- 1Department of Psychiatry, Institute of Biomedical Science, Tokushima University Graduate School, Tokushima, Japan
- 2Department of Psychiatry, Osaka University Graduate School of Medicine, Osaka, Japan
- 3Molecular Research Center for Children's Mental Development, United Graduate School of Child Development, Osaka University, Osaka, Japan
- 4Department of Pathology of Mental Diseases, National Institute of Mental Health, National Center of Neurology and Psychiatry, Tokyo, Japan
Clozapine is an efficacious atypical antipsychotic for treatment-refractory schizophrenia. Clinical response and appearance of adverse events vary among individual patients receiving clozapine, with genetic and non-genetic factors potentially contributing to individual variabilities. Pharmacogenetic studies investigate associations between genetic variants and drug efficacy and toxicity. To date, most pharmacogenetic studies of clozapine have been conducted through candidate gene approaches. A recent advance in technology made it possible to perform comprehensive genetic mapping underlying clinical phenotypes and outcomes, which allow novel findings beyond biological hypotheses based on current knowledge. In this paper, we will summarize the studies on clozapine pharmacogenetics that have extensively examined clinical response and agranulocytosis. While there is still limited evidence on clozapine efficacy, recent genome-wide studies provide further evidence of the involvement of the human leukocyte antigen (HLA) region in clozapine-induced agranulocytosis.
Introduction
Approximately 30% of patients with schizophrenia are treatment-resistant (Meltzer, 1997). Clinical practice guidelines recommend clozapine in treatment-refractory schizophrenia (Warnez and Alessi-Severini, 2014), given that it has been shown to be superior for those resistant to treatment (Siskind et al., 2016). Clozapine may cause serious adverse events, such as agranulocytosis, cardiomyopathy, and myocarditis (Alvir et al., 1993; De Berardis et al., 2012; Alawami et al., 2014), so careful monitoring is needed during clozapine treatment. Clinical response and the presence of adverse events vary among individuals taking clozapine. Although the molecular mechanisms of clozapine action are still unclear (Yamamori et al., 2013, 2014; Hung et al., 2017; Kinoshita et al., 2017; Lee et al., 2017; Nakazawa et al., 2017), twin studies suggest that genetic factors may contribute to the variance in therapeutic and adverse effects of clozapine (Vojvoda et al., 1996; Horácek et al., 2001; Theisen et al., 2001; Wehmeier et al., 2005; Anil Yagcioglu et al., 2011).
Pharmacogenetic studies investigate the associations between genetic variants and drug efficacy and toxicity (Jorgensen and Williamson, 2008). To date, a number of pharmacogenetic studies of clozapine have been reported. In this paper, we summarize the studies on clozapine pharmacogenetics that have extensively examined clinical response and agranulocytosis.
Clinical Response to Clozapine
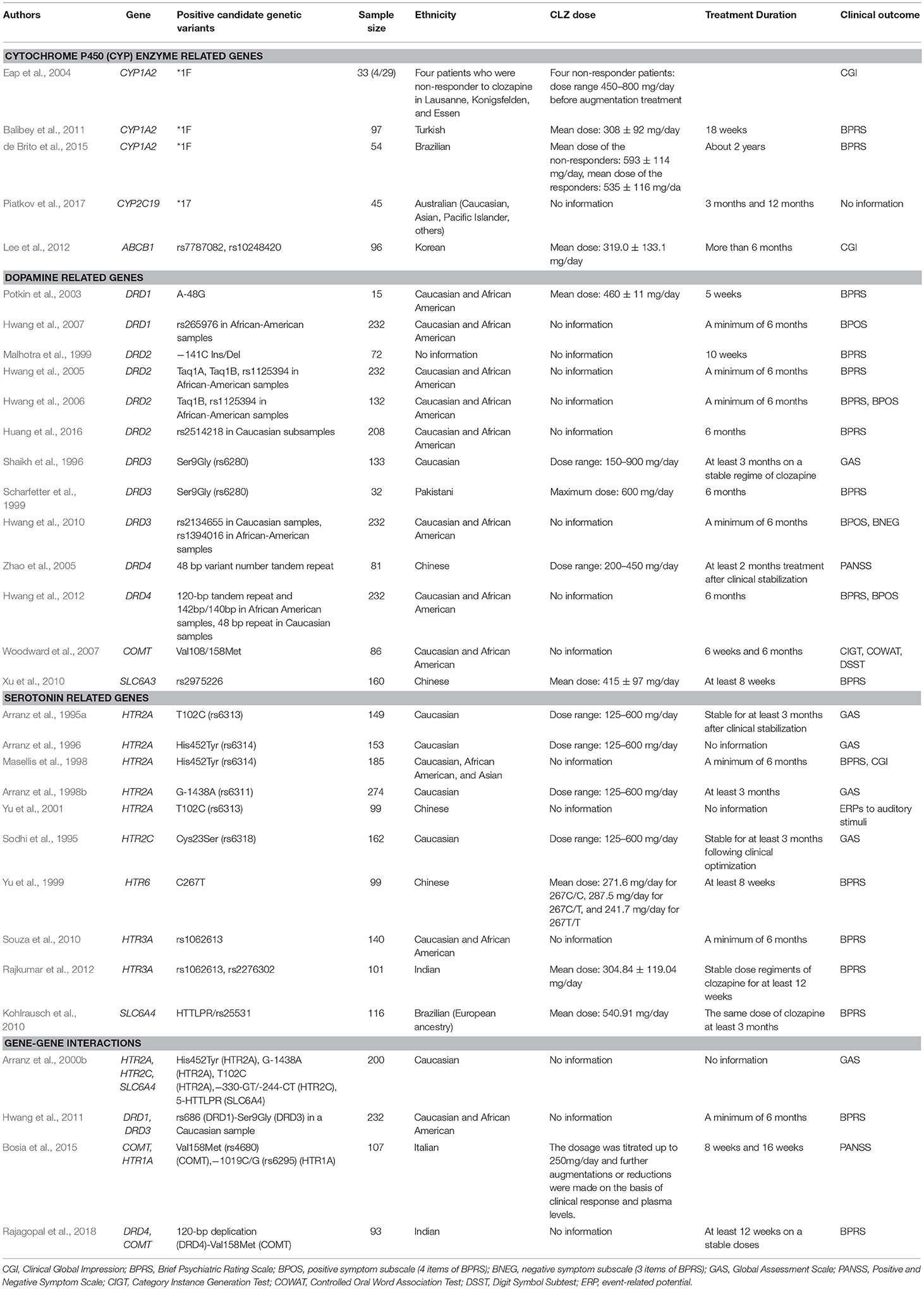
Response rate for clozapine ranges from 32% in the short term to 39% in the long term among those with treatment-resistant schizophrenia (Siskind et al., 2017). Pharmacogenetic studies of clinical response to clozapine have focused on variations in the genes involved in the metabolism of clozapine [pharmacokinetics, e.g., the cytochrome P 450 (CYP) enzyme family] and the affinities of clozapine (pharmacodynamics, e.g., dopaminergic and serotonergic receptors). Positive candidate gene studies of the clinical response to clozapine are shown in Table 1.
Candidate Gene Approach
CYP enzyme related genes
Clozapine is metabolized primarily by CYP1A2, with additional contributions from CYP2C19, CYP2D6, and CYP3A4 (Urichuk et al., 2008). To date, genetic variants in the CYP1A2, CYP2C19, CYP2D6, and CYP3A4 genes as well as in the CYP3A5, CYP3A7, CYP3A43, and ATP binding cassette subfamily B member 1 (ABCB1) genes have been investigated in clozapine response studies (Arranz et al., 1995b; Eap et al., 2004; Balibey et al., 2011; Lee et al., 2012; Rajkumar et al., 2013b; de Brito et al., 2015; Piatkov et al., 2017). Five studies found significant associations between the clozapine treatment response and genetic variants in the CYP1A2, CYP2C19, and ABCB1 genes (Eap et al., 2004; Balibey et al., 2011; Lee et al., 2012; de Brito et al., 2015; Piatkov et al., 2017). Eap and colleagues suggested that the CYP1A2*1F/*1F genotype may be associated with low plasma levels of clozapine and a lack of response to clozapine (N = 4) (Eap et al., 2004). Balibey and colleagues demonstrated that a positive response rate of clozapine was significantly lower in patients carrying the CYP1A2*1F/*1F genotype compared to those with at least one wild type allele for CYP1A2 (N = 97) (Balibey et al., 2011). De Brite and colleagues replicated these findings (N = 54) (de Brito et al., 2015). Piatkov and colleagues demonstrated that homozygote CYP2C19*17 carriers were five times more likely to exhibit improvement on clozapine treatment (N = 45) (Piatkov et al., 2017). Furthermore, Lee and colleagues demonstrated that rs7787082 G and rs10248420 A alleles in the ABCB1 gene were more common in non-responders (N = 96) (Lee et al., 2012).
Dopamine related genes
Clozapine exhibits a relatively high affinity for the dopamine D4 receptor (DRD4) and relatively lower affinities for the dopamine D1 receptor (DRD1), the dopamine D2 receptor (DRD2), and the dopamine D3 receptor (DRD3) (Meltzer, 1994). The combination of relatively high D1, low D2, and very high 5-HT2 receptor occupancy rates is unique to clozapine (Mauri et al., 2014). To date, genetic variants in the DRD1, DRD2, DRD3, DRD4, and dopamine D5 receptor (DRD5) genes as well as in dopamine -related genes, Catechol-O-methyltransferase (COMT) and solute carrier family 6 member 3 (SLC6A3) have been investigated in clozapine response studies, with positive associations observed in the DRD1, DRD2, DRD3, DRD4, COMT, and SLC6A3 genes.
