Neonatal Diet and Gut Microbiome Development After C-Section During the First Three Months After Birth: A Systematic Review
- RECETOX, Faculty of Science, Masaryk University, Brno, Czech Republic
Background: Cesarean section (C-section) delivery imprints fundamentally on the gut microbiota composition with potential health consequences. With the increasing incidence of C-sections worldwide, there is a need for precise characterization of neonatal gut microbiota to understand how to restore microbial imbalance after C-section. After birth, gut microbiota development is shaped by various factors, especially the infant’s diet and antibiotic exposure. Concerning diet, current research has proposed that breastfeeding can restore the characteristic gut microbiome after C-section.
Objectives: In this systematic review, we provide a comprehensive summary of the current literature on the effect of breastfeeding on gut microbiota development after C-section delivery in the first 3 months of life.
Methods: The retrieved data from PubMed, Scopus, and Web of Science were evaluated according to the PICO/PECO strategy. Quality assessment was conducted by the Newcastle–Ottawa Scale.
Results: After critical selection, we identified 14 out of 4,628 studies for the evaluation of the impact of the diet after C-section delivery. The results demonstrate consistent evidence that C-section and affiliated intrapartum antibiotic exposure affect Bacteroidetes abundance and the incapacity of breastfeeding to reverse their reduction. Furthermore, exclusive breastfeeding shows a positive effect on Actinobacteria and Bifidobacteria restoration over the 3 months after birth. None of the included studies detected any significant changes in Lactobacillus abundance in breastfed infants after C-section.
Conclusion: C-section and intrapartum antibiotic exposure influence an infant’s gut microbiota by depletion of Bacteroides, regardless of the infant’s diet in the first 3 months of life. Even though breastfeeding increases the presence of Bifidobacteria, further research with proper feeding classification is needed to prove the restoration effect on some taxa in infants after C-section.
Systematic Review Registration: [www.crd.york.ac.uk/prospero/], identifier [CRD42021287672].
Introduction
The acquisition and development of the intestinal microbiome is a dynamic process shaped by various factors. Starting at birth, the microbial community evolves into a stable and complex microbiome, resembling an adult one by the age of 3 (1–3). Despite current discussion about prenatal contact with microbes in utero, the first major transition of gut microbiota occurs during birth (4, 5). Vaginal delivery (VD) is a natural process where neonates are exposed to bacteria in the maternal birth canal. When either the mother or baby is at risk, cesarean section (C-section) delivery is necessary. Rates of this surgical procedure continue to rise globally, now accounting for more than one in five (21%) of all childbirths, depending on the access of the procedure (6). In contrast to vaginal birth, cesarean section delivery (CS) transmits distinguished gut microbiota from the maternal skin and hospital environment. Moreover, CS neonates are also indirectly exposed to antibiotics (ATB) through intrapartum prophylaxis (IAP). These early-life exposures are associated with reduced microbiota diversity and altered taxonomic distribution of gut microbiota (7, 8). Commonly observed patterns are Bifidobacterium and Bacteroides depletion and Enterococcus, Staphylococcus, Streptococcus, Enterobacter, and Clostridioides increases (9–11). These perturbations potentially lead to long-term effects on health in childhood and later in life (12–14).
Following birth, the microbiome is shaped by complex interactions between the mother, the infant, and their environment (5, 15). One of the major determinants of establishing a healthy gut microbiome is the neonatal diet (16, 17). Breastmilk provides optimal nutrition not only for the infant but also for the intestinal microbial community. It is a complex fluid of nutrients, bioactive compounds, and bacteria that support healthy infant development (18). Nevertheless, breastfeeding is not successfully initiated in the case of every infant. One of the reasons is the mode of delivery. CS influences lactogenesis, delays the establishment of breastfeeding, and discourages the process from the beginning (19, 20). Donor milk is an effective substitute in preterm infants, while formula feeding is common practice in healthy term infants. Although infant formula has a standardized composition, some nutrients, bioactive compounds, or live cells cannot be added to it due to negative interactions, short shelf life, bioavailability, or excessive production costs (21, 22). This, in turn, affects the neonatal gut microbiome. Several studies characterize the microbiome of formula-fed infants as distinct in wider microbiota diversity, resembling the weaning profile. The percentage of Bifidobacterium and Bacteroides is downregulated, Clostridioides, Streptococcus, and Enterococcus are increased; opportunistic pathogens are present (23, 24). The gut microbiome profile after formula feeding notably resembles the profile reported in CS infants; therefore, there is a question to what extent the gut microbiome perturbations in CS infants are a result of birth exposure or the following diet. Moreover, some recent studies declare that breastfeeding restores the distinctive gut microbiome after CS delivery (25–27).
To our knowledge, previous systematic reviews have assessed the impact of the delivery mode or the impact of feeding mode on the infant gut microbiome independently (7, 28, 29), with one exception emphasizing breastfeeding (30). Accordingly, our review aims to systematically assess existing publications describing the influence of delivery mode together with neonatal diet on the gut microbiome. We considered the optimal period to assess the effect of diet on the gut microbiome of CS infants from the first week to three months. The interval ensures that initiated lactation influences the infant’s gut microbiome and that complementary feeding is not implemented into the infant’s diet and impacts the results. Thus, in our systematic review, we compare the microbiome profiles of breastfed CS and VD infants from the first week to three months after birth. Furthermore, we describe the gut microbiome profile in formula-fed CS infants if they were stratified in studies included in our systematic review. This allows us to evaluate the effect of breastfeeding on the CS infant microbiome.
Materials and Methods
This systematic literature review was registered with the International Prospective Register of Systematic Reviews (PROSPERO registration No. CRD42021287672). The research question “Does breastfeeding restore the gut microbiome of healthy full-term infants born by C-section in the first 3 months of life compared to vaginally delivered infants?” was defined using the PICO/PECO strategy (for PICO/PECO elements, see Table 1).
Study Identification
We used PubMed, Scopus, and Web of Science to identify relevant articles on the effect of feeding practice on the gut microbiome in the first month after birth. We searched for the following MeSH terms: “(nutrition OR diet) AND (infant OR neonate OR newborn) AND (gut OR intestinal) AND (microbiome OR microbiota OR bacteria OR microbial)”. Only studies published before 1 September 2021 were included.
Study Selection and Inclusion and Exclusion Criteria
Two of the authors (EP and IK) separately searched the databases using the key search string. Once duplicates were removed, EP and IK independently reviewed the remaining articles by title and abstract to either retain or discard them according to the inclusion and exclusion criteria. This systematic review only includes observational studies of various designs (e.g., prospective, retrospective, cross-sectional, cohort, or case–control). The following studies were excluded: (1) non-human and in vitro studies; (2) studies not in English; (3) studies on preterm infants (<36 weeks), infants with very low birth weight (<1,500 g) or infants suffering from any disease/disorder; (4) studies focusing exclusively on supplementation, probiotics, and prebiotics; (5) studies with results not considering the mode of delivery and feeding together; (6) studies without control groups (breastfed vaginally delivered infants); (7) studies using samples collected only within the first week after birth or after 3 months post-partum; and (8) studies not empowering culture-independent molecular techniques for bacterial detection in stool samples. In the case of uncertainties, the full text was investigated. Any disagreements were resolved through discussion. In addition to the database searches, forward searches of included studies and relevant reviews were performed to identify additional sources.
