- 1Department of Oncology, Suzhou Ninth People’s Hospital, Suzhou Ninth Hospital Affiliated to Soochow University, Suzhou, China
- 2Department of Breast Surgery, Xuzhou Central Hospital, The Affiliated Xuzhou Hospital of Medical College of Southeast University, Xuzhou, China
- 3Department of Critical Care Medicine, The People’s Hospital of Huaiyin, Jinan, China
- 4Department of Pathology, Xuzhou Central Hospital, The Affiliated Xuzhou Hospital of Medical College of Southeast University, Xuzhou, China
Background: The gut microbiota has been significantly associated with differentiated thyroid cancer (DTC). However, the causal relationship between the gut microbiota and DTC remains unexplored.
Methods: Genome-wide association study (GWAS) summary databases were utilized to select exposures and outcomes. The Mendelian randomization (MR) method was employed to investigate the causal relationship between the gut microbiota and DTC. A sensitivity analysis was performed to assess the reliability of the findings.
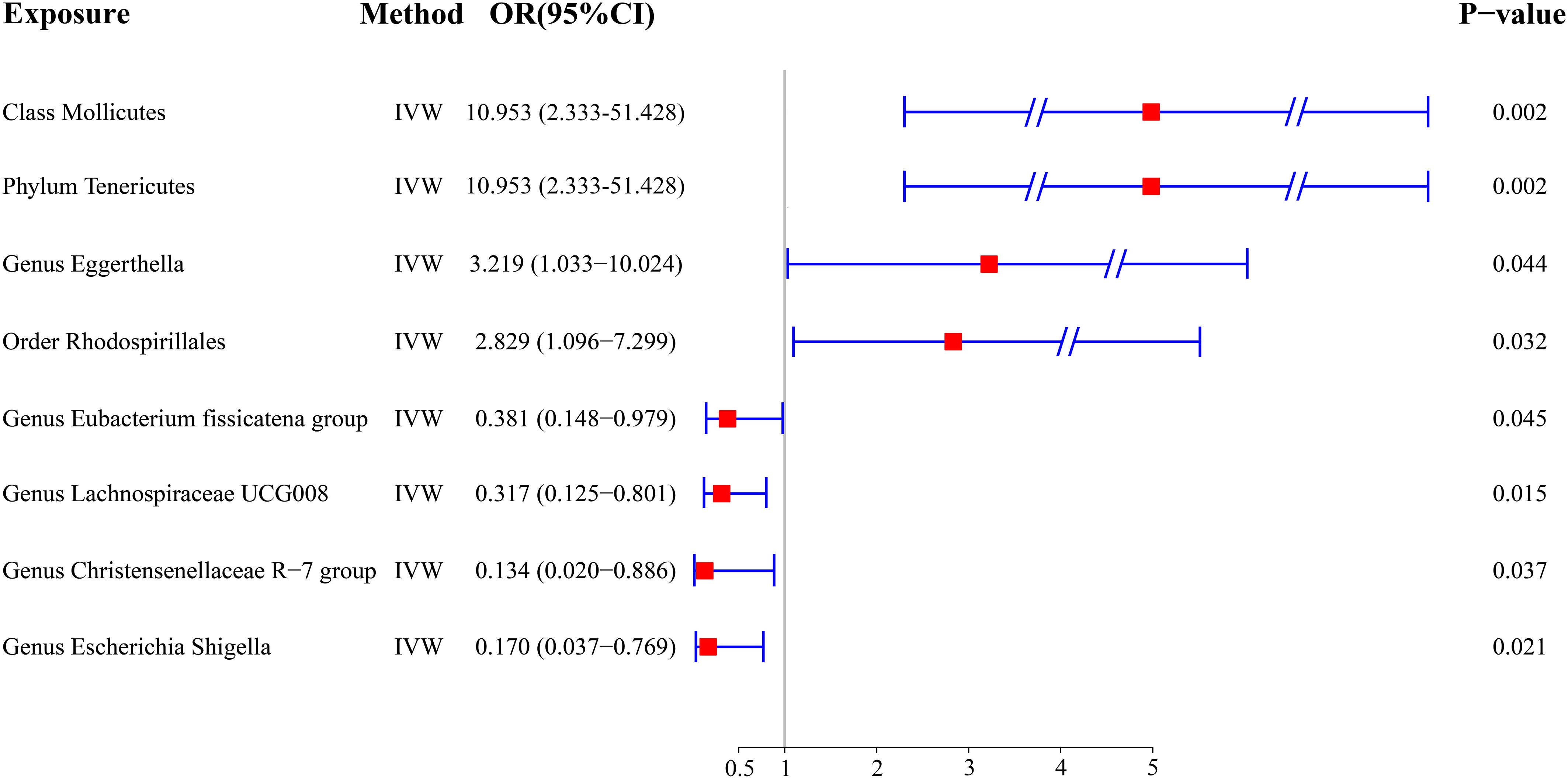
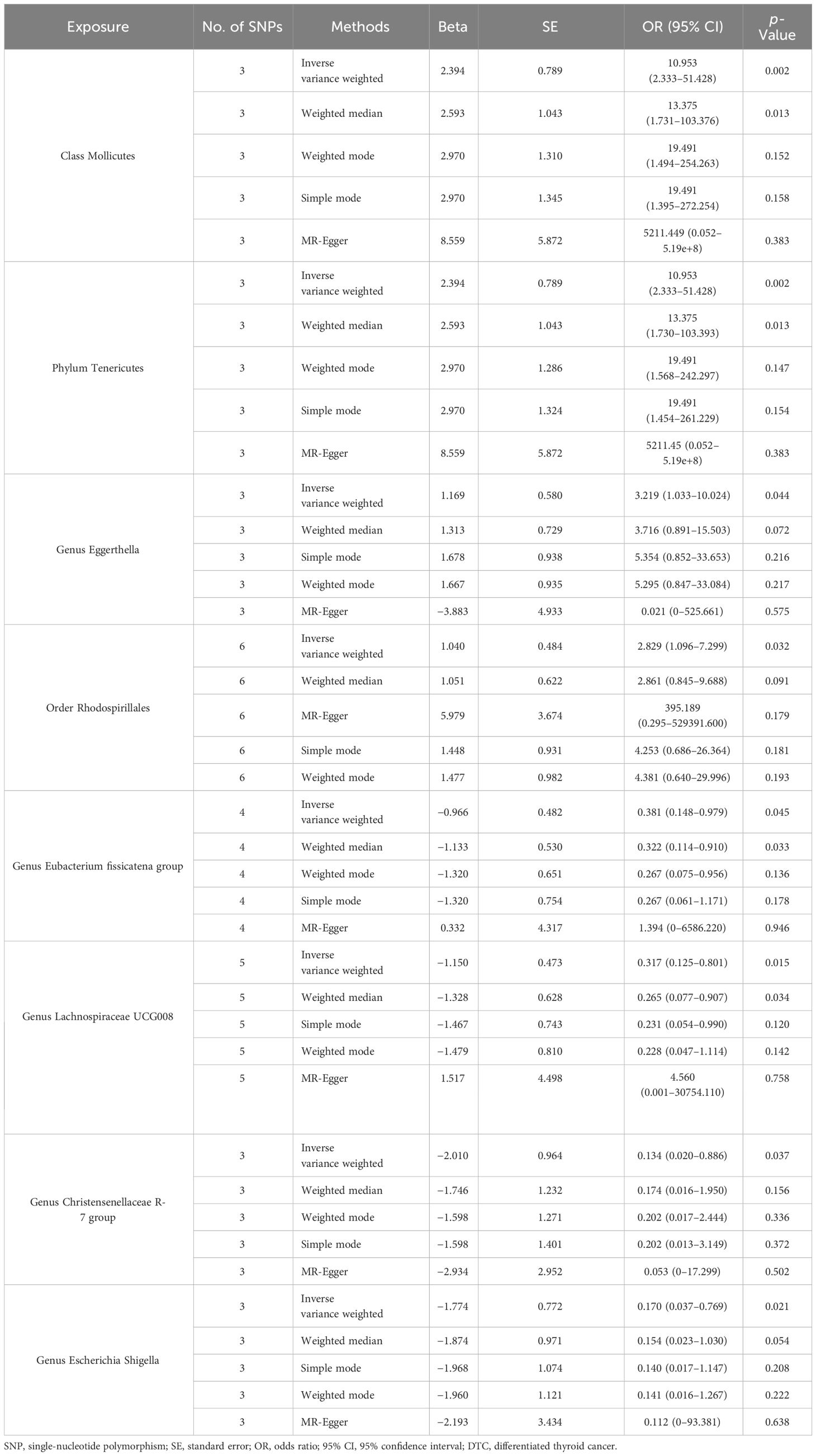
Results: Four bacterial traits were associated with the risk of DTC: Class Mollicutes [odds ratio (OR) = 10.953, 95% confidence interval (95% CI): 2.333–51.428, p = 0.002], Phylum Tenericutes (OR = 10.953, 95% CI: 2.333–51.428, p = 0.002), Genus Eggerthella (OR = 3.219, 95% CI: 1.033–10.024, p = 0.044), and Order Rhodospirillales (OR = 2.829, 95% CI: 1.096–7.299, p = 0.032). The large 95% CI range for the Class Mollicutes and the Phylum Tenericutes may be attributed to the small sample size. Additionally, four other bacterial traits were negatively associated with DTC: Genus Eubacterium fissicatena group (OR = 0.381, 95% CI: 0.148–0.979, p = 0.045), Genus Lachnospiraceae UCG008 (OR = 0.317, 95% CI: 0.125–0.801, p = 0.015), Genus Christensenellaceae R-7 group (OR = 0.134, 95% CI: 0.020–0.886, p = 0.037), and Genus Escherichia Shigella (OR = 0.170, 95% CI: 0.037–0.769, p = 0.021).
Conclusion: These findings contribute to our understanding of the pathological mechanisms underlying DTC and provide novel insights for the clinical treatment of DTC.
