- Department of Leukemia, University of Texas MD Anderson Cancer Center, Houston, TX, United States
Recent advances in therapeutics coupled with steady improvements in supportive care for patients with acute myeloid leukemia (AML) have led to improved outcomes. Despite these advances, even in patients that achieve a complete remission with initial therapy high rates of relapse remain a clinical dilemma. For decades, investigators have attempted strategies of maintenance therapy to prolong both remission duration and overall survival in patients with AML. These approaches have included cytotoxic chemotherapy, immunotherapy, hypomethylating agents, and targeted small molecule therapy. Overall, the evidence in favor of maintenance therapy is limited. Recent strategies, especially with hypomethylating agents have begun to show promise as maintenance therapy in improving clinical outcomes. Ongoing and future studies will continue to elucidate the true role for maintenance therapy options in patients with AML. In this review we summarize prior and ongoing maintenance therapy approaches in AML and highlight some of the most promising strategies.
Introduction
Advances in therapeutics and supportive care for acute myeloid leukemia (AML) have led to steady improvements in the outcomes for patients with AML. For example, dose intensification and novel drug combinations during induction therapy have led to higher response rates and improved survival in patients with newly diagnosed AML (1–6). Consolidation therapy helps to eradicate residual leukemia and reduces the risk of disease relapse (2, 7, 8). Despite these successes, relapse remains a major concern with relapse risk greater than 50% for all adults with high risk AML (9–11). There is a critical need for therapeutic strategies that decrease this relapse risk and improve the survival of patients with AML.
Patients with high risk AML that are ineligible for allogeneic hematopoietic stem cell transplantation (alloSCT) continue to have poor outcomes and low likelihood of cure (12). The most effective post remission therapy in AML continues to be alloSCT, but is not available to all patients with high-risk disease because high rates of complications limit broad applicability to patients with multiple comorbidities and some patients lack suitable donors (8, 13–15). A major reason for the success of alloSCT in maintenance of remission and cure of AML is through the generation of allo-reactive T cells and graft versus leukemia (GVL) effect (16–18). The current standard of care for most patients with AML achieving a CR with induction and consolidation is observation without maintenance therapy, with the exception of acute promyelocytic leukemia where maintenance arsenic trioxide and retinoic acid have shown clear benefit (19, 20). However with the recent completion of the QUAZAR AML-001 clinical study and FDA approval of CC-486 (oral azacitidine) this paradigm maybe set to change (21).
Given the high rate of relapse, there is rationale and need for post remission maintenance therapy to mitigate this risk. Maintenance remains a standard of care for patients with acute lymphoblastic leukemia (22), yet even in ALL maintenance therapy with 6-mercaptopurine, methotrexate, vincristine, and prednisone (POMP) has never been formally tested in randomized studies. Despite this, studies consistently show inferior outcomes in ALL without maintenance therapy (23–25). Clinical trials evaluating maintenance cytotoxic chemotherapy in AML in the past have consistently failed to show a benefit in overall survival while providing occasionally seen benefit in relapse-free survival (26–29).
The goal of maintenance therapy should be to improve overall survival. Improvements in disease-free, relapse-free, or event-free survival are not enough to justify the added exposure to and toxicity from anti-leukemia therapy unless they translate to gains in overall survival. This is especially true when effective salvage treatment options exist. Active therapy where active disease exists is likely to delay relapse; but if observation followed by salvage therapy leads to similar overall survival then we are likely increasing risk of therapy side effects without meaningfully affecting the natural history of a patient’s AML. Relatively few clinical trials have met this bar. With the availability of newer methods to measure minimal residual disease (MRD) after achieving a complete morphologic response, it seems intuitive that this residual disease that persists after induction and consolidation is the source of most relapses. It follows, then, that another quantifiable goal of post remission maintenance therapy is to eradicate MRD. In this review we summarize the clinical trial data on maintenance therapy in adults with AML as well as highlight avenues of promising research.
Maintenance Cytotoxic Chemotherapy
Similar to ALL, the first post-consolidation maintenance approaches in AML involved the continued use of similar cytotoxic chemotherapy to prolong remission. There have been five main randomized studies that investigated maintenance chemotherapy compared with observation in patients with AML that is in a complete remission (CR) (26, 28, 30–32). The Swiss Group for Clinical Cancer Research study randomized 74 patients with AML in remission following induction and consolidation treatment to maintenance therapy with every 8 weeks for 2 years or observation (26). The maintenance regimen consisted of alternating cytarabine (ara-C) and 6-thioguanine with ara-C and prednisone, which showed no difference in relapse or survival compared with observation (26). The German AML Cooperative Group randomized 145 patients with AML after 6-thioguanine, ara-C, and daunorubicin (TAD) induction and consolidation to a maintenance strategy of monthly alternating courses of cytarabine-daunorubicin, cytarabine–6-thioguanine, cytarabine-cyclophosphamide for 3 years (30). This strategy improved relapse-free survival (RFS) but the report did not mention overall survival (OS) (30). In a small study from the SW Leukemia Group, 32 patients were randomly assigned to maintenance therapy with monthly thioguanine and etoposide alternating with lomustine for a total of 6 courses which did not affect remission duration (31). In a study from EORTC and HOVON, 147 patients with AML in remission were randomized to maintenance low dose ara-C (LDAC) or observation (28). Maintenance LDAC improved disease-free survival (DFS) but did not improve overall survival (28). In a Group LAME study the addition of maintenance therapy with continuous oral mercaptopurine and monthly pulses of LDAC for patients in CR failed to improve 5-year DFS and resulted in inferior overall survival attributed to lower rate of response to salvage therapy (32).
Several other studies have incorporated maintenance chemotherapy strategies into their design however these have not included observation-only control arms (33–45). Given the unclear benefit of adding maintenance cytotoxic chemotherapy to patients with AML in a CR, the lack of an observation-only control arm makes these trials difficult to interpret in this setting. Based on the available data there is no consistent role for maintenance cytotoxic chemotherapy in adult AML patients in a CR given the lack of overall survival benefit and inconsistent benefit of RFS/DFS. Many of these studies are plagued by a high level of both treatment and disease heterogeneity and small sample sizes, which make any potential benefit difficult to interpret in the setting of modern AML diagnosis and treatment.
Maintenance Hypomethylating Agents
Recent studies with maintenance hypomethylating agents (HMA) have shown some promise in patients with AML in CR that are not eligible for alloSCT. Three major randomized studies have compared strategies using azacitidine maintenance with observation (21, 46, 47). In the UK NCRI AML16 trial participants achieving a CR were randomized to azacitidine 75 mg/m2 per day on days 1 to 5 for nine 6-week courses compared with observation (46). The addition of maintenance azacitidine did not result in improvement in OS; yet an unplanned subset analysis showed that for patients with no measurable residual disease (MRD) there appeared to be a survival benefit with 5-year OS of 40% in the azacitidine arm and 13% in the observation arm; this was not observed in patients with measurable MRD (46). The HOVON97 phase 3 randomized trial enrolled patients > 60 years of age with AML or MDS in CR/CRi and treated them with azacitidine 50 mg/m2 subcutaneously, days 1–5, every 4 weeks or placebo until disease progression (47). Median disease free survival in the maintenance azacitidine arm was 15.9 months which was improved from the placebo arm (HR: 0.62; 95% CI: 0.41–0.95), however this did not confer an overall survival advantage of maintenance azacitidine (HR: 0.91; 95% CI: 0.58–1.44) (47). In an exploratory multivariate analysis of this study, the DFS benefit of maintenance azacitidine appeared to be limited to patients with a platelet count of at least 100 × 109/L and those in a CR at inclusion, where the DFS benefit was not seen for those with platelet count less than 100 × 109/L and in CRi at inclusion (47).
