- 1Division of Digestive Endoscopy, Shiga University of Medical Science Hospital, Otsu, Japan
- 2Department of Environmental and Preventive Medicine, Faculty of Medicine, Oita University, Yufu, Japan
Complete eradication of Helicobacter pylori is important for preventing the development of gastric cancer. The outcome of H. pylori eradication therapy is mainly dependent on bacterial susceptibility to antimicrobial agents and potent neutralization of intragastric pH across 24 h, especially when using acid-sensitive antimicrobial agents such as clarithromycin (CLR), amoxicillin and sitafloxacin. However, conventional regimens comprising twice-daily doses (bid) of proton pump inhibitors (PPIs) are generally insufficient for maintaining the required gastric acid secretion for 24 h for successful eradication in all H. pylori-positive patients. Further, the increasing prevalence of CLR-resistant strains with each year has led to a decrease in eradication rates of first-line PPI- and CLR-containing therapies in developed countries, including Japan. In 2015, the potassium-competitive acid blocker vonoprazan (VPZ) became clinically available in Japan. VPZ competitively inhibits H+/K+-ATPase activity more potently than PPIs (e.g., omeprazole, lansoprazole, rabeprazole, pantoprazole, and esomeprazole). Therefore, a VPZ-containing H. pylori eradication regimen is expected to increase the eradication rate compared with conventional regimens containing a standard dose of PPI. In fact, a recent meta-analysis that investigated the efficacy of first-line eradication therapy showed that a VPZ-containing regimen achieved a higher eradication rate than a PPI-containing regimen. While the Maastricht V/Florence Consensus Report recommends selecting a bismuth or non-bismuth quadruple therapy and concomitant therapy for patients living in areas with high prevalence of CLR resistance, a VPZ-containing regimen demonstrates effectiveness for patients infected with CLR-resistant strains and patients living in areas where the prevalence of CLR-resistant strains is >15%. As a next step, studies are needed to determine the factors affecting the clinical outcome of VPZ-containing therapy and optimal VPZ-containing alternative regimens for tailored treatments. In this review, we summarize the advantages and disadvantages of VPZ in H. pylori eradication therapy.
Introduction
Rapid and potent acid inhibition after treatment with acid-inhibitory drugs (i.e., proton pump inhibitor [PPI]) is necessary for curing acid-peptic disorders. Treatments that neutralize intragastric pH levels are associated with improved cure rates for peptic ulcers (Barer et al., 1983), gastroesophageal reflux disease (Bell et al., 1992), non-erosive reflux diseases, aspirin-induced and non-steroidal anti-inflammatory drug-induced gastroduodenal mucosal injury (Sugimoto et al., 2012a), and Helicobacter pylori infection (Labenz et al., 1995; Sugimoto et al., 2007; Yang et al., 2011). Therefore, an understanding of methods to suppress gastric acid secretion is important in the treatment of acid-peptic disorders.
The new, potent acid-inhibitory drug vonoprazan (VPZ) recently became clinically available in Japan. VPZ competitively inhibits the binding of potassium ions to H+/K+-ATPase in gastric parietal cells more potently than PPIs (Parsons and Keeling, 2005). VPZ also has two pharmacological advantages over PPIs: it does not require pharmacological activation by gastric acid to inhibit acid secretion, and has a longer half-life (t1/2) due to its slow dissociation kinetics from H+/K+-ATPase (Sugimoto et al., 2004; Scott et al., 2015). While PPIs typically require more than 75–100 h to exert a maximal gastric acid inhibitory effect (Saitoh et al., 2002; Sugimoto et al., 2006), VPZ produces rapid, strong and long-lasting gastric acid inhibition after administration of the first tablet in a dose-dependent manner (Jenkins et al., 2015; Sakurai et al., 2015). At steady state on Day 7, a once daily dose (oid) of VPZ 40 mg displayed sustained and potent acid inhibition throughout a 24-h period (Jenkins et al., 2015). Moreover, a twice daily dose (bid) of VPZ 20 mg, the standard dosage for H. pylori eradication therapy, maintained gastric acid inhibition throughout the 24 h: the pH > 4 and >5 holding time ratio (HTR) was 100 and 99%, respectively, even in H. pylori-negative subjects (Kagami et al., 2016). Therefore, VPZ may be an effective first-line acid-inhibitory drug for patients with acid-peptic disorders.
In Japan, H. pylori eradication therapies are currently limited to regimens comprising an acid-inhibitory drug such as a PPI or VPZ at a standard dose bid, amoxicillin (AMX) 750 mg bid, and clarithromycin (CLR) 200 mg or 400 mg bid for 7 days as a first-line eradication regimen; and PPI or VPZ bid, AMX 750 mg bid, and metronidazole (MNZ) 250 mg bid for 7 days as a second-line eradication regimen. Unfortunately, the frequent use of CLR in general clinical situations has led to an increase in the prevalence of CLR-resistant H. pylori strains in Japan (more than 30%) (Asaka et al., 2001; Murakami et al., 2002), prompting the need for alternative regimens. Because potent neutralization of pH is associated with better outcomes of H. pylori eradication therapy, as it is for other acid-peptic disorders (Labenz et al., 1995; Sugimoto et al., 2007; Yang et al., 2011), VPZ may dramatically improve the decreasing H. pylori eradication rate in Japan.
Here, we discuss the impact of VPZ in H. pylori eradication therapy, as well as some of its disadvantages. We first discuss the general factors influencing the cure rate for H. pylori infection due to H. pylori eradication therapy and association with the outcome of eradication and importance of inhibiting acid secretion. We subsequently discuss the efficacy of VPZ-containing eradication therapy of first-, second- and third-line treatments, and in patients with penicillin allergies.
Possible Factors Contributing to the Outcome of Eradication Therapy for H. pylori Infection
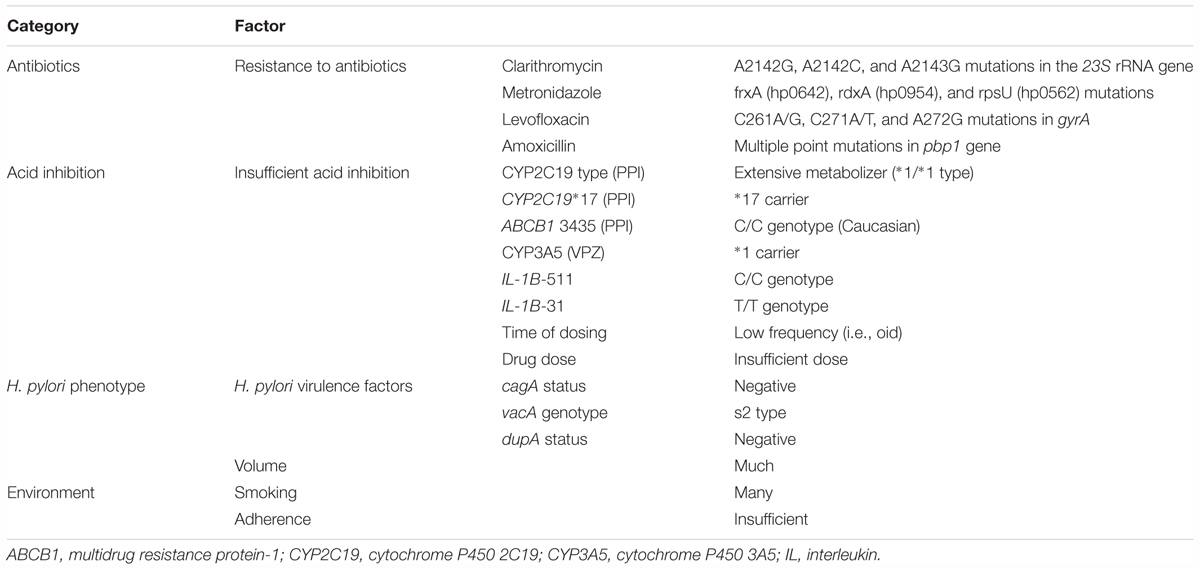
The cure rate for H. pylori infection is affected by several possible factors, as below: antibiotic susceptibility (e.g., CLR, AMX, MNZ and levofloxacin) (Asaka et al., 2001; Furuta et al., 2001; Murakami et al., 2002), insufficient acid inhibition during eradication therapy (e.g., CYP2C19 and CYP3A4/5 genotype, dose of drug, treatment schedule and type of acid-inhibitory drug) (Furuta et al., 2001; Sugimoto et al., 2007; Sugimoto and Yamaoka, 2009), the environment (e.g., smoking), and poor adherence to medication and H. pylori strain with low virulence activity (e.g., cagA-negative strains, vacA s2 genotype and dupA-negative strains) (Sugimoto and Yamaoka, 2009; Shiota et al., 2012; Table 1). Although much attention is focused on the relationship between resistant strains of H. pylori and the success or failure of eradication therapy, potent acid inhibition throughout the 24 h during eradication therapy has become re-recognized as an important outcome of eradication therapy. In fact, the Maastricht V/Florence Consensus Report states that “the use of high dose PPI bid increases the efficacy of triple therapy. Level of evidence: low, and Grade of recommendation: weak” (Malfertheiner et al., 2017).
Control of pH using acid-inhibitory drugs depends on the type of acid-inhibitory drug, dosage (dose and dosing time), combination of drugs (e.g., any of PPI, VPZ and histamine 2 receptor antagonist) and polymorphisms in drug-metabolizing enzyme genes (e.g., cytochrome P450 2C19 (CYP2C19), CYP3A4, and CYP3A5) and polymorphisms in drug transporter genes (e.g., multidrug resistance protein-1 [ABCB1]) that effect the pH during treatment (Table 1; Furuta et al., 1999; Shirai et al., 2001, 2002; Sugimoto et al., 2004, 2005, 2012b; Kodaira et al., 2009; Kagami et al., 2016).