Introduction
Thyroid cancer is the most prevalent malignancy of the endocrine system (1). According to the GLOBOCAN (2020) database, there were 586,202 new cases of thyroid cancer in 2020 worldwide, constituting 3.0% of all cancer incidences (2, 3). Thyroid cancer encompasses four primary pathological classifications: papillary thyroid cancer (PTC), follicular thyroid cancer (FTC), medullary thyroid cancer (MTC), and anaplastic thyroid carcinoma (ATC) (4, 5). Differentiated thyroid cancer (DTC), comprising PTC and FTC, accounts for most thyroid cancer cases and typically presents a favorable prognosis (6). However, DTC is susceptible to local lymph node metastasis, contributing to a recurrence rate of up to 20% within 10 years (7). Certain subtypes, such as the diffuse sclerosing variant (DSV), exhibit relatively high invasiveness and are associated with a dismal prognosis (8). Therefore, a profound comprehension of the mechanisms underlying the onset and development of DTC is imperative.
Accumulating evidence suggests that the gut microbiota plays a crucial role in malignant tumors, including colorectal cancer, lung cancer, and breast cancer (9–11). Yu et al. (12) observed a significant decline in the richness and diversity of the gut microbiota in patients with DTC compared to healthy individuals. They developed a 10-genus microbial signature capable of effectively distinguishing patients with DTC from healthy individuals. Moreover, the gut microbiota is closely associated with the therapeutic response to radioactive iodine (RAI) following thyroidectomy (13). Before RAI treatment, thyroid hormone withdrawal (THW) is typically necessary to stimulate the secretion of thyroid stimulating hormone; however, THW-related complications significantly decrease quality of life. A recent randomized clinical trial showed that probiotics improve multiple symptoms induced by THW, including constipation and excessive weight gain (14). These findings indicate the involvement of the gut microbiota in the progression of DTC. Nevertheless, the causal relationship between the gut microbiota and DTC remains unexplored.
