- 1Institute of Child and Adolescent Health, School of Public Health, Peking University, Beijing, China
- 2Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China
- 3Department of Maternal and Child Health, School of Public Health, Peking University, Beijing, China
Objective: This study systematically evaluated the effectiveness and safety of therapeutic vaccines for precancerous cervical lesions, providing evidence for future research.
Methods: We systematically searched the literature in 10 databases from inception to February 18, 2021. Studies on the effectiveness and safety of therapeutic vaccines for precancerous cervical lesions were included. Then, we calculated the overall incidence rates of four outcomes, for which we used the risk ratio (RR) and 95% confidence interval (95% CI) to describe the effects of high-grade squamous intraepithelial lesions (HSILs) on recurrence.
Results: A total of 39 studies were included, all reported in English, published from 1989 to 2021 in 16 countries. The studies covered 22,865 women aged 15–65 years, with a total of 5,794 vaccinated, and 21 vaccines were divided into six types. Meta-analysis showed that the overall incidence rate of HSIL regression in vaccine therapies was 62.48% [95% CI (42.80, 80.41)], with the highest rate being 72.32% for viral vector vaccines [95% CI (29.33, 99.51)]. Similarly, the overall incidence rates of HPV and HPV16/18 clearance by vaccines were 48.59% [95% CI (32.68, 64.64)] and 47.37% [95% CI (38.00, 56.81)], respectively, with the highest rates being 68.18% [95% CI (45.13, 86.14)] for bacterial vector vaccines and 55.14% [95% CI (42.31, 67.66)] for DNA-based vaccines. In addition, a comprehensive analysis indicated that virus-like particle vaccines after conization reduced the risk of HSIL recurrence with statistical significance compared to conization alone [RR = 0.46; 95% CI (0.29, 0.74)]. Regarding safety, only four studies reported a few severe adverse events, indicating that vaccines for precancerous cervical lesions are generally safe.
Conclusion: Virus-like particle vaccines as an adjuvant immunotherapy for conization can significantly reduce the risk of HSIL recurrence. Most therapeutic vaccines have direct therapeutic effects on precancerous lesions, and the effectiveness in HSIL regression, clearance of HPV, and clearance of HPV16/18 is great with good safety. That is, therapeutic vaccines have good development potential and are worthy of further research.
Systematic Review Registration: PROSPERO https://www.crd.york.ac.uk/PROSPERO/, CRD42021275452.
1 Background
Cervical cancer is a commonly diagnosed cancer of the reproductive system in women. According to the World Health Organization (WHO), in 2020, 604,127 women were newly diagnosed with cervical cancer and 341,831 women died from the disease throughout the world; the disease ranks fourth in incidence and mortality among all female malignant tumors (1). Cervical cancer has become an important public health problem worldwide. In May 2018, the WHO issued a call to action for the elimination of cervical cancer, and in November 2020, the organization launched a global strategy to accelerate its elimination (2).
Precancerous lesions of cervical cancer refer to the obvious lesions of epithelial cells in the cervical transformation area, of which cytological and histological classifications include cervical intraepithelial neoplasia (CIN), atypical squamous cells of unknown significance (ASCUS), high-grade squamous intraepithelial lesions (HSILs), atypical squamous cells–cannot rule out HSIL (ASC-H), low-grade squamous intraepithelial lesions (LSILs), and atypical glandular cells (AGCs), among others (3). CIN1 is also classified as LSIL, and CIN2–3 are classified as HSIL (3). They are mostly caused by long-term or persistent infection of high-risk types of human papillomavirus (HPV) (3). Because precancerous lesions evolve for years before advancing to invasive cervical cancer, early treatments of precancerous lesions are crucial for preventing disease progression, lowering medical expenditures, and reducing the burden of the disease (4, 5). The WHO recommends cryotherapy, large loop excision of the transformation zone [LLETZ or loop electrosurgical excision procedure (LEEP) in the USA], and cold knife conization as treatments (6). However, complications such as intraoperative and postoperative bleeding, incision infection, cervical stenosis, endometriosis, and intestinal injury may occur (7). There are also higher risks of disease recurrence (8, 9).
As emerging treatment measures for precancerous cervical lesions, therapeutic vaccines have made a lot of progress in recent years. On the one hand, some HPV vaccines have already been used in patients for surgical adjuvant immunotherapy to prevent the recurrence of precancerous lesions. Studies have shown that three HPV vaccines already on the market (Cervarix, Gardasil, and Gardasil 9) can significantly reduce the risk of disease recurrence (10, 11), and it is worth noting that these three vaccines have no direct therapeutic effects. On the other hand, unlike these HPV vaccines, which are designed to induce the production of consistently high levels of neutralizing antibodies, there are therapeutic vaccines that can stimulate cell-mediated immune responses to eliminate HPV-infected cells to directly treat precancerous lesions (12). Several kinds of therapeutic vaccine for precancerous cervical lesions have already been developed, including viral vector, bacterial vector, DNA-based, peptide-based, and protein-based vaccines (13–18), but no systematic review or meta-analysis of related content has been conducted. A comprehensive meta-analysis is urgently needed to evaluate the effectiveness of various therapeutic vaccines for precancerous cervical lesions. Therefore, in this study, we systematically evaluated the effectiveness and safety of therapeutic vaccines for cervical cancer precancerous lesions, establishing a reliable evidence-based basis for the actual effects of therapeutic vaccines, and providing reference for future research.
2 Materials and Methods
2.1 Search Strategy
We systematically searched the literature in the PubMed, Embase, Web of Science, Cochrane Library, Proquest, ClinicalTrails.gov, Chinese Biomedical Literature Service System (SinoMed), China National Knowledge Infrastructure (CNKI), Chinese Science and Technology Periodicals (VIP), and WanFang databases from inception to February 18, 2021. We combined three key terms in our search strategy, including their subject headings and synonyms: “precancerous cervical lesions,” “vaccine,” and “therapy” (see detailed search strategy in Supplementary Table S1). Our results are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA, see detailed information in Supplementary Table S2), and this study has been registered with PROSPERO (CRD42021275452).
2.2 Inclusion and Exclusion Criteria
We included published literature related to the effectiveness and safety of therapeutic vaccines in patients with precancerous cervical lesions. Randomized controlled trials (RCTs), non-RCTs, cohort studies, case–control studies, and case series were included. We excluded studies that were not original research, those from which we could not extract outcome data specifically, and those for which we were unable to access the full text. We also excluded mechanism studies, such as animal experiments and in vitro experiments, among others.
2.3 Literature Screening and Data Extraction
Literature screening and data extraction were conducted in a two-person, independent and parallel manner. Five investigators (ShC, XT, KM, SiC, and DL) screened all identified records independently by reading titles and abstracts. If the information in the title and abstract was insufficient, the full text was obtained for review. If there was any disagreement, it was resolved through discussion with a third person.
The same investigators, working in pairs, read the full texts to extract information independently using a predesigned extraction sheet. The extracted information included basic information (first author and publication year), study design (study type and clinical trial stage), vaccine information (types and name), participants (sample size, age, and disease status), effectiveness and safety outcomes (HSIL regression, HPV clearance, HPV16/18 clearance, HSIL recurrence, and adverse events), and corresponding number of participants.
HSIL regression was defined as histopathological regression to either LSIL or normal pathology (15); HPV clearance was defined as viral clearance based on related HPV genotypes (15); HSIL recurrence referred to new rather than residual HSIL that appeared after treatment (19); any adverse events mainly included any grade 1–4 adverse events; and severe adverse events mainly referred to grade 3–4 adverse events. Grade 1–4 adverse events were classified according to the Medical Dictionary for Regulatory Activities (MedDRA) (20) or the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) (13).
2.4 Risk of Bias Assessment
The same five investigators, working in pairs, independently assessed the quality of studies to ensure the reliability of the findings, with disagreements resolved by a third investigator. The Risk of Bias Tool recommended by Cochrane Reviewer’s Handbook (21) was used to assess the quality of RCTs. The Newcastle–Ottawa Scale (NOS) (22) was used to evaluate the quality of cohort studies and case–control studies. For case series studies, we used Recommendations of the National Institute for Clinical Excellence (NICE) (23). For non-RCTs, we used the Bias in Non-randomized Studies - of Interventions (ROBINS) Tool (24).
