- 1Department of Cardio-Thoracic Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Departments of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 3Jiangxi Medical College, Nanchang University, Nanchang, China
- 4Department of Cardiology, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Background and Aims: Aspirin leads to substantial benefits for the secondary prevention of cardiovascular disease (CVD). We aimed to cast more light on aspirin’s role for the primary prevention of CVD.
Methods: Databases were searched for clinical trials comparing aspirin vs. no aspirin use in this meta-analysis. Efficacy and safety profiles were rigorously investigated. Trial sequential analysis (TSA) was used to determine the robustness of the results.
Results: Fourteen studies with 163,840 participants were eligible (mean follow-up 6.2 y). Aspirin intake was found to be associated with 9, 13, and 12% reductions in the risk of cardiovascular events (CV events) (relative risk [RR]: 0.91, 95% confidence intervals [CI]: 0.87–0.96; risk difference (RD): 0.29%; absolute risk percentage (AR%): 7.61%; number needed to treat (NNT): 345), myocardial infarction (RR: 0.87, 95% CI: 0.77–0.97; RD: 0.21%; AR%: 11.11%; NNT: 488) and ischemic stroke (RR: 0.88, 95% CI: 0.80–0.96; RD: 0.21%; AR%: 16.14%; NNT: 476), respectively; aspirin intake was also associated with 40%, 30%, and 57% increases in the risk of major bleeding (RR: 1.40, 95% CI: 1.29–1.53; RD: 0.47%; AR%: 27.85; NNT: 214), intracranial bleeding (RR: 1.30, 95% CI: 1.11–1.52; RD: 0.10%; AR%: 22.99%; NNT: 1,000) and major gastrointestinal bleeding (RR: 1.57, 95% CI: 1.38–1.78; RD: 0.32%; AR%: 36.70%; NNT: 315), respectively. Further, populations with low doses of aspirin intake (≤100 mg), populations <65 y old or populations with body mass index (BMI) ≧ 25 experienced more advantages; high-risk (10-y cardiovascular risk ≧10%) and full diabetic individuals reported hardly clinical benefits.
Conclusion: Aspirin intake was associated with a reduced risk of CV events and an increased incidence of bleeding profiles in primary prevention. It is necessary to identify individual’s CVD risk using clear examinations or assessments before aspirin intake, and truly realize individualized prescription.
Introduction
Currently, many patients are at high risk because their health is influenced by occlusive vascular disease; indeed, a long-term antiplatelet regimen (e.g., aspirin therapy) reduces the yearly risk of worse vascular events (such as nonfatal myocardial infarction, nonfatal stroke and vessel-related death) by almost one-quarter (Antiplatelet Trialists’ Collaboration, 1994). Distinct benefits are observed with respect to the incidence of non-fatal cardiovascular events (CV events), with a small but definitive absolute risk reduction of approximately 10–20 CV events per 1,000 per year. Despite the benefits of aspirin, the absolute risk of major gastrointestinal or other major extracranial bleeding is also increased by an order of magnitude, so in secondary prevention, the benefits exceed the risks (Antithrombotic Trialists’ Collaboration, 2002).
For primary prevention in patients without prior cardiovascular disease (CVD), both the risk without aspirin and absolute benefits of aspirin are smaller than those in secondary prevention. Although rates of death from coronary heart disease (CHD) and stroke in America have significantly decreased, CVD and cerebrovascular disease remain a large health and economic burden (Bibbins-Domingo, 2016). New guidelines suggest that regardless of bleeding risk, the wide use of aspirin is recommended for patients with a moderate risk of CHD, and a low dosage of aspirin (75–100 mg daily) may be reasonably recommended to 40- to 70-year-old adults at high risk of CVD without increasing major bleeding (IIb grade). New guidelines also recommended that age should be considered as a key determinant of the CVD risk, as a daily dose aspirin (alone or in combination with other drugs) has been recommended for all people above a specific age. Low doses of aspirin should not be recommended as primary prevention for 70-year-olds or for individuals with a high risk of bleeding (Pearson et al., 2002; Wald and Law, 2003; Elwood et al., 2005; Bulugahapitiya et al., 2008; Fox et al., 2015a; Bibbins-Domingo, 2016; Piepoli et al., 2016; Grundy et al., 2019; Mortensen and Nordestgaard, 2020). However, a moderate risk of CVD is hard to define, and whether the high CVD risk populations as well as the diabetic populations can get real benefits from aspirin or not.
Deferring the start of long-term aspirin use for primary prevention is a noted alternative that has the main advantage of avoiding an increased risk of slight or major bleeding events but has the disadvantage that the initial manifestation may be a disabling or fatal event. In previous primary prevention trials (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Belch et al., 2008; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Saito et al., 2017; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018), control populations with non-fatal CVD (non-fatal CHD or non-fatal occlusive stroke) would probably be prescribed long-term aspirin use to avoid recurrence, hence helping to compare the efficacy of immediate vs. deferred aspirin use.
A previous meta-analysis (Whitlock et al., 2016) noted that aspirin reduced all-cause mortality, myocardial infarction (MI), and ischemic stroke while increasing the risk of major bleeding; another pooled study (Zheng and Roddick, 2019) showed that aspirin reduced nonfatal MI but did not significantly influence all-cause mortality. Above mentioned studies had heterogeneous results on all-cause mortality because they had involved different number of trials conducted in different time. Another key controversial point was on individuals’ CVD risk classification that whether the higher risk individuals or the lower risk individuals could derive real prevention benefits from aspirin discussed by various guidelines or researchers. Actually, there are a lot of meta-analysis discussing this topic emerging yearly, not so many addressed their “cost-effectiveness”, which is to say if the conclusions are statistically sufficient and robust, no repetitive meta-analyses or further evidence are needed to some extent so that saving the cost on public health.
Given the large number of individuals affected by current studies and guidelines, and less helpful of the impact from no-innovative work on global health policy making, we conducted a comprehensive meta-analysis with the aim to resolve clinical controversial points under intention-to-treat principles and to evaluate the sufficiency of current synthesized evidence using trial sequential method.
Methods
The current study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, the PRISMA Checklist was shown in Supplementary Table S1. The protocol is available in PROSPERO (CRD42019127570).
Data Source and Study Selection
A rigorous search was performed in the PubMed, EMBASE, Cochrane Library, Web of Science and ClinicalTrials.gov databases from inception to February 1, 2020, to retrieve randomized controlled trials (RCTs) relating to aspirin use in patients without prior CVD. The search had no language restrictions. The main key words used were “aspirin”, “cardiovascular disease”, “cardiovascular events”, “coronary heart disease”, and “randomized controlled trials”. Reference lists of the eligible studies and identified meta-analyses were also reviewed (Supplementary Material S1).
The inclusion criteria were as follows: 1) enrolled adult participants (≥18 y) without preexisting CV events [CV events here include peripheral arterial disease, CHD, prior myocardial infarction (MI), ischemic stroke, prior percutaneous coronary intervention, prior coronary artery bypass grafting]; 2) compared aspirin use to no aspirin use (placebo included); 3) had a follow-up no less than 1 year to confirm the high quality of primary studies; 4) provided reliable and available outcome data (at least one primary efficacy outcome of interest was reported); and 5) was an RCT.
