- Department of Oncology, Zigong NO. 4 People's Hospital, Zigong, China
Background and Objective: Both induction chemotherapy (IC) followed by concurrent chemoradiotherapy (CCRT; IC+CCRT) and CCRT plus adjuvant chemotherapy (AC; CCRT+AC) are standard treatments for advanced nasopharyngeal carcinoma (NPC). However, no prospective randomized trials comparing these two approaches have been published yet. We conducted this network meta-analysis to address this clinical question.
Method: We recruited randomized clinical trials involving patients with advanced NPC randomly allocated to IC+CCRT, CCRT+AC, CCRT, or radiotherapy (RT) alone. Pairwise meta-analysis was first conducted, then network meta-analysis was performed using the frequentist approach. Effect size was expressed as hazard ratio (HR) and 95% confidence interval (CI).
Results: Overall, 12 trials involving 3,248 patients were recruited for this study, with 555 receiving IC+CCRT, 840 receiving CCRT+AC, 1,039 receiving CCRT, and 814 receiving radiotherapy (RT) alone. IC+CCRT achieved significantly better overall survival ([HR], 0.69; 95% [CI], 0.51–0.92), distant metastasis-free survival (HR, 0.58; 95% CI, 0.44–0.78), and locoregional recurrence-free survival (HR, 0.67; 95% CI, 0.47–0.98) than CCRT. However, survival outcomes did not significantly differ between IC+CCRT and CCRT+AC, or between CCRT+AC and CCRT arms for all the endpoints. As expected, RT alone is the poorest treatment. In terms of P-score, IC+CCRT ranked best for overall survival (96.1%), distant metastasis-free survival (99.0%) and locoregional recurrence-free survival (87.1%).
Conclusions: IC+CCRT may be a better and more promising treatment strategy for advanced NPC; however, head-to-head randomized trials comparing IC-CCRT with CCRT-AC are warranted.
Background
Nasopharyngeal carcinoma (NPC) arises from the nasopharynx epithelium and achieves the highest incidence among all head and neck cancers in China (1). Worldwide, NPC exhibits an extremely unbalanced distribution with an incidence of 20–50 per 100,000 in Southern China but <1 per 100,000 in most western countries (2, 3). As constrained by its complicated anatomical location, surgery is not available and radiotherapy (RT) has become the only radical curative treatment for NPC. As NPC is also highly sensitive to chemotherapeutic agents, incorporation of chemotherapy with RT has been established as the standard care for stage II-IVA disease. Notably, patients with early disease usually achieve excellent survival outcomes while prognosis of advanced disease still remains poor (4).
Upon the publishing of Intergroup 0099 trial in 1998, this milestone study has established concurrent chemoradiotherapy (CCRT) plus adjuvant chemotherapy (AC) as the standard regimen for advanced NPC since it could provide a 31% increase in overall survival (OS) (5). However, many subsequent studies demonstrated that AC additional to CCRT may be useless (6–8). More importantly, AC brought severe toxicities and many patients could not complete the assigned cycles, which constrains its wide clinical application. Given this, other intensive treatment strategies should be developed. Recently, there is increasing amount of evidence showing that induction chemotherapy (IC), delivered before radiotherapy, is also an effective and promising treatment strategy as it has better compliance rates and facilitates early eradication of micrometastases (9–12). Based on these findings, the National Comprehensive Cancer Network (NCCN) guidelines recommend IC plus CCRT as one of the standard treatments for stage II-IVA disease. However, it still remains unclear which chemotherapy sequence is better as we lack head-to-head trials comparing IC+CCRT with CCRT+AC. In view of the urgent need for effective and less toxic therapies, we conducted this network meta-analysis to compare IC+CCRT with CCRT+AC through extracting data from published clinical randomized trials.
