- Department of Hematology, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Objective: The combination of high-dose cyclophosphamide (HD-Cy) (3g/m2) plus granulocyte colony-stimulating factor (G-CSF) and on-demand plerixafor (PXF) has been considered an effective mobilization regimen of patients with multiple myeloma(MM). However, the daily multi-injection regimen of G-CSF poses challenges. This study delves into the efficiency and cost implications of a novel approach, using HD-Cy alongside pegylated G-CSF (PEG G-CSF) and on-demand PXF. Unlike G-CSF, which necessitates daily injections, the half-life of PEG G-CSF extended allows for a single injection.
Methods: A retrospective analysis was conducted on 350 MM patients, which were categorized based on their mobilization regimens: Cy+PEG G-CSF+/-PXF (n=66), Cy+PEG G-CSF (n=91), Cy+ G-CSF (n=169), and G-CSF+PXF (n=24).
Results: Mobilization with Cy+PEG G-CSF+/-PXF(8.79)yielded a notably higher median CD34+ cell count compared to the other regimens: Cy+PEG G-CSF(4.96), Cy+G-CSF (4.65), and G-CSF+PXF (2.99) (P<0.001). The percentage of patients who achieved >6×106/kg CD34+ cells was significantly higher in the Cy+PEG G-CSF+/-PXF group (77.3%) than in the other mobilization regimens: Cy+PEG G-CSF (41.8%), Cy+ G-CSF (37.3%), and G-CSF+PXF (8.3%) (P<0.001). From a cost perspective, the Cy+PEG G-CSF+/-PXF approach was more economical than the G-CSF+PXF strategy but was marginally costlier than the other two methods. A multivariate assessment highlighted that the combination of Cy+PEG G-CSF with on-demand PXF had a superior potential to achieve the desired harvest (6×106/kg) compared to the Cy+PEG G-CSF protocol without PXF. The incremental cost-effectiveness ratio for each 1% increase in the probability of achieving a successful optimal harvest was $ 97.02 per patient. The incidence of neutropenic fever was 3.0% in the Cy+PEG G-CSF+/-PXF group.
Conclusion: The combination of on-demand PXF with HD-Cy and PEG G-CSF offers a cost-effective approach with a high mobilization success rate, manageable side effects, and the convenience of fewer injections. It stands as a promising mobilization strategy for MM patients.
Introduction
In the current landscape of novel treatments, autologous stem cell transplantation (ASCT) consistently plays a crucial role for eligible newly diagnosed multiple myeloma (MM) patients (1, 2). The advantage of tandem ASCT, especially for those at high risk, underscores the need for an optimal collection of hematopoietic stem cells (HSCs) (3, 4). Additionally, salvage ASCT has shown the potential to enhance overall survival for MM patients experiencing a relapse after an initial ASCT (5). It is important to collect enough stem cells for two or three transplants at the first mobilization. The success of ASCT largely rests on acquiring a significant number of CD34+ cells, ensuring rapid and long-lasting engraftment. While the minimum requirement for CD34+ cells stands at 2 × 106/kg for a single ASCT and 4 × 106/kg for tandem ASCT, many consider the safer benchmarks to be 3 and 6 × 106/kg, respectively (6, 7). For younger patients, aiming for a higher count becomes imperative, anticipating potential needs during relapse. The International Myeloma Working Group (IMWG) recommends a minimum target of 4 × 106 CD34+ cells/kg. However, ideally, securing an average of 8-10×106 CD34+ cells/kg ensures most MM patients are equipped for two ASCTs during their treatment journey (8).
Effective and economical collection of ample HSCs, with minimal complications and fewer apheresis sessions, is the ultimate objective of mobilization. Currently, granulocyte-colony stimulating factor (G-CSF) is a common method, either standalone or paired with chemotherapy, for HSC collection. Yet, G-CSF monotherapy mobilization often faces a high failure rate, identified by a yield falling below 2 × 106 CD34+/kg (9). One previous study indicated that a mere 34% of MM patients relying on G-CSF-only mobilization achieved the target of ≥ 6 × 106/kg CD34+ cells (10). Adding high-dose cyclophosphamide (HD-Cy) to G-CSF does enhance HSC yield (11), but about 30% of patients still struggle to secure more than 4 × 106/kg CD34+ stem cells (12). Regrettably, a smaller fraction even hits the 6 or 8 × 106/kg mark, which is insufficient for two transplants. Failed initial mobilizations often necessitate a subsequent mobilization using backup regimens. However, these can lead to a higher rate of mobilization failure, heightened toxicity, morbidity, and costs (13–15). Consequently, patients who can not mobilize effectively miss out on ASCT and adequate treatment. Plerixafor (PXF), a CXCR4 antagonist, is an effective mobilizing agent. However, the cost of PXF is high. If universally adopted in initial MM patient mobilizations, the costs related to HSC harvesting would surge (16). The strategy of administering PXF ‘on-demand’, following an initial mobilization using either standalone G-CSF or a combination of chemotherapy and G-CSF, targets those patients displaying early indicators of inadequate mobilization. By reserving PXF for these specific cases, potential healthcare costs can be curtailed, making this approach increasingly favored in contemporary medical practice (17, 18). Research suggests that the mobilization efficiency of the combined regimen of Cy, G-CSF, and on-demand PXF surpasses that of the combination of G-CSF and on-demand PXF (19–21). However, the necessity for daily, multiple injections with G-CSF poses a challenge. Pegylated granulocyte colony-stimulating factor (PEG G-CSF) is a pegylated form of G-CSF. Its enhanced half-life (33 vs. 3-4 h) translates to the convenience of a single dose administration (22, 23). Multiple investigations affirm the efficacy of PEG G-CSF in ensuring an optimal HSC yield for ASCT. Notably, these studies suggest that PEG G-CSF not only presents a manageable side-effect profile but also outperforms standard G-CSF in cost-effectiveness and patient convenience (24–27). In addition, Luciano et al. highlighted that a solitary 12 mg dose of PEG G-CSF exhibited superior HSC mobilization capabilities compared to G-CSF. This not only streamlined the mobilization process but also reduced the dependency on PXF for patients diagnosed with either multiple myeloma or lymphoma (25).
Therefore, we postulate that combining HD-Cy with PEG G-CSF and on-demand PXF can enhance the efficiency and convenience of HSC mobilization while curtailing associated costs. Given the current paucity of prospective study on this topic, we offer a retrospective study, drawing from real-world data. Specifically, we contrast the outcomes of four distinct mobilization regimens: Cy+PEG G-CSF+/-PXF, Cy+PEG G-CSF, Cy+G-CSF, and G-CSF+PXF, all administered to MM patients poised for ASCT.
Methods
Patients
This retrospective study compared the use of four different mobilizing strategies in patients with MM eligible for ASCT at The First Affiliated Hospital of Sun Yat-sen University: Cy+PEG G-CSF+/-PXF (n=66) versus Cy+PEG G-CSF (n=91) versus Cy+ G-CSF (n=169) versus G-CSF+PXF (n=24). The analysis included patients with MM eligible for ASCT who underwent the first stem cell mobilization attempt (those with a second mobilization for salvage ASCT were excluded) between August 2008 and March 2023. The eligibility criteria included the following: (i) diagnosed with MM as defined by the International Myeloma Working Groups after induction treatment; (ii) 18–70 years old; (iii) Eastern Cooperative Oncology Group (ECOG) performance status ≤ 3; (iv) normal kidney and live function; and (v) no history of cardiac arrhythmias, congestive heart failure, or severe coronary artery disease. This study was conducted according to the Declaration of Helsinki and approved by the institutional review board of First Affiliated Hospital of Sun Yat-sen University.
