- 1School of Medicine, Baylor College of Medicine, Houston, TX, United States
- 2Section of Ophthalmology, Department of Head and Neck Surgery, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 3Department of Pediatrics, Division of Cancer Medicine, Baylor College of Medicine, Houston, TX, United States
- 4Department of Ophthalmology & Visual Sciences, The University of Texas Medical Branch at Galveston, Galveston, TX, United States
- 5Department of Melanoma Medical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Purpose: Evaluate incidence of second primary malignancies (SPM) after non-acral cutaneous melanoma (NACM), acral lentiginous melanoma (ALM), mucosal melanoma (MM), and uveal melanoma (UM).
Patients and Methods: First primary NACM, ALM, MM, and UM cases diagnosed 2000-2016 were extracted from SEER. Seer*Stat was used to calculate excess absolute risks (EAR) and standardized incidence ratios (SIR) of SPMs relative to a matched cohort from the general population. P-value of 0.05 determined significance.
Results: Inclusion criteria was met by 109,385 patients with NACM, 2166 with ALM, 2498 with MM, and 6250 with UM. Increased incidence of malignancies occurred after NACM (SIR 1.51; 95%CI, 1.49-1.54), ALM (SIR 1.59; 95%CI, 1.40-1.81), MM (SIR 2.14; 95%CI, 1.85-2.45), and UM (SIR 1.24; 95%CI, 1.14-1.34) relative to the general population. Cutaneous melanoma occurred more frequently after NACM (SIR 9.54; 95%CI, 9.27-9.83), ALM (SIR 12.19; 95%CI, 9.70-15.14), MM (SIR 10.05; 95%CI, 7.18-13.68), and UM (SIR 2.91; 95%CI, 2.27-3.66). Patients with initial NACM (SIR 2.44; 95%CI, 1.64-3.51) and UM (SIR 44.34; 95%CI, 29.91-63.29) demonstrated increased incidence of eye and orbit melanoma. Renal malignancies occurred more frequently after NACM (SIR 1.24; 95%CI, 1.11-1.38), MM (SIR 3.54; 95%CI, 1.62-6.72) and UM (SIR 1.68; 95%CI, 1.09-2.48). Increased incidence of thyroid malignancies was observed after NACM (SIR 1.83; 95%CI, 1.61-2.06), ALM (SIR 3.74; 95%CI, 1.71-7.11), MM (SIR 4.40; 95%CI, 1.77-9.06), and UM (SIR 3.79; 95%CI, 2.52-5.47). Increased incidence of lymphoma was observed after NACM (SIR 1.20; 95%CI, 1.09-1.31) and ALM (SIR 2.06; 95%CI, 1.13-3.46).
Conclusion: Patients with NACM, ALM, MM, and UM have increased incidence of SPMs compared to that expected from the general population. Each of these melanoma subtypes had increased occurrence of cutaneous melanoma and thyroid cancer; some, but not all, had increased occurrence of renal malignancies, eye and orbit melanoma, and lymphoma.
Background
Malignant melanoma, a serious and devastating disease, originates from melanocytes within the non-glabrous skin (non-acral cutaneous melanoma), palm and sole glabrous skin (acral lentiginous melanoma), mucosal membranes (mucosal melanoma), and the uvea (uveal melanoma) (1–3). Non-acral cutaneous melanoma (NACM) represents the most common subtype of melanoma, accounting for close to 90% of diagnoses; acral lentiginous melanoma (ALM), mucosal melanoma (MM), and uveal melanoma (UM) largely comprise the remainder of cases (4–6). Despite a shared cell origin, these subtypes differ greatly by genetic composition (1, 7), treatment response (8), and clinical outcomes (4, 6).
NACM generally portends a better prognosis than ALM, MM or UM, with 5-year survival rates of 91.3%, 80.3%, 34.0%, and 78.4% respectively (4, 6). If complete remission is attained, patient care becomes increasingly focused on surveillance for recurrences and management of cancer sequalae; second primary malignancies (SPMs) embody one such sequela. Although incidence of SPMs has been investigated for patients with cutaneous melanoma (CM; encompasses NACM and ALM) (9–11) and UM (12, 13), limited literature exists on SPMs specific to MM (14) and ALM (15). Prior study of SPMs associated with mucosal melanoma (14) are limited solely to those arising from the sinonasal cavity and prior study of SPMs associated with ALM (15) focuses on an exclusively Korean population; both lack site-specific SPM risk investigation. With gaps in current literature, consensus cancer guidelines provided by organizations such as National Comprehensive Cancer Network (NCCN) (16–18), Cancer Care Ontario (CCO) (19), Canadian Medical Association (CMA), and European Society for Medical Oncology (ESMO) (20) provide either no or very limited discussion on SPM risk and follow-up after these malignancies.
