- 1Department of Oncology, Peking University International Hospital, Beijing, China
- 2Department of Laboratory Medicine, Beijing Haidian Hospital, Beijing, China
- 3Department of Medicine, Geneplus-Beijing, Beijing, China
- 4Department of Medical Affairs, Shanghai Junshi Biosciences Co., Ltd., Beijing, China
Primary splenic angiosarcoma (PSA) is a rare malignancy with poor prognosis. At present, little study is available on immunotherapy in PSA. Here, we report a case of a patient with metastatic PSA who was treated with programmed death-1 (PD-1) inhibitors and vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors combined therapy and achieved complete response (CR). The patient was a 57−year−old woman with three liver metastases. She was treated with seven cycles of toripalimab plus anlotinib. Programmed death-ligand 1 (PD-L1) immunohistochemistry and next-generation sequencing was performed, and the PD-L1 tumor proportion score was 75%. Finally, she achieved CR after six cycles of the combined therapy regimen. No serious adverse events were detected. To the best of our knowledge, this is the first clinical evidence that anti-PD-1 plus anti-VEGF therapy might be a promising option for patients with metastatic PSA. However, more clinical trials are needed to verify this conclusion.
Introduction
Primary splenic angiosarcoma (PSA) is a rare and aggressive tumor with poor prognosis and a high rate of liver metastasis. The typical symptom of PSA is left upper abdomen pain (1–3); other symptoms include weakness or fatigue, fever, chest pain, weight loss, and bleeding (1, 4). Splenectomy is the only potentially curative treatment for patients with early-stage PSA. In addition, some patients with distant metastasis may receive emergency splenectomy due to splenic rupture.
Although some case reports have reported the potential benefit of systemic therapy in PSA, traditional chemotherapy have limited efficacy in metastatic PSA (1, 5). In recent years, programmed death-1 (PD-1) inhibitors have significantly improved the long-term survival of patients with various tumors (6, 7). Moreover, vascular endothelial growth factor (VEGF) tyrosine kinase inhibitors (TKIs) have shown promising effects in patients with angiosarcoma (8).
Here, we report a case of a metastatic PSA patient with high expression of programmed death-ligand 1 (PD-L1) who reached complete response (CR) after anti−PD−1 and anti−VEGF combination therapy.
Case Report
The patient was a 57−year−old woman who was diagnosed with PSA in 2020. She presented to a local hospital with left-sided upper abdominal pain for three hours.
There was no bloating, nausea, or vomiting. No family cancer history was noted. Abdominal contrast-enhanced computed tomography (CT) showed spontaneous rupture of a spleen neoplasm and abdominal hemorrhage, three suspicious lesions in the liver were also detected. One day later, an emergency splenectomy was performed. The postoperative immunohistochemical staining results were as follows: CD31(+), CD34(+), EGFR (+), CK (−), P63(−), CK20(−), CK5/6(−), Syn (−), and CK7(−). The final pathologic results confirmed the diagnosis of angiosarcoma (Figure 1). Positron emission tomography-CT (PET-CT) was performed 1 month after surgery, demonstrating three hypermetabolic foci in the liver, which were diagnosed as hepatic metastases and correspond to the same lesions initially found in CT. No other distant metastases were identified.
