- 1Key Laboratory for Experimental Teratology of Ministry of Education and Dept. Immunology, School of Basic Medical Sciences, Cheeloo Medical College, Shandong University, Jinan, Shandong, China
- 2Key Laboratory of Infection and Immunity of Shandong Province, Shandong University, Jinan, Shandong, China
As a transcriptional factor and the negative regulator of alpha fetal protein (AFP), Zinc fingers and homeoboxes 2 (ZHX2) has a well-established role in protection against hepatocellular carcinoma (HCC). However, recent studies have suggested ZHX2 as an oncogene in clear cell renal cell carcinoma (ccRCC) and triple-negative breast cancer (TNBC). Moreover, mounting evidence has illustrated a much broader role of ZHX2 in multiple cellular processes, including cell proliferation, cell differentiation, lipid metabolism, and immunoregulation. This comprehensive review emphasizes the role of ZHX2 in health and diseases which have been more recently uncovered.
Introduction
ZHX2, a member of the ZHX (Zinc fingers and homeoboxes) family, is a ubiquitous transcriptional factor that was first identified as a negative regulator of murine postnatal alpha fetal protein (AFP) (1). In 1977, Roushlatti and colleagues compared serum AFP in different mouse strains and found a gene which they called Regulator of Alpha-fetoprotein (Raf), subsequently renamed Alpha-fetoprotein regulator 1 (Afr1), negatively regulated the AFP expression in adult mice (1, 2). In 2005, Perincheri et al. further refined and identified Zhx2 as the homologous gene of Afr1 by positional cloning (3). Human ZHX2 was first cloned by Nagase et al. from a size-fractionated brain cDNA library in 1998 (2). In 2003, human ZHX2 was then identified as a ZHX1-interacting protein by Kawata et al. (4).
ZHX2 has been extensively studied in cancer development. ZHX2 suppresses the transcription of oncofetal genes AFP (1, 3, 5) and glypican 3 (GPC3), and works as a tumor suppressor gene in HCC (5, 6). Subsequent studies have found that ZHX2 is widely expressed and participates in many types of cancer. Consistent with findings in HCC, low ZHX2 expression correlates with poor prognosis of thyroid cancer (7), multiple myeloma (8–10), and chronic lymphocytic leukemia (11, 12). On the contrary, ZHX2 promotes the development of ccRCC (13–15), TNBC (16), and gastric cancer (17, 18). Beyond regulating cancer development, the latest reports have shown that ZHX2 involves in several other physiological or pathological processes, including cell differentiation and development (19–21), lipid metabolism (22–24), and viral replication (25, 26). Especially, ZHX2 is abundantly expressed in the thymus and spleen (2) and there is clear evidence supporting the involvement of ZHX2 in regulating B cell development (27), NK cell maturation (28), and macrophage polarization (29–31).
In this review, we outline these new advances in ZHX2 mediated regulation in health and diseases. We also discuss the multiple mechanisms involved in controlling ZHX2 expression and transcription.
ZHX2 protein structure and its role as a transcription factor
The human ZHX2 gene is localized on chromosome 8q24.13 and consists of 4 exons (4). The third exon is the sole coding exon of ZHX2 which encodes a protein of 837 amino acid residues (4). Human ZHX2 protein, like the other two family members ZHX1 and ZHX3, contains two Cys-Xaa2-Cys-Xaa12-His-Xaa4-His-type zinc finger domains (Znf) and four homeodomains (HD) (originally thought as five HDs) (4). Besides, ZHX2 contains a proline-rich region (PRR) at position 408 to 440 between HD1 and HD2 (4). The homology of ZHX2 protein in humans and mice is as high as 87%. Kawata et al., in 2003, identified ZHX2 as a ubiquitous transcription factor. ZHX2 interacts with nuclear transcription factor Y subunit alpha (NF-YA) and forms homodimers or heterodimers with ZHX1 or ZHX3 to exert transcriptional inhibitory function (5). The amino acid sequence between residues 195 and 358 containing HD1 is required for homodimerization of ZHX2, and ZHX2 interacts with NF-YA via the region between 263 and 497 residues (4). Similar to full-length ZHX2, truncated ZHX2 containing residues 242-446 (ZHX2(242-446)) but not ZHX2(242-439) maintain the capability to localize in the nuclei and suppress the expression of Cyclin A/E in HCC (6). The decreased nucleic ZHX2 expression significantly correlates with poor survival of HCC patients (6). However, how ZHX2 loses its nuclear localization is completely unknown. More studies are required to define the exact nuclear localization signal (NLS) and the molecules or mechanisms regulating the nuclei translocation of ZHX2.
A growing number of genes have been identified as the ZHX2 targets, most of which are cancer-related. ZHX2 not only negatively controls the transcription of liver cancer marker genes AFP and GPC3, but also inhibits cell proliferation-related genes such as Cdc25 (4), Cyclin A/E (6), and Notch1 (32). In addition, ZHX2 represses transcription of multidrug resistance mutation 1 (MDR1) (33), lipoprotein lipase (LPL) (34), lysine demethylase 2A (KMD2A) (35), and S100 calcium binding protein A14 (S100A14) (7) in HCC and thyroid cancer cells. Although ZHX2 was originally reported to be a ubiquitous transcriptional repressor, recent reports uncover another face of ZHX2 as a transcriptional activator (36, 37). Jiang et al. found that Zhx2 binds Mup promoters and is required for high levels of Mup expression in adult mouse liver (36). ZHX2 also binds to the promoter of phosphatase and tensin homolog (PTEN) and subsequently promotes the transcription of PTEN (37). Strikingly, several non-coding RNAs have been elucidated as the ZHX2 targets, either enhanced or inhibited. ZHX2 represses transcription of H19 (3, 38, 39), the first imprinted non-coding transcript to be identified. In glioma cells, ZHX2 binds to the promoter region of linc00707 and negatively regulates its expression, leading to glioma cells proliferation, migration and invasion, and vasculogenic mimicry (VM) formation (40). On the contrary, ZHX2 increases transcription of miR-24-3p and linc01431, which targets SREBP1c (24) and PRMT1 (26) in hepatocytes respectively.
The mechanism by which ZHX2 controls target gene transcription is not fully understood. ZHX2 was originally known as an NF-YA interacting protein (4) and therefore represses transcription of MDR1, Cdc25, and Notch1 by interacting with NF-YA (4, 6, 32, 33). However, there is no evidence for the presence of NF-YA binding sites in promoter of some other ZHX2-targeted genes, such as Cyclin E, or AFP (5, 6). A global analysis of Zhx2 targets using ChIP-seq in a murine macrophage cell line shows a significant overlap with two known apoptosis regulators Jun (41) and Bcl6 (42), which suggest a strong involvement of Zhx2 in cell apoptosis (30). In ccRCC, ChIP-seq data indicate that the genome-wide chromatin occupancy of ZHX2 overlaps with 75% of p65-binding motifs (13). ZHX2 and RelA/p65 overlapping sites also display a strong enrichment for H3K4me3 and H3K27ac, indicating that ZHX2 colocalizes with NF-κB to active gene promoters (13). In TNBC, the integrated ChIP-seq and gene expression profiling show that ZHX2 and HIF1α co-occupy transcriptional active promoters to promote gene expression (16). These studies suggest that ZHX2 may mainly serve as a transcriptional cofactor, interacting with different coactivators/repressors in different physiological circumstance to control its localization in the genome and downstream transcriptional activity. In addition, the Znf domains of ZHX2 process potential DNA-binding activity, however, whether ZHX2 can bind DNA directly and its consensus binding motif still need to be investigated.
