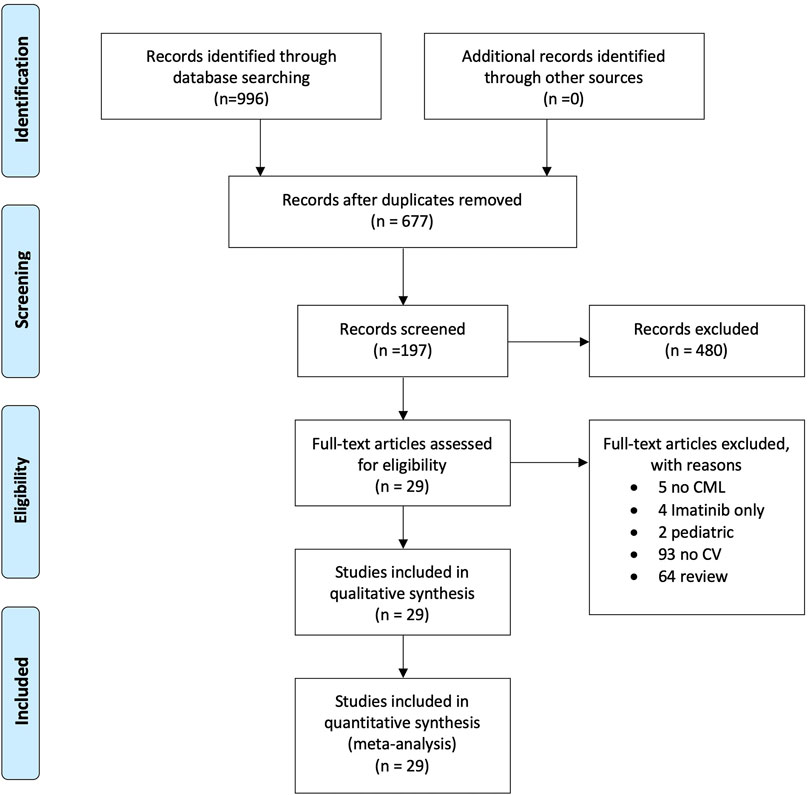
A systematic literature search on PubMed, Web of Sciences, Scopus, and ClinicalTrials.gov was performed to find studies on CML treated with second- or third-generation tyrosine kinase inhibitors and cases of hypertension published from January 1, 2000, to January 30, 2021. Using MeSH headings, we searched for the terms “Chronic Myeloid leukemia,” “CML,” “Tyrosine kinase inhibitors,” “TKI,” “Hypertension,” and “Cardiovascular,” as well as variations thereof. The results were defined using the Preferred Reporting Items for Systematic and Meta-Analyses (PRISMA) statement to identify, select, and determine the eligibility of articles for inclusion in the study. Figure 1 shows the study flow diagram. Quality rating of randomized clinical trials and observational studies was performed using the NIH Study Quality Assessment Tools (Study Quality Assessment, 2020), and the results are shown in Supplementary Table S1. The systematic search strategy is available in Supplementary Table S2.
Inclusion Criteria
Studies were included in this analysis if they were (Faderl et al., 1999) randomized controlled trials or cohort studies of adult patients of at least 18 years old treated with second- or third-generation TKIs (nilotinib, dasatinib, bosutinib, and ponatinib) for chronic, accelerated, and blastic CML phases (Hochhaus et al., 2017); studies reporting hypertension events (Cortes et al., 2016a); single cohort studies or a comparison study of second- or third-generation TKI versus imatinib (Cortes et al., 2018a); indicating the time of exposure to TKI (Hochhaus et al., 2016a); and in the English language. We included conference abstracts only if they met inclusion criteria and sufficient data were available for the prespecified analysis plan. Finally, for some clinical trials, we used the data found at clinicaltrials.gov because they were complete compared to any otherwise published version.
Statistical Analysis
Pooled incidence rates of hypertension (including both single- and double-arm studies) were calculated using a single-proportion random-effect model. The analysis was also carried out to evaluate the duration of TKI exposure. The incidence rate allows taking into account sample size and time to exposure in the estimation of the proportion of cases with the predefined outcome. The incidence rate was calculated based on person-time at exposition (Szklo and Nieto, 2007). For studies that compared the rate of hypertension events between two different TKIs, we measured risk ratio with corresponding 95% CI using the DerSimonian and Laird method for the random-effect model. To assess heterogeneity between the studies, the chi-squared test (for evaluation of heterogeneity between studies statistically; p less than 0.05) and I2 index (to evaluate the heterogeneity of the results) were used with an I2 value <25% reflecting mild heterogeneity, 25–50% reflecting moderate heterogeneity, and >50% reflecting severe heterogeneity (Higgins and Thompson, 2002). The analyses were conducted using STATA version 16.1 and Review Manager 5.4.
Results
Overall, 996 articles were found in the preliminary analysis, and after the subsequent screening, 197 studies were evaluated. Finally, 29 articles were included in the qualitative analysis, with a total sample of 5,533 patients examined. Overall, 29 studies were considered for the quantitative analysis, 28 in the pooled analysis, and 10 in the meta-analysis (Figure 1).
Quality Assessment
The analysis of the risk of bias is reported in Supplementary Table S1.
In our work, we considered both the retrospective analysis and phase 2 and 3 trials reporting clinical cases of new-onset hypertension during TKI treatment. This choice determines lack of homogeneity in the number and type of previous therapy lines and median exposure times. The number of patients considered in each study is highly variable, ranging from 5 to 1,089 patients, and sample justification is rarely given. In some cases, median exposition time was fairly short, making it difficult to see medium- and long-term adverse effects like the one we are considering. Unfortunately, many of them did not distinguish between different grades of hypertension, only reporting the rough number of cases. This is considered an important bias for the analysis, not allowing a good estimate of the severity of hypertension and its potential clinical outcome.
The majority of the studies analyzed lack the details of the randomization process, the selection of the reported outcome, and the mean dose of the drug used. Many studies allowed dose adjustment because of adverse effects or scarce disease control, making it impossible to clearly define whether dose variation can modify the risk of hypertension. Lastly, consideration of potential confounding variables in the planning of the study is not always performed. Overall, the quality of clinical trials reported is fair or good. Only five studies are considered poor the reasons being that the study population was not clearly defined, or adverse effects and responses were not stratified considering different TKI dosages.