Importance of Gastric Acid Inhibition in H. pylori Eradication Therapy
Helicobacter pylori can survive a periplasmic pH of 4.0–8.0 in the gastric mucosa (Scott et al., 1998). When the bacterial urease activity of H. pylori raises the intragastric pH to 4.0–6.0, H. pylori survives into the gastric mucosa but does not divide (Scott et al., 1998). Therefore, the consistent and potent action of acid-inhibitory drugs also enables H. pylori to grow and become more sensitive to antimicrobial agents against H. pylori (Scott et al., 1998). In addition, potent acid inhibition during 24-h increases the stability and bioavailability of acid-sensitive antimicrobial agents by preventing their degradation. Further, PPI and vonoprazan increase gastric mucosal antimicrobial agents concentration (Grayson et al., 1989; Goddard et al., 1996; Scott et al., 1998). Raising the intragastric pH from 3.5 to 5.5 is shown to increase the in vitro antimicrobial efficacy of AMX more than 10-fold (Grayson et al., 1989). The activity of CLR against H. pylori is higher at intragastric pH 7.4 than at intragastric pH 5.0, and activity is intermediate at pH 6.8 (Heifets et al., 1992). Recently, the Maastricht V/Florence Consensus Report recommended first-line eradication therapy using a CLR-containing regimen with PPI/AMX or PPIMNZ and an alternative eradication treatment using bismuth-containing quadruple treatment (PPI/bismuth/MNZ/tetracycline) in areas where prevalence of CLR-resistant strains is low (Level of evidence: high, Grade of recommendation: strong), and bismuth or non-bismuth quadruple treatment and concomitant (PPI/AMX/CLR/nitroimidazole) therapies in areas of high (>15%) CLR resistance (Level of evidence: low, Grade of recommendation: strong) (Malfertheiner et al., 2017). These recommendations indicate that there are numerous opportunities to use acid-sensitive antimicrobial agents around the world, and underlines the need to monitor inhibition of gastric acid secretion in eradication treatment.
However, the question of how strongly gastric acid secretion should be inhibited remains. Previously, we showed, using PPI/AMX/CLR triple therapy, a standard eradication regimen around the world, that the median 24-h pH for successful eradication was higher (6.4) and the median pH < 4 HTR (0.5%) was shorter than that for failed eradication (pH 5.2 and pH < 4 HTR 26.7%) (Sugimoto et al., 2007). Therefore, the degree and duration of acid inhibition during eradication therapy are related to the cure rate of H. pylori, and we concluded that intragastric pH > 4 should be maintained for 24 h and that the 24-h intragastric pH should be higher than 6.0 (Sugimoto et al., 2007).
Unfortunately, treatment with PPI at standard dose bid does not maintain pH values at higher than 4.0 for long enough to accept the above criteria in all patients receiving eradication therapy (Sugimoto et al., 2004). Therefore, it is necessary to identify the factors that affect acid secretion and to determine the optimal drug therapy to enable the inhibition of acid secretion across 24 h. Because VPZ 20 mg bid inhibits acid secretion across 24 h (pH ≥ 4 HTR is 100%), (Kagami et al., 2016) VPZ is an effective acid-inhibitory drug in eradication therapies for H. pylori infection. There is no report to investigate direct association with advantage of vonoprazan use and acid inhibition during vonoprazan-containing eradication therapy for better outcome of H. pylori eradication therapy.
First-Line Vonoprazan-Containing Eradication Therapy
Study Selection
In this review, we searched for all of relevant studies published up until September 2018 that examined the efficacy of the vonoprazan-containing triple H. pylori eradication therapies, using PubMed, EMBASE, and Web of Science. Key words were [“potassium-competitive acid blocker,” “vonoprazan,” or “VPZ”] AND [“H. pylori eradication” or “H. pylori eradication”]. In addition, we examined the references of the screened articles to identify additional studies. All studies published in English were selected, whereas studies are randomized trial and retrospective observational studies. As the first step of study selection, we excluded irrelevant articles by examining the titles and abstracts of the papers. Next, we screened the full-text of all selected studies. The inclusion criteria were (1) patients: H. pylori-positive patients, (2) eradication therapy: vonoprazan-containing triple therapies (first-, second-, and third-line treatments, and in patients with penicillin allergies), and (3) outcome: eradication rate. The exclusion criteria were (1) non-English language and (2) no detail information, such as sample number.
Efficacy of First-Line Vonoprazan-Containing Eradication Therapy
In recent years, several studies have compared eradication rates between VPZ-containing and PPI-containing triple therapies across centers in Japan. As shown in Table 2, up until September 2018, 23 reports had investigated the efficacy of first-line VPZ-containing therapy (21 reports for VPZ/AMX/CLR and 2 reports for VPZ/MNZ/CLR) (Matsumoto et al., 2016; Murakami et al., 2016; Noda et al., 2016; Shichijo et al., 2016; Shinozaki et al., 2016, 2018; Suzuki et al., 2016; Tsujimae et al., 2016; Yamada et al., 2016; Kajihara et al., 2017; Katayama et al., 2017; Maruyama et al., 2017; Nishizawa et al., 2017; Ono et al., 2017; Sakurai et al., 2017; Sue et al., 2017a,b, 2018a; Sugimoto et al., 2017; Tanabe et al., 2017, 2018; Mori et al., 2018; Ozaki et al., 2018), and 19 reports had compared the efficacy between VPZ-containing therapy and PPI-containing therapy, including 3 randomized control trials (Murakami et al., 2016; Maruyama et al., 2017; Sue et al., 2018a) and 16 non-randomized retrospective cohort trials (Matsumoto et al., 2016; Noda et al., 2016; Shichijo et al., 2016; Shinozaki et al., 2016; Suzuki et al., 2016; Tsujimae et al., 2016; Yamada et al., 2016; Kajihara et al., 2017; Nishizawa et al., 2017; Ono et al., 2017; Sakurai et al., 2017; Sue et al., 2017a,b; Mori et al., 2018; Ozaki et al., 2018; Tanabe et al., 2018).
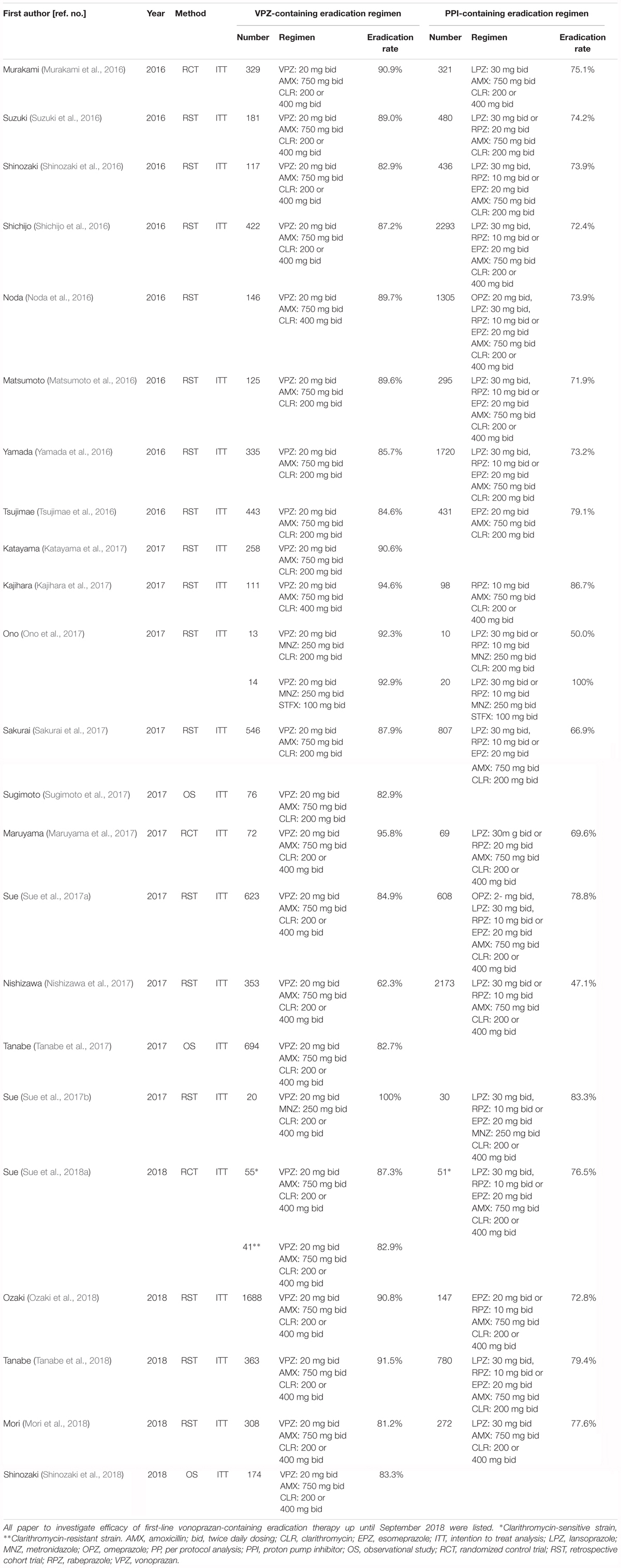
Table 2. Summary of previous studies for the investigation of the efficacy of first-line eradication therapy for H. pylori infection.
