- 1Department of Radiology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, China
- 2Department of Radiology, Shanghai Sixth People’s Hospital, Shanghai, China
- 3Department of Radiology, Nanjing Drum Tower Hospital, Nanjing, China
- 4Institute of Radiation Medicine, Fudan University, Shanghai, China
Background: Abrupt change in the caliber of the main pancreatic duct (MPD) with distal pancreatic atrophy (PA) was considered as one of worrisome features in the International Association of Pancreatology guideline and American College of Gastroenterology guideline for the management of intraductal papillary mucinous neoplasms (IPMNs). However, this feature was not included in other guidelines. Moreover, the association between PA alone and malignancy in IPMNs has not been fully evaluated. In the present study, we investigated the role of image-based PA in identifying malignant IPMNs or invasive carcinoma.
Methods: A total of 186 patients with IPMNs were included for analysis. The tumor size, location, MPD diameter, presence of a mural nodule (MN), and PA were evaluated using magnetic resonance imaging. Demographic information and serum carbohydrate antigen 19-9 and carcinoembryonic antigen (CEA) levels were also collected. IPMNs with high-grade dysplasia and associated invasive carcinoma were regarded as malignant IPMNs.
Results: PA was observed in 34 cases (18.3%). The occurrence of malignant IPMNs or invasive carcinoma in patients with PA were significantly higher than in those without PA (52.9% vs. 22.3%; 44.1% vs. 8.9%, all P < 0.01). Multivariate logistic regression analysis showed that PA was an independently associated factor for malignant IPMNs [odds ratio (OR) = 2.69, 95% confidence interval (CI): 1.07-6.78] or invasive carcinoma (OR = 7.78, 95%CI: 2.62-23.10) after modified with confounders. Subgroup analysis in MPD-involved IPMNs also indicated that PA was an independently associated factor for invasive carcinoma (OR = 9.72, 95%CI: 2.43-38.88). PA had a similar performance with MPD plus MN [the area under the curve (AUC) was both 0.71] in identifying malignancy. PA had a higher performance in identifying invasive carcinoma in MPD-involved IPMNs than MN (AUC = 0.71 vs. 0.65, P = 0.02).
Conclusion: Our data showed that imaging-based PA was associated with malignancy or invasive carcinoma regardless of abrupt change in the caliber of MPD in IPMNs. PA had an acceptable performance in identifying malignant IPMNs.
Introduction
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas is a cystic tumor with papillary growth and mucin secretion in the duct. IPMNs have a great potential of malignant transformation. The management of IPMNs remains a challenge in clinical practice (1) because none of the guidelines is chiefly complete (2). Nowadays, a tumor size ≥3.0 cm, the presence of an enhanced mural nodule (MN), main pancreatic diameter ≥10 mm, thickened/enhancing cyst walls, obstructive jaundice, and elevated carbohydrate antigen 19-9 (CA19-9) levels have been widely used as risk factors to predict malignancy (3). Some models with acceptable performance were also established based on those risk factors (4, 5).
Pancreatic atrophy (PA) is usually occurred in pancreatic ductal adenocarcinoma. Excessive mucin secretion from IPMNs can obstruct the main pancreatic duct (MPD), leading to PA and fibrosis (6). Currently, nine guidelines (2) proposed radiological and clinical criteria to assess the malignant potential of pancreatic IPMNs. Among these, only two guidelines mentioned that PA was a risk factor for malignancy. The International Association of Pancreatology (IAP) guideline (2017) (3) mentioned that abrupt change in the caliber of MPD with distal PA was a worrisome feature of malignant IPMNs, and the American College of Gastroenterology (ACG) clinical guideline (2018) (7) suggested the abrupt change in the caliber of MPD with distal PA as a high-risk characteristic for malignant mucinous pancreatic cysts.
Interestingly, some studies have found that pathological PA was also related to invasive carcinoma or malignant IPMNs (8–11). Saito et al. (8) found that PA and fibrosis were more common in a high grade of IPMNs than that in a low grade (50%-76% vs. 6%). In addition, several studies showed that abrupt MPD caliber change with distal PA reported during radiological examinations was associated with malignant IPMNs or invasive carcinoma in all IPMNs and branch-duct IPMNs (BD-IPMNs) (12–20). By contrast, some studies did not observe such association in univariate or multivariate analysis (21–26). Moreover, whether PA alone (regardless of abrupt MPD caliber change) is associated with malignant IPMNs is still not fully understood. To the best of our knowledge, only one study with a small sample size (n = 55) showed that PA is associated with malignant IPMNs in univariate analysis (13). However, whether PA is an independent risk factor was not investigated. Therefore, the main purpose of this study is to investigate whether image-based PA can independently indicate the presence of malignant lesions in IPMNs.
Materials and Methods
Patients
The retrospective study was approved by the Ethnic Committee of Affiliated Hospital of Nanjing University of Chinese Medicine. A total of 214 patients with pathologically proved IPMNs were found in our institutions during January 2016 to May 2021. Among these patients, 29 patients were excluded for the following reasons: the absence of magnetic resonance imaging (MRI) or MRI examinations at an outside hospital (n = 16); absence of contrast-enhanced examinations (n = 5); and other pancreatic tumors (n = 8). Because MRI or MR cholangiopancreatography (MRCP) has an advantage in identifying BD-IPMNs, we only included patients with MRI examinations. The clinical information, including demographic data (age, gender); tumor biomarkers [CA19-9 and carcinoembryonic antigen (CEA)]; and medical history of diabetes mellitus (DM), pancreatitis, and jaundice, were recorded from the medical system. DM was considered if plasma glucose levels were higher than 7.1 mmol/L or if there is a history of DM.
Imaging Methods and Imaging Analysis
MR scans were performed by using a 3.0-T or 1.5-T unit (Signa HDx 3.0-T; GE Medical Systems, Milwaukee, WI, United States, or Achieva 1.5-T; Philips, Amsterdam, The Netherlands). Conventional axial, sagittal, and coronal T1-weighted turbo spin-echo imaging sequence (without and with gadolinium), fast spin-echo T2-weighted fat-suppressed sequence (echo time/repetition time [TE/TR]: 4,000–8,000/80–90 ms). MRCP was performed using heavily T2-weighted fast acquisition spin echo sequence (TR/TE: 2,400–6,000/500–800 ms). Contrast enhanced imaging was also performed after the intravenous injection of 0.1 mmol/kg gadolinium (2.5 ml/s).
