- 1Department of Hepatobiliary Surgery, Mengchao Hepatobiliary Hospital of Fujian Medical University, Fuzhou, China
- 2Department of Radiation Oncology, Fujian Cancer Hospital, The School of Clinical Medicine, Fujian Medical University, Fuzhou, China
- 3Department of Hepatobiliary Surgery, The Second Hospital Affiliated to Zhejiang University, Hangzhou, China
- 4Department of Hepatobiliary Surgery, The Southwest Hospital Affiliated to the Army Medical University, Chongqing, China
- 5Department of Hepatobiliary Surgery, Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China
- 6Department of Hepatobiliary Surgery, Tongji Hospital Affiliated to Tongji Medical College, Huazhong University of Science & Technology, Wuhan, China
- 7Department of Hepatobiliary Surgery, Beijing Friendship Hospital Affiliated to Capital Medical University, Beijing, China
- 8Department of Hepatobiliary Surgery, The West China Hospital of Sichuan University, Chengdu, China
- 9Department of Hepatobiliary Surgery, Renji Hospital Affiliated to Shanghai Jiaotong University, Shanghai, China
- 10Department of Hepatobiliary Surgery, Xuanwu Hospital Affiliated to Capital Medical University, Beijing, China
- 11Department of Hepatobiliary Surgery, The Affiliated Hospital of Chuanbei Medical University, Nanchong, China
- 12Department of Hepatobiliary Surgery, Tiantan Hospital Affiliated to Capital Medical University, Beijing, China
- 13Department of Hepatobiliary Surgery III, Eastern Hepatobiliary Surgery Hospital, Secondary Military Medical University, Shanghai, China
Background: The clinical value of lymph-node dissection (LND) for intrahepatic carcinoma (ICC) patients with clinically negative lymph node metastasis (LNM) remains unclear; hence we conducted a multi-center study to explore it.
Methods: Patients who were diagnosed ICC with clinically negative LNM and underwent hepatectomy with or without LND from December 2012 to December 2015 were retrospectively collected from 12 hepatobiliary centers in China. Overall survival (OS) was analyzed using the Kaplan–Meier method, and then subgroup analysis was conducted stratified by variables related to the prognosis.
Results: A total of 380 patients were eligible including 106 (27.9%) in the LND group and 274 (72.1%) in the non-LND group. Median OS in the LND group was slightly longer than that in the non-LND group (24.0 vs. 18.0 months, P = 0.30), but a significant difference was observed between the two groups (24.0 vs. 14.0 months, P = 0.02) after a well-designed 1:1 propensity score matching without increased severe complications. And, LND was identified to be one of the independent risk factors of OS (HR = 0.66, 95%CI = 0.46–0.95, P = 0.025). Subgroup analysis in the matched cohort showed that patients could benefit more from LND if they were male, age <60 years, had no HBV infection, with ECOG score <2, CEA ≤5 ug/L, blood loss ≤400 ml, transfusion, major hepatectomy, resection margin ≥1 cm, tumor size >5 cm, single tumor, mass-forming, no satellite, no MVI, and no perineural invasion (all P < 0.05). Furthermore, only patients with pathologically confirmed positive LNM were found to benefit from postoperative adjuvant therapy (P < 0.001).
Conclusion: With the current data, we concluded that LND would benefit the selected ICC patients with clinically negative LNM and might guide the postoperative management.
Introduction
The incidence of intrahepatic cholangiocarcinoma (ICC) has been increasing in recent decades worldwide (1, 2). The long-term outcome of ICC patients receiving surgical resection remains far from satisfactory (3, 4) partly because of the unique lymph node metastasis (LNM) with an incidence of 30 to 40% (5, 6). To conduct lymph node dissection (LND) for ICC patients with clinically positive LNM has gained a consensus, but it remains controversial for patients with clinically negative LNM. The reasons are as follows: 1) survival benefit has not been confirmed yet in a recent meta-analysis (7), and 2) increasing risk of complications related to LND stirs up the surgeons’ hesitation (8, 9).
However, considering that the preoperative imaging is far from enough to ensure an accurate N staging and the postoperative management of ICC is poor, LND has been tried in more and more hepatobiliary centers. Hu et al (10). firstly reported that routine LND would not improve the prognosis of ICC patients with clinically negative LNM in a retrospective study from single center, but the survival benefit was well identified by a recent multi-center study derived from France and Japan (11), which shed light on routine LND for ICC patients with clinically negative LNM.
As is known to all, the incidence of ICC is higher in China than in most part of the world (12). Hence, we want to verify the clinical value of LND for ICC patients with clinically negative LNM using the data from a multi-center study in China and identify the potential beneficiary of regional LND.
Material and Methods
This study was conducted under the ethical guideline of the 1975 Declaration of Helsinki and was approved by Mengchao Hepatobiliary Hospital of Fujian Medical University’s Ethics Committee (No. 2018_048_01). Individual informed consent was waived by the ethics committee mainly because patient medical data including clinicopathological information and follow-up were extracted retrospectively. Data of patients receiving surgical resection between December 2012 and December 2015 were collected from multi-centers in China, including Mengchao Hepatobiliary Hospital of Fujian Medical University, Eastern Hepatobiliary Surgery Hospital, Affiliated Cancer Hospital of Chinese Academy of Medical Sciences, Tongji Hospital Affiliated to Tongji Medical College, Huazhong University of Science & Technology, Beijing Friendship Hospital Affiliated to Capital Medical University, Xuanwu Hospital Affiliated to Capital Medical University, Tiantan Hospital Affiliated to Capital Medical University, the Affiliated Hospital of Chuanbei Medical University, Renji Hospital Affiliated to Shanghai Jiaotong University, the West China Hospital of Sichuan University, the Southwest Hospital Affiliated to the Army Medical University, and the Second Hospital of Zhejiang University.
Eligibility
Patients were enrolled in this study if they 1) had been pathologically diagnosed as ICC, 2) had no LNM identified by preoperative computed tomography (CT)/magnetic resonance imaging (MRI), and 3) underwent an R0 resection with or without regional LND. Patients who had 1) preoperative obstructive jaundice, 2) extrahepatic metastasis, 3) received preoperative adjuvant treatments, and 4) died within one month following resection were excluded in this study.
Interventions
Generally, surgical procedure included hepatectomy with or without LND. Hepatectomy was conducted for patients who met the following criteria: 1) technically resectable tumor with no evidence of extrahepatic metastasis, 2) good general condition, well-tolerated liver function, and sufficient residual liver volume.
LND was conducted on condition that: 1) patient was considered to be most likely with LNM by a multiple discipline team discussion before operation, 2) swollen lymph nodes were detected manually by surgeons in the surgical procedure. LND in this study generally referred to regional LND. The procedure of LND included skeletonization of hepatoduodenal ligament and resection of para hepatic artery lymph nodes at least to the second station, which was a little different from each center.
Adjuvant treatment (AT) including transarterial chemoembolization (TACE), chemotherapy, radiotherapy, or chemoradiotherapy was conducted one month after radical resection to reduce recurrence and improve prognosis. Briefly, the preferred regimen was one or two courses of TACE, 4–6 courses of fluoropyrimide- or gemcitabine-based chemotherapy regimens, and intensity-modulated radiation therapy with a total dose of 45–50 Gy at 1.8–2.0 Gy/fractions, which was a little different from each center.
Definition
Clinically negative LNM was defined as patients without any suspicious or positive LNM determined by contrast CT or MRI (13, 14), and clinically positive LNM was defined as lymph node with short axis diameter >10 mm, central necrosis, and inhomogeneous enhancement (14, 15).
