- 1Comprehensive Breast Health Center, Department of Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
- 2School of Medicine, College of Medicine, National Yang-Ming Chiao Tung University, Taipei, Taiwan
- 3Division of Cancer Prevention, Department of Oncology, Taipei Veterans General Hospital, Taipei, Taiwan
- 4Division of General Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
- 5Division of Transfusion Medicine, Department of Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
- 6Division of Medical Oncology, Department of Oncology, Taipei Veterans General Hospital, Taipei, Taiwan
- 7Institute of Clinical Medicine, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 8Department of Pathology and Laboratory Medicine, Taipei Veterans General Hospital, Taipei, Taiwan
- 9Medical Affairs, Novartis (Taiwan) Co. Ltd, Taipei, Taiwan
- 10Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan
Background: Breast cancer is the most common cancer type that affects women. In hormone receptor–positive (HR+), human epidermal growth factor receptor 2−negative (HER2–) advanced breast cancer (ABC), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) is the most frequently mutated gene associated with poor prognosis. This study evaluated the frequency of PIK3CA mutations in the Taiwanese breast cancer population.
Methodology: This is a retrospective study; patient data were collected for 2 years from a next-generation sequencing database linked to electronic health records (EHRs). The primary endpoint was the regional prevalence of PIK3CA mutation. The secondary endpoints were to decipher the mutation types across breast cancer subtype, menopausal status, and time to treatment failure after everolimus (an mTOR inhibitor) or cyclin-dependent kinase 4/6 (CDK4/6) inhibitor treatment.
Results: PIK3CA mutations were identified in 278 of 728 patients (38%). PIK3CA mutations were reported in 43% of patients with HR−/HER2+ subtype and 42% of patients with HR+/HER2– postmenopausal status. A lower prevalence of PIK3CA mutations was observed in triple-negative (27%) and HR+/HER2– premenopausal patients (29%). The most common mutation was at exon 20 (H1047R mutation, 41.6%), followed by exon 9 (E545K mutation, 18.9% and E542K mutation, 10.3%). Among patients treated with CDK4/6 inhibitors, the median time to treatment failure was 12 months (95% CI: 7-21 months) in the PIK3CA mutation cohort and 16 months (95% CI: 11-23 months) in the PIK3CA wild-type cohort, whereas patients receiving an mTOR inhibitor reported a median time to treatment failure of 20.5 months (95% CI: 8-33 months) in the PIK3CA mutation cohort and 6 months (95% CI: 2-9 months) in the PIK3CA wild-type cohort.
Conclusion: A high frequency of PIK3CA mutations was detected in Taiwanese patients with breast cancer, which was consistent with previous studies. Early detection of PIK3CA mutations might influence therapeutic decisions, leading to better treatment outcomes.
1 Introduction
Breast cancer is the most commonly diagnosed cancer and one of the leading causes of cancer death among women in Taiwan. In 2019, Taiwan reported 14,856 new cases of breast cancer and 2633 deaths that occurred due to breast cancer (1). Despite the wide range of therapeutic interventions available for breast cancer, important current advances are focused on genetic profiling to allow a mutation-driven, targeted, and effective therapeutic approach.
Phosphoinositide 3-kinase (PI3K) is the most frequently disrupted signaling pathway in hormone receptor−positive (HR+) breast cancer (2). Phosphatidylinositol 3-kinase alpha (PI3Kα) is a heterodimeric protein complex composed of the catalytic subunit p110α (coded by the phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha [PIK3CA] gene) and the regulatory subunit p85α (coded by the PIK3R1 gene) (3). Mutations of the PIK3CA gene, inducing hyperactivation of the alpha isoform (p110α) of PI3K, occur in 28% to 46% of patients with HR+/human epidermal growth factor receptor-2–negative (HER2–) advanced breast cancer (ABC) (4, 5). This variant is associated with poor response to HER2 targeted therapy, endocrine therapy, and chemotherapy (6). The presence of an oncogenic PI3K mutation has also been correlated with a worse clinical outcome in patients with ABC receiving cyclin-dependent kinase 4/6 (CDK4/6) inhibitors (7). The main “hotspots” reported for PIK3CA mutations are E542K and E545K of the helical domain on exon 9 and H1047R of the kinase domain on exon 20 (8–10). Advancement in next-generation sequencing (NGS) technologies has made gene sequencing and mutation analysis feasible and effective for clinical application in breast cancer. NGS is helpful in identifying the key mutations for guiding personalized therapy (11). A study conducted by Huang et al. in Taiwanese patients with breast cancer identified PIK3CA as one of the most frequently mutated genes in 38% of the study population, followed by ERBB2 (23%), ESR1 (10%), AKT1 (6%), and BRCA2 (5%) mutations (12).
Alpelisib, a selective PI3K inhibitor, showed efficacy in patients with PIK3CA-mutated HR+/HER2– ABC in the SOLAR-1 trial, with a median progression-free survival (PFS) of 11.0 months (95% confidence interval [CI]: 7.5-14.5) (13). Based on these results, alpelisib was approved in Taiwan since December 2020 for the treatment of postmenopausal women and men, with HR+/HER2–, PIK3CA-mutated, locally advanced or metastatic breast cancer following progression on or after an endocrine-based regimen (14). Because the data on the prevalence of PIK3CA mutations in the Taiwanese population are limited, this study aimed to evaluate the frequency of PIK3CA mutation status, thereby determining the patient pool that might benefit from a personalized treatment plan. Here, we report the results from a single-center observational study that investigated the frequency of PIK3CA mutations in Taiwanese patients with all subtypes of breast cancer using NGS over a period of 24 months.
2 Methods
This is a retrospective, single-center study investigating the prevalence of PIK3CA mutations in female patients diagnosed with breast cancer in Taiwan.
2.1 Trial design
Taipei Veterans General Hospital, Taiwan, has an ongoing project (VGH-TAYLOR) performing comprehensive genomic profiling on tumor tissues from patients with breast cancer via NGS, which is sponsored by the YongLin Healthcare Foundation. The VGH-TAYLOR study aimed to discover potential biomarkers for recurrence, diagnosis, and prognosis of breast cancer that may enable personalized medicine and improvement in breast cancer treatment; the rationale and design of the study protocol have been previously described (15). Patient and genomic data were collected from the NGS database linked with electronic health records (EHRs) to investigate the mutation prevalence in various subtypes and stages of breast cancer.
In the NGS database, medical records of patients with advanced/metastatic breast cancer who were treated with an mTOR inhibitor or CDK4/6 inhibitor, with treatment initiation (index date) from January 1, 2018 to January 30, 2020, were included in the study once the inclusion/exclusion criteria were met. The EHRs were retrieved 3 years prior to the index date, from January 1, 2015 to January 1, 2018, for baseline characteristics and breast cancer recurrence data collection (Figure 1). Patients with information of first prescription of mTOR or CDK4/6 inhibitors during the 4-year EHR data collection period were also included for analysis for time to treatment failure. The study protocol was approved by the ethics committee of the Taipei Veterans General Hospital.