With respect to the DRD1 gene (Potkin et al., 2003; Hwang et al., 2007; Lee et al., 2012), two studies found significant associations between genetic variants and the clozapine response (Potkin et al., 2003; Hwang et al., 2007). Potkin and colleagues demonstrated that rs4532 2/2 carriers were more likely to have a positive clozapine response following 5 weeks of treatment, while rs4532 1/2 carriers demonstrated a diminished clozapine response (N = 15) (Potkin et al., 2003). Hwang and colleagues demonstrated that rs265976 AC carriers were more likely to be non-responders to clozapine with a minimum of 6 months treatment in an African American sample (N = 49). They also investigated an association between three-marker haplotype (rs265981, rs4532, and rs686) and clozapine response, demonstrating that the T-G-A haplotype was associated with poor response in a Caucasian sample (N = 183), while the T-G-G haplotype was associated with better response in an African American sample (N = 49) (Hwang et al., 2007). With respect to the DRD2 gene (Arranz et al., 1998a; Malhotra et al., 1999; Hwang et al., 2005, 2006; Lee et al., 2012), three studies found significant associations between genetic variants in this gene and the clozapine response (Malhotra et al., 1999; Hwang et al., 2005, 2006). Malhotra and colleagues investigated an association between 141C Ins/Del (rs1799732) and clozapine response in a 10 week treatment, demonstrating that Del- subjects had a five-fold greater reduction in psychotic symptoms as compared to Del+ subjects (N = 72) (Malhotra et al., 1999). Additionally, Hwang and colleagues investigated an association between 12 single nucleotide polymorphisms (SNPs) in the DRD2 gene and clozapine response for a minimum of 6 months treatment, identifying 3 SNPs (Taq1A, Taq1B, and rs1125394) only in an African-American sample (N = 49) and several haplotypes in both Caucasian (N = 183) and African-American samples (N = 49) as being predictive of a clozapine response (Hwang et al., 2005). This same group later investigated the effect of the same 12 SNPs in the DRD2 gene on clozapine response evaluated by overall, positive, and negative symptoms in smaller sample set (N = 35) and demonstrated that 2 SNPs (Taq1B and rs1125394) were associated with overall and positive symptom response to clozapine in an African-American sample (Hwang et al., 2006). However, a recent meta-analysis (total N = 596) suggests no association between 141C Ins/Del and a clozapine treatment response (Gressier et al., 2016). With respect to the DRD3 gene (Gaitonde et al., 1996; Shaikh et al., 1996; Malhotra et al., 1998; Scharfetter et al., 1999; Arranz et al., 2000b; Barlas et al., 2009; Hwang et al., 2010; Lee et al., 2012), three studies found significant associations between genetic variants and the clozapine response (Shaikh et al., 1996; Scharfetter et al., 1999; Hwang et al., 2010). Shaikh and colleagues investigated an association between Ser-9-Gly polymorphism (rs6280) in the DRD3 gene and clozapine response for at least 3 months of treatment (N = 79), demonstrating that the genotype Ser-9/Ser-9 was more frequent in the non-responders than in responders (Shaikh et al., 1996). Scharfetter and colleagues replicated this finding in patients treated with clozapine for 6 months (N = 32) (Scharfetter et al., 1999). However, a recent meta-analysis (total N = 852) suggests no association between Ser-9-Gly and a clozapine treatment response (Gressier et al., 2016). Hwang and colleagues demonstrated an association of better clozapine response for a minimum of 6 months with the A allele of rs2134655 in a Caucasian sample (N = 183) and the T allele of rs1394016 in an African-American sample (N = 49) (Hwang et al., 2010). With respect to the DRD4 gene (Shaikh et al., 1993, 1995; Kerwin et al., 1994; Rao et al., 1994; Rietschel et al., 1996; Kohn et al., 1997; Kaiser et al., 2000; Zhao et al., 2005; Hwang et al., 2012; Lee et al., 2012; Rajagopal et al., 2018), two studies found significant associations between genetic variants and the clozapine response (Zhao et al., 2005; Hwang et al., 2012). Zhao and colleagues investigated an association between the 48 bp variant number tandem repeat (VNTR) polymorphism in the DRD4 gene and clozapine response for at least 2 months of treatment after clinical stabilization (N = 81), demonstrating that the frequencies of 5 allele and 5/5 genotype were higher among the non-responders than in responders (N = 81) (Zhao et al., 2005). Hwang and colleagues demonstrated an association between 120-bp 1-copy allele and intron I (G)n 142/140 bp genotype and poor clozapine responders for 6 months of treatment in an African American sample (N = 49), and an association between 48 bp repeat polymorphism and better clozapine response in a Caucasian sample (N = 183) (Hwang et al., 2012). With respect to the DRD5 gene, there were no significant associations between genetic variants and the clozapine response (Hwang et al., 2012). With respect to the COMT gene (Woodward et al., 2007; Bosia et al., 2015; Rajagopal et al., 2018), two studies found a significant association between the COMT Val108/158Met polymorphism (rs4680) and the clozapine response (Woodward et al., 2007; Bosia et al., 2015). Woodward and colleagues investigated an association between this polymorphism and clozapine response for 6 weeks and 6 months of treatment (N = 86), demonstrating that both the Met/Met and Val/Met groups showed greater improvement in attention and verbal fluency domains compared to the Val/Val group (Woodward et al., 2007). Bosia and colleagues demonstrated a greater improvement in the Val/Val group compared to both the Val/Met and Met/Met groups in the negative symptom response for 8 and 16 weeks of treatment with clozapine (N = 107) (Bosia et al., 2015). With respect to the SLC6A3 gene, Xu and colleagues demonstrated that the 71T allele of rs2975226 (T-71A) in the SLC6A3 gene occurred more frequently in the responders than in non-responders following at least 8 weeks of treatment with clozapine (Xu et al., 2010).
Serotonin related genes
Clozapine has a high affinity for the 5-hydroxytryptamine receptor 2A (HTR2A), the 5-hydroxytryptamine receptor 2C (HTR2C), the 5-hydroxytryptamine receptor 6 (HTR6), and the 5-hydroxytryptamine receptor 7 (HTR7) (Meltzer, 1994). To date, genetic variants in the HTR2A, HTR2C, HTR6, and HTR7 genes as well as in 5-hydroxytryptamine receptor 1A (HTR1A), 5-hydroxytryptamine receptor 3A (HTR3A), 5-hydroxytryptamine receptor 3B (HTR3B), 5-hydroxytryptamine receptor 5A (HTR5A), and solute carrier family 6 member 4 (SLC6A4) genes have been investigated in clozapine response studies, with positive associations observed in the HTR2A, HTR2C, HTR6, HTR1A, HTR3A, and SLC6A4 genes.
With respect to the HTR2A gene (Arranz et al., 1995a, 1996, 1998b, 2000b; Masellis et al., 1995, 1998; Nöthen et al., 1995; Malhotra et al., 1996a; Lin et al., 1999; Schumacher et al., 2000; Yu et al., 2001; Lee et al., 2012), six studies found significant associations between genetic variants and the clozapine response (Arranz et al., 1995a, 1996, 1998b, 2000b; Masellis et al., 1998; Yu et al., 2001). Arransz and colleagues investigated an association between the T102C polymorphism (rs6313) in the HTR2A gene and clozapine response for at least 3 months of treatment following clinical stabilization (N = 149), demonstrating that homozygosity for the C102 allele was more frequent in the non-responders than in the responders (Arranz et al., 1995a). Yu and colleagues demonstrated an association between the 102C/C genotypes and higher N100 amplitude following clozapine treatment using event-related potentials to auditory stimuli (N = 98) (Yu et al., 2001). Consistent with these findings, a recent meta-analysis (total N = 868) suggests a significant association between the CC genotype and poor clozapine treatment response (Gressier et al., 2016). Arransz and colleagues also investigated an association between the his452tyr polymorphism (rs6314) in the HTR2A gene and clozapine response (N = 153), demonstrating that the Tyr452 allele occurred more frequently in the non-responders than in the responders (Arranz et al., 1996). Masellis and colleagues replicated this finding in subjects who received clozapine for a minimum of 6 month (N = 185) (Masellis et al., 1998). Consistent with these findings, a recent meta-analysis (total N = 671) suggests a significant association between C allele or C carriers or CC genotype and better clozapine treatment response compared to T allele or T carriers or TT genotype (Gressier et al., 2016). Arranz and colleagues also examined an association between the G-1438A polymorphism (rs6311) in the HTR2A gene and clozapine response (N = 274), demonstrating that homozygosity for the G-1438 allele was more frequent in the non-responders than the responders (Arranz et al., 1998b). However, a recent meta-analysis (total N = 547) suggests no association between the G-1438A polymorphism and clozapine treatment response (Gressier et al., 2016). With respect to the HTR2C gene (Sodhi et al., 1995; Malhotra et al., 1996b; Rietschel et al., 1997; Masellis et al., 1998; Arranz et al., 2000b; Schumacher et al., 2000), two studies found a significant association between genetic variants and the clozapine response (Sodhi et al., 1995; Arranz et al., 2000b). Sodhi and colleagues investigated an association between the Cys23Ser polymorphism (rs6318) in the HTR2C gene and clozapine response for at least 3 months treatment after clinical stabilization (N = 162), demonstrating that Ser allele carriers were more likely to show a response to clozapine (Sodhi et al., 1995). However, a recent meta-analysis suggests no association between the Cys23Ser polymorphism and clozapine treatment response in a Caucasian sample (Gressier et al., 2016). With respect to the HTR6 gene (Yu et al., 1999; Masellis et al., 2001; Lee et al., 2012), one study found a significant association between a genetic variant and the clozapine response (Yu et al., 1999). Yu and colleagues examined the association between the C267T polymorphism in the HTR6 gene and clozapine response for at least 8 weeks of treatment (N = 99), demonstrating that the 267T/T genotype was more frequent in responders than in non-responders (Yu et al., 1999). With respect to the HTR7 gene, there was not a significant association between the pro279leu polymorphism and the clozapine response (Masellis et al., 2001). With respect to the HTR1A gene (Masellis et al., 2001; Bosia et al., 2015), one study found a significant association between a genetic variant and the clozapine response (Bosia et al., 2015). Bosia and colleagues examined the association between the −1019C/G (rs6295) polymorphism in the HTR1A gene and clozapine response for 8 and 16 weeks of treatment (N = 107), demonstrating a greater improvement in the G/G group compared to the C/C group (Bosia et al., 2015). With respect to the HTR3A gene (Arranz et al., 2000b; Gutiérrez et al., 2002; Souza et al., 2010; Lee et al., 2012; Rajkumar et al., 2012), two studies found significant associations between genetic variants and the clozapine response (Souza et al., 2010; Rajkumar et al., 2012). Souza and colleagues demonstrated the T allele of rs1062613 in the HTR3A gene was associated with a good clozapine treatment response when used for at least 6 months (N = 140) (Souza et al., 2010). Similarly, Rajkumar and colleagues investigated an association of rs1062613 and rs2276302 in the HTR3A gene with the clozapine response, demonstrating that the T allele of rs106263 and the G allele of rs2276302 were associated with a good clozapine treatment response when presented with a stable dose for at least 12 weeks (N = 101) (Rajkumar et al., 2012). Consistent with these findings, a recent meta-analysis suggested that there may be an association between the T allele of rs1062613 and an improved response to clozapine (Gressier et al., 2016). With respect to the HTR3B and HTR5A genes (Arranz et al., 2000b; Birkett et al., 2000; Gutiérrez et al., 2002; Souza et al., 2010), there were no significant associations between genetic variants and the clozapine response. With respect to the SLC6A4 gene (Arranz et al., 2000a,b; Schumacher et al., 2000; Tsai et al., 2000; Kohlrausch et al., 2010), two studies found significant associations between genetic variants and the clozapine treatment response (Arranz et al., 2000b; Kohlrausch et al., 2010). Arranz and colleagues demonstrated a significant association between the biallelic polymorphism in the promoter region of the SLC6A4 gene, 5-HTTLPR, and the clozapine response in a Caucasian sample (N = 200) (Arranz et al., 2000b), and they found a similar trend in a larger sample (N = 268) (p = 0.08) (Arranz et al., 2000a). Kohlrausch and colleagues demonstrated that the short allele of HTTLPR/rs25531 occurred more frequently in the non-responders than in responders (Kohlrausch et al., 2010) (N = 116).