One randomized study has compared decitabine maintenance with observation, the ECOG-ACRIN E2906 study was a randomized phase II trial which enrolled AML patients ≥ 60 years of age in CR/CRi after induction and consolidation therapy and randomized them to decitabine 20 mg/m2 on days 1–3 every 28 days for 1 year or observation (48). The study was closed with only 70% of target accrual which limited power; here they showed median disease free survival of 15.3 months in decitabine arm (HR: 0.77, 95% CI: 0.50–1.19) and median overall survival of 25.8 months (HR: 0.69, 95% CI: 0.43–1.09) both favoring the decitabine arm but neither reaching statistical significance (48). A randomized study of decitabine versus conventional care for maintenance therapy (which included LDAC, prolonged intensive chemotherapy, or observation) in patients with acute myeloid leukemia in complete remission was completed which ultimately failed to show a benefit to maintenance decitabine (49).
QUAZAR AML-001 study demonstrated the superiority of CC-486, an oral formulation of azacitidine, over placebo as a maintenance strategy in AML patients >55 years of age in first remission (21). This study enrolled patients who were aged 55 years or older, had AML, and were within 4 months of achieving first complete remission (CR) or complete remission with incomplete blood count recovery (CRi) with intensive induction chemotherapy and were not candidates for HSCT. In this study, CC-486 was given at 300 mg daily on days 1–14 of a 28-day cycle and continued until disease progression, unacceptable toxicity, or alloSCT. CC-486 improved both the relapse free and overall survival compared with placebo with median relapse free survival of 10.2 months (HR: 0.66; 95% CI: 0.53–0.81) and median overall survival of 24.7 months (HR: 0.65; 95% CI: 0.52–0.81) in the CC-486 arm (21). The subgroup analysis for overall survival showed that the point estimates for all subgroups favored the CC-486 arm. With that being said there are a few subgroups that appeared to have preferential benefit from maintenance CC-486: those that were ≥65 years old, those in a CR, those that did not receive consolidation therapy, and those that were MRD positive at study inclusion. The ideal patient for this type of maintenance approach might be an older patient, not a candidate for alloSCT, that was unable to receive any or the intended amount of consolidation therapy that is in an MRD positive state.
Overall, in patients with AML in CR that are ineligible for alloSCT, maintenance HMA approaches look promising. Now for the first time, the investigational agent CC-486 has shown an overall survival benefit using an oral azacitidine regimen; however we await the full publication before declaring this as the de facto standard of care despite recent FDA approval (21, 50). The other maintenance HMA approaches have generally shown promise in delaying relapse, yet they have consistently failed to improve overall survival. This coupled with their parenteral delivery and need for frequent clinic visits likely makes them less desirable for maintenance therapy in the majority of patients.
Maintenance Immunotherapies
Probably the most extensively studied approach to maintenance therapy in patients with AML has been with immunotherapy. In some ways, alloSCT can be considered a type of maintenance therapy in that grafted allogeneic T cells continuously surveille and maintain remission in responders through GVL effect. AlloSCT serves as a proof of concept that AML is an immune responsive malignancy and that harnessing the immune system has the potential to cure AML. However, early approaches with BCG, interleukin-2, and interferon alpha have failed to show consistent benefits of maintenance immunotherapy in AML.
There have been four RCTs on the value of adding BCG vaccination as maintenance therapy in patients with AML. Only one study, which used a combination vaccination approach with BCG and allogeneic AML cells, showed an improvement in both remission duration and OS; however that study only recruited 41 patients (51). The other three RCTs of BCG vaccination approaches failed to show benefits of this approach as maintenance therapy in patients with AML (52–54).
Cytokine therapy with interleukin-2 (IL-2) and interferon alpha (IFNα) have also been studied in a number of RCTs. Seven major RCTs have evaluated the role of IL-2 as maintenance therapy in patients with AML compared with observation (55–61). A patient level meta-analysis of six studies was performed in 2011 (62) and updated in 2015 (63), showed no benefit in terms of DFS or OS with this approach, as did the seventh trial published in 2018. IL-2 plays a crucial role in cytotoxic T and NK cell growth and survival which serves as rationale for its use in stimulating anti-leukemic immune responses. However it is also crucial for regulatory T cell survival, which may help explain the lack of clinical benefits seen, especially with low-dose IL-2 therapy (64–66). IL-2 combined with histamine dihydrochloride (HDC) is approved by the European Medicines Agency for remission maintenance in adult patients with AML in CR1 (67). This is based on the results of a Swedish study of 320 patients with AML in CR randomized to IL-2/HDC or observation which improved leukemia-free survival (LFS), especially in those in CR1 where 3-year LFS was 40% in the IL-2/HDC arm and 26% in the observation arm (68). The benefits in LFS did not translate to improvements in OS (68). Despite the lack of OS benefit, this therapy appeared to be very well tolerated with 92% of non-relapsed patients completing all intended cycles (10) of therapy (68). A German study (NCT01770158) that was set to investigate MRD dynamics with Il-2/HDC therapy in adult AML patients in CR but with MRD, however has been closed due to inability to accrue (NCT01770158). A similarly designed Swedish study (NCT01347996) to evaluate MRD in patients receiving IL-2/HDC has completed but has not yet reported results (NCT01347996).
The other cytokine that has been studied as a maintenance strategy for AML is IFNα, which may have both direct activity against AML blasts as well as indirect action through immune stimulation (69). Despite the biologic rationale, RCTs of IFNα have failed to show a benefit of IFNα as a maintenance strategy in patients with AML in CR (27, 70). A Finnish study randomized patients with AML in CR to either observation, IFNα maintenance, or thioguanine and ara-C maintenance and found no difference in these arms in terms of OS (27). The UK MRC AML11 trial included a maintenance phase (12 months with low-doses IFNα) for 362 older AML patients in CR1, which added no improvement in DFS or OS (70).
Over the last decade the development of immune checkpoint inhibitors that block immune inhibitory molecules, PD-1, PD-L1, and CTLA-4, can induce anti-cancer immune responses and have led to dramatic responses in a number of solid tumors such as melanoma and non-small cell lung cancer (71–73). Following the alloSCT example of T cells mediating graft versus leukemia effect, the use of checkpoint blockade to induce autologous, anti-leukemic T cell responses has rationale. We recently concluded a single arm study of nivolumab maintenance in AML patients in CR, at high risk for relapse but ineligible for alloSCT (74). Fifteen patients were treated with the 12- and 24-month estimated overall survival of 60% and 53%, respectively (74). Two patients of nine with detectable MRD at study entry achieved MRD negativity while on therapy; however most patients with detectable MRD at enrollment had progressive disease with eventual disease recurrence (74). A larger, randomized phase 2 study (NCT02275533) of nivolumab for eradication of MRD in high-risk AML in remission is ongoing and should further clarify this strategy.
The combination of HMA and nivolumab may have additive effects in the maintenance setting.
There is rationale for the combination of HMAs and immune checkpoint blockade, with demethylation of PD1 promoter associated with worse response in MDS and AML and increased expression of immune checkpoint molecules in patients with MDS treated with HMA (75, 76). The combination of nivolumab and azacitidine in the relapsed/refractory AML setting was recently reported and showed an overall response rate of 33%, with the overall response rate in the HMA-naive patients of 58% (77).