Mobilization and apheresis
Four groups of patients received the following mobilization regimens:
Cy+G-CSF: Patients received Cy 3 g/m2 and on the next day, they began G-CSF 300 μg/d, which was continued until collection was complete. Cy+ PEG G-CSF: Patients received Cy 3 g/m2 and one dose of PEG G-CSF 6 mg was administered 24 hours after Cy. Cy+PEG G-CSF+/-PXF: Patients received Cy 3 g/m2 and one dose of PEG G-CSF 6 mg was administered 24 hours after Cy. On-demand plerixafor (20 mg or 0.16 mg/kg in case of reduced renal function) was reserved for patients with an absolute CD34+ cell count of <20 cells/uL before apheresis or patients with a Day 1 CD34+ yield of < 3×106/kg CD34+ cells. We administered mesna, hydration, alkalization of urine, and diuretic to each patient during CTX mobilization to prevent hemorrhagic cystitis. Daily monitoring of CD34+ cells in peripheral blood was performed by flow cytometry, starting from day +8. The flow cytometry laboratories involved in this study regularly participated in the external quality control of the CD34+ cell count. Apheresis began on Days 9-12 when the CD34+ count was >10 cells/uL or the WBC count was >2 × 106/L and continued daily for up to 4 days or until more than or equal to 6 × 106 CD34+ cells/kg were collected.
G-CSF+ PXF: Patients received 300 μg/d G-CSF for 4 consecutive days. All patients received a subcutaneous injection of PXF (20 mg or 0.16 mg/kg in case of reduced renal function) at 22:00 on Day 4, regardless of the CD34+ cell count. Apheresis was started at 9:00 on the 5th day. A collection endpoint of at least 2 × 106/kg CD34+ cells sometimes required more than a single harvesting procedure. The target for 2 ASCT was defined as at least 6 × 106/kg CD34+ cells.
Study endpoints
The primary study endpoint was a comparison of the four mobilization strategies in terms of the percentage of patients achieving the stem cell dose for double ASCT (6×106/kg CD34+). The secondary endpoints were defined as follows: (1) percentage of patients who had a stem cell dose collected ≥2 × 106/kg; (2) total number of stem cells collected and Day 1+Day 2 stem cells collected; (3) rate of on-demand PXF administration; (4) incidence of adverse events occurring during mobilization; (5) factors influencing stem cell mobilization outcomes; and (6) total cost of mobilization and apheresis;(6) incremental cost-effectiveness ratios.
Costs and cost-effectiveness ratio
All charges and quantities for the mobilizing agents and apheresis were collected from the centralized computer billing system of the hospitals. Only direct medical costs were included in our cost analysis, i.e., costs related to mobilizing drugs, hospitalization, apheresis, CD 34 + cell testing, the cost of the infusion of blood components, and the cost of prophylactic antibiotics. The calculation for the incremental cost effectiveness ratio (ICER) refers to the study of Milone et al. (12).
Statistical analysis
Categorical variables are presented as counts and percentages, while continuous variables are expressed as mean ± standard deviation. The normality of data distribution was assessed using the Shapiro-Wilk test. For comparisons between two groups: Data following a normal distribution were analyzed using the T-test. The Wilcoxon test was applied to non-normally distributed data. Proportional data were assessed with the chi-square test or Fisher’s exact test, as appropriate. For comparisons among four groups: Univariate ANOVA was employed to evaluate continuous data (like age) differences. When assessing differences across the groups, Bonferroni correction was applied for pairwise comparisons. Proportional data were evaluated with the chi-square test or Fisher’s exact test. For datasets diverging from normal distribution: The Bonferroni approach was utilized for multiple sample rate pairwise comparisons. The Kruskal-Wallis H test was chosen for non-normally distributed data. To calculate adjusted odds ratios and identify potential confounders, significant variables from univariate analyses were incorporated into multivariate logistic regression models. Two distinct stem cell dose thresholds (2 and 6 ×106/kg) served as dependent variables. A significance level of P < 0.05 was used for all statistical tests. All statistical evaluations were conducted using SPSS version 22.0.
Results
Patient characteristics
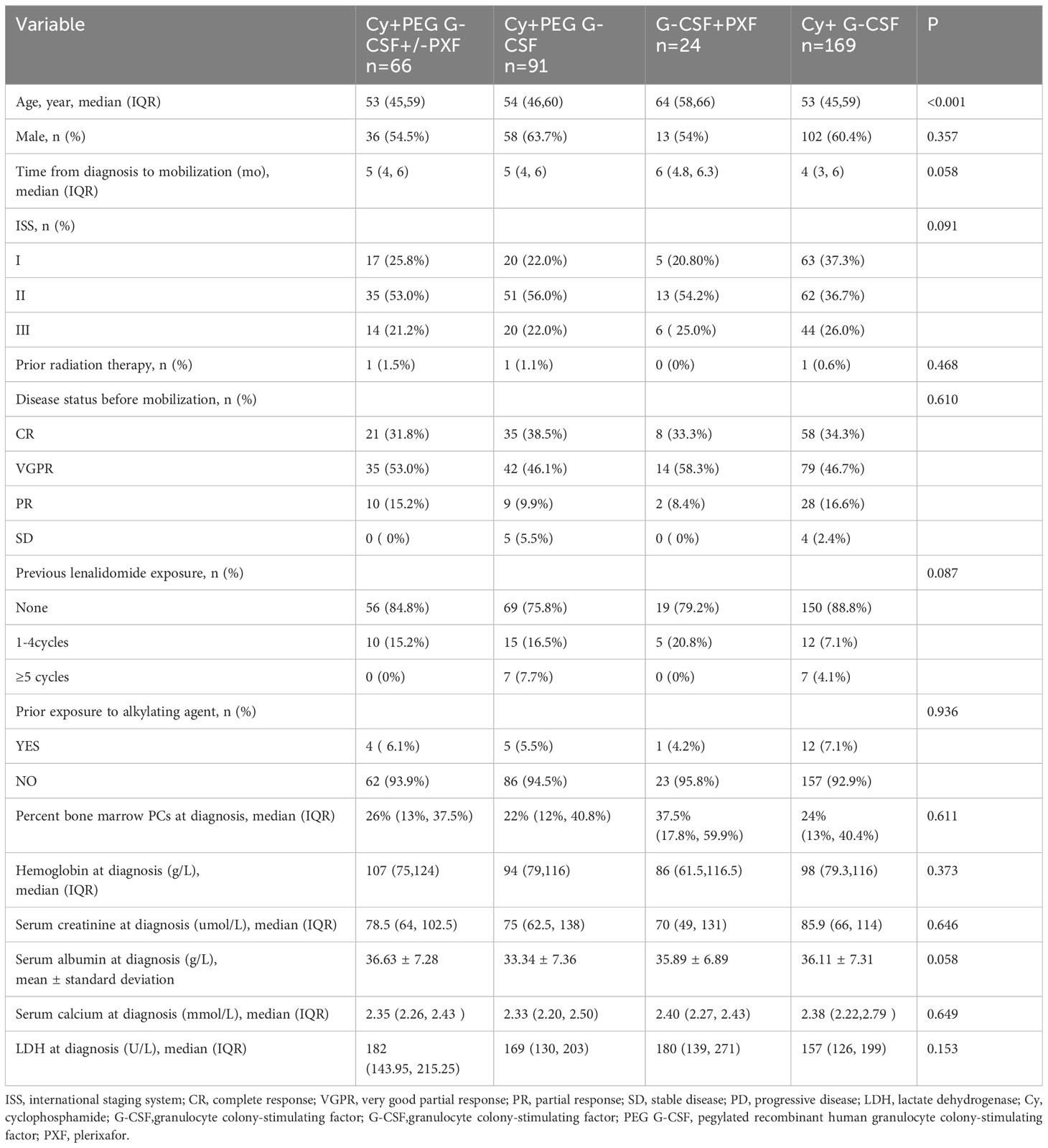
A total of 350 patients met the inclusion criteria of the study, including 66 patients in the Cy+PEG G-CSF+/-PXF group, 91 patients in the Cy+PEG G-CSF group, 169 patients in the Cy+ G-CSF group, and 24 patients in the G-CSF+PXF group. A comprehensive overview of patient demographics is summarized in Table 1. The patients in the G-CSF+PXF group were older than those in the other three groups. Other main characteristics remained largely consistent across all study cohorts.