In the present study, the Mendelian randomization (MR) method was employed to ascertain whether there exists a causal relationship between the gut microbiota and DTC. Our findings may provide crucial insights into the pathological mechanisms underlying DTC.
Patients and methods
Patient data
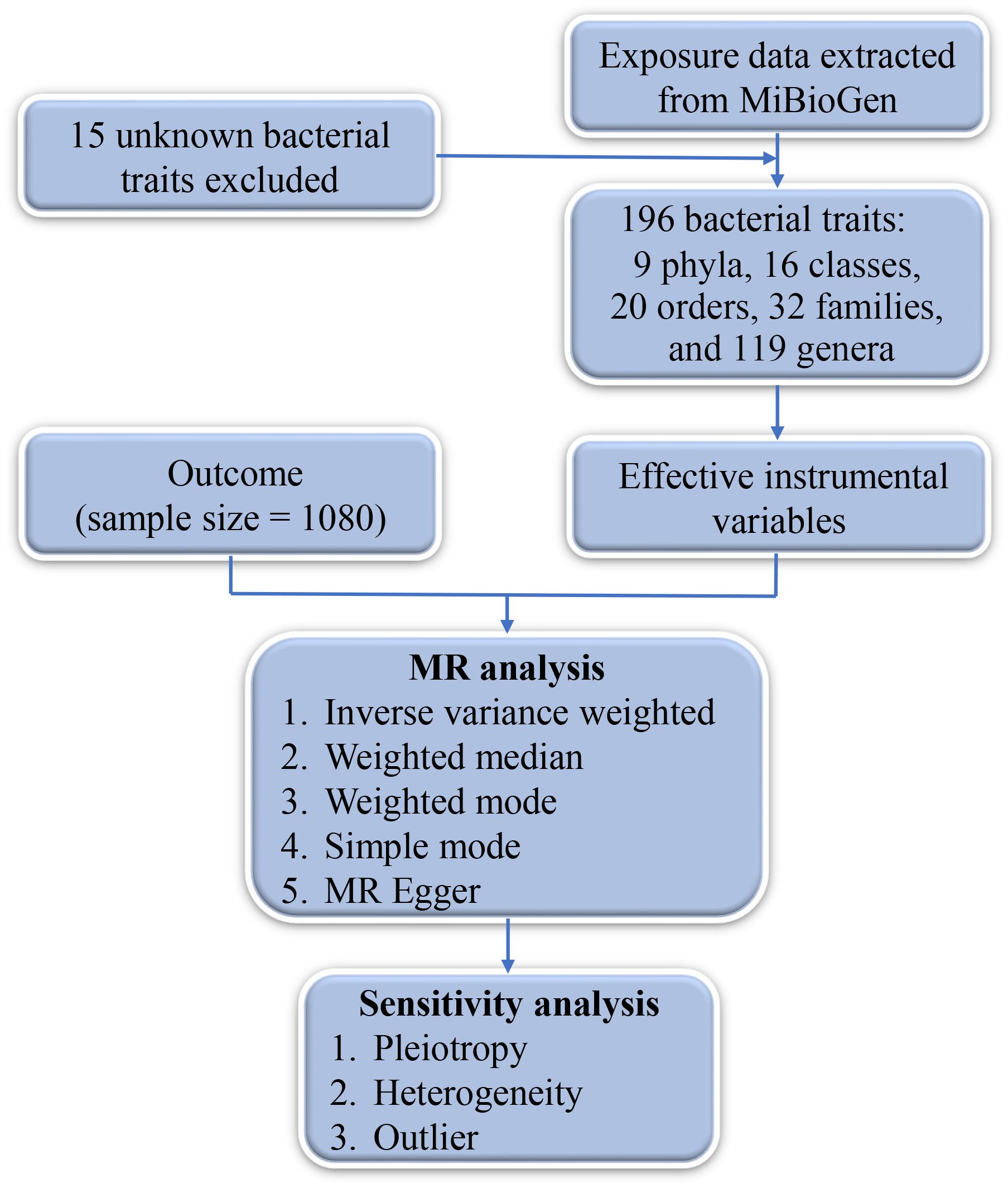
A genome-wide association study (GWAS) dataset of the gut microbiota was downloaded from MiBioGen (https://mibiogen.gcc.rug.nl/menu/main/home/) and utilized as an exposure variable. To investigate the interactive effects of human genetics and gut microbiota, 16S rRNA gene sequencing was performed on 18,340 individuals from 24 different cohorts. Following the exclusion of entries with unknown taxonomic information, a total of 196 bacterial traits were selected, comprising 9 phyla, 16 classes, 20 orders, 32 families, and 119 genera. A GWAS dataset of DTC (GWAS ID: ieu-a-1082) was obtained from the IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/) and employed as the outcome variable (15). Initially, a total of 701 Italian individuals with DTC (median age, 46 years) were included, of whom 649 remained after rigorous quality control measures. All cases were histologically validated as DTC, without further differentiation between PTC and FTC subtypes.
To establish a definitive causality between the gut microbiota and DTC, we employed the following screening criteria for instrumental variables (IVs): 1) single-nucleotide polymorphisms (SNPs) had a strong correlation with exposure (gut microbiota). The level of significance (p-value) was set to p < 1 × 10−5, consistent with the established protocol in prior studies (16, 17). 2) An F-statistic threshold >10 was utilized to mitigate weak IV bias. 3) SNPs exhibiting linkage disequilibrium effects were excluded, employing an R2 cutoff of 0.001 and a clumping window size of 10,000 kb. 4) In cases where SNPs were absent in the outcome, proxy SNPs (R2 > 0.8) were obtained from the 1000 Genomes Project (http://www.internationalgenome.org/). 5) The threshold for allele frequencies was set to 0.01. The study was approved by the Ethics Committee of Suzhou Ninth Hospital Affiliated to Soochow University.
