- 1New Hope Cancer Center, Beijing United Hospital, Beijing, China
- 2Department of Medicine, University of New South Wales, Sydney, NSW, Australia
- 3Cancer Programme, Garvan Institute of Medical Research, Sydney, NSW, Australia
- 4National Health & Medical Research Council (NHMRC) Clinical Trials Centre, Sydney University, Sydney, NSW, Australia
Introduction
The problem of cancer has long been supported by public taxation, private philanthropy and business investment (1), with this support having been both a cause and effect of clinical and scientific breakthroughs – including but not limited to adjuvant therapies, targeted therapies, immunotherapies, digitised imaging technologies, genetic sequencing, and cancer-preventive vaccines. Indeed, the term “oncology” only entered professional usage after proclamation of a War on Cancer by President Nixon in the lead-up to his 1972 re-election (2); this anti-cancer campaign was revived in 2016 by the Cancer Moonshot project, “re-ignition” of which was declared in 2022 by President Biden, with the goal of reducing age-specific cancer deaths by 50% over 25 years (3).
To understand the success [notwithstanding certain caveats (4)] of what has so far been a half-century campaign, it should first be asked why cancer has attracted more funding per unit of disease-specific mortality than have most other health issues; for example, there has been no similar support for a War on Heart Disease, even though cardiovascular problems have long caused higher death rates and health costs than cancer (5). This depth of support for cancer has been attributed to perceptions that a cancer diagnosis presents a unique existential threat [i.e., an “unspeakable” illness (6)] that not only poses lethal risks but also creates spiritually arduous – whether “moralistic” or “militaristic” – uncertainties as to the timing of cancer recurrence, nature of future symptoms, disease response or resistance, and speed (rapidity or slowness) of death (7).
Nonetheless, since resources are limited in any system, even the most serious personal health concerns (8, 9) must ultimately compete for support with societal-level threats (10, 11). During the first two decades (1971-90) of the War on Cancer, such threats included overpopulation, risks of nuclear war, and the HIV pandemic. Concerns over these issues abated over time; in hindsight, this fading of competing threats – akin to a peace dividend (12) – enabled sharper focus on the individual risk of a cancer diagnosis. Looking ahead, although cancer will always loom large as a major worry for personal health (13), the association of this disease with aging [i.e., with a low detriment to species fertility (14)] ensures that its impact on humanity will remain modest (15).
End of the golden age
Competing threats to the traditional support base for cancer lie ahead. Amongst these are the approaching impacts of global population aging, in tandem with falling birth rates (16), on oncology practice. Population aging will increase the aggregate burden of cancer diagnoses (17), even as age-specific cancer prevalence declines due to preventive advances; hence, as populations age over the coming decades, cancer will become commoner, but mainly among adults older than 65 years who will tend to have more age-related frailties than those diagnosed (younger) in the past (18). On the positive side, this greater longevity partly reflects improved disease prevention and wellness (19) (“healthy aging”, “delayed aging”), just as falling fertility may arise to some extent from more effective contraception (20).
Whatever the reasons for population aging, a key consequence for today’s oncologists is that their future (older) patients – who despite healthy aging are likely to have on average more restricted activities of daily living, more tenuous quality of life, and more competing causes of death (18) – may come to value autonomy and life quality relatively more highly than did their survival-focused predecessors (21, 22). The advent of patient-reported outcome measures represents a major step forward in this process of change, signaling as it does a ‘personalisation’ not of treatment targeting but of quality-of-life feedback and optimisation. Although such changes will not transform practice in the present decade, by 2040 evidence of this transition is predicted to become clear (23).
A different threat to human livelihoods is environmental degradation (24). There may seem little that links oncology practice and the causes or effects of environmental decline; yet when all correlates of the latter problem are considered – global warming, weather disasters, species displacements driving risks of disease spread to humans, travel restrictions, decarbonisation costs, energy shortages, rising prices, geopolitical instability – a total disconnect seems unlikely (25). At the least, traditional access to funding and philanthropy is likely to be constrained by competing cost-intensive problems like climate change (26), with the result – amplified by population aging – that resources for cancer become squeezed. Hence, although paradigm-shifting therapeutic advances must continue to add benefit for cancer patients in the future, other emerging challenges are likely to diminish investment in cancer therapeutics over time, especially when expressed as a fraction of world expenditures against all threats.