In order to address this literature gap, we conducted a retrospective analysis of the SEER database to evaluate if patients with NACM, ALM, MM, and UM demonstrate increased incidence for SPMs compared to the general population in the contemporary era (2000-2016). We performed additional analysis to identify specific sites and latency periods with elevated risk for secondary malignancies. We conduct, to the best of our knowledge, the first investigation of site-specific SPM risk after MM and ALM. National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) registries (21), a national population-based cancer database, has been used and validated for such analyses in the past (9–12, 22).
Patients and Methods
Data Source
Cases of melanoma were extracted from the SEER database, which is comprised of up to 21 cancer registries that geographically account for approximately 36.7% of the US population (21). The specific dataset used for this study, “Incidence - SEER 18 Regs excluding AK Research Data, Nov 2018 Sub (2000-2016)”, contained data from 18 registries with cases diagnosed between 2000 and 2016. The SEER program tracks incidence of new tumors and documents demographic, treatment, tumor, and survival data; however, it does not include behavioral risk factors (e.g. smoking, physical inactivity) and comorbid diseases. Institutional review board approval was not required for this study, as it utilized only deidentified data with permission from NCI.
Data Collection
Patients diagnosed with NACM, ALM, MM, and UM between 2000-2016 were included in the study; cases that were not first primary malignancies, were diagnosed by death certificate, were diagnosed by autopsy record, or were of unknown age were excluded from analysis.
Cases of NACM were identified using International Classification of Diseases for Oncology third edition (ICD-O-3) morphological codes 8721/3-8743/3; 8745/3-8790/3 (malignant melanoma excluding malignant melanoma, NOS & acral lentiginous melanoma) and topographical codes C44.0-44.9 (skin). ALM was identified using morphological code 8744/3 (acral lentiginous melanoma) and topographical codes C44.6-C44.7 (skin of upper limb, shoulder, lower limb, and hip). MM cases were identified using morphological codes 8720/3-8790/3 (melanoma) and topographical codes C00.0–C06.9 (lip, tongue, gum, palate, mouth); C09.0–C14.8 (tonsil, oropharynx, nasopharynx, pyriform sinus, hypopharynx); C15.0–C16.9 (esophagus, stomach); C19.9–C21.8 (rectosigmoid junction, rectum, anus/anal canal); C30.0 (nasal cavity); C31.0–C31.9 (accessory sinuses); C51.0–C51.9 (vulva); C52.9–C53.9 (vagina, cervix uteri); C60.0–C63.9 (male genital organs); C64.9–C68.9 (urinary tract). Lastly, UM was identified using morphological codes 8720-8790 (melanoma) and topographical codes C69.2 (retina); C69.3 (choroid); C69.4 (ciliary body, iris). “Retinal” melanomas (0.9%; 56/6250) were included as they most likely represent misclassification of uveal melanoma, a phenomenon described in previous studies (12, 23). ICD-O-3 codes used to identify NACM (6), ALM (6), MM (4), and UM (23) were consistent with prior studies investigating these malignancies. Patient demographics collected included age at diagnosis, race, and sex. Tumor data included laterality, histology, and site of origin.
Statistical Analysis
Demographic and tumor data was tabulated. The multiple primary standardized incidence ratio (MP-SIR) algorithm of the Seer*stat program (version 8.3.6.1) was used to obtain standardized incidence ratios (SIR) and excess absolute risk (EAR) for second primary malignancies in patients with NACM, ALM, MM, and UM compared to a reference group representative of the general population, with similar sex, race (white/unknown, black, other) age-group (5-year interval), and calendar year of diagnosis (5-year interval). The algorithm was then further used to identify specific latency periods in which there was increased incidence of SPMs relative to the reference population. The authors (AL, SP, DSG) examined the site-specific analysis to identify trends across melanoma subtypes.
Analysis was limited to second malignancies only (early exit at next malignancy) to isolate relationship of subsequent malignancies with the first primary. Only malignant neoplasms diagnosed greater than two months after the melanoma diagnosis were considered to be second primaries, in order to distinguish them from concurrent malignancies discovered during screening. The reference population linked to the SEER database is comprised of Census Bureau data, through partnership with the National Center for Health Statistics (https://seer.cancer.gov/popdata/). An alpha level of significance of 0.05 was used for the study, and EAR was calculated per 10,000 individuals. IBM Statistical Product and Service Solutions (SPSS) version 26 and Microsoft Excel version 16.38 were used to conduct descriptive analysis and generate charts.