Major hepatectomy was defined as resection of three or more liver segments according to the Couinaud’s classification, while resection of less than three segments was defined as minor hepatectomy (16).
Radical resection was defined as no tumor residual at surgical margin under microscopy (17). Wide surgical margin was defined as the distance from the surgical margin to tumor edge exceeding 1 cm, while narrow surgical margin was the distance within 1 cm (18).
Histopathological specimens were evaluated by three independent pathologists. The morphological status was grouped as mass-forming (MF), intraductal growth (IG), and periductal infiltrating (PI) (3), and the differentiation was graded as well, moderate and poor.
Patients receiving LND was divided into negative pLNM (without pathologically confirmed LNM) and positive pLNM (with at least one pathologically confirmed LNM), while patients without LND were defined as non-LND.
Perineural invasion was defined as the presence of tumor cells around the nerve (19), and microvascular invasion (MVI) was defined as a portal vein, hepatic vein, or large capsule vessel of the liver tissue adjacent to the tumor edge (20).
Chemoradiotherapy was defined as patients receiving both adjuvant chemotherapy and adjuvant radiotherapy, regardless of whether sequential or simultaneous.
Adverse events (AEs) related to surgery were evaluated according to the Clavien-Dindo classification (21), and Grade III and above were defined as severe AEs.
Follow-Up and Definition of Endpoints
All patients were periodically followed up once every 2–3 months in the first 2 years and then once every 6 months. Routine follow-up items included liver function tests, serum levels of CA19-9, and CEA, and abdominal ultrasound, and a contrast-enhanced CT or MRI was warranted once recurrence was clinically suspected. Recurrence or metastasis was defined as new lesions with radiologic characteristics of ICC (22), and further treatment was immediately adopted whenever recurrence was confirmed.
The primary endpoint was overall survival (OS), which was calculated from the data of resection to either the data of death or the latest follow-up. The secondary endpoint was AE related to surgery including operation time, blood loss, transfusion, and hospital stay, which were extracted from the medical records.
Clinicopathological Variables
Potential variables associated with the prognosis of HCC patients were determined according to previous studies (23). Tumor diameter (<5 cm vs. ≥5 cm) and tumor number (single vs. multiple) were categorized according to the American Joint of Cancer Committee (AJCC) system (23). HBV infection was defined as history of HBV infection, regardless of status of HBsAg and HBV-DNA (23). The data of blood transfusion was extracted from anesthesia records, which typically included intraoperative transfusion of red blood cell and plasma (24). Age (<60 years vs. ≥60 years), blood loss (≤400 vs. >400 ml), surgical margin (wide vs. narrow), MF (no vs. yes), and differentiation (well-moderate vs. poor) were categorized as previous studies reported (23), and preoperative levels of CA19-9 (<37 vs. ≥37 U/ml) and CEA (<5 vs. ≥5 ng/ml) were categorized using cutoff value provided by clinical references (10).
Statistics
Propensity score matching (PSM) was adopted to minify the selection bias (25). Variables such as sex, age, underlying liver disease, ECOG score, and tumor characteristics including size and number were used to be matched using a 1:1 nearest neighbor method with a caliber of 0.20.
Considering that continuous variables in this study were re-defined as categorical variables, all the variables were evaluated by the chi-square test or Fisher’s exact test between the two groups before and after PSM. The clinical efficacy of LND and AT was determined by the Kaplan–Meier method, and medians with hazard ratio and confidence interference (CI) 95% were evaluated using log-rank test. Prognostic factors with P <0.05 using the log-rank test were then enrolled into multivariable Cox proportional hazards model to identify potential independent risk factors. Of note, variate of LND was abandoned due to collinearity might exist between variates of LND and LNM.
Subgroup analyses of sex (female vs. male), age (≤60 vs.>60 years), HBV infection (no vs. yes), the Eastern Cooperative Oncology Group (ECOG) score (<2 vs. ≥2),CA19-9 (≤37 vs. >37 U/mL), CEA (≤5 vs. >5 µg/L), blood loss (≤400 vs. >400 ml), resection margin (≥1 vs. <1 cm), tumor diameter (≤5 vs. >5 cm), tumor number (single vs. multiple), MF (no vs. yes), tumor differentiation (well-moderate vs. poor), satellite (no vs. yes), MVI (no vs. yes), and perineural invasion (no vs. yes) for OS were performed using log-rank test before and after PSM, and then forest plot of subgroup analysis was depicted with each estimated HR and 95% CI.
All statistical analyses were conducted using Rstudio 3.6.1 including packages of “table1”, “MatchIt”, “survminer”, “survival”, “plyr”, “forestplot”. All P values were two sided, and P <0.05 was considered statistically significant.
Results
Initially, 553 patients with ICC underwent resection, but 14 patients (2.5%) were excluded for preoperative obstructive jaundice, 13 (2.3%) were for death within one month, 32 (5.7%) were for extrahepatic metastasis, and 52 (9.4%) were for the preoperative imaging lymph node positive. During the period of follow-up (1–66 months), 32 patients (7.8%) lost to follow-up. In the final, 380 patients with clinically node-negative remained to be analyzed, including 106 (27.9%) in the LND group and 274 (72.1%) in the non-LND group (Figure 1).
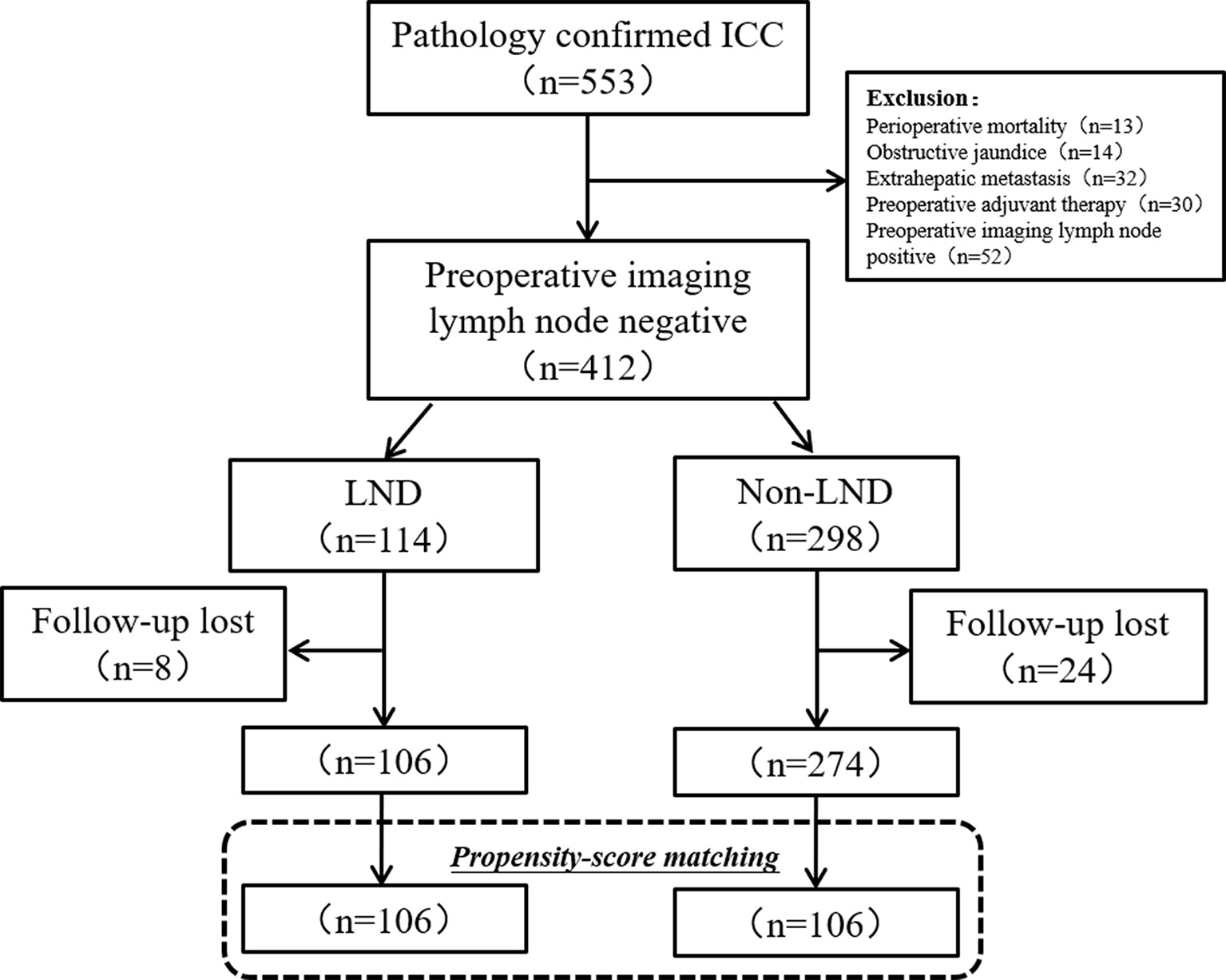
Figure 1 Flow chart of patients’ enrollment. ICC, intrahepatic carcinoma; LND, lymph node dissection.