Statistical analysis
Before conducting MR analysis, palindromic SNPs were excluded to harmonize the effects of the SNPs, and Steiger filtering was performed to ensure the correct directionality of each SNP. Five common MR methods were utilized in this study: inverse variance weighted (IVW), weighted median, weighted mode, simple mode, and MR-Egger methods. The IVW method estimates causal relationships by integrating the effect sizes of multiple genetic variations and applying inverse variance weighting. This approach enhances statistical power, mitigates estimation bias, and improves the accuracy of causal relationship assessment, making it a preferred choice in MR-related studies. The odds ratio (OR) and 95% confidence interval (95% CI) were determined. The MR-Egger intercept test was employed to assess directional horizontal pleiotropy (18). Cochran’s Q statistic was used to assess the heterogeneity in the data (19). MR Pleiotropy Residual Sum and Outlier (MR-PRESSO) analysis was performed to assess the presence of outliers (20). All statistical analyses were conducted using R software (version 4.3.1), primarily utilizing the R packages “TwoSampleMR” (version 0.5.7), “MRPRESSO” (version 1.0), “ieugwasr” (version 0.1.5), and “plinkbinr” (version 0.0.0.9000). A p-value <0.05 indicated statistical significance.
Results
MR analysis
The outcome comprised a total of 1,080 individuals, including 649 DTC cases and 431 controls (Figure 1). The IVW analysis showed that the Class Mollicutes and the Phylum Tenericutes were positively correlated with DTC, suggesting that Mollicutes and Tenericutes are associated with an increased risk of DTC (Figures 2, 3A, B, Table 1; Class Mollicutes, OR = 10.953, 95% CI: 2.333–51.428, p = 0.002; Phylum Tenericutes, OR = 10.953, 95% CI: 2.333–51.428, p = 0.002). The risk effects associated with both entities on DTC were nearly identical, as the Class Mollicutes falls within the Phylum Tenericutes. The weighted median method further confirmed the results (Table 1; Class Mollicutes, OR = 13.375, 95% CI: 1.731–103.376, p = 0.013; Phylum Tenericutes, OR = 13.375, 95% CI: 1.730–103.393, p = 0.013). In addition, no directional horizontal pleiotropy was observed (Supplementary Table 1; Class Mollicutes, Egger intercept = −0.482, p = 0.482; Phylum Tenericutes, Egger intercept = −0.482, p = 0.482). Furthermore, Cochran’s Q test indicated no heterogeneity in individual causal effects (Supplementary Table 2; Class Mollicutes, Cochran’s Q = 0.290, p = 0.590; Phylum Tenericutes, Cochran’s Q = 0.290, p = 0.590). Additionally, the MR-PRESSO analysis did not identify any potential outliers (Supplementary Table 3).
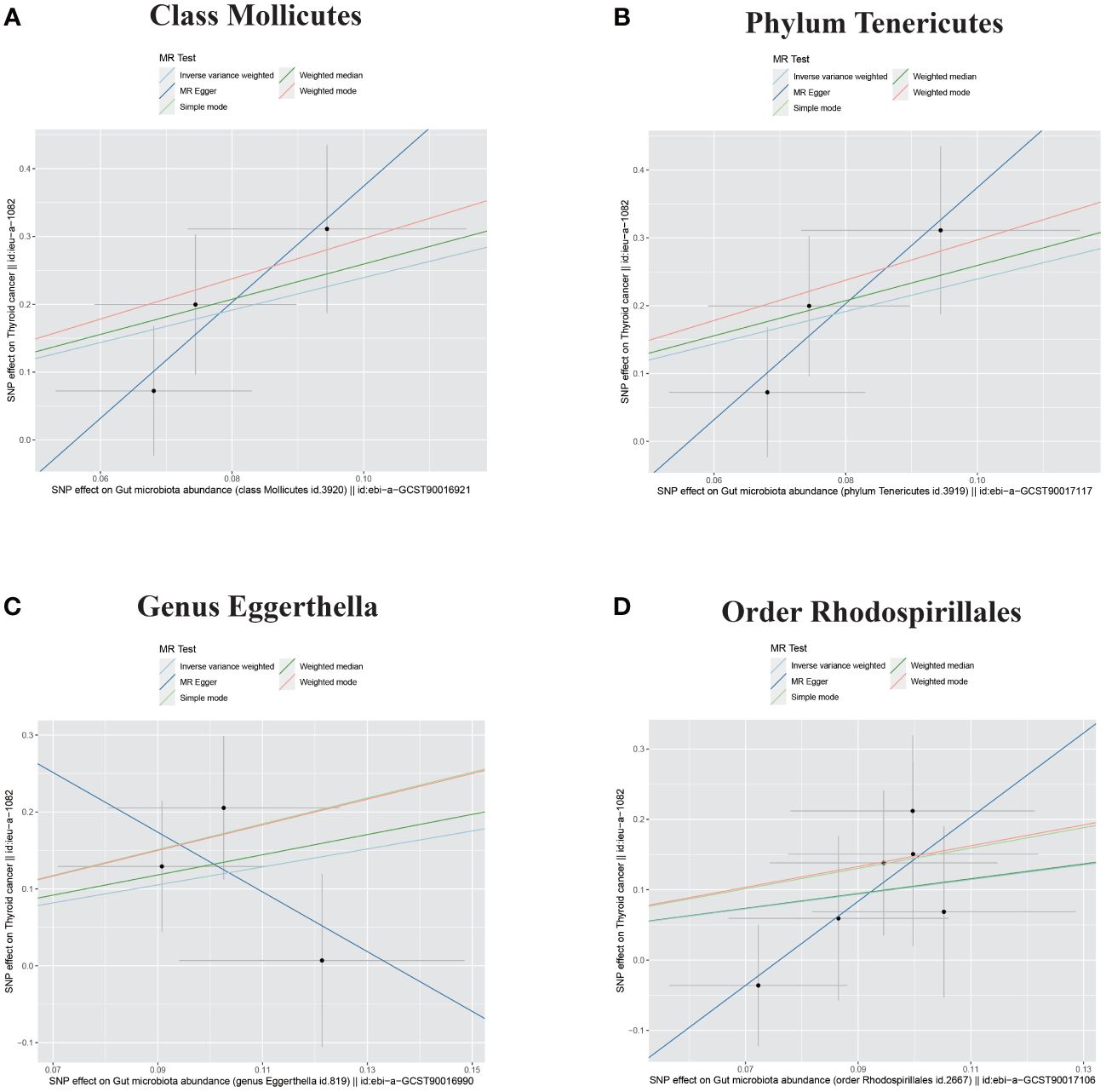
Figure 3 Scatter plots of four bacterial traits positively associated with DTC. (A) Class Mollicutes. (B) Phylum Tenericutes. (C) Genus Eggerthella. (D) Order Rhodospirillales. DTC, differentiated thyroid cancer; MR, Mendelian randomization; SNP, single-nucleotide polymorphism.
