- 1Department of Gastrointestinal Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2Laboratory of Gastric Cancer, State Key Laboratory of Biotherapy/Collaborative Innovation Center of Biotherapy and Cancer Center, West China Hospital, Sichuan University, Chengdu, China
- 3Breast Center, West China Hospital, Sichuan University, Chengdu, China
- 4Medical college, Hebei University of Engineering, Hebei, China
- 5Department of Urology, West China Hospital, Sichuan University, Chengdu, China
Purpose: Studies have reported that breast cancer (BC) patients’ circulating tumor cells (CTCs) have varying results for their diagnostic role. Thus, we conducted a meta-analysis to systematically assess the accuracy of CTCs in the diagnosis of BC.
Methods: A meta-analysis was conducted to evaluate the overall accuracy of CTC detection. A pooled analysis of sensitivity (SEN), specificity (SPE), positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic advantage ratio (DOR) was used to measure diagnostic accuracy. In addition, the area under the summary receiver operating characteristic curve (AUC) was used to discriminate BC from non-BC. An analysis of the threshold effect was calculated using the Spearman correlation coefficient. We calculated the Q and I2 statistics to determine whether the studies were heterogeneous. Sensitivity analysis was performed by removing studies one by one. Publication bias was assessed by Deeks’ funnel plot asymmetry test.
Results: Studies from the PubMed, Cochrane Library, Embase, Web of Science, Wanfang, Vip, and CNKI databases were collected for diagnosing BC from January 2000 to April March 2023. Finally, 8 publications were retrieved in total containing 2014 cases involved in the study. Based on a random-effects model, it was found that the pooled SEN was 0.69 (0.55 - 0.80), SPE was 0.93 (0.60 - 0.99), PLR was 9.5 (1.4 - 65.9), NLR was 0.33 (0.23 - 0.48), DOR was 29 (4 - 205) and the AUC of the summary receiver operating characteristic (SROC) curve was 0.81 (0.77 - 0.84). Some heterogeneity was found in the article, but there was no threshold effect to account for it (P = 0.27). Deek’s funnel plot asymmetry test indicated that no publication bias was observed in this meta-analysis (P = 0.52).
Conclusion: The results of this meta-analysis confirmed that CTCs were an important component of noninvasive methods of confirming BC with SEN of 0.69 (0.55 - 0.80), SPE of 0.93 (0.60 - 0.99) and AUC of 0.81 (0.77 - 0.84).
Background
Among women, BC is the most prevalent cancer and is influenced by lifestyle factors, hormonal factors, reproductive factors, and iatrogenic factors. Furthermore, recent data from 185 countries reported 2.3 million new cases (more than 10% of all cancers) of breast cancer and a mortality rate of 6.9%, and BC ranked the second leading cause of death from cancer among women globally (1, 2). During the past few decades, the morbidity of BC has continued to increase around the world (3). Furthermore, a study showed a significant increase in breast cancer mortality rate in low-income regions, while the decreasing rate mostly belongs to Western Europe, with 37.57 in 1990 to 36.00 in 2015 (4). Due to advances in screening methods and breakthroughs in early diagnosis and treatment, BC survival rates have improved. The conventional diagnostic methodologies of BC include breast biopsy, which is regarded as the gold standard, and imaging methods without high sensitivity to detect BC in the early stage (5). In addition, molecular markers, including CA15-3 and CEA, are common markers for monitoring and follow-up of patients by testing BC patient blood samples, but they have low SEN and SPE (6–8). Thus, it was not suitable for the detection of BC. To improve BC cure rates and reduce BC mortality, early diagnosis remains essential. Thus, it is necessary to explore a new test with high SEN and SPE to diagnose BC in the early stage. Recently, a hot research topic about tumors has been the clinical application of CTCs. CTCs, a subset of tumor cells that circulate within the body due to tumor tissue instability or external physical stimulation, participate in the body’s circulation and then integrate into the peripheral blood circulation (9, 10). The Fourth Edition of the National Comprehensive Cancer Network (NCCN) guidelines has added a new M0 (i+) category, which is defined as “no clinical or radiographic evidence of distant metastases, but the presence of detected tumor cells in the circulation fluids” (11). In addition, CTCs have demonstrated efficacy in the screening of malignant cancers such as prostate, lung, and colorectal cancers (9). In a recent study, CTCs were found to be 76.56% sensitive and 95.4% specific for diagnosing breast cancer using the CytoSorter® (12). Nonetheless, several studies have been conducted on CTCs to diagnose BC with varied results by testing peripheral blood. In addition, current studies have shown that CTC detection positive rates (≥ 1 CTC/7.5 ml) range from 11%~54% for early breast cancer, while ≥1 CTC can be detected in approximately 70% of stage IV BC patients. Thus, a meta-analysis was performed to determine whether CTCs are particularly useful as a diagnostic tool in patients with BC.
Methods
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (13).
Literature search
We conducted a comprehensive computer literature search of abstracts from human studies to identify articles about the effectiveness of CTC tests for diagnosing BC by two independent individuals (Tao Jin and Yao Chen). Electronic databases such as PubMed, Embase, Cochrane Library, Web of Science, Wanfang, CNKI, and Vip were used with the following search terms: “CTCs”, “circulating tumor cells”, “breast cancer”, “breast carcinoma”, “accuracy”, “sensitivity and specificity”, from January 2000 to March 2023, without language limitation. We manually searched references in the included literature to identify studies that met our eligibility criteria, and gray literature was also included in the study.
Literature eligibility
The included studies were screened according to the following criteria: (1) Type of trial: studies applying the method of detecting CTCs to diagnose breast cancer; (2) Diagnostic gold standard: histopathological examination or biopsy results; (3) The literature should include sufficient study data including true positive (TP), false positive (FP), true negative (TN), false negative (FN); (4) patients without other malignant tumors; (5) we chose the article with the most detail or the most recent when more than one article presented the same data or subset of data. Exclusion criteria were (1) insufficient information in the literature to obtain complete diagnostic data from the full text of the literature and (2) reports on cases, reviews, letters, single-arm trials, editorials, and duplicate studies.
