- 1Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai, China
- 2Department of Medical Oncology, Shanghai Medical College, Fudan University, Shanghai, China
- 3Institute of Pathology, Fudan University, Shanghai, China
- 4Department of Medical Oncology, Fudan University Shanghai Cancer Center, Shanghai, China
- 5Wuxi Cancer Institute, Affiliated Hospital of Jiangnan University, Wuxi, China
Background: Cumulative evidence in colorectal cancer (CRC) suggests that patients with human epidermal growth factor receptor 2 (HER2) overexpression or amplification can benefit from anti-HER2 therapy. The purpose of our study was to evaluate HER2 status and its correlation with clinicopathological characteristics and survival according to currently utilized HER2 diagnostic criteria in a large cohort of Chinese CRC patients.
Methods: HER2 protein expression was tested by immunohistochemistry (IHC) in formalin-fixed, paraffin-embedded (FFPE) samples from 4,836 CRC patients in our institution. Breast cancer (BC) and gastroesophageal adenocarcinoma (GEA) criteria, as well as the HERACLES criteria, were used for the determination of HER2 status. Dual-color silver-enhanced in situ hybridization (DSISH) was performed in all IHC 2+~3+ cases determined by BC/GEA criteria.
Results: The HER2 expression rate of IHC (1+~3+) was 7.01% (339/4,836) and 6.02% (291/4,836) in CRCs based on the BC/GEA criteria and the HERACLES criteria, respectively, while combined DSISH results in the HER2 amplification/overexpression ratio of 3.39% (164/4,836) in our cohort. HER2 expression detected by IHC was positively correlated with the female gender, whereas the HER2 overexpression/amplification showed no correlation with any clinicopathological parameter. In addition, no significant correlation was found between HER2 statuses and either disease-free survival or overall survival regardless of the evaluation criterion used. However, patients with HER2 1+ CRC showed a tendency of having the shortest overall survival as compared with any other group of patients according to the HERACLES criteria, and this trend has always existed in the rectal location, T3 stage, and TNM stage II, medium differentiation, and perineural invasion stratified group. Furthermore, the HER2 protein expression was significantly negatively correlated with RAS/BRAF mutations according to the HERACLES criteria.
Conclusion: To our knowledge, this is the largest study of HER2 status in Asian patients with CRC. Our findings suggest that the current most commonly used HERACLES criteria might be too strict for patients with CRC. Future studies are needed to explore the most suitable criteria for screening CRC patients who could benefit from anti-HER2 therapy as much as possible.
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer and the second most common cause of cancer-related death in the world, with an annual death rate exceeding 608,700 according to the National Cancer Institute. According to the latest data released by the International Agency for Research on Cancer (IARC) of the WHO in 2020, CRC ranks as the second most common malignancy and the fifth leading cause of cancer-related deaths in China (https://www.iarc.who.int/faq/latest-global-cancer-data-2020-qa/).
The oncogene human epidermal growth factor receptor 2 (HER2) is localized in chromosome 17q21 and coding transmembrane tyrosine kinase receptor; its amplification plays a pivotal role in oncogenesis and development of human malignancies. HER2 has been identified to be overexpressed and/or amplified in up to 30% of breast cancers (BCs) (1) and 13% of gastroesophageal adenocarcinoma (GEA) (2). HER2-targeted therapy significantly prolongs the survival of patients with HER2-positive BC or advanced gastric cancer, which has become a basic strategy for the first-line treatment of related tumors. Recently, HER2 was also reported to be overexpressed in CRC, with some reporting expression varying in the range of 2.6%–11.2% in China (3–6). In metastatic CRC (mCRC), adding trastuzumab and pertuzumab, two monoclonal antibodies targeting HER2, to the standard chemotherapy can significantly improve response rates and survival in patients with HER2-positive tumors (7, 8). However, there is a wide variation in the HER2 positivity rate, which may be due to territorial diversity, different testing methods, and, more importantly, different scoring criteria for HER2 positivity. Previously, the data regarding HER2 expression in CRC mostly refer to HER2 scoring criteria for BC (American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) 2013) (9) and GEA (GEA criteria) (10). In 2015, Valtorta et al. (11) proposed HER2 test criteria (HERACLES diagnostic criteria) for CRC, which is now used by the National Comprehensive Cancer Network (NCCN) and the Chinese Society of Clinical Oncology (CSCO) guidelines as the diagnostic criteria for HER2 interpretation in CRC. However, there is still a lack of support from large-scale research data on whether these immunohistochemistry (IHC) interpretation criteria can accurately reflect the HER2 status in CRC in China. In addition, the correlation between HER2 and the clinicopathological parameters and prognosis in CRC remains controversial (3–6). These indicate that the positivity and role of HER2 in CRC require further exploration.
In the current study, to detect HER2 overexpression and amplification in patients with CRC (HOLIC), we carried out a large-scale retrospective study in the Chinese population. We aimed to compare the prevalence of HER2 positivity underlying the three HER2 diagnostic criteria (BC, GEA, and HERACLES diagnostic criteria). In addition, we identified HER2 expression status and its correlation with clinicopathological features as well as survival data in patients with CRC.
Materials and Methods
Patient Collection and Sample Preparation
A total of 4,836 consecutive cases of CRC treated by radical surgery without any preoperative therapy during 2011–2014 were retrieved from the Department of Pathology of Fudan University Shanghai Cancer Center (FUSCC). The clinicopathological characteristics and molecular pathological detection analysis results (if any) of each patient were retrieved from the hospital information systems. Among all cases, 59 patients underwent a commercial next-generation sequencing (NGS) detection in the Department of Molecular Pathology. Genomic mutations were identified using the NGS-based YuanSu450 gene panel (OrigiMed, Shanghai, China), which covers the coding exons of 450 cancer-related genes and 64 selected introns in 39 genes that are frequently rearranged in solid tumors. The genes were captured and sequenced with a mean depth of 800× using the Illumina NextSeq 500. Genomic alterations (GAs), including single-nucleotide variants (SNVs), insertion–deletion polymorphisms (Indels), and copy number variation, were identified using MuTect (v.1.17), PINDEL (v.2.04), and Control-FREEC (v9.4), respectively. The pathologic diagnosis, including the pTNM stage, was confirmed by two experienced and independent pathologists. For each sample, 3-µm sections were cut from the selected representative paraffin-embedded tumor blocks for IHC and dual-color silver-enhanced in situ hybridization (DSISH) analyses. The study was approved by the Ethical Committee for Clinical Research at FUSCC.
