- 1Department of Medical Oncology, The Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan
- 2Department of Medical Oncology/Haematology, Kakogawa Central City Hospital, Hyogo, Japan
Background/Aim: Most paratesticular liposarcomas (PLPSs) are well-differentiated liposarcomas (WDLPSs) with favourable prognoses. As such, the rare occurrence of PLPS often leads to its misdiagnosis as a hernia or hydrocele on physical examination. Curative resection of the tumour may not be possible in cases where PLPSs have transformed into dedifferentiated liposarcomas (DDLPSs) owing to a delay in diagnosis. Herein, we describe a case of unresectable paratesticular dedifferentiated liposarcoma (PDDLPS) with poor prognosis due to delayed diagnosis.
Case Report: A 57-year-old man visited our hospital with a chief complaint of a right scrotal mass, which was diagnosed as scrotal hydrocele but without treatment or follow-up. Eight years later, the patient complained of abdominal distension, and a computed tomography scan revealed the presence of retroperitoneal and right scrotal masses. The right scrotal mass was removed, and histopathology revealed DDLPS. The patient was diagnosed with unresectable PDDLPS metastasising to the retroperitoneum, and the left pleura was treated with doxorubicin. After an initial response, pleural effusion and ascites increased during the sixth cycle of chemotherapy. The patient subsequently received eribulin but died 5 months after the initial DDLPS diagnosis.
Conclusion: It is difficult to distinguish PLPS from benign inguinal hernia and hydrocele testis on physical examination. PLPS generally has a considerably good prognosis. However, failure to diagnose WDLPS can be dangerous as it might lead to malignant transformation to DDLPS, which has a poor prognosis. Physicians should consider this malignancy when examining patients with hernias or hydroceles of the inguinal region and should perform ultrasonography or magnetic resonance imaging.
Introduction
Malignant soft tissue tumours usually occur in the extremities or retroperitoneum and rarely in the proximal testicular region (1, 2). The most common subtype accounting for 39%–51% of paratesticular sarcomas is liposarcoma (3–5). Most paratesticular liposarcomas (PLPSs) appear as low-growing, painless, inguinal, or scrotal masses (1, 6, 7). Therefore, they are curatively resectable and thus have a good prognosis. However, the rare occurrence of PLPS often leads to its misdiagnosis as a hernia or hydrocele on physical examination (8, 9). In some cases, due to the transformation of PLPS to dedifferentiated liposarcoma (DDLPS) because of delayed diagnosis, curative resection of the tumour may not be possible. Herein, we describe a case of unresectable paratesticular dedifferentiated liposarcoma (PDDLPS) with poor prognosis due to delayed diagnosis.
Case description
A 57-year-old-man presented to our hospital with the chief complaint of a painless right scrotal mass. On physical examination, his right scrotum was enlarged, but was painless, elastic, and soft. Thus, it was diagnosed as scrotal hydrops and left untreated. Two years later, a magnetic resonance imaging (MRI) scan showed a mass, 6 cm diameter, in his right scrotum with a high signal intensity on T1-weighted image. There was partial heterogeneity inside the mass, and a diagnosis of lipoma was made (Figure 1A). After more than 6 years without treatment, he presented again with the chief complaint of mild abdominal distention and a right scrotal mass. Palpation revealed that the scrotum was slightly firm with a smooth surface swelling. Abdominal ultrasonography revealed a rather heterogeneous, hyperechoic right scrotal mass. Abdominal-pelvic computed tomography (CT) scan showed a 50 × 70 × 68 mm3 substantial mass in the right scrotum and a 10 cm wide mass in the right retroperitoneum (Figures 1B, C). The peritoneum also showed nodules and ascites. There was no elevation of serum human chorionic gonadotropin or alpha-fetoprotein level, and the germ cell tumour was considered unlikely. There was a strong suspicion that the scrotal tumour was malignant. The patient underwent a diagnostic high right radical inguinal orchiectomy, which revealed a yellowish-grey-white nodule that was compressing the testis and epididymis. This nodule was 73 × 50 mm in size and well-demarcated. Histopathological studies indicated that the nodule was composed of a bundle-like proliferation of medium-sized spindle-shaped cells interspersed with low-grade atypical adipoblasts and large adipocytes (Figures 2A–C). Immunostaining was positive for MDM2 and negative for S-100 protein (Figures 2D, E). The patient was diagnosed with liposarcoma with a dedifferentiated component. We diagnosed the retroperitoneal tumour as metastasis of PDDLPS based on CT findings. Five weeks after surgery, a CT scan showed increasing ascites and left pleural effusion (Figure 1D). We aspirated the pleural fluid for a differential diagnosis of the left pleural effusion. The pleural effusion was haemorrhagic but showed no malignant cells. Keeping in mind the clinical presentation, we diagnosed the cause of the left pleural effusion as pleural metastasis of PDDLPS. Therefore, we initiated monotherapy with doxorubicin (DXR) for unresectable dedifferentiated liposarcoma. After three cycles of DXR monotherapy, CT showed resolution of pleural effusion (Figure 1E). However, CT after six cycles again showed an increased pleural effusion (Figure 1F). Hence, we started eribulin as the second line of treatment, but the patient did not respond and died 5 months after the definite diagnosis.