In 2016, a phase III trial of first-line triple therapy in 650 H. pylori-positive subjects showed that the first-line eradication rate was 92.6% (95% confidence interval [CI]: 89.2–95.2%) for the VPZ 20 mg/AMX 750 mg/CLR 200 mg or 400 mg regimen compared to 75.9% (95% CI: 70.9–80.5%) for the lansoprazole/AMX/CLR regimen. There was a difference of 16.7% (95% CI: 11.2–22.1%) in favor of VPZ, confirming the non-inferiority of VPZ (P < 0.0001) (Murakami et al., 2016). In another randomizes control trial including 141 H. pylori-positive patients with VPZ group (VPZ 20 mg, AMX 750 mg, and CLR 200 or 400 mg) or PPI group (rabeprazole 20 mg or lansoprazole 30 mg, AMX 750 mg, and CLR 200 or 400 mg), the eradication rate was significantly higher in VPZ group (95.8 and 95% CI: 88.3–99.1%) than PPI group (69.6 and 95% CI: 57.3–80.1%, P < 0.001) in ITT analysis (Maruyama et al., 2017). In a summary of 21 studies investigating the efficacy of the first-line VPZ/CLR/AMX eradication regimen in 7,469 patients who received VPZ-containing therapy and 12,010 patients who received PPI-containing triple therapy, the studies with eradication rate of more than 85% was 61.2% (13/21 studies) in for VPZ-containing therapy and 0% for PPI-containing therapy (Table 2). Jung et al. (2017) reported that the pooled H. pylori eradication rate determined by intention-to-treat (ITT) analysis is 88.1% (95% CI: 86.1–89.9%) in the vonoprazan-containing triple therapy and 72.8% (95% CI: 71.0–75.4%) in PPI-containing triple therapy, respectively, as meta-analysis using 10 reports. In addition, the incidence of any adverse events was similar between both regimens (pooled relative risk [95% CI] = 1.02 [0.78–1.34]) (Jung et al., 2017). However, because most of included studies were retrospective observational studies, this review may have a problem with the quality of the studies (Jung et al., 2017). Meta-analysis should avid to mix randomized case-control studies with cohorts and observational studies.
As shown in Table 2, first-line triple VPZ-containing therapies (VPZ/AMX/CLR) therefore show superior efficacy in Japanese individuals in terms of H. pylori eradication compared to PPI-containing therapies (Matsumoto et al., 2016; Murakami et al., 2016; Noda et al., 2016; Shichijo et al., 2016; Shinozaki et al., 2016, 2018; Suzuki et al., 2016; Tsujimae et al., 2016; Yamada et al., 2016; Kajihara et al., 2017; Katayama et al., 2017; Maruyama et al., 2017; Nishizawa et al., 2017; Ono et al., 2017; Sakurai et al., 2017; Sue et al., 2017a,b, 2018a; Sugimoto et al., 2017; Tanabe et al., 2017, 2018; Mori et al., 2018; Ozaki et al., 2018). According to a grading system established by Graham et al. (2007) the 69.1–75.0% eradication rate of PPI-containing therapies constitutes an unacceptable grade (grade F). In contrast, the 86.6–91.7% eradication rate of the VPZ-containing therapies reflects an acceptable grade (grade B or C). Although this rate is by no means excellent, it is a positive step for establishing improved treatment methods in the future. These findings suggest that potent acid inhibition using VPZ is a key requirement for a successful therapy, and is effective despite the high rate of CLR-resistance strains in Japanese individuals.
Increasing the duration from 7 to 10–14 days at eradication is known to increase eradication rates by approximately 5% (Calvet et al., 2000; Fuccio et al., 2007). However, given that there is currently no data on the efficacy of eradication regimens on prolonged eradication periods, further studies are needed to clarify the effectiveness of such therapies.
First-Line Vonoprazan-Containing Eradication Therapy for Patients Infected With Clarithromycin-Resistant Strains
Implementation of PPI-containing therapy without culture testing is accepted in many countries, whereas H. pylori is one of infectious diseases, because eradication treatment based on the culture test requires more time and higher costs compared to non-culture empirical treatment. However, because CLR resistance is becoming a global clinical problem for H. pylori eradication in many countries, especially in Japan where the CLR has been used for many patients with bacterial infection, eradication therapy that has been susceptibility tested may be an effective option. CLR is a key antimicrobial agent of current first-line triple H. pylori eradication therapies, exerting its antimicrobial effects by binding to the bacterial ribosome 50S subunit to inhibit protein synthesis. The minimum inhibitory concentration used to define resistance to CLR is generally higher than 1.0 mg/mL (Adamek et al., 1998). The susceptibility to CLR in most H. pylori strains is conferred by a single nucleotide polymorphism at either position 2142 or 2143 (i.e., A2142G and A2143G) in the H. pylori 23S rRNA gene and associated with MIC > 64ug/ml. CLR resistance is therefore a potential confounding factor because CLR resistance significantly affects the efficacy of eradication therapy.
Up until September 2018 (PubMed, EMBASE, Web of Science), six reports had compared the efficacy of first-line VPZ-containing therapy between patients infected with CLR-sensitive and -resistant strains, while 4 reports had compared the efficacy of VPZ/AMX/CLR and PPI (omeprazole 20 mg, lansoprazole 30 mg, rabeprazole 10 mg, or esomeprazole 20 mg)/AMX/CLR therapy (Table 3). In a multicenter prospective randomized clinical trial, the ITT analysis of VPZ/AMX/CLR in the patients infected with CLR-sensitive strain were 87.3% (95% CI: 75.5–94.7%) and that of PPI/AMX/CLR were 76.5% (62.5–87.2%), respectively. No significant difference was observed between the VPZ-containing and PPI-containing regimes in terms of the ITT analysis (P = 0.21) (Sue et al., 2018a). A meta-analysis of five original articles (Matsumoto et al., 2016; Murakami et al., 2016; Noda et al., 2016; Sue et al., 2017a, 2018a) showed that in patients infected with CLR-sensitive H. pylori strains, eradication rates of VPZ- and conventional PPI-containing therapies were similar in two randomized controlled trials (eradication rate: 95.4% [VPZ] and 92.8% [PPI], odds ratio [OR]: 1.63, 95% CI: 0.74–3.61, P = 0.225) (Li et al., 2018). However, VPZ-containing therapy was significantly superior to PPI-containing therapy for patients with CLR-resistant strains in both the randomized controlled trials (eradication rate: 82.0% [VPZ] and 40.0% [PPI], OR: 6.83, 95% CI: 3.63–12.86, P < 0.0001) (Li et al., 2018). Compared to the efficacy of conventional PPI-based therapy, the risk of VPZ-containing therapy determined using non-randomized controlled trials was greater for H. pylori-positive patients infected with CLR-resistant strains (OR: 5.92) than for H. pylori-positive patients infected with CLR-sensitive strains (OR: 2.02) (Dong et al., 2017). Based on this evidence, CLR may be overused given that the combination of VPZ and AMX eradicates approximately 80% of H. pylori without CLR (Li et al., 2018). However, as an eradication rate of 80% is not satisfactory, additional measures are needed to obtain a higher eradication rate in patients infected with H. pylori CLR-resistant strains.
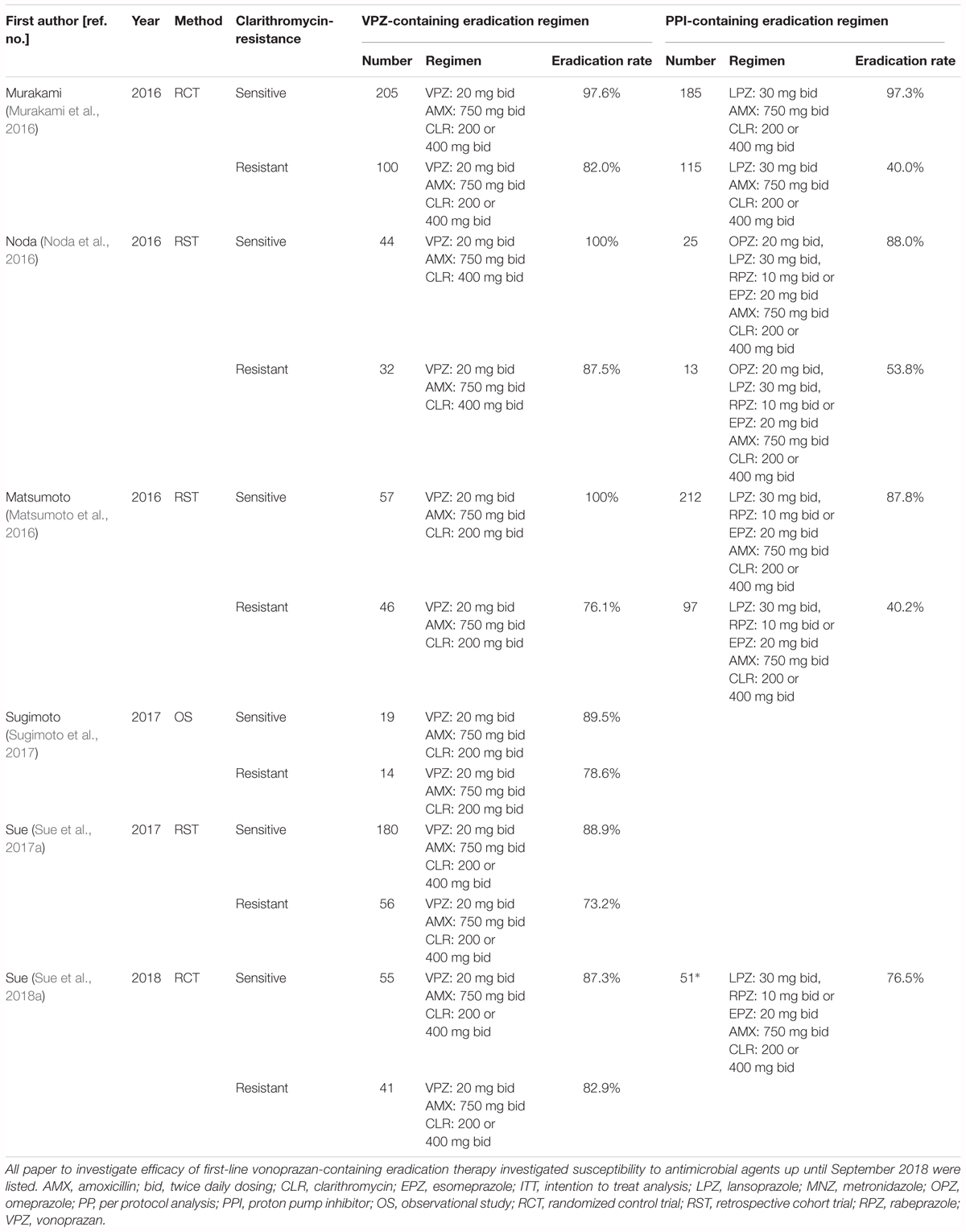
Table 3. Summary of previous studies for the investigation of the efficacy of first-line eradication therapy between clarithromycin-sensitive and -resistant strains.