The following imaging parameters were collected: tumor location (head–neck or body–tail), tumor size, MPD diameter, and the presence of enhanced MN with a size ≥5 mm. The MPD diameter was measured at the point of the maximally dilated pancreatic duct (16). Enhanced MN was considered if there were any enhancing solid papillary protuberances within the lesions (16). PA was considered if the ratio between the MPD diameter and the width of the pancreas parenchyma is larger than 0.5 (27).
Pathological Examinations
IPMN was divided into three subtypes based on the degree of involvement of the pancreatic ductal system: main duct (MD), BD, and mixed type (MT). The pathological grade of IPMN was classified as low-intermediate dysplasia, high-grade dysplasia, and invasive adenocarcinoma according to the World Health Organization guideline. Those IPMNs with high-grade dysplasia and associated invasive carcinoma were regarded as malignant IPMNs.
Statistical Analysis
Data management and statistical analysis were all performed by using SPSS 16.0 (IBM, Armonk, NY, United States). CA19-9, CEA, the tumor size, and MPD diameter were divided into two groups: <37.0 and ≥37.0 U/ml; <5.0 and ≥5.0 ng/ml; ≥3.0 and <3.0 cm; and ≥1.0 and <1.0 cm. Two-tailed independent t tests or the Mann–Whitney U-test were adopted to compare the variables between patients with and without PA. A chi-square test or Fisher exact test was used to compare categorical variables. Univariate and multivariate logistic regression analyses were used to investigate the association between the presence of PA and malignant IPMNs or invasive carcinoma. Receiver operating characteristic (ROC) curves were conducted to show the performance of PA alone or combination with other markers in identifying malignant IPMNs or invasive carcinoma. Two-tailed P-values < 0.05 were considered as a statistical significance.
Results
Characteristics of Subjects
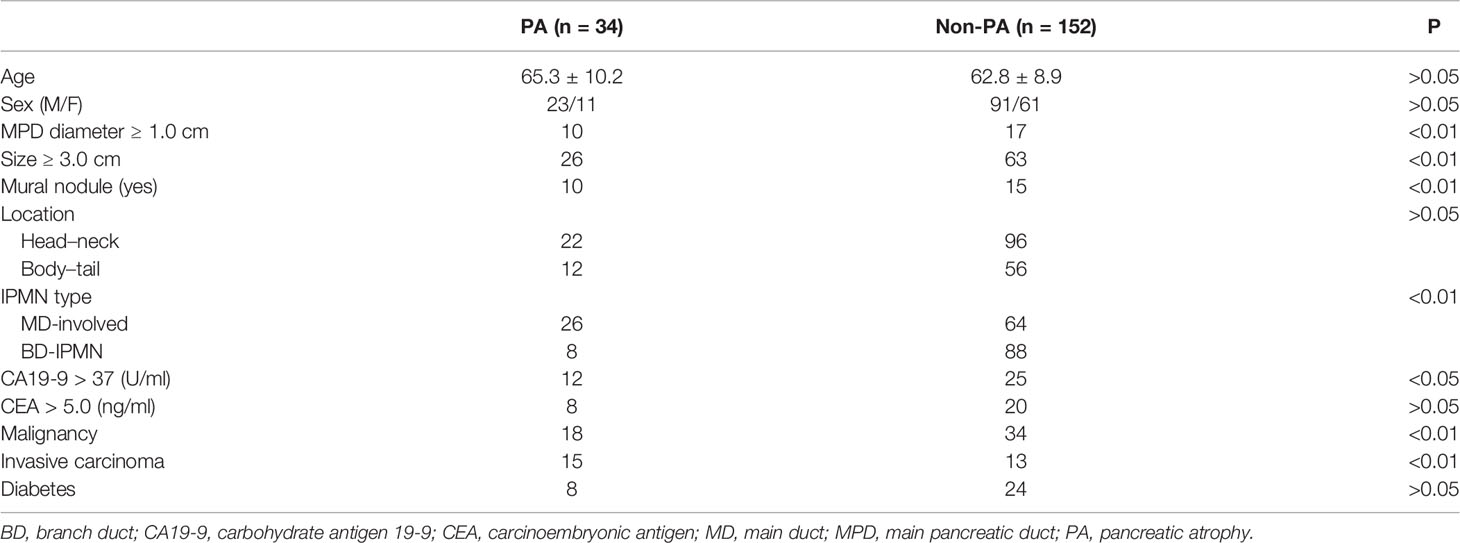
A total of 186 patients met the selection criteria and were included in the analysis. The characteristics of subjects with PA and without PA are shown in Table 1. PA occurred in 34 (18.3%) patients with IPMNs. Factors such as age, sex, CEA level, and DM did not differ between the two groups (all P > 0.05). The prevalence of high CA19-9 levels (>37 U/ml) in patients with PA was significantly higher than those without PA (P < 0.05). Regarding the morphological features of IPMNs, the occurrences of an MPD diameter ≥1.0 cm, cyst size ≥3.0 cm, and MN were more common in patients with PA than those without PA (all P < 0.01), but no such difference was observed in the tumor location (P > 0.05). The incidence of PA was higher in MD-involved IPMNs than that in BD-IPMNs (P < 0.01).
Occurrences of Malignancy and Invasive Carcinoma in Patients With PA
The occurrences of malignancy and invasive carcinoma in patients with PA were all higher than those without PA (52.9% vs. 22.4%; 44.2% vs. 8.6%) (both P < 0.01). Subsequently, we assessed the correlation between the prevalence of PA and grade of IPMNs (Figure 1). The prevalence of PA in all IPMNs and MD-involved IPMNs were increased with tumor the grade (p < 0.01). Such trends were also observed in BD-IPMNs, but no statistical significance was found (P = 0.21). Three cases of IPMNs with or without PA are shown in Figure 2.
