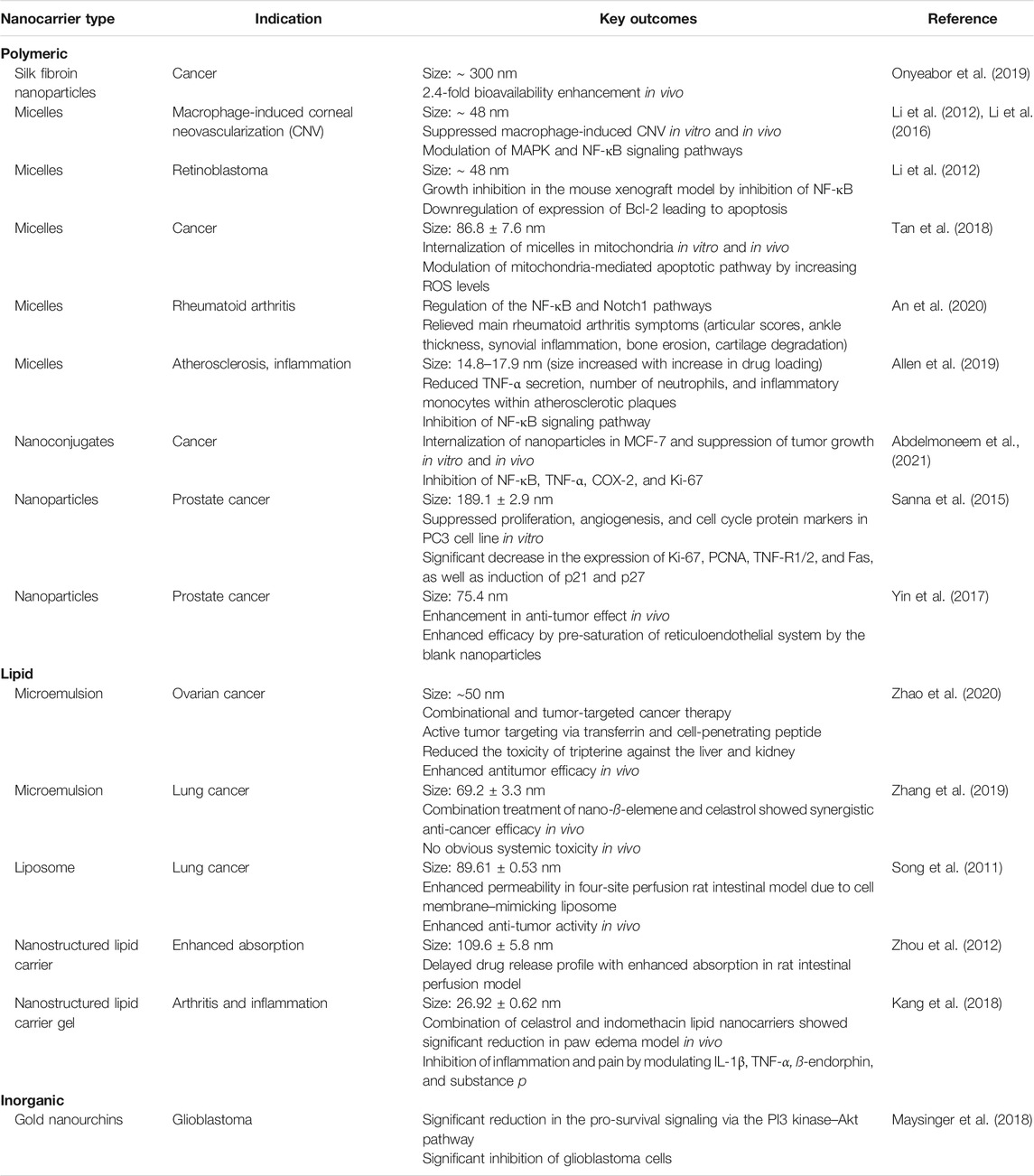
- Department of Pharmaceutical Sciences, College of Pharmacy, Western University of Health Sciences, Pomona, CA, United States
Celastrol (also called tripterine) is a quinone methide triterpene isolated from the root extract of Tripterygium wilfordii (thunder god vine in traditional Chinese medicine). Over the past two decades, celastrol has gained wide attention as a potent anti-inflammatory, anti-autoimmune, anti-cancer, anti-oxidant, and neuroprotective agent. However, its clinical translation is very challenging due to its lower aqueous solubility, poor oral bioavailability, and high organ toxicity. To deal with these issues, various formulation strategies have been investigated to augment the overall celastrol efficacy in vivo by attempting to increase the bioavailability and/or reduce the toxicity. Among these, nanotechnology-based celastrol formulations are most widely explored by pharmaceutical scientists worldwide. Based on the survey of literature over the past 15 years, this mini-review is aimed at summarizing a multitude of celastrol nanoformulations that have been developed and tested for various therapeutic applications. In addition, the review highlights the unmet need in the clinical translation of celastrol nanoformulations and the path forward.
Introduction
Clinical translation of bioactive compounds extracted from medicinal plants has gained substantial interest over the past several years due to their superior pharmacological activities especially as anti-inflammatory, anti-tumor, and neuroprotective agents. One such widely investigated medicinal plant is Tripterygium wilfordii, a perennial vine of the Celastraceae family, commonly known as “thunder god vine” or “lei gong teng.” It is used traditionally in China to treat autoimmune disorders such as rheumatoid arthritis, Crohn’s disease, and type 1 diabetes (Cascão et al., 2017). The plant is rich in phytochemicals that comprise triterpenoids and alkaloids, which are mainly extracted from the root pulp of the plant. Among these phytochemicals, the most abundant and promising bioactive compound is celastrol.
Celastrol, also known as tripterine, is a quinone methide triterpene (Figure 1). It has gained importance over the past two decades due to its potent anti-inflammatory (Pinna et al., 2004; Shaker et al., 2014; Ma et al., 2015), anti-cancer [gastric and ovarian cancers (Xu et al., 2019; Chen et al., 2020), cervical cancer (Zhou et al., 2017), and hepatocellular carcinoma (Wang et al., 2019; Chen et al., 2020; Du et al., 2020)], neuroprotective (Paris et al., 2010; Li et al., 2012; Jiang et al., 2018), and anti-oxidant (Cleren et al., 2005) activities. However, albeit potent, its clinical translation is impeded due to two main disadvantages that are poor water solubility of 0.044 mg/ml at 25°C (BCS class IV drug) (Yang et al., 2019), which limits its bioavailability, and high systemic toxicity resulting from its narrow therapeutic index (Zhang et al., 2014; Shi et al., 2020).
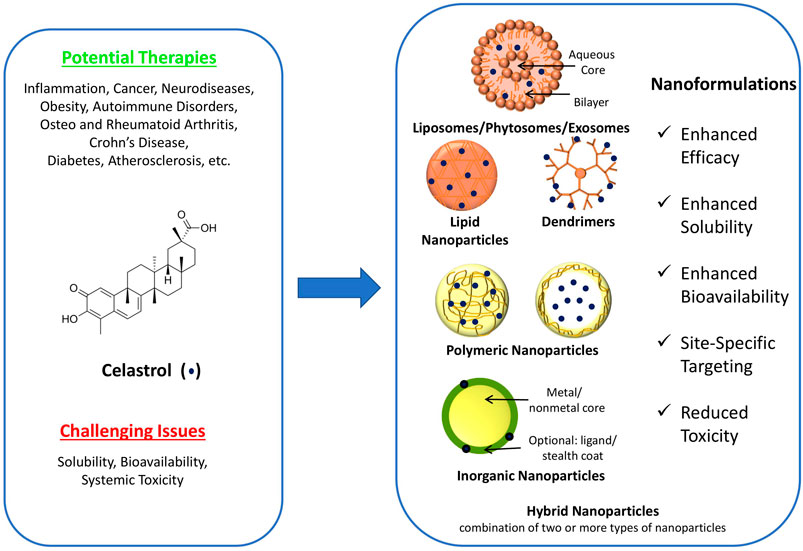
FIGURE 1. Structure of celastrol, potential therapies, challenging issues, and schematic representation of various celastrol nanoformulations [modified from Desai et al. (2012)].
