Value of extracorporeal artificial liver support in pediatric acute liver failure: A single-center experience of over 10 years
- Department of Liver Transplant Intensive Care Unit, Memorial Sisli Hospital, Şişli, Turkey
Purpose: Acute liver failure (ALF) is a life-threatening disease characterized by rapid-onset liver dysfunction, coagulopathy, and encephalopathy in patients without chronic liver disease. Today, the combined application of continuous veno-venous hemodiafiltration (CVVHDF) and plasma exchange (PEX), which are forms of supportive extracorporeal therapy (SECT), with conventional liver therapy in ALF is recommended. This study aims to retrospectively analyze the effects of combined SECT in pediatric patients with ALF.
Materials and Methods: We retrospectively analyzed 42 pediatric patients, followed in the liver transplantation intensive care unit. The patients had ALF and received PEX supportive therapy with combined CVVHDF. The biochemical lab values of the results for the patients before the first combined SECT and after the last combined SECT were analyzed comparatively.
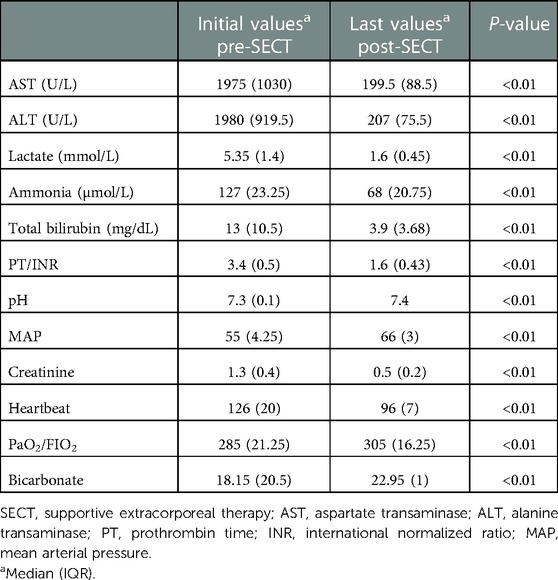
Results: Of the pediatric patients included in our study, 20 were girls and 22 were boys. Liver transplantation was performed in 22 patients, and 20 patients recovered without transplantation. After the discontinuation of combined SECT, all patients had significantly lower serum liver function test results (total bilirubin, alanine transaminase, aspartate transaminase), ammonia, and prothrombin time/international normalized ratio levels than the previous levels (p < 0.01). Hemodynamic parameters (i.e., mean arterial pressure) also improved significantly.
Discussion and Conclusion: Combined CVVHDF and PEX treatment significantly improved biochemical parameters and clinical findings, including encephalopathy, in pediatric patients with ALF. PEX therapy combined with CVVHDF is a proper supportive therapy for bridging or recovery.
Introduction
Acute liver failure (ALF) is a life-threatening disease characterized by rapid-onset liver dysfunction, coagulopathy, and encephalopathy in patients without chronic liver disease. Acute-on-chronic liver failure (ACLF) is used to describe the occurrence of ALF with sudden and life-threatening worsening of clinical conditions in patients with cirrhosis or chronic liver disease. ALF has a mortality rate of up to 90% and is higher in patients younger than 12 months of age (1, 2). In the pathophysiology of the disease, the hypothesis of endogenous intoxication is accepted. Endogenous intoxication causes several metabolic disorders, including hepatic coma and multiorgan failure (MOF) (3–6). Disseminated intravascular coagulation (DIC), acute renal failure (ARF), shock due to the loss of vascular resistance, acute respiratory distress syndrome (ARDS), hepatic encephalopathy (HE), and sepsis-associated infection translocation often develop secondary to ALF. The severity of organ failure is linked to elevated and circulating cytokines and endotoxins, such as TNF-alpha, interleukin (IL)-6, IL-1ß, IL-8, IL-10, IL-2, IL-4, and IFN-γ (7–9). Although some patients with a high probability of death may recover with supportive treatment, emergency liver transplantation (LTX) still is the definitive treatment; however, the availability of suitable organs for LTX is limited. A combination of continuous veno-venous hemodiafiltration (CVVHDF) and plasma exchange (PEX) is applied as a bridging treatment both in recovery without LTX and to prolong treatment until LTX (10). This study aims to retrospectively analyze the effects of combined supportive extracorporeal therapy (SECT) in pediatric patients with ALF.