Results
Baseline Characteristics
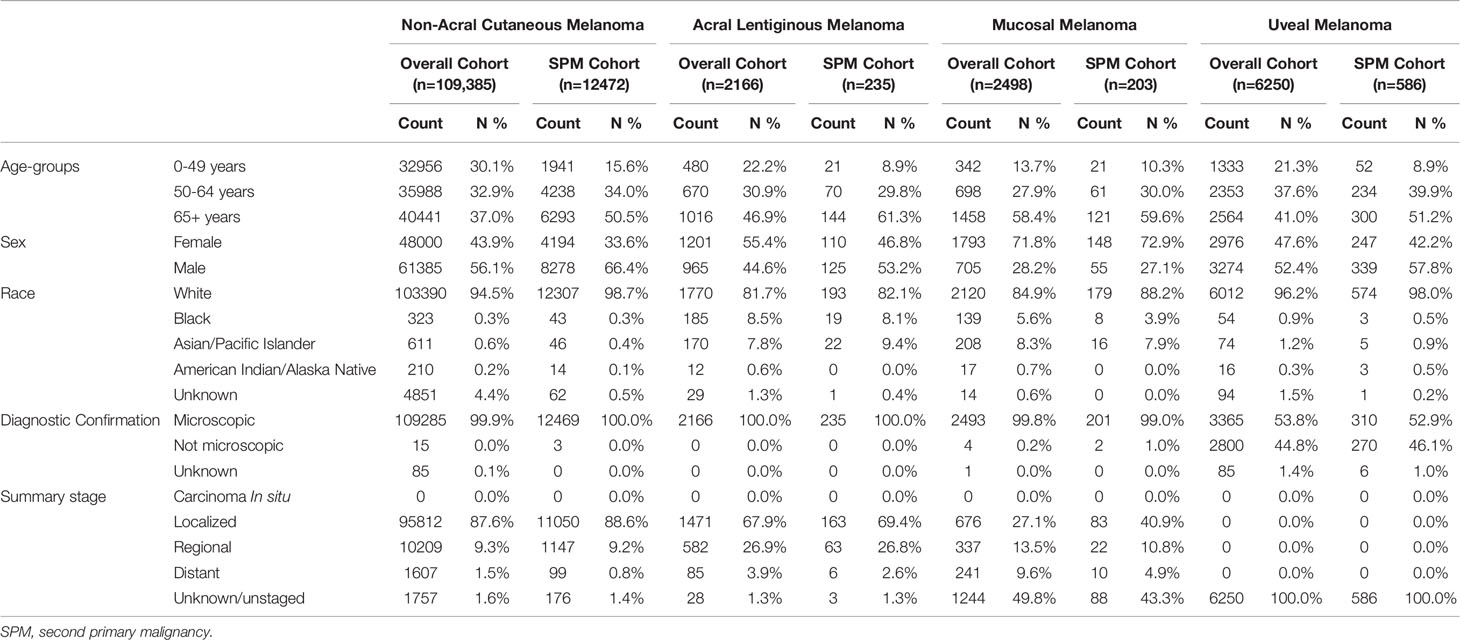
Inclusion criteria was met by 109,385 patients with NACM, 2166 patients with ALM, 2498 patients with MM, and 6250 patients with UM for a total of 120,299 patients. The median (+/- SD) follow-up period for patients with NACM was 5.6 (+/- 4.7) years, with ALM was 4.3 (+/- 4.5) years, with MM was 1.7 (+/- 3.6) years, and with UM was 4.8 (+/- 4.4) years. During this period 11.4% (12472/109,385) of NACM patients, 10.8% (235/2166) of ALM patients, 8.1% (203/2498) of MM patients, and 9.4% (586/6250) of UM patients developed SPMs.
Most patients with initial NACM (56.1%; 61,385/109,385) and UM (52.4%; 3274/6250) were male, whereas most patients with initial ALM (55.4%; 1201/2166) and MM (71.8%; 1793/2498) were female. Majority of patients with NACM (94.5%;103,390/109,385), ALM (81.7%; 1770/2166), MM (84.9%; 2120/2498) and UM (96.2%; 6012/6250) were white. Almost all diagnoses of initial NACM (99.9%; 109,285/109,385), ALM (100%; 2166/2166), and MM (99.8%; 2493/2498) were microscopically confirmed whereas only 53.8% (3365/6250) of UM cases were microscopically confirmed. Additional patient characteristics are displayed in Table 1.
SPM Incidence
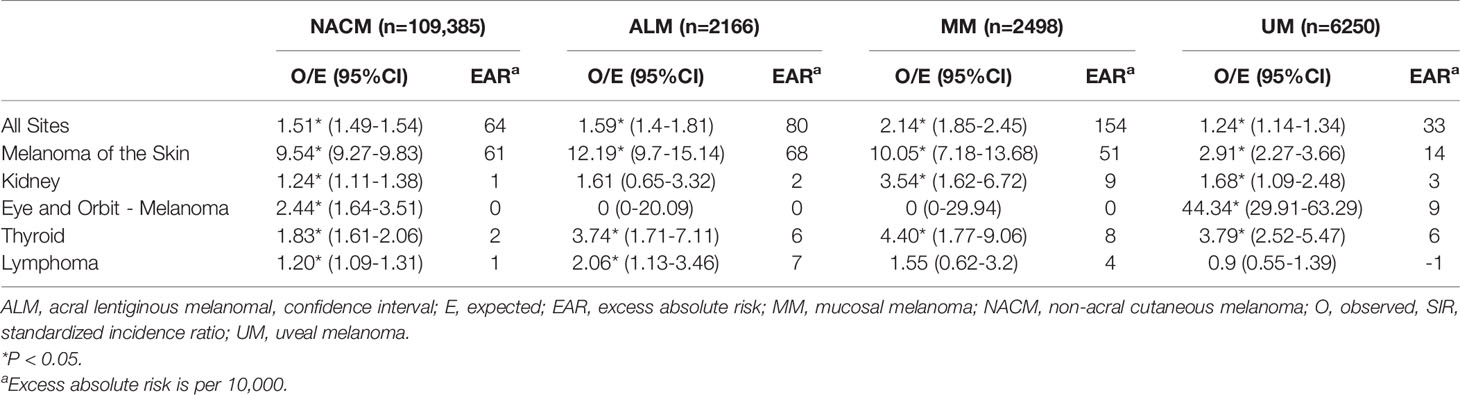
Relative to the general population, an increased incidence of new malignancies was observed in patients with initial NACM (SIR 1.51; 95% CI, 1.49 to 1.54; EAR 64.46), ALM (SIR 1.59; 95% CI, 1.40 to 1.81; EAR 79.56), MM (SIR 2.14; 95% CI, 1.85 to 2.45; EAR 153.59), and UM (SIR 1.24; 95% CI, 1.14 to 1.34; EAR 33.04). Notably, increased incidence of secondary CM, eye and orbit melanoma, kidney cancer, thyroid cancer, and lymphoma were observed across some melanoma subtypes (Table 2 and Figure 1).