Qualitative Analysis
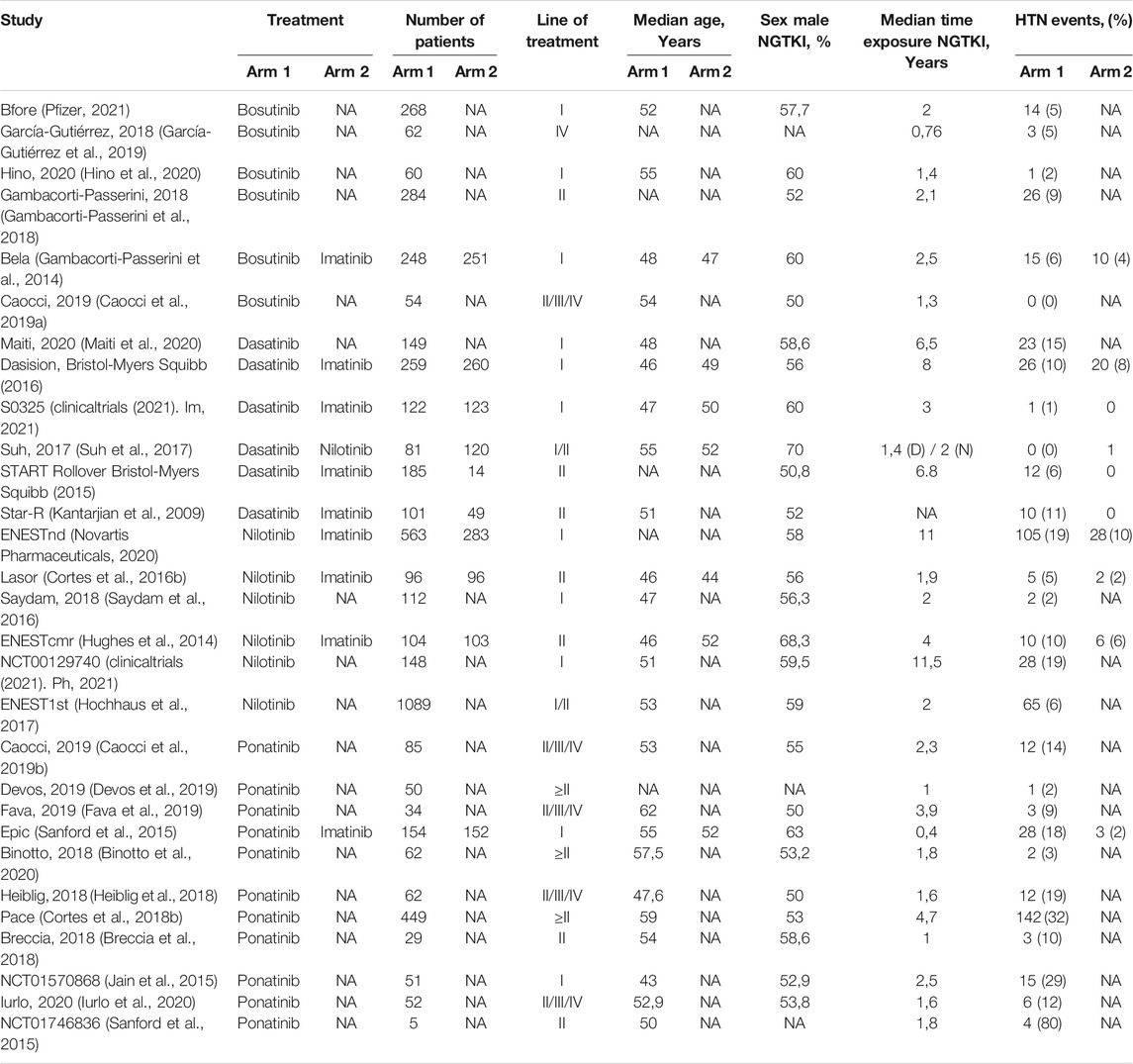
Characteristics of studies are available in Table 1. Overall, seven studies were evaluated for bosutinib. The frequency of hypertensive events varied between 2 and 9%. Among these, four were clinical trials considering patients since the second line of treatment (Gambacorti-Passerini et al., 2014; Gambacorti-Passerini et al., 2018; Hino et al., 2020; Pfizer, 2021). In contrast, only two retrospective articles have analyzed patients treated with subsequent lines (Caocci et al., 2019a; García-Gutiérrez et al., 2019). Almost all the detected studies on dasatinib were clinical trials considering patients on the first or second line of treatment (Maiti et al., 2020; Bristol-Myers Squibb, 2016; Bristol-Myers Squibb, 2015 (clinicaltrials, (2021). Im, 2021; Kantarjian et al., 2009). One article evaluated patients retrospectively collected, including the same line of treatment (Suh et al., 2017). The range of events was between 0 and 15%. Similarly, in the nilotinib setting, only one article reported the incidence of hypertension in patients retrospectively evaluated since the second line of treatment (Suh et al., 2017). The other ones were clinical trials evaluating patients in the first or second line of treatment (Hughes et al., 2014; Cortes et al., 2016b; Hochhaus et al., 2016b; Saydam et al., 2016; Novartis Pharmaceuticals, 2020; clinicaltrials (2021). Ph, 2021). The rate of hypertension was higher, between 5 and 19%. On the contrary, the identified articles on ponatinib were mostly retrospective studies (Breccia et al., 2018; Heiblig et al., 2018; Caocci et al., 2019b; Devos et al., 2019; Fava et al., 2019; Binotto et al., 2020; Iurlo et al., 2020), and as expected, they collected data on treatment lines higher than the third. In the clinical trials evaluated, ponatinib was administered as the first- or second-line treatment (Jain et al., 2015; Sanford et al., 2015; Cortes et al., 2018b; clinicaltrials (2021). Po, 2021). In this case, the frequency of hypertension was significantly increased, varying between 2 and 80%.
Quantitative Assessment
A pooled analysis of the incidence rate of hypertension was carried out considering all the studies with inclusion criteria. Only one study was not included in the analysis because it was not possible to evaluate the time of exposure to dasatinib (Kantarjian et al., 2009). No distinction between observational studies and trials was made. Considering all TKIs, the pooled proportion of hypertension was 10% (95% CI; 0.07–0.13, I2 = 93.42%). Subanalysis for each NGTKI showed a pooled rate of 17% (95% CI; 0.09–0.25, I2 = 93.24%) for ponatinib, 8% (95% CI; 0.04–0.13, I2 = 95.19%) for nilotinib, 8% (95% CI; 0.02–0.14, I2 = 92.87%) for dasatinib, and 5% (95% CI; 0.03–0.08, I2 = 60.63%) for bosutinib (Figure 2). A further analysis was made to evaluate the pooled rate of hypertension when TKIs were used in the first- or second-line treatment versus over the second-line treatment, showing 9% (95% CI; 0.06–0.12, I2 = 92.28%) and 12% (95% CI; 0.03–0.21, I2 = 94.61%), respectively (Figure 3). If the analysis was conducted considering the mean exposure time, the pooled proportion of hypertension was 3% (95% CI; 0.02–0.03, I2 = 89.74%). In the ponatinib subset, the pooled incidence was 8% (95% CI; 0.05–0.11, I2 = 86.80%). A reduction was detected in nilotinib and dasatinib studies, with 2% (95% CI; 0.01–0.02, I2 = 76.82%) and 1% (95% CI; 0.00–0.02, I2 = 81.41%), respectively. A reduction was observed also for bosutinib with 3% (95% CI; 0.02–0.04, I2 = 39.31%) (Supplementary Figure S1). The pooled proportion for lines of treatment subdivision showed a decrease with 2% (95% CI; 0.01–0.03, I2 = 87.36%) and 5% (95% CI; 0.03–0.07, I2 = 74.46%) in the first or second line versus over second line, respectively (Supplementary Figure S2).