Data Collection
After the data extraction study, details were tabulated as follows: study overview (author identification, year of publication, country of origin, population size), subject characteristic (mode of delivery, feeding type), study design (sampling time points, methodology used, amplified region/primers, platform), and key findings (changes in diversity and taxonomical changes). If necessary, authors were additionally contacted to clarify unclear details.
Strategy for Data Synthesis and Quality Assessment
For data synthesis, a descriptive synthesis was applied. Where available and comparable, data on diversity (OTU/ASV counts and Shannon index) were extracted for particular groups. The quality assessment was performed based on Newcastle–Ottawa Scale quality assessment (31). Points were assigned on a nine-point scale for quality factors including (i) comparability of exposed and non-exposed groups; (ii) evidence of microbiome assessment prior to exposure; (iii) record of diet; (iv) confounding factors; and (v) statistical analysis (Supplementary Table 1). The point for representativeness of the exposed cohort was allocated if the cohort of breastfed CS infants was ≥10.
Results
Study Selection and Characteristics
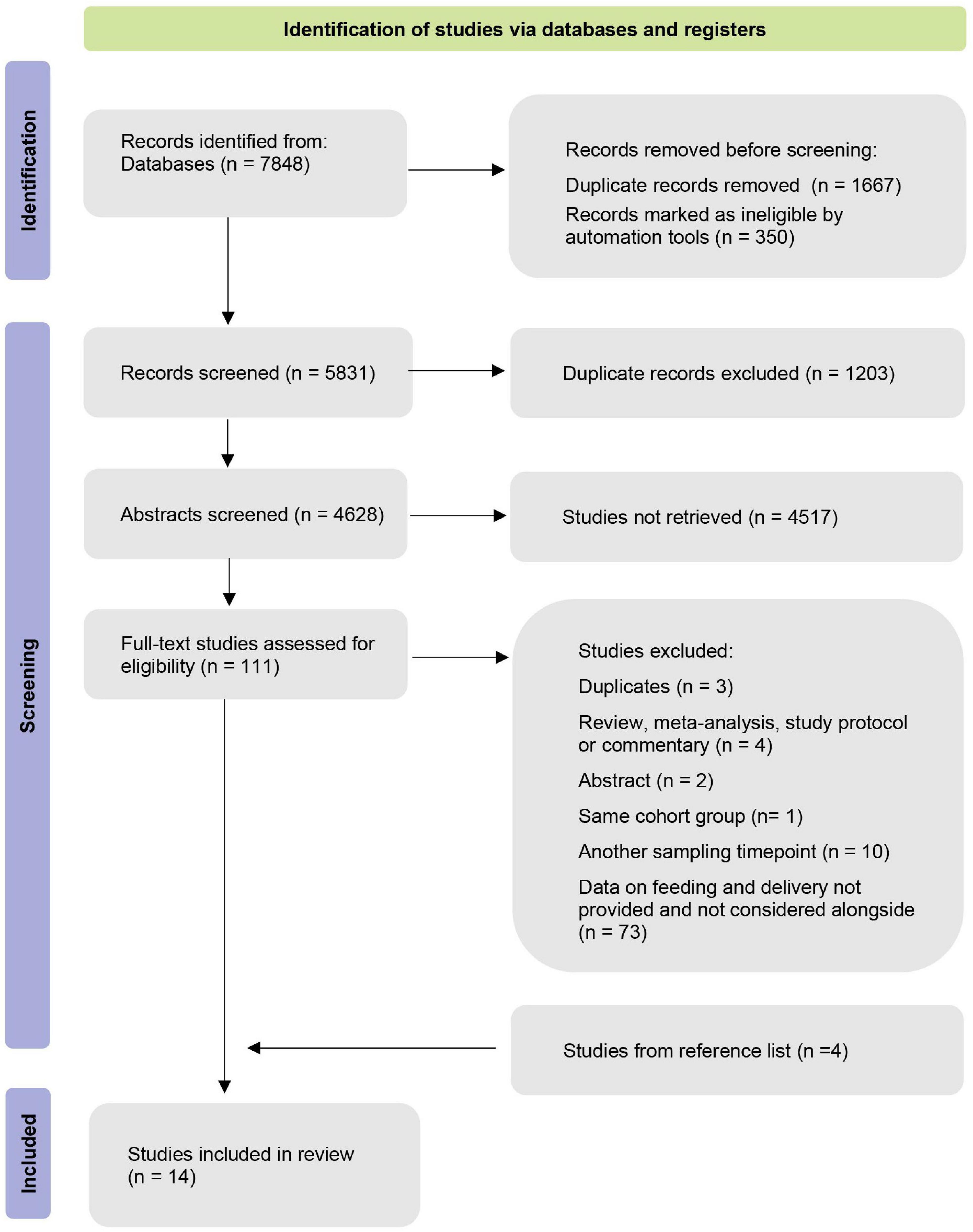
Our database search yielded 7,848 publications. In total, 1,667 duplicates were removed after the duplicate search in Mendeley and Excel. Afterward, we screened the remaining 5,831 records based on their title and abstract. Four additional studies were identified through forward searches in the reference list. Altogether, we selected 111 studies for full-text screening. Finally, 98 studies were excluded based on the defined inclusion/exclusion criteria (Figure 1). The remaining 14 manuscripts were eligible for our systematic review.
Study Characteristics
Overall, 14 selected manuscripts (Table 2) comprise a cohort of 3,091 infants: 626 breastfed CS infants, 134 non-breastfed CS infants, and 1,187 breastfed VD infants. All studies were conducted in high-income countries: six studies from North America (32–37), four from Europe (38–41), three from Asia (26, 27, 42), and one from South America (43). Selected studies were published in the last 10 years between 2014 and 2021. The majority of included studies provided details on study exclusion criteria, covering, for instance, ATB use in postnatal life. Two studies analyzed the effect of intrapartum antibiotic prophylaxis (IAP) in VD, elective, and acute CS delivery (33, 35). Two studies investigated the effect of a mother’s secretor status (40, 43).
