Chemical and biochemical mechanisms underlying the cardioprotective roles of dietary organopolysulfides
- 1Food Science and Technology Programme, Department of Chemistry, National University of Singapore, Singapore, Singapore
- 2National University of Singapore (Suzhou) Research Institute, Jiangsu, China
Foods that are rich in organosulfides are highly regarded for their broad range of functions in disease prevention and health promotion since ancient time yet modern scientific study, particularly clinical studies could not agree with traditional wisdom. One of the complexities is due to the labile nature of organosulfides, which are often transformed to different structures depending on the processing conditions. The recent evidence on polysulfides as H2S donors may open up a new avenue for establishing structure and health promotion activity relationship. To put this development into perspective, we carried out a review on the recent progress on the chemistry and biochemistry of organopolysulfides with emphasis on their cardioprotective property. First, we briefly surveyed the foods that are rich in polysulfides and their structural diversity. This is followed by in-depth discussion on the chemical transformations of polysulfides under various processing conditions. We further reviewed the potential action mechanisms of polysulfides in cardioprotection through: (a) hydrogen sulfide releasing activity; (b) radical scavenging activity; and (c) activity in enzyme inhibition and intervention of gene regulation pathways. Based on the literature trend, we can conclude that the emerging concept of organopolysulfides as naturally occurring H2S donors is intriguing and warrants further research to establish the structure and activity relationship of the organopolysulfides as H2S donors.
Introduction
According to the World Health Organization data, cardiovascular disease (CVD) is the number one cause of deaths and there will be estimated 23.3 million people who will die due to CVD by 2030 globally, which is more than the total population of Australia (1). Finding effective means for reducing the risk factors of CVDs has been a long-standing research theme for decades and much progress has been made and healthy diet is critically important. Fruits and vegetable are the key components of a healthy diet in part because they are rich in bioactive phytochemicals such as polyphenolic antioxidants, anti-inflammatory agents, and poly unsaturated fatty acids, particularly omega-3 fatty acids. For example, flavanols are shown to improve endothelium-mediated vasodilation (2) and European Food Safety Authority has approved the claim that consumption of 200 mg flavanols from cocoa daily can improve blood flow (3). In addition, cumulative evidence has suggested that dietary organosulfur compounds have a wide range of bioactivity, particularly cardiovascular health (4).
Cruciferous vegetables and the Allium family are known for their rich contents of bioactive organosulfur compounds (Figure 1). Cruciferous vegetables are rich in glucosinolates, which undergo hydrolysis by thioglucosidase (myrosinase) to isothiocyanates (5) including sulforaphane in the broccoli (6), benzyl isothiocyanate in garden cress (7), and phenyl-ethyl isothiocyanate in watercress (8). While isothiocyanates from cruciferous vegetables have received great attention because of their potential anti-cancer activity through modulation of phase II enzyme activities (9, 10), the organosulfides in Alliums are well known for their broad spectrum of health promoting benefits, including anti-microbial, anti-cancer, and cardioprotective effects (4, 11). Yet, there is lack of consistent human clinical evidence to support the traditional wisdom. The chemistry of dietary organosulfur compounds is particularly complex because of their sensitivity to structural transformation mediated by the enzymes in the vegetables or during food processing. Consequently, it is a challenge to establish structure and bioactivity relationships. The bioactivity of allicin from garlic has been extensively studied and reviewed. However, human clinical trials in garlic found that allicin has no effect on reducing cholesterol level (12). The other important organosulfides in Alliums are volatile polysulfides readily formed when garlic is processed. They have shown potential for cardiovascular health promotion. Of all the Allium species currently known, garlic (Allium sativum) and onion (Allium cepa) are the most popular species due to various health benefits associated with their consumption. Garlic and onions have been greatly valued for their medicinal uses throughout the history of civilization. A large amount of literature have shown that the organosulfur compounds in these two species are associated with their biological functions against chronic ailments, including cancer, diabetes, and CVDs (4, 11). Yet, the labile chemistry of dietary organosulfides makes it challenging to establish structure and activity relationship. In this review, we summarized recent literatures regarding the organopolysulfide chemistry, biochemistry, and potential action mechanisms for promoting cardiovascular health, particularly as natural donor of H2S. Based on this, the future research directions of dietary organopolysulfides are suggested.