Patients’ Characteristics
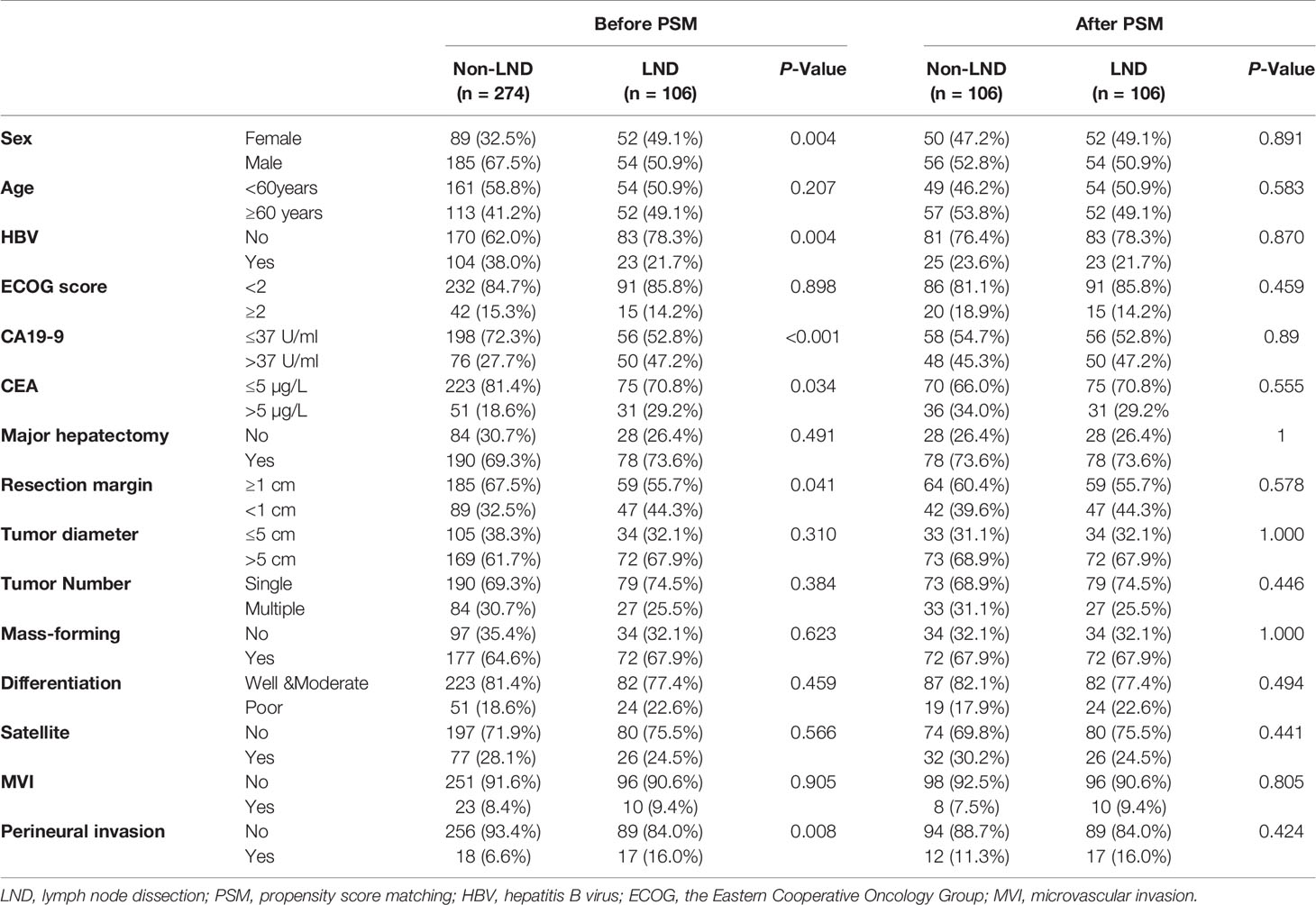
Baseline characteristics of patients were summarized in Table 1 before and after PSM. In the crude cohort, the proportion of female patients, CA19-9 >37 U/ml, CEA >5 µg/L, narrow margin (≤1 cm), and perineural invasion in the LND group was higher than that in the non-LND group (all P < 0.05), but the proportion of HBV infection in the LND group was lower than that in the non-LND group (P = 0.004). However, no significant differences were observed in all the variables after PSM (all P > 0.05).