Public acceptance of this new reality could bring with it a slow change from an individualistic view of health as priceless (27) to a more socially cognizant “doughnut economics” (28), with proposals of this kind having already been made (29). Such a trend is also consistent with the Moonshot initiative which, in contrast to the War on Cancer, is not driven by major new funds; instead, the Moonshot proposes a switch of emphasis limited to an additional 5% of existing funding, with prevention as the priority. A transition of this kind aligns with recommendations to invest in a Culture of Health (30), based on a mindset valuing shared community needs (31, 32) at least as highly as the ‘magic bullet’ hope and hype which has for so long energised cancer research (33).
Steps to a greening of oncology
Different cultures of oncology already exist in different parts of the world, implying that changes are possible in any knowledge system (34). For cancer care to evolve from its golden age values to a more equable and lower-profile green age culture (Table 1), resistance may be eased by educational campaigns which can convince both public and professionals that these selection pressures reflect essential adaptive challenges for modern healthcare (35). A stepwise approach to this cultural transition is suggested below.
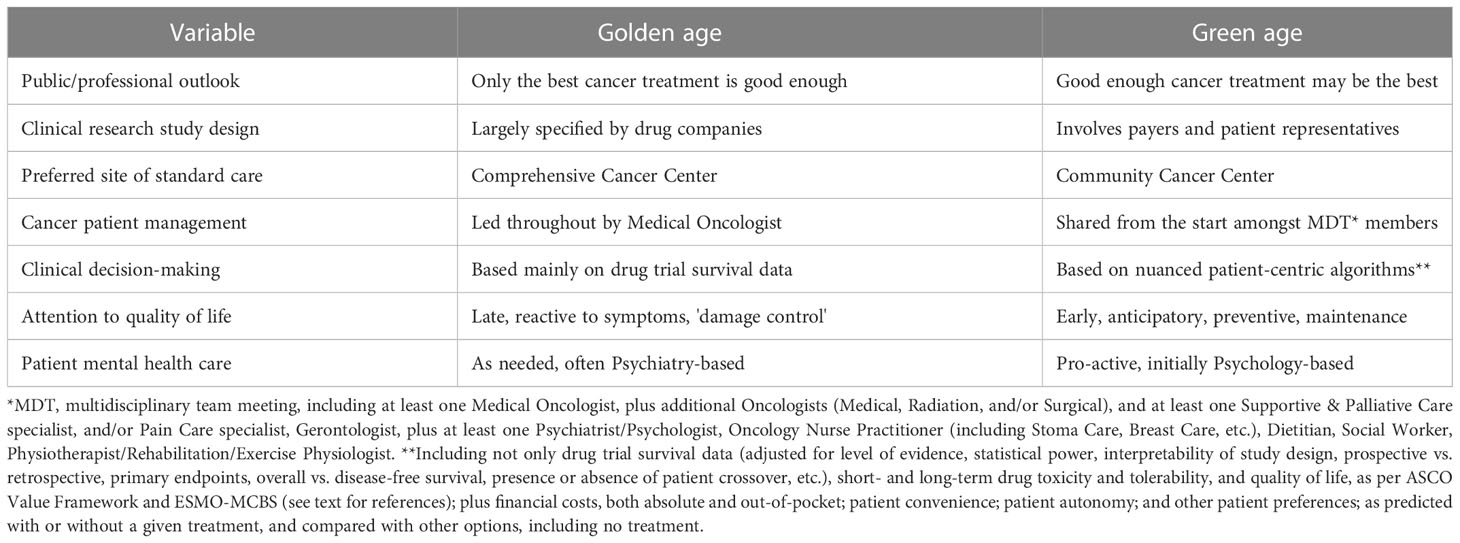
Table 1 Comparison of variables proposed to differ between the past (golden age) and potential future (green age) of oncology.
