- 1Department of Thoracic Surgery, Henan Provincial Chest Hospital, Zhengzhou, China
- 2Department of Oncology, The Affiliated Huai’an Hospital of Xuzhou Medical University and The Second People’s Hospital of Huai’an, Huaian, China
Long noncoding RNAs act essential regulators in lung cancer tumorigenesis. Our research aimed to investigate the potential function and molecular mechanisms of MLK7-AS1 in NSCLC (non-small-cell lung cancer). QRT-PCR results indicated that the MLK7-AS1 expression level was upregulated in NSCLC cells and tissues. MLK7-AS1 strengthened cell migration and invasion in H1299 and A549 cells. Luciferase reporter assay found that MLK7-AS1 functioned as an endogenous sponge for miR-375-3p. Transwell assay results showed that miR-375-3p suppressed cell migration and invasion in H1299 and A549 cells. YWHAZ was confirmed as a target gene of miR-375-3p by Targetscan. YWHAZ overexpression promoted the invasion of H1299 and A549 cells. MLK7-AS1 upregulated YWHAZ expression and enhanced H1299 and A549 cell invasion by sponging miR-375-3p. MLK7-AS1 improved the metastasis ability of A549 in vivo. In conclusion, MLK7-AS1 was identified as a novel oncogenic RNA in NSCLC and can function as a potential therapeutic target for NSCLC treatment.
Introduction
NSCLC is the main cause of thoracic neoplasms in the world (1, 2). Unfortunately, the rate of lung cancer diagnoses is increasing steadily in China (3). Considerable advances in operation and chemoradiotherapy have been achieved, but a high frequency of recurrence in patients with lung cancer remains a problem in lung cancer treatment (4). Understanding the mechanisms of NSCLC development is the cornerstone of solving clinical questions.
Long noncoding RNAs (lncRNAs) are noncoding RNA molecules longer than 200 nucleotides (5, 6). LINC01296 promotes proliferation in NSCLC viamiR-5095/Wnt axis (7). FLVCR1-AS1 downregulation suppresses cell invasion in NSCLC through Wnt/β-catenin signaling pathway (8). LINC00163 overexpression inhibits lung cancer progression by transcriptionally upregulating TCF21 expression (9). SLCO4A1-AS1 accelerates the colorectal cancer development through Wnt signaling pathway (10). lncRNA PVT1 facilitates the tumor progression in gallbladder cancer via the miR-143/HK2 axis (11). MLK7 antisense RNA 1 (MLK7-AS1) was previously identified as an oncogene in several tumors. For example, MLK7−AS1 can enhance ovarian cancer cells invasion by upregulating the expression of YAP1 (12). However, the role of MLK7−AS1 in NSCLC is largely unclarified.
MicroRNAs (miRNAs), small noncoding RNAs of 20–25 nucleotides in length, regulate the expression of downstream targets through post-transcriptional modulation (13, 14). Competing endogenous RNAs (ceRNA) is a vital mechanism regulating the progression of various cancers. In-depth studies have demonstrated that lncRNAs acted as a vital regulatory role in malignancies as competing endogenous RNAs. FLVCR1-AS1 enhances gastric cancer tumorigenesis by sponging miR-155 and targeting c-Myc (15). lncRNA SNHG16 accelerates the cancer cells migration and invasion abilities through sponging miR-520d-3p and targeting STAT3 in hemangioma (16). CeRNA network is the vital mechanism of tumor development (17). lncRNA ZEB1-AS1 promotes TGF-β1-induced invasion of bladder tumor cells via targeting the miR-200b/FSCN1 pathway (18). Thus, exploring the roles of lncRNAs in NSCLC can help scholars understand potential mechanisms from a new perspective.
In this study, we found MLK7−AS1 was upregulated in NSCLC, which indicated MLK7−AS1 was a favorable factor for NSCLC. To further demonstrate the role of MLK7−AS1 and the underlying mechanism in NSCLC, NSCLC cell lines and in vivo models was used, which would contribute to the understanding of the development and progression of NSCLC, thus could provide potential therapeutic target for NSCLC.
Material and Methods
Cell Culture
Three NSCLC lines (i.e., H1299, A549, and H1650) and the normal cell line BEAS-2B were cultured in an incubator (37°C, 5% CO2) and in RPMI1640 (Gibco BRL, Gaithersburg, MD, USA) supplemented with 10% FBS (Gibco BRL, Gaithersburg, MD, USA).
Transwell Migration and Invasion Assay
Transwell migration and invasion assay was performed in accordance with previously described method (19). For the Transwell migration assays, the transfected H1299 and A549 (N=5.5×103) cells were plated in top chambers with a noncoated membrane (Invitrogen, Carlsbad, CA). For the invasion assays, the transfected H1299 and A549 (N=11×103) cells were plated in top chambers with a coated membrane. The number of invading cells was counted after fixed with 4% paraformaldehyde (Beyotime, Shanghai, China).
Cell Transfection and Lentivirus Production
The sequence of YWHAZ was cloned into the pcDNA3.1 vector and the empty vector acted as a negative control. The lentiviral vector for MLK7−AS1 was purchased from Kaiji Gene (Shanghai, China). mimic/inhibitor-miR-375-3p and mimic/inhibitor-NC were purchased from RiboBio (China, Guangzhou). Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was utilized for transfection in accordance with the manufacturer’s instructions.
Nuclear and Cytoplasmic RNA Isolation
Nuclear and cytoplasmic RNA was isolated in accordance with previously described methods (20, 21). The cytoplasm and nuclear RNAs of NSCLC cells were separated and extracted using a nuclear and cytoplasmic RNA purification kit (Invitrogen, Carlsbad, CA). qPCR assay was performed to detect the isolated RNA.
Quantitative Reverse Transcription PCR (qRT-PCR)
The RNA of NSCLC cells was isolated using TRIzol reagent (US, Life Technologies, USA). SYBR Green qRT-PCR on an ABI7300 real-time PCR machine was used to measure the expression levels of mRNAs. YWHAZ and GAPDH expression levels were examined using the following specific primers:
5′CCTGCATGAAGTCTGTAACTGAG3′,
5′GACCTACGGGCTCCTACAACA3′,
5′GGAGCGAGATCCCTCCAAAAT3′, and
5′GGCTGTTGTCATACTTCTCATGG3′.
Western Blot Analysis
The protein (15–20 mg) extracted from cells was used for Western blot analysis. The antibodies utilized in this study included anti-YWHAZ (1:1000; Cell Signaling Technology, USA) and GAPDH (1:1000; Cell Signaling Technology, USA). GAPDH (1:2000; Cell Signaling Technology, USA) was used as a loading control.