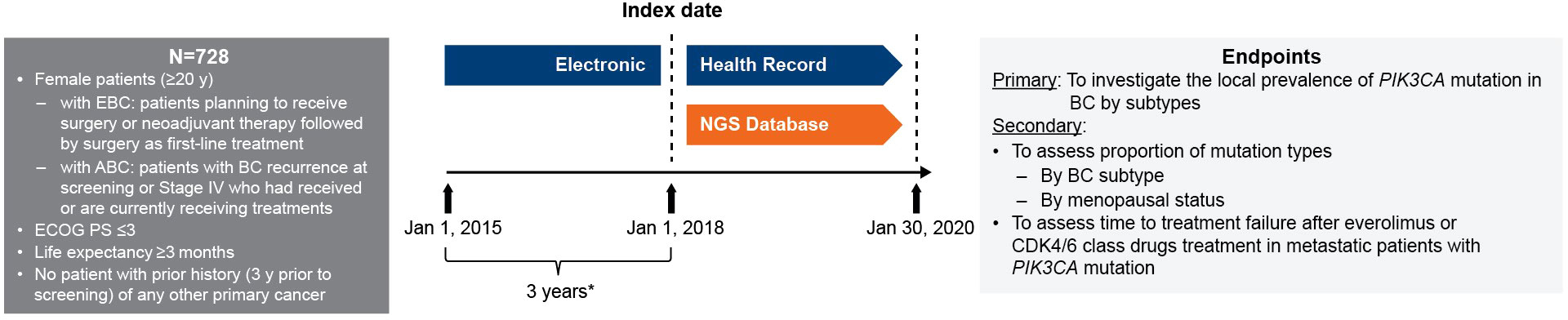
Figure 1 Study design. * Baseline characteristics collection period from HER. ABC, advanced breast cancer; BC, breast cancer; CDK4/6, cyclin-dependent 4 or 6 kinase; EBC, early breast cancer; ECOG PS, Eastern Cooperative Oncology Group performance status; EHC, electronic health record; NGS, next-generation sequencing; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; y, years.
The pathological features of the tissue samples were determined using immunohistochemistry (IHC) assay. At least 1% of nuclei staining–positive results were defined as ER-positive. As per the American Society of Clinical Oncology (ASCO) and College of American Pathologists (CAP) guidelines, IHC 3+ and IHC 2+ with fluorescence in situ hybridization (FISH) amplification indicated HER2 overexpression. An IHC score of 0 to 1+ is called HER2– and a score of 3+ is called HER2+. A FISH HER2:CEP17 signal ratio >2.2 is called amplification whereas, ratio between 1.8-2.2 is equivocal and <1.8 is non-amplification
2.2 Patients
Inclusion criteria were defined as follows: (1) female patients aged ≥20 years; (2) patients with confirmed diagnosis of primary invasive breast cancer who are planning to receive treatment for breast cancer; (3) patients who have breast cancer recurrence at screening or Stage IV patients who have received or are currently receiving treatments for breast cancer; (4) Eastern Cooperative Oncology Group (ECOG) performance status ≤3; and (5) life expectancy ≥3 months.
Patients were excluded if they had a primary cancer other than breast cancer within 5 years prior to screening. Archival samples from the biobank (retrospective cohort) will be withdrawn if: (1) tumor content of the FFPE sample is lower than the specified percentage according to the standard of the central laboratory; (2) FFPE samples failed the DNA/RNA quality check. The criteria of the DNA/RNA quality check will follow the standard of the central laboratory. Enrolled patients will be withdrawn if one of the following conditions occurs: (1) patient withdraws consent; (2) patient refuses to provide specimens for evaluation after enrollment; (3) patient for which all samples/specimens fail the DNA/RNA quality check. The criteria of the DNA/RNA quality check will follow the standard of the central laboratory; (4) patient who does not have sufficient FFPE samples, tissues or blood samples for genetic profiling analysis by principal investigator’s discretion; (5) patient who does not return to the clinical site for more than 6 months (based on their medical records) will be considered as lost to follow-up. However, whether this subject should be withdrawn will be based on the PI’s discretion (15).
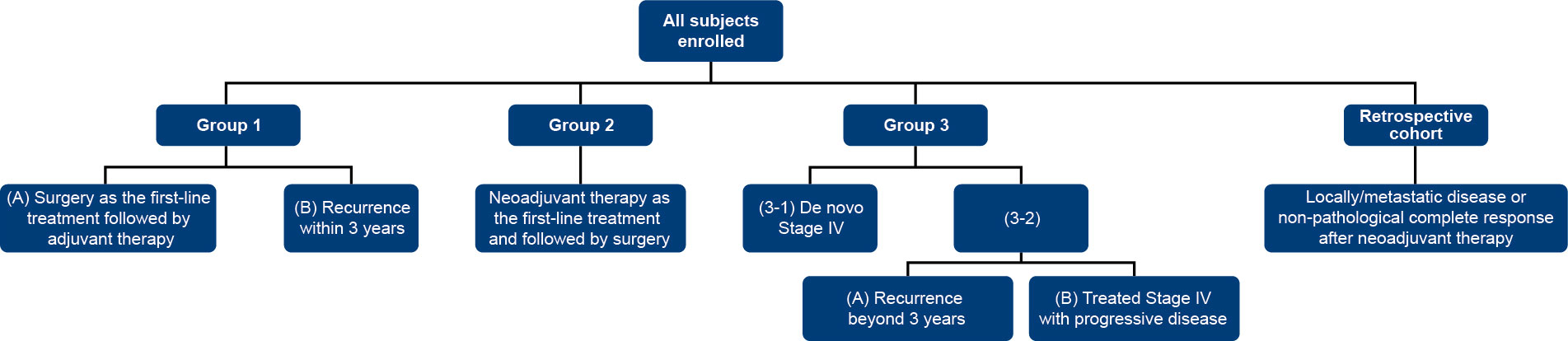
In this study we retrospectively analyze the database from VGH-TAILOR project (Group 1,2,3). Enrolled patients were categorized into 4 major groups, presented in Figure 2.
2.2.1 Group 1
• Group 1A (early breast cancer): patients who were planning to receive surgery as the first-line treatment followed by adjuvant therapy.
• Group1B (advanced breast cancer): patients with recurrence at screening, who had received surgery for primary breast cancer within 3 years prior to screening, and with primary tumor formalin-fixed paraffin-embedded (FFPE) tissues available.
2.2.2 Group 2 (early breast cancer)
Patients who were planning to receive neoadjuvant therapy as the first-line treatment for breast cancer and followed by surgery.
2.2.3 Group 3 (advanced breast cancer)
• Group 3-1: patients diagnosed with de novo and treatment naïve stage IV breast cancer.
• Group 3-2: patients diagnosed with a stage IV breast cancer and with recurrence beyond 3 years after surgery (Group 3-2A) or stage IV subjects who had received or are currently receiving treatments for breast cancer (Group 3-2B).
2.2.4 Retrospective cohort
Samples were collected from the biobank of the patients with local/metastatic disease or non-pathological complete response after neoadjuvant therapy
2.3 Endpoints
The primary endpoint was the regional prevalence of PIK3CA mutation in breast cancer and by breast cancer subtypes in overall patients with breast cancer, patients with HR+/HER2–, HR−/HER2+, HR+/HER2+ and triple-negative breast cancer. Because the pre- and postmenopausal status present different biological and genetic characteristics in HR+/HER2– breast cancer, the prevalence of PIK3CA mutations was further analyzed by menopausal status in HR+/HER2– breast cancer. Menopause is usually a clinical diagnosis made after ≥12 months of amenorrhea (16).
Secondary endpoints were to assess the frequency of mutation types by breast cancer subtype and by menopausal status and time to treatment failure after mTOR or CDK4/6 inhibitor in metastatic patients stratified by PIK3CA mutation status. Time to treatment failure is defined as the time from the date of first dose of treatment of interest to the date of the patient discontinuing treatment for any reason; the Kaplan-Meier (KM) method was used for the estimation.