IVW analysis identified the Genus Eggerthella and the Order Rhodospirillales as risk factors for DTC (Figures 2, 3C, D, Table 1; Genus Eggerthella, OR = 3.219, 95% CI: 1.033–10.024, p = 0.044; Order Rhodospirillales, OR = 2.829, 95% CI: 1.096–7.299, p = 0.032). However, these results lacked support from the weighted median method (Table 1; Genus Eggerthella, OR = 3.716, 95% CI: 0.891–15.503, p = 0.072; Order Rhodospirillales, OR = 2.861, 95% CI: 0.845–9.688, p = 0.091). MR-Egger analysis indicated no directional horizontal pleiotropy (Genus Eggerthella, Egger intercept = 0.523, p = 0.490; Order Rhodospirillales, Egger intercept = −0.455, p = 0.246). Cochran’s Q test revealed no heterogeneity in individual causal effects (Supplementary Table 2; Genus Eggerthella, Cochran’s Q = 1.139, p = 0.286; Order Rhodospirillales, Cochran’s Q = 1.288, p = 0.863).
In addition to the gut microbiota associated with an increased risk of DTC, the microbiota protecting against DTC was identified. IVW analysis revealed that the Genus Eubacterium fissicatena group and the Genus Lachnospiraceae UCG008 were negatively correlated with DTC, indicating their potential protective roles against DTC (Figures 2, 4A, B, Table 1; Genus Eubacterium fissicatena group, OR = 0.381, 95% CI: 0.148–0.979, p = 0.045; Genus Lachnospiraceae UCG008, OR = 0.317, 95% CI: 0.125–0.801, p = 0.015). These results were further supported by the weighted median analysis (Table 1; Genus Eubacterium fissicatena group, OR = 0.322, 95% CI: 0.114–0.910, p = 0.033; Genus Lachnospiraceae UCG008, OR = 0.265, 95% CI: 0.077–0.907, p = 0.034). In addition, no directional horizontal pleiotropy was observed (Supplementary Table 1; Genus Eubacterium fissicatena group, Egger intercept = −0.169, p = 0.790; Genus Lachnospiraceae UCG008, Egger intercept = −0.280, p = 0.593). Cochran’s Q test indicated no heterogeneity in the individual causal effects (Supplementary Table 2; Genus Eubacterium fissicatena group, Cochran’s Q = 4.218, p = 0.121; Genus Lachnospiraceae UCG008, Cochran’s Q = 0.860, p = 0.835).
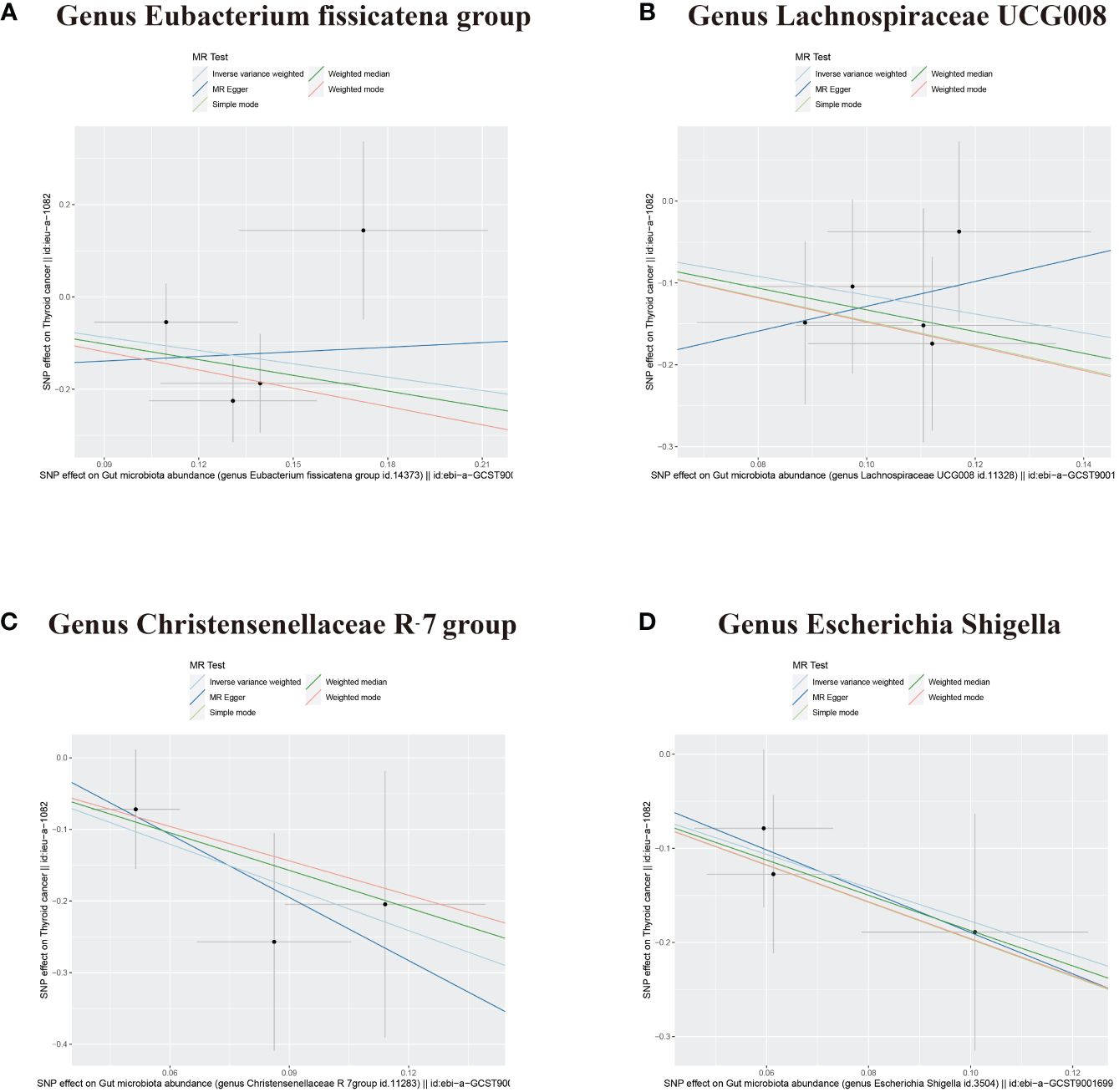
Figure 4 Scatter plots depicting four bacterial traits negatively associated with DTC. (A) Genus Eubacterium fissicatena group. (B) Genus Lachnospiraceae UCG008. (C) Genus Christensenellaceae R-7 group. (D) Genus Escherichia Shigella. DTC, differentiated thyroid cancer; MR, Mendelian randomization; SNP, single-nucleotide polymorphism.
The Genus Christensenellaceae R-7 group and the Genus Escherichia Shigella emerged as protective factors against DTC, as revealed by the IVW analysis (Figures 2, 4C, D, Table 1; Genus Christensenellaceae R-7 group, OR = 0.134, 95% CI: 0.020–0.886, p = 0.037; Genus Escherichia Shigella, OR = 0.170, 95% CI: 0.037–0.769, p = 0.021). However, these results were not supported by the weighted median analysis (Table 1; Genus Christensenellaceae R-7 group, OR = 0.174, 95% CI: 0.016–1.950, p = 0.156; Genus Escherichia Shigella, OR = 0.154, 95% CI: 0.023–1.030, p = 0.054). Moreover, the MR-Egger analysis indicated no directional horizontal pleiotropy (Supplementary Table 1; Genus Christensenellaceae R-7 group, Egger intercept = 0.069, p = 0.796; Genus Escherichia Shigella, Egger intercept = 0.030, p = 0.921). Additionally, Cochran’s Q test revealed no heterogeneity in individual causal effects (Supplementary Table 2; Genus Christensenellaceae R-7 group, Cochran’s Q = 0.353, p = 0.552; Genus Escherichia Shigella, Cochran’s Q = 0.142, p = 0.707).