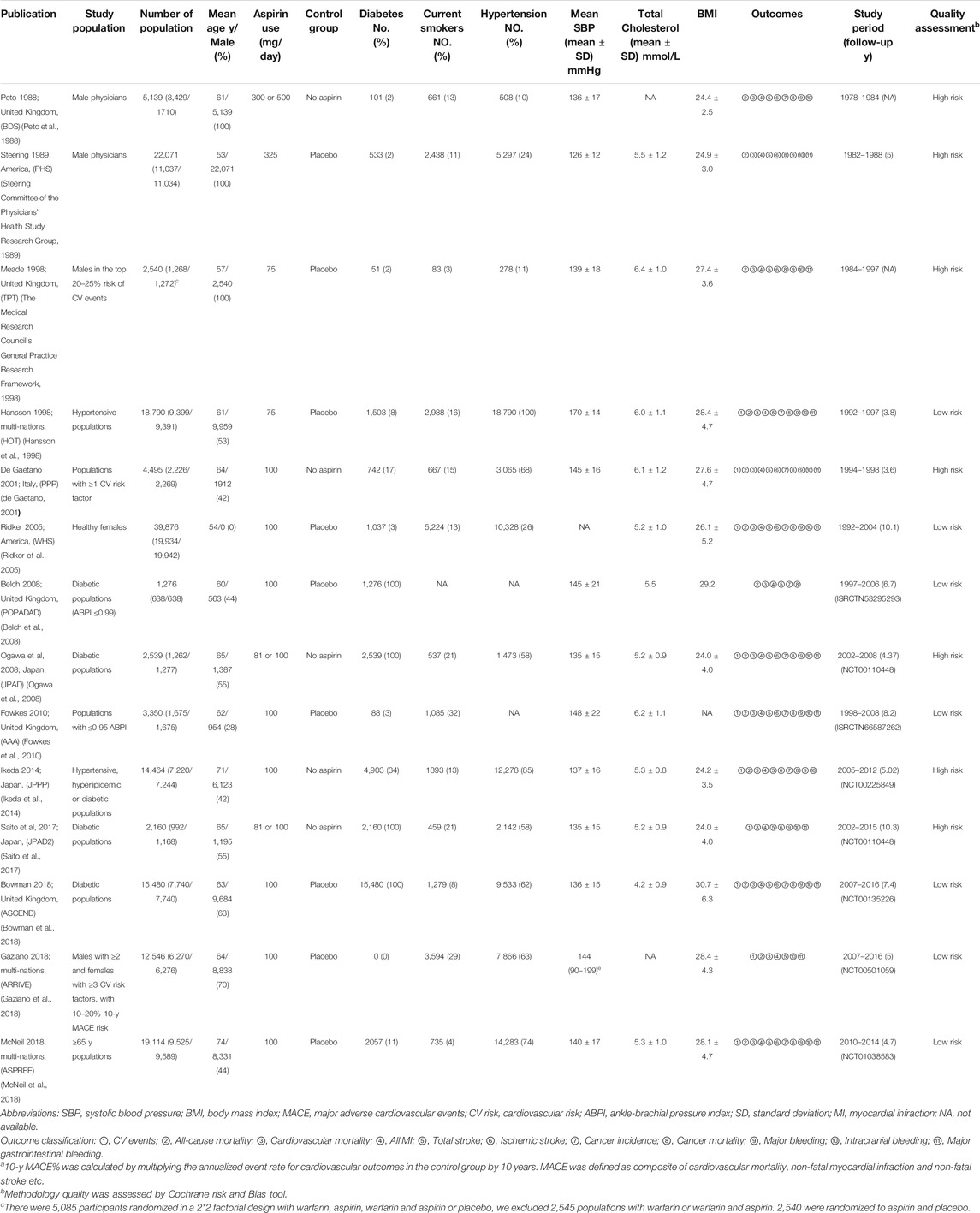
Studies with the most comprehensive outcomes were included to avoid duplications; studies that assessed patients with diabetes but without atherosclerosis were also considered. JPAD (Ogawa et al., 2008) and JPAD2 (Saito et al., 2017) trials were both included for they had different characteristics and proportion of the incorporated individuals as well as the differed follow-up. We excluded pure basic studies, reviews, and animal experiments.
Data Extraction and Outcome Definition
Two authors (Binghao Zhao, Yiping Wei) independently performed the study screening and extracted the baseline characteristics of each eligible trial. The baseline characteristics included demographic characteristics of included populations, clinical information about the intervention/control arms, and essential outcome data as well as the study design. Fully adjusted models for adjusted hazard ratio (HR), odd ratio (OR) and relative risk (RR) of analyzed outcomes were used if the models were available in included studies. Fully adjusted variables were varied, however, mostly included sex, age, country, hypertension, diabetes and smoking status. If some studies used intention-to-treat principles, we extracted the intention-to-treat data. Any discrepancies between the reviewers were resolved by a third author. If there were any missing data, the original authors were contacted.
The primary efficacy outcomes were CV events, all-cause mortality and cardiovascular mortality due to their universal definitions and balance of efficacy and safety, which reduce heterogeneity among eligible studies. The secondary efficacy outcomes were all MI, total stroke, ischemic stroke, cancer incidence and cancer mortality. The safety profile outcomes were major bleeding, intracranial bleeding and major gastrointestinal bleeding, as defined by each eligible trial. Intracranial bleeding was treated as a potential outcome of aspirin use in addition to CV events. All these definitions follow per included study’s definition (Grundy et al., 2019).
Some studies even noted that aspirin increased the probability of cancer mortality, therefore, cancer outcomes were also appointed as exploratory outcome for robust evidence. The 10-y major adverse cardiovascular event rate (10-y MACE%) was extracted and calculated by multiplying the annualized event rate for cardiovascular mortality, nonfatal MI, and nonfatal stroke. A 10-y MACE% ≥ 10% was regarded as high risk; the others were regarded as low risk (Supplementary Material S1).
Study Quality Assessment
Methodological quality assessment was performed by three co-authors (Binghao Zhao, Li Wang, Wenxiong Zhang). We used the Cochrane Risk and Bias Tool (Higgins et al., 2011) recommended by the Cochrane handbook to evaluate the quality of each eligible study. There were several terms regarding the methodological quality of RCTs, and each study could be categorized as low, high or unclear quality; low-quality studies and those with unclear quality had a high risk of bias. Details are provided in the Supplementary Material S1.
Statistical Analysis
For descriptive purposes and statistical convenience, weighted frequencies were calculated for categorical variables using the provided sample size of each trial. Multivariable RRs and 95% confidence intervals (95% CIs) (De Lima Taga and Singer, 2018) for primary/secondary efficacy outcomes of interest and primary safety outcomes were estimated using the DerSimonian-Laird (D-L) random effects model considering the existence of within- and between-study variability. To further illustrate these outcome estimations, risk difference (RD), absolute risk percentage (AR%) and number needed to treat (NNT) were also analyzed. For further statistical purposes, HRs and ORs were considered RRs in this study. Fully adjusted effect sizes (ESs) were logarithmically transformed to stabilize the variance; hence, the data distribution could be normalized.
Between-study heterogeneity and variability were quantified by Cochran’s Q test and I2, whereby an I2 > 50% or a p-value for the Q test <0.10 was considered to represent significant heterogeneity (Higgins et al., 2003). To provide more clinical implications, we conducted comprehensive subgroup analyses mainly focusing on several significant variables, including region (North America vs. Europe vs. Asia vs. multiple nations), individuals’ main age (<65 vs. ≧ 65 y), mean body mass index (BMI) (<25 vs. ≧ 25), aspirin dose taken (≤100 vs. > 100 mg) and 10-y MACE% (low risk vs. high risk). For 10-y MACE%, the computed value of 10-y MACE% < 10% was defined as low risk, but the other populations were high risk. To provide more useful clinical data as well as to investigate the influence of individual studies on final results, we carried out sensitivity analyses by omitting one study each turn.
Publication bias was assessed by funnel plots and Egger’s test (Egger et al., 1997), with p < 0.05 indicating significant bias. All analyses were performed using R project software (version 3.5.3, https://www.r-project.org/, United States) with forest, ggplot2, survminer etc. public packages; a two-sided p < 0.05 was considered statistically significant except where otherwise specified. More details are provided in the Supplementary Material S1.
Trial Sequential Analysis
Previous studies have confirmed that the risk of type 1 error from interim analyses can be reasonably reduced through monitoring boundaries and modifying the p-value. Similar in meta-analyses, random errors caused by sparse data and repetitive testing also enhance the risk of type 1 error. Such a method setting analogous trial sequential monitoring boundaries to meta-analyses is called trial sequential analysis (TSA), is used to determine whether evidence is reliable or conclusive (Wetterslev et al., 2008; Brok et al., 2009). Actually, random errors can be rectified and reduced using TSA software [version 0.9 beta (http://www.ctu.dk/tsa)] because it combines the estimation of the required information size (RIS) with an adjusted threshold for statistical significance. We assumed that if the Z-curve crossed the TSA boundary or entered the futility area, a sufficient effect was obtained, and further studies were not required; otherwise, the amount of evidence was considered insufficient. TSA was performed for a 10% relative risk reduction, conservatively, according to the TSA manual; there was also a 5% (α = 0.05; two-sided) risk of a type 1 error and 80% statistical power. Other parameters were set empirically following default settings.