IHC staining was carried out using the anti-HER2/NEU (4B5) antibody (Ventana Medical Systems, Inc., Tucson, AZ, USA) as the primary antibody against HER2 on a Ventana Benchmark XT automatic staining system according to the optimized manufacturer’s instructions. Two pathologists independently scored HER2 staining based on both the traditional HER2 IHC scoring guidelines for BC and GEA and the HERACLES criteria for CRC (11), and any discrepancy was resolved by a consensus meeting.
To screen for deficient DNA mismatch repair (dMMR), all eligible patients had their paraffin-embedded tumor tissues tested by IHC for the expression of MLH1, MSH2, MSH6, and PMS2. dMMR was considered when there was nuclear negativity in IHC staining for MLH1, MSH2, MSH6, or PMS2, with the presence of protein expression in adjacent normal colonic mucosa and/or stromal cells.
Dual-Color Silver-Enhanced In Situ Hybridization
HER2 amplification was confirmed by DSISH assays. DSISH was carried out on a Ventana Benchmark XT automatic staining system according to Chromosome 17 (CHR17) Probe (Ventana Medical Systems SA). Briefly, 20 tumor nuclei were initially counted per case. The scoring and evaluation for in situ hybridization were performed by counting ERBB2 and CEN17 signals from 100 nuclei per case. Non-tumor tissue (normal colon mucosa) was used as an internal negative control. Samples with an ERBB2/CEN17 ratio ≥2.0 were considered amplified (11). The DSISH was assessed by two independent pathologists separately who were blinded to the IHC scores.
Statistical Analysis
Data were analyzed using SPSS 23.0 software. The correlations between HER2 expression and the investigated parameters were evaluated. Survival analysis was carried out using the Kaplan–Meier method, and the multivariate survival analysis was in proportional hazards regression models described by Cox. The significance tests were two-sided, and a p-value of <0.05 was considered statistically significant.
Results
Human Epidermal Growth Factor Receptor 2 Expression Rate Determined by Immunohistochemistry and Dual-Color Silver-Enhanced In Situ Hybridization
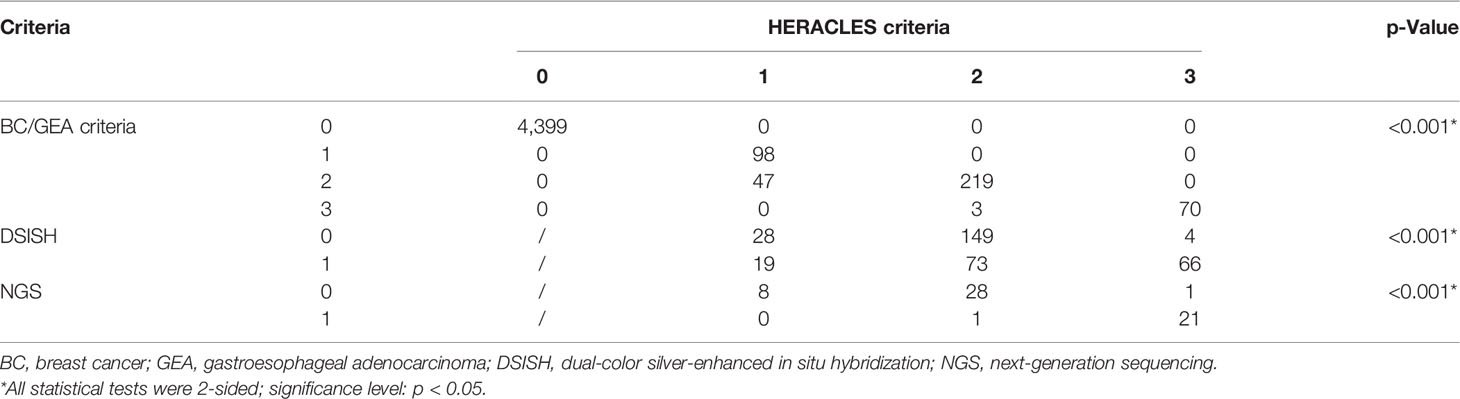
The expression was studied using whole tissue sections. HER2 was observed by lateral or basolateral membrane staining in CRC cells (Figure 1A). According to the BC and GEA HER2 IHC diagnostic criteria, 98 (2.03%) were scored up to HER2 1+, 266 (5.50%) were scored up to but no higher than HER2 2+, and 73 cases (1.51%) were scored up to HER2 3+. Immunonegative cancer cells (HER2 0) were found in 4,399 (90.96%) cases. Thus, the HER2 expression rate of IHC (1+~3+) was 7.01% (339/4,836) in CRCs based on the BC/GEA criteria. However, there was a significant difference between the evaluation results based on the HERACLES criteria and evaluation results based on BC/GEA criteria (p < 0.001; Figure 1B and Table 1). Specifically, 47 samples scored up to but no higher than HER2 2+ according to the BC/GEA criteria was scored up to but no higher than 1+ according to the HERACLES criteria; 3 samples scored up to HER2 3+ according to the BC/GEA criteria was scored up to but not higher than 2+ according to the HERACLES criteria (Figure 1B). Therefore, there was 6.02% (291/4,836) of CRC scored up to HER2 2~3+ according to the HERACLES criteria, which is 0.96% less than that of BC/GEA criteria.