Data extraction and quality assessment
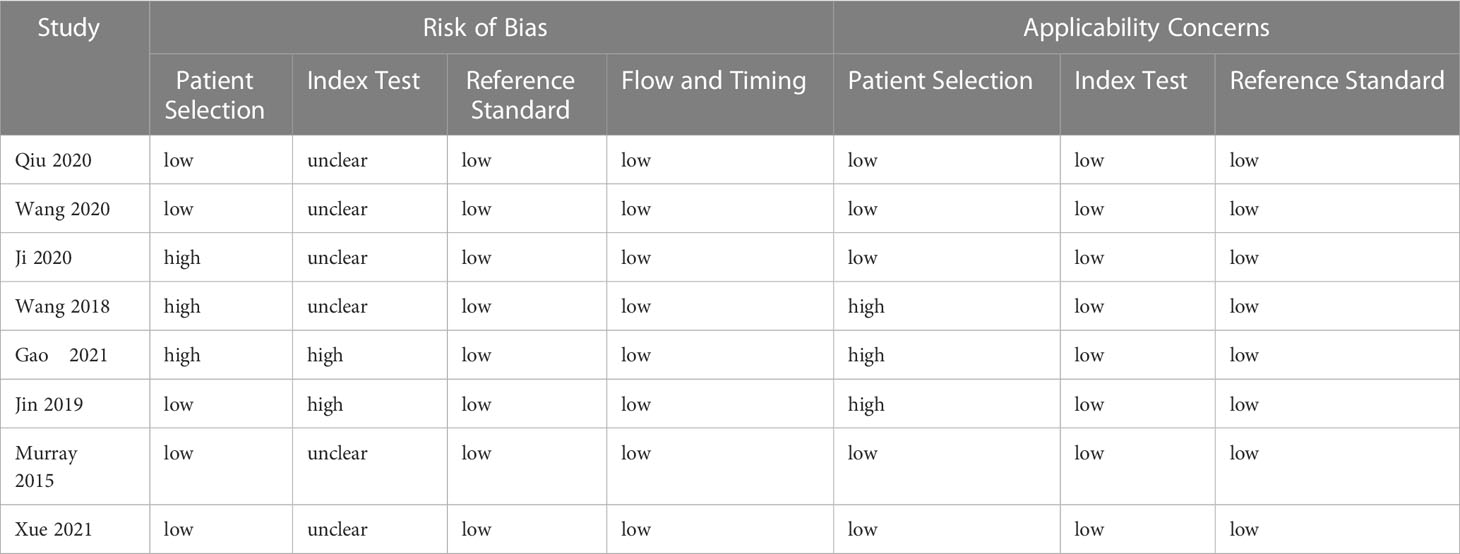
Two independent researchers (Tao Jin and Yao Chen) reviewed all studies. Disagreements between researchers were resolved through discussion and consensus. In the case of disputes, an independent third researcher was responsible for resolving disagreements. The main data information included author, year of publication, country, tumor stage, isolation enrichment method, assay identification method, CTC cutoff, TP, FP, FN, and TN. Data for results not directly reported were derived from estimates of SEN and SPE, along with positive and negative predictive values. Primary outcome measures were pooled estimates of SEN and SPE. Evaluation of the quality of the included literature was carefully conducted using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) (14) by two independent reviewers. The inconsistent evaluation was decided by discussion.
Statistical analysis
The diagnostic accuracy of CTC detection in BC was determined using Stata (version 15.0). Pooled analysis of SEN, SPE, PLR, NLR, and DOR and the corresponding 95% confidence interval (CI) was used to evaluate diagnostic accuracy. The SROC was performed using a bivariate regression approach to identify abnormal examinations that resulted in the expected trade-off between SEN and SPE. In addition, the AUC can summarize the inherent capacity of a test for discriminating BC from non-BC. The threshold effect was analyzed using Spearman correlation coefficients in the heterogeneity analysis. The heterogeneity of the studies was evaluated by the Q test and I2 statistics. I2 values ≥50% indicated substantial heterogeneity; additionally, we considered the difference to be statistically significant at P < 0.05. Sensitivity analysis was performed by a one-by-one exclusion method to determine whether the hypothesis had a significant effect on the results. Deeks’ funnel plot asymmetry was used to assess publication bias, and a significance level of P < 0.05 was considered significant.