Glutamatergic receptors
Accumulating evidence suggests the potential for clozapine to act on glutamatergic neurotransmission (Heresco-Levy, 2003). To date, genetic variants in the solute carrier family 1 member 2 (SLC1A2), solute carrier family 6 member 9 (SLC6A9), glutamate ionotropic receptor AMPA type subunit 1 (GRIA1), glutamate metabotropic receptor 2 (GRM2), and glutamate decarboxylase 1 (GAD1) genes, glutamate ionotropic receptor NMDA type subunit 1 (GRIN1), glutamate ionotropic receptor NMDA type subunit 2A (GRIN2A), glutamate ionotropic receptor NMDA type subunit 2B (GRIN2B), have all been examined in clozapine treatment response studies, with no significant associations observed following correction for multiple testing (Hong et al., 2001; Hwang et al., 2011; Taylor et al., 2016, 2017).
Gene-Gene Interactions
Several trials have been performed to investigate a gene-gene interaction between multiple candidate genes and the clinical response to clozapine. Arranz and colleagues examined an association between 19 genetic polymorphisms in nine clozapine-targeted receptor subtypes and a neurotransmitter transporter and the response to clozapine, demonstrating that the strongest association was with a combination of six polymorphisms (HTR2A T102C, HTR2A his452tyr, HTR2C−330-GT/-244-CT, HTR2C Cys23Ser, SLC6A4 5-HTTLPR, and H2−1018-G/A) (N = 200) (Arranz et al., 2000b). However, Schumacher et al. could not replicate this finding (Schumacher et al., 2000). Hwang and colleagues demonstrated the most straightforward gene-gene- interaction effect of DRD1 rs686 and DRD3 Ser9Gly on the clozapine treatment response when taken for a minimum of 6 months in a Caucasian sample (N = 183) (Hwang et al., 2011). Bosia and colleagues demonstrated an additive effect of COMT Val158Met and HTR1A−1019 C/G on the clozapine treatment response when taken for 8 and 16 weeks (N = 107) (Bosia et al., 2015). Similarly, Rajagopal and colleagues demonstrated a significant gene-gene- interaction effect of DRD4 120-bp duplication and COMT Val158Met on the clozapine response when taken for at least 12 weeks (N = 93) (Rajagopal et al., 2018). Furthermore, Xu and colleagues conducted multivariate interaction analysis using 77 SNPs of 25 genes and demonstrated that the combination of rs6269 in the COMT gene and rs3813929 in the HTR2C gene may work as predictor to improve the clinical antipsychotic response (risperidone, clozapine, quetiapine, and chlorpromazine) in a large sample set (N = 995) (Xu et al., 2016).
Non-candidate Gene Approach
To date, the authors are unaware of any genome-wide association studies (GWAS) of clozapine efficacy that have been published. Recent pharmacogenetics GWAS suggest genetic overlap between antipsychotic response and susceptibility to schizophrenia (Ikeda et al., 2015; Ruderfer et al., 2016), and two genetic variants, identified by GWAS as schizophrenia risk loci (Cross-Disorder Group of the Psychiatric Genomics Consortium., 2013; Schizophrenia Working Group of the Psychiatric Genomics Consortium., 2014), have shown to be associated with a significant clinical response to clozapine (Brandl et al., 2016; Huang et al., 2016). Randl and colleagues demonstrated a significant association between rs2535629 in the inter-alpha-trypsin inhibitor heavy chain H3 (ITIH3) gene and an improvement of negative symptoms after 6 months of clozapine treatment (N = 105) (Brandl et al., 2016). Additionally, Huang and colleagues demonstrated a significant association between rs2514218, located upstream of the DRD2 gene, and clozapine response for 6 months of treatment (N = 208) (Huang et al., 2016).
Clozapine-Induced Agranulocytosis
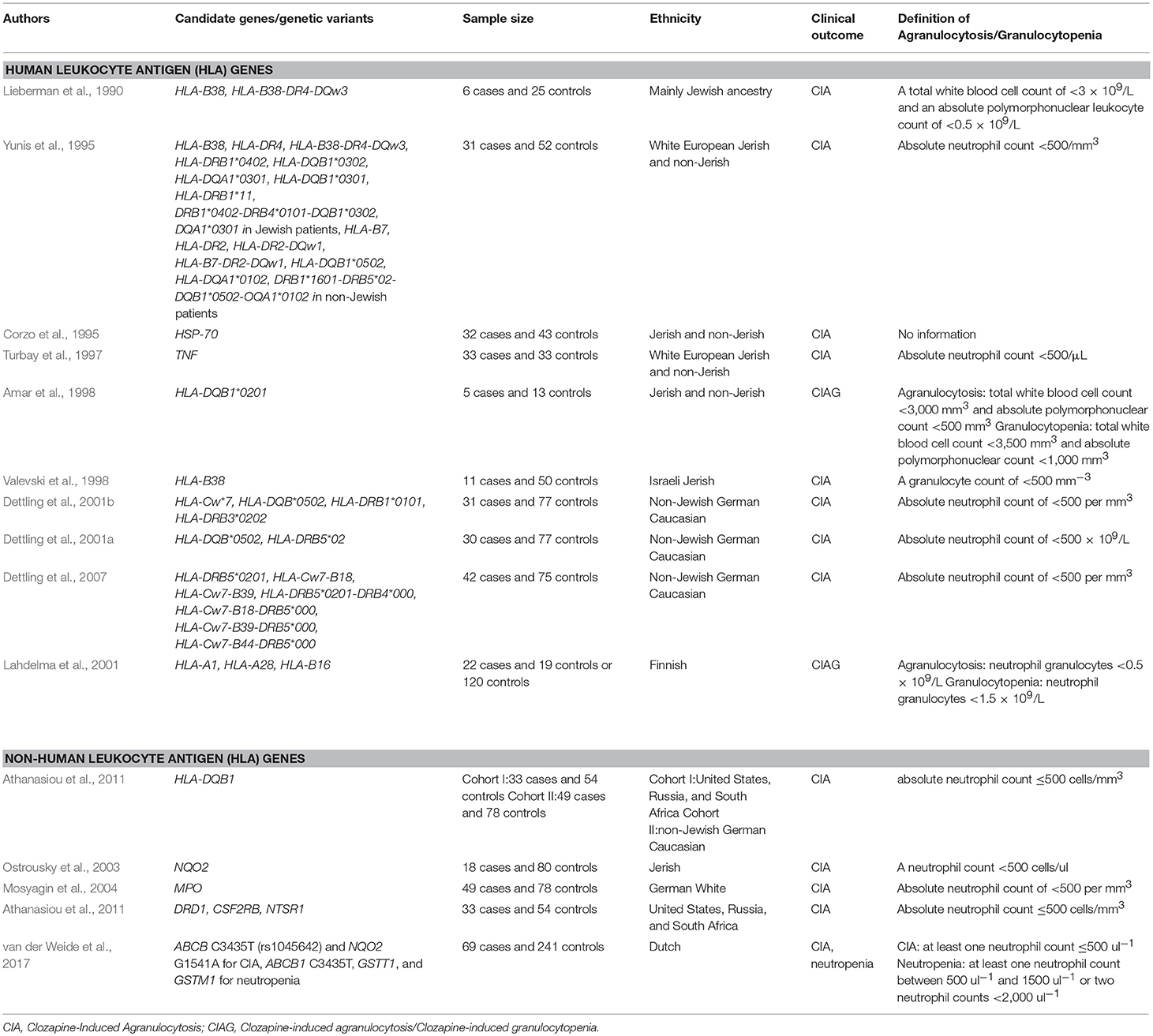
The occurrence of clozapine-induced agranulocytosis (CIA) is 0.8% at 1 year of administration of clozapine with reduced incidence after the first 6 months of clozapine treatment (Alvir et al., 1993). A recent meta-analysis suggests that 3.8% of patients exposed to clozapine will develop mild neutropenia (with an absolute neutrophil count of < 1,500/μl; Myles et al., 2018). A number of genetic studies of CIA and clozapine-induced agranulocytosis/granulocytopenia (CIAG) using samples from patients with schizophrenia have been conducted (Lieberman et al., 1990; Claas et al., 1992; Corzo et al., 1995; Yunis et al., 1995; Theodoropoulou et al., 1997; Turbay et al., 1997; Amar et al., 1998; Valevski et al., 1998; Meged et al., 1999; Dettling et al., 2000, 2001a,b, 2007; Lahdelma et al., 2001; Ostrousky et al., 2003; Mosyagin et al., 2004, 2005; Athanasiou et al., 2011; Anil Yagcioglu et al., 2016; van der Weide et al., 2017), many focusing on genetic variants in the genes within the major histocompatibility complex (MHC) region. Positive candidate gene studies of CIA and CIAG are shown in Table 2.