Results
Study Selection and Characteristics
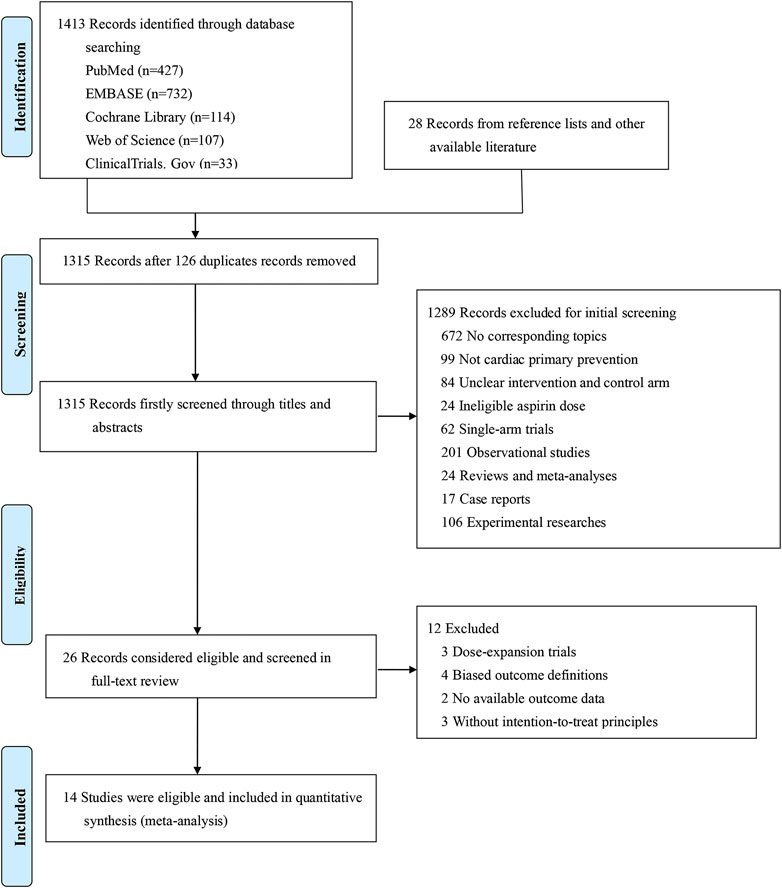
Among 1,441 searched articles (1,423 from database searching and 28 from other available source), we identified 26 studies for full-text review, of which 14 studies were eligible for qualitative and quantitative analyses (Figure 1). The 14 included studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Belch et al., 2008; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Saito et al., 2017; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018) encompassed a total of 163,840 patients and used intention-to-treat principles. The detailed study characteristics are summarized in Table 1.
Two studies (Steering Committee of the Physicians’ Health Study Research Group, 1989; Ridker et al., 2005) were conducted in America, six studies were conducted in Europe (5 (Peto et al., 1988; The Medical Research Council’s General Practice Research Framework, 1998; Belch et al., 2008; Saito et al., 2017; Bowman et al., 2018) in the United Kingdom and 1 (de Gaetano, 2001) in Italy), three studies (Ogawa et al., 2008; Ikeda et al., 2014; Saito et al., 2017) were performed in Japan, and three studies (Hansson et al., 1998; Gaziano et al., 2018; McNeil et al., 2018) were performed in multiple nations. The comparator treatment was a placebo group in nine studies (Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; Ridker et al., 2005; Belch et al., 2008; Fowkes et al., 2010; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018) and was a no aspirin group in five studies. Of note, in addition to aspirin and placebo, six studies used a factorial design, in which 1 (The Medical Research Council’s General Practice Research Framework, 1998) study used warfarin, 2 (de Gaetano, 2001); (Ridker et al., 2005) used vitamin E, 1 (Bowman et al., 2018) prescribed n-3 fatty acid, 1 (Belch et al., 2008) used antioxidants, and 1 (Peto et al., 1988) supplied anti-hypertension drugs. Three studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998) exclusively enrolled male individuals (29,750 males), and one study (Ridker et al., 2005) specially enrolled female individuals (39,876 females). Across the included studies, 78,696 (48%) patients were males. Four studies (Belch et al., 2008; Ogawa et al., 2008; Saito et al., 2017; Bowman et al., 2018) exclusively enrolled diabetic patients (including type I and type II diabetes). The mean BMI of eligible participants was 28.5, and the mean 10-y MACE% was 7.24. The median duration was 8.1 y (4 (de Gaetano, 2001) to 13 (The Medical Research Council’s General Practice Research Framework, 1998; Saito et al., 2017)), and the mean follow-up was 6.2 y. The studies were published between 1988 (Peto et al., 1988) and 2018 (Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018). All studies were written in English, and there was no attempt to ask the primary authors for raw data.
Methodological Quality Assessment
Of the 14 included studies, nine studies used double-blind methods and five studies (Peto et al., 1988; de Gaetano, 2001; Ogawa et al., 2008; Ikeda et al., 2014; Saito et al., 2017) used open-label settings. Three studies (Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; de Gaetano, 2001) had selective reporting or other bias. Of the included studies, 7 (Hansson et al., 1998; Ridker et al., 2005; Belch et al., 2008; Fowkes et al., 2010; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018) were of low risk and 7 (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; de Gaetano, 2001; Ogawa et al., 2008; Ikeda et al., 2014; Saito et al., 2017) were of high risk (Supplementary Figure S1; Supplementary Table S2).
The Primary Efficacy Outcomes
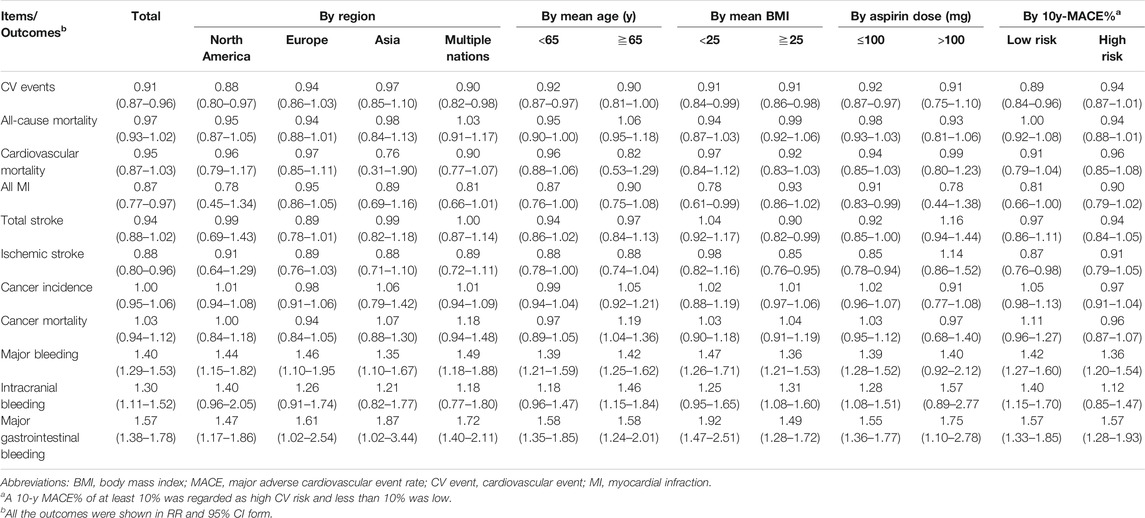
For the primary efficacy outcomes, twelve studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Saito et al., 2017; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018) involving 160,024 individuals reported CV event outcomes, and we found that the use of aspirin was associated with a 9% reduction in CV events (RR: 0.91, 95% CI: 0.87–0.96; p < 0.001; RD: 0.29%; AR%: 7.61%; NNT = 345) compared to no aspirin use, and there was no significant heterogeneity (I2 = 0; p = 0.64). Thirteen studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Belch et al., 2008; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018) including 161,680 individuals examined all-cause mortality outcomes; aspirin use did not lead to a significant reduction in all-cause mortality (RR: 0.97, 95% CI: 0.93–1.02; p = 0.22; RD: 0.04%; AR%: 0.99%; NNT = 2,273), and there was no heterogeneity (I2 = 0; p = 0.60). Fourteen studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Belch et al., 2008; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Saito et al., 2017; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018) (163,840 participants) examined cardiovascular mortality; aspirin use was not significantly associated with cardiovascular mortality reduction (RR: 0.95, 95% CI: 0.87–1.03; p = 0.23; RD: 0.02%; AR%: 1.91%; NNT = 4,348), and there was no significant heterogeneity (I2 = 0; p = 0.57) (Figure 2).