Cohort studies and case–control studies were classified as having low (scores of 7–9), moderate (5–6), and high risk of bias (0–4) with a total possible score of 9. Case series were classified as having low (6–8), moderate (4–5), and high risk of bias (0–3) with a total possible score of 8.
2.5 Statistical Analysis
We calculated the overall incidence rate of effectiveness and safety outcomes. The weight of each study was calculated using the random-effects model directly from the STATA “metaprop” command, and an arcsine transformation was conducted to stabilize variance. Between-study statistical heterogeneity was tested to assess data consistency (the higher the inconsistency, the larger the uncertainty in meta-analysis results) using the I2 and Cochran’s Q homogeneity tests.
For HSIL recurrence, we used the risk ratio (RR) and its 95% confidence interval (CI) to describe effects. When I2 < 50%, indicating low to moderate heterogeneity, a fixed-effects model was used; when I2 > 50%, indicating high heterogeneity, a random-effects model was used.
In addition, we conducted a sensitivity analysis by excluding studies with a high risk of bias. Statistical analysis was performed using STATA 15.1 software.
3 Results
3.1 Basic Characteristics
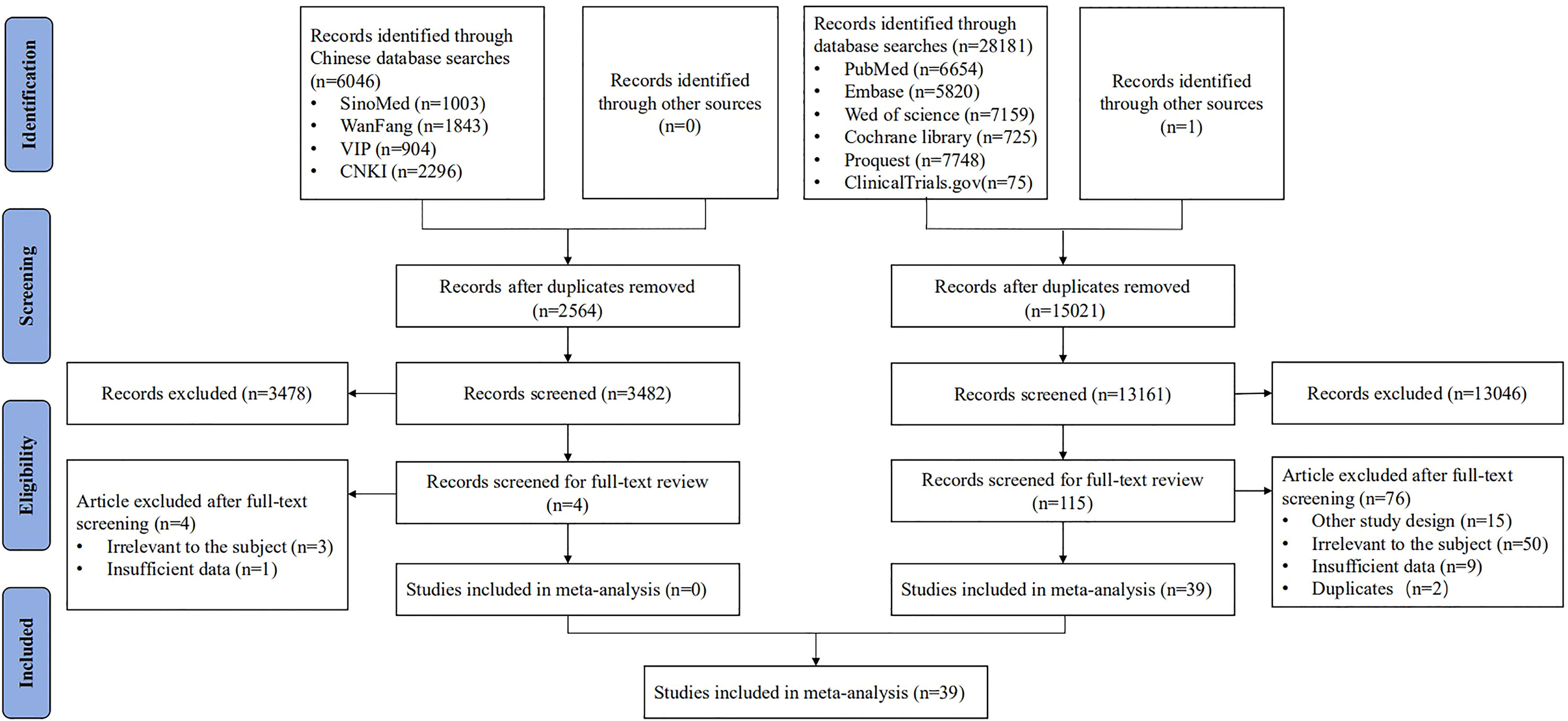
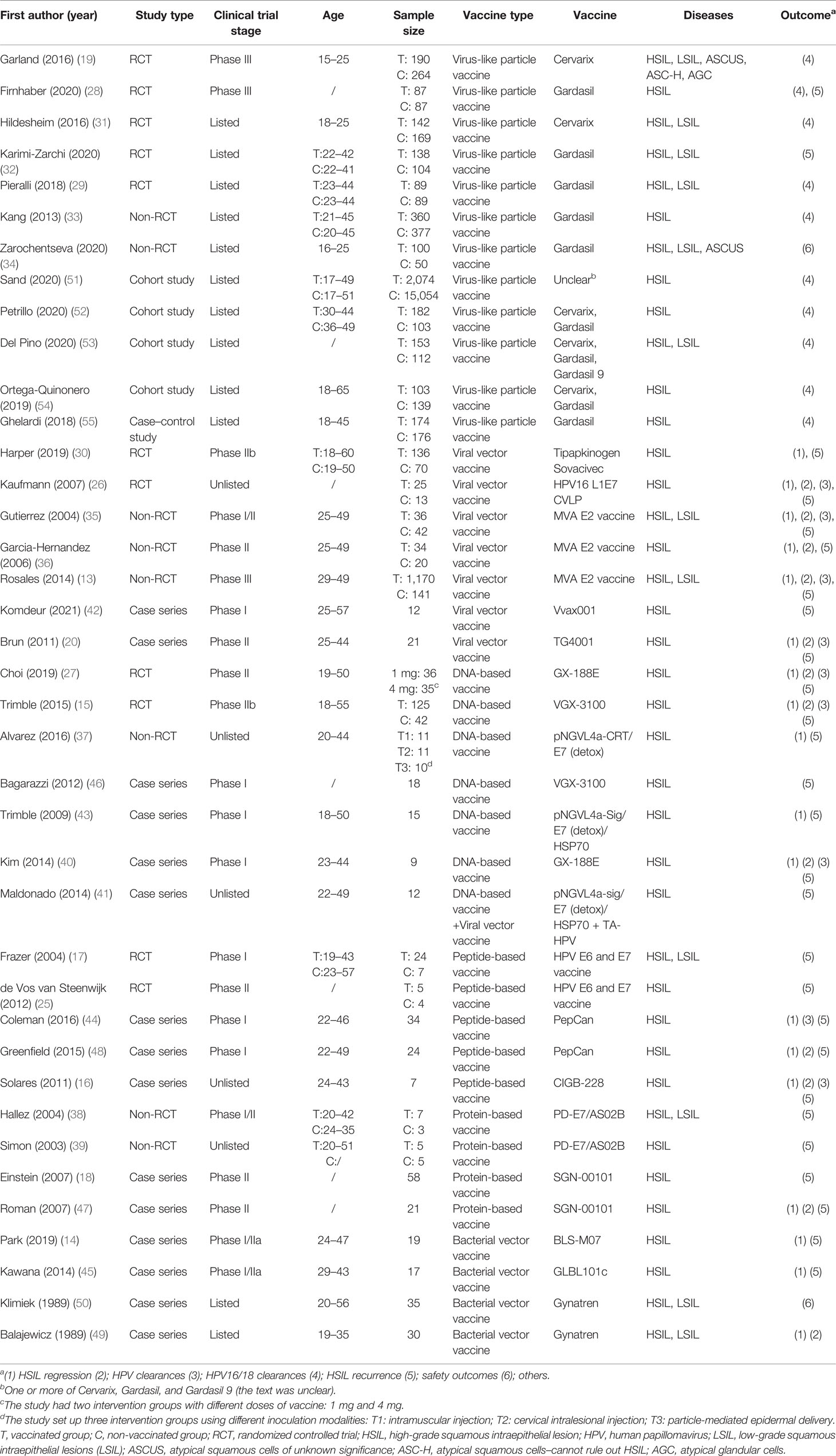
After screening 34,228 articles, 39 studies were included, all reported in English, and published from 1989 to 2021 in 16 countries (Figure 1). The study types included RCTs (n = 11) (15, 17, 19, 25–32), non-RCTs (n = 8) (13, 33–39), case series studies (n = 15) (14, 16, 18, 20, 40–50), cohort studies (n = 4) (51–54), and case–control studies (n = 1) (55). The sample covered 22,865 women aged 15–65, with a total of 5,794 vaccinated. The studies included 21 vaccines divided into six types, including virus-like particle (n = 12) (19, 28, 29, 31–34, 51–55), viral vector (n = 8) (13, 20, 26, 30, 35, 36, 41, 42), DNA-based (n = 7) (15, 27, 37, 40, 41, 43, 46), peptide-based (n = 5) (16, 17, 25, 44, 48), protein-based (n = 4) (18, 38, 39, 47), and bacterial vector vaccines (n = 4) (14, 45, 49, 50). In the included studies, virus-like particle vaccines (Cervarix, Gardasil, and Gardasil 9) were combined with conization to prevent the recurrence of precancerous lesions, whereas other vaccines were used alone as treatment. All studies focused on HSIL as the main disease, and a few studies included other precancerous lesions such as LSIL, ASCUS, ASC-H, and AGC (the basic characteristics of the included studies are shown in Table 1).