Efficacy of stem cell mobilization and apheresis results
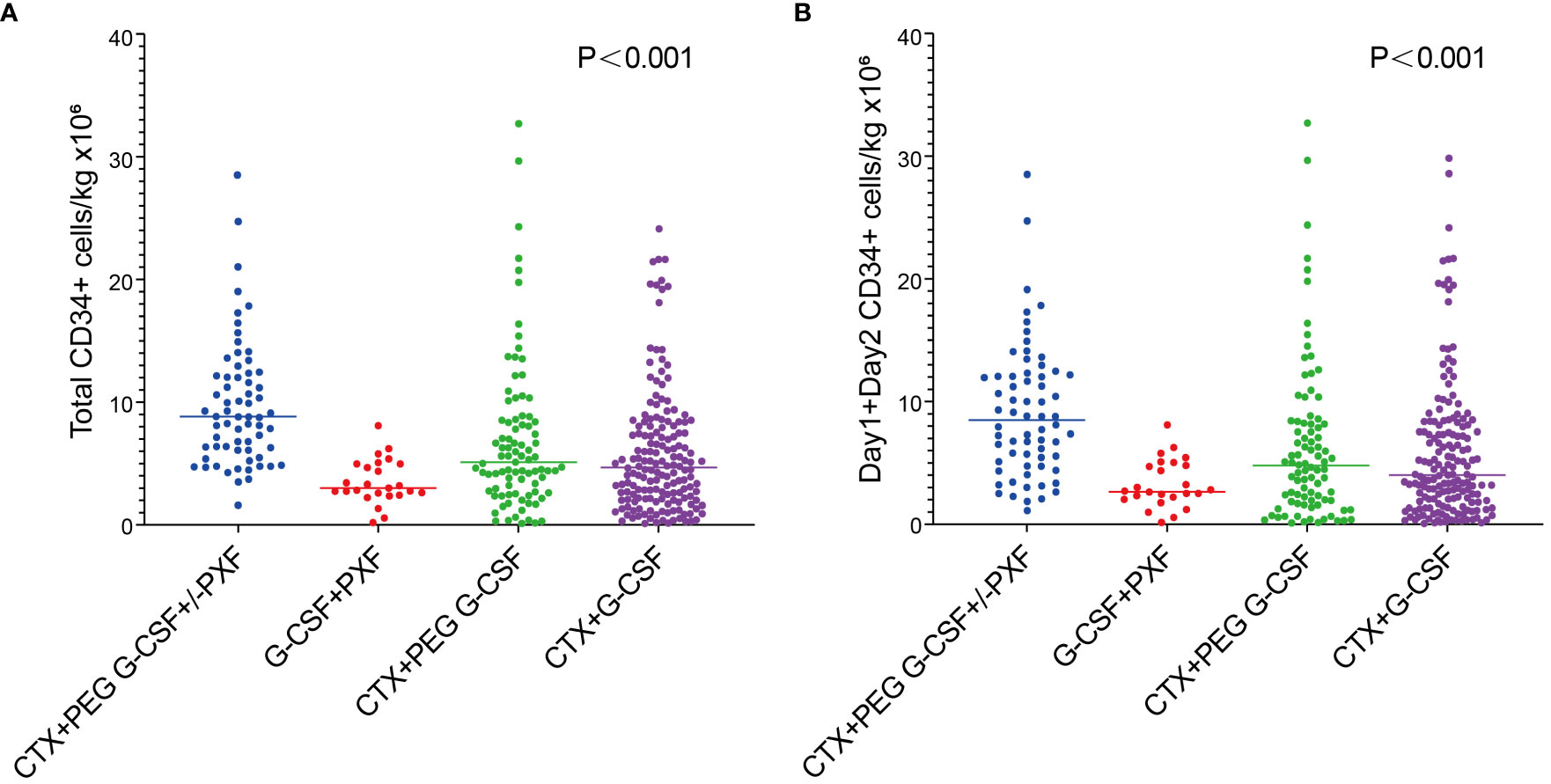
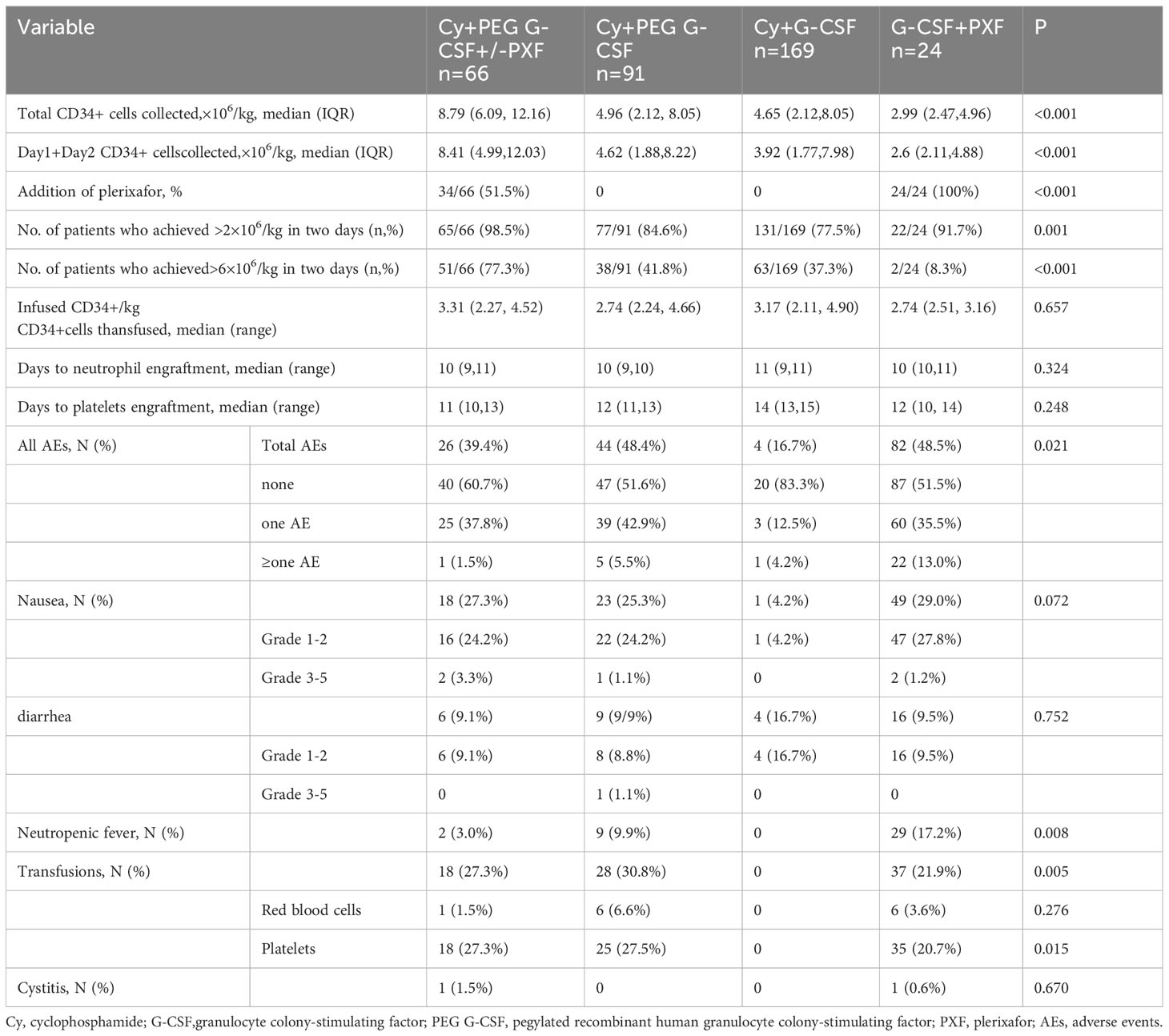
The percentage of patients who achieved > 6 × 106/kg CD34+ cells, the target dose for double ASCT, was markedly higher in the Cy+PEG G-CSF+/-PXF group (77.3%) than in the other mobilization regimens: Cy+PEG G-CSF (41.8%; P<0.001), Cy+ G-CSF (37.3%; P<0.001), and G-CSF+PXF (8.3%; P<0.001). A harvest of at least 2 ×106/kg CD34+ cell, the minimal target dose for single ASCT, was reached in 98.5% of the Cy+PEG G-CSF+/-PXF group, 84.6% of the Cy+PEG G-CSF group, 77.5% of the Cy+G-CSF group, and 91.7% of the G-CSF+PXF group (P= 0.001).The median aggregate CD34+ cell yield (×106/kg) across the groups was as follows: Cy+PEG G-CSF+/-PXF at 8.79, Cy+PEG G-CSF at 4.96, Cy+G-CSF at 4.65, and G-CSF+PXF at 2.99 (P<0.001) (Figure 1A). Assessing the median yields on Day 1 and Day 2 combined, values (×106/kg) were: Cy+PEG G-CSF+/-PXF at 8.41, Cy+PEG G-CSF at 4.62, Cy+G-CSF at 3.92, and G-CSF+PLX at 2.6 (P<0.001) (Figure 1B). In our cohort, PXF was deployed for 51.5% of patients in the Cy+PEG G-CSF+/-PXF group with an adaptive strategy and universally for those in the G-CSF+PXF group. On proceeding to ASCT, the average infused CD34+ cell count (×106/kg) was PEG G-CSF+/-PXF at 3.31, Cy+PEG G-CSF at 2.74, Cy+G-CSF at 3.17, and G-CSF+PXF at 2.74 (P=0.657). There was no statistical difference in the median time to neutrophil (P=0.324)and platelet engraftment across the groups (P=0.248) (Table 2).
Factors influencing stem cell mobilization outcomes
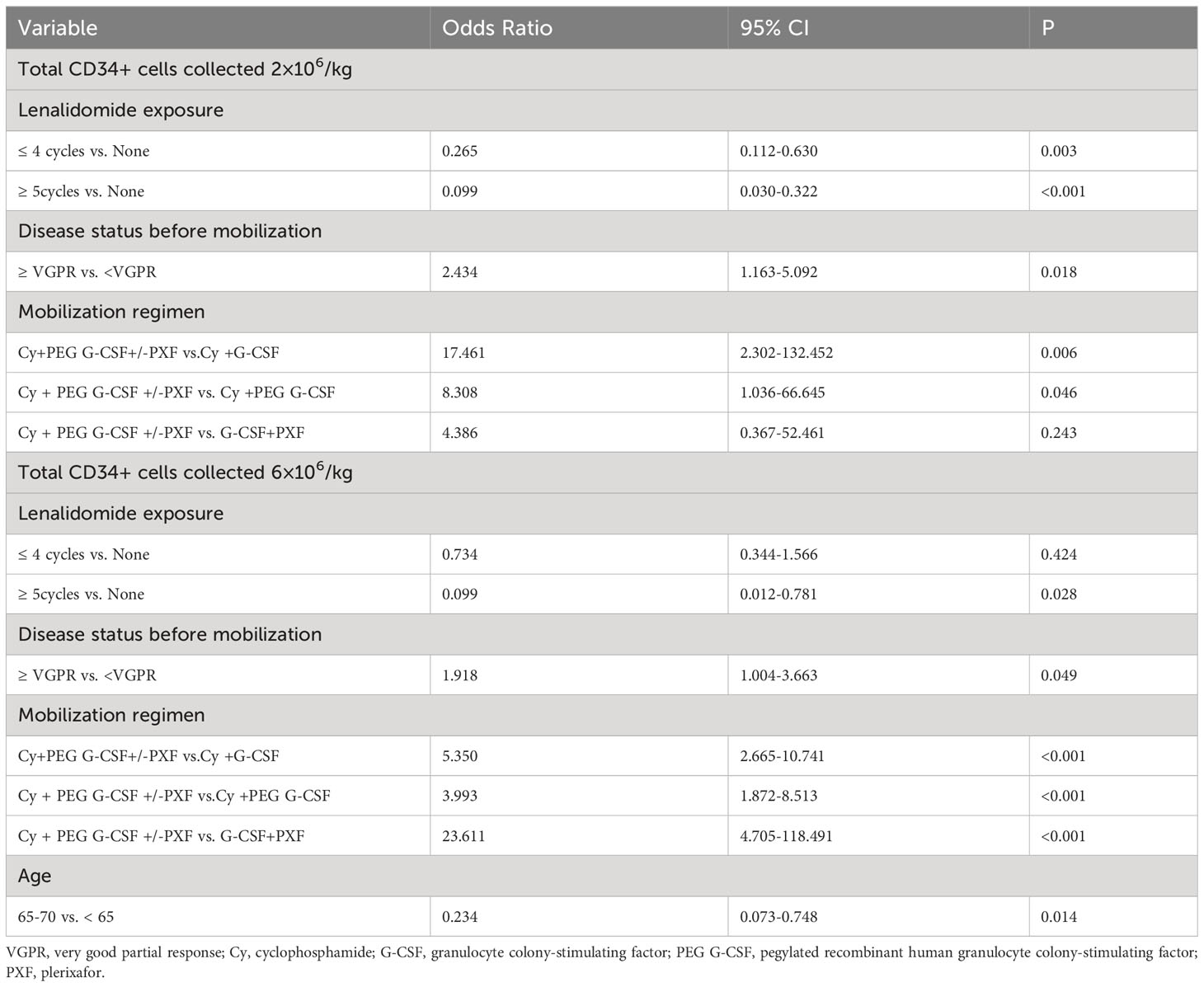