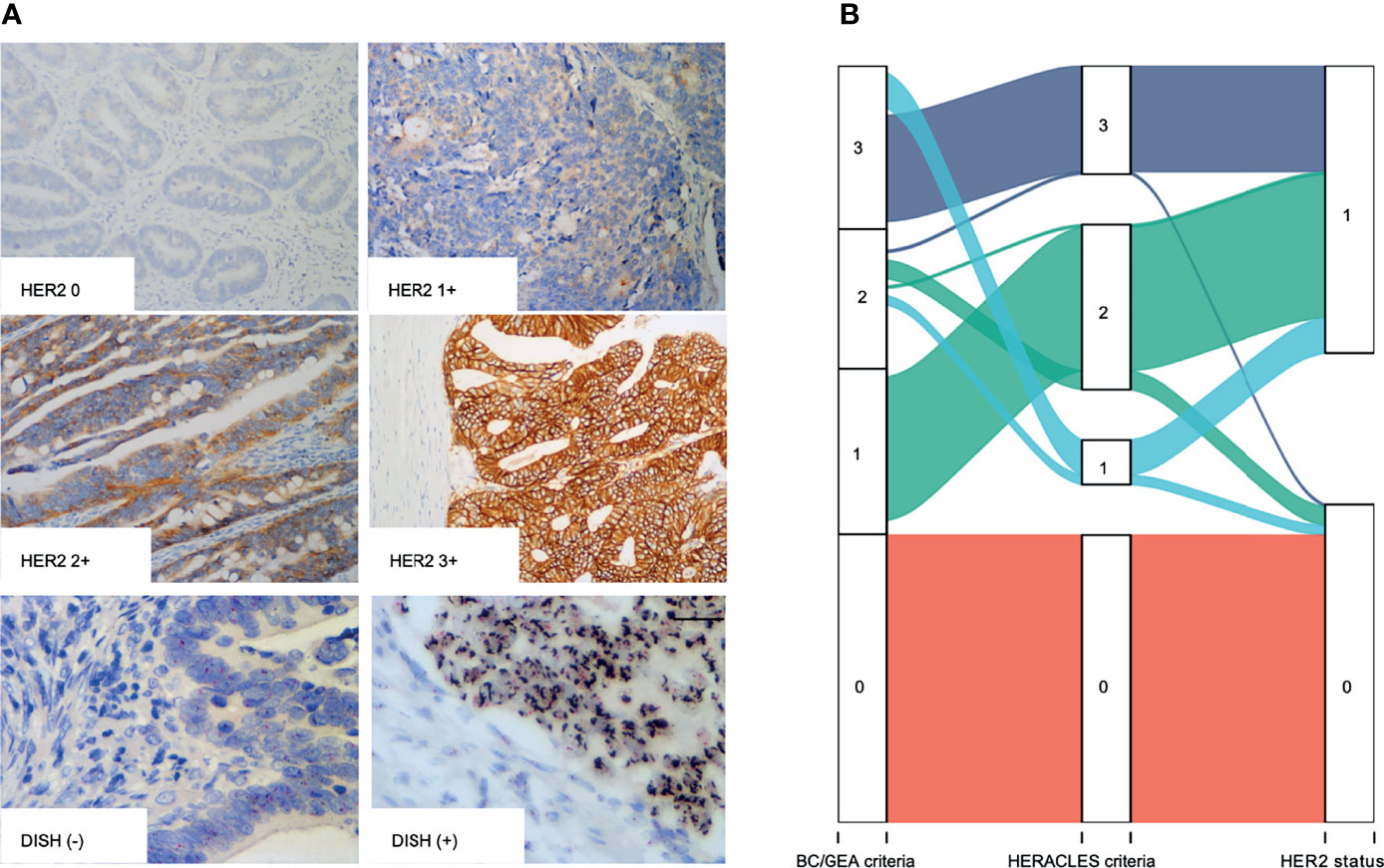
Figure 1 IHC and DSISH staining of HER2. (A) Representative IHC intensity (0, 1+, 2+, and 3+) of tumor cells (original magnification, ×200) and representative negative (DSISH−) and positive (DSISH+) amplification of HER2 gene (original magnification, ×400). (B) Alluvial diagram shows the distribution of each case in different diagnostic criteria. IHC, immunohistochemistry; DSISH, dual-color silver-enhanced in situ hybridization; HER2, human epidermal growth factor receptor 2.
All the 339 cases that showed membranous staining of HER2 2~3+ according to BC/GEA criteria were then subjected to HER2 DSISH analysis. In addition, 50 cases displaying no/weak membranous IHC staining according to BC/GEA criteria were randomly selected for DSISH analysis, and none of these cases showed HER2 gene amplification (Figure 1A). As a result, 181 cases were reported as negative (not amplified), and 158 cases were reported as positive (amplified) by DSISH. Very high consistency in the results between IHC 3+ and DSISH was obtained; 67 (91.78%) of 73 HER2 IHC 3+ cases showed HER2 gene amplification. However, only 91 (34.21%) of 266 HER2 IHC 2+ cases showed HER2 gene amplification (Figure 1B and Table 1). Thus, the HER2 amplification/overexpression ratio was 3.39% (164/4,836) in our cohort. Among these HER2 gene amplification cases reported by DSISH, 19 cases were scored up to HER2 IHC 1+, and 75 cases were scored up to HER2 IHC 2+ according to the HERACLES criteria (Table 1).
Association of Human Epidermal Growth Factor Receptor 2 Expression With Clinicopathological Characteristics and Survival of Patients
To determine the association of HER2 expression with clinicopathological characteristics, all 339 cases enrolled in DSISH analysis were firstly analyzed. Interestingly, in these 339 HER2 IHC 2+~3+ cases, the 158 HER2 DSISH amplified cases were more likely to be distributed in the left colon (p = 0.014), with more regional lymph nodes (p = 0.003) and vascular invasion (p = 0.013) and higher TNM stage (p = 0.029), and were mutually exclusive with dMMR (p = 0.030, Table 2).
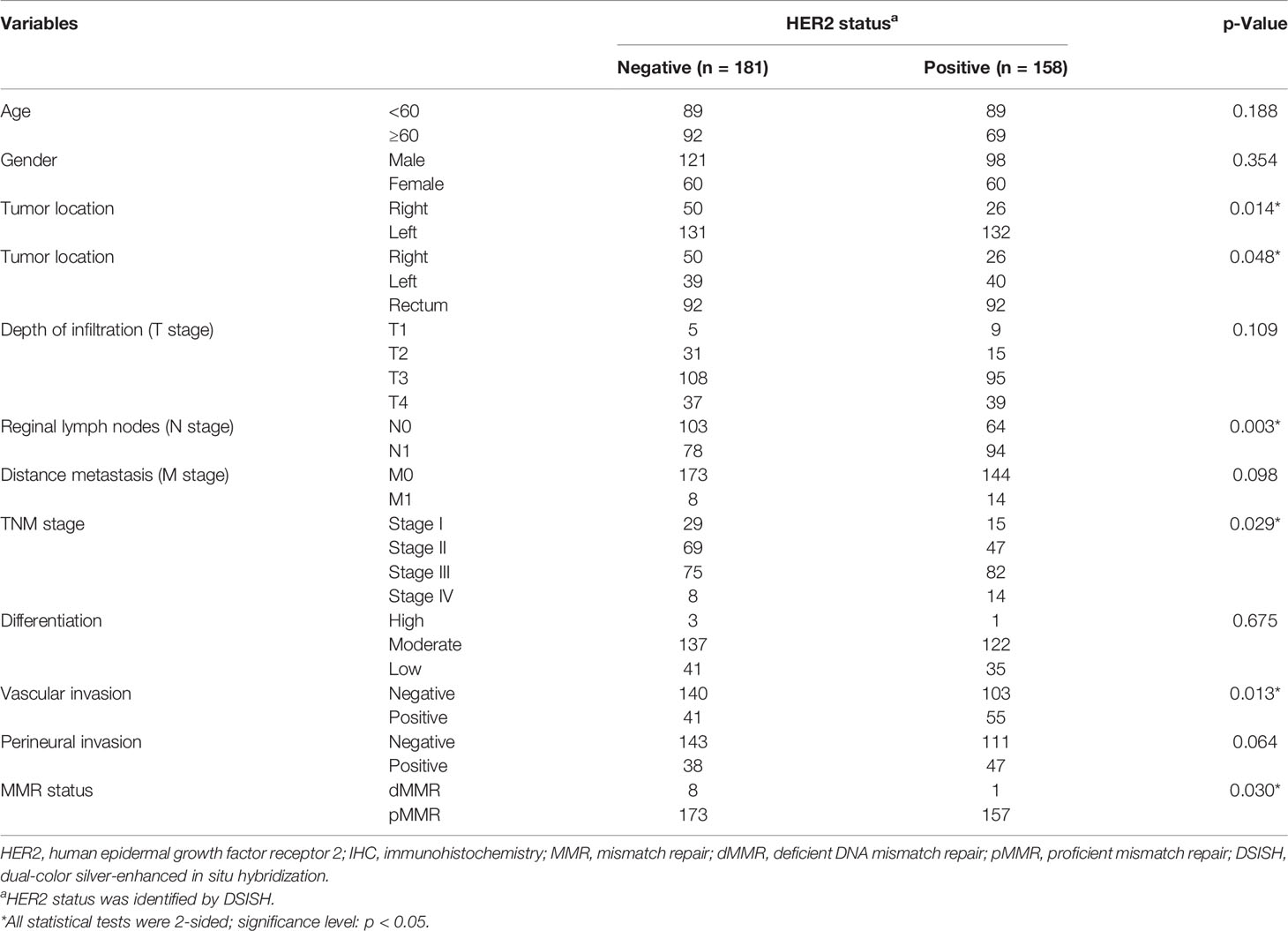
Table 2 Correlation between HER2 expression and clinical parameters in the IHC HER2 2~3+ group (n = 339).