In terms of risk of bias, most of the studies had a low/moderate risk. Among the 11 RCTs, 2 studies were judged to be at high risk of bias for incomplete outcome data (25, 32) and 6 studies lacked enough information to judge the risk of bias (17, 19, 26, 27, 30, 31). Among the eight non-RCTs, five studies were judged to have a high risk of bias for unclear measurements or selective reporting of outcomes (13, 35, 36, 38, 39). The bias scores were 4–7 for all 15 case series and 6–7 for the four cohort studies and one case–control study (Table S3).
3.2 Effectiveness
3.2.1 HSIL Regression
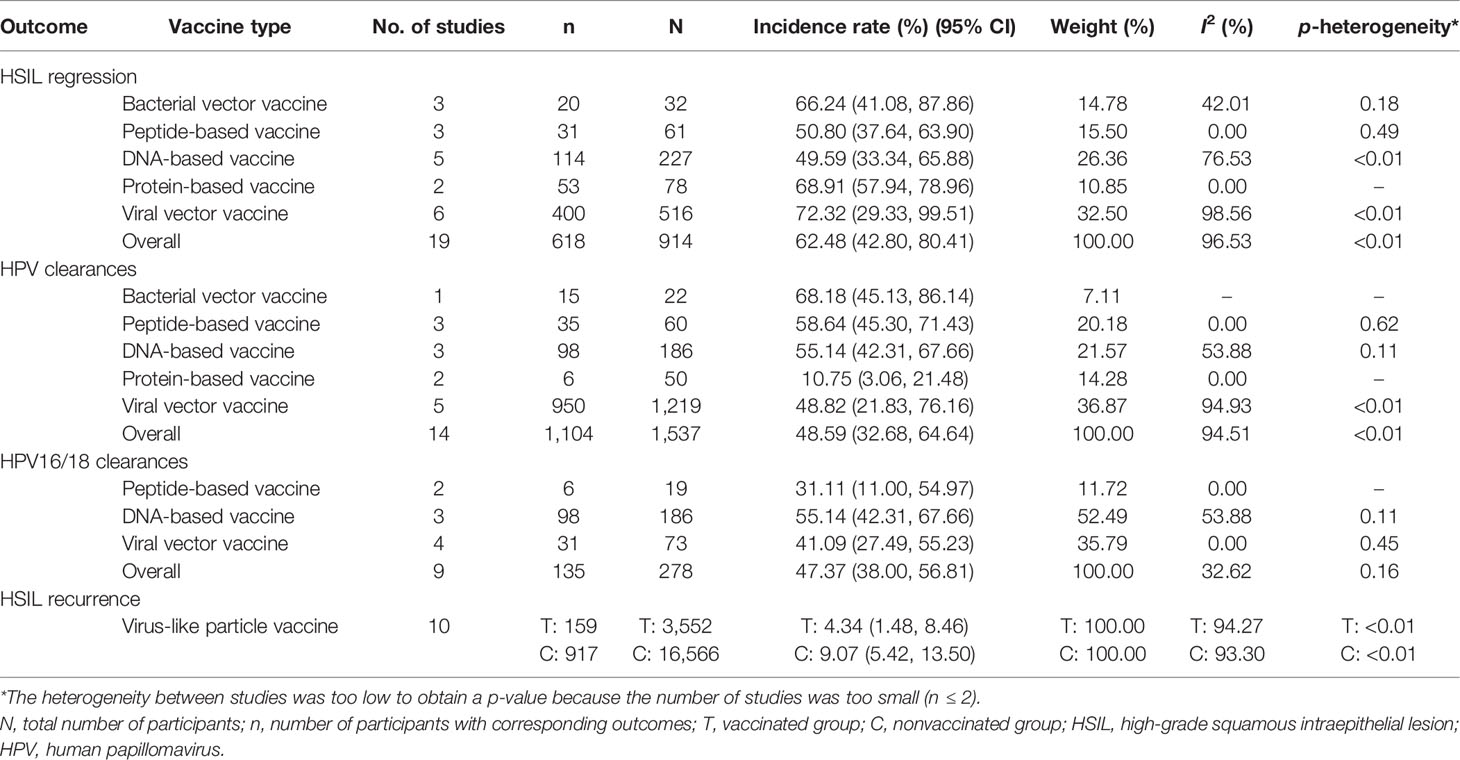
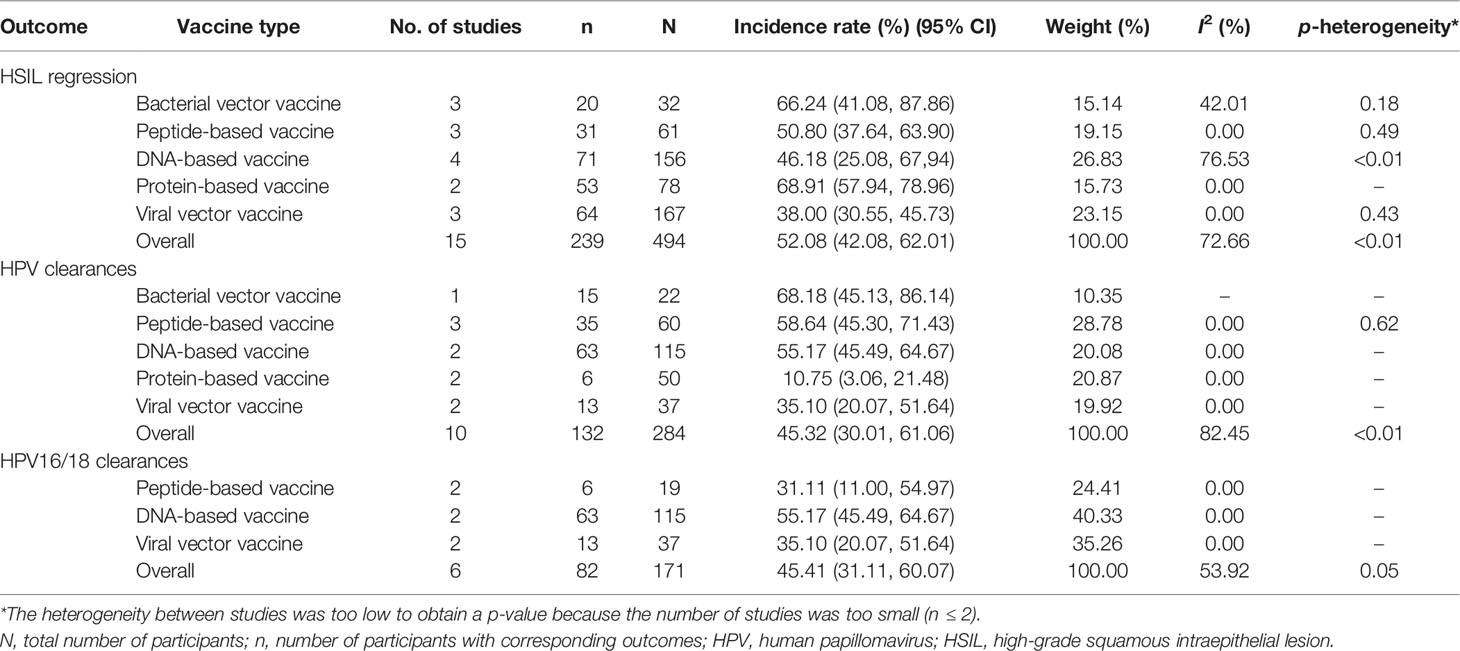
In all, 19 studies reported HSIL regression in 914 patients (13–16, 18, 20, 26, 27, 30, 35–37, 40, 43–45, 47–49). As shown in Table 2, a comprehensive analysis showed that the overall incidence rate of HSIL regression induced by the five types of vaccine was 62.48% [95% CI (42.80, 80.41)]. Viral vector vaccines had the best effect, with an incidence rate of 72.32% [95% CI (29.33, 99.51)], followed by protein-based vaccines at rate of 68.91% [95% CI (57.94, 78.96)]. After removing four studies with a high risk of bias, the overall effect changed to 52.08% [95% CI (42.08, 62.01)], and the heterogeneity among studies changed from 96.53% (p < 0.01) to 72.66% (p < 0.01) (Table 3).
3.2.2 HPV Clearance