The multivariate analysis results are depicted in Table 3. Statistically significant variables from the univariate analysis were gathered to establish multivariate models, considering two distinct stem cell dose thresholds as dependent variables: 2×106/kg and 6×106/kg. Patients who underwent lenalidomide treatment for either ≥ 5 cycles (OR: 0.099; 95% CI: 0.030-0.322;P<0.001) or ≤ 4 cycles (OR: 0.265; 95% CI: 0.112-0.630; P=0.003) were less likely to achieve the 2×106 CD34+ cells/kg, compared to those without any lenalidomide exposure. Having a disease status of ≥ VGPR before mobilization, as opposed to < VGPR, increased the chances of reaching the target of 2×106 CD34+ cells/kg (OR: 2.434; 95% CI: 1.163-5.092; P=0.018). The Cy+PEG G-CSF+/-PXF mobilization strategy demonstrated a higher success rate in achieving the 2×106 CD34+ cells/kg target than the Cy+G-CSF approach. Yet, no significant difference was identified between the Cy+PEG G-CSF+/-PXF and G-CSF+PXF regimens. Regarding the higher threshold of 6×106 CD34+ cells/kg: A pre-mobilization disease status of ≥ VGPR increased the probability of success (OR: 1.918; 95% CI: 1.004-3.663; P=0.049), as did the utilization of Cy + PEG G-CSF +/-PXF for mobilization. Patients aged between 65 and 70 were less likely to achieve this benchmark when compared to those aged < 65 (OR: 0.234; 95% CI: 0.073-0.748; P=0.014). Further, a longer lenalidomide treatment duration (≥ 5 cycles) diminished the likelihood of reaching this goal when compared to no treatment (OR: 0.099; 95% CI: 0.012-0.781; P=0.028). Interestingly, the difference between no lenalidomide exposure and exposure for ≤ 4 cycles was not statistically significant for this threshold.
