- 1Department of Gastroenterology, Zhongnan Hospital of Wuhan University, Hubei Clinical Center and Key Laboratory for Intestinal and Colorectal Diseases, Wuhan, China
- 2Department of Pathology, Zhongnan Hospital of Wuhan University, Wuhan, China
Plexiform fibromyxoma (PF) is a rare mesenchymal tumor of which the pathogenesis and molecular changes are still unclear. Histologically, it is characterized by a cluster of bland spindle or ovoid cells growing in the mucoid or fibromyxoid stroma rich in small blood vessels. At present, surgical resection is the primary treatment for PF.
Introduction
Gastric plexiform fibromyxoma (PF) is a rare mesenchymal tumor initially reported as a “plexiform angiomyxoid myofibroblastic tumor (PAMT)” by Takahashi et al. in 2007 (1). In 2010, the World Health Organization (WHO) recommended its current name (2). Due to the lack of typical clinical symptoms, endoscopic manifestations, and imaging features, it is difficult to discriminate PF from gastrointestinal stromal tumors and other gastrointestinal mesenchymal tumors. Therefore,PF is often missed in diagnosis or misdiagnosed as gastrointestinal stromal tumor (GIST) by medical professionals. To date, the diagnosis of PF still relies on histological examination of the lesion. This report aims to deepen the understanding of this rare tumor by presenting the first case ofof endoscopic submucosal excavation (ESE)-based diagnosis of PF. In addition, we performed a systematic review of the literature to better understand this pathology to design appropriate treatment and follow-up strategy for patients with PF.
Case report
A 45-year-old woman who suffered from mild abdominal distension and discomfort for more than 10 years received a gastroscopy in the hospital. The result indicated a submucosal apophysis with a rough surface in the lesser curvature of the gastric antrum. The patient was admitted to the Department of Gastroenterology for further examination and treatment. Her medical history showed a mediastinal cyst, thyroid nodule, right breast nodule (BI-RADS type 3), bilateral mammary hyperplasia, and helicobacter pylori infection. Her family history suggested no obvious risks of gastrointestinal tumors. The routine physical examination results seemed normal. The laboratory testing revealed increases in uric acid (377.4 μ mol/l), low-density lipoprotein (3.51mmol/L), thyrotropin (6.0461ulU/ml), Anti-Tg (51.57IU/ml), and A-TPO (233.76IU/mL). No obvious abnormalities were present in the complete blood count, urinalysis, stool analysis, procalcitonin test, and coagulation tests. An enhanced computed tomography scan showed no remarkable changes in the stomach. An upper gastrointestinal endoscopy revealed a submucosal protrusion with a rough surface on the lesser curvature side of the gastric antrum (Figure 1A). The lesion was recorded by a real-time 20-MHz ultrasonic probe. An endoscopic ultrasonography (EUS) indicated the clear gastric wall and a hypoechoic lesion located in the gastric submucosa, with a section size of about 7.7 × 4.1mm (Figure 1B). Subsequently, ESE was performed to remove the lesion under gastroscopy (Figures 1C, D): Multiple submucosal injections were conducted at the periphery of the lesion, and the white tumor mass was found to originate from the submucosal layer. Then it was gradually peeled off and completely removed, followed by closure of the wound with hemoclips. Finally the resected lesion size was 1.6*1.5*0.2cm. The pathological examination revealed that the resected tissue was irregular nodular hyperplasia consisting of spindle fibroblasts and myofibroblast-like cells (Figures 2A, B). The Immunohistochemistry indicated that the tumor was positive for vimentin, CD34, and SDHB but negative for smooth muscle actin (SMA), Desmin, DOG-1, CD117, cytokeratin, ALK-1, and S-100 protein (Figures 2C–L). Approximately 2% of the tumor cells expressed the proliferation marker Ki-67. Based on these findings, the lesion was diagnosed as gastric PF. The patient was alive without any recurrence or metastasis of the tumor after 3 months of follow-up.
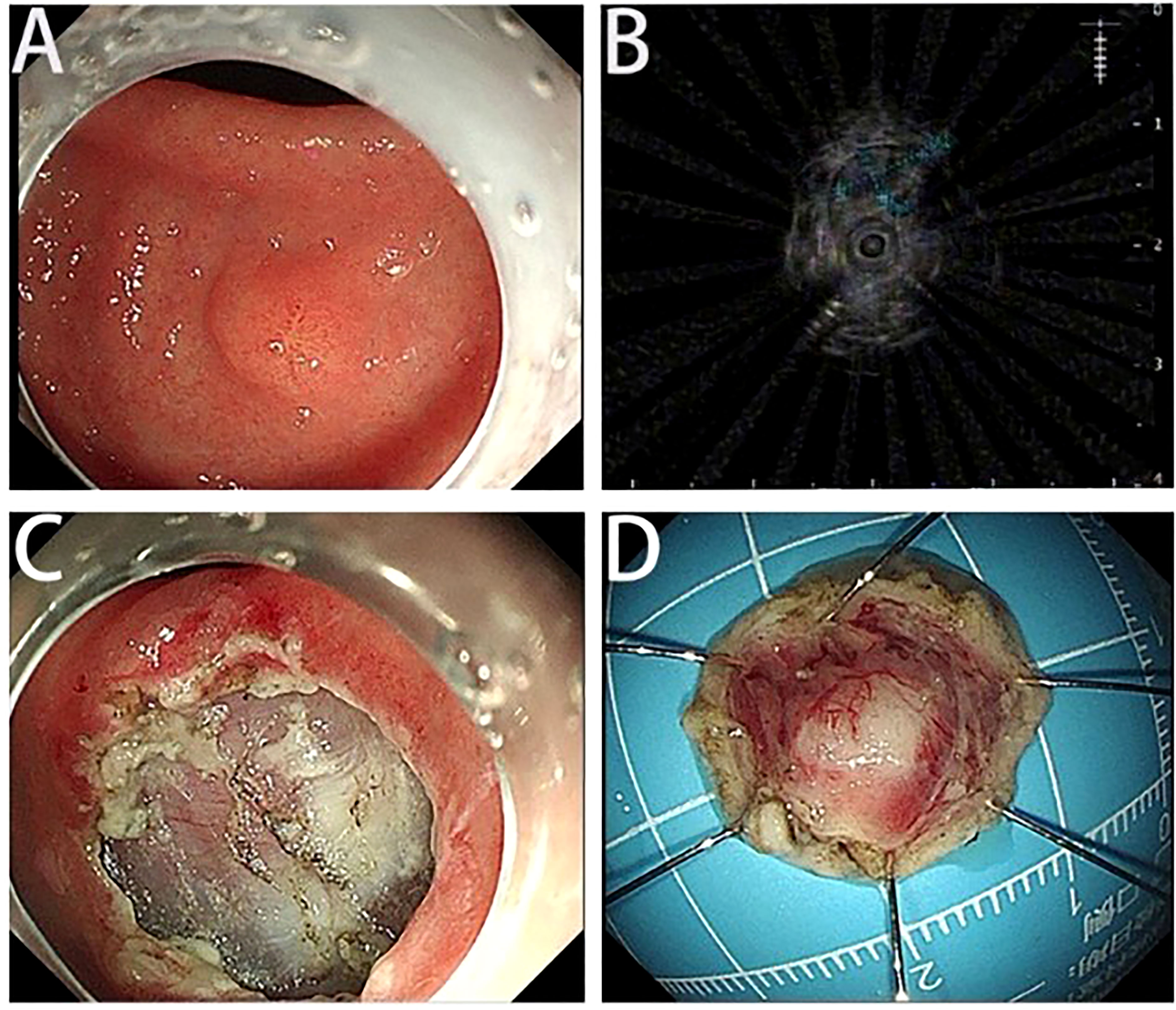
Figure 1 Endoscopic and endoscopic ultrasound findings and ESE images. (A) The upper gastrointestinal endoscopy shows a submucosal protrusion at the lesser curvature of gastric antrum. The tumor surface is covered by normal gastric mucosa. (B) The EUS shows the hypoechoic lesion originating from the submucosa. (C) The wounds after ESE. (D) The resected tumor tissue.
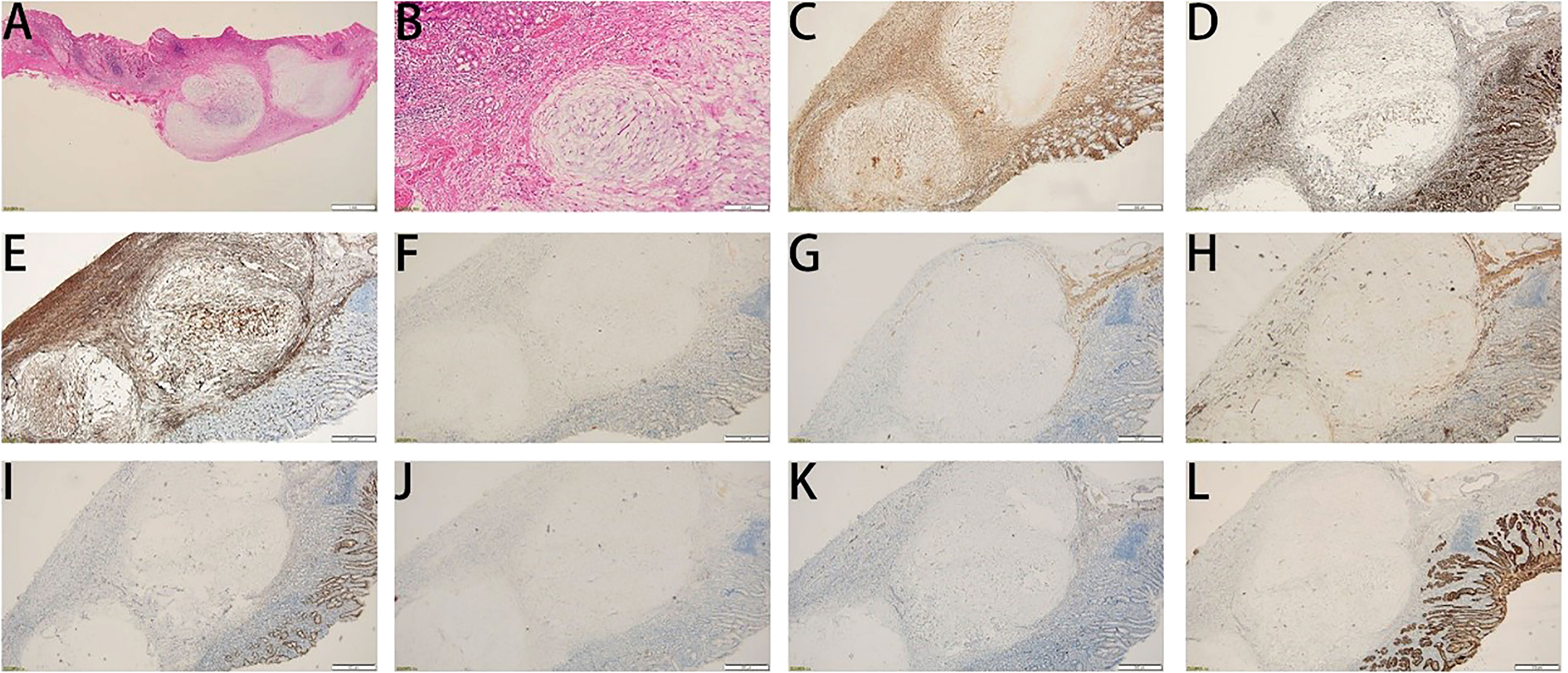
Figure 2 Histological features of the tumor. (A, B) Hematoxylin and eosin staining. (A) Magnification: 10 ×; (B) Magnification: 40 ×. (C-L) Immunohistochemical staining for vimentin (C), SDHB (D), CD34 (E), CD117 (F), DEMSIN (G), SMA (H), DOG1 (I), ALK (J), S100 (K), and CK (L).