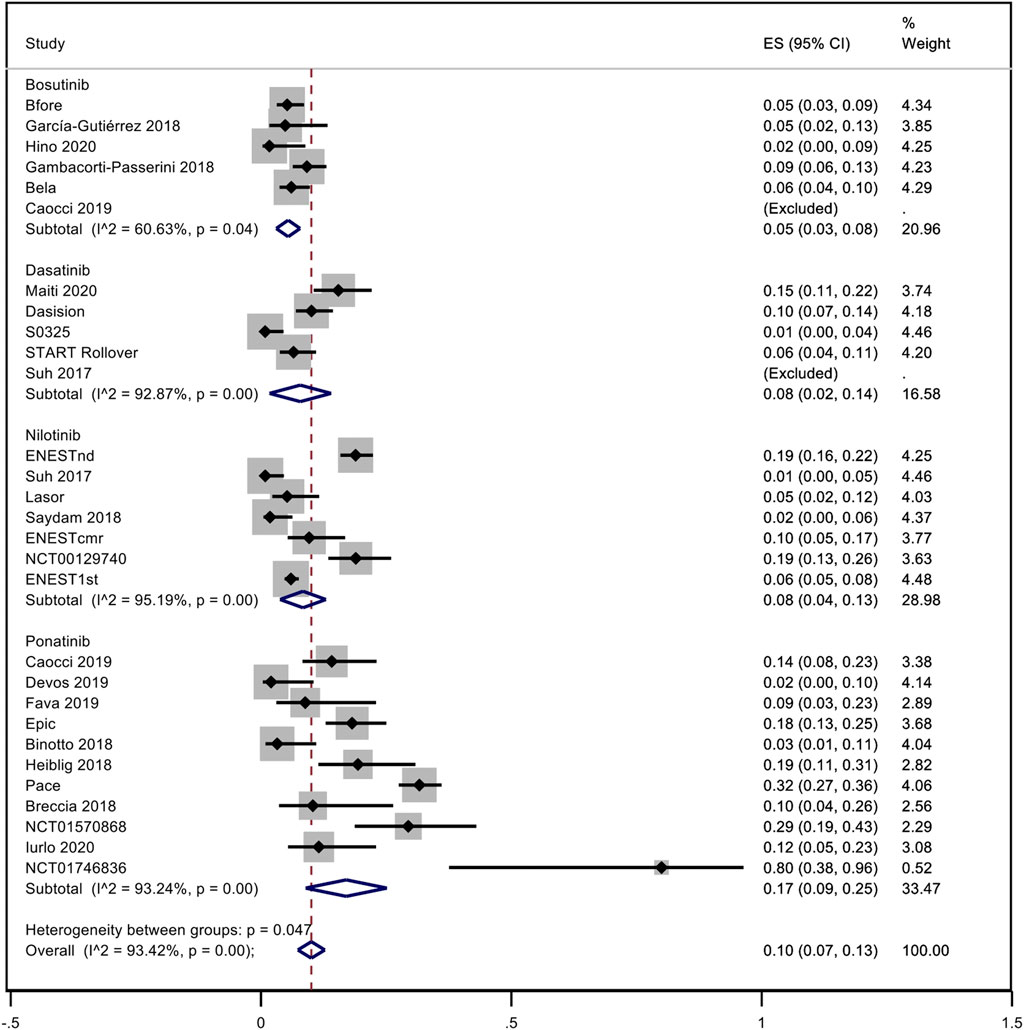
FIGURE 2. Pooled incidence rate of hypertension in patients treated with second- or third-generation TKI.
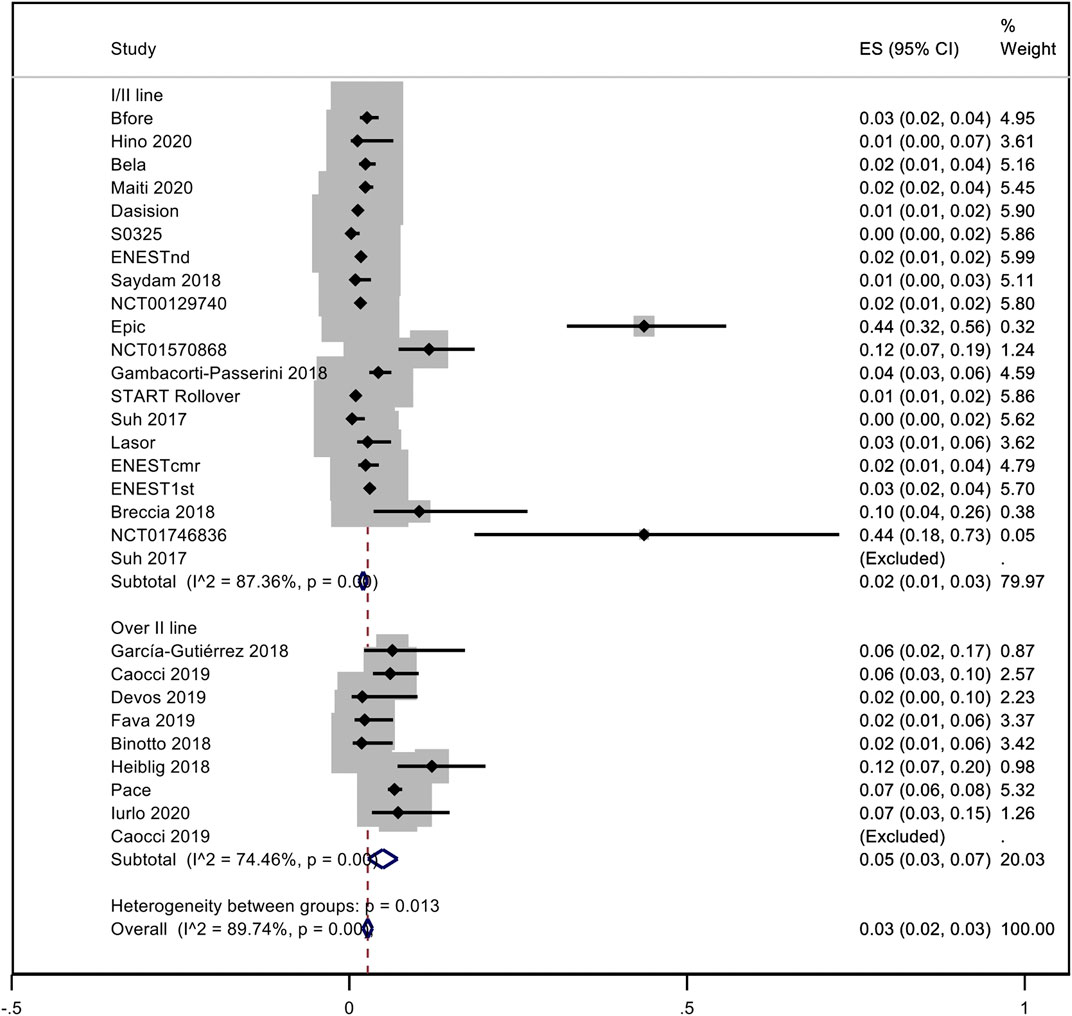
FIGURE 3. Pooled rate of hypertension when TKI were used in the first- or second-line versus over second-line.
