Case report: Early thrombosis in left atrial during transcatheter closure of ASD in a child with favorable outcome after use of GPIIb/IIIa receptor antagonist and heparin
- 1Department of Pediatric Cardiology, Children’s Medical Center, Hunan Provincial People's Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, China
- 2Department of Pediatric Orthopedics, Hunan Provincial People's Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, China
- 3Department of Ultrasound, Hunan Provincial People's Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, China
- 4Department of Cardiology, Children’s Medical Center, Hunan Provincial People's Hospital, The First Affiliated Hospital of Hunan Normal University, Changsha, China
Background: Acute thrombus in atrial septal defect occluders is a rare complication that requires aggressive, effective, and safe management. Tirofiban, a platelet glycoprotein IIb/IIIa receptor antagonist, is widely used for the management of thromboembolic diseases, such as coronary heart disease and stroke. To date, there is no report using the GPIIb/IIIa receptor antagonist tirofiban for the management of ASD closure-related thrombosis in children.
Case presentation: Herein, we reported a case of a 5-year-old girl with ASD who presented with acute thrombus on the left disc of the occluder device immediately after transcatheter closure of ASD. The thrombus was successfully dissolved 24 h after a combined infusion of heparin and tirofiban, followed by 1 months of aspirin and clopidogrel and 5 months of aspirin alone. No thromboembolism or hemorrhage events occurred during follow-up for more than 2 years.
Conclusion: The continuous infusion of GPIIb/IIIa receptor antagonist tirofiban combined with heparin may have beneficial effects for the management of thrombosis during ASD closure procedure.
Introduction
Atrial septal defect (ASD) is one of the most common congenital heart diseases (CHD), accounting for approximately 6%–10% of the CHD population (1, 2). Over the years, percutaneous transcatheter closure of ASD has replaced the traditional thoracotomy, becoming the preferred treatment for secondary ASD (3–7). Since King TD first performed transcatheter closure of ASD in 1976 (3), there has been rapid development of materials and equipment used for transcatheter closure techniques. The accessibility, hospitalization and complications have been largely improved. However, infrequent adverse events can still occur related to ASD closure, such as movement or embolization of the closure device, pericardial effusion, arrhythmias, thrombosis, mitral insufficiency and vascular injury (8–10).
Thrombus formation during closure of ASD is a rare but severe complication. Tirofiban, a platelet glycoprotein IIb/IIIa receptor antagonist, is widely used for the management of thromboembolic diseases, such as coronary heart disease and stroke. Nonetheless, tirofiban is rarely administrated for ASD closure-related thrombosis, especially for children. Here we report a single case of acute thrombosis during ASD closure with favorable outcome, treated with combination of tirofiban and heparin.
Case presentation
A 5-year-old girl was admitted to our hospital on 17 August 2020 with a 3-year history of a heart murmur. She had no history of other cardiovascular, hematologic, or neurological diseases and no medication use.
The child was developing normally, without shortness of breath or cyanosis. Fixed splitting of the second heart sound and 2/6 systolic blowing murmur could be heard in the second intercostal space on the left sternal border. Blood examination showed the following: WBC 9.64 (×109/L), N 4.45 (×109/L), L 4.58 (×109/L), Hb 136 (g/L), Plt 393 (×109/L), and CRP <0.499. Biochemistry tests showed no abnormalities. Coagulation function were: TT:10.1 s, INR:0.90, FIB:3.00 g/L, APTT:25.4 s, TT:17.2 s, D-Dimer:0.45 mg/L, FDP:6.12 ug/ml, AT-IIIAg:92.7%, showing no abnormalities.
Preoperative preparation was completed. Intravenous general anesthesia was performed, and intravenous heparin anticoagulation was 100 u/kg. The intraoperative examination showed Qp : Qs ratio of 1.5 : 1 and pulmonary arterial pressure of 30 mmHg. Intraoperative transthoracic echocardiography (TTE) showed central secundum ASD (II) with a defect diameter of 8 mm, and the marginal conditions were suitable for closure.
