- Department of Thoracic Surgery, Ganzhou People’s Hospital, Jiangxi Medical College, Nanchang University, Ganzhou, Jiangxi, China
With the increasing implementation of early lung cancer screening and the increasing emphasis on physical examinations, the early-stage lung cancer detection rate continues to rise. Visceral pleural invasion (VPI), which denotes the tumor’s breach of the elastic layer or reaching the surface of the visceral pleura, stands as a pivotal factor that impacts the prognosis of patients with non-small cell lung cancer (NSCLC) and directly influences the pathological staging of early-stage cases. According to the latest 9th edition of the TNM staging system for NSCLC, even when the tumor diameter is less than 3 cm, the final T stage remains T2a if VPI is present. There is considerable controversy within the guidelines regarding treatment options for stage IB NSCLC, especially among patients exhibiting VPI. Moreover, the precise determination of VPI is important in guiding treatment selection and prognostic evaluation in individuals with NSCLC. This article aims to provide a comprehensive review of the current status and advancements in studies pertaining to stage IB NSCLC accompanied by VPI.
1 Introduction
As per the latest Global Burden of Cancer report released by the International Agency for Research on Cancer in 2022, the estimated number of new malignant cases worldwide in 2020 was around 19 million, with approximately 10 million cancer-related deaths. Lung cancer stands as one of the primary contributors to cancer mortality, accounting for roughly 18% of all cancer-related deaths. It is projected that the global incidence of new cancer cases will reach 28.4 million by 2040 (1). With the increasing focus on early lung cancer screening and physical examinations, the detection rate of early-stage lung cancer has witnessed a continuous rise. Presently, lung cancer remains the most prevalent and lethal malignancy in the world (1). Therefore, early detection, diagnosis, and treatment play a vital role in patient prognosis. However, there is still ample room for further lung cancer prevention and treatment advancements.
The TNM staging system for non-small cell lung cancer (NSCLC) has undergone multiple revisions since its inception in the 1960s, with accurate staging playing a crucial role in prognostic assessment and treatment decision-making. Although there is room for further improvement in precise staging, it is widely recognized as a critical foundation for prognostic evaluation and treatment guidance. Rami-Porta et al. (2) proposed comprehensive revisions to T staging, elucidating the objectives of these revisions and highlighting the value and significance of T staging in guiding precise treatment for NSCLC. They emphasized the importance of distinguishing between visceral pleural invasion (VPI) and parietal pleural invasion. Numerous previous studies have demonstrated that VPI is associated with more aggressive disease behavior and poor prognostic factors (3–6).
According to the 8th edition of the TNM staging system for NSCLC by the Union for International Cancer Control (UICC) and the American Joint Committee on Cancer (AJCC), even if the maximum diameter of the primary tumor is ≤3 cm, the presence of VPI leads to an upstaging of T-stage to T2a, resulting in the transition from stage IA to stage IB in terms of pathological staging (7). Currently, there is a contentious debate regarding the optimal approach to surgical resection and postoperative adjuvant therapy for patients with stage IB NSCLC exhibiting VPI, with inconsistent guidelines on this matter. This article aims to comprehensively review the current status and progress of research about the diagnosis, treatment, and prognosis of stage IB NSCLC with VPI.
2 Visceral pleural invasion
2.1 Definition of visceral pleural invasion
The pleura is comprised of two layers: the visceral pleura, which envelops the surface of the lungs, and the parietal pleura, which adheres to the inner surface of the chest wall. These layers create a closed pleural cavity (8). This article focuses on the structure of the visceral pleura, specifically exploring its characteristics in diseases.
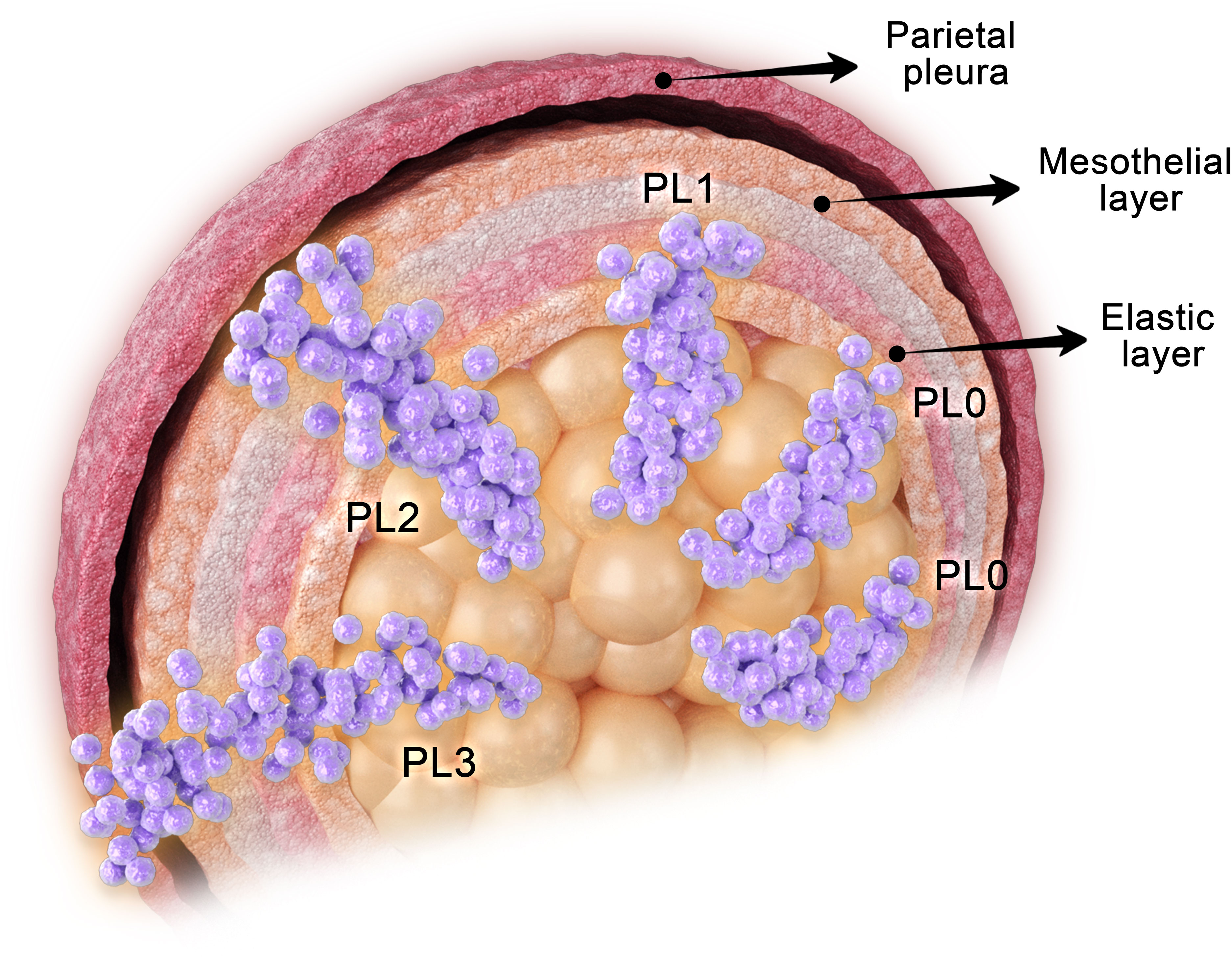
Histologically, the visceral pleura can be divided into five layers: the mesothelial layer, the submesothelial connective tissue layer, the outer elastic layer, the inner elastic layer, and the deep connective tissue layer, in sequential order from outermost to innermost (9). VPI is defined as the infiltration and penetration of tumor tissue through the elastic layer of the visceral pleura, which also encompasses invasion of the pleural surface (10–13). To avoid confusion with pathological tumor staging (pTNM), some researchers have proposed using the abbreviation “PL” instead of “P” to denote the pleura (13).
2.2 Diagnosis of visceral pleural invasion
The detection and assessment of VPI in NSCLC can be accomplished through various methods. Preoperative chest computed tomography (CT) images provide essential information for diagnosing VPI. Certain CT image features, such as pleural retraction and pleural indentation, enhance the accuracy of early detection of VPI in peripheral NSCLC (14, 15). Onoda et al. (16) conducted a study to evaluate the correlation between pleural markers on CT scans and VPI, involving 221 patients with peripheral NSCLC who underwent surgical resection. The labeling of pleural abnormalities on CT scans improved the predictive precision of VPI when the tumor was ≤3 cm in diameter and did not directly contact the pleural surface. Moreover, clinicopathological characteristics like age, histological type, and tumor differentiation level can serve as additional indicators for diagnosing VPI (17). A prediction model that combines preoperative CT findings with pathological features yields a more accurate prognosis of VPI (18).