Discussion
Most studies on the gut microbiota have predominantly focused on malignant tumors of the digestive tract (21). Gradually, it has been acknowledged that the gut microbiota also impacts non-gastrointestinal tumors through various mechanisms, including inflammation and immunoregulation, metabolic pathways, and bacterial translocation. For example, Bacteroides fragilis induces the differentiation of Treg cells, thereby promoting the formation of an immunosuppressive microenvironment through the production of immunosuppressive factors such as IL-10 and TGF-β, ultimately contributing to the development of gliomas (22, 23). Additionally, Ruminococcus sp. DSM_100440 has been found to convert androgen precursors into androgens, expediting the progression of castration-resistant prostate cancer (CRPC) (24). In this study, we identified eight bacterial traits significantly associated with DTC. Among these, four bacterial traits (Class Mollicutes, Phylum Tenericutes, Genus Eggerthella, and Order Rhodospirillales) were associated with the risk of DTC, while the remaining four traits (Genus Eubacterium fissicatena group, Genus Lachnospiraceae UCG008, Genus Christensenellaceae R-7 group, and Genus Escherichia Shigella) exhibited a protective effect against DTC. These findings contribute to our comprehension of the role of the gut microbiota in non-gastrointestinal tumors and offer a novel avenue for DTC treatment.
Christensenellaceae is widely distributed throughout the digestive tract and is intricately linked to human health (25). We found that the Genus Christensenellaceae R-7 group had a significant negative causality with DTC. Consistent with our findings, Lu et al. showed that the Genus Christensenellaceae R-7 group is significantly associated with DTC (26). They suggested that the Genus Christensenellaceae R-7 group may promote the occurrence and development of DTC by regulating lipid metabolism, as indicated by the marked inhibition of lipid digestion and steroid biosynthesis pathways. Similarly, a reduced abundance of the Genus Christensenellaceae R-7 group has been observed in patients with colorectal cancer (27), further underscoring the potential of this genus as a probiotic.
The Family Lachnospiraceae has been reported to play a protective role against colorectal carcinogenesis by bolstering the tumor immunosurveillance function of CD8+ T cells (28). Additionally, it has been demonstrated to serve a protective role in the regulation of radiation-induced intestinal damage (29). However, Zheng et al. (13) elucidated a greater abundance of the Family Lachnospiraceae in patients with DTC exhibiting a non-excellent response to RAI compared to those exhibiting an excellent response. We speculate that the absence of further taxonomic classification within the Lachnospiraceae family may have contributed to these discrepant findings. A genus-level analysis showed that the abundance of the Genus Lachnospiraceae UCG010 is significantly higher in patients with DTC exhibiting an excellent response to RAI than in those exhibiting a non-excellent response, suggesting that the Genus Lachnospiraceae UCG010 is a protective factor. Similarly, our analysis indicated that Lachnospiraceae UCG008 exerts protective effects against DTC.