Control of ZHX2 expression
ZHX2 expression is tightly regulated under different circumstances. A computational network study indicates ZHX2 as one of the most regulated transcription factors in myeloid cells to avoid an avalanche of transcriptional events (31). In Hodgkin lymphoma (HL), a chromosomal rearrangement far upstream region of ZHX2 gene results in the transcriptional silence of ZHX2, and two transcription factors, homeodomain protein MSX1 and bZIP protein XBP1, are identified to directly regulate ZHX2 expression (11). Furthermore, human ZHX2 is lower expressed in fetal liver, increased after birth, and silenced in HCC (43–45). Consequently, multiple mechanisms are revealed to control ZHX2 expression at different levels (Figure 1):
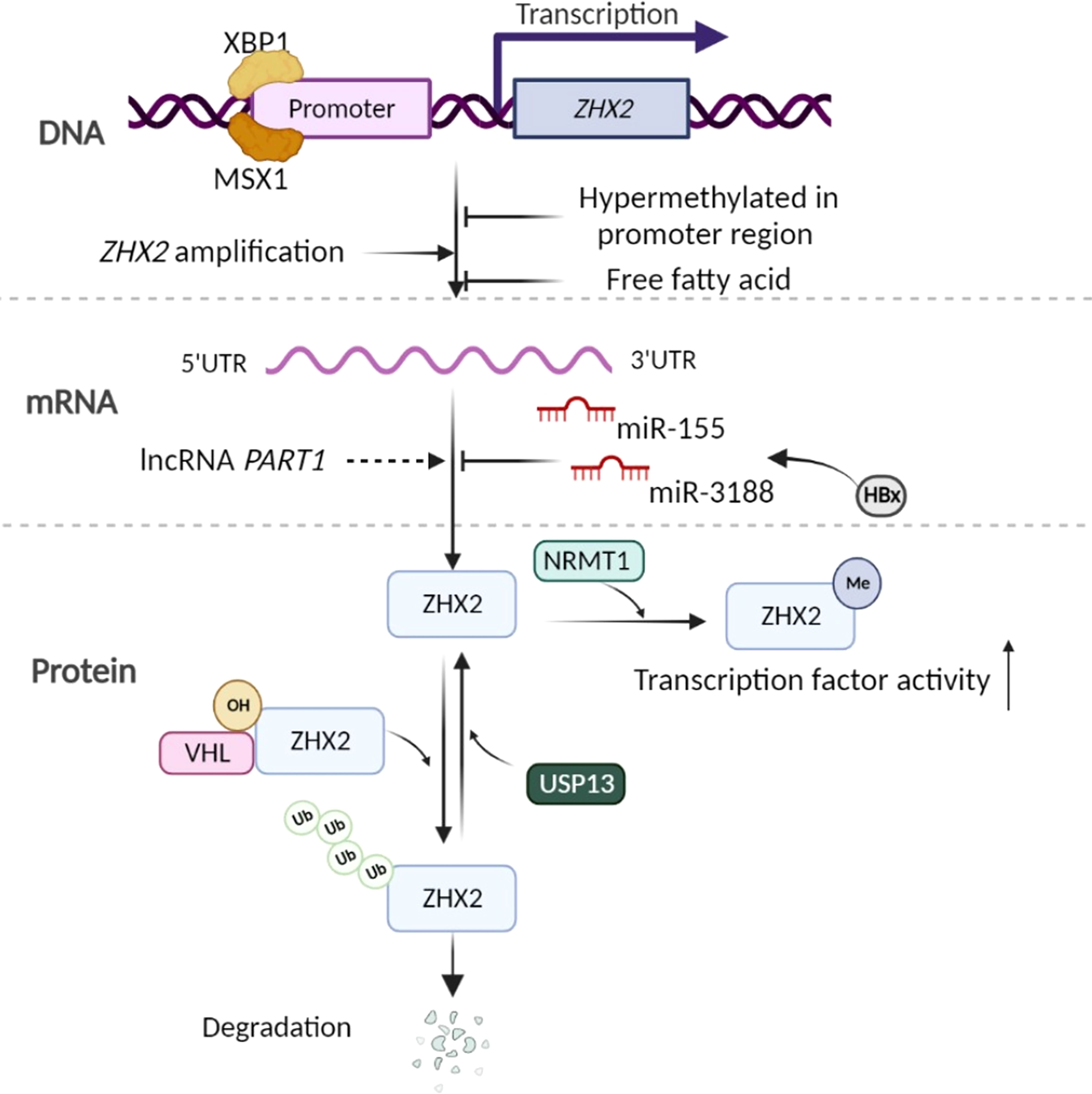
Figure 1 Control of ZHX2 expression. At the gene and transcription level, some transcription factors, hypermethylation of ZHX2 promotor, and cellular stimuli such as free fatty acid are known to regulate ZHX2 transcription. Concurrently, ZHX2 gene amplification contributes to its enhanced expression in cancer. At the post-transcription level, miR-155 and miR-3188 upregulated by HBx inhibit ZHX2 mRNA translation, but lncRNA PART1 promotes ZHX2 mRNA level by altering the miRNA landscape. At the PTMs level, hydroxylated ZHX2 protein is recognized and degraded by E3 ubiquitin ligase VHL, which is inhibited by USP13-induced deubiquitination, while NRMT1-mediated Nα-methylation of ZHX2 promotes its transcription factor activity. Created using Biorender.com.
At the ZHX2 gene transcription level- Lv et al. found that ZHX2 promoter region is hypermethylated in HCC, suggesting that the hypermethylation-mediated silencing of ZHX2 is an epigenetic event involved in HCC (45). In addition, copy number analysis showed that ZHX2 gene is amplified in various cancers, including ovarian cancer (~40%) and breast cancer (~15%). The ZHX2 copy number significantly correlates with enhanced ZHX2 expression (16). Wu et al. (34) and Zhao et al. (37)found that Zhx2 expression can be repressed by free fatty acid in hepatocytes. Constantly, hepatic Zhx2 is reduced in mice with fatty liver, indicating that ZHX2 could be regulated by the metabolic microenvironment. This is consistent with a previous computational network study indicating ZHX2 as one of the most regulated transcription factors in myeloid cells (31). The detailed mechanisms regulating ZHX2 expression in different circumstances need to be further studied.
At the post-transcription level- microRNAs (miRNAs) are short non-coding RNAs that regulate gene expression post-transcriptionally. They generally bind to the 3’-UTR (untranslated region) of their target mRNAs and reduce protein production by destabilizing mRNA or translational silencing (46, 47). HBV-encoded proteins, particularly a well-known oncogenic protein HBx, drive the high expression of miR-155, which binds to seed sites in the 3’-UTR of the ZHX2 mRNA and inhibit its translation (48). Similarly, HBx promotes CREB-mediated activation of miR-3188 to repress ZHX2 expression, leading to activated Notch signaling in HCC (32). While in TNBC, lncRNA PART1 promotes ZHX2 transcription (49).
At the posttranslational modifications (PTMs) level- Zhang et al. report that inactivation of the von Hippel-Lindau (VHL) E3 ubiquitin ligase in ccRCC leads to the accumulation of ZHX2 protein and its nuclear localization. ZHX2 protein hydroxylation at proline 427, 440, and 464 allows VHL to bind and promote its protein degradation (13). However, a deubiquitinase USP13 inhibits the ubiquitination of ZHX2 and enhances its stability (15). A recent study found that the N-terminal methylation (Nα-methylation) of ZHX2 by the methyltransferase NRMT1 regulates its transcription factor activity and its occupancy on targeted promoters (50). Up to now, whether there are other PTMs and their roles in ZHX2 trafficking, stability, and transcriptional activity are less clear.
ZHX2 in cancer-a context-dependent tumor repressor or driver?
ZHX2 is initially identified as an AFP repressor and a tumor repressor in HCC (3, 5). Whereafter, abnormal expression of ZHX2 is reported in multiple types of tumor (6, 8, 11). Furthermore, ZHX2 expression is closely related to the malignancy and poor prognosis of B-cell lymphoma (11, 12), myeloma (8–10), lung cancer (51), and thyroid cancer (7), suggesting that ZHX2 plays an important role in tumorigenesis and cancer development. Interestingly, latest studies reported that ZHX2 functions as an oncogene in ccRCC (13, 14) and TNBC (16). Likewise, Jiang et al. reported that the whole-body knockout of Zhx2 results in reduced liver tumors in diethylnitrosamine (DEN)-induced HCC mice (52). Therefore, ZHX2 is abnormally expressed in multiple tumors and plays different roles, either acting as a tumor suppressor or oncogene in a context-dependent manner (Figure 2). Here, we outline the role of ZHX2 in multiple tumors.