Technical aspects
Plasma exchange
PEX can be performed by centrifugation or filtration-based mechanisms. Centrifugation separates the blood into its components by density (specific gravity), while in filtration, components of the blood are separated according to their size by passing through a perforated membrane, according to the pore diameter of the membrane. Centrifugation and filtration-based systems are similar in terms of safety, efficiency, and therapeutic effect. As the shaped cells of the blood are returned to the patient, the plasma is eliminated and replaced with fresh-frozen plasma (FFP), albumin, or another liquid. The use of FFP allows for the replacement of important and highly beneficial substances, such as albumin and coagulation factors, in addition to substances that act on mediators of inflammation. Typical PEX therapy replaces 1–1.5 times the patient's estimated plasma volume. Plasma volume is calculated from the estimated total blood volume using common physiological variables, such as an individual's sex, height, weight, body muscle mass, and hematocrit. An exchange of 1–1.5 times the plasma volume removes approximately 70% of the substances from the intravascular space.
Continuous veno-venous hemodiafiltration
CVVHDF combines two continuous renal replacement therapies (CRRTs): hemodialysis and hemofiltration. It can clear both low- and medium-to-high-molecular-weight molecules by combining diffusion and convective methods. Diffusion is the movement of a solute along a semipermeable membrane according to a concentration gradient. The clearance depends on the concentration gradient, the size of the solute, protein binding, and the surface area of the filter. Diffusion eases the clearance of low-molecular-weight solutes, such as urea and creatinine. Convection involves the movement of water and dissolved solutes across a semipermeable membrane due to a pressure gradient. Depending on the membrane porosity, medium-to-large molecules, such as cytokines, can be scavenged by convection.
Materials and methods
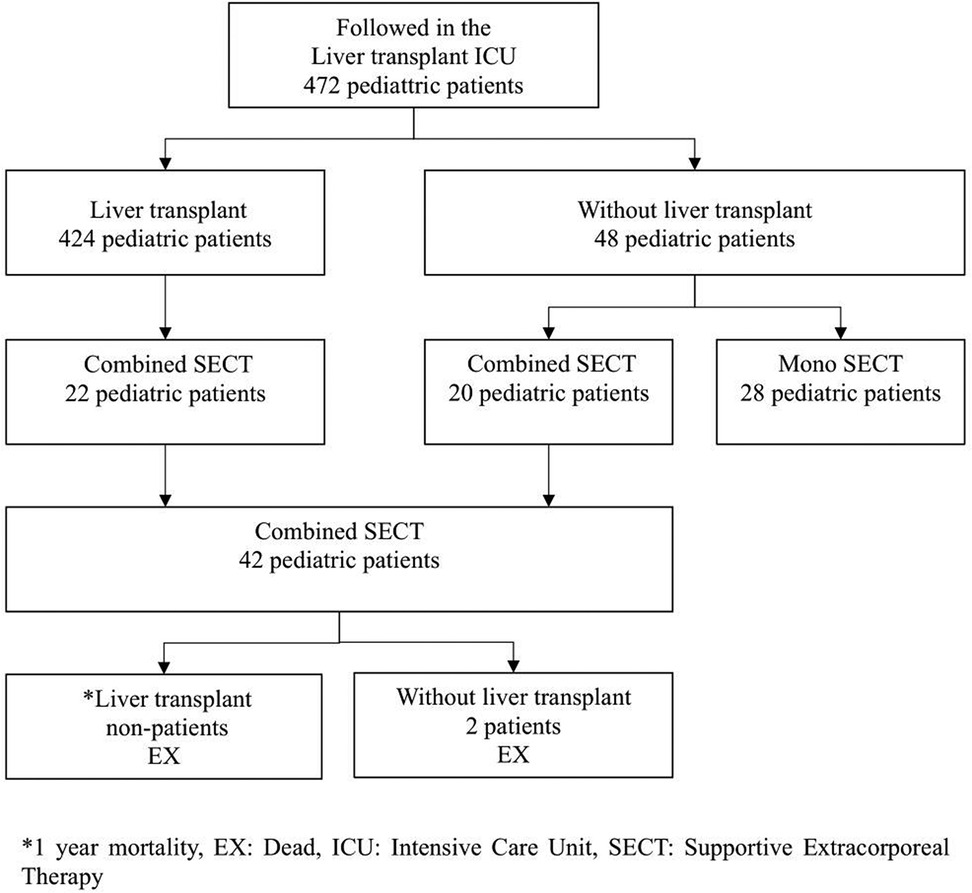
The data of 424 pediatric patients who underwent LTX and 48 patients who followed up without transplantation were retrospectively analyzed. The data were collected from the intensive care unit (ICU) of Memorial Sisli Hospital, Istanbul, Turkey, where an average of 100 LTXs per year were performed between January 2010 and January 2020. In this ICU, pre- and post-transplant support and treatment were performed.