The reported therapeutic dose of celastrol against various mouse models is in the range of 3–5 mg/kg (Yang et al., 2006). At these doses, though effective, systemic toxicities including cardiotoxicity (Liu et al., 2019), hepatotoxicity (Jin et al., 2019), and nephrotoxicity (Wu et al., 2018) have been reported, whereas lower doses, though safe, show limited efficacy. To overcome the toxicity issues while achieving the desired therapeutic efficacy, various drug delivery approaches have been investigated that include combination with other chemotherapeutic agents such as afatinib, axitinib, and gefitinib (Zhang et al., 2014; Choi et al., 2016; Gao et al., 2019; Lee et al., 2019; Zhao et al., 2019; Dai et al., 2020), combination with traditional Chinese medicines such as betulinic and ellagic acids (An et al., 2015; Duan et al., 2019), overcoming multidrug resistance (Metselaar et al., 2019), nanoparticulate drug delivery systems (Qi et al., 2014; Hakala et al., 2020; Li et al., 2020), and combination with nucleic acid (Huang et al., 2017). Among these, nanoparticulate drug delivery systems/nanoformulations of celastrol have been widely reported as a promising strategy to effectively deliver drug at the target site rendering enhanced efficacy and safety.
Furthermore, celastrol is classified as a BCS class IV molecule (exhibiting low solubility and permeability), and therefore, solubility and permeability enhancement strategies are the most effective in improving the bioavailability of the drug. In this context, nanoformulations, owing to its smaller size and targeting potential, offer advantages of enhanced solubility (high surface-to-volume ratio) and permeability, both of which are advantageous parameters in enhancing the bioavailability of celastrol. Thus, in view of a multitude of advantages offered by the nanotechnology, celastrol nanoformulations have been widely explored and reported in the literature. Specifically, celastrol nanoformulations have shown significant benefits in several therapeutic applications against prostate cancer, breast and pancreatic cancers, non-small-cell lung cancer, ovarian cancer, and human colon cancer and other applications in treating rheumatoid arthritis, polycystic kidney disease, inflammation, and Parkinson’s disease (schematically depicted in Figure 1) (Abbas et al., 2007; Salminen et al., 2010; Yadav et al., 2010; Mou et al., 2011; Kim et al., 2013; Chang et al., 2018; Qi et al., 2018; Zha et al., 2018; Lin et al., 2019; Song et al., 2019; Wang et al., 2019; Yan et al., 2020). This review summarizes such state-of-the-art therapeutic applications of celastrol nanoformulations in the subsequent sections. For this, peer-reviewed publications over the past 15 years in the area of celastrol nanoformulations were searched, categorized based on the therapeutic application, and summarized to develop the comprehensive mini-review as presented.
Celastrol Nanoformulations and Their Therapeutic Applications
Cancer
NF-κB inhibition is the most commonly reported pharmacological mechanism of celastrol’s anti-cancer activity (Elhasany et al., 2020). Celastrol also inhibits M2-like polarized tumor-associated macrophages that are involved in tumor metastasis. In an in vivo study (Yang et al., 2018), the expression of M2-like genes by quantitative real-time PCR showed that genes including MRC1, Arg1, Fizz1, Mgl2, and CD11c were up-regulated by IL-13 administration, which was greatly reduced by celastrol co-administration. Other molecular targets include liver X receptor α and ATP-binding cassette transporter A1 (Zhang et al., 2020), microRNA-21 (Yao et al., 2019), androgen receptor/microRNA-101 (Guo et al., 2015), lipoprotein receptor-1 (Gu et al., 2013), microRNA-33a-5p/E2F7 transcription factor (Liu et al., 2020), PI3K–Akt–mTOR signaling (Li et al., 2018), mitochondrial ubiquitination (Hu et al., 2017), CXC chemokine receptor type 4 (Yadav et al., 2010), peroxisome proliferator–activated receptor α signaling (Zhao et al., 2020), and transforming growth factor β1 (Kang et al., 2013). In spite of multi-target anti-cancer potency of celastrol, its clinical translation has not been realized due to poor bioavailability, inadequate tumor targeting, and high toxicity. The nanoformulations developed and investigated to overcome these challenges for various cancers are described below, and additional nanoformulations not discussed in detail are summarized in Table 1.
Cancer tumorigenesis and metastasis is induced by multiple mechanisms including migration, invasion, and angiogenesis. The tumor microenvironment (TME) plays a key role in these carcinogenic mechanisms, and multiple strategies have been investigated to alter the TME in order to treat cancers. Among various pharmacological responses, celastrol is reported to inhibit NLRP3 inflammasome, which in turn impedes the macrophage potency to promote migration and invasion of melanoma cells (Lee et al., 2019). To effectively deliver celastrol for treatment of melanoma, self-assembling amphiphilic polymer/celastrol prodrug nanoparticles were developed by chemically conjugating celastrol to the diblock polymer methoxy-poly(ethylene glycol)-b-poly(L-lysine) (Li et al., 2020). This celastrol prodrug underwent self-assembly to form stable micellar nanoparticles (103.1 ± 10.7 nm) due to hydrophobic and electrostatic interactions between the drug and the polymer. An in vivo study in the B16F10 mouse melanoma model showed significant uptake of the nanoparticle formulation due to the enhanced permeability and retention (EPR) effect that resulted in tumor growth reduction and lowered toxicity compared to that of celastrol alone, confirming the potential of functionalized nanoparticle-mediated drug targeting as a safe and effective tool (Li et al., 2020). In another investigation, a celastrol nanoemulsion was reported to downregulate programmed cell death-ligand 1, eliciting strong immunogenic cell death in a bilateral tumor model. This can be viewed as a promising avenue of chemotherapy-induced cancer immunotherapy (Qiu et al., 2021). Celastrol was also reported to have inhibitory effect on tumor-associated fibroblasts that play a critical role in desmoplastic melanoma. In view of the strong anti-fibroblast and immunomodulatory effects of celastrol, it has also been combined with potent anti-cancer drugs to achieve simultaneous chemo-immunotherapy in melanoma treatment. For instance, Liu et al. developed TME-responsive targeted aminoethylanisamide polymeric nanoparticles comprising a drug combination of the anti-cancer agent mitoxantrone and celastrol in a 5:1 ratio. The nanoparticles exhibited a size of 112 ± 6 nm with > 75% drug encapsulation for both drugs. In vivo melanoma tumor model studies confirmed inhibition of cancer progression/metastasis and TME immunosuppression, confirming the hypothesis synergistic anti-cancer efficacy with combination drug nanoparticles of mitoxantrone and celastrol (Liu et al., 2018). Similarly, bio-mimicking polyethylene glycol–poly(lactic-co-glycolic acid) (PEG–PLGA) nanoparticles coated with the neutrophil membrane showed higher internalization and apoptosis in the murine melanoma B16F10 cell line as compared to uncoated nanoparticles. This coating also helped to increase the biodistribution in the tumor xenograft model (Zhou et al., 2019).
Celastrol has also been reported to have potent activity against ovarian cancer via mechanisms that include intracellular accumulation of reactive oxygen species (ROS), apoptosis, cell cycle arrest (G2/M phase), and ultimately cell growth inhibition (Xu et al., 2019). For instance, an in vitro study with the ovarian cancer SKOV3 cell line showed proportional increase in intracellular ROS concentration with increased exposure to celastrol, confirming the ROS responsiveness of celastrol (Xu et al., 2019). To enhance the clinical efficacy with reduction in toxicity, various nanoformulations have been investigated. Furthermore, to enhance ovarian cancer targeting, Niu et al. prepared celastrol nanoparticles using the poly(lactic-co-glycolic acid)–poly(ethylene glycol) methyl ether (PLGA–mPEG) polymer and coated them with folic acid for active tumor targeting. Folate receptors upregulate in tumor tissue, and hence, the use of folic acid–coated nanoparticles enables enhanced uptake via active targeting. The prepared nanoparticles displayed the encapsulation efficiency of 95% with a particle size of 155 nm and showed significant enhancement in ROS levels’ inhibitory potential against SKOV3 cells with prolonged treatment time (Niu et al., 2020). The folate receptor tumor targeting approach was also investigated by Law et al. wherein they developed folic acid–functionalized celastrol-conjugated gold–polymer nanoparticles to achieve active targeting against breast cancer. The developed nanoparticles showed significant enhancement in apoptosis in 2D and 3D breast tumor models compared to celastrol alone. The nanoparticles also exhibited higher cellular uptake efficiency and lower colony-forming assay units, confirming enhanced uptake of the nanoparticles leading to improved efficacy (Law et al., 2020). Celastrol nanosuspension with a size of 147.9 nm has also been developed and investigated for breast cancer treatment. Celastrol was stabilized in an amorphous form in the nanosuspension, hence enhancing its dissolution significantly to 69.2% in 48 h. Compared to intravenous injection of the anticancer drug paclitaxel, the oral and intravenous treatment with celastrol nanosuspension showed similar and higher tumor inhibition rates, respectively. Hence, the unique property of nanoformulations to enhance dissolution of poorly soluble drugs such as celastrol can be explored to enhance solubility and in turn the in vivo efficacy (Huang et al., 2020).