1. Win the battle of ideas over good-enough cancer treatment
Change in oncology is slowed by the idea that drug treatment choice – whether right [i.e., best, usually the latest [36)] or wrong (anything other than the best) – is a main determinant of disease outcome. As shown by the small absolute size of most clinical trial benefits, however, the reality is that cancer biology still tends to be the main factor affecting outcomes, with choices between approved treatments [or differences in national expenditures thereon (37)] usually making only modest differences (38). The modern incrementalist culture may exploit patients’ fear of death (39) by perpetuating the norm that only the best is good enough (40), leading to the paradox that a disproportionate amount of oncology costs are generated in the last months of life (41). Such thinking may reflect a survival-of-the-fittest instinct, whereby any threat triggers a safety-first (kill it before it kills you) reaction; yet in the real world less extreme responses may not only suffice, but also prove less morbid (42).
2. Re-prioritise from research-centered to patient-centered practice
Focusing solely on the disease-modifying potential of therapies – with the potentially gratifying but often undetectable benefits of immune checkpoint therapy being a case in point (43) – distracts from the importance of factoring in other decision-making criteria such as treatment tolerability, safety, convenience, cost, options of delaying treatment (e.g., by later sequential or crossover drug use), and so on (44). One way to break this cycle, and thus to assess the validity or otherwise of good-enough treatment, is to quantify all factors pertinent to a cancer patient’s predicted length and quality of life with or without the prescription of a treatment (45). If this exercise becomes possible to score – a technical quantum leap not yet reduced to practice – a good-enough cancer therapy could, paradoxically, deliver superior overall (i.e., holistic) outcomes compared to the best cancer treatment as determined by survival data. One step towards this has been the use of clinical trial or gene expression data to predict when addition of adjuvant cytotoxic therapy to hormone therapy delivers such a low absolute breast cancer survival increment as to be not recommendable as a standard of care (46). Validated algorithms which can quantify a given patients’ preferences and priorities thus seem a prerequisite for progress.
3. Abandon zero-sum mindsets
Career success in newer subspecialties, such as oncology, has long been favoured by a tight focus on established (homophilic) goals and colleagues (47) – the so-called silo effect – rather than by more collaborative approaches (48). Yet if it is assumed that the problems of oncology do not overlap with those of other important contextual issues, such as the aging population or environmental deterioration, then a zero-sum interaction is assured; that is, any competition for resources between these fields will proceed on an ‘I-win-you-lose’ basis. In contrast, if common goals can be discerned, win-win scenarios for mutual benefit, and hence for co-resourcing, could be pursued (49, 50). Examples of integrated initiatives for cancer patients and the public include:
● Smoking elimination, clean air, ambient toxin reduction (e.g., radon, asbestos)
● Addiction prevention, mental resilience promotion
● Vaccination drives (e.g., HPV, HBV)
● Exercise programs (51)
● Ideal body weight education
● Food/beverage labeling improvements
● More multidisciplinary hospital-based care
● More community-based care for standard clinical problems
● User-friendly software development to aid more nuanced decision-making
4. Question self-reinforcing feedback loops
A risk of any golden age is that it selects for its own survival. In oncology, therapeutic progress has always been similarly sought by physicians, patients, pharmas, philanthropists, and the press; the only stakeholders who are motivated to query such progress are the third-party payers (52). This near-unanimity over the desirability of constant progress has made objective debate difficult (53), with the careers of critics prone to damage (54). The best solution to this problem will be to develop and validate quantitative metrics that can value holistic (i.e., qualitative) patient-centered variables, thus extending and refining the meaning of cost-efficacy (55).
5. Broaden decision-making and management
This quest to validate predictors of therapeutic value has made progress with upgradings of both the American Society of Clinical Oncology (ASCO) Value Framework Net Benefit Score (56) and the European Society of Medical Oncology (ESMO) Magnitude of Clinical Benefit Scale (MCBS) (57). The pros and cons of these sophisticated tools have been compared (58), and reveal promising complementarity in the questions addressed (59). Implementation of these algorithms remains labour-intensive, however, hampering patient inputs and clinical adoption (60). Broadening management to include routine early involvement of non-oncology multidisciplinary experts should help to dilute what is now, for many standard-setting cancer patients, an overly specialised approach to care (61). Better patient experiences could also result from moving standard care away from specialised cancer centers into general hospitals or local communities, assisted where needed by telehealth communications, while also evolving towards value-based reimbursement systems (62). More promotion of oncologists for excellence in communication or education (63) could be another step towards a flatter service structure. Finally, more attention to the mental resilience of patients, will also add value to patients’ well-being, in part by reducing reliance on test results as critical arbiters of survivorship (64).