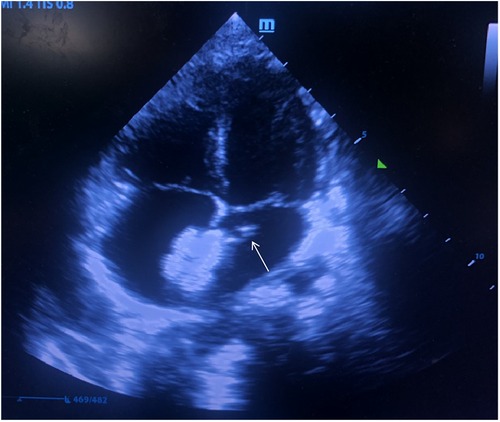
A 12 mm Amplatzer atrial septal occluder (Starway Medical Technology, Inc. Beijing, China) was initially deployed in the atrial septum within 40 min after femoral vein puncture. Due to the unsatisfactory shape of the occluder by the first release, the left disc of the occluder was recollected into the delivery sheath and was released again. The procedure time from the recollection to the repositioning of occluder was estimated 15 min. After a second complete release of the occluder and withdrawal of the delivery sheath, the bedside TTE revealed a slightly hyperechoic mass (approximately 6.5 × 3 mm in size) attached to the edge of the left atrial disc of the deployed occluder, oscillating with the movement of cardiac cycle (Figure 1). An acute thrombosis was swiftly considered by the ultrasonographer and cardiologists.
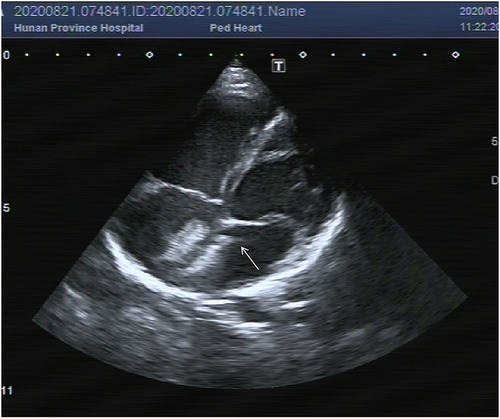
After obtaining the informed consent, platelet glycoprotein IIb/IIIa receptor antagonist tirofiban 0.4 ug/kg/min was pumped immediately lasting for 30 min. Then 0.10 ug/kg/min tirofiban was used for continuous pumping for 24 h. After 24 h, TTE showed that the thrombus had disappeared, and the absorption rate was over 90% (Figure 2). During the infusion, the child had transient mild headache and chest tightness, lasting for about 3 h. No other discomfort or adverse events occurred, such as vomiting, bleeding or oozing at the puncture site. Since the headache and chest tightness are both common symptoms after general anesthesia, no particular intervention was given and the symptoms resolved without recurrence in the follow up.
Re-examination of coagulation function showed the following: blood routine showed WBC 8.29 (×109/L), Hb 126 (g/L), and Plt 294 (×109/L). Postoperative re-examination of coagulation function: PT: 10.7 s, INR: 0.93, FIB: 1.9 g/L, APTT: 26.8 s, TT: 17.2 s, D-Dimer: 0.89 mg/L, FDP: 6.30 ug/ml, AT-III Ag: 105.3%.
Tirofiban was discontinued 24 h later and replaced by aspirin and clopidogrel for anticoagulation. One month later, TTE showed that the occluder was in a good position, and the thrombus on the left disc of the occluder was completely dissolved (Figure 3). Aspirin alone was continued in the subsequent 5 months. TTE was then performed again at 3 months, 6 months, 1 year, and 2 years after surgery showing no recurrence of thrombus formation or bleeding events.
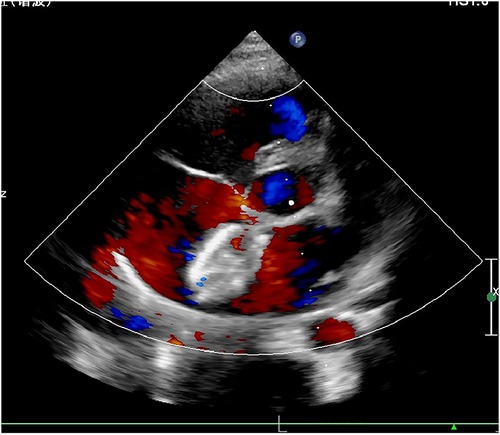
Figure 3. Transthoracic echocardiography at 1-month post-procedure showed that the thrombus was completely absorbed.
Discussion
Thrombus formation on occluder device or in cardiac cavities is a relatively rare but severe complication relating to ASD closure. It can result in systemic embolism with detachment and activity of the thrombus both in the early and late follow-up if not promptly and effectively treated. Studies indicate that the incidence of ASD closure-related thrombosis is estimated 0.7% to 1% (11–16). Nevertheless, ASD closure-related thrombosis is still a serious adverse event which should not be neglected.
ASD closure-related thrombosis is more frequently seen in delayed than in early stage after the occluder release (5, 9, 12, 17, 18). However, thrombosis can occur during device implantation and any stage after occluder release (13, 19).