Non-small-cell lung cancer is the most predominant lung carcinoma, and tyrosine kinase inhibitors (TKIs) are classically used for chemotherapy. Celastrol has gained particular attention in treatment of this type of cancer due to its serine threonine protein kinase (Akt) inhibitory potential, which is proven to be very effective if combined with TKIs. Particularly, Xie et al. developed a nano-product comprising the TKI gefitinib and celastrol along with a fluorescent diagnostic probe. The combination nano-prodrug approach not only allowed fluorescence and optoacoustic tumor imaging but was also proven to be superior, exhibiting significant tumor inhibition in an orthotopic mouse tumor model (Xie et al., 2020). In another study (Zhao et al., 2018), a nanoformulation comprising celastrol-loaded glucolipid-like conjugates tagged with avb3-ligand tetraiodothyroacetic acid was developed to inhibit the NF-kB signaling pathway in lung and breast metastatic cancer cells. The targeted nanoformulation was selectively taken up by the cells via the avb3 receptor–mediated interaction. The study showed reduction in the apoptotic marker MMP-9 in vivo, confirming that the prepared celastrol-loaded micelles suppressed breast tumor invasion and lung metastasis. In addition, self-assembled micelles containing covalently conjugated celastrol–PEG–ginsenoside Rh2 were developed for endosomal/lysosomal delivery. The formulation showed significant enhancement in the bioavailability due to introduction of PEG that imparted stealth (long circulation) properties to the nanoparticles and showed synergistic anti–lung cancer activity due to the combination approach (Li et al., 2017).
In addition to the use of folic acid for active tumor targeting, multiple other active targeting strategies have also been reported in this area. For instance, glucose was used as an affinity ligand to decorate mesoporous silica nanoparticles for the delivery of celastrol with high specificity to HeLa and A549 cancer cells. To further increase the specificity, poly(ethylene imine) was surface-branched on the nanoparticles that increased the overall positive charge and hence the cellular uptake (Niemelä et al., 2015). In another interesting study, theranostic (combining therapeutics with diagnostics) nanoparticles incorporated with a drug combination of celastrol and sulfasalazine were developed for targeted breast cancer management. Specifically, SPION-tagged amphiphilic zein–chondroitin sulfate micelles were used to achieve simultaneous CD44–tumor-targeted drug delivery of celastrol and sulfasalazine along with MRI. The combination nanoplatform showed highest efficacy compared to non-targeted and free drug treatment groups confirming its superiority (Elhasany et al., 2020). Furthermore, folate receptor–targeted liposomes carrying combination of celastrol and irinotecan have also been reported for lung and breast cancer treatment (Soe et al., 2018). The liposomes exhibited improved cellular uptake and apoptosis when tested in multiple cancer cell lines (MCF-7, MDA-MB-231, A549). The in vivo studies in MDA-MB-231 tumor-bearing female BALB/c nude mice confirmed highest suppression with the liposomal treatment group (Soe et al., 2018). Another vesicular nanoformulation investigated for lung cancer treatment was celastrol exosomes that showed efficacy against A549 and H1299 lung cancer cells. Additionally, when tested in a xenograft model, exosomal celastrol presented enhanced anti-tumor efficacy compared to free celastrol and was devoid of kidney and liver toxicity, confirming its promise in lung cancer treatment (Aqil et al., 2016).
Celastrol has also been proven to be effective against prostate cancer. For example, investigation of celastrol poly(ε-caprolactone) nanoparticles against prostate cancer cell lines (LNCaP, DU-145, and PC3) revealed significant inhibition (IC50 < 2 µM) with modulation of apoptotic proteins (Sanna et al., 2015). In another study, polycaprolactone polymeric tripterine nanoparticles were prepared with a size of about 75 nm. The nanoparticles were proven to elicit significant tumor reduction compared to the free drug in LNCaP cell BALB/c mice xenograft model (Yin et al., 2017). Lipid nanocarriers have also been investigated for this purpose. For example, Chen et al. developed nanostructured lipid carriers (NLCs) of celastrol and coated them with the cell-penetrating peptide to achieve active tumor targeting. The NLCs (size: 126.7 ± 9.2 nm) showed a controlled drug release profile with enhance absorption in vivo due to NLCs’ colloidal form and nanosize. Specifically, the NLC formulation showed 484.75% enhancement in bioavailability compared to a plain drug (Chen et al., 2012). The similar group further investigated the efficacy of the targeted NLCs in the prostate cancer model in vivo. The studies confirmed enhanced anti-tumor effect and reduction in tumor markers (necrosis factor-alpha, interleukin-6 cytokine) compared to plain drug control in a dose-dependent manner (Yuan et al., 2013).
Celastrol has also been proven effective against pancreatic carcinoma (Cao et al., 2019). Celastrol-loaded neutrophil-mimicking nanoparticles were demonstrated to achieve pancreas-specific drug delivery by overcoming the blood–pancreas barrier in vivo. For this, the poly(ethylene glycol) methyl ether-block-poly(lactic-co-glycolic acid) polymer was used as a naive neutrophil membrane coating. The coating induced neutrophil-like properties to the nanoparticles that enhanced their uptake by the cells both in vitro and in vivo. In vitro evaluation of these nanoparticles in the lipopolysaccharide-stimulated RAW264.7 macrophages and L929 cells showed marked cellular uptake and internalization. Furthermore, the in vivo anti-tumor efficacy study in the female pancreatic cancer mice model proved enhanced and site-specific anti-tumor activity. Similarly, celastrol-loaded silk fibroin (SF) nanoparticles were synthesized and studied in human pancreatic cancer cells (MIA PaCa-2 and PANC-1). SF nanoparticles showed lower IC50 values in both the cell lines compared to free celastrol (Ding et al., 2017).
To target celastrol for solid tumor treatment, mesoporous silica nanoparticles capped with PEGylated polyaminoacid were prepared for mitochondria-targeted delivery of celastrol. The targeted nanoparticles were shown to have enhanced efficacy in the SCC-7 cancer cell–bearing xenograft tumor mice model (Choi et al., 2018). In a study to examine apoptotic effects of celastrol on cancer cells, SW620 colorectal cancer cells both in vitro and in nude mice were conducted along with biosafety studies in zebrafish and xenograft mice models. The prepared dendrimer bioconjugate of celastrol showed a particle size of 40 nm (spherical in shape) and induced apoptosis in the colorectal cancer cells in vitro and in mice with reduction in local and systemic toxicity (Ge et al., 2020). To summarize, a plethora of celastrol nanoformulations have shown their potential to enhance the efficacy and safety profile of celastrol in cancer treatment.
Osteoarthritis and Rheumatoid Arthritis
Celastrol is a potent therapeutic agent investigated for the treatment of osteoarthritis and rheumatoid arthritis. It elicits treatment benefit by regulating functions of Th1 and Th2 cells, fibroblasts, macrophages, and endothelial cells that play critical roles in the etiology and pathogenesis of arthritis. In addition, celastrol inhibits numerous inflammatory chemokines that include mundane T cells expressed and secreted, monocyte chemoattractant protein 1, macrophage inflammatory proteins, and growth-regulated oncogene/keratinocyte chemoattractant. In addition to these molecular targets, celastrol also modulates the function of metalloprotein, JNK and MEK1 pathways (Song et al., 2019). Celastrol nanoformulations have been reported to further enhance the anti-inflammatory efficacy while offering promising safety profile. For example, celastrol-loaded palmitic acid–modified bovine serum albumin (PAB) nanoparticles and bovine serum albumin (BSA) nanoparticles were developed and tested for anti-inflammatory response in the AIA rats for scavenger receptor-A targeting via intravenous injection treatment (Gong et al., 2020). The celastrol PAB nanoparticles significantly improved rheumatoid arthritis symptoms at a lower dose with fewer toxic effects compared to the celastrol BSA nanoparticles. Furthermore, mechanistically, celastrol PAB nanoparticles were proven to enhance scavenger receptor-A targeting due to high electronegativity (excipients: BSA and palmitic acid) compared to celastrol BSA nanoparticles (excipient: BSA) (Gong et al., 2020). In another study, phytosomes with a combination of celastrol and selenium were administered via oral gavage to treat arthritis in male AIA rats. These phytosomes enhanced the transepithelial transport of drugs due to smaller phytosomal size (126 nm) and enhanced nanoparticle transmembrane diffusion. This enhanced uptake resulted in significant alleviation of the arthritis symptoms and also lowered the inflammatory factors (Zhu et al., 2020).