Results
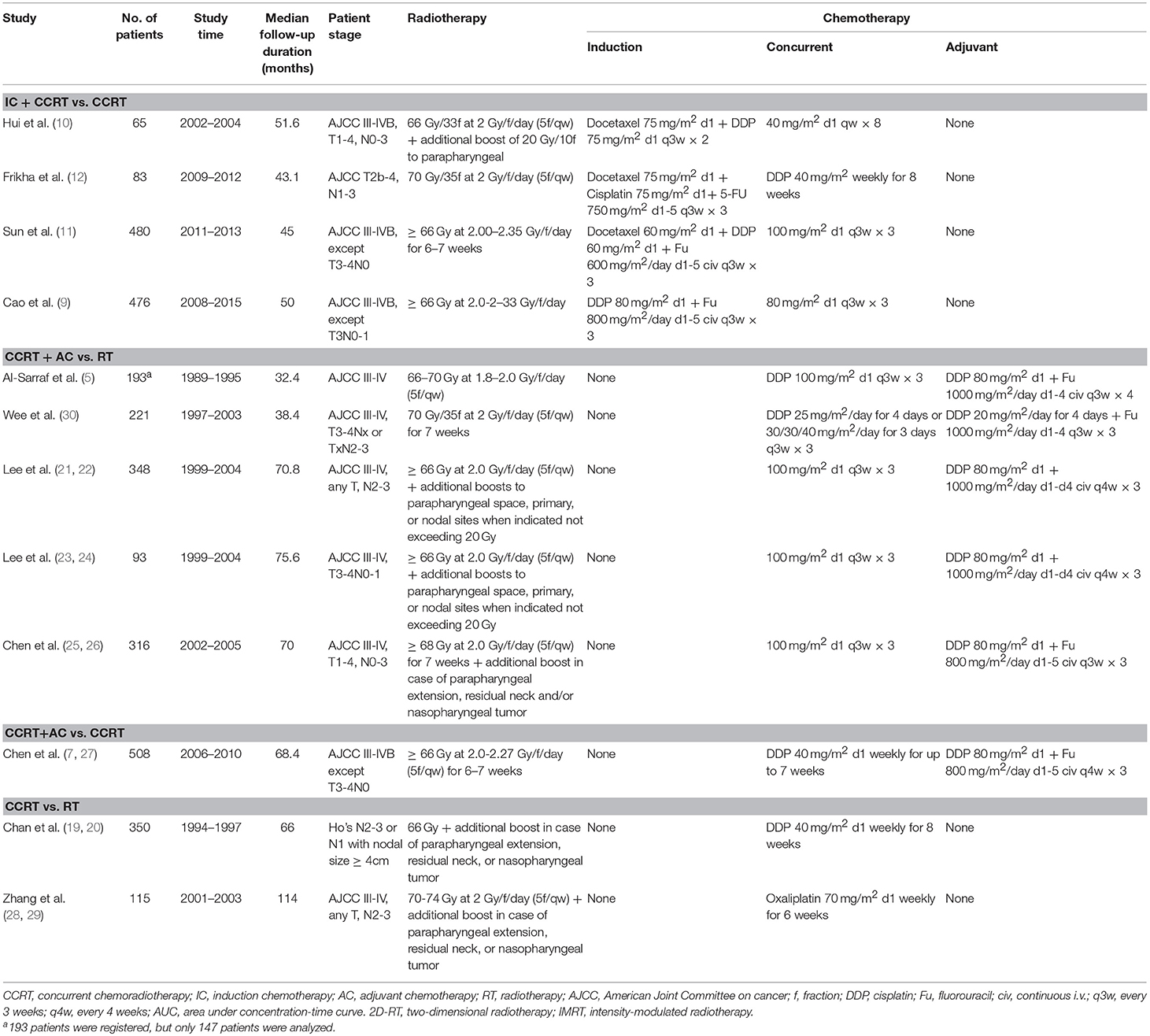
Baseline Information of Recruited Trials
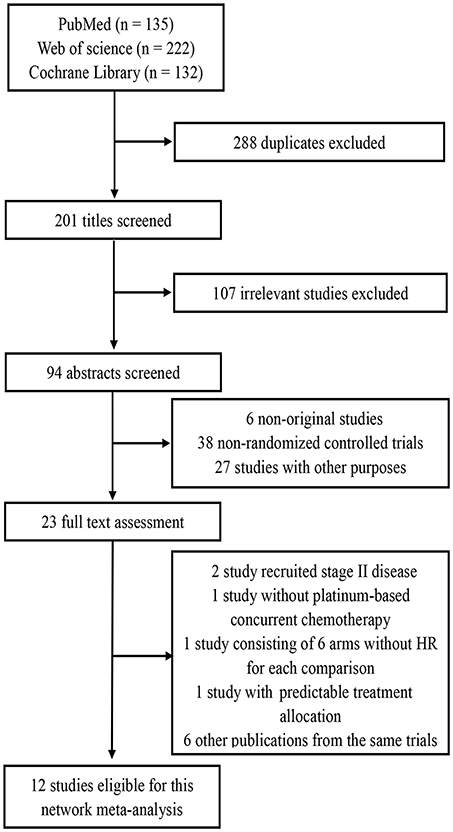
By the last literature searching (May 2018), we in total identified 24 potentially eligible clinical trials. Flow chart of studies inclusion was presented in Figure 1. The study by Lin et al. (13) was not included because HRs and 95% CI was not provided in original text. Two studies involving stage II NPC were excluded (14, 15). Due to the one-side 95% CI reported in the study by Tan et al. (16) and unknown HR for each treatment comparison in the study by Lee et al. (17), we therefore excluded these two studies. We also excluded the study by Kwong et al. (18) because uracil + tegafur was used as the concurrent chemotherapy regimen; however, this study would be included in the sensitivity analysis. Additionally, six studies updated their long follow-up data: Chan et al. (19, 20), Lee et al. (21, 22), Lee et al. (23, 24), Chen et al. (25, 26), Chen et al. (7, 27), and Zhang et al. (28, 29). Finally, 12 studies (5, 9–12, 19, 22, 23, 26–28, 30) were included for the current study. Notably, we excluded two treatment arms receiving accelerated-fraction radiotherapy in the study by Lee et al. (23, 24) because they did not meet the inclusion criterion of conventional-fraction radiotherapy. The basic information of the 12 studies are summarized in Table 1. In total, 3,248 patients were randomly allocated with 555 receiving IC+CCRT, 840 receiving CCRT+AC, 1,039 receiving CCRT, and 814 receiving RT alone. Quality assessment of the 12 studies was summarized in Supplementary Table S1.