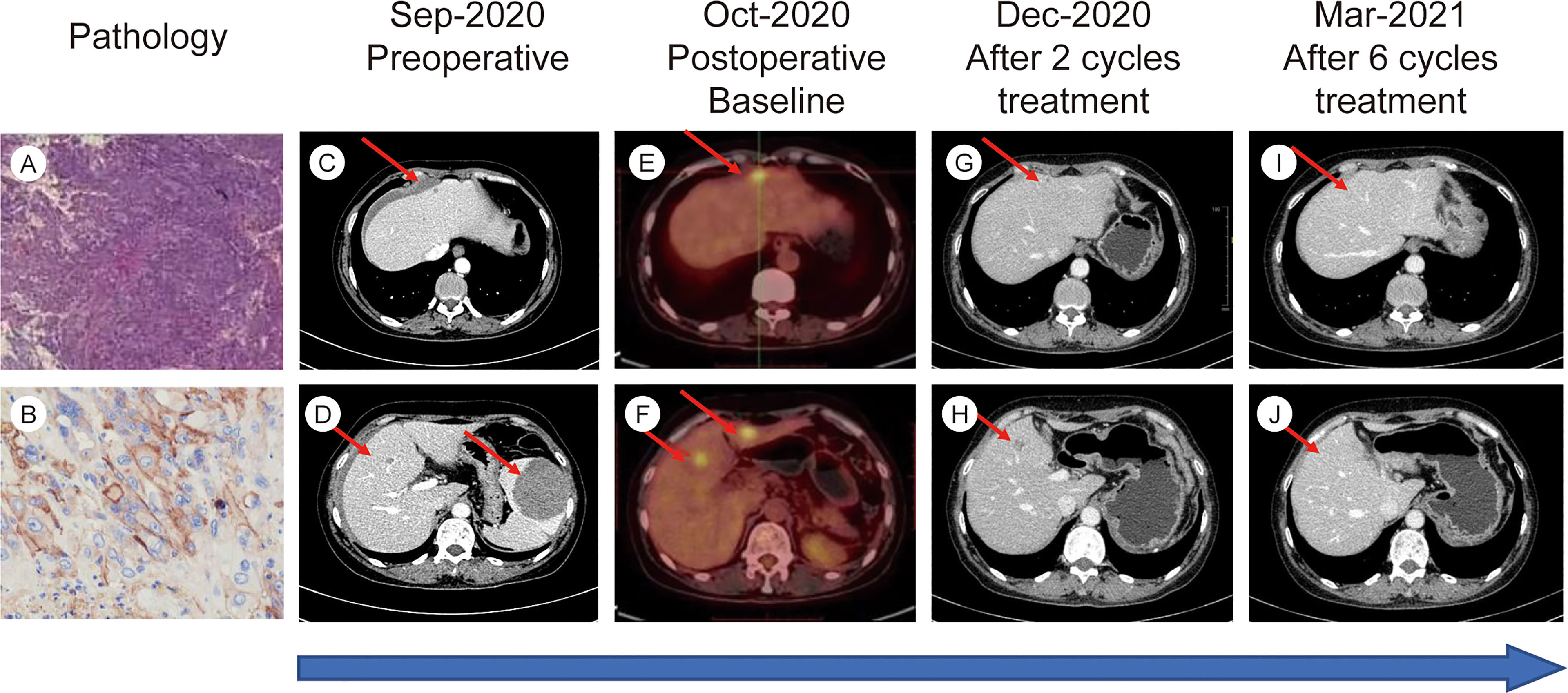
Figure 1 (A) H&E stain from postoperative tumor tissue (10X magnification). (B) Immunohistochemical staining for PD-L1 in postoperative tumor tissue. (C–J) Timelines of imaging changes.
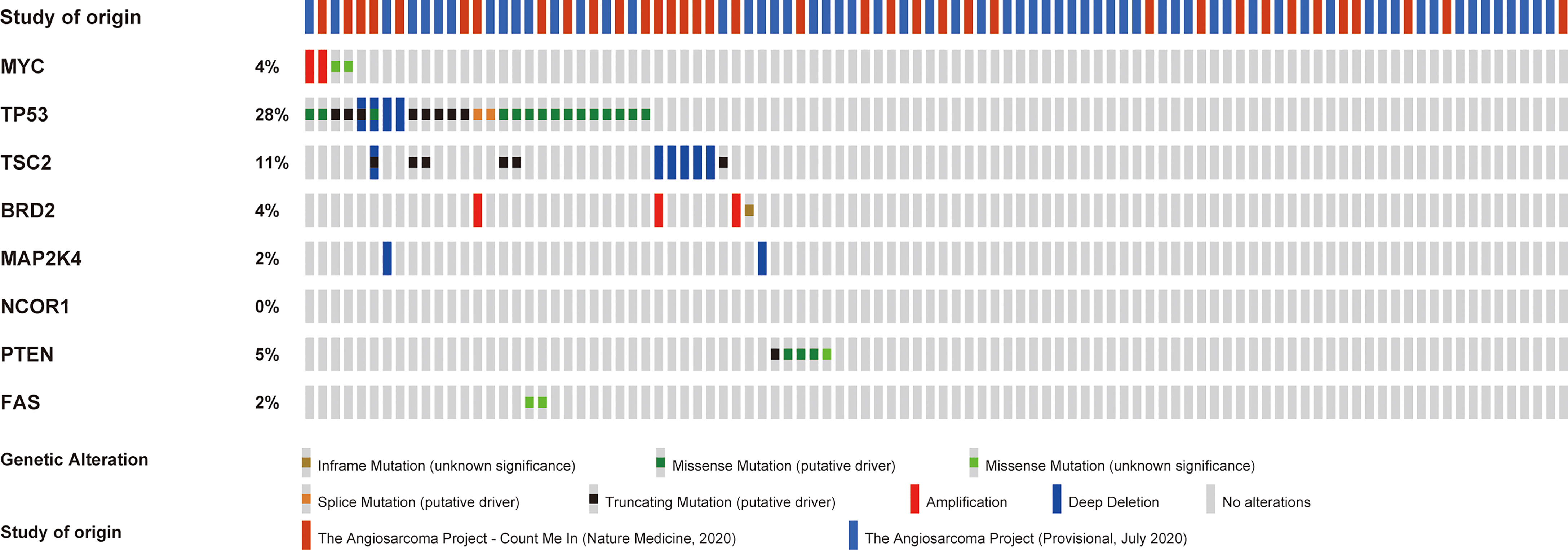
One month later, she was referred to our hospital for further care. PD-L1 staining with a 22C3 antibody was performed, and the results indicated high PD-L1 expression [tumor proportion score (TPS)=75%]. Moreover, we performed next-generation sequencing of 1021 cancer-related genes using tumor tissue and matched lymphocyte samples. The results suggested microsatellite-stable (MSS). No germline pathogenic or likely pathogenic variants were identified, but eight somatic mutations (MYC, TP53, TSC2, BRD2, MAP2K4, NCOR1, PTEN, FAS) were found in this patient. She finally received combined anti-VEGF and anti-PD-1 inhibitor treatment in 3-week cycles, with 240 mg toripalimab admitted intravenously on day 1 of each cycle and 10 mg anlotinib given daily on days 1 to 14 of each cycle. Adverse reactions, including grade 1 myelosuppression and grade 2 diarrhea, were noted. However, no serious adverse events were observed in this patient.
The efficacy of the combination treatment was assessed using CT and magnetic resonance imaging scans. Tumor load was evaluated at baseline and after every two cycles of treatment using response evaluation criteria in solid tumors (RECIST) guidelines (version1.1). In total, seven cycles of the combined treatment were delivered. The patient exhibited a favorable response to our combined regimen. The evaluation of efficacy after two and four cycles was partial response. The tumor shrank approximately 70% after four cycles of treatment. Surprisingly, our patient finally