Luciferase Reporter Assay
Luciferase reporter assay was performed in accordance with previously described procedures (22). Wt-pmirGLO-, MLK7−AS1, wt-pmirGLO-YWHAZ, and their corresponding mutated vectors were constructed. Wt-pmirGLO or mut-pmirGLO was cotransfected into cells with miR-375-3p inhibitor or miR-375-3p mimic by using Lipofectamine 2000. Luciferase activity was detected 48h after transfection.
In Vivo Study
Six-week-old female nude mice were obtained from Medical Center of Yangzhou University. (Yangzhou, China). The A549 cell line stably overexpressing MLK7-AS1 was established. A lung metastasis mice model was also established with the intra-splenic injection of 5×106 stably overexpressing MLK7-AS1 or LV-NC cells. After 24 days, lung colonization capacity was evaluated. The number of lung metastatic foci was counted via H&E staining. This work was approved by the Medical Ethics Committee of Henan Provincial Chest Hospital.
Statistical Analysis
GraphPad Prism 5.0 and SPSS 13.0 were used to analyze data. Statistical data were expressed as mean ± standard deviation. Differences were considered significant at P<0.05.
Results
MLK7-AS1 Promoted the Migration and Invasion of NSCLC cells
MLK7-AS1 expression was detected by qPCR in NSCLC cell lines, namely, H1299, A549, H1650 compared with normal human bronchial epithelium BEAS-2B. As shown in Figure 1A. The expression level of MLK7-AS1 was upregulated NSCLC cell lines. Moreover, the expression level of MLK7-AS1 was upregulated in NSCLC tissues than in adjacent tissues (N=25) (Figure 1B). To explore the biological functions of MLK7-AS1, overexpression (LV-MLK7-AS1) and knockdown (sh-MLK7-AS1) assays were performed in H1299 and A549 cells. The efficiency of LV-MLK7-AS1 and sh-MLK7-AS1 was determined using qRT-PCR (Figures 1C, D). Wound-healing assays results indicated that MLK7-AS1 overexpression promoted the cells migration and silencing of MLK7-AS1 weakened the cells migration in H1299 and A549 (Figures 1E, F). Moreover, transwell assays indicated that the cell migration in H1299 and A549 was strengthened by MLK7-AS1 upregulation but was weakened by MLK7-AS1 downregulation (Figures 1G, H). The cell invasion in H1299 and A549 was promoted by MLK7-AS1 overexpression but was suppressed by MLK7-AS1 knockout knockdown (Figures 1I, J). These results demonstrated that the MLK7-AS1 strengthened the NSCLC cells migration and invasion.
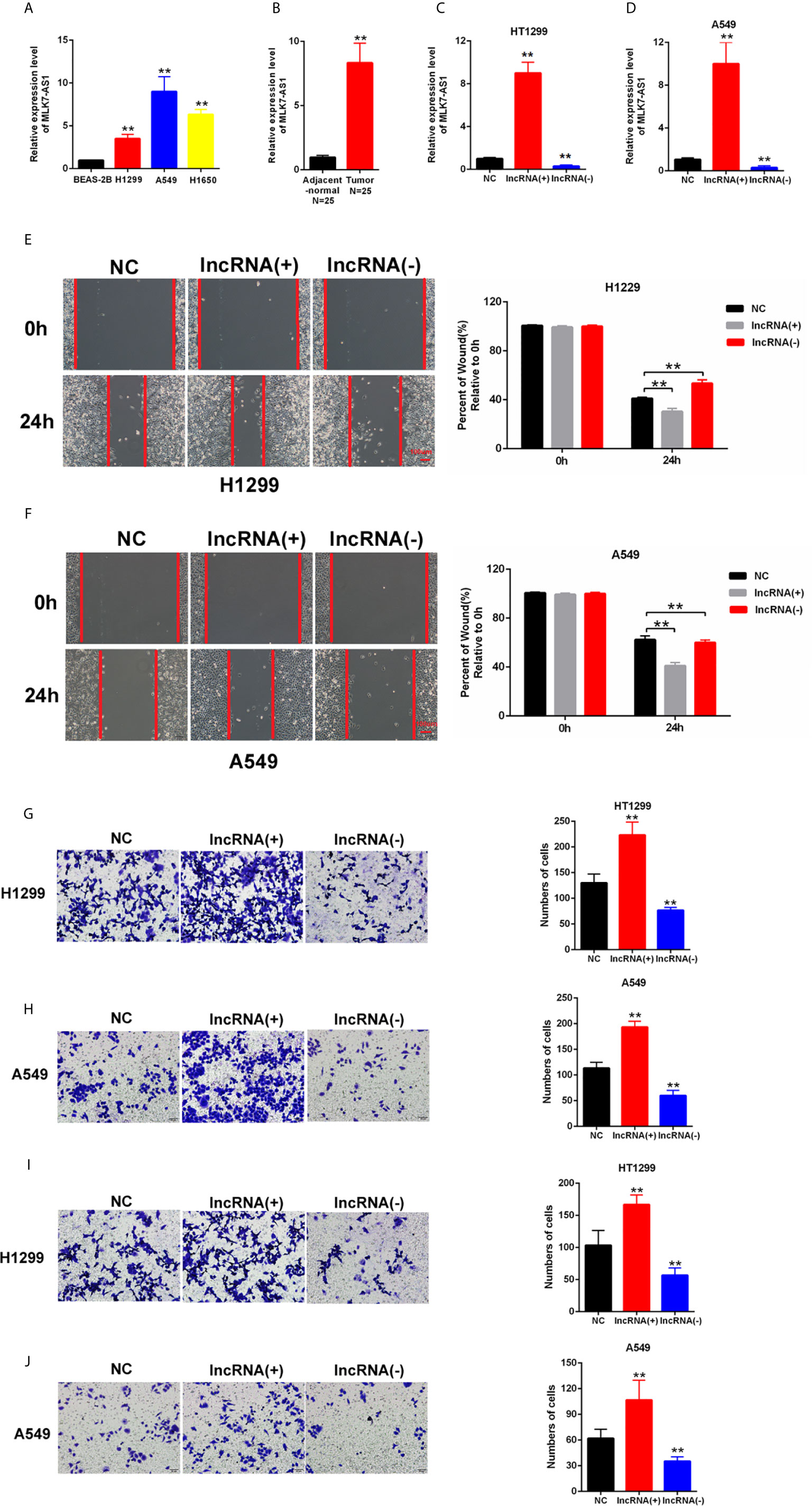
Figure 1 Upregulated MLK7-AS1 enhanced the migration and invasion of NSCLC cells. (A) MLK7-AS1 expression level was upregulated in the NSCLC cell lines compared with that in the normal line. (B) MLK7-AS1 expression level was upregulated in NSCLC tissues compared with that in tumor-adjacent normal pairs (N=25). (C, D) LV-MLK7-AS1 and sh-MLK7-AS1 efficiency was measured through qRT-PCR. (E, F) Effect of MLK7-AS1 on migration detected through wound-healing assays. LV-MLK7-AS1 promoted cell migration, whereas sh-MLK7-AS1 inhibited cell migration in H1229 and A549. (G, H) Effect of MLK7-AS1 on migration detected through Transwell assays. LV-MLK7-AS1 strengthened cell migration, whereas sh-MLK7-AS1 suppressed cell migration in H1229 and A549. (I, J) Effect of MLK7-AS1 on invasion detected through Transwell assays. LV-MLK7-AS1 increased cell invasion, but sh-MLK7-AS1 decreased cell invasion in H1229 and A549. Data indicate the mean ± SD, n = 3. **P < 0.01 vs. control.