Candidate Gene Approach
Human leukocyte antigen (HLA) genes
In patients developing CIA (N = 6) compared with controls (N = 25), Lieberman and colleagues demonstrated that the occurrence of HLA-B38 as well as the haplotype of HLA-B38, DR4, and DQw3, was more frequent (Lieberman et al., 1990). The same laboratory subsequently analyzed them separately by Ashkenazi Jewish patients and non-Jewish patients, conforming their previous finding in the Ashkenazi Jewish samples and demonstrating a significant association of HLA-B7 and DR2 with CIA in non-Jewish patients (Yunis et al., 1995). They also demonstrated that the variants in the heat-shock protein 70 (HSP-70) and tumor necrosis factor (TNF) genes were associated with CIA (Corzo et al., 1995; Turbay et al., 1997). Additionally, Amar and colleagues demonstrated that HLA-DQB1*020 significantly occurred more frequently in subjects who developed CIAG compared to those who did not develop those complications (5 CIAG cases vs. 13 controls) (Amar et al., 1998). Valevski and colleagues replicated the involvement of HLA-B38 in CIA in Jewish Israeli samples (11 CIA cases vs. 50 controls) (Valevski et al., 1998). Dettling and colleagues demonstrated a significant association of HLA-Cw*7, DQB*0502, DRB1*0101, and DRB3*0202 with CIA in non-Jewish Caucasian samples (31 CIA cases vs. 77 controls) (Dettling et al., 2001b). The same laboratory also demonstrated a significantly higher frequency of HLA-DRB5*02 and HLA-DQB*0502 in patients who developed CIA (Dettling et al., 2001a). Later, the same laboratory performed an association study of CIA by combining the HLA class I and class II genetic variants, demonstrating that HLA-DRDB5*0201 and several haplotypes of the HLA genetic variants were associated with CIA (Dettling et al., 2007). Furthermore, Lahdelma and colleagues demonstrated that the HLA-B16 allele occurred more frequently in patients who developed CIAG (22 CIAG cases vs. 120 healthy controls) (Lahdelma et al., 2001), while Athanasiou and colleagues demonstrated that the HLA-DQB1 “REC 21G” occurred more frequently in patients who developed CIA compared with controls in two independent cohorts (Athanasiou et al., 2011).
Non-human leukocyte antigen (HLA) genes
Ostrousky and colleagues demonstrated that four polymorphisms in the dihydronicotinamide riboside quinone oxidoreductase 2 (NQO2) gene were associated with CIA (18 CIA cases vs. 80 controls) (Ostrousky et al., 2003). Additionally, Mosyagin and colleagues demonstrated that myeloperoxidase (MPO)−463AA carriers occurred more frequently in patients who developed CIA compared with AG and GG-carriers combined (49 CIA cases vs. 78 controls) (Mosyagin et al., 2004). Furthermore, Athanasiou and colleagues showed that DRD1, neurotensin receptor 1 (NTSR1), and β chain of colony-stimulating factor 2 receptor (CSF2RB) as well as HLA-DQB1 and HLA-C were associated with CIA (33 CIA cases vs. 54 controls) (Athanasiou et al., 2011). Van der Weide and colleagues demonstrated that NQO2 154AA and ABC1 3435TT occurred more frequently in patients who developed CIA compared with controls (31 CIA cases vs. 241 controls) (van der Weide et al., 2017). They also showed that for patients who developed neutropenia (N = 38), compared to controls (N = 241), ABCB1 3435TT and homozygosity for glutathione S-transferase theta 1 (GSTT1)null occurred more frequently, but glutathione S-transferase mu 1 (GSTM1)null occurred less frequently (van der Weide et al., 2017).
Non-candidate Gene Approach
Tiwari and colleagues conducted exome sequence analysis and did not find any genetic variants associated with CIA in Finnish patients after Bonferroni correction (13 CIA cases and 11 cases with severe neutropenia vs. 26 controls) (Tiwari et al., 2014). Meanwhile, Goldstein and colleagues conducted GWAS, whole-exome sequencing, and HLA allele imputation and were able to show that HLA-B 158T and HLA-DQB1 126Q were associated with CIAG (Goldstein et al., 2014). Legge and colleagues utilized GWAS, imputed HLA alleles, exome array, and copy-number variation analyses in a European population and subsequently combined the data of Goldstein et al. (2014) and Legge et al. (2017). In their meta-analysis of GWAS, they demonstrated that rs149104283, an intronic transcript of SLCO1B3 and SLCO1B7, was associated with clozapine-induced neutropenia. The authors of this paper conducted GWAS of CIAG in Japanese samples (50 CIAG cases vs. 2,905 controls) and identified rs1800625 in the HLA region as the CIAG candidate locus (Saito et al., 2016). A classical HLA analysis was subsequently conducted, demonstrating a significant association of HLA-B*59:01 with CIAG. However, we failed to replicate the risk SNPs on clozapine-associated neutropenia previously identified by Legge et al. (2017) in a Japanese population (Saito et al., 2017).
Discussion
In the present article, we summarize clozapine pharmacogenetic studies, separated by clinical response and CIA. To date, pharmacogenetic studies of clozapine in schizophrenia have been mostly conducted through candidate gene approaches. Genetic variants in the CYP enzyme family, dopamine, and serotonin receptor genes have been extensively examined as pharmacokinetic and pharmacodynamics candidates related to clozapine efficacy. Although there is limited evidence, the most recent meta-analyses indicate that only three SNPs (rs6313 and rs6314 in the HTR2A gene and rs1062613 in the HT3A gene) are associated with a significant clozapine response (Gressier et al., 2016). Genetic variants in the genes within the MHC region have been extensively examined with respect to CIA. The results of these candidate studies indicate the HLA variants are implicated in developing CIA. A comprehensive screening of genetic variants linked to a significant clinical response to clozapine and CIA is critical because the selection of candidate genes is restricted to current knowledge about underlying biological mechanisms (Adkins et al., 2011). Recently, the field of clozapine pharmacogenetics has shifted from a candidate gene approach to a genome-wide approach. A genome-wide approach has successfully identified novel genes that are associated with CIA and provided further evidence of the involvement of the HLA variants in CIA (Goldstein et al., 2014; Saito et al., 2016). On the other hand, there are no known GWAS looking at the clinical response to clozapine, although there are several GWAS looking at the antipsychotic treatment response in patients with schizophrenia (McClay et al., 2011; Drögemöller et al., 2016; Ruderfer et al., 2016; Li et al., 2017; Yu et al., 2018). Yu and colleagues conducted GWAS in a large cohort (n = 3,792) and detected five loci (CNTNAP5, MEGF10, PCDH7, SLC1A1, and TNIK) associated with general antipsychotic treatment response (Yu et al., 2018). Interestingly, these loci did not include DRD2, although D2 receptor blockade in the brain is a general pharmacodynamic property of antipsychotics (Mauri et al., 2014).
Pharmacogenetic testing has the potential to help improve patient outcome, lower healthcare costs, and increase patient medication adherence (Gardner et al., 2014). Pharmacogenetic testing in psychiatry is not yet a standard of practice, however, its utilization is steadily increasing (Eum et al., 2016; Fabbri et al., 2018). Several studies have, indeed, assessed the clinical utility for risk genetic variants of CIA. The sensitivity and specificity of the HLA-DQB1 6672G.>C polymorphism for CIA in patients treated with clozapine, identified though candidate approach, was 21.5 and 98.4%, respectively (Athanasiou et al., 2011). The sensitivity and specificity of the HLA-B*59:01 for CIA, identified by GWAS, was 31.8 and 95.3%, respectively (Saito et al., 2016) and the sensitivity and specificity of HLA-B 158T and HLA-DQB1 126Q polymorphisms for CIA, identified by GWAS and whole-exome sequencing (Goldstein et al., 2014), was 41 and 85%, respectively (Girardin et al., 2018). Clinical application guidelines require HLA allele testing for CIA to have a sensitivity of ~50% (Girardin et al., 2018), therefore none of these have reached an acceptable threshold for clinical application. Conversely, we examined the diagnostic performance of non-risk allele (alleles except for HLA-B*59:01) on non-CIA among CIG patients and demonstrated a moderate, positive predictive value for detecting non-CIA subjects in the CIG group without the risk allele, suggesting a potential candidate for re-challenging with clozapine treatment in a Japanese population (Saito et al., 2016). Based on this finding, a re-challenging with clozapine following neutropenia in a patient with a low risk of CIAG (HLA-B*52:01/52:01) was successfully conducted (Yamaki et al., 2017). The decision regarding clozapine re-challenge or withdrawal in case of CIAG should be based on careful consideration of risk factors, which can be facilitated by genetic testing in the future (Wicinski and Weclewicz, 2018). Further efforts to identify strong and reproducible genetic variants related to the clinical response to clozapine and CIA are needed to develop appropriate pharmacogenetic testing of clozapine.
The major issue of pharmacogenetics studies is inconsistent findings among studies. The discrepancies between these studies might be caused by statistical issues (i.e., sample size, multiple testing) and methodological issues (i.e., study design, phenotype definition, genetic polymorphism, population stratification) (Ross et al., 2012). Indeed, each sample size of the clozapine pharmacogenetic studies was relatively small. Large samples are needed to have enough statistical power to detect the effects of genetic variants on clinical outcomes by creating a clozapine consortium (Saito et al., 2016, 2017) or performing meta-analyses (Gressier et al., 2016; Legge et al., 2017). A sample size of more than 900 participants will be needed in a pharmacogenetic study if a common variant is anticipated with a large effect (Ross et al., 2012). Additionally, most of the clozapine pharmacogenetic studies did not adjust for the multiple statistical comparisons, resulting in type I error. To adjust for multiple testing, a false discovery rate (FDR) correction will be useful. Furthermore, selection bias and information bias are confounding factors in both prospective cohort and case-control studies (Ross et al., 2012). Clinical responses to clozapine have been determined by several different evaluation scales, including the Clinical Global Impressions Improvement (CGI-I) score, the global assessment scale (GAS), the Brief Psychiatric Rating Scale (BPRS), and Positive and Negative Symptom Scale (PANSS). Linkage disequilibrium (LD) varies among ethnic population, which may affect cross-subpopulation comparisons when causal SNPs are not directly genotyped but rather captures by proxy SNPs (Ross et al., 2012). Population stratification occurs when ethnic subpopulations within the entire study population differ in terms of genotype frequency and risk of disease (Thomas and Witte, 2002). In addition, clozapine doses and treatment length as well as types of antipsychotics preceding clozapine administration differ among studies. Although the clozapine treatment length in clinical response studies ranges from 5 weeks to more than 6 months, Lieberman and colleagues reported that 76% of patients responded to clozapine administered up to 52 weeks and slow clozapine responders experienced 70% of their total improvement after 12 weeks of treatment with clozapine (Lieberman et al., 1994). Standardized treatment protocols and evaluations of clinical outcomes will be needed. Furthermore, both non-genetic and genetic factors play an important role in the clinical outcome. For example, age at onset, gender, severity of the illness, negative symptoms, extrapyramidal side effects, clozapine concentration, history of catatonia, smoking, hyper-somnolence, and cognitive deficits have all been associated with the clozapine treatment response (Perry et al., 1991; Lieberman et al., 1994; Miller et al., 1994; Umbricht et al., 2002; Semiz et al., 2007; Rajkumar et al., 2013a). To take these factors in analyses will be needed.
