- 1Special Medicine Department, School of Basic Medicine, Qingdao University, Qingdao, China
- 2Clinical Medicine Department, Medical College, Qingdao University, Qingdao, China
- 3Physiology Department, Medical College, Qingdao University, Qingdao, China
Background: Several studies have reported that hyperinsulinemia plays a part in the etiology of breast cancer. However, no consensus has been reached. Therefore, we conducted a meta-analysis to explore the role of insulin and C-peptide in breast cancer.
Methods: A systematic search in PubMed, Embase, and The Cochrane Library was conducted up to September, 2020. Standardized mean differences (SMDs) with 95% confidence intervals (CIs) were used to measure effect sizes. Publication bias was assessed using the Egger test. Stability of these results was evaluated using sensitivity analyses.
Results: Fourteen articles including 27,084 cases and five articles including 2,513 cases were extracted for serum insulin levels and C-peptide levels. We found that C-peptide levels were positively associated with breast cancer with overall SMD = 0.37 (95% CI = 0.09–0.65, I2 = 89.1%). Subgroup analysis by control source illustrated a positive relationship between breast cancer and C-peptide levels in population-based control. Subgroup analysis by C-peptide level indicated a positive correlation between breast cancer and C-peptide levels no matter C-peptide levels in case group is ≤3 ng/ml or >3 ng/ml. Subgroup analysis by age showed that C-peptide level positively correlated to breast cancer in women between the ages of 50 and 60. However, we did not identify any relationship between breast cancer and insulin levels (SMD = 0.22, 95% CI = −0.06–0.50, I2 = 97.3%).
Conclusion: This meta-analysis demonstrated that C-peptide levels were positively related to breast cancer in women, and no relationship between insulin levels and breast cancer was found.
Introduction
There is a high mortality of breast cancer in women aged 20 to 50 years, with an incidence rate up to 10.4% of all cancers (1). The yearly incidence of the disease is increasing while the related mortality is steadily decreasing (2). Many aspects, including genetic, environmental, and hormonal factors, were involved in breast cancer. Obesity, diabetes, and other metabolic disorders have long been known to have a relationship with increased breast cancer incidence (3). Currently, the relationship between causative factor of metabolic diseases and breast cancer has been extensively studied. Metabolic hormones such as c-peptide and insulin are believed to play a role in the occurrence and development of breast cancer. The findings, however, have been inconsistent (4).
Insulin is a kind of protein growth factor secreted by the pancreas, which has physiological functions such as regulating cellular metabolism, glucose consumption, and cardiovascular homeostasis (5). There are several lines of evidence to suggest that insulin levels are positively related to breast cancer incidence. Insulin is a potent hormone that activates various pathways to drive the occurrence and progression of breast cancer (6). Obesity-induced insulin resistance is a risk factor for aggressive breast cancer biology in post-menopausal women (7). High serum insulin levels can drive breast cancer incidence, and this explains the association between obesity and breast cancer (8). However, Cordero-Franco et al. (9) reported an absence of a link between IR and breast cancer incidence, which is similar to the results presented by Mink et al. (10) and Muti et al. (11) who found no relationship between increased breast cancer risk and insulin.