Adverse events
Within our cohort, AEs were observed in different frequencies among the treatment groups: 39.4% (26 patients) in the Cy + PEG G-CSF +/-PXF group, 48.4% (44 patients) in the Cy + PEG G-CSF group, 48.52% (82 patients) in the Cy +G-CSF group, and 16.67% (4 patients) in the G-CSF+PXF group. The predominant AEs encountered were nausea and diarrhea, with most being of mild intensity. The majority of AEs were categorized as grades 1-2 based on the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Instances of neutropenic fever varied among groups: 3.0% in the Cy + PEG G-CSF +/-PXF group, 9.9% in the Cy + PEG G-CSF group, and 17.2% in the Cy + G-CSF group. Importantly, no severe (grade 4 or 5) infections were identified (Table 2). Red blood cell transfusions were administered when hemoglobin levels dropped below 60 g/L, and platelet transfusions were provided when platelet counts descended below 20×109/µl. Transfusion requirements were as follows: Red blood cell transfusions were necessary for 1.5% of patients in the Cy + PEG G-CSF +/-PXF group, 6.6% in the Cy + PEG G-CSF group, and 3.6% in the Cy + G-CSF group. For platelet transfusions, 27.3% of patients in the Cy + PEG G-CSF +/-PXF group, 27.5% in the Cy + PEG G-CSF group, and 20.7% in the Cy + G-CSF group required the intervention. Hemorrhagic cystitis was a rare complication, appearing in only 1.5% of patients in the Cy + PEG G-CSF (+/-PXF) group and 0.6% in the Cy + G-CSF group. In affected patients, the condition resolved in under 48 hours with adequate hydration and bladder irrigation.
Financial analysis and incremental cost-effectiveness ratios
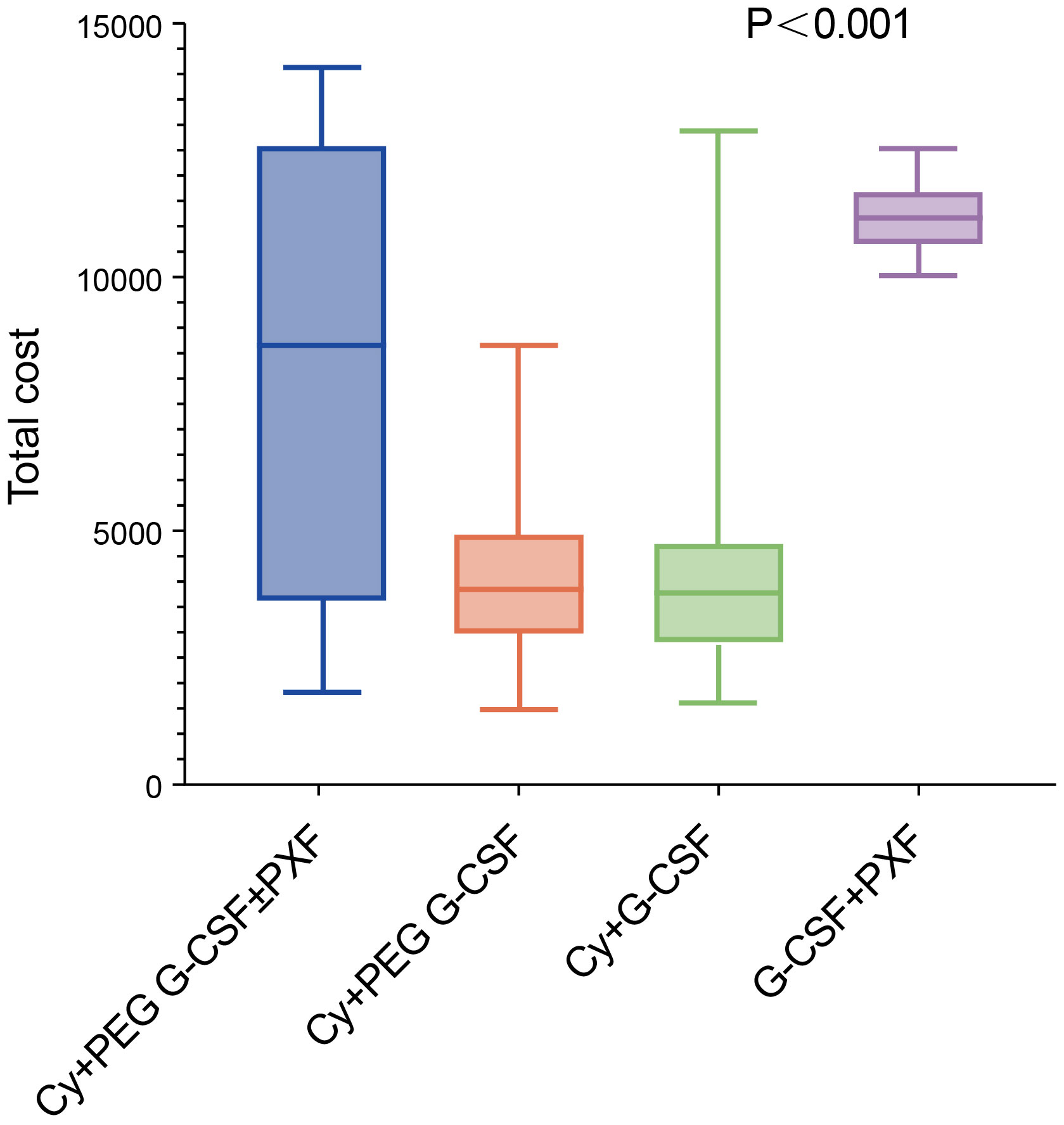
The total cost of mobilization and apheresis using Cy +PEG G-CSF +/-PXF was significantly lower at $ 5,929.52 (1,727.07 -11,711.29)compared to the G-CSF+PXF group at $11,146.86
(10,563.68-11,195.07) and significantly higher than the Cy+ PEG G-CSF group at $ 2,485.31 (1,959.00-2,991.23) and the Cy+ G-CSF group at $ 1,880.81(1,637.50 -2,474.67) (P< 0.001) (Figure 2). From an economic perspective, when considering the enhancement in success rates, the Cy + PEG G-CSF +/-PXF group had an incremental cost-effectiveness ratio (ICER) of $247.78 for each 1% rise in achieving the minimum threshold of 2×106 CD34+ cell apheresis harvest (designated as ICER-1). Furthermore, for attaining the higher benchmark of 6×106 CD34+ cell harvest, the ICER stood at $97.02 per 1% increase (ICER-2) (Table 4).