Results
Literature search results
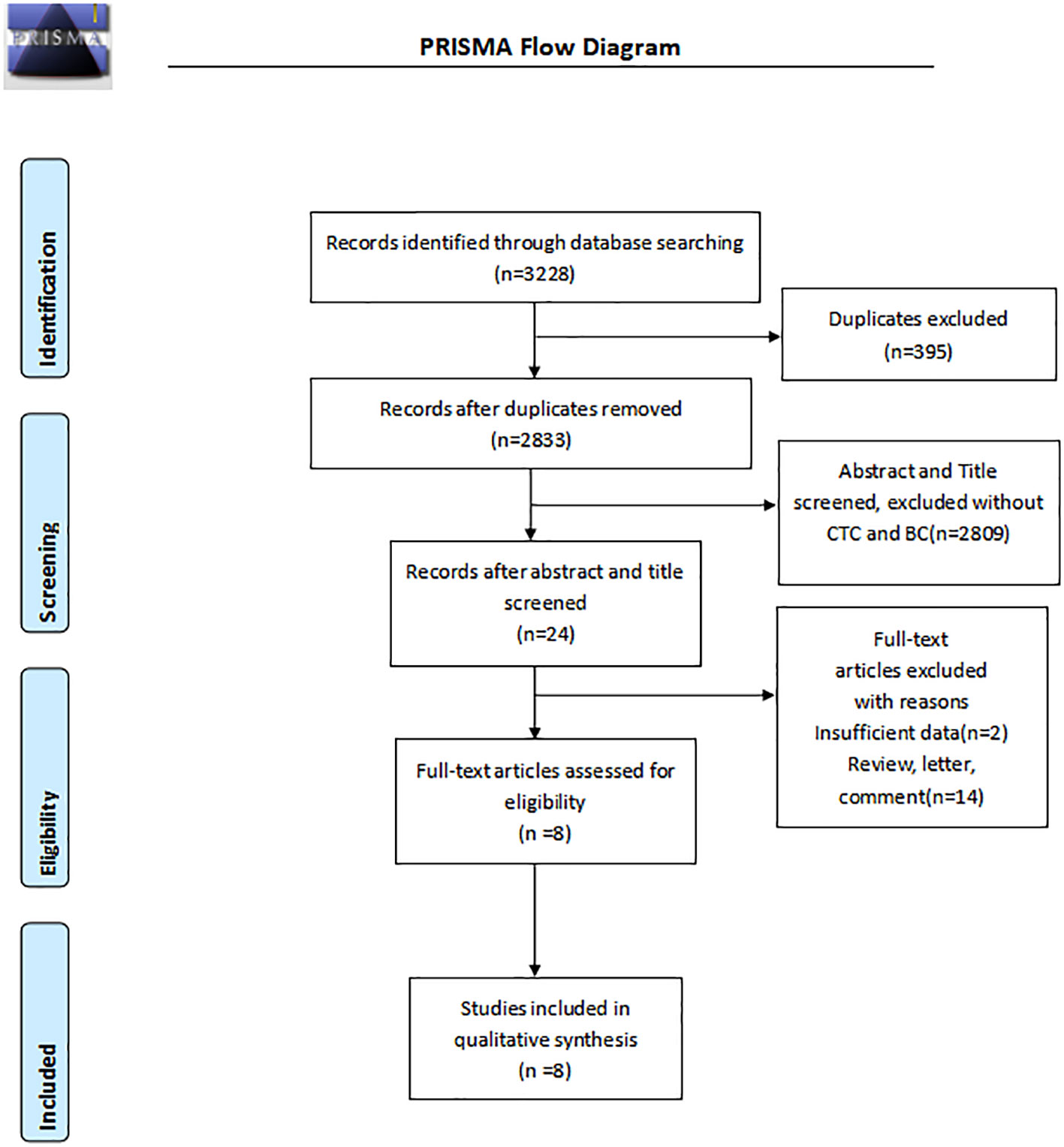
A total of 3225 pieces of literature were retrieved through electronic databases. After excluding duplicates and irrelevant studies, we carefully and independently reviewed the titles and abstracts. Finally, eight studies (12, 15–21), including 2014 cases, met the requirements through careful screening by two independent researchers after reading the full text in detail. The flow diagram in Figure 1 illustrates the process of searching for eligible studies.
Basic characteristics and quality assessment
A summary of the basic characteristics of the included studies is provided in Table 1. All patients were diagnosed with stage I to IV disease. Seven studies were from Asia, and one study was from Western countries. Four, three, and one articles set the CTC cutoff as 2, 1, and 1.5, respectively. The enrichment methods of CTCs included negative enrichment, density gradient centrifugation, CytoSorter, immunomagnetic bead, and CellSearch. Most of the articles used imFISH to identify CTCs. Table 2 presents the results of the QUADAS-2 assessment. Patient selection and index tests accounted for the majority of bias risks.
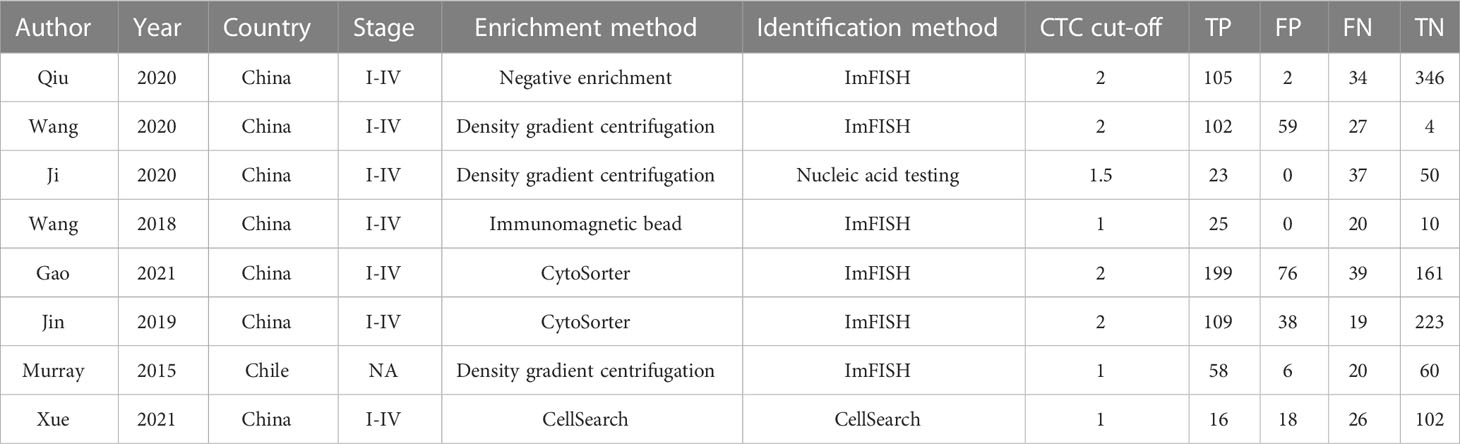
Table 1 Main characteristics of studies included in the meta-analysis of the diagnostic accuracy of CTCs detection in BC.