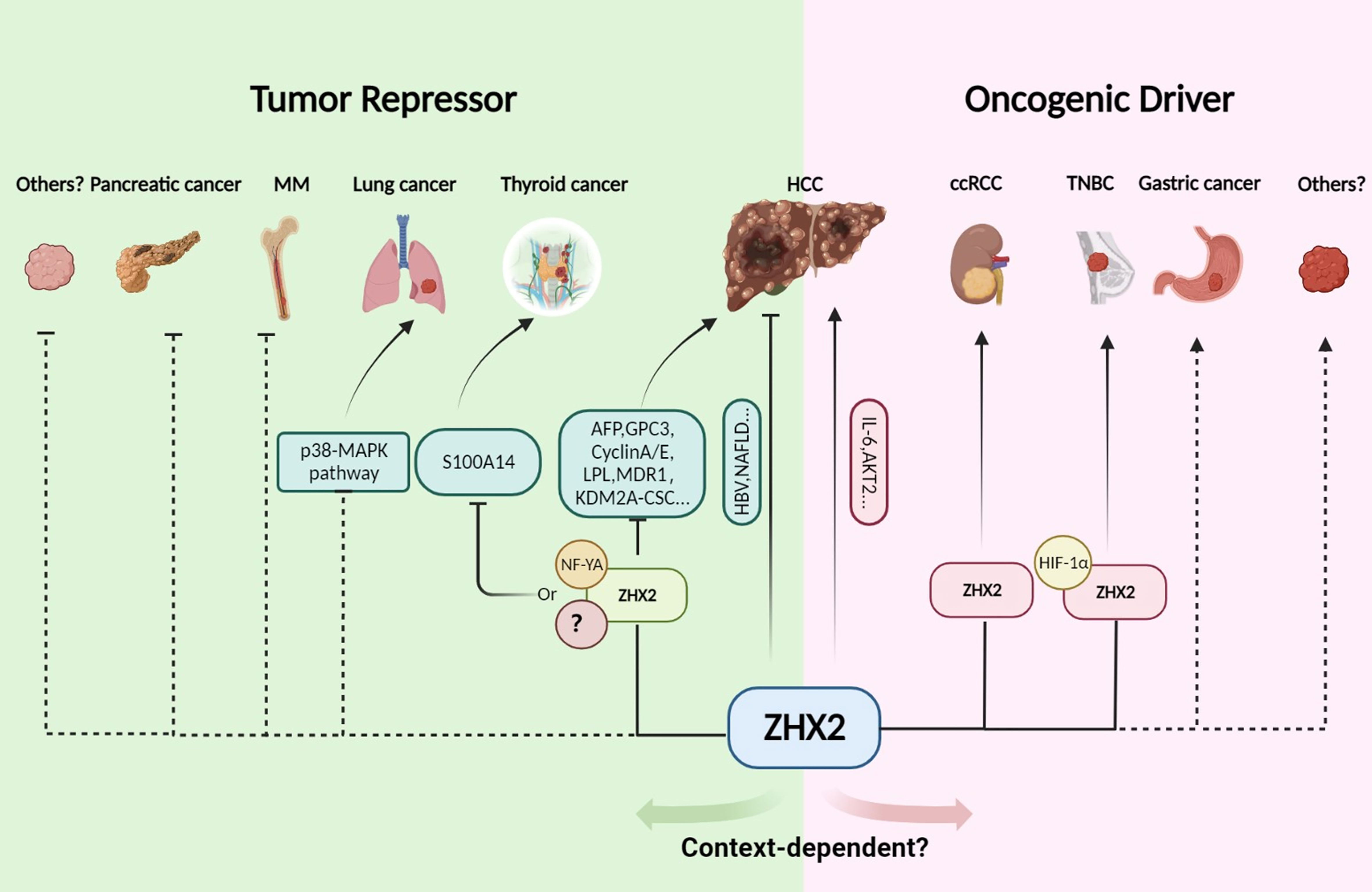
Figure 2 The tumor repressor or driver role of ZHX2 in cancer. In HCC, ZHX2 has a context-dependent role. ZHX2 inhibits HCC via multiple mechanisms, but whole body knockout of Zhx2 reduces DEN-induced liver tumors indicating its complex roles. In HCC, lung cancer, multiple myeloma, HL, and thyroid cancer, ZHX2 acts as a tumor suppressor and transcriptionally represses AFP, GPC3, Cyclin A/E, LPL, KDM2A, and S100A14 expression via interacting with NF-YA or other unknown partners to restrict cancer progress. However, in ccRCC and TNBC, ZHX2 plays an oncogenic driver role by interacting with p65 and HIF1α to activate oncogenic signaling. Created using Biorender.com.
ZHX2 as a tumor suppressor in HCC and other cancers
ZHX2 regulates the posttranscriptional silencing of oncofetal genes AFP, and GPC3, both of which are expressed in fetal liver, silenced after birth, and reactivated in HCC (43–45). These suggest that ZHX2 contributes to hepatocarcinogenesis as a tumor suppressor. Consistently, our previous study showed that the nuclear ZHX2 is reduced in human HCC tissues compared with adjacent nontumor tissues and nuclear ZHX2 represses HCC cell growth by inhibition of cell cycle genes (Cyclin A and Cyclin E), demonstrating for the first time the tumor suppressor activity of ZHX2 in HCC (6). In accordance, another study detected the hypermethylation of ZHX2 promoter and the silencing of ZHX2 expression in HCC tissues (45). Subsequent studies further illustrated the critical role of ZHX2 as a tumor suppressor in HCC with a variety of etiologies, including NASH-related HCC (34, 37) and HBV-related HCC (25, 32). However, there is conflicting data on the role of ZHX2 in HCC. Hu et al. reported increased ZHX2 staining in HCC tissues and higher ZHX2 expression in poorly differentiated and metastasis samples, indicating that ZHX2 might promote HCC progression (53). Jiang et al. recently showed that whole body Zhx2 knockout (Zhx2KO) leads to dramatically reduced liver cancer in DEN-induced HCC mouse model, indicating the oncogenic role of ZHX2 in DEN-induced liver tumor model (52). Interestingly, compared with Zhx2KO mice, DEN induces more tumors in liver-specific Zhx2 knock-out mice (Zhx2Δliv) (52). These data suggest that ZHX2 expression in non-parenchymal cells plays a critical role in liver carcinogenesis. Therefore, although most studies support the conclusion that ZHX2 works as a tumor suppressor in HCC, the exact role of ZHX2 in HCC needs to be further defined and ZHX2 expression in non-parenchymal cells should be deeply investigated.
The tumor suppressor role of ZHX2 has also been demonstrated in many other types of tumors including hematological tumors and solid tumors. Spectral karyotyping identified chromosomal rearrangement far upstream region of ZHX2 gene in Hodgkin lymphoma and this aberration results in ZHX2 silencing (11, 12). Low ZHX2 is associated with poor prognosis in chronic lymphocytic leukemia and multiple myeloma (MM) (8, 54), while higher ZHX2 mRNA correlates with better overall survival in patients with breast cancer (55) and thyroid cancer (7). ZHX2 inhibits proliferation and promotes apoptosis of lung cancer cells by inhibiting the p38-MAPK signaling pathway (51). Integrative bioinformatics analyses reveal that a miRNA-related SNP (rs3802266-G), which creates a stronger binding site for miR-181-a-2-3p in 3’UTR of ZHX2 mRNA and consequently reduces ZHX2 expression, was significantly associated with increased risk of pancreatic cancer (56).
ZHX2 not only inhibits tumor growth but also suppresses tumorigenesis and tumor development through multiple mechanisms. Cancer stem cells (CSCs) are critical determinants of tumor relapse and therapeutic resistance (57). ZHX2 counteracts liver cancer stem cell traits by inhibiting KDM2A-mediated demethylation of H3K36 at the promoter region of stemness-associated transcription factors, such as NANOG, SOX2, and OCT4 (35). Furthermore, ZHX2 inhibits thyroid cancer metastasis (7) and is responsible for reduced chemotherapy resistance in HCC (33). ZHX2 enhances the cytotoxicity of anti-cancer drugs in HCC via transcriptional repression of MDR1 leading to decreased drug efflux (33). Consistently, a clinical study shows a positive correlation between high ZHX2 expression and longer survival in MM patients (8). However, a recent in vitro study shows that treatment of proteasome inhibitor bortezomib (BTZ) leads to enhanced ZHX2 expression which in turn promotes BTZ resistance in cultured MM cells (58). All these data reveal a widespread restriction role of ZHX2 in tumor development at multiple dimensions, including tumor cell proliferation, metastasis, stemness, and chemotherapeutic resistance.