In addition, a comparative analysis between NGTKI and imatinib was made, with the results shown in Figure 4. Overall, a significantly increased risk of hypertension was detected in NGTKI compared to imatinib, with a risk ratio (RR) of 1.84 (95% CI; 1.24–2.71, I2 = 39.93%, z = 3.05, p = 0.002). Analysis by the subgroup showed a trend of increased risk of hypertension, without significant results, for bosutinib and dasatinib with an RR of 1.11 (95% CI; 0.64–1.93, I2 = 25%, z = 0.39, p = 0.70) and 1.50 (95% CI; 0.89–2.54, I2 = 0%, z = 1.52, p = 0.13), respectively. Nilotinib is associated with an increased significant risk of hypertension with an RR of 2 (95% CI; 1.39–2.88, I2 = 0%, z = 3.73, p = 0.0002). The greater risk is found with ponatinib RR 9.21 (95% CI; 2.86–29.66, z = 3.72, p = 0.0002).
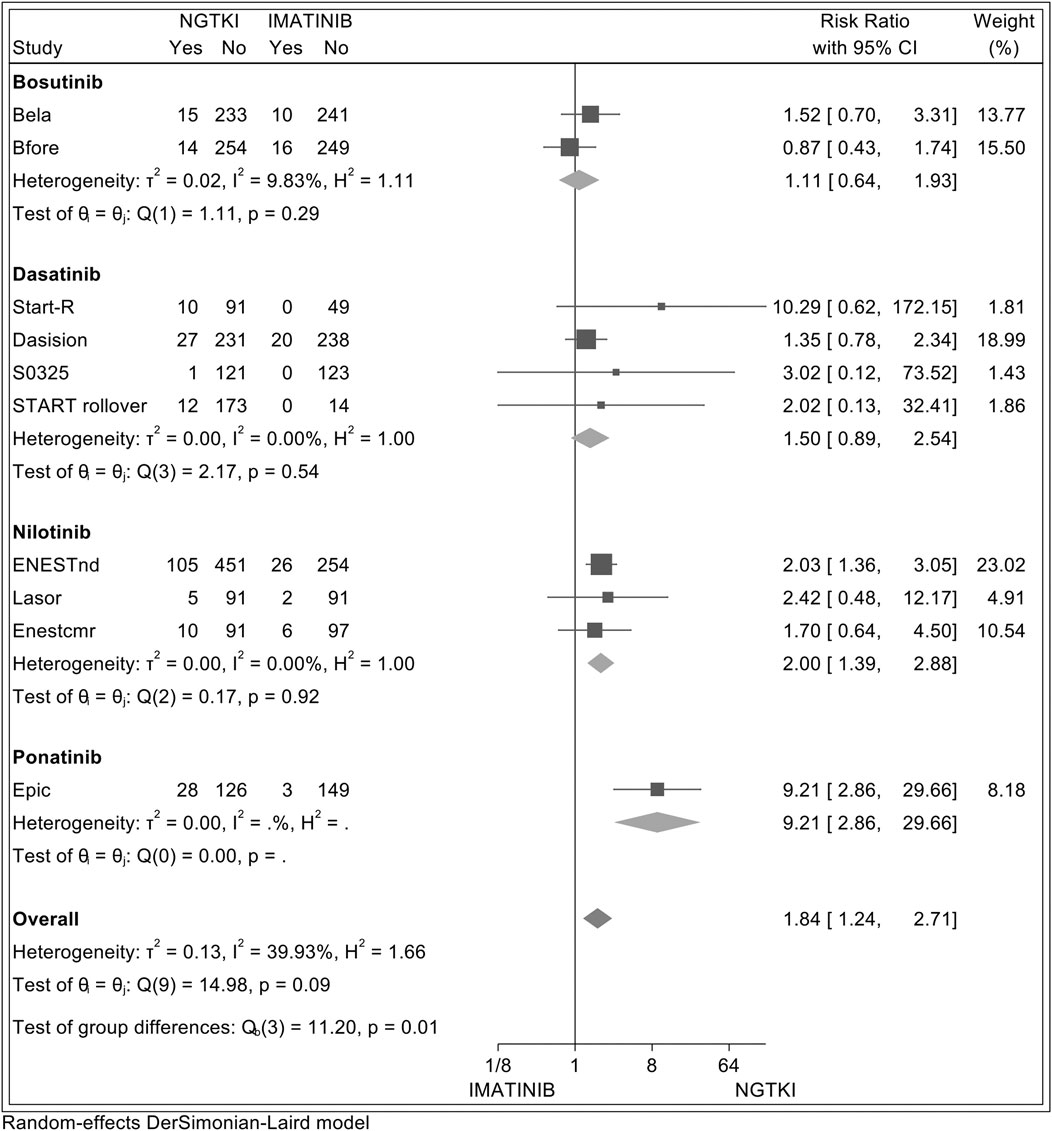
FIGURE 4. Forest plot showing random-effect meta-analysis of hypertension between imatinib and subsequent-generation TKI.
Discussion
The targeted approach with TKI has revolutionized the treatment of CML, and been able to ensure a life expectancy for patients similar to that of the general population (Bower et al., 2016). Unfortunately, off-target side effects are increasing with the use of these drugs; in particular CV toxicities are leading to significant morbidity and mortality (Damrongwatanasuk and Fradley, 2017). Nowadays, the risk of CV events is well established with nilotinib and ponatinib due to an increase in occlusive events, including myocardial infarction (MI), cerebrovascular accidents (CVAs), and peripheral arterial disease (PAOD) (Medeiros et al., 2018). Hypertension, if not appropriately managed, can be strongly associated with high incidence of CV events (Williams et al., 2018) and can represent a leading cause of CV-related mortality (Lewington et al., 2002). In CML patients, an increase in hypertensive events has been reported with ponatinib, with an incidence of 20–30% in pivotal trials (Lipton et al., 2016; Cortes et al., 2018b). The hypertensive complication is not surprising, given the significant inhibition of ponatinib on vascular endothelial growth factor 2 (VEGFR2) (Ai et al., 2018). VEGF signaling plays a key role in angiogenesis; blocking this pathway not only has antitumor effects but also leads to accelerated hypertension possibly via decreased nitric oxide bioavailability, increased endothelin-1 production, or microvascular rarefaction (Herrmann, 2020). Hypertension is a common event also with nilotinib (Herrmann, 2020). It exerts direct proatherogenic and antiangiogenic effects on vascular endothelial cells, which may contribute to the development of damage in the vascular tissue (Hadzijusufovic et al., 2017). Instead, weak data are available with other TKIs. Another important mechanism associated with hypertension development is the renin–angiotensin system (RAS), which may be found in a circulating form or as a specific tissue expression. Particularly, local bone marrow RAS plays a crucial role in proliferative events, mobilization, angiogenesis, and fibrosis. This has been associated with hypertension development and with atheromatic vascular disease. Furthermore, angiotensin II would appear to favor erythroid proliferation and stimulate differentiation of hematopoietic CD34 progenitors(56). Thus, hypertension and CML could have with RAS an interesting common ground.