Twenty ALF pediatric patients and 22 ALF developing liver transplant recipient candidates who received a combination of CVVHDF and PEX supportive therapy were included in the study. The group consisting of 22 patients was a candidate for liver transplantation according to different etiology and the Pediatric End-Stage Liver Disease (PELD) score and was on the organ transplantation waiting list. This group, which was CLF, converted to ALF for various reasons during the waiting period for transplant. In other words, it was ACLF. The living donor of this group was ready or being prepared and was on the waiting list. The other group consisting of 20 pediatric patients developed ALF due to different etiologies. The inclusion criteria of these two groups were combined SECT (CVVHDF + PEX) until recovery or liver transplant. Patients with ALF who did not undergo combined SECT or who only underwent mono SECT were excluded from the study (Figure 1, Table 1). A SECT protocol used in the ICU was applied (Figure 2) (11).
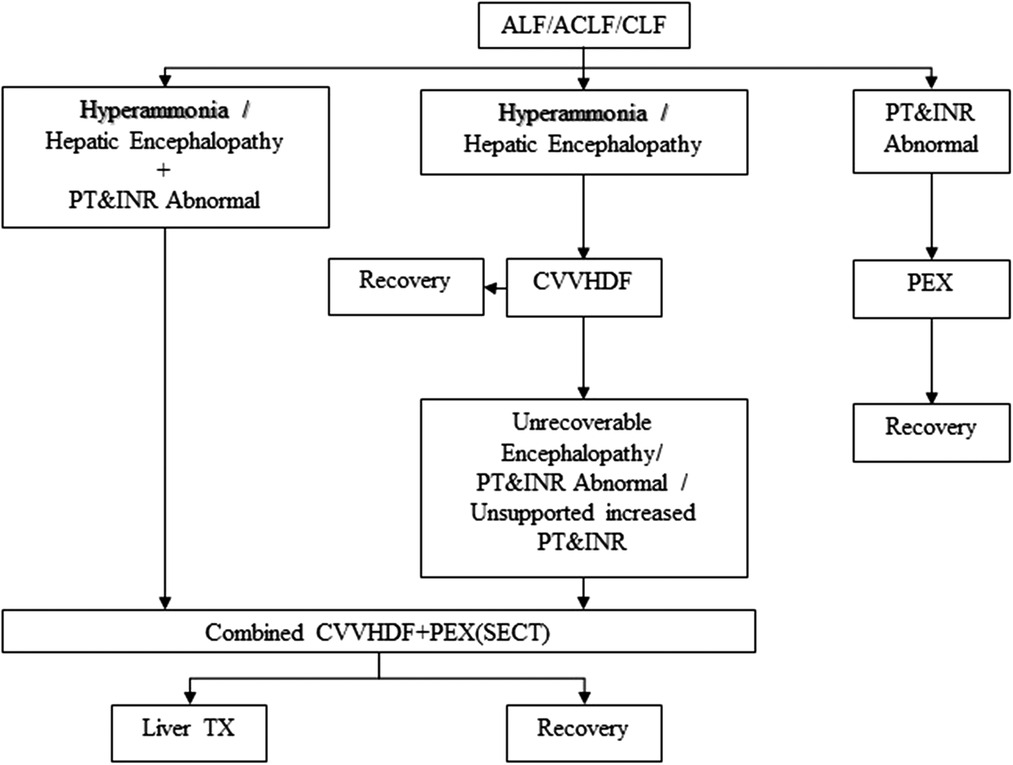
Figure 2. ECT protocol for ALF, ACLF, or CLF. ECT, extracorporeal therapy; ALF, acute liver failure; ACLF, acute-on-chronic liver failure; CVVHDF, continuous veno-venous hemodiafiltration; CLF, chronic liver failure; PEX, plasma exchange; PT, prothrombin time; INR, international normalized ratio; Liver TX, liver transplantation; SECT, supportive extracorporeal therapy.
CVVHDF was performed using a renal replacement machine (Fresenius Medical Care, Bad Homburg, Germany) and a hemodiafiltration kit (Multifiltrate Kit midi CVVHDF 400-Pediatric). Continuous 24 h/day citrate-Ca local circuit anticoagulation was performed. The blood flow rate was 3–5 ml/kg/min, the dialysate flow rate was 180–300 ml/kg/h, and the filtration rate after dilution was 36–60 ml/kg/h. The dialysate and replacement solutions used were MultiBic, MultiPLUS, Ci-Ca Dialysate, Ci-Ca Dialysate-K2 PLUS, Ci-Ca Dialysate-K4 PLUS, and 4% sodium citrate. The procedure was continued until LTX or recovery of encephalopathy.
PEX was performed using a continuous renal replacement machine (Fresenius Medical Care, Bad Homburg, Germany) and the Fresenius Plasma Exchange Kit (multiFiltrate Kit MPS P1 DRY-Pediatric). Sessions used an equal volume of FFP replacement without anticoagulant administration by calculating 30 cc/min, 50 cc/kg, and 1.5 volumes. The treatment was applied twice a day for 2–4 h. The PEX circuit was connected as a side flow to the CVVHDF circuit and removed after each treatment session; the CVVHDF continued.