Oncogenic role of ZHX2 in ccRCC, TNBC, and other tumors
Despite the apparent tumor repression role of ZHX2 in HCC and other cancer types, a number of studies have illustrated that ZHX2 can function as an oncogene. Recently, Zhang et al. reported in Science that the loss of tumor suppressor gene VHL in ccRCC leads to the accumulation of ZHX2 protein in the nuclear, which is correlated with poor survival in patients (13, 59). Mechanistically, ZHX2 interacts with RelA/p65 and promotes oncogenic signaling at least partially via activating NF-κB signaling (13). ChIP-seq and gene expression profiling show that 75% of p65 binding sites overlap with those of ZHX2 and their overlapping sites display a strong enrichment of H3K4me3 and H3K27ac (13). In addition, Zhu et al. reported that ZHX2 promotes cell growth and migration through activating MEK/ERK pathway and mediates Sunitinib resistance by regulating the autophagy in ccRCC (14). A similar phenomenon is found in studies of multiple osteosarcoma and gastric cancer (17, 18), where high expression of ZHX2 shows a significant correlation with poor survival. Further, a recent study clarified that ZHX2 functions as a cofactor of the HIF1α to promote HIF1α oncogenic signaling in TNBC (16).
Together, accumulated data demonstrate the critical role of ZHX2 in cancer, either as a tumor suppressor or as an oncogene. However, the detailed mechanism underlying the context-dependent role of ZHX2 in tumors remains largely unknown. Further investigation is required to define the genetic and environmental contexts that influence ZHX2 interaction networks and put genetic interaction networks into different tumors context.
Beyond cancer — other biological roles for ZHX2
Besides the complicated roles in tumors, recent studies suggest the involvement of ZHX2 in the regulation of cell differentiation, HBV replication, lipid homeostasis, and immune responses (Figure 3). In agreement, ZHX2 has been reported in the occurrence of chronic hepatitis B (CHB) (32, 48), metabolism-related diseases (30, 37), nerve-related diseases (19, 60, 61), and immune-related diseases (29) (Figure 3). We will discuss the role of ZHX2 in different physiological and pathological processes here.
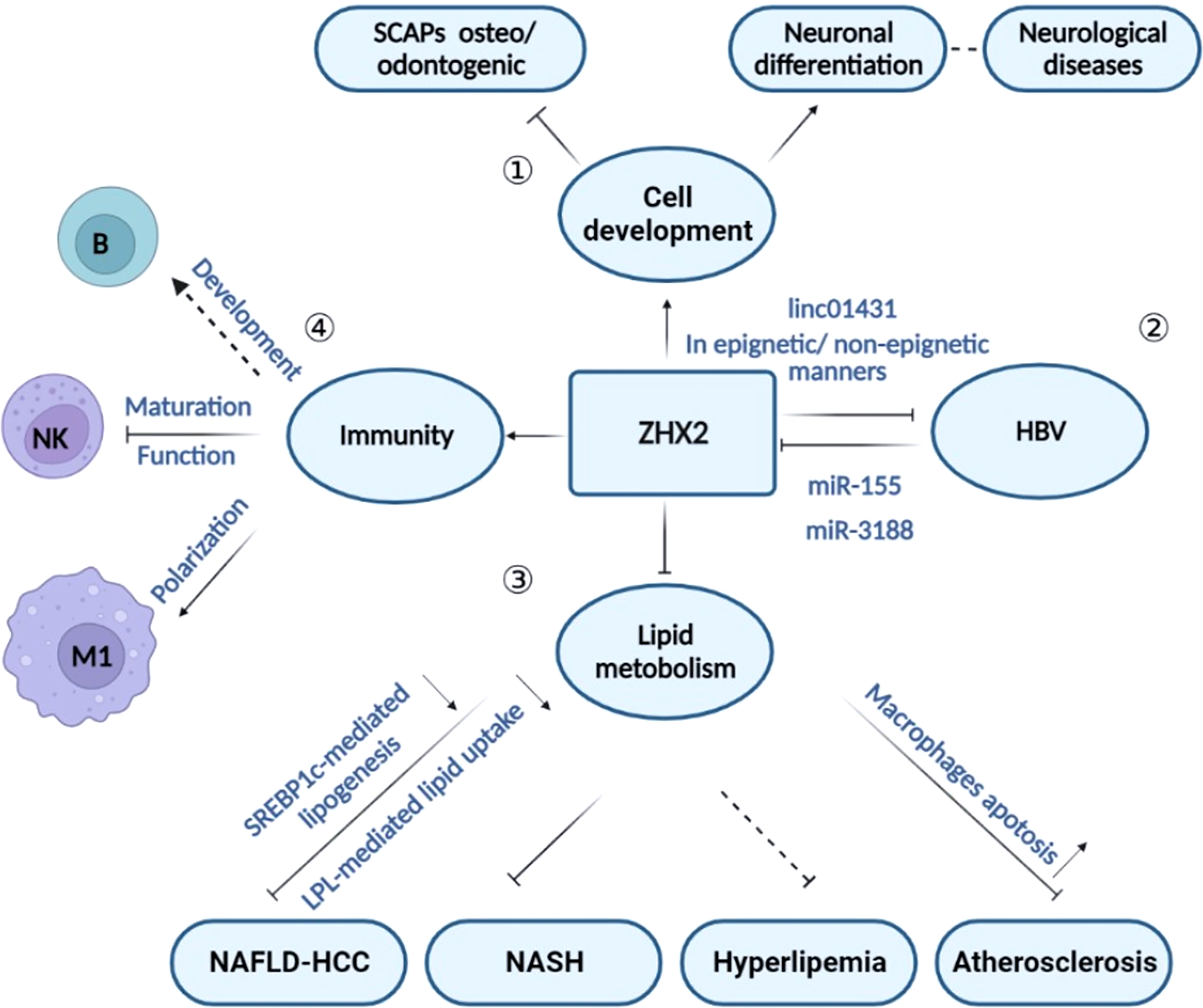
Figure 3 The role of ZHX2 in different physiological and pathological processes. ① Through regulating cell development, ZHX2 is implicated in inhibiting neuronal differentiation and promoting osteo/odontogenic differentiation of stem cells from SCAPs. ② ZHX2 restricts HBV replication via CBP/p300 and linc01431-mediated epigenetic repression or via inhibiting viral promoter activity in non-epigenetic manners. However, HBx protein reduces ZHX2 expression by upregulating miR-155 and miR-3188 expression. ③ ZHX2 is a critical regulator in lipid hemostasis and plays roles in atherosclerosis, NASH, and NAFLD-HCC progress. ④ ZHX2 is involved in immune regulation by influencing the development of multiple immune cell subsets.
ZHX2 in development
The first evidence indicating the involvement of ZHX2 in development comes from the critical role of Zhx2 in the postnatal repression of Afp and Gpc3 in mice (3). In agreement, the dynamic expression of hepatic Zhx2 has been found during liver development and after hepatectomy (21). Zhx2 is low in fetal liver and increases after birth, while Zhx2 expression is significantly declined 24 hours after hepatectomy and then reverses to normal level (21). Therefore, ZHX2 might be a potential therapeutic target in different liver diseases which cause liver injury.
Several studies have illustrated the participation of ZHX2 in regulation of cell development of different origins, such as neurons, blood cells, and bipolar cells. Altered ZHX2 expression has been detected during erythroid differentiation (62) and B cell development (27). Concurrently, ZHX2 is responsible for macrophage polarization (29) and NK cell’s terminal maturation (28). In the nervous system, ZHX2 interacts with Ephrin-B and regulates neural progenitor maintenance (19). Genome-wide analyses identified inherited CNVs (copy number variations) that affect non-genic intervals upstream ZHX2 in autism spectrum disorder (ASD) patients (61). Exome sequencing in subjects with familial corticobasal degeneration (CBD) shows that mutations in ZHX2 gene may cause its structural changes, indicating the possible involvement of ZHX2 in corticobasal degeneration (63). In the process of tooth root development, ZHX2 knockdown reduces the mineralization of stem cells from the apical papilla (SCAPs) and promotes SCAPs proliferation (20). Also, Zhx2 participates in the regulation of bipolar cell subset fate determination during retinal development (64). Collectively, accumulating evidence demonstrated that ZHX2 is strongly involved in the developmental processes of different cells, which is consistent with the acknowledged ZHX2-mediated transcription of stemness genes. However, much work is required to better understand the exact roles and mechanisms of ZHX2 in organogenesis and tissue repair.