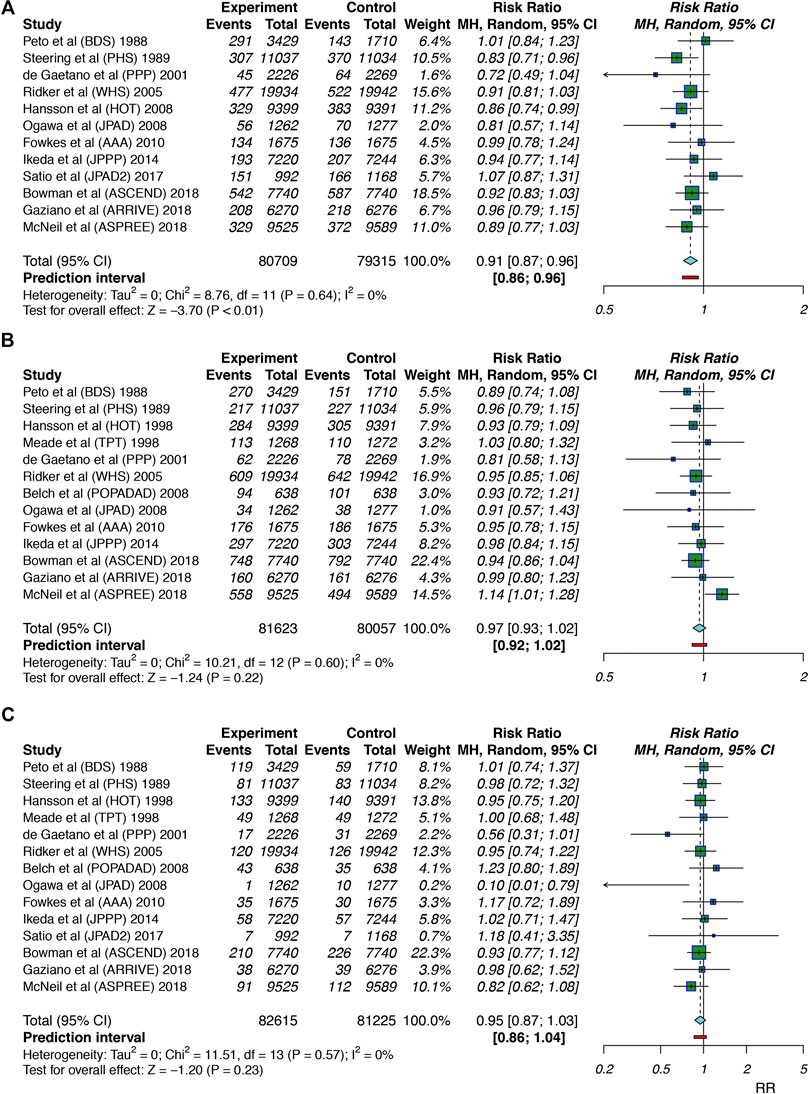
FIGURE 2. Summary forest plots for the primary efficacy outcomes. (A) Forest plot for CV events. (B) Forest plot for all-cause mortality. (C) Forest plot for cardiovascular mortality.
The Secondary Efficacy Outcomes
Regarding the secondary efficacy outcomes, fourteen studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Belch et al., 2008; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Saito et al., 2017; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018) with 163,840 individuals revealed that aspirin intake was associated with a 13% reduction in all MIs (RR: 0.87, 95% CI: 0.77–0.97; p = 0.02; RD: 0.21%; AR%: 11.11%; NNT = 488), and there was significant heterogeneity (I2 = 58%; p < 0.01). Eleven studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; de Gaetano, 2001; Ridker et al., 2005; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Saito et al., 2017; Bowman et al., 2018; McNeil et al., 2018) (131,228 individuals) revealed that aspirin intake was associated with a 12% risk reduction in ischemic stroke (RR: 0.88, 95% CI: 0.80–0.96; p < 0.01; RD: 0.21%; AR%: 16.14%; NNT = 476), and there was no significant heterogeneity (I2 = 0; p = 0.62). Fourteen studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Belch et al., 2008; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Saito et al., 2017; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018) (163,840 individuals) revealed that aspirin use was not significantly associated with total stroke (RR: 0.94, 95% CI: 0.88–1.02; p = 0.13; RD: 0.09%; AR%: 5.30%; NNT = 1,111), and there was no significant heterogeneity (I2 = 0; p = 0.59).
Furthermore, we explored the cancer outcomes. Ten studies (Peto et al., 1988; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Belch et al., 2008; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Bowman et al., 2018; McNeil et al., 2018) including 124,523 participants and 12 studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Belch et al., 2008; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Bowman et al., 2018; McNeil et al., 2018) including 149,134 participants reported cancer incidence and cancer mortality, respectively. There was no significant difference in cancer incidence (RR: 1.00, 95% CI: 0.95–1.06; p = 0.87; RD: 0.02%; AR%: 0.28%; NNT = 5,000) or cancer mortality (RR: 1.03, 95% CI: 0.94–1.12; p = 0.87; RD: 0.07%; AR%: 3.41%; NNT = 1,449) between the aspirin use and no aspirin use groups, and there was no significant heterogeneity (I2 = 36%, p = 0.12; I2 = 21%, p = 0.24, respectively). Aspirin showed the potential to increase the risk of cancer mortality (Supplementary Figure S2).
The Safety Profile Outcomes
Safety profiles outcomes included major bleeding, intracranial bleeding and major gastrointestinal bleeding. Twelve studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Saito et al., 2017; Bowman et al., 2018; McNeil et al., 2018) including 150,397 patients examined major bleeding events; aspirin use was found to significantly increase the risk of major bleeding by 40% (RR: 1.40, 95% CI: 1.29–1.53; p < 0.01; RD: 0.47%; AR%: 27.85%; NNT = 214), and there was no significant heterogeneity (I2 = 0%; p = 0.54). Thirteen studies (Peto et al., 1988; Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Ogawa et al., 2008; Fowkes et al., 2010; Ikeda et al., 2014; Saito et al., 2017; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018) (162,934 participants) examined intracranial bleeding; aspirin use was associated with a 30% increase in intracranial bleeding (RR: 1.30, 95% CI: 1.11–1.52; p < 0.01; RD: 0.10%; AR%: 22.99%; NNT = 1,000), and there was no heterogeneity (I2 = 0%; p = 0.84). Eleven trials (Steering Committee of the Physicians’ Health Study Research Group, 1989; The Medical Research Council’s General Practice Research Framework, 1998; Hansson et al., 1998; de Gaetano, 2001; Ridker et al., 2005; Ogawa et al., 2008; Fowkes et al., 2010; Saito et al., 2017; Bowman et al., 2018; Gaziano et al., 2018; McNeil et al., 2018) (143,340 participants) examined major gastrointestinal bleeding; aspirin intake was associated with a 57% increase in major gastrointestinal bleeding (RR: 1.57, 95% CI: 1.38–1.78; p < 0.01; RD: 0.32%; AR%: 36.70%; NNT = 315), and there was no heterogeneity (I2 = 0%; p = 0.57). The finding that aspirin use significantly increased the risk of bleeding events led us to identify the proper indicators for balancing the benefits and harm of clinical routines (Figure 3).
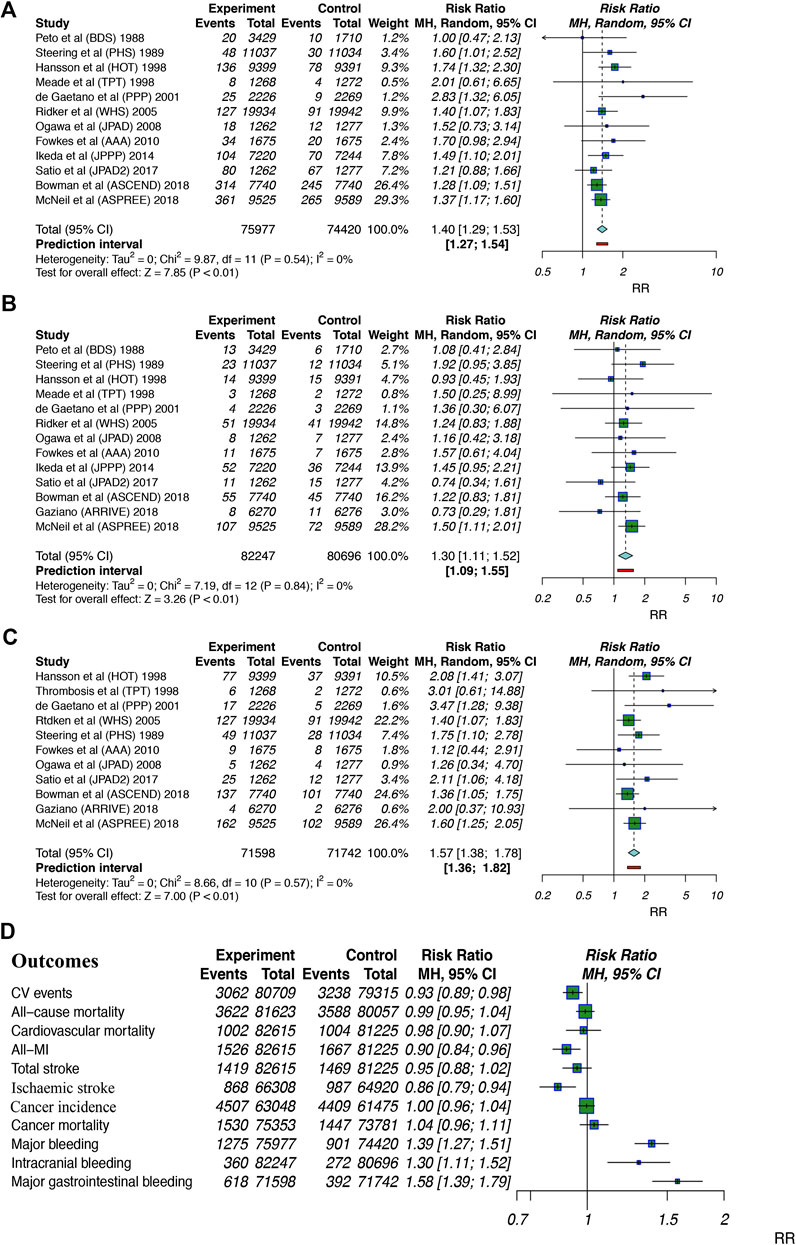
FIGURE 3. Summary forest plots for the outcomes of bleeding. (A) Forest plot for major bleeding. (B) Forest plot for intracranial bleeding. (C) Forest plot for major gastrointestinal bleeding. (D) Forest plot for summarized outcomes analyzed in the current study. MI, myocardial infarction; 95% CI, 95% confidence interval.