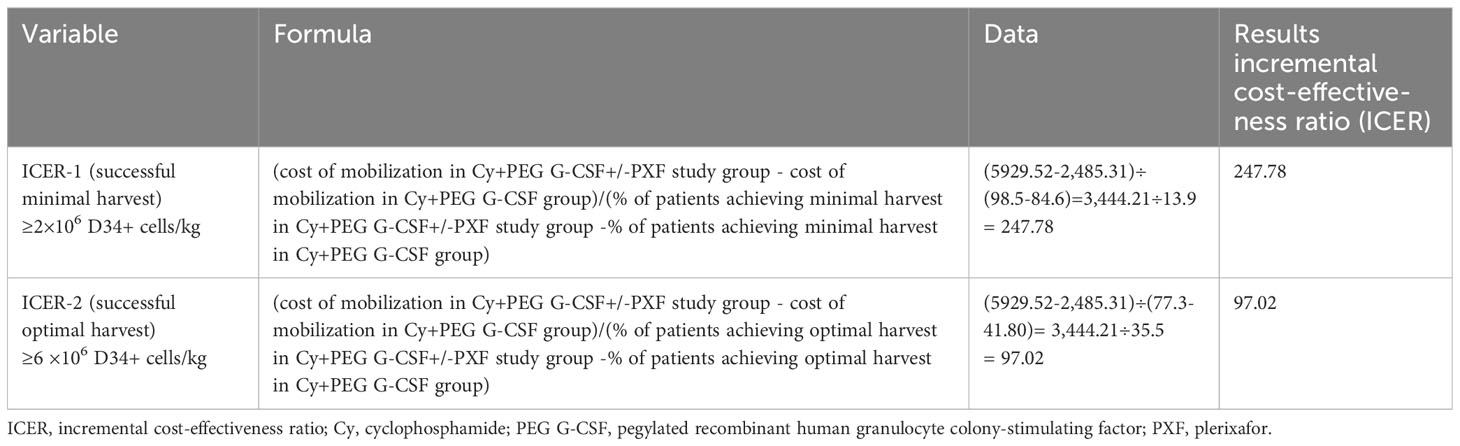
Table 4 Incremental cost-effectiveness ratio (ICER) of the on-demand PXF group compared to the control group.
Discussion
While newer therapeutic agents have emerged, offering profound responses, ASCT continues to be the cornerstone for eligible newly diagnosed MM patients. Securing two or more sufficient stem cell grafts ensures the possibility of tandem ASCT or rescue ASCT for appropriate MM patients. Historically, two primary stem cell mobilization techniques prevailed: utilizing growth factors like G-CSF by itself or combined with chemotherapy. However, these techniques do not uniformly benefit all patients (12, 28).
The integration of high-dose cyclophosphamide (Cy) (3 g/m2) with G-CSF, supplemented by PXF as needed, has gained traction as an effective mobilization strategy. Yet, the G-CSF regimen demands daily, multiple injections. On the other hand, PEG G-CSF requires only a one-time injection at a 6 mg dosage, given its prolonged serum duration. This makes PEG G-CSF not only more user-friendly but also reduces discomfort.
In this study, the mobilizing strategy of Cy+PEG G-CSF+/-PXF obtained results statistically superior to those obtained using Cy+ PEG G-CSF, Cy+G-CSF, and G-CSF+PXF. To the best of our knowledge, this is the first study to compare Cy+PEG G-CSF +/-PXF with other mobilization schemes. Our data suggests that adding on-demand PXF to Cy and PEG G-CSF improved mobilization outcomes in comparison to the control groups (Cy+ PEG G-CSF and Cy+G-CSF). A striking 77.3% of patients in the Cy+PEG G-CSF+/-PXF group achieved the benchmark of >6×106/kg—sufficient for two transplants—manifestly surpassing the Cy+PEG G-CSF (41.8%; P<0.001) and Cy+G-CSF groups (37.3%; P<0.001). Furthermore, these commendable outcomes necessitated limited PXF interventions (only 51.5%), contributing to an impressive cost-effectiveness ratio. Notably, our data discerned the superiority of Cy+PEG G-CSF+/-PXF over G-CSF+PXF. Prior research indicated a substantial failure rate with G-CSF monotherapy mobilization (9, 10). Echoing these findings, and we also noted a high rate of mobilization failure in this group. Consequently, we integrated PXF as a standard measure, ensuring its administration to all patients receiving G-CSF, irrespective of their precollection stem cell count. Our study found that even if all patients in the G-CSF group received PXF, the mobilization effect of Cy+PEG G-CSF+/-PXF was still better than that of G-CSF+PXF. It is imperative to acknowledge the age discrepancy across our study groups. The cohort undergoing G-CSF+ PXF predominantly comprised elderly participants, while the remaining groups were relatively younger. Recognized determinants, such as age (29, 30), prior lenalidomide exposure (31), and pre-mobilization disease status (32), undeniably influence mobilization and harvest outcomes in MM. Hence, the pronounced efficacy of Cy+PEG G-CSF+/-PXF might be attributed, at least partially, to the younger age profile in this group compared to the G-CSF+PXF patients. However, our multivariable logistic regression analysis further validated the superior HSC mobilization and harvest outcomes of the Cy+PEG G-CSF+/-PXF cohort. In this analysis, the differences in age, lenalidomide exposure, and disease status before mobilization were taken into account. It highlighted that even when every patient in the G-CSF+PXF group was consistently treated with PXF, the mobilization efficacy of Cy+PEG G-CSF+/-PXF remained superior to G-CSF+PXF. A significant consideration was the financial implications of universally administering PXF, given that it is administered to all patients in the G-CSF+PXF group. Our findings indicated a higher percentage of patients achieving the desired stem cell yield (≥6 × 106 CD34+ cells/kg, suitable for tandem transplantation) in the Cy+PEG G-CSF+/-PXF cohort compared to G-CSF+PXF as documented in earlier studies (77% vs. 72%) (10).