For survival analysis, the follow-up information of all 164 HER2 amplification/overexpression patients and finally tumor-specific survival data in a total of 122 patients was obtained. Subsequently, 135 patients from the total 4,399 HER2-negative cases were matched using the propensity score matching (PSM) method. The correlation of HER2 expression with clinicopathological characteristics in these 257 patients was also analyzed. The result showed that regardless of the evaluation criterion used, HER2 expression was positively correlated with the female gender (all p < 0.05). However, after categorizing HER2 expression status as negative or positive, there were no statistically significant associations with any clinicopathological data (Table 3).
The median follow-up time was 75.17 months (range: 0.23–119.27 months), and 113 patients (44%) died of cancer, while the remaining 144 patients (56%) were still alive at the end of the study period. A total of 123 patients (47.1%) patients relapsed; 52.1% of patients survived without a tumor. There was no significant correlation between HER2-positive and HER2-negative patients on either overall survival (OS; Figures 2A–C) or disease-free survival (DFS; Figures 2D–F).
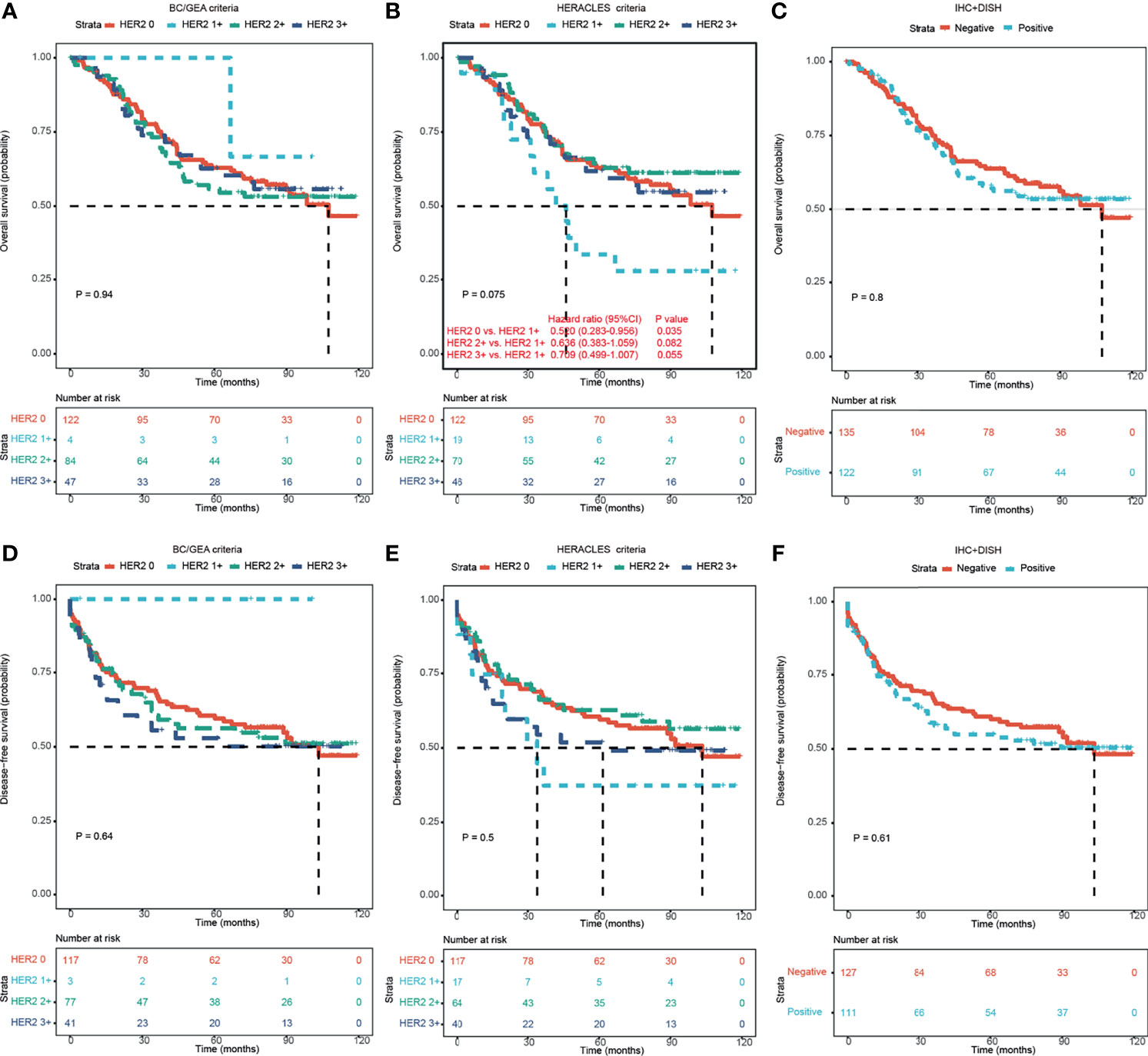
Figure 2 Kaplan–Meier survival analyses of HER2 expression in CRC patients according to different criteria. (A–C) Kaplan–Meier OS analysis of HER2 expression in CRC patients according to BC/GEA criteria (A), HERACLES criteria (B), and HER2 expression status (C). (D–F) Kaplan–Meier DFS analysis of HER2 expression in CRC patients according to BC/GEA criteria (D), HERACLES criteria (E), and HER2 expression status (F). HER2, human epidermal growth factor receptor 2; CRC, colorectal cancer; OS, overall survival; BC, breast cancer; GEA, gastroesophageal adenocarcinoma; DFS, disease-free survival.