Discussion
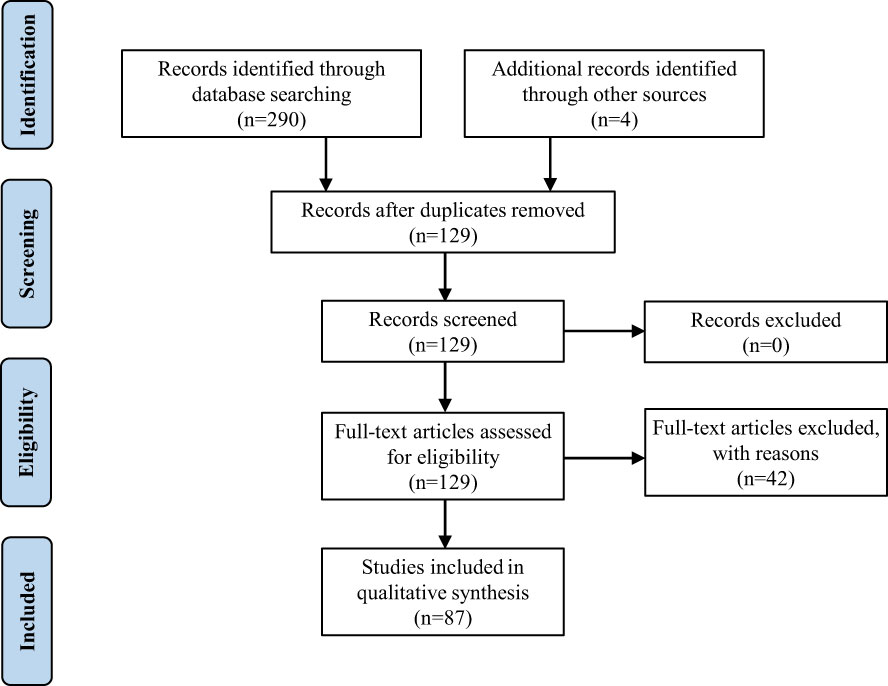
The present study reports the diagnosis of PF for a 45-year-old woman. Due to the rarity and importance of PF, we performed a retrospective review of the literature on which to base the treatment and follow-up of this pathology. Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (i.e., “PRISMA”) guidelines, we searched the keywords “plexiform fibromyxoma” and “plexiform angiomyxoid myofibroblastic tumor” in PubMed, Web of Science, Embase, and Cochrane in January 2023, and eventually found 260 articles. Only articles published in English with available abstracts were included. Moreover, we screened the reference lists of relevant studies and identified other 4 potentially eligible studies. Finally, we selected 87 articles including 139 patients (Supplement Table 1) as shown in the PRISMA flow diagram (Figure 3) (1, 3–88). We extracted the following factors from each article: the first author, date of publication, country, patient number, age, symptoms, tumor size, tumor location, diagnosis, treatment, and follow-up. Data management and analysis were performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA). The data were presented as proportions for categorical variables, mean ± standard deviation, or median (interquartile range) for continuous variables. The Student’s t-test or nonparametric Mann-Whitney U test, where applicable, was used to compare the differences of continuous variables between groups. The Chi-square (χ2) test was used to compare categorical data.
Given the fact that only 33.3% of PF cases were reported by 2015 (approximately 8 years after the initial report of the disorder), the remarkably increased PF incidence in recent years might be attributable to the growing understanding of PF by clinicians and pathologists. The patients’ages ranged from 5 to 81 years (mean age 43.38± 18.26 years; median age 45 years). This age distribution was in approximate accordance with previous reviews (89). Furthermore, PF appeared more frequently in patients between 40-60 years old (38.4%) and 20-40 years old (26.4%). It was rare in patients >75 years old (0.7%). The tumor diameter ranged from 0.8 to 18cm (average 5.23± 3.72 cm), and 61.2% of tumors were 2-6 cm in diameter. Notably, 56.1% of patients were women and 43.9% were men, suggesting a slight female predominance. Interestingly, the age and size distribution of the tumor were significantly independent of gender, as shown by an independent Student’s t-test (p=0.774) and Pearson’s chi-squared test(p=0.360). The condition of the tumor surface was reported in 87 cases: 61 (70.1%) were ulcerated, 26(29.9%) were nonulcerated (normal or eroded mucosa). Ulceration of the tumor was significantly associated with hemorrhage-related signs or symptoms (p=0.001). The difference in tumor size between ulcerative lesions and nonulcerative lesions was not statistically significant (p=0.597).
In 138 reported cases, the most frequent location of PF was the gastric antrum (including pylorus and gastric angle, N=101;73.2%), followed by gastric body (N=16;11.6%), stomach (inside location unspecified, N=6; 4.3%), gastric fundus (N=4;2.9%), duodenum (N=3;2.2%), small bowel(N=3;2.2%), esophagus(N=2; 1.4%), gallbladder (N=1; 0.7%), and mediastinum (N=1; 0.7%).The clinical manifestations ranged from asymptomatic to nonspecific gastrointestinal (GI) symptoms and hemorrhagic gastrointestinal presentations. The most common symptom was abdominal pain. Other clinical presentations included bloating, abdominal discomfort, bleeding, anemia, melena and weight loss. Different manifestations may arise when PF occurred with other diseases or resided in other sites. For example, a 16-year-old girl with mediastinum PF experienced chest pain, shortness of breath, and finger numbness (21). A 35-year-old woman with PF and polycystic ovary syndrome had a cushingoid appearance and amenorrhea (9).