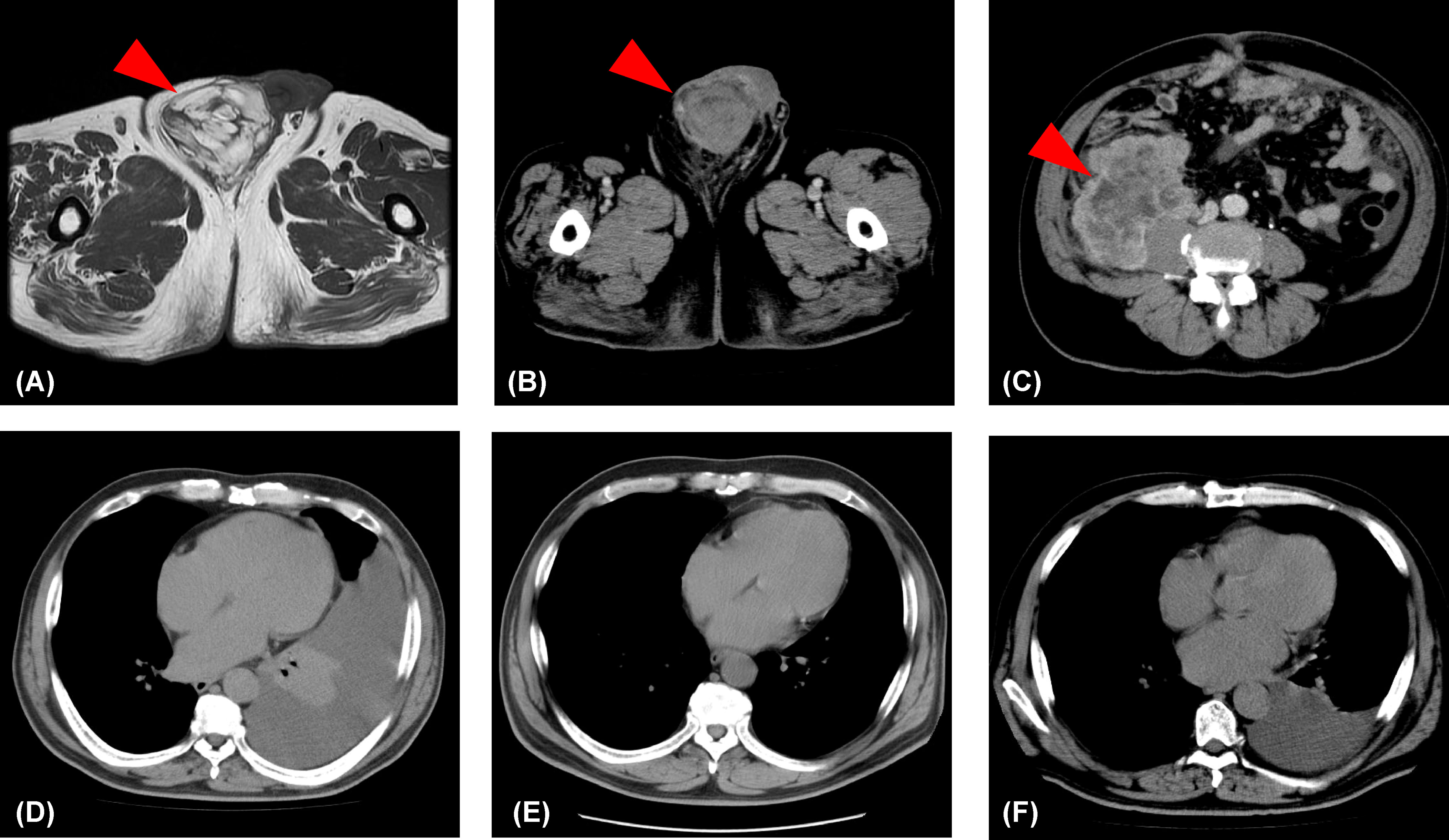
Figure 1 Changes in the lesions identified by imaging. (A) T1-weighted image showing a lipoma (red arrowhead) in the right scrotum. (B) An abdominal-pelvic computed tomography scan showing a substantial mass (>5 cm; red arrowhead) in the right scrotum. (C) An abdominal-pelvic computed tomography scan showing a mass (>10 cm; red arrowhead) in the right retroperitoneum. (D) A 5-week postoperative computed tomography scan showing a left pleural effusion. (E) A computer tomography scan showing no pleural effusion following three cycles of doxorubicin monotherapy. (F) A computer tomography scan showing increased pleural effusion following six cycles of doxorubicin monotherapy.