Interestingly, according to the HERACLES criteria, patients with HER2 1+ showed significantly shorter OS than patients with the other three HER2 expression statuses (p = 0.035 vs. HER2 0, p = 0.082 vs. HER2 2+, and p = 0.055 vs. HER2 3+; Figure 2B). According to the HERACLES criteria, when patients with clinicopathological characteristics were categorized, patients with HER2 1+ showed significantly shorter OS than patients with the other three HER2 expression statuses in the rectal location, T3 stage, TNM stage II, moderate differentiation, and perineural invasion groups (Figure 3).
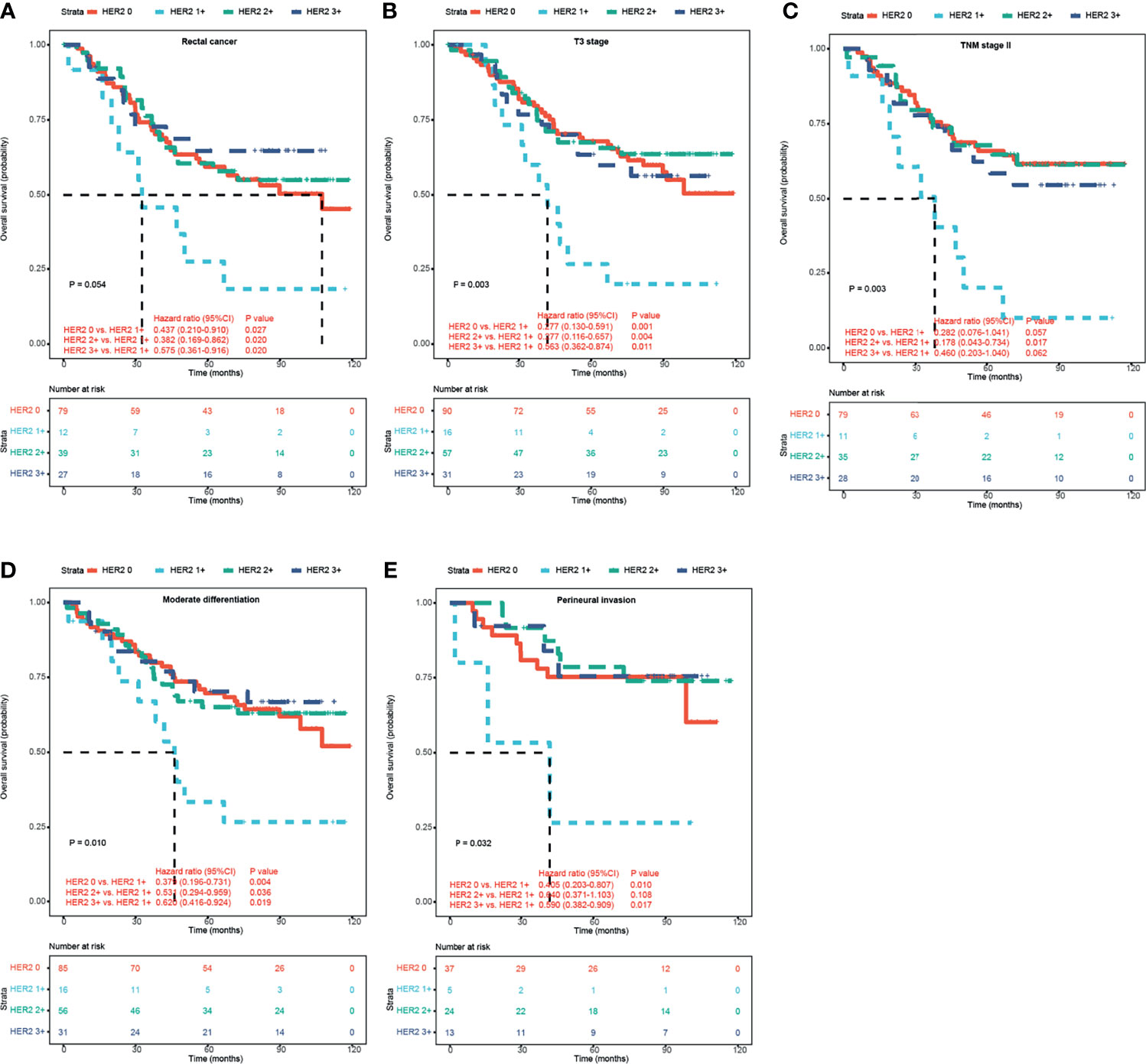
Figure 3 Kaplan–Meier survival analyses of HER2 expression in CRC patients according to HERACLES criteria. Kaplan–Meier OS analysis of patients with rectal cancer (A), T3 stage (B), TNM stage II (C), moderate differentiation (D), and perineural invasion (E) based on HER2 expression status. HER2, human epidermal growth factor receptor 2; CRC, colorectal cancer.
Human Epidermal Growth Factor Receptor 2 and Common Gene Status Alteration
Among these 164 cases that were determined as HER2 positive by DSISH/IHC, KRAS, NRAS, and BRAF (RAS/BRAF) mutation data determined by a commercialized NGS method were available in a total of 59 patients from the clinical molecular pathology data. Among them, 23 (38.98%) HER2-positive cases showed wild-type RAS/BRAF status. In the other 39 cases with RAS/BRAF mutations, 33 (20.63%) harbored a KRAS mutation, 3 (1.88%) harbored an NRAS mutation, and 4 (2.5%) harbored a BRAF mutation.
In these 59 cases with second-generation sequencing data, the protein expression of HER2 according to the HERACLES criteria was as follows: 8 cases of HER2 1+, 29 cases of HER2 2+, and 22 cases of HER2 3+. Further chi-square test showed that HER2 protein expression was significantly correlated with RAS/BRAF, RARA, TOP2A, FAT3, BRAF, and CDK12 alterations (Figure 4). In particular, the HER2 protein expression was significantly negatively correlated with RAS/BRAF and FAT3 mutation whereas positively correlated with RARA, TOP2A, and CDK12 alterations. Among these alterations, RAS, BRAF, and FAT3 are involved in gene mutations; RARA and TOP2A are involved in gene amplification; and CDK12 are involved in both gene mutation and amplification. By classifying these patients into two groups using tumor mutational burden (TMB) >10 (12) or >28 (13) as the cutoff value, no significant correlation between TMB and HER2 protein expression was found.