Conclusion
The choice of a War on Cancer as an encore to the Apollo moon landings signalled the zenith for cancer as an existential human threat, kicking off a golden age for the science and practice of oncology. Times have since changed, however, as population aging and planetary threats have altered the trajectory of public concerns. The field of oncology is likely to feel these pressures to adapt within the next two decades, and the healthcare changes that result over the next fifty years could prove to be just as important and beneficial as the paradigm shifts that preceded these.
A global approach, based on public and professional education (65), will be needed to bring about economics-based system changes that can adapt to the disruptive evolutionary era ahead (66). Crosstalk with all stakeholders – including, though not limited to, patients, physicians, advocacy groups, governments, insurers, and pharmas – will necessarily precede such changes. The 20th century paradigm of ever more resources (ultimately derived from the environment) being harnessed to deliver ever more personalised oncologic increments (ultimately benefiting individuals more than populations) may prove to be less sustainably applicable to the 21st century world, where an imbalance between human demand and ecosystem fragility has become evident. Constructive change will require that concepts such as ‘greater good’, ‘big picture’ and ‘longer term’ come to be pursued more systematically than the self-interested priorities of the past.
Author contributions
RE wrote the first draft with inputs from FL and YG, then the second draft was further appraised and critiqued by FL and YG. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sam D, Cheung WY. A population-level comparison of cancer-related and non-cancer-related health care costs using publicly available provincial administrative data. Curr Oncol (2019) 26:94–7. doi: 10.3747/co.26.4399
2. Kennedy BJ. Medical oncology: The new subspecialty. Med Pediatr Oncol (1975) 1:23–7. doi: 10.1002/mpo.2950010105
3. Ledford H. Cancer ‘moonshot’ has lofty new goal: Halve deaths in 25 years. Nature (2022) 602:561. doi: 10.1038/d41586-022-00376-0
4. Sonnenschein C, Soto AM. Over a century of cancer research: Inconvenient truths and promising leads. PloS Biol (2020) 18:e3000670. doi: 10.1371/journal.pbio.3000670
5. Muka T, Imo D, Jaspers L, Colpani V, Chaker L, van der Lee SJ, et al. The global impact of non-communicable diseases on healthcare spending and national income: A systematic review. Eur J Epidemiol (2015) 30:251–77. doi: 10.1007/s10654-014-9984-2
6. Pierotti MA. The molecular understanding of cancer: from the unspeakable illness to a curable disease. Ecancermedicalscience (2017) 11:747. doi: 10.3332/ecancer.2017.747
7. Curran L, Sharpe L, MacCann C, Butow P. Testing a model of fear of cancer recurrence or progression: The central role of intrusions, death anxiety and threat appraisal. J Behav Med (2020) 43:225–36. doi: 10.1007/s10865-019-00129-x