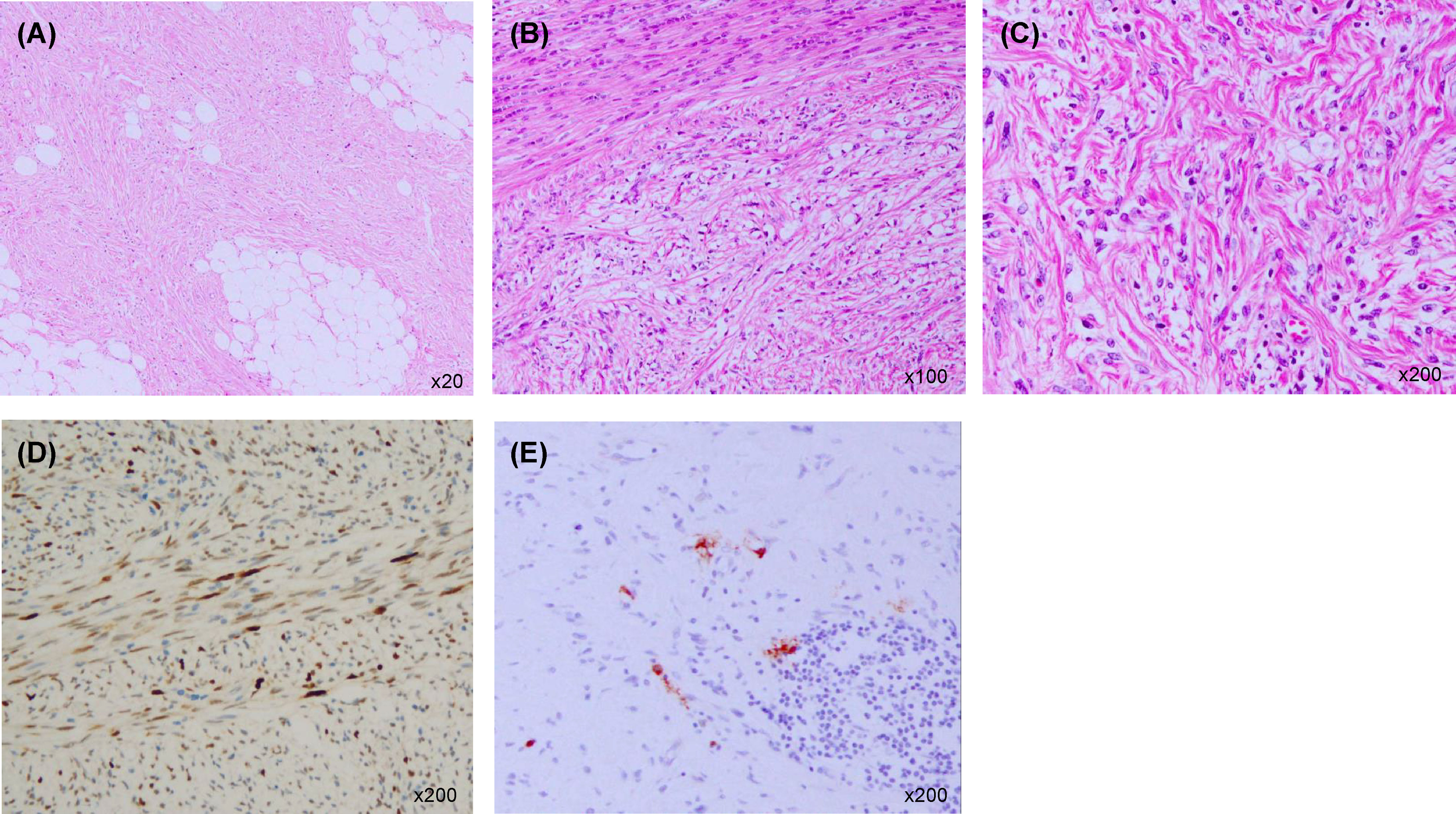
Figure 2 Histopathological examination of the right paratesticular mass. (A) Large adipocytes (haematoxylin and eosin staining ×20). (B) A bundle-like proliferation of medium-sized spindle-shaped cells interspersed with low-grade atypical adipoblasts (haematoxylin and eosin staining ×100). (C) Fibrosarcoma-like dedifferentiated liposarcoma (haematoxylin and eosin staining ×200). (D) Immunostaining positive for MDM2 (×200). (E) Immunostaining negative for S-100 protein (×200).