Studies have demonstrated that the use of white light patterns in combination with autofluorescence can enhance the sensitivity and accuracy of intraoperative diagnosis of VPI during thoracoscopic surgery (19). However, relying solely on intraoperative visualization may lead to significant errors in determining VPI, necessitating postoperative histopathology for confirmation. Conventional hematoxylin and eosin staining may occasionally present challenges in clearly distinguishing between PL0 and PL1 stages, and it may even miss instances of tumor penetration through the elastic fiber layer. Elastic staining plays a crucial role in providing significant prognostic insights and facilitating accurate pathologic staging in cases where pleural invasion remains undetected by 10% hematoxylin and eosin staining (20). Common techniques for elastic staining include Victoria blue, Gomori’s aldehyde-fuchsin, or Weigert’s resorcin-fuchsin (21). some researchers advocate for the utilization of elastic staining assays to aid in identifying the extent of disruption in the elastic fiber layer (13, 22–24) (Figures 1A-D). Furthermore, recent findings have demonstrated that the utilization of dual-block elastin staining can effectively identify patients with more pronounced VPI (25).
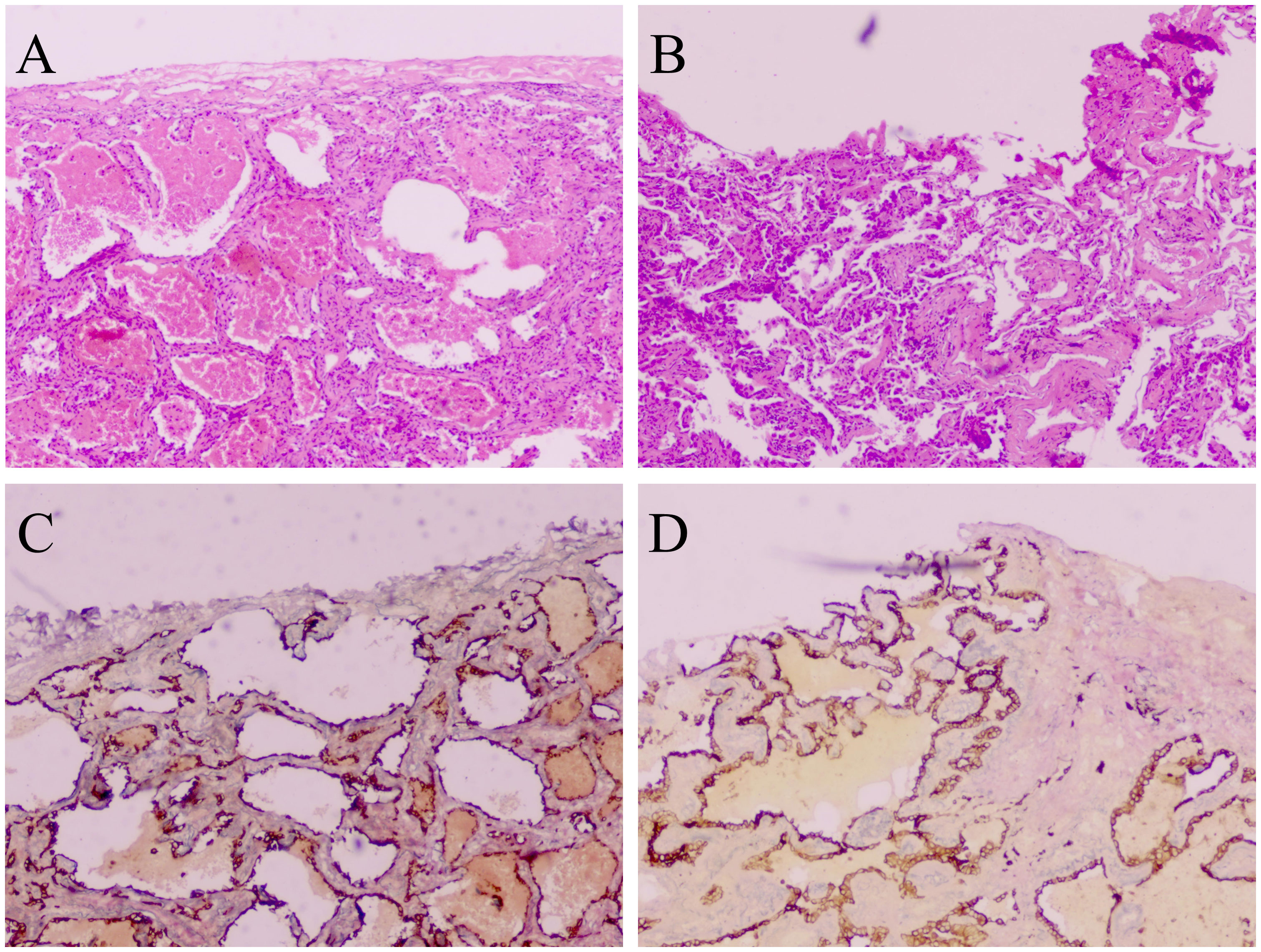
Figure 1 VPI is confined to the elastic layer without penetration of the pleural surface (PL1), stained with hematoxylin and eosin (A). VPI involving penetration of the pleural surface (PL2), stained with hematoxylin and eosin (B). VPI is confined to the elastic layer without penetration of the pleural surface (PL1), stained with elastic staining (C). VPI involving penetration of the pleural surface (PL2), stained with elastic staining (D). A-D: 40 × magnification.
2.3 Grading of visceral pleural invasion
In 1988, Hammar introduced a grading system for VPI in his book ‘Pulmonary Pathology’ (26). Subsequently, the Japanese Lung Cancer Society widely adopted Hammar’s grading scheme. However, when the sixth edition of the UICC/AJCC TNM staging system for NSCLC was released in 1997, although VPI was recognized as a crucial factor for improved staging, Hammar’s classification was not used to precisely define it (27).
As a result, the seventh edition of the TNM staging system for NSCLC in 2009 proposed the ‘Modified Hammar classification’. The modified Hammar classification categorizes the extent of VPI as follows: p0 indicates no breach of the elastic fiber layer by the tumor; p1 signifies tumor infiltration beyond the elastic lamina without exposure of the pleural surface; and p2 denotes tumor invasion of the pleural surface of the visceral layer, specifically the mesothelium (28). This grading criterion for VPI has been in use since then. However, in a study by Warth et al. (29) involving 173 NSCLC patients without lymph node metastasis, no significant differences were observed when analyzing survival rates based on the degree of VPI alone. This suggests that assessing the degree of VPI solely based on the elastic fiber layer may not be sufficient.
Overall, the extent of VPI impacts the prognosis following resection of NSCLC, and in clinical practice, it is recommended to categorize VPI into three grades: PL0, PL1, and PL2 (30) (Figure 2).
3 Visceral pleural invasion and tumor staging
Accurate staging of NSCLC is crucial for the effective treatment and prognostic management of patients. VPI plays a significant role in the unfavorable prognosis of NSCLC and is a crucial component of the staging system (10, 22, 31, 32). Precise staging of early-stage NSCLC relies heavily on the accurate assessment of VPI (22). In the sixth edition of the staging system, any tumor that invaded the visceral pleura was classified as T2 (27). However, the seventh edition, proposed in 2009, focused more on detailed tumor size thresholds and did not adequately address the adverse effect of VPI (28). The 8th edition of the TNM staging system for NSCLC classified tumors as T2a when their diameter was less than 3 cm but accompanied by VPI (7).
It is important to note that researchers have gradually recognized the risk factor of VPI. Before the release of the seventh edition of the staging system, Shimizu et al. (11) concluded from a retrospective analysis of 1653 patients with T1, T2, and T3 NSCLCs that tumors with a diameter greater than 3 cm and VPI should be upgraded to T3 status. Some scholars have also proposed an improved classification for the pT2 category: T2a (tumor diameter 3-5 cm and P0-1) and T2b (tumor diameter >5 cm or P2) (33). However, these recommendations were not adopted in the seventh edition of the staging system. In 2007, Hung et al. (34) conducted a retrospective analysis of 445 resected patients with stage I NSCLC with a diameter less than 3 cm. The study concluded that VPI did not significantly affect the overall and disease-free survival (DFS)rates. Therefore, they suggested that NSCLC with VPI of less than 3 cm in diameter should be categorized as T1 rather than T2.
Before the release of the 8th edition of the staging system for NSCLC, Nitadori et al. (35) conducted a study that revealed no significant correlation between VPI and prognosis in patients with tumor diameters smaller than 2 cm. However, a significant correlation was observed in patients with tumor diameters ranging from 2 to 3 cm. Kudo et al. (36) recommended that the subsequent version of the TNM staging system for NSCLC classify tumors with diameters of 3.1-5 cm, accompanied by VPI, as T2b. Furthermore, some scholars have proposed that T1 tumors with VPI should be upgraded to T2a, T2a tumors with VPI should be classified as T2b, and T2b tumors with VPI should be categorized as T3 (37, 38). In 2012, Fibla et al. (39) conducted a survival analysis of 289 patients with stage IB (T2aN0M0) NSCLC according to the seventh edition of the AJCC classification. They divided the patients into three groups based on tumor size and the presence of VPI. The study revealed that patients with stage IB tumors measuring more than 3 cm but less than or equal to 5 cm, along with VPI, had a significantly worse prognosis compared to patients with “T2a” tumors solely based on VPI or tumor size. Therefore, it is recommended to upgrade these patients from the current IB stage to stage IIA. Shim et al. (12) suggested upgrading the T stage to T3 when the tumor diameter exceeds 5 cm but is less than 7 cm and accompanied by VPI, aligning with the eighth edition of the T staging system.