Brief Survey of Organosulfides Rich Foods
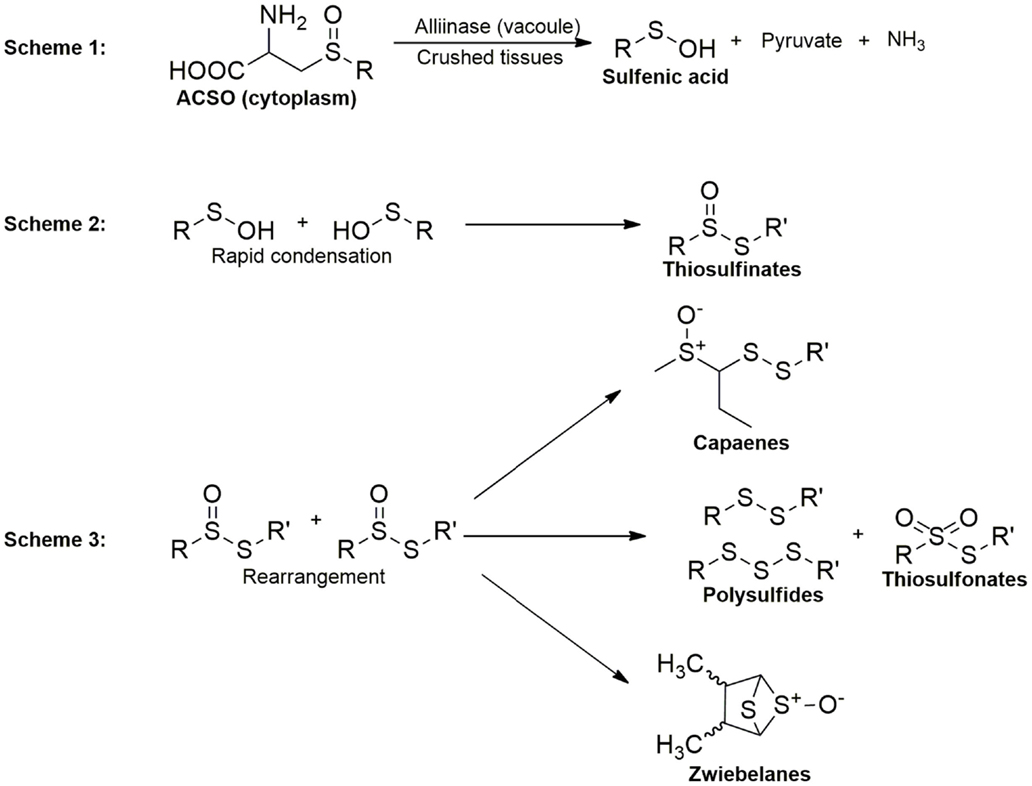
The organosulfides in Alliums are classified into two major groups: (1) oil-soluble polysulfides and (2) water-soluble thiosulfinates, the intermediate formed upon the reaction of the vacuolar enzyme alliinase with the non-volatile S-alk(en)yl-l-cysteine-sulfoxides (ACSOs) present in the cell cytoplasm when Alliums are crushed. Thiosulfinates from garlic and onions are known for their wide biological activities, including antithrombotic, antihypertensive, antioxidant, antibacterial, and antifungal effects and these biological properties have been reviewed elsewhere (13, 14). Organopolysulfides (di-, tri-, and tetrasulfides) are the major OSCs in the oil-soluble components of Alliums. The formation of these lipophilic compounds starts with the alliinase-ACSO reaction, which produces highly unstable intermediates sulfenic acids, pyruvate, and ammonia. Condensation of sulfenic acids leads to the formation of thiosulfinates, which undergo further rearrangements into polysulfides and other OSCs, including cepaenes and zwiebelanes (Figure 2) (15). Alliinase is the key enzyme that facilitates the formation of the oil-soluble OSCs. Due to the compartmentalization of this enzyme and the ACSOs, cell rupture by cutting or maceration is necessary to facilitate their release (16) for the reaction to take place.