Second-Line Vonoprazan-Containing Eradication Therapy
Characteristics of patients who require second-line therapy in Japan are infection with a CLR-resistant strain, CYP2C19 extensive metabolizer (EM) phenotype, and poor adherence when first-line treatment was performed. The second-line regimen PPI/AMX/MNZ is currently covered by the Japanese National Health Insurance system, and while the prevalence of patients with H. pylori infected with MNZ resistance strain in Japan is 5–12%, the success rate of this second-line regimen has remained constant at approximately 90% (Tsujimae et al., 2016; Yamada et al., 2016; Nishizawa et al., 2017; Ono et al., 2017; Sakurai et al., 2017; Sue et al., 2017a). Japan differs from many other countries in its prevalence rate of the H. pylori MNZ-resistant strain, and the high eradication rate following second-line treatment containing MNZ is thought to be country-specific.
Up until September 2018 (PubMed, EMBASE, Web of Science), twelve reports have investigated the efficacy of second-line VPZ-containing therapy and 5 reports have retrospectively compared the efficacy between VPZ/AMX/MNZ and PPI/AMX/MNZ therapy (Table 4). However, a randomized controlled trial on the efficacy of VPZ/AMX/MNZ as a second-line regimen has not been conducted. Murakami et al. (2016) reported that the eradication rate of second-line VPZ-containing eradication therapy (VPZ 20 mg/AMX 750 mg/MNZ 250 mg, bid, 7 days) was high (98.0 and 95% CI: 89.4–99.9%, n = 50) in a phase III trial, ranging from 71.8 to 98.0% in 12 reports (Table 4; Murakami et al., 2016; Tsujimae et al., 2016; Yamada et al., 2016; Katayama et al., 2017; Nishizawa et al., 2017; Sakurai et al., 2017; Sue et al., 2017a; Sugimoto et al., 2017; Tanabe et al., 2017; Ozaki et al., 2018). However, there is no significant difference in eradication rates between PPI and VPZ among comparable trials in real-world clinical settings (Tsujimae et al., 2016; Yamada et al., 2016; Nishizawa et al., 2017; Ono et al., 2017; Sakurai et al., 2017; Sue et al., 2017a). A meta-analysis reported in 2017 showed that the eradication rate of VPZ-containing regimens using ITT analysis was similar to that for PPI-containing regimens (83.4% [VPZ] and 81.2% [PPI], OR: 1.04, 95% CI: 0.77–1.42, P = 0.79) (Dong et al., 2017). Per protocol (PP) analysis showed comparable results to the ITT analysis (89.3% vs. 90.1%, P = 0.06). In addition, the eradication rate is similar among various gastrointestinal diseases (Yamada et al., 2016). The above findings suggest that there is no advantage of using VPZ in second-line treatment. This may be because although AMX is an acid-sensitive antimicrobial agent, MNZ is not an acid-sensitive antimicrobial agent, and potent acid inhibition is not required to increase the stability and bioavailability of MNZ. The mechanism of its antimicrobial effect of MNZ is independent of the bacteria’s stationary or growth phase distribution. In addition, failure to eradicate H. pylori using the VPZ/AMX/CLR regimen as first-line therapy likely limits the efficacy of VPZ in second-line therapy containing MNZ.
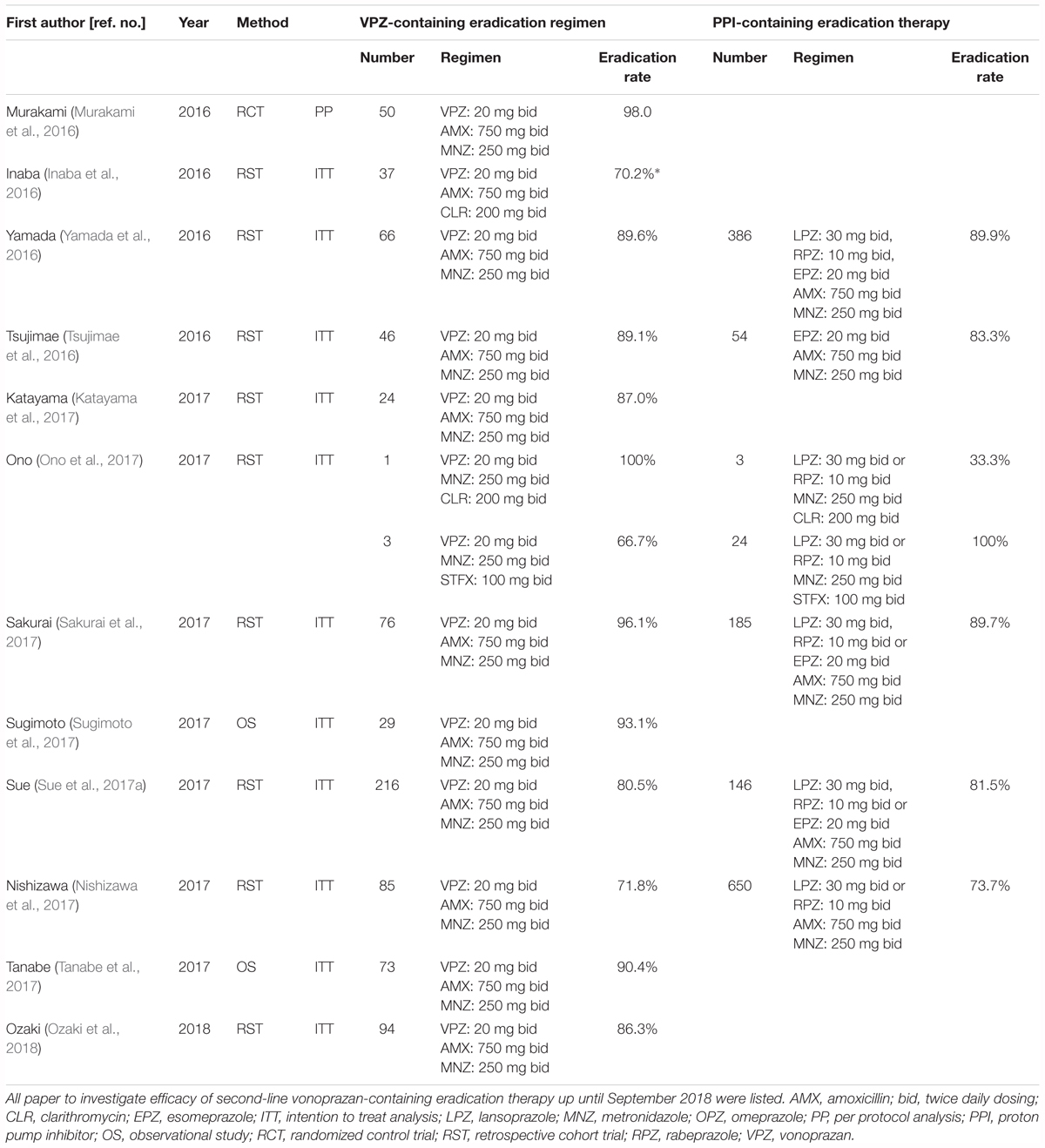
Table 4. Summary of previous studies for the investigation of the efficacy of second-line eradication therapy for H. pylori infection.
In general, second-line therapy with VPZ/AMX/MNZ is well tolerated and patients show good compliance. During the second-line eradication phase, the incidence of treatment-emergent adverse events is 4.0–16.0% (Murakami et al., 2016; Nishizawa et al., 2017). Therefore, in Japan, instead of VPZ/AMX/MNZ, the PPI/AMX/MNZ regimen may be recommended as a second-line treatment due to cost-effectiveness and similar efficacy and safety.
Third-Line Vonoprazan-Containing Eradication Therapy
The bismuth-containing quadruple regimen and/or sequential or concomitant regimens recommended by the Maastricht V/Florence Consensus Report cannot be used as eradication treatment in Japan because bismuth is not an approved medical drug. Further, third-line eradication therapy is not accepted by the Japanese National Health Insurance system. Because the H. pylori strain that infects most patients who receive third-line therapy is resistant to CLR and MNZ, the eradication therapy using PPI/AMX/sitafloxacin (STFX) or PPI/MNZ/STFX, where the PPI is selected, is the main third-line regimen in Japan (Murakami et al., 2013; Furuta et al., 2014a; Sugimoto et al., 2015; Mori et al., 2016). STFX is one of a new quinolone antibacterial agent with anticipated efficacy due to its low MIC for H. pylori, including for levofloxacin (LVFX)-resistant strains. Interestingly, STFX has antimicrobial effects to inhibit DNA gyrase and topoisomerase IV, enzymes that are involved in bacteria DNA replication, transcription, DNA repair and recombination (Zhanel et al., 2002). In particular, topoisomerase IV, which consists of ParC and ParE subunits, has an essential role in partitioning replicated chromosomes and is more sensitive than DNA gyrase to some quinolones, such as levofloxacin and ciprofloxacin (Appelbaum and Hunter, 2000). STFX can overcome the resistance of H. pylori strains with gyrA mutations in vitro, (Suzuki et al., 2009) and the low rate of STFX-resistant strains of less than 10% is a strong motivator for its use in eradication therapy (Sugimoto et al., 2015; Mori et al., 2016). Indeed, the effectiveness of STFX-containing eradication therapy has been reported in patients receiving third-line treatment (Murakami et al., 2009, 2013; Suzuki et al., 2009; Hirata et al., 2012; Matsuzaki et al., 2012; Furuta et al., 2014a,b).