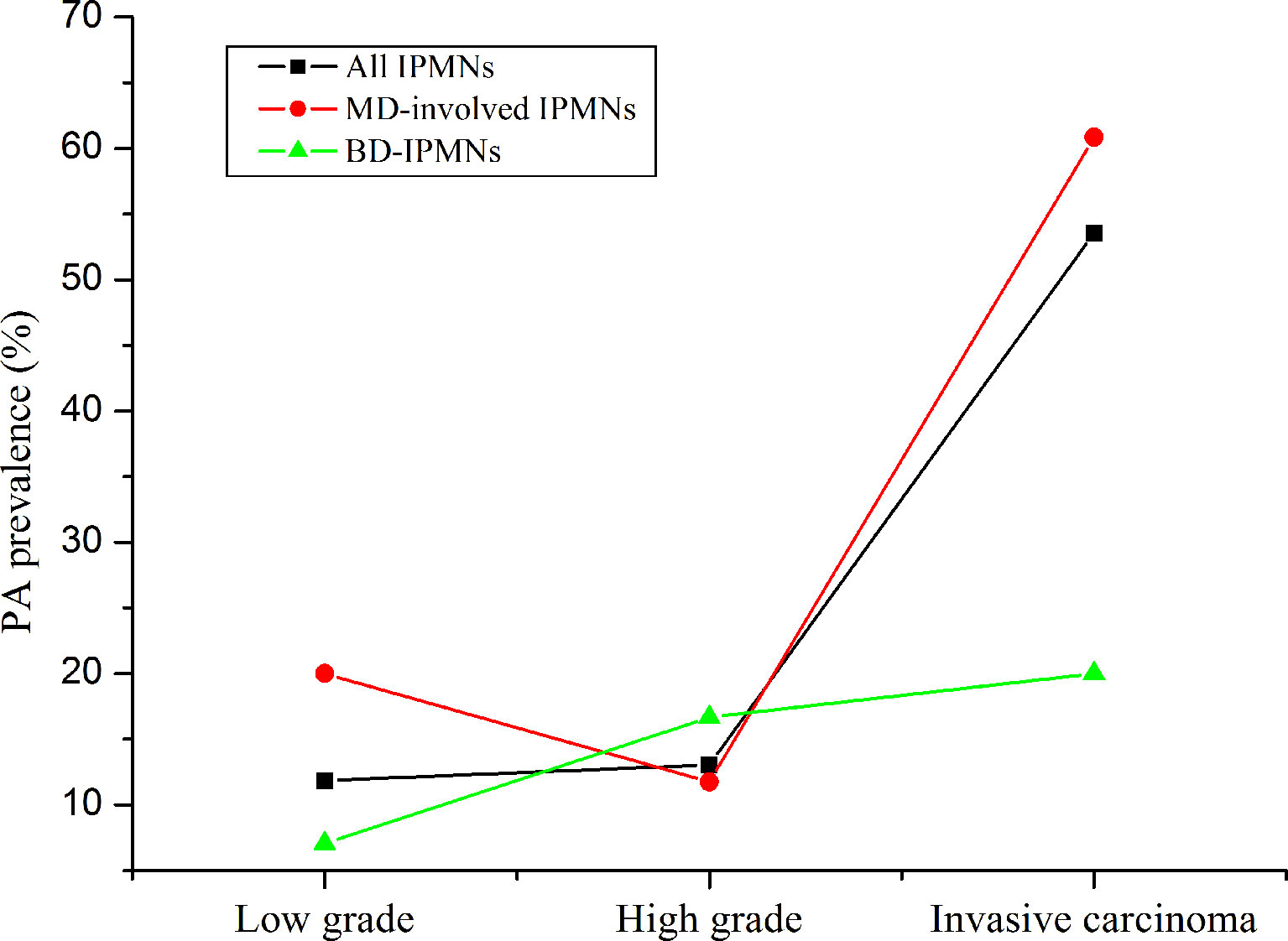
Figure 1 The prevalence of pancreatic atrophy in all intraductal papillary mucinous neoplasms (IPMNs), MD-involved IPMN, and BD-IPMNs. The p-values for trends were less than 0.01 and was 0.21. “Low” means low and intermediate grade.
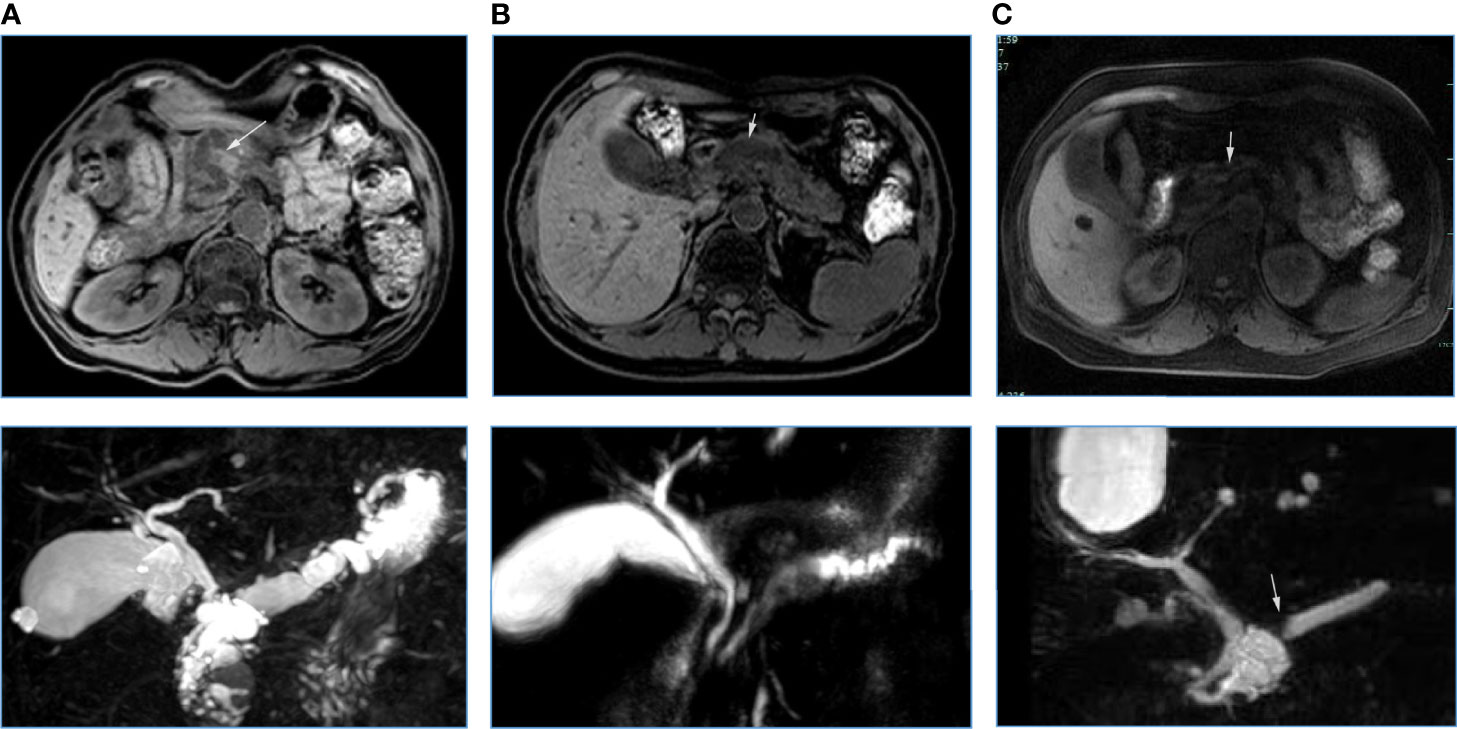
Figure 2 Three cases of IPMNs with (A, B) or without (C) pancreatic atrophy. A: A 67-year-old woman with IPMN-derived invasive carcinoma in pancreatic head (white arrow). Atrophy occurred in the pancreatic body and tail. Pancreatic duct dilatation occurred (below). B: A 61-year old man with low-grade IPMN in the pancreatic head. Pancreatic duct dilatation occurred (white arrow), but pancreatic atrophy was not observed. C: A 57-year old woman with low–moderate grade of IPMN in pancreatic head. An abrupt change in the caliber of the main pancreatic duct (bottom white arrow) with body pancreatic atrophy was observed (the ratio between the main pancreatic duct diameter and the width of the total gland was 0.54, white arrow).