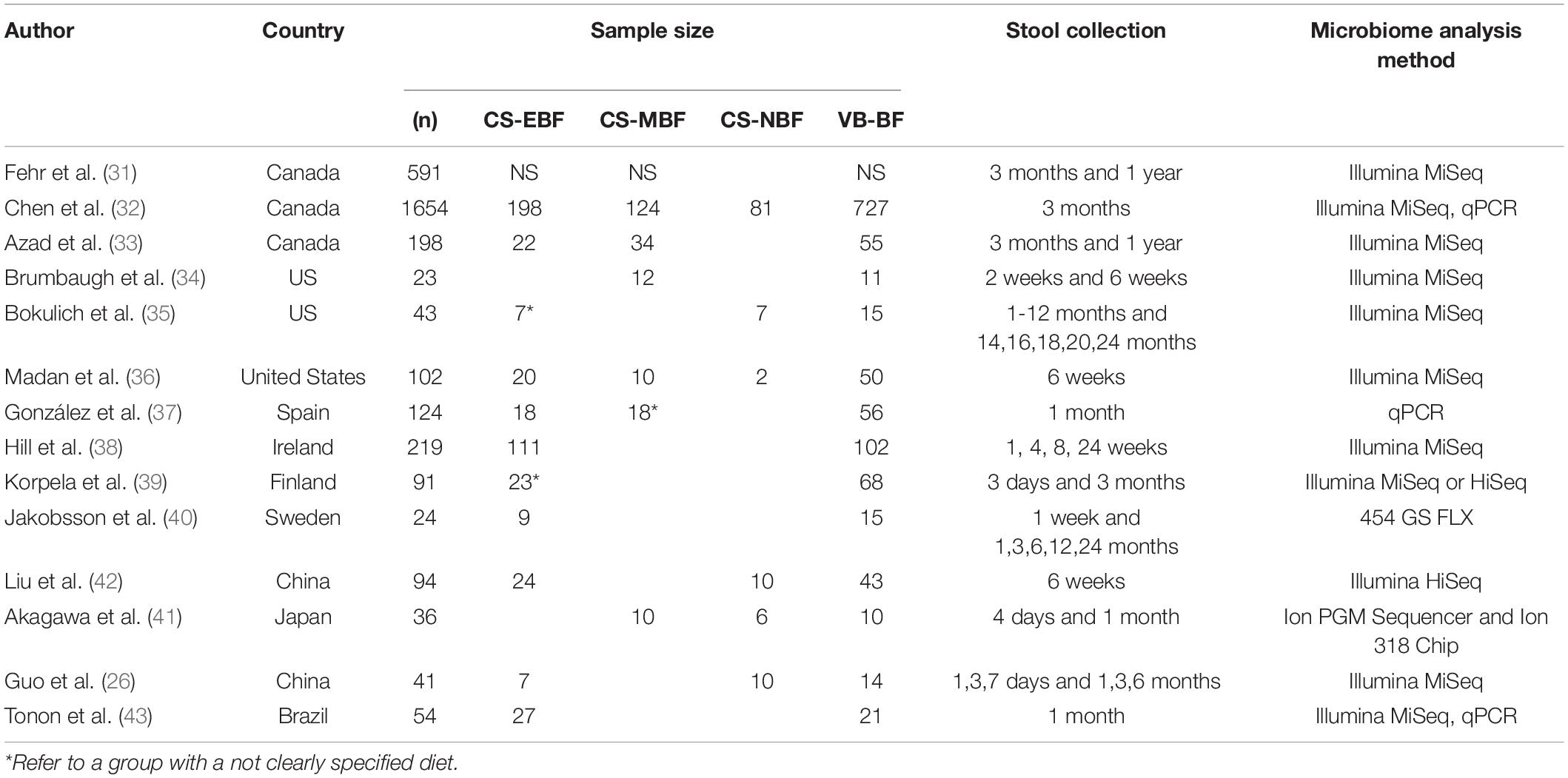
Table 2. Characteristics of the studies. CS-EBF indicates C-section exclusively breastfed infants, CS-MBF indicates C-section mix-fed infants, CS-NBF indicates C-section non-breastfed infants, and VB-BF indicates vaginally delivered breastfed infants.
Fecal samples were collected at various time points during the follow-up, except for five studies with only one time point of sample collection (33, 37, 38, 42, 43). For the evaluation of fecal samples, three studies applied the qPCR technique (33, 38, 43), 10 studies used Illumina MiSeq, and one used the Illumina HiSeq NGS platform, predominantly targeting V4 and V3–V4 regions. The remaining two studies are Ion Torrent and 454 sequencing (27, 41).
The feeding mode was categorized into diverse groups and the approach in the definition of breastfeeding differed between studies. In total, nine studies described the group of exclusively breastfed infants. The rest of the studies defined breastfed group as a group fed with breast milk by more than 80% (27) or as a mix of breastfed and partially breastfed infants (35, 40).
The quality assessment (Newcastle–Ottawa Scale) of selected studies is presented in Supplementary Table 1. A total of seven studies were awarded six points, and six studies were awarded more than seven points. Within the very strict inclusion criteria in our systematic review, no studies obtained five points or less. Also, no study achieved a maximum of nine points.
Data Evaluation
Gut Microbiome in the First 2 Weeks of an Infant’s Life
Bacteroidetes (35, 41) and Bacteroides (39) were significantly increased in VD infants compared to CS infants in all studies analyzing breastfed infants only. Only two studies highlighted a higher relative proportion of Firmicutes dominating in CS infants (39, 41). Hill et al. (39) showed a significant decrease in Actinobacteria and pointed to differences in the proportion of Bifidobacteria in the first week between breastfed CS and VD infants (19 vs. 48%). However, the difference was not significant due to the high inter-individual variation between infants at this timepoint. Only one study pointed to a significant difference in the Shannon index between CS and VD breastfed groups (39), whereas other studies in the first 2 weeks did not find any significant difference in microbial diversity.
Only Guo et al. (26) described the effect of formula feeding in CS infants for the first week of the infant’s life. This study did not identify any differences in the gut microbiome pattern.
Gut Microbiome in the First Month of the Infant’s Life
In the first month, CS breastfed infants maintained a characteristic significant reduction of Bacteroidetes (35, 43) and Bacteroides (27, 35–37, 39, 43) compared to VD breastfed infants. Some studies described Bacteroides as the only increased genus in CS exclusively breastfed infants at 1 month (27, 36, 37). Nevertheless, filling the void left by Bacteroides, some studies underlined other increased genera in breastfed CS infants, more specifically significantly increased Akkermansia (the most abundant genus of Verrucomicrobia) (43), Kluyvera (usually the most abundant genus of Proteobacteria) (43), and Enterococcus (41). Liu et al. (42) reported an increased Enterococcus and Veillonella abundance in mix-fed CS infants; however, they did not observe any significant difference in gut microbiota in exclusively breastfed CS infants compared to exclusively breastfed VD. Studies applying either the qPCR technique (38, 43) or 16S rRNA sequencing (27, 35–37, 39, 41) did not reveal any significant differences in Bifidobacterium spp. abundance in breastfed CS and breastfed VD infants. Only Hill et al. (39) presented a significant difference in the relative proportion of Actinobacteria, yet the relative proportion of Bifidobacterium was insignificant at all time points. Data presented by Gonzales et al. (38) showed an insignificantly lower abundance of Bifidobacterium.
The microbiome composition in formula-fed CS infants shifted from the first week to the first month and resulted in a significantly lower abundance of Bifidobacteria than breastfed CS and VD infants in the first month (26). This is in agreement with data presented by Bokulich et al. (36). Gonzales et al. (38) did not reveal any significant differences in bacterial groups in formula-fed CS and VD infants by qPCR. However, the values of Enterococcus abundance in CS formula-fed infants were the highest compared to other feeding/delivery groups. Bokulich et al. (38) showed a higher abundance of Firmicutes, Clostridiales, and Proteobacteria. Liu et al. (40) reported that the absence of exclusive breastfeeding significantly increased Enterococcus, Veillonella, and Faecalibacterium abundance; two studies did not describe differences in any taxa, except Bacteroides in formula-fed CS infants compared to other feeding types. However, these studies had a very small sample size of formula-fed CS infants (27, 37). Concerning Bacteroides, the abundance remains lower in studies including breastfed and formula-fed CS infants, regardless of feeding type (27, 36, 38).