Overall, 14 studies reported HPV clearance in 1,537 patients (13, 15, 16, 18, 20, 26, 27, 35, 36, 40, 44, 47–49), among which 9 reported HPV16/18 clearance in 278 patients (Table 2) (13, 15, 16, 20, 26, 27, 35, 40, 44). A comprehensive analysis indicated that the overall incidence rate was 48.59% [95% CI (32.68, 64.64)]. Bacterial vector vaccines had the best effect with a rate of 68.18% [95% CI (45.13, 86.14)], followed by peptide-based vaccines at a rate of 58.64% (95% CI (45.30, 71.43)]. After removing four studies with a high risk of bias, the overall effect changed to 45.32% [95% CI (30.01, 61.06)], and the heterogeneity among studies changed from 94.51% (p < 0.01) to 82.45% (p < 0.01) (Table 3). For HPV16/18 clearance specifically, the overall incidence rate was 47.37% [95% CI (38.00, 56.81)], and DNA-based vaccines had the best effect at a rate of 55.14% [95% CI (42.31, 67.66)]. After removing three studies with a high risk of bias, the overall effect changed to 45.41% [95% CI (31.11, 60.07)], and the heterogeneity among studies changed from 32.62% (p = 0.16) to 53.92% (p < 0.01) (Table 3).
3.2.3 HSIL Recurrence
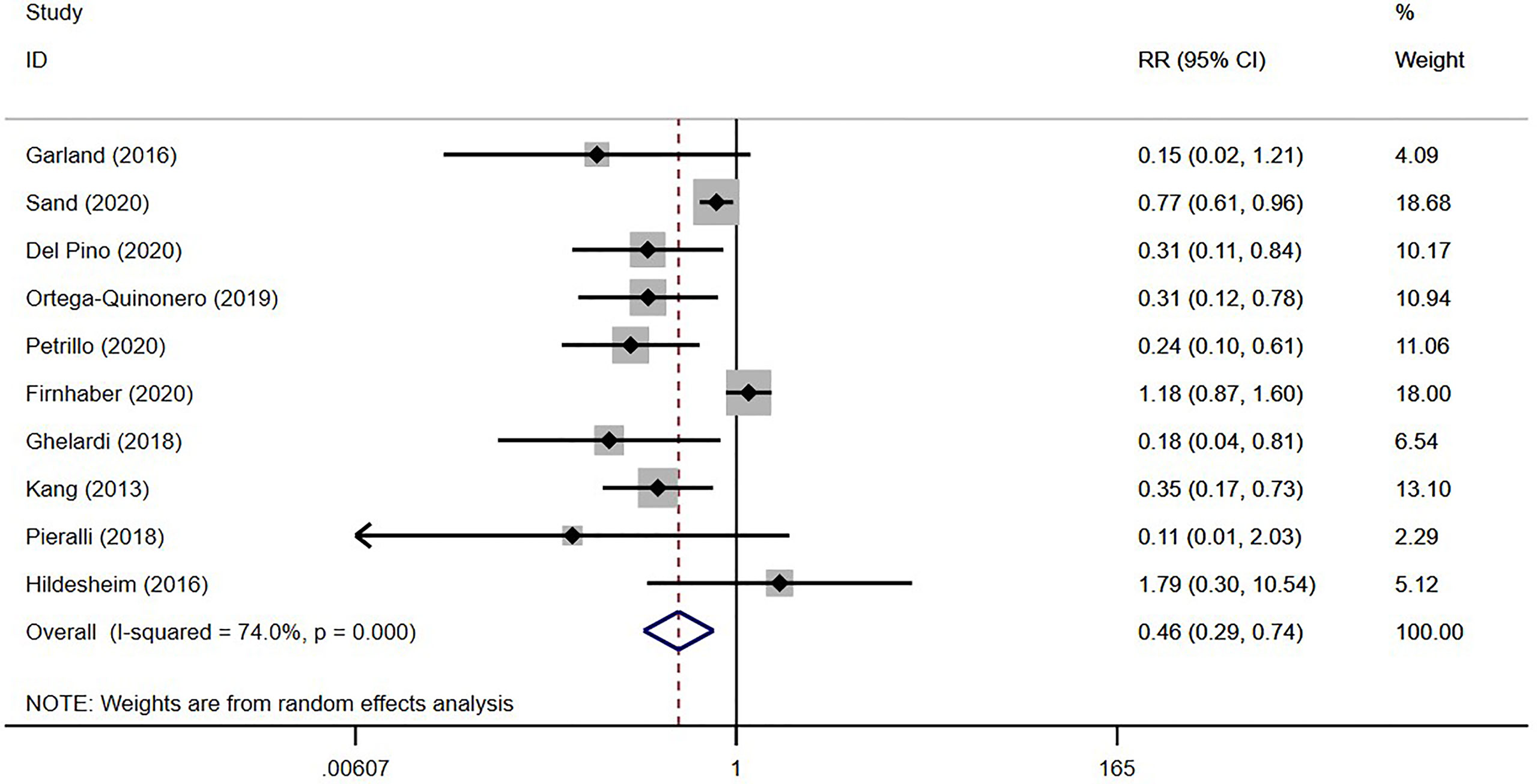
In all, 10 studies reported HSIL recurrence in 20,118 patients (19, 28, 29, 31, 33, 51–55), with 3,552 vaccinated. As shown in Table 2 and Figure 2, the virus-like particle vaccines (Cervarix, Gardasil, and Gardasil 9) reduced the risk of recurrent HSIL after conization with an RR of 0.46 [95% CI (0.29, 0.74)], compared to conization alone. All of these 10 studies had low or moderate risk of bias, and the heterogeneity among studies was 74.00% (p < 0.01).