According to the eighth edition of the staging system for NSCLC, tumors with a diameter of 3 cm or less that invade the visceral pleura are classified as stage T2 (7). Yang et al. (6) conducted a study in 2017 that supported this staging system, suggesting that early-stage tumors with diameters less than 3 cm and VPI should be classified as a higher T stage. Furthermore, their findings indicated that tumors with diameters greater than 3 cm should be classified into higher T stages. However, some researchers and scholars have proposed that, in the absence of further large-scale multicenter studies on NSCLC, there is no need to upgrade patients with VPI and a maximum tumor diameter of 3 cm or less to the T2 stage (40).
In addition, Qi et al. (41) suggested considering escalation to the pT2b stage for early-stage NSCLCs with a diameter between 3.1 and 4.0 cm accompanied by VPI. Cai et al. (42) reached a comparable finding through an analysis of an extensive patient database comprising individuals with early-stage non-small cell lung cancer. Their conclusion suggests that for this specific population, characterized by a diameter of less than 3 cm, it should be classified as a T2a stage, exhibiting a more favorable prognosis. The relationship between the degree of VPI and tumor stage has been extensively explored in numerous relevant studies. A retrospective study revealed that in patients with NSCLC without lymph node metastasis and with a tumor diameter of 2 cm or less, tumors with PL1 features should be classified as stage T1, while tumors with PL2 features should be classified as stage T2 (43). T2a-PL2 tumors were categorized as stage T2b, indicating a poorer prognosis (44). Another study reached a similar conclusion that for tumors of 3 cm or less in NSCLC, tumors with PL1 features should still be classified as stage T1 rather than T2 (45). However, a different perspective exists for lymph node-negative NSCLC of 3 cm or less: there is a belief that patients with PL1 features should be classified as stage pT2a to improve staging accuracy (46).
To summarize, the staging of tumors in these patients should involve a comprehensive assessment of both tumor diameter and the extent of VPI, to develop an integrated staging approach. Particularly for patients with tumor sizes below 3 cm, along with concomitant VPI (PL1), it might be suitable to categorize them within a stage exhibiting a more favorable prognosis. We anticipate further studies to contribute additional evidence for the subsequent iteration of TNM staging for NSCLC.
4 Visceral pleural invasion and the nature of the nodule
Extensive research has demonstrated a substantial association between NSCLC with the presence of VPI and the characteristics of the nodules. In patients who underwent resection for stage I NSCLC, the ground glass component has been identified as a more reliable prognostic indicator (47). However, in cases of mixed ground-glass nodules, VPI did not serve as a prognostic predictor (48). Among patients with solid nodules, those without VPI exhibited a more favorable prognosis compared to individuals with pure glass nodules and mixed ground-glass nodules. Notably, the study did not further analyze the subgroups of PL1 and PL2 about VPI (47).
In a retrospective analysis of CT imaging involving 115 patients with pure ground-glass nodules, Zhao et al. (49) discovered that lung adenocarcinomas appearing as pure ground-glass nodules were typically not accompanied by VPI. Furthermore, multiple studies have revealed that VPI impacts the prognosis of patients with solid nodules, although its significance may vary among certain individuals with solid nodules (48, 50, 51). While VPI can be observed in both pure and mixed ground-glass nodules, it is more frequently observed in ground-glass nodules with a diameter exceeding 2 cm. Additionally, PL2 invasion is less common in patients with ground-glass nodules (52).
5 Visceral pleural invasion and lymph node involvement
In exploring the connection between VPI tumors and lymph node involvement, researchers have made notable contributions. Zhang et al. (53) discovered that among 740 peripheral upper lobe lung tumors, only 7 exhibited lower mediastinal lymph node metastasis, and all of these 7 tumors were characterized by VPI. VPI stands as a significant prognosticator for lymph node metastasis in clinical stage IA NSCLC (54). The optimal extent of lymph node clearance varies depending on the presence of VPI, with VPI(+) tumors necessitating a more extensive lymph node dissection compared to VPI(-) tumors of the same T1 size (55). However, it has also been demonstrated that the existence of VPI does not demonstrate a substantial correlation with lymph node involvement (56).
In summary, both VPI and lymph node involvement serve as indicators of unfavorable tumor prognosis. Although limited evidence exists regarding the relationship between the two, further studies are anticipated in the future.
6 Stage IB and surgical approach
Surgical resection remains the preferred treatment option for patients diagnosed with stage I and II NSCLC (57). Extensive research has been conducted on the various approaches to surgical resection in early-stage NSCLC cases involving VPI.
In 1995, Martini et al. (58) examined 598 patients with stage I NSCLC who underwent surgical resection and discovered that sublobar resection led to higher recurrence rates and lower survival rates, regardless of the histologic type. However, Koike et al. (59) conducted a retrospective analysis of 328 patients with clinical stage IA NSCLC who underwent wedge resection or segmental resection. They found that segmental resection could be the preferred sublobar resection approach for clinical stage IA NSCLC, specifically for patients with tumors lacking definite pleural invasion. In 2022, Song et al. (60) conducted a propensity-matched analysis and competing risk analysis of 2,717 cases of NSCLC surgical modalities. They identified lobectomy as the preferred surgical approach for NSCLC cases with VPI. Furthermore, due to the higher incidence of lymph node metastasis in tumors with VPI, more extensive lymph node dissection is required (36). Recent retrospective studies have reported that in early-stage NSCLC cases with tumors ≤3 cm in diameter and VPI, lobectomy outperforms sublobar resection in terms of survival prognosis (61, 62).
However, certain studies have indicated that for stage IB NSCLC, the prognosis of sublobar resection is comparable to that of lobectomy. In cases where VPI is discovered after segmental resection in patients with stage I NSCLC, continuing with lobectomy is unlikely to yield additional survival benefits (63, 64).
7 Stage IB and adjuvant chemotherapy
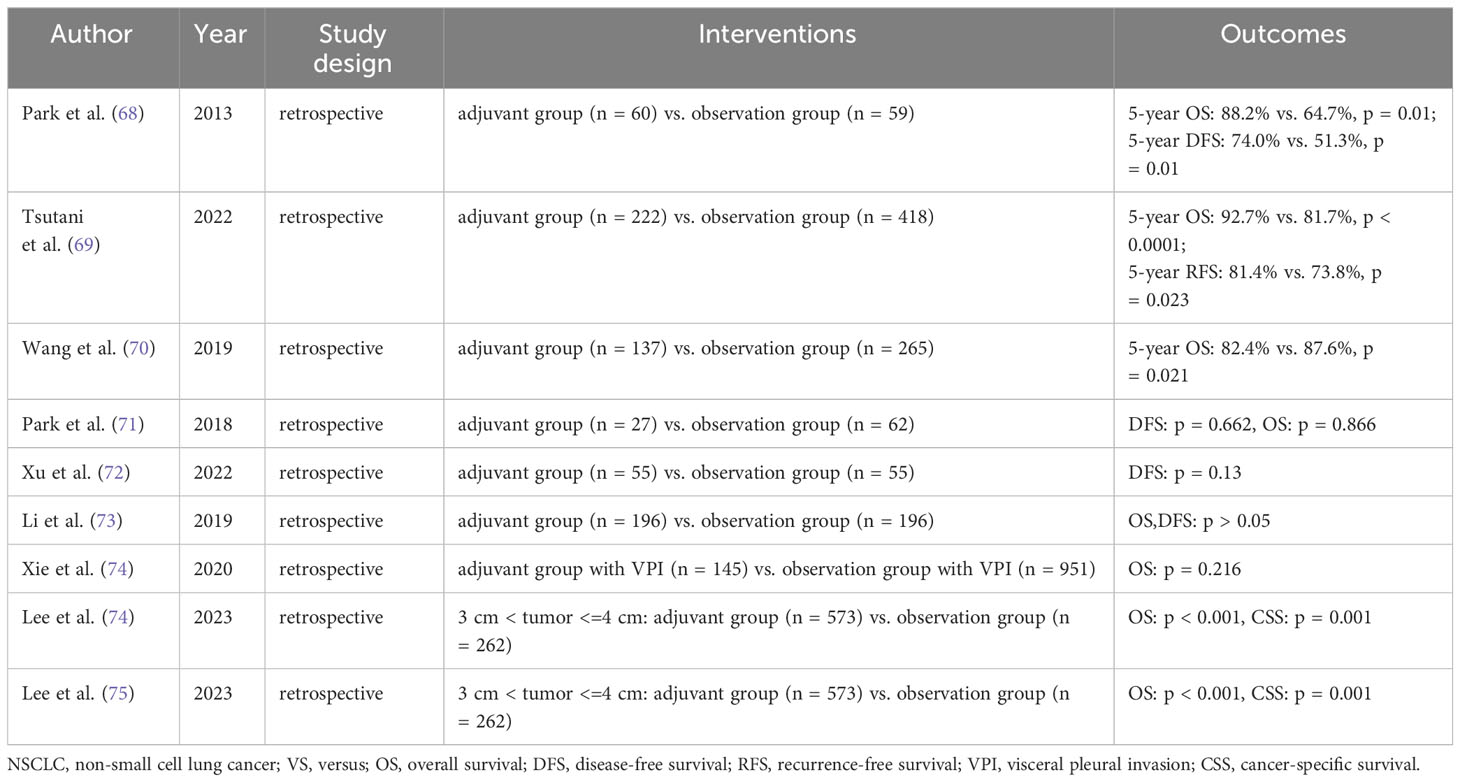
Adjuvant chemotherapy (ACT) treatment regimens incorporating platinum-based chemotherapeutic agents as the primary component have demonstrated enhanced prognostic outcomes for overall survival (OS) in patients with completely resected NSCLC. Furthermore, they have shown improved survival rates in individuals with early-stage NSCLC who exhibit high-risk factors for recurrence (65–69). In comparison to the seventh edition of the TNM staging system for NSCLC, the eighth edition of the staging system better identifies those patients with early-stage NSCLC who would benefit from platinum-based ACT, particularly those classified as stage II (70). However, the utilization of ACT in patients with stage IB NSCLC remains a topic of considerable controversy. In recent years, numerous clinical studies have investigated the potential benefits of ACT for patients with stage IB NSCLC (Table 1).