ZHX2 and HBV infection
HBV is one of the well-known risk factors for HCC. According to the WHO (World Health Organization), almost one-third of the world’s population has been infected with HBV at some point in their lives (65, 66). HBV infects more than 250 million individuals worldwide, and almost 1 million die annually from complications of persistent infection, cirrhosis, and HCC (66).
As a liver cancer suppressor, ZHX2 expression is significantly decreased in tumor tissue from HBV-positive HCC patients and liver from HBV transgenic mice (48). Further studies show that HBV infection, especially the viral protein HBx reduces ZHX2 expression via upregulation of an oncomiR miR-155 (48) or CREB-mediated activation of miR-3188 (32), leading to liver cancer progression. In turn, ZHX2 serves as a novel restriction factor against HBV replication via regulating HBV promoter activities and cccDNA modifications. In vitro and in vivo experiments confirm that ZHX2 significantly inhibits HBc, HBsAg, and HBeAg expression (25), while overexpression of ZHX2 eliminates HBx-mediated proliferation of HCC cells (48). Mechanistically, ZHX2 binds to cccDNA and reduces the expression of histone regulator genes p300/CBP, leading to epigenetic repression of cccDNA (25). Alternatively, ZHX2 increases the expression of linc01431, a novel noncoding RNA for HBV restriction, which competitively binds with PRMT1 to block HBx-mediated degradation and enhances the occupancy of PRMT1 on cccDNA, thereby repressing cccDNA transcription (26). All in all, ZHX2 and HBV are mutually regulated by each other during HBV infection.
ZHX2 and lipid metabolism
Interestingly, a study in mice using the QTL (quantitative trait locus) mapping strategy identified Zhx2 as a novel regulator of plasma levels of lipids, including triglyceride (TG) (23), indicating a potential role of Zhx2 in lipid metabolism. Compared with other mouse strains, BALB/cJ mice that harbor Zhx2 defect exhibit decreased serum lipid levels and resistance to atherosclerosis when fed a high-fat diet (30). Constantly, altered hepatic transcript levels of several genes affecting lipid homeostasis, including Lpl, are detected in BALB/cJ mice (23). Notably, further research shows that ZHX2 inhibits the uptake of exogenous lipids in hepatocytes by transcriptional repression of LPL expression, which leads to cell growth retardation, and suppresses the progression of NAFLD to HCC (34). Concurrently, it has been found that ZHX2 increases transcription of miR-24-3p which binds to SREBP1c mRNA to promote its degradation, thereby inhibiting SREBP1c-mediated lipid de novo synthesis (24). The involvement of ZHX2 in fatty liver disease is further confirmed by a recent study showing that Zhx2 deficiency in hepatocytes exacerbates NASH progression by transcriptional activation of Pten (37). Collectively, ZHX2 is a critical regulator of lipid metabolism, while we still need more studies to fully delineate the downstream network contributing to ZHX2-mediated lipid regulation.
ZHX2 and immune regulation
ZHX2 is abundantly expressed in thymus and spleen (2), and increasing studies have shown that ZHX2 affects the development and function of different immune cells and participates in the progression of a variety of immune-related diseases.
ZHX2 is involved in the process of B-cell differentiation
A study using gene expression profiling describes an interesting expression pattern of ZHX2 in B lymphoid cells. Similar to essential transcription factors PAX5 and E2A, ZHX2 is turned on during the transition from hematopoietic stem cells (HSCs) into early-B and shows a further increase in pro-B and later stages (27). Recently, Nagel et al. confirmed that ZHX2 is significantly upregulated in B cells while ZHX1 is downregulated. The reduced expression of ZHX2 together with the activation of ZHX1 may contribute to the deregulated B-cell differentiation phenotype in HL (67). However, to date, there were no reports about the role of ZHX2 in B cell development and functions. Interestingly, a genome-wide association study reveals rs10108684, the intronic SNP of ZHX2, as one of the eight top-scoring associations between SNPs and vaccinia antibody levels in African-Americans, strongly suggesting the critical involvement of ZHX2 in B cell-mediated antibody production (68). In summary, ZHX2 shows a dynamic expression pattern during B cell development but its function in B cell maturation is completely unknown and requires further studies.
ZHX2 inhibits NK cell maturation and function
Natural killer (NK) cells are primarily involved in innate immunity and possess important functional properties in anti-viral and anti-tumor responses (69–71). NK cells are derived from hematopoietic stem cells (HSC) via a series of developmental stages, including common lymphoid progenitor (CLP), NK cell precursors (NKP), immature NK cells and mature NK cells (72, 73) Multiple internal pathways and external factors contribute to the development of NK cells from HSCs (73). Tan et al. recently showed that ZHX2 significantly restricts the terminal maturation and effector functions of NK cells both in vivo and in vitro (28). Mechanistically, ZHX2 controls NK cell maturation and function via two related pathways. ZHX2 down-regulates the responsiveness of NK cells to IL-15, the cytokine crucial for NK cell development and survival (74). On the other hand, ZHX2 controls the transcription of Zeb2, a transcription factor identified as a major driver of CD27low NK cell maturation (75, 76). It has been reported that Zeb2 directly or indirectly modulates IL-15-mediated survival and development of NK cells (77, 78). Zeb2 might be associated with ZHX2-mediated regulation of IL-15 signaling (77, 78). Accumulation of immature NK cells has been reported in different tumors (79). The contribution of ZHX2 in the dysregulation of tumor-infiltrating NK cells strengthens ZHX2 as an immune checkpoint regulating NK cells. Targeting ZHX2 has great potential in NK cell-based cancer immunotherapy.
ZHX2 is a critical regulator of macrophages
Macrophages are a key subset of phagocytic cells that readily engulf and degrade dying/dead cells as well as invading bacteria and viruses (80). Macrophages are distributed widely in the body tissues and play a vital role in development, tissue homeostasis and repair, and immunity (81). Macrophages are highly plastic cells that usually present different polarization states in response to local milieu stimuli (82, 83). Recently, a computational network study indicates ZHX2 as one of the most regulated transcription factors in myeloid cells to avoid an avalanche transcription event (31) Our previous study showed that Zhx2 is an important transcription factor that regulates macrophage polarization via reprogramming macrophage glucose metabolism (29). Zhx2 deletion in macrophages significantly attenuates systemic inflammation in mice, prolongs mice survival, attenuates pulmonary injury and reduces proinflammatory cytokines in septic mice (29). Specifically, loss of Zhx2 confers macrophage tolerance to LPS-induced sepsis, accompanied by reduced levels of pro-inflammatory cytokines and lactate release (29). Mechanistically, Zhx2 enhances the production of proinflammatory cytokines in macrophages by promoting glycolysis in a Pfkfb3-dependent manner (29). Accordingly, BALB/cJ strain mice are less likely to develop atherosclerosis, and this resistance to atherosclerosis can be repeated in BALB/c mice by the transfer of bone marrow-derived macrophages from BALB/cJ mice (30). That is, ZHX2 promotes macrophage survival and proinflammatory functions in atherosclerotic lesions (30). In addition, tumor-associated macrophages (TAMs) are critical modulators of the tumor microenvironment (84). The important role of ZHX2-mediated pro-inflammatory polarization of macrophages suggests that targeting ZHX2 to modulate TAM may be a promising strategy for anti-tumor immunotherapy.