Our analysis showed that the pooled hypertension rate of the second- and third-generation TKI is 10%, and it confirms the higher proportion in the ponatinib subgroup. The exposuretime correction shows a reduction in the proportion of hypertension incidence, more evident for dasatinib and nilotinib. A recent analysis of the Food and Drug Administration (FDA) adverse event reporting system database highlighted that ponatinib was the only TKI related to hypertension, with a median time to onset estimated at 53 days (Cirmi et al., 2020). Comparison with first-generation imatinib highlights the increased risk of hypertension events in patients treated with NGTKI, especially with nilotinib and ponatinib. Recently, a real-life monocentric experience showed an increased incidence of cardiovascular events in patients treated with nilotinib and dasatinib compared to the imatinib group, in particular with an increased incidence of hypertension of 7 and 4%, respectively (Novo et al., 2020). Exposure to more than two lines of treatment can be another important element of increased risk in hypertension events. This consideration finds similar results in the incidence of thrombotic events (Chai-Adisaksopha et al., 2016; Caocci et al., 2019c; Caocci et al., 2020). A pooled analysis of major arterial events showed, indeed, a higher rate in patients treated with a subsequent line of TKI compared with those treated with single-line treatment (Chai-Adisaksopha et al., 2016). These findings confirmed that more attention should be given to patients treated with multiple TKI lines. Practical recommendation emphasizes that patients, before starting NGTKI, should be assessed for increased risk of hypertension and associated comorbidities such as cardiovascular disease, diabetes, and kidney disease and for patient characteristics, including race and age. Moreover, early signs of arterial hypertension during TKI treatment should be investigated and treated early. In this context, reaching a treatment-free remission (TFR) could be, therefore, a fair compromise in those patients with high Sokal risk score but an unfavorable cardiovascular profile (Breccia et al., 2020). So far, different treatments are available in the management of hypertension (Abruzzese et al., 2014). The use of dihydropyridine calcium channel blockers and renin–angiotensin system inhibitors (RASi) would be preferable due to the strong selectivity for the vascular compartment (Hayman et al., 2012). In addition, the bone marrow RAS is finely implicated in the development of hypertension (Ciftciler and Haznedaroglu, 2020). In fact, recently, RAS inhibitors (RASi) have been associated with a reduction in CV events in CML patients treated with NGTKI (Mulas et al., 2020). Taken together, the use of RASi could play an important role in these patients.
Our study has some limitations. The principal limitation was the high level of heterogeneity among the studies that did not allow a univocal interpretation of the results. Another limitation was the bias of inclusion criteria in clinical trials, where cardiovascular events were a criterion of exclusion. In addition, data about the time of exposure and patient characteristics were missing in the retrospective studies. In the PACE study, 68% of patients developed increased blood pressure at the 48-month follow-up, with some cases of hypertensive crisis reported. We chose to consider only new diagnosis of hypertension, which was 32% at the 5-year follow-up (Cortes et al., 2018b). In retrospective studies, this distinction was not always possible.
In conclusion, NGTKIs are associated with higher incidence of hypertension. Timely recognition and treatment would allow a reduced risk of developing cardiovascular events.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors without undue reservation.
Author Contributions
OM and GC were responsible for conception and design. OM, GC, BM, and GN collected and assembled the data. OM conducted the statistical analysis. OM and GC wrote the manuscript.
Funding
This article was carried out within the framework of the research project financed by P.O.R. SARDEGNA F.S.E. 2014–2020—Asse III “Istruzione e Formazione, Obiettivo Tematico: 10, Obiettivo Specifico: 10.5, Azione dell’accordo fi Partenariato: 10.5.12” “Avviso di chiamata per il finanziamento di Progetti di ricerca—Anno 2017.”
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.674748/full#supplementary-material
References
Abruzzese, E., Breccia, M., and Latagliata, R. (2014). Second-generation Tyrosine Kinase Inhibitors in First-Line Treatment of Chronic Myeloid Leukaemia (CML). BioDrugs 28 (1), 17–26. doi:10.1007/s40259-013-0056-z
Aghel, N., Delgado, D. H., and Lipton, J. H. (2017). Cardiovascular Toxicities of BCR-ABL Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia: Preventive Strategies and Cardiovascular Surveillance. Vhrm Vol. 13, 293–303. doi:10.2147/vhrm.s108874
Ai, N., Chong, C.-M., Chen, W., Hu, Z., Su, H., Chen, G., et al. (2018). Ponatinib Exerts Anti-angiogenic Effects in the Zebrafish and Human Umbilical Vein Endothelial Cells via Blocking VEGFR Signaling Pathway. Oncotarget 9 (62), 31958–31970. doi:10.18632/oncotarget.24110
Binotto, Gianni., Castagnetti, Fausto., Gugliotta, Gabriele., Abruzzese, Elisabetta., Iurlo, Alessandra., Stagno, Fabio., et al. (2020). Ponatinib 15mg Daily, Combining Efficacy and Tolerability. A Retrospective Survey in Italy. [Internet]. [cited 2021 Feb 16]. Available from: https://library.ehaweb.org/eha/2018/stockholm/215436/gianni.binotto.ponatinib.15.mg.daily.combining.efficacy.and.tolerability.a.html.
Bower, H., Björkholm, M., Dickman, P. W., Höglund, M., Lambert, P. C., and Andersson, T. M.-L. (2016). Life Expectancy of Patients with Chronic Myeloid Leukemia Approaches the Life Expectancy of the General Population. Jco 34 (24), 2851–2857. doi:10.1200/jco.2015.66.2866
Breccia, M., Abruzzese, E., Castagnetti, F., Bonifacio, M., Gangemi, D., Sorà, F., et al. (2018). Ponatinib as Second-Line Treatment in Chronic Phase Chronic Myeloid Leukemia Patients in Real-Life Practice. Ann. Hematol. 97 (9), 1577–1580. doi:10.1007/s00277-018-3337-2
Breccia, M., Efficace, F., Colafigli, G., Scalzulli, E., Di Prima, A., Martelli, M., et al. (2020). Tyrosine Kinase Inhibitor Discontinuation in the Management of Chronic Myeloid Leukemia: a Critical Review of the Current Practice. Expert Rev. Hematol. 13 (12), 1311–1318. doi:10.1080/17474086.2021.1852924
Bristol-Myers Squibb (2016). An Open-Label, Randomized, Multicenter Phase III Trial of Dasatinib (SPRYCEL®) vs. Standard Dose Imatinib (400 Mg) in the Treatment of Subjects with Newly Diagnosed Chronic Phase Philadelphia Chromosome Positive Chronic Myeloid Leukemia [Internet]. clinicaltrials.Gov. [cited 2021 Feb 15]. Report No.: NCT00481247. Available from: https://clinicaltrials.gov/ct2/show/NCT00481247.