Aside from glucosinolates and isothiocyanates, S-methyl-l-cysteine sulfoxide (MCSO, or metthiin), is present in vegetables of genus Brassica (17). MCSO significantly contributes to the typical spicy and pungent aromas of culinary processed Brassica (18). Similar to Alliums, enzymatic catabolism of MCSO in Brassicas generates other sensory-active sulfur compounds including dimethyl thiosulfonate, dimethyl thiosulfinate, and dimethyl sulfides (19). The enzyme responsible, termed cystine lyase (EC 4.4.1.8), behaves similarly as the alliinase in garlic, except that it can also hydrolyze l-cystine (20). Hydrolysis of MCSO generates highly reactive methyl sulfenic acid, which condenses to generate methyl methanethiosulfinate. Subsequently, thermal degradation of methyl methanethiosulfinate forms volatile polysulfides, majority of which are composed of dimethyl disulfide and dimethyl trisulfide (18). The occurrence, concentration, and distribution of MCSO in cruciferous vegetables are well documented (18, 19, 21, 22) and its biological functions have recently been reviewed in Ref. (23). In general, MSCO is found at about 1–2% dry weight in vegetables that belong to Brassicaceae, especially those of the genus Brassica (24). Although MCSO is universally present in Brassicas, factors associated to species and varietal differences influence the concentration and distribution of MCSO in these plants (23). Other factors affecting MCSO concentrations include environmental conditions, nutrient availability, harvest timing, and storage practices (25–27). While is it generally accepted that MCSO and other thermally generated breakdown products such as S-methyl methanethiosulphinate and S-methyl methanethiosulfonate, contribute to the typical flavor of processed cruciferous vegetables, their cardiovascular effect, although limited, has been reported (28, 29).