According to existing reports, the risk factors of ASD closure-related thrombosis are occluder device type (13, 20), atrial fibrillation, atrial septal aneurysm (13, 19), and prothrombin and/or platelet activation abnormality (21). Krumsdorf et al. (13) claimed that Amplatzer occluder was not associated with thrombosis in compare with other 7 types, based on retrospective analysis of 1,000 cases. Interestingly, the child of our case received an Amplatzer occluder and had no pre-existing risk factors. Fiarresga et al. (5) and Eren et al. (22) speculated that the thrombus was already formed in the delivery sheath when the left disc was first released. With the retraction of the occluder, a portion of the thrombus can attach to the left disc and thus be taken to the heart.
There is no current consensus regarding the therapeutic strategy for ASD closure-related thrombosis due to the rare occurrence. Antithrombotic agents are considered suitable options in both early and late stages of the thrombosis. Yorgun et al. (19) reported successful dissolution of early thrombus on an Amplatzer closure device using unfractionated heparin (UFH) and subsequent aspirin combined with warfarin. Willcoxson et al. (23) reported a case of acute ASD closure-related thrombosis in a 12-year-old boy receiving combined use of Abciximab and heparin for 12 h. With subsequent substitution with Aspirin and Clopidogrel, the child's thrombus completely dissolved in two weeks after the closure procedure.
Aside from antithrombotic therapeutics, to aspirate the thrombus by the transport catheter under close monitoring of TEE guidance (5, 22). If a more hazardous event is considered, such as large thrombus, device problem, or failure of medicinal intervention, surgery to remove the thrombus or the closure device should be prompted (24, 25).
Tirofiban is a reversible non-peptide platelet GPIIb/IIIa receptor antagonist that blocks fibrinogen binding to GPIIb/IIIa receptors to inhibit platelet aggregation and can prevent platelet thrombosis (26). In recent years, increasing evidence suggests tirofiban's role as a preferable option and recommendation for thromboembolic diseases, such as coronary heart disease and stroke, in various versions of guideline (27–30).
Limited number of reports indicated tirofiban's possible efficiency in thromboembolic complications relating to ASD closure in adults. Yazıłoğlu et al. (31) reported successful treatment with tirofiban for acute thrombus on the left disc in a patient with homozygous factor V Leiden mutation. The patient subsequently received lifelong warfarin therapy without recurrent thrombus. Vanderheyden M et al. (32) reported a patient with a 1-cm thrombus in both left and right atriums occurred 6 months after PFO closure. Interestingly, the thrombus was completely dissolved after 48 h of continuous intravenous infusion with recombinant tissue plasminogen activator and tirofiban, succeeding to failure by combined use of aspirin, clopidogrel and enoxistin for a week. Unfortunately, there is no report regarding tirofiban in treating ASD closure-related thrombosis in children.
In this case, the child achieved favorable outcome by combined use of tirofiban and heparin after experiencing acute thrombosis during ASD closure procedure. Our explanation for the outcome are: (1) the size of the thrombus was relatively small and we detected the thrombus immediately; (2) the activation of platelets was considered a pivotal mechanism underlying acute thrombosis (21), the combination of tirofiban and heparin was thought to induce stronger antithrombotic effect than use either of the two drugs.
Although the incidence of ASD closure-related is rare, the mobility and potential fragility of thrombus imply high risk of embolism. Therefore, effective and safe approaches to the management are required. In this case, our patient avoided rescuing surgical intervention and had a favorable outcome after treatment with combined use of tirofiban and heparin. We hope our experience will provide insights into the management of thrombosis relating to CHD interventional treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the ethic committee of Hunan Provincial People's Hospital, the First Affiliated Hospital of Hunan Normal University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
HW drafted the initial manuscript, carried out the initial analysis. JW, YZ, ZL, and XX conceptualized and designed the study, coordinated and supervised the data collection. ZZ and YY performed and analyzed the study, XhX and LL did the follow-up with the patients. LL and JH reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study is supported by grant from National Natural Science Foundation of China (8167022).