achieved CR after six cycles of treatment (Figure 1). PET-CT was performed to confirm CR after the seventh cycle, and no 18F-fluorodeoxyglucose elevation was detected in the original metastatic sites. The patient declined further treatment for personal reasons. Three months after the final cycle of treatment, imaging examinations revealed no evidence of tumor relapse. As the influence of COVID-19, the patient did not come to our hospital regularly for re-examination since then.
Discussion
PSA is a kind of malignancy that derived from the splenic vascular endothelium with an extremely low incidence. Fewer than 300 cases of PSA have been reported to date. The average age at diagnosis is 50–60 years (9, 10). As previously reported, the median survival time of metastatic PSA is approximately one year. Rupture of the spleen and distant metastasis are considered poor prognostic factors (11).
Because of the rarity of the disease, there is no standard treatment protocol. Furthermore, there are no randomized clinical trial data to support a systemic treatment regimen for metastatic PSA. Based on the literature of other sarcomas, some researchers have attempted several first-line chemotherapy regimens, including paclitaxel, anthracycline, doxorubicin, and ifosfamide (12–15). Unfortunately, the overall response rate was relatively low.
Combination treatment with antiangiogenic drugs and immune checkpoint inhibitors has been demonstrated to be effective in multiple malignancies (16). The underlying mechanism involves normalization of the tumor vessels through anti-VEGF therapy, which might improve the infiltration of tumors by activating effector T cells and subsequently convert the immunosuppressive tumor microenvironment (TME) into an immune-active TME (17, 18).