The abundance of Mollicutes/Tenericutes was significantly increased in various tumor tissues, including gastric and lung cancers (30, 31). Employing animal models, Lee et al. (32) elucidated a positive correlation between gut Mollicutes/Tenericutes and tumor burden in colitis-associated cancer. To the best of our knowledge, our study is the first to demonstrate the causal relationship between gut Mollicutes/Tenericutes and DTC. The large 95% CI range observed for the Class Mollicutes and Phylum Tenericutes may be attributed to the small sample size. Further studies are warranted to elucidate the pathogenic mechanisms of Mollicutes/Tenericutes.
This study has a few limitations. First, it included only one cohort comprising 1,080 participants, potentially resulting in a limited number of valid SNPs. Studies involving larger cohorts are necessary to establish more robust causal links between the gut microbiota and DTC. Second, although taxonomic classification has identified approximately 1,000 species of gut microbiota (33), our analysis was limited to 119 genera, precluding consideration at the species level. Third, due to the small sample size, DTC was treated as a single entity in this study. In fact, DTC can be classified into various subtypes, each exhibiting distinct biological behaviors. In future studies, histopathological subclassifications will be pivotal for refining precision treatments for DTC. Fourth, potential unaddressed confounding factors could impact the accuracy and generalizability of our findings. Finally, discrepancies observed across MR methods raise concerns regarding the robustness of certain associations, while the lack of functional insights leaves unanswered questions regarding biological mechanisms.
In summary, our investigation revealed that eight bacterial traits exert a significant causal effect on DTC. These findings enhance our comprehension of the pathological mechanisms underlying DTC and provide a novel avenue for its treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethical statement
The experiments were ethically approved by the Suzhou Ninth People’s Hospital.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Suzhou Ninth Hospital Affiliated to Soochow University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
SH: Data curation, Formal analysis, Methodology, Validation, Software, Writing – original draft. FF: Conceptualization, Data curation, Formal analysis, Investigation, Writing – original draft. CT: Data curation, Investigation, Methodology, Resources, Validation, Writing – original draft. LW: Conceptualization, Data curation, Formal analysis, Supervision, Writing – original draft. XL: Conceptualization, Formal analysis, Supervision, Visualization, Writing – review & editing. MS: Conceptualization, Data curation, Methodology, Software, Supervision, Writing – review & editing. LY: Conceptualization, Investigation, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2024.1375525/full#supplementary-material
Supplementary Table 1 | Results of the pleiotropy test.
Supplementary Table 2 | Results of the heterogeneity test.
Supplementary Table 3 | Results of the MRPRESSO test.
References
1. Liu H, Deng H, Zhao Y, Li C, Liang Y. LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J Exp Clin Cancer Res. (2018) 37:279. doi: 10.1186/s13046-018-0950-9
2. Chen W, Li J, Peng S, Hong S, Xu H, Lin B, et al. Association of total thyroidectomy or thyroid lobectomy with the quality of life in patients with differentiated thyroid cancer with low to intermediate risk of recurrence. JAMA Surg. (2022) 157:200–9. doi: 10.1001/jamasurg.2021.6442
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
4. Wang Z, Tang C, Wang Y, Yin Z, Rixiati Y. Inclusion of the number of metastatic lymph nodes in the staging system for medullary thyroid cancer: validating a modified american joint committee on cancer tumor-node-metastasis staging system. Thyroid. (2022) 32:536–43. doi: 10.1089/thy.2021.0571
5. Wang Z, Fan X, Zha X, Xu Y, Yin Z, Rixiati Y, et al. A proposed modified staging system for medullary thyroid cancer: A SEER analysis with multicenter validation. Oncologist. (2023). 29:e59-e67. doi: 10.1093/oncolo/oyad165
6. Wirth LJ, Durante C, Topliss DJ, Winquist E, Robenshtok E, Iwasaki H, et al. Lenvatinib for the treatment of radioiodine-refractory differentiated thyroid cancer: treatment optimization for maximum clinical benefit. Oncologist. (2022) 27:565–72. doi: 10.1093/oncolo/oyac065
7. Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. (2007) 28:742–62. doi: 10.1210/er.2007-0007
8. Malandrino P, Russo M, Regalbuto C, Pellegriti G, Moleti M, Caff A, et al. Outcome of the diffuse sclerosing variant of papillary thyroid cancer: A meta-analysis. Thyroid. (2016) 26:1285–92. doi: 10.1089/thy.2016.0168
9. Kong C, Liang L, Liu G, Du L, Yang Y, Liu J, et al. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut. (2023) 72:1129–42. doi: 10.1136/gutjnl-2022-327156
10. Xi Y, Liu F, Qiu B, Li Y, Xie X, Guo J, et al. Analysis of gut microbiota signature and microbe-disease progression associations in locally advanced non-small cell lung cancer patients treated with concurrent chemoradiotherapy. Front Cell Infect Microbiol. (2022) 12:892401. doi: 10.3389/fcimb.2022.892401
11. Nandi D, Parida S, Sharma D. The gut microbiota in breast cancer development and treatment: The good, the bad, and the useful! Gut Microbes. (2023) 15:2221452. doi: 10.1080/19490976.2023.2221452
