- 1Center for Novel and Exploratory Clinical Trials (Y-NEXT), Yokohama City University Hospital, Yokohama, Japan
- 2Department of Neurosurgery, Graduate School of Medicine, Yokohama City University, Yokohama, Japan
Meningioma is the most common primary neoplasm of the central nervous system (CNS). Generally, these tumors are benign and have a good prognosis. However, treatment can be challenging in cases with aggressive variants and poor prognoses. Among various prognostic factors that have been clinically investigated, bone invasion remains controversial owing to a limited number of assessments. Recent study reported that bone invasion was not associated with WHO grades, progression, or recurrence. Whereas, patients with longer-recurrence tended to have a higher incidence of bone invasion. Furthermore, bone invasion may be a primary preoperative predictor of the extent of surgical resection. Increasing such evidence highlights the potential of translational studies to understand bone invasion as a prognostic factor of meningiomas. Therefore, this mini-review summarizes recent advances in pathophysiology and diagnostic modalities and discusses future research directions and therapeutic strategies for meningiomas with bone invasion.
Introduction
Meningiomas
Meningioma is the most common primary neoplasm of the central nervous system (CNS) in adults, originating from arachnoid cap cells covering the CNS. They are classified according to histopathological characteristics and have a broad morphological spectrum, reflected in 15 subtypes. (1–3). The World Health Organization (WHO) has also classified meningiomas into three grades (1–3), similar to other CNS tumors, linked to overall expected clinical-biological behaviors. Most tumors are WHO grade 1, which are slow-growing with benign features and a comparatively good prognosis. WHO grades 2 and 3 (4, 5) have local brain invasiveness and cellular features, including higher mitosis and atypia. In general, symptomatic cases of any WHO grade are surgically treated, and to date, there is no consensus on the effectiveness of pharmacotherapy, including chemotherapy (6). Hence, Simpson’s grade, based on the extent of surgical resection, has been considered a good tumor recurrence indicator in addition to WHO grading (7). Simpson grade I is defined as complete removal, including resection of the underlying bone and associated dura. However, meningiomas classified as WHO grade 1 and Simpson grade I sometimes recur in long-term follow-up, often requiring additional treatments, such as secondary surgery or salvage radiosurgery, which can be challenging and potentially lead to morbidity (8). Therefore, recent studies have highlighted the importance of long-term recurrence prediction with a different viewpoint than WHO grade and developing diagnostic and therapeutic options for such recurrent cases.
Meningioma With Bone Invasion
Meningiomas are categorized inconsistently based on their location (9). Sometimes, meningiomas grow extracerebrally, corresponding with the tumor’s origin. Tumors arising from locations other than the subdural compartment have been termed ectopic, extracranial, extraneuraxial, extradural, or intraosseous meningiomas (9, 10).
Primary intraosseous meningioma usually describes tumors that develop mainly from the calvarium and are unequivocally excluded from the subdural component (11). Contrastingly, many unrecognizable meningioma synonyms and subtypes secondarily extend into the adjacent bone, such as secondary intraosseous meningioma, meningioma with bone infiltration, and meningioma with bone invasion. (In this review, they are consistently noted as bone-invasive meningiomas to avoid confusion). In general, bone invasive meningiomas can be preoperatively diagnosed by conventional radiographic modalities, such as magnetic resonance imaging (MRI) and computed tomography (CT). They are histopathologically confirmed after surgery, since the preoperative judgment of bone involvement is sometimes ambiguous (Figures 1A–D). Meningioma en plaque, a relatively uncommon and unique form accounting for 2-9% of meningiomas, is often accompanied by hyperostosis in the middle fossa and sphenoid wing, with an incidence rate of 13–49% (12–14). Nevertheless, hyperostosis is seen less in other meningiomas except for lymphoplasmacyte-rich meningioma, a rare histologic subtype (WHO grade 1), which can arise as an en-plaque meningioma, and is characterized by a prominent infiltration of plasma cells and lymphocytes with a variable proportion of meningothelial elements (15–17). To date, hyperostosis has been thought as due to direct tumor invasion to adjacent bone and reactive hypervascularity of the periosteum leading to benign formation, and thus can often be classified as bone-invasive meningiomas (18–22).
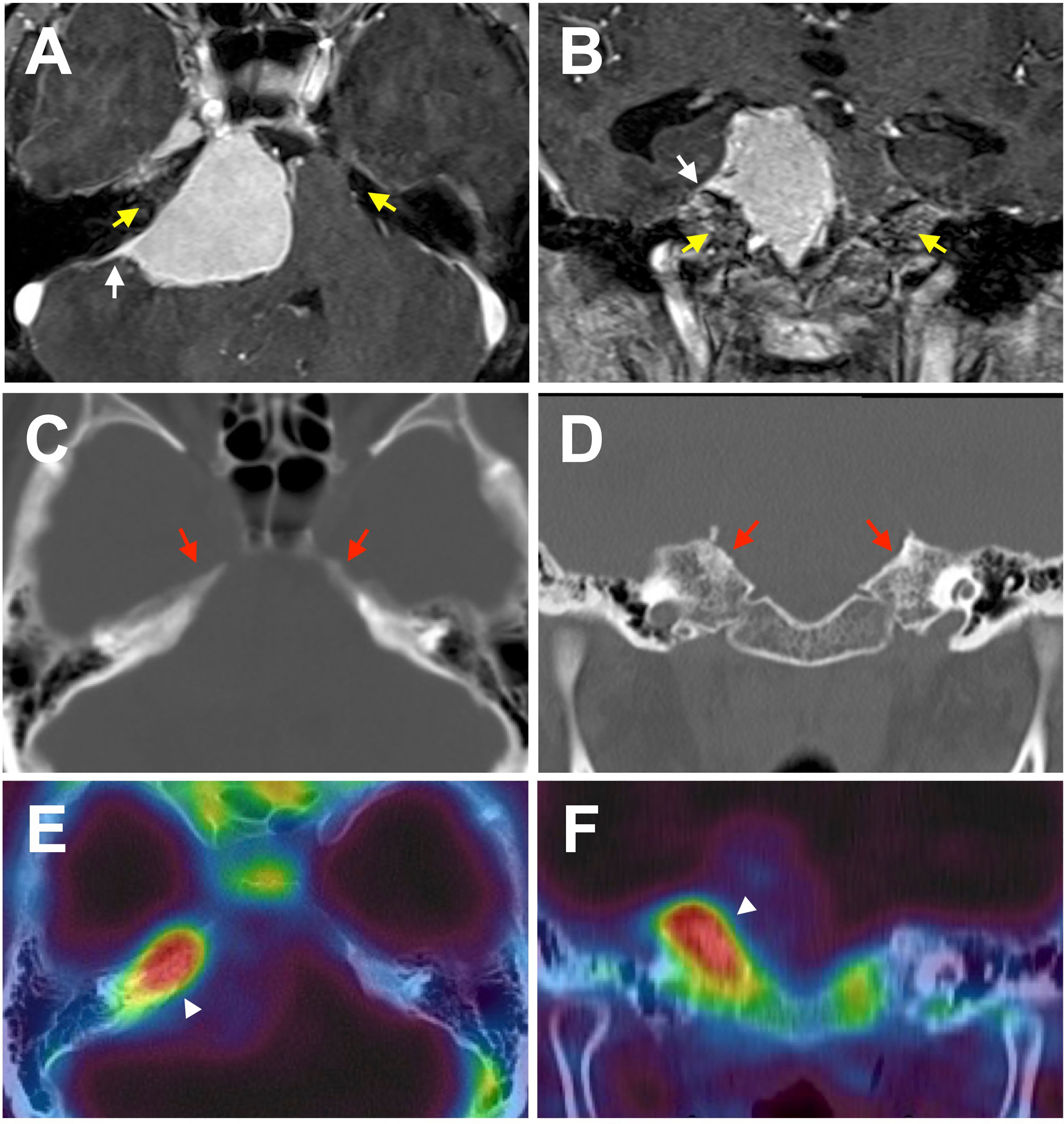
Figure 1 A representative case of meningioma with bone invasion. Axial (A, C, E) and coronal (B, D, F) images demonstrating a petroclival meningioma. (A, B) T1-post contrast MRI shows a characteristic dural tail (white arrows). Enhancement in the adjacent bone is ambiguous, and no obvious laterality is found (yellow arrows). (C, D) Non-contrast bone CT does not reveal a hyperostosis with tumor-associated laterality. (E, F) However, F18 fluoride PET/CT fusion image indicates prominent uptake in the adjacent bone suggesting bone invasion of the tumor (white arrowheads).
In addition to histopathological aggressiveness and surgical extension, accumulating evidence suggests that bone invasion could predict recurrence and is possibly associated with reduced progression-free and overall survival, even in WHO grade 1 or 2 cases that surgically achieved total removal (Gabeau-Lacet et al.: Simpson I-III in WHO grade 1, Abdelzaher et al.: Simpson grade I-II in WHO grade 2, Lemee et al.: Simpson grade I-III in WHO grade 1-3) (23–25). However, due to the limited assessability, bone invasion as a recurrent predictor remains less understood, and is therefore not reflected in the WHO grading criteria. Taken together, these facts strongly suggest that further integrative study of bone invasive meningioma may provide deeper understanding of bone invasive meningioma and improve the long-term prognosis.
Current Issues
The rarity of bone-invasive meningioma may contribute to the limited number of assessments. Thus, bone-invasive meningiomas has not been well described compared with primary intraosseous meningioma (9, 26–28). Another obstacle is the lack of a standard assessment method for bone-invasive meningiomas, except tissue histopathology. In other words, diagnostic options for meningiomas with bone invasion have been less studied. In addition, the specific mechanism of cellular infiltration and the molecular background characteristics are ambiguous. Overall, these facts result in the underdevelopment of therapeutic alternatives for invasion, except for direct microscopic surgery.
However, clinical evidence of bone-invasive meningioma is increasing, emphasizing the importance of further studies to understand bone invasion as an independent prognostic factor or a preoperative factor related to the extent of surgical resection. Several diagnostic modalities have been developed for meningiomas, including bone invasion. Furthermore, recent molecular biology advances exploring therapeutic targets provide future opportunities to reorganize meningioma issues (3, 29).
Aims
Therefore, this mini-review briefly summarizes recent advances in the clinical knowledge of bone-invasive meningioma as a long-term recurrent predictor and introduces potent diagnostic options and molecular pathophysiology. Finally, we discuss future research directions and therapeutic strategies for meningiomas with bone invasion.