Accuracy of CTCs in the diagnosis of BC
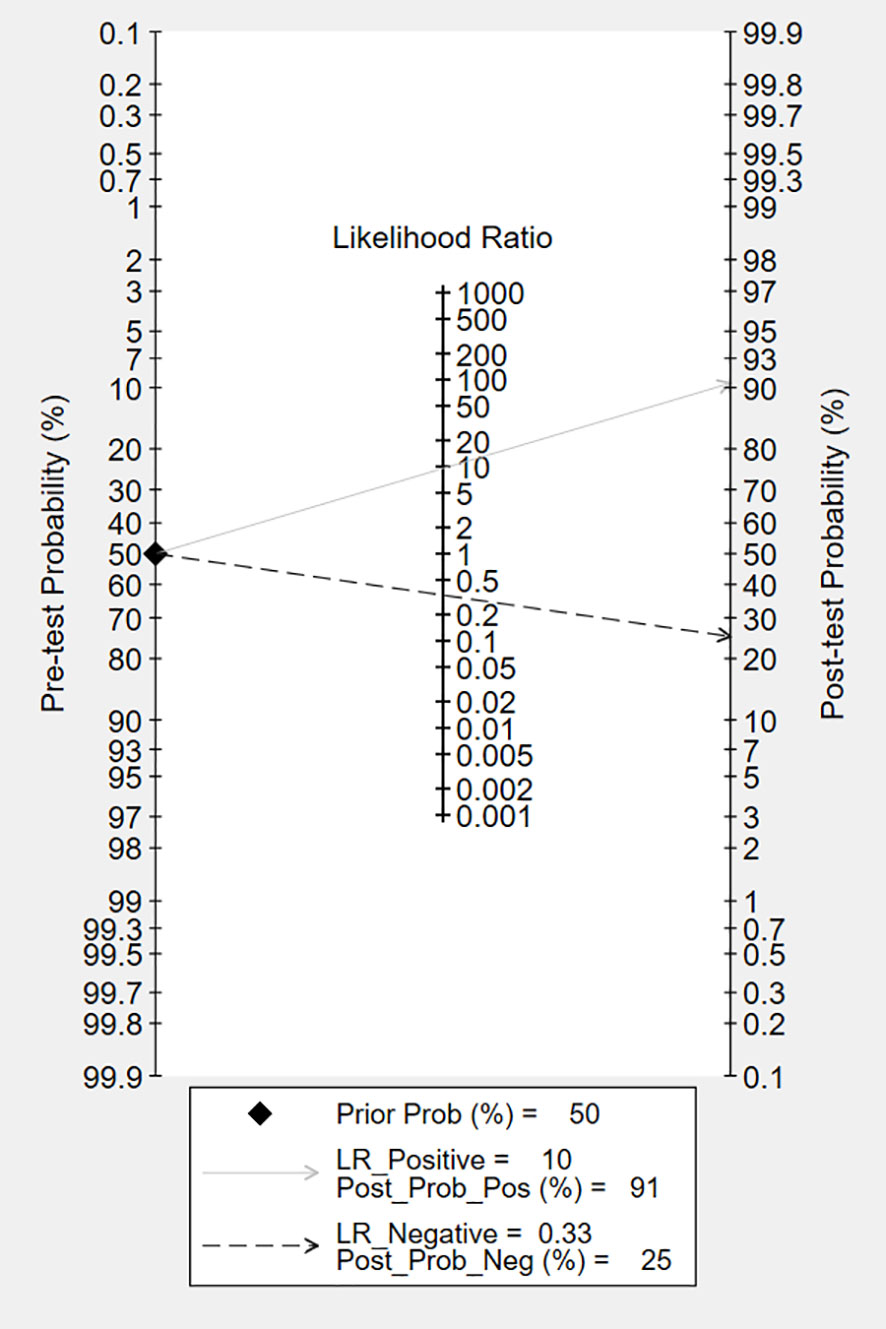
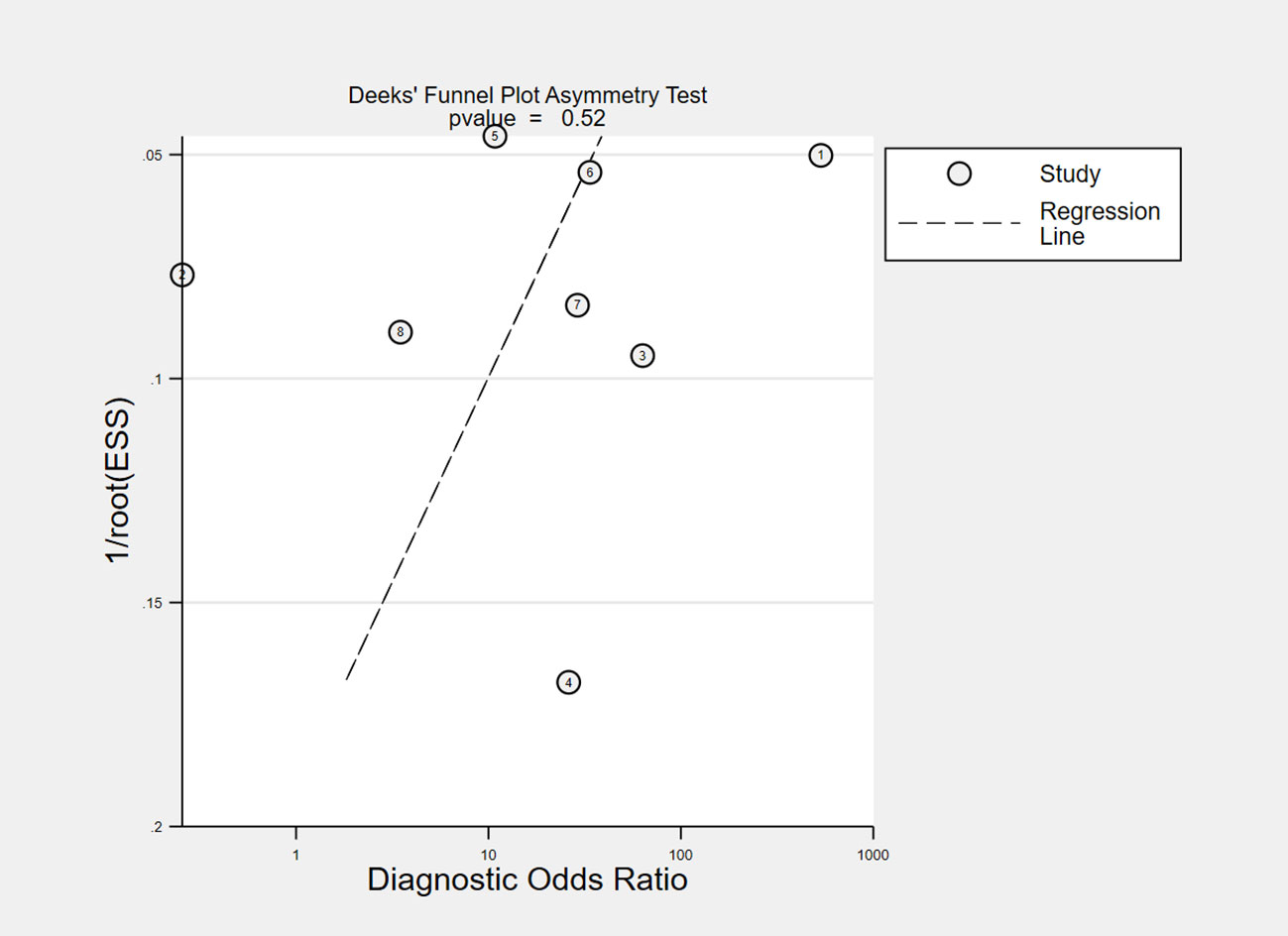
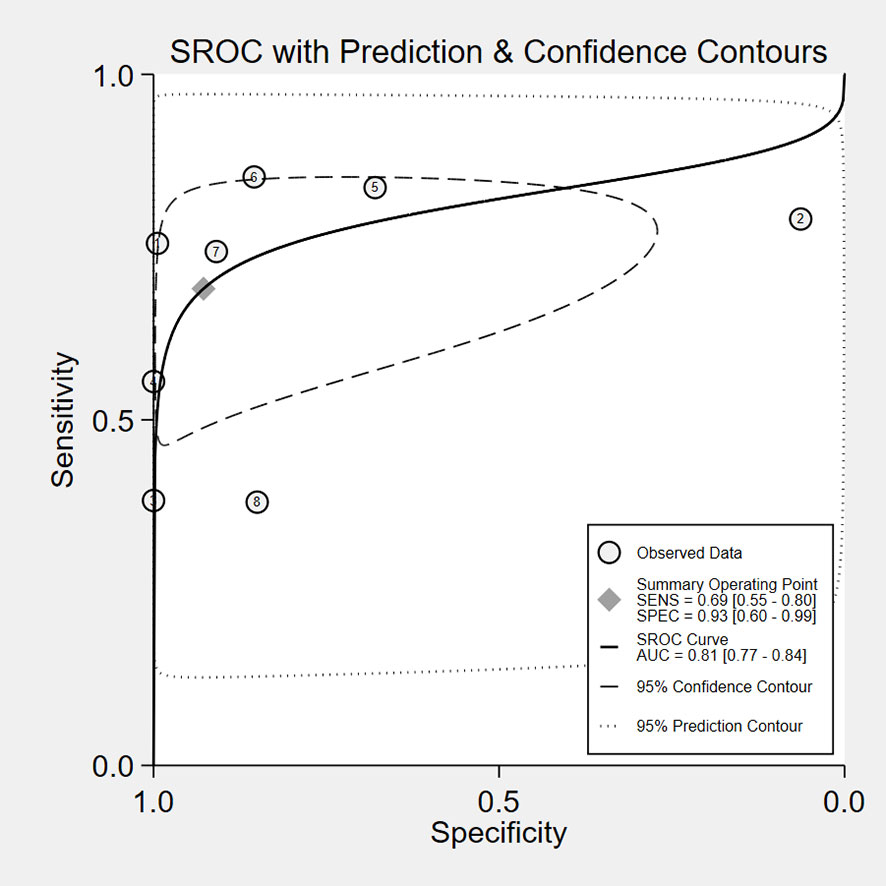
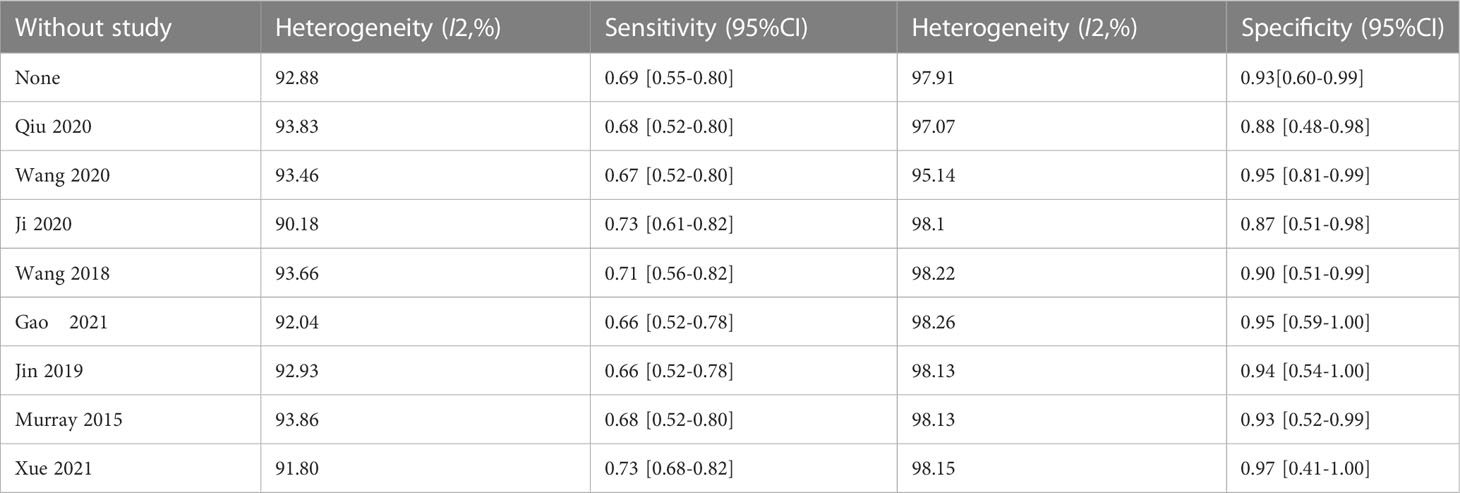
The overall accuracy of CTCs in diagnosing BC was as follows: SEN, 0.69 (0.55 - 0.80); SPE, 0.93 (0.60 - 0.99) (Figure 2); PLR, 9.5 (1.4 - 65.9); NLR, 0.33 (0.23 - 0.48); and DOR, 29 (4 - 205). Figure 3 shows the SROC plot with a 95% CI. The AUC for BC was 0.81 (0.77 - 0.84). The percentage of heterogeneity caused by the threshold effect was 0.27, while the coefficient of correlation in the mixed model was -0.52, which meant no significant influence of the threshold effect. Figure 4 presents the Fagan plot, showing that the prior-test probability of BC was 50%. Furthermore, the posttest probability of BC, given a negative result, was 25%, while 91% had a positive result for CTC detection in this meta-analysis. Deek’s funnel plot asymmetry test demonstrated that the slope coefficient P value was 0.52, suggesting that there was no significant publication bias (Figure 5). Sensitivity analysis (Table 3) showed a slight change when removing articles one by one, indicating that the results were robust.