miR-375-3p Was Predicted as a Direct Target of MLK7-AS1
To explore whether competing endogenous RNA (ceRNA) was involved in the regulation of MLK7-AS1 in NSCLC, FISH assays were performed in H1299 and A549. MLK7-AS1 mainly localized in the cytoplasm (Figure 2A). QRT-PCR also indicated that MLK7-AS1 mainly expressed in the cytoplasm of H1299 and A549 cells (Figures 2B, C). The potential target miRNAs for MLK7-AS1 were predicted using lncBase (lncBase/Experimental/?r=lncBase) and StarBase v2.0 (http://starbase.sysu.edu.cn/). Among many candidates, miR-375-3p could act as a tumor suppressor in several tumors (23). Thus, miR-375-3p was identified as a prior candidate for MLK7-AS1. LV-MLK7-AS1 and sh-MLK7-AS1 were transfected in NSCLC cells to explore the relationship between MLK7-AS1 and miR-375-3p, which revealed that LV-MLK7-AS1 downregulated the miR-375-3p expression, whereas sh-MLK7-AS1 upregulated the miR-375-3p expression in H1299 and A549 cells (Figures 2D, E). StarBase v2.0 was used to predict the specific binding site between MLK7-AS1 and miR-375-3p, which result was shown in Figure 2F. Moreover, dual-luciferase reporter assays were performed in H1299 and A549. As shown in Figures 2G, H, luciferase activity could be inhibited by mimic-miR-375-3p but could be promoted by inhibitor-miR-375-3p in WT-MLK7-AS1 reporter. However, the mutant-type reporter gene (MT-MLK7-AS1 reporter) was not inhibited or increased in the luciferase activity by mimic-or inhibitor-miR-375-3p. In addition, we examined the expression levels of miR-375-3p in lung cancer tissues (n=22), and made the correlation analysis with the expression levels of MLK7-AS1 (Figure 2I). We found that the expression levels of miR-375-3p were negatively correlated with the expression levels of MLK7-AS1. A RIP assay was performed to examine whether MLK7-AS1 and miR-375-3p are in the same RISC complex. Then, RIP assays indicated that MLK7-AS1 and miR-375-3p were enriched in Ago2 compared with control IgG. Subsequently, miR-375-3p got a significant enrichment in the MLK7-AS1 pull down pellets compared with control IgG. Moreover, after transfection with inhibitor-miR-20a-5p, the expression levels of MLK7-AS1 and miR-375-3p enriched in Ago2 were downregulated (Figures 2J–M). Thus, miR-375-3p was a direct target of MLK7-AS1.
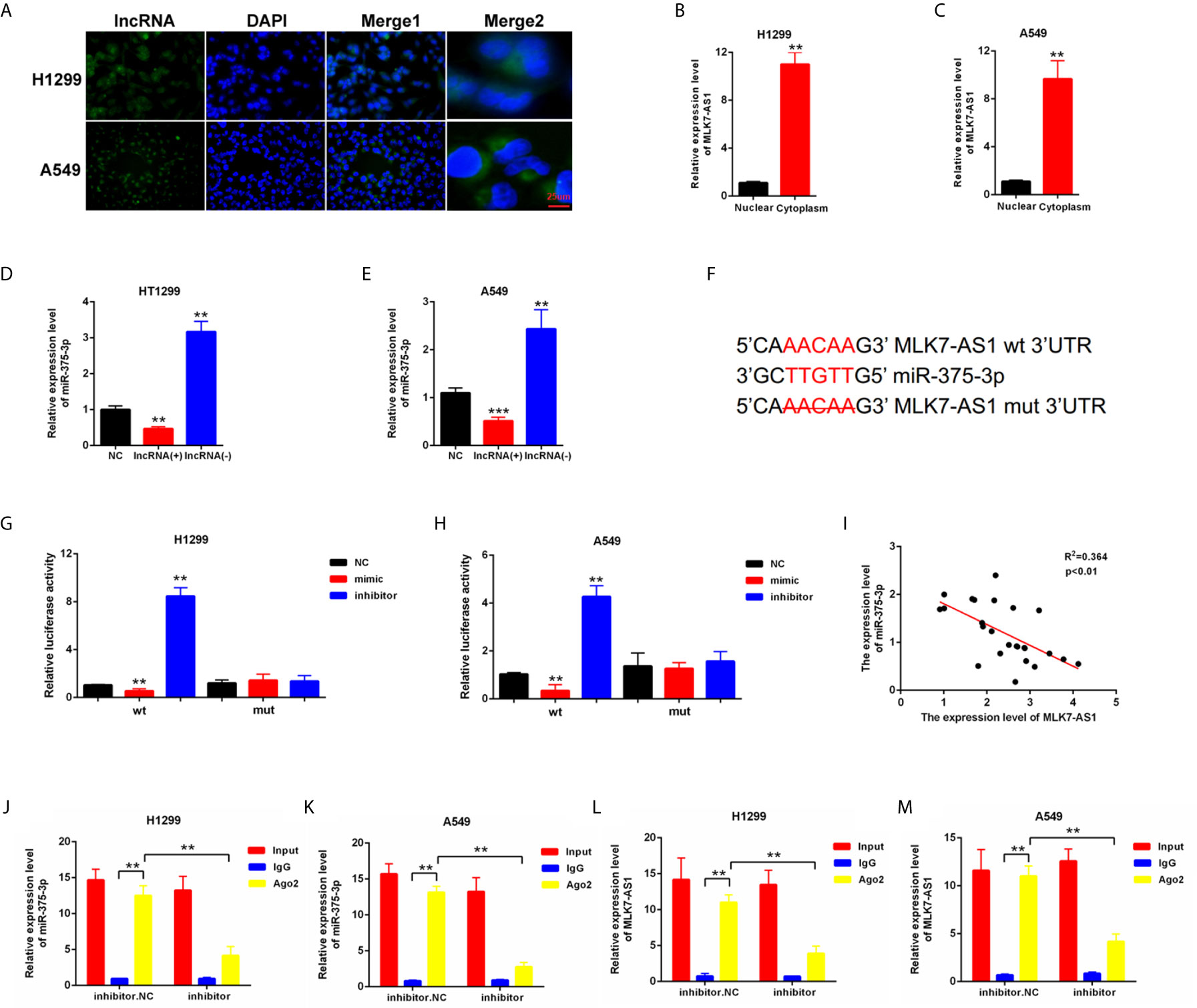