Subgroup Analysis for Further Clinical Implications
Subgroups involving region, mean age, mean BMI, aspirin dosage in the intervention arm and 10-y MACE% were constructed, and subgroup analyses were performed (Table 2). We observed that populations with a dosage of ≤100 mg/day experienced more benefits with respect to CV events, MI, total stroke and ischemic stroke than those with a dosage >100 mg/day. Individuals with a BMI ≧ 25 seemed experience more aspirin-induced benefits with respect to cardiovascular and cerebrovascular outcomes (CV events, RR: 0.91, 95% CI: 0.86–0.98; total stroke, RR: 0.90, 95% CI: 0.82–0.99; ischemic stroke, RR: 0.85, 95% CI: 0.76–0.95) than individuals with a BMI < 25 with similar bleeding events. Aspirin-induced cardiovascular benefits were consistently found in participants with a mean age < 65 y; however, they were not as robust in the patients with a mean age ≥ 65 y, with only one statistically significant outcome for CV events (RR: 0.90, 95% CI: 0.81–1.00). Participants with a low 10-y MACE% risk had the potential to obtain more cardiovascular advantages from aspirin use than those with a high 10-y MACE% risk. There was no significant difference in cardiovascular outcomes and bleeding events between patients from different regions. Across the subgroup analyses, aspirin still had no statistically significant effects on cancer incidence or mortality. All of the above results are presented in Table 2.
Sensitivity Analysis
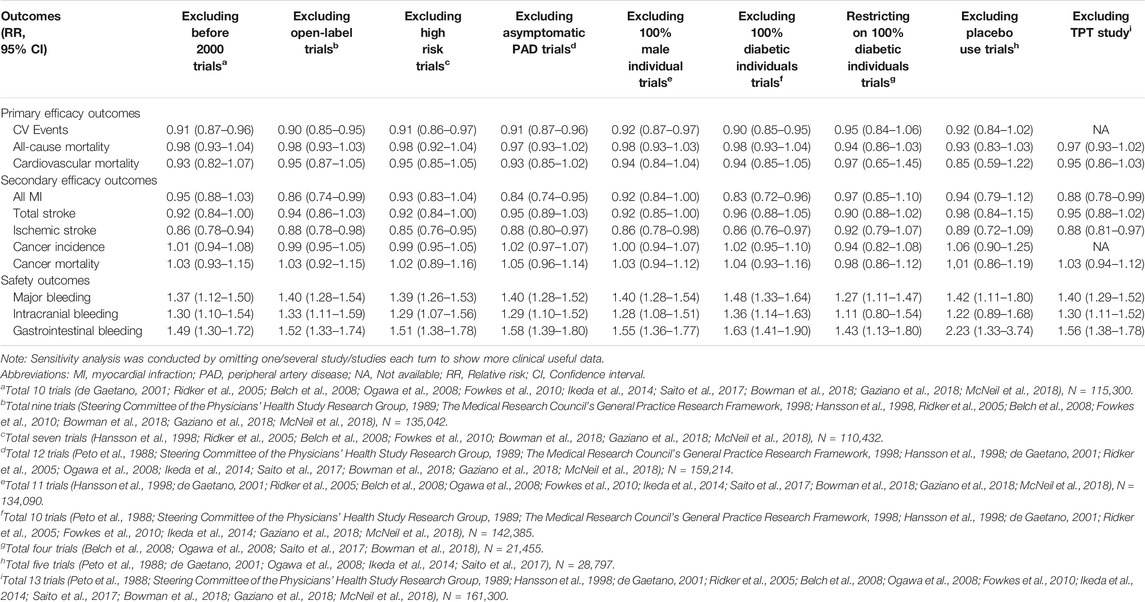
In sensitivity analyses, many variables were classified into different subgroups. To better eliminate bias and heterogeneous interactions (TPT (The Medical Research Council’s General Practice Research Framework, 1998) trial was excluded for warfarin use), we used the inverse variance (IV) statistical method. Most of the results were consistent with the primary results and remained robust through sensitivity analyses. Interestingly, we observed increased aspirin-induced benefits for cardiac outcomes (CV events, RR: 0.90, 95% CI: 0.85–0.95; all MI, RR: 0.83, 95% CI: 0.72–0.96; ischemic stroke, RR: 0.86, 95% CI: 0.76–0.97) among trials with diabetic and nondiabetic patients compared to the trials involving only diabetic patients. We also observed aspirin-induced benefits when excluding patients with asymptomatic peripheral artery disease (PAD). Furthermore, after excluding trials published before 2000, the cardiovascular benefits were still obvious. No effects on cancer were found across sensitivity analyses (Table 3). The omission process as well as the results of the heterogeneity analyses can be found in Table 3 and Supplementary Material S2–S12.
These findings implied that aspirin use among diabetic individuals may not lead to the primary prevention of CVD because diabetes, which is known as a risk factor for CVD, might indirectly enhance the CV risk estimated by the MACE; similarly, the efficacy of aspirin use in studies including both diabetic and nondiabetic patients was excellent. Second, diagnosis technology is developing over time, which means that more patients with potential or asymptomatic CVD could be properly diagnosed and excluded before entering clinical trials or taking aspirin for “primary prevention”. Therefore, the preferable role of aspirin in the primary prevention of CVD would be highlighted, especially in recently published studies (after 2000). Finally, early screening for PAD was equally important to help identify individuals who may not benefit from aspirin.
Trial Sequential Analysis
In TSA, we observed the Z-curve cross the trial sequential analysis boundary (TSA boundary) for CV events, all MI, ischemic stroke, major bleeding, intracranial bleeding and major gastrointestinal bleeding outcomes under conditions of 5% relative risk reduction, 5% for two-sided type 1 error risk, 80% statistical power and 5% control event incidence. The Z-curve did not cross the traditional boundary or the TSA boundary but crossed the futility boundary for cardiovascular mortality. The Z-curve crossed the traditional and futility boundaries but did not cross the TSA boundary for all-cause mortality. These findings showed that conclusions on the abovementioned outcomes were robust and were hardly modified with additional related trials. However, the Z-curve did not cross the TSA boundary or the futility boundary for total stroke, cancer incidence and cancer mortality, which suggested that additional studies should be conducted to evaluate those effects (Figure 4; Supplementary Figure S3).