C-peptide is a small peptide that connects two proinsulin molecule chains and is dissociated before insulin is released. It is secreted in equal molar quantities from pancreatic beta-cells in the insulin cycle (12). C-peptide is a biological by-product of insulin processing; however, due to its stability in plasma, c-peptide is also clinically used to evaluation of beta-cell function (13). Recent work highlighted the role of C-peptide in diabetes and also in many related complications such as cancer (14). Pisani in 2008 reported that the 26% elevated breast cancer incidence was linked to the elevated insulin or C-peptide blood levels (15), while Autier (16) reported no association between C-peptide levels and breast cancer incidence.
Because many studies link obesity, insulin resistance to breast cancer, we need to clarify the status of insulin or c-peptide in breast cancer biology. To do so, we conducted a meta-analysis and investigated whether they play a crucial role in breast cancer biology.
Methods
Search Strategy
Databases including PubMed, Cochrane Library, and Embase were searched by authors ML and LS independently until September, 2020. Three keywords, including medical subject heading terms and free-text terms, were used in the search strategy to find the related studies. In addition, the list of references to relevant studies was manually checked to add additional studies. Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines were followed in the present meta-analysis.
Study Selection and Criteria
The inclusion criteria were as follows: (1) case–control study, nested case–control study or cohort study; (2) new diagnoses with breast cancer pathologically prior to receiving any treatment; (3) adequate data to calculate standardized mean difference (SMD) with 95% confidence interval (CI); and (4) blood samples were collected after fasting overnight and before any therapeutic intervention. The exclusion criteria were as follows: (1) reviews, comments, protocols, meeting abstracts, case report, or letters; (2) no control subjects or other essential information; (3) patients in the studies had previous malignancies or co-existing diseases such as obesity and diabetes; and (4) articles not published in English.
Data Extraction and Quality Assessment
Three researchers extracted useful information and data and evaluated the availability of individual studies independently.
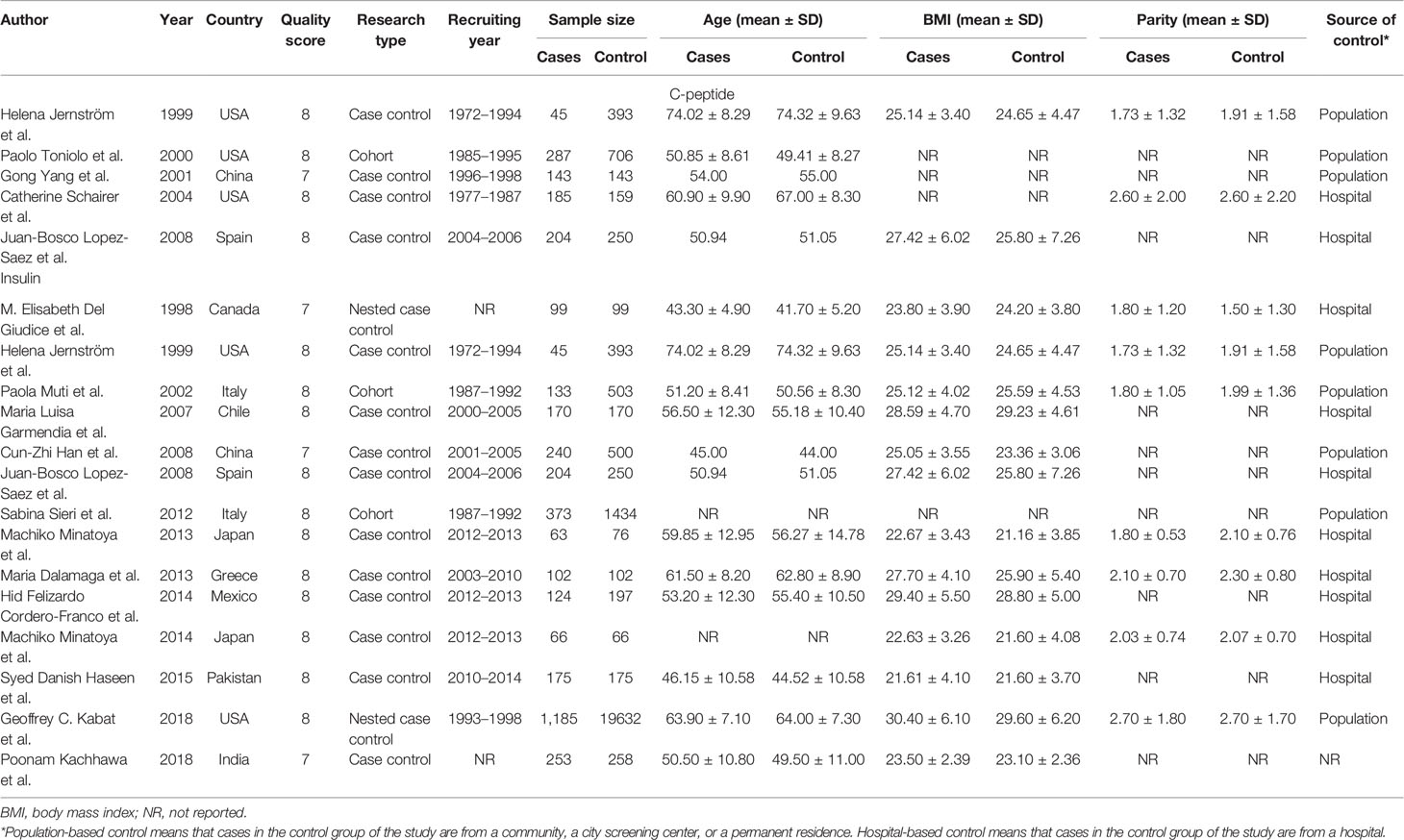
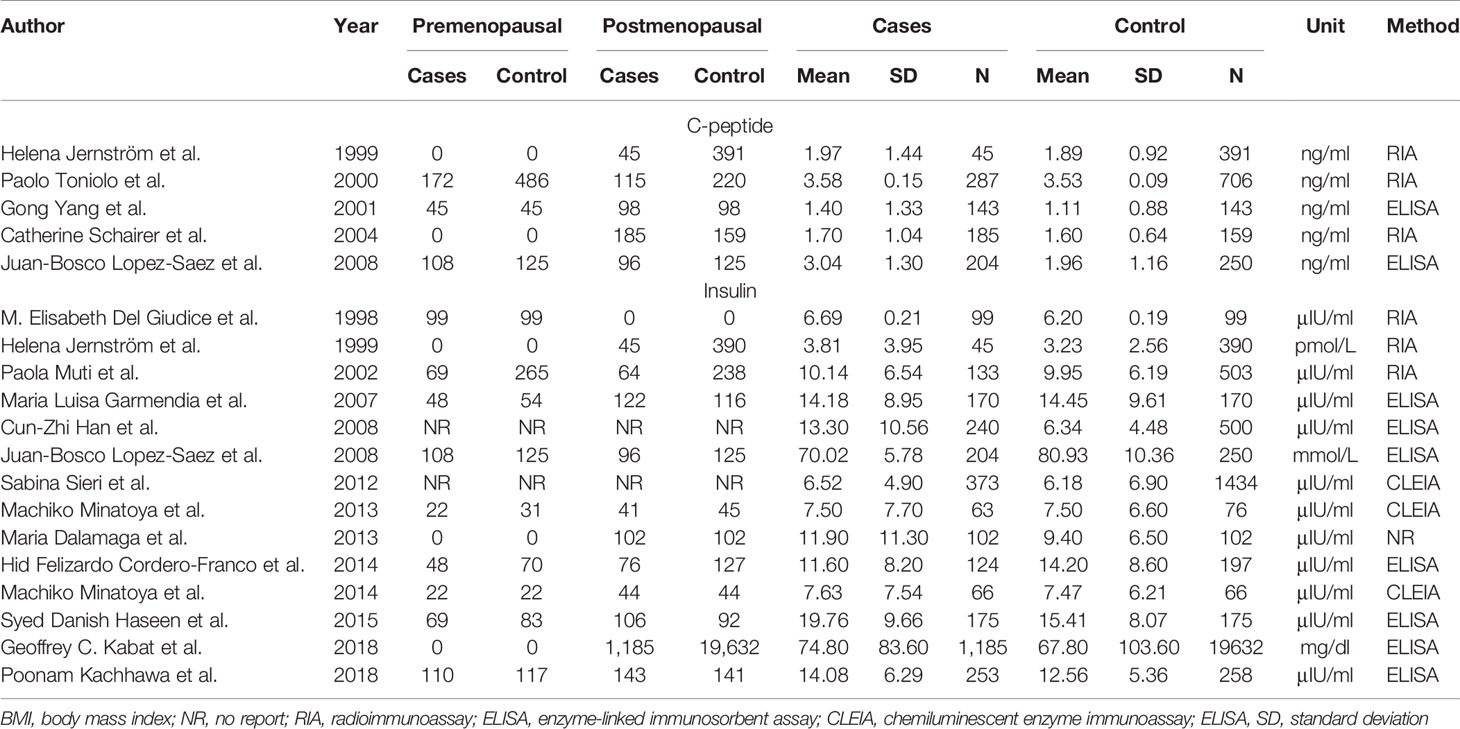
Items were collected as follows: first author’s name, time of publication, countries, research type, serum insulin or C-peptide levels, measuring method, sample size, patient age, body mass index (BMI), parity, source of cases in the control group in the study (control source), and menstrual status. All discrepancies were resolved by consensus.
We used the Newcastle–Ottawa Scale (NOS) to judge the availability of all studies (17). We graded each article with a range from zero (the lowest) to nine (the highest), and if the score is ≥7, it is considered high-quality research.
Statistical Analysis
Data from the included studies were combined for meta-analysis using Stata 16.0 (StataCorp LLC, College Station, TX, USA). The SMDs and 95% CI of each study were calculated according to the sample size, and the mean and standard deviation (SD) of insulin or c-peptide levels. The I2 statistic was used to investigate heterogeneity among studies (18), and a random-effect model was selected. To identify the source of heterogeneity, subgroup analysis was conducted according to control source, C-peptide level, country, measuring method, menopausal status, and age. Sensitivity analysis was also conducted to evaluate the stability of the analysis. Egger’s test was used to evaluate publication bias.
Results
Literature Search
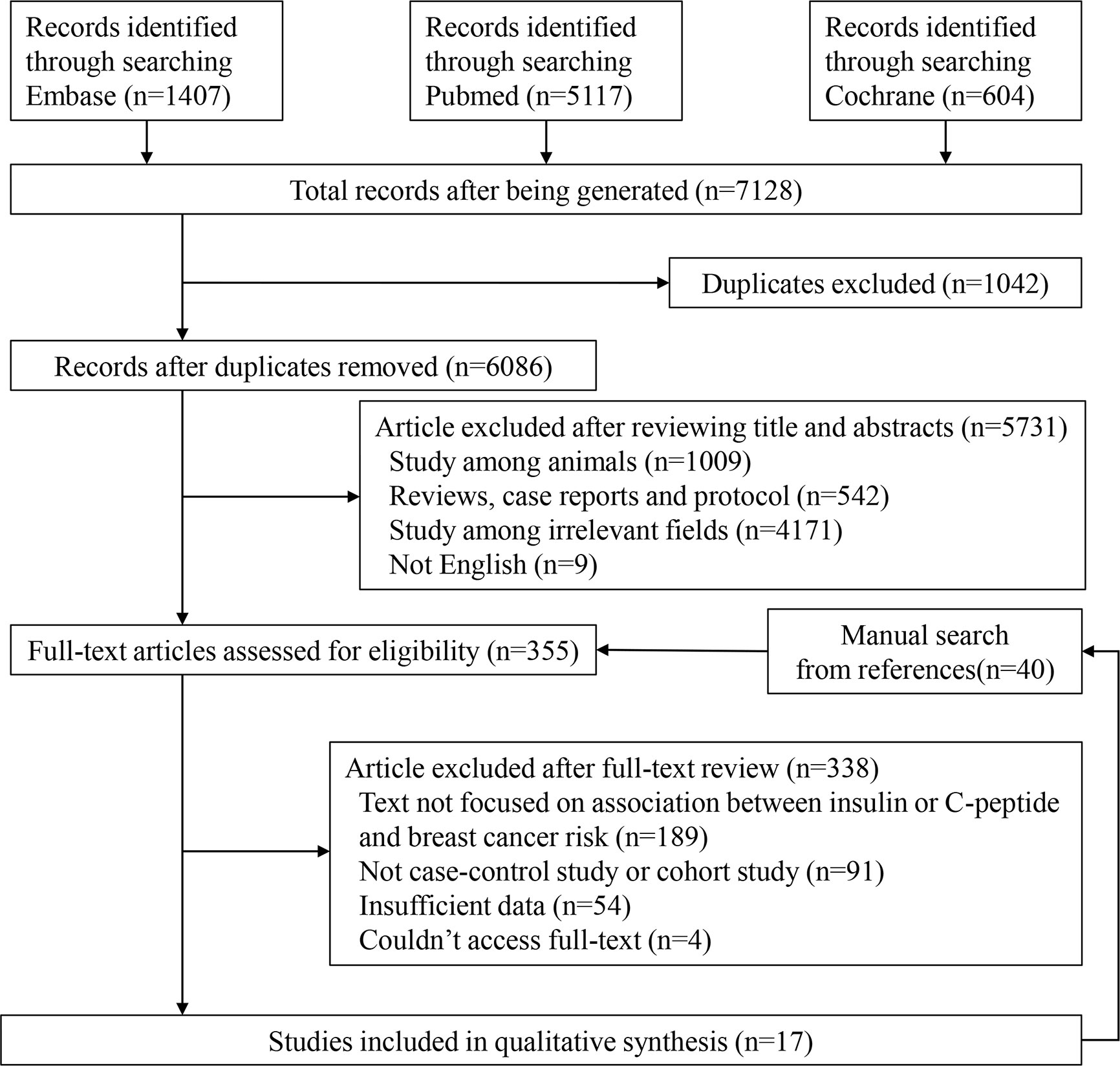
We presented detailed steps for the literature search in Figure 1. The original literature retrieval was 7,128; however, 1,042 were eliminated because of duplication. After independently selecting titles and abstracts, the two reviewers excluded 5,731 articles and 355 articles were evaluated for eligibility. Ultimately, 17 studies were included (9, 11, 19–33). We found a total 40 articles from references of relevant studies and showed them in the flow chart, but they were all included in the retrieved articles (Figure 1).