Bone Invasion as a Predictor of Recurrence
In a surgical series of WHO grade 2 (atypical) meningiomas, as expected, several studies reported a significant association between bone invasion and progression, multiple recurrences, and poor outcomes, even in patients who underwent gross total resection (23, 30–32). In contrast, a surgical series of non-neurofibromatosis type 2 (NF2) cases (WHO grade 1; N = 118, grade 2 or 3; N = 26) reported that bone invasion, dural tails (identified by conventional MRI), and reactive hyperostosis (assessed by CT) were not associated with WHO grades, progression, or recurrence (33). Additionally, in a recent large series of WHO grade 1 studies, such as Corniola et al. (N = 1352) and Haddad et al. (N = 239), bone invasion was not associated with progression or recurrence (34, 35). However, patients with post-median recurrence (>24 months after treatment) tended to have a higher incidence of histopathological bone invasion (38.5% vs. 16.9% without recurrence, p = 0.064). Furthermore, Cox regression analysis identified an independent relationship between recurrence and incomplete (subtotal) resection, even in WHO grade 1 tumors with a consistent Simpson’s grade (35). Therefore, a long-term clinico-radiological study with histopathological assessment of bone invasion may be preferable to understand how bone invasion affects the recurrence of WHO grade 1 meningioma.
Bone Invasion as a Preoperative Factor to Determine the Extent of Surgical Resection
As previously mentioned, the extent of surgical resection quantified by Simpson grade is the main predictor of recurrence. Microsurgery is “tailor-made” according to the size, surrounding structure, and anatomical location of the tumor, yet complete resection is rarely achieved. Therefore, preoperative factors for determining the extent of surgical resection are also important for predicting the prognosis during the early therapeutic stage (35, 36).
A recent surgical series incorporating retrospectively and prospectively collected data included 1469 meningiomas of all three WHO grades (1, 92.3%; 2, 5.2%; 3, 2.2%) and analyzed predictive factors related to the surgical extent of resection (25). In the largest study among a similar series, bone invasion (definition not addressed) was observed in 18.7% of cases and significantly associated with lower rates of a low Simpson’s grade (not defined) and gross total removal (GTR: defined as a Simpson grade I-III resection in this report) [odds ratio: 0.85 (0.73–0.99) and 0.55 (0.73–0.99), respectively]. Based on these results and the classification and regression tree recursive partitioning analysis, the authors demonstrated that the extent of resection could be very low for symptomatic cases, followed by bone invasion as the second main predictor [GTR; 79% (903/1130) of the cases without bone invasion]. Considering the surgical selection bias underlying asymptomatic cases, as the authors addressed, bone invasion would be a primary preoperative predictor of the extent of surgical resection. Furthermore, bone invasion may be an indirect predictor of meningioma recurrence (37–40).
Bone Invasion and Clinicopathological Grading
Prior investigations have identified that aggressive imaging features are associated with clinicopathologically high-grade meningiomas and, therefore, increase the risk of progression or recurrence (41–45). To date, increasing findings remind us that bone invasion is a unique characteristic, partly resembling a high-grade phenotype, despite not being included in any WHO grading criteria of meningioma (5). As previously described, the incidence of histopathologically confirmed bone invasion in WHO grade 1 tended to be higher in the subgroup of post-median recurrence (>24 months; 38.5%) than in those with early recurrence (<24 months; 16.9%) (35). Nevertheless, another study of 304 cases (grade 1, N = 227; 2, N = 77; 3, N = 5) demonstrated a negative association between histopathological bone invasion and the WHO grade (46). These results suggest that long-term tumor recurrence-related bone invasion may be slower than grade 2 or 3 due to different mechanisms from ordinal histopathological aggressiveness, such as mitosis (Figure 3) (4, 20, 47). Since the meningioma characteristics vary tremendously and provide confusing results that are difficult to adopt into clinical practice, molecular biology research of bone-invasive meningioma may help identify therapeutic targets and understand the clinicopathological background, for instance, related to slower recurrence (33).
The findings detailed above highlight two emerging issues: 1) accurately providing a preoperative diagnosis of meningioma with bone invasion, especially for WHO grade 1 and 2) treating these patients without long-term morbidity. Additionally, an ongoing issue is whether meningiomas, including high-grade and/or bone invasive cases, benefit from early irradiation. Biological and diagnostic updates may be helpful in the future to clarify these issues (32, 47, 48).
Biology and Diagnosis
Radiological and Histopathological Diagnosis
There is no doubt that a suspected case of WHO grade 1 meningioma identified by MRI should be diagnosed and followed up. In addition, histopathological classification generally helps facilitate a clinico-biological diagnosis, although it is not mandatory in all cases (4). However, in meningiomas with bone invasion, the judgment of bone involvement is sometimes ambiguous as it is difficult to preoperatively diagnose whether the tumor has invaded the adjacent bone using conventional radiographic modalities, such as CT and MRI (Figures 1A–D). Nevertheless, progress has been made in several areas of meningioma diagnoses (48). Previously, bone invasion was only postoperatively detected by histopathology in suspected cases of bone resection.
Hyperostosis of the bone adjacent to the meningioma, observable on CT with a bone window, has been well-described, with many reports addressing the possible causes. A primary theory is that cellular/tissue invasion of bone indicates hyperostosis (19, 49). Specifically, histopathological studies have clearly shown invasion of the tumor tissue to adjacent bone in areas of characteristic hyperostosis, possibly associated with strong somatostatin receptor subtype 2A (SSR2A) reactivity (12, 18, 50, 51). Moreover, a photodynamic diagnosis combined with histological study demonstrated the reactive fluorescence signal from the dipole to the inner table at the stump of the cranial window along with dense tumor-cells (52). Then, meningioma tissue invades lamellar bone trabeculae (53) (Figure 2). However, some false-negative and -positive hyperostosis cases have been diagnosed using conventional radiography (19, 51, 54). Thus, more accurate diagnostic modalities are required for meningiomas with bone invasion.
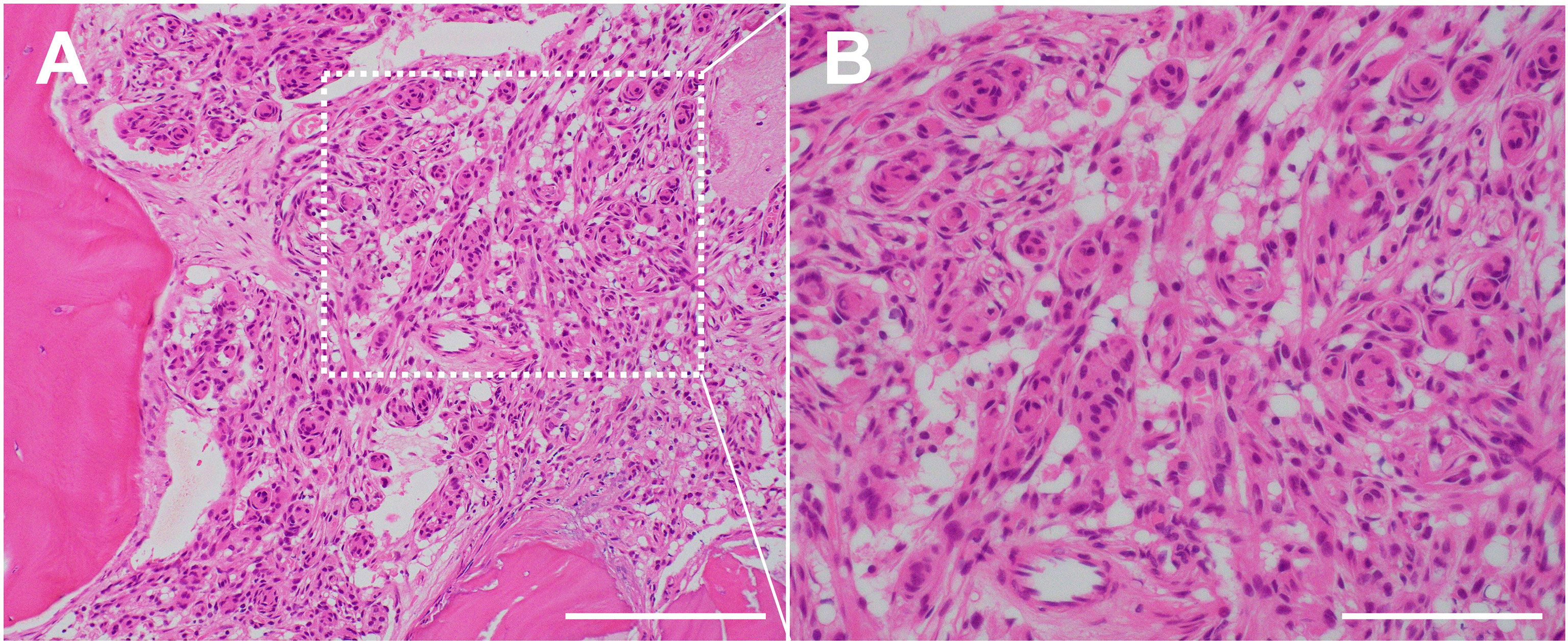
Figure 2 Histopathology of the case of bone invasive meningioma shown in Figure 1. (A) H&E staining demonstrating a cellular/tissue invasion into bone trabecula. ×200 magnification. Scale bar = 200 μm. (B) H&E staining demonstrating a proliferation of tumor cells with round to oval nuclei. Whorl formation of the tumor cells suggests meningothelial meningioma (WHO grade 1). ×400 magnification, Scale bar = 100 μm.
The aforementioned facts strongly suggest that the pathogenesis and molecular mechanisms underlying the cellular/tissue invasion of bone are poorly understood. This is potentially due to the high molecular and genetic heterogeneity of meningioma. Further studies for the microenvironment including bony tropism, osteolytic activity, and vascular remodeling between meningioma and the adjacent bone, that is “meningioma-bone niche”, may help in deeper understanding and future development of molecular-based therapies (55) (related to “protein expression” section) (Supplementary Figure 1).
There is growing evidence that molecular or metabolic imaging using scintigraphy or positron emission tomography (PET) is suitable for meningioma detection. Regarding bone-invasive meningioma, Gay et al. detected SSR2 via pre- and postoperative scintigraphy with a radiolabeled somatostatin analog ([111In-DTPA] octreotide) and intraoperative radio detection using a handheld gamma probe in 18 cases of meningioma en plaque. They reported that SSR2 radiodetection might help guide the surgical removal of bone invasive meningioma en plaque, pre- and postoperative management, and follow-up of meningioma with bone invasion that MRI failed to detect (56). Another study reported five cases of meningioma en plaque without previous bone decalcification, showing that all cases histopathologically were strongly positive for SSR2 and associated with intralesional features similar to oncogenic osteomalacia (51). These findings suggest a considerable limitation in the conventional radiographic assessment of meningioma with bone invasion, particularly when postoperative images are difficult to interpret and other biological and clinical implications may be provided, possibly linked to SSR2A expression. PET, recently developed using some somatostatin analogs, may also help detect bone invasion in meningioma (57, 58).
Whole-body 18F fluoride PET/CT has primarily been used in the context of possible bone metastases. Interestingly, some authors have incidentally found intense intracranial focal radiotracer accumulation in intracranial meningiomas in patients with a history of cancer (59–61). It has been suggested that 18F fluoride PET/CT may allow for the detection of bone invasion in meningiomas (Figures 1E, F; Figure 2) (62, 63). Nevertheless, the accumulation of 18F fluoride theoretically indicates pathological bone diseases that affect osteoblast activity, osteoclast-osteoblast interaction, and bone perfusion. Therefore, 18F fluoride PET can detect various metabolic, autoimmune, and osteogenic bone disorders (64). However, it is necessary to remember that 18F fluoride PET may provide false-positive lesions rather than bone invasion of meningiomas.