3.3 Safety
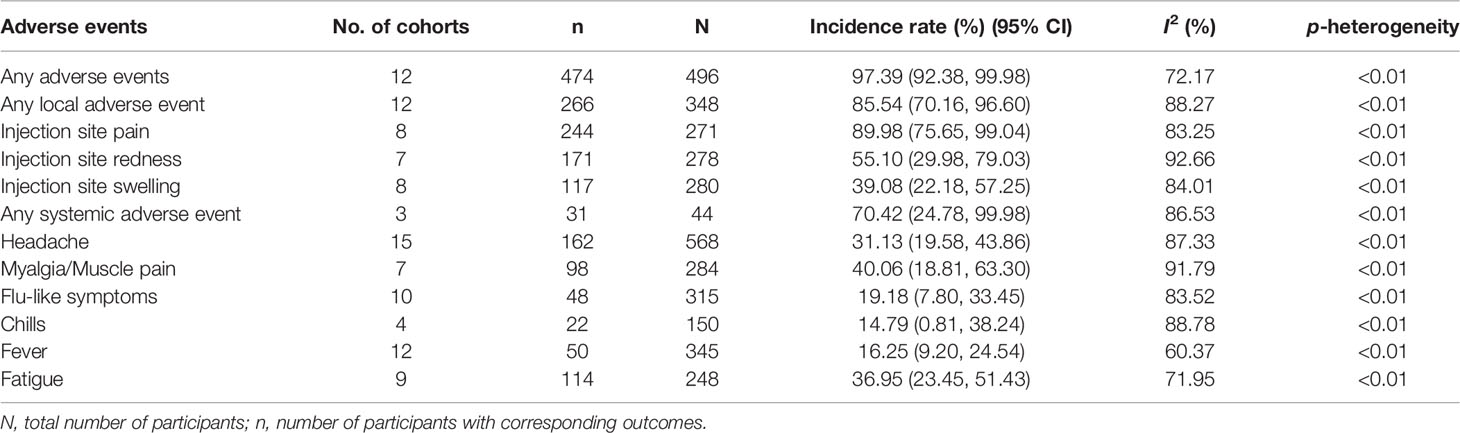
The incidence rates of adverse events after receiving therapeutic vaccines for precancerous lesions of cervical cancer are shown in Table 4. The rate of any adverse event was 97.39% [95% CI (92.38, 99.98)]. The rates of any local or systemic adverse events were 85.54% [95% CI (70.16, 96.60)] and 70.42% [95% CI (24.78, 99.98)], respectively, among which injection site pain [89.98%, 95% CI (75.65, 99.04)], injection site redness [55.10%, 95% CI (29.98, 79.03)], and injection site swelling [39.08%, 95% CI (22.18, 57.25)] were common local adverse events and myalgia/muscle pain [40.06%, 95% CI (18.81, 63.30)], fatigue [36.95%, 95% CI (23.45, 51.43)], and headache [31.13%, 95% CI (19.58, 43.86)] were common systemic adverse events. In addition, four studies (15, 17, 25, 30) reported severe adverse events after vaccination: Trimble et al. (15) reported that 2/125 participants treated with VGX-3100 discontinued because of pain; Frazer et al. (17) reported that 1 participant with severe local pain, 1 participant with severe swelling and redness at the injection site, 3 participants with severe tiredness, and 4 participants discontinued because of adverse events after being treated with the HPV E6 and E7 vaccine; de Vos van Steenwijk et al. (25) reported that 2/5 participants discontinued because of adverse events after being treated with the HPV E6 and E7 vaccine; Harper et al. (30) reported a relatively high number of severe adverse events (among 136 patients): 40 with a grade 3 injection site reaction, 1 with lymphadenopathy, and 2 discontinued for adverse events after being treated with the Tipapkinogen Sovacivec vaccine (Table 5).
4 Discussion
To the best of our knowledge, this study is the first meta-analysis on the effectiveness and safety of therapeutic vaccines for precancerous lesions of cervical cancer. We found that therapeutic vaccines for precancerous cervical lesions could be mainly divided into the virus-like particle vaccines listed as adjuvant immunotherapies to prevent recurrence without direct therapeutic effects, and several other unlisted vaccines with direct therapeutic effects on precancerous lesions. Regarding HSIL recurrence, virus-like particle vaccines along with conization significantly reduced the risk of recurrence compared to conization only. In addition, the overall rates of effectiveness of vaccines were 50%–72% (virus vector vaccines were the best) for HSIL regression, 49–68% (bacterial vector vaccines were the best) for HPV clearance except for protein-based vaccines, and 31%–55% (DNA-based vaccines were the best) for HPV16/18 clearance. Safety analysis showed that the incidence rate of adverse events of therapeutic vaccines was high (97.39%), but most of them were mild local adverse events, and severe adverse events were rare.
Studies have reported recurrence rates of 5.3% (56) to 8% (57) for HSIL within 12 months after conization. Therefore, preventing HSIL recurrence is of great significance for improving patient prognosis and quality of life. Prophylactic HPV vaccination after conization is effective for preventing HSIL recurrence (10, 11, 58). A recent meta-analysis showed that the risk of recurrent HSIL was significantly reduced by HPV vaccination [RR = 0.41; 95% CI (0.27, 0.64)], and subgroup analysis showed that it had a more obvious protective effect on HPV16/18 infections [RR = 0.37; 95% CI (0.17, 0.80)], while patient age, vaccination time, and follow-up time had no significant effects on the recurrence rate (10). Our results are similar [RR = 0.46; 95% CI (0.29, 0.74)]. However, due to the inconsistent study designs of the included articles, we could not conduct further analysis of specific vaccines, such as a network meta-analysis to compare the effectiveness of preventing HSIL recurrence among Cervarix, Gardasil, and Gardasil 9.
For the treatment of HSIL, LEEP is currently recommended by the WHO (6). In a previous meta-analysis, the HPV clearance rates of LEEP and cold knife conization for HSIL were 64.71% and 72.27%, respectively. The risks of major bleeding were 0.23% and 0.86%, while the risks of major infections were 0.13% and 0.09%, and the risks of pelvic infectious disease were 0.14% and 0.14%, respectively (56). Another meta-analysis showed that the reporting rate of residual lesions after LEEP was 11.2%, and that after cold knife conization was 6.1% (9). We found that the current therapeutic vaccines for precancerous cervical lesions had an average effectiveness of 62.48% for HSIL regression (the highest was 72.32%), and the average level of HPV clearance was 48.59% (the highest was 68.18%). However, we noted that protein vaccines had great effectiveness in HSIL regression but extremely low pooled effectiveness in HPV clearance. Original articles for calculating pooled effectiveness were two case series studies about SGN-00101 (18, 47). The reason may be that HPV clearance was not clearly associated with HSIL regression or with the immune response evoked by the vaccines, as the virus was not easily removed completely and might be susceptible to reinfection (47). Moreover, another possible reason was that the sample size of the two studies was small and the follow-up time was short (18, 47). In addition, most vaccines are safe, with a high incidence of mild adverse events and a very low incidence of severe adverse events. One article reported 40/136 patients with a grade 3 injection site reaction after being treated with the Tipapkinogen Sovacivec vaccine (30). However, no explanation was given in that study, and the vaccine studied has not yet been marketed. In sum, although the effectiveness of therapeutic vaccines was not as good as that of conization, the gap was not large, and the vaccines showed better safety. That is, therapeutic vaccines have good development potential, and are worthy of further research.
Global HPV screening and preventive HPV vaccination programs have reached great achievements in preventing HPV infection and related diseases (59). However, due to the gap between developed countries and developing countries in terms of screening and access to vaccines, existing HPV vaccines do not eliminate preexisting infections, resulting in a global disease burden for cervical cancer and other related cancers. It is particularly important to continuously explore immunotherapy methods for HPV and related diseases, including therapeutic vaccines. To date, although no specific therapeutic vaccines for precancerous cervical lesions have been marketed, and vaccine treatments have not been included in clinical guidelines (4), numerous clinical trials have shown that some vaccines have great potential for the treatment of precancerous cervical lesions. However, research progress on therapeutic vaccines for precancerous lesions is slow. This may be because the development of vaccines requires more human, material, financial, and time investment, and at the same time faces many difficulties in terms of safety, immunogenicity, HPV polytype, and mutagenicity (60, 61).