Currently, many experts advocate for a risk-adjusted approach, incorporating PXF “on-demand”, as the mainstream protocol for stem cell mobilization. The synergy of high-dose cyclophosphamide (Cy) (3 g/m2), PEG G-CSF, and on-demand PXF in our investigation aligns closely with outcomes from the CTX+G-CSF+/-PXF arm in previous randomized trials. A retrospective study by Beatrice et al. evaluated HSC mobilization in 422 MM patients. Their cohorts included 188 patients administered with low-dose Cy (LD-Cy, defined as 2 g/m2), 163 with intermediate to high-dose Cy (ID-Cy, designated as 3 g/m2), and 71 patients with G-CSF monotherapy. Although they did not specify the percentage reaching >6×106/kg CD34+ cells, they reported an impressive 90.4% in the LD-Cy and 91.1% in the ID-Cy groups achieving a minimum HSC dose of 4×106 (20). In a retrospective analysis, Andrew et al. assessed 398 patients mobilized with either Cy (4 g/m2) combined with G-CSF or G-CSF as a standalone therapy. Both groups had PXF introduced on-demand. Notably, 94% of the participants in their study achieved the desired yield of 4×106 CD34+/kg (19). Similarly, Giuseppe Milone and his team evaluated 111 MM patients undergoing mobilization with Cy at 4 g/m2 and G-CSF, revealing that 84.6% reached the >4×106/kg threshold (12). In our research, utilizing Cy plus PEG G-CSF in conjunction with on-demand PXF, a remarkable 93.4% met the same >4×106/kg criterion. However, we should note the variations across these studies, both in the target stem cell collection thresholds and the respective algorithms employed for on-demand PXF. Different algorithms might influence the proportion of patients necessitating PXF. However, the percentage of patients in our study who needed PXF (51.5%) was higher than the percentage of patients in previous studies who needed PXF (12.3%-28%) (18, 20, 21). However, previous studies have used multiple injections of PXF until the target stem cells are collected. In our study, PXF was given as a single dose at a fixed dose. This inherent difference makes direct comparisons between mobilization outcomes and the economic ramifications of Cy+PEG G-CSF+/-PXF and Cy+G-CSF +/- PXF a challenge. A subsequent analysis did endorse the efficacy of a solitary, pre-set PXF dose for stem cell mobilization. Significantly, more patients proceeded with successful ASCT post-PXF administration: a leap from 59.6% pre-plerixafor to 90% post-plerixafor (P<0.001) (33). The financial upside of this approach is evident. By restricting the drug’s administration to a single on-demand, fixed-dose, we mitigated ancillary costs, circumvented potential additional apheresis sessions, and minimized the likelihood of secondary mobilization treatments. The financial analysis revealed that the mobilization and apheresis costs associated with the Cy+PEG G-CSF+/-PXF regimen were markedly less than those of the G-CSF+PXF approach. Since 100% of patients received PXF in the G-CSF+PXF group, when PXF was added to mobilization with G-CSF only, the increase in costs related to the use of PXF was $8,517 per patient. Our study also found that the total cost of mobilization and apheresis using Cy+PEG G-CSF+/-PXF was significantly higher than that of the Cy+PEG G-CSF group. In our assessment, the additional expenditure linked to PXF’s integration was $3,444 per patient – a figure that remains financially reasonable. The incremental cost-effectiveness ratio for each 1% increase in the probability of achieving a successful optimal harvest (>6×106/kg) was $97.02 per patient. In the context of the on-demand PXF regimen, while the initial mobilization does carry a steeper price due to PXF’s inclusion, subsequent “rescue” mobilizations are more economical. Thus, when balancing out initial and follow-up mobilization expenses, the on-demand approach proves cost-efficient overall.
Chemotherapy-related mobilization raises legitimate toxicity concerns. Yet, in our study, there were no instances of treatment-induced fatalities prior to ASCT. Overall, the side effects linked to the Cy+PEG G-CSF+/-PXF regimen were controllable. The most frequently reported AEs were nausea and vomiting, with the vast majority being mild. The bulk of these AEs were classified as grades 1-2. Furthermore, only a scant 3.0% of participants experienced neutropenic fever, which, in most instances, was of short duration. Only one case of hemorrhagic cystitis was reported in the patients treated with cyclophosphamide (1.5%) but resolved quickly with a less than 48-hour admission.
Given our findings, we advocate for the application of Cy+PEG G-CSF+/-PXF for those patients in which tandem transplantation is planned or if ASCT at relapse is considered. On the other hand, In contrast, in more fragile patients or if a single ASCT is recommended for age, the G-CSF+PXF combination seems to be a more suitable alternative. Our research underscores that for MM patients earmarked for ASCT, incorporating on-demand PXF with HD-Cy and PEG G-CSF not only ensures a robust stem cell yield but is also a cost-effective and safe mobilization approach.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Clinical Research and Animal Ethics Committee of The First Affiliated Hospital of Sun Yat-sen University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
L-QH: Writing – original draft. J-RL: Writing – review & editing. J-LG: Formal analysis, Methodology, Writing – review & editing. M-LC: Writing – review & editing. L-FK: Writing – review & editing. B-HH: Writing – original draft. W-YZ: Writing – original draft. JL: Conceptualization, Methodology, Resources, Supervision, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank all the staff involved in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kumar SK, Callander NS, Adekola K, Anderson L, Baljevic M, Campagnaro E, et al. Multiple myeloma, version 3.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2020) 18:1685–717. doi: 10.6004/jnccn.2020.0057
2. Rajkumar SV. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am J Hematol (2020) 95(5):548–67. doi: 10.1002/ajh.25791
3. Cavo MF, Gay M, Beksac L, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol (2020) 7(6):e456–68. doi: 10.1016/S2352-3026(20)30099-5
4. Cavo M, Goldschmidt H, Rosinol L, Moreau P, Petrucci MT, Blau W, et al. Double vs single autologous stem-cell transplantation for newly diagnosed multiple myeloma: long-term follow-up (10-Years) analysis of randomized phase 3 studies. Blood (2018) 132:124–4. doi: 10.1182/blood-2018-99-112899
5. Cook GAJ, Ashcroft DA, Cairns CD, Williams CD, Brown JM, Cavenagh JD, et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol (2016) 3(7):e340–351. doi: 10.1016/S2352-3026(16)30049-7
6. Kaufman JL. Roundtable: How I treat a newly diagnosed patient with high-risk myeloma. ” Hematol Am Soc Hematol Educ Program (2019) 2019(1):120–4. doi: 10.1182/hematology.2019000015
7. Sevindik OGS, Korkmaz, Altuntas F. Current status of art mobilization in Myeloma. Transfus Apher Sci (2017) 56(6):850–3. doi: 10.1016/j.transci.2017.11.028
8. Giralt SEA, Stadtmauer JL, Harousseau A, Palumbo A, Bensinger W, Comenzo RL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100). Leukemia (2009) 23(10):1904–12. doi: 10.1038/leu.2009.127
9. Alegre AJ, Tomás C, Martínez-Chamorro JJ, Gil-Fernández JJ, Fernández-Villalta MJ, Arranz R, et al. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transplant (1997) 20(3):211–7. doi: 10.1038/sj.bmt.1700867
10. DiPersio JF, Stadtmauer A, Nademanee IN, Micallef NM, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood (2009) 113(23):5720–6. doi: 10.1182/blood-2008-08-174946