Radiomics is a novel imaging technique in the medical field, providing data regarding the biological properties and heterogeneity of the tumor by extracting many high-throughput imaging features (65, 66). Recently, radiomics has presented the possibility of accurately predicting meningioma grades and histological subtypes (67). Furthermore, preoperative imaging has the potential for predicting meningioma bone invasion (68). Zhang et al. evaluated 490 meningioma cases, of which 213 were bone-invasive meningioma primarily defined by surgeons (WHO grade 1; N = 448, 2; N = 38, 3; N = 4; the subtypes were not reported). They reported that radiomics contributed to the amelioration of clinical decision-making and bone invasion meningioma predictions, indicating that future radiomics studies with histopathologically diagnosed cases may be worthwhile to determine the value of radiomics for preoperatively diagnosing bone invasion meningioma (68, 69).
Cytogenesis and Genomics
As previously mentioned, even histologically benign meningiomas may show invasive behavior in the adjacent bone, resulting in repeated recurrences. This phenomenon can occur even after complete macroscopic resection (7, 70, 71). These are some of the main reasons for accelerating cytogenetics of meningioma, which has been best studied in humans (72) and well-summarized in the literature (73). Briefly, meningiomas typically have a normal karyotype or losses, which are mostly monosomy, but on rare occasions, there are deletions of the tumor suppressor gene NF2 located on chromosome 22 (74). Additionally, recent studies using next-generation sequencing approach have identified several mutations, such as TRAF7, KLF4, AKT1, SMO, and PIK3CA, with an interesting finding that mutations of these genes occur to a large degree without concurrent alteration of NF2, and that the clinical outcome and recurrence rate are associated with genomic subgroups (75, 76).
Most recently, technological developments have suggested that a higher rate of malignant meningiomas may be induced by increasing hypodiploidy, complex ablations, and even epigenetics (77–80). Furthermore, certain characteristics have been correlated with histological subtypes, especially copy number alterations and mutations, suggesting a greater potential for gene therapy (58, 80). Cytogenetics of lymphoplasmacyte-rich meningioma, a rare type of WHO grade 1 arising as an en plaque meningioma, is worth investigating, to develop therapeutic strategies for bone invasive meningioma.
Even in the era of genomics, epigenomics, and transcriptomics, there are currently no valuable cytogenetic and genetic recurrent predictors for meningioma, including bone-invasive meningiomas. Therefore, approaches combining histology, multi-”omics” patterns including radiomics and genomics, and radiological data may open a window to biology-based diagnostics for meningioma, perhaps leading to stratification of the recurrent risk and aggressive behavior of such tumors.
Protein Expression
Previous studies have revealed the presence of receptors in meningioma tissues (81–87). In particular, SSR2 has been reported in meningioma tissues, thus is being considered for clinical applications based on its molecular characteristics. SSR2 is one of the most studied molecules in bone-invasive meningioma, especially for diagnostic applications. The vascular endothelial growth factor (VEGF) pathway, including VEGF and its receptors, is involved in the dynamic blood vessel structures under normal conditions and cooperates with growth, recurrence, and development of edema of meningioma through their neovascularization effect when overexpressed (88). Although nothing has been reported regarding the VEGF pathway in meningiomas with bone invasion, this angiogenic molecular system is now thought to be a therapeutic target (Supplementary Figure 1).
Matrix metalloproteinases (MMPs), a family of calcium-dependent zinc-containing peptidases, are assumed to promote tumor cell growth and invasion (89). To date, the functional role of MMPs in meningioma biology is complex and unclear. Previous studies focused on the role of MMP2 in meningiomas with tumor recurrence and brain invasion, and produced contradictory results (55). MMP2 expression was found to be different depending on histopathological subtypes (90). A study that used high-throughput tissue microarray on bone invasive meningiomas demonstrated that key proteins are differentially expressed, and that the anatomical location of bone invasion is a key determinant of the expression pattern of MMP2, together with osteopontin (OPN) and integrin beta-1 (ITGB1) (55, 91).
Proteomics is a widely accepted screening approach for broad protein profiles that directly analyzes proteins expressed by a cell, tissue, or tumor type. Proteomic approaches for meningioma arose in 2000s, and several methods have been used to demonstrate molecular patterns (92–101). However, few studies have reported on the proteome of benign meningiomas (102, 103). Furthermore, the proteomics of bone-invasion meningioma, first described by Wibom et al., has even fewer reports. Wibom et al. evaluated 42 WHO grade 1 meningiomas (13 fibrous, 29 meningothelial, 16 bone invasive, and 26 noninvasive) by mass spectroscopy, demonstrating that the protein expression pattern distinguishes invasiveness and histological type of meningioma. Furthermore, Mukherjee et al. compared liquid chromatography-mass spectrometry-based protein profiles between WHO grades 1 and 2, including bone invasion, and indicated possible intratumoral heterogeneity, thus requiring close follow-up (104). Nevertheless, proteomics is growing as a characterizing tool for meningiomas, and features of bone-invasion tumors have yet to be identified. In addition to gene-related “omics” studies, proteomic analysis can be useful for the molecular characterization of bone invasion meningiomas.
Treatment
Surgery
Longitudinal volumetric studies have determined that meningiomas grow by approximately 1 cm3 annually. Moreover, there is a significant risk of progression for younger patients (<60 years) and those with larger tumors at the initial diagnosis (>25 mm), tumors without calcification, and tumors at specific locations (e.g., non-skull base) (69, 105). Thus, Oya et al. suggested surgical resection for asymptomatic tumors with a worsening Simpson grade after conservative management if they grow under conservative management (106). In other words, preoperative factors are essential for determining the extent of surgical resection, and bone invasion may be a preoperative factor related to incomplete resection, in addition to tumor location in the skull-base (25, 33, 35). Taken together, in cases of suspected meningioma with bone invasion, maximal resection of the adjacent bone would be preferable (107). Although, meningioma surgery is sometimes challenging due to anatomical circumstances (e.g., venous sinus involvement, arterial or cranial nerve envelopment, and extensive involvement of the base of the skull), especially in skull base cases (108–111). To achieve maximal resection of meningiomas, including the adjacent bone, a multidisciplinary surgical strategy combined with preoperative embolization may help (112). Considering that patients with bone invasion may be comparatively older and the invasive component of the bone may not be too aggressive, the risk and benefit balance must be assessed to establish a certain case selection and future surgical strategy (113–117).
Intraoperative assistance to detect the suspected bone invasion margin can be key for “complete” resection during meningioma surgery. Growing experience has demonstrated the usefulness of fluorescence guidance using 5-aminolevulinic acid (5-ALA) in meningioma surgery, especially in cases of bone invasion, in addition to intraoperative radio detection of somatostatin analog using a handheld gamma probe (52, 118–126). However, as Scheichel et al. reported, the accumulation result has a positive predictive value of 100% and a negative predictive value of 83% of 5-ALA fluorescence in meningioma bone invasion, demonstrating that it may help to improve the extent of resection. However, further studies are necessary to investigate the rate of false-negative fluorescence and its effect on progression-free survival (PFS) (126).
Recent studies have reported proliferation and invasiveness differences between meningiomas located in the skull base and other areas. Furthermore, the genetic background may differ depending on the location, even in non-NF2 meningiomas (127–130). Given that skull-base meningioma may be less biologically aggressive than those in other locations, extensive bony resection may be too challenging even after meningioma surgery has considerably improved, especially in skull-base cases. Thus far, it is unclear whether surgical resection plays a central role in meningioma treatment, and radiological follow-up is favorable in cases with suspected bone invasion. Therefore, patients with bone invasion may need additional treatments and future medical therapy in addition to those with WHO grades 2 and 3.
Radiosurgery
Radiosurgery is an alternative for small to medium-sized symptomatic or recurrent meningiomas. Patients with large or post-surgical remaining tumors are also eligible for fractionated radiosurgery (4, 58, 131). To date, therapeutic strategies combining surgery and (fractionated) radiosurgery have been developed. However, details regarding its use based on the WHO grade, tumor size, and the anatomical location remain controversial (111).
Radiosurgery for the bone-invading component of meningiomas has been less studied. However, accumulating evidence highlights that adjuvant radiosurgery improves local control in WHO grade 2 meningiomas irrespective of the initial resection extent compared to observation only. Furthermore, bone invasion might be associated with multiple recurrences. (32, 132). One study evaluated a cohort with mixed WHO grades who underwent irradiation, reporting that PFS did not differ between cases with and without bone invasion. These results suggest that radiation may influence meningioma tissue invading the bone (48). However, the major problem with radiosurgery for bone invading meningioma is target delineation (133). Brastianos et al. suggested that radiation is unnecessary for the dural tail unless they contain suspicious nodular enhancement because they are typically composed of benign and hypervascular tissue. Additionally, WHO grade 1 and radiographically presumed grade 1 meningiomas require a 0–5 mm clinical target volume margin. In contrast, in cases of WHO grade 2 and 3 meningiomas, hyperostosis or direct bone invasion should be included in the gross tumor volume with an additional margin of 3–5 mm (134). Future prospective studies combining radiosurgery with reproducible target planning and image, and histopathology-based therapeutic strategies are needed to set up target delineation for bone-invasive meningiomas.
Recent progress in advanced radiation therapies has resulted in possibilities for the development of future treatments for bone-invasive meningiomas, especially high-grade meningiomas (135–145).
Proton beam therapy and photon radiation therapy are shown to be safe and effective for meningioma treatment (146–149). Intensity-modulated radiation therapy (IMRT) provides some benefits, such as higher dose conformality and improved target coverage, without the contraindications of conventional radiosurgery. It has also demonstrated preferable results for the treatment of meningioma causing visual impairment by minimizing toxicity to the adjacent nervous structure (148). Boron neutron capture therapy (BNCT) is a targeted radiotherapy that enables the selective elimination of malignant cells and the sparing of surrounding normal cells. Although evidence of BNCT for meningioma treatment is not as robust, recent studies have shown relatively good local control and favorable survival along with an acceptable safety profile for recurrent and refractory high-grade meningioma (139, 144). Photodynamic therapy (PDT) adopts a photosensitizer (PS) accumulated into tumor tissue or hypervascular lesion. Irradiation of the PS with a laser at a specific wavelength causes a photochemical reaction and produces singlet oxygen, resulting in cellular injury of the target (141). This mechanism causes an inherent selectivity of the procedure. Since the laser light can only penetrate a few millimeters of tissue, therapeutic potential of PDT is limited for the tumor located in deeper areas (150). Thus, PDT is lacking sufficient clinical evidence for meningioma treatment. However, studies suggest adequate effectiveness in the treatment of high-grade meningioma, in an in vitro environment (141, 142). Although the effectiveness of these modalities for bone-invasive meningioma is not well understood, appropriate applications should be studied according to modality-specific advantages and disadvantages (136, 142, 151).
These findings suggest that radiosurgery for bone invasion remains controversial but may show greater potential for prognosis, and further prospective studies are warranted.