Study Characteristics
A total of five articles reporting c-peptide and breast cancer were enrolled in the meta-analysis. These articles were published from 1999 to 2008 and included 2,513 women in total, of which 864 had newly diagnosed breast cancer patients in the case groups and 1,649 were people without breast cancer in control group (four case–control studies and one cohort study). There are 14 articles published in 1998 to 2018 including 27,084 women reporting information of insulin and breast cancer, of which 3,232 were newly diagnosed breast cancer patients in the case group, and 23,852 were those without breast cancer in the control group (ten case–control study, two nested case–control study, and two cohort study).
Two articles were used by both meta-analyses. Serum c-peptide and insulin in all studies were measured after overnight fasting by enzyme linked immunosorbent assay (ELISA), Radioimmunoassay (RIA), and chemiluminescence enzyme immunoassay (CLEIA). The three authors independently reviewed and cross-checked the articles, and all agreed that the research was qualified. The information was presented in Tables 1 and 2.
Overall Analysis and Subgroup Analysis
As shown in Figure 2, C-peptide levels were significantly positively related to breast cancer (SMD = 0.37, 95% CI: 0.09–0.65). However, there was considerable heterogeneity among the studies (I2 = 89.1%, P < 0.001). Therefore, we performed subgroup analysis to explore the source of heterogeneity by country, test methods, control source, and menopausal status in our dataset.
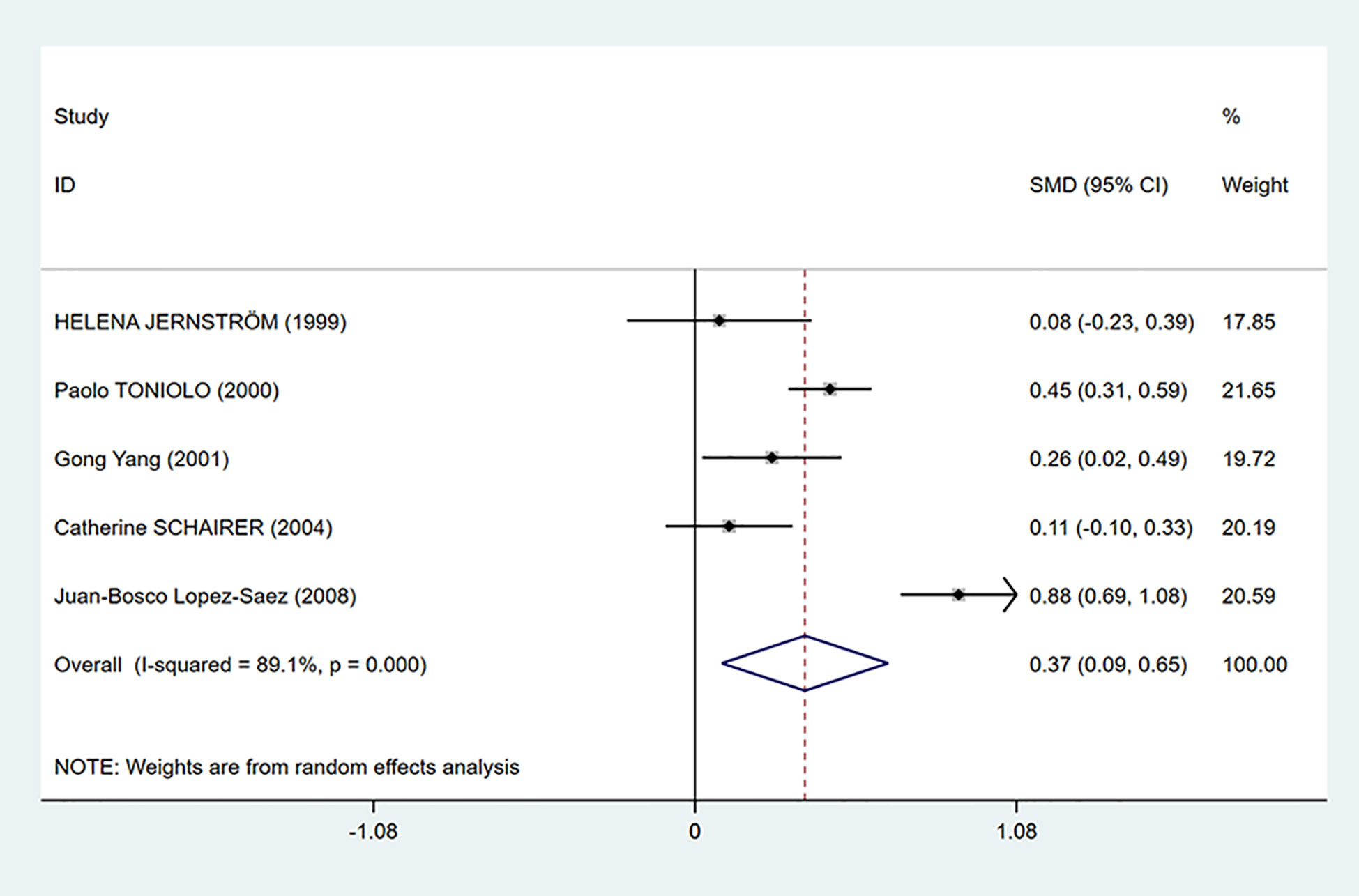
Figure 2 Overall forest plot of meta-analysis on the association between C-peptide and breast cancer risk. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Summary estimates were analyzed using a random-effects model. Zero is not included in this confidence interval, and diamond is on the right of zero, which indicates that C-peptide levels were positively associated with breast cancer risk.
We performed a subgroup analysis according to the source of cases in the control groups. Population-based control means that cases in the control group of the study are from communities, city screening centers, or permanent residences. Hospital-based control means that cases in control group of the study are from a hospital. As we can see in Figure 3, the mean C-peptide levels of breast cancer patients were significantly higher in the population-based control group (SMD = 0.30, 95% CI: 0.09–0.51, P = 0.006), which meant that c-peptide levels were positively correlated with breast cancer in this group. In addition, the heterogeneity of population-based control group decreased (I2 = 64.1%, P = 0.062).
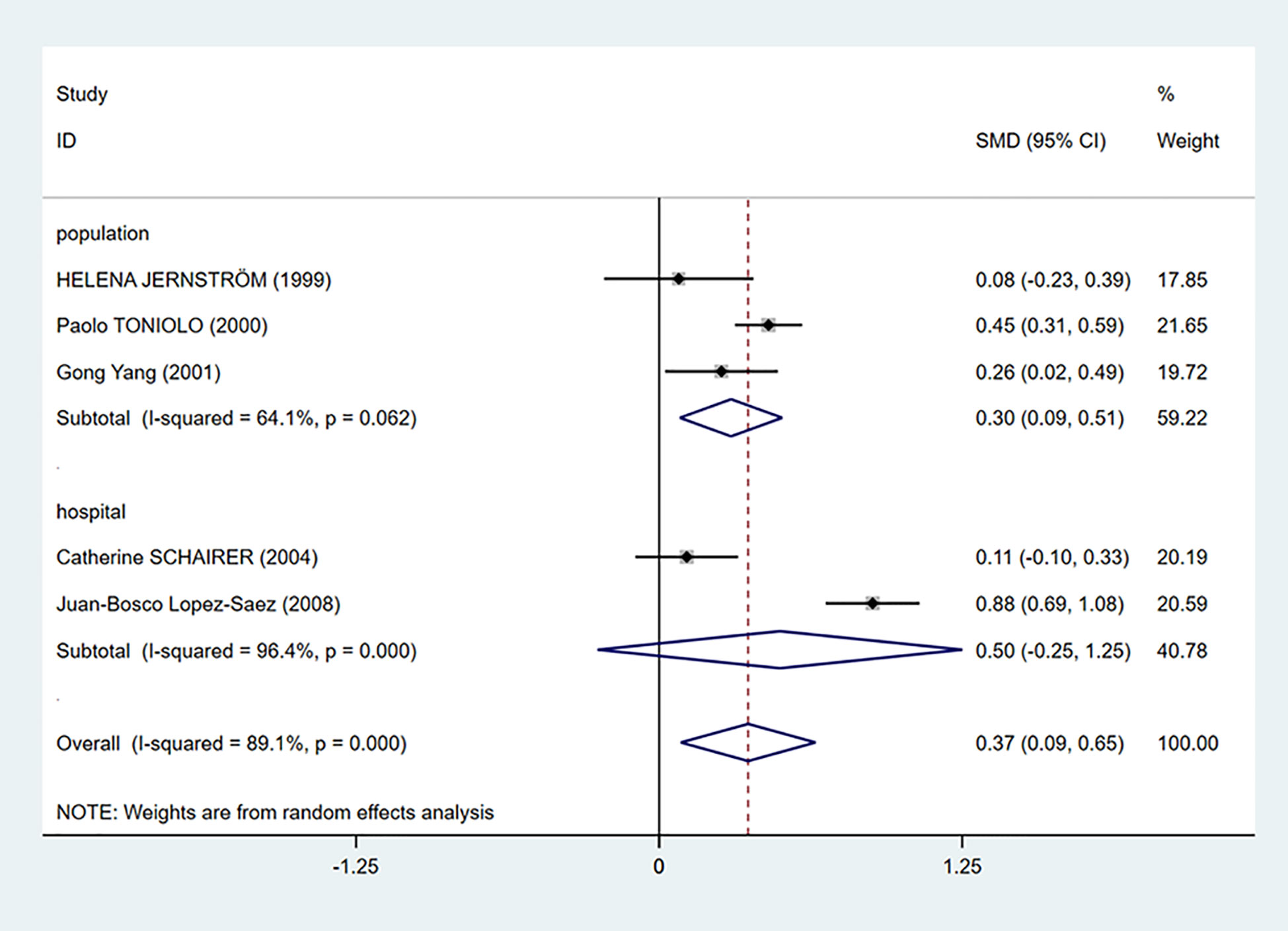
Figure 3 Subgroup analysis of the association between C-peptide and breast cancer in different control sources. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Summary estimates were analyzed using a random-effects model. C-peptide levels are positively associated with breast cancer risk in population-based control group. No association is presented in hospital-based control group.