Vital findings were obtained from noninvasive and invasive monitoring recordings in the ICU, and laboratory values were obtained before and immediately after the combined CVVHDF + PEX treatment. PEX treatment with CVVHDF was calculated as hours and the number of treatments.
The retrospective study was approved by the institutional review board of Memorial Sisli Hospital with a date of 03/06/2022 and number 521/22. In addition, these approvals were included in intensive care hospitalization approvals. Patient data were obtained from the hospital information system. This study was carried out at Memorial Sisli Hospital Liver Transplant Intensive Care Unit.
Statistical analyses
SPSS version 21 (IBM Corp., Armonk, NY, United States) was used for all statistical analyses. Kolmogorov–Smirnoff analysis was used to analyze the normality of the study data. The mean values were used for normally distributed data, and the median (IQR) values were used for non-normally distributed data. A comparison of combined CVVHDF + PEX pre- and post-session laboratory values was performed using the Wilcoxon test. The Mann–Whitney U test was used to compare the data of patients with and without LTX. A P-value less than 0.05 (P < 0.05) was considered statistically significant in this study.
Results
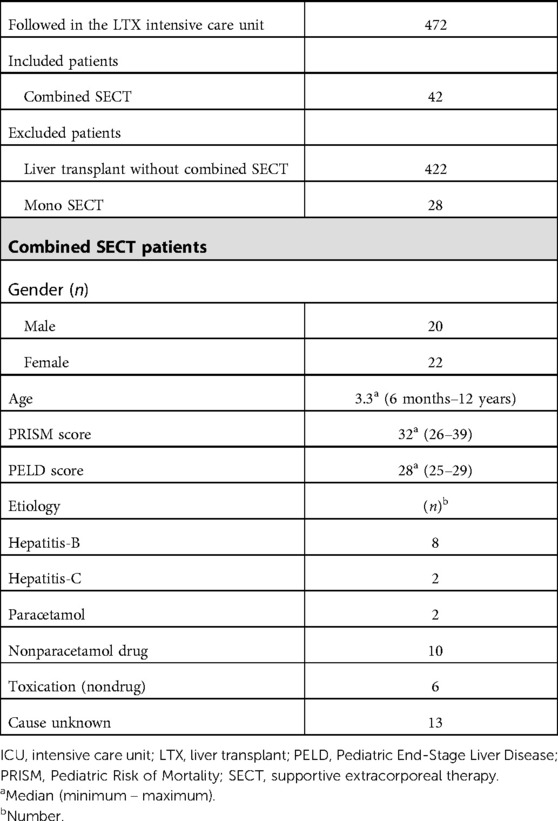
When the patients were admitted to the ICU, inclusion and exclusion criteria, demographic data, the Pediatric Mortality Risk (PRISM) score, the PELD score, and etiology were recorded (Table 1). All patients included in the study had encephalopathy grade 3 or 4 according to the West Haven classification. The median duration of combined SECT applied to pediatric patients was 8 days. PEX sessions were applied to all patients twice a day. The mean number of sessions was 12.39, with a minimum of 6 and a maximum of 25 sessions. The median duration of CVVHDF was 168.96 h (7.04 days), with a minimum of 74 h and a maximum of 311 h.
The LTX candidates had significantly higher serum total bilirubin values before the first combined SECT than the survivors (recovery ALF). However, alanine transaminase (ALT) and aspartate transaminase (AST) values were significantly lower. The survivors had significantly lower serum ammonia, prothrombin time (PT)/international normalized ratio (INR), and total bilirubin values after combined SECT than the LTX candidates (Table 2). In all pediatric patients (22 liver transplanted and 20 nontransplanted patients), serum liver function test (total bilirubin, ALT, AST), ammonia, and PT/INR levels were significantly lower than the first levels after combined SECT. Hemodynamic parameters (i.e., mean arterial pressure, MAP) also improved significantly (Table 3). The serum liver function test (total bilirubin, ALT, AST) values, ammonia, and PT/INR levels were significantly lower after combined SECT than the first levels in patients with and without LTX. Hemodynamic parameters (i.e., MAP) also improved significantly (Table 4).
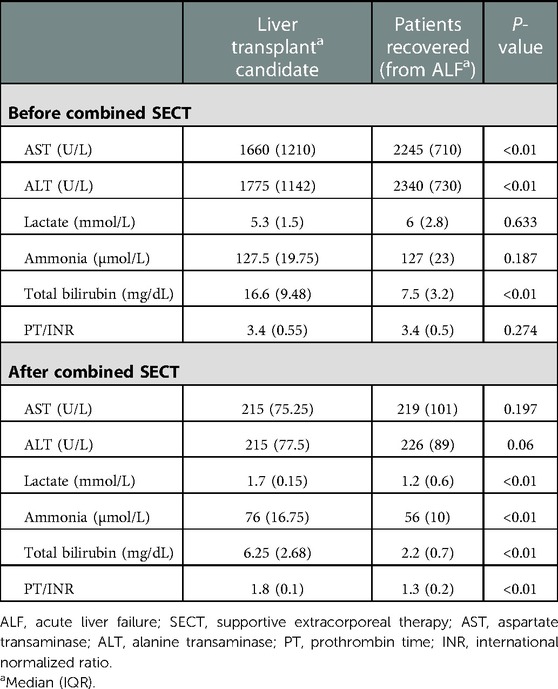
Table 2. Laboratory values of pediatric patients before the first combined SECT and after the last combined SECT.