Conclusions and perspectives
As a transcription factor, ZHX2 transcriptionally regulates the expression of a series of genes that participate in cell proliferation, differentiation, and metabolism homeostasis. Accordingly, ZHX2 has a broader role in regulating multiple physiological and pathological processes, including cell development, immune regulation, cancer development, and metabolism-related diseases. Significantly, ZHX2 exerts its roles in a context-dependent manner. The exact mechanisms controlling the switch of ZHX2 function in health and diseases are still not clear. Nevertheless, it remains uncertain whether ZHX2 interacts with DNA directly or indirectly via other transcription factors to exert its transcriptional regulation role. Future research needs to be focused on ZHX2 structure, protein interactome, and high throughput screening to clarify its transcriptional regulation and identify new targeted genes. Equally important, the mechanisms that regulate ZHX2 expression are still uncertain. Accumulated studies have suggested that different stimuli regulate ZHX2 expression at different levels including transcription, post-transcription, and posttranslational modification levels. However, the mechanisms are not yet precisely understood. Moreover, in addition to hydroxylation, ubiquitination, and Nα-methylation, other PTMs that determine the biological function and nucleocytoplasmic shuttling of ZHX2 under different circumstances need to be further explored.
Author contributions
NL, ZW, and CM designed and prepared the manuscript and the figures. CM gave guidance on the outline and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by grants from the National key research and development program (2021YFC2300603), the National Science Foundation of China (Key program 81830017, and 81902051), Taishan Scholarship (No.tspd20181201), Major Basic Research Project of Shandong Natural Science Foundation (No.ZR2020ZD12), Key Research and Development Program of Shandong (2019GSF108238).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Olsson M. G, Lindahl, Ruoslahti E. Genetic control of alpha-fetoprotein synthesis in the mouse. J Exp Med (1977) 145(4):819–27. doi: 10.1084/jem.145.4.819
2. Nagase T, Ishikawa K, Suyama M, Kikuno R, Hirosawa M, Miyajima N, et al. Prediction of the coding sequences of unidentified human genes. XII. the complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res (1998) 5(6):355–64. doi: 10.1093/dnares/5.6.355
3. Perincheri S, Dingle RW, Peterson ML, Spear BT. Hereditary persistence of alpha-fetoprotein and H19 expression in liver of BALB/cJ mice is due to a retrovirus insertion in the Zhx2 gene. Proc Natl Acad Sci U.S.A. (2005) 102(2):396–401. doi: 10.1073/pnas.0408555102
4. Kawata H, Yamada K, Shou Z, Mizutani T, Yazawa T, Yoshino M, et al. Zinc-fingers and homeoboxes (ZHX) 2, a novel member of the ZHX family, functions as a transcriptional repressor. Biochem J (2003) 373(Pt 3):747–57. doi: 10.1042/BJ20030171
5. Shen H, Luan F, Liu H, Gao L, Liang X, Zhang L, et al. ZHX2 is a repressor of alpha-fetoprotein expression in human hepatoma cell lines. J Cell Mol Med (2008) 12(6B):2772–80. doi: 10.1111/j.1582-4934.2008.00233.x
6. Yue X, Zhang Z, Liang X, Gao L, Zhang X, Zhao D, et al. Zinc fingers and homeoboxes 2 inhibits hepatocellular carcinoma cell proliferation and represses expression of cyclins a and e. Gastroenterology (2012) 142(7):1559–70.e2. doi: 10.1053/j.gastro.2012.02.049
7. Zhang Y, Sun M, Gao L, Liang X, Ma C, Lu J, et al. ZHX2 inhibits thyroid cancer metastasis through transcriptional inhibition of S100A14. Cancer Cell Int (2022) 22(1):76. doi: 10.1186/s12935-022-02499-w
8. Armellini A, Sarasquete ME, Garcia-Sanz R, Chillon MC, Balanzategui A, Alcoceba M, et al. Low expression of ZHX2, but not RCBTB2 or RAN, is associated with poor outcome in multiple myeloma. Br J Haematol (2008) 141(2):212–5. doi: 10.1111/j.1365-2141.2007.06956.x
9. Shaughnessy J Jr., Zhan F, Barlogie B, Stewart AK. Gene expression profiling and multiple myeloma. Best Pract Res Clin Haematol (2005) 18(4):537–52. doi: 10.1016/j.beha.2005.02.003
10. Legartova S, Harnicarova-Horakova A, Bartova E, Hajek R, Pour L, Kozubek S. Expression of RAN, ZHX-2, and CHC1L genes in multiple myeloma patients and in myeloma cell lines treated with HDAC and dnmts inhibitors. Neoplasma (2010) 57(5):482–7. doi: 10.4149/neo_2010_05_482
11. Nagel S, Schneider B, Meyer C, Kaufmann M, Drexler HG, Macleod RA. Transcriptional deregulation of homeobox gene ZHX2 in Hodgkin lymphoma. Leuk Res (2012) 36(5):646–55. doi: 10.1016/j.leukres.2011.10.019
12. Nagel S, Schneider B, Rosenwald A, Meyer C, Kaufmann M, Drexler HG, et al. T (4,8)(q27;q24) in Hodgkin lymphoma cells targets phosphodiesterase PDE5A and homeobox gene ZHX2. Genes Chromosomes Cancer (2011) 50(12):996–1009. doi: 10.1002/gcc.20920
13. Zhang J, Wu T, Simon J, Takada M, Saito R, Fan C, et al. VHL substrate transcription factor ZHX2 as an oncogenic driver in clear cell renal cell carcinoma. Science (2018) 361(6399):290–95. doi: 10.1126/science.aap8411
14. Zhu L, Ding R, Yan H, Zhang J, Lin Z. ZHX2 drives cell growth and migration via activating MEK/ERK signal and induces sunitinib resistance by regulating the autophagy in clear cell renal cell carcinoma. Cell Death Dis (2020) 11(5):337. doi: 10.1038/s41419-020-2541-x
15. Xie H, Zhou J, Liu X, Xu Y, Hepperla AJ, Simon JM, et al. USP13 promotes deubiquitination of ZHX2 and tumorigenesis in kidney cancer. Proc Natl Acad Sci U.S.A. (2022) 119(36):e2119854119. doi: 10.1073/pnas.2119854119
16. Fang W, Liao C, Shi R, Simon JM, Ptacek TS, Zurlo G, et al. ZHX2 promotes HIF1alpha oncogenic signaling in triple-negative breast cancer. Elife (2021) 10:e70412. doi: 10.7554/eLife.70412
17. You Y, Bai F, Li H, Ma Y, Yao L, Hu J, et al. Prognostic value and therapeutic implications of ZHX family member expression in human gastric cancer. Am J Transl Res (2020) 12(7):3376–88.