The Association Between PA and Malignant IPMNs
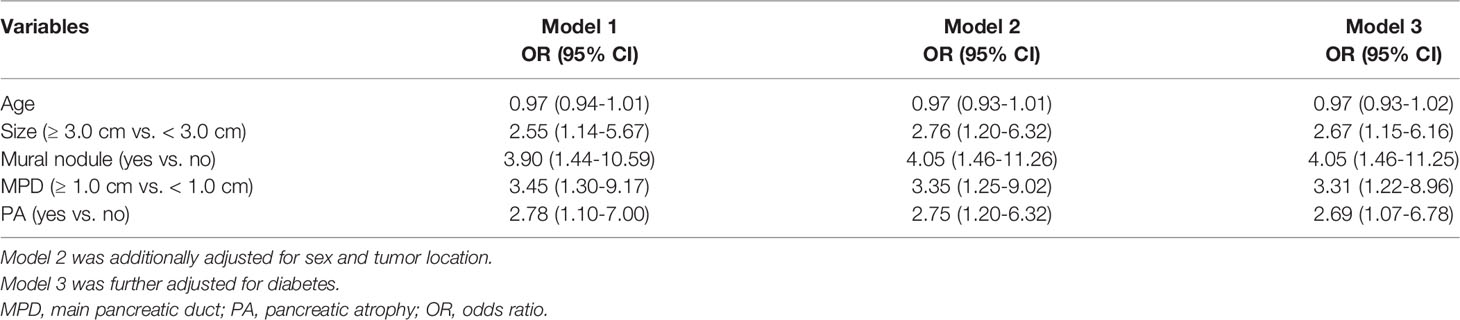
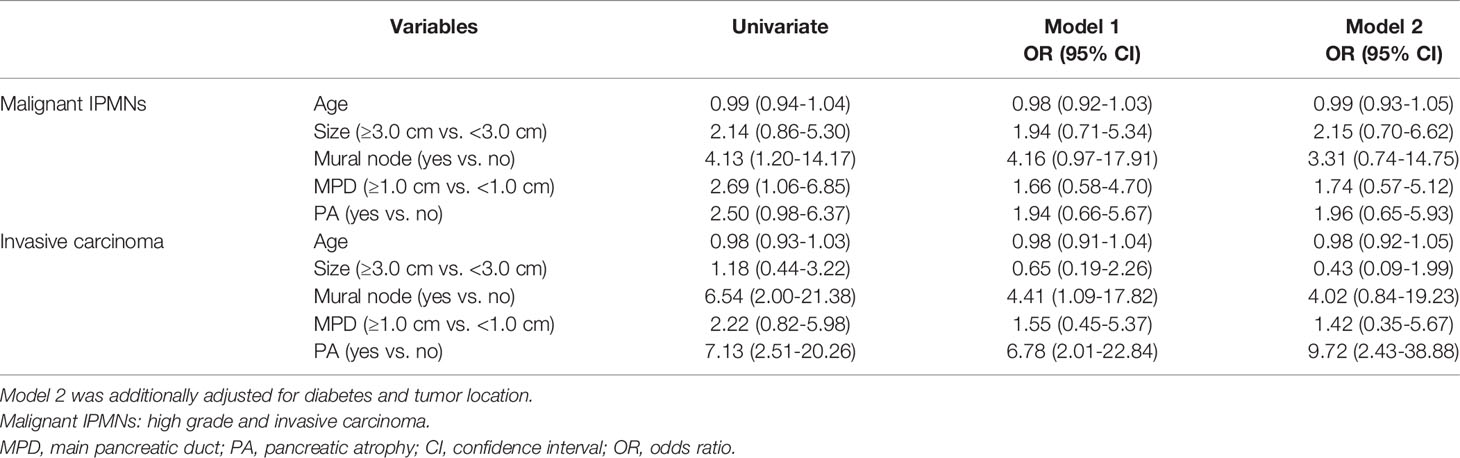
Next, we observed the association between PA and malignancy or invasive carcinoma in IPMNs (Tables 2, 3). Both univariate and multivariate logistic regression models showed that the occurrence of PA was an independent risk factor for malignancy (OR = 7.78, 95% CI: 2.62-23.10) or invasive carcinoma (OR = 2.69, 95% CI: 1.07-6.78) in IPMNs after being adjusted with confounders such as the tumor size, presence of MN, and MPD dilatation. The occurrence of the cyst size ≥3.0 cm and MPD diameter ≥1.0 cm were significantly associated with a higher risk of malignancy in IPMNs but not with invasive carcinoma.