Discussion
We report a case in which a right scrotal mass was initially misdiagnosed as a benign disease, and was later diagnosed as unresectable PDDLPS after 8 years of no treatment. The patient died 5 months after the definitive diagnosis.
More than half of PLPSs are well-differentiated liposarcomas (WDLPSs), and distant metastasis is extremely rare (1, 10, 11). However, some WDLPSs may transform into DDLPS; their rates of transformation to DDLPS are 6% in primary extremity WDLPS and 28% in paratesticular WDLPS (PWDLPS) (12). The time to dedifferentiation is 2–25 years (12). The distant metastasis rate for PDDLPS is 5%–10% higher than that for PWDLPS (1, 11, 13) (Table 1). Therefore, the prognosis is also worse for PDDLPS than for PWDLPS (10).
Liposarcomas, similar to other malignant soft tissue tumours, tend to occur in the extremities and retroperitoneum (1, 12, 14). PLPSs, in particular, are rare, accounting for 4.4% of liposarcomas (15). However, paratesticular metastases of malignant tumours are rare, and the most common primary site of metastasis is from solid tumours, such as gastric cancer (16). Paratesticular metastasis of primary retroperitoneal DDLPS is extremely rare (1).
This case showed not only a dedifferentiated component within the paratesticular tumour but also a highly differentiated component. Therefore, it is quite plausible that the PWDLPS transformed into PDDLPS and metastasised to the retroperitoneum and pleura in this patient.
First-line chemotherapy regimens containing DXR are recommended for liposarcomas with distant metastases. The response rate of DDLPS is 24%, the median progression-free survival (PFS) is 4 months, and the median overall survival (OS) is 25 months (17). Although eribulin is effective as a second-line treatment for liposarcoma, the response rate in DDLPS is 0%, the median PFS is 2 months, and the median OS is 8 months (18). Thus, there are limited effective treatments for dedifferentiated liposarcoma, including second-line chemotherapy. In our case, pleural effusion was resolved after DXR administration but worsened after approximately 4 months and did not respond to eribulin; the patient died only 5 months after diagnosis.
On physical examination, it is difficult to distinguish PLPS from benign inguinal hernia and hydrocele testis; however, PLPS has a considerably better prognosis as most PLPSs are WDLPSs with no possibility of metastasis and possible surgical resection. However, failure to timely diagnose WDLPS can be dangerous and may lead to malignant transformation into DDLPS, which has a poor prognosis. Ultrasonography shows a uniform hypoechoic area in the case of hydrocele testis but a heterogeneous hyperechoic area in the case of liposarcoma. In addition, MRI shows high signal intensity on T1-weighted images in the case of a fat-fitting inguinal hernia and lipoma and a low signal intensity in the case of liposarcoma. Hence, physicians should consider this malignancy when examining patients with hernias or hydroceles of the inguinal region and should perform ultrasonography or MRI.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional Review Board Kakogawa Central City Hospital Ethics Committee. The patient’s family provided their written informed consent to participate in this study.