Sitafloxacin is also an acid-sensitive antimicrobial agent, whose stability and bioavailability is increased by potent acid inhibition. Therefore, a VPZ/STFX-containing H. pylori eradication regimen is expected to increase the eradication rate compared with a PPI/STFX-containing eradication regimen. As shown in Table 5, two reports have investigated the efficacy of third-line VPZ-containing therapy (Sugimoto et al., 2017; Sue et al., 2018b). In a randomized case-controlled trial, patients were divided into a VPZ group (VPZ/AMX/STFX bid for 7 days) or a PPI group (PPI standard dose/AMX/STFX bid for 7 days) (Sue et al., 2018b). Although sample number was limited (n = 63), ITT analysis showed that the eradication rates were 75.8% (95% CI: 57.7–88.9%) in the VPZ group and 53.3% (95% CI: 34.3–71.7%) in the PPI group, respectively (Sue et al., 2018b). No significant difference in the frequency of adverse events was evident between the VPZ-containing and PPI- containing regimens. Therefore, although an eradication rate of around 80% remains unsatisfactory, the VPZ/AMX/STFX regimen is more effective than PPI/AMX/STFX as a third-line regimen in patients in whom CLR-containing first-line and MNZ-containing second-line therapies failed to eradicate H. pylori. However, because, as mentioned above, H. pylori was eradicated in 99% or more patients who received both VPZ-containing first- and second-line treatments, the number of H. pylori-positive patients requiring third-line therapy is expected to be considerably reduced, to less than 1%.
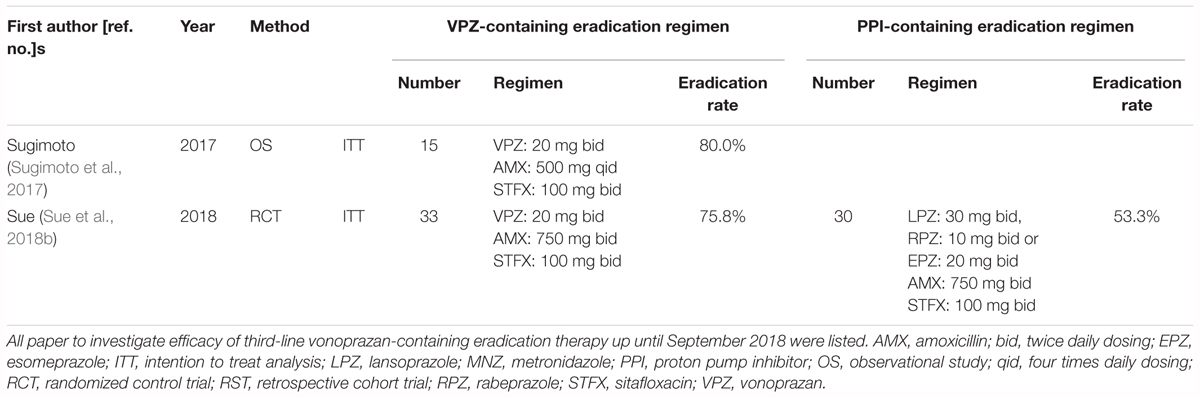
Table 5. Summary of previous studies for the investigation of the efficacy of third-line eradication therapy for H. pylori infection.
Vonoprazan-Containing Eradication Therapy in Patients With Penicillin Allergies
Amoxicillin is the most effective and popular drug used for H. pylori infection. Therefore, most recommended regimens for H. pylori infection include AMX as a key antimicrobial agent. However, because AMX-containing regimens cannot be used by patients with penicillin allergies, eradication regimens that do not contain penicillin derivatives or agents with beta-lactam rings are selected for H. pylori eradication in these patients in Japan (Harris et al., 1996; Furuta et al., 2014b). The incidence of penicillin allergies is 3–7% in Japan (Muranaka et al., 1973). For patients who are allergic to penicillin, the Maastricht V/Florence Consensus Report recommends selecting a PPI/CLR/MNZ regimen in areas with low rates of CLR resistance and bismuth-based quadruple therapy in areas of high CLR resistance (Malfertheiner et al., 2017). However, the PPI/CLR/MNZ regimen is associated with an unacceptably low eradication rate, from 55 to 64% according to PP analysis, in patients with penicillin allergies in areas with high rates of CLR resistance (Gisbert et al., 2005, 2010). In contrast, STFX-based triple therapy is reportedly effective in patients with penicillin allergies. Therefore, a PPI/STFX/MNZ regimen may be potentially useful in patients with penicillin allergies, especially in areas with high CLR resistance rates when PPI is used (Murakami et al., 2009, 2013; Suzuki et al., 2009; Hirata et al., 2012; Matsuzaki et al., 2012; Furuta et al., 2014a,b).
Two reports have investigated the efficacy of VPZ-containing therapy in patients with penicillin allergies (Table 6). Ono et al. (2017) reported that the eradication rate of VPZ/MNZ/STFX and VPZ/MNZ/CLR was 92.3% (n = 17) and 92.9% (n = 14), respectively. Sue et al. (2017b) reported that the efficacy of 7-day first-line treatment with VPZ/MNZ/CLR for H. pylori eradication was 100% (95% CI: 86.1–100%; n = 20). Because the first-line eradication rate of a VPZ-containing regimen in a CLR-resistant population is around 80% (Murakami et al., 2016), VPZ/STFX/MNZ may potentially be effective in patients with penicillin allergies in areas with a high CLR resistance rate, while VPZ/CLR/MNZ may be effective in areas with a low CLR resistance rate.
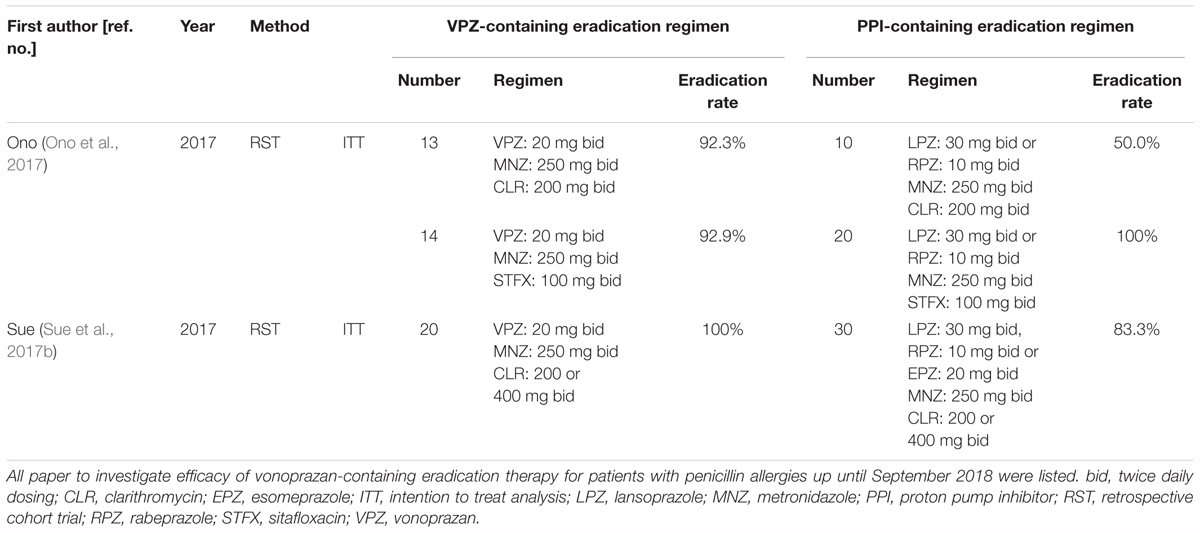
Table 6. Summary of previous studies for the investigation of the efficacy of first-line eradication therapy for patients with penicillin allergies.
Vonoprazan-Containing Eradication Therapy and CYP3A4/5 Genotype
Vonoprazan is primarily metabolized to its inactive form by CYP3A4/5, and partially by CYP2B6, CYP2C19, and CYP2D6 (Yamasaki et al., 2016). Although the association between plasma VPZ levels and CYP3A5 genotype is obscure, the elimination rate of VPZ and the formation rate of its major metabolites are significantly correlated with enzyme activity of CYP3A4/5 (Yamasaki et al., 2016), suggesting that CYP3A4/5 activity affects the pharmacokinetics of VPZ, and therefore different clinical outcomes for H. pylori eradication among patients with different CYP3A4/5 genotypes. We previously reported that in first-line eradication treatment, the eradication rate in CYP3A5 ∗1 carriers was 72.7% (95% CI 54.5–86.7%), which was significantly lower than that in the CYP3A5∗3/∗3 type (90.7 and 95% CI 69.4–94.1%, P = 0.039) (Sugimoto et al., 2017). In univariate analysis of this study, carriage of CYP3A5 ∗3/∗3 type was a positive predictive factor for outcome of eradication (OR: 3.656, 95% CI 1.014–13.190, P = 0.048) (Sugimoto et al., 2017). CYP3A5 ∗3/∗3 type may therefore be a significant positive prognostic predictor of successful eradication when VPZ is used in first-line therapy containing CLR and AMX.
Tailored Eradication Therapy for H. pylori Infection Based on Culture Test
Determining the antibiotic susceptibility using either culture or genetic testing or both is useful to increase the eradication rate, particularly in populations with a high rate of infection with drug-resistant strains. Tailored eradication therapies are promising for significantly increasing successful outcomes compared to standard therapies, particularly in areas with a high prevalence of CLR-resistant strains (Furuta et al., 2007; Kawai et al., 2008; Sugimoto et al., 2014; Ferenc et al., 2017; Cho et al., 2018). A tailored treatment regimen based on susceptibility to CLR (PPI/AMX/CLR for patients infected with CLR-sensitive strains and PPI/AMX/MNZ for those with CLR-resistant strains) achieved a 94.3% eradication rate, which is significantly higher than that achieved with standard treatment (71.4%) (Kawai et al., 2008). Currently, no study has examined the efficacy of VPZ-containing tailored eradication therapy based on susceptibility to CLR. Because the eradication rate of VPZ-containing triple regimen in patients infected with CLR -resistant strains is around 80%, tailored eradication is expected to improve the effectiveness of these therapies.