The final diagnosis of PF still relies on histological and immunohistochemical examination of the lesion. Histologically, the typical characteristics of PF include spindle-shaped bland tumor cells arranged characteristically in a plexiform or multinodular pattern, separated by myxoid stroma and rich blood vessels, rare cytological atypia, and mitosis. Immunohistochemistry indicates that PF is diffusively positive for vimentin, muscle-specific actin (MSA), and smooth muscle actin (SMA). The tumor cells may be variably positive for desmin, CD10, and caldesmon. Other markers such as CD117, DOG-1, S100, CD34, β-catenin, anaplastic lymphoma kinase (ALK), and cytokeratin were almost negative. However, how to distinguish between PF and benign lesions before or during surgery to avoid overtreatment is still confusing. Moreover, it is difficult to distinguish PF from other SMT with malignant tendencies such as GIST, the most common SMT (61). EUS is useful to identify SMT and it tells the size, origin layer, echo pattern, lesion margin, and phenotype of SMT (90). Importantly, EUS recognizes the tumor location which is crucial for diagnosis. EUS not only identifies extramural compression and intramural lesions but reveals the nature of lesions (90). In a prospective study of EUS performance, the criteria for predicting malignancy included a tumor size of more than 3 cm, an inhomogeneous pattern, irregular outer margins, and lymph nodes larger than 10 mm in diameter (91). EUS suggests that PF predominantly originates from the submucosal or muscularis propria and is hypoechoic with mild heterogeneity. Hyperechoic lesions were scarce in PF cases (39, 79). However, EUS alone is insufficient to make a definitive diagnosis in most cases, especially those with hypoechoic lesions (90, 91). Therefore, EUS-guided fine needle aspiration (EUS-FNA) plus immunohistochemical analysis is needed for diagnosis. The EUS-based diagnostic traits of GIST include 1) Mildly hypoechoic and well-delineated homogeneous lesion in continuity with the fourth hypoechoic sonographic layer (muscularis propria). 2) EUS-FNA cytology shows that most GIST cells are spindle cells with elongated to wavy nuclei (53). 3) Few GIST tumors show plexiform or nodular growth. 4) GIST tumors are positive for DOG-1 and CD117 and have mutations of the KIT or PDGFRA gene (92), which are not seen in all the 139 cases involved in our study. It is also necessary to distinguish between PF and gastrointestinal leiomyoma. Gastrointestinal leiomyoma involves esophageal and gastric leiomyomas. EUS shows that gastrointestinal leiomyoma is a well-circumscribed hypoechoic homogeneous lesion in the second (muscularis mucosae) or fourth layer (muscularis propria) (90). Microscopically, a leiomyoma comprises irregular fascicular smooth muscle cells with bright eosinophilic cytoplasm and blunt-ended nuclei. Gastrointestinal leiomyoma is positive for SMA and desmin. Furthermore, PF must be discriminated against schwannomas. Schwannomas are tumors of neural origin and are mainly located in the proximal portion of the stomach. On EUS, schwannomas look similar to PF or GISTs. However, schwannomas are typically diffusively and strongly positive for S100 (93). It should be noted that the results of EUS-FNA could be affected by the tumor size, location, number of punctures, tumor nature, patient condition, and experience of pathologists.
Of all the 139 reported cases, 117 cases offered treatment, with most performed for surgical resection (87.2%), only 11.1% for endoscopic treatment, and two cases adopted to biopsy-only without resection. In our case, we initially considered benign submucosal lesions based on contrast-enhanced CT and EUS. So we decided to remove the tumor through ESE rather than surgical resection. Previous studies reported no recurrence or metastasis of PF after resection in the follow-up, suggesting PF as a benign mesenchymal tumor. Recently, PF recurrence and metastasis (liver and bilateral ovary metastasis) were reported one year after ESD and the patient died 4 months after total gastrectomy (81). This is the first case of fatal PF recurrence and distant metastasis. Furthermore, vascular and viscera invasion have also been reported in individual cases (6, 88). Therefore, whether PF is benign requires further investigation. Follow-up data were available for 88 cases, the uneventful or alive duration ranged from 0.75 to 306 months, with a median of 14 months. We found that surgical resection was performed for all patients with bleeding symptoms (including hematemesis, upper gastrointestinal bleeding, melena), but the difference in treatment between with bleeding symptoms and with non-bleeding patients was not statistically significant(p=0.074). When discussing the influencing factors of treatment methods, we found that the difference in tumor location between surgical and nonsurgical treatment was not statistically significant (P =0.184) while the size and surface condition of lesions were (P=0.001, P=0.005). Due to its good prognosis and based on relevant guidelines on SMT, we recommend that if there are no clinically malignant features, such as irregular margins, ulceration, and/or growth during endoscopic follow-up, gastric PF less than 2 cm in diameter could be removed by endoscopy. If PF is larger than 2 cm in diameter and displays high-risk features and SMT increases in size, surgical resection is highly recommended. It is noteworthy that this case is relatively complicated in the presence of the mediastinal cyst. Whether there is a correlation between the two entities needs further evaluation
In conclusion, gastric PF is an extremely rare mesenchymal tumor. Till now, surgical resection is the primary treatment option for this disease. The final diagnosis of PF depends on the pathology and immunohistochemistry after resection. However, it is always necessary to make preoperative pathological diagnosis for all SMT cases. In this text, EUS - FNA samples and subsequent careful morphological evaluation and immunohistochemical staining make it possible to diagnose PF before or during operation. Previous studies reported no recurrence or metastasis of PF after resection in the follow-up, suggesting PF as a benign mesenchymal tumor. However, the recently reported cases of PF recurrence and distant metastasis and multiple organ invasion deserve our more consideration about whether it is a benign disease. In the future, research regarding the pathogenesis and molecular changes of tumor development and effective treatment methods are required for improving the diagnosis and treatment of PF.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZX and ZZ analyzed the clinical data, and prepared the initial manuscript. ZX and ZZ contributed equally to this study. HW and FW performed the operation together. WG performed pathological diagnosis. FZ designed the study, and revised the manuscript critically. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by National Natural Science Foundation of China (grant number 82070569).
Acknowledgments
We would like to thank all the medical staff involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1090259/full#supplementary-material
Supplementary Table 1 | Characteristics of studies included in the review.
References
1. Takahashi Y, Shimizu S, Ishida T, Aita K, Toida S, Fukusato T, et al. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg Pathol (2007) 31(5):724–8. doi: 10.1097/01.pas.0000213448.54643.2f