8. Hood B. The self illusion: How the social brain creates identity. New York: Oxford University Press (2012).
9. Allen SE. Cancer survivors: The success story that’s straining health care. IEEE Pulse (2017) 8:14–7. doi: 10.1109/MPUL.2016.2629999
10. Badulescu D, Simut R, Badulescu A, Badulescu AV. The relative effects of economic growth, environmental pollution and non-communicable diseases on health expenditures in European union countries. Int J Environ Res Public Health (2019) 16:5115. doi: 10.3390/ijerph16245115
11. Bae EY, Lim MK, Lee B, Bae G. Who should be given priority for public funding? Health Policy (2020) 124:1108–14. doi: 10.1016/j.healthpol.2020.06.010
12. Al Mandhari A, Ghaffar A, Etienne CF. Harnessing the peace dividends of health. BMJ Glob Health (2021) 6:e006287. doi: 10.1136/bmjgh-2021-006287
13. Vehling S, Kissane DW. Existential distress in cancer: Alleviating suffering from fundamental loss and change. Psychooncology (2018) 27:2525–30. doi: 10.1002/pon.4872
15. Jasienska G, Bribiescas RG, Furberg AS, Helle S, Nunez-de la Mora A. Human reproduction and health: an evolutionary perspective. Lancet (2017) 390:510–20. doi: 10.1016/S0140-6736(17)30573-1
16. Pantazis A, Clark SJ. A parsimonious characterization of change in global age-specific and total fertility rates. PLoS One (2018) 13:e0190574. doi: 10.1371/journal.pone.0190574
17. Nolen SC, Evans MA, Fischer A, Corrada MM, Kawas CH, Bota DA. Cancer-incidence, prevalence and mortality in the oldest-old. a comprehensive review. Mech Ageing Dev (2017) 164:113–26. doi: 10.1016/j.mad.2017.05.002
18. Gu YF, Lin FP, Epstein RJ. How aging of the global population is changing oncology. Ecancermedicalscience (2021) 15:ed119. doi: 10.3332/ecancer.2021.ed119
19. Kemoun P, Ader I, Planat-Benard V, Dray C, Fazilleau N, Monsarrat P, et al. A gerophysiology perspective on healthy ageing. Ageing Res Rev (2022) 73:101537. doi: 10.1016/j.arr.2021.101537
20. Gotmark F, Andersson M. Human fertility in relation to education, economy, religion, contraception, and family planning programs. BMC Public Health Oxford UK (2020) 20:265. doi: 10.1186/s12889-020-8331-7
21. Gawande A. Being mortal: medicine and what matters in the end. New York: Metropolitan Books (2014).
22. Philipp R, Mehnert A, Lo C, Muller V, Reck M, Vehling S. Characterizing death acceptance among patients with cancer. Psychooncology (2019) 28:854–62. doi: 10.1002/pon.5030
23. Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet (2018) 392:2052–90. doi: 10.1016/S0140-6736(18)31694-5
24. Sage RF. Global change biology: A primer. Glob Chang Biol (2020) 26:3–30. doi: 10.1111/gcb.14893
25. Frumkin H, Haines A. Global environmental change and noncommunicable disease risks. Annu Rev Public Health (2019) 40:261–82. doi: 10.1146/annurev-publhealth-040218-043706
26. Iyer HS, DeVille NV, Stoddard O, Cole J, Myers SS, Li H, et al. Sustaining planetary health through systems thinking: Public health’s critical role. SSM Popul Health (2021) 15:100844. doi: 10.1016/j.ssmph.2021.100844
28. Raworth K. Doughnut economics: 7 ways to think like a 21st-century economist. Vermont: Chelsea Green (2017).
29. Farrelly C. Responsible biology, aging populations and the 50th anniversary of the “War on cancer”. Biogerontology (2021) 22:429–40. doi: 10.1007/s10522-021-09925-y
30. Tziraki-Segal C, De Luca V, Santana S, Romano R, Tramontano G, Scattola P, et al. Creating a culture of health in planning and implementing innovative strategies addressing non-communicable chronic diseases. Front Sociol (2019) 4:9. doi: 10.3389/fsoc.2019.00009
31. Prasad V. Our best weapons against cancer are not magic bullets. Nature (2020) 577:451. doi: 10.1038/d41586-020-00116-2
32. Marrauld L, Rambaud T, Egneli M, Geist JN.THESHIFTPROJECT, « DÉCARBONER LA SANTÉ POUR SOIGNER DURABLEMENT »: LE RAPPORT DU SHIFT PROJECT. Paris, France: The Shift Project (2021).
33. Sanchez NS, Mills GB, Mills Shaw KR. Precision oncology: Neither a silver bullet nor a dream. Pharmacogenomics (2017) 18:1525–39. doi: 10.2217/pgs-2017-0094
34. Haynes A, Rychetnik L, Finegood D, Irving M, Freebairn L, Hawe P. Applying systems thinking to knowledge mobilisation in public health. Health Res Policy Syst (2020) 18:134. doi: 10.1186/s12961-020-00600-1
35. Hensher M, Tisdell J, Canny B, Zimitat C. Health care and the future of economic growth: exploring alternative perspectives. Health Econ Policy Law (2020) 15:419–39. doi: 10.1017/S1744133119000276
36. Danzon PM. Drug pricing and value in oncology. Recent Results Cancer Res (2019) 213:153–67. doi: 10.1007/978-3-030-01207-6_10
37. Hermanowski T, Bystrov V, Staszewska-Bystrova A, Szafraniec-Burylo SI, Rabczenko D, Kolasa K, et al. Analysis of trends in life expectancies and per capita gross domestic product as well as pharmaceutical and non-pharmaceutical healthcare expenditures. Acta Pol Pharm (2015) 72:1045–50.