Operative and Postoperative Outcomes
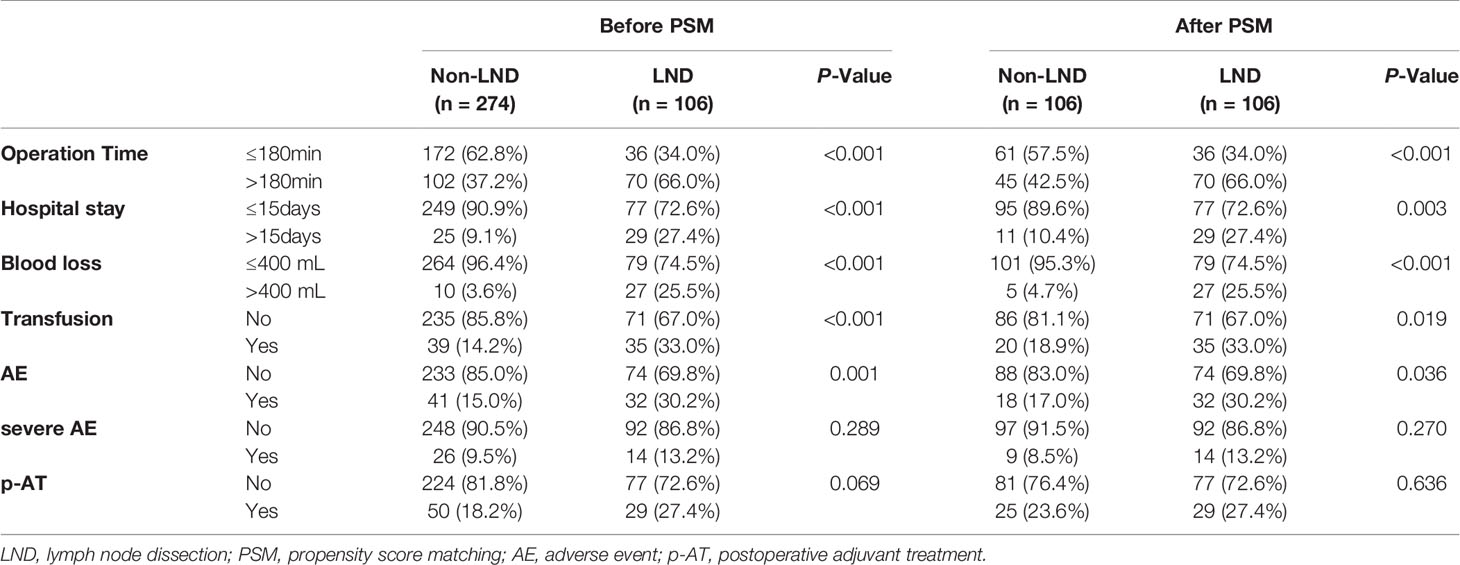
The median number of lymph node dissection was 3.5 (1–39). The proportions of operation time >180 min, hospital stay >15 days, blood loss >400 ml, transfusion, and the total AE related to the surgery in the LND group were significantly higher than those in the non-LND group (all P < 0.05, Table 2) before and after PSM, but no significant differences were observed in the incidences of severe AEs between the two groups before and after PSM (both P > 0.05). Of note, the proportion of patients receiving p-AT in the LND group was slightly higher than that in the non-LND group (27.4 vs. 18.2%, P = 0.069) before PSM, but they were comparable in the matched cohort (27.4 vs. 23.6%, P = 0.636).
Long-Term Outcomes
In the crude cohort, median OS in the LND group was slightly longer than that in the non-LND group (24.0 vs. 18.0 months, P = 0.30, Figure 2A), and the 1-, 2-, and 3-year survival rates in the two groups were 66.0 vs. 67.5%, 53.8 vs. 48.5%, 50.0 vs. 40.1%, respectively. However, a significant difference was observed in the term of median OS between the two groups after PSM (24.0 vs. 14.0 months, P = 0.02, Figure 2B), and the 1-, 2-, and 3-year survival rates in the two groups were 66.0 vs. 60.4%, 53.8 vs. 37.7%, 50.0 vs. 34.0%, respectively.
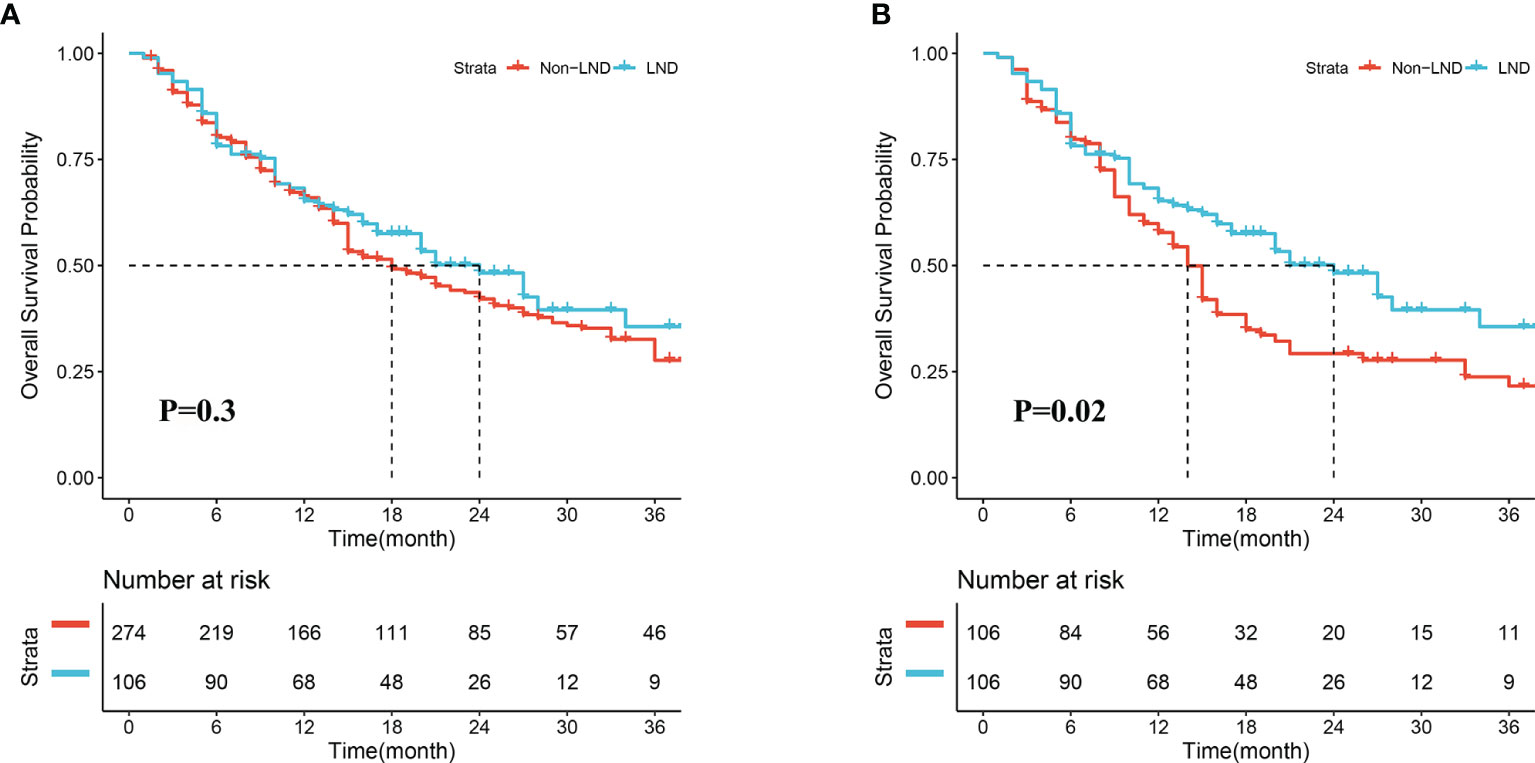
Figure 2 Overall survival of patients receiving lymph node dissection (LND) or not before (A) and after propensity score matching (B).