Bristol-Myers Squibb (2015). Dasatinib in Chronic Myelogenous Leukemia or Philadelphia Chromosome Positive Acute Lymphoblastic Leukemic Subjects Who Are Experiencing Clinical Benefit on Current START or CA180-039 Protocols: Long Term Safety and Efficacy Analysis [Internet]. clinicaltrials.Gov. [cited 2021 Feb 15]. Report No.: results/NCT00982488. Available from: https://clinicaltrials.gov/ct2/show/results/NCT00982488.
Caocci, G., Mulas, O., Abruzzese, E., Iurlo, A., Annunziata, M., Orlandi, E. M., et al. (2019). Incidence and Evaluation of Predisposition to Cardiovascular Toxicity in Chronic Myeloid Leukemia Patients Treated with Bosutinib in the Real-Life Practice. Ann. Hematol. 98 (8), 1885–1890. doi:10.1007/s00277-019-03705-y
Caocci, G., Mulas, O., Abruzzese, E., Luciano, L., Iurlo, A., Attolico, I., et al. (2019). Arterial Occlusive Events in Chronic Myeloid Leukemia Patients Treated with Ponatinib in the Real‐life Practice Are Predicted by the Systematic Coronary Risk Evaluation (SCORE) Chart. Hematological Oncol. 37 (3), 296–302. doi:10.1002/hon.2606
Caocci, G., Mulas, O., Annunziata, M., Luciano, L., Abruzzese, E., Bonifacio, M., et al. (2020). Long-term Mortality Rate for Cardiovascular Disease in 656 Chronic Myeloid Leukaemia Patients Treated with Second- and Third-Generation Tyrosine Kinase Inhibitors. Int. J. Cardiol. 301, 163–166. doi:10.1016/j.ijcard.2019.10.036
Caocci, G., Mulas, O., Bonifacio, M., Abruzzese, E., Galimberti, S., Orlandi, E. M., et al. (2019). Recurrent Arterial Occlusive Events in Patients with Chronic Myeloid Leukemia Treated with Second- and Third-Generation Tyrosine Kinase Inhibitors and Role of Secondary Prevention. Int. J. Cardiol. 288, 124–127. doi:10.1016/j.ijcard.2019.04.051
Chai-Adisaksopha, C., Lam, W., and Hillis, C. (2016). Major Arterial Events in Patients with Chronic Myeloid Leukemia Treated with Tyrosine Kinase Inhibitors: a Meta-Analysis. Leuk. Lymphoma 57 (6), 1300–1310. doi:10.3109/10428194.2015.1091929
Ciftciler, R., and Haznedaroglu, I. C. (2020). Pathobiological Interactions of Local Bone Marrow Renin-Angiotensin System and Central Nervous System in Systemic Arterial Hypertension. Front. Endocrinol. (Lausanne). 11, 425. doi:10.3389/fendo.2020.00425
Cirmi, S., El Abd, A., Letinier, L., Navarra, M., and Salvo, F. (2020). Cardiovascular Toxicity of Tyrosine Kinase Inhibitors Used in Chronic Myeloid Leukemia: An Analysis of the FDA Adverse Event Reporting System Database (FAERS). Cancers 12 (4). doi:10.3390/cancers12040826
clinicaltrials (2021). Imatinib Mesylate or Dasatinib in Treating Patients with Previously Untreated Chronic Phase Chronic Myelogenous Leukemia - Study Results. - ClinicalTrials.gov [Internet]. [cited 2021 Feb 18]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT00070499.
clinicaltrials (2021). Phase II Nilotinib with Newly Diagnosed Chronic Phase Chronic Myelogenous Leukemia. (CML) - Study Results - ClinicalTrials.gov [Internet]. [cited 2021 Feb 18]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT00129740.
clinicaltrials (2021). Ponatinib in Newly Diagnosed Chronic Myeloid Leukemia. (CML) (EPIC) - Study Results - ClinicalTrials.gov [Internet]. [cited 2021 Feb 18]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT01650805.
Cortes, J. E., De Souza, C. A., Ayala, M., Lopez, J. L., Bullorsky, E., Shah, S., et al. (2016). Switching to Nilotinib versus Imatinib Dose Escalation in Patients with Chronic Myeloid Leukaemia in Chronic Phase with Suboptimal Response to Imatinib (LASOR): a Randomised, Open-Label Trial. Lancet Haematol. 3 (12), e581–e591. doi:10.1016/s2352-3026(16)30167-3
Cortes, J. E., Gambacorti-Passerini, C., Deininger, M. W., Mauro, M. J., Chuah, C., Kim, D.-W., et al. (2018). Bosutinib versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results from the Randomized BFORE Trial. Jco 36 (3), 231–237. doi:10.1200/jco.2017.74.7162
Cortes, J. E., Kim, D.-W., Pinilla-Ibarz, J., le Coutre, P. D., Paquette, R., Chuah, C., et al. (2018). Ponatinib Efficacy and Safety in Philadelphia Chromosome-Positive Leukemia: Final 5-year Results of the Phase 2 PACE Trial. Blood 132 (4), 393–404. doi:10.1182/blood-2016-09-739086
Cortes, J. E., Kim, D.-W., Pinilla-Ibarz, J., le Coutre, P., Paquette, R., Chuah, C., et al. (2013). A Phase 2 Trial of Ponatinib in Philadelphia Chromosome-Positive Leukemias. N. Engl. J. Med. 369 (19), 1783–1796. doi:10.1056/nejmoa1306494
Cortes, J. E., Saglio, G., Kantarjian, H. M., Baccarani, M., Mayer, J., Boqué, C., et al. (2016). Final 5-Year Study Results of DASISION: The Dasatinib versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. Jco 34 (20), 2333–2340. doi:10.1200/jco.2015.64.8899
Damrongwatanasuk, R., and Fradley, M. G. (2017). Cardiovascular Complications of Targeted Therapies for Chronic Myeloid Leukemia. Curr. Treat. Options. Cardiovasc. Med. 19 (4). doi:10.1007/s11936-017-0524-8
Deininger, M. W., Shah, N. P., Altman, J. K., Berman, E., Bhatia, R., Bhatnagar, B., et al. (2020). Chronic Myeloid Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. JNCCN J. Natl. Compr. Cancer Netw. 18 (10), 1385–1415. doi:10.6004/jnccn.2020.0047
Devos, T., Havelange, V., Theunissen, K., Meers, S., Benghiat, F. S., Gadisseur, A., et al. (2019). Real-Life Outcomes of Ponatinib Treatment in Patients with Chronic Myeloid Leukemia (CML) and Philadelphia Chromosome-Positive Acute Lymphoblastic Leukemia (Ph+ ALL): Data from a Nationwide Belgian Registry. Blood 134 (Suppl. ment_1), 4161. doi:10.1182/blood-2019-127923