Potential Benefits or Limitations of Using VPZ in Populations Outside Japan
Japan is the first country to approve the use of VPZ for clinically acid-peptic disorders, all of studies investigated efficacy of VPZ-containing eradication therapy were reported from Japan. The data on the inhibitory effect on acid secretion of VPZ in Westerners is limited. A recent study showed that at steady state on Day 7, VPZ 40 mg once daily displayed sustained acid inhibition during a 24-h and intragastric pH > 4 HTR in 100% of Japanese and 93.2% of British individuals (Jenkins et al., 2015). Therefore, the effect of suppressing acid secretion of VPZ may differ between Japanese and Westerners. However, acid inhibitory effects of VPZ administration have advantage in patients with CYP2C19 EM, refractory genotype to PPI therapy, suggesting that VPZ will be expected to show usability to Western population with high prevalence of CYP2C19 EMs.
Conclusion
This review focused on the efficacy of VPZ-containing H. pylori eradication therapy in relation to intragastric pH. We discussed the factors effecting the therapeutic outcomes of VPZ-containing H. pylori eradication therapy and summarized previous findings using first-, second- and third-line therapies. VPZ-containing triple therapy shows high efficacy in terms of H. pylori eradication compared to PPI-containing therapy, especially in patients infected with CLR-resistant strains who received VPZ/AMX/CLR as first-line therapy, patients who received VPZ/AMX/STFX as third-line therapy, and patients with penicillin allergies who received VPZ/AMX/STFX. Importantly, however, efficacy is similar between VPZ-containing and PPI-containing eradication therapy in patients infected with CLR-sensitive strains who received VPZ/AMX/CLR as first-line therapy and patients who received VPZ/AMX/MNZ as second-line therapy. We described the potential of a culture test-based and patients’ pharmacogenomics-based tailored treatment for achieving an eradication rate exceeding 95%. No report to investigate direct association with advantage of vonoprazan use and acid inhibition during vonoprazan-containing eradication therapy using a pH monitoring study consists. Although one of factors affecting H. pylori eradication is potent acid inhibition during PPI-containing eradication therapy, it is required to clarify whether theoretical advantages of vonoprazan is caused by potent acid inhibition during eradication therapy as same as PPI-containing eradication therapy.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adamek, R. J., Suerbaum, S., Pfaffenbach, B., and Opferkuch, W. (1998). Primary and acquired Helicobacter pylori resistance to clarithromycin, metronidazole, and amoxicillin–influence on treatment outcome. Am. J. Gastroenterol. 93, 386–389.
Appelbaum, P. C., and Hunter, P. A. (2000). The fluoroquinolone antibacterials: past, present and future perspectives. Int. J. Antimicrob. Agents 16, 5–15. doi: 10.1016/S0924-8579(00)00192-8
Asaka, M., Sugiyama, T., Kato, M., Satoh, K., Kuwayama, H., Fukuda, Y., et al. (2001). A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter 6, 254–261. doi: 10.1046/j.1523-5378.2001.00037.x
Barer, D., Ogilvie, A., Henry, D., Dronfield, M., Coggon, D., French, S., et al. (1983). Cimetidine and tranexamic acid in the treatment of acute upper-gastrointestinal-tract bleeding. N. Engl. J. Med. 308, 1571–1575. doi: 10.1056/NEJM198306303082606
Bell, N. J., Burget, D., Howden, C. W., Wilkinson, J., and Hunt, R. H. (1992). Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion 51(Suppl. 1), 59–67. doi: 10.1159/000200917
Calvet, X., Garcia, N., Lopez, T., Gisbert, J. P., Gene, E., and Roque, M. (2000). A meta-analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Aliment. Pharmacol. Ther. 14, 603–609. doi: 10.1046/j.1365-2036.2000.00744.x
Cho, J. H., Jeon, S. R., Kim, H. G., Jin, S. Y., and Park, S. (2018). Cost-effectiveness of a tailored Helicobacter pylori eradication strategy based on the presence of a 23S ribosomal RNA point mutation that causes clarithromycin resistance in Korean patients. J. Gastroenterol. Hepatol. doi: 10.1111/jgh.14383 [Epub ahead of print].
Dong, S. Q., Singh, T. P., Wei, X., Yao, H., and Wang, H. L. (2017). Review: a Japanese population-based meta-analysis of vonoprazan versus PPI for Helicobacter pylori eradication therapy: is superiority an illusion? Helicobacter 22:e12438. doi: 10.1111/hel.12438
Ferenc, S., Gnus, J., Koscielna, M., Kinda, M., Yarka, A., Stewart, L., et al. (2017). High antibiotic resistance of Helicobacter pylori and its effect on tailored and empiric eradication of the organism in Lower Silesia, Poland. Helicobacter 22:e12365. doi: 10.1111/hel.12365
Fuccio, L., Minardi, M. E., Zagari, R. M., Grilli, D., Magrini, N., and Bazzoli, F. (2007). Meta-analysis: duration of first-line proton-pump inhibitor based triple therapy for Helicobacter pylori eradication. Ann. Intern. Med. 147, 553–562. doi: 10.7326/0003-4819-147-8-200710160-00008
Furuta, T., Ohashi, K., Kosuge, K., Zhao, X. J., Takashima, M., Kimura, M., et al. (1999). CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin. Pharmacol. Ther. 65, 552–561. doi: 10.1016/S0009-9236(99)70075-5
Furuta, T., Shirai, N., Kodaira, M., Sugimoto, M., Nogaki, A., Kuriyama, S., et al. (2007). Pharmacogenomics-based tailored versus standard therapeutic regimen for eradication of H. pylori. Clin. Pharmacol. Ther. 81, 521–528. doi: 10.1038/sj.clpt.6100043
Furuta, T., Shirai, N., Takashima, M., Xiao, F., Hanai, H., Sugimura, H., et al. (2001). Effect of genotypic differences in CYP2C19 on cure rates for Helicobacter pylori infection by triple therapy with a proton pump inhibitor, amoxicillin, and clarithromycin. Clin. Pharmacol. Ther. 69, 158–168. doi: 10.1067/mcp.2001.113959
Furuta, T., Sugimoto, M., Kodaira, C., Nishino, M., Yamade, M., Uotani, T., et al. (2014a). Sitafloxacin-based third-line rescue regimens for Helicobacter pylori infection in Japan. J. Gastroenterol. Hepatol. 29, 487–493. doi: 10.1111/jgh.12442
Furuta, T., Sugimoto, M., Yamade, M., Uotani, T., Sahara, S., Ichikawa, H., et al. (2014b). Eradication of H. pylori infection in patients allergic to penicillin using triple therapy with a PPI, metronidazole and sitafloxacin. Intern. Med. 53, 571–575.