18. Cheng A, Guo X, Dai X, Wang Z. Upregulation of ZHX2 predicts poor prognosis and is correlated with immune infiltration in gastric cancer. FEBS Open Bio (2021) 11(6):1785–98. doi: 10.1002/2211-5463.13160
19. Wu C, Qiu R, Wang J, Zhang H, Murai K, Lu Q. ZHX2 interacts with ephrin-b and regulates neural progenitor maintenance in the developing cerebral cortex. J Neurosci (2009) 29(23):7404–12. doi: 10.1523/JNEUROSCI.5841-08.2009
20. Wan F, Gao L, Lu Y, Ma H, Wang H, Liang X, et al. Proliferation and osteo/odontogenic differentiation of stem cells from apical papilla regulated by zinc fingers and homeoboxes 2: An in vitro study. Biochem Biophys Res Commun (2016) 469(3):599–605. doi: 10.1016/j.bbrc.2015.11.135
21. Weng MZ, Zhuang PY, Hei ZY, Lin PY, Chen ZS, Liu YB, et al. ZBTB20 is involved in liver regeneration after partial hepatectomy in mouse. Hepatob Pancreat Dis (2014) 13(1):48–54. doi: 10.1016/s1499-3872(14)60006-0
22. Clinkenbeard EL, Turpin C, Jiang J, Peterson ML, Spear XXXB.T. Liver size and lipid content differences between BALB/c and BALB/cJ mice on a high-fat diet are due, in part, to Zhx2. Mamm Genome (2019) 30(7-8):226–36.doi: 10.1007/s00335-019-09811-6
23. Gargalovic PS, Erbilgin A, Kohannim O, Pagnon J, Wang X, Castellani L, et al. Quantitative trait locus mapping and identification of Zhx2 as a novel regulator of plasma lipid metabolism. Circ Cardiovasc Genet (2010) 3(1):60–7. doi: 10.1161/CIRCGENETICS.109.902320
24. Yu X, Lin Q, Wu Z, Zhang Y, Wang T, Zhao S, et al. ZHX2 inhibits SREBP1c-mediated de novo lipogenesis in hepatocellular carcinoma via miR-24-3p. J Pathol (2020) 252(4):358–70. doi: 10.1002/path.5530
25. Xu L, Wu Z, Tan S, Wang Z, Lin Q, Li X, et al. Tumor suppressor ZHX2 restricts hepatitis b virus replication via epigenetic and non-epigenetic manners. Antiviral Res (2018) 153:114–23. doi: 10.1016/j.antiviral.2018.03.008
26. Sun Y, Teng Y, Wang L, Zhang Z, Chen C, Wang Y, et al. LINC01431 promotes histone H4R3 methylation to impede HBV covalently closed circular DNA transcription by stabilizing PRMT1. Adv Sci (Weinh) (2022) 9(16):e2103135. doi: 10.1002/advs.202103135
27. Hystad ME, Myklebust JH, Bo TH, Sivertsen EA, Rian E, Forfang L, et al. Characterization of early stages of human b cell development by gene expression profiling. J Immunol (2007) 179(6):3662–71. doi: 10.4049/jimmunol.179.6.3662
28. Tan S, Guo X, Li M, Wang T, Wang Z, Li C, et al. Transcription factor Zhx2 restricts NK cell maturation and suppresses their antitumor immunity. J Exp Med (2021) 218(9):e20210009. doi: 10.1084/jem.20210009
29. Wang Z, Kong L, Tan S, Zhang Y, Song X, Wang T, et al. Zhx2 accelerates sepsis by promoting macrophage glycolysis via Pfkfb3. J Immunol (2020) 204(8):2232–41. doi: 10.4049/jimmunol.1901246
30. Erbilgin A, Seldin MM, Wu X, Mehrabian M, Zhou Z, Qi H, et al. Transcription factor Zhx2 deficiency reduces atherosclerosis and promotes macrophage apoptosis in mice. Arterioscler Thromb Vasc Biol (2018) 38(9):2016–27. doi: 10.1161/ATVBAHA.118.311266
31. Espinal-Enriquez J, Gonzalez-Teran D, Hernandez-Lemus E. The transcriptional network structure of a myeloid cell: A computational approach. Int J Genomics (2017) 2017:4858173. doi: 10.1155/2017/4858173
32. Zhou SJ, Deng YL, Liang HF, Jaoude JC, Liu FY. Hepatitis b virus X protein promotes CREB-mediated activation of miR-3188 and notch signaling in hepatocellular carcinoma. Cell Death Differ (2017) 24(9):1577–87. doi: 10.1038/cdd.2017.87
33. Ma H, Yue X, Gao L, Liang X, Yan W, Zhang Z, et al. ZHX2 enhances the cytotoxicity of chemotherapeutic drugs in liver tumor cells by repressing MDR1 via interfering with NF-YA. Oncotarget (2015) 6(2):1049–63. doi: 10.18632/oncotarget.2832
34. Wu Z, Ma H, Wang L, Song X, Zhang J, Liu W, et al. Tumor suppressor ZHX2 inhibits NAFLD-HCC progression via blocking LPL-mediated lipid uptake. Cell Death Differ (2020) 27(5):1693–708. doi: 10.1038/s41418-019-0453-z
35. Lin Q, Wu Z, Yue X, Yu X, Ma C. ZHX2 restricts hepatocellular carcinoma by suppressing stem cell-like traits through KDM2A-mediated H3K36 demethylation. EBioMedicine (2020) 53:102676. doi: 10.1016/j.ebiom.2020.102676
36. Jiang J, Creasy KT, Purnell J, Peterson ML, Spear BT. Zhx2 (zinc fingers and homeoboxes 2) regulates major urinary protein gene expression in the mouse liver. J Biol Chem (2017) 292(16):6765–74. doi: 10.1074/jbc.M116.768275
37. Zhao Y, Gao L, Jiang C, Chen J, Qin Z, Zhong F, et al. The transcription factor zinc fingers and homeoboxes 2 alleviates NASH by transcriptional activation of phosphatase and tensin homolog. Hepatology (2022) 75(4):939–54. doi: 10.1002/hep.32165
38. Spear BT, Jin L, Ramasamy S, Dobierzewska A. Transcriptional control in the mammalian liver: liver development, perinatal repression, and zonal gene regulation. Cell Mol Life Sci (2006) 63(24):2922–38. doi: 10.1007/s00018-006-6258-5
39. Bartolomei MS, Tilghman SM. Genomic imprinting in mammals. Annu Rev Genet (1997) 31:493–525. doi: 10.1146/annurev.genet.31.1.493
40. Yu S, Ruan X, Liu X, Zhang F, Wang D, Liu Y, et al. HNRNPD interacts with ZHX2 regulating the vasculogenic mimicry formation of glioma cells via linc00707/miR-651-3p/SP2 axis. Cell Death Dis (2021) 12(2):153. doi: 10.1038/s41419-021-03432-1
41. Shaulian E, Karin M. AP-1 as a regulator of cell life and death. Nat Cell Biol (2002) 4(5):E131–6. doi: 10.1038/ncb0502-e131
42. Kurosu T, Fukuda T, Miki T, Miura O. BCL6 overexpression prevents increase in reactive oxygen species and inhibits apoptosis induced by chemotherapeutic reagents in b-cell lymphoma cells. Oncogene (2003) 22(29):4459–68. doi: 10.1038/sj.onc.1206755
43. Luan F, Liu P, Ma H, Yue X, Liu J, Gao L, et al. Reduced nucleic ZHX2 involves in oncogenic activation of glypican 3 in human hepatocellular carcinoma. Int J Biochem Cell Biol (2014) 55:129–35. doi: 10.1016/j.biocel.2014.08.021
44. Morford LA, Davis C, Jin L, Dobierzewska A, Peterson ML, Spear BT. The oncofetal gene glypican 3 is regulated in the postnatal liver by zinc fingers and homeoboxes 2 and in the regenerating liver by alpha-fetoprotein regulator 2. Hepatology (2007) 46(5):1541–7. doi: 10.1002/hep.21825
45. Z. Lv, Zhang M, Bi J, Xu F, Hu S, Wen J. Promoter hypermethylation of a novel gene, ZHX2, in hepatocellular carcinoma. Am J Clin Pathol (2006) 125(5):740–6. doi: 10.1309/09B4-52V7-R76K-7D6K
46. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell (2009) 136(2):215–33. doi: 10.1016/j.cell.2009.01.002
47. Iwasaki S, Kobayashi M, Yoda M, Sakaguchi Y, Katsuma S, Suzuki T, et al. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol Cell (2010) 39(2):292–9. doi: 10.1016/j.molcel.2010.05.015
48. Song X, Tan S, Wu Z, Xu L, Wang Z, Xu Y, et al. HBV suppresses ZHX2 expression to promote proliferation of HCC through miR-155 activation. Int J Cancer (2018) 143(12):3120–30. doi: 10.1002/ijc.31595
49. Cruickshank BM, Wasson MD, Brown JM, Fernando W, Venkatesh J, Walker OL, et al. LncRNA PART1 promotes proliferation and migration, is associated with cancer stem cells, and alters the miRNA landscape in triple-negative breast cancer. Cancers (Basel) (2021) 13(11):2644. doi: 10.3390/cancers13112644
50. Conner MM, Parker HV, Falcone DR, Chung G, Schaner Tooley CE. Novel regulation of the transcription factor ZHX2 by n-terminal methylation. Transcription (2022) 13(1-3):1–15. doi: 10.1080/21541264.2022.2079184
51. Tian X, Wang Y, Li S, Yue W, Tian H. ZHX2 inhibits proliferation and promotes apoptosis of human lung cancer cells through targeting p38MAPK pathway. Cancer biomark (2020) 27(1):75–84. doi: 10.3233/CBM-190514
52. Jiang J, Turpin C, Qiu GS, Xu M, Lee E, Hinds TD Jr., et al. Zinc fingers and homeoboxes 2 is required for diethylnitrosamine-induced liver tumor formation in C57BL/6 mice. Hepatol Commun (2022). doi: 10.1002/hep4.2106
53. Hu S, Zhang M, Lv Z, Bi J, Dong Y, Wen J. Expression of zinc-fingers and homeoboxes 2 in hepatocellular carcinogenesis: a tissue microarray and clinicopathological analysis. Neoplasma (2007) 54(3):207–11.