To further investigate the source of heterogeneity, we separated five studies into two groups according to the mean C-peptide level in the case group: ≤3 ng/ml and >3 ng/ml group. We found that C-peptide and breast cancer had positive correlations in both groups. The heterogeneity in the ≤3 ng/ml group was decreased dramatically (I2 = 0.0%, P = 0.577), while the heterogeneity in the >3 ng/ml group increased (Figure 4).
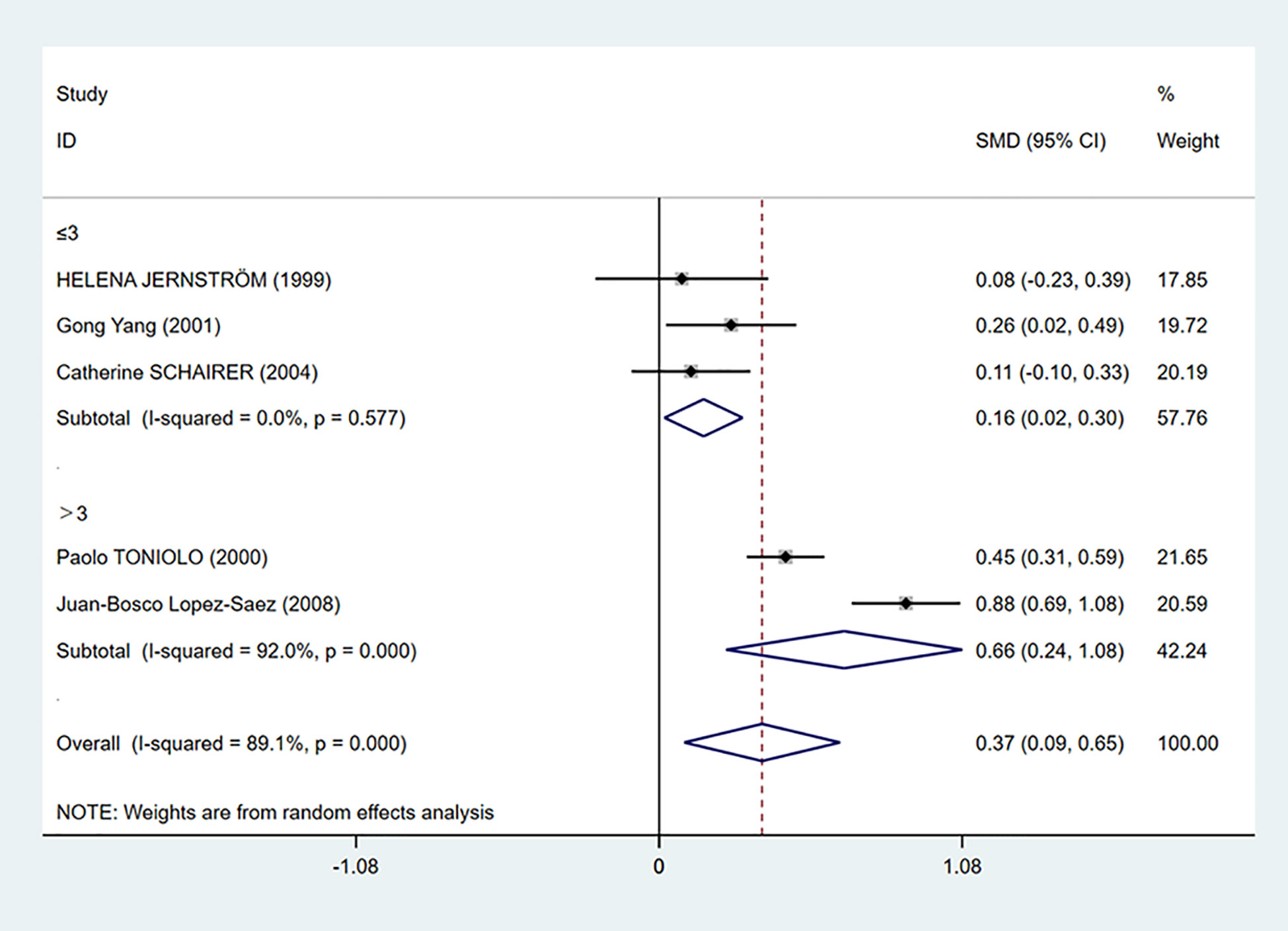
Figure 4 Subgroup analysis of the association between C-peptide levels and breast cancer in C-peptide ≤3 or >3 ng/ml. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Summary estimates were analyzed using a random-effects model. C-peptide levels are positively associated with breast cancer risk in both ≤3 and >3 ng/ml groups.
We also performed a subgroup analysis based on age (Figure 5). Even though there was still heterogeneity, it was obvious that C-peptide level was positively related to breast cancer in women between the ages of 50 and 60 (I2 = 89.6%, P = 0.000).
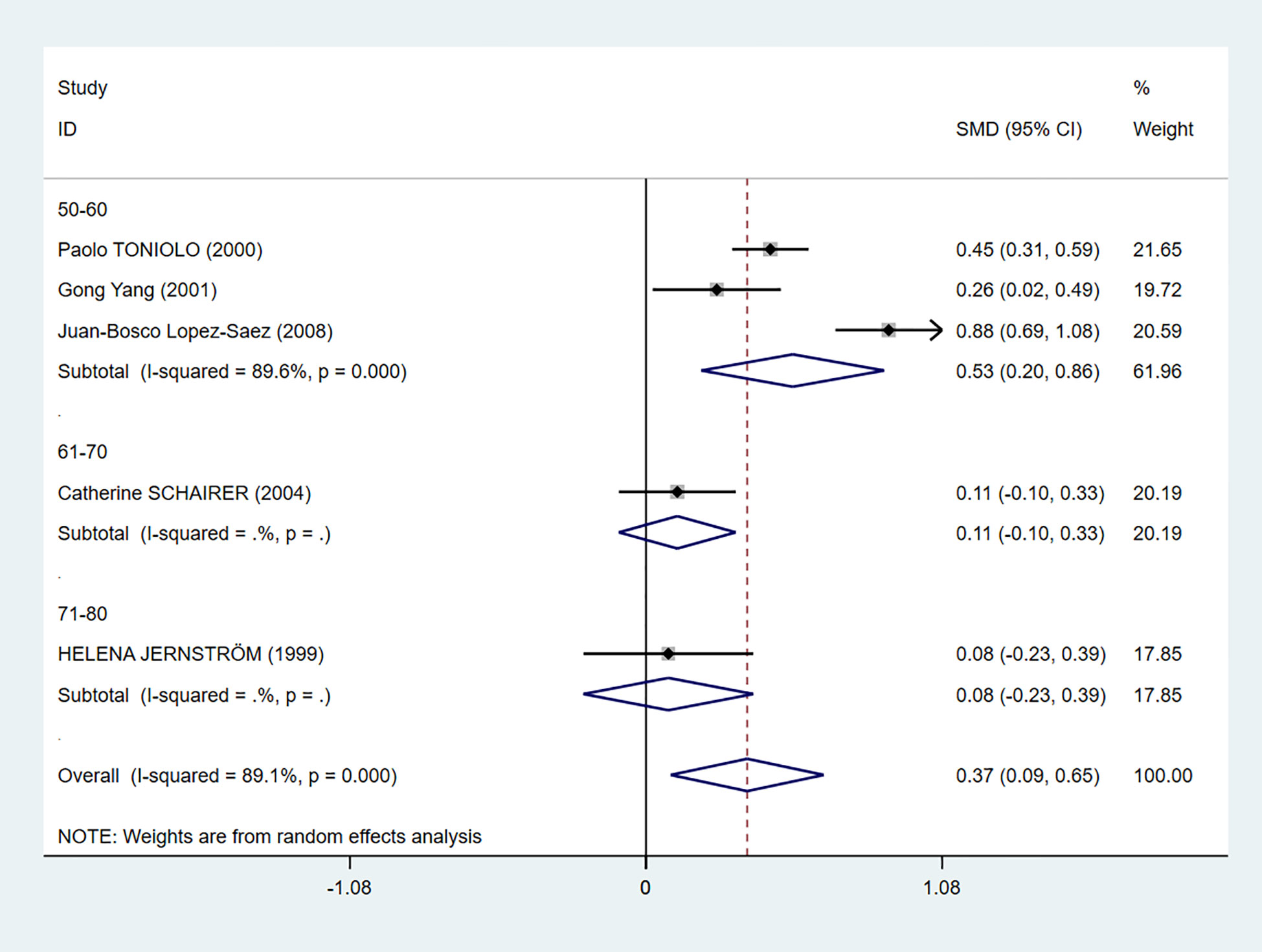
Figure 5 Subgroup analysis of the association between C-peptide and breast cancer in different ages. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Summary estimates were analyzed using a random-effects model. C-peptide levels are positively associated with breast cancer risk in the age 50–60 group.