The Association Between PA and Invasive Carcinoma in MD-Involved IPMNs
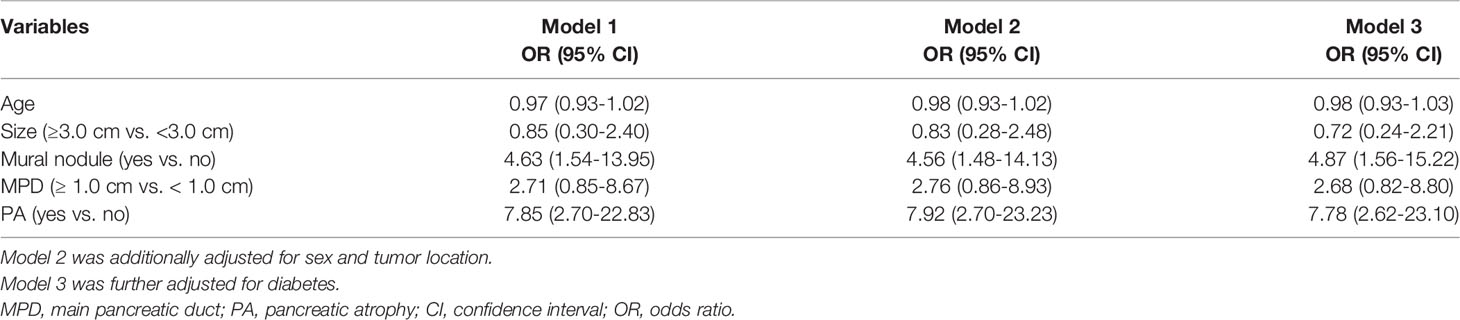
We examined whether the correlation between PA and malignancy or invasive carcinoma was present in MD-involved IPMN patients (Table 4). Univariable analysis showed that PA were associated with invasive carcinoma (OR = 6.54, 95% CI: 2.00-21.38; OR = 7.13, 95% CI: 2.51-20.26), but not with malignant IPMNs. Multivariate analysis further demonstrated that PA were independently associated with invasive carcinoma (OR = 9.72, 95% CI: 2.43-38.88).
ROC Curves in Identifying Malignancy or Invasive Carcinoma
The ROC curves in identifying malignancy or invasive carcinoma are presented in Figure 3. PA had a similar performance with MPD plus MN (the AUC was both 0.71). The combined MPD diameter, MN, and PA had a higher performance (AUC = 0.78) than that of the MPD diameter plus MN or PA alone (P = 0.03) in identifying invasive carcinoma in all IPMNs.
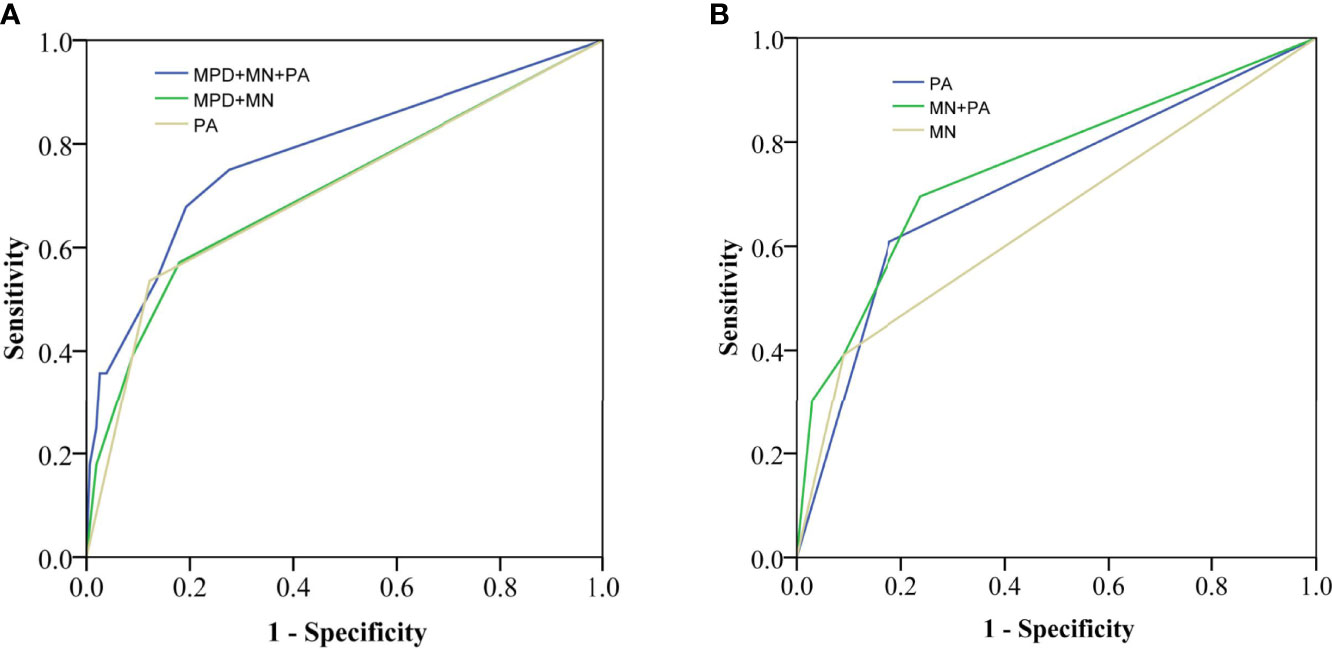
Figure 3 Receiver operating characteristic (ROC) curves for identifying invasive carcinoma in all IPMNs (A) [area under the curve (AUC) = 0.78 vs. 0.71, 0.71] and MD-involved IPMNs (B) (AUC = 0.76 vs. 0.65, 0.71). MPD, main pancreatic duct; MN, mural nodule; PA, pancreatic atrophy.
PA also had acceptable performance in identifying invasive carcinoma in MD-involved IPMNs (AUC = 0.71), which was higher than that of MN (AUC = 0.65) (P = 0.02). Moreover, the AUC of MN plus PA (AUC = 0.76) was higher than that of MN (AUC = 0.65) or PA (AUC = 0.71) alone in MD-involved IPMNs.
Discussion
An abrupt change in the caliber of MPD with distal PA has been regarded as a risk factor for malignant IPMNs or feature for surgical resection in IAP and ACG guidelines (3, 7). However, little is known about the association between PA alone and malignant IPMNs. In the present study, our data demonstrated that PA alone was an associated factor for malignant IPMNs or invasive carcinoma. PA was also associated with invasive carcinoma in MD-involved IPMNs. Moreover, PA alone, PA plus MN, and PA Plus MN and MPD all had acceptable performance in identifying malignant IPMNs and invasive carcinoma (AUC > 0.70).