In conclusion, a number of clozapine pharmacogenetic studies have been performed based on candidate gene approaches. However, there is heterogeneity across studies and their results have been inconsistent. Reproducible genetic variants with large effect related to the clinical response to clozapine and CIA have not been detected so far. This field is beginning to shift from candidate gene approaches to more a comprehensive strategy, such as GWAS and whole genome sequencing, which will make it possible not only to identify novel genetic variants related to clinical outcomes, but also to analyze the effects of multiple genes on clinical outcomes. Extensive effort is required to apply pharmacogenetic information in clinical practice for a personalized medicine strategy of clozapine treatment.
Author Contributions
SN and HU selected the articles and wrote the first draft of the manuscript. RH and TO supervised and contributed to the editing the manuscript. All authors have approved the final manuscript.
Funding
This work was supported in part by Japan Agency for Medical Research and development, AMED (JP15dk0310058, JP16dk0307065, SN and RH).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Adkins, D. E., Aberg, K., McClay, J. L., Bukszár, J., Zhao, Z., Jia, P., et al. (2011). Genomewide pharmacogenomic study of metabolic side effects to antipsychotic drugs. Mol. Psychiatry 16, 321–332. doi: 10.1038/mp.2010.14
Alawami, M., Wasywich, C., Cicovic, A., and Kenedi, C. (2014). A systematic review of clozapine induced cardiomyopathy. Int. J. Cardiol. 176, 315–320. doi: 10.1016/j.ijcard.2014.07.103
Alvir, J. M., Lieberman, J. A., Safferman, A. Z., Schwimmer, J. L., and Schaaf, J. A. (1993). Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N. Engl. J. Med. 329, 162–167. doi: 10.1056/NEJM199307153290303
Amar, A., Segman, R. H., Shtrussberg, S., Sherman, L., Safirman, C., Lerer, B., et al. (1998). An association between clozapine-induced agranulocytosis in schizophrenics and HLA-DQB1*0201. Int. J. Neuropsychopharmacol. 1, 41–44. doi: 10.1017/S1461145798001023
Anil Yagcioglu, A. E., Ilhan, B. Ç., Göktaş, M. T., Babaoglu, M. O., Uz, E., and Yazici, M. K. (2011). Agranulocytosis related to clozapine in monozygotic twins and association with allelic variants of multidrug resistance gene MDR1. J. Clin. Psychopharmacol. 31, 247–249. doi: 10.1097/JCP.0b013e31821084dc
Anil Yagcioglu, A. E., Yoca, G., Ayhan, Y., Karaca, R. Ö., Çevik, L., Müderrisoglu, A., et al. (2016). Relation of the allelic variants of multidrug resistance gene to agranulocytosis associated with clozapine. J. Clin. Psychopharmacol. 36, 257–261. doi: 10.1097/JCP.0000000000000495
Arranz, M. J., Bolonna, A. A., Munro, J., Curtis, C. J., Collier, D. A., and Kerwin, R. W. (2000a). The serotonin transporter and clozapine response. Mol. Psychiatry 5, 124–125. doi: 10.1038/sj.mp.4000652
Arranz, M. J., Collier, D., Sodhi, M., Ball, D., Roberts, G., Price, J., et al. (1995a). Association between clozapine response and allelic variation in 5-HT2A receptor gene. Lancet 346, 281–282. doi: 10.1016/S0140-6736(95)92168-0
Arranz, M. J., Collier, D. A., Munro, J., Sham, P., Kirov, G., Sodhi, M., et al. (1996). Analysis of a structural polymorphism in the 5-HT2A receptor and clinical response to clozapine. Neurosci. Lett. 217, 177–178. doi: 10.1016/0304-3940(96)13094-9
Arranz, M. J., Dawson, E., Shaikh, S., Sham, P., Sharma, T., Aitchison, K., et al. (1995b). Cytochrome P4502D6 genotype does not determine response to clozapine. Br. J. Clin. Pharmacol. 39, 417–420. doi: 10.1111/j.1365-2125.1995.tb04471.x
Arranz, M. J., Li, T., Munro, J., Liu, X., Murray, R., Collier, D. A., et al. (1998a). Lack of association between a polymorphism in the promoter region of the dopamine-2 receptor gene and clozapine response. Pharmacogenetics 8, 481–484. doi: 10.1097/00008571-199812000-00004
Arranz, M. J., Munro, J., Birkett, J., Bolonna, A., Mancama, D., Sodhi, M., et al. (2000b). Pharmacogenetic prediction of clozapine response. Lancet 355, 1615–1616. doi: 10.1016/S0140-6736(00)02221-2
Arranz, M. J., Munro, J., Owen, M. J., Spurlock, G., Sham, P. C., Zhao, J., et al. (1998b). Evidence for association between polymorphisms in the promoter and coding regions of the 5-HT2A receptor gene and response to clozapine. Mol. Psychiatry 3, 61–66. doi: 10.1038/sj.mp.4000348
Athanasiou, M. C., Dettling, M., Cascorbi, I., Mosyagin, I., Salisbury, B. A., Pierz, K. A., et al. (2011). Candidate gene analysis identifies a polymorphism in HLA-DQB1 associated with clozapine-induced agranulocytosis. J. Clin. Psychiatry 72, 458–463. doi: 10.4088/JCP.09m05527yel
Balibey, H., Basoglu, C., Lundgren, S., Babaoglu, M. O., Yasar, U., Herken, H., et al. (2011). CYP1A2*1F polymorphism decreases clinical response to clozapine in patients with schizophrenia. Bull. Clin. Psychopharmacol. 21, 93–99. doi: 10.5455/bcp.20110622071701
Barlas, I. O., Cetin, M., Erdal, M. E., Semiz, U. B., Basoglu, C., Ay, M. E., et al. (2009). Lack of association between DRD3 gene polymorphism and response to clozapine in Turkish schizoprenia patients. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 56–60. doi: 10.1002/ajmg.b.30770
Birkett, J. T., Arranz, M. J., Munro, J., Osbourn, S., Kerwin, R. W., and Collier, D. A. (2000). Association analysis of the 5-HT5A gene in depression, psychosis and antipsychotic response. Neuroreport 11, 2017–2020. doi: 10.1097/00001756-200006260-00042
Bosia, M., Lorenzi, C., Pirovano, A., Guglielmino, C., Cocchi, F., Spangaro, M., et al. (2015). COMT Val158Met and 5-HT1A-R−1019 C/G polymorphisms: effects on the negative symptom response to clozapine. Pharmacogenomics 16, 35–44. doi: 10.2217/pgs.14.150
Brandl, E. J., Lett, T. A., Chowdhury, N. I., Tiwari, A. K., Bakanidze, G., Meltzer, H. Y., et al. (2016). The role of the ITIH3 rs2535629 variant in antipsychotic response. Schizophr. Res. 176, 131–135. doi: 10.1016/j.schres.2016.06.032
Claas, F. H., Abbott, P. A., Witvliet, M. D., D'Amaro, J., Barnes, P. M., et al. (1992). No direct clinical relevance of the human leucocyte antigen (HLA) system in clozapine-induced agranulocytosis. Drug Saf. 7(Suppl. 1), 3–6. doi: 10.2165/00002018-199200071-00004
Corzo, D., Yunis, J. J., Salazar, M., Lieberman, J. A., Howard, A., and Krupp, P. (1995). The major histocompatibility complex region marked by HSP70-1 and HSP70-2 variants is associated with clozapine-induced agranulocytosis in two different ethnic groups. Blood 86, 3835–3840.
Cross-Disorder Group of the Psychiatric Genomics Consortium. (2013). Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381, 1371–1379. doi: 10.1016/S0140-6736(12)62129-1
De Berardis, D., Serroni, N., Campanella, D., Olivieri, L., Ferri, F., Carano, A., et al. (2012). Update on the adverse effects of clozapine: focus on myocarditis. Curr. Drug Saf. 7, 55–62. doi: 10.2174/157488612800492681
de Brito, R. B., de Carvalho Araújo, L., Diniz, M. J. A., de Castro Georg, R., Nabout, J. C., Vianelo, R. P., et al. (2015). The CYP1A2−163C > A polymorphism is associated with super-refractory schizophrenia. Schizophr. Res. 169, 502–503. doi: 10.1016/j.schres.2015.10.018
Dettling, M., Cascorbi, I., Opgen-Rhein, C., and Schaub, R. (2007). Clozapine-induced agranulocytosis in schizophrenic Caucasians: confirming clues for associations with human leukocyte class I and II antigens. Pharmacogenomics J. 7, 325–332. doi: 10.1038/sj.tpj.6500423
Dettling, M., Cascorbi, I., Roots, I., and Mueller-Oerlinghausen, B. (2001a). Genetic determinants of clozapine-induced agranulocytosis: recent results of HLA subtyping in a non-jewish caucasian sample. Arch. Gen. Psychiatry 58, 93–94. doi: 10.1001/archpsyc.58.1.93
Dettling, M., Sachse, C., Müller-Oerlinghausen, B., Roots, I., Brockmöller, J., Rolfs, A., et al. (2000). Clozapine-induced agranulocytosis and hereditary polymorphisms of clozapine metabolizing enzymes: no association with myeloperoxidase and cytochrome P4502D6. Pharmacopsychiatry 33, 218–220. doi: 10.1055/s-2000-8359
Dettling, M., Schaub, R. T., Mueller-Oerlinghause, B., Roots, I., and Cascorbi, I. (2001b). Further evidence of human leukocyte antigen-encoded susceptibility to clozapine-induced agranulocytosis independent of ancestry. Pharmacogenetics 11, 135–141. doi: 10.1097/00008571-200103000-00004
Drögemöller, B. I., Emsley, R., Chiliza, B., van der Merwe, L., Wright, G. E., Daya, M, et al. (2016). The identification of novel genetic variants associated with antipsychotic treatment response outcomes in first-episode schizophrenia patients. Pharmacogenet. Genomics 26, 235–242. doi: 10.1097/FPC.0000000000000213
Eap, C. B., Bender, S., Jaquenoud Sirot, E., Cucchia, G., Jonzier-Perey, M., Baumann, P., et al. (2004). Nonresponse to clozapine and ultrarapid CYP1A2 activity: clinical data and analysis of CYP1A2 gene. J. Clin. Psychopharmacol. 24, 214–219. doi: 10.1097/01.jcp.0000116646.91923.2f
Eum, S., Lee, A. M., and Bishop, J. R. (2016). Pharmacogenetic tests for antipsychotic medications: clinical implications and considerations. Dialogues Clin. Neurosci. 18, 323–337.