2.4 Sample preparation
The genetic profiles were determined through NGS of FFPE samples. For screening and recruitment of patients for Group 1B and Group 3-2, paired FFPE primary and recurrent tumor samples were collected and sequenced, and for Group 2, paired FFPE diagnostic and post-neoadjuvant specimens were assayed.
The preparation of FFPE was done under standard conditions at the trial site. The DNA/RNA extraction and hematoxylin and eosin (H&E) staining were performed in accordance with the laboratory manual under the guidance of a certified pathologist in the central laboratory. Quality checks for DNA/RNA were performed as per the manual of the Thermo Fisher™ Oncomine™ (TMO) Comprehensive Assay requirement (see below) and additional samples were collected in case of failure. Of the seven unstained FFPE sections retrieved (per subject), one section was prepared for H&E staining and six sections were prepared for TMO comprehensive assay.
2.5 Oncomine™ comprehensive assay (TMO comprehensive assay)
Oncomine™ comprehensive assay (TMO comprehensive assay, Thermo Fisher Scientific, Waltham, MA) was used to profile thousands of variants across 161 cancer-relevant genes using FFPE tissues (17). Analyses of TMO comprehensive assay included identification of genes and detection of mutation types such as frameshift, missense, synonymous, single nucleotide variation (SNV), insertion/deletion (Indel), and copy number variation (CNV) observed in individual subject.
Amplicon libraries were constructed with multiplex polymerase chain reaction (PCR) primers for preparation of DNA and RNA (for fusion genes) from FFPE samples. Sequencing was performed with the Ion Gene Studio S5 System and Ion 540 Chips. Raw data process, alignment, and variant calling were performed with Torrent Suite™ Software, with variant calling using the Torrent Variant Caller plug-in. Further management was proceeded by Ion Reporter™ Software with workflow “Oncomine Comprehensive v3 - w3.2 - DNA and Fusions - Single Sample” version 5.10 selected and filter chain “Oncomine Variants” version 5.10 applied. Reference genome was hg19.
2.6 Variables
Preindex variables, including demographics of all patients, menopausal status, primary diagnosis, previous medical history/comorbidities, and family history of cancer, were extracted from the NGS database, whereas the treatment response of previous breast cancer was taken from the EHRs.
Postindex variables in the follow-up period, including breast cancer genetic mutations, treatment, and treatment response from previous lines if any, were extracted from the NGS database, and breast cancer recurrence data were also extracted from the EHRs.
2.7 Statistical analysis
All statistical tests were conducted with a two-sided alternative hypothesis, and a significance level of 0.05 and a P value of <0.05 were considered statistically significant, unless otherwise specified. Mutations in the PIK3/AKT/mTOR pathway were deciphered. The prevalence of PIK3CA mutations was calculated as the number of PIK3CA mutations divided by the number of patients with breast cancer in the populations stated above. The 95% CIs were presented as appropriate. Analysis of the primary endpoint was descriptive in nature, and no statistical hypothesis or testing was performed. The enrolled set was adopted for both primary and secondary endpoints unless otherwise specified.
3 Results
3.1 Patient demographics and baseline characteristics
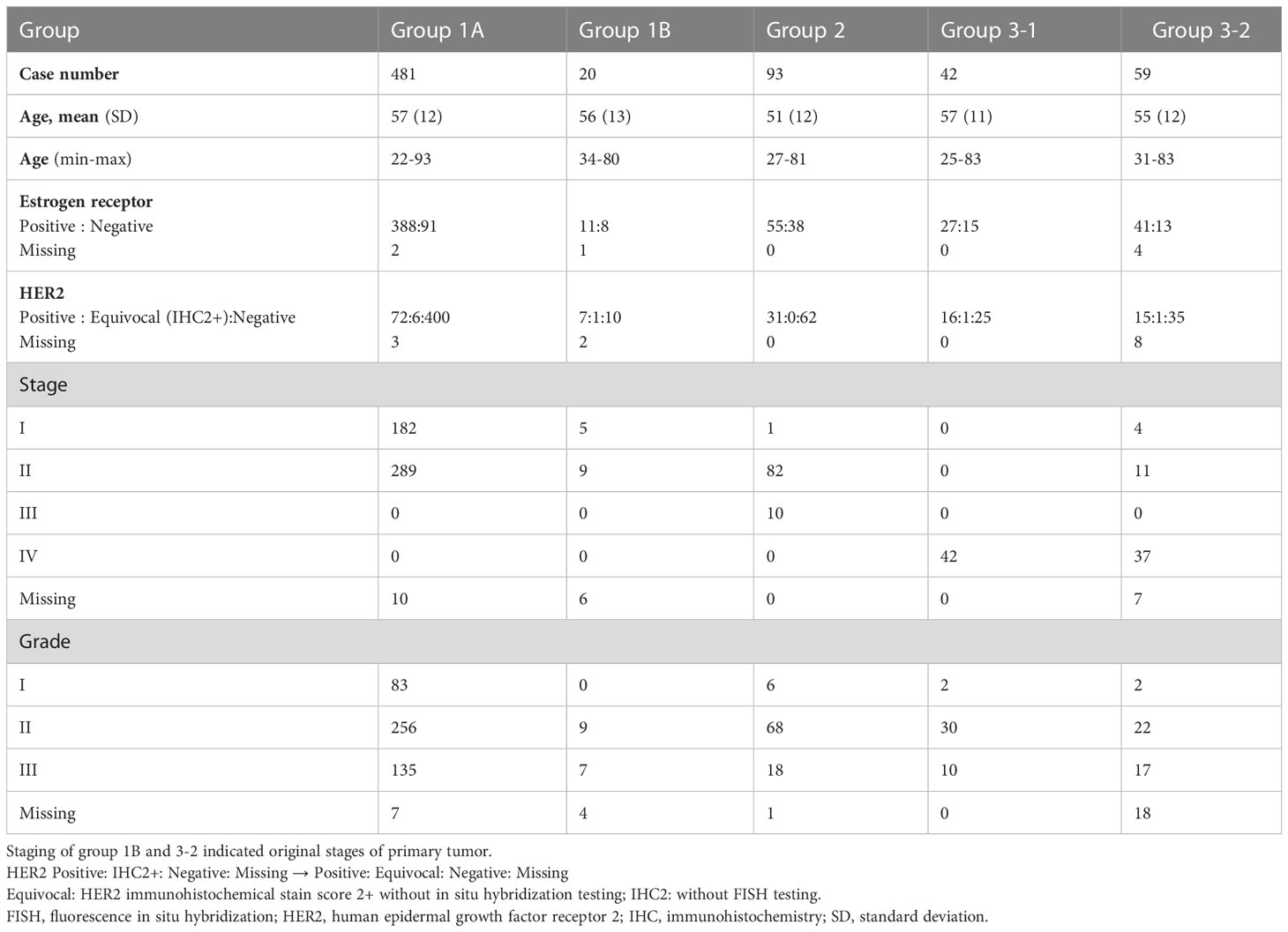
A total of 728 patients were included for the PIK3CA mutation analysis, most of whom were categorized into Group 1A (481 patients, 66.07%), followed by Group 2 (93 patients, 12.77%), Group 3-2 (59 patients, 8.10%), Group 3-1 (42 patients, 5.77%), and Group 1B (20 patients, 2.75%), please refer to Figure 2 for what each group stands for. The mean age in all the groups at the time of testing was 51 to 57 years of age. A total of 548 patients (76.2%) with HR+ and 149 patients (20.8%) with HER2+ status were reported. Patient demographic characteristics are detailed in Table 1.