Univariate and Multivariate Analyses of Prognosis Factors Associated With Overall Survival
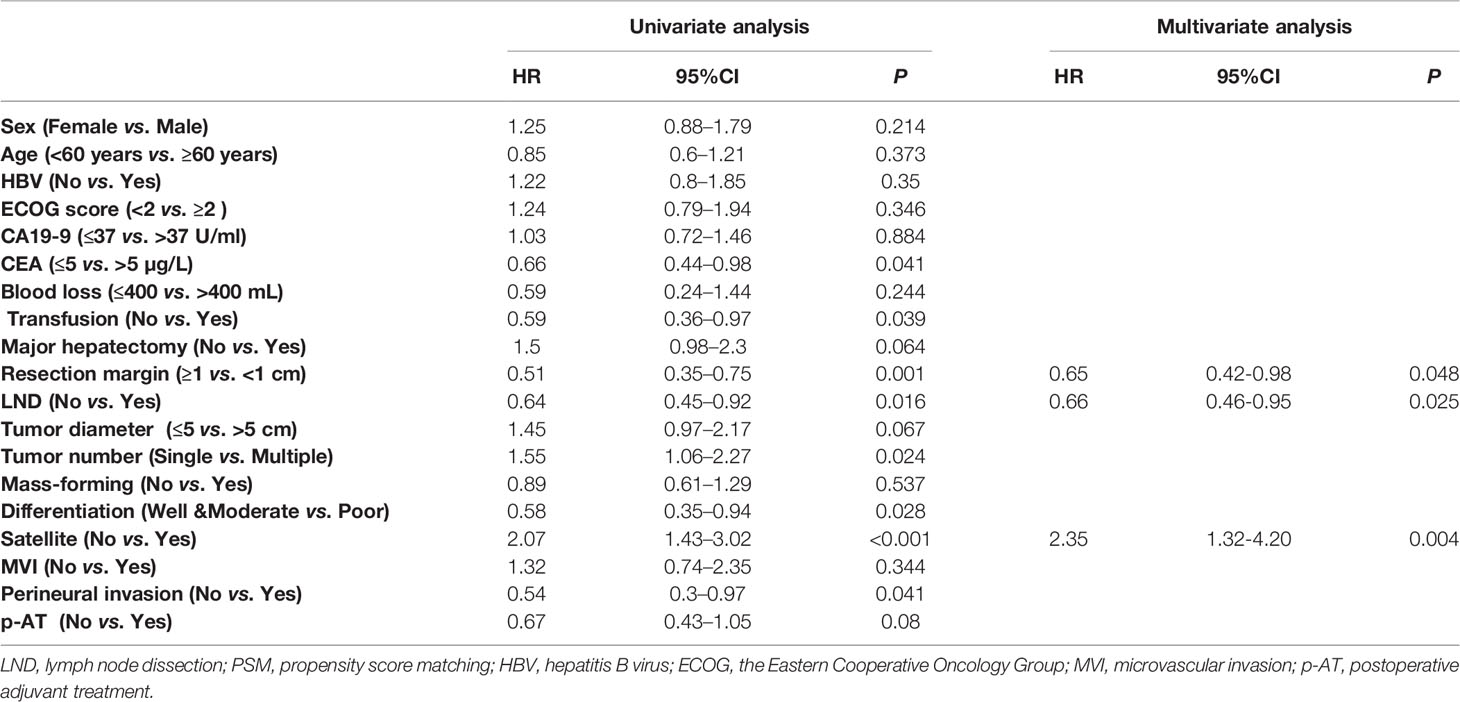
In the matched cohort, preoperative levels of CEA, transfusion, resection margin, LND, tumor number, tumor differentiation, satellite, and perineural invasion were identified to be associated with OS (all P < 0.05, Table 3) using univariate analysis. And then, LND (HR = 0.66, 95%CI = 0.46–0.95, P = 0.025), resection margin (HR = 0.65, 95%CI = 0.42–0.98, P = 0.048), and satellite (HR = 2.35, 95%CI = 1.32–4.20, P = 0.004) were independent risk factors for OS using multivariate analysis. Details were depicted in Table 3.
Subgroup Analysis of Overall Survival Stratified by Risk Factors
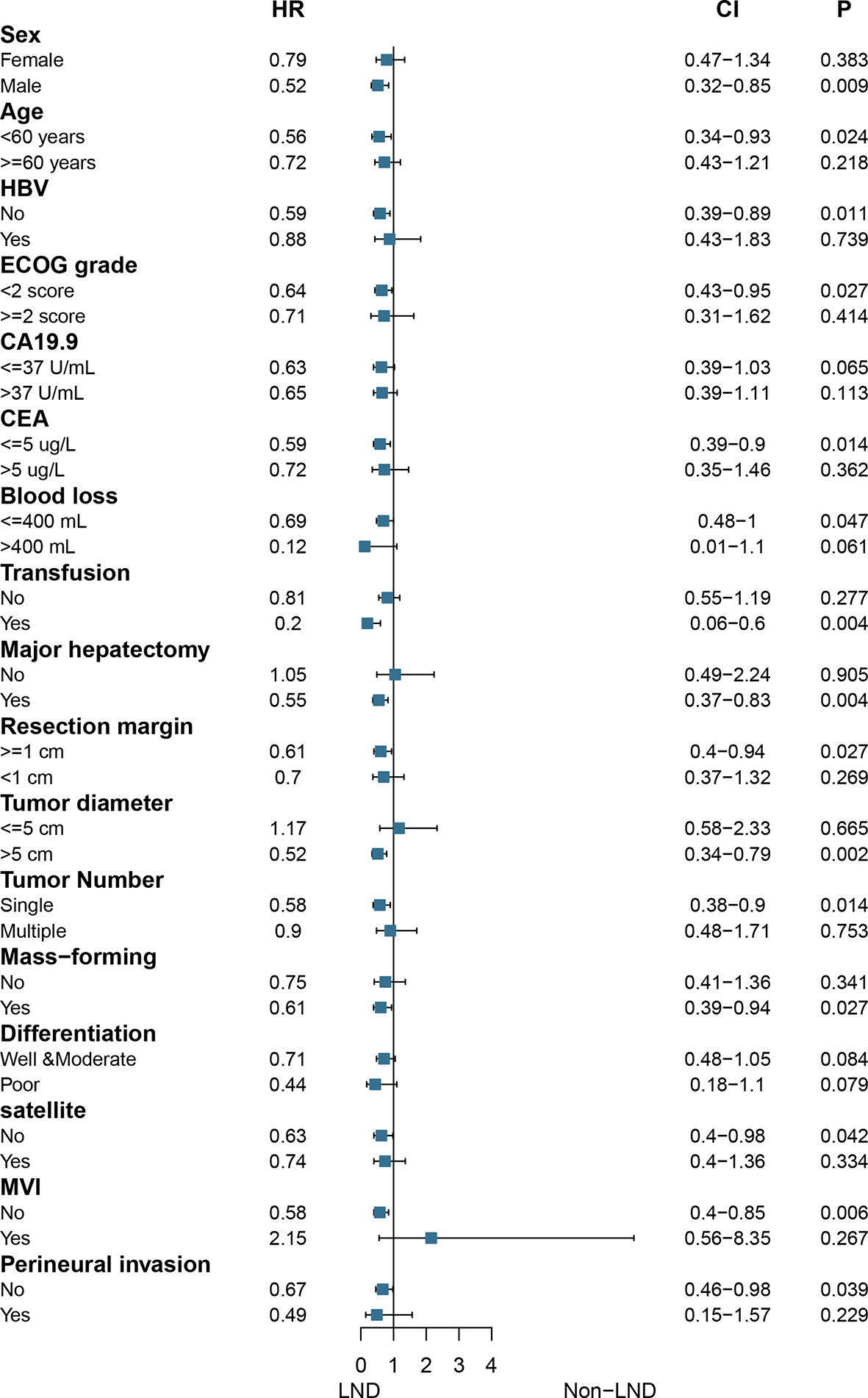
In the matched cohort, subgroup analysis showed that only patients with the following characteristics would benefit from LND: male, age <60 years, no HBV infection, ECOG score <2, CEA ≤5 ug/L, blood loss ≤400 ml, transfusion, major hepatectomy, resection margin ≥1 cm, tumor size >5 cm, single tumor, MF, no satellite, no MVI, and no perineural invasion (all P < 0.05, Figure 3).