11. Gertz MA, Kumar MQ, Lacy A, Dispenzieri A, Hayman SR, Buadi FK, et al. Comparison of high-dose CY and growth factor with growth factor alone for mobilization of stem cells for transplantation in patients with multiple myeloma. Bone Marrow Transplant (2009) 43(8):619–25. doi: 10.1038/bmt.2008.369
12. Milone G, Martino M, Leotta S, Spadaro A, Zammit V, Cupri A, et al. Cost-effectiveness of on-demand plerixafor added to chemotherapy and granulocyte-colony stimulating factor for peripheral blood stem cell mobilization in multiple myeloma. Leuk Lymphoma (2018) 59(1):42–8. doi: 10.1080/10428194.2017.1324161
13. Pusic IS, Jiang S, Landua GL, Geoffrey LU, Rettig MP, Cashen AF, et al. Impact of mobilization and remobilization strategies on achieving sufficient stem cell yields for autologous transplantation. Biol Blood Marrow Transplant (2008) 14(9):1045–56. doi: 10.1016/j.bbmt.2008.07.004
14. Vose JM, Coiffier P, Corradini I, Corradini P, Khouri I, Sureda A, et al. Advances in mobilization for the optimization of autologous stem cell transplantation. Leuk Lymphoma (2009) 50(9):1412–21. doi: 10.1080/10428190903096701
15. Goterris RJ, Hernández-Boluda A, Teruel C, Gómez C, Lis MJ, Terol MJ, et al. Impact of different strategies of second-line stem cell harvest on the outcome of autologous transplantation in poor peripheral blood stem cell mobilizers. Bone Marrow Transplant (2005) 36(10):847–53. doi: 10.1038/sj.bmt.1705147
16. Awan FS, Kochuparambil DE, Falconer A, Cumpston A, Leadmon S, Watkins K, et al. Comparable efficacy and lower cost of PBSC mobilization with intermediate-dose cyclophosphamide and G-CSF compared with plerixafor and G-CSF in patients with multiple myeloma treated with novel therapies. Bone Marrow Transplant (2013) 48(10):1279–84. doi: 10.1038/bmt.2013.52
17. Chabannon CF, Bijou JM, Miclea N, Milpied N, Grouin J-M, Mohty M. A nationwide survey of the use of plerixafor in patients with lymphoid Malignancies who mobilize poorly demonstrates the predominant use of the ldquo]on-demand[rdquo scheme of administration at French autologous hematopoietic stem cell transplant programs. Transfusion (2015) 55(9):2149–57. doi: 10.1111/trf.13141
18. Chow EK, Rao WA, Wood D, Covington D, Armistead PM, Coghill J, et al. Effectiveness of an algorithm-based approach to the utilization of plerixafor in patients undergoing chemotherapy-based stem cell mobilization. Biol Blood Marrow Transplant (2014) 20(7):1064–8. doi: 10.1016/j.bbmt.2014.03.023
19. Johnsrud AA, Ladha L, Muffly P, Shiraz P, Goldstein G, Osgood V, et al. Stem cell mobilization in multiple myeloma: comparing safety and efficacy of cyclophosphamide +/- plerixafor versus granulocyte colony-stimulating factor +/- plerixafor in the lenalidomide era. Transplant Cell Ther (2021) 27(7):590.e591–590.e598. doi: 10.1016/j.jtct.2021.04.016
20. Zannetti BA, Saraceni C, Cellini E, Fabbri E, Federica Monaco F, Guarini A, et al. Low-Dose Cyclophosphamide versus Intermediate-High-Dose Cyclophosphamide versus Granulocyte Colony-Stimulating Factor Alone for Stem Cell Mobilization in Multiple Myeloma in the Era of Novel Agents: A Multicenter Retrospective Study. Transplant Cell Ther (2021) 27(3):244.e241–244.e248. doi: 10.1016/j.jtct.2020.12.009
21. Vaxman IE, Muchtar E, Jacob P, Kapoor P, Kumar S, Dispenzieri A, et al. The efficacy and safety of chemotherapy-based stem cell mobilization in multiple myeloma patients who are poor responders to induction: the mayo clinic experience. Transplant Cell Ther (2021) 27(9):770.e771–770.e777. doi: 10.1016/j.jtct.2021.06.016
22. Molineux GO, Kinstler B, Briddell C, Hartley C, McElroy P, Kerzic P, et al. A new form of Filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Exp Hematol (1999) 27(12):1724–34. doi: 10.1016/s0301-472x(99)00112-5
23. Molineux G. Pegfilgrastim: using pegylation technology to improve neutropenia support in cancer patients. Anticancer Drugs (2003) 14(4):259–64. doi: 10.1097/00001813-200304000-00002
24. Hosing CM, Qazilbash P, Kebriaei S, Giralt S, Davis MS, Popat U, et al. Fixed-dose single agent pegfilgrastim for peripheral blood progenitor cell mobilisation in patients with multiple myeloma. Br J Haematol (2006) 133(5):533–7. doi: 10.1111/j.1365-2141.2006.06054.x
25. Costa LJ, Kramer KR, Hogan CD, Butcher CD, Littleton A, Shoptawet KB, et al. Pegfilgrastim- versus filgrastim-based autologous hematopoietic stem cell mobilization in the setting of preemptive use of plerixafor: efficacy and cost analysis. Transfusion (2012) 52(11):2375–81. doi: 10.1111/j.1537-2995.2012.03579.x
26. Ding XW, Huang Y, Peng H, Fan HQ, Zhu YQ, Liu XL, et al. Pegfilgrastim improves the outcomes of mobilization and engraftment in autologous hematopoietic stem cell transplantation for the treatment of multiple myeloma. Ann Hematol (2020) 99(6):1331–9. doi: 10.1007/s00277-019-03800-0
27. Watts NL, Marques DB, Peavey R, Innis-Shelton R, Saad A, Stasi A, et al. Mobilization of hematopoietic progenitor cells for autologous transplantation using pegfilgrastim and plerixafor: efficacy and cost implications. Biol Blood Marrow Transplant (2019) 25(2):233–8. doi: 10.1016/j.bbmt.2018.09.005
28. Laszlo DG, Marcacci M, Martino D, Radice D, Rabascio C, BLucchetti B, et al. A comparison of chemo-free strategy with G-CSF plus plerixafor on demand versus intermediate-dose cyclophosphamide and G-CSF as PBSC mobilization in newly diagnosed multiple myeloma patients: An Italian explorative cost Analysis. Transfus Apher Sci (2020) 59(5):102819. doi: 10.1016/j.transci.2020.102819
29. Lee KH, Jung SJ, Kim JH, Jang JH, Kim K, Kimet WS, et al. Incidence and risk factors of poor mobilization inadult autologous peripheral blood stem cell transplantation: a single-centre experience.Vox Sang (2014) 107(4):407–15. doi: 10.1111/vox.12183
30. Ozkurt ZN, Yegin E, Suyani SZ, Aki SZ, Acar K, Yagci M, et al. Factors affecting stem cell mobilization for autologous hematopoietic stem cell transplantation. J Clin Apher (2010) 25(5):280–6. doi: 10.1002/jca.20246
31. Paripati HA, Stewart S, Cabou A, Dueck A, Zepeda VJ, Pirooz N, et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia (2008) 22(6):1282–4. doi: 10.1038/sj.leu.2405100
32. Ataca Atilla PS, Bakanay O, Demirer T. How to manage poor mobilizers for high dose chemotherapy and autologous stem cell transplantation? Transfus Apher Sci (2017) 56(2):190–8. doi: 10.1016/j.transci.2016.11.005
33. Greil CG, Ihorst C, Kiote-Schmidt S, Hildenbeutel S, Kühbach K, Bosse R, et al. Stem cell mobilization in poor mobilizers with multiple myeloma or lymphoma before and after introduction of plerixafor: a single-center comparative analysisusing a cost-efficient single fixed-dose schedule. Leuk Lymphoma (2018) 59(7):1722–5. doi: 10.1080/10428194.2017.1393673
Keywords: pegylated granulocyte colony-stimulating factor, cyclophosphamide, plerixafor, stem cell mobilization, multiple myeloma
Citation: Hou L-q, Liu J-R, Gu J-L, Chen M-L, Kuang L-F, Huang B-H, Zou W-y and Li J (2024) On-demand plerixafor added to high-dose cyclophosphamide and pegylated recombinant human granulocyte colony-stimulating factor in the mobilization of patients with multiple myeloma: a treatment with high effectiveness, convenient, and affordable cost. Front. Oncol. 13:1306367. doi: 10.3389/fonc.2023.1306367
Received: 03 October 2023; Accepted: 28 December 2023;
Published: 17 January 2024.
Edited by:
Gabor Mikala, Central Hospital of Southern Pest, HungaryReviewed by:
Szabolcs Modok, University of Szeged, HungaryLászló Váróczy, University of Debrecen, Hungary
Copyright © 2024 Hou, Liu, Gu, Chen, Kuang, Huang, Zou and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Li, ljuan@mail.sysu.edu.cn
 Li-qiong Hou
Li-qiong Hou Jun-Ru Liu
Jun-Ru Liu Jing-Li Gu
Jing-Li Gu Juan Li
Juan Li