Acknowledgments
We thank Tao Zhan from The First Hospital of Changsha for his constructive advice and generous help for the writing and revision of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Liu Y, Chen S, Zühlke L, Black GC, Choy MK, Li N, et al. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. (2019) 48(2):455–63. doi: 10.1093/ije/dyz009
2. Bouma BJ, Mulder BJ. Changing landscape of congenital heart disease. Circ Res. (2017) 120(6):908–22. doi: 10.1161/CIRCRESAHA.116.309302
3. Mills NL, King TD. Nonoperative closure of left-to-right shunts. J Thorac Cardiovasc Surg. (1976) 72(3):371–8. doi: 10.1016/S0022-5223(19)40065-2
4. Fischer G, Stieh J, Uebing A, Hoffmann U, Morf G, Kramer HH. Experience with transcatheter closure of secundum atrial septal defects using the amplatzer septal occluder: a single centre study in 236 consecutive patients. Heart. (2003) 89(2):199–204. doi: 10.1136/heart.89.2.199
5. Fiarresga A, De Sousa L, Martins JD, Ramos R, Paramés F, Freitas I, et al. Percutaneous closure of atrial septal defects: a decade of experience at a reference center. Rev Port Cardiol. (2010) 29(5):767–80. PMID: 20866006
6. Han Y, Zhang X, Zhang F. Transcatheter and intraoperative device closure of atrial septal defect in infants under three years of age. J Cardiothorac Surg. (2020) 15(1):9. doi: 10.1186/s13019-020-1063-z
7. Özbay YŞ, Eker R, Dindar A, Aydoğan Ü, Nişli K. Transcatheter closure of atrial septal defect in children: single-center experience, mid-term follow-up results. Turk Arch Pediatr. (2022) 57(4):406–12. doi: 10.5152/TurkArchPediatr.2022.21307
8. Kaya MG, Baykan A, Dogan A, Inanc T, Gunebakmaz O, Dogdu O, et al. Intermediate-term effects of transcatheter secundum atrial septal defect closure on cardiac remodeling in children and adults. Pediatr Cardiol. (2010) 31(4):474–82. doi: 10.1007/s00246-009-9623-y
9. Bonou M, Lampropoulos KM, Barbetseas J. Thrombus formation 10 years after placement of an atrial septal secundum defect closure device. Eur Heart J. (2012) 33(6):704. doi: 10.1093/eurheartj/ehr313
10. Tanghöj G, Odermarsky M, Naumburg E, Liuba P. Early complications after percutaneous closure of atrial septal defect in infants with procedural weight less than 15 kg. Pediatr Cardiol. (2017) 38(2):255–63. doi: 10.1007/s00246-016-1507-3
11. Chessa M, Butera G, Carminati M. Risk of thrombus formation on devices used to close transcatheter atrial septal defect and patent foramen ovale. J Am Coll Cardiol. (2004) 44(8):1712; author reply 1714–6. doi: 10.1016/j.jacc.2004.07.029
12. Sen T, Astarcioglu MA, Kilit C, Amasyali B. Successful thrombolytic treatment of a mobile thrombus on atrial septal defect occluder device. Acta Clin Belg. (2016) 71(5):334–6. doi: 10.1080/17843286.2015.1119963
13. Krumsdorf U, Ostermayer S, Billinger K, Trepels T, Zadan E, Horvath K, et al. Incidence and clinical course of thrombus formation on atrial septal defect and patient foramen ovale closure devices in 1,000 consecutive patients. J Am Coll Cardiol. (2004) 43(2):302–9. doi: 10.1016/j.jacc.2003.10.030
14. Clem A, Awadallah S, Amin Z. Safety, feasibility, results, and economic impact of common interventional procedures in a low-volume region of the United States. Pediatr Cardiol. (2017) 38(7):1332–6. doi: 10.1007/s00246-017-1664-z
15. Almanla A, Charafeddine F, Abutaqa M, Mustafa H, Tabbakh A, Hussein HB, et al. Transcatheter closure of atrial septal defects: comparable experience and outcomes between developing and developed countries. Pediatr Cardiol. (2019) 40(3):610–5. doi: 10.1007/s00246-018-2034-1
16. Cao C, Li R, Huang J, Zhao Y, Wang Z, Xie Y, et al. Feasibility of transcatheter closure of Secundum atrial septal defect in low weight infants under 2-year-old from a 3-year retrospective cohort study. Am J Cardiol. (2020) 132:133–9. doi: 10.1016/j.amjcard.2020.06.011
17. Sherman JM, Hagler DJ, Cetta F. Thrombosis after septal closure device placement: a review of the current literature. Catheter Cardiovasc Interv. (2004) 63(4):486–9. doi: 10.1002/ccd.20220