Miscellaneous
Celastrol nanoformulations have been studied to benefit in treatment of obesity (Liu et al., 2015), diabetes, lipid accumulation, psoriasis (Zhou et al., 2011), etc. For example, celastrol-loaded polyethylene glycol–polycaprolactone nanomicelles effectively ameliorated body weight, lipid accumulation, diabetes, and metabolic dysfunction in diet-induced obese mice. Furthermore, histopathological examination of the high-fat-diet–induced obese mice model confirmed that the treatment with celastrol nanoformulation did not result in any anal irritation or intestinal disturbance otherwise seen in control or plain celastrol–treated animals. Hence, celastrol nanomicelles can be deemed more effective and safer (Zhao et al., 2019). Celastrol has also been proven to be effective in treatment of renal diseases (Tang et al., 2018). But its severe systemic toxicity limits its use. Guo et al. prepared mesangial cell–targeting celastrol nanoparticles using human serum albumin for ameliorating the effects of mesangioproliferative glomerulonephritis (MsPGN). They confirmed the selective uptake of celastrol nanoparticles via in vivo fluorescence imaging and semiquantitative fluorescence intensity measurement of the kidneys (excised 5 min after tail vein injection of nanoparticles), and the nanoparticles with size 95 nm showed maximum uptake by the kidneys (Guo et al., 2017). The in vivo evaluation clearly showed that the formulation not only reduced the systemic toxicity but also minimized the off-targeting effects of celastrol. It also showed potent effects against proteinuria, inflammation, glomerular hypercellularity, and extracellular matrix (ECM) deposition in an anti-Thy1.1 nephritis rat model. This was attributed to the anti-inflammatory, anti-proliferative, and anti-fibrotic mechanisms, highlighting celastrol as a promising agent for the treatment of MsPGN such as IgA nephropathy (Guo et al., 2017). Multiple studies as described earlier have shown that nanoparticles enhance the bioavailability of celastrol. In one such specific study, Freag et al. encapsulated celastrol in a self-assembled phospholipid-based phytosomal nanocarrier system. The in vivo pharmacokinetic data in rabbits indicated that the phytosomes increased the bioavailability and Cmax by fourfold and fivefold, respectively, compared to the celastrol suspension. The authors attributed the bioavailability enhancement to meticulous use of phospholipids that not only retain cell membrane fluidity but also potentially enhance the rate and extent of intestinal drug absorption and enhancement in the aqueous solubility of celastrol by incorporating it in a nanoformulation (Freag et al., 2018).
Conclusion and Future Prospects
Medicinal plants containing bioactive constituents are a great resource for modern drug development, and Tripterygium wilfordii is one of them. Its major constituent celastrol has numerous pharmacological actions including anti-inflammatory, anti-obesity, anti-diabetic, and anti-cancer activities (Cascão et al., 2017; Chen et al., 2018; Hou et al., 2020; Yan et al., 2020; Lu et al., 2021). However, there are multiple challenges in translating traditional herbal medicines and their active constituents to modern drug therapies. In particular, celastrol presents issues of low solubility, bioavailability, and toxicity (Zha et al., 2018). For seven decades, numerous attempts have been made to overcome the problems of celastrol delivery, and recently, nanotechnology-based formulations have shown great promise in enhancing its overall pharmacological efficacy and safety. Celastrol nanoformulations (enhanced permeation, retention, tumor targeting, and controlled drug release) can be looked upon as a promising avenue toward successful clinical translation of this potent bioactive agent toward treatment of various human diseases (Fang and Tang, 2020). More importantly, the universal challenge of clinical translation of nanomedicine needs more attention, and as rightly pointed out by Sun et al. (2020), pharmaceutical scientists, engineers, chemists, and material scientists must work in synergy to develop stable, scalable, and effective nanoformulations. Furthermore, regulatory authorities worldwide are developing specific guidelines to streamline the approval of nanomedicine-based products that would help in successful clinical translation of these formulations in the near future (Paradise, 2019).
Author Contributions
JW conceived and proposed the idea. PW and PD compiled the manuscript. SP and JW reviewed and revised the manuscript.
Funding
The funding was provided by the Western University of Health Sciences.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abbas, S., Bhoumik, A., Dahl, R., Vasile, S., Krajewski, S., Cosford, N. D. P., et al. (2007). Preclinical Studies of Celastrol and Acetyl Isogambogic Acid in Melanoma. Clin. Cancer Res. 13 (22 Pt 1), 6769–6778. doi:10.1158/1078-0432.Ccr-07-1536
Abdelmoneem, M. A., Abd Elwakil, M. M., Khattab, S. N., Helmy, M. W., Bekhit, A. A., Abdulkader, M. A., et al. (2021). Lactoferrin-dual Drug Nanoconjugate: Synergistic Anti-tumor Efficacy of Docetaxel and the NF-Κb Inhibitor Celastrol. Mater. Sci. Eng. C 118, 111422. doi:10.1016/j.msec.2020.111422
Allen, S. D., Liu, Y.-G., Kim, T., Bobbala, S., Yi, S., Zhang, X., et al. (2019). Celastrol-loaded PEG-B-PPS Nanocarriers as an Anti-inflammatory Treatment for Atherosclerosis. Biomater. Sci. 7 (2), 657–668. doi:10.1039/c8bm01224e
An, L., Li, Z., Shi, L., Wang, L., Wang, Y., Jin, L., et al. (2020). Inflammation-Targeted Celastrol Nanodrug Attenuates Collagen-Induced Arthritis through NF-Κb and Notch1 Pathways. Nano Lett. 20 (10), 7728–7736. doi:10.1021/acs.nanolett.0c03279
An, N., Li, H. Y., and Zhang, X. M. (2015). Growth Inhibitive Effect of Betulinic Acid Combined with Tripterine on MSB-1 Cells and its Mechanism. Poult. Sci. 94 (12), 2880–2886. doi:10.3382/ps/pev267
Aqil, F., Kausar, H., Agrawal, A. K., Jeyabalan, J., Kyakulaga, A.-H., Munagala, R., et al. (2016). Exosomal Formulation Enhances Therapeutic Response of Celastrol against Lung Cancer. Exp. Mol. Pathol. 101 (1), 12–21. doi:10.1016/j.yexmp.2016.05.013