In addition, we conducted subgroup analysis according to country, measuring method, and menopausal status, but no source of heterogeneity was found (Table 3).
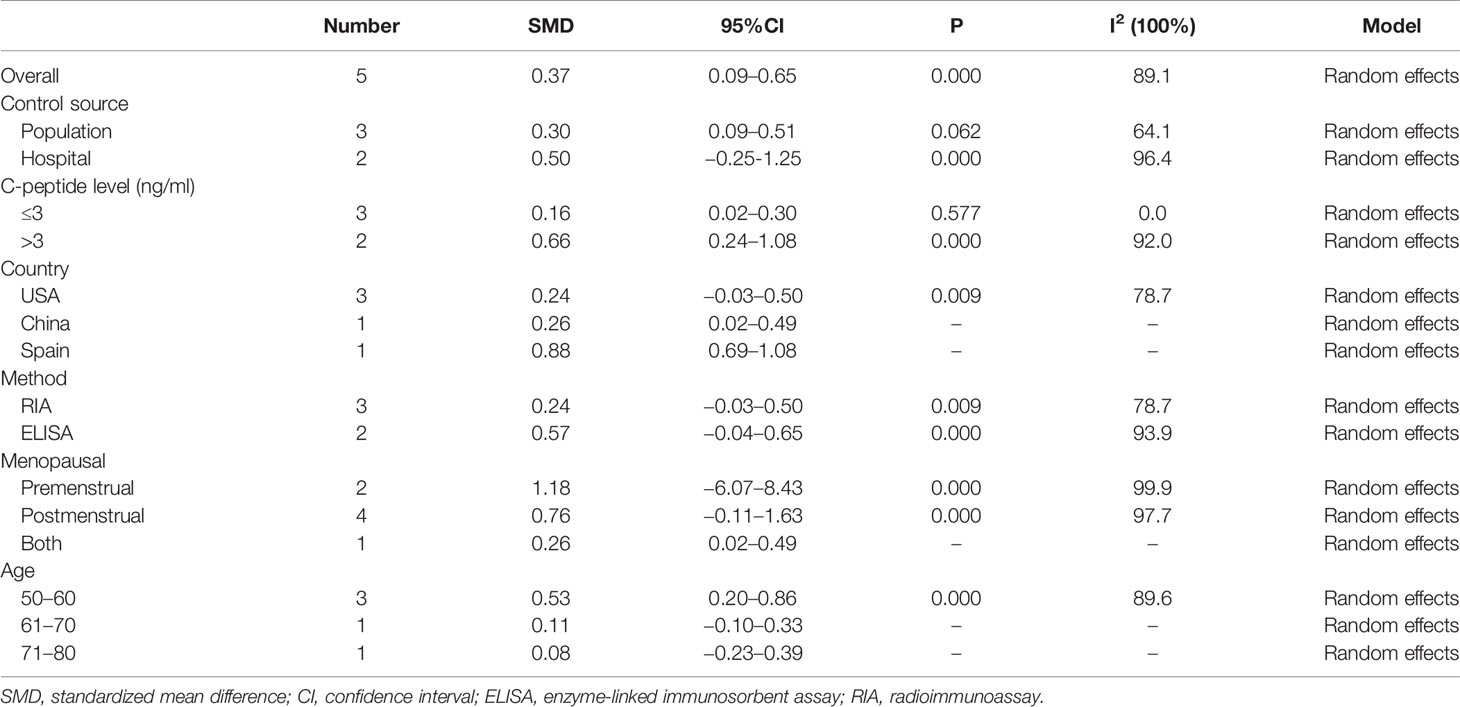
Table 3 The pooled and subgroup results of the serum C-peptide levels in breast cancer patients compared with the control groups.
As shown in Figure 6, insulin levels were irrelevant to breast cancer (SMD = 0.22, 95% CI: −0.06–0.50) and considerable heterogeneity exists among studies (I2 = 97.3%, P = 0.000).
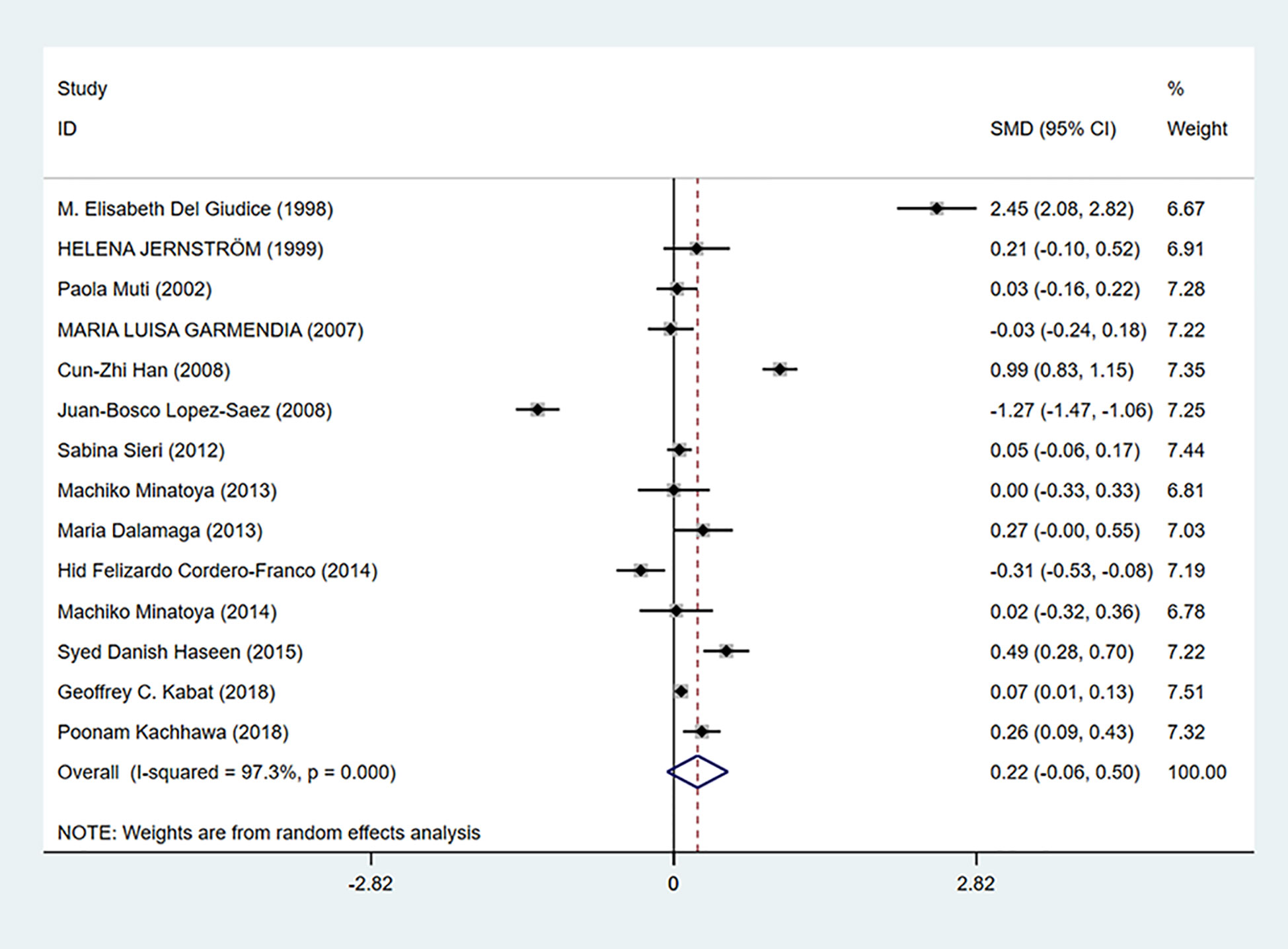
Figure 6 Overall forest plot of meta-analysis on the association between insulin and breast cancer risk. The size of the square box is proportional to the weight that each study contributes in the meta-analysis. The overall estimate and CI are marked by a diamond. Summary estimates were analyzed using a random-effects model. Zero is included in this confidence interval, which indicates that insulin levels had no association with breast cancer risk.