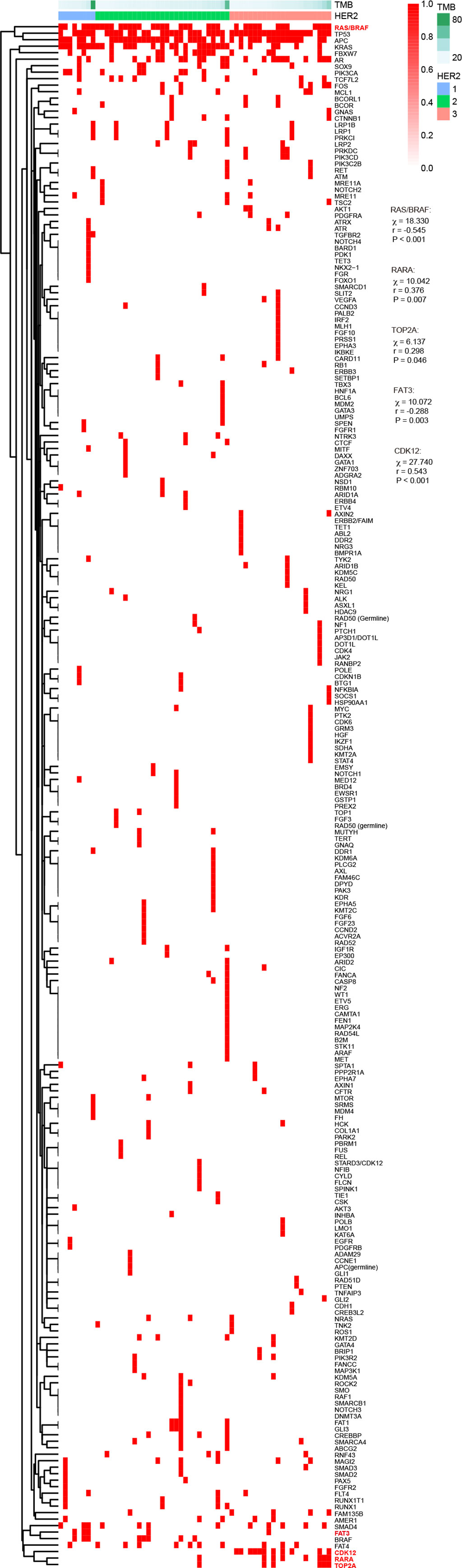
Figure 4 Association of HER2 expression with TMB and gene mutation according to different criteria. HER2, human epidermal growth factor receptor 2; TMB, tumor mutational burden.
The HER2 status was also determined by NGS in these 59 cases. However, it was found that for HER2 amplification, the agreement rate between DSISH and NGS was only 37.9%; only 22 HER2-positive cases also showed HER2 amplification in NGS results. By NGS, 21 cases of HER2 3+ scored by the HERACLES criteria was determined as HER2 positive, whereas all 8 cases of HER2 1+ and 28 cases of HER2 2+ scored by the HERACLES criteria were determined as HER2 negative, with a significant statistical difference (p < 0.001; Table 1).
Discussion
In this study, we detected the HER2 status in CRC patients on a large scale with a total of 4,836 patients hospitalized during 2011–2014. We found that there was a significant difference between the evaluation results based on the HERACLES criteria and BC/GEA criteria. In particular, 47 samples scored as 2+ determined by BC/GEA criteria would be scored down to 1+ determined by the HERACLES criteria. In the general diagnosis process of HER2 detection, these cases would not be subsequently detected by DSISH to verify the HER2 status. However, in fact, we performed DSISH analysis in these HER2 1+ cases determined by the HERACLES criteria, and some of the cases (19/47) turned out to be HER2 positive. As we all know, DSISH is the gold standard for detecting the HER2 status. Our results suggested that relying solely on the HERACLES criteria to interpret the HER2 status might lead to missing a subset of HER2-positive CRC patients and deprive them of anti-HER2 therapy. On the other hand, for HER2 amplification, the agreement rate between DSISH and NGS was only 38.9%; only 23 positive cases showed consistent results with NGS results. It is suggested that NGS may be less sensitive to HER2-positive detection than IHC/DSISH of tissues; larger samples are needed for further validation.
The relationship between HER2 overexpression and clinicopathological features in CRC is still unclear. Meng et al. (14) found no correlation between HER2 positivity and clinicopathological parameters. Zhang et al. (6) showed that in Chinese patients with stage I to III CRC, HER2 amplification was associated with deeper intestinal wall tumor invasion and later TNM staging. In our cohort, the HER2 status has no significant association with clinicopathological features except for gender. We found that HER2 expression detected by IHC was positively correlated with the female gender, while there is no difference when we categorized the HER2 status as negative or positive based on the results of DSISH. The reasons for the inconsistent results may be attributed to about 55% of HER2 2~3+ male cases being identified as HER2 negative subsequently by DSISH, and to some extent, the percent of female cases increased in the HER2-positive group. Interestingly, when focused on those 339 patients who were scored as HER2 2~3+ according to BC/GEA criteria and then determined by DSISH, the clinicopathological feature distribution in these 339 patients showed that HER2 amplification was positively associated with tumor invasion, lymphatic metastasis, and advanced TNM staging. This suggested that once HER2 becomes expressed in CRC (which was determined by IHC), HER2 amplification is more likely to appear in patients with advanced clinical stages.
In our cohort, we obtained KRAS, NRAS, and BRAF gene statuses (determined by NGS) of 59 HER2-positive cases from their clinical data and found that 36 (38.98%) HER2-positive cases have RAS/BRAF mutation. According to the results of the Mypathway study (15), trastuzumab combined with pertuzumab treatment in RAS/RAF wild-type patients tends to obtain a higher overall response rate (ORR) efficiency, which suggests that the combined detection of HER2 and RAS/BRAF status can help in screening patients who may benefit from dual-targeted anti-HER2 therapy. It has been reported that HER2-positive and KRAS, NRAS, and BRAF mutations are mutually exclusive in CRC (16). Similarly, when determining the HER2 status according to the HERACLES criteria, the HER2 protein expression was also significantly negatively correlated with RAS/BRAF status in our cohort. This indicates that HER2-positive CRC patients are more likely to be RAS/RAF wild-type. Some studies have found that activation of HER2 signaling causes drug resistance to anti-EGFR therapy in a fraction of mCRC patients with RAS wild-type tumors (17, 18). Kras gain-of-function is one of the most frequent mutations in colon cancers, which has been shown to correlate well with poorer survival. Whether HER2 has a regulatory role in CRC needs to be further studied. Besides, the HER2 protein expression was also significantly negatively correlated with FAT3 alterations whereas positively correlated with RARA, TOP2A, and CDK12 alterations. Although the functional mechanisms behind these associations are still unclear, they provide some perspectives for the compatibility of targeted drugs.