Fabbri, C., Zohar, J., and Serretti, A. (2018). Pharmacogenetic tests to guide drug treatment in depression: comparison of the available testing kits and clinical trials. Prog. Neuropsychopharmacol. Biol. Psychiatry 86, 36–44. doi: 10.1016/j.pnpbp.2018.05.007
Gaitonde, E. J., Morris, A., Sivagnanasundaram, S., McKenna, P. J., Hunt, D. M., and Mollon, J. D. (1996). Assessment of association of D3 dopamine receptor MscI polymorphism with schizophrenia: analysis of symptom ratings, family history, age at onset, and movement disorders. Am. J. Med. Genet. 67, 455–458. doi: 10.1002/(SICI)1096-8628(19960920)67:5<455::AID-AJMG3>3.0.CO;2-J
Gardner, K. R., Brennan, F. X., Scott, R., and Lombard, J. (2014). The potential utility of pharmacogenetic testing in psychiatry. Psychiatry J. 2014:730956. doi: 10.1155/2014/730956
Girardin, F. R., Poncet, A., Perrier, A., Vernaz, N., Pletscher, M., F Samer, C., et al. (2018). Cost-effectiveness of HLA-DQB1/HLA-B pharmacogenetic-guided treatment and blood monitoring in US patients taking clozapine. Pharmacogenomics J. doi: 10.1038/s41397-017-0004-2. [Epub ahead of print].
Goldstein, J. I., Jarskog, L. F., Hilliard, C., Alfirevic, A., Duncan, L., Fourches, D., et al. (2014). Clozapine-induced agranulocytosis is associated with rare HLA-DQB1 and HLA-B alleles. Nat. Commun. 5:4757. doi: 10.1038/ncomms5757
Gressier, F., Porcelli, S., Calati, R., and Serretti, A. (2016). Pharmacogenetics of clozapine response and induced weight gain: a comprehensive review and meta-analysis. Eur. Neuropsychopharmacol. 26, 163–185. doi: 10.1016/j.euroneuro.2015.12.035
Gutiérrez, B., Arranz, M. J., Huezo-Diaz, P., Dempster, D., Matthiasson, P., Travis, M., et al. (2002). Novel mutations in 5-HT3A and 5-HT3B receptor genes not associated with clozapine response. Schizophr. Res. 58, 93–97. doi: 10.1016/S0920-9964(02)00205-0
Heresco-Levy, U. (2003). Glutamatergic neurotransmission modulation and the mechanisms of antipsychotic atypicality. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 1113–1123. doi: 10.1016/j.pnpbp.2003.09.007
Hong, C. J., Yu, Y. W., Lin, C. H., Cheng, C. Y., and Tsai, S. J. (2001). Association analysis for NMDA receptor subunit 2B (GRIN2B) genetic variants and psychopathology and clozapine response in schizophrenia. Psychiatr. Genet. 11, 219–222. doi: 10.1097/00041444-200112000-00007
Horácek, J., Libiger, C., Höschl, K., Borzova, I., and Hendrychová, J. (2001). Clozapine-induced concordant agranulocytosis in monozygotic twins. Int. J. Psychiatry Clin. Pract. 5, 71–73. doi: 10.1080/136515001300225231
Huang, E., Maciukiewicz, M., Zai, C. C., Tiwari, A. K., Li, J., Potkin, S. G., et al. (2016). Preliminary evidence for association of genome-wide significant DRD2 schizophrenia risk variant with clozapine response. Pharmacogenomics 17, 103–109. doi: 10.2217/pgs.15.155
Hung, Y. P., Wang, C. S., Yen, C. N., Chang, H. C., Chen, P. S., Lee, I. H., et al. (2017). Role of cytokine changes in clozapine-induced fever: a cohort prospective study. Psychiatry Clin. Neurosci. 71, 395–402. doi: 10.1111/pcn.12508
Hwang, R., Shinkai, T., De Luca, V., Müller, D. J., Ni, X., Macciardi, F., et al. (2005). Association study of 12 polymorphisms spanning the dopamine D(2) receptor gene and clozapine treatment response in two treatment refractory/intolerant populations. Psychopharmacology 181, 179–187. doi: 10.1007/s00213-005-2223-5
Hwang, R., Shinkai, T., De Luca, V., Ni, X., Potkin, S. G., Lieberman, J. A., et al. (2007). Association study of four dopamine D1 receptor gene polymorphisms and clozapine treatment response. J. Psychopharmacol. 21, 718–727. doi: 10.1177/0269881106072341
Hwang, R., Shinkai, T., Deluca, V., Macciardi, F., Potkin, S., Meltzer, H. Y., et al. (2006). Dopamine D2 receptor gene variants and quantitative measures of positive and negative symptom response following clozapine treatment. Eur. Neuropsychopharmacol. 16, 248–259. doi: 10.1016/j.euroneuro.2005.09.004
Hwang, R., Souza, R. P., Tiwari, A. K., Zai, C. C., Müller, D. J., Potkin, S. G., et al. (2011). Gene-gene interaction analyses between NMDA receptor subunit and dopamine receptor gene variants and clozapine response. Pharmacogenomics 12, 277–291. doi: 10.2217/pgs.10.182
Hwang, R., Tiwari, A. K., Zai, C. C., Felsky, D., Remington, E., Wallace, T., et al. (2012). Dopamine D4 and D5 receptor gene variant effects on clozapine response in schizophrenia: replication and exploration. Prog. Neuropsychopharmacol. Biol. Psychiatry 37, 62–75. doi: 10.1016/j.pnpbp.2011.11.018
Hwang, R., Zai, C., Tiwari, A., Müller, D. J., Arranz, M. J., Morris, A. G., et al. (2010). Effect of dopamine D3 receptor gene polymorphisms and clozapine treatment response: exploratory analysis of nine polymorphisms and meta-analysis of the Ser9Gly variant. Pharmacogenomics J. 10, 200–218. doi: 10.1038/tpj.2009.65
Ikeda, M., Yoshimura, R., Hashimoto, R., Kondo, K., Saito, T., Shimasaki, A., et al. (2015). Genetic overlap between antipsychotic response and susceptibility to schizophrenia. J. Clin. Psychopharmacol. 35, 85–88. doi: 10.1097/JCP.0000000000000268
Jorgensen, A. L., and Williamson, P. R. (2008). Methodological quality of pharmacogenetic studies: issues of concern. Stat. Med. 27, 6547–6569. doi: 10.1002/sim.3420
Kaiser, R., Könneker, M., Henneken, M., Dettling, M., Müller-Oerlinghausen, B., Roots, I., et al. (2000). Dopamine D4 receptor 48-bp repeat polymorphism: no association with response to antipsychotic treatment, but association with catatonic schizophrenia. Mol. Psychiatry 5, 418–424. doi: 10.1038/sj.mp.4000729
Kerwin, R. W., Pilowsky, L., Munro, J., Shaikh, S., Gill, M., and Collier, D. (1994). Functional neuroimaging and pharmacogenetic studies of clozapine's action at dopamine receptors. J. Clin. Psychiatry 55(Suppl. B):57–62.
Kinoshita, M., Numata, S., Tajima, A., Yamamori, H., Yasuda, Y., Fujimoto, M., et al. (2017). Effect of clozapine on DNA methylation in peripheral leukocytes from patients with treatment-resistant schizophrenia. Int. J. Mol. Sci. 18:E632. doi: 10.3390/ijms18030632
Kohlrausch, F. B., Salatino-Oliveira, A., Gama, C. S., Lobato, M. I., Belmonte-de-Abreu, P., and Hutz, M. H. (2010). Influence of serotonin transporter gene polymorphisms on clozapine response in Brazilian schizophrenics. J. Psychiatr. Res. 44, 1158–1162. doi: 10.1016/j.jpsychires.2010.04.003
Kohn, Y., Ebstein, R. P., Heresco-Levy, U., Shapira, B., Nemanov, L., Gritsenko, I., et al. (1997). Dopamine D4 receptor gene polymorphisms: relation to ethnicity, no association with schizophrenia and response to clozapine in Israeli subjects. Eur. Neuropsychopharmacol. 7, 39–43. doi: 10.1016/S0924-977X(96)00380-X
Lahdelma, L., Ahokas, A., Andersson, L. C., Suvisaari, J., Hovatta, I., Huttunen, M. O., et al. (2001). Human leukocyte antigen-A1 predicts a good therapeutic response to clozapine with a low risk of agranulocytosis in patients with schizophrenia. J. Clin. Psychopharmacol. 21, 4–7. doi: 10.1097/00004714-200102000-00002
Lee, B. J., Marchionni, L., Andrews, C. E., Norris, A. L., Nucifora, L. G., Wu, Y. C., et al. (2017). Analysis of differential gene expression mediated by clozapine in human postmortem brains. Schizophr. Res. 185, 58–66. doi: 10.1016/j.schres.2016.12.017
Lee, S. T., Ryu, S., Kim, S. R., Kim, M. J., Kim, S., Kim, J. W., et al. (2012). Association study of 27 annotated genes for clozapine pharmacogenetics: validation of preexisting studies and identification of a new candidate gene, ABCB1, for treatment response. J. Clin. Psychopharmacol. 32, 441–448. doi: 10.1097/JCP.0b013e31825ac35c
Legge, S. E., Hamshere, M. L., Ripke, S., Pardinas, A. F., Goldstein, J. I., Rees, E., et al. (2017). Genome-wide common and rare variant analysis provides novel insights into clozapine-associated neutropenia. Mol. Psychiatry 22, 1502–1508. doi: 10.1038/mp.2016.97
Li, Q., Wineinger, N. E., Fu, D. J., Libiger, O., Alphs, L., Savitz, A., et al. (2017). Genome-wide association study of paliperidone efficacy. Pharmacogenet. Genomics 27, 7–18. doi: 10.1097/FPC.0000000000000250
Lieberman, J. A., Safferman, A. Z., Pollack, S., Szymanski, S., Johns, C., Howard, A., et al. (1994). Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am. J. Psychiatry 151, 1744–1752. doi: 10.1176/ajp.151.12.1744
Lieberman, J. A., Yunis, J., Egea, E., Canoso, R. T., Kane, J. M., and Yunis, E. J. (1990). HLA-B38, DR4, DQw3 and clozapine-induced agranulocytosis in Jewish patients with schizophrenia. Arch. Gen. Psychiatry 47, 945–948. doi: 10.1001/archpsyc.1990.01810220061007
Lin, C. H., Tsai, S. J., Yu, Y. W., Song, H. L., Tu, P. C., Sim, C. B., et al. (1999). No evidence for association of serotonin-2A receptor variant (102T/C) with schizophrenia or clozapine response in a Chinese population. Neuroreport 10, 57–60. doi: 10.1097/00001756-199901180-00011
Malhotra, A. K., Buchanan, R. W., Kim, S., Kestler, L., Breier, A., Pickar, D., et al. (1999). Allelic variation in the promoter region of the dopamine D2 receptor gene and clozapine response. Schizoohr. Res. 36, 92–93.