Gisbert, J. P., Gisbert, J. L., Marcos, S., Olivares, D., and Pajares, J. M. (2005). Helicobacter pylori first-line treatment and rescue options in patients allergic to penicillin. Aliment. Pharmacol. Ther. 22, 1041–1046. doi: 10.1111/j.1365-2036.2005.02687.x
Gisbert, J. P., Perez-Aisa, A., Castro-Fernandez, M., Barrio, J., Rodrigo, L., Cosme, A., et al. (2010). Helicobacter pylori first-line treatment and rescue option containing levofloxacin in patients allergic to penicillin. Dig. Liver Dis. 42, 287–290. doi: 10.1016/j.dld.2009.06.007
Goddard, A. F., Jessa, M. J., Barrett, D. A., Shaw, P. N., Idstrom, J. P., Cederberg, C., et al. (1996). Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology 111, 358–367. doi: 10.1053/gast.1996.v111.pm8690200
Graham, D. Y., Lu, H., and Yamaoka, Y. (2007). A report card to grade Helicobacter pylori therapy. Helicobacter 12, 275–278. doi: 10.1111/j.1523-5378.2007.00518.x
Grayson, M. L., Eliopoulos, G. M., Ferraro, M. J., and Moellering, R. C. Jr. (1989). Effect of varying pH on the susceptibility of Campylobacter pylori to antimicrobial agents. Eur. J. Clin. Microbiol. Infect. Dis. 8, 888–889. doi: 10.1007/BF01963775
Harris, A. W., Pryce, D. I., Gabe, S. M., Karim, Q. N., Walker, M. M., Langworthy, H., et al. (1996). Lansoprazole, clarithromycin and metronidazole for seven days in Helicobacter pylori infection. Aliment. Pharmacol. Ther. 10, 1005–1008. doi: 10.1046/j.1365-2036.1996.100272000.x
Heifets, L. B., Lindholm-Levy, P. J., and Comstock, R. D. (1992). Clarithromycin minimal inhibitory and bactericidal concentrations against Mycobacterium avium. Am. Rev. Respir. Dis. 145(4 Pt 1), 856–858. doi: 10.1164/ajrccm/145.4_Pt_1.856
Hirata, Y., Ohmae, T., Yanai, A., Sakitani, K., Hayakawa, Y., Yoshida, S., et al. (2012). Sitafloxacin resistance in Helicobacter pylori isolates and sitafloxacin-based triple therapy as a third-line regimen in Japan. Int. J. Antimicrob. Agents 39, 352–355. doi: 10.1016/j.ijantimicag.2011.12.002
Inaba, T., Iwamuro, M., Toyokawa, T., and Okada, H. (2016). Letter: promising results of Helicobacter pylori eradication with vonoprazan-based triple therapy after failure of proton pump inhibitor-based triple therapy. Aliment. Pharmacol. Ther. 43, 179–180. doi: 10.1111/apt.13462
Jenkins, H., Sakurai, Y., Nishimura, A., Okamoto, H., Hibberd, M., Jenkins, R., et al. (2015). Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment. Pharmacol. Ther. 41, 636–648. doi: 10.1111/apt.13121
Jung, Y. S., Kim, E. H., and Park, C. H. (2017). Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 46, 106–114. doi: 10.1111/apt.14130
Kagami, T., Sahara, S., Ichikawa, H., Uotani, T., Yamade, M., Sugimoto, M., et al. (2016). Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment. Pharmacol. Ther. 43, 1048–1059. doi: 10.1111/apt.13588
Kajihara, Y., Shimoyama, T., and Mizuki, I. (2017). Analysis of the cost-effectiveness of using vonoprazan-amoxicillin-clarithromycin triple therapy for first-line Helicobacter pylori eradication. Scand. J. Gastroenterol. 52, 238–241. doi: 10.1080/00365521.2016.1250157
Katayama, Y., Toyoda, K., Kusano, Y., Suda, T., Adachi, S., Terauchi, I., et al. (2017). Efficacy of vonoprazan-based second-line Helicobacter pylori eradication therapy in patients for whom vonoprazan-based first-line treatment failed. Gut 66, 752–753. doi: 10.1136/gutjnl-2016-312028
Kawai, T., Yamagishi, T., Yagi, K., Kataoka, M., Kawakami, K., Sofuni, A., et al. (2008). Tailored eradication therapy based on fecal Helicobacter pylori clarithromycin sensitivities. J. Gastroenterol. Hepatol. 23(Suppl. 2), S171–S174. doi: 10.1111/j.1440-1746.2008.05408.x
Kodaira, C., Sugimoto, M., Nishino, M., Yamade, M., Shirai, N., Uchida, S., et al. (2009). Effect of MDR1 C3435T polymorphism on lansoprazole in healthy Japanese subjects. Eur. J. Clin. Pharmacol. 65, 593–600. doi: 10.1007/s00228-009-0625-8
Labenz, J., Stolte, M., Blum, A. L., Jorias, I., Leverkus, F., Sollbohmer, M., et al. (1995). Intragastric acidity as a predictor of the success of Helicobacter pylori eradication: a study in peptic ulcer patients with omeprazole and amoxicillin. Gut 37, 39–43. doi: 10.1136/gut.37.1.39
Li, M., Oshima, T., Horikawa, T., Tozawa, K., Tomita, T., Fukui, H., et al. (2018). Systematic review with meta-analysis: vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter 23:e12495. doi: 10.1111/hel.12495
Malfertheiner, P., Megraud, F., O’Morain, C. A., Gisbert, J. P., Kuipers, E. J., Axon, A. T., et al. (2017). Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 66, 6–30. doi: 10.1136/gutjnl-2016-312288
Maruyama, M., Tanaka, N., Kubota, D., Miyajima, M., Kimura, T., Tokutake, K., et al. (2017). Vonoprazan-based regimen is more useful than PPI-based one as a first-line Helicobacter pylori eradication: a randomized controlled trial. Can. J. Gastroenterol. Hepatol. 2017:4385161. doi: 10.1155/2017/4385161
Matsumoto, H., Shiotani, A., Katsumata, R., Fujita, M., Nakato, R., Murao, T., et al. (2016). Helicobacter pylori eradication with proton pump inhibitors or potassium-competitive acid blockers: the effect of clarithromycin resistance. Dig. Dis. Sci. 61, 3215–3220. doi: 10.1007/s10620-016-4305-0
Matsuzaki, J., Suzuki, H., Nishizawa, T., Hirata, K., Tsugawa, H., Saito, Y., et al. (2012). Efficacy of sitafloxacin-based rescue therapy for Helicobacter pylori after failures of first- and second-line therapies. Antimicrob. Agents Chemother. 56, 1643–1645. doi: 10.1128/AAC.05941-11
Mori, H., Suzuki, H., Matsuzaki, J., Tsugawa, H., Fukuhara, S., Miyoshi, S., et al. (2016). Efficacy of 10-day sitafloxacin-containing third-line rescue therapies for Helicobacter pylori strains containing the gyrA mutation. Helicobacter 21, 286–294. doi: 10.1111/hel.12286
Mori, N., Nishiura, Y., Suga, D., Moritani, I., Yamanaka, Y., Ooya, Y., et al. (2018). Second-line triple therapy in failures with vonoprazan-based triple therapy for eradication of Helicobacter pylori. Biomed. Rep. 9, 169–174. doi: 10.3892/br.2018.1111
Murakami, K., Furuta, T., Ando, T., Nakajima, T., Inui, Y., Oshima, T., et al. (2013). Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. J. Gastroenterol. 48, 1128–1135. doi: 10.1007/s00535-012-0731-8
Murakami, K., Okimoto, T., Kodama, M., Tanahashi, J., Fujioka, T., Ikeda, F., et al. (2009). Sitafloxacin activity against Helicobacter pylori isolates, including those with gyrA mutations. Antimicrob. Agents Chemother. 53, 3097–3099. doi: 10.1128/AAC.01552-08
Murakami, K., Sakurai, Y., Shiino, M., Funao, N., Nishimura, A., and Asaka, M. (2016). Vonoprazan, a novel potassium-competitive acid blocker, as a component of first-line and second-line triple therapy for Helicobacter pylori eradication: a phase III, randomised, double-blind study. Gut 65, 1439–1446. doi: 10.1136/gutjnl-2015-311304
Murakami, K., Sato, R., Okimoto, T., Nasu, M., Fujioka, T., Kodama, M., et al. (2002). Eradication rates of clarithromycin-resistant Helicobacter pylori using either rabeprazole or lansoprazole plus amoxicillin and clarithromycin. Aliment. Pharmacol. Ther. 16, 1933–1938. doi: 10.1046/j.1365-2036.2002.01368.x
Muranaka, M., Okumura, H., Takeda, K., Koizumi, K., and Igarashi, H. (1973). Population studies on drug hypersensitivities. Acta Allergol. 28, 50–61. doi: 10.1111/j.1398-9995.1973.tb02565.x
Nishizawa, T., Suzuki, H., Fujimoto, A., Kinoshita, H., Yoshida, S., Isomura, Y., et al. (2017). Effects of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J. Clin. Biochem. Nutr. 60, 208–210. doi: 10.3164/jcbn.16-86
Noda, H., Noguchi, S., Yoshimine, T., Goji, S., Adachi, K., Tamura, Y., et al. (2016). A novel potassium-competitive acid blocker improves the efficacy of clarithromycin-containing 7-day triple therapy against Helicobacter pylori. J. Gastrointestin. Liver Dis. 25, 283–288. doi: 10.15403/jgld.2014.1121.253.7hp
Ono, S., Kato, M., Nakagawa, S., Mabe, K., and Sakamoto, N. (2017). Vonoprazan improves the efficacy of Helicobacter pylori eradication therapy with a regimen consisting of clarithromycin and metronidazole in patients allergic to penicillin. Helicobacter 22:e12374. doi: 10.1111/hel.12374
Ozaki, H., Harada, S., Takeuchi, T., Kawaguchi, S., Takahashi, Y., Kojima, Y., et al. (2018). Vonoprazan, a novel potassium-competitive acid blocker, should be used for the Helicobacter pylori eradication therapy as first choice: a large sample study of vonoprazan in real world compared with our randomized control trial using second-generation proton pump inhibitors for Helicobacter pylori eradication therapy. Digestion 97, 212–218. doi: 10.1159/000485097
Parsons, M. E., and Keeling, D. J. (2005). Novel approaches to the pharmacological blockade of gastric acid secretion. Expert Opin. Investig. Drugs 14, 411–421. doi: 10.1517/13543784.14.4.411
Saitoh, T., Fukushima, Y., Otsuka, H., Hirakawa, J., Mori, H., Asano, T., et al. (2002). Effects of rabeprazole, lansoprazole and omeprazole on intragastric pH in CYP2C19 extensive metabolizers. Aliment. Pharmacol. Ther. 16, 1811–1817. doi: 10.1046/j.1365-2036.2002.01348.x