3.2 Prevalence and characteristics of PIK3CA mutations
Overall, 403 of 728 patients (55%) had mutations in the PI3K/AKT/mTOR pathway, of which the 3 most commonly detected mutations were PIK3CA (57%), AKT3 (14%), and PTEN (10%) (Figure 3).
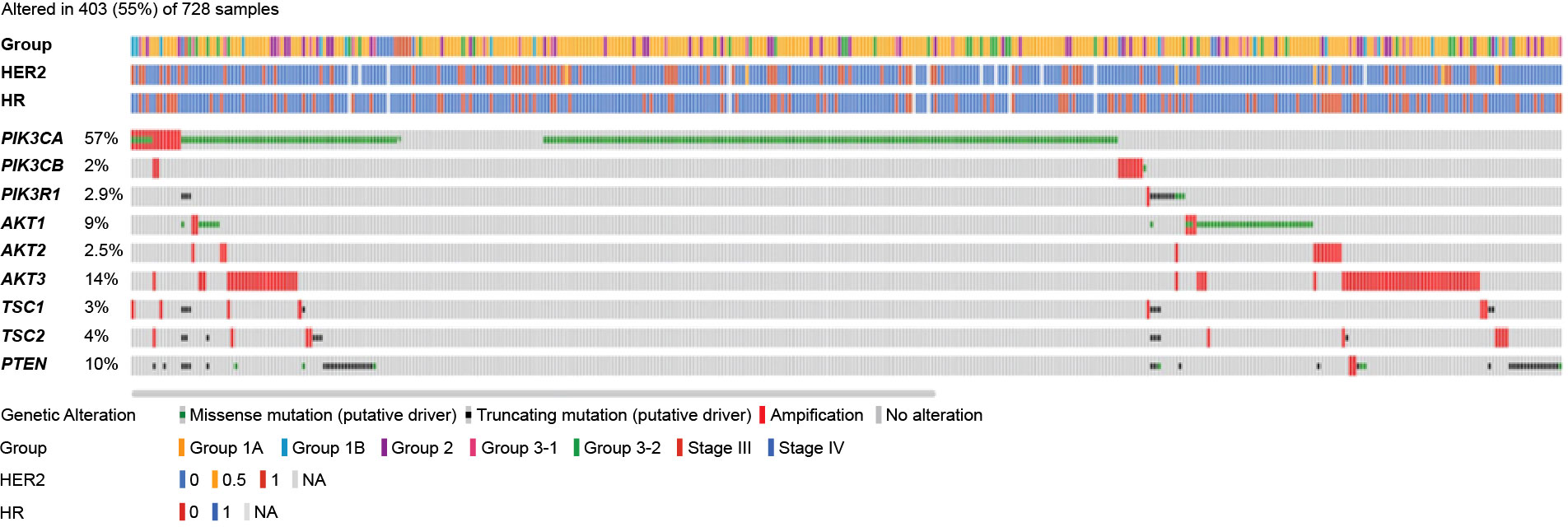
Figure 3 Mutation map of genes in the PI3K/AKT/mTOR pathway. AKT, serine/threonine kinase; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; mTOR, mammalian target of rapamycin; PI3K, phosphoinositide 3-kinase; PIK3CA/PIK3CB, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha/beta; PIK3R1, phosphoinositide-3-kinase regulatory subunit 1; PTEN, phosphatase and TENsin homolog; TSC, tuberous sclerosis complex.
A total of 278 of 728 patients (38%) harbored PIK3CA mutations. With respect to the IHC phenotypes, 29 patients (43.0%) were HR−/HER2+, 96 patients (42.0%) were HR+/HER2– (postmenopausal), and 34 patients (41.0%) were HR+/HER2+. The prevalence of PIK3CA mutation was relatively lower in triple-negative breast cancer (27.0%) and premenopausal patients with HR+/HER2– status (29.0%). The prevalence of PIK3CA mutation by IHC status is presented in Figure 4.
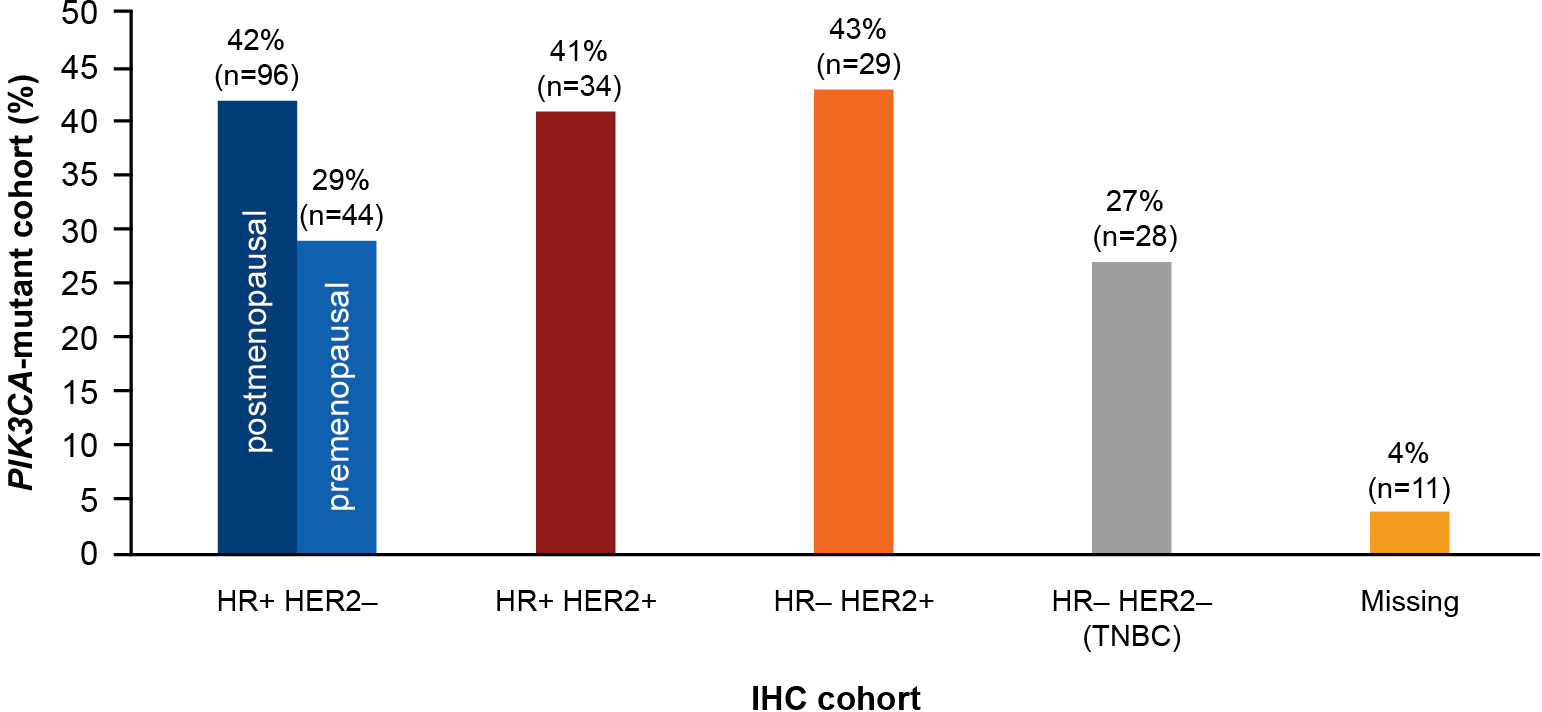
Figure 4 Prevalence of PIK3CA mutation in each IHC cohort. HR, hormone receptor; HER2, human epidermal growth factor receptor 2; IHC, immunohistochemistry; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; TNBC, triple-negative breast cancer.