2. Bosman FT. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer (2010). 417 p.
3. Rau TT, Hartmann A, Dietmaier W, Schmitz J, Hohenberger W, Hofstaedter F, et al. Plexiform angiomyxoid myofibroblastic tumour: Differential diagnosis of gastrointestinal stromal tumour in the stomach. J Clin pathol (2008) 61(10):1136–7. doi: 10.1136/jcp.2008.059162
4. Galant C, Rousseau E, Ho Minh Duc DK, Pauwels P. Re: Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am J Surg pathol (2008) 32(12):1910. doi: 10.1097/PAS.0b013e3181838fa9
5. Yoshida A, Klimstra DS, Antonescu CR. Plexiform angiomyxoid tumor of the stomach. Am J Surg pathol (2008) 32(12):1910–2. doi: 10.1097/PAS.0b013e3181838fd1
6. Miettinen M, Makhlouf HR, Sobin LH, Lasota J. Plexiform fibromyxoma: A distinctive benign gastric antral neoplasm not to be confused with a myxoid GIST. Am J Surg Pathol (2009) 33(11):1624–32. doi: 10.1097/PAS.0b013e3181ae666a
7. Pailoor J, Mun KS, Chen CTC, Pillay B. Plexiform angiomyxoid myofibroblastic tumour of the stomach. Pathology (2009) 41(7):698–9. doi: 10.3109/00313020903305753
8. Kishi H, Monteiro MRMB, Santo GC. Gastric plexiform fibromyxoma: A case report. Histopathology (2010) 57:102–3. doi: 10.1111/j.1365-2559.2010.03637.x
9. Sing Y, Subrayan S, Mqadi B, Ramdial PK, Reddy J, Moodley MS, et al. Gastric plexiform angiomyxoid myofibroblastic tumor. Pathol Int (2010) 60(9):621–5. doi: 10.1111/j.1440-1827.2010.02569.x
10. Takahashi Y, Suzuki M, Fukusato T. Plexiform angiomyxoid myofibroblastic tumor of the stomach. World J gastroenterol (2010) 16(23):2835–40. doi: 10.3748/wjg.v16.i23.2835
11. Tan CYS, Santos LD, Biankin A. Plexiform angiomyxoid myofibroblastic tumour of the stomach: A case report. Pathology (2010) 42(6):581–3. doi: 10.3109/00313025.2010.508739
12. Wang WY, Li JN, Li GD. Plexiform angiomyxoid myofibroblastic tumour of the gastric fundus: Successful diagnosis and treatment by endoscopy. J Clin pathol (2010) 63(6):569–70. doi: 10.1136/jcp.2010.076646
13. Kim A, Bae YK, Shin HC, Choi JH. Plexiform angiomyxoid myofibroblastic tumor of the stomach: A case report. J Korean Med sci (2011) 26(11):1508–11. doi: 10.3346/jkms.2011.26.11.1508
14. Majdoub W, Sellami-Dhouib R, Abbes I, Doghri R, Driss M, Sassi S, et al. A rare benign gastric tumour. Virchows Archiv (2011) 459:S177–S8. doi: 10.1007/s00428-011-1113-y
15. Bl R, Yin W, Liu XL, Wei HM, Sheng WQ, Wang J. Plexiform angiomyxoid myofibroblastic tumor of stomach. Chin J Pathol (2012) 41(11):756–60. doi: 10.3760/cma.j.issn.0529-5807.2012.11.010
16. Kang Y, Jung W, Do IG, Lee EJ, Lee MH, Kim KM, et al. Plexiform angiomyxoid myofibroblastic tumor of the stomach: Report of two cases and review of the literature. Korean J Pathol (2012) 46(3):292–6. doi: 10.4132/KoreanJPathol.2012.46.3.292
17. Li P, Zhang Q, Jia X, Li Q, Li Z, Wang Z. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Open J Pathol (2012) 02(04):147–9. doi: 10.4236/ojpathology.2012.24027
18. Schulz T, Drgac J, Chmelar C, Hohler T, Agaimy A, Vieth M. Plexiform angiomyxoid myofibroblastic tumour of the stomach. Pathologe (2012) 33(1):65–8. doi: 10.1007/s00292-011-1548-6
19. Wang LM, Chetty R. Selected unusual tumors of the stomach: A review. Int J Surg Pathol (2012) 20(1):5–14. doi: 10.1177/1066896911429300
20. Baek S-H, Yoon J-H, Kim J-Y. Plexiform angiomyxoid myofibroblastic tumor of the stomach: Report of a case and review of the literature. J Korean Soc Radiol (2014) 70(1):47–52. doi: 10.3348/jksr.2014.70.1.47
21. Duckworth LV, Gonzalez RS, Martelli M, Liu C, Coffin CM, Reith JD. Plexiform fibromyxoma: Report of two pediatric cases and review of the literature. Pediatr Dev Pathol (2014) 17(1):21–7. doi: 10.2350/13-09-1373-OA.1
22. Ikemura M, Maeda E, Hatao F, Aikou S, Seto Y, Fukayama M. Plexiform angiomyxoid myofibroblastic tumor (PAMT) of the stomach. a case report focusing on its characteristic growth pattern. Int J Clin Exp pathol (2014) 7(2):685–9.
23. Lee PWT, Yau DTW, Lau PPL, Chan JKC. Plexiform fibromyxoma (plexiform angiomyxoid myofibroblastic tumor) of stomach: An unusual presentation as a fistulating abscess. Int J Surg Pathol (2014) 22(3):286–90. doi: 10.1177/1066896913492198
24. Li PF, Yang SL, Wang CC, Li YM, Geng M. Presence of smooth muscle cell differentiation in plexiform angiomyxoid myofibroblastic tumor of the stomach: A case report. Int J Clin Exp Pathol (2014) 7(2):823–7.
25. Sakamoto K, Hirakawa M, Atsumi K, Mimori K, Shibata K, Tobo T, et al. A case of gastric plexiform fibromyxoma: Radiological and pathological findings. Japanese J Radiol (2014) 32(7):431–6. doi: 10.1007/s11604-014-0315-z
26. Banerjee N, Gupta S, Dash S, Ghosh S. Plexiform angiomyxoid myofibroblastic tumour of the duodenum: A rare entity. BMJ Case Rep (2015) 2015. doi: 10.1136/bcr-2015-210004
27. Fassan M, Salmaso R, Saraggi D, Alaggio R, Guido M, Balsamo L, et al. Plexiform fibromyxoma of the gallbladder. Pathologica (2015) 107(3-4):181–4.
28. Lu B, Ye W, Liu H. A rare gastric tumor in a young woman. gastric plexiform angiomyxoid myofibroblastic tumor. Gastroenterology (2015) 149(2):294–5. doi: 10.1053/j.gastro.2015.03.050
29. Ni Z, Xie XM, Xu H. Plexiform fibromyxoma of the stomach: Report of one case and review of the literature. World Chin J Digestol (2015) 23(31):5085–8. doi: 10.11569/wcjd.v23.i31.5085
30. Wei J, Liu W, Sun A. Plexiform angiomyxoid myofibroblastic tumor of stomach: Report of a case. Zhonghua bing li xue za zhi Chin J pathol (2015) 44(1):61–2.