Cao, X., Hu, Y., Luo, S., Wang, Y., Gong, T., Sun, X., et al. (2019). Neutrophil-mimicking Therapeutic Nanoparticles for Targeted Chemotherapy of Pancreatic Carcinoma. Acta Pharmaceutica Sinica B 9 (3), 575–589. doi:10.1016/j.apsb.2018.12.009
Cascão, R., Fonseca, J. E., and Moita, L. F. (2017). Celastrol: A Spectrum of Treatment Opportunities in Chronic Diseases. Front. Med. 4 (69), 4. doi:10.3389/fmed.2017.00069
Chang, M.-Y., Hsieh, C.-Y., Lin, C.-Y., Chen, T.-D., Yang, H.-Y., Chen, K.-H., et al. (2018). Effect of Celastrol on the Progression of Polycystic Kidney Disease in a Pkd1-Deficient Mouse Model. Life Sci. 212, 70–79. doi:10.1016/j.lfs.2018.09.047
Chen, S.-R., Dai, Y., Zhao, J., Lin, L., Wang, Y., and Wang, Y. (2018). A Mechanistic Overview of Triptolide and Celastrol, Natural Products from Tripterygium Wilfordii Hook F. Front. Pharmacol. 9 (104). doi:10.3389/fphar.2018.00104
Chen, X., Hu, X., Hu, J., Qiu, Z., Yuan, M., and Zheng, G. (2020). Celastrol-Loaded Galactosylated Liposomes Effectively Inhibit AKT/c-Met-Triggered Rapid Hepatocarcinogenesis in Mice. Mol. Pharmaceutics 17 (3), 738–747. doi:10.1021/acs.molpharmaceut.9b00428
Chen, X., Zhao, Y., Luo, W., Chen, S., Lin, F., Zhang, X., et al. (2020). Celastrol Induces ROS-Mediated Apoptosis via Directly Targeting Peroxiredoxin-2 in Gastric Cancer Cells. Theranostics 10 (22), 10290–10308. doi:10.7150/thno.46728
Chen, Y., Yuan, L., Zhou, L., Zhang, Z. H., Cao, W., and Wu, Q. (2012). Effect of Cell-Penetrating Peptide-Coated Nanostructured Lipid Carriers on the Oral Absorption of Tripterine. Int. J. Nanomedicine 7, 4581–4591. doi:10.2147/IJN.S34991
Choi, J. Y., Gupta, B., Ramasamy, T., Jeong, J.-H., Jin, S. G., Choi, H.-G., et al. (2018). PEGylated Polyaminoacid-Capped Mesoporous Silica Nanoparticles for Mitochondria-Targeted Delivery of Celastrol in Solid Tumors. Colloids Surf. B: Biointerfaces 165, 56–66. doi:10.1016/j.colsurfb.2018.02.015
Choi, J. Y., Ramasamy, T., Kim, S. Y., Kim, J., Ku, S. K., Youn, Y. S., et al. (2016). PEGylated Lipid Bilayer-Supported Mesoporous Silica Nanoparticle Composite for Synergistic Co-delivery of Axitinib and Celastrol in Multi-Targeted Cancer Therapy. Acta Biomater. 39, 94–105. doi:10.1016/j.actbio.2016.05.012
Cleren, C., Calingasan, N. Y., Chen, J., and Beal, M. F. (2005). Celastrol Protects against MPTP- and 3-nitropropionic Acid-Induced Neurotoxicity. J. Neurochem. 94 (4), 995–1004. doi:10.1111/j.1471-4159.2005.03253.x
Dai, C. H., Zhu, L. R., Wang, Y., Tang, X. P., Du, Y. J., Chen, Y. C., et al. (2020). Celastrol Acts Synergistically with Afatinib to Suppress Non‐small Cell Lung Cancer Cell Proliferation by Inducing Paraptosis. J. Cel. Physiol. 236, 4538–4554. doi:10.1002/jcp.30172
Desai, P. P., Date, A. A., and Patravale, V. B. (2012). Overcoming Poor Oral Bioavailability Using Nanoparticle Formulations - Opportunities and Limitations. Drug Discov. Today Tech. 9 (2), e87–e95. doi:10.1016/j.ddtec.2011.12.001
Ding, B., Wahid, M. A., Wang, Z., Xie, C., Thakkar, A., Prabhu, S., et al. (2017). Triptolide and Celastrol Loaded Silk Fibroin Nanoparticles Show Synergistic Effect against Human Pancreatic Cancer Cells. Nanoscale 9 (32), 11739–11753. doi:10.1039/C7NR03016A
Du, S., Song, X., Li, Y., Cao, Y., Chu, F., Durojaye, O. A., et al. (2020). Celastrol Inhibits Ezrin-Mediated Migration of Hepatocellular Carcinoma Cells. Sci. Rep. 10 (1), 11273. doi:10.1038/s41598-020-68238-1
Duan, J., Zhan, J.-C., Wang, G.-Z., Zhao, X.-C., Huang, W.-D., and Zhou, G.-B. (2019). The Red Wine Component Ellagic Acid Induces Autophagy and Exhibits Anti-lung Cancer Activity In Vitro and In Vivo. J. Cel. Mol. Med. 23 (1), 143–154. doi:10.1111/jcmm.13899
Elhasany, K. A., Khattab, S. N., Bekhit, A. A., Ragab, D. M., Abdulkader, M. A., Zaky, A., et al. (2020). Combination of Magnetic Targeting with Synergistic Inhibition of NF-Κb and Glutathione via Micellar Drug Nanomedicine Enhances its Anti-tumor Efficacy. Eur. J. Pharmaceutics Biopharmaceutics 155, 162–176. doi:10.1016/j.ejpb.2020.08.004
Fang, G., and Tang, B. (2020). Current Advances in the Nano-Delivery of Celastrol for Treating Inflammation-Associated Diseases. J. Mater. Chem. B 8 (48), 10954–10965. doi:10.1039/D0TB01939A
Freag, M. S., Saleh, W. M., and Abdallah, O. Y. (2018). Self-assembled Phospholipid-Based Phytosomal Nanocarriers as Promising Platforms for Improving Oral Bioavailability of the Anticancer Celastrol. Int. J. Pharmaceutics 535 (1-2), 18–26. doi:10.1016/j.ijpharm.2017.10.053
Gao, Y., Zhou, S., Pang, L., Yang, J., Li, H. J., Huo, X., et al. (2019). Celastrol Suppresses Nitric Oxide Synthases and the Angiogenesis Pathway in Colorectal Cancer. Free Radic. Res. 53 (3), 324–334. doi:10.1080/10715762.2019.1575512
Ge, P., Niu, B., Wu, Y., Xu, W., Li, M., Sun, H., et al. (2020). Enhanced Cancer Therapy of Celastrol In Vitro and In Vivo by Smart Dendrimers Delivery with Specificity and Biosafety. Chem. Eng. J. 383, 123228. doi:10.1016/j.cej.2019.123228
Gong, T., Tan, T., Zhang, P., Li, H., Deng, C., Huang, Y., et al. (2020). Palmitic Acid-Modified Bovine Serum Albumin Nanoparticles Target Scavenger Receptor-A on Activated Macrophages to Treat Rheumatoid Arthritis. Biomaterials 258, 120296. doi:10.1016/j.biomaterials.2020.120296
Gu, L., Bai, W., Li, S., Zhang, Y., Han, Y., Gu, Y., et al. (2013). Celastrol Prevents Atherosclerosis via Inhibiting LOX-1 and Oxidative Stress. PLoS One 8 (6), e65477. doi:10.1371/journal.pone.0065477
Guo, J., Huang, X., Wang, H., and Yang, H. (2015). Celastrol Induces Autophagy by Targeting AR/miR-101 in Prostate Cancer Cells. PLoS One 10 (10), e0140745. doi:10.1371/journal.pone.0140745
Guo, L., Luo, S., Du, Z., Zhou, M., Li, P., Fu, Y., et al. (2017). Targeted Delivery of Celastrol to Mesangial Cells Is Effective against Mesangioproliferative Glomerulonephritis. Nat. Commun. 8 (1), 878. doi:10.1038/s41467-017-00834-8
Hakala, T. A., Davies, S., Toprakcioglu, Z., Bernardim, B., Bernardes, G. J. L., and Knowles, T. P. J. (2020). A Microfluidic Co‐Flow Route for Human Serum Albumin‐Drug-Nanoparticle Assembly. Chem. Eur. J. 26 (27), 5965–5969. doi:10.1002/chem.202001146
Hou, W., Liu, B., and Xu, H. (2020). Celastrol: Progresses in Structure-Modifications, Structure-Activity Relationships, Pharmacology and Toxicology. Eur. J. Med. Chem. 189, 112081. doi:10.1016/j.ejmech.2020.112081