There were some limitations to this study that should be mentioned. First, although 39 studies were included, 21 vaccines have been researched in these studies, which means that there is little literature in regard to each vaccine; thus, we only conducted subgroup analysis by vaccine type and risk of bias, and it was difficult to further analyze the age of patients, the severity of precancerous lesions, and the HPV infection status for heterogeneity analysis. Second, because most of the articles that included control groups studied different vaccines and different primary outcome measures, we only calculated the overall incidence rates of most outcomes and did not conduct comparative analyses, including comparison with the HSIL regression that occurs naturally. Previous meta-analysis showed that the pooled rate of lesion regression in untreated CIN2 patients within 24 months was as high as 50%, with high heterogeneity among included studies (62). However, there was little direct evidence for the comparison between therapeutic vaccines and the untreated group; thus, it was difficult to evaluate the effectiveness of therapeutic vaccines in comparison with untreated patients. There was also a lack of comparative studies between different therapeutic vaccines, indicating that high-quality prospective original studies need to be carried out to provide more evidence support for clinical decision-making in the future.
5 Conclusion
At present, many studies on therapeutic vaccines for precancerous cervical lesions have been carried out, involving various types of vaccine. Virus-like particle vaccines as an adjuvant immunotherapy for conization can significantly reduce the risk of HSIL recurrence. Most therapeutic vaccines have direct therapeutic effects on precancerous lesions, and the effectiveness in HSIL regression, clearance of HPV, and clearance of HPV16/18 is great. Although the effectiveness of therapeutic vaccines may not be as good as that of conization, the gap is not large, and the vaccines have good safety profiles. That is, therapeutic vaccines have good development potential and are worthy of further research.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
FS conceived and designed the study. ShC carried out the literature searches. ShC, XT, KM, DL, and SiC extracted the data, and assessed study quality. ShC performed the statistical analysis. ShC, XT, and KM wrote the manuscript. FS, XZ, PL, ShC, XT, and KM revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Science and Technology Major Project (2021YFC2301601), the National Natural Science Foundation of China (72074011), and the Special Project for Director, China Center for Evidence Based Traditional Chinese Medicine (2020YJSZX-2). The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the paper. No payment was received by any of the authors for the preparation of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.918331/full#supplementary-material
References
1. World Health Organization. Estimated Number of Incident Cases and Deaths Worldwide, Females, All Ages . Available at: https://gco.iarc.fr (Accessed 29 January 2022).
2. World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem . Available at: https://www.who.int/publications/i/item/9789240014107 (Accessed 15 February 2022).
3. World Health Organization. Comprehensive Cervical Cancer Control: A Guide to Essential Practice - Second Edition . Available at: https://www.who.int/reproductivehealth/publications/cancers/cervical-cancer-guide/en/ (Accessed 15 February 2022).
4. World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention, Second Edition: Use of mRNA Tests for Human Papillomavirus (HPV) . Available at: https://www.who.int/publications/i/item/9789240040434 (Accessed 15 February 2022).
5. Li T, Wang C. High Risk HPV Test Combined With TCT in the Screening of Cervical Intraepithelial Neoplasia. Chin Gen Pract (2021) 24(09):1106–10. doi: 10.12114/j.issn.1007-9572.2021.00.443
6. World Health Organization. Treatment of Cervical Intraepithelial Neoplasia 2–3 and Adenocarcinoma in Situ: Cryotherapy, Large Loop Excision of the Transformation Zone, and Cold Knife Conization . Available at: https://www.who.int/reproductivehealth/publications/cancers/treatment_CIN_2-3/en/ (Accessed 15 February 2022).
7. Liu M, Han X. Research Progress on Treatment and Prognosis Prediction of High-Grade Cervical Intraepithelial Neoplasia. J Of Int Obstetrics And Gynecol (2021) 48(03):337–42. doi: 10.12280/gjfckx.20200855
8. Kalliala I, Athanasiou A, Veroniki AA, Salanti G, Efthimiou O, Raftis N, et al. Incidence and Mortality From Cervical Cancer and Other Malignancies After Treatment of Cervical Intraepithelial Neoplasia: A Systematic Review and Meta-Analysis of the Literature. Ann Oncol (2020) 31(2):213–27. doi: 10.1016/j.annonc.2019.11.004
9. Hurtado-Roca Y, Becerra-Chauca N, Malca M. Efficacy and Safety of Cryotherapy, Cold Cone or Thermocoagulation Compared to LEEP as a Therapy for Cervical Intraepithelial Neoplasia: Systematic Review. Rev saúde pública (2020) 54:27. doi: 10.11606/s1518-8787.2020054001750
10. Jentschke M, Kampers J, Becker J, Sibbertsen P, Hillemanns P. Prophylactic HPV Vaccination After Conization: A Systematic Review and Meta-Analysis. Vaccine (2020) 38(41):6402–9. doi: 10.1016/j.vaccine.2020.07.055
11. Lichter K, Krause D, Xu J, Tsai SHL, Hage C, Weston E, et al. Adjuvant Human Papillomavirus Vaccine to Reduce Recurrent Cervical Dysplasia in Unvaccinated Women: A Systematic Review and Meta-Analysis. Obstetrics gynecol (New York 1953) (2020) 135(5):1070–83. doi: 10.1097/AOG.0000000000003833
12. Wang R, Pan W, Jin L, Huang W, Li Y, Wu D, et al. Human Papillomavirus Vaccine Against Cervical Cancer: Opportunity and Challenge. Cancer Lett (2020) 471:88–102. doi: 10.1016/j.canlet.2019.11.039
13. Rosales R, López-Contreras M, Rosales C, Magallanes-Molina JR, Gonzalez-Vergara R, Arroyo-Cazarez JM, et al. Regression of Human Papillomavirus Intraepithelial Lesions is Induced by MVA E2 Therapeutic Vaccine. Hum Gene Ther (2014) 25(12):1035–49. doi: 10.1089/hum.2014.024
14. Park YC, Ouh YT, Sung MH, Park HG, Kim TJ, Cho CH, et al. A Phase 1/2a, Dose-Escalation, Safety and Preliminary Efficacy Study of Oral Therapeutic Vaccine in Subjects With Cervical Intraepithelial Neoplasia 3. J Gynecologic Oncol (2019) 30(6):e88. doi: 10.3802/jgo.2019.30.e88
15. Trimble CL, Morrow MP, Kraynyak KA, Shen X, Dallas M, Yan J, et al. Safety, Efficacy, and Immunogenicity of VGX-3100, a Therapeutic Synthetic DNA Vaccine Targeting Human Papillomavirus 16 and 18 E6 and E7 Proteins for Cervical Intraepithelial Neoplasia 2/3: A Randomised, Double-Blind, Placebo-Controlled Phase 2b Trial. Lancet (2015) 386(10008):2078–88. doi: 10.1016/S0140-6736(15)00239-1
16. Solares AM, Baladron I, Ramos T, Valenzuela C, Borbon Z, Fanjull S, et al. Safety and Immunogenicity of a Human Papillomavirus Peptide Vaccine (CIGB-228) in Women With High-Grade Cervical Intraepithelial Neoplasia: First-In-Human, Proof-of-Concept Trial. ISRN Obstetrics Gynecol (2011) 2011:292951. doi: 10.5402/2011/292951
17. Frazer IH, Quinn M, Nicklin JL, Tan J, Perrin LC, Ng P, et al. Phase 1 Study of HPV16-Specific Immunotherapy With E6E7 Fusion Protein and ISCOMATRIX Adjuvant in Women With Cervical Intraepithelial Neoplasia. Vaccine (2004) 23(2):172–81. doi: 10.1016/j.vaccine.2004.05.013
18. Einstein MH, Kadish AS, Burk RD, Kim MY, Wadler S, Streicher H, et al. Heat Shock Fusion Protein-Based Immunotherapy for Treatment of Cervical Intraepithelial Neoplasia III. Gynecologic Oncol (2007) 106(3):453–60. doi: 10.1016/j.ygyno.2007.04.038
19. Garland SM, Paavonen J, Jaisamrarn U, Naud P, Salmerón J, Chow SN, et al. Prior Human Papillomavirus-16/18 AS04-Adjuvanted Vaccination Prevents Recurrent High Grade Cervical Intraepithelial Neoplasia After Definitive Surgical Therapy: Post-Hoc Analysis From a Randomized Controlled Trial. Int J Cancer (2016) 139(12):2812–26. doi: 10.1002/ijc.30391
20. Brun JL, Dalstein V, Leveque J, Mathevet P, Raulic P, Baldauf JJ, et al. Regression of High-Grade Cervical Intraepithelial Neoplasia With TG4001 Targeted Immunotherapy. Am J Obstetrics Gynecol (2011) 204(2):169.e1–8. doi: 10.1016/j.ajog.2010.09.020
21. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. he Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. Bmj (2011) 343:d5928. doi: 10.1136/bmj.d5928
22. The Ottawa Hospital. The Newcastle-Ottawa Scale (NOS) for Assessing The Quality of Nonrandomised Studies in Meta-Analyses . Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed 02 August 2021).