There have been several case reports regarding the combination therapy of antiangiogenic drugs and immune checkpoint inhibitors in sarcomas. A patient with metastatic undifferentiated pleomorphic sarcoma received pembrolizumab and pazopanib after multiple lines of therapy and had a partial response for 9 months (19). In addition, a patient with recurrent intestinal follicular dendritic cell sarcoma received sintilimab plus lenvatinib as third-line treatment and achieved a progression-free survival of 7 months (20). Several clinical trials have also explored the combination therapy in sarcoma. A phase II single-arm study of pembrolizumab plus lenvatinib in previously treated classic Kaposi sarcoma is in progress (https://www.clinicaltrialsregister.eu/ctr-search/search?query=2020-004426-36).Toripalimab is a newly developed monoclonal antibody that blocks PD-1. Clinical trial data have exhibited a promising antitumor activity of toripalimab in metastatic sarcoma and other malignancies (21, 22). Anlotinib is a novel TKI targeting VEGF1-3 and has shown encouraging effects in sarcoma (23, 24). A Phase II clinical trial (NCT04172805) is aimed to test the safety and effectiveness of anlotinib and toripalimab in soft tissue sarcoma. However, the results have not been published (https://clinicaltrials.gov/ct2/show/NCT04172805). In our patient, we innovatively attempted toripalimab and anlotinib combined therapy for metastatic PSA, and the regimen was surprisingly effective and well-tolerated.
High expression of PD-L1 predicts better efficacy of immunotherapy in several cancers (25, 26). A previous study found that the positive rate of PD-L1 was approximately 60% in angiosarcoma, and the differentiation level of the tumor was significantly associated with the PD-L1 status (27). The TPS of our patient was as high as 75% and the tumor rapidly decreased in size after several cycles of combined treatment.
Of note, we performed next-generation sequencing of tumor tissue and further analyzed angiosarcoma datasets from The Cancer Genome Atlas (TCGA). TCGA data showed the mutation frequencies of these genes in angiosarcoma patients (Figure 2). We further analyzed the correlation between the expression of these genes and the tumor mutational burden (TMB) in angiosarcoma cases from the TCGA database. Finally, we observed that PTEN mutation was negatively correlated with TMB (Figure 3).
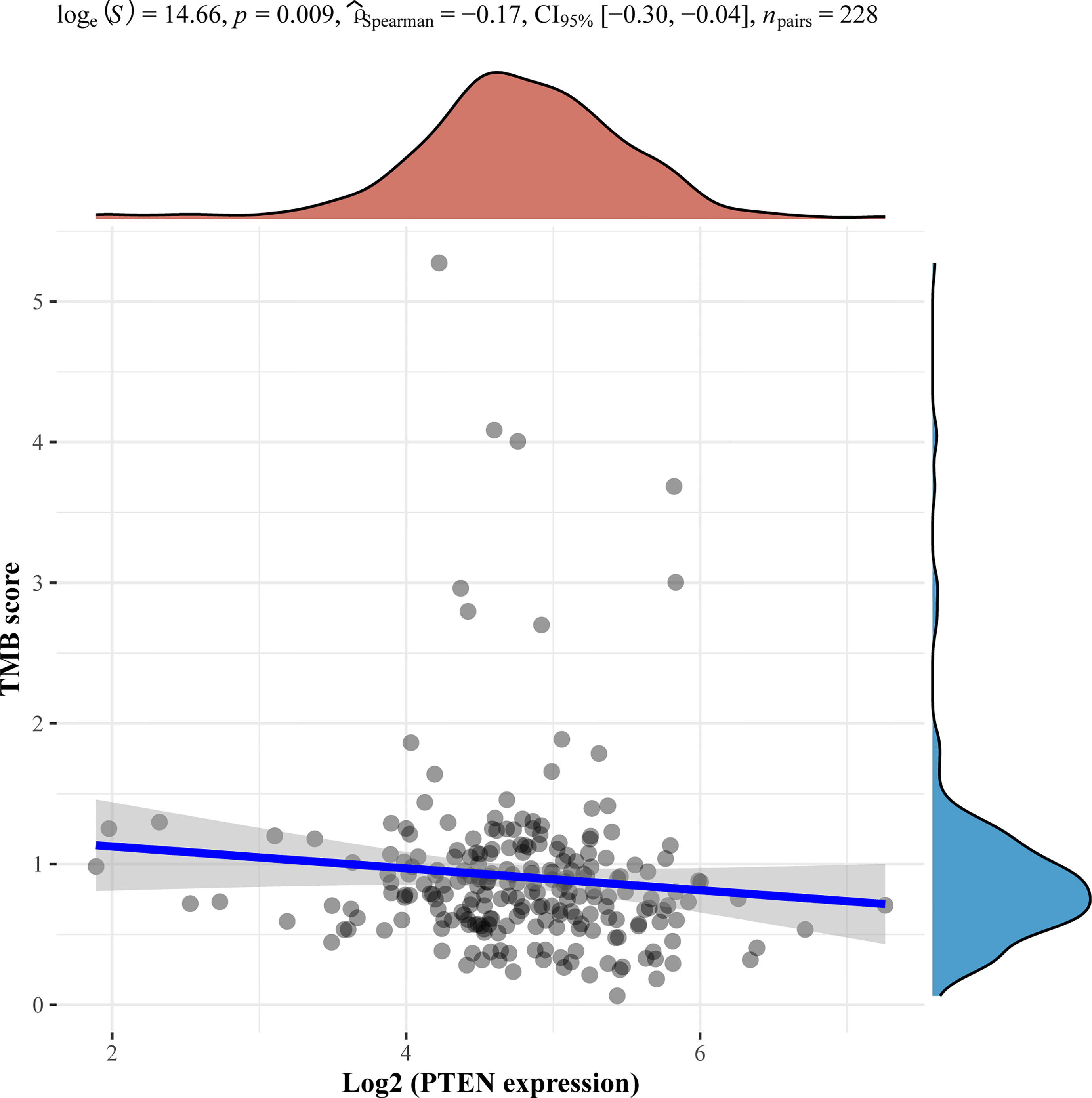
Figure 3 Correlation analysis of PTEN gene expression and TMB. The horizontal axis in the figure represents the expression distribution of the gene, and the ordinate is the expression distribution of the TMB score. The density curve on the right represents the distribution trend of the TMB score; the upper density curve represents the distribution trend of the gene; the top side represents the correlation p value, correlation coefficient and correlation calculation method.
We also found that some gene mutations of our patients were correlated with the efficacy of immunotherapy. TSC2 was associated with T cell exhaustion inhibition, which upregulated PD-L1 on tumors (28). The TP53 mutation status was related to the survival benefit of PD-1 inhibitors in non-small cell lung cancer (29). NCOR1 mutation was reported as a potential positive biomarker to predict the efficacy of immunotherapy in bladder cancer (30). However, we also identified negative predictors of immunotherapy in our patient, such as PTEN mutation (31). This gene alteration reduces CD8-positive T cells in the immune microenvironment, leading to an inadequate response to PD-1 inhibitors.