Fachi, M. M., Tonin, F. S., Leonart, L. P., Aguiar, K. S., Lenzi, L., Figueiredo, B. C., et al. (2018). Comparative Efficacy and Safety of Tyrosine Kinase Inhibitors for Chronic Myeloid Leukaemia: A Systematic Review and Network Meta-Analysis. Eur. J. Cancer 104, 9–20. doi:10.1016/j.ejca.2018.08.016
Faderl, S., Talpaz, M., Estrov, Z., O'Brien, S., Kurzrock, R., and Kantarjian, H. M. (1999). The Biology of Chronic Myeloid Leukemia. N. Engl. J. Med. 341 (3), 164–172. doi:10.1056/nejm199907153410306
Fava, C., Dragani, M. E., Boggione, P., Paoloni, F., Luciano, L., Gozzini, A., et al. (2019). Preliminary Results of CML1214, a Survey on Ponatinib Compassionate Use in Italy by the Gimema CML Working Party. Blood 134 (Suppl. ment_1), 2931. doi:10.1182/blood-2019-125052
Gambacorti-Passerini, C., Cortes, J. E., Lipton, J. H., Dmoszynska, A., Wong, R. S., Rossiev, V., et al. (2014). Safety of Bosutinib versus Imatinib in the Phase 3 BELA Trial in Newly Diagnosed Chronic Phase Chronic Myeloid Leukemia. Am. J. Hematol. 89 (10), 947–953. doi:10.1002/ajh.23788
Gambacorti-Passerini, C., Cortes, J. E., Lipton, J. H., Kantarjian, H. M., Kim, D.-W., Schafhausen, P., et al. (2018). Safety and Efficacy of Second-Line Bosutinib for Chronic Phase Chronic Myeloid Leukemia over a Five-Year Period: Final Results of a Phase I/II Study. Haematologica 103 (8), 1298–1307. doi:10.3324/haematol.2017.171249
García-Gutiérrez, V., Milojkovic, D., Milojkovic, D., Hernandez-Boluda, J. C., Claudiani, S., Martin Mateos, M. L., et al. (2019). Safety and Efficacy of Bosutinib in Fourth-Line Therapy of Chronic Myeloid Leukemia Patients. Ann. Hematol. 98 (2), 321–330. doi:10.1007/s00277-018-3507-2
Hadzijusufovic, E., Albrecht-Schgoer, K., Huber, K., Hoermann, G., Grebien, F., Eisenwort, G., et al. (2017). Nilotinib-induced Vasculopathy: Identification of Vascular Endothelial Cells as a Primary Target Site. Leukemia 31 (11), 2388–2397. doi:10.1038/leu.2017.245
Haguet, H., Graux, C., Mullier, F., Dogné, J-M., and Douxfils, J. (2020). Long-term Survival, Vascular Occlusive Events and Efficacy Biomarkers of First-Line Treatment of CML: A Meta-Analysis. Cancers 12 (5). doi:10.3390/cancers12051242
Hayman, S. R., Leung, N., Grande, J. P., and Garovic, V. D. (2012). VEGF Inhibition, Hypertension, and Renal Toxicity. Curr. Oncol. Rep. 14 (4), 285–294. doi:10.1007/s11912-012-0242-z
Heiblig, M., Rea, D., Chrétien, M.-L., Charbonnier, A., Rousselot, P., Coiteux, V., et al. (2018). Ponatinib Evaluation and Safety in Real-Life Chronic Myelogenous Leukemia Patients Failing More Than Two Tyrosine Kinase Inhibitors: the PEARL Observational Study. Exp. Hematol. 67, 41–48. doi:10.1016/j.exphem.2018.08.006
Herrmann, J. (2020). Vascular Toxic Effects of Cancer Therapies. Nat. Rev. Cardiol. 17 (8), 503–522. doi:10.1038/s41569-020-0347-2
Higgins, J. P. T., and Thompson, S. G. (2002). Quantifying Heterogeneity in a Meta-Analysis. Statist. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Hino, M., Matsumura, I., Fujisawa, S., Ishizawa, K., Ono, T., Sakaida, E., et al. (2020). Phase 2 Study of Bosutinib in Japanese Patients with Newly Diagnosed Chronic Phase Chronic Myeloid Leukemia. Int. J. Hematol. 112 (1), 24–32. doi:10.1007/s12185-020-02878-x
Hochhaus, A., Baccarani, M., Silver, R. T., Schiffer, C., Apperley, J. F., Cervantes, F., et al. (2020). European LeukemiaNet 2020 Recommendations for Treating Chronic Myeloid Leukemia. Leukemia 34 (4), 966–984. doi:10.1038/s41375-020-0776-2
Hochhaus, A., Larson, R. A., Guilhot, F., Radich, J. P., Branford, S., Hughes, T. P., et al. (2017). Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N. Engl. J. Med. 376, 917–927. doi:10.1056/NEJMoa1609324
Hochhaus, A., Rosti, G., Cross, N. C. P., Steegmann, J. L., le Coutre, P., Ossenkoppele, G., et al. (2016). Frontline Nilotinib in Patients with Chronic Myeloid Leukemia in Chronic Phase: Results from the European ENEST1st Study. Leukemia 30 (1), 57–64. doi:10.1038/leu.2015.270
Hochhaus, A., Saglio, G., Hughes, T. P., Larson, R. A., Kim, D.-W., Issaragrisil, S., et al. (2016). Long-term Benefits and Risks of Frontline Nilotinib vs Imatinib for Chronic Myeloid Leukemia in Chronic Phase: 5-year Update of the Randomized ENESTnd Trial. Leukemia 30 (5), 1044–1054. doi:10.1038/leu.2016.5
Hughes, T. P., Lipton, J. H., Spector, N., Cervantes, F., Pasquini, R., Clementino, N. C. D., et al. (2014). Deep Molecular Responses Achieved in Patients with CML-CP Who Are Switched to Nilotinib after Long-Term Imatinib. Blood 124 (5), 729–736. doi:10.1182/blood-2013-12-544015
Iurlo, A., Cattaneo, D., Malato, A., Accurso, V., Annunziata, M., Gozzini, A., et al. (2020). Low‐dose Ponatinib Is a Good Option in Chronic Myeloid Leukemia Patients Intolerant to Previous TKIs. Am. J. Hematol. 95 (10), E260–E263. doi:10.1002/ajh.25908
Jain, P., Kantarjian, H., Jabbour, E., Gonzalez, G. N., Borthakur, G., Pemmaraju, N., et al. (2015). Ponatinib as First-Line Treatment for Patients with Chronic Myeloid Leukaemia in Chronic Phase: A Phase 2 Study. Lancet Haematol. 2 (9), e376–e383. doi:10.1016/s2352-3026(15)00127-1
Kantarjian, H., Pasquini, R., Lévy, V., Jootar, S., Holowiecki, J., Hamerschlak, N., et al. (2009). Dasatinib or High-Dose Imatinib for Chronic-phase Chronic Myeloid Leukemia Resistant to Imatinib at a Dose of 400 to 600 Milligrams Daily. Cancer 115 (18), 4136–4147. doi:10.1002/cncr.24504