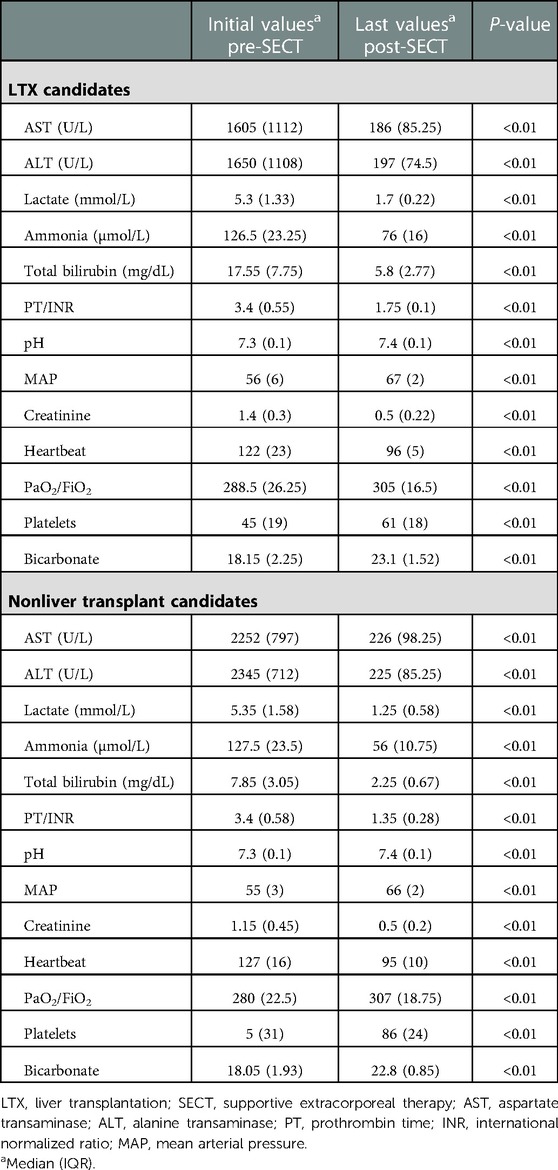
Table 4. LTX candidates (underwent liver TX after the last SECT) and nonliver transplant candidates.
Hypernatremia (five patients) and metabolic acidosis (four patients) developed during PEX in the combined SECT. These improved with hemodiafiltration in SECT. Additionally, four patients had mild skin rash due to FFP used during PEX; the procedure was stopped for these patients. Fresh-frozen plasma in the process was replaced with a new one. Once the skin rashes passed, the procedure continued.
Discussion and conclusions
We evaluated a group of 22 liver transplanted and 20 nontransplanted pediatric patients who underwent combined SECT and followed up in the LTX ICU. In our study, encephalopathy could not be permanently reduced below grade 3 in 22 patients who underwent LTX; however, it showed a fluctuating course, and there was no increase in the encephalopathy grade or worsening of hepatic coma. This shows that the electrolyte and fluid balance needed to control intracranial pressure (ICP), as reported in the literature, is controlled tightly and precisely. Therefore, combined SECT can be used as a bridge to stabilize candidates for LTX. Encephalopathy in the 18 patients who recovered clinically regressed to grade 1 and below; however, two patients who could not recover had no indication for transplantation or could not find a suitable donor and became ex-patients (dead). The grade of encephalopathy in the two ex-patients could not be permanently reduced below grade 3. A significant improvement was achieved in laboratory (biochemical) values and vital signs in all patients who underwent combined SECT and were followed up in the intensive care unit. However, there was no clinical recovery in patients whose encephalopathy grade could not be permanently decreased. Therefore, we believe that the main factor in determining the prognosis is encephalopathy, which is permanently reduced to grade 1 or lower with combined SECT.
Permanent clinical improvement, including the improvement of encephalopathy as well as a low PT/INR value is observed in surviving patients (recovered patients), and has been achieved with consistently high ammonia clearance, as reported in the literature. We believe that this was due to high functional liver capacity or regeneration of liver tissue (12). In addition, the recovery of patients in the paracetamol-induced ALF group without the need for LTX is consistent with the literature (13).