Publication Bias and Sensitivity Analysis
Egger’s test was used to measure publication bias (P = 0.48 for c-peptide and P = 0.59 for insulin). We found no publication bias among the articles included in this meta-analysis (Figure 7). The sensitivity analysis demonstrated the stability of our meta-analysis (Figure 8).
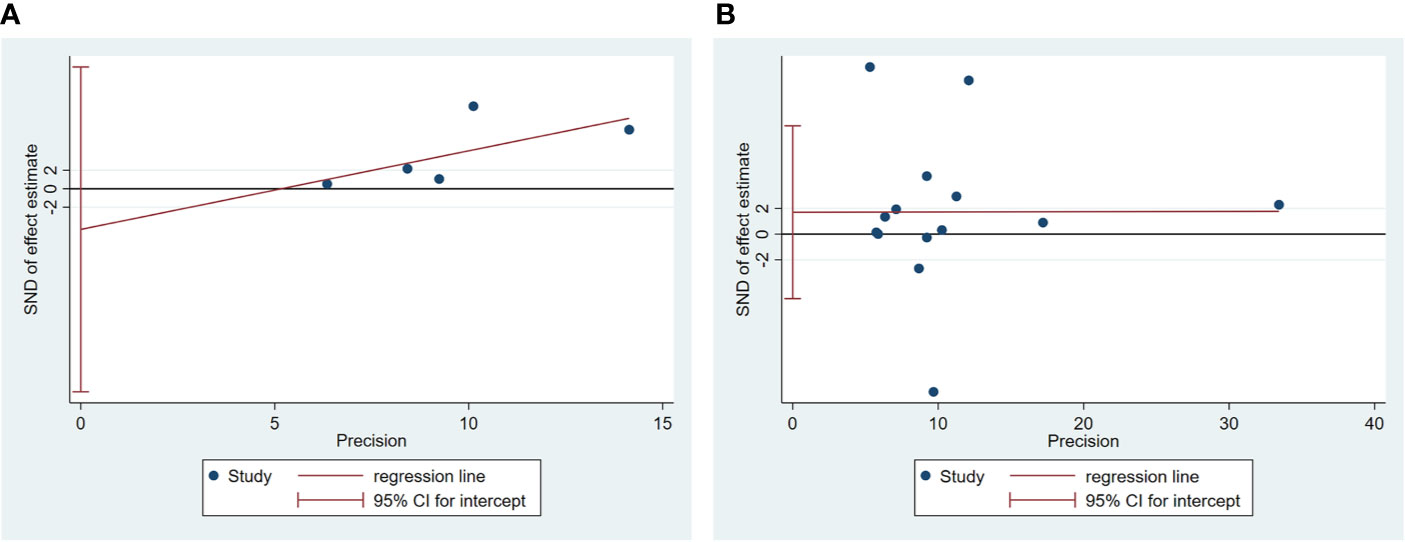
Figure 7 Egger’s linear regression for publication bias about association between C-peptide (A) or insulin (B) and breast cancer. Each circle represents a separate study pertaining to the indicated association. The circles imply that an asymmetrical distribution is not present, suggesting that no publication biases were present.
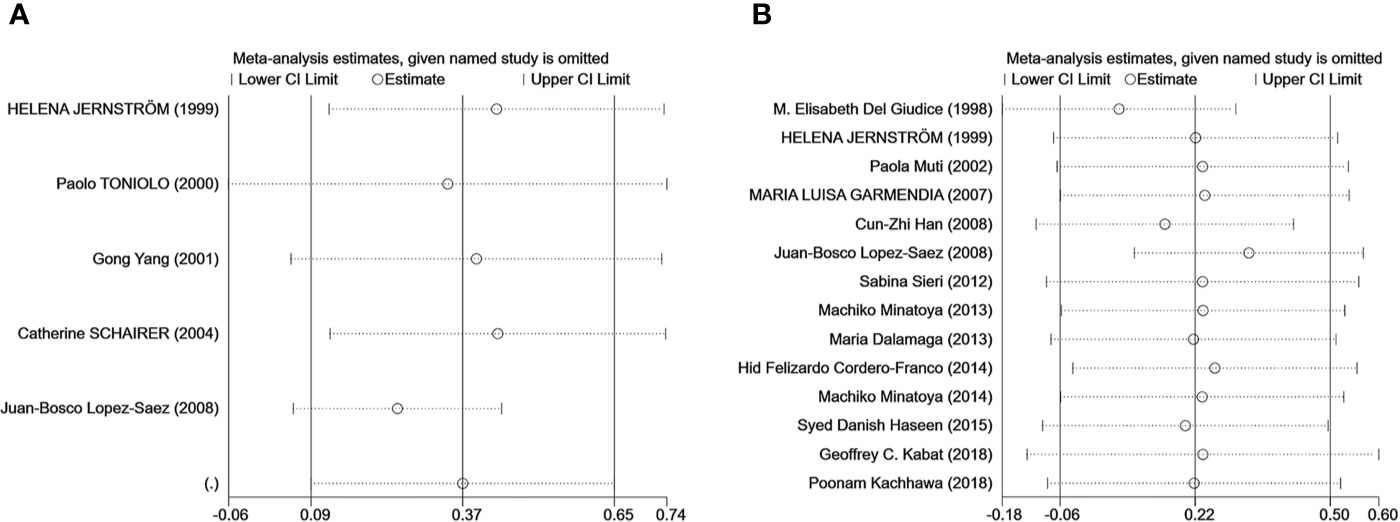
Figure 8 The result of the sensitivity analysis on the association between C-peptide (A) or insulin (B) and breast cancer. Sensitivity analyses for the influence of individual studies on the summary SMD. The vertical axis indicates the overall SMD, and the two vertical axes indicate its 95% CI. Every hollow round indicates the pooled SMD when the left study is omitted in this meta-analysis. The two ends of every broken line represent the respective 95% CI.
Discussion
The overall conclusion of this study was consistent with those of previous articles indicating no relationship between serum insulin and breast cancer. Autier et al. (16) found that serum insulin levels showed a non-significant increase in the risk of breast cancer in a previous meta-analysis. The meta-analysis of Hernandez et al. (4) also found that insulin had little effect on the incidence of breast cancer. However, several epidemiologic studies (34–36), but not all (10, 37), showed that high levels of insulin cycling were related to increased breast cancer incidence in premenopausal and postmenopausal women. Some laboratory studies showed that insulin, like its associated growth factors, may have mitotic effects on both normal and neoplastic breast epithelial cells (38, 39). This could be caused by the characteristics of insulin that its half time in serum is short. Metabolism of insulin in blood is complex and more study is needed in order to clarify the relationship among insulin, metabolism and cancer.
As shown in the results, we analyzed the correlation between C-peptide and breast cancer. Although this study drew the same conclusion as previous studies reporting that C-peptide is of great importance in breast cancer progression (4, 20), there was considerable heterogeneity among studies (I2 = 89.1%). We found no publication bias among the included articles, and sensitivity analysis showed the superior reliability of this meta-analysis, which demonstrated that the conclusion is reliable.