The occurrence of PA in IPMNs is not uncommon. Two recent radiological studies showed that 23% of IPMN patients had PA in computed tomography (CT) images (16, 17), which was similar to the occurrence of the MPD size ≥1.0 cm. Our data showed that 18.2% of IPMN patients had PA that was consistent with those previous results. Moreover, a recent study reported that PA occurred in 30% IPMN patients (20). Therefore, the role of PA in IPMNs should draw people’s attention extensively.
Several guidelines have showed malignant features or surgical indications for IPMNs (2), such as MN or enhanced solid component, MPD > 1.0 cm, and tumor size ≥ 3.0 cm. The diagnostic performances of the 2017 revised International Consensus Guidelines have been validated by some studies (16, 17, 28). Lee et al. (16) indicated that the diagnostic performance of CT and MRI (based on high-risk stigmata and worrisome features) were both good, AUC = 0.83 for CT and AUC = 0.86 for MRI. Min et al. reported that the diagnostic accuracy of high-risk stigmata (MPD diameter ≥10 mm, MN ≥5 mm, and obstructive jaundice) was 73.7% for CT and 75.4% for MRI (n = 175). Several nomogram models based on clinical, high-risk stigmata and/or worrisome features also indicated an excellent discrimination performance (4, 29, 30). However, abrupt change in the caliber of MPD with distal PA or PA alone was not included in those models. Our data showed that the combined model of MPD plus MN had a similar discrimination performance (AUC = 0.71) to previous data (AUC = 0.69) (30). The addition of PA significantly improved the performance of the combined model of MPD plus MN in identifying malignant IPMNs (AUC = 0.78 vs. 0.71, P < 0.05). The performance of PA plus MPD and MN was comparable to those reported models (4, 29).
A single biomarker in isolation was also used to identify malignancy in IPMNs. Sugimoto et al. showed that the diagnostic performance of an MPD diameter cut-off of 7.2 mm for malignant neoplasms was acceptable (AUC = 0.70, 95% CI: 0.59-0.81). Kim et al. (31) demonstrated that the accuracy was 73.8% for elevated serum CA19-9, 73.3% for MPD >5 mm, and 77.7% for MN in identifying malignant IPMNs. A recent study indicates that mucin 5AC (MUC5AC) in a circulating extracellular vesicle can predict high-grade IPMNs with an AUC of 0.73 and predict invasive carcinoma with an AUC of 0.91 (32). Our previous study also reported that serum ferritin had acceptable performance (AUC = 0.67) (33). In the present study, our data further showed that the diagnostic power of PA alone was comparable to that of MPD or MN (AUC = 0.71) and the serum biomarkers of CA19-9 or serum ferritin or a circulating extracellular vesicle in identifying malignant IPMNs but lower than that of the circulating extracellular vesicle in identifying invasive carcinoma (32).
CT, MRI, and endoscopic ultrasonography (EUS) are all useful for IPMN diagnosis or malignancy identification. A recent meta-analysis showed that contrast-enhanced EUS had good performance (accuracy of 89.6%, sensitivity of 88.2%, and specificity of 79.1%) in identifying a mural nodule (34). EUS also has a great role in preoperative biopsy. Crinò et al. reported a new EUS-guided cyst-wall biopsy in pancreatic cysts (35), which can be used for pathological examinations. Moreover, EUS-guided confocal laser endomicroscopy has great potential in identifying high-grade dysplasia/adenocarcinoma with high sensitivity and accuracy (36, 37). However, we did not observe the role of EUS for PA evaluation because EUS is not routinely performed for IPMNs in China. Because MRI or MRCP may have an advantage in differentiating the IPMN type and some CT examinations of our patients were performed outside, we only included those patients with MRI examinations.
Our study has several limitations. First, only the patients who underwent surgery and MRI examinations were included, which may cause selection bias. The generalization of our results should be confirmed by other studies, even though pathological studies have shown that PA was related to invasive carcinoma or malignant IPMNs (8, 11). Second, PA was evaluated by radiological examinations, not by pathological examinations. Third, some worrisome features, such as elevated CA19-9 levels and thickened enhancing cyst walls, were not considered as confounders because of the small sample size of IPMNs with PA. Finally, we did not analyze the association between PA and malignancy in BD-IPMNs because the incidence of PA was low in BD-IPMNs.
In conclusion, the occurrence of PA in IPMNs was common. PA was associated with malignant IPMNs and invasive carcinoma. PA alone or combined with MPD and MN had an acceptable performance in predicting malignancy in IPMNs. Our data support that PA could be regarded as one of the associated factors or worrisome features in the guidelines.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The retrospective study was approved by the Ethic Committee of Affiliated Hospital of Nanjing University of Chinese Medicine. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
CW and XiaoC participated in the design of the study. TL, XinC and XiaoC wrote the manuscript. TL, XinC, JL, YC, and WC collected and analyzed the data. TL, XinC, CW and ZW contributed to interpretation of data and preparation of the manuscript. All authors read and approved the final manuscript.
Funding
Peak academic talent training fund of Jiangsu Province Hospital of Chinese Medicine(y2018rc04); Science and Technology Development Plan fund of Chinese Medicine of Jiangsu Province (ZD201907) and National Natural Science Foundation of China (No. 81773460).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Chuangen Guo (the First Affiliated Hospital of Zhejiang University) for data collection.