12. Yu X, Jiang W, Kosik RO, Song Y, Luo Q, Qiao T, et al. Gut microbiota changes and its potential relations with thyroid carcinoma. J Adv Res. (2022) 35:61–70. doi: 10.1016/j.jare.2021.04.001
13. Zheng L, Zhang L, Tang L, Huang D, Pan D, Guo W, et al. Gut microbiota is associated with response to (131)I therapy in patients with papillary thyroid carcinoma. Eur J Nucl Med Mol Imaging. (2023) 50:1453–65. doi: 10.1007/s00259-022-06072-5
14. Lin B, Zhao F, Liu Y, Wu X, Feng J, Jin X, et al. Randomized clinical trial: probiotics alleviated oral-gut microbiota dysbiosis and thyroid hormone withdrawal-related complications in thyroid cancer patients before radioiodine therapy following thyroidectomy. Front Endocrinol (Lausanne). (2022) 13:834674. doi: 10.3389/fendo.2022.834674
15. Kohler A, Chen B, Gemignani F, Elisei R, Romei C, Figlioli G, et al. Genome-wide association study on differentiated thyroid cancer. J Clin Endocrinol Metab. (2013) 98:E1674–81. doi: 10.1210/jc.2013-1941
16. Li R, Guo Q, Zhao J, Kang W, Lu R, Long Z, et al. Assessing causal relationships between gut microbiota and asthma: evidence from two sample Mendelian randomization analysis. Front Immunol. (2023) 14:1148684. doi: 10.3389/fimmu.2023.1148684
17. Wang J, Luo R, Zhao X, Xia D, Liu Y, Shen T, et al. Association between gut microbiota and primary ovarian insufficiency: a bidirectional two-sample Mendelian randomization study. Front Endocrinol (Lausanne). (2023) 14:1183219. doi: 10.3389/fendo.2023.1183219
18. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan NA, Thompson JR. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: the role of the I2 statistic. Int J Epidemiol. (2016) 45:1961–74. doi: 10.1093/ije/dyw220
19. Wu F, Huang Y, Hu J, Shao Z. Mendelian randomization study of inflammatory bowel disease and bone mineral density. BMC Med. (2020) 18:312. doi: 10.1186/s12916-020-01778-5
20. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7
21. Grochowska M, Perlejewski K, Laskus T, Radkowski M. The role of gut microbiota in gastrointestinal tract cancers. Arch Immunol Ther Exp (Warsz). (2022) 70:7. doi: 10.1007/s00005-021-00641-6
22. Albulescu R, Codrici E, Popescu ID, Mihai S, Necula LG, Petrescu D, et al. Cytokine patterns in brain tumour progression. Mediators Inflammation. (2013) 2013:979748. doi: 10.1155/2013/979748
23. Zitvogel L, Galluzzi L, Viaud S, Vetizou M, Daillere R, Merad M, et al. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. (2015) 7:271ps1. doi: 10.1126/scitranslmed.3010473
24. Pernigoni N, Zagato E, Calcinotto A, Troiani M, Mestre RP, Cali B, et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science. (2021) 374:216–24. doi: 10.1126/science.abf8403
25. Waters JL, Ley RE. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. (2019) 17:83. doi: 10.1186/s12915-019-0699-4
26. Lu G, Yu X, Jiang W, Luo Q, Tong J, Fan S, et al. Alterations of gut microbiome and metabolite profiles associated with anabatic lipid dysmetabolism in thyroid cancer. Front Endocrinol (Lausanne). (2022) 13:893164. doi: 10.3389/fendo.2022.893164
27. Peters BA, Dominianni C, Shapiro JA, Church TR, Wu J, Miller G, et al. The gut microbiota in conventional and serrated precursors of colorectal cancer. Microbiome. (2016) 4:69. doi: 10.1186/s40168-016-0218-6
28. Zhang X, Yu D, Wu D, Gao X, Shao F, Zhao M, et al. Tissue-resident Lachnospiraceae family bacteria protect against colorectal carcinogenesis by promoting tumor immune surveillance. Cell Host Microbe. (2023) 31:418–432 e8. doi: 10.1016/j.chom.2023.01.013
29. Guo H, Chou WC, Lai Y, Liang K, Tam JW, Brickey WJ, et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science. (2020) 370:eaay9097. doi: 10.1126/science.aay9097
30. Nascimento Araujo CD, Amorim AT, Barbosa MS, Alexandre J, Campos GB, Macedo CL, et al. Evaluating the presence of Mycoplasma hyorhinis, Fusobacterium nucleatum, and Helicobacter pylori in biopsies of patients with gastric cancer. Infect Agent Cancer. (2021) 16:70. doi: 10.1186/s13027-021-00410-2
31. Zhuo M, An T, Zhang C, Wang Z. Characterization of microbiota in cancerous lung and the contralateral non-cancerous lung within lung cancer patients. Front Oncol. (2020) 10:1584. doi: 10.3389/fonc.2020.01584
32. Lee JG, Lee YR, Lee AR, Park CH, Han DS, Eun CS. Role of the global gut microbial community in the development of colitis-associated cancer in a murine model. BioMed Pharmacother. (2021) 135:111206. doi: 10.1016/j.biopha.2020.111206
Keywords: causality, gut microbiota, differentiated thyroid cancer, Mendelian randomization, genome-wide association study
Citation: Hu S, Tang C, Wang L, Feng F, Li X, Sun M and Yao L (2024) Causal relationship between gut microbiota and differentiated thyroid cancer: a two-sample Mendelian randomization study. Front. Oncol. 14:1375525. doi: 10.3389/fonc.2024.1375525
Received: 24 January 2024; Accepted: 08 April 2024;
Published: 26 April 2024.
Edited by:
Diogo Alpuim Costa, CUF Oncologia, PortugalReviewed by:
Rafael De Cicco, Doctor Arnaldo Cancer Institute, BrazilFrancesca Pirini, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), Italy
Pedro Ribeiro, Centro Laboratorial Germano de Sousa, Portugal
Copyright © 2024 Hu, Tang, Wang, Feng, Li, Sun and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijun Yao, yaolijun109@126.com; Mingyu Sun, 645396517@qq.com; Xiaoxin Li, 563810043@qq.com
†These authors have contributed equally to this work
 Shaojun Hu1†
Shaojun Hu1† Chuangang Tang
Chuangang Tang Mingyu Sun
Mingyu Sun Lijun Yao
Lijun Yao