Gut Microbiome in Three Months of an Infant’s Life
In the third month of life, in fants born by CS were still characterized by a significant reduction in Bacteroidetes (33, 34, 40, 41) and Bacteroides (32–34, 36, 40, 41). Korpela et al. (40) reported significantly reduced Actinobacteria. Regarding Bifidobacteria, Chen et al. (31) revealed differences between exclusively breastfed infants delivered by elective CS and emergency CS. However, CS breastfed infants compared to VD infants did not significantly differ in most of the 16S rDNA amplicon sequencing (32, 34, 40, 41) or qPCR studies (33). Moreover, Chen et al. (33) described a decreased abundance of Clostridium and Enterococcus genus in CS compared to VD breastfed infants. Azad et al. (34) reported an increase in Clostridium taxa in exclusively breastfed infants after emergency CS but not after elective CS. Fehr et al. (32) presented significant differences between exclusively breastfed CS and VD infants in Bacteroides uniformis, Enterococcus, and Veillonella dispar. Korpela et al. (40) highlighted the remaining increased abundance of Verrucomicrobia from one month in CS breastfed infants of secretor mothers; two studies describing a similar trend in the first month did not find any significant difference at 3 months (36, 41).
When exclusive breastfeeding is reduced to partial breastfeeding, the gut microbiota profile of CS infants differed in Bacteroides, Clostridium paraputrificum, Enterococcus, Lachnospiraceae, and Veillonella dispar abundance (32). Azad et al. (34) pointed to a significantly lower abundance of Bacteroidetes and a higher abundance of Clostridiales in partially breastfed infants. Bokulich et al. (36) characterized the gut microbiota of formula-fed infants at 3 months by increased Ruminococcaceae and Lachnospiraceae. Absolute quantification of Bifidobacterium by qPCR revealed lower absolute quantities in CS formula-fed infants than VD breastfed infants (33). Bokulich et al. (36) showed an increased abundance of Proteobacteria in gut microbiota both in CS formula-fed and CS exclusively breastfed infants.
The main findings of significant differences in the gut bacterial abundance within the first 3 months of an infant’s life are presented in Table 3.
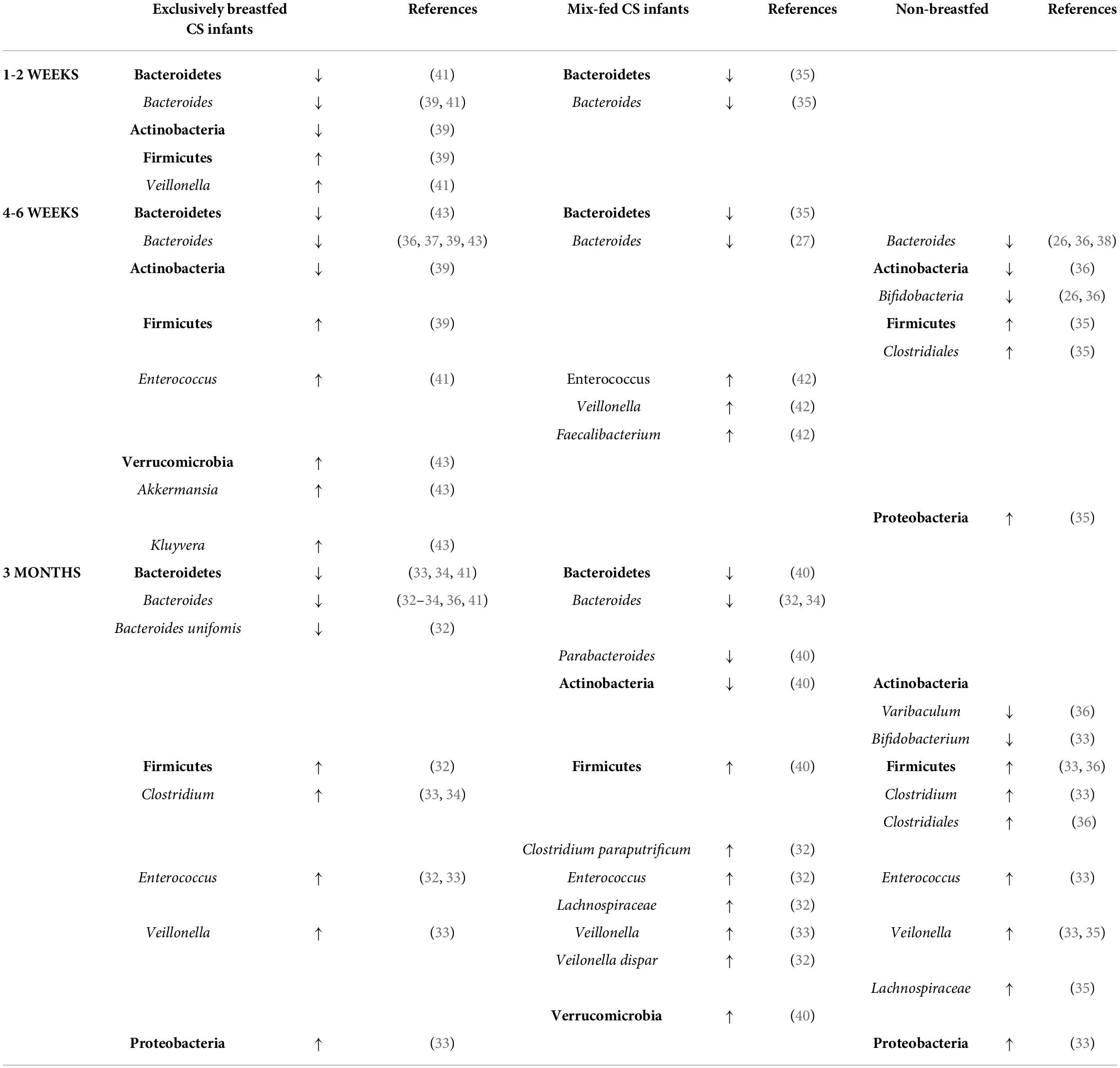
Table 3. Main findings of significant differences in the gut bacterial abundance of exclusively breastfed, mix-fed, and non-breastfed CS infants compared to breastfed VD infants.
Discussion
Reduced Bacteroides Abundance in Cesarean Section Delivery Breastfed Infants
Our systematic review reports several findings in gut microbiome development in the first 3 months of an infant’s life. First, all studies presented data on the reduced relative abundance of Bacteroides. This concurs well with most of the studies presenting delayed colonization of Bacteroides as a fundamental characteristic of CS-delivered infants (10, 44, 45), indicating the essential role of attributes of cesarean delivery in the establishment of early gut microbiota. It is noted that Bacteroides and Parabacteroides are most frequently transmitted from the mother to neonates through vaginal birth (15, 46). In addition to that, Bacteroides depletion is associated with maternal IAP. The effect of IAP is supported by studies describing the administration of IAP in vaginal delivery, where a similar trend of Bacteroides reduction is reported (32, 33, 45, 47). Both maternal transmission and IAP cause substantially lower colonization of Bacteroides from the first day after birth in CS infants. This is in agreement with recent findings by Mitchell et al. (48). Interestingly, they emphasize the presence of reduced Bacteroides species during the first week of life, followed by the disappearance of Bacteroides species in the second week. With these results, they point to the stability of acquired microbiome composition after C-section. More specifically, Bacteroides stability could be shattered by the competition of co-occurred taxa present after initial birth seeding, by lack of supporting factors like breastfeeding, and by differences in diversity. In their study, CS infants were less likely to have B. fragilis or B. thetaiotaomicron than VD infants.