Medical Therapies
Compared with surgery and radiosurgery, studies on the clinical application of medical therapies against meningiomas are growing slowly, and some have promising results. However, the European Association of Neuro-Oncology recommended only experimental systemic therapy, with a “C” level class of evidence. Thus, no specific recommendations are provided (58, 73). The National Comprehensive Cancer Network recommends using alpha-interferon, somatostatin receptor agonists, and VEGF inhibitors to treat meningioma (152). However, their efficacy is limited. Thus far, there is no established evidence for their use, and more studies are required to unravel the mechanistic roles in bone-invasive meningiomas and across the entire meningioma spectrum (58, 78, 80, 153–155).
In vitro cell culture is widely used for oncological investigations, including meningiomas (156). This model provides self-mitogenic agents, autocrine mechanisms, and several molecules for developing novel systemic therapies for meningioma in the future. Studies have demonstrated the effects of signaling suppression on tumor invasion and cell proliferation, highlighting the importance of exploring novel non-toxic drugs for aggressive meningiomas (157–159). However, importantly, cell lines may harbor genomic and transcriptomic alterations, confounding translational research (160). Therefore, primary tumor culture should be performed rather than using transcriptionally different cell lines to understand the molecular mechanisms underlying meningioma invasion and cell proliferation for clinical applications, although there are limitations in availability and logistical concerns (160). Primary culture and specific cell lines have not been established for invasive bone meningiomas. A bone-like culture system formed with minerals structuring pores and a bone-like mechanical environment, similar to those used for other bone tumor research (161), and related assessment methodologies may help specify the molecular characteristics and further provide information on a novel concept of a meningioma-bone niche (Supplementary Figure 1).
Summary and Therapeutic Perspectives
The abundance of clinical results and advancing research technologies have prompted the exploration of the biological characteristics of bone-invasive meningiomas. Studies have confirmed a significant association between bone invasion and incomplete resection, possibly affecting long-term recurrence and outcomes (Figure 3). Moreover, radio detection and fluorescence-guided 5-ALA are confirmed intraoperative assistance tools. If metabolic imaging, such as 18F fluoride PET, is available in addition to a precise combination of CT and MRI, suspected bone invasion can be diagnosed preoperatively. However, postoperative histopathology of adjacent bone remains a crucial part for definitive diagnosis. Advanced preoperative diagnostic modalities, such as radiomics and PET with SSR may play a central role in developing a surgical strategy for suspected cases of bone invasion.
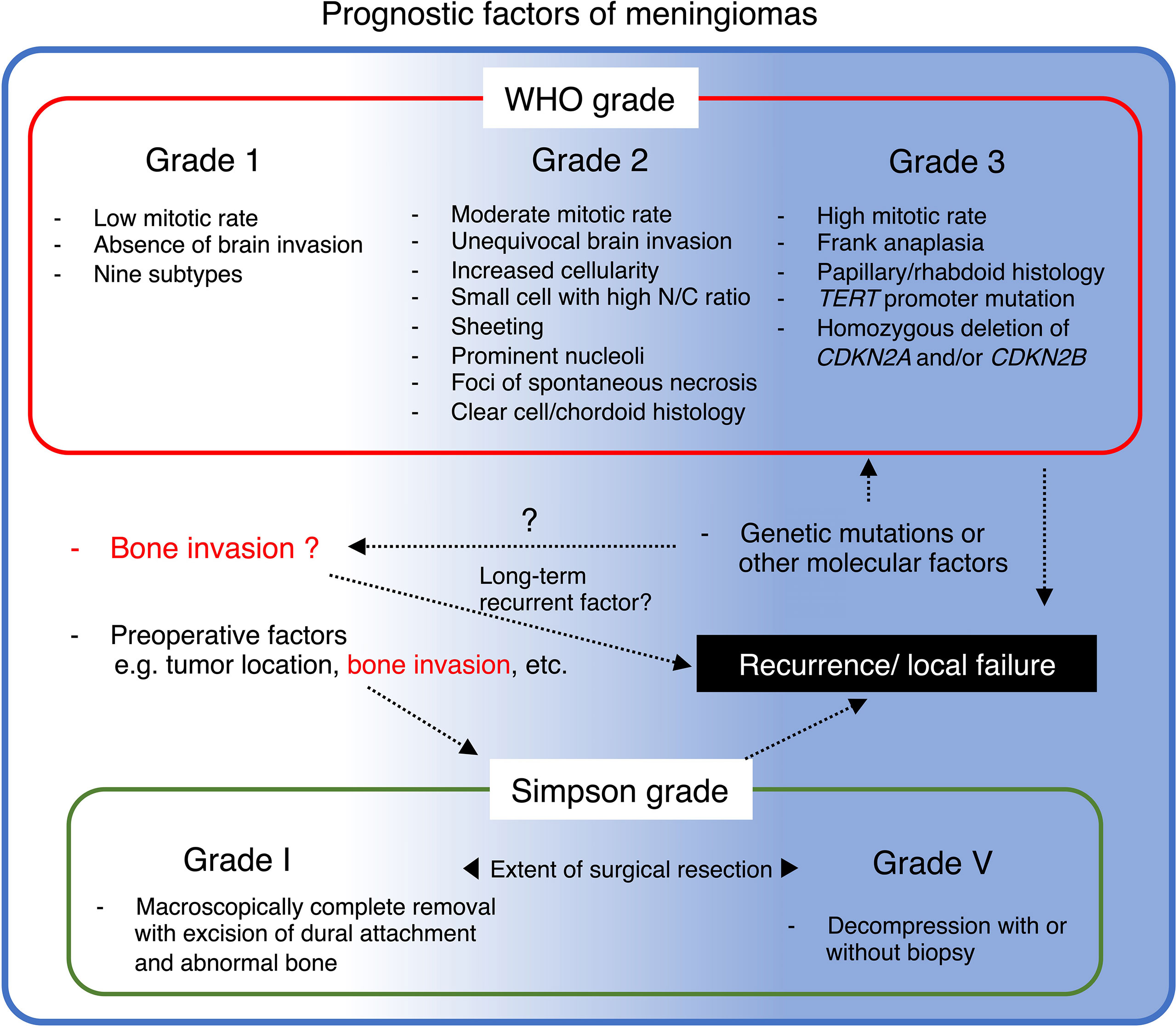
Figure 3 Summary of prognostic factors of meningioma and their potential relationship with bone invasion.
Combined direct surgery and radiosurgery is also becoming more common, and advanced radiations, such as IMRT, BNCT, and PDT, might be good candidates for treating bone-invasive meningioma in the clinic. The specific genomic pattern of bone-invasive meningioma has not been detected. However, proteomics suggests that the protein profile of bone-invasive meningioma is more heterogeneous than that of non-invasive tumors, requiring closer follow-up. Although there is no medical therapy to treat meningiomas, including bone-invasive cases, some medical therapies are promising druggable targets, and their implementation in clinical practice is under consideration. An in vitro cell culture model would be a good option to test potential therapeutic targets in bone-invasive meningioma. However, primary culture should be used rather than a transcriptionally different cell line. Bone-like culture systems used for other bone tumor research may help specify the molecular characteristics and mechanisms in meningioma-bone niche, and effects of therapeutic agents for bone-invasive meningiomas.
Translating emerging clinical and basic research knowledge into clinical management remains incipient. Thus, similar to other biomedical research fields, “a valley of death exists between basic and clinical research” (162). The clinicopathological characteristics of bone-invasive meningioma are divergent, and it is challenging to commit to a long-term result when treating these tumors. However, collaborative efforts between basic science and clinics and among clinical experts, such as surgeons, radiosurgeons, radiologists, pathologists, clinician-scientists familiar with basic research, and statisticians, would help cross the valley (163).
Author Contributions
Conception and design, drafted and/or critically revised the work, funding acquisition, Final approval of manuscript: HT; Revised the manuscript: HT and TY. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by Japan Society for the Promotion of Science “KAKENHI” (20K09330) (HT).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Shoji Yamanaka, Department of Pathology, and Dr. Hiromichi Iwashita, Division of Anatomical and Surgical Pathology, Yokohama City University Hospital, for the histopathological diagnosis. This work is dedicated to the memory of Prof. Nobutaka Kawahara.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.895374/full#supplementary-material
Supplementary Figure 1 | “Meningioma-bone niche”, a microenvironment, in which meningioma shows bone invasion. SSR2A, strong somatostatin receptor subtype 2A; VEGFR, vascular endothelial growth factor receptor; VEGF, vascular endothelial growth factor; MMP2, matrix metalloproteinase; OPN, osteopontin; ITGB1, integrin beta-1.
Abbreviations
CNS, central nervous system; WHO, World Health Organization; NF2, neurofibromatosis type 2; CT, computer tomography; MRI, magnetic resonance imaging; 5-ALA, 5-aminolevulinic acid; GTR, gross total removal; OR, odds ratio; PET, positron emission tomography; SSR2, somatostatin receptor subtype 2; VEGF, vascular endothelial growth factor; IMRT, intensity modulated radiation therapy; BNCT, boron neutron capture therapy; PDT, photodynamic therapy.
References
1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol (2007) 114:97–109. doi: 10.1007/s00401-007-0243-4
2. Ostrom QT, Cioffi G, Gittleman H, Patil N, Waite K, Kruchko C, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012-2016. Neuro Oncol (2019) 21:v1–v100. doi: 10.1093/neuonc/noz150
3. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
4. Goldbrunner R, Minniti G, Preusser M, Jenkinson MD, Sallabanda K, Houdart E, et al. EANO Guidelines for the Diagnosis and Treatment of Meningiomas. Lancet Oncol (2016) 17:e383–391. doi: 10.1016/S1470-2045(16)30321-7
5. WHO. World Health Organization Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer (2021).
6. Chamberlain MC. The Role of Chemotherapy and Targeted Therapy in the Treatment of Intracranial Meningioma. Curr Opin Oncol (2012) 24:666–71. doi: 10.1097/CCO.0b013e328356364d
7. Simpson D. The Recurrence of Intracranial Meningiomas After Surgical Treatment. J Neurol Neurosurg Psychiatry (1957) 20:22–39. doi: 10.1136/jnnp.20.1.22
8. Zang KD. Meningioma: A Cytogenetic Model of a Complex Benign Human Tumor, Including Data on 394 Karyotyped Cases. Cytogenet Cell Genet (2001) 93:207–20. doi: 10.1159/000056986
9. Lang FF, Macdonald OK, Fuller GN, Demonte F. Primary Extradural Meningiomas: A Report on Nine Cases and Review of the Literature From the Era of Computerized Tomography Scanning. J Neurosurg (2000) 93:940–50. doi: 10.3171/jns.2000.93.6.0940