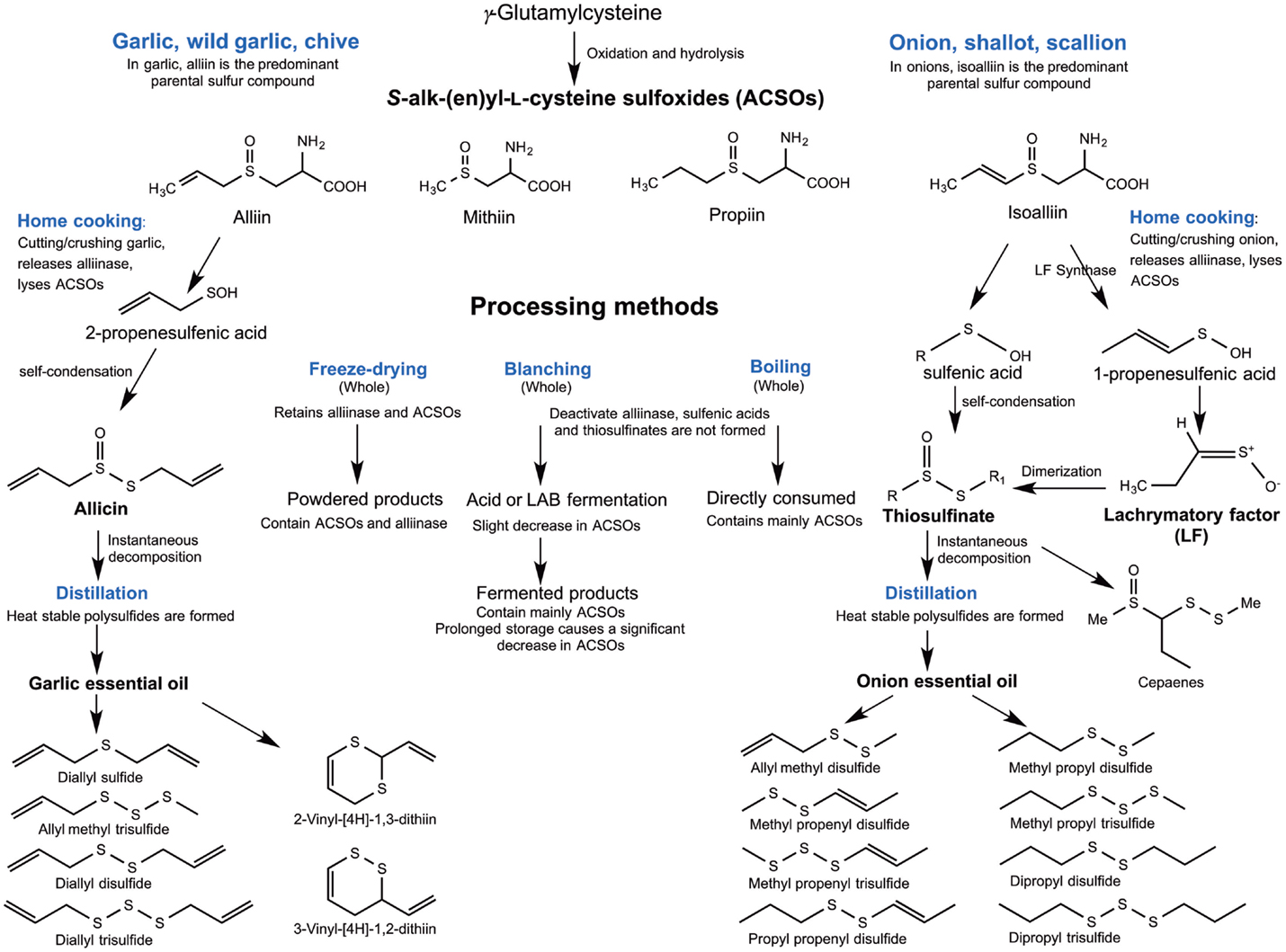
Garlic has been used for centuries as a traditional remedy to treat infectious diseases (30, 31). One of the main sulfur-containing compounds present in raw garlic is γ-glutamylcysteine. It has been proposed that γ-glutamylcysteine, along with glutathione are the starting compounds, which undergo hydrolysis and oxidation leading to the biosynthesis of ACSOs (13). In garlic, S-allyl-l-cysteine sulfoxide (alliin) is the predominant ACSO, while S-methyl-l-cysteine sulfoxide (methiin) and S-propenyl-l-cysteine sulfoxide (isoalliin) are present in smaller amounts (32). Another major compound formed upon hydrolysis of garlic is S-allylcysteine (SAC), a water-soluble thiosulfinate produced during aqueous garlic extraction catalyzed by the enzyme γ-glutamyl transpeptidase (33). Some of the health-beneficial functions of garlic are attributed to its organosulfur components, including the main ACSO precursor alliin and allicin, a thiosulfinate resulting from the lyses of alliin by alliinase. However, allicin is a transient compound that rapidly undergoes non-enzymatic decomposition into numerous oil-soluble polysulfides such as diallyl monosulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), diallyl tetrasulfide, and allyl methyl trisulfide (34, 35). The main components of garlic oil (Table 1) obtained by steam and hydrodistillation are DADS, DATS, allyl methyl trisulfide, and 2-vinyl-4H-1,3-dithiin (36–40). All eight thiosulfinates containing combinations of methyl, 1-propenyl, and 2-propenyl substituents are present in garlic homogenates, which explains the presence of lipid-soluble polysulfides with similar substituent combinations in distilled garlic oil (41).