Relevant research has indicated that VPI is a significant prognostic factor for stage IB NSCLC. However, even in high-risk patients, ACT has shown no impact on the prognosis of stage IB NSCLC (71). Furthermore, studies have demonstrated that ACT may not contribute to improved DFS in individuals with completely resected stage IB NSCLC (72, 73).
Incorporating a database of stage IB NSCLCs from 2010 to 2015, Xie et al. (74) conducted a multifactorial analysis using Cox proportional risk regression. Their findings revealed that even in patients with VPI, ACT did not confer benefits to those with stage IB NSCLC. Additionally, a study by Lee et al. (75) observed that ACT improved OS in patients with stage IB lung adenocarcinoma and tumor diameters larger than 3 cm but ≤4 cm. However, for patients with tumor diameters ≤3 cm and VPI, ACT did not contribute to a survival advantage. It has been reported that excessive use of ACT can be avoided in patients with lymph node-negative NSCLC and tumor diameters of ≤3 cm (45). In conclusion, ACT did not significantly enhance long-term survival in patients with stage IB NSCLC accompanied by VPI.
Significantly, emerging evidence suggests that the presence of VPI is an independent prognostic factor associated with unfavorable outcomes in patients diagnosed with stage I NSCLC. Consequently, a more aggressive therapeutic approach, such as ACT, may be warranted for individuals presenting with VPI (76–79). Specifically, ACT could be a viable treatment option for patients with stage IB NSCLC, particularly those with high-risk factors including VPI, vascular invasion, advanced age, and poorly differentiated tumors (80, 81). Moreover, ACT has demonstrated the potential to enhance long-term prognosis in patients with stage IB NSCLC who have a history of previous malignancies (82).
In the case of lymph node-negative NSCLC, the combined influence of VPI and tumor size exhibits a synergistic effect on survival. A study conducted by Zhang et al. (83) found no significant association between ACT and improved 5-year survival in patients diagnosed with stage IB-IIA NSCLC. However, for patients with tumor diameters ranging from 3 to 4 cm and concurrent VPI, ACT may confer potential benefits. A similar conclusion was reached by Wightman et al. (84), suggesting that ACT should be particularly considered for patients with tumor diameters of 3-4 cm and the presence of VPI. Additionally, ACT has been reported to enhance survival in a substantial cohort of T2N0M0 patients who underwent complete resection, with significant outcomes observed in patients with tumors measuring less than 4 cm in diameter (85). It is worth noting that ACT is recommended for patients with stage IB disease with VPI, particularly when the tumor diameter is ≥4 cm (86, 87).
Building upon these studies, Hou et al. (88) conducted a comprehensive investigation in 2022 involving 1050 patients with NSCLC characterized by pathologic T2N0M0 staging. The patients were divided into two groups: those who received ACT and those who did not. The results indicated that ACT only improved OS in patients with tumor diameters exceeding 4 cm (OS: P = 0.003; DFS: P = 0.013). Furthermore, ACT exhibited a significant 5-year survival benefit in patients with wild-type epithelial growth factor receptor (EGFR) (P = 0.022). Therefore, in patients with tumor diameters greater than 4 cm and the presence of wild-type EGFR, ACT may offer a survival advantage. Additionally, in patients diagnosed with N0 NSCLC, PL2 emerges as a crucial prognostic factor closely associated with recurrence and poorer overall survival, thereby justifying the consideration of ACT (89).
Nevertheless, further investigation is required to fully comprehend the significance of ACT in patients presenting with VPI. Consequently, there is an imperative to develop relevant prognostic markers and models to identify patients who may not necessitate adjuvant therapy and to identify those who could benefit from such treatment. Numerous factors have been assessed, including VPI, which may influence the selection of ACT. However, the majority of currently available data are retrospective, limiting the applicability of these markers in present clinical practice (90).
Some scholars have identified risk factors influencing postoperative recurrence and have constructed predictive models for recurrence, aiming to inform the selection of ACT for stage IB NSCLC patients (91–96). However, relatively limited research has been conducted on predictive models for postoperative recurrence in patients with stage IB NSCLC featuring VPI. Ren et al. (97) developed a scale that provides personalized predictions of recurrence-free survival following resection in patients with stage I lung adenocarcinoma. High-risk patients with a score ≥245 may derive benefits from postoperative ACT. Similarly, the identification of >4 circulating tumor cells defines a high-risk subgroup, offering a novel strategy for optimal clinical decision-making in stage IB lung adenocarcinoma (98). Furthermore, nomograms offer more accurate prognostic predictions for patients with resected stage IB NSCLC (81). ACT has demonstrated superior OS compared to non-ACT in patients with stage IB NSCLC. Nomogram modeling provides individualized predictions of OS in patients following surgical resection. Patients with tumor sizes ranging from 2 cm to 4 cm may represent potential candidates for adjuvant chemotherapy (99).
8 Stage IB and targeted therapy
In recent years, extensive research has been conducted on adjuvant targeted therapy following surgery for early to mid-stage NSCLC. Targeted therapy, overall, offers significant advantages over chemotherapy and is better tolerated by patients. A global multicenter phase III double-blind study known as ADAURA (100) has confirmed the considerable extension of DFS in patients with EGFR mutation-positive NSCLC (stages IB to IIIA) through the use of osimertinib. Notably, stage IB patients treated with osimertinib experienced a remarkable 60% reduction in the risk of disease progression or death. Another phase II clinical study (GASTO1003, CORIN), led by Wang et al. (101), involved 128 surgically resected stage IB patients (based on the 7th edition of TNM staging). The patients were randomly assigned to either the icotinib group (63 patients) or the observation group (65 patients). The icotinib group received one year of targeted therapy with icotinib, commencing six weeks after the surgery, while the observation group underwent observation until disease progression or intolerable adverse effects. The study concluded that postoperative adjuvant icotinib therapy significantly prolonged DFS by three years in patients with completely resected stage IB NSCLC harboring EGFR mutations. These findings were consistent when applying the 8th edition of the TNM staging system.
In summary, the CORIN study aligns with the results of the ADAURA trial, providing further evidence of the efficacy and tolerability of adjuvant EGFR-TKI as a postoperative treatment for patients with stage IB NSCLC with EGFR mutations.
9 Stage IB and immunotherapy
In the past, some studies related to immunotherapy for early-stage NSCLC have been conducted, but there are almost no studies on immunotherapy for patients with stage IB with VPI. In terms of neoadjuvant immunotherapy, the Checkmate 816 (102) study revolutionized the field of neoadjuvant immunotherapy by demonstrating that combining three cycles of preoperative nivolumab with chemotherapy significantly increased the rate of pathological complete remission compared to chemotherapy alone. Similarly, the keynote671 (103) study revealed that neoadjuvant pembrolizumab, combined with chemotherapy before resection, followed by adjuvant pembrolizumab, led to significant improvements in event-free survival, major pathologic response, and pathological complete remission when compared to neoadjuvant chemotherapy alone followed by surgery. Turning to adjuvant immunotherapy, Felip et al. (104) conducted the IMpower010 study, a randomized phase 3 trial, which demonstrated that adjuvant immunotherapy with atezolizumab, following adjuvant chemotherapy, significantly enhanced DFS in patients with completely resected stage II-IIIA NSCLC. This study offered a promising therapeutic option for surgically resected early-stage NSCLC patients. However, it did not provide detailed survival data specifically for stage IB. Subsequent findings from the randomized phase III clinical trial PEARLS/KEYNOTE-091 indicated that pembrolizumab substantially improved DFS compared to placebo, potentially serving as a novel therapeutic approach for stage IB-IIIA NSCLC after complete resection (105).
Overall, numerous studies have demonstrated the advantages of immunotherapy; nevertheless, the current research on immunotherapy for stage IB patients remains limited. Furthermore, although previous studies addressed this stage, their classification was based on the 7th edition of the TNM staging system. According to the 8th edition, the majority of stage IB patients should be reclassified as stage IIA.