Traditional Pairwise Comparison
Figure 2 presents the results of pairwise meta-analysis. Heterogeneity between treatment arms only existed in CCRT vs. RT for DMFS (I2 = 55.9%), and a random-effects model was then applied. Compared with CCRT, IC+CCRT was associated with significantly improved OS (HR, 0.65; 95% CI, 0.43–0.83), DMFS (HR, 0.57; 95% CI, 0.39–0.75), and LRFS (HR, 0.63; 95% CI, 0.36–0.89). Undoubtedly, CCRT+AC achieved better OS (HR, 0.63; 95% CI, 0.53–0.74), DMFS (HR, 0.51; 95% CI, 0.39–0.64), and LRFS (HR, 0.48; 95% CI, 0.32–0.64) than RT alone. Similarly, CCRT could prolong OS (HR, 0.75; 95% CI, 0.58–0.91) and DMFS (HR, 0.61; 95% CI, 0.42–0.81) compared with RT alone. Consistent with the original study, no significant differences between CCRT+AC and CCRT were observed in terms of OS, DMFS and LRFS.
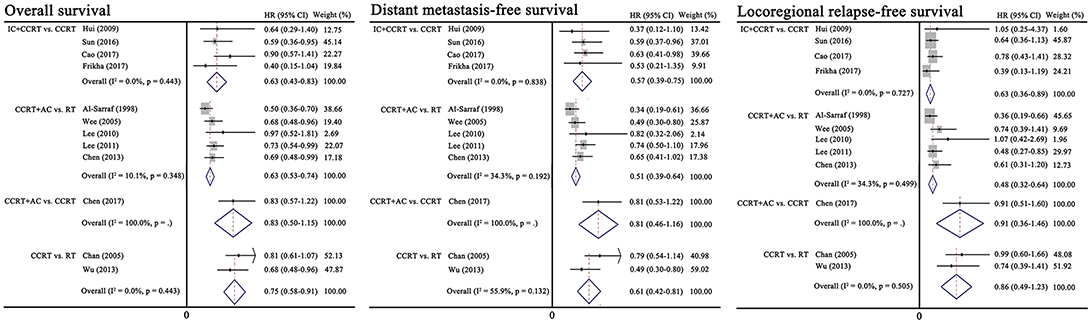
Figure 2. Results of traditional pairwise meta-analysis. CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; AC, adjuvant chemotherapy; RT, radiotherapy; HR, hazard ratio; CI, confidence interval.
Multiple Network Comparison
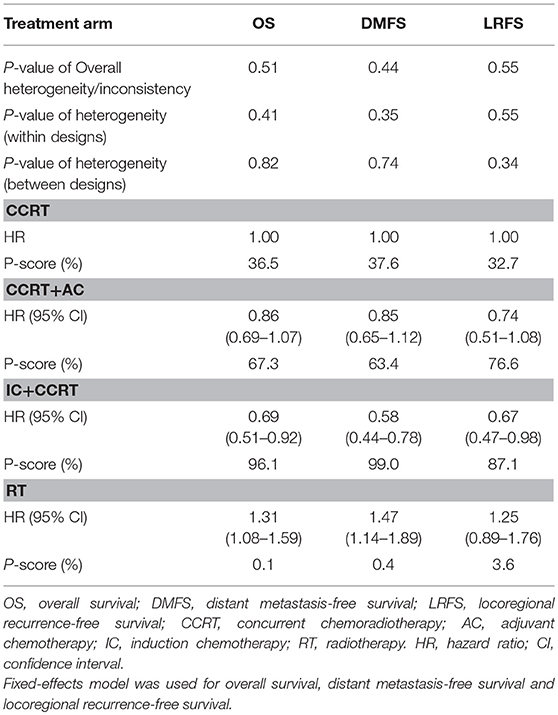
Figure 3 presented the network analysis of the four treatment arms (IC+CCRT, CCRT+AC, CCRT, and RT). In the multiple comparison, CCRT arm was treated as the reference group, and results network meta-analysis are summarized in Table 2. There is no inconsistency or heterogeneity neither between nor within studies (P > 0.1 for all rates). Thus, a fixed-effects model was used. The forest plots of multiple treatment comparisons with different reference groups were presented in Figure 4.
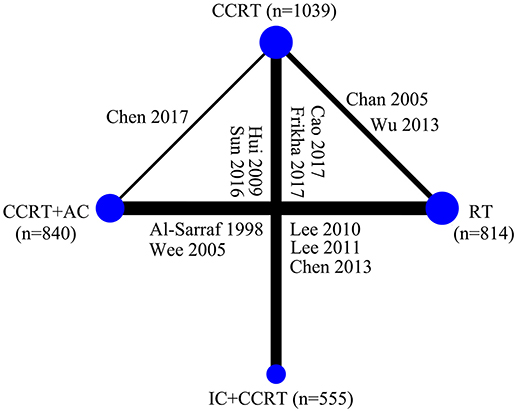
Figure 3. Graphical presentation of the trial network for overall survival. The width of the lines between nodes is proportional to the number of comparisons. Only two treatment arms receiving conventional-fraction radiotherapy in the study by Lee et al. (23) were included in this study. CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; AC, adjuvant chemotherapy; RT, radiotherapy.