Many case reports of liver failure describe rapid progression to MOF with the development of systemic infection, brain edema, circulatory failure, abnormal coagulation, and metabolic complications (14). Various clinical studies have shown the ability of CRRT techniques, such as CVVHDF, to reduce inflammation and mediators of inflammation in septic or MOF patients (14, 15). CRRT can be used safely in even the most critical cases associated with circulatory failures, such as cerebral edema (16). If performed continuously, CRRT can effectively remove the densely accumulated substances from the body, not only intravascularly but also extravascularly. Acute kidney injury requiring CRRT is a common manifestation of MOF in ALF; it can develop in 30%–50% of these patients. In addition to removing excess fluid volume from the body, CRRT ensures the excretion of endogenous toxins that cannot be excreted renally. It produces a clinical improvement in patients who are hemodynamically unstable, develop encephalopathy, and go through an extensive inflammation process due to increased and uncleared cytokines. Furthermore, it is widely used to reduce the risk of increased ICP and cerebral edema in patients with severe hyperammonemia (17).
Plasma exchange clears circulating inflammatory cytokines, endotoxins, and neurotoxins formed in patients with ALF. It lowers bilirubin and ammonia levels, corrects coagulopathy, and regulates the antimicrobial response, thereby reducing transplants, with hemodynamic improvement (17, 18). These reduced substances have been reported to be important mediators in developing both HE and MOF in ALF. Mao et al. showed that bilirubin, coagulopathy, and ammonia levels were corrected with PEX treatment (19, 20). Combined SECT is not a new practice; simultaneous dialysis and PEX were first introduced in 1999 and these have been reported in the literature as serial or parallel connected tandem procedures (21). According to Matsubara et al. an artificial liver support system, such as continuous hemofiltration with PEX, can effectively remove medium-molecular-weight substances and is thus effective in treating hepatic coma (22–24). Yoshiba et al. reported that combining PEX and hemodiafiltration could improve hepatic coma in many patients; using this artificial liver support system, the survival rate in 67 patients with fulminant hepatitis was 56% (25). Compared with previous combined SECT reports, the transplant-free survival was similar. On the other hand, although the causative etiology is different, in 38% of pediatric ALF patients who do not undergo liver TX, Kathemann et al. reported a 59% mortality rate in those who did not undergo SECT and were left to recover spontaneously (26). These patients should be supported in the ICU until their liver function is restored or LTX is performed, with various types of monitoring and measures, including intensive treatment.
We believe that our multidisciplinary approach to LTX, performed at the proper time, is effective in preventing mortality and ensuring neurological stability. We attributed the low mortality rate in our study to the use of early and aggressive artificial liver support.
Many studies have shown that rapid, large-volume FFP administration can produce side effects such as hypernatremia, metabolic alkalosis, and colloid osmotic pressure (COP) during the PEX procedure. Furthermore, it has been reported that large-volume FFP administration can lead to complications, such as brain and pulmonary edema (27). The first randomized control trial (RCT) describing the benefits of the PEX procedure in patients with ALF was conducted in 2016 by Larsen et al. (28). Persistent cerebral edema and hepatic coma in patients with ALF are important because they show the abrupt development of irreversible cerebral damage. This situation was also reported by Bernal and Wendon as a contraindication for LTX (29). Since 2007, the combined use of PEX and CVVHDF has been ongoing in our unit. By using this method, we efficiently remove hepatic toxins and minimize the side effects of PEX administration alone, such as hypernatremia, COP, and metabolic alkalosis (30, 31).
Nevertheless, there is a need for randomized controlled studies on this subject since our study is single-center and retrospective and there is no control group.
Combined CVVHDF and PEX supportive treatment significantly improved the biochemical parameters and clinical findings, including encephalopathy. Furthermore, it was found to have a major effect on patient survival, with 4.76% mortality and 42.8% transplant-free survival. Therefore, we believe that this combined therapy can be used as supportive therapy for recovery or bridging purposes in patients with ALF or candidates for LTX while waiting for liver regeneration or a suitable donor.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethics review and approval/written informed consent was not required as per local legislation and institutional requirements.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Acknowledgments
The author thanks the Apheresis specialist technicians, intensive care nurses, and assistant doctors for their participation. In addition, the author expresses his respect to Munci Kalayoğlu, the founder of the Liver Transplantation Center, who, as always, did not spare his support in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SECT, supportive extracorporeal therapy; ALF, acute liver failure; CVVHDF, continuous veno-venous hemodiafiltration; CRRT, continuous renal replacement therapy; PEX, plasma exchange; LTX, liver transplantation; ACLF, acute-on-chronic liver failure; MOF, multiorgan failure; ICU, intensive care unit; MAP, mean arterial pressure; ALT, alanine transaminase; AST, aspartate transaminase; PT, prothrombin time; INR, international normalized ratio; COP, colloid osmotic pressure.