The majority of the PIK3CA mutations were clustered in exon 9 and exon 20, helical and kinase domains, respectively. Among 278 patients with PIK3CA mutations, the most frequently observed mutations were in exon 20 (H1047R, 41.6%) and exon 9 (E545K, 18.9% and E542K, 10.3%); these are presented in Figure 5A.
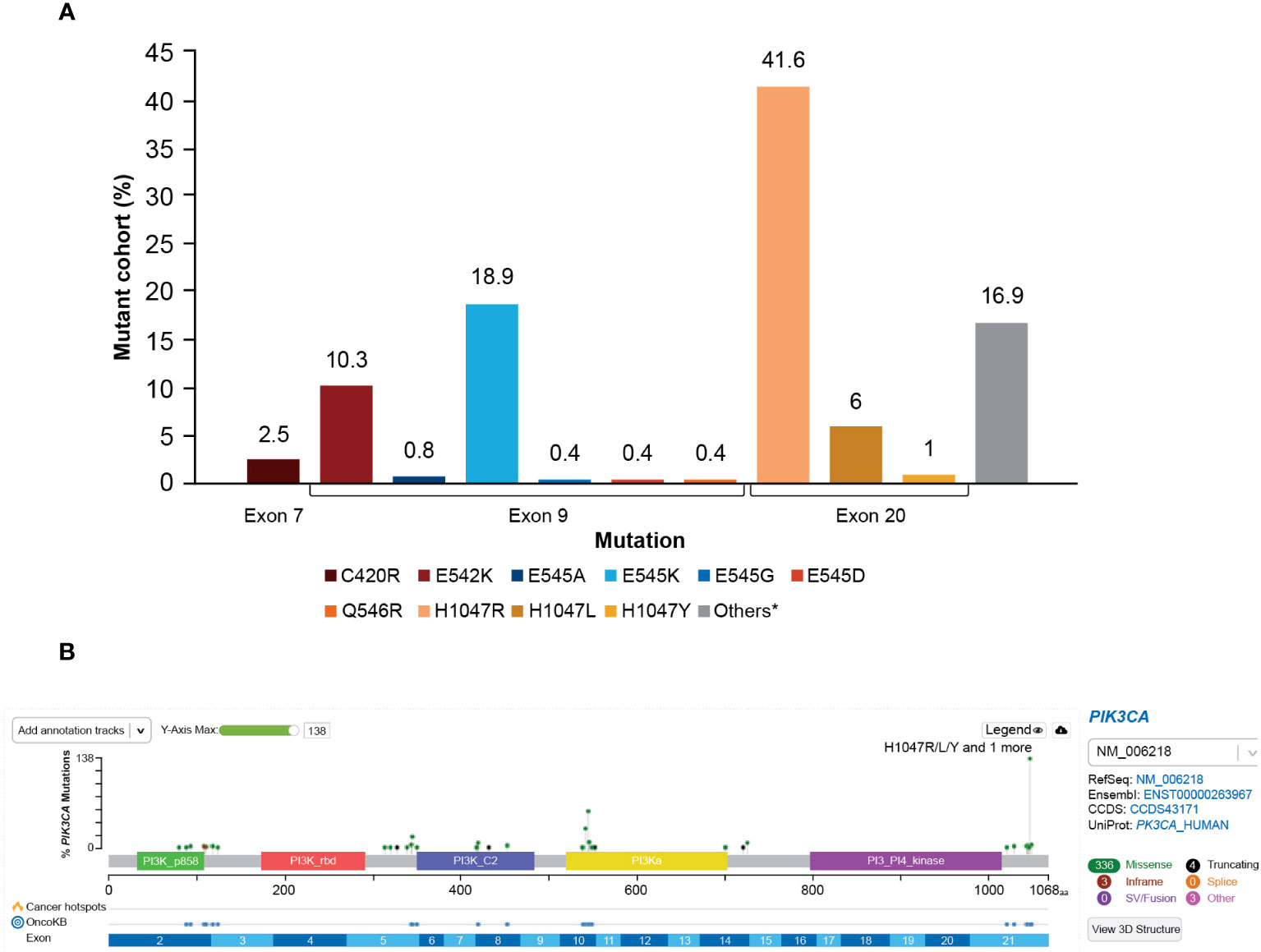
Figure 5 (A) Distribution of PIK3CA variants frequency. *A total of 16.9% of mutations in patients from this study were not captured by the therascreen PIK3CA Rotor-Gene Q (RGQ) PCR Kit. The most frequent mutations not captured were E726K (2.8%) and N345K (5.3%). Mutation Q546E was not detected (0%) at exon 9. (B) OncoPrinter plot of PIK3CA mutations. PI3K, phosphoinositide 3-kinase; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha.
The distribution of PIK3CA mutation types is presented in Figure 5B. A total of 27 of 278 patients (9.7%) harbored multiple PIK3CA mutations within one sample. Mutual exclusivity was observed between PIK3CA and AKT1 (P<0.001), PIK3CA and AKT3 (P<0.001), and PIK3CA and PIK3R1 (P=0.007).
3.3 PIK3CA mutations in patients with HR+/HER2– breast cancer by menopausal status
PIK3CA hotspot mutations (H1047R, E545K, and E542K) were found in 63.5% of premenopausal patients with breast cancer and in 56.2% of postmenopausal patients with HR+/HER2– breast cancer. Among the premenopausal PIK3CA-mutated patients, H1047R and E545K mutations were the most common (27% each), followed by the E542K mutation (9.5%). A similar distribution was seen in postmenopausal patients, where the H1047R mutation was the most common mutation (32%), followed by E545K (14.5%) and E542K mutations (9.7%).