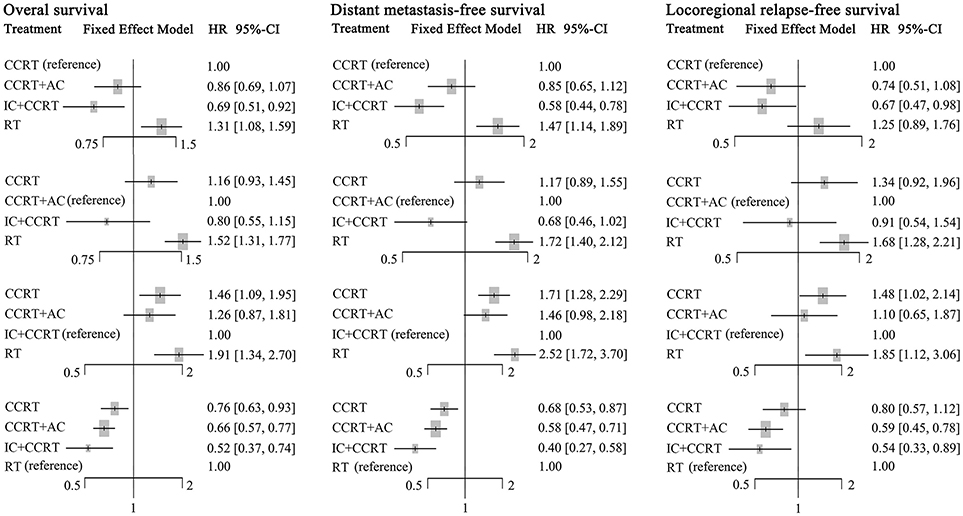
Figure 4. Forest plot of network meta-analysis for overall survival, distant metastasis-free survival, and locoregional recurrence-free survival with different reference groups. CCRT, concurrent chemoradiotherapy; IC, induction chemotherapy; AC, adjuvant chemotherapy; RT, radiotherapy.
Compared to CCRT, IC+CCRT achieved significantly better OS (HR, 0.69; 95% CI, 0.51–0.92), DMFS (HR, 0.58; 95% CI, 0.44–0.78), and LRFS (HR, 0.67; 95% CI, 0.47–0.98). However, no significant survival differences were found between CCRT+AC and CCRT, or CCRT+AC and IC+CCRT (Supplementary Tables S2–S4). Notably, RT alone always led to significantly poorer survival outcomes compared with the other three treatments except RT vs. CCRT for LRFS (HR, 1.25; 95% CI, 0.89–1.76).
The corresponding P-scores of IC+CCRT, CCRT+AC, CCRT, and RT treatment arms were 96.1%, 67.3, 36.5, and 0.1% for OS; 99.0, 63.4, 37.6, and 0.4% for DMFS; 87.1, 76.6, 32.7, and 3.6% for LRFS, indicating IC+CCRT has the highest probability of being the best treatment in terms of OS, DMFS, and LRFS.
Sensitivity Analysis
We further performed sensitivity analysis after including the study by Kwong et al. (18) to validate our findings; and the results are shown in the Supplementary Results (Supplementary Figures S1–S3, Supplementary Tables S5,S6). Notably, the conclusions remained valid after including this study. More importantly, IC+CCRT was even found to be superior to CCRT+AC with regard to DMFS (HR, 0.63; 95% CI, 0.43–0.93). Similarly, IC+CCRT still provided the highest benefit on OS, DMFS, and LRFS. These results indicated that IC+CCRT may be better than CCRT+AC.
Discussion
In our current study, we applied frequentist method to conduct multiple treatment comparisons between IC+CCRT, CCRT+AC, CCRT, and RT in advanced NPC based on all available information extracted from the published studies. We found that IC+CCRT was superior to CCRT and RT, and provided the largest OS, DMFS, and LRFS benefits. While no significant difference was observed between IC+CCRT and CCRT+AC. Further sensitivity analysis after including the study by Kwong et al. (18) also yield similar results. Notably, no inconsistency and heterogeneity were observed between these comparisons for all end-points, indicating that our findings are robust.
The role of chemotherapy in managing advanced NPC has changed greatly over the last two decades. Before the Intergroup 0099 study (5), radiotherapy alone is the only care for both early and advanced disease. Later on, CCRT+AC was proven better than RT alone in improving OS and this regimen has deemed the standard treatment for advanced NPC. However, a meta-analysis conducted by Baujat et al. (6) revealed this survival benefit mainly came from concurrent chemotherapy during RT. Moreover, the study by Chen et al. (7) found that AC additional to CCRT may be useless and this conclusion was further proven by long-term follow-up outcomes (27). Consequently, CCRT with or without AC was recommended by the NCCN guidelines. Although this regimen was applied, distant metastasis still remains the main failure pattern for advanced NPC (31). Therefore, novel treatments like IC was introduced. However, we still know little about the efficacy difference between these treatment modalities. In our study, we aimed at addressing this issue.