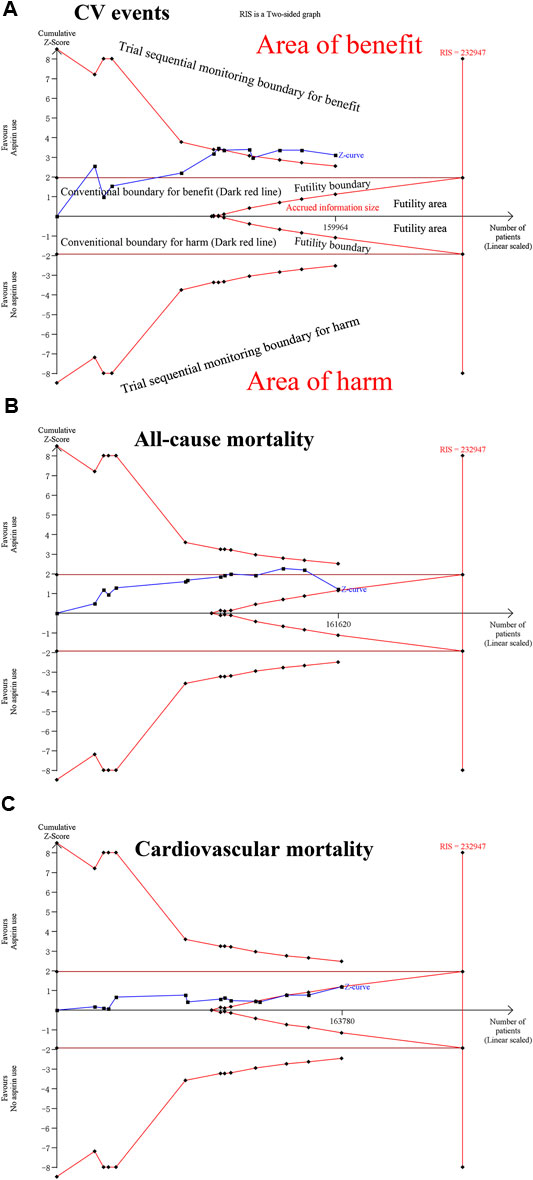
FIGURE 4. Trial sequential analysis of CV events, all-cause mortality, and cardiovascular mortality under 5% relative risk reduction, 5% for two-sided type 1 error risk, 80% statistical power and 5% control event incidence conditions. (A) For CV events. (B) For all-cause mortality. (C) For cardiovascular mortality.
Egger’s test revealed no significant publication bias for CV events (p = 0.882), all-cause mortality (p = 0.362), CV mortality (p = 0.390), major bleeding (p = 0.126), intracranial bleeding (p = 0.236), or major gastrointestinal bleeding (p = 0.152) (Supplementary Figure S4).
Discussion
As one of the most widely used drugs worldwide, aspirin celebrated its 121st birthday in 2020 and the remarkable store is still going on (Vranckx et al., 2018). In this study, aspirin was observed to be significantly associated with a 9, 13, and 12% reduction in the risk of CV events, all-MI and ischemic stroke, respectively; however, aspirin was associated with a 40, 30, and 57% increase in the risk of bleeding profiles, including major bleeding, intracranial bleeding and major gastrointestinal bleeding, respectively. No causal outcomes were found in all-cause mortality, cardiovascular mortality, total stroke, cancer incidence or cancer mortality. Low doses of aspirin (≤100 mg) might offer more clinical benefits than high doses of aspirin; individuals who are <65 y old and have a BMI ≥ 25 demonstrated stronger effects of aspirin on the primary prevention of CVD; the data indicated that aspirin did not confer benefits in the high 10-y MACE% risk group. The results were not significantly modified after excluding asymptomatic PAD trials and trials with only diabetic individuals. Besides recommendations from contemporary guidelines, we hypothesized that aspirin might be prescribed depending on body size (BMI), that is, individuals with varied BMI should take different dose of aspirin, for we observing significant differences between <25 and ≧25 BMI, ≤100 and >100 aspirin intake groups on few intended CV outcomes (Rothwell et al., 2018). It is still crucial to perform complete screening and examinations on large populations to evaluate populations’ CVD risk, hence quantifying their probability of obtaining real benefits from aspirin. This study provides further insights through updated data on comprehensive subgroup and sensitivity analyses to display potential utility on CVD primary prevention. Indeed, the one-dose-fits-all intake strategy is unlikely optimal, and a more tailored and wise dosing approach is called for to maximize substantial benefits and reduce potential risk.
The endorsed role of aspirin in the primary prevention of ischemic events (all-MI, ischemic stroke) has been supported by several studies (Fox et al., 2015b). The potential mechanism for preventing ischemic events is based on the inhibition of thrombus propagation and plaque rupture (Cleland, 2013). This study also suggested a beneficial role of aspirin in all-MI and ischemic stroke outcomes. Notably, only two eligible trials (HOT and PHS) (Steering Committee of the Physicians’ Health Study Research Group, 1989; Hansson et al., 1998) exhibited significant risk reduction in all-MI; however, their conducting time was rather early, and no significant risk reduction was observed in cardiovascular mortality and all-cause mortality under the long follow-up period. Because the two trials were conducted early, researchers could not properly emphasize the biases from risk factors such as smoking status, blood glucose, blood cholesterol level or blood pressure. Another concern is that almost 50% of MIs are considered to be clinically silent; accordingly, it is not easy to ascertain the clinical benefit from long-term aspirin use through this endpoint (Zhang et al., 2016). It may be that all CV events are assessed to be proper endpoints to evaluate all these cases. Some studies have suggested that populations with substantially increased CVD risk may benefit from preventive aspirin use, and guidelines from the US Preventive Services Task Force also suggested prescribing low doses of aspirin in adults aged 50–59 years with a CVD risk of at least 10% (Guirguis-Blake et al., 2016), which was in contrast to our findings that low-risk individuals seemed to obtain more clinical benefits. We used the 10-y MACE% to reflect participants’ CVD risk and hypothesized that the CVD risk of participants tended to be overestimated due to the lack of agreement on unified risk calculators in primary trials (Rana et al., 2016). For example, the ARRIVE trial (Gaziano et al., 2018) mixed predicted and observed CVD risk, such that the enrolled moderate risk populations had a standard risk of 17.3% as estimated by American Heart Association (AHA)/American College of Cardiology (ACC) 10-y CV risk estimated criteria (Allan et al., 2013; Rana et al., 2016) but had an observed CVD risk rate of 6.9%. Similarly, the ASPREE trial (McNeil et al., 2018) enrolled patients who were older than 65 or 70 y old; the CVD risk of these older patients was hard to evaluate, and the reported 10-y MACE% of 7.8% differed from the 8.3% figure found herein, although both 10-y MACE% were less than 10%. The reason for this discrepancy was that MACE in the ASPREE trial was defined as a composite of fatal coronary heart disease, nonfatal MI and fatal or nonfatal ischemic stroke, which differed from the unified definition. In this study, CV event risk was reduced by 11% in the low 10-y MACE% risk group.
Guidelines driven by the AHA/American Diabetes Association (ADA) recommend aspirin use in diabetic populations with intermediate risk (5–10% 10-y MACE%) for primary prevention (Fox et al., 2015b). JPAD (Ogawa et al., 2008) and ASCEND (Bowman et al., 2018) trials specifically incorporated diabetic populations, but the cardiovascular benefits seemed to be higher in the ASCEND trial. The total proportion of statin use was 75% in the ASCEND trial vs. 25% in the JPAD trial, which might have resulted in higher benefits seen in the ASCEND trial. Additionally, this study indicated fewer CVD benefits among populations with diabetes, which was supported by recent European Society of Cardiology guidelines recommending against aspirin use in diabetic populations who have no history of CVD (Piepoli et al., 2016). Routine aspirin use was not enough for primary prevention among individuals with a high risk of CVD; at that time, blood pressure and blood glucose were controlled, cholesterol levels were reduced with statins, and physical activity and healthy eating were reduced are also necessary. Aspirin use increased the risk of bleeding profiles but was not associated with cardiovascular mortality considering that deaths caused by bleeding were rare. Since the strategy to reduce harm of long-term aspirin use is not understood from current evidence, prescribing proton pump inhibitors (PPIs) might limit the risk of major gastrointestinal bleeding and enhance the benefit-risk ratio toward intended populations (Fowkes et al., 2010). Aspirin appears to be not associated with all-cause mortality; however, several trials revealed that aspirin reduced the risk of colorectal cancer (RR: 0.73, 95% CI: 0.69–0.78), squamous-cell oesophageal cancer (RR: 0.67, 95% CI: 0.57–0.79), gastric cancer (RR: 0.64, 95% CI: 0.51–0.82) and pancreatic cancer (RR: 0.78, 95% CI: 0.68–0.89) (Bosetti et al., 2020). At this time, the reduction in cancer mortality appeared after 5 y of follow-up, and this result was not duplicated in the ASCEND trial (Bowman et al., 2018). Current findings suggest a neutral role of aspirin in cancer outcomes; therefore, no suggestions could be made regarding benefit-risk balance from current evidence.