Figure 1 Standardized incidence ratios of secondary malignancies grouped by category. Standardized incidence ratios of overall secondary malignancies (A), secondary cutaneous melanoma (B), secondary eye and orbit melanoma (C), secondary kidney malignancies (D), secondary thyroid malignancies (E), and secondary lymphoma (F) following first primary melanomas. ALM, acral lentiginous melanoma; MM, mucosal melanoma, NACM, non-acral cutaneous melanoma; UM, uveal melanoma. *p<0.05.
CM occurred more frequently in patients with initial NACM (SIR 9.54; 95% CI, 9.27 to 9.83; EAR 60.72), ALM (SIR 12.19; 95% CI, 9.70 to 15.14; EAR 68.41), MM (SIR 10.05; 95% CI, 7.18 to 13.68; EAR 51.23), and UM (SIR 2.91; 95% CI, 2.27 to 3.66; EAR 13.75) than expected from the general population. On the other hand, only patients with initial NACM (SIR 2.44; 95% CI, 1.64 to 3.51; EAR 0.26) and UM (SIR 44.34; 95% CI, 29.91 to 63.29; EAR 8.54) demonstrated increased incidence of eye and orbit melanoma, whereas patients with ALM (SIR 0.00; 95% CI, 0.00 to 20.09; EAR -0.17) and MM (SIR 0.00; 95% CI, 0.00 to 29.94; EAR -0.18) demonstrated no significant difference from the reference population. Renal malignancies were noted to occur more frequently in patients with NACM (SIR 1.24; 95% CI, 1.11 to 1.38; EAR 0.96), MM (SIR 3.54; 95% CI, 1.62 to 6.72; EAR 9.19) and UM (SIR 1.68; 95% CI, 1.09 to 2.48; EAR 2.95), but not ALM (SIR 1.61; 95% CI, 0.65 to 3.32; EAR 2.41). Increased incidence of thyroid malignancies was observed in patients with initial NACM (SIR 1.83; 95% CI, 1.61 to 2.06; EAR 1.79), ALM (SIR 3.74; 95% CI, 1.71 to 7.11; EAR 5.99), MM (SIR 4.40; 95% CI, 1.77 to 9.06; EAR 7.69), and UM (SIR 3.79; 95% CI, 2.52 to 5.47; EAR 6.00). Lastly, increased incidence of lymphoma was observed in patients with initial NACM (SIR 1.20; 95% CI, 1.09 to 1.31; EAR 1.17) and ALM (SIR 2.06; 95% CI, 1.13 to 3.46; EAR 6.55) but not MM (SIR 1.55; 95% CI, 0.62 to 3.20; EAR 3.55) or UM (SIR 0.90; 95% CI, 0.55 to 1.39; EAR -0.63).
SPM Latency Analysis
Patients with NACM demonstrated elevated incidence of overall SPMs during the first year (2-11 months) following diagnosis (SIR 2.12; 95% CI 2.03 to 2.21), 1-5 years following diagnosis (SIR 1.57; 95% CI 1.53 to 1.61), 5-10 years following diagnosis (SIR 1.30; 95% CI 1.25 to 1.34), and greater than 10 years following diagnosis (SIR 1.21; 95% CI 1.15 to 1.28). Similarly, patients with MM had increased incidence of overall SPMs during the first year (2-11 months) following diagnosis (SIR 2.38; 95% CI 1.81 to 3.08), 1-5 years following diagnosis (SIR 2.12; 95% CI 1.72 to 2.58), 5-10 years following diagnosis (SIR 1.81; 95% CI 1.24 to 2.56), and greater than 10 years following diagnosis (SIR 2.24; 95% CI 1.23 to 3.76). In contrast, those with ALM only had increased incidence of overall SPMs the first year following diagnosis (SIR 1.98; 95% CI 1.45 to 2.66) and 1-5 years following diagnosis (SIR 1.72; 95% CI 1.43 to 2.06). Those with UM demonstrated elevated incidence of overall SPMs during the first year following diagnosis (SIR 1.53; 95% CI 1.24 to 1.86; EAR 69.12), 1-5 years following diagnosis (SIR 1.21; 95% CI 1.07 to 1.37; EAR 28.62), and 5-10 years following diagnosis (SIR 1.20; 95% CI 1.02 to 1.39; EAR 27.78). High-risk latency periods further differed by SPM types (Table 3 and Supplementary Material).