This shows the importance of Bacteroides in an infant’s health and the gut microbiome. Previous studies refer to the effect of Bacteroides fragilis, Bacteroides vulgatus, or Bacteroides thetaiotaomicron on the immune system by activating T-cell-dependent immune responses (49–52). B. uniformis is also involved in glycan metabolization and its abundance increases in response to breastfeeding. Martin et al. (44) showed a lower probability to detect B. fragilis and B. uniformis during the first 3 months of life in CS infants. In this systematic review, Fehr et al. (33) reported a significant reduction of B. uniformis in CS infants, not only at 3 months but also at 1 year.
There is no agreement on how long the reduction of Bacteroides lasts (5, 7). Our systematic review showed a significant decrease in Bacteroides within 3 months. However, the reduction is often traced to at least 6 months of age. In addition, some studies presented Bacteroides as the only differentially abundant genera persisting over 3 months in breastfed CS infants and even up to 1 year (6, 9, 36). Most importantly, our systematic review observed that even exclusive breastfeeding did not equalize the difference in Bacteroides abundance after initial birth seeding within 3 months. Compared to formula-fed CS infants, exclusive breastfeeding positively influences the abundance of Bacteroides in CS infants but still does not restore the significant difference. Thus, the length of breastfeeding accelerates Bacteroides recovery within the first year (34, 41).
Effect of Breastfeeding on Bifidobacteria
Another widely discussed genus Bifidobacterium is regarded as a key intestinal taxon in early life. Bifidobacteria generate a low-pH environment, produce antimicrobial polysaccharides, and consequently influence the presence of other microbes (53, 54). A reduction in Bifidobacteria, related perturbations in interlinked microbes, and disruption of their functions at an early age are associated with immune and metabolic disorders (55–57). Regarding the Bifidobacteria depletion, the aberrant numbers have been described in CSes and after ATB treatment. All species and strains of Bifidobacterium are sensitive to IAP exposure (58–60). Stearns et al. (61) reported that every hour of IAP administration decreases the abundance of Bifidobacteria at 12 weeks. This is in agreement with the study of Chen et al. (33) who indicated a negative effect of IAP on VD exclusively breastfed infants at 3 months. The systematic review of the colonization pattern after CS delivery done by Rutayisire et al. (7) affirms the association between C-section and lower abundance of Bifidobacteria from birth to 3 months of life. Moreover, it is generally accepted by the scientific community that the genus Bifidobacterium dominates in the gut microbiota of VD breastfed infants (56) and that breastfeeding supports the growth and presence of Bifidobacteria (62). Therefore, it raises the question of how C-sections affect the abundance of Bifidobacteria in exclusively breastfed infants and if breastfeeding has the power to restore the effect of initial CS seeding and IAP on Bifidobacteria.
The evidence presented by our systematic review shows a significant reduction in the phylum level of Actinobacteria (39, 40) but an insignificant decrease in Bifidobacterium abundance in breastfed CS infants. This interpretation contrasts with studies not stratifying the feeding mode in C-section delivery. Moreover, the importance of breastfeeding is supported by the results in formula-fed CS infants, in which Bifidobacteria significantly decreased in abundance in the first month (36) and third month (33, 36).
The principal factor in the selective growth of intestinal Bifidobacterium spp. is human milk oligosaccharides (HMOs). HMOs are essential components of breast milk, which plays an important role in an infant’s gut microbiome development. They act as prebiotics, anti-adhesive, antimicrobial, and antibiofilm agents (63, 64). HMO concentrations vary widely between mothers and are associated with multiple factors (65). One characteristic is genetic secretor status, which determines the synthesis of fucosylated HMO absent or depleted in the milk of non-secretor mothers. These HMOs are degraded by enzymes possessed by strains of Bifidobacteria and Bacteroides (66, 67), microbes decreased in CS infants. In addition, the HMO profile and secretor status differ between populations (ranging from 65% to 98%) (68). Because of this, it is relevant to consider the secretor status in studies describing the effect of delivery and feeding mode on an infant’s gut microbiome. Korpela et al. (40) noted that CS intestinal microbiota may be detrimental if the infants are breastfed by a non-secretor mother.
In the same way, there is a need to consider geographical location in the comparison of results for genetic characteristics and also for cultural variations in feeding practices, hygiene, and lifestyle (69, 70). For instance, Princisval et al. (30) summarized the variations of proportion in Bifidobacteria favored in the northeast a south/southeast Asia, or Bacteroides more abundant in Central Europe. Half of the studies included in this systematic review represent CS infants from North America, only three studies were conducted in Asia, and one in South America. Given this, more studies are needed from various populations for a better understanding of the topic.
The intraindividual spectrum of HMOs in breast milk creates a stark contrast to the infant formula with few prebiotics. Even though the composition of infant formula evolved immensely over the last decade, it is not feasible to mimic the bioactive compounds present in breast milk. Different prebiotic mixtures of galactooligosaccharides (GOS) and fructooligosaccharides (FOS) are applied in an infant’s formula for the bifidogenic effect (71–74). Furthermore, commercially available HMO 2′-fucosyllactose (2′FL) and lacto-N-neotetraose (LNnT) were recently introduced. In consideration of the availability and recent introduction of products with HMO on the market, few studies evaluated the effect of formula with HMO on an infant’s gut microbiome. To date, the evidence suggests that even partial formula feeding in the first days after birth negatively influences the gut microbial composition (32).
Potential Effect on Firmicutes and Verrucomicrobia
Studies included in our systematic review underlined an increased abundance of Firmicutes. The seeding distinction after C-section favors the growth and colonization of Enterococcus, Clostridia, or Veillonella. These organisms opportunistically take advantage of the depleted taxa and successfully outcompete other bacteria. Interestingly, if we consider the presence of breastfeeding in the first month of a CS infant’s life, no alterations were observed in Clostridiales taxa. Concerning the reported increase in Firmicutes, five of 13 studies employing a broad range of molecular techniques did not prove any significant differences in other taxa than Bacteroides.
The missing contact with maternal vaginal microbiota during birth may be a possible reason for the higher abundance of some genera of the Firmicutes phylum. Studies investigating the role of CS on newborn gut microbiota development often discuss the acquisition of Lactobacillus (75, 76). Our systematic review did not show any significant reduction of Lactobacillus in CS breastfed infants. This lends support to findings that exclusive breastfeeding is positively associated with the abundance of Lactobacillus taxa (77, 78) and that Lactobacillus species from breast milk quickly restore their abundance in infants’ gut microbiota after CS.
Furthermore, one of the components of breast milk microbiota is the genus Akkermansia belonging to the phylum Verrucomicrobia. Both Tonon et al. (43) and Korpela et al. (40) describe their increased abundance in the gut microbiota of exclusively breastfed infants in secretory mothers. They also suggest that the higher abundance of Akkermansia and Bacteroides in CS infants of secretory mothers potentially decreases the risk of allergies.
Nevertheless, regarding the consistent significant differences in Bacteroides, other taxa belonging to Firmicutes, Proteobacteria, and Verrucomicrobia, and their changes in abundance are not supported by more than one study within the given timepoint, except for the increase of Enterococcus and Clostridia supported by two studies.
Limitations and Strengths
After the systematic literature search, 14 studies stratified breastfeeding status within CS delivery. However, the categorization of feeding practices is not uniform. Some studies classify infants as breastfed if this practice dominates. Other studies strictly distinguished between exclusive and partial breastfeeding. Moreover, the status of breastfeeding is evaluated according to the exact feeding practice at the moment of the sample collection. However, the history and use of formula, most importantly in the first days after birth, are not considered. Notably, the most recent studies observed that the gut microbiota profile of mix-fed infants resembles more formula-fed infants. Even small interventions in breastfeeding may affect the gut microbiota development in the first days of neonatal life (26, 32). The number of studies on formula-fed participants is inadequate, and some included studies presenting negligible sample sizes limit the statistical power. This again emphasizes the importance to record feeding habits and uniform the categorization into exclusive breastfeeding, partial breastfeeding, and exclusive formula feeding when evaluating microbiota development. An adequate number of studies could support the changes in feeding practices after C-section and in the neonatal ward, where formula feeding is a common practice.