Figure 2 miR-375-3p was predicted as a direct target of MLK7-AS1. (A) FISH assays were conducted to detect the location of MLK7-AS1 in H1299 and A549. MLK7-AS1 was mainly localized in the cytoplasm. (B, C) qRT-PCR was performed to determine the location of MLK7-AS1 in H1299 and A549. MLK7-AS1 expression level was higher in the cytoplasm than in nucleus. (D, E) qRT-PCR results showed that MLK7-AS1 upregulation decreased the miR-375-3p expression level, whereas MLK7-AS1 downregulation increased the miR-375-3p expression level. (F) Direct binding sites between MLK7-AS1 and miR-375-3p were presented. (G, H) Luciferase reporter assay was performed to confirm the direct binding relationship between MLK7-AS1 and miR-375-3p. (I) The regression analysis of correlation between the expression of MLK7-AS1 and miR-375-3p in lung cancer tissues (n=22). (J–M) After RIP assay in H1299 or A549, the levels of MLK7-AS1 and miR-375-3p were respectively quantified by RT-qPCR. Data indicate the mean ± SD, n = 3. **P < 0.01, ***P < 0.001 vs. control.
miR-375-3p Suppressed the Migration and Invasion of NSCLC Cells
H1299 and A549 were transfected with mimic/inhibitor of miR-375-3p to investigate the role of miR-375-3p in NSCLC cells. The efficiency of mimic-miR-375-3p and inhibitor-miR-375-3p was measured through qRT-PCR (Figures 3A–D). The cell migration was inhibited by mimic-miR-375-3p and was enhanced by inhibitor-miR-375-3p in H1299 and A549 (Figures 3E–H). The cell invasion was inhibited by mimic-miR-375-3p and was enhanced by inhibitor-miR-375-3p in H1299 and A549 (Figures 3I–L). Thus, miR-375-3p suppressed cell migration and invasion in H1299 and A549.
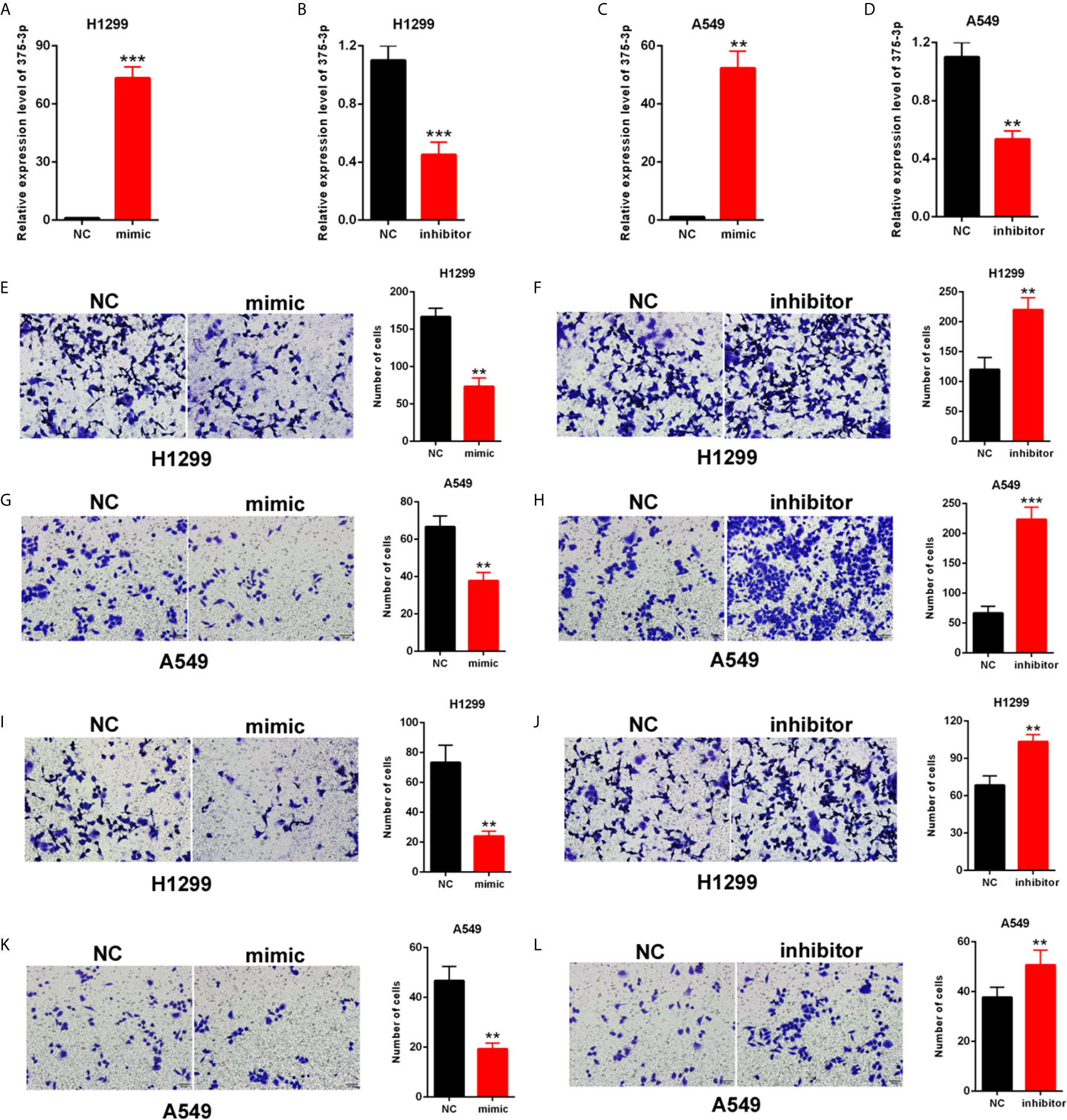
Figure 3 Effects of miR-375-3p on the migration and invasion of NSCLC cells. (A, B) Efficiency of mimic/inhibitor-miR-375-3p was determined through qPCR in H1299. (C, D) Efficiency of mimic/inhibitor-miR-375-3p was determined through qRT-PCR in A549. (E–H) Effect of miR-375-3p on migration was detected through Transwell assays in H1299 and A549. Cell migration was promoted by miR-375-3p-mimic but was suppressed by miR-375-3p-inhibitor. (I–L) Effects of miR-375-3p on invasion detected through Transwell assays in H1299 and A549. Cell invasion was enhanced by miR-375-3p-mimic but was suppressed by miR-375-3p-inhibitor in H1299 and A549. Data indicate the mean ± SD, n = 3. **P < 0.01, ***P < 0.001 vs. control.