23. Dalziel K, Round A, Stein K, Garside R, Castelnuovo E, Payne L. Do the Findings of Case Series Studies Vary Significantly According to Methodological Characteristics? Health Technol Assess (Winchester England) (2005) 9(2):1–146. doi: 10.3310/hta9020
24. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A Tool for Assessing Risk of Bias in non-Randomised Studies of Interventions. Bmj (2016) 355:i4919. doi: 10.1136/bmj.i4919
25. de Vos van Steenwijk PJ, Ramwadhdoebe TH, Löwik MJ, van der Minne CE, Berends-van der Meer DM, Fathers LM, et al. A Placebo-Controlled Randomized HPV16 Synthetic Long-Peptide Vaccination Study in Women With High-Grade Cervical Squamous Intraepithelial Lesions. Cancer immunol immunother (2012) 61(9):1485–92. doi: 10.1007/s00262-012-1292-7
26. Kaufmann AM, Nieland JD, Jochmus I, Baur S, Friese K, Gabelsberger J, et al. Vaccination Trial With HPV16 L1E7 Chimeric Virus-Like Particles in Women Suffering From High Grade Cervical Intraepithelial Neoplasia (UN 2/3). Int J Cancer (2007) 121(12):2794–800. doi: 10.1002/ijc.23022
27. Choi YJ, Hur SY, Kim TJ, Hong SR, Lee JK, Cho CH, et al. A Phase 2, Prospective, Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients With Cervical Intraepithelial Neoplasia 3. Clin Cancer Res (2019) 26(7):1616–23. doi: 10.1158/1078-0432.CCR-19-1513
28. Firnhaber C, Swarts A, Jezile V, Mulongo M, Goeieman B, Williams S, et al. HPV Vaccination Prior to Loop Electroexcision Procedure Does Not Prevent Recurrent Cervical High Grade Squamous Intraepithelial Lesions in Women Living With HIV: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin Infect Dis (2020) 73(7):e2211–6. doi: 10.1093/cid/ciaa1456
29. Pieralli A, Bianchi C, Auzzi N, Fallani MG, Bussani C, Fambrini M, et al. Indication of Prophylactic Vaccines as a Tool for Secondary Prevention in HPV-Linked Disease. Arch Gynecol Obstet (2018) 298(6):1205–10. doi: 10.1007/s00404-018-4926-y
30. Harper DM, Nieminen P, Donders G, Einstein MH, Garcia F, Huh WK, et al. The Efficacy and Safety of Tipapkinogen Sovacivec Therapeutic HPV Vaccine in Cervical Intraepithelial Neoplasia Grades 2 and 3: Randomized Controlled Phase II Trial With 2.5years of Follow-Up. Gynecologic Oncol (2019) 153(3):521–9. doi: 10.1016/j.ygyno.2019.03.250
31. Hildesheim A, Gonzalez P, Kreimer AR, Wacholder S, Schussler J, Rodriguez AC, et al. Impact of Human Papillomavirus (HPV) 16 and 18 Vaccination on Prevalent Infections and Rates of Cervical Lesions After Excisional Treatment. Am J Obstetrics Gynecol (2016) 215(2):212.e1–212.e15. doi: 10.1016/j.ajog.2016.02.021
32. Karimi-Zarchi M, Allahqoli L, Nehmati A, Kashi AM, Taghipour-Zahir S, Alkatout I. Can the Prophylactic Quadrivalent HPV Vaccine be Used as a Therapeutic Agent in Women With CIN? A Randomized Trial. BMC Public Health (2020) 20(1):274. doi: 10.1186/s12889-020-8371-z
33. Kang WD, Choi HS, Kim SM. Is Vaccination With Quadrivalent HPV Vaccine After Loop Electrosurgical Excision Procedure Effective in Preventing Recurrence in Patients With High-Grade Cervical Intraepithelial Neoplasia (CIN2-3)? Gynecologic Oncol (2013) 130(2):264–8. doi: 10.1016/j.ygyno.2013.04.050
34. Zarochentseva NV, Belaiya JM, Malinovskaya VV. Combined Use of Interferon Alpha-2B Drugs With Tetravalent Vaccine Against Reinfection in HPV Female Patients. Electronic J Gen Med (2020) 17(6):1–5. doi: 10.29333/ejgm/8369
35. Gutierrez CMC, Tinoco A, Navarro T, Contreras ML, Cortes RR, Calzado P, et al. Therapeutic Vaccination With MVA E2 can Eliminate Precancerous Lesions (CIN 1, CIN 2, and CIN 3) Associated With Infection by Oncogenic Human Papillomavirus. Hum Gene Ther (2004) 15(5):421–31. doi: 10.1089/10430340460745757
36. Garcia-Hernandez E, Gonzalez-Sanchez JL, Andrade-Manzano A, Contreras ML, Padilla S, Guzman CC, et al. Regression of Papilloma High-Grade Lesions (CIN 2 and CIN 3) is Stimulated by Therapeutic Vaccination With MVA E2 Recombinant Vaccine. Cancer Gene Ther (2006) 13(6):592–7. doi: 10.1038/sj.cgt.7700937
37. Alvarez RD, Huh WK, Bae S, Lamb LS, Conner MG, Boyer J, et al. A Pilot Study of Pngvl4a-CRT/E7(detox) for the Treatment of Patients With HPV16 + Cervical Intraepithelial Neoplasia 2/3 (CIN2/3). Gynecologic Oncol (2016) 140(2):245–52. doi: 10.1016/j.ygyno.2015.11.026
38. Hallez S, Simon P, Maudoux F, Doyen J, Noël JC, Beliard A, et al. Phase I/II Trial of Immunogenicity of a Human Papillomavirus (HPV) Type 16 E7 Protein-Based Vaccine in Women With Oncogenic HPV-Positive Cervical Intraepithelial Neoplasia. Cancer Immunol Immunother (2004) 53(7):642–50. doi: 10.1007/s00262-004-0501-4
39. Simon P, Buxant F, Hallez S, Burny A, Fayt I, Anaf V, et al. Cervical Response to Vaccination Against HPV16 E7 in Case of Severe Dysplasia. Eur J Obstetrics Gynecol Reprod Biol (2003) 109(2):219–23. doi: 10.1016/S0301-2115(03)00093-9
40. Kim TJ, Jin HT, Hur SY, Yang HG, Seo YB, Hong SR, et al. Clearance of Persistent HPV Infection and Cervical Lesion by Therapeutic DNA Vaccine in CIN3 Patients. Nat Commun (2014) 5:5317. doi: 10.1038/ncomms6317
41. Maldonado L, Teague JE, Morrow MP, Jotova I, Wu TC, Wang C, et al. Intramuscular Therapeutic Vaccination Targeting HPV16 Induces T Cell Responses That Localize in Mucosal Lesions. Sci Transl Med (2014) 6(221):221ra13. doi: 10.1126/scitranslmed.3007323
42. Komdeur FL, Singh A, van de Wall S, Meulenberg JJM, Boerma A, Hoogeboom BN, et al. First-In-Human Phase I Clinical Trial of an SFV-Based RNA Replicon Cancer Vaccine Against HPV-Induced Cancers. Mol Ther J Am Soc Gene Ther (2021) 29(2):611–25. doi: 10.1016/j.ymthe.2020.11.002
43. Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, et al. A Phase I Trial of a Human Papillomavirus DNA Vaccine for HPV16+Cervical Intraepithelial Neoplasia 2/3. Clin Cancer Res (2009) 15(1):361–7. doi: 10.1158/1078-0432.CCR-08-1725
44. Coleman HN, Greenfield WW, Stratton SL, Vaughn R, Kieber A, Moerman-Herzog AM, et al. Human Papillomavirus Type 16 Viral Load is Decreased Following a Therapeutic Vaccination. Cancer Immunol Immunother (2016) 65(5):563–73. doi: 10.1007/s00262-016-1821-x
45. Kawana K, Adachi K, Kojima S, Taguchi A, Tomio K, Yamashita A, et al. Oral Vaccination Against HPV E7 for Treatment of Cervical Intraepithelial Neoplasia Grade 3 (CIN3) Elicits E7-Specific Mucosal Immunity in the Cervix of CIN3 Patients. Vaccine (2014) 32(47):6233–9. doi: 10.1016/j.vaccine.2014.09.020
46. Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, et al. Immunotherapy Against HPV16/18 Generates Potent TH1 and Cytotoxic Cellular Immune Responses. Sci Trans Med (2012) 4(155):155ra138. doi: 10.1126/scitranslmed.3004414
47. Roman LD, Wilczynski S, Muderspach LI, Bumett AF, O'Meara A, Brinkman JA, et al. A Phase II Study of Hsp-7 (SGN-00101) in Women With High-Grade Cervical Intraepithelial Neoplasia. Gynecologic Oncol (2007) 106(3):558–66. doi: 10.1016/j.ygyno.2007.05.038
48. Greenfield WW, Stratton SL, Myrick RS, Vaughn R, Donnalley LM, Coleman HN, et al. A Phase I Dose-Escalation Clinical Trial of a Peptide-Based Human Papillomavirus Therapeutic Vaccine With Candida Skin Test Reagent as a Novel Vaccine Adjuvant for Treating Women With Biopsy-Proven Cervical Intraepithelial Neoplasia 2/3. OncoImmunology (2015) 4(10):e1031439. doi: 10.1080/2162402X.2015.1031439
49. Balajewicz M, Dembowska J, Klimek R. Test of Immunopotentialization in Colposcopy–a Clinical Evaluation. Eur J Obstet Gynecol Reprod Biol (1989) 33(3):253–7. doi: 10.1016/0028-2243(89)90138-x
50. Klimiek R, Dembowska J, Balajewicz M, Plechanow J. Effect of Immunopotentialization on Rate of Vaginal Smear Normalization According to Appearance of Cervical Intraepithelial Neoplasia. Int J Gynecol Obstetrics (1989) 28(1):41–4. doi: 10.1016/0020-7292(89)90542-0
51. Sand FL, Kjaer SK, Frederiksen K, Dehlendorff C. Risk of Cervical Intraepithelial Neoplasia Grade 2 or Worse After Conization in Relation to HPV Vaccination Status. Int J Cancer J Int du Cancer (2020) 147(3):641–7. doi: 10.1002/ijc.32752
52. Petrillo M, Dessole M, Tinacci E, Saderi L, Muresu N, Capobianco G, et al. Efficacy of HPV Vaccination in Women Receiving LEEP for Cervical Dysplasia: A Single Institution’s Experience. Vaccines (2020) 8(1):45. doi: 10.3390/vaccines8010045
53. Del Pino M, Martí C, Torras I, Henere C, Munmany M, Marimon L, et al. HPV Vaccination as Adjuvant to Conization in Women With Cervical Intraepithelial Neoplasia: A Study Under Real-Life Conditions. Vaccines (2020) 8(2):245. doi: 10.3390/vaccines8020245
54. Ortega-Quinonero P, Remezal-Solano M, Carazo-Diaz MC, Prieto-Merino D, Urbano-Reyess MI, Garcia de Guadiana-Romualdo L, et al. Impact of the Human Papillomavirus Vaccination on Patients Who Underwent Conization for High-Grade Cervical Intraepithelial Neoplasia. Eur J Gynaecol Oncol (2019) 40(3):402–7. doi: 10.12892/ejgo4628.2019
55. Ghelardi A, Parazzini F, Martella F, Pieralli A, Bay P, Tonetti A, et al. SPERANZA Project: HPV Vaccination After Treatment for CIN2+. Gynecologic Oncol (2018) 151(2):229–34. doi: 10.1016/j.ygyno.2018.08.033
56. Santesso N, Mustafa RA, Wiercioch W, Kehar R, Gandhi S, Chen Y, et al. Systematic Reviews and Meta-Analyses of Benefits and Harms of Cryotherapy, LEEP, and Cold Knife Conization to Treat Cervical Intraepithelial Neoplasia. Int J gynecol obstetrics (2015) 132(3):266–71. doi: 10.1016/j.ijgo.2015.07.026
57. Arbyn M, Ronco G, Anttila A, Meijer CJLM, Poljak M, Ogilvie G, et al. Evidence Regarding Human Papillomavirus Testing in Secondary Prevention of Cervical Cancer. Vaccine (2012) 30(Suppl 5):F88–99. doi: 10.1016/j.vaccine.2012.06.095
58. Bartels HC, Postle J, Rogers AC, Brennan D. Prophylactic Human Papillomavirus Vaccination to Prevent Recurrence of Cervical Intraepithelial Neoplasia: A Meta-Analysis. Int J gynecol Cancer (2020) 30(6):777–82. doi: 10.1136/ijgc-2020-001197
59. Goodman A. HPV Testing as a Screen for Cervical Cancer. Bmj (2015) 350:h2372. doi: 10.1136/bmj.h2372
60. Wang Y, Zhang H, Yu J, Li C, Lu Y. Advances of Human Papillomavirus Therapeutic Vaccine. J Int Oncol (2017) 44(7):526–30. doi: 10.3760/cma.j.issn.1673-422X.2017.07.012
61. Hua C, Sun S, Cheng H, Han R. Advance in Human Papillomavirus Major Capsid Protein L1-Based Vaccines. Chin J Microbiol Immunol (2019) 39(10):788–93. doi: 10.3760/cma.j.issn.0254-5101.2019.010.011
Keywords: precancerous cervical lesions, human papillomavirus, therapeutic vaccines, effectiveness, safety
Citation: Cai S, Tan X, Miao K, Li D, Cheng S, Li P, Zeng X and Sun F (2022) Effectiveness and Safety of Therapeutic Vaccines for Precancerous Cervical Lesions: A Systematic Review and Meta-Analysis. Front. Oncol. 12:918331. doi: 10.3389/fonc.2022.918331
Received: 12 April 2022; Accepted: 10 May 2022;
Published: 06 June 2022.
Edited by:
Zong Sheng Guo, Roswell Park Comprehensive Cancer Center, United StatesReviewed by:
Mary Schwartz, Houston Methodist Hospital, United StatesShu-Hsing Cheng, Tao Yuan General Hospital, Taiwan
Copyright © 2022 Cai, Tan, Miao, Li, Cheng, Li, Zeng and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Sun, sunfeng@bjmu.edu.cn
 Shan Cai
Shan Cai Xiaoyu Tan2
Xiaoyu Tan2 Feng Sun
Feng Sun