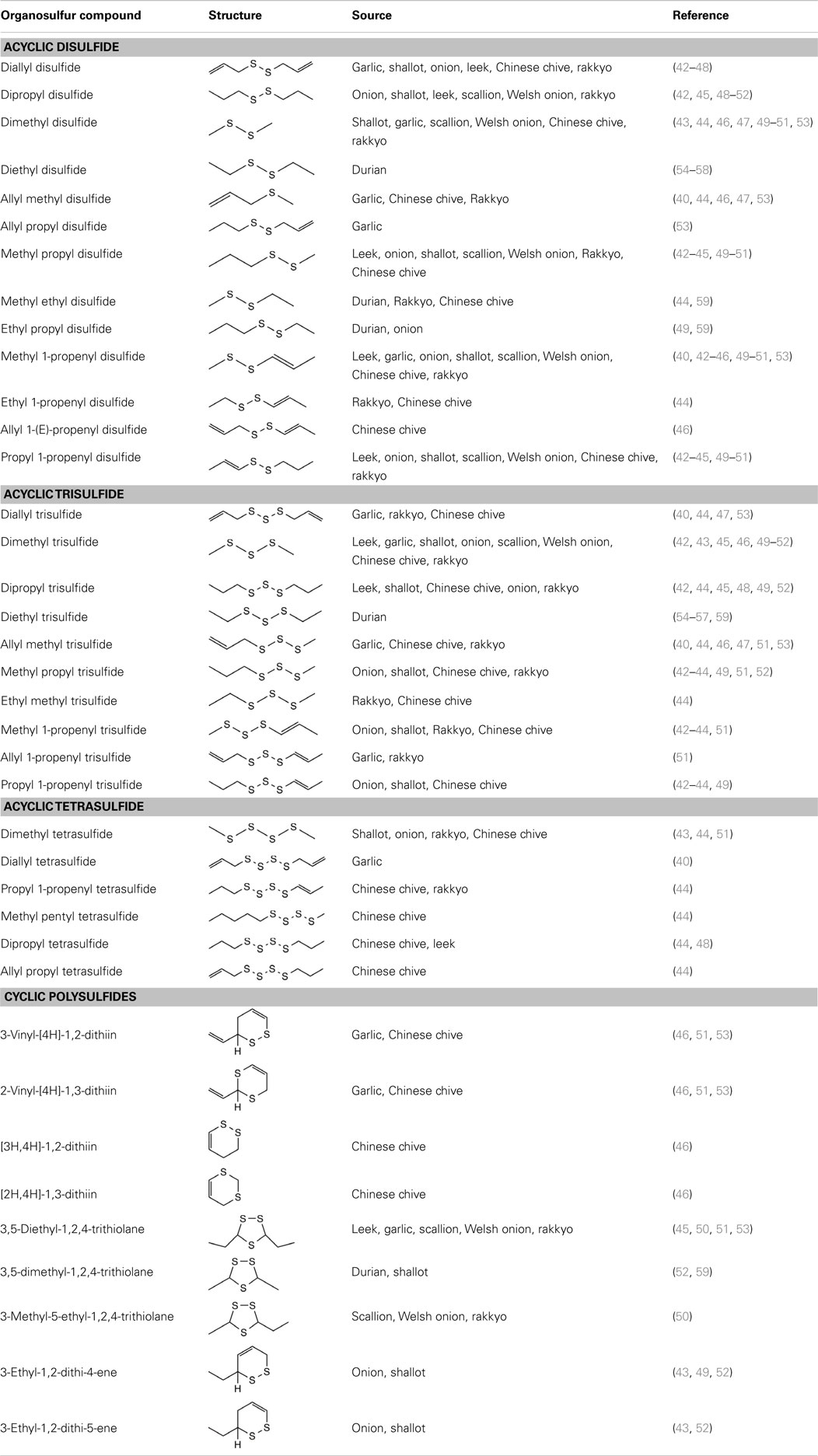
Table 1. Acyclic and cyclic organopolysulfides from the oil-soluble components of common dietary sources.