An additional limitation is the lack of published data from microbiome analysis that prevent our intention of comparing alpha or beta diversity and conducting any meta-analysis. Moreover, studies differ in chosen sequencing platforms and targeted hypervariable regions of the 16S rRNA gene. Some studies underline the use of universal primers and their underestimation of Bifidobacteria abundance (33).
The strengths of our systematic review are the strict inclusion criteria. Critically, only studies with a control group of vaginally delivered breastfed infants were included. Essential components of the review are the data that combine the information about the number of enrolled infants, mode of delivery, and type of diet. Moreover, our systematic review included some studies with gut microbiome profiles of vaginally delivered infants after ATB treatment. These results emphasize the detrimental effect of ATB treatment, regardless of the delivery mode. Furthermore, we considered the heterogeneity in feeding classification and distinguished between exclusively breastfed, partially breastfed, and non-breastfed infants. To evaluate the dynamics of gut microbiota development in breastfed infants, the results were divided according to the time sampling and were compared to the gut microbiota of formula-fed infants.
Finally, with the increasing incidence of C-section delivery worldwide, our presented data help to characterize the microbial imprint of C-section in breastfed infants. Our results demonstrate the benefit of breastfeeding, which can modify IAP-induced intestinal microbiota changes. However, further research is needed to evaluate diet-induced changes in an infant’s microbiota and also the impact of IAP administration.
Summary
Unique changes are evident in CS breastfed infants after C-section in gut microbiota development over 3 months after birth. Breastfeeding does not demonstrate the ability to restore the depletion of Bacteroides after C-section with affiliated administration of IAP. This in turn may be reflected in the increase in Firmicutes abundance. Furthermore, breastfeeding in CS infants showed a positive influence on Bifidobacteria, which dominate in the healthy gut microbiota of VD breastfed infants and are very sensitive to IAP.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
EP participated in literature search, data extraction, and quality assessment. EP wrote the manuscript and designed the figures. IK participated in literature search, data extraction, quality assessment, and reviewed the manuscript. VT supervised and reviewed the manuscript for clarity. All authors contributed to the article and approved the submitted version.
Funding
We thank the Operational Program Research, Development, and Education - project CETOCOEN PLUS project (CZ.02.1.01/0.0/0.0/15_003/0000469) for financial support.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Research Infrastructure RECETOX RI (LM2018121) financed by the Ministry of Education, Youth and Sports, and Operational Program Research, Development, and Education—project CETOCOEN Excellence (CZ.02.1.01/0.0/0.0/17_043/0009632) for supportive background. This study was supported by the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No. 857560. This publication reflects only the authors’ view, and the European Commission is not responsible for any use that may be made of the information it contains.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.941549/full#supplementary-material
Supplementary Table 1 | Quality assessment.
References
1. Fallani M, Young D, Scott J, Norin E, Amarri S, Adam R, et al. Intestinal microbiota of 6-week-old infants across Europe: geographic influence beyond delivery mode, breast-feeding, and antibiotics. J Pediatr Gastroenterol Nutr. (2010) 51:77–84. doi: 10.1097/MPG.0B013E3181D1B11E
2. Tanaka M, Nakayama J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int. (2017) 66:515–22. doi: 10.1016/J.ALIT.2017.07.010
3. Chong CYL, Bloomfield FH, O’Sullivan JM. Factors affecting gastrointestinal microbiome development in neonates. Nutrients. (2018) 10:274. doi: 10.3390/NU10030274
4. Stinson LF, Payne MS, Keelan JA. Planting the seed: origins, composition, and postnatal health significance of the fetal gastrointestinal microbiota. Crit Rev Microbiol. (2016) 43:352–69. doi: 10.1080/1040841X.2016.1211088
5. Moore RE, Townsend SD. Temporal development of the infant gut microbiome. Open Biol. (2019) 9:190128. doi: 10.1098/RSOB.190128
6. Betran AP, Ye J, Moller A-B, Souza JP, Zhang J. Trends and projections of caesarean section rates: global and regional estimates. BMJ Global Health. (2021) 6:5671. doi: 10.1136/bmjgh-2021-005671
7. Rutayisire E, Huang K, Liu Y, Tao F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: a systematic review. BMC Gastroenterol. (2016) 16:86. doi: 10.1186/s12876-016-0498-0
8. Stewart CJ, Ajami NJ, O’Brien JL, Hutchinson DS, Smith DP, Wong MC, et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. (2018) 562:583. doi: 10.1038/S41586-018-0617-X
9. Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, et al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. (2015) 17:690–703. doi: 10.1016/J.CHOM.2015.04.004
10. Shao Y, Forster SC, Tsaliki E, Vervier K, Strang A, Simpson N, et al. Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature. (2019) 574:117–21. doi: 10.1038/s41586-019-1560-1
11. Hoang DM, Levy EI, Vandenplas Y. The impact of caesarean section on the infant gut microbiome. Acta Paediatr. (2021) 110:60–7. doi: 10.1111/APA.15501
12. Stokholm J, Thorsen J, Chawes BL, Schjørring S, Krogfelt KA, Bønnelykke K, et al. Cesarean section changes neonatal gut colonization. J Allergy Clin Immunol. (2016) 138: 881–889.e2. doi: 10.1016/J.JACI.2016.01.028
13. O’Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol. (2016) 7:925. doi: 10.3389/FMICB.2016.00925
14. Tamburini S, Shen N, Wu HC, Clemente JC. The microbiome in early life: implications for health outcomes. Nat Med. (2016) 22:713–22. doi: 10.1038/nm.4142
15. Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe. (2018) 24: 133–145.e5. doi: 10.1016/J.CHOM.2018.06.005
16. Guaraldi F, Salvatori G. Effect of breast and formula feeding on gut microbiota shaping in newborns. Front Cell Infect Microbiol. (2012) 2:94. doi: 10.3389/FCIMB.2012.00094
17. Walker WA, Iyengar RS. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr Res. (2015) 77:220–8. doi: 10.1038/pr.2014.160
18. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. (2013) 60:49. doi: 10.1016/J.PCL.2012.10.002
19. Wu Y, Wang Y, Huang J, Zhang Z, Wang J, Zhou L, et al. The association between caesarean delivery and the initiation and duration of breastfeeding: a prospective cohort study in China. Eur J Clin Nutr. (2018) 72:1644–54. doi: 10.1038/S41430-018-0127-9