10. Shuangshoti S. Primary Meningiomas Outside the Central Nervous System. New York: Raven Press (1991).
11. Van Tassel P, Lee YY, Ayala A, Carrasco CH, Klima T. Case Report 680. Intraosseous Meningioma of the Sphenoid Bone. Skeletal Radiol (1991) 20:383–6. doi: 10.1007/BF01267669
12. Akutsu H, Sugita K, Sonobe M, Matsumura A. Parasagittal Meningioma En Plaque With Extracranial Extension Presenting Diffuse Massive Hyperostosis of the Skull. Surg Neurol (2004) 61:165–9; discussion 169. doi: 10.1016/S0090-3019(03)00521-4
13. Basu K, Majumdar K, Chatterjee U, Ganguli M, Chatterjee S. En Plaque Meningioma With Angioinvasion. Indian J Pathol Microbiol (2010) 53:319–21. doi: 10.4103/0377-4929.64306
14. Simas NM, Farias JP. Sphenoid Wing En Plaque Meningiomas: Surgical Results and Recurrence Rates. Surg Neurol Int (2013) 4:86. doi: 10.4103/2152-7806.114796
15. Kunimatsu A, Kunimatsu N, Kamiya K, Katsura M, Mori H, Ohtomo K. Variants of Meningiomas: A Review of Imaging Findings and Clinical Features. Jpn J Radiol (2016) 34:459–69. doi: 10.1007/s11604-016-0550-6
16. Nambirajan A, Sharma MC, Garg K, Sriram S, Boorgula MT, Suri V. Large Dural-Based Mass With Bony Hyperostosis in a 16-Year-Old Male: IgG4-Related Disease Mimicking Lymphoplasmacyte-Rich Meningioma. Childs Nerv Syst (2019) 35:1423–7. doi: 10.1007/s00381-019-04187-z
17. de Almeida GB, Januario G. Lymphoplasmacyte-Rich Meningioma: A Rare Histologic Variant of Benign Meningioma With Atypical Bone Invasion. Radiol Case Rep (2022) 17:922–7. doi: 10.1016/j.radcr.2021.12.059
18. Bonnal J, Thibaut A, Brotchi J, Born J. Invading Meningiomas of the Sphenoid Ridge. J Neurosurg (1980) 53:587–99. doi: 10.3171/jns.1980.53.5.0587
19. Pieper DR, Al-Mefty O, Hanada Y, Buechner D. Hyperostosis Associated With Meningioma of the Cranial Base: Secondary Changes or Tumor Invasion. Neurosurgery (1999) 44:742–6; discussion 746-747. doi: 10.1097/00006123-199904000-00028
20. Bikmaz K, Mrak R, Al-Mefty O. Management of Bone-Invasive, Hyperostotic Sphenoid Wing Meningiomas. J Neurosurg (2007) 107:905–12. doi: 10.3171/JNS-07/11/0905
21. Sun SQ, Kim AH, Cai C, Murphy RK, Dewees T, Sylvester P, et al. Management of Atypical Cranial Meningiomas, Part 1: Predictors of Recurrence and the Role of Adjuvant Radiation After Gross Total Resection. Neurosurgery (2014) 75:347–54; discussion 354-345; quiz 355. doi: 10.1227/NEU.0000000000000461
22. Watts J, Box G, Galvin A, Brotchie P, Trost N, Sutherland T. Magnetic Resonance Imaging of Meningiomas: A Pictorial Review. Insights Imaging (2014) 5:113–22. doi: 10.1007/s13244-013-0302-4
23. Gabeau-Lacet D, Aghi M, Betensky RA, Barker FG, Loeffler JS, Louis DN. Bone Involvement Predicts Poor Outcome in Atypical Meningioma. J Neurosurg (2009) 111:464–71. doi: 10.3171/2009.2.JNS08877
24. Abdelzaher E, El-Gendi SM, Yehya A, Gowil AG. Recurrence of Benign Meningiomas: Predictive Value of Proliferative Index, BCL2, P53, Hormonal Receptors and HER2 Expression. Br J Neurosurg (2011) 25:707–13. doi: 10.3109/02688697.2010.522743
25. Lemee JM, Corniola MV, Da Broi M, Joswig H, Scheie D, Schaller K, et al. Extent of Resection in Meningioma: Predictive Factors and Clinical Implications. Sci Rep (2019) 9:5944. doi: 10.1038/s41598-019-42451-z
26. Elder JB, Atkinson R, Zee CS, Chen TC. Primary Intraosseous Meningioma. Neurosurg Focus (2007) 23:E13. doi: 10.3171/FOC-07/10/E13
27. McGuire TP, Palme CE, Perez-Ordonez B, Gilbert RW, Sandor GK. Primary Intraosseous Meningioma of the Calvaria: Analysis of the Literature and Case Report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (2007) 104:e34–41. doi: 10.1016/j.tripleo.2007.03.023
28. Ilica AT, Mossa-Basha M, Zan E, Vikani A, Pillai JJ, Gujar S, et al. Cranial Intraosseous Meningioma: Spectrum of Neuroimaging Findings With Respect to Histopathological Grades in 65 Patients. Clin Imaging (2014) 38:599–604. doi: 10.1016/j.clinimag.2014.05.013
29. Ho A, Tang H. Editorial: Meningioma: From Basic Research to Clinical Translational Study. Front Oncol (2021) 11:750690. doi: 10.3389/fonc.2021.750690
30. Nowak A, Dziedzic T, Krych P, Czernicki T, Kunert P, Marchel A. Benign Versus Atypical Meningiomas: Risk Factors Predicting Recurrence. Neurol Neurochir Pol (2015) 49:1–10. doi: 10.1016/j.pjnns.2014.11.003
31. Shakir SI, Souhami L, Petrecca K, Mansure JJ, Singh K, Panet-Raymond V, et al. Prognostic Factors for Progression in Atypical Meningioma. J Neurosurg (2018) 129:1240–8. doi: 10.3171/2017.6.JNS17120
32. Zima L, Baine MJ, Sleightholm R, Wang B, Punsoni M, Aizenberg M, et al. Pathologic Characteristics Associated With Local Recurrence of Atypical Meningiomas Following Surgical Resection. J Clin Med Res (2021) 13:143–50. doi: 10.14740/jocmr4444
33. Hwang WL, Marciscano AE, Niemierko A, Kim DW, Stemmer-Rachamimov AO, Curry WT, et al. Imaging and Extent of Surgical Resection Predict Risk of Meningioma Recurrence Better Than WHO Histopathological Grade. Neuro Oncol (2016) 18:863–72. doi: 10.1093/neuonc/nov285
34. Corniola MV, Lemee JM, Meling TR. Histological Transformation in Recurrent WHO Grade I Meningiomas. Sci Rep (2020) 10:11220. doi: 10.1038/s41598-020-68177-x
35. Haddad AF, Young JS, Kanungo I, Sudhir S, Chen JS, Raleigh DR, et al. WHO Grade I Meningioma Recurrence: Identifying High Risk Patients Using Histopathological Features and the MIB-1 Index. Front Oncol (2020) 10:1522. doi: 10.3389/fonc.2020.01522
36. Kawahara N, Suenaga J, Yoshida S, Takase H, Murata H. Surgical Management of Sphenoid Ridge Meningioma. J Neurol Surg B Skull Base (2016) 77:A032. doi: 10.1055/s-0036-1579821
37. Gallagher MJ, Jenkinson MD, Brodbelt AR, Mills SJ, Chavredakis E. WHO Grade 1 Meningioma Recurrence: Are Location and Simpson Grade Still Relevant? Clin Neurol Neurosurg (2016) 141:117–21. doi: 10.1016/j.clineuro.2016.01.006
38. Gousias K, Schramm J, Simon M. The Simpson Grading Revisited: Aggressive Surgery and its Place in Modern Meningioma Management. J Neurosurg (2016) 125:551–60. doi: 10.3171/2015.9.JNS15754
39. Voss KM, Spille DC, Sauerland C, Suero Molina E, Brokinkel C, Paulus W, et al. The Simpson Grading in Meningioma Surgery: Does the Tumor Location Influence the Prognostic Value? J Neurooncol (2017) 133:641–51. doi: 10.1007/s11060-017-2481-1
40. Sicking J, Voss KM, Spille DC, Schipmann S, Holling M, Paulus W, et al. The Evolution of Cranial Meningioma Surgery-a Single-Center 25-Year Experience. Acta Neurochir (Wien) (2018) 160:1801–12. doi: 10.1007/s00701-018-3617-6
41. Hashiba T, Hashimoto N, Maruno M, Izumoto S, Suzuki T, Kagawa N, et al. Scoring Radiologic Characteristics to Predict Proliferative Potential in Meningiomas. Brain Tumor Pathol (2006) 23:49–54. doi: 10.1007/s10014-006-0199-4
42. Hsu CC, Pai CY, Kao HW, Hsueh CJ, Hsu WL, Lo CP. Do Aggressive Imaging Features Correlate With Advanced Histopathological Grade in Meningiomas? J Clin Neurosci (2010) 17:584–7. doi: 10.1016/j.jocn.2009.09.018
43. Kawahara Y, Nakada M, Hayashi Y, Kai Y, Hayashi Y, Uchiyama N, et al. Prediction of High-Grade Meningioma by Preoperative MRI Assessment. J Neurooncol (2012) 108:147–52. doi: 10.1007/s11060-012-0809-4
44. Lin BJ, Chou KN, Kao HW, Lin C, Tsai WC, Feng SW, et al. Correlation Between Magnetic Resonance Imaging Grading and Pathological Grading in Meningioma. J Neurosurg (2014) 121:1201–8. doi: 10.3171/2014.7.JNS132359
45. Liu Y, Chotai S, Chen M, Jin S, Qi ST, Pan J. Preoperative Radiologic Classification of Convexity Meningioma to Predict the Survival and Aggressive Meningioma Behavior. PLoS One (2015) 10:e0118908. doi: 10.1371/journal.pone.0118908
46. Saygın I, Çakır E. Retrospective Analysis of Meningioma and Alternative Method of Grading. Erciyes Med J (2020) 42:380–5. doi: 10.14744/etd.2020.69851
47. Kim D, Niemierko A, Hwang WL, Stemmer-Rachamimov AO, Curry WT, Barker FG, et al. Histopathological Prognostic Factors of Recurrence Following Definitive Therapy for Atypical and Malignant Meningiomas. J Neurosurg (2018) 128:1123–32. doi: 10.3171/2016.11.JNS16913
48. Zwirner K, Paulsen F, Schittenhelm J, Gepfner-Tuma I, Tabatabai G, Behling F, et al. Integrative Assessment of Brain and Bone Invasion in Meningioma Patients. Radiat Oncol (2019) 14:132. doi: 10.1186/s13014-019-1341-x
49. Marosi C, Hassler M, Roessler K, Reni M, Sant M, Mazza E, et al. Meningioma. Crit Rev Oncol Hematol (2008) 67:153–71. doi: 10.1016/j.critrevonc.2008.01.010
50. Kashimura H, Beppu T, Wada T, Yoshida Y, Suzuki M, Ogawa A. [A Case of Meningioma En Plaque: Review of 73 Cases]. No Shinkei Geka (1997) 25:1097–100.
51. Matschke J, Addo J, Bernreuther C, Zustin J. Osseous Changes in Meningioma En Plaque. Anticancer Res (2011) 31:591–6.