10 Visceral pleural invasion and prognosis and survival
Extensive research has been conducted to investigate the relationship between VPI and prognosis. One study indicated that VPI does not significantly impact OS in patients with early-stage NSCLC (106). Building upon this finding, Seok et al. (107) conducted a retrospective study in 2017, examining the survival of 90 patients with N0 NSCLC and concurrent VPI. Ultimately, they concluded that the extent of VPI may not affect the prognosis of N0 NSCLC patients who undergo surgical resection with VPI. However, this study has certain limitations, including a small sample size and a short postoperative follow-up period, which may introduce errors in the results.
On the contrary, Oyama et al. (108) held a different perspective. They included 1,488 patients with NSCLC who underwent surgical resection and examined the extent and location of VPI. By comparing various clinicopathological factors, they found a significant difference in the prognosis of patients with PL0 and PL1-3 tumors. Additionally, they observed no difference in prognosis among the PL1, PL2, and PL3 groups. However, it was also demonstrated that patients with PL0 and PL1 degrees of VPI exhibited similar survival rates, suggesting that these two groups could be considered negative for VPI, while the PL2 group had an impact on patient survival (56).
In 2015, Adachi et al. (109) further identified, through a retrospective study, that VPI influenced postoperative survival in patients with NSCLC with N0 or N1 metastases, rather than the extent of invasion. Building upon previous studies, David et al. (110) analyzed 1166 patients with pN0M0 NSCLC who underwent lobectomy, including 214 patients with VPI and 952 patients without VPI. The results revealed that the impact of VPI on survival varied based on tumor size, with weak correlations between VPI and OS or DFS in tumors smaller than 5 cm.
Regarding stage IB NSCLC patients with concurrent VPI, differences in tumor location did not affect patient prognosis (111). Additionally, total pleural adhesions were not found to be a risk factor for recurrence after lobectomy for stage I NSCLC with VPI (112). A study by Ahn et al. (113) revealed that percutaneous lung puncture biopsy did not significantly increase the risk of pleural recurrence in stage I NSCLC; however, VPI was identified as the cause of pleural recurrence.
11 Limitations and prospects
VPI is a significant negative prognostic factor in NSCLC. Despite numerous studies conducted on this topic, there are still limitations and opportunities for improvement. For tumors with a diameter of less than 3 cm, the presence of VPI leads to a final pathological stage of IB. Although studies have examined the extent of VPI, the impact of different degrees of invasion on patient prognosis and tumor staging for small tumors remains unclear. Moreover, there is a lack of research on tumor penetration of the inner and outer elastic layers of the visceral pleural, which hampers our understanding of the relationship between these two levels of invasion and prognosis. Therefore, further data collection is necessary.
The existing studies on this subject have yielded varied results, emphasizing the need for comprehensive investigations in the future. Such studies should consider multiple factors, including VPI, histological type of NSCLC, tumor differentiation, nodule characteristics, lymph node involvement, inflammatory markers, tumor gene mutations (such as EGFR gene mutations), and the degree of VPI. By collectively exploring the impact of these factors on the survival prognosis of this patient population, we can gain a more comprehensive understanding.
Furthermore, accurate determination of VPI is crucial. While elastic staining is widely acknowledged as a diagnostic method for VPI, its utilization in actual pathology remains relatively uncommon, and there is no consensus on the choice of staining method. Considering the importance of VPI in guiding clinical staging and postoperative adjuvant therapy, a combination of elastic staining and immunohistochemistry can be considered for diagnosing VPI.
12 Summary
In conclusion, VPI plays a pivotal role in the unfavorable prognosis of individuals diagnosed with NSCLC. The accurate identification of VPI holds the utmost importance in determining prognostic outcomes and guiding therapeutic interventions for this condition. To precisely evaluate the influence of VPI on the survival and prognosis of patients with stage IB NSCLC, a comprehensive examination of various factors, including tumor characteristics, surgical approaches, and the nature of the nodule, is warranted. However, previous studies have predominantly focused on the prognostic impact of VPI alone, neglecting the exploration of comprehensive factors encompassing tumor-related aspects, surgical techniques, and the degree of VPI. We strongly believe that future large-scale multicenter prospective studies will enable a more accurate prediction of prognosis in patients with stage IB NSCLC featuring VPI.
Author contributions
ZR: Writing – original draft. XZ: Writing – original draft. CX: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
NSCLC, non-small cell lung cancer; VPI, visceral pleural invasion; CT, computed tomography; ACT, adjuvant chemotherapy; DFS, disease-free survival; OS, overall survival; EGFR, epithelial growth factor receptor.
References
1. Deo SVS, Sharma J, Kumar S. Globocan 2020 report on global cancer burden: challenges and opportunities for surgical oncologists. Ann Surg Oncol (2022) 29(11):6497–500. doi: 10.1245/s10434-022-12151-6
2. Rami-Porta R, Bolejack V, Crowley J, Ball D, Kim J, Lyons G, et al. The iaslc lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the tnm classification for lung cancer. J Thorac Oncol (2015) 10(7):990–1003. doi: 10.1097/jto.0000000000000559
3. Kang JH, Kim KD, Chung KY. Prognostic value of visceral pleura invasion in non-small cell lung cancer. Eur J Cardiothorac Surg (2003) 23(6):865–9. doi: 10.1016/s1010-7940(03)00119-2
4. Chen T, Luo J, Wang R, Gu H, Gu Y, Huang Q, et al. Visceral pleural invasion predict a poor survival among lung adenocarcinoma patients with tumor size ≤ 3cm. Oncotarget (2017) 8(39):66576–83. doi: 10.18632/oncotarget.16476
5. Tian D, Pei Y, Zheng Q, Zhang J, Li S, Wang X, et al. Effect of visceral pleural invasion on the prognosis of patients with lymph node negative non-small cell lung cancer. Thorac Cancer (2017) 8(2):97–105. doi: 10.1111/1759-7714.12412
6. Yang X, Sun F, Chen L, Shi M, Shi Y, Lin Z, et al. Prognostic value of visceral pleural invasion in non-small cell lung cancer: A propensity score matching study based on the seer registry. J Surg Oncol (2017) 116(3):398–406. doi: 10.1002/jso.24677
7. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest (2017) 151(1):193–203. doi: 10.1016/j.chest.2016.10.010
8. Charalampidis C, Youroukou A, Lazaridis G, Baka S, Mpoukovinas I, Karavasilis V, et al. Pleura space anatomy. J Thorac Dis (2015) 7(Suppl 1):S27–32. doi: 10.3978/j.issn.2072-1439.2015.01.48
9. Wang NS. Anatomy of the pleura. Clin Chest Med (1998) 19(2):229–40. doi: 10.1016/s0272-5231(05)70074-5
10. Osaki T, Nagashima A, Yoshimatsu T, Yamada S, Yasumoto K. Visceral pleural involvement in nonsmall cell lung cancer: prognostic significance. Ann Thorac Surg (2004) 77(5):1769–73. doi: 10.1016/j.athoracsur.2003.10.058
11. Shimizu K, Yoshida J, Nagai K, Nishimura M, Yokose T, Ishii G, et al. Visceral pleural invasion classification in non-small cell lung cancer: A proposal on the basis of outcome assessment. J Thorac Cardiovasc Surg (2004) 127(6):1574–8. doi: 10.1016/j.jtcvs.2003.11.017
12. Shim HS, Park IK, Lee CY, Chung KY. Prognostic significance of visceral pleural invasion in the forthcoming (Seventh) edition of tnm classification for lung cancer. Lung Cancer (2009) 65(2):161–5. doi: 10.1016/j.lungcan.2008.11.008
13. Travis WD, Brambilla E, Rami-Porta R, Vallières E, Tsuboi M, Rusch V, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the tnm classification for lung cancer. J Thorac Oncol (2008) 3(12):1384–90. doi: 10.1097/JTO.0b013e31818e0d9f
14. Hsu JS, Han IT, Tsai TH, Lin SF, Jaw TS, Liu GC, et al. Pleural tags on ct scans to predict visceral pleural invasion of non-small cell lung cancer that does not abut the pleura. Radiology (2016) 279(2):590–6. doi: 10.1148/radiol.2015151120
15. Qi LP, Li XT, Yang Y, Chen JF, Wang J, Chen ML, et al. Multivariate analysis of pleural invasion of peripheral non-small cell lung cancer-based computed tomography features. J Comput Assist Tomogr (2016) 40(5):757–62. doi: 10.1097/rct.0000000000000439
16. Onoda H, Higashi M, Murakami T, Tao H, Yokoyama S, Kunihiro Y, et al. Correlation between pleural tags on ct and visceral pleural invasion of peripheral lung cancer that does not appear touching the pleural surface. Eur Radiol (2021) 31(12):9022–9. doi: 10.1007/s00330-021-07869-y
17. Deng HY, Li G, Luo J, Alai G, Zhuo ZG, Lin YD. Novel biologic factors correlated to visceral pleural invasion in early-stage non-small cell lung cancer less than 3 cm. J Thorac Dis (2018) 10(4):2357–64. doi: 10.21037/jtd.2018.03.185
18. Iizuka S, Kawase A, Oiwa H, Ema T, Shiiya N, Funai K. A risk scoring system for predicting visceral pleural invasion in non-small lung cancer patients. Gen Thorac Cardiovasc Surg (2019) 67(10):876–9. doi: 10.1007/s11748-019-01101-x
19. Takizawa H, Kondo K, Kawakita N, Tsuboi M, Toba H, Kajiura K, et al. Autofluorescence for the diagnosis of visceral pleural invasion in non-small-cell lung cancer. Eur J Cardiothorac Surg (2018) 53(5):987–92. doi: 10.1093/ejcts/ezx419
20. Bunker ML, Raab SS, Landreneau RJ, Silverman JF. The diagnosis and significance of visceral pleural invasion in lung carcinoma. Histologic Predictors Role Elastic Stains Am J Clin Pathol (1999) 112(6):777–83. doi: 10.1093/ajcp/112.6.777
21. Xie LW, Wang J. Evaluating three elastic-fiber staining methods for detecting visceral pleural invasion in lung adenocarcinoma patients. Clin Lab (2021) 67(7):1570–1575. doi: 10.7754/Clin.Lab.2020.200851
22. Butnor KJ, Cooper K. Visceral pleural invasion in lung cancer: recognizing histologic parameters that impact staging and prognosis. Adv Anat Pathol (2005) 12(1):1–6. doi: 10.1097/01.pap.0000151266.26814.02
23. Taube JM, Askin FB, Brock MV, Westra W. Impact of elastic staining on the staging of peripheral lung cancers. Am J Surg Pathol (2007) 31(6):953–6. doi: 10.1097/PAS.0b013e31802ca413
24. Jung G, Hwang HS, Jang SJ, Ro JY. Are elastic stain and specialty sign out necessary to evaluate pleural invasion in lung cancers? Ann Diagn Pathol (2012) 16(4):250–4. doi: 10.1016/j.anndiagpath.2011.10.006
25. Li S, Huang Y, Zhang L, Dong Z, Wu W, Zhang W, et al. Clinical significance of dual-block elastic stain evaluating visceral pleural invasion in peripheral non-small cell lung cancer. Int J Surg Pathol (2023) 31(2):175–83. doi: 10.1177/10668969221098089
27. Frederick L, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. Ajcc cancer staging manual. Berlin/Heidelberg, Germany: Springer Science & Business Media (2002).