Lenalidomide is an orally bioavailable cereblon modulator, approved for the treatment of myelodysplastic syndrome and multiple myeloma. Lenalidomide, which as shown activity in AML, has also been shown to enhance natural killer (NK) cell cytotoxicity, cytokine secretion, and immune synapse formation that may favor anti-leukemia immunity. A recent single-arm phase II study of lenalidomide maintenance in patients with high-risk AML in CR1 or CR2, ineligible for SCT, reported interesting results (78). Among 28 patients, with a median follow-up of 22.3 months, the median CR duration was 18.7 months and the 2-year OS was 63%, surpassing historical controls (78). The benefit was most pronounced in patients with non-secondary AML and those who had undetectable MRD (78). While promising, a larger, randomized study is needed to confirm its benefit.
Despite intense focus of research over decades and strong preclinical and clinical rationale, maintenance immunotherapies have been generally disappointing. These studies have mostly suffered from heterogeneous patient populations and small sample sizes. None have shown a clear benefit in overall survival and only IL-2/HDC has a major drug approval in maintenance therapy. Ongoing efforts with checkpoint inhibitors potentially combined with HMAs or other agents such as targeted bispecific antibodies may show benefits in the future.
Maintenance Targeted Therapies
FLT3 inhibitors have been incorporated into studies that included maintenance FLT3-inhibitors in their design, in treating patients with FLT3 mutated AML. In general, it has been difficult within these studies, to ascertain the relative benefit of the maintenance phase of FLT3 inhibitors compared with the benefit in the induction and consolidation phases. With that caveat, the RATIFY trial assessed midostaurin at a dose of 50 mg twice daily added to induction and consolidation therapy and then followed by 12 months of maintenance midostaurin compared to standard therapy that did not include maintenance therapy (2). Addition of midostaurin to standard therapy resulted in significant improvements in both OS (74.7 months vs 26.0 months) and event-free survival (EFS)(8.0 months vs 3.0 months) which were also significant when censoring for alloSCT (2). In an unplanned post-hoc analysis of the RATIFY trial, the relative benefit of maintenance midostaurin on DFS could not be definitively ascertained. Here the authors looked at patients that went on to the maintenance phase of treatment, the point estimate for DFS hazard ratio was 0.83 when comparing midostaurin maintenance to placebo but with a wide confidence interval consistent with both large benefit as well as potential harm (95%CI, 0.48–1.43) (79). Additionally, a recently completed single arm German-Austrian AML Study Group trial investigated midostaurin added to chemotherapy and continued as a single-agent maintenance therapy in AML with FLT3-ITD (80). Here, 34% of the trial population (97 patients) went on to receive maintenance midostaurin either after alloSCT or consolidation and in a propensity matched analysis of the overall trial population compared with historical controls midostaurin improved EFS (80). While still unclear how much benefit the maintenance phase is adding, if treating patients per the RATIFY protocol, continuation of midostaurin maintenance post consolidation is recommended. Similarly, the SORAML trial randomized patients with FLT3 mutated AML to standard therapy with or without sorafenib (at a dose of 400 mg twice daily); for patients in CR1 the protocol added maintenance sorafenib or placebo (81). Patients that received sorafenib had improved EFS of 21 months vs 9.5 months but no improvement in OS (81). A similar trial assessed sorafenib added to standard therapy in older patients with FLT3 altered AML, however in this cohort sorafenib did not appear to improve EFS or OS (82). Gilteritinib which has shown improved outcomes in relapsed/refractory FLT3 mutated AML (83) is also being studied in an ongoing phase 2 study (NCT02927262) where patients are randomized to receive gilteritinib or placebo for a 2-year period after completion of induction/consolidation chemotherapy (83).
Multiple other targeted therapies are being tested in patients with AML with specific molecular alterations, some of which include a maintenance targeted-therapy phase. Many of these studies are single arm studies and when compared with placebo they usually include the targeted therapy in induction, consolidation, and maintenance phases making the specific contribution of the maintenance phase less clear. For example an ongoing phase 1 study (NCT02632708) of patients with IDH1 or IDH2 mutated AML receive ivosidenib (for IDH1 mutated) or enasidenib (for IDH2 mutated) combined with standard therapy for newly diagnosed AML and can continue ivosidenib or enasidenib maintenance until relapse, unacceptable toxicity, or alloSCT. Venetoclax, a bcl-2 inhibitor which has demonstrated improved outcomes in combination with HMAs for older and unfit patients with newly diagnosed AML is also being tested in a phase 2 study (NCT03466294) where patients are treated with azacitidine and venetoclax until MRD negativity is achieved and followed by venetoclax maintenance. A second study is studying a lower dose of azacitidine and venetoclax as post-consolidation maintenance therapy regardless of the type of induction therapy received (NCT04062266). Dasatinib added to intensive induction and consolidation chemotherapy and administered as single agent for 1-year maintenance for first-line patients with core binding factor showed activity in phase Ib/IIa testing and is currently being evaluated in a larger phase 3 study (NCT02013648) (84).
Targeted therapy approaches hold tremendous promise in treating patients with AML. Incorporation of maintenance continuation phases with the oral targeted therapy until relapse, unacceptable toxicity, or alloSCT is an attractive strategy. For example, this was the approval path for midostaurin in patients with FLT3-mutated AML. However, the relative contribution of the maintenance phase is unclear and will be similarly unclear if the same strategy is used for other targeted therapies. Ideally, building in a second randomization into front-line trials studying maintenance vs. observation-only could help answer these questions definitely. This approach becomes a bit more challenging after the recent approval of CC-486 as AML maintenance given the question of an appropriate control arm.
Post Allogenic Stem Cell Transplant Maintenance Therapies
AML relapse after alloSCT remains a major concern, with approximately 40% of AML patients relapsing post alloSCT and face a dismal prognosis (85–87). We consider maintenance therapy in this setting to mean treatment of patients with negative MRD with the goal of maintaining remission to allow time for or to cooperate with the graft versus leukemia effect to eradicate residual leukemic cells.
The best studied approaches to date are targeted therapies with FLT3 inhibitors. The only published RCT in this setting is the SORMAIN trial which randomized 83 patients with FLT3-ITD–positive AML in CR after alloSCT to 24 months of sorafenib (n = 43) or placebo (n = 40) (88). This study showed an improvement in RFS in favor of the sorafenib group with a HR 0.39 (95% CI, 0.18–0.85) (88). With incomplete OS follow-up there was not a statistically significant benefit in OS, but 2-year landmark survival analysis was improved in the sorafenib arm with 91% of patients in the sorafenib arm alive compared with 66% of patients alive in the placebo arm corresponding to a HR for death of 0.241 (95% CI, 0.08–0.74) (88). A similar phase 2 RCT, RADIUS (NCT01883362) using midostaurin as post alloSCT maintenance has completed but not yet reported. A much larger (n=356) phase 3 RCT of gilteritinib (NCT02997202) as a maintenance strategy in the post alloSCT setting is underway. In the ADMIRAL study, patients undergoing alloSCT that continued gilteritinib appeared to have improved survival (83). Other targeted therapy maintenance strategies are being tested such as the use of enasidenib maintenance after alloSCT in patients with IDH2 mutated myeloid neoplasms (NCT03515512).