Effects of Lymph Node Dissection on the Postoperative Management of Intrahepatic Carcinoma
In the matched cohort, 43 patients receiving LND were present with positive pLNM, 63 receiving LND were negative pLNM, and 106 receiving hepatectomy only were non-LND. Median OS of patients with negative pLNM was significantly longer than those in the patients with positive pLNM, and non-LND (28.0 vs. 10.0 months, P < 0.001; 28.0 vs. 14.0 months, P < 0.001; respectively, Figure 4A), and no significant difference was observed between patients with positive pLNM and non-LND (P > 0.05, Figure 4A).
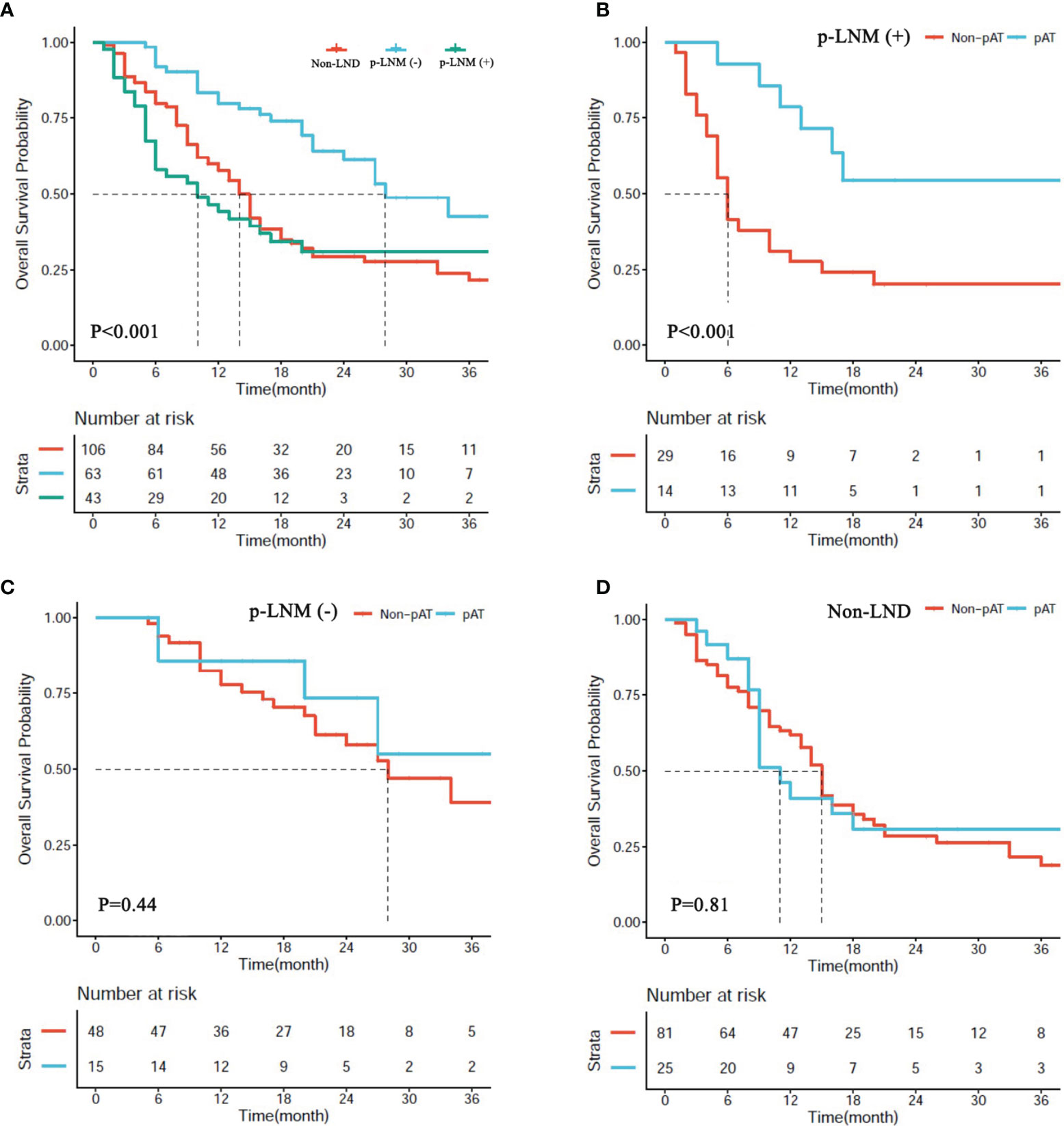
Figure 4 (A) Overall survival (OS) of patients with hepatectomy alone (non-LND), lymph node metastasis (p-LNM+), and no lymph node metastasis (p-LNM−). (B–D) OS of patients with p-LNM+, p-LNM−, and non-LND receiving postoperative adjuvant treatment or not.
In the matched cohort, 54 patients (25.5%) received AT following resection including 14 patients (32.6%) in the subgroup of positive pLNM, 15 (23.8%) in the subgroup of negative pLNM, and 25 (23.6%) in the subgroup of non-LND. The benefit of AT was observed in the subgroup of positive pLNM (P < 0.001, Figure 4B), but not in the subgroups of negative pLNM and non-LND (P = 0.81, Figure 4C; P = 0.41, Figure 4D; respectively).
Discussion
LND is a precondition of LNM diagnosed by pathology (3), which is one of the most prominent risk factors associated with recurrence and poor prognosis in ICC patients following surgical resection (26, 27), but its efficacy for patients with clinically node-negative remains controversial. In this study, the clinical value of LND for patients with clinically node-negative was evaluated using a large-scale, well-matched cohort of patients derived from 12 highly experienced hepatobiliary centers in China. Results showed that LND was associated with a prolonged median OS of ICC patients with clinically node-negative, which was accordant with the recent study from France and Japan (11).
A high incidence of LNM is the unique characteristic of ICC (5, 6), but routine LND has not been recommended in the guidelines or expert consensus on ICC. In fact, the proportion of patients who received LND is considerably low (8, 28); the reasons might be as follows: 1) previous studies found that LND did not bring the benefit for ICC patients (7); and 2) LND was generally along with prolonged surgery time and increased surgical risk (7, 8). In addition, LND is conducted in highly experienced hepatobiliary centers, and the extent of LND was far from standardization, which is determined by each surgeon. Therefore, LND was not routinely conducted in the hepatectomy for ICC patients.
Preoperative lymph node staging is the determining factor for LND or not, but the current clinical lymph node staging is unsatisfactory. The sensitivity and specificity of contrast CT are reported to be 35.0–50.0% and 77.0–92.0% (29, 30), while as for MRI, they are 34.0–78.2% and 37.4–74.5% (31, 32). In this study, 43 of 106 patients (40.6%) with clinically negative LNM receiving LND were confirmed to be present with LNM by postoperative pathology, which indicated that preoperative imaging alone could not be enough to decide whether LND should be conducted for patients with ICC, especially for those with clinically negative LNM.
However, results from the only two published studies were apparently different from each other (10, 11). In the current multi-center study, LND was found to prolong the median OS of patients with clinically node-negative in a well-designed cohort (P < 0.05), which was coincident with the previous multi-center study. Further analysis showed that patients would be much more likely to benefit from LND if they were male, age <60 years, had no HBV infection, with ECOG score <2, CEA ≤5 ug/L, major hepatectomy, wide resection margin, MF, single tumor, tumor size >5 cm, no satellite, no perineural invasion, and no MVI (all P < 0.05), which indicated that not all patients will benefit from LND. Hence, we concluded that LND should be conducted in selected patients with clinically negative LNM.