YWHAZ Was a Direct Target Gene of miR-375-3p
The potential target genes of miR-375-3p were predicted by StarBase v2.0. Among many candidates, YWHAZ was identified as an oncogene in several tumors (24, 25). Thus, YWHAZ was chosen as a prior candidate for miR-375-3p. As shown in Figures 4A–H, results indicated that the mRNA and protein expression level of YWHAZ was downregulated after transfection with mimic-miR-375-3p and was upregulated after transfection with inhibitor-miR-375-3p. The analysis through StarBase v2.0 revealed the direct binding sites between miR-375-3p and YWHAZ (Figure 4I). Moreover, dual-luciferase reporter experiments were performed in H1299 and A549. The luciferase activity was inhibited by mimic-miR-375-3p and was enhanced by inhibitor-miR-375-3p in WT-YWHAZ reporter. However, the mutant-type reporter gene (MT- YWHAZ reporter) was not inhibited or enhanced by mimic-miR-375-3p or inhibitor-miR-375-3p (Figures 4J, K). We found that the expression levels of YWHAZ were negatively correlated with the expression levels of miR-375-3p in lung cancer tissues (n=22, Figure 4L). As such, YWHAZ was a direct target gene of miR-375-3p.
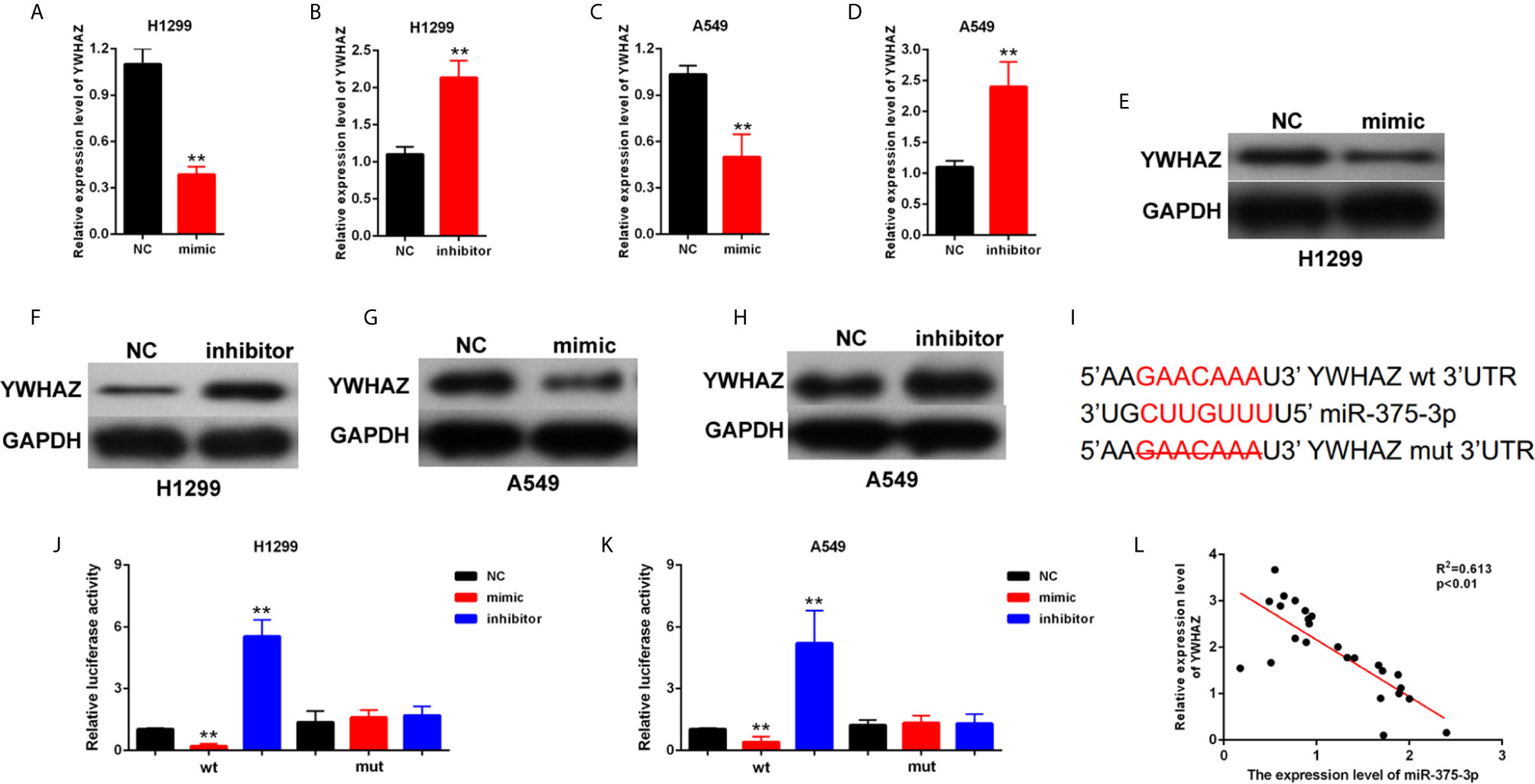
Figure 4 YWHAZ was a direct target gene of miR-375-3p. (A–D) qRT-PCR results indicated that the expression level of YWHAZ was decreased by miR-375-3p-mimic but was increased by miR-375-3p-inhibitor. (E–H) Western blot results revealed that the expression level of YWHAZ was increased by miR-375-3p overexpression but was decreased by miR-375-3p. (I) Direct binding sites between miR-375-3p and YWHAZ were presented. (J, K) Luciferase reporter assays were performed in H1299 and A549. Direct binding relationship between miR-375-3p and YWHAZ was confirmed. (L) The regression analysis of correlation between the expression of miR-375-3p and YWHAZ in lung cancer tissues (n=22). Data indicate the mean ± SD, n = 3. **P < 0.01 vs. control.