Currently, there are conflicting studies on the relationship between HER2 positivity and the prognosis of CRC. Meng et al. (14) showed that compared with patients with HER2-negative CRC, patients with HER2-positive CRC had worse 5-year DFS and OS in the overall group of 119 patients with CRC. Liu et al. (3) also found that patients with HER2-positive CRC showed an association with shorter DFS in patients with stage II–III CRC. On the contrary, Wang et al. (4) found that patients with HER2-positive CRC showed no association with DFS or OS in the overall group of 1,058 patients with CRC. Similarly, by matching 122 HER2-positive patients with 135 HER2-negative patients using the PSM method, we found no significant correlations between HER2-positive rates and either DFS or OS regardless of the evaluation criterion used. The different research conclusions may be affected by the treatment that the patients received as well as the involved samples. In our study, firstly, we used 254 cases to ensure sufficient power to test, which is to our knowledge the largest sample size to analyze the prognostic significance in CRC. Secondly, we used the PSM to control our known confounding bias as much as possible to verify this conclusion, with a higher level of evidence. Our results might be closer to the real situation.
Interestingly, according to HERACLES diagnostic criteria, patients with HER2 1+ CRC showed the shortest OS as compared with any other group of patients. In the rectal location, T3 stage, TNM stage II, medium differentiation, and perineural invasion stratified group, the same conclusion is suggested. It is well known that HER2 can promote tumor cell proliferation, survival, and angiogenesis by activating downstream oncogenic signaling pathways (19). Therefore, it is not difficult to understand that the prognosis of HER2 1+ patients is worse than that of HER2 0 patients. In addition, consistent with the previous study (16), our study also showed that the RAS/BRAF mutation rate of patients with HER2 1+ is significantly higher than that of patients with HER2 2~3+. Although we failed to obtain the clinical therapeutic data of these patients with HER2 1~3+, in clinical practice, RAS/BRAF wild-type patients are routinely treated with EGFR monoclonal antibodies such as Erbitux. We suspect that this may be the reason why the prognosis of HER2 1+ patients is worse than that of HER2 2~3+ patients. Although our sample size of HER2 1+ patients is small, based on the clinical benefit of HER2 1+, our results suggested that special attention should be paid to patients with HER2 low-expressing CRC. More future studies are needed to break the treatment bottleneck of this subgroup of CRC patients with relatively poor biological behavior.
In recent years, a number of clinical studies have shown that dual-target anti-HER2 therapy, novel anti-HER2 tyrosine kinase inhibitor, antibody–drug conjugate (ADC) drugs, and bispecific antibodies might become a promising anti-HER2 treatment in HER2-positive mCRC (15, 20). Among these trials, DESTINY-CRC01 is the only trial that enrolled patients with HER2 low-expressing mCRC (21). The previous study has demonstrated a promising preliminary antitumor activity of T-DXd in patients with HER2 low BC (objective response rate: 37.0%) (22). However, no patients with HER2 2+ or HER2 1+ mCRC had a confirmed objective response to T-DXd.
Limitations of the study include that the treatment information of the patients is not obtained. There was a lack of efficacy data of anti-HER2 treatment. Additionally, we did not perform NGS analysis in the matching peripheral blood to verify the consistency of HER2 amplification with tissue. A recent clinical trial showed that identification of the HER2 status by circulating tumor DNA (ctDNA) showed similar accuracy to conventional tissue genotyping (23). Although this suggests that ctDNA has good application value in identifying patients who benefit from the dual-HER2 blockade and monitor treatment response, it is still necessary to conduct a large number and detailed comparative analyses on the expression profile of HER2 in tissues and ctDNA and to comprehensively judge the clinical applicability of ctDNA genotyping on HER2 amplification.
In summary, we conducted a large-scale HER2 status detection in the Chinese CRC patients and found that when scored by the HERACLES Criteria, patients with HER2 1+ CRC had the worst prognosis. Also, our study suggested that the current most commonly used HERACLES criteria might be too strict for patients with CRC. Future studies are needed to explore the most suitable criteria for screening CRC patients who could benefit from anti-HER2 therapy as much as possible.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethical Committee for Clinical Research at Fudan University Shanghai Cancer Center. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SN and XiW conceived the study, performed the literature search and bioinformatics analysis, and prepared the figures. XuW, WW, TC, MZ, and JC helped with data collection, analysis, and interpretation. MX, ZH, CJ, WW, and DH wrote and revised the manuscript. The authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81972249, 81802367, 81802361, and 82172702), Clinical Research Project of Shanghai Shenkang Hospital Development Center (SHDC2020CR4068), Shanghai Clinical Science and Technology Innovation Project of Municipal Hospital (SHDC12020102), Fudan University Double First-class Original Research Personalized Support Project (XM03190634), the Natural Science Foundation of Shanghai (21ZR1414900), Shanghai Science and Technology Development Fund (19MC1911000), Clinical Research Project of Shanghai Municipal Health Committee (20194Y0348), Shanghai “Rising Stars of Medical Talents” Youth Development Program Youth Medical Talents—Specialist Program (SHWSRS (2020)_087) and Shanghai Anticancer Association EYAS project (SACA-CY19B10).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human Breast Cancer: Correlation of Relapse and Survival With Amplification of the HER-2/Neu Oncogene. Science (1987) 235(4785):177–82. doi: 10.1126/science.3798106
2. Sheng WQ, Huang D, Ying JM, Lu N, Wu HM, Liu YH, et al. HER2 Status in Gastric Cancers: A Retrospective Analysis From Four Chinese Representative Clinical Centers and Assessment of Its Prognostic Significance. Ann Oncol: Off J Eur Soc Med Oncol (2013) 24(9):2360–4. doi: 10.1093/annonc/mdt232