Table 3 Risk of second primary malignancy distributed by time from diagnosis of first primary malignancy.
Discussion
Using a national cancer database, we analyzed 120,299 patients with various melanoma subtypes and found an elevated incidence of SPMs relative to the general population. Notably, all four melanoma subtypes (NACM, ALM, MM, UM) demonstrated increased risk of secondary CM and thyroid cancer, and some but not all melanoma subtypes demonstrated increased risk for secondary renal malignancies (NACM, MM, UM), eye and orbit melanoma (NACM, UM), and lymphoma (NACM, ALM).
A biologic rationale exists for the findings in our study. CM and thyroid cancers commonly harbor oncogenic mutations of the mitogen-activated protein kinase (MAPK) pathway (24–29). Renal cancers share immunogenicity and BAP1 aberrations with CM and UM (30–35). Lymphomas and melanomas are associated with decreased immune surveillance (36–43).
Although historic and smaller retrospective analyses of the SEER database have examined SPMs following CM (9, 10) and UM (12), herein we provide, to the best of our knowledge, the first investigation of site-specific SPM risk after MM and ALM. Moreover, through analysis of UM in a larger and more contemporary cohort, we highlight increased incidence of secondary thyroid malignancies, a finding undetected in prior investigation (12). Bradford, et al. (9) and Spanogle, et al. (10) investigated incidence of SPMs following CM in various subsets of the SEER database and both found increased incidence of secondary CM, eye and orbit melanoma, thyroid cancer, renal cancer, and lymphoma. Similarly, Vakharia, et al. (11) in their investigation of secondary malignancies excluding CM demonstrated increased risk for these sites. Their findings (9–11) were consistent with our findings of SPMs following NACM. Laíns, et al. (12) investigated risk of second primary malignancies following UM and found increased incidence for secondary CM, eye and orbit melanoma, and renal cancer but not a significant increase in thyroid cancer. However, the study (12) showed a strong trend of increased thyroid cancer (SIR 2.06, 95% CI 0.99 to 3.78) in a cohort of 3976 patients, which with increased power may have captured a significant result similar to our study.
Despite a growing body of literature on SPMs, national consensus guidelines such as NCCN (16–18) (US), Canada CCO (19) (Canada), CMA (Canada) and ESMO (20) (Europe) sparsely address secondary malignancies in their follow-up recommendations. Canadian (19) and European (20) guidelines discuss only an increased risk for secondary cutaneous malignancies after initial CM and the importance of long-term dermatological surveillance; these guidelines lack SPM follow-up recommendations specific to ALM, MM, and UM. American consensus guidelines provide slightly more insight on non-cutaneous SPMs following CM by discussing the role of genetic testing in determining SPM risk and by providing guidance on when to consider such testing (16). However, American guidelines do not remark on the increased incidence of lymphoma or thyroid cancer (16). Moreover, these guidelines state that CM is not associated with an increased risk for UM (17).
Indeed, developing follow-up recommendations poses a challenge as cost, clinical benefit, and burden of increased health-care visits must all be balanced. Nonetheless, increased awareness of the associations studied herein are paramount to guiding appropriate clinician judgement when caring for melanoma patients; discussion of up-to-date evidence in national guidelines can improve patient care and long-term health outcomes. With a more appropriate index of suspicion, lesions (e.g. renal cyst, thyroid nodule) and atypical findings discovered during diagnostic or surveillance imaging that may otherwise have been dismissed as benign may instead be deemed to warrant additional follow-up. Furthermore, symptoms concerning for an associated SPM (e.g. visual flashers and floaters) can be interpreted more appropriately and potentially lead to earlier diagnosis. The high-risk latency periods identified by this study may provide additional clinical insight when deciding further management for patients presenting with these signs or symptoms.
The authors propose that cost effective screening such as total body skin exams be recommended for patients with all subtypes (NACM, ALM, MM, UM) of melanoma; prior studies (44, 45) support the economic efficiency of targeted screening strategies in high-risk groups. The authors suggest that patients with NACM should receive routine complete eye exams (including dilated fundus exam) at least as often as recommend for asymptomatic adults without risk factors for ocular disease by American Academy of Ophthalmology (AAO) guidelines (46): every 5 to 10 years when less than 40 years of age, every 2 to 4 years when between 40 and 54 years of age, every 1 to 3 years when between 55 and 64 years of age, and every 1 to 2 years when 65 or more years of age. Surveillance for SPM in patients undergoing screening measures versus those who do not undergo these screening exams may further elucidate the role and feasibility of monitoring for the development of cancer in these patients. Moreover, it would be prudent to identify high-risk groups and factors through prediction models for subsequent melanoma, such as those described by Cust et al. (47), to help guide effective recommendations.