Malhotra, A. K., Goldman, D., Buchanan, R. W., Rooney, W., Clifton, A., Kosmidis, M. H., et al. (1998). The dopamine D3 receptor (DRD3) Ser9Gly polymorphism and schizophrenia: a haplotype relative risk study and association with clozapine response. Mol. Psychiatry 3, 72–75. doi: 10.1038/sj.mp.4000288
Malhotra, A. K., Goldman, D., Ozaki, N., Breier, A., Buchanan, R., and Pickar, D. (1996a). Lack of association between polymorphisms in the 5-HT2A receptor gene and the antipsychotic response to clozapine. Am. J. Psychiatry 153, 1092–1094. doi: 10.1176/ajp.153.8.1092
Malhotra, A. K., Goldman, D., Ozaki, N., Rooney, W., Clifton, A., Buchanan, R. W., et al. (1996b). Clozapine response and the 5HT2C Cys23Ser polymorphism. Neuroreport 7, 2100–2102. doi: 10.1097/00001756-199609020-00007
Masellis, M., Basile, V., Meltzer, H. Y., Lieberman, J. A., Sevy, S., Macciardi, F. M., et al. (1998). Serotonin subtype 2 receptor genes and clinical response to clozapine in schizophrenia patients. Neuropsychopharmacology 19, 123–132. doi: 10.1016/S0893-133X(98)00007-4
Masellis, M., Basile, V. S., Meltzer, H. Y., Lieberman, J. A., Sevy, S., Goldman, D. A., et al. (2001). Lack of association between the T–>C 267 serotonin 5-HT6 receptor gene (HTR6) polymorphism and prediction of response to clozapine in schizophrenia. Schizophr. Res. 47, 49–58. doi: 10.1016/S0920-9964(00)00016-5
Masellis, M., Paterson, A. D., Badri, F., Lieberman, J. A., Meltze, H. Y., Cavazzoni, P., et al. (1995). Genetic variation of 5-HT2A receptor and response to clozapine. Lancet 346:1108. doi: 10.1016/S0140-6736(95)91785-3
Mauri, M. C., Paletta, S., Maffini, M., Colasanti, A., Dragogna, F., and Altamura, A. C. (2014). Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 13, 1163–1191.
McClay, J. L., Adkins, D. E., Aberg, K., Stroup, S., Perkins, D. O., Vladimirov, V. I., et al. (2011). Genome-wide pharmacogenomic analysis of response to treatment with antipsychotics. Mol. Psychiatry 16, 76–85. doi: 10.1038/mp.2009.89
Meged, S., Stein, D., Sitrota, P., Melamed, Y., Elizur, A., Shmuelian, I., et al. (1999). Human leukocyte antigen typing, response to neuroleptics, and clozapine-induced agranulocytosis in jewish Israeli schizophrenic patients. Int. Clin. Psychopharmacol. 14, 305–312. doi: 10.1097/00004850-199909000-00005
Meltzer, H. Y. (1994). An overview of the mechanism of action of clozapine. J. Clin. Psychiatry 55(Suppl. B), 47–52.
Meltzer, H. Y. (1997). Treatment-resistant schizophrenia–the role of clozapine. Curr. Med. Res. Opin. 14, 1–20. doi: 10.1185/03007999709113338
Miller, D. D., Fleming, F., Holman, T. L., and Perry, P. J. (1994). Plasma clozapine concentrations as a predictor of clinical response: a follow-up study. J. Clin. Psychiatry 55(Suppl. B), 117–121.
Mosyagin, I., Cascorbi, I., Schaub, R., Krüger, T., and Dettling, M. (2005). Drug-induced agranulocytosis: impact of different fcgamma receptor polymorphisms? J. Clin. Psychopharmacol. 25, 435–440. doi: 10.1097/01.jcp.0000177551.13714.33
Mosyagin, I., Dettling, M., Roots, I., Mueller-Oerlinghausen, B., and Cascorbi, I (2004). Impact of myeloperoxidase and NADPH-oxidase polymorphisms in drug-induced agranulocytosis. J. Clin. Psychopharmacol. 24, 613–617. doi: 10.1097/01.jcp.0000144891.52858.a6
Myles, N., Myles, H., Xia, S., Large, M., Kisely, S., Galletly, C., et al. (2018). Acta Psychiatr. Scand. doi: 10.1111/acps.12898. [Epub ahead of print].
Nakazawa, T., Kikuchi, M., Ishikawa, M., Yamamori, H., Nagayasu, K., Matsumoto, T., et al. (2017). Differential gene expression profiles in neurons generated from lymphoblastoid B-cell line-derived iPS cells from monozygotic twin cases with treatment-resistant schizophrenia and discordant responses to clozapine. Schizophr. Res. 181, 75–82. doi: 10.1016/j.schres.2016.10.012
Nöthen, M. M., Rietschel, M., Erdmann, J., Oberländer, H., Möller, H. J., Nober, D., et al. (1995). Genetic variation of the 5-HT2A receptor and response to clozapine. Lancet 346, 908–909.
Ostrousky, O., Meged, S., Loewenthal, R., Valevski, A., Weizman, A., Carp, H., et al. (2003). NQO2 gene is associated with clozapine-induced agranulocytosis. Tissue Antigens 62, 483–491. doi: 10.1046/j.1399-0039.2003.00133.x
Perry, P. J., Miller, D. D., Arndt, S. V., and Cadoret, R. J. (1991). Clozapine and norclozapine plasma concentrations and clinical response of treatment-refractory schizophrenic patients. Am. J. Psychiatry 148, 231–235.
Piatkov, I., Caetano, D., Assur, Y., Lau, S. L., Coelho, M., Jones, T., et al. (2017). CYP2C19*17 protects against metabolic complications of clozapine treatment. World J. Biol. Psychiatry 18, 521–527. doi: 10.1080/15622975.2017.1347712
Potkin, S. G., Basile, V. S., Jin, Y., Masellis, M., Badri, F., Keator, D., et al. (2003). D1 receptor alleles predict PET metabolic correlates of clinical response to clozapine. Mol. Psychiatry 8, 109–113. doi: 10.1038/sj.mp.4001191
Rajagopal, V. M., Rajkumar, A. P., Jacob, K. S., and Jacob, M. (2018). Gene-gene interaction between DRD4 and COMT modulates clinical response to clozapine in treatment-resistant schizophrenia. Pharmacogenet. Genomics 28, 31–35. doi: 10.1097/FPC.0000000000000314
Rajkumar, A. P., Poonkuzhali, B., Kuruvilla, A., Jacob, M., and Jacob, K. S. (2013a). Clinical predictors of serum clozapine levels in patients with treatment-resistant schizophrenia. Int. Clin. Psychopharmacol. 28, 50–56. doi: 10.1097/YIC.0b013e32835ac9da
Rajkumar, A. P., Poonkuzhali, B., Kuruvilla, A., Srivastava, A., Jacob, M., and Jacob, K. S. (2012). Outcome definitions and clinical predictors influence pharmacogenetic associations between HTR3A gene polymorphisms and response to clozapine in patients with schizophrenia. Psychopharmacology 224, 441–449. doi: 10.1007/s00213-012-2773-2
Rajkumar, A. P., Poonkuzhali, B., Kuruvilla, A., Srivastava, A., Jacob, M., and Jacob, K. S. (2013b). Association between CYP1A2 gene single nucleotide polymorphisms and clinical responses to clozapine in patients with treatment-resistant schizophrenia. Acta Neuropsychiatr. 25, 2–11. doi: 10.1111/j.1601-5215.2012.00638.x
Rao, P. A., Pickar, D., Gejman, P. V., Ram, A., Gershon, E. S., and Gelernter, J. (1994). Allelic variation in the D4 dopamine receptor (DRD4) gene does not predict response to clozapine. Arch. Gen. Psychiatry 51, 912–917.
Rietschel, M., Naber, D., Fimmers, R., Möller, H. J., Propping, P., and Nöthen, M. M. (1997). Efficacy and side-effects of clozapine not associated with variation in the 5-HT2C receptor. Neuroreport 8, 1999–2003.
Rietschel, M., Naber, D., Oberländer, H., Holzbach, R., Fimmers, R., Eggermann, K., et al. (1996). Efficacy and side-effects of clozapine: testing for association with allelic variation in the dopamine D4 receptor gene. Neuropsychopharmacology 15, 491–496.
Ross, S., Anand, S. S., Joseph, P., and Par,é, G. (2012). Promises and challenges of pharmacogenetics: an overview of study design, methodological and statistical issues. JRSM Cardiovasc. Dis. 1:cvd.2012.012001. doi: 10.1258/cvd.2012.012001
Ruderfer, D. M., Charney, A. W., Readhead, B., Kidd, B. A., Kähler, A. K., Kenny, P. J., et al. (2016). Polygenic overlap between schizophrenia risk and antipsychotic response: a genomic medicine approach. Lancet Psychiatry 3, 350–357. doi: 10.1016/S2215-0366(15)00553-2
Saito, T., Ikeda, M., Hashimoto, R., Iwata, N., Yamamori, H., et al. (2017). transethnic replication study to assess the association between clozapine-induced agranulocytosis/granulocytopenia and genes at 12p12.2 in a Japanese population. Biol. Psychiatry 82, e9–e10. doi: 10.1016/j.biopsych.2016.12.009
Saito, T., Ikeda, M., Mushiroda, T., Ozeki, T., Kondo, K., Shimasaki, A., et al. (2016). pharmacogenomic study of clozapine-induced agranulocytosis/granulocytopenia in a Japanese population. Biol. Psychiatry 80, 636–642. doi: 10.1016/j.biopsych.2015.12.006
Scharfetter, J., Chaudhry, H. R., Hornik, K., Fuchs, K., Sieghart, W., Kasper, S., et al. (1999). Dopamine D3 receptor gene polymorphism and response to clozapine in schizophrenic Pakastani patients. Eur. Neuropsychopharmacol. 10, 17–20.