IC+CCRT achieved significantly better OS, DMFS, and LRFS than CCRT in both the pairwise and network meta-analyses, Which was different from the findings by Ribassin-Majed et al. (32). Undoubtedly, the inclusion of the lastest three IC studies (9, 11, 12) could add the weight of IC+CCRT in the network loop, resulting in better efficacy than CCRT alone. Another possible reason contributing to the survival difference between these two groups may be the difference of radiotherapy technique since almost all patients in IC+CCRT arm received intensity-modulated radiotherapy (IMRT) while some patients in CCRT arm received conventional radiotherapy. Therefore, we could conclude that IC+CCRT is better than CCRT and should be considered prior to CCRT. Although IC+CCRT was not found to be better than CCRT in other head and neck cancers (33), we should not apply this result to NPC because NPC has extremely different biological behaviors and is more sensitive to radiotherapy and chemotherapy compared with other head and neck cancers. It should be noted that the delivery of IC should be selective. Recently, two studies (34, 35) revealed additional IC to CCRT may be useless in T3-4N0-1 patients, indicating that only high-risk patients (defined as patients with N2-3 category, overall stage IVA or high pre-treatment Epstein-Barr virus DAN load) may benefit from IC. Moreover, the IC regimen also plays a key role. Docetaxel plus cisplatin with fluorouracil (TPF) has been proven to be superior to PF in head and neck cancer (36–38). Moreover, gemcitabine with cisplatin (GP) has been proven superior to PF in recurrent or metastatic NPC (39). Therefore, selection of effective IC regimens for high-risk patients should be a priority.
Similar to the results of original studies (7, 27), survival outcomes did not significantly differ between CCRT+AC and CCRT treatment arms, suggesting the value of adding AC to CCRT may be limited. Notably, all the included studies regarding CCRT+AC used the recommended AC regimen, cisplatin with fluorouracil. However, this combined AC regimen did not improve survival outcomes compared with either single-agent regimen individually in head and neck cancer (40). In addition, compliance to three cycles of AC was poor (5, 7, 21, 25, 30) and many patients also require dose reductions. Therefore, it is reasonable to infer the adjuvant PF regimen additional to CCRT is not good enough to further improve survival outcomes. Other regimens like GP or single-agent maintenance therapy should be further investigated.
CCRT may be inadequate for high-risk patients with advanced NPC; additional cycles of chemotherapy are worth being investigated (41). Therefore, either IC+CCRT or CCRT+AC may be a better choice than CCRT alone. However, we lack head-to-head clinical trials comparing IC+CCRT with CCRT+AC. In this study, survival outcomes did not differ significantly between CCRT+AC and IC+CCRT for any end-point. However, after including the study by Kwong et al. (18), IC+CCRT achieved better DMFS than CCRT-AC, which was inconsistent with the finding by Ribassin-Majed et al (32). The main reason as we discuss above is the inclusion of three new trials which achieved positive results and added the weight of IC+CCRT in the network loop. Therefore, IC+CCRT may be a little better than, or at least as efficacious as, CCRT+AC. In light of efficacy, it is reasonable to recommend IC+CCRT as the preferred treatment for advanced NPC. There may be another concern about IC+CCRT that IC may affect compliance with subsequent radiotherapy. Since our study was not based on individualized patient data, we, therefore, could not conclude on this. However, from historical data (9, 11, 12, 16), IC may have no impact on the compliance to radiotherapy. Actually, patients receiving or not receiving IC have same completion rate of radiotherapy at clinical practice. However, it should be pointed that compliance of concomitant chemotherapy might be impacted by IC.
Undoubtedly, RT was always poorer than IC+CCRT, CCRT+AC, and CCRT for almost all end-points. Thus, RT alone should not be recommended whenever possible. Notably, the rank of each treatment was indicated by the P-score in multiple treatment comparison. Although differences in effect size between different treatment arms were small and non-significant, a treatment ranking probability would still have been generated without definitive statistical meaning. Therefore, we should interpret the P-score discreetly, and clinical treatment strategies should not only refer to it.
Our study also had limitations: HRs and corresponding 95% CIs were mainly extracted from the original studies, which may produce reporting bias. Radiotherapy technique varied between different treatment arms which may affect the results of our study, and this issue should be solved by future individualized study data. Also, the role of hyperfractionated or accelerated hyperfractionated radiotherapy needs further investigation. Moreover, endpoints did not include PFS as the definitions of PFS varied between studies. To minimize these limitations, we set strict inclusion criteria and three investigators independently reviewed and extracted data. Furthermore, sensitivity analysis confirmed the findings were valid.
Materials and Methods
Literature Searching Strategy
First, we searched the English datasets including PubMed, Web of Science, and the Cochrane Library using the following items: “nasopharyngeal carcinoma” and “induction chemotherapy” or “neoadjuvant chemotherapy” or “adjuvant chemotherapy” or “concurrent chemoradiotherapy” or “radiotherapy.” Study type was restricted to clinical trial. Two investigators (ML and WY) performed the searching independently to identify all potentially eligible studies. Furthermore, we will also retrieve the National Knowledge Infrastructure and WanFang database to include any related Chinese references. Supplementary Method showed the detailed process of literature searching. The institutional ethical review board of Zigong NO. 4 People's Hospital approved our current study. All study methods were performed in accordance with our center guidelines.