Hu, M., Luo, Q., Alitongbieke, G., Chong, S., Xu, C., Xie, L., et al. (2017). Celastrol-Induced Nur77 Interaction with TRAF2 Alleviates Inflammation by Promoting Mitochondrial Ubiquitination and Autophagy. Mol. Cel. 66 (1), 141–153. doi:10.1016/j.molcel.2017.03.008
Huang, T., Wang, Y., Shen, Y., Ao, H., Guo, Y., Han, M., et al. (2020). Preparation of High Drug-Loading Celastrol Nanosuspensions and Their Anti-breast Cancer Activities In Vitro and In Vivo. Sci. Rep. 10 (1), 8851. doi:10.1038/s41598-020-65773-9
Huang, W., Chen, L., Kang, L., Jin, M., Sun, P., Xin, X., et al. (2017). Nanomedicine-based Combination Anticancer Therapy between Nucleic Acids and Small-Molecular Drugs. Adv. Drug Deliv. Rev. 115, 82–97. doi:10.1016/j.addr.2017.06.004
Jiang, M., Liu, X., Zhang, D., Wang, Y., Hu, X., Xu, F., et al. (2018). Celastrol Treatment Protects against Acute Ischemic Stroke-Induced Brain Injury by Promoting an IL-33/ST2 axis-mediated Microglia/macrophage M2 Polarization. J. Neuroinflammation 15 (1), 78. doi:10.1186/s12974-018-1124-6
Jin, C., Wu, Z., Wang, L., Kanai, Y., and He, X. (2019). CYP450s-Activity Relations of Celastrol to Interact with Triptolide Reveal the Reasons of Hepatotoxicity of Tripterygium Wilfordii. Molecules 24 (11), 2162. doi:10.3390/molecules24112162
Kang, H., Lee, M., and Jang, S.-W. (2013). Celastrol Inhibits TGF-β1-Induced Epithelial-Mesenchymal Transition by Inhibiting Snail and Regulating E-Cadherin Expression. Biochem. Biophysical Res. Commun. 437 (4), 550–556. doi:10.1016/j.bbrc.2013.06.113
Kang, Q., Liu, J., Zhao, Y., Liu, X., Liu, X.-Y., Wang, Y.-J., et al. (2018). Transdermal Delivery System of Nanostructured Lipid Carriers Loaded with Celastrol and Indomethacin: Optimization, Characterization and Efficacy Evaluation for Rheumatoid Arthritis. Artif. Cell Nanomedicine, Biotechnol. 46 (Suppl. 3), S585–S597. doi:10.1080/21691401.2018.1503599
Kim, J. H., Lee, J. O., Lee, S. K., Kim, N., You, G. Y., Moon, J. W., et al. (2013). Celastrol Suppresses Breast Cancer MCF-7 Cell Viability via the AMP-Activated Protein Kinase (AMPK)-induced P53-polo like Kinase 2 (PLK-2) Pathway. Cell Signal. 25 (4), 805–813. doi:10.1016/j.cellsig.2012.12.005
Law, S., Leung, A. W., and Xu, C. (2020). Folic Acid-Modified Celastrol Nanoparticles: Synthesis, Characterization, Anticancer Activity in 2D and 3D Breast Cancer Models. Artif. Cell Nanomedicine, Biotechnol. 48 (1), 542–559. doi:10.1080/21691401.2020.1725025
Lee, H. E., Lee, J. Y., Yang, G., Kang, H. C., Cho, Y.-Y., Lee, H. S., et al. (2019). Inhibition of NLRP3 Inflammasome in Tumor Microenvironment Leads to Suppression of Metastatic Potential of Cancer Cells. Sci. Rep. 9 (1), 12277. doi:10.1038/s41598-019-48794-x
Lee, Y., Kim, S. Y., and Lee, C. (2019). Axl Is a Novel Target of Celastrol that Inhibits Cell Proliferation and Migration, and Increases the Cytotoxicity of Gefitinib in EGFR Mutant Non-small C-ell L-ung C-ancer C-ells. Mol. Med. Rep. 19 (4), 3230–3236. doi:10.3892/mmr.2019.9957
Li, J., Jia, Y., Zhang, P., Yang, H., Cong, X., An, L., et al. (2020). Celastrol Self-Stabilized Nanoparticles for Effective Treatment of Melanoma. Ijn 15, 1205–1214. doi:10.2147/IJN.S232603
Li, P., Zhou, X., Qu, D., Guo, M., Fan, C., Zhou, T., et al. (2017). Preliminary Study on Fabrication, Characterization and Synergistic Anti-lung Cancer Effects of Self-Assembled Micelles of Covalently Conjugated Celastrol-Polyethylene Glycol-Ginsenoside Rh2. Drug Deliv. 24 (1), 834–845. doi:10.1080/10717544.2017.1326540
Li, X., Zhu, G., Yao, X., Wang, N., Hu, R., Kong, Q., et al. (2018). Celastrol Induces Ubiquitin-dependent Degradation of mTOR in Breast Cancer Cells. Ott 11, 8977–8985. doi:10.2147/ott.S187315
Li, Y., He, D., Zhang, X., Liu, Z., Zhang, X., Dong, L., et al. (2012). Protective Effect of Celastrol in Rat Cerebral Ischemia Model: Down-Regulating P-JNK, P-C-Jun and NF-Κb. Brain Res. 1464, 8–13. doi:10.1016/j.brainres.2012.04.054
Li, Y., Li, Z., Wu, J., Li, J., Yao, L., Zhang, Y., et al. (2012). Antitumor Activity of Celastrol Nanoparticles in a Xenograft Retinoblastoma Tumor Model. Ijn 7, 2389–2398. doi:10.2147/IJN.S29945
Li, Z., Yao, L., Li, J., Zhang, W., Wu, X., Liu, Y., et al. (2012). Celastrol Nanoparticles Inhibit Corneal Neovascularization Induced by Suturing in Rats. Int. J. Nanomedicine 7, 1163–1173. doi:10.2147/IJN.S27860
Li, Z., Guo, Z., Chu, D., Feng, H., Zhang, J., Zhu, L., et al. (2020). Effectively Suppressed Angiogenesis-Mediated Retinoblastoma Growth Using Celastrol Nanomicelles. Drug Deliv. 27 (1), 358–366. doi:10.1080/10717544.2020.1730522
Li, Z., Li, J., Zhu, L., Zhang, Y., Zhang, J., Yao, L., et al. (2016). Celastrol Nanomicelles Attenuate Cytokine Secretion in Macrophages and Inhibit Macrophage-Induced Corneal Neovascularization in Rats. Ijn 11, 6135–6148. doi:10.2147/ijn.S117425
Lin, M.-W., Lin, C. C., Chen, Y.-H., Yang, H.-B., and Hung, S.-Y. (2019). Celastrol Inhibits Dopaminergic Neuronal Death of Parkinson's Disease through Activating Mitophagy. Antioxidants 9 (1), 37. doi:10.3390/antiox9010037
Liu, C., Zhang, C., Wang, W., Yuan, F., He, T., Chen, Y., et al. (2019). Integrated Metabolomics and Network Toxicology to Reveal Molecular Mechanism of Celastrol Induced Cardiotoxicity. Toxicol. Appl. Pharmacol. 383, 114785. doi:10.1016/j.taap.2019.114785
Liu, J., Lee, J., Salazar Hernandez, M. A., Mazitschek, R., and Ozcan, U. (2015). Treatment of Obesity with Celastrol. Cell 161 (5), 999–1011. doi:10.1016/j.cell.2015.05.011
Liu, P., Wang, M., Tang, W., Li, G., and Gong, N. (2020). Circ_SATB2 Attenuates the Anti-tumor Role of Celastrol in Non-small-cell Lung Carcinoma through Targeting miR-33a-5p/E2F7 Axis. Ott 13, 11899–11912. doi:10.2147/ott.S279434
Liu, Q., Chen, F., Hou, L., Shen, L., Zhang, X., Wang, D., et al. (2018). Nanocarrier-Mediated Chemo-Immunotherapy Arrested Cancer Progression and Induced Tumor Dormancy in Desmoplastic Melanoma. ACS Nano 12 (8), 7812–7825. doi:10.1021/acsnano.8b01890
Lu, Y., Liu, Y., Zhou, J., Li, D., and Gao, W. (2021). Biosynthesis, Total Synthesis, Structural Modifications, Bioactivity, and Mechanism of Action of the Quinone‐methide Triterpenoid Celastrol. Med. Res. Rev. 41, 1022–1060. doi:10.1002/med.21751