References
1. Farrell JJ. Editorial: Stopping Pancreatic Cyst Surveillance? Am J Gastroenterol (2017) 112(7):1162–4. doi: 10.1038/ajg.2017.163
2. Tanaka M. Clinical Management and Surgical Decision-Making of Ipmn of the Pancreas. Methods Mol Biol (2019) 1882:9–22. doi: 10.1007/978-1-4939-8879-2_2
3. Tanaka M, Fernández-Del Castillo C, Kamisawa T, Jang JY, Levy P, Ohtsuka T, et al. Revisions of International Consensus Fukuoka Guidelines for the Management of Ipmn of the Pancreas. Pancreatology (2017) 17(5):738–53. doi: 10.1016/j.pan.2017.07.007
4. Jang JY, Park T, Lee S, Kim Y, Lee SY, Kim SW, et al. Proposed Nomogram Predicting the Individual Risk of Malignancy in the Patients With Branch Duct Type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann Surg (2017) 266(6):1062–8. doi: 10.1097/SLA.0000000000001985
5. Shimizu Y, Hijioka S, Hirono S, Kin T, Ohtsuka T, Kanno A, et al. New Model for Predicting Malignancy in Patients With Intraductal Papillary Mucinous Neoplasm. Ann Surg (2020) 272(1):155–62. doi: 10.1097/SLA.0000000000003108
6. Ban S, Naitoh Y, Mino-Kenudson M, Sakurai T, Kuroda M, Koyama I, et al. Intraductal Papillary Mucinous Neoplasm (Ipmn) of the Pancreas: Its Histopathologic Difference Between 2 Major Types. Am J Surg Pathol (2006) 30(12):1561–9. doi: 10.1097/01.pas.0000213305.98187.d4
7. Elta GH, Enestvedt BK, Sauer BG, Lennon AM. Acg Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol (2018) 113(4):464–79. doi: 10.1038/ajg.2018.14
8. Saito M, Imada H, Suzuki T, Sata N, Yasuda Y, Maetani I, et al. Distinct Patterns of Peritumoral Histological Findings in Subtypes of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann Diagn Pathol (2015) 19(5):347–52. doi: 10.1016/j.anndiagpath.2015.07.005
9. Yagi Y, Masuda A, Zen Y, Takenaka M, Toyama H, Sofue K, et al. Predictive Value of Low Serum Pancreatic Enzymes in Invasive Intraductal Papillary Mucinous Neoplasms. Pancreatology (2016) 16(5):893–9. doi: 10.1016/j.pan.2016.06.663
10. Takenaka M, Masuda A, Shiomi H, Yagi Y, Zen Y, Sakai A, et al. Chronic Pancreatitis Finding by Endoscopic Ultrasonography in the Pancreatic Parenchyma of Intraductal Papillary Mucinous Neoplasms Is Associated With Invasive Intraductal Papillary Mucinous Carcinoma. Oncology (2017) 93 Suppl 1:61–8. doi: 10.1159/000481232
11. Nakahodo J, Fukumura Y, Saito T, Mitomi H, Saiura A, Fujisawa T, et al. Radiological and Pathological Assessment of the 2017 Revised International Association of Pancreatology Consensus Guidelines for Intraductal Papillary Mucinous Neoplasm, With an Emphasis on the Gastric Pyloric Gland Type. Pancreas (2020) 49(2):216–23. doi: 10.1097/MPA.0000000000001487
12. Strauss A, Birdsey M, Fritz S, Schwarz-Bundy BD, Bergmann F, Hackert T, et al. Intraductal Papillary Mucinous Neoplasms of the Pancreas: Radiological Predictors of Malignant Transformation and the Introduction of Bile Duct Dilation to Current Guidelines. Br J Radiol (2016) 89(1061):20150853. doi: 10.1259/bjr.20150853
13. Chiu SS, Lim JH, Lee WJ, Chang KT, Oh DK, Lee KT, et al. Intraductal Papillary Mucinous Tumour of the Pancreas: Differentiation of Malignancy and Benignancy by Ct. Clin Radiol (2006) 61(9):776–83. doi: 10.1016/j.crad.2006.04.008
14. Hwang DW, Jang JY, Lee SE, Lim CS, Lee KU, Kim SW. Clinicopathologic Analysis of Surgically Proven Intraductal Papillary Mucinous Neoplasms of the Pancreas in Snuh: A 15-Year Experience at a Single Academic Institution. Langenbecks Arch Surg (2012) 397(1):93–102. doi: 10.1007/s00423-010-0674-6
15. Yu S, Takasu N, Watanabe T, Fukumoto T, Okazaki S, Tezuka K, et al. Validation of the 2012 Fukuoka Consensus Guideline for Intraductal Papillary Mucinous Neoplasm of the Pancreas From a Single Institution Experience. Pancreas (2017) 46(7):936–42. doi: 10.1097/MPA.0000000000000874
16. Lee JE, Choi SY, Min JH, Yi BH, Lee MH, Kim SS, et al. Determining Malignant Potential of Intraductal Papillary Mucinous Neoplasm of the Pancreas: Ct Versus Mri by Using Revised 2017 International Consensus Guidelines. Radiology (2019) 293(1):134–43. doi: 10.1148/radiol.2019190144
17. Hwang JA, Choi SY, Lee JE, Kim SS, Lee S, Moon JY, et al. Pre-Operative Nomogram Predicting Malignant Potential in the Patients With Intraductal Papillary Mucinous Neoplasm of the Pancreas: Focused on Imaging Features Based on Revised International Guideline. Eur Radiol (2020) 30(7):3711–22. doi: 10.1007/s00330-020-06736-6
18. Chai L, Zhu N, Wang Q, Wang T, Chai W. Assessment of Malignancy Potential in Intraductal Papillary Mucinous Neoplasms of the Pancreas on Mdct. Acad Radiol (2021) 28(5):679–86. doi: 10.1016/j.acra.2020.03.042
19. Kim M, Mi Jang K, Kim SH, Doo Song K, Jeong WK, Kang TW, et al. Diagnostic Accuracy of Diffusion Restriction in Intraductal Papillary Mucinous Neoplasm of the Pancreas in Comparison With "High-Risk Stigmata" of the 2012 International Consensus Guidelines for Prediction of the Malignancy and Invasiveness. Acta Radiol (2017) 58(10):1157–66. doi: 10.1177/0284185116685921
20. Fang X, Liu F, Li J, Cao K, Wang T, Zhang H, et al. Computed Tomography Nomogram to Predict a High-Risk Intraductal Papillary Mucinous Neoplasm of the Pancreas. Abdom Radiol (NY) (2021) 46(11):5218–28. doi: 10.1007/s00261-021-03247-w
21. Yagi Y, Masuda A, Zen Y, Shiomi H, Toyama H, Sofue K, et al. Pancreatic Inflammation and Atrophy Are Not Associated With Pancreatic Cancer Concomitant With Intraductal Papillary Mucinous Neoplasm. Pancreatology (2018) 18(1):54–60. doi: 10.1016/j.pan.2017.12.007