The organosulfide profile of onion oil includes polysulfides with different combinations of methyl, propyl, and 1-propenyl substituents, except for the compound with 1-propenyl and 1-propene substituents on either side of the -S-S(=O)- group of a thiosulfinate (60). 1-Propenyl 1-propenethiosulfinate (CH3-CH =CH-SS(O)CH =CH-CH3) has not been detected in Allium tissues possibly due to its oxidation to bissulfine or cyclization to zwiebelanes and a possible reaction with sulfenic acids to form cepaenes (15, 32).
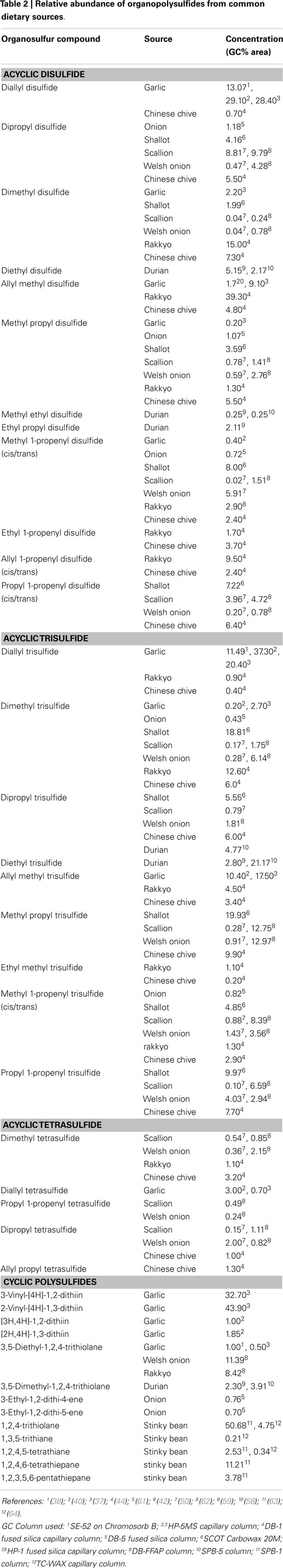
Distilled oils and solvent extracts from other Allium species are also known for their predominantly high contents of organosulfur compounds. These species include Chinese chive (Allium tuberosum Rottl. ex.), leek (Allium porrum L.), shallot (Allium cepa L. Aggregatum Group), rakkyo (Allium chinense G. Don), scallion (Allium fistulosum L. var caispitosum), and Welsh onions (Allium fistulosum L. var maichuon) (Figure 1). Summarized in Table 2 are the concentrations of the various oil-soluble polysulfides from their respective sources. Apparently, the abundance of the individual compounds depends on which Allium species they are found, although it is well known that garlic oil is rich in allyl polysulfides while onion oil is typically characterized by their high amounts of polysulfides with propyl substituents. While varietal differences may have effects on the levels of polysulfides, it is rather premature to conclude on this yet due to limited literature available.
Shallot is an important Allium species commonly used in many Asian diets. High amounts of organosulfides have been reported in the distilled and solvent extracted oils of shallot (42). Data from our lab also shown that hydrodistilled oil from shallot originating from Vietnam contain high amounts of onion-type polysulfides (52). Distilled oil and solvent extracts of Welsh onions and scallions consists 82–87% of organosulfides (50, 62). Similarly, organosulfides from the essential oils of Chinese chive and rakkyo comprised 88–94% of their total volatiles (44). Twelve varieties of solvent extracted oil from Chinese chive were found to contain disulfides, trisulfides, and vinyldithiins formed enzymatically from methiin as their precursor (46). The individual polysulfides in these less popular Allium species are listed in Table 1.