MLK7-AS1 Upregulated the YWHAZ Expression Level and Promoted Invasion by Sponging miR-375-3p
YWHAZ was overexpressed in H1299 and A549 cells (Figures 5A–D). We found that the upregulation of YWHAZ promoted cell invasion in H1299 and A549 cells (Figures 5E, F). Restore experiments were performed in H1299 and A549, and the cells were cotransfected with mimic-miR-375-3p or LV-MLK7-AS1. miR-375-3p overexpression could restore the upregulation of YWHAZ in NSCLC cells after transfection with LV-MLK7-AS1 (Figures 5G–J). mimic-miR-375-3p could restore the improvement of invasion ability in NSCLC cells after transfection with LV-MLK7-AS1 (Figures 5K, L). Lastly, the stable MLK7-AS1-overexpression in A549 cell line was established. Lung metastasis models were established by inoculation of A549 cells. As shown in Figure 5M, MLK7-AS1 overexpression could enhance the metastasis ability of A549 cells. Thus, these results suggested that MLK7-AS1 upregulated the YWHAZ expression level and enhanced the invasion by acting as miR-375-3p sponge in NSCLC.
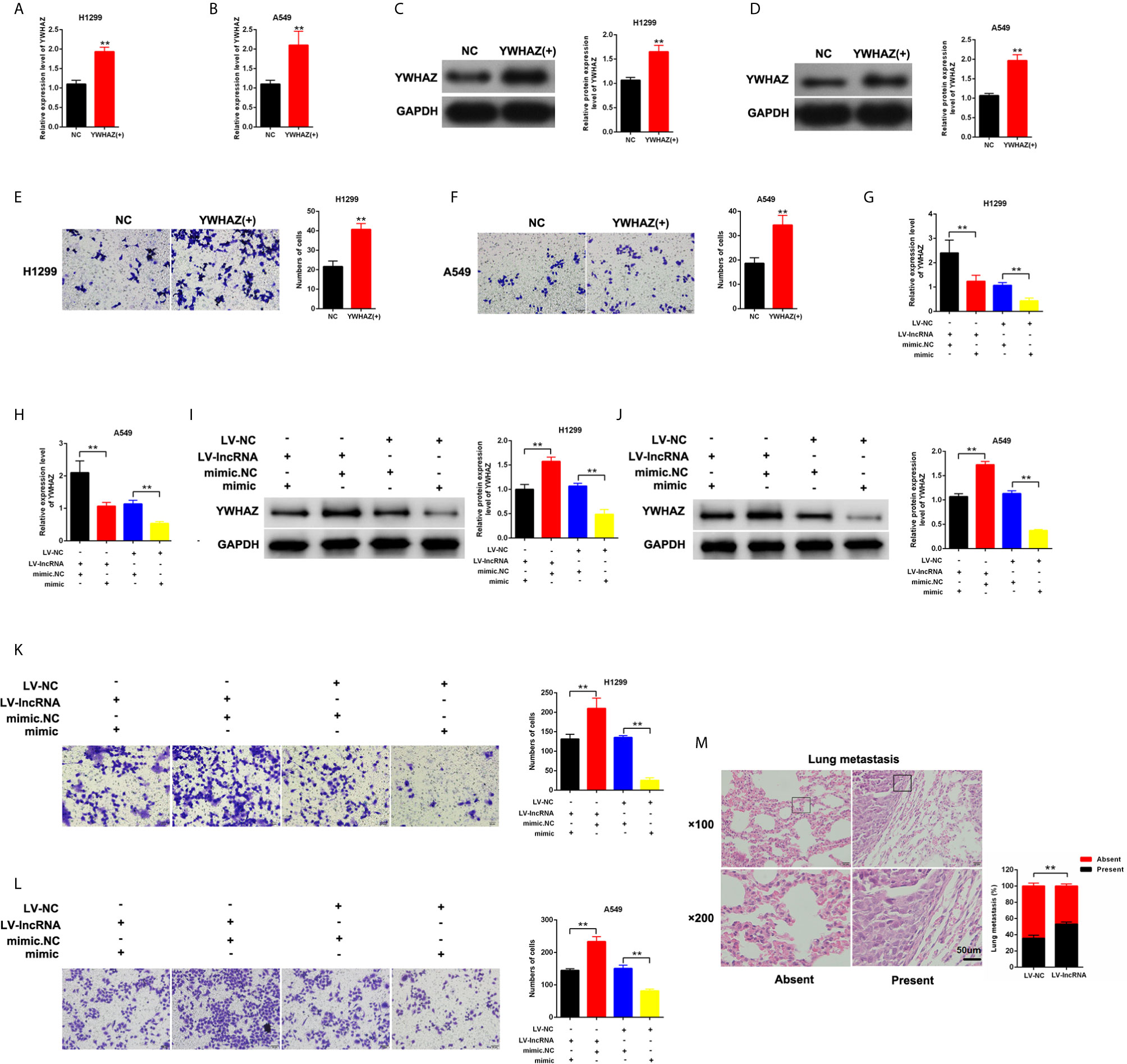
Figure 5 MLK7-AS1 upregulated YWHAZ expression and promoted the invasion by acting as miR-375-3p sponge. (A, B) Efficiency of pcDNA3.1-YWHAZ was determined through qPCR in H1229 and A549 cells. (C, D) Western blot results showed that YWHAZ gene was successfully overexpressed after transfection with pcDNA3.1-YWHAZ. (E, F) Overexpression of YWHAZ enhanced cell invasion in H1229 and A549. (G, H) mRNA expression level of YWHAZ was detected through qPCR. H1299 and A549 cells were transfected with LV-MLK7-AS1 or mimic-miR-375-3p. (I, J) Protein expression level of YWHAZ was detected by western blot. H1299 and A549 cells were transfected with LV-MLK7-AS1 or mimic-miR-375-3p. (K, L) Invasion was detected through Transwell assays. Cells were transfected with LV-MLK7-AS1 or mimic-miR-375-3p. (M) Typical images for the lung metastasis of a mouse model. The percentage of mice with or without metastatic nodules in the lungs was counted. Data indicate the mean ± SD, n = 3., **P < 0.01 vs. control.