3.4 Time to treatment failure in patients with PIK3CA mutation after CDK4/6 inhibitors or mTOR inhibitor
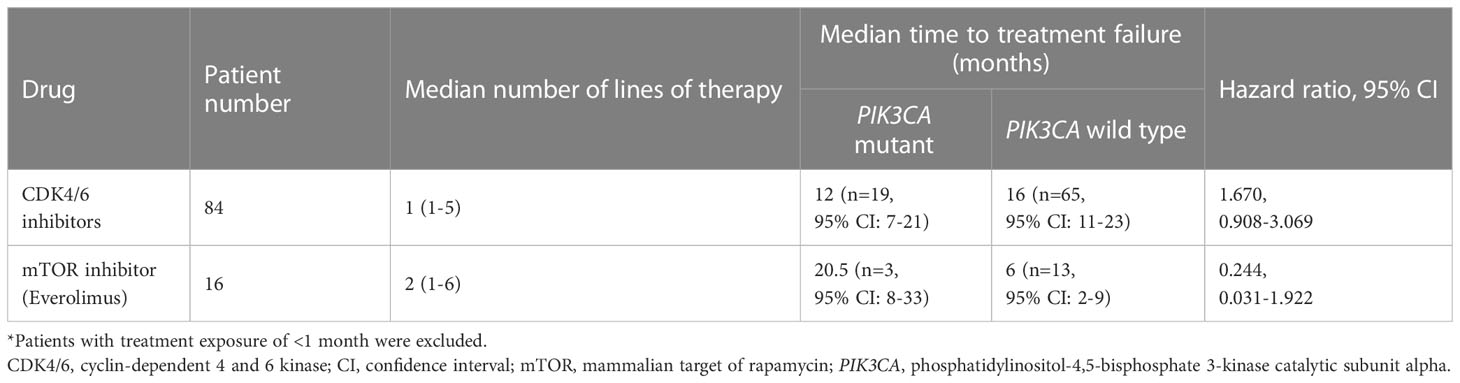
A total of 19 patients (22.62%) with advanced breast cancer who received CDK4/6 inhibitors (n=84, ribociclib=47, palbociclib=32, abemaciclib=5) and 3 patients (18.75%) who received an mTOR inhibitor (n=16) were reported to have a PIK3CA mutation. For patients treated with CDK4/6 inhibitors, the median time to treatment failure was 16 months (95% CI: 11-23 months) in the PIK3CA wild-type cohort and 12 months (95% CI: 7-21 months) in the PIK3CA mutation cohort, with a hazard ratio (HR) of 1.670 (95% CI: 0.908-3.069) (Figure 6A; Table 2). The data demonstrated a trend of shorter treatment duration with CDK4/6 inhibitors in patients with PIK3CA mutation. For patients receiving mTOR inhibitor, the median time to treatment failure was 20.5 months (95% CI: 8-33 months) in the PIK3CA mutation cohort and 6 months (95% CI: 2-9 months) in the PIK3CA wild-type cohort, with an HR of 0.244 (95% CI: 0.031-1.922) (Figure 6B; Table 2).
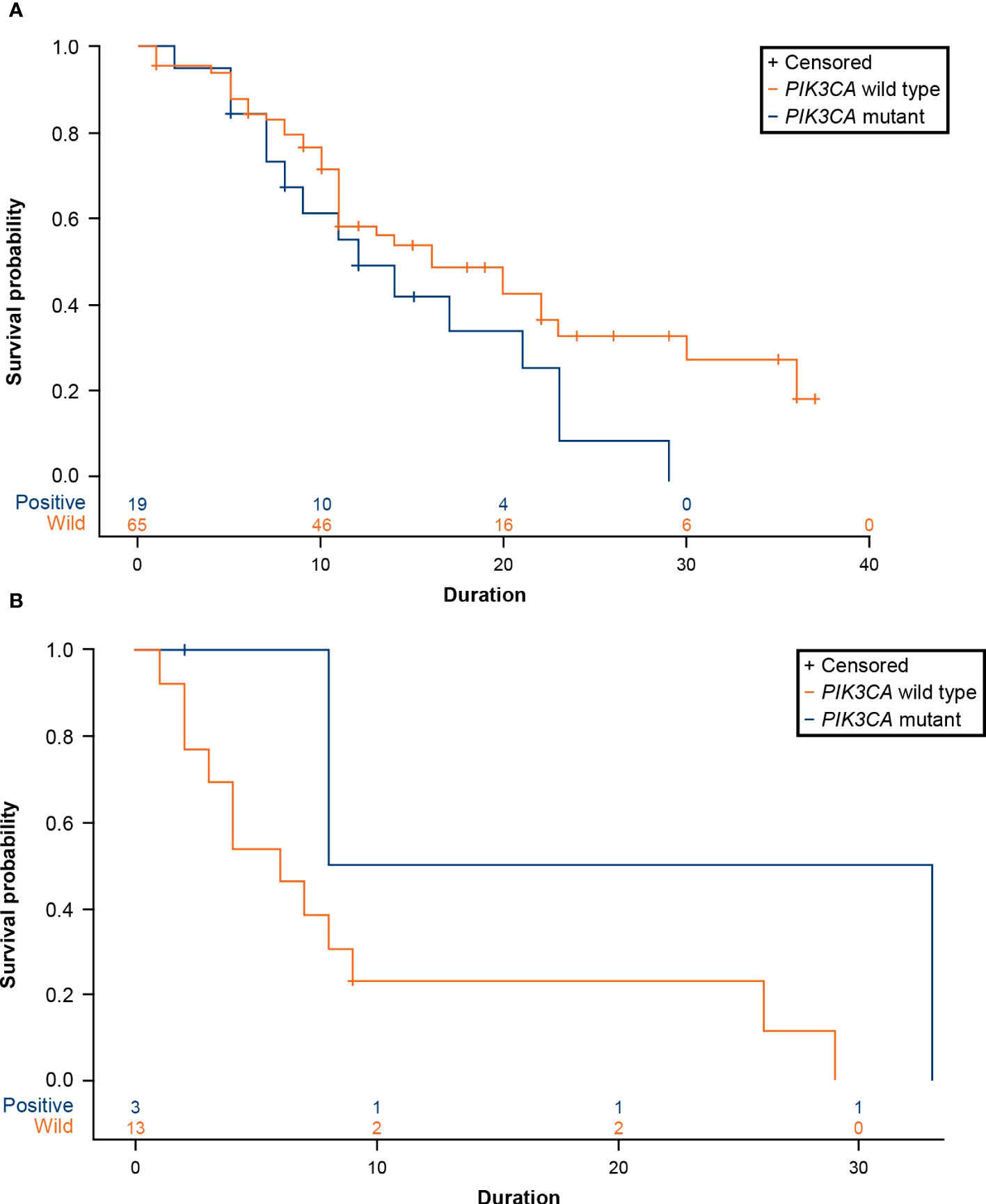
Figure 6 (A) Product-limit survival estimates of CDK4/6 inhibitors. (B) Product-limit survival estimates of everolimus (an mTOR inhibitor). CDK4/6, cyclin-dependent 4 and 6 kinase; mTOR, mammalian target of rapamycin; PIK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha.
4 Discussion
PIK3CA mutations are significantly associated with breast cancer occurrence and provide a growth advantage to cancer cells, which leads to progression and drug resistance (18). Hence, early detection of PIK3CA mutations may help in identifying patients who might benefit from a more effective personalized targeted therapy (19). In the present study, we reported the prevalence of PIK3CA mutations in Taiwanese patients with breast cancer stratified by IHC subtypes using an NGS database. In addition, we assessed the impact of PIK3CA mutation status on time to treatment failure of CDK4/6 and mTOR inhibitors in patients with metastatic breast cancer.
In this study, genes in PIK3/AKT/mTOR pathway were deciphered, and PIK3CA mutations were identified as the most frequent genetic alterations observed in 38% of Taiwanese patients with breast cancer. Similarly, an earlier study by Huang et al. reported PIK3CA mutation as the most frequent mutation occurring in 38% of Taiwanese patients with breast cancer (12). The differences in the PIK3CA mutation frequency observed between the 2 studies could be attributed to the different sampling sizes and techniques of analysis.