References
1. Nafady-Hego H, Li Y, Ohe H, Zhao X, Satoda N, Sakaguchi S, et al. The generation of donor-specific CD4+ CD25++ CD45RA+ naive regulatory T cells in operationally tolerant patients after pediatric living-donor liver transplantation. Transplantation. (2010) 90(12):1547–55. doi: 10.1097/TP.0b013e3181f9960d
2. Deep A, Alexander EC, Bulut Y, Fitzpatrick E, Grazioli S, Heaton N, et al. Advances in medical management of acute liver failure in children: promoting native liver survival. Lancet Child Adolesc Health. (2022) 6(10):725–37. doi: 10.1016/S2352-4642(22)00190-0
3. Ozdemir FN, Tutal E, Sezer S, Gür G, Bilgic A, Haberal M. Effect of supportive extracorporeal treatment in liver transplantation recipients and advanced liver failure patients. Hemodial Int. (2006) 10(S2):S28–32. doi: 10.1111/j.1542-4758.2006.00113.x
4. Sharma S, Agarwal S, Saraya A, Choudhury A, Mahtab MA, Alam MS, et al. Acute variceal bleeding portends poor outcomes in patients with acute-on-chronic liver failure: a propensity score matched study from the APASL ACLF Research Consortium (AARC). Hepatol Int. (2022) 16(5):1234–43. doi: 10.1007/s12072-022-10372-1
5. Xie J, Xiao W, Lin J. Effect of oXiris-CVVH on the clinical outcomes of patients with septic shock: an inverse probability of treatment-weighted analysis. Blood Purif. (2022) 51(12):972–89. doi: 10.1159/000524088
6. Bellomo R, Homel P, Brendolan A, Dan M, Piccinni P, La Greca G. Effects of different doses in continuous veno-venous hemofiltration on outcomes of acute renal failure: a prospective randomized trial. Lancet. (2000) 356(9223):26–30. doi: 10.1016/S0140-6736(00)02430-2
7. Biancofiore G, Bindi LM, Urbani L, Catalano G, Mazzoni A, Scatena F, et al. Combined twice-daily plasma exchange and continuous veno-venous hemodiafiltration for bridging severe acute liver failure. Transplant Proc. (2003) 35(8):3011–4. doi: 10.1016/j.transproceed.2003.10.077
8. Thanh NT, Dat NT, Thinh TN, Phuong NTM, Thanh MTH, Bao NT, et al. Therapeutic plasma exchange and continuous renal replacement therapy in pediatric dengue-associated acute liver failure: a case series from Vietnam. Transfus Apher Sci. (2022):103617. doi: 10.1016/j.transci.2022.103617. [Epub ahead of print]
9. Fujiwara K, Oda S, Abe R, Yokosuka O. On-line hemodiafiltration or high-flow continuous hemodiafiltration is one of the most effective artificial liver support devices for acute liver failure in Japan. J Hepatobiliary Pancreat Sci. (2015) 22(3):246–7. doi: 10.1002/jhbp.204
10. Iwai H, Nagaki M, Naito T, Ishiki Y, Murakami N, Sugihara JI, et al. Removal of endotoxin and cytokines by plasma exchange in patients with acute hepatic failure. Crit Care Med. (1998) 26(5):873–6. doi: 10.1097/00003246-199805000-00021
11. Ocak I, Topaloğlu S, Acarli K. Posthepatectomy liver failure. Turk J Med Sci. (2020) 50(6):1491–503. doi: 10.3906/sag-2006-31
12. Maiwall R, Moreau R. Plasma exchange for acute on chronic liver failure: is there a light at the end of the tunnel? Hepatol Int. (2016) 10(3):387–9. doi: 10.1007/s12072-016-9703-z
13. Rotundo L, Pyrsopoulos N. Liver injury induced by paracetamol and challenges associated with intentional and unintentional use. World J Hepatol. (2020) 12(4):125. doi: 10.4254/wjh.v12.i4.125
14. Mandal AK, King KE, Humphreys SL, Maley WR, Burdick JF, Klein AS. Plasmapheresis: an effective therapy for primary allograft nonfunction after liver transplantation. Transplantation. (2000) 70(1):216–20. PMID: 10919607
15. Saraiva IE, Ortiz-Soriano VM, Mei X, Gianella FG, Woc WS, Zamudio R, et al. Continuous renal replacement therapy in critically ill patients with acute on chronic liver failure and acute kidney injury: a retrospective cohort study. Clin Nephrol. (2020) 93(4):187–94. doi: 10.5414/CN109983
16. Ide K, Muguruma T, Shinohara M, Toida C, Enomoto Y, Matsumoto S, et al. Continuous veno-venous hemodiafiltration and plasma exchange in infantile acute liver failure. Pediatr Crit Care Med. (2015) 16(8):e268–74. doi: 10.1097/PCC.0000000000000511