Discussion
NSCLC is a highly aggressive tumor and has a poor five-year survival rate. Metastasis and recurrence are the important negative prognostic factors of NSCLC. Understanding the molecular mechanism of NSCLC development is helpful to address its poor survival rate. lncRNAs are important regulators of NSCLC progression. lncRNA NEF inhibits NSCLC proliferation by targeting glucose transport (26). LINC00702 inhibits tumor growth and invasion in NSCLC via the miR-510/PTEN axis (27). A novel lncRNA BC200 regulates the PI3K/AKT pathway and promoted the development of NSCLC (28).
In this study, MLK7-AS1 was identified as a vital regulator in NSCLC. Firstly, we found that MLK7-AS1 was upregulated in H1299, A549 and H1650 cell lines and NSCLC tissues, which suggested that MLK7-AS1 might participate in the progression of NSCLC. In order to study the role of MLK7-AS1 in NSCLC, overexpression and knockdown of MLK7-AS1 were performed. We found that LV-MLK7-AS1 strengthened the invasion of H1299 and A549, whereas, sh-MLK7-AS1 weakened the invasion of NSCLC cells. In-depth researches have proven that lncRNAs acted as an important regulatory role in malignancies as competing endogenous RNAs[14]. Then, miR-375-3p was identified as a direct target of MLK7-AS1. Previous studies suggested that miR-375-3p participates in tumor development. miR-375-3p may act as a tumor suppressor by targeting LAMC1 in HNSCC (29). However, the role of miR-375-3p in NSCLC is unknown.
Overexpression and knockdown assays were performed in NSCLC cells. Transwell assays indicated that the invasion of the NSCLC cells could be suppressed by mimic-miR-375-3p but could be enhanced by inhibitor-miR-375-3p. The binding between lncRNA and miRNA is according to bases of complementary matching principle (29). Then, dual-luciferase reporter assays were performed in H1299 and A549. miR-375-3p regulated the luciferase activity in WT-MLK7-AS1 reporter. But, the luciferace activity of the mutant-type reporter gene was not decreased or increased by miR-375-3p. Thus, miR-375-3p was a direct target of MLK7-AS1. By binding to the 3’UTR region of the coding gene, miRNAs downregulated the target genes expression (27). YWHAZ was predicted as a target gene for miR-375-3p. Results indicated that the mRNA and protein expression of YWHAZ were upregulated by mimic-miR-375-3p but was downregulated by inhibitor-miR-375-3p in H1299 and A549. Dual-luciferase reporter assays indicated that YWHAZ was a direct target gene for miR-375-3p. The role of YWHAZ in NSCLC was further investigated. YWHAZ enhances metastasis and is related to the poor survival in hepatocellular carcinoma (30). YWHAZ strengthens the gastric cancer cells growth ability by suppressing cell apoptosis and autophagy (24).
However, the role of YWHAZ in NSCLC is unclear. YWHAZ was overexpressed in NSCLS cells via pcDNA3.1-YWHAZ transfection. YWHAZ overexpression promoted the cell invasion in H1299 and A549. The effect of miR-375-3p in MLK7-AS1 function was investigated. The NSCLC cells were transfected with LV-MLK7-AS1 or mimic-miR-375-3p. Restore experiments confirmed that MLK7-AS1 promoted the cell invasion and upregulated the YWHAZ expression by sponging miR-375-3p. The effect of MLK7-AS1 was explored in vivo. A lung metastasis mouse model was established, and the MLK7-AS1 overexpression enhanced the metastasis ability of NSCLC cells in vivo.
Conclusion
In summary, our study reported that MLK7-AS1 was upregulated in NSCLC and can promote cell invasion in vitro and in vivo through upregulating miR-375-3p/YWHAZ axis. MLK7-AS1 might act as a potential diagnostic biomarker and therapeutic target for NSCLC.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The animal study was reviewed and approved by Henan Provincial Chest Hospital.
Author Contributions
WW and HY designed and revised the study. JJ performed the experiments and prepared the manuscript. JS and HY collected and analyzed the data. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
qPCR, Quantitative reverse transcription polymerase chain reaction; lncRNAs, long noncoding RNAs; ceRNA, competing endogenous RNA; NSCLC, non-small-cell lung cancer.
References
1. Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, et al. The lung microenvironment: an important regulator of tumour growth and metastasis. Nat Rev Cancer (2019) 19:9–31. doi: 10.1038/s41568-018-0081-9
2. Lee WH, Loo CY, Ghadiri M, Leong CR, Young PM, Traini D. The potential to treat lung cancer via inhalation of repurposed drugs. Adv Drug Delivery Rev (2018) 133:107–30. doi: 10.1016/j.addr.2018.08.012
3. Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res (2015) 27:1. doi: 10.3978/j.issn.1000-9604.2015.02.07
4. Mahvi DA, Liu R, Grinstaff MW, Colson YL, Raut CP. Local Cancer Recurrence: The Realities, Challenges, and Opportunities for New Therapies. CA Cancer J Clin (2018) 68:488–505. doi: 10.3322/caac.21498
5. Chang ZW, Jia YX, Zhang WJ, Song LJ, Gao M, Li MJ, et al. LncRNA-TUSC7/miR-224 affected chemotherapy resistance of esophageal squamous cell carcinoma by competitively regulating DESC1. J Exp Clin Cancer Res (2018) 37:56. doi: 10.1186/s13046-018-0724-4
6. Sun J, Hu J, Wang G, Yang Z, Zhao C, Zhang X, et al. LncRNA TUG1 promoted KIAA1199 expression via miR-600 to accelerate cell metastasis and epithelial-mesenchymal transition in colorectal cancer. J Exp Clin Cancer Res (2018) 37:106. doi: 10.1186/s13046-018-0771-x
7. Hu X, Duan L, Liu H, Zhang L. Long noncoding RNA LINC01296 induces non-small cell lung cancer growth and progression through sponging miR-5095. Am J Transl Res (2019) 11:895–903.
8. Lin H, Shangguan Z, Zhu M, Bao L, Zhang Q, Pan S. lncRNA FLVCR1-AS1 silencing inhibits lung cancer cell proliferation, migration, and invasion by inhibiting the activity of the Wnt/beta-catenin signaling pathway. J Cell Biochem (2019) 120:10625–32. doi: 10.1002/jcb.28352
9. Guo X, Wei Y, Wang Z, Liu W, Yang Y, Yu X, et al. LncRNA LINC00163 upregulation suppresses lung cancer development though transcriptionally increasing TCF21 expression. Am J Cancer Res (2018) 8:2494–506.