Ma, X., Xu, L., Alberobello, A. T., Gavrilova, O., Bagattin, A., Skarulis, M., et al. (2015). Celastrol Protects against Obesity and Metabolic Dysfunction through Activation of a HSF1-Pgc1α Transcriptional Axis. Cel. Metab. 22 (4), 695–708. doi:10.1016/j.cmet.2015.08.005
Maysinger, D., Moquin, A., Choi, J., Kodiha, M., and Stochaj, U. (2018). Gold Nanourchins and Celastrol Reorganize the Nucleo- and Cytoskeleton of Glioblastoma Cells. Nanoscale 10 (4), 1716–1726. doi:10.1039/C7NR07833A
Metselaar, D. S., Meel, M. H., Benedict, B., Waranecki, P., Koster, J., Kaspers, G. J. L., et al. (2019). Celastrol-induced Degradation of FANCD2 Sensitizes Pediatric High-Grade Gliomas to the DNA-Crosslinking Agent Carboplatin. EBioMedicine 50, 81–92. doi:10.1016/j.ebiom.2019.10.062
Mou, H., Zheng, Y., Zhao, P., Bao, H., Fang, W., and Xu, N. (2011). Celastrol Induces Apoptosis in Non-small-cell Lung Cancer A549 Cells through Activation of Mitochondria- and Fas/FasL-Mediated Pathways. Toxicol. Vitro 25 (5), 1027–1032. doi:10.1016/j.tiv.2011.03.023
Niemelä, E., Desai, D., Nkizinkiko, Y., Eriksson, J. E., and Rosenholm, J. M. (2015). Sugar-decorated Mesoporous Silica Nanoparticles as Delivery Vehicles for the Poorly Soluble Drug Celastrol Enables Targeted Induction of Apoptosis in Cancer Cells. Eur. J. Pharmaceutics Biopharmaceutics 96, 11–21. doi:10.1016/j.ejpb.2015.07.009
Niu, W., Wang, J., Wang, Q., and Shen, J. (2020). Celastrol Loaded Nanoparticles with ROS-Response and ROS-Inducer for the Treatment of Ovarian Cancer. Front. Chem. 8, 8. doi:10.3389/fchem.2020.574614
Onyeabor, F., Paik, A., Kovvasu, S., Ding, B., Lin, J., Wahid, M. A., et al. (2019). Optimization of Preparation and Preclinical Pharmacokinetics of Celastrol-Encapsulated Silk Fibroin Nanoparticles in the Rat. Molecules 24 (18), 3271. doi:10.3390/molecules24183271
Paradise, J. (2019). Regulating Nanomedicine at the Food and Drug Administration. AMA J. Ethics 21 (4), E347–E355. doi:10.1001/amajethics.2019.347
Paris, D., Ganey, N. J., Laporte, V., Patel, N. S., Beaulieu-Abdelahad, D., Bachmeier, C., et al. (2010). Reduction of β-amyloid Pathology by Celastrol in a Transgenic Mouse Model of Alzheimer's Disease. J. Neuroinflammation 7, 17. doi:10.1186/1742-2094-7-17
Pinna, G. F., Fiorucci, M., Reimund, J.-M., Taquet, N., Arondel, Y., and Muller, C. D. Celastrol Inhibits Pro-inflammatory Cytokine Secretion in Crohn's Disease Biopsies. Biochem. Biophysical Res. Commun. (2004) 322(3):778–786. doi: doi: doi:10.1016/j.bbrc.2004.07.186
Qi, X., Qin, J., Ma, N., Chou, X., and Wu, Z. (2014). Solid Self-Microemulsifying Dispersible Tablets of Celastrol: Formulation Development, Charaterization and Bioavailability Evaluation. Int. J. Pharmaceutics 472 (1-2), 40–47. doi:10.1016/j.ijpharm.2014.06.019
Qi, Y., Wang, R., Zhao, L., Lv, L., Zhou, F., Zhang, T., et al. (2018). Celastrol Suppresses Tryptophan Catabolism in Human Colon Cancer Cells as Revealed by Metabolic Profiling and Targeted Metabolite Analysis. Biol. Pharm. Bull. 41 (8), 1243–1250. doi:10.1248/bpb.b18-00171
Qiu, N., Liu, Y., Liu, Q., Chen, Y., Shen, L., Hu, M., et al. (2021). Celastrol Nanoemulsion Induces Immunogenicity and Downregulates PD-L1 to Boost Abscopal Effect in Melanoma Therapy. Biomaterials 269, 120604. doi:10.1016/j.biomaterials.2020.120604
Salminen, A., Lehtonen, M., Paimela, T., and Kaarniranta, K. (2010). Celastrol: Molecular Targets of Thunder God Vine. Biochem. biophysical Res. Commun. 394 (3), 439–442. doi:10.1016/j.bbrc.2010.03.050
Sanna, V., Chamcheu, J. C., Pala, N., Mukhtar, H., Sechi, M., and Siddiqui, I. A. (2015). Nanoencapsulation of Natural Triterpenoid Celastrol for Prostate Cancer Treatment. Ijn 10, 6835–6846. doi:10.2147/IJN.S93752
Shaker, M. E., Ashamallah, S. A., and Houssen, M. E. (2014). Celastrol Ameliorates Murine Colitis via Modulating Oxidative Stress, Inflammatory Cytokines and Intestinal Homeostasis. Chemico-Biological Interactions 210, 26–33. doi:10.1016/j.cbi.2013.12.007
Shi, J., Li, J., Xu, Z., Chen, L., Luo, R., Zhang, C., et al. (2020). Celastrol: A Review of Useful Strategies Overcoming its Limitation in Anticancer Application. Front. Pharmacol. 11, 11. doi:10.3389/fphar.2020.558741
Soe, Z. C., Thapa, R. K., Ou, W., Gautam, M., Nguyen, H. T., Jin, S. G., et al. (2018). Folate Receptor-Mediated Celastrol and Irinotecan Combination Delivery Using Liposomes for Effective Chemotherapy. Colloids Surf. B: Biointerfaces 170, 718–728. doi:10.1016/j.colsurfb.2018.07.013
Song, J., Shi, F., Zhang, Z., Zhu, F., Xue, J., Tan, X., et al. (2011). Formulation and Evaluation of Celastrol-Loaded Liposomes. Molecules 16 (9), 7880–7892. doi:10.3390/molecules16097880
Song, X., Zhang, Y., Dai, E., Du, H., and Wang, L. (2019). Mechanism of Action of Celastrol against Rheumatoid Arthritis: A Network Pharmacology Analysis. Int. Immunopharmacology 74, 105725. doi:10.1016/j.intimp.2019.105725
Sun, D., Zhou, S., and Gao, W. (2020). What Went Wrong with Anticancer Nanomedicine Design and How to Make it Right. ACS Nano 14 (10), 12281–12290. doi:10.1021/acsnano.9b09713
Tan, Y., Zhu, Y., Zhao, Y., Wen, L., Meng, T., Liu, X., et al. (2018). Mitochondrial Alkaline pH-Responsive Drug Release Mediated by Celastrol Loaded Glycolipid-like Micelles for Cancer Therapy. Biomaterials 154, 169–181. doi:10.1016/j.biomaterials.2017.07.036
Tang, M., Cao, X., Zhang, K., Li, Y., Zheng, Q-Y., Li, G-Q., et al. (2018). Celastrol Alleviates Renal Fibrosis by Upregulating Cannabinoid Receptor 2 Expression. Cell Death Dis. [Internet] 9 (6), 601. doi:10.1038/s41419-018-0666-y
Wang, G., Xiao, Q., Wu, Y., Wei, Y. j., Jing, Y., Cao, X. r., et al. (2019). Design and Synthesis of Novel Celastrol Derivative and its Antitumor Activity in Hepatoma Cells and Antiangiogenic Activity in Zebrafish. J. Cel. Physiol. 234 (9), 16431–16446. doi:10.1002/jcp.28312
Wang, S., Ma, K., Zhou, C., Wang, Y., Hu, G., Chen, L., et al. (2019). LKB1 and YAP Phosphorylation Play Important Roles in Celastrol-Induced β-catenin Degradation in Colorectal Cancer. Ther. Adv. Med. Oncol. 11, 175883591984373. doi:10.1177/1758835919843736
Wu, M., Chen, W., Yu, X., Ding, D., Zhang, W., Hua, H., et al. (2018). Celastrol Aggravates LPS-Induced Inflammation and Injuries of Liver and Kidney in Mice. Am. J. Transl. Res. 10 (7), 2078–2086.