3. Liu F, Ren C, Jin Y, Xi S, He C, Wang F, et al. Assessment of Two Different HER2 Scoring Systems and Clinical Relevance for Colorectal Cancer. Virchows Arch (2020) 476(3):391–8. doi: 10.1007/s00428-019-02668-9
4. Wang XY, Zheng ZX, Sun Y, Bai YH, Shi YF, Zhou LX, et al. Significance of HER2 Protein Expression and HER2 Gene Amplification in Colorectal Adenocarcinomas. World J Gastrointest Oncol (2019) 11(4):335–47. doi: 10.4251/wjgo.v11.i4.335
5. Shan L, Lv Y, Bai B, Huang X, Zhu H. Variability in HER2 Expression Between Primary Colorectal Cancer and Corresponding Metastases. J Cancer Res Clin Oncol (2018) 144(11):2275–81. doi: 10.1007/s00432-018-2744-z
6. Zhang X, Wu J, Wang L, Zhao H, Li H, Duan Y, et al. HER2 and BRAF Mutation in Colorectal Cancer Patients: A Retrospective Study in Eastern China. PeerJ (2020) 8:e8602. doi: 10.7717/peerj.8602
7. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. To GATI. Trastuzumab in Combination With Chemotherapy Versus Chemotherapy Alone for Treatment of HER2-Positive Advanced Gastric or Gastro-Oesophageal Junction Cancer (ToGA): A Phase 3, Open-Label, Randomised Controlled Trial. Lancet (London England) (2010) 376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X
8. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of Chemotherapy Plus a Monoclonal Antibody Against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N Engl J Med (2001) 344(11):783–92. doi: 10.1056/NEJM200103153441101
9. Kwak Y, Yun S, Nam SK, Seo AN, Lee KS, Shin E, et al. Comparative Analysis of the EGFR, HER2, C-MYC, and MET Variations in Colorectal Cancer Determined by Three Different Measures: Gene Copy Number Gain, Amplification Status and the 2013 ASCO/CAP Guideline Criterion for HER2 Testing of Breast Cancer. J Trans Med (2017) 15(1):167. doi: 10.1186/s12967-017-1265-x
10. Conradi LC, Styczen H, Sprenger T, Wolff HA, Rodel C, Nietert M, et al. Frequency of HER-2 Positivity in Rectal Cancer and Prognosis. Am J Surg Pathol (2013) 37(4):522–31. doi: 10.1097/PAS.0b013e318272ff4d
11. Valtorta E, Martino C, Sartore-Bianchi A, Penaullt-Llorca F, Viale G, Risio M, et al. Assessment of a HER2 Scoring System for Colorectal Cancer: Results From a Validation Study. Modern Pathol: an Off J United States Can Acad Pathol Inc (2015) 28(11):1481–91. doi: 10.1038/modpathol.2015.98
12. Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, et al. Analysis of 100,000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational Burden. Genome Med (2017) 9(1):34. doi: 10.1186/s13073-017-0424-2
13. Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F, et al. Effect of Combined Immune Checkpoint Inhibition vs Best Supportive Care Alone in Patients With Advanced Colorectal Cancer: The Canadian Cancer Trials Group CO.26 Study. JAMA Oncol (2020) 6(6):831–8. doi: 10.1001/jamaoncol.2020.0910
14. Meng X, Wang R, Huang Z, Zhang J, Feng R, Xu X, et al. Human Epidermal Growth Factor Receptor-2 Expression in Locally Advanced Rectal Cancer: Association With Response to Neoadjuvant Therapy and Prognosis. Cancer Sci (2014) 105(7):818–24. doi: 10.1111/cas.12421
15. Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, et al. Pertuzumab Plus Trastuzumab for HER2-Amplified Metastatic Colorectal Cancer (MyPathway): An Updated Report From a Multicentre, Open-Label, Phase 2a, Multiple Basket Study. Lancet Oncol (2019) 20(4):518–30. doi: 10.1016/S1470-2045(18)30904-5
16. Cancer Genome Atlas N. Comprehensive Molecular Characterization of Human Colon and Rectal Cancer. Nat (2012) 487(7407):330–7. doi: 10.1038/nature11252
17. Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, et al. A Molecularly Annotated Platform of Patient-Derived Xenografts (“Xenopatients”) Identifies HER2 as an Effective Therapeutic Target in Cetuximab-Resistant Colorectal Cancer. Cancer Discov (2011) 1(6):508–23. doi: 10.1158/2159-8290.cd-11-0109
18. Tu J, Yu Y, Liu W, Chen S. Significance of Human Epidermal Growth Factor Receptor 2 Expression in Colorectal Cancer. Exp Ther Med (2015) 9(1):17–24. doi: 10.3892/etm.2014.2063
19. Moasser MM. The Oncogene HER2: Its Signaling and Transforming Functions and Its Role in Human Cancer Pathogenesis. Oncogene (2007) 26(45):6469–87. doi: 10.1038/sj.onc.1210477
20. Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-Targeted Therapy With Trastuzumab and Lapatinib in Treatment-Refractory, KRAS Codon 12/13 Wild-Type, HER2-Positive Metastatic Colorectal Cancer (HERACLES): A Proof-of-Concept, Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol (2016) 17(6):738–46. doi: 10.1016/S1470-2045(16)00150-9
21. Siena S, Di Bartolomeo M, Raghav K, Masuishi T, Loupakis F, Kawakami H, et al. Trastuzumab Deruxtecan (DS-8201) in Patients With HER2-Expressing Metastatic Colorectal Cancer (DESTINY-CRC01): A Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol (2021) 22(6):779–89. doi: 10.1016/S1470-2045(21)00086-3
22. Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, et al. Antitumor Activity and Safety of Trastuzumab Deruxtecan in Patients With HER2-Low-Expressing Advanced Breast Cancer: Results From a Phase Ib Study. J Clin Oncol: Off J Am Soc Clin Oncol (2020) 38(17):1887–96. doi: 10.1200/jco.19.02318
Keywords: colorectal cancer, HER2, methodology, amplification, prognosis
Citation: Ni S, Wang X, Chang J, Sun H, Weng W, Wang X, Tan C, Zhang M, Wang L, Huang Z, Huang D, Xu M and Sheng W (2022) Human Epidermal Growth Factor Receptor 2 Overexpression and Amplification in Patients With Colorectal Cancer: A Large-Scale Retrospective Study in Chinese Population. Front. Oncol. 12:842787. doi: 10.3389/fonc.2022.842787
Received: 24 December 2021; Accepted: 14 March 2022;
Published: 28 April 2022.
Edited by:
Qi Sun, The First Affiliated Hospital of Xi’an Jiaotong University, ChinaReviewed by:
Jiandi Zhang, Quanticision Diagnostics, Inc., United StatesLin Zhang, University of California, San Diego, United States
Copyright © 2022 Ni, Wang, Chang, Sun, Weng, Wang, Tan, Zhang, Wang, Huang, Huang, Xu and Sheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiqi Sheng, shengweiqi2006@163.com; Midie Xu, xumd27202003@sina.com
†These authors have contributed equally to this work
‡These authors share senior authorship
 Shujuan Ni
Shujuan Ni Xin Wang
Xin Wang Jinjia Chang
Jinjia Chang Hui Sun1,2,3
Hui Sun1,2,3 Weiwei Weng
Weiwei Weng Lei Wang
Lei Wang Zhaohui Huang
Zhaohui Huang Dan Huang
Dan Huang Midie Xu
Midie Xu Weiqi Sheng
Weiqi Sheng