Maintenance HMA approaches post-SCT have also been studied. A recently completed study of azacitidine maintenance in high-risk AML and MDS patients post-transplant failed to show a benefit in RFS or OS (89). In an early phase clinical trial, 30 patients with AML received CC-486 (oral azacitidine) maintenance therapy after alloSCT with a 1 year relapse rate of 21% (90). A phase 3 RCT, the AMADEUS study (NCT04173533) of this approach is currently underway. Several other small, single arm studies have tested maintenance HMA in the post alloSCT setting with varying success (91–95). A study combining venetoclax with azacitidine as maintenance therapy post alloSCT is ongoing (NCT04128501).
Immunotherapy approaches have also been studied. Cellular therapy options that have been tested include prophylactic donor lymphocyte infusion (DLI) and NK cells. Prophylactic DLI tested in 62 patients resulted in a 5-year PFS of 65% and OS of 80% (96). Prophylactic NK cell infusion was tested but did not appear to improve relapse rates compared with historical controls (97). A randomized phase 2 vaccination strategy with GVAX (an autologous cancer vaccine with GM-CSF) is currently underway (NCT01773395). Lenalidomide, an immunomodulatory molecule with particular activity in myeloid malignancies with loss of chromosome 5q (del5q), has been tested in a phase 2 LENAMAINT trial, in which 10 patients with del5q AML or MDS were treated with lenalidomide after alloSCT (98). This trial was stopped prematurely because of a signal that lenalidomide increased GVHD with 6/10 patients developing grades 3–4 GVHD within the first two cycles of lenalidomide (98).
Conclusion
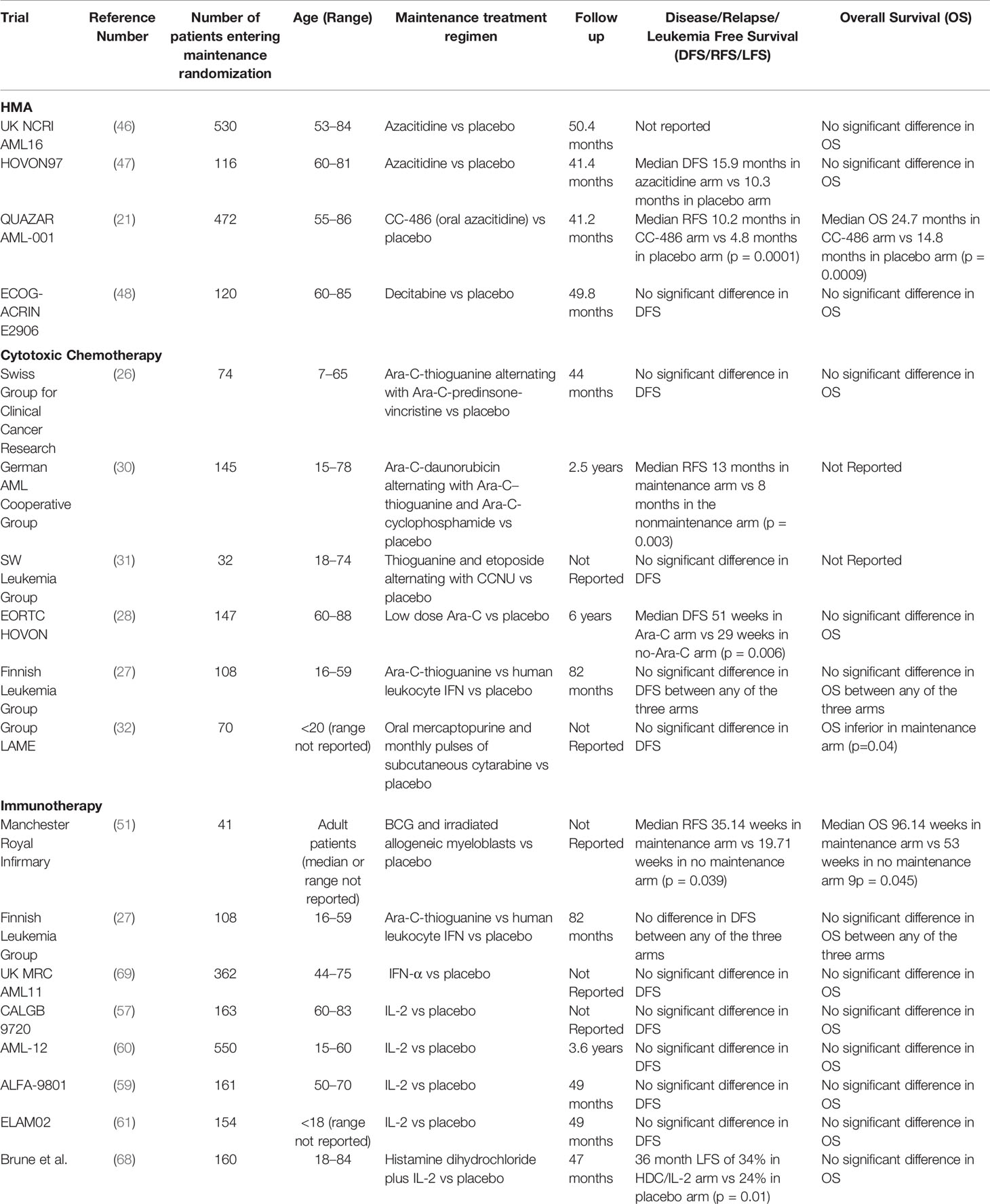
Despite decades of intense study, optimal maintenance therapy in AML has remained elusive. Improvements in overall survival and quality of life (QoL) remain the gold standard bar to achieve for maintenance approaches in patients with AML. Very few maintenance studies have incorporated QoL assessment and outcomes into their design. While we suspect that QoL will be generally diminished when comparing maintenance therapy with observation, this remains to be seen and there may be underappreciated QoL benefits in eliminating residual leukemia, even if not detectable. For now, the goal of maintenance therapy should remain to improve overall survival, but the time to read out survival improvement among patients in remission can slow progress in this arena. Surrogate measures such as eradication of MRD and improvements in relapse-free survival can be early thresholds of success that highlight promising approaches. While randomized trials are the benchmark for clinical benefit, carefully conducted pilot phase II trials with surrogate markers and historical comparisons can also be useful to quickly identify new paradigms. Table 1 highlights and summarizes the studies which have tested maintenance strategies in placebo controlled randomized studies and their effect on disease, relapse, or leukemia free survival and overall survival.
The biggest advance in AML maintenance currently has been the approval of CC-486, oral azacitidine, demonstrating improvement in both RFS and OS for patients in CR1 that are ineligible for alloSCT. While this is encouraging, the data needs to be examined further and the experience needs to be built upon. When discussing maintenance therapy in AML going forward, it will be important to clarify the role of post induction consolidation. In the QUAZAR-AML-001 study the majority of patients enrolled received either 0 or 1 cycle of consolidation therapy before starting CC-486 (21). Similarly in the UK NCRI AML16 trial, OS was improved incrementally by consolidation or maintenance but not by both (46). With agents like CC-486 or HMAs, are we simply providing a prolonged lower-intensity consolidation (instead of repeated cycles of HiDAC), or is there a benefit to long term maintenance after optimal consolidation therapy? A post-hoc analysis of the QUAZAR-AML-001 study has attempted to answer this question and suggests a benefit of CC-486 regardless of amount of consolidation received, however the overall survival benefit was not statistically longer in the patients receiving one of ≥2 consolidation cycles (99). Targeted therapies have great promise in personalizing AML therapy and improving outcomes. Incorporation of individualized targeted therapies systematically as maintenance post-consolidation is the next frontier. In the post alloSCT setting, the use of maintenance sorafenib appears to improve both DFS and likely OS when added to patients with FLT3-ITD altered AML. The use of midostaurin in FLT3 altered AML in the frontline setting showed improved OS and maintenance midostaurin may also benefit those that responded to induction and consolidation.