31. Dixit JD, Sharief SA, Goyal MK, Khan S, Kauser L. Plexiform angiomyxoid myofibroblastic tumor (PAMT) of stomach with synchronous bilateral cystic ovarian neoplasms, a rare case presentation. Indian J Surg Oncol (2016) 7(1):82–5. doi: 10.1007/s13193-015-0454-4
32. Inoue Y, Gunji S, Obama K, Okabe H, Sakai Y. Laparoscopy endoscopy cooperative surgery for gastric plexiform fibromyxoma: A case report. Surg Case Rep (2016) 2(1):119. doi: 10.1186/s40792-016-0249-z
33. Jonaitis L, Kiudelis M, Slepavicius P, Poskienė L, Kupcinskas L. Plexiform angiomyxoid myofibroblastic tumor of stomach: A rare case. World J gastrointestinal endosc (2016) 8(18):674–8. doi: 10.4253/wjge.v8.i18.674
34. Kane JR, Lewis N, Lin R, Villa C, Larson A, Wayne JD, et al. Plexiform fibromyxoma with cotyledon−like serosal growth: A case report of a rare gastric tumor and review of the literature. Oncol Letters (2016) 11(3):2189–94. doi: 10.3892/ol.2016.4185
35. Morris MW, Sullivan L, Sawaya DE, Steiner MA, Nowicki MJ. Gastric plexiform fibromyxoma tumor in a child - case report and review of the literature. J Pediatr Surg Case Rep (2016) 4:38–41. doi: 10.1016/j.epsc.2015.11.002
36. Nagahisa Y, Itou T, Okita C, Yamakawa T, Chen K, Kouda Y, et al. Laparoscopic and endoscopic cooperative surgery for plexiform angiomyxoid myofibroblastic tumor. Case Rep gastroenterol (2016) 10(2):302–7. doi: 10.1159/000446764
37. Quero G, Musarra T, Carrato A, Fici M, Martini M, Tos APD, et al. Unusual focal keratin expression in plexiform angiomyxoid myofibroblastic tumor a case report and review of the literature. Medicine (2016) 95(28):e4207. doi: 10.1097/MD.0000000000004207
38. Spans L, Fletcher CDM, Antonescu CR, Rouquette A, Coindre JM, Sciot R, et al. Recurrent MALAT1-GLI1 oncogenic fusion and GLI1 up-regulation define a subset of plexiform fibromyxoma. J Pathol (2016) 239(3):335–43. doi: 10.1002/path.4730
39. Li X, Li S, Xiong S, Wang Z, Zhang H. A rare case of plexiform angiomyxoid myofibroblastic tumor in the stomach which was diagnosed at the earliest stage in the literature. Gastroenterol Rep (2016) 6(4):313–6. doi: 10.1093/gastro/gow035
40. Akai H, Kiryu S, Shinozaki M, Ohta Y, Nakano Y, Yasaka K, et al. Computed tomography and magnetic resonance imaging of a plexiform angiomyxoid myofibroblastic tumor: A case report. BMC Med imaging (2017) 17(1):7. doi: 10.1186/s12880-017-0180-1
41. Chen CJ, Yen HH, Wu PR. Plexiform fibromyxoma of stomach: A rare case report of the sonographic and pathological evaluation. Ultrasound Med Biol (2017) 43:S169. doi: 10.1016/j.ultrasmedbio.2017.08.1561
42. Hu GM, Chen HP, Liu QY, Wei JG, Feng YK, Fu WJ, et al. Plexiform fibromyxoma of the stomach: A clinicopathological study of 10 cases. Int J Clin Exp Pathol (2017) 10(11):10926–33.
43. Kawara F, Tanaka S, Yamasaki T, Morita Y, Ohara Y, Okabe Y, et al. Gastric plexiform fibromyxoma resected by endoscopic submucosal dissection after observation of chronological changes: A case report. World J Gastrointest Oncol (2017) 9(6):263–7. doi: 10.4251/wjgo.v9.i6.263
44. Kim SM, An JY, Choi MG, Lee JH, Sohn TS, Kim KM, et al. Plexiform angiomyxoid myofibroblastic tumor of the stomach: A rare case. J Gastric Cancer (2017) 17(3):277–81. doi: 10.5230/jgc.2017.17.e22
45. Li L, Lin FZ, Zhang PX, Han CH. Plexiform angiomyxoid myofibroblastic tumor of the stomach: A case report. Diagn Cytopathol (2017) 45(1):55–8. doi: 10.1002/dc.23572
46. Moris D, Spanou E, Sougioultzis S, Dimitrokallis N, Kalisperati P, Delladetsima I, et al. Duodenal plexiform fibromyxoma as a cause of obscure upper gastrointestinal bleeding: A case report. Med (Baltimore) (2017) 96(1):e5883. doi: 10.1097/MD.0000000000005883
47. Szurian K, Till H, Amerstorfer E, Hinteregger N, Mischinger HJ, Liegl-Atzwanger B, et al. Rarity among benign gastric tumors: Plexiform fibromyxoma - report of two cases. World J gastroenterol (2017) 23(31):5817–22. doi: 10.3748/wjg.v23.i31.5817
48. Wambura C, Surani S. Plexiform fibromyxoma: A rare gastric tumor. Case Rep gastrointestinal Med (2017) 2017:4014565. doi: 10.1155/2017/4014565
49. Yang MX, Zhao ZH, Yang JF, Chen B, Shen XZ, Wei JG, et al. Imaging findings of gastric plexiform fibromyxoma with a cystic change. Med (United States) (2017) 96(52):e8967. doi: 10.1097/MD.0000000000008967
50. Zhou J, Xu JJ, Jiang GZ, Ma YH, Qi JW, Li WC, et al. Gastrointestinal stromal tumor with a PDGFRA mutation masquerading as gastric plexiform fibromyxoma: A comparative clinicopathological study of two cases. Oncol Letters (2017) 13(2):887–92. doi: 10.3892/ol.2016.5486
51. Gonzalez-Cordero PL, Vara-Brenes D, Pecero-Hormigo MDC, Mateos-Rodríguez JM, Molina-Infante J, Martínez Mateo YA, et al. Plexiform fibromyxoma, a rare mesenchymal gastric tumor. Gastroenterologia y hepatologia (2017) 41(3):166–7. doi: 10.1016/j.gastrohep.2017.03.003
52. Kabincong C, Ramlan AH, Mustapha NRN. Plexiform fibromyxoma: A rare benign gastric tumour. Malaysian J Pathol (2018) 40(3):383–4.