To further identify the source of the heterogeneity, we did subgroup analysis using several factors, including country, measuring method, control source, age, and menopausal status, which may increase variability of results. In the subgroup analysis by control source, we found that the mean C-peptide levels of breast cancer patients were higher in the population-based control subgroup and the heterogeneity decreased significantly, which means that serum C-peptide level was positively related to breast cancer in this group. Nevertheless, the hospital-based control subgroup showed no relationship between C-peptide and breast cancer level. This situation may be explained by the fact that control patients from hospital suffered from diseases even though they were not diagnosed, which amplified the heterogeneity of the hospital control source group. Although we excluded studies that included patients with a combination of other diseases in the Methods section, we cannot guarantee the quality of each patient in each article.
Furthermore, we performed a subgroup analysis in which studies were divided into two groups according to the C-peptide levels of breast cancer patients. The results showed that even though the heterogeneity in the ≤3 ng/ml group decreased dramatically, C-peptide level in the >3 ng/ml group was higher than that in the ≤3 ng/ml group. It was reported that insulin resistance, characterized by elevated C-peptide levels, was associated with central obesity and several other diseases, including cancer (38, 40). This is consistent with the result in this meta-analysis to the fact that the higher C-peptide level, the greater the risk of breast cancer. However, we did not find the source of heterogeneity when performing subgroup according to country, test method or menopausal status.
In subgroup analysis according to age, C-peptide level was positively related to breast cancer in women between the ages of 50 and 60 (Figure 5). It is reported that 67.8% breast cancer women are at the age of 41–60 (41), which may be for the reason that women are just entering menopause, and metabolism is in a state of disorder.
However, our results were inconsistent with those of a previous meta-analysis reporting that C-peptide did not play a role in breast cancer progression. Hernandez et al. (42) found that elevated fasting insulin or C-peptide levels were not related to breast cancer in women. Autier in 2013 reported that they did not find any results to locate a positive relationship between insulin or C-peptide levels and breast cancer incidence (16). They calculated summary relative risks and 95% CI applied to the relative risk associated with the highest versus lowest quantile of serum concentrations. However, we employed the sample size, the mean, and SD to calculate SMDs and 95% CIs for each study, which is the better way to do meta-analysis of continuous variable (43). In addition, some studies reported that C-peptide correlated positively with breast cancer risk, but only among postmenopausal women (44). In our analysis, two articles reported data about postmenopausal women, so there were more cases in postmenopausal women than that in premenopausal women, which may explain our conclusion. In addition, insulin and C-peptide are synthesized in islets by enzymatic hydrolysis of proinsulin and released into the blood circulation in equal moles. C-peptide has a longer half-life in plasma than insulin; therefore, it can serve as a marker to more accurately reflect the relationship between metabolism and breast cancer (45).
There are some limitations in our meta-analysis. First, in terms of medical ethics, some studies have relatively limited sample sizes. Second, because of the limited number of included studies, we were unable to conduct an adequate combinatorial analysis during the subgroup analysis nor were we able to analyze many other parameters that might reflect breast cancer (e.g., breast cancer type, breast cancer stage, and BMI, etc.). Third, unmeasured and residual confounding may result in confusion in relation to the conclusion. Finally, the analysis was also limited by the quality of the individual studies, and many other important factors affecting breast cancer incidence could not be analyzed. Given the limitations of our meta-analysis, further large-scale studies and sufficient samples are needed in order to present a convincing link between insulin and C-peptide and breast cancer risk.
Conclusion
The current meta-analysis suggests that C-peptide levels were positively related to breast cancer risk in women, and no relationship between insulin and breast cancer was found.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Author Contributions
ML and LS designed the program, searched and reviewed the studies, and were in charge of the manuscript. ML, JY, and DZ assessed the studies, extracted the data, and wrote part of the manuscript. LS, CZ, and YLiu extracted the data and wrote part of the manuscript. QL, HW, KS, YLi, ZM, and DL reviewed and edited the manuscript. JD directed the project, contributed to the discussion, reviewed, and edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants (to JD) from the National Natural Science Foundation of China (No. 31872791) and Natural Science Foundation of Shandong Province of China (No. ZR2019MC046).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Rui Wang and Yingchang Pang in Qingdao University, and Zhao Tian in Beijing University for helpful advice during the conceiving of the program and the creation of the manuscript.
Abbreviations
SMDs, standardized mean differences; CIs, confidence intervals; BMI, body mass index; NOS, Newcastle–Ottawa Scale; SD, standard deviation; ORs, odds ratios.
References
1. Siegel RL, Miller KD. Cancer statistics, 2019. CA Cancer J Clin (2019) 69(1):7–34. doi: 10.3322/caac.21551
2. Iacoviello L, Bonaccio M, de Gaetano G, Donati MB. Epidemiology of breast cancer, a paradigm of the “common soil” hypothesis. Semin Cancer Biol (2020) S1044-579X(20)30043-2. doi: 10.1016/j.semcancer.2020.02.010
3. Peairs KS, Barone BB, Snyder CF, Yeh HC, Stein KB, Derr RL, et al. Diabetes mellitus and breast cancer outcomes: a systematic review and meta-analysis. J Clin Oncol (2011) 29(1):40–6. doi: 10.1200/jco.2009.27.3011
4. Shen J, Hernandez D, Ye Y, Wu X, Chow WH, Zhao H. Metabolic hormones and breast cancer risk among Mexican American Women in the Mano a Mano Cohort Study. Sci Rep (2019) 9(1):9989. doi: 10.1038/s41598-019-46429-9
5. Formica V, Tesauro M, Cardillo C, Roselli M. Insulinemia and the risk of breast cancer and its relapse. Diabetes Obes Metab (2012) 14(12):1073–80. doi: 10.1111/j.1463-1326.2012.01614.x
6. Yee LD, Mortimer JE, Natarajan R, Dietze EC, Seewaldt VL. Metabolic Health, Insulin, and Breast Cancer: Why Oncologists Should Care About Insulin. Front Endocrinol (Lausanne) (2020) 11:58. doi: 10.3389/fendo.2020.00058
7. Sánchez-Jiménez F, Pérez-Pérez A, de la Cruz-Merino L, Sánchez-Margalet V. Obesity and Breast Cancer: Role of Leptin. Front Oncol (2019) 9:596. doi: 10.3389/fonc.2019.00596
8. Atoum MF, Alzoughool F, Al-Hourani H. Linkage Between Obesity Leptin and Breast Cancer. Breast Cancer (Auckl) (2020) 14:1178223419898458. doi: 10.1177/1178223419898458
9. Cordero-Franco HF, Salinas-Martínez AM, Abundis A, Espinosa-Flores EM, Vázquez-Lara J, Guerrero-Romero F. The effect of insulin resistance on breast cancer risk in Latinas of Mexican origin. Metab Syndr Relat Disord (2014) 12(9):477–83. doi: 10.1089/met.2014.0071
10. Mink PJ, Shahar E, Rosamond WD, Alberg AJ, Folsom AR. Serum insulin and glucose levels and breast cancer incidence: the atherosclerosis risk in communities study. Am J Epidemiol (2002) 156(4):349–52. doi: 10.1093/aje/kwf050
11. Muti P, Quattrin T, Grant BJ, Krogh V, Micheli A, Schünemann HJ, et al. Fasting glucose is a risk factor for breast cancer: a prospective study. Cancer Epidemiol Biomarkers Prev (2002) 11(11):1361–8.
12. Yaribeygi H, Maleki M, Sathyapalan T, Sahebkar A. The effect of C-peptide on diabetic nephropathy: A review of molecular mechanisms. Life Sci (2019) 237:116950. doi: 10.1016/j.lfs.2019.116950
13. Yosten GL, Kolar GR. The Physiology of Proinsulin C-Peptide: Unanswered Questions and a Proposed Model. Physiol (Bethesda) (2015) 30(4):327–32. doi: 10.1152/physiol.00008.2015
14. Souto SB, Campos JR, Fangueiro JF, Silva AM, Cicero N, Lucarini M, et al. Multiple Cell Signalling Pathways of Human Proinsulin C-Peptide in Vasculopathy Protection. Int J Mol Sci (2020) 21(2):645. doi: 10.3390/ijms21020645
15. Pisani P. Hyper-insulinaemia and cancer, meta-analyses of epidemiological studies. Arch Physiol Biochem (2008) 114(1):63–70. doi: 10.1080/13813450801954451
16. Autier P, Koechlin A, Boniol M, Mullie P, Bolli G, Rosenstock J, et al. Serum insulin and C-peptide concentration and breast cancer: a meta-analysis. Cancer Causes Control (2013) 24(5):873–83. doi: 10.1007/s10552-013-0164-6
17. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
19. Lopez-Saez JB, Martinez-Rubio JA, Alvarez MM, Carrera CG, Dominguez Villar M, de Lomas Mier AG, et al. Metabolic profile of breast cancer in a population of women in southern Spain. Open Clin Cancer J (2008) 2:1–6. doi: 10.2174/1874189400802010001
20. Jernström H, Barrett-Connor E. Obesity, weight change, fasting insulin, proinsulin, C-peptide, and insulin-like growth factor-1 levels in women with and without breast cancer: the Rancho Bernardo Study. J Womens Health Gend Based Med (1999) 8(10):1265–72. doi: 10.1089/jwh.1.1999.8.1265
21. Yang G, Lu G, Jin F, Dai Q, Best R, Shu XO, et al. Population-based, case-control study of blood C-peptide level and breast cancer risk. Cancer Epidemiol Biomarkers Prev (2001) 10(11):1207–11.