22. Poiraud C, El Amrani M, Barbier L, Chiche L, Mabrut JY, Bachellier P, et al. Total Pancreatectomy for Presumed Intraductal Papillary Mucinous Neoplasms: A Multicentric Study of the French Surgical Association (Afc). Ann Surg (2018) 268(5):823–30. doi: 10.1097/SLA.0000000000002944
23. Jang KM, Kim SH, Min JH, Lee SJ, Kang TW, Lim S, et al. Value of Diffusion-Weighted Mri for Differentiating Malignant From Benign Intraductal Papillary Mucinous Neoplasms of the Pancreas. AJR Am J Roentgenol (2014) 203(5):992–1000. doi: 10.2214/AJR.13.11980
24. Djordjevic V, Grubor N, Kovac JD, Micev M, Milic N, Knezevic D, et al. Comparison of Preoperative Evaluation With the Pathological Report in Intraductal Papillary Mucinous Neoplasms: A Single-Center Experience. J Clin Med (2021) 10(4):678. doi: 10.3390/jcm10040678
25. Serafini S, Friziero A, Sperti C, Vallese L, Grego A, Piangerelli A, et al. The Ratio of C-Reactive Protein to Albumin Is an Independent Predictor of Malignant Intraductal Papillary Mucinous Neoplasms of the Pancreas. J Clin Med (2021) 10(10):2058. doi: 10.3390/jcm10102058
26. Watanabe Y, Niina Y, Nishihara K, Okayama T, Tamiya S, Nakano T. Neutrophil-To-Lymphocyte Ratio and Mural Nodule Height as Predictive Factors for Malignant Intraductal Papillary Mucinous Neoplasms. Acta Chir Belg (2018) 118(4):239–45. doi: 10.1080/00015458.2018.1427329
27. Karasawa E, Goldberg HI, Moss AA, Federle MP, London SS. CT pancreatogram in carcinoma of the Pancreas and Chronic Pancreatitis. Radiology (1983) 148(2):489–93. doi: 10.1148/radiology.148.2.6867347
28. Min JH, Kim YK, Kim SK, Kim H, Ahn S. Intraductal Papillary Mucinous Neoplasm of the Pancreas: Diagnostic Performance of the 2017 International Consensus Guidelines Using Ct and Mri. Eur Radiol (2021) 31(7):4774–84. doi: 10.1007/s00330-020-07583-1
29. Attiyeh MA, Fernández-Del Castillo C, Al Efishat M, Eaton AA, Gönen M, Batts R, et al. Development and Validation of a Multi-Institutional Preoperative Nomogram for Predicting Grade of Dysplasia in Intraductal Papillary Mucinous Neoplasms (Ipmns) of the Pancreas: A Report From the Pancreatic Surgery Consortium. Ann Surg (2018) 267(1):157–63. doi: 10.1097/SLA.0000000000002015
30. Kim HS, Song W, Choo W, Lee S, Han Y, Bassi C, et al. Development, Validation, and Comparison of a Nomogram Based on Radiologic Findings for Predicting Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas: An International Multicenter Study. J Hepatobiliary Pancreat Sci (2021). doi: 10.1002/jhbp.962
31. Kim JR, Jang JY, Kang MJ, Park T, Lee SY, Jung W, et al. Clinical Implication of Serum Carcinoembryonic Antigen and Carbohydrate Antigen 19-9 for the Prediction of Malignancy in Intraductal Papillary Mucinous Neoplasm of Pancreas. J Hepatobiliary Pancreat Sci (2015) 22(9):699–707. doi: 10.1002/jhbp.275
32. Yang KS, Ciprani D, O'Shea A, Liss AS, Yang R, Fletcher-Mercaldo S, et al. Extracellular Vesicle Analysis Allows for Identification of Invasive Ipmn. Gastroenterology (2021) 160(4):1345–58.e11. doi: 10.1053/j.gastro.2020.11.046
33. Zhuge X, Zhou H, Chen L, Chen H, Chen X, Guo C. The Association Between Serum Ferritin Levels and Malignant Intraductal Papillary Mucinous Neoplasms. BMC Cancer (2021) 21(1):1253. doi: 10.1186/s12885-021-08986-z
34. Lisotti A, Napoleon B, Facciorusso A, Cominardi A, Crinò SF, Brighi N, et al. Contrast-Enhanced EUS for the Characterization of Mural Nodules Within Pancreatic Cystic Neoplasms: Systematic Review and Meta-Analysis. Gastrointest Endosc (2021) 94(5):881–9.e5. doi: 10.1016/j.gie.2021.06.028
35. Crinò SF, Bernardoni L, Gabbrielli A, Capelli P, Salvia R, Rusev BC, et al. Beyond Pancreatic Cyst Epithelium: Evidence of Ovarian-Like Stroma in EUS-Guided Through-The-Needle Micro-Forceps Biopsy Specimens. Am J Gastroenterol (2018) 113(7):1059–60. doi: 10.1038/s41395-018-0124-6
36. Krishna SG, Hart PA, DeWitt JM, DiMaio CJ, Kongkam P, Napoleon B, et al. EUS-Guided Confocal Laser Endomicroscopy: Prediction of Dysplasia in Intraductal Papillary Mucinous Neoplasms (With Video). Gastrointest Endosc (2020) 91(3):551–63.e5. doi: 10.1016/j.gie.2019.09.014
37. Machicado JD, Chao WL, Carlyn DE, Pan TY, Poland S, Alexander VL, et al. High Performance in Risk Stratification of Intraductal Papillary Mucinousneoplasms By Confocal Laser Endomicroscopy Image Analysis With Convolutional Neural Networks (With Video). Gastrointest Endosc (2021) 94(1):78–87.e2. doi: 10.1016/j.gie.2020.12.054
Keywords: pancreatic atrophy, intraductal papillary mucinous neoplasms, pancreas, malignancy, invasive carcinoma
Citation: Lin T, Chen X, Liu J, Cao Y, Cui W, Wang Z, Wang C and Chen X (2022) MRI-Based Pancreatic Atrophy Is Associated With Malignancy or Invasive Carcinoma in Intraductal Papillary Mucinous Neoplasm. Front. Oncol. 12:894023. doi: 10.3389/fonc.2022.894023
Received: 11 March 2022; Accepted: 06 May 2022;
Published: 03 June 2022.
Edited by:
Marco Rengo, Sapienza University of Rome, ItalyReviewed by:
Giovanni Morana, ULSS2 Marca Trevigiana, ItalyStefano Francesco Crinò, University of Verona, Italy
Copyright © 2022 Lin, Chen, Liu, Cao, Cui, Wang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao Chen, chxwin@163.com; Cheng Wang, wangcheng940215@163.com
†These authors have contributed equally to this work
 Tingting Lin
Tingting Lin Xin Chen2†
Xin Chen2† Wenjing Cui
Wenjing Cui Cheng Wang
Cheng Wang Xiao Chen
Xiao Chen