Organosulfur compounds are also present in the seeds of Parkia speciosa Hassk, commonly known as “petai” or stinky bean because of its unpleasant smell. In addition to its culinary uses, stinky bean is believed to have anti-microbial (65, 66), antioxidant (67–69), hypoglycemic (70), antiulcer (71), and antihypertensive (69) effects. Cyclic polysulfides are the major components of cooked petai (63). Earlier reports indicate that cyclic polysulfides [1,2,4-trithiolane (1), 1,3,5-trithiane (2), 1,2,4,6-tetrathiepane (5), and 1,2,3,5,6-pentathiepane (lenthionine) (7), 1,2,4,5,7,8-hexathionine] were the major constituents of stinky bean (Figure 3) (65, 68). In another study, hydrogen sulfide was found as the most abundant (41.3%) headspace constituent of stinky bean (64). Other cyclic polysulfides that have been found from stinky bean, include 3,5-dimethyl-1,2,4-trithiolane (3), 1,2,4,5-tetrathiane (4), 1,2,4,5-tetrathiocane (6), 1,2,3,4,5,6-hexathiepane (8), and 1,2,4,5,7,8-hexathionane (9).
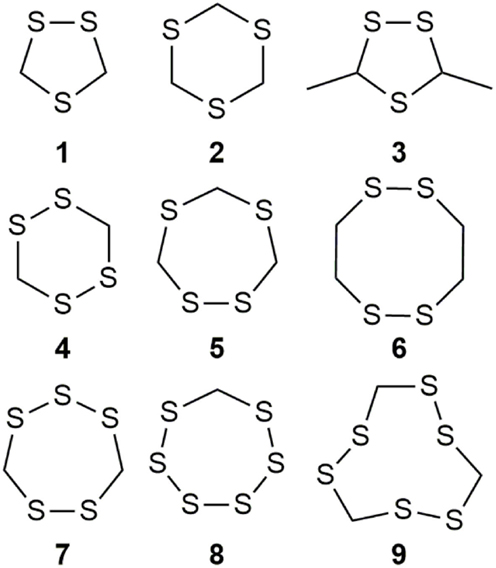
Figure 3. Cyclic organopolysulfides from stinky bean. 1, 1,2,4-trithiolane; 2, 1,3,5-trithiane; 3, 3,5-dimethyl-1,2,4-trithiolane; 4, 1,2,4,5-tetrathiane; 5, 1,2,4,6-tetrathiepane; 6, 1,2,4,5-tetrathiocane; 7, 1,2,3,5,6-pentathiepane (lenthionine); 8, 1,2,3,4,5,6-hexathiepane; 9, 1,2,4,5,7,8-hexathionane.
Of the edible fruits, durian (Durio zibethinus Murray) is perhaps the only tropical fruit known for its organosulfur contents. Durian, dubbed as the king of tropical fruits, is an exotic fruit with extensive popularity in Southeast Asia due to its distinct taste, odor, and texture. Of the 108 volatiles compounds determined in durian, 18 organosulfides were identified, making sulfurous compounds the second major volatile constituents, after ester group (56). Durian from Indonesia was reported to contain 17 (55) and 43 (72) organosulfides in two individual studies, with some common organosulfides detected such as S-ethyl thioacetate, diethyl disulfide, and 3,5-dimethyl-1,2,4-trithiolane. The volatile constituents of three different durian varieties D15, D28, and D74 were compared and it was found that D28 has the highest organosulfides content (54). In a separate study, organosulfides were the predominant compounds in durian in terms of quantity, contributing to more than 50% of the total volatiles (57).
Overall, there are various natural organosulfide sources including durian, the Allium vegetables, and stinky bean with characteristically high amounts of organosulfides. The formation of garlic- and onion-type OSCs follow a similar reaction mechanism with the ACSO-alliinase reaction as the main step after Alliums are crushed. Except for garlic and onions, the biological functions of the OSCs from these dietary sources are not yet fully explored.