Despite the prior shortcomings of maintenance trials in AML, the recent success coupled with improvements in diagnosing, classifying, and treating AML make room for new approaches to maintenance therapy. In the non-alloSCT setting, stratifying patients based on the presence of detectable MRD at the time of consideration of maintenance therapy will help identify patients at high risk for relapse which will allow for better selected patients for trials of maintenance therapy. The continued development of better molecularly and immunologically targeted agents may allow for safer treatment and improved outcomes.
Author Contributions
Both PR and TK conceived and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Dr. Reville is supported by grants from the National Institutes of Health, USA (NIH grants T32CA009666).
Conflict of Interest
Dr. Kadia reports personal fees from: Novartis, Pfizer, Abbvie, Genentech, JAZZ, Agios and grants from: Pfizer, BMS, Abbvie, Genetech, JAZZ, Amgen, Astra Zeneca, Astellas, Celleknos, Genfleet, DeltaFlyPharma.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Burnett AK, Russell NH, Hills RK, Kell J, Cavenagh J, Kjeldsen L, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood (2015) 125(25):3878–85. doi: 10.1182/blood-2015-01-623447
2. Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N Engl J Med (2017) 377(5):454–64. doi: 10.1056/NEJMoa1614359
3. Borthakur G, Cortes JE, Estey EE, Jabbour E, Faderl S, O’Brien S, et al. Gemtuzumab ozogamicin with fludarabine, cytarabine, and granulocyte colony stimulating factor (FLAG-GO) as front-line regimen in patients with core binding factor acute myelogenous leukemia. Am J Hematol (2014) 89(10):964–8. doi: 10.1002/ajh.23795
4. Nazha A, Kantarjian H, Ravandi F, Huang X, Choi S, Garcia-Manero G, et al. Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients≤ 60 years with newly diagnosed acute myeloid leukemia. Am J Hematol (2013) 88(11):961–6. doi: 10.1002/ajh.23544
5. Burnett AK, Russell NH, Hills RK, Hunter AE, Kjeldsen L, Yin J, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol (2013) 31(27):3360–8. doi: 10.1200/JCO.2012.47.4874
6. Willemze R, Suciu S, Meloni G, Labar B, Marie J-P, Halkes CJM, et al. High-Dose Cytarabine in Induction Treatment Improves the Outcome of Adult Patients Younger Than Age 46 Years With Acute Myeloid Leukemia: Results of the EORTC-GIMEMA AML-12 Trial. J Clin Oncol (2014) 32(3):219–28. doi: 10.1200/JCO.2013.51.8571
7. Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive Postremission Chemotherapy in Adults with Acute Myeloid Leukemia. N Engl J Med (1994) 331(14):896–903. doi: 10.1056/NEJM199410063311402
8. Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA (2009) 301(22):2349–61. doi: 10.1001/jama.2009.813
9. Röllig C, Bornhäuser M, Thiede C, Taube F, Kramer M, Mohr B, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol (2011) 29(20):2758–65. doi: 10.1200/JCO.2010.32.8500
10. Almeida AM, Ramos F. Acute myeloid leukemia in the older adults. Leuk Res Rep (2016) 6:1–7. doi: 10.1016/j.lrr.2016.06.001
11. Rashidi A, Weisdorf DJ, Bejanyan N. Treatment of relapsed/refractory acute myeloid leukaemia in adults. Br J Haematol (2018) 181(1):27–37. doi: 10.1111/bjh.15077
12. Vasu S, Kohlschmidt J, Mrózek K, Eisfeld A-K, Nicolet D, Sterling LJ, et al. Ten-year outcome of patients with acute myeloid leukemia not treated with allogeneic transplantation in first complete remission. Blood Adv (2018) 2(13):1645–50. doi: 10.1182/bloodadvances.2017015222
13. Cassileth PA, Harrington DP, Appelbaum FR, Lazarus HM, Rowe JM, Paietta E, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. N Engl J Med (1998) 339(23):1649–56. doi: 10.1056/NEJM199812033392301
14. Cornelissen JJ, van Putten WLJ, Verdonck LF, Theobald M, Jacky E, Daenen SMG, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood (2007) 109(9):3658–66. doi: 10.1182/blood-2006-06-025627
15. Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the US registry. N Engl J Med (2014) 371(4):339–48. doi: 10.1056/NEJMsa1311707
16. Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood (1990) 75(3):555–62. doi: 10.1182/blood.V75.3.555.555
17. Marmont AM, Horowitz MM, Gale RP, Sobocinski K, Ash RC, van Bekkum DW, et al. T-cell depletion of HLA-identical transplants in leukemia. Blood (1991) 78(8):2120–30. doi: 10.1182/blood.V78.8.2120.bloodjournal7882120
18. Sweeney C, Vyas P. The Graft-Versus-Leukemia Effect in AML. Front Oncol (2019) 9:1217. doi: 10.3389/fonc.2019.01217
19. Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med (2013) 369(2):111–21. doi: 10.1056/NEJMoa1300874
20. Liang B, Zheng Z, Shi Y, Chen J, Hu X, Qian H, et al. Maintenance therapy with all-trans retinoic acid and arsenic trioxide improves relapse-free survival in adults with low- to intermediate-risk acute promyelocytic leukemia who have achieved complete remission after consolidation therapy. OncoTargets Ther (2017) 10:2305–13. doi: 10.2147/OTT.S135013
21. Wei AH, Dohner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, et al. The QUAZAR AML-001 Maintenance Trial: Results of a Phase III International, Randomized, Double-Blind, Placebo-Controlled Study of CC-486 (Oral Formulation of Azacitidine) in Patients with Acute Myeloid Leukemia (AML) in First Remission. Blood (2019) 134(Supplement_2):LBA–3. doi: 10.1182/blood-2019-132405
22. Terwilliger T, Abdul-Hay M. Acute lymphoblastic leukemia: a comprehensive review and 2017 update. Blood Cancer J (2017) 7(6):e577. doi: 10.1038/bcj.2017.53
23. Cuttner J, Mick R, Budman DR, Mayer RJ, Lee EJ, Henderson ES, et al. Phase III trial of brief intensive treatment of adult acute lymphocytic leukemia comparing daunorubicin and mitoxantrone: a CALGB Study. Leukemia (1991) 5(5):425–31.
24. Ellison RR, Mick R, Cuttner J, Schiffer CA, Silver RT, Henderson ES, et al. The effects of postinduction intensification treatment with cytarabine and daunorubicin in adult acute lymphocytic leukemia: a prospective randomized clinical trial by Cancer and Leukemia Group B. J Clin Oncol (1991) 9(11):2002–15. doi: 10.1200/JCO.1991.9.11.2002
25. Cassileth PA, Andersen JW, Bennett JM, Hoagland HC, Mazza JJ, O’Connell MC, et al. Adult acute lymphocytic leukemia: the Eastern Cooperative Oncology Group experience. Leukemia (1992) 6 Suppl 2:178–81.