52. Morofuji Y, Matsuo T, Hayashi Y, Suyama K, Nagata I. Usefulness of Intraoperative Photodynamic Diagnosis Using 5-Aminolevulinic Acid for Meningiomas With Cranial Invasion: Technical Case Report. Neurosurgery (2008) 62:102–3; discussion 103-104. doi: 10.1227/01.neu.0000317378.22820.46
53. Abdelzaher E, El Deeb NM, Gowil AG, Yehya A. Biological and Demographic Profile of Meningiomas in a Cohort of Egyptian Patients: Impact on Tumor Recurrence. Sci World J (2013) 2013:375139. doi: 10.1155/2013/375139
54. Min JH, Kang SH, Lee JB, Chung YG, Lee HK. Hyperostotic Meningioma With Minimal Tumor Invasion Into the Skull. Neurol Med Chir (Tokyo) (2005) 45:480–3. doi: 10.2176/nmc.45.480
55. Salehi F, Jalali S, Alkins R, Lee JI, Lwu S, Burrell K, et al. Proteins Involved in Regulating Bone Invasion in Skull Base Meningiomas. Acta Neurochir (Wien) (2013) 155:421–7. doi: 10.1007/s00701-012-1577-9
56. Gay E, Vuillez JP, Palombi O, Brard PY, Bessou P, Passagia JG. Intraoperative and Postoperative Gamma Detection of Somatostatin Receptors in Bone-Invasive En Plaque Meningiomas. Neurosurgery (2005) 57:107–113; discussion 107-113. doi: 10.1227/01.NEU.0000163490.15578.FF
57. Galldiks N, Lohmann P, Albert NL, Tonn JC, Langen KJ. Current Status of PET Imaging in Neuro-Oncology. Neurooncol Adv (2019) 1:vdz010. doi: 10.1093/noajnl/vdz010
58. Goldbrunner R, Stavrinou P, Jenkinson MD, Sahm F, Mawrin C, Weber DC, et al. EANO Guideline on the Diagnosis and Management of Meningiomas. Neuro Oncol (2021) 23(11):1821–34. doi: 10.1093/neuonc/noab150
59. Derlin T, Mester J, Klutmann S. Abnormal F-18 Fluoride Uptake in Intracranial Meningiomas on PET/CT. Clin Nucl Med (2010) 35:806–7. doi: 10.1097/RLU.0b013e3181ef0a9e
60. Zacchi SR, Duarte PS, Coura Filho GB, Sapienza MT, Buchpiguel CA. Incidental Finding of Anterior Cranial Fossa Meningioma on 18F-Fluoride PET/CT. Clin Nucl Med (2013) 38:913–5. doi: 10.1097/RLU.0b013e3182a77c49
61. Teo TY, Menda Y, Mcneely P, Kahn D, Graham M. Incidental Meningioma Detected on 18F-Fluoride With PET/CT During Initial Staging for Prostate Cancer. Clin Nucl Med (2015) 40:596–7. doi: 10.1097/RLU.0000000000000802
62. Tateishi U, Tateishi K, Shizukuishi K, Shishikura A, Murata H, Inoue T, et al. 18f-Fluoride PET/CT Allows Detection of Hyperostosis and Osseous Involvement in Meningioma: Initial Experience. Clin Nucl Med (2013) 38:e125–131. doi: 10.1097/RLU.0b013e318279fd79
63. Tateishi U, Tateishi K, Hino-Shishikura A, Torii I, Inoue T, Kawahara N. Multimodal Approach to Detect Osseous Involvement in Meningioma: Additional Value of (18)F-Fluoride PET/CT for Conventional Imaging. Radiology (2014) 273:521–8. doi: 10.1148/radiol.14132118
64. Park PSU, Raynor WY, Sun Y, Werner TJ, Rajapakse CS, Alavi A. (18)F-Sodium Fluoride PET as a Diagnostic Modality for Metabolic, Autoimmune, and Osteogenic Bone Disorders: Cellular Mechanisms and Clinical Applications. Int J Mol Sci (2021) 22:6504. doi: 10.3390/ijms22126504.
65. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, Van Stiphout RG, Granton P, et al. Radiomics: Extracting More Information From Medical Images Using Advanced Feature Analysis. Eur J Cancer (2012) 48:441–6. doi: 10.1016/j.ejca.2011.11.036
66. Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding Tumour Phenotype by Noninvasive Imaging Using a Quantitative Radiomics Approach. Nat Commun (2014) 5:4006. doi: 10.1038/ncomms5006
67. Park YW, Oh J, You SC, Han K, Ahn SS, Choi YS, et al. Radiomics and Machine Learning may Accurately Predict the Grade and Histological Subtype in Meningiomas Using Conventional and Diffusion Tensor Imaging. Eur Radiol (2019) 29:4068–76. doi: 10.1007/s00330-018-5830-3
68. Zhang J, Sun J, Han T, Zhao Z, Cao Y, Zhang G, et al. Radiomic Features of Magnetic Resonance Images as Novel Preoperative Predictive Factors of Bone Invasion in Meningiomas. Eur J Radiol (2020) 132:109287. doi: 10.1016/j.ejrad.2020.109287
69. Hashimoto N, Rabo CS, Okita Y, Kinoshita M, Kagawa N, Fujimoto Y, et al. Slower Growth of Skull Base Meningiomas Compared With non-Skull Base Meningiomas Based on Volumetric and Biological Studies. J Neurosurg (2012) 116:574–80. doi: 10.3171/2011.11.JNS11999
70. Sanson M, Cornu P. Biology of Meningiomas. Acta Neurochir (Wien) (2000) 142:493–505. doi: 10.1007/s007010050462
71. Yamasaki F, Yoshioka H, Hama S, Sugiyama K, Arita K, Kurisu K. Recurrence of Meningiomas. Cancer (2000) 89:1102–10. doi: 10.1002/1097-0142(20000901)89:5<1102::AID-CNCR20>3.0.CO;2-L
72. Ruttledge MH, Xie YG, Han FY, Peyrard M, Collins VP, Nordenskjold M, et al. Deletions on Chromosome 22 in Sporadic Meningioma. Genes Chromosomes Cancer (1994) 10:122–30. doi: 10.1002/gcc.2870100207
73. Ogasawara C, Philbrick BD, Adamson DC. Meningioma: A Review of Epidemiology, Pathology, Diagnosis, Treatment, and Future Directions. Biomedicines (2021) 9:319. doi: 10.3390/biomedicines9030319
74. Preusser M, Brastianos PK, Mawrin C. Advances in Meningioma Genetics: Novel Therapeutic Opportunities. Nat Rev Neurol (2018) 14:106–15. doi: 10.1038/nrneurol.2017.168
75. Bi WL, Zhang M, Wu WW, Mei Y, Dunn IF. Meningioma Genomics: Diagnostic, Prognostic, and Therapeutic Applications. Front Surg (2016) 3:40. doi: 10.3389/fsurg.2016.00040
76. Youngblood MW, Miyagishima DF, Jin L, Gupte T, Li C, Duran D, et al. Associations of Meningioma Molecular Subgroup and Tumor Recurrence. Neuro Oncol (2021) 23:783–94. doi: 10.1093/neuonc/noaa226
77. Sahm F, Schrimpf D, Stichel D, Jones DTW, Hielscher T, Schefzyk S, et al. DNA Methylation-Based Classification and Grading System for Meningioma: A Multicentre, Retrospective Analysis. Lancet Oncol (2017) 18:682–94. doi: 10.1016/S1470-2045(17)30155-9
78. Sanson M, Kalamarides M. Epigenetics: A New Tool for Meningioma Management? Lancet Oncol (2017) 18:569–70. doi: 10.1016/S1470-2045(17)30153-5
79. Hemmer S, Urbschat S, Oertel J, Ketter R. Deletions in the 17q Chromosomal Region and Their Influence on the Clonal Cytogenetic Evolution of Recurrent Meningiomas. Mol Cytogenet (2019) 12:22. doi: 10.1186/s13039-019-0434-4
80. Moussalem C, Massaad E, Minassian GB, Ftouni L, Bsat S, Houshiemy MNE, et al. Meningioma Genomics: A Therapeutic Challenge for Clinicians. J Integr Neurosci (2021) 20:463–9. doi: 10.31083/j.jin2002049
81. Blankenstein MA, Berns PM, Blaauw G, Mulder E, Thijssen JH. Search for Estrogen Receptors in Human Meningioma Tissue Sections With a Monoclonal Antibody Against the Human Estrogen Receptor. Cancer Res (1986) 46:4268s–70s.
82. Reubi JC, Lang W, Maurer R, Koper JW, Lamberts SW. Distribution and Biochemical Characterization of Somatostatin Receptors in Tumors of the Human Central Nervous System. Cancer Res (1987) 47:5758–64.
83. Reubi JC, Horisberger U, Lang W, Koper JW, Braakman R, Lamberts SW. Coincidence of EGF Receptors and Somatostatin Receptors in Meningiomas But Inverse, Differentiation-Dependent Relationship in Glial Tumors. Am J Pathol (1989) 134:337–44.
84. Carroll RS, Glowacka D, Dashner K, Black PM. Progesterone Receptor Expression in Meningiomas. Cancer Res (1993) 53:1312–6.
85. Carroll RS, Zhang J, Dashner K, Sar M, Wilson EM, Black PM. Androgen Receptor Expression in Meningiomas. J Neurosurg (1995) 82:453–60. doi: 10.3171/jns.1995.82.3.0453
86. Wernicke AG, Dicker AP, Whiton M, Ivanidze J, Hyslop T, Hammond EH, et al. Assessment of Epidermal Growth Factor Receptor (EGFR) Expression in Human Meningioma. Radiat Oncol (2010) 5:46. doi: 10.1186/1748-717X-5-46
87. Rutkowski R, Reszec J, Hermanowicz A, Chrzanowski R, Lyson T, Mariak Z, et al. Correlation of Leptin Receptor Expression With BMI in Differential Grades of Human Meningiomas. Oncol Lett (2016) 11:2515–9. doi: 10.3892/ol.2016.4272
88. Preusser M, Hassler M, Birner P, Rudas M, Acker T, Plate KH, et al. Microvascularization and Expression of VEGF and its Receptors in Recurring Meningiomas: Pathobiological Data in Favor of Anti-Angiogenic Therapy Approaches. Clin Neuropathol (2012) 31:352–60. doi: 10.5414/NP300488
89. Duffy MJ, McCarthy K. Matrix Metalloproteinases in Cancer: Prognostic Markers and Targets for Therapy (Review). Int J Oncol (1998) 12:1343–8. doi: 10.3892/ijo.12.6.1343
90. Rooprai HK, Van Meter TE, Robinson SD, King A, Rucklidge GJ, Pilkington GJ. Expression of MMP-2 and -9 in Short-Term Cultures of Meningioma: Influence of Histological Subtype. Int J Mol Med (2003) 12:977–81. doi: 10.3892/ijmm.12.6.977
91. Ichimura S, Takahara K, Fujii K. Fibrous Meningioma With Skull Invasion. J Neurosci Rural Pract (2019) 10:707–10. doi: 10.1055/s-0039-3399600
92. Monleon D, Morales JM, Gonzalez-Darder J, Talamantes F, Cortes O, Gil-Benso R, et al. Benign and Atypical Meningioma Metabolic Signatures by High-Resolution Magic-Angle Spinning Molecular Profiling. J Proteome Res (2008) 7:2882–8. doi: 10.1021/pr800110a
93. Saydam O, Senol O, Schaaij-Visser TB, Pham TV, Piersma SR, Stemmer-Rachamimov AO, et al. Comparative Protein Profiling Reveals Minichromosome Maintenance (MCM) Proteins as Novel Potential Tumor Markers for Meningiomas. J Proteome Res (2010) 9:485–94. doi: 10.1021/pr900834h
94. Kim JH, Lee SK, Yoo YC, Park NH, Park DB, Yoo JS, et al. Proteome Analysis of Human Cerebrospinal Fluid as a Diagnostic Biomarker in Patients With Meningioma. Med Sci Monit (2012) 18:BR450–460. doi: 10.12659/MSM.883538
95. Cui GQ, Jiao AH, Xiu CM, Wang YB, Sun P, Zhang LM, et al. Proteomic Analysis of Meningiomas. Acta Neurol Belg (2014) 114:187–94. doi: 10.1007/s13760-013-0253-z
96. Abbritti RV, Polito F, Cucinotta M, Lo Giudice C, Caffo M, Tomasello C, et al. Meningiomas and Proteomics: Focus on New Potential Biomarkers and Molecular Pathways. Cancer Genomics Proteomics (2016) 13:369–79.