54. Maciel NIG, Filiu-Braga LDC, Neves FAR, Rego EM, Lucena-Araujo AR, Saldanha-Araujo F. Low expression of ZHX1 and ZHX2 impacts on the prognosis of chronic lymphocytic leukemia. biomark Res (2021) 9(1):10. doi: 10.1186/s40364-021-00263-2
55. You Y, Ma Y, Wang Q, Ye Z, Deng Y, Bai F. Attenuated ZHX3 expression serves as a potential biomarker that predicts poor clinical outcomes in breast cancer patients. Cancer Manag Res (2019) 11:1199–210. doi: 10.2147/CMAR.S184340
56. Ke J, Peng X, Mei S, Tian J, Ying P, Yang N, et al. Evaluation of polymorphisms in microRNA-binding sites and pancreatic cancer risk in Chinese population. J Cell Mol Med (2020) 24(3):2252–59. doi: 10.1111/jcmm.14906
57. Yamashita T, Wang XW. Cancer stem cells in the development of liver cancer. . J Clin Invest (2013) 123(5):1911–8. doi: 10.1172/JCI66024
58. Jiang J, Sun Y, Xu J, Xu T, Xu Z, Liu P. ZHX2 mediates proteasome inhibitor resistance via regulating nuclear translocation of NF-kappaB in multiple myeloma. Cancer Med (2020) 9(19):7244–52. doi: 10.1002/cam4.3347
59. Chen Y, Zhu L, Xue S, Shi J, He C, Zhang Q. Novel VHL substrate targets SFMBT1 and ZHX2 may be important prognostic predictors in patients with ccRCC. Oncol Lett (2021) 21(5):379. doi: 10.3892/ol.2021.12640
60. Guedj F, Pennings JL, Wick HC, Bianchi DW. Analysis of adult cerebral cortex and hippocampus transcriptomes reveals unique molecular changes in the Ts1Cje mouse model of down syndrome. Brain Pathol (2015) 25(1):11–23. doi: 10.1111/bpa.12151
61. Walker S, Scherer SW. Identification of candidate intergenic risk loci in autism spectrum disorder. BMC Genomics (2013) 14:499. doi: 10.1186/1471-2164-14-499
62. De Andrade T, Moreira L, Duarte A, Lanaro C, De Albuquerque D, Saad S, et al. Expression of new red cell-related genes in erythroid differentiation. Biochem Genet (2010) 48(1-2):164–71. doi: 10.1007/s10528-009-9310-y
63. Fekete R, Bainbridge M, Baizabal-Carvallo JF, Rivera A, Miller B, Du P, et al. Exome sequencing in familial corticobasal degeneration. Parkinsonism Relat Disord (2013) 19(11):1049–52. doi: 10.1016/j.parkreldis.2013.06.016
64. Kawamura Y, Yamanaka K, Poh B, Kuribayashi H, Koso H, Watanabe S. The role of Zhx2 transcription factor in bipolar cell differentiation during mouse retinal development. Biochem Biophys Res Commun (2018) 503(4):3023–30. doi: 10.1016/j.bbrc.2018.08.088
65. Global Burden of Disease Liver Cancer C, Akinyemiju T, Abera S, Ahmed M, Alam N, Alemayohu MA, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: Results from the global burden of disease study 2015. JAMA Oncol (2017) 3(12):1683–91. doi: 10.1001/jamaoncol.2017.3055
66. Razavi-Shearer D, Gamkrelidze I, Nguyen MH, Chen DS, Van Damme P, Abbas Z, et al. Global prevalence, treatment, and prevention of hepatitis b virus infection in 2016: a modelling study. Lancet Gastroenterol (2018) 3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6
67. Nagel S, Ehrentraut S, Meyer C, Kaufmann M, Drexler HG, MacLeod RA. Aberrantly expressed OTX homeobox genes deregulate b-cell differentiation in Hodgkin lymphoma. PloS One (2015) 10(9):e0138416. doi: 10.1371/journal.pone.0138416
68. Ovsyannikova IG, Kennedy RB, O'Byrne M, Jacobson RM, Pankratz VS, Poland GA. Genome-wide association study of antibody response to smallpox vaccine. Vaccine (2012) 30(28):4182–9. doi: 10.1016/j.vaccine.2012.04.055
69. SchartonKersten TM, Sher A. Role of natural killer cells in innate resistance to protozoan infections. Curr Opin Immunol (1997) 9(1):44–51. doi: 10.1016/S0952-7915(97)80157-4
70. Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer (2016) 16(1):7–19. doi: 10.1038/nrc.2015.5
71. Sheppard S, Sun JC. Virus-specific NK cell memory. J Exp Med (2021) 218(4):e20201731. doi: 10.1084/jem.20201731
72. Dogra P, Rancan C, Ma W, Toth M, Senda T, Carpenter DJ, et al. Tissue determinants of human NK cell development, function, and residence. Cell (2020) 180(4):749–63.e13. doi: 10.1016/j.cell.2020.01.022
73. Huntington ND, Vosshenrich CA, Santo JPDi. Developmental pathways that generate natural-killer-cell diversity in mice and humans. Nat Rev Immunol (2007) 7(9):703–14. doi: 10.1038/nri2154
74. Marcais A, Cherfils-Vicini J, Viant C, Degouve S, Viel S, Fenis A, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol (2014) 15(8):749–57. doi: 10.1038/ni.2936
75. van Helden MJ, Goossens S, Daussy C, Mathieu AL, Faure F, Marcais A, et al. Terminal NK cell maturation is controlled by concerted actions of T-bet and Zeb2 and is essential for melanoma rejection. J Exp Med (2015) 212(12):2015–25. doi: 10.1084/jem.20150809
76. Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, et al. The transcription factors T-bet and eomes control key checkpoints of natural killer cell maturation. Immunity (2012) 36(1):55–67. doi: 10.1016/j.immuni.2011.11.016
77. Wang X, Zhao XY. Transcription factors associated with IL-15 cytokine signaling during NK cell development. Front Immunol (2021) 12:610789. doi: 10.3389/fimmu.2021.610789
78. Wang Y, Zhang Y, Yi P, Dong W, Nalin AP, Zhang J, et al. The IL-15-AKT-XBP1s signaling pathway contributes to effector functions and survival in human NK cells. Nat Immunol (2019) 20(1):10–7. doi: 10.1038/s41590-018-0265-1
79. Krneta T, Gillgrass A, Chew M, Ashkar AA. The breast tumor microenvironment alters the phenotype and function of natural killer cells. Cell Mol Immunol (2016) 13(5):628–39. doi: 10.1038/cmi.2015.42
80. Nagata S. Apoptosis and clearance of apoptotic cells. Annu Rev Immunol (2018) 36:489–517. doi: 10.1146/annurev-immunol-042617-053010
81. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol (2011) 11(11):723–37. doi: 10.1038/nri3073
82. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol (2008) 8(12):958–69. doi: 10.1038/nri2448
83. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest (2012) 122(3):787–95. doi: 10.1172/JCI59643
Keywords: ZHX2, tumor repressor, oncogene, cell differentiation, lipid metabolism, immunoregulation
Citation: Li N, Wu Z and Ma C (2022) ZHX2 in health and disease. Front. Oncol. 12:1038890. doi: 10.3389/fonc.2022.1038890
Received: 07 September 2022; Accepted: 04 November 2022;
Published: 18 November 2022.
Edited by:
Li Wu, Tsinghua University, ChinaReviewed by:
Xiangzhi Li, School of Life Sciences, Shandong University, ChinaJacob T. Jackson, The University of Melbourne, Australia
Copyright © 2022 Li, Wu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunhong Ma, machunhong@sdu.edu.cn
†These authors have contributed equally to this work
 Na Li1†
Na Li1† Zhuanchang Wu
Zhuanchang Wu Chunhong Ma
Chunhong Ma