22. Schairer C, Hill D, Sturgeon SR, Fears T, Pollak M, Mies C, et al. Serum concentrations of IGF-I, IGFBP-3 and c-peptide and risk of hyperplasia and cancer of the breast in postmenopausal women. Int J Cancer (2004) 108(5):773–9. doi: 10.1002/ijc.11624
23. Toniolo P, Bruning PF, Akhmedkhanov A, Bonfrer JM, Koenig KL, Lukanova A, et al. Serum insulin-like growth factor-I and breast cancer. Int J Cancer (2000) 88(5):828–32. doi: 10.1002/1097-0215(20001201)88:5<828::aid-ijc22>3.0.co;2-8
24. Del Giudice ME, Fantus IG, Ezzat S, McKeown-Eyssen G, Page D, Goodwin PJ. Insulin and related factors in premenopausal breast cancer risk. Breast Cancer Res Treat (1998) 47(2):111–20. doi: 10.1023/a:1005831013718
25. Haseen SD, Khanam A, Sultan N, Idrees F, Akhtar N, Imtiaz F. Elevated fasting blood glucose is associated with increased risk of breast cancer: outcome of case-control study conducted in Karachi, Pakistan. Asian Pac J Cancer Prev (2015) 16(2):675–8. doi: 10.7314/apjcp.2015.16.2.675
26. Kachhawa P, Kachhawa K, Agrawal D, Sinha V, Sarkar PD, Kumar S. Association of Dyslipidemia, Increased Insulin Resistance, and Serum CA 15-3 with Increased Risk of Breast Cancer in Urban Areas of North and Central India. J Midlife Health (2018) 9(2):85–91. doi: 10.4103/jmh.JMH_77_17
27. Minatoya M, Kutomi G, Asakura S, Otokozawa S, Sugiyama Y, Ohnishi H, et al. Relationship of serum isoflavone, insulin and adiponectin levels with breast cancer risk. Breast Cancer (2015) 22(5):452–61. doi: 10.1007/s12282-013-0502-2
28. Minatoya M, Kutomi G, Shima H, Asakura S, Otokozawa S, Ohnishi H, et al. Relation of serum adiponectin levels and obesity with breast cancer: a Japanese case-control study. Asian Pac J Cancer Prev (2014) 15(19):8325–30. doi: 10.7314/apjcp.2014.15.19.8325
29. Kabat GC, Kim MY, Lane DS, Zaslavsky O, Ho GYF, Luo J, et al. Serum glucose and insulin and risk of cancers of the breast, endometrium, and ovary in postmenopausal women. Eur J Cancer Prev (2018) 27(3):261–8. doi: 10.1097/cej.0000000000000435
30. Han CZ, Du LL, Jing JX, Zhao XW, Tian FG, Shi J, et al. Associations among lipids, leptin, and leptin receptor gene Gin223Arg polymorphisms and breast cancer in China. Biol Trace Elem Res (2008) 126(1-3):38–48. doi: 10.1007/s12011-008-8182-z
31. Dalamaga M, Karmaniolas K, Papadavid E, Pelekanos N, Sotiropoulos G, Lekka A. Hyperresistinemia is associated with postmenopausal breast cancer. Menopause (2013) 20(8):845–51. doi: 10.1097/GME.0b013e31827f06dc
32. Sieri S, Muti P, Claudia A, Berrino F, Pala V, Grioni S, et al. Prospective study on the role of glucose metabolism in breast cancer occurrence. Int J Cancer (2012) 130(4):921–9. doi: 10.1002/ijc.26071
33. Garmendia ML, Pereira A, Alvarado ME, Atalah E. Relation between insulin resistance and breast cancer among Chilean women. Ann Epidemiol (2007) 17(6):403–9. doi: 10.1016/j.annepidem.2007.01.037
34. Bruning PF, Bonfrèr JM, van Noord PA, Hart AA, de Jong-Bakker M, Nooijen WJ. Insulin resistance and breast-cancer risk. Int J Cancer (1992) 52(4):511–6. doi: 10.1002/ijc.2910520402
35. Hirose K, Toyama T, Iwata H, Takezaki T, Hamajima N, Tajima K. Insulin, insulin-like growth factor-I and breast cancer risk in Japanese women. Asian Pac J Cancer Prev (2003) 4(3):239–46.
36. Malin A, Dai Q, Yu H, Shu XO, Jin F, Gao YT, et al. Evaluation of the synergistic effect of insulin resistance and insulin-like growth factors on the risk of breast carcinoma. Cancer (2004) 100(4):694–700. doi: 10.1002/cncr.20023
37. Kaaks R, Lundin E, Rinaldi S, Manjer J, Biessy C, Söderberg S, et al. Prospective study of IGF-I, IGF-binding proteins, and breast cancer risk, in northern and southern Sweden. Cancer Causes Control (2002) 13(4):307–16. doi: 10.1023/a:1015270324325
38. Rose DP, Komninou D, Stephenson GD. Obesity, adipocytokines, and insulin resistance in breast cancer. Obes Rev (2004) 5(3):153–65. doi: 10.1111/j.1467-789X.2004.00142.x
39. Kaaks R. Nutrition, insulin, IGF-1 metabolism and cancer risk: a summary of epidemiological evidence. Novartis Found Symp (2004) 262:247–60; discussion 260-68.
40. Kelly TA, Strupp HH. Patient and therapist values in psychotherapy: perceived changes, assimilation, similarity, and outcome. J Consult Clin Psychol (1992) 60(1):34–40. doi: 10.1037//0022-006x.60.1.34
41. Wong FY, Tham WY, Nei WL, Lim C, Miao H. Age exerts a continuous effect in the outcomes of Asian breast cancer patients treated with breast-conserving therapy. Cancer Commun (Lond) (2018) 38(1):39. doi: 10.1186/s40880-018-0310-3
42. Hernandez AV, Guarnizo M, Miranda Y, Pasupuleti V, Deshpande A, Paico S, et al. Association between insulin resistance and breast carcinoma: a systematic review and meta-analysis. PLoS One (2014) 9(6):e99317. doi: 10.1371/journal.pone.0099317
43. Pan H, Deng LL, Cui JQ, Shi L, Yang YC, Luo JH, et al. Association between serum leptin levels and breast cancer risk: An updated systematic review and meta-analysis. Med (Baltimore) (2018) 97(27):e11345. doi: 10.1097/md.0000000000011345
44. Verheus M, Peeters PH, Rinaldi S, Dossus L, Biessy C, Olsen A, et al. Serum C-peptide levels and breast cancer risk: results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer (2006) 119(3):659–67. doi: 10.1002/ijc.21861
Keywords: breast cancer, C-peptide, insulin, review, meta-analysis
Citation: Li M, Song L, Yuan J, Zhang D, Zhang C, Liu Y, Lin Q, Wang H, Su K, Li Y, Ma Z, Liu D and Dong J (2020) Association Between Serum Insulin and C-Peptide Levels and Breast Cancer: An Updated Systematic Review and Meta-Analysis. Front. Oncol. 10:553332. doi: 10.3389/fonc.2020.553332
Received: 18 April 2020; Accepted: 08 October 2020;
Published: 29 October 2020.
Edited by:
Lowell L. Hart, Wake Forest Baptist Medical Center, United StatesReviewed by:
Camila O. Dos Santos, Cold Spring Harbor Laboratory, United StatesDezhi Mu, Sichuan University, China
Copyright © 2020 Li, Song, Yuan, Zhang, Zhang, Liu, Lin, Wang, Su, Li, Ma, Liu and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Dong, dongjing6@hotmail.com
†These authors have contributed equally to this work
 Manwen Li
Manwen Li Limin Song
Limin Song Junhua Yuan
Junhua Yuan Di Zhang
Di Zhang Caishun Zhang
Caishun Zhang Yuan Liu
Yuan Liu Qian Lin
Qian Lin Haidan Wang
Haidan Wang Kaizhen Su
Kaizhen Su Yanrun Li
Yanrun Li Zhengye Ma
Zhengye Ma Defeng Liu
Defeng Liu Jing Dong
Jing Dong