Lewington, S., Clarke, R., Qizilbash, N., Peto, R., and Collins, R. (2002). Age-specific Relevance of Usual Blood Pressure to Vascular Mortality: a Meta-Analysis of Individual Data for One Million Adults in 61 Prospective Studies. Lancet 360 (9349), 1903–1913. doi:10.1016/s0140-6736(02)11911-8
Lipton, J. H., Chuah, C., Guerci-Bresler, A., Rosti, G., Simpson, D., Assouline, S., et al. (2016). Ponatinib versus Imatinib for Newly Diagnosed Chronic Myeloid Leukaemia: an International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 17 (5), 612–621. doi:10.1016/s1470-2045(16)00080-2
Maiti, A., Cortes, J. E., Patel, K. P., Masarova, L., Borthakur, G., Ravandi, F., et al. (2020). Long‐term Results of Frontline Dasatinib in Chronic Myeloid Leukemia. Cancer 126 (7), 1502–1511. doi:10.1002/cncr.32627
Medeiros, B. C., Possick, J., and Fradley, M. (2018). Cardiovascular, Pulmonary, and Metabolic Toxicities Complicating Tyrosine Kinase Inhibitor Therapy in Chronic Myeloid Leukemia: Strategies for Monitoring, Detecting, and Managing. Blood Rev. 32 (4), 289–299. doi:10.1016/j.blre.2018.01.004
Mulas, O., Caocci, G., Stagno, F., Bonifacio, M., Annunziata, M., Luciano, L., et al. (2020). Renin Angiotensin System Inhibitors Reduce the Incidence of Arterial Thrombotic Events in Patients with Hypertension and Chronic Myeloid Leukemia Treated with Second- or Third-Generation Tyrosine Kinase Inhibitors. Ann. Hematol. 99 (7), 1525–1530. doi:10.1007/s00277-020-04102-6
Novartis Pharmaceuticals (2020). A Phase III Multi-center, Open-Label, Randomized Study of Imatinib versus Nilotinib in Adult Patients with Newly Diagnosed Philadelphia Chromosome Positive (Ph+) Chronic Myelogenous Leukemia in Chronic Phase. (CML-CP) [Internet]. clinicaltrials.gov[cited 2021 Feb 15]. Report No.: NCT00471497. Available from: https://clinicaltrials.gov/ct2/show/NCT00471497.
Novo, G., Di Lisi, D., Bronte, E., Macaione, F., Accurso, V., Badalamenti, G., et al. (2020). Cardiovascular Toxicity in Cancer Patients Treated with Tyrosine Kinase Inhibitors: A Real-World Single-Center Experience. Oncology 98 (7), 445–451. doi:10.1159/000505486
Pfizer, A. (2021). MULTICENTER PHASE 3 RANDOMIZED, OPEN-LABEL STUDY of BOSUTINIB versus IMATINIB IN ADULT PATIENTS with NEWLY DIAGNOSED CHRONIC PHASE CHRONIC MYELOGENOUS LEUKEMIA [Internet]. clinicaltrials.Gov. [cited 2021 Feb 15]. Report No.: results/NCT02130557. Available from: https://clinicaltrials.gov/ct2/show/results/NCT02130557.
Piepoli, M. F., Hoes, A. W., Agewall, S., Albus, C., Brotons, C., Catapano, A. L., et al. (2016). 2016 European Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 37 (29), 2315–2381. doi:10.1093/eurheartj/ehw106
Sanford, D., Kantarjian, H., Skinner, J., Jabbour, E., and Cortes, J. (2015). Phase II Trial of Ponatinib in Patients with Chronic Myeloid Leukemia Resistant to One Previous Tyrosine Kinase Inhibitor. Haematologica 100 (12), e494–e495. doi:10.3324/haematol.2015.132845
Saydam, G., Haznedaroglu, I. C., Kaynar, L., Yavuz, A. S., Ali, R., Guvenc, B., et al. (2016). Outcomes with Frontline Nilotinib Treatment in Turkish Patients with Newly Diagnosed Philadelphia Chromosome-Positive Chronic Myeloid Leukemia in Chronic Phase. Expert Opin. Pharmacother. 17 (14), 1851–1858. doi:10.1080/14656566.2016.1219338
Steegmann, J. L., Baccarani, M., Breccia, M., Casado, L. F., García-Gutiérrez, V., Hochhaus, A., et al. (2016). European LeukemiaNet Recommendations for the Management and Avoidance of Adverse Events of Treatment in Chronic Myeloid Leukaemia. Leukemia 30 (8), 1648–1671. doi:10.1038/leu.2016.104
Study Quality Assessment Tools | NHLBI, NIH (2020). Study Quality Assessment Tools | NHLBI, NIH. [Internet]. [cited 2020 Sep 30]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
Suh, K. J., Lee, J. Y., Shin, D.-Y., Koh, Y., Bang, S.-M., Yoon, S.-S., et al. (2017). Analysis of Adverse Events Associated with Dasatinib and Nilotinib Treatments in Chronic-phase Chronic Myeloid Leukemia Patients outside Clinical Trials. Int. J. Hematol. 106 (2), 229–239. doi:10.1007/s12185-017-2225-1
Szklo, M., and Nieto, F. J. (2007). Epidemiology: Beyond the Basics. 2nd Edn. Boston: Jones and Bartlett Publishers, 154–187.
Valent, P., Hadzijusufovic, E., Hoermann, G., Füreder, W., Schernthaner, G.-H., Sperr, W. R., et al. (2017). Risk Factors and Mechanisms Contributing to TKI-Induced Vascular Events in Patients with CML. Leuk. Res. 59, 47–54. doi:10.1016/j.leukres.2017.05.008
Williams, B., Mancia, G., Spiering, W., Rosei, E. A., Azizi, M., Burnier, M., et al. (2018). [2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH)]. G Ital. Cardiol. (Rome) 19 (33), 3S–73S. doi:10.1714/3026.30245
Keywords: chronic myeloid, leukemia, tyrosine kinase inhibitor, hypertension, cardiovascular
Citation: Mulas O, Caocci G, Mola B and La Nasa G (2021) Arterial Hypertension and Tyrosine Kinase Inhibitors in Chronic Myeloid Leukemia: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12:674748. doi: 10.3389/fphar.2021.674748
Received: 12 March 2021; Accepted: 03 August 2021;
Published: 22 September 2021.
Edited by:
Husain Yar Khan, Wayne State University, United StatesReviewed by:
Santa Cirmi, University of Bari Aldo Moro, ItalyIbrahim C. Haznedaroglu, Hacettepe University Hospital, Turkey
Copyright © 2021 Mulas, Caocci, Mola and La Nasa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Caocci, giovanni.caocci@unica.it
 Olga Mulas
Olga Mulas Giovanni Caocci
Giovanni Caocci Brunella Mola
Brunella Mola Giorgio La Nasa
Giorgio La Nasa