Study Inclusion Criteria
Brief inclusion criteria of our study were as follow: (1) newly diagnosed advanced NPC without metastasis; (2) randomized controlled phase II/III trials; (3) patients received conventional-fractionation and radical radiotherapy; (4) concurrent chemotherapy should be platinum-based regimens. Supplementary Method presented the detailed information on study inclusion criteria. In our present study, we mainly recruited four treatment arms (CCRT+AC, IC+CCRT, CCRT, and RT alone) to conduct multiple network comparisons.
Study Review and Data Acquisition
In order to assess the quality of the recruited trials, the following items were reviewed to score each study according to Jadad/Oxford quality scoring system(42): randomization procedure, blinding principle, intention-to-treat principle, allocation concealment, and patient dropout. The study information such as included patients, study time, radiotherapy, and chemotherapy regimens, follow-up duration, and survival outcomes were extracted. Three investigators (ML, WY, and J-DM) performed the review process and data acquisition separately, and any discrepancies would be resolved by consensus.
Study Endpoints
Survival outcomes were shown as hazard ratios (HRs) and corresponding confidence intervals (CIs) which were extracted from original studies or an individualized data meta-analysis (43) using the method proposed by Parmar et al. (44). Study endpoints included OS, distant metastasis-free survival (DMFS) and locoregional recurrence-free survival (LRFS). Given the different definition of progression-free survival (PFS) in the trials, we did not included it into analysis.
Statistical Method
First, we conducted pairwise meta-analysis comparison between each treatment group was using Stata 12.0 (StataCorp LP, College Station, TX, USA). Treatment effects were presented by HRs and corresponding 95% CIs. Study heterogeneity was determined using the I2 statistic or χ2 test. An I2 statistic > 50% or the P-value of χ2 test < 0.1 indicated statistically heterogeneity; otherwise, no heterogeneity exist between studies. Then, we performed network comparisons using the R (version 3.3.3; R Foundation, Vienna, Austria) netmeta package (45, 46). The frequentist approach (45) was adopted to carry out the network meta-analysis. Before multiple comparison, heterogeneity or inconsistency between treatment arms was assessed by Q test (45). If no significant heterogeneity existed (P > 0.1), fixed-effects model would be employed; otherwise, the random-effects model would be used. Finally, each treatment arm was ranked based on their corresponding P-score (47). A P-score of 100% suggested that treatment is the best, and a P-score of 0% indicated the worst treatment. Toxicity between different arms were compared using the χ2 test. A two-sided P < 0.05 was considered significant. Detailed process of multiple network comparison was shown in Supplementary Method.
Conclusion
In summary, this network meta-analysis demonstrates IC+CCRT is superior to CCRT and provides highest benefit on OS, DMFS, and LRFS benefits LRFS. Therefore, IC+CCRT may be a better choice for advanced NPC at clinical practice. Head-to-head clinical trials comparing IC+CCRT with CCRT+AC are warranted to validate our findings.
Author Contributions
ML and WY conceived and designed the experiments and conducted literature searching. ML, WY, J-DM, X-BZ, and D-KC extracted study data and performed analysis. ML, WY, LX, and L-FX contributed to reagents, materials, and analysis tools. ML, WY, and Y-BS wrote the paper, WY and YG contributed to quality control and review of the manuscript. All authors approved the final version of this manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00597/full#supplementary-material
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. (2016) 66:115–32. doi: 10.3322/caac.21338
2. Wei WI, Sham JS. Nasopharyngeal carcinoma. Lancet (2005) 365:2041–54. doi: 10.1016/S0140-6736(05)66698-6
3. Zhang LF, Li YH, Xie SH, Ling W, Chen SH, Liu Q, et al. Incidence trend of nasopharyngeal carcinoma from 1987 to 2011 in Sihui County, Guangdong Province, South China: an age-period-cohort analysis. Chin J Cancer (2015) 34:350–7. doi: 10.1186/s40880-015-0018-6
4. Yi JL, Gao L, Huang XD, Li SY, Luo JW, Cai WM, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. (2006) 65:161–8. doi: 10.1016/j.ijrobp.2005.12.003
5. Al-Sarraf M, LeBlanc M, Giri PG, Fu KK, Cooper J, Vuong T, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. (1998) 16:1310–7. doi: 10.1200/JCO.1998.16.4.1310
6. Baujat B, Audry H, Bourhis J, Chan AT, Onat H, Chua DT, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. (2006) 64:47–56. doi: 10.1016/j.ijrobp.2005.06.037
7. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: a phase 3 multicentre randomised controlled trial. Lancet Oncol. (2012) 13:163–71. doi: 10.1016/S1470-2045(11)70320-5
8. Chen YP, Wang ZX, Chen L, Liu X, Tang LL, Mao YP, et al. A Bayesian network meta-analysis comparing concurrent chemoradiotherapy followed by adjuvant chemotherapy, concurrent chemoradiotherapy alone and radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. (2015) 26:205–11. doi: 10.1093/annonc/mdu507
9. Cao SM, Yang Q, Guo L, Mai HQ, Mo HY, Cao KJ, et al. Neoadjuvant chemotherapy followed by concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase III multicentre randomised controlled trial. Eur J Cancer (2017) 75:14–23. doi: 10.1016/j.ejca.2016.12.039
10. Hui EP, Ma BB, Leung SF, King AD, Mo F, Kam MK, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. (2009) 27:242–9. doi: 10.1200/JCO.2008.18.1545
11. Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY, et al. Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol. (2016) 17:1509–20. doi: 10.1016/S1470-2045(16)30410-7
12. Frikha M, Auperin A, Tao Y, Elloumi F, Toumi N, Blanchard P, et al. A randomized trial of induction docetaxel-cisplatin-5FU followed by concomitant cisplatin-RT versus concomitant cisplatin-RT in nasopharyngeal carcinoma (GORTEC 2006-02). Ann Oncol. (2017) 29:731–6. doi: 10.1093/annonc/mdx770
13. Lin JC, Jan JS, Hsu CY, Liang WM, Jiang RS, Wang WY. Phase III study of concurrent chemoradiotherapy versus radiotherapy alone for advanced nasopharyngeal carcinoma: positive effect on overall and progression-free survival. J Clin Oncol. (2003) 21:631–7. doi: 10.1200/JCO.2003.06.158
14. Chen QY, Wen YF, Guo L, Liu H, Huang PY, Mo HY, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: phase III randomized trial. J Natl Cancer Inst. (2011) 103:1761–70. doi: 10.1093/jnci/djr432
15. Fountzilas G, Ciuleanu E, Bobos M, Kalogera-Fountzila A, Eleftheraki AG, Karayannopoulou G, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: a randomized phase II study conducted by the Hellenic Cooperative Oncology Group (HeCOG) with biomarker evaluation. Ann Oncol. (2012) 23:427–35. doi: 10.1093/annonc/mdr116
16. Tan T, Lim WT, Fong KW, Cheah SL, Soong YL, Ang MK, et al. Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. (2015) 91:952–60. doi: 10.1016/j.ijrobp.2015.01.002
17. Lee AW, Ngan RK, Tung SY, Cheng A, Kwong DL, Lu TX, et al. Preliminary results of trial NPC-0501 evaluating the therapeutic gain by changing from concurrent-adjuvant to induction-concurrent chemoradiotherapy, changing from fluorouracil to capecitabine, and changing from conventional to accelerated radiotherapy fractionation in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer (2015) 121:1328–38. doi: 10.1002/cncr.29208
18. Kwong DL, Sham JS, Au GK, Chua DT, Kwong PW, Cheng AC, et al. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial study. J Clin Oncol. (2004) 22:2643–53. doi: 10.1200/JCO.2004.05.173
19. Chan AT, Leung SF, Ngan RK, Teo PM, Lau WH, Kwan WH, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. (2005) 97:536–9. doi: 10.1093/jnci/dji084
20. Chan AT, Teo PM, Ngan RK, Leung TW, Lau WH, Zee B, et al. Concurrent chemotherapy-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: progression-free survival analysis of a phase III randomized trial. J Clin Oncol. (2002) 20:2038–44. doi: 10.1200/JCO.2002.08.149
21. Lee AW, Lau WH, Tung SY, Chua DT, Chappell R, Xu L, et al. Preliminary results of a randomized study on therapeutic gain by concurrent chemotherapy for regionally-advanced nasopharyngeal carcinoma: NPC-9901 Trial by the Hong Kong Nasopharyngeal Cancer Study Group. J Clin Oncol. (2005) 23:6966–75. doi: 10.1200/JCO.2004.00.7542
22. Lee AW, Tung SY, Chua DT, Ngan RK, Chappell R, Tung R, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. (2010) 102:1188–98. doi: 10.1093/jnci/djq258
23. Lee AW, Tung SY, Chan AT, Chappell R, Fu YT, Lu TX, et al. A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiother Oncol. (2011) 98:15–22. doi: 10.1016/j.radonc.2010.09.023
24. Lee AW, Tung SY, Chan AT, Chappell R, Fu YT, Lu TX, et al. Preliminary results of a randomized study (NPC-9902 Trial) on therapeutic gain by concurrent chemotherapy and/or accelerated fractionation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. (2006) 66:142–51. doi: 10.1016/j.ijrobp.2006.03.054
25. Chen Y, Liu MZ, Liang SB, Zong JF, Mao YP, Tang LL, et al. Preliminary results of a prospective randomized trial comparing concurrent chemoradiotherapy plus adjuvant chemotherapy with radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma in endemic regions of china. Int J Radiat Oncol Biol Phys. (2008) 71:1356–64. doi: 10.1016/j.ijrobp.2007.12.028