The role of LND for node-negative ICC is not just to ensure an accurate N staging. In this study, LND was found to bring survival benefit for node-negative ICC in a matched cohort, and patients receiving LND are much more likely to receive p-AT (LND vs. non-LND: 27.4 vs. 18.2%, P = 0.069). Further analysis showed that positive pLNM patients suffered worse prognosis compared with those with negative pLNM and non-LND (P < 0.05), but only patients with positive LNM were found to benefit from postoperative adjuvant treatments (P < 0.05), which indicated that LND might also play an important role in the postoperative management of ICC.
Of note, we performed further analysis not according to the N staging but based on the pathological lymph node status in this study. Firstly, at least six lymph nodes should be harvested according to the 8th AJCC guidelines (3), but the median harvested nodes were four (2–8) with the proportion of harvested lymph nodes exceeding six of 12.5% in the previous multi-center study (33). As for ICC with clinically negative LNM, it is considered to be fewer. In the current study, the median harvested nodes were 3.5 (1–39). Among the 106 patients who received LND, 30 patients (28.3%) with harvested lymph nodes exceeding six, and only 15 patients (14.2%) were diagnosed as N1. But among the remaining 76 patients (71.7%) with harvested lymph nodes <6, there were 28 patients (26.4%) who were found to have pathological LNM. In our opinion, it is acceptable that postoperative treatment could be guided according to the pathological lymph node status rather than N staging.
The optimal p-AT for resected ICC patients has not been determined, and it lacks clinical trial data to support a standard regimen in the postoperative management. Using the data from the multi-center study, we established a nomogram model (23), and found that only patients with “middle risk” could benefit from p-AT, which was confirmed by our recent meta-analysis based on the retrospective studies (34). Currently, adjuvant capecitabine chemotherapy with a duration of 6 months is recommended for patients with resected biliary tract cancer by ASCO guideline (35); while the 8th NCCN guideline suggests observation or systemic therapy for ICC patients with R0 resection and fluoropyrimidine-/gemcitabine-based chemotherapy for patients with R1 or pLNM (3). In our previous multi-center study (23), a total of 77 patients received postoperative adjuvant treatment, including 32 (41.6%) patients received TACE, 21 (27.3%) received chemotherapy, 10 (13.0%) received radiotherapy, and 14 (18.2%) received chemoradiotherapy, but only postoperative chemotherapy and TACE were found to benefit ICC patients (both P < 0.05). However, in the era of novel chemotherapy, targeted drug like apatinib, immune checkpoint inhibitors like pembrolizumab, and intensity modulated radiation therapy (35, 36), the postoperative management of ICC with positive pLNM is very promising.
There were several limitations in this study. Firstly, selection bias and recalling bias were hard to avoid in a retrospective study, although a well-designed PSM was conducted. Imaging modalities, perioperative management, and surgical procedures varied from each center, and detailed data on chemotherapy drugs and dose, radiotherapy modality and dosage, and treatment after recurrence were missing. Second, it seems insufficient to make a decision on LND based on the current CT or MRI. In the current study, the false negative rate of LNM was as high as 40.6%. Hence, PET-CT or laparoscopic exploration is strongly recommended if possible. The third limitation was the extension of LND as previously reported. Considering that the extension of LND was mostly determined by each surgeon and most of the data on the detailed extension of LND were hard to collect, we collected the number of harvested lymph nodes from the pathology reports. Even so, the median harvested lymph nodes of 3.5 were far from six to conduct N staging according to the 8th AJCC guideline. In future, a prospective multi-center trial should be conducted, in which the extension of LND and the least harvested number should be well designed and implemented.
Conclusion
With the current data, we concluded that LND could not only improve the prognosis of selected ICC patients with clinically negative LNM, but also guide the postoperative management. However, patients intended to undergo LND should be prudently selected.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: All data included in this study are available upon request by contact with the corresponding author (YZ, lamp197311@126.com).
Ethics Statement
The studies involving human participants were reviewed and approved by the Mengchao Hepatobiliary Hospital of Fujian Medical University’s Ethics Committee (No. 2018_048_01). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LW, JYL, SZ, XB, JMW, WG, FL, JW, YMZ, JDL, SC, and WZ offered the data. QK, LW, and ZL acquired, analyzed, and interpreted the data. QK and LW drafted the article. YYZ conceptualized and designed the study, critically revised the article, and gave the final approval. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Fujian Provincial Medical Center of Hepatobiliary, Science and Technology project of Fuzhou (Grant number: 2019-SZ-47).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer JL declared a shared affiliation, with no collaboration, with several of the authors QK, LW, ZL, YZ to the handling editor at the time of the review.
Acknowledgments
We thank all the patients who participated in this multi-center study.
Abbreviations
ICC, intrahepatic cholangiocarcinoma; LND, lymph node dissection; LNM, lymph node metastasis; pLNM, pathologically confirmed lymph node metastasis; PSM, propensity score matching; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, the Eastern Cooperative Oncology Group; MF, mass forming; MVI, microvascular invasion; p-AT, postoperative adjuvant therapy; TACE, transarterial chemoembolization; CT, computed tomography; MRI, magnetic resonance imaging.
References
1. Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol (2019) 71:104–14. doi: 10.1016/j.jhep.2019.03.013
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
3. Benson AB, D’Angelica MI, Abbott DE, Abrams TA, Alberts SR, Anaya DA, et al. Guidelines Insights: Hepatobiliary Cancers, Version 2.2019. J Natl Compr Canc Netw (2019) 17:302–10. doi: 10.6004/jnccn.2019.0019
4. Cillo U, Fondevila C, Donadon M, Gringeri E, Mocchegiani F, Schlitt HJ, et al. Surgery for cholangiocarcinoma. Liver Int (2019) 39Suppl 1:143–55. doi: 10.1111/liv.14089
5. de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol (2011) 29:3140–5. doi: 10.1200/JCO.2011.35.6519
6. Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, et al. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol (2009) 16:3048–56. doi: 10.1245/s10434-009-0631-1
7. Zhou R, Lu D, Li W, Tan W, Zhu S, Chen X, et al. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? A systematic review and meta-analysis. HPB (Oxford) (2019) 21:784–92. doi: 10.1016/j.hpb.2018.12.011
8. Kim DH, Choi DW, Choi SH, Heo JS, Kow AW. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery (2015) 157:666–75. doi: 10.1016/j.surg.2014.11.006
9. Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Surgical Management of Intrahepatic Cholangiocarcinoma in Patients with Cirrhosis: Impact of Lymphadenectomy on Peri-Operative Outcomes. World J Surg (2018) 42:2551–60. doi: 10.1007/s00268-017-4453-1
10. Hu J, Chen FY, Zhou KQ, Zhou C, Cao Y, Sun HC, et al. Intrahepatic cholangiocarcinoma patients without indications of lymph node metastasis not benefit from lymph node dissection. Oncotarget (2017) 8:113817–27. doi: 10.18632/oncotarget.22852
11. Yoh T, Cauchy F, Le Roy B, Seo S, Taura K, Hobeika C, et al. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: A multicenter study. Surgery (2019) 166:975–82. doi: 10.1016/j.surg.2019.06.025
12. Shen F, Xie ZH, Xia Y, Wu MC. Progress on surgical treatment of intrahepatic cholangiocarcinoma. Zhonghua Wai Ke Za Zhi (2019) 57:241–6. doi: 10.3760/cma.j.issn.0529-5815.2019.04.001
13. Seo S, Hatano E, Higashi T, Nakajima A, Nakamoto Y, Tada M, et al. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts lymph node metastasis, P-glycoprotein expression, and recurrence after resection in mass-forming intrahepatic cholangiocarcinoma. Surgery (2008) 143:769–77. doi: 10.1016/j.surg.2008.01.010
14. Zhu Y, Mao Y, Chen J, Qiu Y, Wang Z, He J. Preoperative Computed Tomography Features of Intrahepatic Cholangiocarcinoma for Predicting Lymph Node Metastasis and Overall Survival. J Comput Assist Tomogr (2019) 43:729–35. doi: 10.1097/RCT.0000000000000922
15. Ji GW, Zhu FP, Zhang YD, Liu XS, Wu FY, Wang K, et al. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur Radiol (2019) 29:3725–35. doi: 10.1007/s00330-019-06142-7
16. Weber SM, Ribero D, O’Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic cholangiocarcinoma: expert consensus statement. HPB (Oxford) (2015) 17:669–80. doi: 10.1111/hpb.12441
17. Nitsche U, Maak M, Schuster T, Kunzli B, Langer R, Slotta-Huspenina J, et al. Prediction of prognosis is not improved by the seventh and latest edition of the TNM classification for colorectal cancer in a single-center collective. Ann Surg (2011) 254:793–800; discussion 800-1. doi: 10.1097/SLA.0b013e3182369101
18. Spolverato G, Yakoob MY, Kim Y, Alexandrescu S, Marques HP, Lamelas J, et al. The Impact of Surgical Margin Status on Long-Term Outcome After Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol (2015) 22:4020–8. doi: 10.1245/s10434-015-4472-9
19. Shirai K, Ebata T, Oda K, Nishio H, Nagasaka T, Nimura Y, et al. Perineural invasion is a prognostic factor in intrahepatic cholangiocarcinoma. World J Surg (2008) 32:2395–402. doi: 10.1007/s00268-008-9726-2
20. Hu LS, Weiss M, Popescu I, Marques HP, Aldrighetti L, Maithel SK, et al. Impact of microvascular invasion on clinical outcomes after curative-intent resection for intrahepatic cholangiocarcinoma. J Surg Oncol (2019) 119:21–9. doi: 10.1002/jso.25305
21. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae
22. Hu LS, Zhang XF, Weiss M, Popescu I, Marques HP, Aldrighetti L, et al. Recurrence Patterns and Timing Courses Following Curative-Intent Resection for Intrahepatic Cholangiocarcinoma. Ann Surg Oncol (2019) 26:2549–57. doi: 10.1245/s10434-019-07353-4
23. Wang L, Deng M, Ke Q, Lou J, Zheng S, Bi X, et al. Postoperative adjuvant therapy following radical resection for intrahepatic cholangiocarcinoma: A multicenter retrospective study. Cancer Med (2020) 9:2674–85. doi: 10.1002/cam4.2925
24. Zhou PY, Tang Z, Liu WR, Tian MX, Jin L, Jiang XF, et al. Perioperative blood transfusion does not affect recurrence-free and overall survivals after curative resection for intrahepatic cholangiocarcinoma: a propensity score matching analysis. BMC Cancer (2017) 17:762. doi: 10.1186/s12885-017-3745-z
25. Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, et al. Reporting and Guidelines in Propensity Score Analysis: A Systematic Review of Cancer and Cancer Surgical Studies. J Natl Cancer Inst (2017) 109(8):djw323. doi: 10.1093/jnci/djw323
26. Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg (2014) 149:565–74. doi: 10.1001/jamasurg.2013.5137
27. Kim SH, Han DH, Choi GH, Choi JS, Kim KS. Oncologic Impact of Lymph Node Dissection for Intrahepatic Cholangiocarcinoma: a Propensity Score-Matched Study. J Gastrointest Surg (2019) 23:538–44. doi: 10.1007/s11605-018-3899-2
28. Vitale A, Moustafa M, Spolverato G, Gani F, Cillo U, Pawlik TM. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol (2016) 113:685–91. doi: 10.1002/jso.24213
29. Adachi T, Eguchi S, Beppu T, Ueno S, Shiraishi M, Okuda K, et al. Prognostic Impact of Preoperative Lymph Node Enlargement in Intrahepatic Cholangiocarcinoma: A Multi-Institutional Study by the Kyushu Study Group of Liver Surgery. Ann Surg Oncol (2015) 22:2269–78. doi: 10.1245/s10434-014-4239-8
30. Grobmyer SR, Wang L, Gonen M, Fong Y, Klimstra D, D’Angelica M, et al. Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann Surg (2006) 244:260–4. doi: 10.1097/01.sla.0000217606.59625.9d
31. Lu W, Tang ZH, Quan ZW. Viewpoint of systematic lymphadenectomy for intrahepatic cholangiocarcinoma patients. Zhonghua Wai Ke Za Zhi (2019) 57:247–52. doi: 10.3760/cma.j.issn.0529-5815.2019.04.002
32. Songthamwat M, Chamadol N, Khuntikeo N, Thinkhamrop J, Koonmee S, Chaichaya N, et al. Evaluating a preoperative protocol that includes magnetic resonance imaging for lymph node metastasis in the Cholangiocarcinoma Screening and Care Program (CASCAP) in Thailand. World J Surg Oncol (2017) 15:176. doi: 10.1186/s12957-017-1246-9
33. Bagante F, Spolverato G, Weiss M, Alexandrescu S, Marques HP, Aldrighetti L, et al. Assessment of the Lymph Node Status in Patients Undergoing Liver Resection for Intrahepatic Cholangiocarcinoma: the New Eighth Edition AJCC Staging System. J Gastrointest Surg (2018) 22:52–9. doi: 10.1007/s11605-017-3426-x
34. Ke Q, Lin N, Deng M, Wang L, Zeng Y, Liu J. The effect of adjuvant therapy for patients with intrahepatic cholangiocarcinoma after surgical resection: A systematic review and meta-analysis. PLoS One (2020) 15:e0229292. doi: 10.1371/journal.pone.0229292
35. Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, et al. Adjuvant Therapy for Resected Biliary Tract Cancer: ASCO Clinical Practice Guideline. J Clin Oncol (2019) 37:1015–27. doi: 10.1200/JCO.18.02178
Keywords: intrahepatic cholangiocarcinoma, lymph node dissection, node-negative, overall survival, propensity score matching
Citation: Ke Q, Wang L, Lin Z, Lou J, Zheng S, Bi X, Wang J, Guo W, Li F, Wang J, Zheng Y, Li J, Cheng S, Zhou W and Zeng Y (2021) Prognostic Value of Lymph Node Dissection for Intrahepatic Cholangiocarcinoma Patients With Clinically Negative Lymph Node Metastasis: A Multi-Center Study From China. Front. Oncol. 11:585808. doi: 10.3389/fonc.2021.585808
Received: 21 July 2020; Accepted: 28 January 2021;
Published: 11 March 2021.
Edited by:
Marco Scarpa, University Hospital of Padua, ItalyReviewed by:
Zhimin Geng, Xi’an Jiaotong University, ChinaJingfeng Liu, First Affiliated Hospital of Fujian Medical University, China
Alfredo Guglielmi, University of Verona, Italy
Copyright © 2021 Ke, Wang, Lin, Lou, Zheng, Bi, Wang, Guo, Li, Wang, Zheng, Li, Cheng, Zhou and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongyi Zeng, lamp197311@126.com
†These authors have contributed equally to this work
 Qiao Ke
Qiao Ke Lei Wang2†
Lei Wang2† Jianying Lou
Jianying Lou Xinyu Bi
Xinyu Bi Yongyi Zeng
Yongyi Zeng