Surprisingly, our patient quickly achieved CR during toripalimab plus anlotinib treatment and had no serious adverse events. Our case suggests that anti-PD-1 plus anti-VEGF therapy might be a promising option for metastatic PSA patients with high expression of PD-L1. However, clinical trials are warranted to confirm the efficacy of this regimen for these patients.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
Ethical approval was obtained from the Peking University International Hospital Research Ethics Committee. The patient provided her written informed consent to participate in this study. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
WX drafted the manuscript. XN revised the manuscript. All authors read and approved the final manuscript.
Conflict of Interest
Author WG was employed by Geneplus-Beijing. Co-author XN and HZ were full-time employee of Shanghai Junshi Biosciences Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Melissa Crawford, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/) for editing the English text of a draft of this manuscript.
References
1. Neuhauser TS, Derringer GA, Thompson LD, Fanburg-Smith JC, Miettinen M, Saaristo A, et al. Splenic Angiosarcoma: A Clinicopathologic and Immunophenotypic Study of 28 Cases. Modern Pathol (2000) 13(9):978–87. doi: 10.1038/modpathol.3880178
2. Falk S, Krishnan J, Meis J. Primary Angiosarcoma of the Spleen. A Clinicopathologic Study of 40 Cases. Am J Surg Pathol (1993) 17(10):959–70. doi: 10.1097/00000478-199310000-00001
3. Valbuena JR, Levenback C, Mansfield P, Liu J. Angiosarcoma of the Spleen Clinically Presenting as Metastatic Ovarian Cancer. A Case Report and Review of the Literature. Ann Diagn Pathol (2005) 9(5):289–92. doi: 10.1016/j.anndiagpath.2005.03.007
4. Peckova K, Michal M, Hadravsky L, Damjanov I, Miesbauerova M, et al. Littoral Cell Angioma of the Spleen: A Study of 25 Cases With Confirmation of Frequent Association With Visceral Malignancies. Histopathology (2016) 69(5):762–74. doi: 10.1111/his.13026
5. Sordillo EM, Sordillo PP, Hajdu SI. Primary Hemangiosarcoma of the Spleen: Report of Four Cases. Med Pediatr Oncol (1981) 9(4):319–24. doi: 10.1002/mpo.2950090403
6. Mahoney KM, Freeman GJ, McDermott DF. The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma. Clin Ther (2015) 37(4):764–82. doi: 10.1016/j.clinthera.2015.02.018
7. Sui H, Ma N, Wang Y, Li H, Liu X, Su Y, et al. Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies. J Immunol Res (2018) 2018:6984948. doi: 10.1155/2018/6984948
8. Young RJ, Woll P, Staton C, Reed M, Brown N. Vascular-Targeted Agents for the Treatment of Angiosarcoma. Cancer Chemother Pharmacol (2014) 73(2):259–70. doi: 10.1007/s00280-013-2345-0
9. Despoina M, Dionysios D, Georgios A, Konstantinos S, Efstratios K, Adamantia Z-S. Primary Angiosarcoma of the Spleen: An Oncological Enigma. Case Rep Oncol Med (2014) 2014:193036. doi: 10.1155/2014/193036
10. Fotiadis C, Georgopoulos I, Stoidis C, Patapis P. Primary Tumors of the Spleen. Int J Biomed Sci: IJBS (2009) 5(2):85.