The frequency of PIK3CA mutations varied by breast cancer subtype and menopausal status. A high prevalence of PIK3CA mutations was observed in patients with HR−/HER2+ status (43%) and HR+/HER2– postmenopausal status (42%), followed by HR+/HER2+ patients (41%). A comparatively lower prevalence was seen in HR+/HER2– premenopausal patients (29%), followed by patients with triple-negative breast cancer (27%). Although there is no consensus about a predisposition of PIK3CA mutation by breast cancer subtype, several studies have reported that 13.3% to 61.5% of HR+/HER2– ABC tumors and 12% to 25% of HER2+ tumors had PIK3CA mutations, whereas triple-negative breast cancer harbors the lowest rates of PI3KCA mutations (20, 21). A recent-published study (AURORA, BIG 14-01) analyzed 339 patients treated with first-line endocrine therapy plus CDK4/6 inhibitor, and found that PIK3CA mutation rate was 40%, which was similar to our findings (42% in HR+/HER2– patients). These mutations were not associated with first-line outcome (HR: 1.07 [95% CI: 0.66-1.75], P=0.65) (22). In our study with limited case numbers (n=84) and heterogenous characteristics (CDK4/6 inhibitors not purely first-line), it is difficult to compare the outcome in patients with PIK3CA mutations in these two studies. In our study, the most commonly reported hotspot mutations were H1047R (41.6%), followed by E545K (18.9%) and E542K (10.3%). These results were consistent with prior findings that reported H1047R, E545K, and E542K as the most common hotspots for PIK3CA mutations (5, 8, 9).
In the present study, 27 patients were reported with multiple PIK3CA mutations. Of note, a prior report by Vasan et al. indicated markedly increased sensitivity of multiple PIK3CA-mutant tumors to PI3K inhibitors, compared with a single hotspot mutation (23). In the Taiwanese population, PIK3CA and PIK3R1 mutations were observed to be mutually exclusive. This mutual exclusivity of PIK3CA and PIK3R1 mutations was reported earlier, leading to oncogenesis and activation of the PI3K pathway (24).
In a recent study by Pavithran et al., the presence of PIK3CA mutations was associated with reduced sensitivity to CDK4/6 inhibitors (25). In the present study, the median time to treatment failure of CDK4/6 inhibitors was much lower in patients with PIK3CA mutations than those without PIK3CA mutations (12 months vs 16 months; HR: 1.670; 95% CI: 0.908-3.069). However, this profile was shorter than that in another study that reported a time to treatment failure of 19.7 months where they used CDK4/6 inhibitors as the first-line therapy (26). This difference may be attributed to the discrepancy in patient characteristics, follow-up period, or sample size (24). Findings from the MONALEESA-7 study evaluating ribociclib in premenopausal or perimenopausal women with HR+/HER2– ABC showed a shorter PFS in patients with PIK3CA mutations than those without, although this difference was not statistically significant (27). Collectively, multiple studies demonstrated numerical trends towards shorter PFS in patients with PIK3CA mutations, which might indicate that the presence of PIK3CA mutations may influence the sensitivity to CDK4/6 inhibitors.
Mutations in PIK3CA often result in hyperactivation of the PI3K/mammalian target of rapamycin (mTOR) pathway and may predict response to mTOR inhibitors. In the present study, the median time to treatment failure of an mTOR inhibitor (everolimus) was comparably longer in patients with PIK3CA mutations than those without (20.5 months vs 6 months; HR: 0.244; 95% CI: 0.031-1.922). Further research is required to validate these findings owing to the small sample size of the PIK3CA-mutant cohort (n=3). A combined analysis from BOLERO-1 and BOLERO-3 suggested that patients having tumors with PIK3CA mutations or hyperactive PI3K pathway derived PFS benefit from everolimus, whereas patients having tumors without these molecular alterations did not (28). Moynahan et al. reported that this survival benefit with everolimus was maintained irrespective of the type of PIK3CA genotype in BOLERO-2 (29). This further supports the findings of the current study. The earlier detection of PIK3CA mutations may help oncologists in treatment decisions and thereby help in providing an effective personalized targeted therapy to the patients.
The present study has a few limitations that are worth noting. This was a retrospective, single-center study and was prone to selection bias. Therefore, the actual frequency of genetic alterations observed in the study may not fully represent the general population in Taiwan. Lastly, the small sample size of patients receiving targeted therapies warrants further studies to validate these findings, especially in the case of time to treatment failure.
5 Conclusion
Consistent with previous studies, we identified a high prevalence of PIK3CA mutations in 38% of the Taiwanese patients with breast cancer (12). The lower prevalence in premenopausal patients and patients with triple-negative breast cancer warrants further studies. Most of the mutations were in exon 9 and exon 20, with H1047R, E545K, and E542K being the hotspots. A longer time to treatment failure in wild-type PIK3CA cohorts treated with CDK4/6 inhibitors was reported, which demonstrated the better efficacy of CDK4/6 inhibitors in wild-type PIK3CA cohorts than that in the PIK3CA-mutant cohort. Everolimus, an mTOR inhibitor, reported a longer time to treatment failure in the PIK3CA-mutant cohort and demonstrated better efficacy. Cumulatively, this indicated variations in the prevalence of PIK3CA mutation based on breast cancer IHC phenotype. Detection of mutations at an earlier stage can help in making appropriate therapeutic decisions, thus saving time and resulting in better outcomes for the Taiwanese breast cancer population.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by ethics committee of the Taipei Veterans General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
All authors critically reviewed, provided feedback at each stage of the manuscript, approved the final version and agreed to be accountable for all aspects of the work.
Acknowledgments
The authors would like to express their gratitude to the Taiwan Clinical Oncology Research Foundation, the Melissa Lee Cancer Foundation, the YongLin Healthcare Foundation, and Mr Morris Chang for their kind support during the study. The authors thank the patients and study investigators involved. The authors thank Caamin Arora for medical writing assistance.
Conflict of interest
Author JL is full time employee of Novartis.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declare his study was supported by Novartis Pharmaceuticals Corporation. The funder was involved in the study design and medical writing assistance was provided by Caamin Arora, M Pharm, from Novartis Pharmaceuticals Corporation, India.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Shih N-C, Kung P-T, Kuo W-Y, Tsai W-C. Association of treatment delay and stage with mortality in breast cancer: a nationwide cohort study in Taiwan. Sci Rep (2022) 12(1):18915. doi: 10.1038/s41598-022-23683-y
2. Hosford SR, Miller TW. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and PI3K/AKT/mTOR pathways. Pharmgenomics Pers Med (2014) 7:203–15. doi: 10.2147/PGPM.S52762
3. Vasan N, Toska E, Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann Oncol (2019) 30(Suppl_10):x3–x11. doi: 10.1093/annonc/mdz281
4. André F, Ciruelos EM, Juric D, Loibl S, Campone M, Mayer IA, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol (2021) 32(2):208–17. doi: 10.1016/j.annonc.2020.11.011
5. Martínez-Sáez O, Chic N, Pascual T, Adamo B, Vidal M, González-Farré B, et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res (2020) 22(1):45. doi: 10.1186/s13058-020-01284-9
6. Rodon J, Dienstmann R, Serra V, Tabernero J. Development of PI3K inhibitors: lessons learned from early clinical trials. Nat Rev Clin Oncol (2013) 10(3):143–53. doi: 10.1038/nrclinonc.2013.10
7. Del Re M, Crucitta S, Lorenzini G, De Angelis C, Diodati L, Cavallero D, et al. PI3K mutations detected in liquid biopsy are associated to reduced sensitivity to CDK4/6 inhibitors in metastatic breast cancer patients. Pharmacol Res (2021) 163:105241. doi: 10.1016/j.phrs.2020.105241
8. Zhao L, Vogt PK. Helical domain and kinase domain mutations in p110α of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc Natl Acad Sci (2008) 105(7):2652–7. doi: 10.1073/pnas.0712169105
9. Mangone FR, Bobrovnitchaia IG, Salaorni S, Manuli E, Nagai MA. PIK3CA exon 20 mutations are associated with poor prognosis in breast cancer patients. Clinics (Sao Paulo) (2012) 67(11):1285–90. doi: 10.6061/clinics/2012(11)11
10. Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA (2005) 102(3):802–7. doi: 10.1073/pnas.0408864102
11. Tripathy D, Harnden K, Blackwell K, Robson M. Next generation sequencing and tumor mutation profiling: are we ready for routine use in the oncology clinic? BMC Med (2014) 12(1):140. doi: 10.1186/s12916-014-0140-3
12. Huang C-C, Tsai Y-F, Liu C-Y, Chao T-C, Lien P-J, Lin Y-S, et al. Comprehensive molecular profiling of Taiwanese breast cancers revealed potential therapeutic targets: prevalence of actionable mutations among 380 targeted sequencing analyses. BMC Cancer (2021) 21(1):199. doi: 10.1186/s12885-021-07931-4
13. André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-mutated, hormone receptor–positive advanced breast cancer. New Engl J Med (2019) 380(20):1929–40. doi: 10.1056/NEJMoa1813904
14. Taiwan Food and Drug Administration: Assessment Report . Available at: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiUytLHwtL5AhXqglYBHbAEBDwQFnoECCIQAQ&url=https%3A%2F%2Fwww.fda.gov.tw%2Ftc%2Fincludes%2FGetFile.ashx%3Fmid%3D189%26id%3D36084%26t%3Ds&usg=AOvVaw1atEjQQExVG92i10vpWwob (Accessed August 19, 2022).