20. Hobbs AJ, Mannion CA, McDonald SW, Brockway M, Tough SC. The impact of caesarean section on breastfeeding initiation, duration and difficulties in the first four months postpartum. BMC Pregnancy Childbirth. (2016) 16:90. doi: 10.1186/S12884-016-0876-1
21. Azad MB, Nickel NC, Bode L, Brockway M, Brown A, Chambers C, et al. Breastfeeding and the origins of health: interdisciplinary perspectives and priorities. Matern Child Nutr. (2021) 17:17. doi: 10.1111/MCN.13109
22. Lönnerdal B. Bioactive proteins in human milk: health, nutrition, and implications for infant formulas. J Pediatr. (2016) 173:S4–9. doi: 10.1016/J.JPEDS.2016.02.070
23. Ma J, Li Z, Zhang W, Zhang C, Zhang Y, Mei H, et al. Comparison of gut microbiota in exclusively breast-fed and formula-fedes: a study of 91 term infants. Sci Rep. (2020) 10:15792. doi: 10.1038/s41598-020-72635-x
24. Chin N, Méndez-Lagares G, Taft DH, Laleau V, Kieu H, Narayan NR, et al. Transient effect of infant formula supplementation on the intestinal microbiota. Nutrients. (2021) 13:807. doi: 10.3390/NU13030807
25. Savino F, Roana J, Mandras N, Tarasco V, Locatelli E, Tullio V. Faecal microbiota in breast-fed infants after antibiotic therapy. Acta Paediatr. (2011) 100:75–8. doi: 10.1111/J.1651-2227.2010.01988.X
26. Guo C, Zhou Q, Li M, Zhou L, Xu L, Zhang Y, et al. Breastfeeding restored the gut microbiota in caesarean section infants and lowered the infection risk in early life. BMC Pediatr. (2020) 20:532. doi: 10.1186/s12887-020-02433-x
27. Akagawa S, Tsuji S, Onuma C, Akagawa Y, Yamaguchi T, Yamagishi M, et al. Effect of delivery mode and nutrition on gut microbiota in neonates. Ann Nutr Metab. (2019) 74:132–9. doi: 10.1159/000496427
28. Shaterian N, Abdi F, Ghavidel N, Alidost F. Role of cesarean section in the development of neonatal gut microbiota: a systematic review. Open Med. (2021) 16:624–39. doi: 10.1515/MED-2021-0270
29. Ho NT, Li F, Lee-Sarwar KA, Tun HM, Brown BP, Pannaraj PS, et al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun. (2018) 9:4169. doi: 10.1038/s41467-018-06473-x
30. Princisval L, Rebelo F, Williams BL, Coimbra AC, Crovesy L, Ferreira AL, et al. Association between the mode of delivery and infant gut microbiota composition up to 6 months of age: a systematic literature review considering the role of breastfeeding. Nutr Rev. (2021) 80:113–27. doi: 10.1093/NUTRIT/NUAB008
31. Ottawa Hospital Research Institute. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-ANALYSES. (2021). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed February 21, 2022).
32. Fehr K, Moossavi S, Sbihi H, Boutin RCT, Bode L, Robertson B, et al. Breastmilk feeding practices are associated with the co-occurrence of bacteria in mothers’ milk and the infant gut: the child cohort study. Cell Host Microbe. (2020) 28: 285–297.e4. doi: 10.1016/J.CHOM.2020.06.009
33. Chen YY, Zhao X, Moeder W, Tun HM, Simons E, Mandhane PJ, et al. Impact of maternal intrapartum antibiotics, and caesarean section with and without labour on bifidobacterium and other infant gut microbiota. Microorganisms. (2021) 9:1847. doi: 10.3390/MICROORGANISMS9091847
34. Azad MB, Konya T, Persaud RR, Guttman DS, Chari RS, Field CJ, et al. Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study. BJOG. (2016) 123:983–93. doi: 10.1111/1471-0528.13601
35. Brumbaugh DE, Arruda J, Robbins K, Ir D, Santorico SA, Robertson CE, et al. Mode of delivery determines neonatal pharyngeal bacterial composition and early intestinal colonization. J Pediatr Gastroenterol Nutr. (2016) 63:320–8. doi: 10.1097/MPG.0000000000001124
36. Bokulich NA, Chung J, Battaglia T, Henderson N, Jay M, Li H, et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci Transl Med. (2016) 8:343ra82. doi: 10.1126/scitranslmed.aad7121
37. Madan JC, Hoen AG, Lundgren SN, Farzan SF, Cottingham KL, Morrison HG, et al. Association of cesarean delivery and formula supplementation with the intestinal microbiome of 6-week-old infants. JAMA Pediatr. (2016) 170:212–9. doi: 10.1001/JAMAPEDIATRICS.2015.3732
38. González S, Selma-Royo M, Arboleya S, Martínez-Costa C, Solís G, Suárez M, et al. Levels of predominant intestinal microorganisms in 1 month-old full-termes and weight gain during the first year of life. Nutrients. (2021) 13:2412. doi: 10.3390/NU13072412
39. Hill CJ, Lynch DB, Murphy K, Ulaszewska M, Jeffery IB, O’Shea CA, et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome. (2017) 5:4. doi: 10.1186/s40168-016-0213-y
40. Korpela K, Salonen A, Hickman B, Kunz C, Sprenger N, Kukkonen K, et al. Fucosylated oligosaccharides in mother’s milk alleviate the effects of caesarean birth on infant gut microbiota. Sci Rep. (2018) 8:13757. doi: 10.1038/S41598-018-32037-6
41. Jakobsson HE, Abrahamsson TR, Jenmalm MC, Harris K, Quince C, Jernberg C, et al. Decreased gut microbiota diversity, delayed bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut. (2014) 63:559–66. doi: 10.1136/gutjnl-2012-303249
42. Liu Y, Qin S, Song Y, Feng Y, Lv N, Xue Y, et al. The perturbation of infant gut microbiota caused by cesarean delivery is partially restored by exclusive breastfeeding. Front Microbiol. (2019) 10:598. doi: 10.3389/FMICB.2019.00598/BIBTEX
43. Tonon KM, Morais TB, Taddei CR, Araújo-Filho HB, Abrão ACFV, Miranda A, et al. Gut microbiota comparison of vaginally and cesarean born infants exclusively breastfed by mothers secreting a1-2 fucosylated oligosaccharides in breast milk. PLoS One. (2021) 16:e0246839. doi: 10.1371/journal.pone.0246839
44. Martin R, Makino H, Yavuz AC, Ben-Amor K, Roelofs M, Ishikawa E, et al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One. (2016) 11:e0158498. doi: 10.1371/JOURNAL.PONE.0158498
45. Long G, Hu Y, Tao E, Chen B, Shu X, Zheng W, et al. The influence of cesarean section on the composition and development of gut microbiota during the first 3 months of life. Front Microbiol. (2021) 12:691312. doi: 10.3389/fmicb.2021.691312
46. Yassour M, Jason E, Hogstrom LJ, Arthur TD, Tripathi S, Siljander H, et al. Strain-level analysis of mother-to-child bacterial transmission during the first few months of life. Cell Host Microbe. (2018) 24:146. doi: 10.1016/J.CHOM.2018.06.007
47. Yasmin F, Tun HM, Konya TB, Guttman DS, Chari RS, Field CJ, et al. Cesarean section, formula feeding, and infant antibiotic exposure: separate and combined impacts on gut microbial changes in later infancy. Front Pediatr. (2017) 5:200. doi: 10.3389/FPED.2017.00200/FULL
48. Mitchell CM, Mazzoni C, Hogstrom L, Bryant A, Bergerat A, Cher A, et al. Delivery mode affects stability of early infant gut microbiota. Cell Rep Med. (2020) 1:100156. doi: 10.1016/J.XCRM.2020.100156
49. Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. (2008) 453:620–5. doi: 10.1038/nature07008
50. Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AGP, et al. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. (2004) 5:104–12. doi: 10.1038/NI1018
51. Cuív PÓ, De Wouters T, Giri R, Mondot S, Smith WJ, Blottière HM, et al. The gut bacterium and pathobiont Bacteroides vulgatus activates NF-κB in a human gut epithelial cell line in a strain and growth phase dependent manner. Anaerobe. (2017) 47:209–17. doi: 10.1016/j.anaerobe.2017.06.002
52. Troy EB, Kasper DL. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci. (2010) 15:25. doi: 10.2741/3603
53. Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. (2011) 469:543–7. doi: 10.1038/nature09646
54. Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, et al. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev. (2017) 81:e36–17. e00036-17 doi: 10.1128/MMBR.00036-17
55. Forbes JD, Azad MB, Vehling L, Tun HM, Konya TB, Guttman DS, et al. Association of exposure to formula in the hospital and subsequent infant feeding practices with gut microbiota and risk of overweight in the first year of life. JAMA Pediatr. (2018) 172:e181161. doi: 10.1001/JAMAPEDIATRICS.2018.1161
56. Saturio S, Nogacka AM, Alvarado-jasso GM, Salazar N, de los Reyes-Gavilán CG, Gueimonde M, et al. Role of bifidobacteria on infant health. Microorganisms. (2021) 9:2415. doi: 10.3390/MICROORGANISMS9122415
57. Stuivenberg GA, Burton JP, Bron PA, Reid G. Why are bifidobacteria important for infants? Microorganisms. (2022) 10:278. doi: 10.3390/MICROORGANISMS10020278
58. Saturio S, Suárez M, Mancabelli L, Fernández N, Mantecón L, de los Reyes-Gavilán CG, et al. Effect of intrapartum antibiotics prophylaxis on the bifidobacterial establishment within the neonatal gut. Microorganisms. (2021) 9:1867. doi: 10.3390/MICROORGANISMS9091867/S1
59. Nogacka A, Salazar N, Suárez M, Milani C, Arboleya S, Solís G, et al. Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates. Microbiome. (2017) 5:93. doi: 10.1186/S40168-017-0313-3
60. Corvaglia L, Tonti G, Martini S, Aceti A, Mazzola G, Aloisio I, et al. Influence of intrapartum antibiotic prophylaxis for group b streptococcus on gut microbiota in the first month of life. J Pediatr Gastroenterol Nutr. (2016) 62:304–8. doi: 10.1097/MPG.0000000000000928
61. Stearns JC, Simioni J, Gunn E, McDonald H, Holloway AC, Thabane L, et al. Intrapartum antibiotics for GBS prophylaxis alter colonization patterns in the early infant gut microbiome of low risk infants. Sci Rep. (2017) 7:16527. doi: 10.1038/s41598-017-16606-9
62. Lee SA, Lim JY, Kim BS, Cho SJ, Kim NY, Kim OB, et al. Comparison of the gut microbiota profile in breast-fed and formula-fed Korean infants using pyrosequencing. Nutr Res Pract. (2015) 9:242. doi: 10.4162/NRP.2015.9.3.242
63. Chambers SA, Townsend SD. Like mother, like microbe: human milk oligosaccharide mediated microbiome symbiosis. Biochem Soc Trans. (2020) 48:1139. doi: 10.1042/BST20191144
64. Zhang S, Li T, Xie J, Zhang D, Pi C, Zhou L, et al. Gold standard for nutrition: a review of human milk oligosaccharide and its effects on infant gut microbiota. Microb Cell Fact. (2021) 20:108. doi: 10.1186/S12934-021-01599-Y
65. Azad MB, Robertson B, Atakora F, Becker AB, Subbarao P, Moraes TJ, et al. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J Nutr. (2018) 148:1733–42. doi: 10.1093/JN/NXY175
66. Marcobal A, Barboza M, Sonnenburg ED, Pudlo N, Martens EC, Desai P, et al. Bacteroides in the Infant gut consume milk oligosaccharides via mucus-utilization pathways. Cell Host Microbe. (2011) 10:507. doi: 10.1016/J.CHOM.2011.10.007
67. Lewis ZT, Totten SM, Smilowitz JT, Popovic M, Parker E, Lemay DG, et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome. (2015) 3:13. doi: 10.1186/S40168-015-0071-Z
68. McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am J Clin Nutr. (2017) 105:1086–100. doi: 10.3945/AJCN.116.139980
69. Quin C, Gibson DL. Human behavior, not race or geography, is the strongest predictor of microbial succession in the gut bacteriome of infants. Gut Microbes. (2020) 11:1143. doi: 10.1080/19490976.2020.1736973
70. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. (2012) 486:222. doi: 10.1038/NATURE11053
71. Salvini F, Riva E, Salvatici E, Boehm G, Jelinek J, Banderali G, et al. Specific prebiotic mixture added to starting infant formula has long-lasting bifidogenic effects. J Nutr. (2011) 141:1335. doi: 10.3945/JN.110.136747
72. Sierra C, Bernal MJ, Blasco J, Martínez R, Dalmau J, Ortuño I, et al. Prebiotic effect during the first year of life in healthy infants fed formula containing GOS as the only prebiotic: a multicentre, randomised, double-blind and placebo-controlled trial. Eur J Nutr. (2015) 54:89. doi: 10.1007/S00394-014-0689-9
73. Ben XM, Li J, Feng ZT, Shi SY, Lu YD, Chen R, et al. Low level of galacto-oligosaccharide in infant formula stimulates growth of intestinal bifidobacteria and lactobacilli. World J Gastroenterol. (2008) 14:6564. doi: 10.3748/WJG.14.6564
74. Borewicz K, Suarez-Diez M, Hechler C, Beijers R, de Weerth C, Arts I, et al. The effect of prebiotic fortified infant formulas on microbiota composition and dynamics in early life. Sci Rep. (2019) 9:2434. doi: 10.1038/s41598-018-38268-x
75. Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. (2010) 107:11971–5. doi: 10.1073/pnas.1002601107
76. Nagpal R, Tsuji H, Takahashi T, Kawashima K, Nagata S, Nomoto K, et al. Sensitive quantitative analysis of the meconium bacterial microbiota in healthy term infants born vaginally or by cesarean section. Front Microbiol. (2016) 7:1997. doi: 10.3389/FMICB.2016.01997/ABSTRACT
77. Levin AM, Sitarik AR, Havstad SL, Fujimura KE, Wegienka G, Cassidy-Bushrow AE, et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci Rep. (2016) 6:31775. doi: 10.1038/srep31775
Keywords: breastfeeding, delivery mode, C-section, infant, nutrition, microbiome, bacteria
Citation: Pivrncova E, Kotaskova I and Thon V (2022) Neonatal Diet and Gut Microbiome Development After C-Section During the First Three Months After Birth: A Systematic Review. Front. Nutr. 9:941549. doi: 10.3389/fnut.2022.941549
Received: 11 May 2022; Accepted: 15 June 2022;
Published: 26 July 2022.
Edited by:
Christophe Lacroix, ETH Zürich, SwitzerlandReviewed by:
Imad Omar Al Kassaa, Lebanese University, LebanonCarol Huang, University of Calgary, Canada
Copyright © 2022 Pivrncova, Kotaskova and Thon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vojtech Thon, vojtech.thon@recetox.muni.cz
 Eliska Pivrncova
Eliska Pivrncova  Vojtech Thon
Vojtech Thon