28. Goldstraw P. The 7th edition of tnm in lung cancer: what now? J Thorac Oncol (2009) 4(6):671–3. doi: 10.1097/JTO.0b013e31819e7814
29. Warth A, Muley T, Herpel E, Pfannschmidt J, Hoffmann H, Dienemann H, et al. A histochemical approach to the diagnosis of visceral pleural infiltration by non-small cell lung cancer. Pathol Oncol Res (2010) 16(1):119–23. doi: 10.1007/s12253-009-9201-x
30. Wang T, Zhou C, Zhou Q. Extent of visceral pleural invasion affects prognosis of resected non-small cell lung cancer: A meta-analysis. Sci Rep (2017) 7(1):1527. doi: 10.1038/s41598-017-01845-7
31. Shimizu K, Yoshida J, Nagai K, Nishimura M, Ishii G, Morishita Y, et al. Visceral pleural invasion is an invasive and aggressive indicator of non-small cell lung cancer. J Thorac Cardiovasc Surg (2005) 130(1):160–5. doi: 10.1016/j.jtcvs.2004.11.021
32. Zhang X, Xie J, Hu S, Peng W, Xu B, Li Y, et al. Prognostic value of visceral pleural invasion in the stage pt(1-2)N(2)M(0) non-small cell lung cancer: A study based on the seer registry. Curr Probl Cancer (2021) 45(1):100640. doi: 10.1016/j.currproblcancer.2020.100640
33. Sakakura N, Mori S, Okuda K, Fukui T, Hatooka S, Shinoda M, et al. Subcategorization of lung cancer based on tumor size and degree of visceral pleural invasion. Ann Thorac Surg (2008) 86(4):1084–90. doi: 10.1016/j.athoracsur.2008.04.117
34. Hung JJ, Wang CY, Huang MH, Huang BS, Hsu WH, Wu YC. Prognostic factors in resected stage I non-small cell lung cancer with a diameter of 3 cm or less: visceral pleural invasion did not influence overall and disease-free survival. J Thorac Cardiovasc Surg (2007) 134(3):638–43. doi: 10.1016/j.jtcvs.2007.04.059
35. Nitadori JI, Colovos C, Kadota K, Sima CS, Sarkaria IS, Rizk NP, et al. Visceral pleural invasion does not affect recurrence or overall survival among patients with lung adenocarcinoma ≤ 2 cm: A proposal to reclassify T1 lung adenocarcinoma. Chest (2013) 144(5):1622–31. doi: 10.1378/chest.13-0394
36. Kudo Y, Saji H, Shimada Y, Nomura M, Matsubayashi J, Nagao T, et al. Impact of visceral pleural invasion on the survival of patients with non-small cell lung cancer. Lung Cancer (2012) 78(2):153–60. doi: 10.1016/j.lungcan.2012.08.004
37. Kawase A, Yoshida J, Miyaoka E, Asamura H, Fujii Y, Nakanishi Y, et al. Visceral pleural invasion classification in non-small-cell lung cancer in the 7th edition of the tumor, node, metastasis classification for lung cancer: validation analysis based on a large-scale nationwide database. J Thorac Oncol (2013) 8(5):606–11. doi: 10.1097/JTO.0b013e31828632b8
38. Liu QX, Deng XF, Zhou D, Li JM, Min JX, Dai JG. Visceral pleural invasion impacts the prognosis of non-small cell lung cancer: A meta-analysis. Eur J Surg Oncol (2016) 42(11):1707–13. doi: 10.1016/j.ejso.2016.03.012
39. Fibla JJ, Cassivi SD, Brunelli A, Decker PA, Allen MS, Darling GE, et al. Re-evaluation of the prognostic value of visceral pleura invasion in stage ib non-small cell lung cancer using the prospective multicenter acosog Z0030 trial data set. Lung Cancer (2012) 78(3):259–62. doi: 10.1016/j.lungcan.2012.09.010
40. Yip R, Ma T, Flores RM, Yankelevitz D, Henschke CI. Survival with parenchymal and pleural invasion of non-small cell lung cancers less than 30 mm. J Thorac Oncol (2019) 14(5):890–902. doi: 10.1016/j.jtho.2019.01.013
41. Qi M, Bian D, Zhang J, Zhu X, Zhou C, Zhang L. The modification of T description according to visceral pleural invasion and tumor size from 3.1 Cm to 4.0 Cm in non-small cell lung cancer: A retrospective analysis based on the seer database. Lung Cancer (2021) 158:47–54. doi: 10.1016/j.lungcan.2021.06.003
42. Cai JS, Wang X. Investigation of early-stage non-small cell lung cancer patients with different T2 descriptors: real word data from a large database. Lung (2023) 201(4):415–23. doi: 10.1007/s00408-023-00635-5
43. Fang P, Cheng J, Lu Y, Fu L. Rethinking the selection of pathological T-classification for non-small-cell lung cancer in varying degrees of visceral pleural invasion: A seer-based study. Front Surg (2022) 9:902710. doi: 10.3389/fsurg.2022.902710
44. Sakakura N, Mizuno T, Kuroda H, Arimura T, Yatabe Y, Yoshimura K, et al. The eighth tnm classification system for lung cancer: A consideration based on the degree of pleural invasion and involved neighboring structures. Lung Cancer (2018) 118:134–8. doi: 10.1016/j.lungcan.2018.02.009
45. Liang RB, Li P, Li BT, Jin JT, Rusch VW, Jones DR, et al. Modification of pathologic T classification for non-small cell lung cancer with visceral pleural invasion: data from 1,055 cases of cancers ≤ 3 cm. Chest (2021) 160(2):754–64. doi: 10.1016/j.chest.2021.03.022
46. Cai JS, Dou XM. Non-small cell lung cancer surpassing the elastic layer should remain classified as pt2a. Semin Thorac Cardiovasc Surg (2023) 35(3):583–93. doi: 10.1053/j.semtcvs.2022.04.009
47. Wang C, Wu Y, Li J, Ren P, Gou Y, Shao J, et al. Distinct clinicopathologic factors and prognosis based on the presence of ground-glass opacity components in patients with resected stage I non-small cell lung cancer. Ann Transl Med (2020) 8(18):1133. doi: 10.21037/atm-20-4971
48. Fu F, Zhang Y, Wen Z, Zheng D, Gao Z, Han H, et al. Distinct prognostic factors in patients with stage I non-small cell lung cancer with radiologic part-solid or solid lesions. J Thorac Oncol (2019) 14(12):2133–42. doi: 10.1016/j.jtho.2019.08.002
49. Zhao Q, Wang JW, Yang L, Xue LY, Lu WW. Ct diagnosis of pleural and stromal invasion in Malignant subpleural pure ground-glass nodules: an exploratory study. Eur Radiol (2019) 29(1):279–86. doi: 10.1007/s00330-018-5558-0
50. Hattori A, Suzuki K, Matsunaga T, Takamochi K, Oh S. Visceral pleural invasion is not a significant prognostic factor in patients with a part-solid lung cancer. Ann Thorac Surg (2014) 98(2):433–8. doi: 10.1016/j.athoracsur.2014.04.084
51. Okada S, Hattori A, Matsunaga T, Takamochi K, Oh S, Inoue M, et al. Prognostic value of visceral pleural invasion in pure-solid and part-solid lung cancer patients. Gen Thorac Cardiovasc Surg (2021) 69(2):303–10. doi: 10.1007/s11748-020-01470-8
52. Zhao LL, Xie HK, Zhang LP, Zha JY, Zhou FY, Jiang GN, et al. Visceral pleural invasion in lung adenocarcinoma ≤3 cm with ground-glass opacity: A clinical, pathological and radiological study. J Thorac Dis (2016) 8(7):1788–97. doi: 10.21037/jtd.2016.05.90
53. Zhang Y, Fu F, Wen Z, Deng L, Wang S, Li Y, et al. Segment location and ground glass opacity ratio reliably predict node-negative status in lung cancer. Ann Thorac Surg (2020) 109(4):1061–8. doi: 10.1016/j.athoracsur.2019.10.072
54. Ding N, Mao Y, Gao S, Xue Q, Wang D, Zhao J, et al. Predictors of lymph node metastasis and possible selective lymph node dissection in clinical stage ia non-small cell lung cancer. J Thorac Dis (2018) 10(7):4061–8. doi: 10.21037/jtd.2018.06.129
55. Wo Y, Zhao Y, Qiu T, Li S, Wang Y, Lu T, et al. Impact of visceral pleural invasion on the association of extent of lymphadenectomy and survival in stage I non-small cell lung cancer. Cancer Med (2019) 8(2):669–78. doi: 10.1002/cam4.1990