38. Tannock I, Presley CJ, Saltz LB. Value-added decisions in oncology. Am Soc Clin Oncol Educ Book (2019) 39:122–31. doi: 10.1200/EDBK_238831
39. Menzies RF, Menzies RG. Mortals: How the fear of death shaped human society. Sydney: Allen & Unwin (2021).
40. Engelberg AB, Avorn J, Kesselheim AS. A new way to contain unaffordable medication costs - exercising the government’s existing rights. N Engl J Med (2022) 386:1104–6. doi: 10.1056/NEJMp2117102
41. Dvortsin E, Gout-Zwart J, Eijssen EL, van Brussel J, Postma MJ. Comparative cost-effectiveness of drugs in early versus late stages of cancer; review of the literature and a case study in breast cancer. PloS One (2016) 11:e0146551. doi: 10.1371/journal.pone.0146551
42. Milo DS. Good enough: The tolerance for mediocrity in nature and society. Cambridge, MA USA: Harvard University Press (2019).
43. Pichler M, Steyrer J. Cost-effectiveness analysis of the use of immunotherapy in metastatic solid tumours in Austria by applying the ESMO-magnitude of clinical benefit scale (ESMO-MCBS) version 1.1. ESMO Open (2021) 6:100198. doi: 10.1016/j.esmoop.2021.100198
44. Schnog JB, Samson MJ, Gans ROB, Duits AJ. An urgent call to raise the bar in oncology. Br J Cancer (2021) 125:1477–85. doi: 10.1038/s41416-021-01495-7
45. Walsh S, de Jong EEC, van Timmeren JE, Ibrahim A, Compter I, Peerlings J, et al. Decision support systems in oncology. JCO Clin Cancer Inform (2019) 3:1–9. doi: 10.1200/CCI.18.00001
46. Caparica R, Brandao M, Piccart M. Systemic treatment of patients with early breast cancer: Recent updates and state of the art. Breast (2019) 48 Suppl 1:S7–S20. doi: 10.1016/S0960-9776(19)31115-4
47. Woelmer WM, Bradley LM, Haber LT, Klinges DH, Lewis ASL, Mohr EJ, et al. Ten simple rules for training yourself in an emerging field. PloS Comput Biol (2021) 17:e1009440. doi: 10.1371/journal.pcbi.1009440
48. Akerlof KL. Beyond the sheltering academic silo: Norms for scientists’ participation in policy. Prog Mol Biol Transl Sci (2022) 188:29–44. doi: 10.1016/bs.pmbts.2021.11.007
49. Malhi Y, Franklin J, Seddon N, Solan M, Turner MG, Field CB, et al. Climate change and ecosystems: Threats, opportunities and solutions. Philos Trans R Soc Lond B Biol Sci (2020) 375:20190104. doi: 10.1098/rstb.2019.0104
50. Bretti S, Porcile G, Romizi R, Palazzo S, Oliani C, Crispino S, et al. “Green oncology”: The Italian medical oncologists’ challenge to reduce the ecological impact of their clinical activity. Tumori (2014) 100:e94–7. doi: 10.1177/1578.17246
51. Kennedy MA, Bayes S, Newton RU, Zissiadis Y, Spry NA, Taaffe DR, et al. Implementation barriers to integrating exercise as medicine in oncology: An ecological scoping review. J Cancer Surviv (2022) 16:865–81. doi: 10.1007/s11764-021-01080-0
52. Nabhan C, Phillips EG, Feinberg BA. Value in oncology: It is in the eyes of the beholder. J Natl Compr Canc Netw (2019) 17:2–5. doi: 10.6004/jnccn.2018.7092
53. Hordern J, Maughan T, Feiler T, Morrell L, Horne R, Sullivan R. The ‘molecularly unstratified’ patient: A focus for moral, psycho-social and societal research. BioMed Hub 2 (2017) 2(suppl. 1):146–53. doi: 10.1159/000480422