26. Sauter C, Berchtold W, Fopp M, Gratwohl A, Imbach P, Maurice P, et al. Acute myelogenous leukaemia: maintenance chemotherapy after early consolidation treatment does not prolong survival. Lancet (1984) 1(8373):379–82. doi: 10.1016/S0140-6736(84)90424-0
27. Palva IP, Almqvist A, Elonen E, Hänninen A, Jouppila J, Järventie G, et al. Value of maintenance therapy with chemotherapy or interferon during remission of acute myeloid leukaemia. Eur J Haematol (1991) 47(3):229–33. doi: 10.1111/j.1600-0609.1991.tb01560.x
28. Löwenberg B, Suciu S, Archimbaud E, Haak H, Stryckmans P, de Cataldo R, et al. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy–the value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly: final report. European Organization for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group. J Clin Oncol (1998) 16(3):872–81. doi: 10.1200/JCO.1998.16.3.872
29. Robles C, Kim KM, Oken MM, Bennett JM, Letendre L, Wiernik PH, et al. Low-dose cytarabine maintenance therapy vs observation after remission induction in advanced acute myeloid leukemia: an Eastern Cooperative Oncology Group Trial (E5483). Leukemia (2000) 14(8):1349–53. doi: 10.1038/sj.leu.2401850
30. Büchner TH, Urbanitz D, Hiddemann W, Rühl H, Ludwig WD, Fischer J, et al. Intensified induction and consolidation with or without maintenance chemotherapy for acute myeloid leukemia (AML): two multicenter studies of the German AML Cooperative Group. J Clin Oncol (1985) 3(12):1583–9. doi: 10.1200/JCO.1985.3.12.1583
31. Johnson SA, Prentice AG, Phillips MJ. Treatment of acute myeloid leukaemia with early intensive induction therapy. Acta Oncol (1988) 27(5):527–9. doi: 10.3109/02841868809093582
32. Perel Y, Auvrignon A, Leblanc T, Vannier JP, Michel G, Nelken B, et al. Group LAME of the French Society of Pediatric Hematology and Immunology. Impact of addition of maintenance therapy to intensive induction and consolidation chemotherapy for childhood acute myeloblastic leukemia: results of a prospective randomized trial, LAME 89/91. J Clin Oncol (2002) 20(12):2774–81. doi: 10.1200/JCO.2002.07.300
33. Rees JK, Gray RG, Swirsky D, Hayhoe FG. Principal results of the Medical Research Council’s 8th acute myeloid leukaemia trial. Lancet (1986) 2(8518):1236–41. doi: 10.1016/S0140-6736(86)92674-7
34. Jehn U, Knüppel W, Wilmanns W. Intensive maintenance treatment in acute myelogenous leukemia (AML): single institution experience of a multicenter randomized trial. Onkologie (1988) 11(1):13–7. doi: 10.1159/000216473
35. Jehn U, Group ELC, Zittoun R, Suciu S, Fiere D, Haanen C, et al. A Randomized Comparison of Intensive Maintenance Treatment for Adult Acute Myelogenous Leukemia Using Either Cyclic Alternating Drugs or Repeated Courses of the Induction-Type Chemotherapy: AML-6 Trial of the EORTC Leukemia Cooperative Group. Acute Leukemias II (1990) 1990:277–84. doi: 10.1007/978-3-642-74643-7_50
36. Montastruc M, Reiffers J, Stoppa AM, Sotto JJ, Corront B, Marit G, et al. Treatment of acute myeloid leukemia in elderly patients: the influence of maintenance therapy (BGM 84 protocol). Nouv Rev Fr Hematol (1990) 32(2):147–52.
37. Stein RS, Vogler WR, Winton EF, Cohen HJ, Raney MR, Bartolucci A. Therapy of acute myelogenous leukemia in patients over the age of 50: a randomized Southeastern Cancer Study Group trial. Leuk Res (1990) 14(10):895–903. doi: 10.1016/0145-2126(90)90179-D
38. Büchner T, Hiddemann W, Wörmann B, Löffler H, Maschmeyer G, Hossfeld D, et al. Longterm effects of prolonged maintenance and of very early intensification chemotherapy in AML: data from AMLCG. Leukemia (1992) 6 Suppl 2:68–71.
39. Cassileth PA, Lynch E, Hines JD, Oken MM, Mazza JJ, Bennett JM, et al. Varying intensity of postremission therapy in acute myeloid leukemia. Blood (1992) 79(8):1924–30. doi: 10.1182/blood.V79.8.1924.bloodjournal7981924
40. Ohno R, Kobayashi T, Tanimoto M, Hiraoka A, Imai K, Asou N, et al. Randomized study of individualized induction therapy with or without vincristine, and of maintenance—intensification therapy between 4 or 12 courses in adult acute myeloid leukemia. AML-87 study of the Japan adult leukemia study group. Cancer (1993) 71(12):3888–95. doi: 10.1002/1097-0142(19930615)71:12<3888::AID-CNCR2820711216>3.0.CO;2-G
41. Jehn U. Long-term outcome of postremission chemotherapy for adults with acute myeloid leukemia using different dose-intensities. Leuk Lymphoma (1994) 15(1-2):99–112. doi: 10.3109/10428199409051684
42. Hewlett J, Kopecky KJ, Head D, Eyre HJ, Elias L, Kingsbury L, et al. A prospective evaluation of the roles of allogeneic marrow transplantation and low-dose monthly maintenance chemotherapy in the treatment of adult acute myelogenous leukemia (AML): a Southwest Oncology Group study. Leukemia (1995) 9(4):562–9.
43. Büchner T, Hiddemann W, Berdel WE, Wörmann B, Schoch C, Fonatsch C, et al. 6-Thioguanine, cytarabine, and daunorubicin (TAD) and high-dose cytarabine and mitoxantrone (HAM) for induction, TAD for consolidation, and either prolonged maintenance by reduced monthly TAD or TAD-HAM-TAD and one course of intensive consolidation by sequential HAM in adult patients at all ages with de novo acute myeloid leukemia (AML): a randomized trial of the German AML Cooperative Group. J Clin Oncol (2003) 21(24):4496–504. doi: 10.1200/JCO.2003.02.133
44. Miyawaki S, Sakamaki H, Ohtake S, Emi N, Yagasaki F, Mitani K, et al. A randomized, postremission comparison of four courses of standard-dose consolidation therapy without maintenance therapy versus three courses of standard-dose consolidation with maintenance therapy in adults with acute myeloid leukemia. Cancer (2005) 104(12):2726–34. doi: 10.1002/cncr.21493
45. Krug U, Berdel WE, Gale RP, Haferlach C, Schnittger S, Müller-Tidow C, et al. Increasing intensity of therapies assigned at diagnosis does not improve survival of adults with acute myeloid leukemia. Leukemia (2016) 30(6):1230–6. doi: 10.1038/leu.2016.25
46. Burnett A, Russell N, Freeman S, Kjeldsen L, Milligan D, Pocock C, et al. A comparison of limited consolidation chemotherapy therapy or not, and demethylation maintenance or not in older patients with AML and high risk MDS: Long term results of the UK NCRI AML16 trial. Haematologica (2015) 100(S1):513.