18. Xiong W, Tang L, Long W, Liu J, Song H. Case report: bi-atrial thrombus after occlusion of atrial septal defect with acute cerebral infarction and pulmonary embolism. Front Cardiovasc Med. (2022) 9:987538. doi: 10.3389/fcvm.2022.987538
19. Yorgun H, Canpolat U, Kaya EB, Aytemir K, Oto A. Thrombus formation during percutaneous closure of an atrial septal defect with an amplatzer septal occluder. Tex Heart Inst J. (2011) 38(4):427–30. PMID: 21841876; PMCID: PMC3147222
20. Anzai H, Child J, Natterson B, Krivokapich J, Fishbein MC, Chan VK, et al. Incidence of thrombus formation on the CardioSEAL and the amplatzer interatrial closure devices. Am J Cardiol. (2004) 93(4):426–31. doi: 10.1016/j.amjcard.2003.10.036
21. Rodés-Cabau J, Palacios A, Palacio C, Girona J, Galve E, Evangelista A, et al. Assessment of the markers of platelet and coagulation activation following transcatheter closure of atrial septal defects. Int J Cardiol. (2005) 98(1):107–12. doi: 10.1016/j.ijcard.2004.03.022
22. Eren NK, Akyildiz ZI, Acet H, Ertas F, Nazli C, Ergene O. Thrombus formation on the delivery sheath during transcatheter atrial septal defect closure. Tex Heart Inst J. (2009) 36(6):624–5. PMID: 20069098; PMCID: PMC2801952
23. Willcoxson FE, Thomson JD, Gibbs JL. Successful treatment of left atrial disk thrombus on an amplatzer atrial septal defect occluder with Abciximab and heparin. Heart. (2004) 90(5):e30. doi: 10.1136/hrt.2003.027946
24. Acar P, Aggoun Y, Abdel-Massih T. Images in cardiology: thrombus after transcatheter closure of ASD with an amplatzer septal occluder assessed by three-dimensional echocardiographic reconstruction. Heart. (2002) 88(1):52. doi: 10.1136/heart.88.1.52-a
25. Kovacevic P, Srdanovic I, Ivanovic V, Rajic J, Petrovic N, Velicki L. Late complications of transcatheter atrial septal defect closure requiring urgent surgery. Postepy Kardiol Interwencyjnej. (2017) 13(4):335–8. doi: 10.5114/aic.2017.71617
26. Disli OM, Erdil N, Akca B, Otlu YO, Battaloglu B. Large thrombus formation from right atrial incision site after closure of atrial septal defect. Korean Circ J. (2013) 43(12):842–4. doi: 10.4070/kcj.2013.43.12.842
27. Wu KK, Matijevic-Aleksic N. Molecular aspects of thrombosis and antithrombotic drugs. Crit Rev Clin Lab Sci. (2005) 42(3):249–77. doi: 10.1080/10408360590951171
28. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50(12):e344–418. doi: 10.1161/STR.0000000000000211
29. Zhou X, Wu X, Sun H, Li J. Efficacy and safety of eptifibatide versus tirofiban in acute coronary syndrome patients: a systematic review and meta-analysis. J Evid Based Med. (2017) 10(2):136–44. doi: 10.1111/jebm.12253
30. Sullivan AE, Nanna MG, Wang TY, Bhatt DL, Angiolillo DJ, Mehran R, et al. Bridging antiplatelet therapy after percutaneous coronary intervention: jACC review topic of the week. J Am Coll Cardiol. (2021) 78(15):1550–63. doi: 10.1016/j.jacc.2021.08.013
31. Yazıcıoğlu V, Sahin M, Karaca O, Türkmen M. Acute thrombus formation on an amplatzer device during transcatheter closure of an atrial septal defect in a patient with homozygous factor V Leiden mutation. Turk Kardiyol Dern Ars. (2011) 39(7):587–90. doi: 10.5543/tkda.2011.01527
Keywords: ASD, thrombosis, complications, tirofiban, children, case report, catheterization
Citation: Wang H, Zhu Z, Liu Z, Yuan Y, Xu X, Liu L, Wen J, Xia X, Zhang Y and He J (2023) Case report: Early thrombosis in left atrial during transcatheter closure of ASD in a child with favorable outcome after use of GPIIb/IIIa receptor antagonist and heparin. Front. Pediatr. 11:1138717. doi: 10.3389/fped.2023.1138717
Received: 6 January 2023; Accepted: 24 February 2023;
Published: 13 March 2023.
Edited by:
Nazmi Narin, Izmir Katip Celebi University, TürkiyeReviewed by:
Endale Tefera, University of Botswana, BotswanaAnna Olasinska-Wisniewska, Poznan University of Medical Sciences, Poland
© 2023 Wang, Zhu, Liu, Yuan, Xu, Liu, Wen, Xia, Zhang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liping Liu 190606755@qq.com
Specialty Section: This article was submitted to Pediatric Cardiology, a section of the journal Frontiers in Pediatrics
 Hui Wang
Hui Wang Zhiwei Zhu1
Zhiwei Zhu1  Yonghua Yuan
Yonghua Yuan Liping Liu
Liping Liu Jie Wen
Jie Wen