Author contributions
Conceptualisation: HS, and AO. Methodology: HS, and AO. Investigation: HS, YI, and AO. Data curation: HS, YI, and AO. Writing—original draft preparation: HS. Writing—review and editing: HS, YI, and AO. Supervision: AO. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Montgomery E, Fisher C. Paratesticular liposarcoma: A clinicopathologic study. Am J Surg Pathol (2003) 27:40–7. doi: 10.1097/00000478-200301000-00005
2. Crago AM, Brennan MF. Principles in management of soft tissue sarcoma. Adv Surg (2015) 49:107–22. doi: 10.1016/j.yasu.2015.04.002
3. Rodríguez D, Barrisford GW, Sanchez A, Preston MA, Kreydin EI, Olumi AF. Primary spermatic cord tumors: disease characteristics, prognostic factors, and treatment outcomes. Urol Oncol (2014) 32:52.e19–25. doi: 10.1016/j.urolonc.2013.08.009
4. Fagundes MA, Zietman AL, Althausen AF, Coen JJ, Shipley WU. The management of spermatic cord sarcoma. Cancer (1996) 77:1873–6. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1873
5. Coleman J, Brennan MF, Alektiar K, Russo P. Adult spermatic cord sarcomas: management and results. Ann Surg Oncol (2003) 10:669–75. doi: 10.1245/aso.2003.11.014
6. Schwartz SL, Swierzewski SJ 3rd, Sondak VK, Grossman HB. Liposarcoma of the spermatic cord: report of 6 cases and review of the literature. J Urol (1995) 153:154–7. doi: 10.1097/00005392-199501000-00055
7. Folpe AL, Weiss SW. Paratesticular soft tissue neoplasms. Semin Diagn Pathol (2000) 17:307–18. doi: 10.1007/978-3-319-27617-5_10
8. Noguchi H, Naomoto Y, Haisa M, Yamatsuji T, Shigemitsu K, Uetsuka H, et al. Retroperitoneal liposarcoma presenting a indirect inguinal hernia. Acta Med Okayama (2001) 55:51–4. doi: 10.18926/AMO/32032
9. Hassan JM, Quisling SV, Melvin WV, Sharp KW. Liposarcoma of the spermatic cord masquerading as an incarcerated inguinal hernia. Am Surg (2003) 69:163–5.
10. Kamitani R, Matsumoto K, Takeda T, Mizuno R, Oya M. Optimal treatment strategy for paratesticular liposarcoma: retrospective analysis of 265 reported cases. Int J Clin Oncol (2020) 25:2099–106. doi: 10.1007/s10147-020-01753-3
11. Morozumi K, Kawasaki Y, Kaiho Y, Kawamorita N, Fujishima F, Watanabe M, et al. Dedifferentiated liposarcoma in the spermatic cord finally diagnosed at 7th resection of recurrence: A case report and bibliographic consideration. Case Rep Oncol (2017) 10:713–9. doi: 10.1159/000479364
12. Weiss SW, Rao VK. Well-differentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites. a follow-up study of 92 cases with analysis of the incidence of “dedifferentiation”. Am J Surg Pathol (1992) 16:1051–8. doi: 10.1097/00000478-199211000-00003
13. Kryvenko ON, Rosenberg AE, Jorda M, Epstein JI. Dedifferentiated liposarcoma of the spermatic cord: A series of 42 cases. Am J Surg Pathol (2015) 39:1219–25. doi: 10.1097/PAS.0000000000000426
14. Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: A clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol (1997) 21:271–81. doi: 10.1097/00000478-199703000-00002
15. Hare HF, Cerny MJ Jr. Soft tissue sarcoma. A Rev 200 cases. Cancer (1963) 16:1332–7. doi: 10.1002/1097-0142(196310)16:10<1332
16. Algaba F, Santaularia JM, Villavicencio H. Metastatic tumor of the epididymis and spermatic cord. Eur Urol (1983) 9:56–9. doi: 10.1159/000474045
17. Livingston JA, Bugano D, Barbo A, Lin H, Madewell JE, Wang WL, et al. Role of chemotherapy in dedifferentiated liposarcoma of the retroperitoneum: defining the benefit and challenges of the standard. Sci Rep (2017) 7:11836. doi: 10.1038/s41598-017-12132-w
18. Demetri GD, Schöffski P, Grignani G, Blay JY, Maki RG, Van Tine BA’, et al. Activity of eribulin in patients with advanced liposarcoma demonstrated in a subgroup analysis from a randomized phase III study of eribulin versus dacarbazine. J Clin Oncol (2017) 35:3433–9. doi: 10.1200/JCO.2016.71.6605
Keywords: paratesticular dedifferentiated liposarcoma, poor prognosis, delayed diagnosis, ultrasonography, magnetic resonance imaging, well-differentiated liposarcoma
Citation: Suto H, Inui Y and Okamura A (2022) Case report: Paratesticular dedifferentiated liposarcoma with poor prognosis. Front. Oncol. 12:1085794. doi: 10.3389/fonc.2022.1085794
Received: 31 October 2022; Accepted: 15 November 2022;
Published: 01 December 2022.
Edited by:
Haoran Liu, Stanford University, United StatesReviewed by:
Bingbing Hou, First Affiliated Hospital of Anhui Medical University, ChinaRahul Gupta, Synergy Institute of Medical Sciences, India
Ketan Vagholkar, Padmashree Dr. D.Y. Patil University, India
Copyright © 2022 Suto, Inui and Okamura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hirotaka Suto, hirotaka.suto@jfcr.or.jp
 Hirotaka Suto
Hirotaka Suto Yumiko Inui2
Yumiko Inui2