Added Value and Limitations
Contrast to prior similar studies, current study has several innovations. Mahmoud et al. (Mahmoud et al., 2019) conducted a TSA meta-analysis, the authors mainly focused on CVD-related outcomes including all-cause mortality, all MI, bleeding events. Comparing to Mahmoud et al. (Mahmoud et al., 2019), current study is more comprehensive because we also investigated cancer outcomes. Study from Mahmoud et al. (Mahmoud et al., 2019) included 11 RCTs, in our prospective, it was not enough, trials like POPADAD (Belch et al., 2008), AAA (Fowkes et al., 2010) were not reasonably included. Also, several 10y-MACE% values presented in that study were not in consistent with current study, for example ASCEND (Bowman et al., 2018), ARRIVE (Gaziano et al., 2018) and ASPREE (McNeil et al., 2018). 10y-MACE% for BDS (Peto et al., 1988) and TPT (The Medical Research Council’s General Practice Research Framework, 1998) was also absent in Mahmoud et al. (Mahmoud et al., 2019) study. Lin et al. (Lin et al., 2019) investigated the role of low-dose of aspirin on CVD primary prevention, they demonstrated low-dose aspirin had no role in all MI, but did reduce stroke incidence, which was in contrast to findings from current paper (that aspirin might significantly reduce all MI incidence instead of total stroke, ischemic stroke could be reasonably reduced). Current study had included more comprehensive RCTs than Lin et al. (Lin et al., 2019), subgroup analyses aiming to low-dose of aspirin (<100 mg/d) were also conducted. This study clearly pinpointed low CVD risk individuals might get more clinical benefits than the high risk from aspirin. Only one TSA for MACE outcome in Lin et al. (Lin et al., 2019) was far enough to draw robust conclusions. Major controversial issues from current study and Gelbenegger et al. (Gelbenegger et al., 2019) were the outcomes on diabetic populations, this study supported there were no substantial benefits of aspirin on diabetic populations primary prevention. POPADAD (Belch et al., 2008), JPAD (Ogawa et al., 2008), JPAD2 (Saito et al., 2017) and ASCEND (Bowman et al., 2018) were special trials conducted on full diabetic populations (100% diabetic individuals), to our great knowledge, it was more proper to investigate the intended results on the four trials, data stem from calculation on other small diabetic-proportion trials (Ridker et al., 2005; Ikeda et al., 2014) would add extra reporting bias. Zheng et al. (Zheng and Roddick, 2019) also performed a similar research, however, no TSA results were revealed and merits from network meta-analysis methods seemed not so obvious. Overall, current study with particular subgroup and sensitivity analyses clearly addressed the less priority of aspirin on high 10y-MACE% risk and diabetic populations, such populations may need more aggressive therapy or combined pharmaceutical intervention. We believe these results add new evidence to the discussion on aspirin primary prevention in CVD and may arouse new disputes.
Limitations were also detected. First, definitions of reported outcomes were different, reflecting advances in CVD diagnosis and treatment. To best overcome this heterogeneity, we defined unified primary and secondary efficacy outcomes and safety profiles and then properly extracted the required data in eligible studies. Second, aspirin use in the included studies was not consistent with the major dose of 75–100 mg. Importantly, more clinical benefits with bleeding risk were found in trials restricted to ≤100 mg/d intake. Third, several trials (BDS (1998), PHS (1989), TPT (1998), HOT (1998)) were published rather early, and thus, some examinations and screening methods may not have been as accurate as expected. This contributed to an overestimated 10-y MACE%. Long-term follow-up studies are welcomed to better characterize individuals who may benefit from aspirin for primary prevention outweighing the unexpected bleeding events. Objective influence on all-cause mortality and cancer incidence should be re-evaluated. Considering no individual-patients-data was involved, therefore, a more precise study based on individual data is quite encouraged.
Conclusion
Aspirin intake was associated with reduced risk of CV events, all MI, and ischemic stroke, and was associated with increased incidences of major bleeding, intracranial bleeding, and major gastrointestinal bleeding in the primary prevention of CVD. The use was not associated with an increased risk of all-cause mortality, cardiovascular mortality, total stroke, cancer incidence or cancer mortality. No substantial benefits with respect to CVD were observed in the diabetic and high 10-y MACE% risk group populations. A one-dose-fits-all strategy is not optimal, and BMI may be a potential indicator to guide aspirin prescription. It is also necessary to identify individuals who may benefit from aspirin by more accurate cardiovascular-relating examinations. Overall, the benefits and harm of aspirin for primary prevention should be re-evaluated. Based on these findings, we believe it is not yet the time to quit the aspirin era.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
All authors designed and conducted this review. BZ wrote the paper. QW, LW, CL, YD, JX, YW, and WZ helped the study design. BZ, YW, and WZ revised the statistical methodology. BZ and WZ had primary responsibility for the final content. All authors read and approved the final manuscript. Notably, BZ and WZ equally share the corresponding authorship.
Funding
This study was supported by National Natural Science Foundation of China (NSFC), with no commercial entity involved (grant no 81560345). The NSFC had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No other disclosures were reported.
Acknowledgments
The authors thank Wenbin Ma, MD, PhD (Departments of Neurosurgery, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College), for his writing instructions and statistical guidance.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.592116/full#supplementary-material
References
Allan, G. M., Nouri, F., Korownyk, C., Kolber, M. R., Vandermeer, B., and McCormack, J. (2013). Agreement among cardiovascular disease risk calculators. Circulation 127 (19), 1948–1956. doi:10.1161/CIRCULATIONAHA.112.000412
Antiplatelet Trialists’ Collaboration (1994). Collaborative overview of randomised trials of antiplatelet therapy–III: reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. Antiplatelet trialists’ collaboration. BMJ 308 (6923), 235–246. doi:10.1136/bmj.308.6923.235
Antithrombotic Trialists’ Collaboration (2002). Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324 (7329), 71–86. doi:10.1136/bmj.324.7329.71
Belch, J., MacCuish, A., Campbell, I., Cobbe, S., Taylor, R., Prescott, R., et al. (2008). The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ 337, a1840. doi:10.1136/bmj.a1840
Bibbins-Domingo, K. (2016). Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann. Intern. Med. 164 (12), 836–845. doi:10.7326/M16-0577
Bosetti, C., Santucci, C., Gallus, S., Martinetti, M., and La Vecchia, C. (2020). Aspirin and the risk of colorectal and other digestive tract cancers: an updated meta-analysis through 2019. Ann. Oncol. 31 (5), 558–568. doi:10.1016/j.annonc.2020.02.012
Bowman, L., Mafham, M., Wallendszus, K., Stevens, W., Buck, G., Barton, J., et al. (2018). Effects of aspirin for primary prevention in persons with diabetes mellitus. N. Engl. J. Med. 379 (16), 1529–1539. doi:10.1056/NEJMoa1804988
Brok, J., Thorlund, K., Wetterslev, J., and Gluud, C. (2009). Apparently conclusive meta-analyses may be inconclusive–trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int. J. Epidemiol. 38 (1), 287–298. doi:10.1093/ije/dyn188
Bulugahapitiya, U., Siyambalapitiya, S., Sithole, J., Fernando, D. J., and Idris, I. (2008). Age threshold for vascular prophylaxis by aspirin in patients without diabetes. Heart 94 (11), 1429–1432. doi:10.1136/hrt.2008.150698
Cleland, J. G. (2013). Is aspirin useful in primary prevention? Eur. Heart J. 34 (44), 3412–3418. doi:10.1093/eurheartj/eht287
de Gaetano, G. (2001). Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomised trial in general practice. Collaborative group of the primary prevention project. Lancet 357 (9250), 89–95. doi:10.1016/s0140-6736(00)03539-x