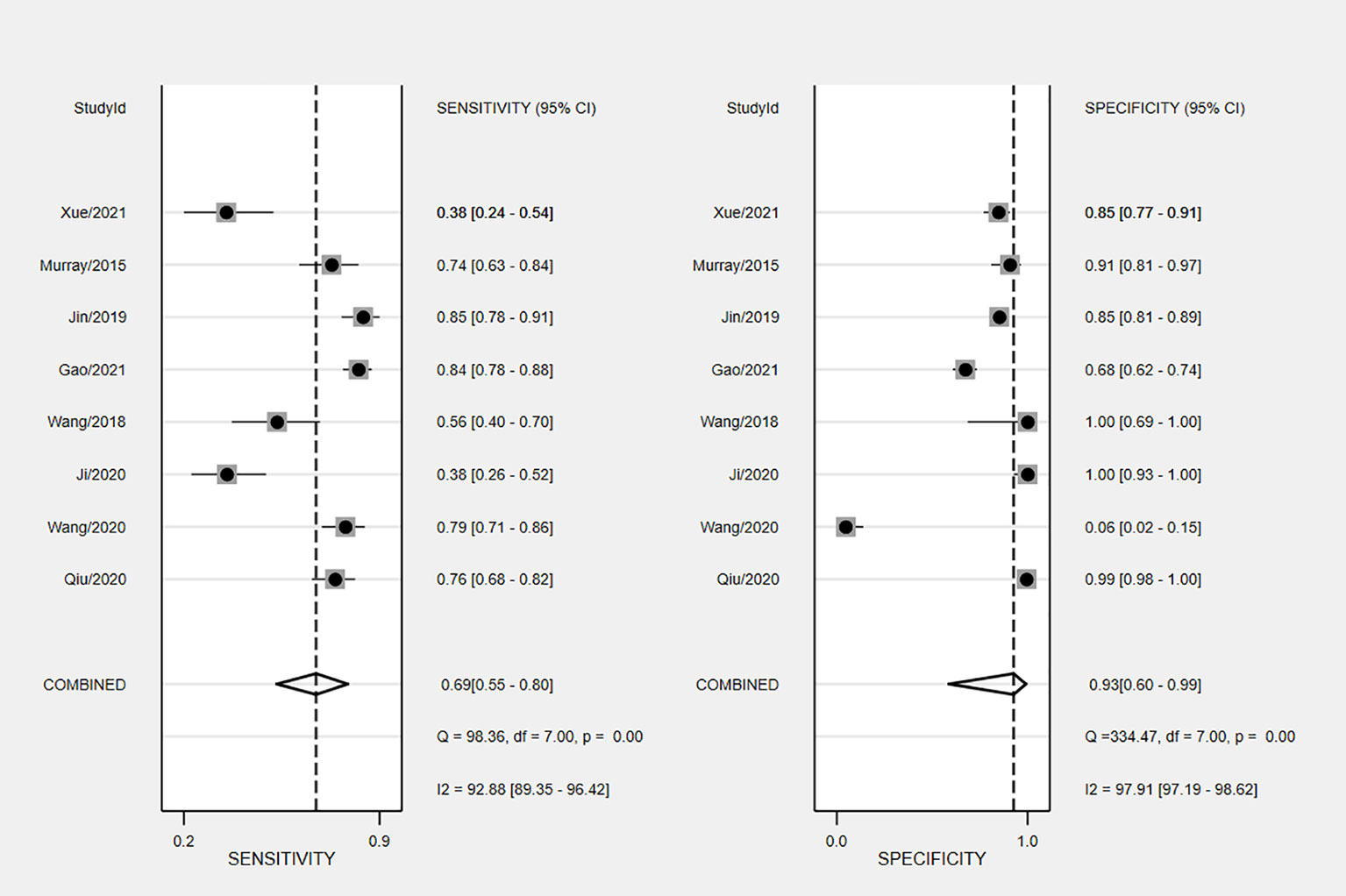
Figure 4 Fagan nomogram plot analysis for the evaluation of CTCs as a diagnostic tool for detecting BC.