Limitations in the Study Design
An important limitation of the SEER database and our study is the possibility of miscoding a recurrence as a second primary malignancy when pathologic evaluation is unavailable and tumor location is identical. This most directly impacts the calculation of secondary eye and orbit melanoma after initial UM (6.67% cases without microscopic confirmation), as UM is largely diagnosed clinically through examination and imaging rather than pathologically (48); indeed, this finding is more likely representative of recurrences rather than true SPMs. Another important limitation is the inability of Seer.Stat’s MP-SIR algorithm to analyze site-specific secondary malignancy incidence beyond those preset in the software. As a result, we were unable to provide analysis on incidence of NACM and ALM as secondary malignancies, and instead had them grouped as secondary CM. Other limitations include other possible miscoding and inability to account for variables not included within the database. Additionally, patients that moved to a geographical area not covered by SEER could be lost to follow-up leading to underreporting. Despite these limitations, however, the national database has been validated for SPM analyses (9–12, 22).
Conclusions
We found patients with NACM, ALM, MM, and UM to have increased incidence of SPMs compared to that expected from the general population. Each of these melanoma subtypes had increased occurrence of secondary CM and thyroid cancer; some, but not all, had increased occurrence of secondary renal malignancies, eye and orbit melanoma, and lymphoma. These patients may benefit from cost-effective screening methods such as full body skin exams. Patients with NACM should, at a minimum, receive age-appropriate comprehensive eye screening per national guidelines. Increased awareness of these associations is prudent to guiding clinical follow-up and additional studies are necessary to identify best-practice screening guidelines.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Conception: SP. Study design: AL, DG, and SP. Statistical analysis: AL. Data interpretation: AL, DG, and SP. Manuscript preparation: AL, DG, and SP. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.853076/full#supplementary-material
References
1. Bastian BC. The Molecular Pathology of Melanoma: An Integrated Taxonomy of Melanocytic Neoplasia. Annu Rev Pathol Mech Dis (2014) 9(1):239–71. doi: 10.1146/annurev-pathol-012513-104658
2. Jovanovic P, Mihajlovic M, Djordjevic-Jocic J, Vlajkovic S, Cekic S, Stefanovic V. Ocular Melanoma: An Overview of the Current Status. Int J Clin Exp Pathol (2013) 6(7):1230–44.
3. Mihajlovic M, Vlajkovic S, Jovanovic P, Stefanovic V. Primary Mucosal Melanomas: A Comprehensive Review. Int J Clin Exp Pathol (2012) 5(8):739–53.
4. Bishop KD, Olszewski AJ. Epidemiology and Survival Outcomes of Ocular and Mucosal Melanomas: A Population-Based Analysis. Int J Cancer (2014) 134(12):2961–71. doi: 10.1002/ijc.28625
5. McLaughlin CC, Wu XC, Jemal A, Martin HJ, Roche LM, Chen VW. Incidence of Noncutaneous Melanomas in the U.S. Cancer (2005) 103(5):1000–7. doi: 10.1002/cncr.20866
6. Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral Lentiginous Melanoma: Incidence and Survival Patterns in the United States, 1986-2005. Arch Dermatol (2009) 145(4):427–34. doi: 10.1001/archdermatol.2008.609
7. Curtin JA, Busam K, Pinkel D, Bastian BC. Somatic Activation of KIT in Distinct Subtypes of Melanoma. J Clin Oncol (2006) 24(26):4340–6. doi: 10.1200/JCO.2006.06.2984
8. Shoushtari AN, Munhoz RR, Kuk D, Ott PA, Johnson DB, Tsai KK, et al. The Efficacy of Anti-PD-1 Agents in Acral and Mucosal Melanoma. Cancer (2016) 122(21):3354–62. doi: 10.1002/cncr.30259
9. Bradford PT, Freedman DM, Goldstein AM, Tucker MA. Increased Risk of Second Primary Cancers After a Diagnosis of Melanoma. Arch Dermatol (2010) 146(3):265–72. doi: 10.1001/archdermatol.2010.2
10. Spanogle JP, Clarke CA, Aroner S, Swetter SM. Risk of Second Primary Malignancies Following Cutaneous Melanoma Diagnosis: A Population-Based Study. J Am Acad Dermatol (2010) 62(5):757–67. doi: 10.1016/j.jaad.2009.07.039
11. Vakharia PP, Kelm RC, Orrell KA, Patel KR, Singam V, Ali Y, et al. Risks for Noncutaneous Second Primary Malignancy in Cutaneous Malignant Melanoma Survivors: An Analysis of Data From the Surveillance, Epidemiology, and End Results (SEER) Program. Int J Dermatol (2020) 59(4):463–8. doi: 10.1111/ijd.14781
12. Laíns I, Bartosch C, Mondim V, Healy B, Kim IK, Husain D, et al. Second Primary Neoplasms in Patients With Uveal Melanoma: A SEER Database Analysis. Am J Ophthalmol (2016) 165:54–64. doi: 10.1016/j.ajo.2016.02.022
13. Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Second Primary Cancers After Enrollment in the COMS Trials for Treatment of Choroidal Melanoma: COMS Report No. 25. Arch Ophthalmol (2005) 123(5):601–4. doi: 10.1001/archopht.123.5.601
14. Ganti A, Plitt MA, Kuan EC, Kuhar HN, Batra PS, Tajudeen BA. Risk of Second Primary Malignancy in Patients With Sinonasal Tumors: A Population-Based Cohort Study. Int Forum Allergy Rhinol (2018) 8(6):756–62. doi: 10.1002/ALR.22092
15. Bae SH, Seon HJ, Choi YD, Shim HJ, Lee JB, Yun SJ. Other Primary Systemic Cancers in Patients With Melanoma: Analysis of Balanced Acral and Nonacral Melanomas. J Am Acad Dermatol (2016) 74(2):333–40. doi: 10.1016/J.JAAD.2015.09.047
16. NCCN Clinical Practice Guidelines in Oncology. Cutaneous Melanoma (Version 3.2020) Plymouth Meeting, PA: National Comprehensive Cancer Network (2020).