47. Huls G, Chitu DA, Havelange V, Jongen-Lavrencic M, van de Loosdrecht AA, Biemond BJ, et al. Azacitidine maintenance after intensive chemotherapy improves DFS in older AML patients. Blood (2019) 133(13):1457–64. doi: 10.1182/blood-2018-10-879866
48. Foran JM, Sun Z, Claxton DF, Lazarus HM, Arber DA, Rowe JM, et al. Maintenance Decitabine (DAC) Improves Disease-Free (DFS) and Overall Survival (OS) after Intensive Therapy for Acute Myeloid Leukemia (AML) in Older Adults, Particularly in FLT3-ITD-Negative Patients: ECOG-ACRIN (EA) E2906 Randomized Study. Blood (2019) 134(Supplement_1):115. doi: 10.1182/blood-2019-129876
49. Boumber Y, Kantarjian H, Jorgensen J, Wen S, Faderl S, Castoro R, et al. A randomized study of decitabine versus conventional care for maintenance therapy in patients with acute myeloid leukemia in complete remission. Leukemia (2012) 26(11):2428–31. doi: 10.1038/leu.2012.153
50. Center for Drug E. Research. FDA approves Onureg (azacitidine tablets) for acute myeloid leukemia. FDA (2020).
51. Zuhrie SR, Harris R, Freeman CB, MacIver JE, Geary CG, Delamore IW, et al. Immunotherapy alone vs no maintenance treatment in acute myelogenous leukaemia. Br J Cancer (1980) 41(3):372–7. doi: 10.1038/bjc.1980.60
52. Harris R, Zuhrie SR, Freeman CB, Taylor GM, MacIver JE, Geary CG, et al. Active immunotherapy in acute myelogenous leukaemia and the induction of second and subsequent remissions. Br J Cancer (1978) 37(2):282–8. doi: 10.1038/bjc.1978.37
53. Summerfield GP, Gibbs TJ, Bellingham AJ. Immunotherapy using BCG during remission induction and as the sole form of maintenance in acute myeloid leukaemia. Br J Cancer (1979) 40(5):736–42. doi: 10.1038/bjc.1979.254
54. Omura GA, Vogler WR, Lefante J, Silberman H, Knospe W, Gordon D, et al. Treatment of acute myelogenous leukemia: influence of three induction regimens and maintenance with chemotherapy or BCG immunotherapy. Cancer (1982) 49(8):1530–6. doi: 10.1002/1097-0142(19820415)49:8<1530::AID-CNCR2820490804>3.0.CO;2-1
55. Blaise D, Attal M, Reiffers J, Michallet M, Bellanger C, Pico JL, et al. Randomized study of recombinant interleukin-2 after autologous bone marrow transplantation for acute leukemia in first complete remission. Eur Cytokine Netw (2000) 11(1):91–8.
56. Kolitz JE, Hars V, DeAngelo DJ, Allen SL, Shea TC, Vij R, et al. Phase III Trial of Immunotherapy with Recombinant Interleukin-2 (rIL-2) Versus Observation in Patients< 60 Years with Acute Myeloid Leukemia (AML) in First Remission (CR1): Preliminary Results from Cancer and Leukemia Group B (CALGB) 19808. Blood (2007) 110(11):157. doi: 10.1182/blood.V110.11.157.157
57. Baer MR, George SL, Caligiuri MA, Sanford BL, Bothun SM, Mrózek K, et al. Low-dose interleukin-2 immunotherapy does not improve outcome of patients age 60 years and older with acute myeloid leukemia in first complete remission: Cancer and Leukemia Group B Study 9720. J Clin Oncol (2008) 26(30):4934–9. doi: 10.1200/JCO.2008.17.0472
58. Lange BJ, Smith FO, Feusner J, Barnard DR, Dinndorf P, Feig S, et al. Outcomes in CCG-2961, a children’s oncology group phase 3 trial for untreated pediatric acute myeloid leukemia: a report from the children’s oncology group. Blood (2008) 111(3):1044–53. doi: 10.1182/blood-2007-04-084293
59. Pautas C, Merabet F, Thomas X, Raffoux E, Gardin C, Corm S, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol (2010) 28(5):808–14. doi: 10.1200/JCO.2009.23.2652
60. Willemze R, Suciu S, Mandelli F, Halkes SJM, Marie J-P, Labar B, et al. High Dose (HD-AraC) Vs Standard Dose Cytosine Arabinoside (SD-AraC) During Induction and IL-2 Vs Observation After Consolidation/Autologous Stem Cell Transplantation in Patients with Acute Myelogenous Leukemia (AML): Final Report of the AML-12 Trial of EORTC and GIMEMA Leukemia Groups on the Value of Low Dose IL-2 Maintenance. Blood (2011) 118(21):3612–. doi: 10.1182/blood.V118.21.3612.3612
61. Petit A, Ducassou S, Leblanc T, Pasquet M, Rousseau A, Ragu C, et al. Maintenance Therapy With Interleukin-2 for Childhood AML: Results of ELAM02 Phase III Randomized Trial. Hemasphere (2018) 2(6):e159–e. doi: 10.1097/HS9.0000000000000159
62. Buyse M, Squifflet P, Lange BJ, Alonzo TA, Larson RA, Kolitz JE, et al. Individual patient data meta-analysis of randomized trials evaluating IL-2 monotherapy as remission maintenance therapy in acute myeloid leukemia. Blood (2011) 117(26):7007–13. doi: 10.1182/blood-2011-02-337725
63. Mao C, Fu X-H, Yuan J-Q, Yang Z-Y, Huang Y-F, Ye Q-L, et al. Interleukin-2 as maintenance therapy for children and adults with acute myeloid leukaemia in first complete remission. Cochrane Database Syst Rev (2015) (11):CD010248. doi: 10.1002/14651858.CD010248.pub2
64. Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol (2018) 18(10):648–59. doi: 10.1038/s41577-018-0046-y
65. Tahvildari M, Dana R. Low-Dose IL-2 Therapy in Transplantation, Autoimmunity, and Inflammatory Diseases. J Immunol (2019) 203(11):2749–55. doi: 10.4049/jimmunol.1900733
66. Sander FE, Nilsson M, Rydström A, Aurelius J, Riise RE, Movitz C, et al. Role of regulatory T cells in acute myeloid leukemia patients undergoing relapse-preventive immunotherapy. Cancer Immunol Immunother (2017) 66(11):1473–84. doi: 10.1007/s00262-017-2040-9
68. Brune M, Castaigne S, Catalano J, Gehlsen K, Ho AD, Hofmann W-K, et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood (2006) 108(1):88–96. doi: 10.1182/blood-2005-10-4073
69. Anguille S, Lion E, Willemen Y, Van Tendeloo VFI, Berneman ZN, J. EL. Interferon-α in acute myeloid leukemia: an old drug revisited. Leukemia (2011) 25(5):739–48. doi: 10.1038/leu.2010.324
70. Goldstone AH, Burnett AK, Wheatley K, Smith AG, Hutchinson RM, Clark RE, et al. Attempts to improve treatment outcomes in acute myeloid leukemia (AML) in older patients: the results of the United Kingdom Medical Research Council AML11 trial. Blood (2001) 98(5):1302–11. doi: 10.1182/blood.V98.5.1302
71. Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med (2010) 363(8):711–23. doi: 10.1056/NEJMoa1003466
72. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
73. Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med (2015) 373(1):23–34. doi: 10.1056/NEJMoa1504030
74. Kadia TM, Cortes JE, Ghorab A, Ravandi F, Jabbour E, Daver NG, et al. Nivolumab (Nivo) maintenance (maint) in high-risk (HR) acute myeloid leukemia (AML) patients. J Clin Onc (2018) 36(15_suppl):7014–. doi: 10.1200/JCO.2018.36.15_suppl.7014