Discussion
Breast ultrasound and mammography are currently the main methods for screening BC, but with low SEN, they are easily influenced by breast density, and the incidence of false negatives and false positives is high (22). Serological markers such as carbohydrate antigen CA153 and carcinoembryonic antigen (CEA) have the characteristics of noninvasiveness, nonradiation, and low price but still have low SEN and SPE. Thus, they are not suitable for the early diagnosis of BC (23). CTCs are cancer cells that contain unique biomarkers and are commonly found in blood samples from individuals with solid tumors but often not in healthy populations. The prognostic relevance of CTCs in many types of metastatic cancer has already been demonstrated (24–26). According to the eighth AJCC cancer staging manual, BC patients with CTCs are at a greater risk for poor outcomes (27). In recent years, CTC detection has been proven to be helpful in the diagnosis of lung cancer (28), bladder cancer, urothelial cancer (29), pancreatic cancer (30), and so on. Furthermore, CTCs in peripheral blood have been used to diagnose BC in a limited number of studies, with varying results. Consequently, we conducted the first meta-analysis to assess the diagnostic value of CTC detection in the peripheral blood of BC patients.
The results of the study, including 2014 individuals from 8 diagnostic accuracy studies, proved that CTCs had high clinical utility in the diagnosis of breast cancer, with a pooled SEN of 0.69 (0.55-0.80), a pooled SPE of 0.93 (0.60-0.99), a pooled LNR of 9.5 (1.4-65.9), a pooled NLR of 0.33 (0.23-0.48), and a total AUC of the SROC curve of 0.81. These results show that the overall accuracy of CTCS in the early diagnosis of BC is relatively good. After sensitivity analysis, the results of the literature we included were stable, indicating that our meta-analysis results are of reference significance. By using the DOR, a diagnostic test evaluation indicator, we could compare the likelihood of positive results between patients with and without the condition. In the present analysis, the pooled DOR was 29 (95% CI, 4–205), indicating that in comparison to patients who do not test positive for CTCs, those who test positive have 29 times the likelihood of developing BC. Based on the above results, CTCs might be helpful as a diagnostic method for BC screening, which is in accordance with a prior study (12). However, the included studies have different CTC detection methodologies, as well as different sensitivity levels, resulting in a varying CTC cutoff value for the same clinical application (31). To date, CellSearch® has been the only CTC system approved by the Food and Drug Administration (FDA). However, CellSearch® had low rates of CTC detection in BC, approximately 40–50% in metastatic BC and just under 30% in early-stage BC (32). There was only one study (21) using CellSearch® in our meta-analysis. Another study (12) reported the CytoSorter® CTC detection system. CytoSorter® was shown to be superior to CellSearch® in detecting CTCs in BC patients at stages II and III, with a detection rate of over 90% (32). Due to the lack of uniform detection standards for CTCs, clinical practice does not consider CTCs to be a standard routine diagnostic tool. Thus, more research is required to determine the criteria for CTC detection. Based on the use of different threshold values in the included studies, we used Spearman’s correlation coefficient to analyze threshold effects and found that there was no connection between thresholds and heterogeneity.
This study has some limitations. First, on account of the relatively small number of cases in this study, we failed to determine the potential source of this study due to the relatively high heterogeneity of this study. Second, in various studies, cutoff values differ, which has an impact on our results, and there is a need for further research on CTCS’s optimal cutoff point. In addition, seven out of eight studies were conducted in Asia, and the electronic databases included regional databases, which could cause bias in the results. It would be beneficial to conduct more international prospective multicenter research on this topic.
Conclusion
This meta-analysis showed that CTCs can be used as a helpful tool in BC screening and early diagnosis, with better sensitivity and specificity. To clarify the accuracy of CTCs as BC diagnostic indicators, more high-quality prospective studies are needed.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TJ, YC and J-QY. The first draft of the manuscript was written by TJ, and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet (2021) 397(10286):1750–69. doi: 10.1016/S0140-6736(20)32381-3
2. Daly AA, Rolph R, Cutress RI, Copson ER. A review of modifiable risk factors in young women for the prevention of breast cancer. Breast Cancer (Dove Med Press) (2021) 13:241–57. doi: 10.2147/BCTT.S268401
3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
4. Azamjah N, Soltan-Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate: A 25-year study. Asian Pac J Cancer Prev (2019) 20(7):2015–20. doi: 10.31557/APJCP.2019.20.7.2015
5. Zhao S, Gai X, Wang Y, Liang W, Gao H, Zhang K, et al. Diagnostic values of carcinoembryonic antigen, cancer antigen 15-3 and cancer antigen 125 levels in nipple discharge. Chin J Physiol (2015) 58(6):385–92. doi: 10.4077/CJP.2015.BAD336
6. Chu WG, Ryu DW. Clinical significance of serum CA15-3 as a prognostic parameter during follow-up periods in patients with breast cancer. Ann Surg Treat Res (2016) 90(2):57–63. doi: 10.4174/astr.2016.90.2.57
7. Wu SG, He ZY, Zhou J, Sun JY, Li FY, Lin Q, et al. Serum levels of CEA and CA15-3 in different molecular subtypes and prognostic value in Chinese breast cancer. Breast (2014) 23(1):88–93. doi: 10.1016/j.breast.2013.11.003
8. Brooks M. Breast cancer screening and biomarkers. Methods Mol Biol (2009) 472:307–21. doi: 10.1007/978-1-60327-492-0_13
9. Farshchi F, Hasanzadeh M. Microfluidic biosensing of circulating tumor cells (CTCs): Recent progress and challenges in efficient diagnosis of cancer. BioMed Pharmacother (2021) 134:111153. doi: 10.1016/j.biopha.2020.111153
10. Zhong X, Zhang H, Zhu Y, Liang Y, Yuan Z, Li J, et al. Circulating tumor cells in cancer patients: Developments and clinical applications for immunotherapy. Mol Cancer (2020) 19(1):15. doi: 10.1186/s12943-020-1141-9
11. Gradishar WJ, Anderson BO, Balassanian R, Blair SL, Burstein HJ, Cyr A, et al. Breast cancer, version 4.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2018) 16(3):310–20. doi: 10.6004/jnccn.2018.0012
12. Jin L, Zhao W, Zhang J, Chen W, Xie T, Wang L, et al. Evaluation of the diagnostic value of circulating tumor cells with CytoSorter(®) CTC capture system in patients with breast cancer. Cancer Med (2020) 9(5):1638–47. doi: 10.1002/cam4.2825
13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. Bmj (2009) 339:b2700. doi: 10.1136/bmj.b2700
14. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
15. Murray NP, Miranda R, Ruiz A, Droguett E. Diagnostic yield of primary circulating tumor cells in women suspected of breast cancer: The BEST (Breast early screening test) study. Asian Pac J Cancer Prev (2015) 16(5):1929–34. doi: 10.7314/APJCP.2015.16.5.1929
16. Gao Y, Fan WH, Duan C, Zhao W, Zhang J, Kang X. Enhancing the screening efficiency of breast cancer by combining conventional medical imaging examinations with circulating tumor cells. Front Oncol (2021) 11:643003. doi: 10.3389/fonc.2021.643003
17. Fuqing J, Mengwan L, Yulong Y, Yonggang L. CTCs, ctDNA detection and diagnostic value in breast cancer patients. Hainan Med [Chinese Journal] (2020) 31(07):838–41.