17. Inoue K, Watanabe T, Maruoka N, Kuroki Y, Takahashi H, Yoshiba M. Japanese-style intensive medical care improves prognosis for acute liver failure and the perioperative management of liver transplantation. Transplant Proc. (2010) 42(10):4109–12. doi: 10.1016/j.transproceed.2010.09.073
18. Jain V, Dhawan A. Extracorporeal liver support systems in paediatric liver failure. J Pediatr Gastroenterol Nutr. (2017) 64(6):855–63. doi: 10.1097/MPG.0000000000001500
19. Mao WL, Chen Y, Chen YM, Li LJ. Changes of serum cytokine levels in patients with acute on chronic liver failure treated by plasma exchange. J Clin Gastroenterol. (2011) 45(6):551–5. doi: 10.1097/MCG.0b013e3181faefa3
20. Mao WL, Lou YF, Ye B, Lin S, Chen YM, Chen Y. Changes in peripheral CD4+ CD25high regulatory T cells in the acute-on-chronic liver failure patients with plasma exchange treatment. Inflammation. (2012) 35(2):436–44. doi: 10.1007/s10753-011-9333-5
21. Yokoi T, Oda S, Shiga H, Matsuda KI, Sadahiro T, Nakamura M, et al. Efficacy of high-flow dialysate continuous hemodiafiltration in the treatment of fulminant hepatic failure. Transfus Apher Sci. (2009) 40(1):61–70. doi: 10.1016/j.transci.2008.11.006
22. Matsubara S. Combination of plasma exchange and continuous hemofiltration as temporary metabolic support for patients with acute liver failure. Artif Organs. (1994) 18(5):363–6. doi: 10.1111/j.1525-1594.1994.tb02217.x
23. Matsubara S, Okabe K, Ouchi K, Miyazaki Y, Yajima Y, Suzuki H, et al. Continuous removal of middle molecules by hemofiltration in patients with acute liver failure. Crit Care Med. (1990) 18(12):1331–8. doi: 10.1097/00003246-199012000-00005
24. Trepatchayakorn S, Chaijitraruch N, Chongsrisawat V, Chanakul A, Kongkiattikul L, Samransamruajkit R. Therapeutic plasma exchange with continuous renal replacement therapy for pediatric acute liver failure: a case series from Thailand. Indian J Crit Care Med. (2021) 25(7):812–6. doi: 10.5005/jp-journals-10071-23896
25. Yoshiba M, Inoue K, Sekiyama K, Koh I. Favorable effect of new artificial liver support on survival of patients with fulminant hepatic failure. Artif Organs. (1996) 20(11):1169–72. doi: 10.1111/j.1525-1594.1996.tb00657.x
26. Kathemann S, Bechmann LP, Sowa JP, Manka P, Dechêne A, Gerner P, et al. Etiology, outcome and prognostic factors of childhood acute liver failure in a German single center. Ann Hepatol. (2015) 14(5):722–8. doi: 10.1016/S1665-2681(19)30767-7
27. Mao W, Ye B, Lin S, Fu Y, Chen Y, Chen Y. Prediction value of model for end-stage liver disease scoring system on prognosis in the acute on chronic liver failure patients with plasma exchange treatment. ASAIO J. (2010) 56(5):475–8. doi: 10.1097/MAT.0b013e3181e6bf13
28. Larsen FS, Schmidt LE, Bernsmeier C, Rasmussen A, Isoniemi H, Patel VC, et al. High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol. (2016) 64(1):69–78. doi: 10.1016/j.jhep.2015.08.018
29. Bernal W, Wendon J. Acute liver failure. N Engl J Med. (2013) 369(26):2525–34. doi: 10.1056/NEJMra1208937
Keywords: acute liver failure (ALF), continuous renal replacement therapy (CRRT), hepatic encephalopathy (HE), liver transplantation (LTX), plasma exchange (PEX)
Citation: Ocak I (2023) Value of extracorporeal artificial liver support in pediatric acute liver failure: A single-center experience of over 10 years. Front. Pediatr. 11:979619. doi: 10.3389/fped.2023.979619
Received: 27 June 2022; Accepted: 19 January 2023;
Published: 13 February 2023.
Edited by:
Kevin Watt, University of Utah, United StatesReviewed by:
Hitoshi Hirose, Thomas Jefferson University, United StatesAli Canbay, University Hospital Bochum GmbH, Germany
Gazi Arslan, Dokuz Eylül University, Turkey
© 2023 Ocak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ilhan Ocak ilhanocak.md@gmail.com
Specialty Section: This article was submitted to Pediatric Critical Care, a section of the journal Frontiers in Pediatrics
 Ilhan Ocak
Ilhan Ocak