75. Ørskov AD, Treppendahl MB, Skovbo A, Holm MS, Friis LS, Hokland M, et al. Hypomethylation and up-regulation of PD-1 in T cells by azacytidine in MDS/AML patients: A rationale for combined targeting of PD-1 and DNA methylation. Oncotarget (2015) 6(11):9612–26. doi: 10.18632/oncotarget.3324
76. Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng Q-R, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia (2014) 28(6):1280. doi: 10.1038/leu.2013.355
77. Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discovery (2019) 9(3):370–83. doi: 10.1158/2159-8290.CD-18-0774
78. Aboudalle I, Kantarjian HM, Ravandi F, Jabbour EJ, Daver NG, Estrov ZE, et al. Phase 2 Study of Lenalidomide Maintenance for Patients with High-Risk Acute Myeloid Leukemia in Remission. Blood (2018) 132(Supplement 1):2714–. doi: 10.1182/blood-2018-99-117427
79. Larson RA, Mandrekar SJ, Sanford BL, Laumann K, Geyer SM, Bloomfield CD, et al. An Analysis of Maintenance Therapy and Post-Midostaurin Outcomes in the International Prospective Randomized, Placebo-Controlled, Double-Blind Trial (CALGB 10603/RATIFY [Alliance]) for Newly Diagnosed Acute Myeloid Leukemia (AML) Patients with FLT3 Mutations. Blood (2017) 130(Supplement 1):145–. doi: 10.1182/blood.V130.Suppl_1.145.145
80. Schlenk RF, Weber D, Fiedler W, Salih HR, Wulf G, Salwender H, et al. Midostaurin added to chemotherapy and continued single-agent maintenance therapy in acute myeloid leukemia with FLT3-ITD. Blood (2019) 133(8):840–51. doi: 10.1182/blood-2018-08-869453
81. Röllig C, Serve H, Hüttmann A, Noppeney R, Müller-Tidow C, Krug U, et al. Addition of sorafenib versus placebo to standard therapy in patients aged 60 years or younger with newly diagnosed acute myeloid leukaemia (SORAML): a multicentre, phase 2, randomised controlled trial. Lancet Oncol (2015) 16(16):1691–9. doi: 10.1016/S1470-2045(15)00362-9
82. Serve H, Krug U, Wagner R, Sauerland MC, Heinecke A, Brunnberg U, et al. Sorafenib in combination with intensive chemotherapy in elderly patients with acute myeloid leukemia: results from a randomized, placebo-controlled trial. J Clin Oncol (2013) 31(25):3110–8. doi: 10.1200/JCO.2012.46.4990
83. Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med (2019) 381(18):1728–40. doi: 10.1056/NEJMoa1902688
84. Paschka P, Schlenk RF, Weber D, Benner A, Bullinger L, Heuser M, et al. Adding dasatinib to intensive treatment in core-binding factor acute myeloid leukemia-results of the AMLSG 11-08 trial. Leukemia (2018) 32(7):1621–30. doi: 10.1038/s41375-018-0129-6
85. Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood (2012) 119(6):1599–606. doi: 10.1182/blood-2011-08-375840
86. Thanarajasingam G, Kim HT, Cutler C, Ho VT, Koreth J, Alyea EP, et al. Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transpl (2013) 19(12):1713–8. doi: 10.1016/j.bbmt.2013.09.011
87. Bejanyan N, Weisdorf DJ, Logan BR, Wang H-L, Devine SM, de Lima M, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transpl (2015) 21(3):454–9. doi: 10.1016/j.bbmt.2014.11.007
88. Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J Clin Oncol (2020) 38(26):2993–3002. doi: 10.1200/JCO.19.03345
89. Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Popat U, et al. A phase 3 randomized study of 5-azacitidine maintenance vs observation after transplant in high-risk AML and MDS patients. Blood Adv (2020) 4(21):5580–8. doi: 10.1182/bloodadvances.2020002544
90. de Lima M, Oran B, Champlin RE, Papadopoulos EB, Giralt SA, Scott BL, et al. CC-486 Maintenance after Stem Cell Transplantation in Patients with Acute Myeloid Leukemia or Myelodysplastic Syndromes. Biol Blood Marrow Transpl (2018) 24(10):2017–24. doi: 10.1016/j.bbmt.2018.06.016
91. Jabbour E, Giralt S, Kantarjian H, Garcia-Manero G, Jagasia M, Kebriaei P, et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer (2009) 115(9):1899–905. doi: 10.1002/cncr.24198
92. de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer (2010) 116(23):5420–31. doi: 10.1002/cncr.25500
93. Oshikawa G, Kakihana K, Saito M, Aoki J, Najima Y, Kobayashi T, et al. Post-transplant maintenance therapy with azacitidine and gemtuzumab ozogamicin for high-risk acute myeloid leukaemia. Br J Haematol (2015) 169(5):756–9. doi: 10.1111/bjh.13248
94. Pusic I, Choi J, Fiala MA, Gao F, Holt M, Cashen AF, et al. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biol Blood Marrow Transpl (2015) 21(10):1761–9. doi: 10.1016/j.bbmt.2015.05.026
95. Craddock C, Jilani N, Siddique S, Yap C, Khan J, Nagra S, et al. Tolerability and Clinical Activity of Post-Transplantation Azacitidine in Patients Allografted for Acute Myeloid Leukemia Treated on the RICAZA Trial. Biol Blood Marrow Transpl (2016) 22(2):385–90. doi: 10.1016/j.bbmt.2015.09.004
96. Krishnamurthy P, Potter VT, Barber LD, Kulasekararaj AG, Lim ZY, Pearce RM, et al. Outcome of Donor Lymphocyte Infusion after T Cell–depleted Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndromes. Biol Blood Marrow Transpl (2013) 19(4):562–8. doi: 10.1016/j.bbmt.2012.12.013
97. Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, et al. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transpl (2013) 48(3):433–8. doi: 10.1038/bmt.2012.162
98. Sockel K, Bornhaeuser M, Mischak-Weissinger E, Trenschel R, Wermke M, Unzicker C, et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica (2012) 97(9):e34–5. doi: 10.3324/haematol.2012.067629
99. Wei A, Roboz GJ, Dombret H, Döhner H, Schuh AC, Montesinos P, et al. CC-486 Improves Overall Survival (OS) and Relapse-Free Survival (RFS) for Patients with Acute Myeloid Leukemia (AML) in First Remission after Intensive Chemotherapy (IC), Regardless of Amount of Consolidation Received: Results from the Phase III QUAZAR AML-001 Maintenance Trial. Blood (2020) 136(Supplement 1):38–40. doi: 10.1182/blood-2020-138498
Keywords: acute myeloid leukemia, maintenance therapy, cancer, targeted therapy, chemotherapy, immunotherapy
Citation: Reville PK and Kadia TM (2021) Maintenance Therapy in AML. Front. Oncol. 10:619085. doi: 10.3389/fonc.2020.619085
Received: 19 October 2020; Accepted: 14 December 2020;
Published: 02 February 2021.
Edited by:
Alessandro Isidori, AORMN Hospital, ItalyReviewed by:
Kendra Lynn Sweet, Moffitt Cancer Center, United StatesAlbert Oriol, Catalan Institute of Oncology, Spain
Copyright © 2021 Reville and Kadia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tapan M. Kadia, tkadia@mdanderson.org
 Patrick K. Reville
Patrick K. Reville Tapan M. Kadia*
Tapan M. Kadia*