11. Chen X, Li H, Wang F, Liu H. Early Detection and Integral Resection Are Keys to Extend Survival in Patients Suffered From Primary Angiosarcoma of the Spleen: A Care-Compliant Case Report and Literature Review. Medicine (2018) 97(5):e9718. doi: 10.1097/MD.0000000000009718
12. Ferreira BP, Rodler ET, Loggers ET, Pollack SM, Jones RL. Systemic Therapy in Primary Angiosarcoma of the Spleen. Rare Tumors (2012) 4(4):178–80. doi: 10.4081/rt.2012.e55
13. Vakkalanka B, Milhem M. Paclitaxel as Neoadjuvant Therapy for High Grade Angiosarcoma of the Spleen: A Brief Report and Literature Review. Clin Med Insights: Oncol (2010) 4:107–10. CMO. S5329. doi: 10.4137/CMO.S5329
14. Skubitz KM, Haddad PA. Paclitaxel and Pegylated-Liposomal Doxorubicin Are Both Active in Angiosarcoma. Cancer: Interdiscip Int J Am Cancer Soc (2005) 104(2):361–6. doi: 10.1002/cncr.21140
15. Lewcun JA, Pameijer C, Kass R, Cream L, Hershock D, Brooks AJ, et al. Doxorubicin, Paclitaxel, and Cisplatin Based Chemotherapy for the Treatment of Angiosarcoma: Two Case Reports. Int J Surg Case Rep (2020) 68:83–7. doi: 10.1016/j.ijscr.2020.02.036
16. Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Vascular Normalization as an Emerging Strategy to Enhance Cancer Immunotherapy. Cancer Res (2013) 73(10):2943–8. doi: 10.1158/0008-5472.CAN-12-4354
17. Pitt JM, Vétizou M, Daillère R, Roberti MP, Yamazaki T, Routy B, et al. Resistance Mechanisms to Immune-Checkpoint Blockade in Cancer: Tumor-Intrinsic and-Extrinsic Factors. Immunity (2016) 44(6):1255–69. doi: 10.1016/j.immuni.2016.06.001
18. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing Cancer Immunotherapy Using Antiangiogenics: Opportunities and Challenges. Nat Rev Clin Oncol (2018) 15(5):325–40. doi: 10.1038/nrclinonc.2018.29
19. Arora S, Rastogi S, Shamim SA, Barwad A, Sethi M. Good and Sustained Response to Pembrolizumab and Pazopanib in Advanced Undifferentiated Pleomorphic Sarcoma: A Case Report. Clin Sarcoma Res (2020) 10(1):1–6. doi: 10.1186/s13569-020-00133-9
20. Jiang M, Lei Y, Zhao S. Unexpected Favorable Outcome to PD-1 Antibody Plus Lenvatinib in a Patient With Recurrent Intestinal Follicular Dendritic Cell Sarcoma: A Case Report and Literature Review. Front Immunol (2021) 12:653319. doi: 10.3389/fimmu.2021.653319
21. Yang J, Dong L, Yang S, Han X, Han Y, Jiang S, et al. Safety and Clinical Efficacy of Toripalimab, a PD-1 mAb, in Patients With Advanced or Recurrent Malignancies in a Phase I Study. Eur J Cancer (2020) 130:182–92. doi: 10.1016/j.ejca.2020.01.028
22. Tang B, Chi Z, Chen Y, Liu X, Wu D, Chen J, et al. Safety, Efficacy, and Biomarker Analysis of Toripalimab in Previously Treated Advanced Melanoma: Results of the POLARIS-01 Multicenter Phase II Trial. Clin Cancer Res (2020) 26(16):4250–9. doi: 10.1158/1078-0432.CCR-19-3922