17. NCCN Clinical Practice Guidelines in Oncology. Uveal Melanoma (Version 1.2020) Plymouth Meeting, PA: National Comprehensive Cancer Network (2020).
18. NCCN Clinical Practice Guidelines in Oncology. Head and Neck Cancers (Version 2.2020) Plymouth Meeting, PA: National Comprehensive Cancer Network (2020).
19. Rajagopal S, Souter LH, Baetz T, McWhirter E, Knight G, Rosen CF, et al. Follow-Up of Patients With Cutaneous Melanoma Who Were Treated With Curative Intent. Toronto (ON): Cancer Care Ontario (2015).
20. Michielin O, Van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U, Committee G. Cutaneous Melanoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up †. (2019) 30(12):1884–901. doi: 10.1093/annonc/mdz411
21. Surveillance, Epidemiology, and End Results (SEER) Program. Available at: www.seer.cancer.gov.
22. Vasudevan V, Cheung MC, Yang R, Zhuge Y, Fischer AC, Koniaris LG, et al. Pediatric Solid Tumors and Second Malignancies: Characteristics and Survival Outcomes. J Surg Res (2010) 160(2):184–9. doi: 10.1016/j.jss.2009.05.030
23. Aronow ME, Topham AK, Singh AD. Uveal Melanoma: 5-Year Update on Incidence, Treatment, and Survival (SEER 1973-2013). Ocul Oncol Pathol (2018) 4(3):145–51. doi: 10.1159/000480640
24. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF Gene in Human Cancer. Nat 2002 4176892 (2002) 417(6892):949–54. doi: 10.1038/nature00766
25. Flaherty KT, McArthur G. BRAF, a Target in Melanoma. Cancer (2010) 116(21):4902–13. doi: 10.1002/CNCR.25261
26. Xing M. Molecular Pathogenesis and Mechanisms of Thyroid Cancer. Nat Rev Cancer 2013 133 (2013) 13(3):184–99. doi: 10.1038/nrc3431
27. Pozdeyev N, Lund G, McDermott MT. Molecular Pathogenesis of Thyroid Cancer and Oncogenes in Thyroid Cancer. Thyroid Cancer (2016) 2016:17–30. doi: 10.1007/978-1-4939-3314-3_3
28. Xing M. BRAF Mutation in Thyroid Cancer. Endocr Relat Cancer (2005) 12(2):245–62. doi: 10.1677/ERC.1.0978
29. Kim SJ, Lee J, Soh EY. The Clinical Significance of the BRAF Mutation in Patients With Papillary Thyroid Cancer. J Endocr Surg (2017) 17(4):175–83. doi: 10.16956/JES.2017.17.4.175
30. Foretová L, Navrátilová M, Svoboda M, Házová J, Vašíčková P, Sťahlová EH, et al. BAP1 Syndrome - Predisposition to Malignant Mesothelioma, Skin and Uveal Melanoma, Renal and Other Cancers. Klin Onkol (2019) 32(Supplementum2):2S118–22. doi: 10.14735/AMKO2019S118
31. Shao YF, Debenedictis M, Yeaney G, Singh AD. Germ Line BAP1 Mutation in Patients With Uveal Melanoma and Renal Cell Carcinoma. Ocul Oncol Pathol (2021) 7(5):340–5. doi: 10.1159/000516695
32. Walpole S, Pritchard AL, Cebulla CM, Pilarski R, Stautberg M, Davidorf FH, et al. Comprehensive Study of the Clinical Phenotype of Germline BAP1 Variant-Carrying Families Worldwide. JNCI J Natl Cancer Inst (2018) 110(12):1328–41. doi: 10.1093/JNCI/DJY171
33. Bertolotto C, Lesueur F, Giuliano S, Strub T, de Lichy M, Bille K, et al. A SUMOylation-Defective MITF Germline Mutation Predisposes to Melanoma and Renal Carcinoma. Nature (2011) 480(7375):94–8. doi: 10.1038/nature10539
34. Laitman Y, Newberg J, Molho RB, Jin DX, Friedman E. The Spectrum of Tumors Harboring BAP1 Gene Alterations. Cancer Genet (2021) 256-257:31–5. doi: 10.1016/J.CANCERGEN.2021.03.007