56. Tanju S, Erus S, Selçukbiricik F, İliaz S, Kapdağlı M, Bulutay P, et al. Level of pleural invasion effects on prognosis in lung cancer. Tumori (2019) 105(2):155–60. doi: 10.1177/0300891618792463
57. Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and ii non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: american college of chest physicians evidence-based clinical practice guidelines. Chest (2013) 143(5 Suppl):e278S–313S. doi: 10.1378/chest.12-2359
58. Martini N, Bains MS, Burt ME, Zakowski MF, McCormack P, Rusch VW, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg (1995) 109(1):120–9. doi: 10.1016/s0022-5223(95)70427-2
59. Koike T, Koike T, Yoshiya K, Tsuchida M, Toyabe S. Risk factor analysis of locoregional recurrence after sublobar resection in patients with clinical stage ia non-small cell lung cancer. J Thorac Cardiovasc Surg (2013) 146(2):372–8. doi: 10.1016/j.jtcvs.2013.02.057
60. Song X, Xie Y, Zhu Y, Lou Y. Is lobectomy superior to sub-lobectomy in non-small cell lung cancer with pleural invasion? A population-based competing risk analysis. BMC Cancer (2022) 22(1):541. doi: 10.1186/s12885-022-09634-w
61. Huang W, Deng HY, Lin MY, Xu K, Zhang YX, Yuan C, et al. Treatment modality for stage ib peripheral non-small cell lung cancer with visceral pleural invasion and ≤3 cm in size. Front Oncol (2022) 12:830470. doi: 10.3389/fonc.2022.830470
62. Yu Y, Huang R, Wang P, Wang S, Ling X, Zhang P, et al. Sublobectomy versus lobectomy for long-term survival outcomes of early-stage non-small cell lung cancer with a tumor size ≤2 cm accompanied by visceral pleural invasion: A seer population-based study. J Thorac Dis (2020) 12(3):592–604. doi: 10.21037/jtd.2019.12.121
63. Mathey-Andrews C, Abruzzo AR, Venkateswaran S, Potter AL, Senthil P, Beqari J, et al. Segmentectomy vs lobectomy for early non-small cell lung cancer with visceral pleural invasion. Ann Thorac Surg (2023). doi: 10.1016/j.athoracsur.2023.06.020
64. Choi SY, Moon MH, Moon Y. The prognosis of small-sized non-small cell lung cancer with visceral pleural invasion after sublobar resection. Transl Cancer Res (2020) 9(10):6431–43. doi: 10.21037/tcr-20-1995
65. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med (2004) 350(4):351–60. doi: 10.1056/NEJMoa031644
66. Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le Chevalier T, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet (2010) 375(9722):1267–77. doi: 10.1016/s0140-6736(10)60059-1
67. Malhotra J, Mhango G, Gomez JE, Smith C, Galsky MD, Strauss GM, et al. Adjuvant chemotherapy for elderly patients with stage I non-small-cell lung cancer ≥4 cm in size: an seer-medicare analysis. Ann Oncol (2015) 26(4):768–73. doi: 10.1093/annonc/mdv008
68. Park SY, Lee JG, Kim J, Byun GE, Bae MK, Lee CY, et al. Efficacy of platinum-based adjuvant chemotherapy in T2an0 stage ib non-small cell lung cancer. J Cardiothorac Surg (2013) 8:151. doi: 10.1186/1749-8090-8-151
69. Tsutani Y, Imai K, Ito H, Miyata Y, Ikeda N, Nakayama H, et al. Adjuvant chemotherapy for high-risk pathologic stage I non-small cell lung cancer. Ann Thorac Surg (2022) 113(5):1608–16. doi: 10.1016/j.athoracsur.2021.04.108
70. Wang J, Wu N, Lv C, Yan S, Yang Y. Should patients with stage ib non-small cell lung cancer receive adjuvant chemotherapy? A comparison of survival between the 8th and 7th editions of the ajcc tnm staging system for stage ib patients. J Cancer Res Clin Oncol (2019) 145(2):463–9. doi: 10.1007/s00432-018-2801-7
71. Park HJ, Park HS, Cha YJ, Lee S, Jeung HC, Cho JY, et al. Efficacy of adjuvant chemotherapy for completely resected stage ib non-small cell lung cancer: A retrospective study. J Thorac Dis (2018) 10(4):2279–87. doi: 10.21037/jtd.2018.03.184
72. Xu F, Chen HC, Xu H, Li J, Hao X, Xing P, et al. Adjuvant chemotherapy in patients with recurrence after completely resected stage ib lung adenocarcinoma: propensity-matched analysis in a cohort of 147 recurrences. Thorac Cancer (2022) 13(22):3105–13. doi: 10.1111/1759-7714.14659
73. Li X, Zhang C, Sun Z, Yang F, Xiao R, Sui X, et al. Propensity-matched analysis of adjuvant chemotherapy for completely resected stage ib non-small-cell lung cancer patients. Lung Cancer (2019) 133:75–82. doi: 10.1016/j.lungcan.2019.04.024
74. Xie J, Zhang X, Hu S, Peng WD, Xu B, Li Y, et al. Effects of adjuvant chemotherapy on survival of patients with stage ib non-small cell lung cancer with visceral pleural invasion. J Cancer Res Clin Oncol (2020) 146(9):2231–9. doi: 10.1007/s00432-020-03276-w
75. Lee PH, Chiang CJ, Tseng JS, Zheng ZR, Chen KC, Chu CH, et al. Adjuvant chemotherapy compared with observation in patients with T2an0 stage ib lung adenocarcinoma. Front Oncol (2023) 13:1096683. doi: 10.3389/fonc.2023.1096683
76. Chang YL, Lin MW, Shih JY, Wu CT, Lee YC. The significance of visceral pleural surface invasion in 321 cases of non-small cell lung cancers with pleural retraction. Ann Surg Oncol (2012) 19(9):3057–64. doi: 10.1245/s10434-012-2354-y
77. Lakha S, Gomez JE, Flores RM, Wisnivesky JP. Prognostic significance of visceral pleural involvement in early-stage lung cancer. Chest (2014) 146(6):1619–26. doi: 10.1378/chest.14-0204
78. Jiang L, Liang W, Shen J, Chen X, Shi X, He J, et al. The impact of visceral pleural invasion in node-negative non-small cell lung cancer: A systematic review and meta-analysis. Chest (2015) 148(4):903–11. doi: 10.1378/chest.14-2765
79. Huang H, Wang T, Hu B, Pan C. Visceral pleural invasion remains a size-independent prognostic factor in stage I non-small cell lung cancer. Ann Thorac Surg (2015) 99(4):1130–9. doi: 10.1016/j.athoracsur.2014.11.052
80. Choi J, Oh JY, Lee YS, Min KH, Shim JJ, Choi SI, et al. Clinical efficacy of adjuvant chemotherapy in stage ib (< 4 cm) non-small cell lung cancer patients with high-risk factors. Korean J Intern Med (2022) 37(1):127–36. doi: 10.3904/kjim.2020.011
81. Xu Y, Wan B, Zhu S, Zhang T, Xie J, Liu H, et al. Effect of adjuvant chemotherapy on survival of patients with 8th edition stage ib non-small cell lung cancer. Front Oncol (2021) 11:784289. doi: 10.3389/fonc.2021.784289
82. Zhou K, Zhao Y, Liang L, Cao J, Lin H, Peng Z, et al. Adjuvant chemotherapy may improve long-term outcomes in stage ib non-small cell lung cancer patients with previous Malignancies: A propensity score-matched analysis. Front Oncol (2022) 12:938195. doi: 10.3389/fonc.2022.938195
83. Zhang P, Duan J, Bai H, Wang Z, Gao S, Tan F, et al. Influence of adjuvant chemotherapy on survival for patients with stage ib and iia non-small cell lung cancer. Thorac Cancer (2021) 12(1):30–9. doi: 10.1111/1759-7714.13685
84. Wightman SC, Lee JY, Ding L, Atay SM, Shemanski KA, McFadden PM, et al. Adjuvant chemotherapy for visceral pleural invasion in 3-4-cm non-small-cell lung cancer improves survival. Eur J Cardiothorac Surg (2022) 62(1):ezab498. doi: 10.1093/ejcts/ezab498
85. Morgensztern D, Du L, Waqar SN, Patel A, Samson P, Devarakonda S, et al. Adjuvant chemotherapy for patients with T2n0m0 nsclc. J Thorac Oncol (2016) 11(10):1729–35. doi: 10.1016/j.jtho.2016.05.022