18. Ruijuan W, Xueming X, Huanhong Z, Qiang L, Linfang S, Junjie Z, et al. Clinical value of peripheral blood circulation tumor cell detection in breast cancer patients. Fujian Med Journal[Chinese Journal] (2020) 42(05):42–5.
19. Xiaoyang Q, Xuan W, Jun L, Xiaofen Z, Zhiqiang C. The application value of peripheral blood circulation tumor cells combined with CA153 detection in breast cancer screening and staging prediction. J Huazhong Univ Sci Technol (Medical Edition)[Chinese Journal] (2020) 49(03):331–7.
20. Jian W, Xu L, Li WZJ, Tao Z, Rongjin S. A study on the relationship between peripheral blood circulation tumor cells and the clinical pathological features of breast cancer. Chin J Oncol Surgery[Chinese Journal] (2021) 13(03):242–5.
21. Paizura S, Ayyu E, Irina Wenting X, Chenguang Z, Weihua J, et al. Clinical value study of peripheral blood circulation of tumor cells with different fragment length cf DNA in the diagnosis of breast cancer. J Clin Military Medicine[Chinese Journal] (2021) 49(12):1372–4.
22. Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American cancer society. Jama (2015) 314(15):1599–614. doi: 10.1001/jama.2015.12783
23. Gao J, Zhang Q, Xu J, Guo L, Li X. Clinical significance of serum miR-21 in breast cancer compared with CA153 and CEA. Chin J Cancer Res (2013) 25(6):743–8.
24. Wang H, Stoecklein NH, Lin PP, Gires O. Circulating and disseminated tumor cells: diagnostic tools and therapeutic targets in motion. Oncotarget (2017) 8(1):1884–912. doi: 10.18632/oncotarget.12242
25. Giordano A, Giuliano M, De Laurentiis M, Arpino G, Jackson S, Handy BC, et al. Circulating tumor cells in immunohistochemical subtypes of metastatic breast cancer: lack of prediction in HER2-positive disease treated with targeted therapy. Ann Oncol (2012) 23(5):1144–50. doi: 10.1093/annonc/mdr434
26. Liu XR, Shao B, Peng JX, Li HP, Yang YL, Kong WY, et al. Identification of high independent prognostic value of nanotechnology based circulating tumor cell enumeration in first-line chemotherapy for metastatic breast cancer patients. Breast (2017) 32:119–25. doi: 10.1016/j.breast.2017.01.007
27. Xie N, Hu Z, Tian C, Xiao H, Liu L, Yang X, et al. In vivo detection of CTC and CTC plakoglobin status helps predict prognosis in patients with metastatic breast cancer. Pathol Oncol Res (2020) 26(4):2435–42. doi: 10.1007/s12253-020-00847-7
28. Maly V, Maly O, Kolostova K, Bobek V. Circulating tumor cells in diagnosis and treatment of lung cancer. In Vivo (2019) 33(4):1027–37. doi: 10.21873/invivo.11571
29. Msaouel P, Koutsilieris M. Diagnostic value of circulating tumor cell detection in bladder and urothelial cancer: Systematic review and meta-analysis. BMC Cancer (2011) 11:336. doi: 10.1186/1471-2407-11-336
30. Chang Y, Min J, Jarusiewicz JA, Actis M, Yu-Chen Bradford S, Mayasundari A, et al. Degradation of janus kinases in CRLF2-rearranged acute lymphoblastic leukemia. Blood (2021) 138(23):2313–26. doi: 10.1182/blood.2020006846
31. Krawczyk N, Banys M, Hartkopf A, Hagenbeck C, Melcher C, Fehm T. Circulating tumor cells in breast cancer. Ecancermedicalscience (2013) 7:352. doi: 10.3332/ecancer.2013.352
Keywords: breast cancer, diagnosis, circulating tumor cells, CTCs, meta - analysis
Citation: Jin T, Chen Y, Chen Q-Y, Xiong Y and Yang J-Q (2023) Circulating tumor cells in peripheral blood as a diagnostic biomarker of breast cancer: A meta-analysis. Front. Oncol. 13:1103146. doi: 10.3389/fonc.2023.1103146
Received: 20 November 2022; Accepted: 10 March 2023;
Published: 22 March 2023.
Edited by:
Ming Yi, Zhejiang University, ChinaReviewed by:
Marcus Vetter, University Hospital of Basel, SwitzerlandNan Wen, Radboud University Medical Centre, Netherlands
Yin Tao, The People’s Hospital of Jianyang City, China
Copyright © 2023 Jin, Chen, Chen, Xiong and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji-Qiao Yang, jqyangscu@163.com
†These authors share first authorship
 Tao Jin1,2†
Tao Jin1,2† Yang Xiong
Yang Xiong Ji-Qiao Yang
Ji-Qiao Yang