97. Bassiri K, Ferluga S, Sharma V, Syed N, Adams CL, Lasonder E, et al. Global Proteome and Phospho-Proteome Analysis of Merlin-Deficient Meningioma and Schwannoma Identifies PDLIM2 as a Novel Therapeutic Target. EBioMedicine (2017) 16:76–86. doi: 10.1016/j.ebiom.2017.01.020
98. Gupta S, Mukherjee S, Syed P, Pandala NG, Choudhary S, Singh VA, et al. Evaluation of Autoantibody Signatures in Meningioma Patients Using Human Proteome Arrays. Oncotarget (2017) 8:58443–56. doi: 10.18632/oncotarget.16997
99. Dunn J, Ferluga S, Sharma V, Futschik M, Hilton DA, Adams CL, et al. Proteomic Analysis Discovers the Differential Expression of Novel Proteins and Phosphoproteins in Meningioma Including NEK9, HK2 and SET and Deregulation of RNA Metabolism. EBioMedicine (2019) 40:77–91. doi: 10.1016/j.ebiom.2018.12.048
100. Papaioannou MD, Djuric U, Kao J, Karimi S, Zadeh G, Aldape K, et al. Proteomic Analysis of Meningiomas Reveals Clinically Distinct Molecular Patterns. Neuro Oncol (2019) 21:1028–38. doi: 10.1093/neuonc/noz084
101. Bender L, Somme F, Ruhland E, Cicek AE, Bund C, Namer IJ. Metabolomic Profile of Aggressive Meningiomas by Using High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance. J Proteome Res (2020) 19:292–9. doi: 10.1021/acs.jproteome.9b00521
102. Wibom C, Moren L, Aarhus M, Knappskog PM, Lund-Johansen M, Antti H, et al. Proteomic Profiles Differ Between Bone Invasive and Noninvasive Benign Meningiomas of Fibrous and Meningothelial Subtype. J Neurooncol (2009) 94:321–31. doi: 10.1007/s11060-009-9865-9
103. Barkhoudarian G, Whitelegge JP, Kelly DF, Simonian M. Proteomics Analysis of Brain Meningiomas in Pursuit of Novel Biomarkers of the Aggressive Behavior. J Proteomics Bioinform (2016) 9:53–7. doi: 10.4172/jpb.1000389
104. Mukherjee S, Biswas D, Gadre R, Jain P, Syed N, Stylianou J, et al. Comprehending Meningioma Signaling Cascades Using Multipronged Proteomics Approaches & Targeted Validation of Potential Markers. Front Oncol (2020) 10:1600. doi: 10.3389/fonc.2020.01600
105. Oya S, Kim SH, Sade B, Lee JH. The Natural History of Intracranial Meningiomas. J Neurosurg (2011) 114:1250–6. doi: 10.3171/2010.12.JNS101623
106. Oya S, Fukushima Y, Lee J, Matsui T, Saito N. [Current Evidence for the Management of Intracranial Meningiomas]. Jpn. J Neurosurg (Tokyo) (2016) 25:654–9. doi: 10.7887/jcns.25.654
107. Meling TR, Da Broi M, Scheie D, Helseth E, Smoll NR. Meningioma Surgery-Are We Making Progress? World Neurosurg (2019) 125:e205–13. doi: 10.1016/j.wneu.2019.01.042
108. Kawahara N, Sasaki T, Nibu K, Sugasawa M, Ichimura K, Nakatsuka T, et al. Dumbbell Type Jugular Foramen Meningioma Extending Both Into the Posterior Cranial Fossa and Into the Parapharyngeal Space: Report of 2 Cases With Vascular Reconstruction. Acta Neurochir (Wien) (1998) 140:323–30; discussion 330-321. doi: 10.1007/s007010050105
109. Takase H, Kawasaki T, Tateishi K, Yokoyama TA, Murata H, Kawahara N. Characteristics and Surgical Strategies for Posterior Clinoid Process Meningioma: Two Case Reports and Review of the Literature. Neurosurg Rev (2017) 40:163–9. doi: 10.1007/s10143-016-0774-z
110. Sato M, Tateishi K, Murata H, Kin T, Suenaga J, Takase H, et al. Three-Dimensional Multimodality Fusion Imaging as an Educational and Planning Tool for Deep-Seated Meningiomas. Br J Neurosurg (2018) 32:509–15. doi: 10.1080/02688697.2018.1485877
111. Huntoon K, Toland AMS, Dahiya S. Meningioma: A Review of Clinicopathological and Molecular Aspects. Front Oncol (2020) 10:579599. doi: 10.3389/fonc.2020.579599
112. Manaka H, Sakata K, Tatezuki J, Shinohara T, Shimohigoshi W, Yamamoto T. Safety and Efficacy of Preoperative Embolization in Patients With Meningioma. J Neurol Surg B Skull Base (2018) 79:S328–33. doi: 10.1055/s-0038-1667043
113. Rushing EJ, Bouffard JP, Mccall S, Olsen C, Mena H, Sandberg GD, et al. Primary Extracranial Meningiomas: An Analysis of 146 Cases. Head Neck Pathol (2009) 3:116–30. doi: 10.1007/s12105-009-0118-1
114. Burkhardt JK, Zinn PO, Graenicher M, Santillan A, Bozinov O, Kasper EM, et al. Predicting Postoperative Hydrocephalus in 227 Patients With Skull Base Meningioma. Neurosurg Focus (2011) 30:E9. doi: 10.3171/2011.3.FOCUS117
115. Oya S, Kawai K, Nakatomi H, Saito N. Significance of Simpson Grading System in Modern Meningioma Surgery: Integration of the Grade With MIB-1 Labeling Index as a Key to Predict the Recurrence of WHO Grade I Meningiomas. J Neurosurg (2012) 117:121–8. doi: 10.3171/2012.3.JNS111945
116. Shah AH, Patel N, Raper DM, Bregy A, Ashour R, Elhammady MS, et al. The Role of Preoperative Embolization for Intracranial Meningiomas. J Neurosurg (2013) 119:364–72. doi: 10.3171/2013.3.JNS121328
117. Przybylowski CJ, Zhao X, Baranoski JF, Borba Moreira L, Gandhi S, Chapple KM, et al. Preoperative Embolization Versus No Embolization for WHO Grade I Intracranial Meningioma: A Retrospective Matched Cohort Study. J Neurosurg (2020) 134:693–700. doi: 10.3171/2020.1.JNS19788
118. Kajimoto Y, Kuroiwa T, Miyatake S, Ichioka T, Miyashita M, Tanaka H, et al. Use of 5-Aminolevulinic Acid in Fluorescence-Guided Resection of Meningioma With High Risk of Recurrence. Case Report. J Neurosurg (2007) 106:1070–4. doi: 10.3171/jns.2007.106.6.1070
119. Coluccia D, Fandino J, Fujioka M, Cordovi S, Muroi C, Landolt H. Intraoperative 5-Aminolevulinic-Acid-Induced Fluorescence in Meningiomas. Acta Neurochir (Wien) (2010) 152:1711–9. doi: 10.1007/s00701-010-0708-4
120. Della Puppa A, Scienza R. 5-Aminolevulinic Acid-Guided Resection of Bone-Invasive Meningiomas. Neurosurg Focus (2013) 35:E6. doi: 10.3171/2012.6.FOCUS12236
121. Della Puppa A, Rustemi O, Gioffre G, Troncon I, Lombardi G, Rolma G, et al. Predictive Value of Intraoperative 5-Aminolevulinic Acid-Induced Fluorescence for Detecting Bone Invasion in Meningioma Surgery. J Neurosurg (2014) 120:840–5. doi: 10.3171/2013.12.JNS131642
122. Millesi M, Kiesel B, Mischkulnig M, Martinez-Moreno M, Wohrer A, Wolfsberger S, et al. Analysis of the Surgical Benefits of 5-ALA-Induced Fluorescence in Intracranial Meningiomas: Experience in 204 Meningiomas. J Neurosurg (2016) 125:1408–19. doi: 10.3171/2015.12.JNS151513
123. Potapov AA, Goryaynov SA, Okhlopkov VA, Shishkina LV, Loschenov VB, Savelieva TA, et al. Laser Biospectroscopy and 5-ALA Fluorescence Navigation as a Helpful Tool in the Meningioma Resection. Neurosurg Rev (2016) 39:437–47. doi: 10.1007/s10143-015-0697-0
124. Rustemi O, Della Puppa A. Hyperostosis and Osteolysis in Skull Base Meningiomas: Are Different Nuances of 5-ALA Fluorescence Related to Different Invasion Patterns? J Neurosurg Sci (2019) 63:484–5. doi: 10.23736/S0390-5616.17.04244-8
125. Valdes PA, Millesi M, Widhalm G, Roberts DW. 5-Aminolevulinic Acid Induced Protoporphyrin IX (ALA-PpIX) Fluorescence Guidance in Meningioma Surgery. J Neurooncol (2019) 141:555–65. doi: 10.1007/s11060-018-03079-7
126. Scheichel F, Popadic B, Kitzwoegerer M, Ungersboeck K, Marhold F. Fluorescence-Guided Resection in Bone and Soft Tissue Infiltrating Meningiomas. Acta Neurochir (Wien) (2020) 162:605–11. doi: 10.1007/s00701-019-04179-7
127. Kane AJ, Sughrue ME, Rutkowski MJ, Shangari G, Fang S, Mcdermott MW, et al. Anatomic Location is a Risk Factor for Atypical and Malignant Meningiomas. Cancer (2011) 117:1272–8. doi: 10.1002/cncr.25591
128. Brastianos PK, Horowitz PM, Santagata S, Jones RT, Mckenna A, Getz G, et al. Genomic Sequencing of Meningiomas Identifies Oncogenic SMO and AKT1 Mutations. Nat Genet (2013) 45:285–9. doi: 10.1038/ng.2526
129. Clark VE, Erson-Omay EZ, Serin A, Yin J, Cotney J, Ozduman K, et al. Genomic Analysis of non-NF2 Meningiomas Reveals Mutations in TRAF7, KLF4, AKT1, and SMO. Science (2013) 339:1077–80. doi: 10.1126/science.1233009
130. Williams SR, Juratli TA, Castro BA, Lazaro TT, Gill CM, Nayyar N, et al. Genomic Analysis of Posterior Fossa Meningioma Demonstrates Frequent AKT1 E17K Mutations in Foramen Magnum Meningiomas. J Neurol Surg B Skull Base (2019) 80:562–7. doi: 10.1055/s-0038-1676821