23. Wang H-y, Chu J-f, Zhang P, Wang J-q, Yan Z, Yao S-n, et al. Safety and Efficacy of Chemotherapy Combined With Anlotinib Plus Anlotinib Maintenance in Chinese Patients With Advanced/Metastatic Soft Tissue Sarcoma. OncoTargets Ther (2020) 13:1561. doi: 10.2147/OTT.S235349
24. Liu Z, Yao W, Zhao Y, Liu O, Zhang P, Ge H. Efficacy and Safety of Anlotinib Combined With Liposomal Doxorubicin Followed by Anlotinib Maintenance in Metastatic Soft Tissue Sarcomas. Cancer Manage Res (2021) 13:1009. doi: 10.2147/CMAR.S286322
25. Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 Inhibitors as a Form of Cancer Immunotherapy: A Comprehensive Review of Registration Trials and Future Considerations. J Immunother Cancer (2018) 6(1):1–18. doi: 10.1186/s40425-018-0316-z
26. Jiang Y, Chen M, Nie H, Yuan Y. PD-1 and PD-L1 in Cancer Immunotherapy: Clinical Implications and Future Considerations. Hum Vaccines Immunother (2019) 15(5):1111–22. doi: 10.1080/21645515.2019.1571892
27. Botti G, Scognamiglio G, Marra L, Pizzolorusso A, Di Bonito M, De Cecio R, et al. Programmed Death Ligand 1 (PD-L1) Expression in Primary Angiosarcoma. J Cancer (2017) 8(16):3166. doi: 10.7150/jca.19060
28. Liu H-J, Lizotte PH, Du H, Speranza MC, Lam HC, Vaughan S, et al. TSC2-Deficient Tumors Have Evidence of T Cell Exhaustion and Respond to Anti–PD-1/Anti–CTLA-4 Immunotherapy. JCI Insight (2018) 3(8):e98674. doi: 10.1172/jci.insight.98674
29. Assoun S, Theou-Anton N, Nguenang M, Cazes A, Danel C, Abbar B, et al. Association of TP53 Mutations With Response and Longer Survival Under Immune Checkpoint Inhibitors in Advanced Non-Small-Cell Lung Cancer. Lung Cancer (2019) 132:65–71. doi: 10.1016/j.lungcan.2019.04.005
30. Lin A, Qiu Z, Zhang J, Luo P. Effect of NCOR1 Mutations on Immune Microenvironment and Efficacy of Immune Checkpoint Inhibitors in Patient With Bladder Cancer. Front Immunol (2021) 12:248. doi: 10.3389/fimmu.2021.630773
Keywords: primary splenic angiosarcoma, PD-1 inhibitor, immunotherapy, anti-VEGF therapy, complete remission
Citation: Xu W, Wang K, Gu W, Nie X, Zhang H, Tang C, Lin L and Liang J (2022) Case Report: Complete Remission With Anti−PD−1 and Anti−VEGF Combined Therapy of a Patient With Metastatic Primary Splenic Angiosarcoma. Front. Oncol. 12:809068. doi: 10.3389/fonc.2022.809068
Received: 04 November 2021; Accepted: 01 February 2022;
Published: 03 March 2022.
Edited by:
Salem Chouaib, Institut Gustave Roussy, FranceReviewed by:
Luigi Cerbone, Gustave Roussy Cancer Campus, FranceCarolina Alves Costa Silva, Gustave Roussy Cancer Campus, France
Copyright © 2022 Xu, Wang, Gu, Nie, Zhang, Tang, Lin and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Lin, linli010120@163.com; Chuanhao Tang, gallanttang@126.com; Jun Liang, liangjun1959@aliyun.com
†These authors have contributed equally to this work and share first authorship
 Weiran Xu
Weiran Xu Kai Wang2†
Kai Wang2†