Schizophrenia Working Group of the Psychiatric Genomics Consortium. (2014). Biological insights from 108 schizophrenia-associated genetic loci. Nature 511, 421–427. doi: 10.1038/nature13595
Schumacher, J., Schulze, T. G., Wienker, T. F., Rietschel, M., and Nöthen, M. M. (2000). Pharmacogenetics of the clozapine response. Lancet 356, 506–507. doi: 10.1016/S0140-6736(05)74176-3
Semiz, U. B., Cetin, M., Basoglu, C., Ebrinc, S., Uzun, O., Herken, H., et al. (2007). Clinical predictors of therapeutic response to clozapine in a sample of Turkish patients with treatment-resistant schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 31, 1330–1336. doi: 10.1016/j.pnpbp.2007.06.002
Shaikh, S., Collier, D., Kerwin, R. W., Pilowsky, L. S., Gill, M., Xu, W. M., et al. (1993). Dopamine D4 receptor subtypes and response to clozapine. Lancet 341:116.
Shaikh, S., Collier, D. A., Sham, P., Pilowsky, L., Sharma, T., Lin, L. K., et al. (1995). Analysis of clozapine response and polymorphisms of the dopamine D4 receptor gene (DRD4) in schizophrenic patients. Am. J. Med. Genet. 60, 541–545.
Shaikh, S., Collier, D. A., Sham, P. C., Ball, D., Aitchison, K., Vallada, H., et al. (1996). Allelic association between a Ser-9-Gly polymorphism in the dopamine D3 receptor gene and schizophrenia. Hum. Genet. 97, 714–719.
Siskind, D., McCartney, L., Goldschlager, R., and Kisely, S. (2016). Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry 209, 385–392. doi: 10.1192/bjp.bp.115.177261
Siskind, D., Siskind, V., and Kisely, S. (2017). Clozapine response rates among people with treatment-resistant schizophrenia: data from a systematic review and meta-analysis. Can. J. Psychiatry 62, 772–777. doi: 10.1177/0706743717718167
Sodhi, M. S., Arranz, M. J., Curtis, D., Ball, D. M., Sham, P., Roberts, G. W., et al. (1995). Association between clozapine response and allelic variation in the 5-HT2C receptor gene. Neuroreport 7, 169–172.
Souza, R. P., de Luca, V., Meltzer, H. Y., Lieberman, J. A., and Kennedy, J. L. (2010). Influence of serotonin 3A and 3B receptor genes on clozapine treatment response in schizophrenia. Pharmacogenet. Genomics 20, 274–276. doi: 10.1097/FPC.0b013e328337ce3e
Taylor, D. L., Tiwari, A. K., Lieberman, J. A., Potkin, S. G., Meltzer, H. Y., Knight, J., et al. (2016). Genetic association analysis of N-methyl-D-aspartate receptor subunit gene GRIN2B and clinical response to clozapine. Hum. Psychopharmacol. 31, 121–134. doi: 10.1002/hup.2519
Taylor, D. L., Tiwari, A. K., Lieberman, J. A., Potkin, S. G., Meltzer, H. Y., Knight, J., et al. (2017). Pharmacogenetic analysis of functional glutamate system gene variants and clinical response to clozapine. Mol Neuropsychiatry 2, 185–197. doi: 10.1159/000449224
Theisen, F. M., Cichon, S., Linden, A., Martin, M., Remschmidt, H., and Hebebrand, J. (2001). Clozapine and weight gain. Am. J. Psychiatry 158:816. doi: 10.1176/appi.ajp.158.5.816
Theodoropoulou, S., Pappa, H., Lykouras, L., Papageorgiou, G., Papasteriades, C., and Sakalis, G. (1997). Human leukocyte antigen system in clozapine-induced agranulocytosis. Neuropsychobiology 36, 5–7.
Thomas, D. C., and Witte, J. S. (2002). Point: population stratification: a problem for case-control studies of candidate-gene associations? Cancer Epidemiol. Biomarkers Prev. 11, 505–512.
Tiwari, A. K., Need, A. C., Lohoff, F. W., Zai, C. C., Chowdhury, N. I., Müller, D. J., et al. (2014). Exome sequence analysis of Finnish patients with clozapine-induced agranulocytosis. Mol. Psychiatry 19, 403–405. doi: 10.1038/mp.2013.74
Tsai, S. J., Hong, C. J., Yu, Y. W., Lin, C. H., Song, H. L., Lai, H. C., et al. (2000). Association study of a functional serotonin transporter gene polymorphism with schizophrenia, psychopathology and clozapine response. Schizophr. Res. 44, 177–181. doi: 10.1016/S0920-9964(99)00170-X
Turbay, D., Lieberman, J., Alper, C. A., Delgado, J. C., Corzo, D., Yunis, J. J., et al. (1997). Tumor necrosis factor constellation polymorphism and clozapine-induced agranulocytosis in two different ethnic groups. Blood 89, 4167–4174.
Umbricht, D. S., Wirshing, W. C., Wirshing, D. A., McMeniman, M., Schooler, N. R., Marder, S. R., et al. (2002). Clinical predictors of response to clozapine treatment in ambulatory patients with schizophrenia. J. Clin. Psychiatry 63, 420–424.
Urichuk, L., Prior, T. I., Dursun, S., and Baker, G. (2008). Metabolism of atypical antipsychotics: involvement of cytochrome p450 enzymes and relevance for drug-drug interactions. Curr. Drug Metab. 9, 410–418. doi: 10.2174/138920008784746373
Valevski, A., Klein, T., Gazit, E., Meged, S., Stein, D., Elizur, A., et al. (1998). HLA-B38 and clozapine-induced agranulocytosis in Israeli Jewish schizophrenic patients. Eur. J. Immunogenet. 25, 11–13.
van der Weide, K., Loovers, H., Pondman, K., Bogers, J., van der Straaten, T., Langemeijer, E., et al. (2017). Genetic risk factors for clozapine-induced neutropenia and agranulocytosis in a Dutch psychiatric population. Pharmacogenomics J. 17, 471–478. doi: 10.1038/tpj.2016.32
Vojvoda, D., Grimmell, K., Sernyak, M., and Mazure, C. M. (1996). Monozygotic twins concordant for response to clozapine. Lancet 347:61.
Warnez, S., and Alessi-Severini, S. (2014). Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry 14:102. doi: 10.1186/1471-244X-14-102
Wehmeier, P. M., Gebhardt, S., Schmidtke, J., Remschmidt, H., Hebebrand, J., and Theisen, F. M. (2005). Clozapine: weight gain in a pair of monozygotic twins concordant for schizophrenia and mild mental retardation. Psychiatry Res. 133, 273–276. doi: 10.1016/j.psychres.2004.02.018
Wicinski, M., and Weclewicz, M. M. (2018). Clozapine-induced agranulocytosis/granulocytopenia: mechanisms and monitoring. Curr. Opin. Hematol. 25, 22–28. doi: 10.1097/MOH.0000000000000391
Woodward, N. D., Jayathilake, K., and Meltzer, H. Y. (2007). COMT val108/158met genotype, cognitive function, and cognitive improvement with clozapine in schizophrenia. Schizophr. Res. 90, 86–96. doi: 10.1016/j.schres.2006.10.002
Xu, M., Xing, Q., Li, S., Zheng, Y., Wu, S., Gao, R., et al. (2010). Pharacogenetic effects of dopamine transporter gene polymorphisms on response to chlorpromazine and clozapine and on extrapyramidal syndrome in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 1026–1032. doi: 10.1016/j.pnpbp.2010.05.017
Xu, Q., Wu, X., Li, M., Huang, H., Minica, C., Yi, Z., et al. (2016). Association studies of genomic variants with treatment response to risperidone, clozapine, quetiapine and chlorpromazine in the Chinese Han population. Pharmacogenomics J. 16, 357–365. doi: 10.1038/tpj.2015.61
Yamaki, N., Hishimoto, A., Otsuka, I., Sasada, T., Boku, S., Saito, T., et al. (2017). Optimizing outcomes in clozapine rechallenge following neutropenia using human leukocyte antigen typing: a case report. Psychiatry Clin. Neurosci. 71, 289–290. doi: 10.1111/pcn.12505
Yamamori, H., Hashimoto, R., Fujita, Y., Numata, S., Yasuda, Y., Fujimoto, M., et al. (2014). Changes in plasma D-serine, L-serine, and glycine levels in treatment-resistant schizophrenia before and after clozapine treatment. Neurosci. Lett. 582, 93–98. doi: 10.1016/j.neulet.2014.08.052
Yamamori, H., Hashimoto, R., Ishima, T., Kishi, F., Yasuda, Y., Ohi, K., et al. (2013). Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment-resistant schizophrenia treated with clozapine. Neurosci. Lett. 556, 37–41. doi: 10.1016/j.neulet.2013.09.059
Yu, H., Yan, H., Wang, L., Li, J., Tan, L., Deng, W., et al. (2018). Five novel loci associated with antipsychotic treatment response in patients with schizophrenia: a genome-wide association study. Lancet Psychiatry 5, 327–338. doi: 10.1016/S2215-0366(18)30049-X
Yu, Y. W., Tsai, S. J., Lin, C. H., Hsu, C. P., Yang, K. H., and Hong, C. J. (1999). Serotonin-6 receptor variant (C267T) and clinical response to clozapine. Neuroreport 10, 1231–1233.
Yu, Y. W., Tsai, S. J., Yang, K. H., Lin, C. H., Chen, M. C., and Hong, C. J. (2001). Evidence for an association between polymorphism in the serotonin-2A receptor variant (102T/C) and increment of N100 amplitude in schizophrenics treated with clozapine. Neuropsychobiology 43, 79–82. doi: 10.1159/000054871
Yunis, J. J., Corzo, D., Salazar, M., Lieberman, J. A., Howard, A., and Yunis, E. J. (1995). HLA associations in clozapine-induced agranulocytosis. Blood 86, 1177–1183.
Keywords: clozapine, schizophrenia, clinical response, agranulocytosis, pharmacogenetics, SNP, GWAS, review
Citation: Numata S, Umehara H, Ohmori T and Hashimoto R (2018) Clozapine Pharmacogenetic Studies in Schizophrenia: Efficacy and Agranulocytosis. Front. Pharmacol. 9:1049. doi: 10.3389/fphar.2018.01049
Received: 11 June 2018; Accepted: 30 August 2018;
Published: 26 September 2018.
Edited by:
Mirko Manchia, Università degli Studi di Cagliari, ItalyReviewed by:
Alfredo Meneses, Centro de Investigación y de Estudios Avanzados (CINVESTAV), MexicoAndrea De Bartolomeis, Università degli Studi di Napoli Federico II, Italy
Copyright © 2018 Numata, Umehara, Ohmori and Hashimoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shusuke Numata, shu-numata@umin.ac.jp
 Shusuke Numata
Shusuke Numata Hidehiro Umehara1
Hidehiro Umehara1 Ryota Hashimoto
Ryota Hashimoto