131. Kondziolka D, Mathieu D, Lunsford LD, Martin JJ, Madhok R, Niranjan A, et al. Radiosurgery as Definitive Management of Intracranial Meningiomas. Neurosurgery (2008) 62:53–8; discussion 58-60. doi: 10.1227/01.NEU.0000311061.72626.0D
132. Lee G, Lamba N, Niemierko A, Kim DW, Chapman PH, Loeffler JS, et al. Adjuvant Radiation Therapy Versus Surveillance After Surgical Resection of Atypical Meningiomas. Int J Radiat Oncol Biol Phys (2021) 109:252–66. doi: 10.1016/j.ijrobp.2020.08.015
133. Gehler B, Paulsen F, Oksuz MO, Hauser TK, Eschmann SM, Bares R, et al. [68ga]-DOTATOC-PET/CT for Meningioma IMRT Treatment Planning. Radiat Oncol (2009) 4:56. doi: 10.1186/1748-717X-4-56
134. Brastianos PK, Galanis E, Butowski N, Chan JW, Dunn IF, Goldbrunner R, et al. Advances in Multidisciplinary Therapy for Meningiomas. Neuro Oncol (2019) 21:i18–31. doi: 10.1093/neuonc/noy136
135. Uy NW, Woo SY, Teh BS, Mai WY, Carpenter LS, Chiu JK, et al. Intensity-Modulated Radiation Therapy (IMRT) for Meningioma. Int J Radiat Oncol Biol Phys (2002) 53:1265–70. doi: 10.1016/S0360-3016(02)02823-7
136. Kawabata S, Hiramatsu R, Kuroiwa T, Ono K, Miyatake S. Boron Neutron Capture Therapy for Recurrent High-Grade Meningiomas. J Neurosurg (2013) 119:837–44. doi: 10.3171/2013.5.JNS122204
137. Cornelius JF, Slotty PJ, El Khatib M, Giannakis A, Senger B, Steiger HJ. Enhancing the Effect of 5-Aminolevulinic Acid Based Photodynamic Therapy in Human Meningioma Cells. Photodiagnosis Photodyn Ther (2014) 11:1–6. doi: 10.1016/j.pdpdt.2014.01.001
138. Kantz S, Sohn M, Troeller A, Reiner M, Weingandt H, Alber M, et al. Impact of MLC Properties and IMRT Technique in Meningioma and Head-and-Neck Treatments. Radiat Oncol (2015) 10:184. doi: 10.1186/s13014-015-0447-z
139. Takeuchi K, Kawabata S, Hiramatsu R, Matsushita Y, Tanaka H, Sakurai Y, et al. Boron Neutron Capture Therapy for High-Grade Skull-Base Meningioma. J Neurol Surg B Skull Base (2018) 79:S322–7. doi: 10.1055/s-0038-1666837
140. Golub D, Kwan K, Knisely JPS, Schulder M. Possible Abscopal Effect Observed in Frontal Meningioma After Localized IMRT on Posterior Meningioma Resection Cavity Without Adjuvant Immunotherapy. Front Oncol (2019) 9:1109. doi: 10.3389/fonc.2019.01109
141. Ichikawa M, Akimoto J, Miki Y, Maeda J, Takahashi T, Fujiwara Y, et al. Photodynamic Therapy With Talaporfin Sodium Induces Dose- and Time-Dependent Apoptotic Cell Death in Malignant Meningioma HKBMM Cells. Photodiagnosis Photodyn Ther (2019) 25:29–34. doi: 10.1016/j.pdpdt.2018.10.022
142. Nakahara Y, Ito H, Masuoka J, Abe T. Boron Neutron Capture Therapy and Photodynamic Therapy for High-Grade Meningiomas. Cancers (Basel) (2020) 12:1334. doi: 10.3390/cancers12051334
143. Takahashi T, Misawa S, Suzuki S, Saeki N, Shinoda Y, Tsuneoka Y, et al. Possible Mechanism of Heme Oxygenase-1 Expression in Rat Malignant Meningioma KMY-J Cells Subjected to Talaporfin Sodium-Mediated Photodynamic Therapy. Photodiagnosis Photodyn Ther (2020) 32:102009. doi: 10.1016/j.pdpdt.2020.102009
144. Takai S, Wanibuchi M, Kawabata S, Takeuchi K, Sakurai Y, Suzuki M, et al. Reactor-Based Boron Neutron Capture Therapy for 44 Cases of Recurrent and Refractory High-Grade Meningiomas With Long-Term Follow-Up. Neuro Oncol (2022) 24:90–8. doi: 10.1093/neuonc/noab108
145. Tom MC, Kotecha R. Beating a Benchmark: Boron Neutron Capture Therapy for Recurrent and Refractory Meningiomas. Neuro Oncol (2022) 24:99–100. doi: 10.1093/neuonc/noab217
146. Slater JD, Loredo LN, Chung A, Bush DA, Patyal B, Johnson WD, et al. Fractionated Proton Radiotherapy for Benign Cavernous Sinus Meningiomas. Int J Radiat Oncol Biol Phys (2012) 83:e633–637. doi: 10.1016/j.ijrobp.2012.01.079
147. Weber DC, Schneider R, Goitein G, Koch T, Ares C, Geismar JH, et al. Spot Scanning-Based Proton Therapy for Intracranial Meningioma: Long-Term Results From the Paul Scherrer Institute. Int J Radiat Oncol Biol Phys (2012) 83:865–71. doi: 10.1016/j.ijrobp.2011.08.027
148. Maclean J, Fersht N, Bremner F, Stacey C, Sivabalasingham S, Short S. Meningioma Causing Visual Impairment: Outcomes and Toxicity After Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys (2013) 85:e179–186. doi: 10.1016/j.ijrobp.2012.10.032
149. Harding R, Trnkova P, Weston SJ, Lilley J, Thompson CM, Short SC, et al. Benchmarking of a Treatment Planning System for Spot Scanning Proton Therapy: Comparison and Analysis of Robustness to Setup Errors of Photon IMRT and Proton SFUD Treatment Plans of Base of Skull Meningioma. Med Phys (2014) 41:111710. doi: 10.1118/1.4897571
150. Gunaydin G, Gedik ME, Ayan S. Photodynamic Therapy-Current Limitations and Novel Approaches. Front Chem (2021) 9:691697. doi: 10.3389/fchem.2021.691697
151. Hefti M, Holenstein F, Albert I, Looser H, Luginbuehl V. Susceptibility to 5-Aminolevulinic Acid Based Photodynamic Therapy in WHO I Meningioma Cells Corresponds to Ferrochelatase Activity. Photochem Photobiol (2011) 87:235–41. doi: 10.1111/j.1751-1097.2010.00821.x
152. Karsy M, Guan J, Cohen A, Colman H, Jensen RL. Medical Management of Meningiomas: Current Status, Failed Treatments, and Promising Horizons. Neurosurg Clin N Am (2016) 27:249–60. doi: 10.1016/j.nec.2015.11.002
153. Kawahara N. [Update Knowledge for Brain Tumors(7)Meningioma]. No Shinkei Geka (2016) 44:415–30. doi: 10.11477/mf.1436203301
154. Delgado-Lopez PD, Cubo-Delgado E, Gonzalez-Bernal JJ, Martin-Alonso J. A Practical Overview on the Molecular Biology of Meningioma. Curr Neurol Neurosci Rep (2020) 20:62. doi: 10.1007/s11910-020-01084-w
155. Shao Z, Liu L, Zheng Y, Tu S, Pan Y, Yan S, et al. Molecular Mechanism and Approach in Progression of Meningioma. Front Oncol (2020) 10:538845. doi: 10.3389/fonc.2020.538845
156. Pallini R, Casalbore P, Mercanti D, Maggiano N, Larocca LM. Phenotypic Change of Human Cultured Meningioma Cells. J Neurooncol (2000) 49:9–17. doi: 10.1023/A:1006436903976
157. Graillon T, Romano D, Defilles C, Saveanu A, Mohamed A, Figarella-Branger D, et al. Octreotide Therapy in Meningiomas: In Vitro Study, Clinical Correlation, and Literature Review. J Neurosurg (2017) 127:660–9. doi: 10.3171/2016.8.JNS16995
158. Das A, Martinez Santos JL, Alshareef M, Porto GBF, Infinger LK, Vandergrift WA 3rd, et al. In Vitro Effect of Dovitinib (TKI258), a Multi-Target Angiokinase Inhibitor on Aggressive Meningioma Cells. Cancer Invest (2020) 38:349–55. doi: 10.1080/07357907.2020.1773844
159. Uhlmann EJ, Rabinovsky R, Varma H, El Fatimy R, Kasper EM, Moore JM, et al. Tumor-Derived Cell Culture Model for the Investigation of Meningioma Biology. J Neuropathol Exp Neurol (2021) 80:1117–24. doi: 10.1093/jnen/nlab111
160. Batchu S. Caution Using Meningioma Cell Lines as Tumour Models. Cancer Invest (2020) 38:535–6. doi: 10.1080/07357907.2020.1821210
161. Eriksson TM, Day RM, Fedele S, Salih VM. The Regulation of Bone Turnover in Ameloblastoma Using an Organotypic In Vitro Co-Culture Model. J Tissue Eng (2016) 7:2041731416669629. doi: 10.1177/2041731416669629
162. Butler D. Translational Research: Crossing the Valley of Death. Nature (2008) 453:840–2. doi: 10.1038/453840a
Keywords: meningioma, bone invasion, recurrence, translational study, long-term
Citation: Takase H and Yamamoto T (2022) Bone Invasive Meningioma: Recent Advances and Therapeutic Perspectives. Front. Oncol. 12:895374. doi: 10.3389/fonc.2022.895374
Received: 13 March 2022; Accepted: 01 June 2022;
Published: 30 June 2022.
Edited by:
Hailiang Tang, Fudan University, ChinaReviewed by:
Nils Ole Schmidt, University Medical Center Regensburg, GermanyShuyu Hao, Capital Medical University, China
Copyright © 2022 Takase and Yamamoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hajime Takase, htakase@yokohama-cu.ac.jp; orcid.org/0000-0001-5813-1386
 Hajime Takase
Hajime Takase Tetsuya Yamamoto2
Tetsuya Yamamoto2