Xie, X., Zhan, C., Wang, J., Zeng, F., and Wu, S. (2020). An Activatable Nano‐Prodrug for Treating Tyrosine‐Kinase‐Inhibitor‐Resistant Non‐Small Cell Lung Cancer and for Optoacoustic and Fluorescent Imaging. Small 16 (38), 2003451. doi:10.1002/smll.202003451
Xu, L.-N., Zhao, N., Chen, J.-Y., Ye, P.-P., Nan, X.-W., Zhou, H.-H., et al. (2019). Celastrol Inhibits the Growth of Ovarian Cancer Cells In Vitro and In Vivo. Front. Oncol. 9, 2. doi:10.3389/fonc.2019.00002
Yadav, V. R., Sung, B., Prasad, S., Kannappan, R., Cho, S.-G., Liu, M., et al. (2010). Celastrol Suppresses Invasion of colon and Pancreatic Cancer Cells through the Downregulation of Expression of CXCR4 Chemokine Receptor. J. Mol. Med. 88 (12), 1243–1253. doi:10.1007/s00109-010-0669-3
Yan, F., Wu, Z., Li, Z., and Liu, L. (2020). Celastrol Inhibits Migration and Invasion of Triple-Negative Breast Cancer Cells by Suppressing Interleukin-6 via Downregulating Nuclear Factor-Κb (NF-Κb). Med. Sci. Monit. 26, e922814. doi:10.12659/MSM.922814
Yang, H., Chen, D., Cui, Q. C., Yuan, X., and Dou, Q. P. (2006). Celastrol, a Triterpene Extracted from the Chinese "Thunder of God Vine," Is a Potent Proteasome Inhibitor and Suppresses Human Prostate Cancer Growth in Nude Mice. Cancer Res. 66 (9), 4758–4765. doi:10.1158/0008-5472.CAN-05-4529
Yang, H., Pan, Z., Jin, W., Zhao, L., Xie, P., Chi, S., et al. (2019). Preparation, Characterization and Cytotoxic Evaluation of Inclusion Complexes between Celastrol with Polyamine-Modified β-cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 95 (1), 147–157. doi:10.1007/s10847-019-00933-7
Yang, Y., Cheng, S., Liang, G., Honggang, L., and Wu, H. (2018). Celastrol Inhibits Cancer Metastasis by Suppressing M2-like Polarization of Macrophages. Biochem. Biophysical Res. Commun. 503 (2), 414–419. doi:10.1016/j.bbrc.2018.03.224
Yao, S. S., Han, L., Tian, Z. B., Yu, Y. N., Zhang, Q., Li, X. Y., et al. (2019). Celastrol Inhibits Growth and Metastasis of Human Gastric Cancer Cell MKN45 by Down‐regulating microRNA‐21. Phytotherapy Res. 33 (6), 1706–1716. doi:10.1002/ptr.6359
Yin, J., Wang, P., Yin, Y., Hou, Y., and Song, X. (2017). Optimization on Biodistribution and Antitumor Activity of Tripterine Using Polymeric Nanoparticles through RES Saturation. Drug Deliv. 24 (1), 1891–1897. doi:10.1080/10717544.2017.1410260
Yuan, L., Liu, C., Chen, Y., Zhang, Z., Zhou, L., and Qu, D. (2013). Antitumor Activity of Tripterine via Cell-Penetrating Peptide-Coated Nanostructured Lipid Carriers in a Prostate Cancer Model. Int. J. Nanomedicine 8, 4339–4350. doi:10.2147/IJN.S51621
Zha, J., Zhang, Q., Li, M., Wang, J.-R., and Mei, X. (2018). Improving Dissolution Properties by Polymers and Surfactants: A Case Study of Celastrol. J. Pharm. Sci. 107 (11), 2860–2868. doi:10.1016/j.xphs.2018.07.008
Zhang, C.-j., Zhu, N., Long, J., Wu, H.-t., Wang, Y.-x., Liu, B.-y., et al. (2020). Celastrol Induces Lipophagy via the LXRα/ABCA1 Pathway in clear Cell Renal Cell Carcinoma. Acta Pharmacol. Sin. doi:10.1038/s41401-020-00572-6
Zhang, Q., Tian, X., and Cao, X. (2019). Transferrin-functionalised Microemulsion Co-delivery of β-elemene and Celastrol for Enhanced Anti-lung Cancer Treatment and Reduced Systemic Toxicity. Drug Deliv. Transl. Res. 9 (3), 667–678. doi:10.1007/s13346-019-00623-4
Zhang, X., Zhang, T., Zhou, X., Liu, H., Sun, H., Ma, Z., et al. (2014). Enhancement of Oral Bioavailability of Tripterine through Lipid Nanospheres: Preparation, Characterization, and Absorption Evaluation. J. Pharm. Sci. 103 (6), 1711–1719. doi:10.1002/jps.23967
Zhao, H., Chen, M., Zhao, Z., Zhu, L., and Yuan, S. (2020). A Multicomponent-Based Microemulsion for Boosting Ovarian Cancer Therapy through Dual Modification with Transferrin and SA-R6h4. Drug Deliv. Transl. Res. doi:10.1007/s13346-020-00859-5
Zhao, J., Luo, D., Zhang, Z., Fan, N., Wang, Y., Nie, H., et al. (2019). Celastrol-loaded PEG-PCL Nanomicelles Ameliorate Inflammation, Lipid Accumulation, Insulin Resistance and Gastrointestinal Injury in Diet-Induced Obese Mice. J. Controlled Release 310, 188–197. doi:10.1016/j.jconrel.2019.08.026
Zhao, Q., Tang, P., Zhang, T., Huang, J.-F., Xiao, X.-R., Zhu, W.-F., et al. (2020). Celastrol Ameliorates Acute Liver Injury through Modulation of PPARα. Biochem. Pharmacol. 178, 114058. doi:10.1016/j.bcp.2020.114058
Zhao, Y., Tan, Y., Meng, T., Liu, X., Zhu, Y., Hong, Y., et al. (2018). Simultaneous Targeting Therapy for Lung Metastasis and Breast Tumor by Blocking the NF-Κb Signaling Pathway Using Celastrol-Loaded Micelles. Drug Deliv. 25 (1), 341–352. doi:10.1080/10717544.2018.1425778
Zhou, L., Chen, Y., Zhang, Z., He, J., Du, M., and Wu, Q. (2012). Preparation of Tripterine Nanostructured Lipid Carriers and Their Absorption in Rat Intestine. Pharmazie 67 (4), 304–310.
Zhou, L.-L., Lin, Z.-X., Fung, K.-P., Cheng, C. H. K., Che, C.-T., Zhao, M., et al. (2011). Celastrol-induced Apoptosis in Human HaCaT Keratinocytes Involves the Inhibition of NF-Κb Activity. Eur. J. Pharmacol. 670 (2-3), 399–408. doi:10.1016/j.ejphar.2011.09.014
Zhou, X., Yu, R., Cao, X., Zhang, Z.-R., and Deng, L. (2019). Bio-Mimicking Nanoparticles for Targeted Therapy of Malignant Melanoma. J. Biomed. Nanotechnol. 15 (5), 993–1004. doi:10.1166/jbn.2019.2739
Zhou, Y., Li, W., Wang, M., Zhang, X., Zhang, H., Tong, X., et al. (2017). Competitive Profiling of Celastrol Targets in Human Cervical Cancer HeLa Cells via Quantitative Chemical Proteomics. Mol. Biosyst. 13 (1), 83–91. doi:10.1039/C6MB00691D
Keywords: celastrol, nanoformulations, targeting, bioavailability, anti-inflammatory, anti-autoimmune, anti-cancer, anti-oxidant
Citation: Wagh PR, Desai P, Prabhu S and Wang J (2021) Nanotechnology-Based Celastrol Formulations and Their Therapeutic Applications. Front. Pharmacol. 12:673209. doi: 10.3389/fphar.2021.673209
Received: 27 February 2021; Accepted: 10 May 2021;
Published: 11 June 2021.
Edited by:
Jamal Arif, Shaqra University, Saudi ArabiaReviewed by:
Wei Gao, Capital Medical University, ChinaMohammed Kuddus, University of Hail, Saudi Arabia
Syed Misbahul Hasan, Integral University, India
Copyright © 2021 Wagh, Desai, Prabhu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffrey Wang, jwang@westernu.edu
†These authors have contributed equally to this work
 Pushkaraj Rajendra Wagh
Pushkaraj Rajendra Wagh Preshita Desai
Preshita Desai Sunil Prabhu
Sunil Prabhu Jeffrey Wang
Jeffrey Wang