Sakurai, K., Suda, H., Ido, Y., Takeichi, T., Okuda, A., Hasuda, K., et al. (2017). Comparative study: vonoprazan and proton pump inhibitors in Helicobacter pylori eradication therapy. World J. Gastroenterol. 23, 668–675. doi: 10.3748/wjg.v23.i4.668
Sakurai, Y., Mori, Y., Okamoto, H., Nishimura, A., Komura, E., Araki, T., et al. (2015). Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects–a randomised open-label cross-over study. Aliment. Pharmacol. Ther. 42, 719–730. doi: 10.1111/apt.13325
Scott, D., Weeks, D., Melchers, K., and Sachs, G. (1998). The life and death of Helicobacter pylori. Gut 43(Suppl. 1), S56–S60. doi: 10.1136/gut.43.2008.S56
Scott, D. R., Munson, K. B., Marcus, E. A., Lambrecht, N. W., and Sachs, G. (2015). The binding selectivity of vonoprazan (TAK-438) to the gastric H+, K+ -ATPase. Aliment. Pharmacol. Ther. 42, 1315–1326. doi: 10.1111/apt.13414
Shichijo, S., Hirata, Y., Niikura, R., Hayakawa, Y., Yamada, A., Mochizuki, S., et al. (2016). Vonoprazan versus conventional proton pump inhibitor-based triple therapy as first-line treatment against Helicobacter pylori: a multicenter retrospective study in clinical practice. J. Dig. Dis. 17, 670–675. doi: 10.1111/1751-2980.12398
Shinozaki, S., Nomoto, H., Kondo, Y., Sakamoto, H., Hayashi, Y., Yamamoto, H., et al. (2016). Comparison of vonoprazan and proton pump inhibitors for eradication of Helicobacter pylori. Kaohsiung J. Med. Sci. 32, 255–260. doi: 10.1016/j.kjms.2016.04.009
Shinozaki, S., Osawa, H., Sakamoto, H., Hayashi, Y., Kobayashi, Y., Miura, Y., et al. (2018). Pre-treatment with proton pump inhibitors decreases the success of primary Helicobacter pylori eradication using a vonoprazan-based regimen. Kaohsiung J. Med. Sci. 34, 456–460. doi: 10.1016/j.kjms.2018.03.009
Shiota, S., Nguyen, L. T., Murakami, K., Kuroda, A., Mizukami, K., Okimoto, T., et al. (2012). Association of Helicobacter pylori dupA with the failure of primary eradication. J. Clin. Gastroenterol. 46, 297–301. doi: 10.1097/MCG.0b013e318243201c
Shirai, N., Furuta, T., Moriyama, Y., Okochi, H., Kobayashi, K., Takashima, M., et al. (2001). Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment. Pharmacol. Ther. 15, 1929–1937. doi: 10.1046/j.1365-2036.2001.01108.x
Shirai, N., Furuta, T., Xiao, F., Kajimura, M., Hanai, H., Ohashi, K., et al. (2002). Comparison of lansoprazole and famotidine for gastric acid inhibition during the daytime and night-time in different CYP2C19 genotype groups. Aliment. Pharmacol. Ther. 16, 837–846. doi: 10.1046/j.1365-2036.2002.01229.x
Sue, S., Kuwashima, H., Iwata, Y., Oka, H., Arima, I., Fukuchi, T., et al. (2017a). The superiority of vonoprazan-based first-line triple therapy with clarithromycin: a prospective multi-center cohort study on Helicobacter pylori eradication. Intern. Med. 56, 1277–1285. doi: 10.2169/internalmedicine.56.7833
Sue, S., Suzuki, N., Shibata, W., Sasaki, T., Yamada, H., Kaneko, H., et al. (2017b). First-line Helicobacter pylori eradication with vonoprazan, clarithromycin, and metronidazole in patients allergic to penicillin. Gastroenterol. Res. Pract. 2017:2019802. doi: 10.1155/2017/2019802
Sue, S., Ogushi, M., Arima, I., Kuwashima, H., Nakao, S., Naito, M., et al. (2018a). Vonoprazan- vs proton-pump inhibitor-based first-line 7-day triple therapy for clarithromycin-susceptible Helicobacter pylori: a multicenter, prospective, randomized trial. Helicobacter 23:e12456. doi: 10.1111/hel.12456
Sue, S., Shibata, W., Sasaki, T., Kaneko, H., Irie, K., Kondo, M., et al. (2018b). Randomized trial of vonoprazan- versus PPI-based third-line triple therapy with sitafloxacin for Helicobacter pylori. J. Gastroenterol. Hepatol. doi: 10.1111/jgh.14456 [Epub ahead of print].
Sugimoto, M., Ban, H., Hira, D., Kamiya, T., Otsuka, T., Inatomi, O., et al. (2017). Letter: CYP3A4/5 genotype status and outcome of vonoprazan-containing Helicobacter pylori eradication therapy in Japan. Aliment. Pharmacol. Ther. 45, 1009–1010. doi: 10.1111/apt.13959
Sugimoto, M., Furuta, T., Shirai, N., Ikuma, M., Hishida, A., and Ishizaki, T. (2006). Initial 48-hour acid inhibition by intravenous infusion of omeprazole, famotidine, or both in relation to cytochrome P450 2C19 genotype status. Clin. Pharmacol. Ther. 80, 539–548. doi: 10.1016/j.clpt.2006.08.010
Sugimoto, M., Furuta, T., Shirai, N., Kajimura, M., Hishida, A., Sakurai, M., et al. (2004). Different dosage regimens of rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotype status. Clin. Pharmacol. Ther. 76, 290–301. doi: 10.1016/j.clpt.2004.06.008
Sugimoto, M., Furuta, T., Shirai, N., Kodaira, C., Nishino, M., Ikuma, M., et al. (2007). Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter 12, 317–323. doi: 10.1111/j.1523-5378.2007.00508.x
Sugimoto, M., Furuta, T., Shirai, N., Nakamura, A., Kajimura, M., Hishida, A., et al. (2005). Comparison of an increased dosage regimen of rabeprazole versus a concomitant dosage regimen of famotidine with rabeprazole for nocturnal gastric acid inhibition in relation to cytochrome P450 2C19 genotypes. Clin. Pharmacol. Ther. 77, 302–311. doi: 10.1016/j.clpt.2004.10.010
Sugimoto, M., Nishino, M., Kodaira, C., Yamade, M., Uotani, T., Ikuma, M., et al. (2012a). Impact of acid inhibition on esophageal mucosal injury induced by low-dose aspirin. Digestion 85, 9–17. doi: 10.1159/000329295
Sugimoto, M., Shirai, N., Nishino, M., Kodaira, C., Uotani, T., Yamade, M., et al. (2012b). Rabeprazole 10 mg q.d.s. decreases 24-h intragastric acidity significantly more than rabeprazole 20 mg b.d. or 40 mg o.m., overcoming CYP2C19 genotype. Aliment. Pharmacol. Ther. 36, 627–634. doi: 10.1111/apt.12014
Sugimoto, M., Sahara, S., Ichikawa, H., Kagami, T., Uotani, T., and Furuta, T. (2015). High Helicobacter pylori cure rate with sitafloxacin-based triple therapy. Aliment. Pharmacol. Ther. 42, 477–483. doi: 10.1111/apt.13280
Sugimoto, M., Uotani, T., Sahara, S., Ichikawa, H., Yamade, M., Sugimoto, K., et al. (2014). Efficacy of tailored Helicobacter pylori eradication treatment based on clarithromycin susceptibility and maintenance of acid secretion. Helicobacter 19, 312–318. doi: 10.1111/hel.12128
Sugimoto, M., and Yamaoka, Y. (2009). Virulence factor genotypes of Helicobacter pylori affect cure rates of eradication therapy. Arch. Immunol. Ther. Exp. 57, 45–56. doi: 10.1007/s00005-009-0007-z
Suzuki, H., Nishizawa, T., Muraoka, H., and Hibi, T. (2009). Sitafloxacin and garenoxacin may overcome the antibiotic resistance of Helicobacter pylori with gyrA mutation. Antimicrob. Agents Chemother. 53, 1720–1721. doi: 10.1128/AAC.00049-09
Suzuki, S., Gotoda, T., Kusano, C., Iwatsuka, K., and Moriyama, M. (2016). The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am. J. Gastroenterol. 111, 949–956. doi: 10.1038/ajg.2016.182
Tanabe, H., Ando, K., Sato, K., Ito, T., Goto, M., Sato, T., et al. (2017). Efficacy of vonoprazan-based triple therapy for Helicobacter pylori eradication: a multicenter study and a review of the literature. Dig. Dis. Sci. 62, 3069–3076. doi: 10.1007/s10620-017-4664-1
Tanabe, H., Yoshino, K., Ando, K., Nomura, Y., Ohta, K., Satoh, K., et al. (2018). Vonoprazan-based triple therapy is non-inferior to susceptibility-guided proton pump inhibitor-based triple therapy for Helicobacter pylori eradication. Ann. Clin. Microbiol. Antimicrob. 17:29. doi: 10.1186/s12941-018-0281-x
Tsujimae, M., Yamashita, H., Hashimura, H., Kano, C., Shimoyama, K., Kanamori, A., et al. (2016). A comparative study of a new class of gastric acid suppressant agent named Vonoparazan versus esomeprazole for the eradication of Helicobacter pylori. Digestion 94, 240–246. doi: 10.1159/000454762
Yamada, S., Kawakami, T., Nakatsugawa, Y., Suzuki, T., Fujii, H., Tomatsuri, N., et al. (2016). Usefulness of vonoprazan, a potassium ion-competitive acid blocker, for primary eradication of Helicobacter pylori. World J. Gastrointest. Pharmacol. Ther. 7, 550–555. doi: 10.4292/wjgpt.v7.i4.550
Yamasaki, H., Kawaguchi, N., Nonaka, M., Takahashi, J., Morohashi, A., Hirabayashi, H., et al. (2016). In vitro metabolism of TAK-438, vonoprazan fumarate, a novel potassium-competitive acid blocker. Xenobiotica 47, 1027–1034. doi: 10.1080/00498254.2016.1203505
Yang, J. C., Wang, H. L., Chern, H. D., Shun, C. T., Lin, B. R., Lin, C. J., et al. (2011). Role of omeprazole dosage and cytochrome P450 2C19 genotype in patients receiving omeprazole-amoxicillin dual therapy for Helicobacter pylori eradication. Pharmacotherapy 31, 227–238. doi: 10.1592/phco.31.3.227
Keywords: Helicobacter pylori, eradication therapy, vonoprazan, intragastric pH, clarithromycin
Citation: Sugimoto M and Yamaoka Y (2019) Role of Vonoprazan in Helicobacter pylori Eradication Therapy in Japan. Front. Pharmacol. 9:1560. doi: 10.3389/fphar.2018.01560
Received: 24 October 2018; Accepted: 21 December 2018;
Published: 15 January 2019.
Edited by:
Raffaele Capasso, University of Naples Federico II, ItalyReviewed by:
Franco Harald Falcone, University of Nottingham, United KingdomAlejandro Piscoya, Universidad San Ignacio de Loyola, Peru
Copyright © 2019 Sugimoto and Yamaoka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mitsushige Sugimoto, sugimo@belle.shiga-med.ac.jp
 Mitsushige Sugimoto
Mitsushige Sugimoto Yoshio Yamaoka
Yoshio Yamaoka