53. Rohit M, Bhatt A, Cruise M, Wearsch PA, Goldblum JR, Sturgis CD. Endoscopic ultrasound FNA: An illustrated review of spindle cell neoplasms of the upper gastrointestinal tract including a novel case of gastric plexiform fibromyxoma. Diagn Cytopathol (2018) 46(9):730–8. doi: 10.1002/dc.24040
54. Wang F, Yan XG, Peng F, Tang CF, Liu D, Song JJ, et al. Plexiform fibromyxoma of the stomach: A case report and review of the literature. Int J Clin Exp Med (2018) 11(3):2770–7.
55. Zhang WG, Xu LB, Xiang YN, Duan CH. Plexiform fibromyxoma of the small bowel: A case report. World J Clin cases (2018) 6(15):1067–72. doi: 10.12998/wjcc.v6.i15.1067
56. Jang KY, Park HS, Kim KM, Lee H, Kim CY. Plexiform fibromyxoma of the stomach: Fine needle aspiration cytology and histological correlation. Cytopathology (2018) 30(2):241–4. doi: 10.1111/cyt.12624
57. Djurić Z, Stojšić Z, Radulović S, Janković R, Milovanović IS. Plexiform fibromyxoma: A rare benign gastric tumor. J Pediatr Gastroenterol Nutr (2018) 68(4):e67. doi: 10.1097/00005176-900000000-96892
58. Banerjee S, de la Torre J, Burgoyne AM, Tipps AMP, Savides TJ, Sicklick JK. Gastric plexiform fibromyxoma. J Gastrointestinal Surg (2019) 23(9):1936–9. doi: 10.1007/s11605-019-04132-0
59. Fukazawa M, Koga H, Hiroshige S, Matsumoto T, Nakazono Y, Yoshikawa Y. Pediatric plexiform fibromyxoma. Med (United States) (2019) 98(3):e14186. doi: 10.1097/MD.0000000000014186
60. Kobori I, Katayama Y, Hayashi K, Fujimoto Y, Kaneko M, Kitahama A, et al. Uninodular fibromyxomatous gastric tumor resected by endoscopic submucosal dissection. Internal Med (2019) 58(14):2015–8. doi: 10.2169/internalmedicine.2370-18
61. Lai JP, Kresak JL, Cao DF, Zhang DW, Zhang SR, Leon ME, et al. Gastric plexiform fibromyxoma: A great mimic of gastrointestinal stromal tumor (GIST) and diagnostic pitfalls. J Surg Res (2019) 239:76–82. doi: 10.1016/j.jss.2019.01.062
62. Li JW, Gao HQ, Lv MX, Ma YY, Wang MF. Gastric plexiform fibromyxoma: A rare case in a 5-year-old male. Pediatr Blood Cancer (2019) 66(5):e27638. doi: 10.1002/pbc.27638
63. Patel H, Masood M, Preston SR, Bagwan IN. A rare case of submucosal gastric tumor. Digestive Liver Disease (2019) 51(5):744–. doi: 10.1016/j.dld.2018.11.014
64. Arslan ME, Li H, Jennings TA, Lee EC, Nigam A, Lee H. Frequency of plexiform fibromyxoma relative to gastrointestinal stromal tumor: A single center study. Ann Diagn Pathol (2020) 48:151568. doi: 10.1016/j.anndiagpath.2020.151568
65. Gan Y, Hammoud G, Esebua M. A rare case of plexiform fibromyxoma in stomach: FNA diagnosis with histological correlation and differential diagnoses. Ann Diagn Pathol (2020) 44:151453. doi: 10.1016/j.anndiagpath.2019.151453
66. Hong YP, Yu J, Wang CY, Su YR, Chen C, Deng WH, et al. Plexiform fibromyxoma of the stomach. J gastrointestinal Surg Off J Soc Surg Alimentary Tract (2020) 24(4):909–12. doi: 10.1007/s11605-019-04238-5
67. Nasralla A, Alwabari M, Alsaif O, Amr SS. Gastric plexiform fibromyxoma arising in the cardia in an adolescent Male: A rare tumor with an unusual location. Case Rep Surg (2020) 2020:9037960. doi: 10.1155/2020/9037960
68. Pei JY, Tan B, Liu P, Cao GH, Wang ZS, Qu LL. Gastric plexiform fibromyxoma: A case report. World J Clin cases (2020) 8(22):5639–44. doi: 10.12998/wjcc.v8.i22.5639
69. Tang J, Liu F. Plexiform fibromyxoma: A rare mesenchymal tumor found in the esophagus. Am J Gastroenterol (2020) 115(5):648. doi: 10.14309/ajg.0000000000000491
70. Vieites Branco I, Silva JC, Pinto F, Pires F, Almeida A. Rare mesenchymal antral gastric tumors: Case reports of glomus tumor and plexiform fibromyxoma. Radiol Case Rep (2020) 15(1):71–6. doi: 10.1016/j.radcr.2019.10.006
71. Perry L, McCann C, Schwartz J, Gott M, Senatore P, Slotman G. Plexiform angiomyxoid myofibroblastic tumor of the stomach. Am Surgeon (2020) 89(1):145–6. doi: 10.1177/0003134820951487
72. Alkaddour A, Cartelle AL, Yap JE. Plexiform fibromyxoma: A perplexing condition of the stomach. Am J Gastroenterol (2021) 116(SUPPL):S1298. doi: 10.14309/01.ajg.0000786128.22983.e3
73. Álvarez CM, Martínez JMO, Martínez ET, Carrera MST, Díaz MJF, Rivas BM, et al. Gastric plexiform fibromyxoma, an uncommon mesenchymal tumor. Rev Espanola Enfermedades Digestivas (2021) 113(3):183–5. doi: 10.17235/reed.2020.7048/2020
74. Gelonch LM, Gallego JIA, Letamendia EE, Enriquez-Navascues JM. Giant gastric plexiform fibromyxoma. Cirugia Espanola (2021) 99(4):306–. doi: 10.1016/j.cireng.2021.03.003
75. Li ZY, Jiang QM, Guo DF, Peng YL, Zhang J, Chen XY. Gastric plexiform fibromyxoma with two different growth patterns on histological images: A case report. J Gastric Cancer (2021) 21(2):213–9. doi: 10.5230/jgc.2021.21.e17