10. Yu J, Han Z, Sun Z, Wang Y, Zheng M, Song C. LncRNA SLCO4A1-AS1 facilitates growth and metastasis of colorectal cancer through beta-catenin-dependent Wnt pathway. J Exp Clin Cancer Res (2018) 37:222. doi: 10.1186/s13046-018-0896-y
11. Chen J, Yu Y, Li H, Hu Q, Chen X, He Y, et al. Long non-coding RNA PVT1 promotes tumor progression by regulating the miR-143/HK2 axis in gallbladder cancer. Mol Cancer (2019) 18:33. doi: 10.1186/s12943-019-0947-9
12. Yan H, Li H, Li P, Li X, Lin J, Zhu L, et al. Long noncoding RNA MLK7-AS1 promotes ovarian cancer cells progression by modulating miR-375/YAP1 axis. J Exp Clin Cancer Res (2018) 37:237. doi: 10.1186/s13046-018-0910-4
13. Huang T, Yin L, Wu J, Gu JJ, Wu JZ, Chen D, et al. MicroRNA-19b-3p regulates nasopharyngeal carcinoma radiosensitivity by targeting TNFAIP3/NF-kappaB axis. J Exp Clin Cancer Res (2016) 35:188. doi: 10.1186/s13046-016-0465-1
14. Wang R, Sun Y, Yu W, Yan Y, Qiao M, Jiang R, et al. Downregulation of miRNA-214 in cancer-associated fibroblasts contributes to migration and invasion of gastric cancer cells through targeting FGF9 and inducing EMT. J Exp Clin Cancer Res (2019) 38:20. doi: 10.1186/s13046-018-0995-9
15. Liu Y, Guo G, Zhong Z, Sun L, Liao L, Wang X, et al. Long non-coding RNA FLVCR1-AS1 sponges miR-155 to promote the tumorigenesis of gastric cancer by targeting c-Myc. Am J Transl Res (2019) 11:793–805.
16. Zhao W, Fu H, Zhang S, Sun S, Liu Y. LncRNA SNHG16 drives proliferation, migration, and invasion of hemangioma endothelial cell through modulation of miR-520d-3p/STAT3 axis. Cancer Med (2018) 7:3311–20. doi: 10.1002/cam4.1562
17. Chen D, Sun Q, Cheng X, Zhang L, Song W, Zhou D, et al. Genome-wide analysis of long noncoding RNA (lncRNA) expression in colorectal cancer tissues from patients with liver metastasis. Cancer Med (2016) 5:1629–39. doi: 10.1002/cam4.738
18. Hua Z, Ma K, Liu S, Yue Y, Cao H, Li Z. LncRNA ZEB1-AS1 facilitates ox-LDL-induced damage of HCtAEC cells and the oxidative stress and inflammatory events of THP-1 cells via miR-942/HMGB1 signaling. Life Sci (2020) 247:117334. doi: 10.1016/j.lfs.2020.117334
19. Yang L, Gao Q, Wu X, Feng F, Xu K. Long noncoding RNA HEGBC promotes tumorigenesis and metastasis of gallbladder cancer via forming a positive feedback loop with IL-11/STAT3 signaling pathway. J Exp Clin Cancer Res (2018) 37:186. doi: 10.1186/s13046-018-0847-7
20. Liu D, Gao M, Wu K, Zhu D, Yang Y, Zhao S. LINC00152 facilitates tumorigenesis in esophageal squamous cell carcinoma via miR-153-3p/FYN axis. BioMed Pharmacother (2019) 112:108654. doi: 10.1016/j.biopha.2019.108654
21. Liu H, Deng H, Zhao Y, Li C, Liang Y. LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J Exp Clin Cancer Res (2018) 37:279. doi: 10.1186/s13046-018-0950-9
22. Geng W, Tang H, Luo S, Lv Y, Liang D, Kang X, et al. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res (2019) 11:780–92.
23. Jamali L, Tofigh R, Tutunchi S, Panahi G, Borhani F, Akhavan S, et al. Circulating microRNAs as diagnostic and therapeutic biomarkers in gastric and esophageal cancers. J Cell Physiol (2018) 233:8538–50. doi: 10.1002/jcp.26850
24. Guo F, Jiao D, Sui GQ, Sun LN, Gao YJ, Fu QF, et al. Anticancer effect of YWHAZ silencing via inducing apoptosis and autophagy in gastric cancer cells. Neoplasma (2018) 65:693–700. doi: 10.4149/neo_2018_170922N603
25. Liu S, Jiang H, Wen H, Ding Q, Feng C. Knockdown of tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ) enhances tumorigenesis both in vivo and in vitro in bladder cancer. Oncol Rep (2018) 39:2127–35. doi: 10.3892/or.2018.6294
26. Chang L, Xu W, Zhang Y, Gong F. Long non-coding RNA-NEF targets glucose transportation to inhibit the proliferation of non-small-cell lung cancer cells. Oncol Lett (2019) 17:2795–801. doi: 10.3892/ol.2019.9919
27. Yu W, Li D, Ding X, Sun Y, Liu Y, Cong J, et al. LINC00702 suppresses proliferation and invasion in non-small cell lung cancer through regulating miR-510/PTEN axis. Aging (Albany NY) (2019) 11:1471–85. doi: 10.18632/aging.101846
28. Gao BB, Wang SX. LncRNA BC200 regulates the cell proliferation and cisplatin resistance in non-small cell lung cancer via PI3K/AKT pathway. Eur Rev Med Pharmacol Sci (2019) 23:1093–101. doi: 10.26355/eurrev_201902_16999
29. Cen WN, Pang JS, Huang JC, Hou JY, Bao WG, He RQ, et al. The expression and biological information analysis of miR-375-3p in head and neck squamous cell carcinoma based on 1825 samples from GEO, TCGA, and peer-reviewed publications. Pathol Res Pract (2018) 214:1835–47. doi: 10.1016/j.prp.2018.09.010
Keywords: non-small-cell lung cancer, noncoding RNA, MLK7-AS1, miR-375-3p, YWHAZ
Citation: Jia J, Sun J, Wang W and Yong H (2021) Long Noncoding RNA MLK7-AS1 Promotes Non-Small-Cell Lung Cancer Migration and Invasion via the miR-375-3p/YWHAZ Axis. Front. Oncol. 11:626036. doi: 10.3389/fonc.2021.626036
Received: 04 November 2020; Accepted: 05 March 2021;
Published: 22 April 2021.
Edited by:
Xiaochen Wang, University of Texas Southwestern Medical Center, United StatesReviewed by:
Xianlin Xu, Nanjing Medical University, ChinaTuoye Xu, Massachusetts General Hospital, United States
Copyright © 2021 Jia, Sun, Wang and Yong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei Yong, cjshazjj@163.com
 Jingzhou Jia1
Jingzhou Jia1 Jiwei Sun
Jiwei Sun Wenbo Wang
Wenbo Wang