De Lima Taga, M. F., and Singer, J. M. (2018). Simple linear regression with interval censored dependent and independent variables. Stat. Methods Med. Res. 27 (1), 198–207. doi:10.1177/0962280215626467
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Elwood, P., Morgan, G., Brown, G., and Pickering, J. (2005). Aspirin for everyone older than 50? For BMJ 330 (7505), 1440–1441. doi:10.1136/bmj.330.7505.1440
Fowkes, F. G., Price, J. F., Stewart, M. C., Butcher, I., Leng, G. C., Pell, A. C., et al. (2010). Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. J. Am. Med. Assoc. 303 (9), 841–848. doi:10.1001/jama.2010.221
Fox, C. S., Golden, S. H., Anderson, C., Bray, G. A., Burke, L. E., de Boer, I. H., et al. (2015a). Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American heart association and the American diabetes association. Circulation 38 (9), 1777–1803. doi:10.1161/CIR.0000000000000230
Fox, C. S., Golden, S. H., Anderson, C., Bray, G. A., Burke, L. E., de Boer, I. H., et al. (2015b). Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American heart association and the American diabetes association. Circulation 132 (8), 691–718. doi:10.1161/CIR.0000000000000230
Gaziano, J. M., Brotons, C., Coppolecchia, R., Cricelli, C., Darius, H., Gorelick, P. B., et al. (2018). Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 392 (10152), 1036–1046. doi:10.1016/S0140-6736(18)31924-X
Gelbenegger, G., Postula, M., Pecen, L., Halvorsen, S., Lesiak, M., Schoergenhofer, C., et al. (2019). Aspirin for primary prevention of cardiovascular disease: a meta-analysis with a particular focus on subgroups. BMC Med. 17 (1), 198. doi:10.1186/s12916-019-1428-0
Grundy, S. M., Stone, N. J., Bailey, A. L., Beam, C., Birtcher, K. K., Blumenthal, R. S., et al. (2019). 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of cardiology/American heart association task force on clinical practice guidelines. Circulation 139 (25), e1082–e1143. doi:10.1161/CIR.0000000000000625
Guirguis-Blake, J. M., Evans, C. V., Senger, C. A., O’Connor, E. A., and Whitlock, E. P. (2016). Aspirin for the primary prevention of cardiovascular events: a systematic evidence review for the U.S. preventive services task force. Ann. Intern. Med. 164 (12), 804–813. doi:10.7326/M15-2113
Hansson, L., Zanchetti, A., Carruthers, S. G., Dahlof, B., Elmfeldt, D., Julius, S., et al. (1998). Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) randomised trial. HOT Study Group. Lancet 351 (9118), 1755–1762. doi:10.1016/s0140-6736(98)04311-6
Higgins, J. P., Altman, D. G., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Ikeda, Y., Shimada, K., Teramoto, T., Uchiyama, S., Yamazaki, T., Oikawa, S., et al. (2014). Low-dose aspirin for primary prevention of cardiovascular events in Japanese patients 60 years or older with atherosclerotic risk factors: a randomized clinical trial. J. Am. Med. Assoc. 312 (23), 2510–2520. doi:10.1001/jama.2014.15690
Lin, M. H., Lee, C. H., Lin, C., Zou, Y. F., Lu, C. H., Hsieh, C. H., et al. (2019). Low-dose aspirin for the primary prevention of cardiovascular disease in diabetic individuals: a meta-analysis of randomized control trials and trial sequential analysis. J. Clin. Med. 8 (5), 609. doi:10.3390/jcm8050609
Mahmoud, A. N., Gad, M. M., Elgendy, A. Y., Elgendy, I. Y., and Bavry, A. A. (2019). Efficacy and safety of aspirin for primary prevention of cardiovascular events: a meta-analysis and trial sequential analysis of randomized controlled trials. Eur. Heart J. 40 (7), 607–617. doi:10.1093/eurheartj/ehy813
McNeil, J. J., Wolfe, R., Woods, R. L., Tonkin, A. M., Donnan, G. A., Nelson, M. R., et al. (2018). Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N. Engl. J. Med. 379 (16), 1509–1518. doi:10.1056/NEJMoa1805819
Mortensen, M. B., and Nordestgaard, B. G. (2020). 2019 vs. 2016 ESC/EAS statin guidelines for primary prevention of atherosclerotic cardiovascular disease. Eur. Heart J. 41, 3005–3015. doi:10.1093/eurheartj/ehaa150
Ogawa, H., Nakayama, M., Morimoto, T., Uemura, S., Kanauchi, M., Doi, N., et al. (2008). Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. J. Am. Med. Assoc. 300 (18), 2134–2141. doi:10.1001/jama.2008.623
Pearson, T. A., Blair, S. N., Daniels, S. R., Eckel, R. H., Fair, J. M., Fortmann, S. P., et al. (2002). AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American heart association science advisory and coordinating committee. Circulation 106 (3), 388–391. doi:10.1161/01.cir.0000020190.45892.75
Peto, R., Gray, R., Collins, R., Wheatley, K., Hennekens, C., Jamrozik, K., et al. (1988). Randomised trial of prophylactic daily aspirin in British male doctors. Br. Med. J. 296 (6618), 313–316. doi:10.1136/bmj.296.6618.313
Piepoli, M. F., Hoes, A. W., Agewall, S., Albus, C., Brotons, C., Catapano, A. L., et al. (2016). European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European association for cardiovascular prevention & rehabilitation (EACPR). Eur. Heart J. 37 (29), 2315–2381. doi:10.1093/eurheartj/ehw106
Rana, J. S., Tabada, G. H., Solomon, M. D., Lo, J. C., Jaffe, M. G., Sung, S. H., et al. (2016). Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J. Am. Coll. Cardiol. 67 (18), 2118–2130. doi:10.1016/j.jacc.2016.02.055
Ridker, P. M., Cook, N. R., Lee, I. M., Gordon, D., Gaziano, J. M., Manson, J. E., et al. (2005). A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N. Engl. J. Med. 352 (13), 1293–1304. doi:10.1056/NEJMoa050613
Rothwell, P. M., Cook, N. R., Gaziano, J. M., Price, J. F., Belch, J. F. F., Roncaglioni, M. C., et al. (2018). Effects of aspirin on risks of vascular events and cancer according to bodyweight and dose: analysis of individual patient data from randomised trials. Lancet 392 (10145), 387–399. doi:10.1016/S0140-6736(18)31133-4
Saito, Y., Okada, S., Ogawa, H., Soejima, H., Sakuma, M., Nakayama, M., et al. (2017). Low-dose aspirin for primary prevention of cardiovascular events in patients with type 2 diabetes mellitus: 10-Year follow-up of a randomized controlled trial. Circulation 135 (7), 659–670. doi:10.1161/CIRCULATIONAHA.116.025760
Steering Committee of the Physicians’ Health Study Research Group (1989). Final report on the aspirin component of the ongoing physicians’ health study. N. Engl. J. Med. 321 (3), 129–135. doi:10.1056/NEJM198907203210301
The Medical Research Council’s General Practice Research Framework (1998). Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The medical research council’s general practice research framework. Lancet 351 (9098), 233–241. doi:10.1016/S0140-6736(97)11475-1
Vranckx, P., Valgimigli, M., Jüni, P., Hamm, C., Steg, P. G., Heg, D., et al. (2018). Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet 392 (10151), 940–949. doi:10.1016/S0140-6736(18)31858-0
Wald, N. J., and Law, M. R. (2003). A strategy to reduce cardiovascular disease by more than 80%. BMJ 326 (7404), 1419. doi:10.1136/bmj.326.7404.1419
Wetterslev, J., Thorlund, K., Brok, J., and Gluud, C. (2008). Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J. Clin. Epidemiol. 61 (1), 64–75. doi:10.1016/j.jclinepi.2007.03.013
Whitlock, E. P., Burda, B. U., Williams, S. B., Guirguis-Blake, J. M., and Evans, C. V. (2016). Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. preventive services task force. Ann. Intern. Med. 164 (12), 826–835. doi:10.7326/M15-2112
Zhang, Z. M., Rautaharju, P. M., Prineas, R. J., Rodriguez, C. J., Loehr, L., Rosamond, W. D., et al. (2016). Race and sex differences in the incidence and prognostic significance of silent myocardial infarction in the atherosclerosis risk in communities (ARIC) study. Circulation 133 (22), 2141–2148. doi:10.1161/CIRCULATIONAHA.115.021177
Keywords: aspirin, primary prevention, cardiovascular disease, secondary study, trial sequential analysis
Citation: Zhao B, Wu Q, Wang L, Liao C, Dong Y, Xu J, Wei Y and Zhang W (2021) Pros and Cons of Aspirin for the Primary Prevention of Cardiovascular Events: A Secondary Study of Trial Sequential Analysis. Front. Pharmacol. 11:592116. doi: 10.3389/fphar.2020.592116
Received: 14 August 2020; Accepted: 24 November 2020;
Published: 14 January 2021.
Edited by:
Raymond Noordam, Leiden University Medical Center, NetherlandsCopyright © 2021 Zhao, Wu, Wang, Liao, Dong, Xu, Wei and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxiong Zhang, zwx123dr@126.com
 Binghao Zhao
Binghao Zhao Qian Wu
Qian Wu Li Wang
Li Wang Chen Liao
Chen Liao Yifei Dong
Yifei Dong Jingsong Xu
Jingsong Xu Yiping Wei1
Yiping Wei1 Wenxiong Zhang
Wenxiong Zhang