26. Chen Y, Sun Y, Liang SB, Zong JF, Li WF, Chen M, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma from endemic regions of China. Cancer (2013) 119:2230–8. doi: 10.1002/cncr.28049
27. Chen L, Hu CS, Chen XZ, Hu GQ, Cheng ZB, Sun Y, et al. Adjuvant chemotherapy in patients with locoregionally advanced nasopharyngeal carcinoma: long-term results of a phase 3 multicentre randomised controlled trial. Eur J Cancer (2017) 75:150–8. doi: 10.1016/j.ejca.2017.01.002
28. Wu X, Huang PY, Peng PJ, Lu LX, Han F, Wu SX, et al. Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. (2013) 24:2131–6. doi: 10.1093/annonc/mdt163
29. Zhang L, Zhao C, Peng PJ, Lu LX, Huang PY, Han F, et al. Phase III study comparing standard radiotherapy with or without weekly oxaliplatin in treatment of locoregionally advanced nasopharyngeal carcinoma: preliminary results. J Clin Oncol. (2005) 23:8461–8. doi: 10.1200/JCO.2004.00.3863
30. Wee J, Tan EH, Tai BC, Wong HB, Leong SS, Tan T, et al. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. (2005) 23:6730–8. doi: 10.1200/JCO.2005.16.790
31. Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. (2011) 80:661–8. doi: 10.1016/j.ijrobp.2010.03.024
32. Ribassin-Majed L, Marguet S, Lee AWM, Ng WT, Ma J, Chan ATC, et al. What is the best treatment of locally advanced nasopharyngeal carcinoma? an individual patient data network meta-analysis. J Clin Oncol. (2017) 35:498–505. doi: 10.1200/JCO.2016.67.4119
33. Vidal L, Ben Aharon I, Limon D, Cohen E, Popovtzer A. Role of induction chemotherapy prior to chemoradiation in head and neck squamous cell cancer-systematic review and meta-analysis. Cancer J. (2017) 23:79–83. doi: 10.1097/PPO.0000000000000253
34. Lan XW, Xiao Y, Zou XB, Zhang XM, OuYang PY, Xie FY. Outcomes of adding induction chemotherapy to concurrent chemoradiotherapy for stage T3N0-1 nasopharyngeal carcinoma: a propensity-matched study. Onco Targets Ther. (2017) 10:3853–60. doi: 10.2147/OTT.S133917
35. Wu LR, Yu HL, Jiang N, Jiang XS, Zong D, Wen J, et al. Prognostic value of chemotherapy in addition to concurrent chemoradiotherapy in T3-4N0-1 nasopharyngeal carcinoma: a propensity score matching study. Oncotarget (2017) 8:76807–15. doi: 10.18632/oncotarget.20014
36. Pointreau Y, Garaud P, Chapet S, Sire C, Tuchais C, Tortochaux J, et al. Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst. (2009) 101:498–506. doi: 10.1093/jnci/djp007
37. Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. (2007) 357:1705–15. doi: 10.1056/NEJMoa070956
38. Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. (2007) 357:1695–704. doi: 10.1056/NEJMoa071028
39. Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet (2016) 388:1883–92. doi: 10.1016/S0140-6736(16)31388-5
40. Jacobs C, Lyman G, Velez-Garcia E, Sridhar KS, Knight W, Hochster H, et al. A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol. (1992) 10:257–63. doi: 10.1200/JCO.1992.10.2.257
41. Lin JC, Liang WM, Jan JS, Jiang RS, Lin AC. Another way to estimate outcome of advanced nasopharyngeal carcinoma–is concurrent chemoradiotherapy adequate? Int J Radiat Oncol Biol Phys. (2004) 60:156–64. doi: 10.1016/j.ijrobp.2004.03.002
42. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12.
43. Blanchard P, Lee A, Marguet S, Leclercq J, Ng WT, Ma J, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. Lancet Oncol. (2015) 16:645–55. doi: 10.1016/S1470-2045(15)70126-9
44. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. (1998) 17:2815–34.
45. Rucker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods (2012) 3:312–24. doi: 10.1002/jrsm.1058
46. Rucker, G, Schwarzer, G, Krahn, U, König, J,. Netmeta: Network Meta-Analysis Using Frequentist Methods. Available online at: http://cran.r-project.org/web/packages/netmeta/index.html
Keywords: nasopharyngeal carcinoma, concurrent chemoradiotherapy, induction chemotherapy, adjuvant chemotherapy, network meta-analysis
Citation: Liu M, You W, Song Y-B, Miao J-D, Zhong X-B, Cai D-K, Xu L, Xie L-F and Gao Y (2018) The Changing Role of Chemotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma: A Updated Systemic Review and Network Meta-Analysis. Front. Oncol. 8:597. doi: 10.3389/fonc.2018.00597
Received: 06 September 2018; Accepted: 26 November 2018;
Published: 19 December 2018.
Edited by:
Lisa Francesca Licitra, Istituto Nazionale dei Tumori (IRCCS), ItalyReviewed by:
Panagiotis Balermpas, UniversitätsSpital Zürich, SwitzerlandEdgar K. Selzer, Medical University of Vienna, Austria
Copyright © 2018 Liu, You, Song, Miao, Zhong, Cai, Xu, Xie and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei You, youweizg@126.com
†These authors have contributed equally to this work
 Mei Liu†
Mei Liu† Wei You
Wei You