54. Harris R. Tweeting oncologist draws ire and admiration for calling out hype. Shots: Health News NPR (2018).
55. Grossmann N, Wolf S, Rothschedl E, Wild C. Twelve years of European cancer drug approval-a systematic investigation of the ‘magnitude of clinical benefit’. ESMO Open (2021) 6:100166. doi: 10.1016/j.esmoop.2021.100166
56. American Society of Clinical Oncology. ASCO value in cancer care (2022). Available at: https://www.asco.org/news-initiatives/current-initiatives/cancer-care-initiatives/value-cancer-care.
57. Kiesewetter B, Cherny NI, Boissel N, Cerisoli F, Dafni U, de Vries EGE, et al. EHA evaluation of the ESMO-magnitude of clinical benefit scale version 1.1 (ESMO-MCBS v1.1) for haematological malignancies. ESMO Open (2020) 5:e000611. doi: 10.1136/esmoopen-2019-000611
58. Saluja R, Everest L, Cheng S, Cheung M, Chan KKW. Assessment of whether the American society of clinical oncology’s value framework and the European society for medical oncology’s magnitude of clinical benefit scale measure absolute or relative clinical survival benefit: An analysis of randomized clinical trials. JAMA Oncol (2019) 5:1188–94. doi: 10.1001/jamaoncol.2019.0818
59. Cherny NI, de Vries EGE, Dafni U, Garrett-Mayer E, McKernin SE, Piccart M, et al. Comparative assessment of clinical benefit using the ESMO-magnitude of clinical benefit scale version 1.1 and the ASCO value framework net health benefit score. J Clin Oncol (2019) 37:336–49. doi: 10.1200/JCO.18.00729
60. Gyawali B, de Vries EGE, Dafni U, Amaral T, Barriuso J, Bogaerts J, et al. Biases in study design, implementation, and data analysis that distort the appraisal of clinical benefit and ESMO-magnitude of clinical benefit scale (ESMO-MCBS) scoring. ESMO Open (2021) 6:100117. doi: 10.1016/j.esmoop.2021.100117
61. Tahara DC, Green RP. Strategic re-design of team-based patient-focused health care services. Adv Health Care Manag (2014) 16:3–22. doi: 10.1108/S1474-823120140000016000
62. Cox JV, Ward JC, Hornberger JC, Temel JS, McAneny BL. Community oncology in an era of payment reform. Am Soc Clin Oncol Educ Book (2014), e447–52. doi: 10.14694/EdBook_AM.2014.34.e447
63. Villaire M, Mayer G. Health literacy: The low-hanging fruit in health care reform. J Health Care Finance (2009) 36:55–9.
64. Mayer DK, Nasso SF, Earp JA. Defining cancer survivors, their needs, and perspectives on survivorship health care in the USA. Lancet Oncol (2017) 18:e11–8. doi: 10.1016/S1470-2045(16)30573-3
65. Wardill HR, Cheung YT, Boltong A, Charalambous A, Koczwara B, Lustberg M, et al. ‘Share your views’-international consultation informs a patient engagement strategy for the multinational association of supportive care in cancer. Support Care Cancer (2022) 30:9953–61. doi: 10.1007/s00520-022-07366-y
Keywords: oncology, health economics, sustainability, demographics, population aging
Citation: Epstein RJ, Gu Y and Lin FPY (2023) Can cancer go green? It’s up to us. Front. Oncol. 13:1074091. doi: 10.3389/fonc.2023.1074091
Received: 01 December 2022; Accepted: 17 January 2023;
Published: 22 February 2023.
Edited by:
Yingying Xu, Beihang University, ChinaReviewed by:
Christian Chabannon, Aix-Marseille Université, FranceCopyright © 2023 Epstein, Gu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Richard J. Epstein, r.epstein@unsw.edu.au
 Richard J. Epstein
Richard J. Epstein Yanfei Gu1
Yanfei Gu1 Frank P. Y. Lin
Frank P. Y. Lin