76. Lin M, Song L, Qin SM, Li DS, Hou G, Li XM. Plexiform fibromyxoma case report and literature review. Medicine (2021) 100(36):e27164. doi: 10.1097/MD.0000000000027164
77. Ma SF, Wang J, Lu ZJ, Shi CY, Yang DH, Lin J. Plexiform fibromyxoma: a clinicopathological and immunohistochemical analysis of two cases with a literature review. J Int Med Res (2021) 49(8):3000605211027878. doi: 10.1177/03000605211027878
78. Mustafa T, Suarez Y, Damani T. Plexiform angiomyxoid myofibroblastic tumor (PAMT) of the stomach: An extremely rare mesenchymal tumor masquerading as gastrointestinal stromal tumor or leiomyoma. J gastrointestinal Surg Off J Soc Surg Alimentary Tract (2021) 25(12):3265–7. doi: 10.1007/s11605-021-05069-z
79. Wu JD, Chen YX, Luo C, Xu FH, Zhang L, Hou XH, et al. Plexiform angiomyxoid myofibroblastic tumor treated by endoscopic submucosal dissection: A case report and review of the literature. World J gastroenterol (2021) 27(31):5288–96. doi: 10.3748/wjg.v27.i31.5288
80. Almeida JI, Lima C, Pinto P, Armas I, Santos T, Freitas C. Spontaneous hemoperitoneum as a rare presentation of gastric lesions: Two case reports. Int J Surg Case Rep (2022) 91:106769. doi: 10.1016/j.ijscr.2022.106769
81. Ayyanar P, Nayak HK, Samal SC, Kar M, Mishra P, Patra S. Recurrent plexiform angiomyxoid myofibroblastic tumour (PAMT) of the stomach with aggressive behaviour. Pathology (2022) 54(5):650–4. doi: 10.1016/j.pathol.2021.09.010
82. Ebi M, Nagao K, Sugiyama T, Yamamoto K, Saito T, Kurahashi S, et al. Gastric plexiform fibromyxoma resected using nonexposed endoscopic wall-inversion surgery: A case report. Case Rep gastroenterol (2022) 16(1):159–64. doi: 10.1159/000522411
83. Higashi M, Hamada T, Sasaki K, Tsuruda Y, Shimonosono M, Kitazono I, et al. Esophageal plexiform fibromyxoma: A case report with molecular analysis for MALAT1-GLI1 fusion. Pathol Res Pract (2022) 233:153878. doi: 10.1016/j.prp.2022.153878
84. Junquera Alonso E, Terroba Alonso M, Cerrella Cano C, Cáceres Pieter CE, Parapar Álvarez L, Seoane Blanco L, et al. Endoscopic submucosal dissection of gastric plexiform fibromyxoma, an alternative to the traditional approach. Rev espanola enfermedades digestivas organo oficial la Sociedad Espanola Patologia Digestiva (2022) 114(6):367–8. doi: 10.17235/reed.2022.8601/2022
85. Lu SY, Guo L. Duodenal plexiform fibromyxoma: A case report. Asian J surg (2022) 45(11):2554–5. doi: 10.1016/j.asjsur.2022.05.150
86. Zhang R, Xia LG, Huang KB, Chen ND. Huge gastric plexiform fibromyxoma presenting as pyemia by rupture of tumor: A case report. World J Clin cases (2022) 10(7):2253–60. doi: 10.12998/wjcc.v10.i7.2253
87. Zhao X, Li X, Huang X, Shang L, Zhang J, Wu J. Gastric plexiform fibromyxoma resected by endoscopic submucosal dissection: A case report and review of literature. Hum Pathology: Case Rep (2022) 23. doi: 10.1016/j.ehpc.2020.200468
88. Krishnan SK, Chirukandath R, Zachariah T, Thomas RS. An unusual stomach tumour: Plexiform angiomyxoid fibroma stomach-a case report. Indian J Surg Oncol (2022) 13(4):691–5. doi: 10.1007/s13193-022-01625-4
89. Su HA, Yen HH, Chen CJ. An update on clinicopathological and molecular features of plexiform fibromyxoma. Can J Gastroenterol hepatol (2019) 2019:3960920. doi: 10.1155/2019/3960920
90. Landi B, Palazzo L. The role of endosonography in submucosal tumours. Best Pract Res Clin Gastroenterol (2009) 23(5):679–701. doi: 10.1016/j.bpg.2009.05.009
91. Rösch T, Kapfer B, Will U, Baronius W, Strobel M, Lorenz R, et al. Accuracy of endoscopic ultrasonography in upper gastrointestinal submucosal lesions: A prospective multicenter study. Scandinavian J gastroenterol (2002) 37(7):856–62. doi: 10.1080/gas.37.7.856.862
92. Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clinics North America (2013) 42(2):399–415. doi: 10.1016/j.gtc.2013.01.001
Keywords: plexiform fibromyxoma (PF), endoscopic submucosal excavation (ESE), systematic review, mesenchymal tumor, case report
Citation: Xia Z, Zhou Z, Guo W, Wang H, Wang F and Zhou F (2023) Endoscopic submucosal excavation for gastric plexiform fibromyxoma: A case report and systematic review of literature. Front. Oncol. 13:1090259. doi: 10.3389/fonc.2023.1090259
Received: 05 November 2022; Accepted: 13 March 2023;
Published: 24 March 2023.
Edited by:
Zaheer Nabi, Asian Institute of Gastroenterology, IndiaReviewed by:
Harshal Mandavdhare, Post Graduate Institute of Medical Education and Research (PGIMER), IndiaFilipe De Castro E. Borges, Champalimaud Foundation, Portugal
Copyright © 2023 Xia, Zhou, Guo, Wang, Wang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Zhou, fengzhou@whu.edu.cn
†These authors have contributed equally to this work
 Ziqin Xia
Ziqin Xia Zhidai Zhou
Zhidai Zhou Wei Guo
Wei Guo Hongling Wang1
Hongling Wang1 Feng Zhou
Feng Zhou