35. Louie BH, Kurzrock R. BAP1: Not Just a BRCA1-Associated Protein. Cancer Treat Rev (2020) 90. doi: 10.1016/J.CTRV.2020.102091
36. Lund I, Hernandez-Ilizaliturri FJ, Torka P. Frequency and Timing of Other Primary Cancers in Patients With Chronic Lymphocytic Leukemia (CLL): A 17-Year Longitudinal Study. Leuk Lymphoma (2022) 13:1–10. doi: 10.1080/10428194.2021.2012662
37. Jhunjhunwala S, Hammer C, Delamarre L. Antigen Presentation in Cancer: Insights Into Tumour Immunogenicity and Immune Evasion. Nat Rev Cancer (2021) 21(5):298–312. doi: 10.1038/S41568-021-00339-Z
38. Vinay DS, Ryan EP, Pawelec G, Talib WH, Stagg J, Elkord E, et al. Immune Evasion in Cancer: Mechanistic Basis and Therapeutic Strategies. Semin Cancer Biol (2015) 35(Suppl):S185–98. doi: 10.1016/J.SEMCANCER.2015.03.004
39. Eddy K, Chen S. Overcoming Immune Evasion in Melanoma. Int J Mol Sci (2020) 21(23):1–48. doi: 10.3390/IJMS21238984
40. Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell (2017) 168(4):707–23. doi: 10.1016/J.CELL.2017.01.017
41. Liu WR, Shipp MA. Signaling Pathways and Immune Evasion Mechanisms in Classical Hodgkin Lymphoma. Blood (2017) 130(21):2265–70. doi: 10.1182/BLOOD-2017-06-781989
42. Upadhyay R, Hammerich L, Peng P, Brown B, Merad M, Brody JD. Lymphoma: Immune Evasion Strategies. Cancers (Basel) (2015) 7(2):736–62. doi: 10.3390/CANCERS7020736
43. Menter T, Tzankov A. Mechanisms of Immune Evasion and Immune Modulation by Lymphoma Cells. Front Oncol (2018) 8:54. doi: 10.3389/FONC.2018.00054
44. Matsumoto M, Secrest A, Anderson A, Saul MI, Ho J, Kirkwood JM, et al. Estimating the Cost of Skin Cancer Detection by Dermatology Providers in a Large Health Care System. J Am Acad Dermatol (2018) 78(4):701–9.e1. doi: 10.1016/j.jaad.2017.11.033
45. Freedberg KA, Geller AC, Miller DR, Lew RA, Koh HK. Screening for Malignant Melanoma: A Cost-Effectiveness Analysis. J Am Acad Dermatol (1999) 41(5 I):738–45. doi: 10.1016/S0190-9622(99)70010-1
46. Feder RS, Olsen TW, Prum BE Jr, Summers CG, Olson RJ, Williams RD, et al. Comprehensive Adult Medical Eye Evaluation. Ophthalmology (2016) 123(1):P209–36. doi: 10.1016/j.ophtha.2015.10.047
47. Cust AE, Badcock C, Smith J, Thomas NE, Haydu LE, Armstrong BK, et al. A Risk Prediction Model for Development of Subsequent Primary Melanoma in a Population-Based Cohort. Br J Dermatol (2020) 182(5):1148. doi: 10.1111/BJD.18524
Keywords: second primary malignancies, melanoma, melanoma subtypes, uveal melanoma, non-acral cutaneous melanoma, acral lentiginous melanoma, mucosal melanoma, standardized incidence ratios
Citation: Loya A, Gombos DS and Patel SP (2022) Second Primary Malignancies in Patients With Melanoma Subtypes: Analysis of 120,299 Patients From the SEER Database (2000-2016). Front. Oncol. 12:853076. doi: 10.3389/fonc.2022.853076
Received: 12 January 2022; Accepted: 21 February 2022;
Published: 18 March 2022.
Edited by:
James L. Fisher, The Ohio State University, United StatesReviewed by:
Paolo Del Fiore, Istituto Oncologico Veneto (IRCCS), ItalyAmarinder Singh Thind, University of Wollongong, Australia
Copyright © 2022 Loya, Gombos and Patel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sapna P. Patel, sppatel@mdanderson.org
 Asad Loya
Asad Loya Dan S. Gombos2,3,4
Dan S. Gombos2,3,4 Sapna P. Patel
Sapna P. Patel