86. De Giglio A, Di Federico A, Gelsomino F, Ardizzoni A. Prognostic relevance of pleural invasion for resected nsclc patients undergoing adjuvant treatments: A propensity score-matched analysis of seer database. Lung Cancer (2021) 161:18–25. doi: 10.1016/j.lungcan.2021.08.017
87. Strauss GM, Herndon JE 2nd, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage ib non-small-cell lung cancer: calgb 9633 with the cancer and leukemia group B, radiation therapy oncology group, and north central cancer treatment group study groups. J Clin Oncol (2008) 26(31):5043–51. doi: 10.1200/jco.2008.16.4855
88. Hou X, Yang MZ, Li JB, Tan ZH, Long H, Fu JH, et al. Who are the real high-risk patients with pathological T2n0m0 non-small-cell lung cancer that can benefit from adjuvant chemotherapy? ESMO Open (2022) 7(3):100508. doi: 10.1016/j.esmoop.2022.100508
89. Hung JJ, Jeng WJ, Hsu WH, Chou TY, Lin SF, Wu YC. Prognostic significance of the extent of visceral pleural invasion in completely resected node-negative non-small cell lung cancer. Chest (2012) 142(1):141–50. doi: 10.1378/chest.11-2552
90. Gadgeel SM, Bepler G. Prognostic and predictive markers for personalized adjuvant therapy for non-small-cell lung cancer patients. Future Oncol (2013) 9(12):1909–21. doi: 10.2217/fon.13.160
91. Zhang Z, Xie S, Cai W, Hong ZN, Yang C, Lin Y, et al. A nomogram to predict the recurrence-free survival and analyze the utility of chemotherapy in stage ib non-small cell lung cancer. Transl Lung Cancer Res (2022) 11(1):75–86. doi: 10.21037/tlcr-21-1038
92. Zhai W, Gong L, Zheng Y, Yan Q, Lai R, Liang D, et al. Ground glass opacity and adjuvant chemotherapy in pathological stage ib-iia lung adenocarcinoma. Front Oncol (2022) 12:851276. doi: 10.3389/fonc.2022.851276
93. Sui Q, Liang J, Hu Z, Xu X, Chen Z, Huang Y, et al. The clinical prognostic factors of patients with stage ib lung adenocarcinoma. Transl Cancer Res (2021) 10(11):4727–38. doi: 10.21037/tcr-21-1174
94. Song X, Xie Y, Deng H, Yu F, Wang S, Lou Y. A novel nomogram for identifying candidates for adjuvant chemotherapy in patients with stage ib non-small cell lung cancer. Cancer Control (2023) 30:10732748231177541. doi: 10.1177/10732748231177541
95. Zuo Z, Zhang G, Song P, Yang J, Li S, Zhong Z, et al. Survival nomogram for stage ib non-small-cell lung cancer patients, based on the seer database and an external validation cohort. Ann Surg Oncol (2021) 28(7):3941–50. doi: 10.1245/s10434-020-09362-0
96. Wang F, Li P, Li F. Nomogram for predicting the relationship between the extent of visceral pleural invasion and survival in non-small-cell lung cancer. Can Respir J (2021) 2021:8816860. doi: 10.1155/2021/8816860
97. Qian J, Xu J, Wang S, Qian F, Yang W, Zhang B, et al. Adjuvant chemotherapy candidates in stage I lung adenocarcinomas following complete lobectomy. Ann Surg Oncol (2019) 26(8):2392–400. doi: 10.1245/s10434-019-07366-z
98. Ren L, Zhong X, Liu W, Xu D, Lei Y, Zhou J, et al. Clinical significance of a circulating tumor cell-based classifier in stage ib lung adenocarcinoma: A multicenter, cohort study. Ann Surg (2023) 277(2):e439–e48. doi: 10.1097/sla.0000000000004780
99. Tu Z, Tian T, Chen Q, Li C. Overall survival analyses following adjuvant chemotherapy or nonadjuvant chemotherapy in patients with stage ib non-small-cell lung cancer. J Oncol (2021) 2021:8052752. doi: 10.1155/2021/8052752
100. Wu YL, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected egfr-mutated non-small-cell lung cancer. N Engl J Med (2020) 383(18):1711–23. doi: 10.1056/NEJMoa2027071
101. Ou W, Li N, Wang BX, Zhu TF, Shen ZL, Wang T, et al. Adjuvant icotinib versus observation in patients with completely resected egfr-mutated stage ib nsclc (Gasto1003, corin): A randomised, open-label, phase 2 trial. EClinicalMedicine (2023) 57:101839. doi: 10.1016/j.eclinm.2023.101839
102. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med (2022) 386(21):1973–85. doi: 10.1056/NEJMoa2202170
103. Wakelee H, Liberman M, Kato T, Tsuboi M, Lee SH, Gao S, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med (2023) 389(6):491–503. doi: 10.1056/NEJMoa2302983
104. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage ib-iiia non-small-cell lung cancer (Impower010): A randomised, multicentre, open-label, phase 3 trial. Lancet (2021) 398(10308):1344–57. doi: 10.1016/s0140-6736(21)02098-5
105. O’Brien M, Paz-Ares L, Marreaud S, Dafni U, Oselin K, Havel L, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage ib-iiia non-small-cell lung cancer (Pearls/keynote-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol (2022) 23(10):1274–86. doi: 10.1016/s1470-2045(22)00518-6
106. Hung JJ, Jeng WJ, Hsu WH, Lin SF, Hsieh CC, Huang BS, et al. Prognostic factors in pathological stage ib nonsmall cell lung cancer greater than 3 cm. Eur Respir J (2010) 36(6):1355–61. doi: 10.1183/09031936.00014109
107. Seok Y, Jeong JY, Lee E. Extent of visceral pleural invasion and the prognosis of surgically resected node-negative non-small cell lung cancer. Thorac Cancer (2017) 8(3):197–202. doi: 10.1111/1759-7714.12424
108. Oyama M, Miyagi Maeshima A, Tochigi N, Tsuta K, Kawachi R, Sakurai H, et al. Prognostic impact of pleural invasion in 1488 patients with surgically resected non-small cell lung carcinoma. Jpn J Clin Oncol (2013) 43(5):540–6. doi: 10.1093/jjco/hyt039
109. Adachi H, Tsuboi M, Nishii T, Yamamoto T, Nagashima T, Ando K, et al. Influence of visceral pleural invasion on survival in completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg (2015) 48(5):691–7. doi: 10.1093/ejcts/ezu515
110. David E, Thall PF, Kalhor N, Hofstetter WL, Rice DC, Roth JA, et al. Visceral pleural invasion is not predictive of survival in patients with lung cancer and smaller tumor size. Ann Thorac Surg (2013) 95(6):1872–7. doi: 10.1016/j.athoracsur.2013.03.085
111. Ren J, Ren J, Wang K, Xu Y, Zhu M, Ren T, et al. The location of visceral pleural invasion in stage ib patients with non-small cell lung cancer: comparison and prognosis. Eur J Surg Oncol (2023) 49(5):950–7. doi: 10.1016/j.ejso.2023.01.022
112. Moon Y, Choi SY, Moon MH. The prognosis of stage I non-small cell lung cancer with visceral pleural invasion and whole pleural adhesion after video-assisted thoracoscopic lobectomy: A single center retrospective study. J Thorac Dis (2020) 12(10):5729–38. doi: 10.21037/jtd-20-1840
Keywords: non-small cell lung cancer, stage IB, visceral pleural invasion, adjuvant chemotherapy, treatment
Citation: Ruan Z, Zhuo X and Xu C (2024) Diagnosis, treatment, and prognosis of stage IB non-small cell lung cancer with visceral pleural invasion. Front. Oncol. 13:1310471. doi: 10.3389/fonc.2023.1310471
Received: 09 October 2023; Accepted: 28 December 2023;
Published: 15 January 2024.
Edited by:
Quincy Siu-chung Chu, University of Alberta, CanadaReviewed by:
Jing-Sheng Cai, Peking University People’s Hospital, ChinaYushun Gao, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Fabrizio Minervini, University of Lucerne, Switzerland
Copyright © 2024 Ruan, Zhuo and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenyang Xu, xuchenyang1973@163.com
 Zegang Ruan
Zegang Ruan Chenyang Xu
Chenyang Xu