15. Liu CY, Huang CC, Tsai YF, Chao TC, Lien PJ, Lin YS, et al. VGH-TAYLOR: Comprehensive precision medicine study protocol on the heterogeneity of Taiwanese breast cancer patients. Future Oncol (2021) 17(31):4057–69 . doi: 10.2217/fon-2021-0131
16. National Comprehensive Cancer Network. Breast Cancer (Version 1.2023) (2023). Available at: https://www.nccn.org/store/Login/Register.aspx.
17. Hovelson DH, McDaniel AS, Cani AK, Johnson B, Rhodes K, Williams PD, et al. Development and validation of a scalable next-generation sequencing system for assessing relevant somatic variants in solid tumors. Neoplasia (2015) 17(4):385–99. doi: 10.1016/j.neo.2015.03.004
18. Fusco N, Malapelle U, Fassan M, Marchiò C, Buglioni S, Zupo S, et al. PIK3CA mutations as a molecular target for hormone receptor-positive, HER2-negative metastatic breast cancer. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.644737
19. Ferreira-Gonzalez A. Plasma PIK3CA mutation testing in advanced breast cancer patients for personalized medicine: A value proposition. J Appl Lab Med (2020) 5(5):1076–89. doi: 10.1093/jalm/jfaa117
20. Anderson EJ, Mollon LE, Dean JL, Warholak TL, Aizer A, Platt EA, et al. A systematic review of the prevalence and diagnostic workup of PIK3CA mutations in HR+/HER2- metastatic breast cancer. Int J Breast Cancer (2020) 2020:3759179. doi: 10.1155/2020/3759179
21. Castaneda CA, Lopez-Ilasaca M, Pinto JA, Chirinos-Arias M, Doimi F, Neciosup SP, et al. PIK3CA mutations in Peruvian patients with HER2-amplified and triple negative non-metastatic breast cancers. Hematology/Oncol Stem Cell Ther (2014) 7(4):142–8. doi: 10.1016/j.hemonc.2014.09.007
22. Sotiriou C, Ignatiadis M, Crestani TA, Venet D, Tyekucheva S, Irrthum A, et al. Clinico-molecular characteristics associated with outcomes in breast cancer patients treated with CDK4/6 inhibitors: Results from the AURORA Molecular Screening Initiative. J Clin Oncol (2023) 41(Suppl 16):1019. doi: 10.1200/JCO.2023.41.16_suppl.1019
23. Vasan N, Razavi P, Johnson JL, Shao H, Shah H, Antoine A, et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kα inhibitors. Science (2019) 366(6466):714–23. doi: 10.1126/science.aaw9032
24. Huang CS, Liu CY, Lu TP, Huang CJ, Chiu JH, Tseng LM, et al. Targeted sequencing of Taiwanese breast cancer with risk stratification by the concurrent genes signature: A feasibility study. J Pers Med (2021) 11(7):1–9. doi: 10.3390/jpm11070613
25. Pavithran K, Jayamohanan H, Jose WM, Soman S, Vijaykumar DK, Ariyannur PS, et al. PI3K mutation is associated with reduced sensitivity to CDK4/6 inhibitors in metastatic breast cancer. Ann Oncol (2021) 32(5):S457–515. doi: 10.1016/j.annonc.2021.08.539
26. Giridhar KV, Choong GM, Leon-Ferre RA, O’Sullivan CC, Ruddy KJ, Haddad TC, et al. Abstract P6-18-09: Clinical management of metastatic breast cancer (MBC) after CDK 4/6 inhibitors: A retrospective single-institution study. Poster Session Abstracts (2019) 79(Suppl 4):P6–18–09. doi: 10.1158/1538-7445.SABCS18-P6-18-09
27. Bardia A, Su F, Solovieff N, Im S-A, Sohn J, Lee KS, et al. Genomic profiling of premenopausal HR+ and HER2– metastatic breast cancer by circulating tumor DNA and association of genetic alterations with therapeutic response to endocrine therapy and ribociclib. JCO Precis Oncol (2021) 5:1408–20. doi: 10.1200/PO.20.00445
28. André F, Hurvitz S, Fasolo A, Tseng L-M, Jerusalem G, Wilks S, et al. Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2–overexpressing metastatic breast cancers: combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J Clin Oncol (2016) 34(18):2115–24. doi: 10.1200/JCO.2015.63.9161
Keywords: advanced breast cancer, PIK3CA mutations, hotspot mutations, next-generation sequencing, Taiwanese population
Citation: Chao T-C, Tsai Y-F, Liu C-Y, Lien P-J, Lin Y-S, Feng C-J, Chen Y-J, Lai J-I, Hsu C-Y, Lynn JJ, Huang C-C and Tseng L-M (2023) Prevalence of PIK3CA mutations in Taiwanese patients with breast cancer: a retrospective next-generation sequencing database analysis. Front. Oncol. 13:1192946. doi: 10.3389/fonc.2023.1192946
Received: 24 March 2023; Accepted: 25 July 2023;
Published: 15 August 2023.
Edited by:
Deniz Can Guven, Hacettepe University, TürkiyeReviewed by:
Baran Akagündüz, Erzincan University, TürkiyeWei-Pang Chung, National Cheng Kung University Hospital, Taiwan
Copyright © 2023 Chao, Tsai, Liu, Lien, Lin, Feng, Chen, Lai, Hsu, Lynn, Huang and Tseng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Cheng Huang, chishenh74@gmail.com; Ling-Ming Tseng, lmtseng@vghtpe.gov.tw
†These authors have contributed equally to this work
 Ta-Chung Chao1,2,3
Ta-Chung Chao1,2,3 Jiun-I. Lai
Jiun-I. Lai Chi-Cheng Huang
Chi-Cheng Huang Ling-Ming Tseng
Ling-Ming Tseng