Transformations of Dietary Organosulfides Under Different Processing Conditions
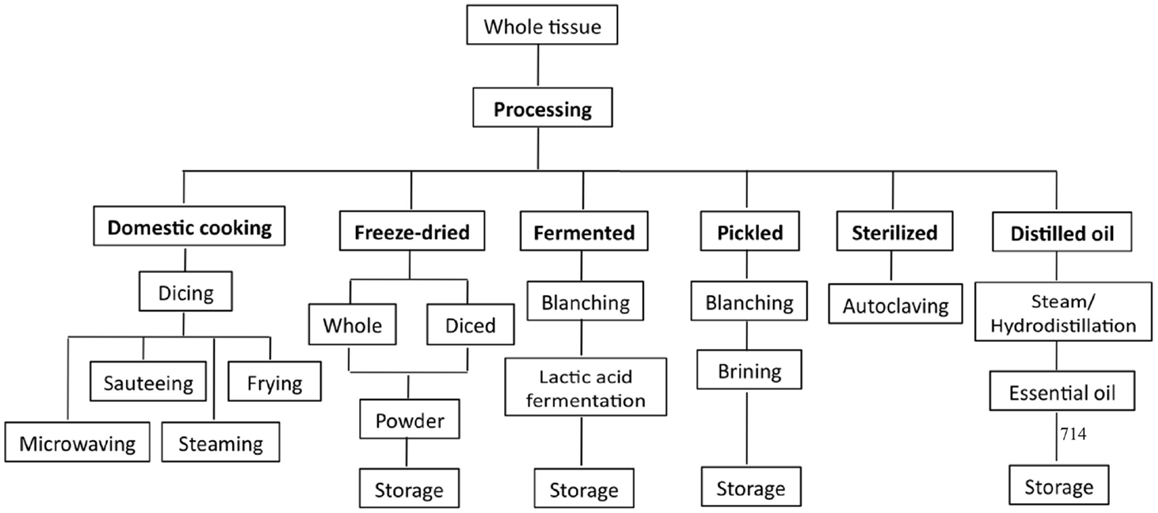
Unlike fruits, vegetables are commonly cooked before they are consumed, except in the case where they are added as ingredients to salads. In general, cooking methods that involve heating (i.e., boiling, steaming, and microwaving) are commonly used to prepare homemade dishes. The most common commercially available form of Allium products is the raw form. However, other forms of Allium products have increased their popularity and availability in the international market (11). Common Allium products available in the market include powdered or dried garlic flakes, garlic and onion essential oils, naturally fermented garlic or aged garlic extract (AGE), pickled fermented and unfermented garlic, and pastes (73). An overview of the various processed forms of Allium products is shown in Figure 4.
The study on the chemical transformation of single organosulfide would shed some light on the key reaction principles and the factors governing the transformation in complex food matrix. Sulfoxides are known to undergo β-elimination reaction upon heating and this property has been applied in organic synthesis of olefins sulfenic acids as by-products (74). In principle, ASCOs can form sulfenic acid through thermal elimination perhaps at higher temperature cooking in food matrix. However, this has not been reported in the literature yet. Instead, the focus is on alliinase catalyzed β-elimination reaction at room temperature. The resulting sulfenic acid quickly dehydrates to form allicin, which may undergo β-elimination again to regenerate sulfenic acid and 2-propenethial. The latter leads to the formation of cyclic sufides through Diels–Alder reaction (75). Formation of disulfides and polysulfides from allicin was proposed to be mediated by acid (76) (Figure 5). Beta-elimination from methyl substituted sulfoxide, S-methyl-l-cysteine sulfoxide, were shown to give rise to primarily dimethyl disulfides, which are responsible for the characteristic flavors of Alliums (18). Cyclic sulfides from stinky beans are not as common. Chemically, they are likely to be formed from formaldehyde and hydrogen sulfide followed by oxidation. In biological system, their formation mechanisms remain to be elucidated (63–65).
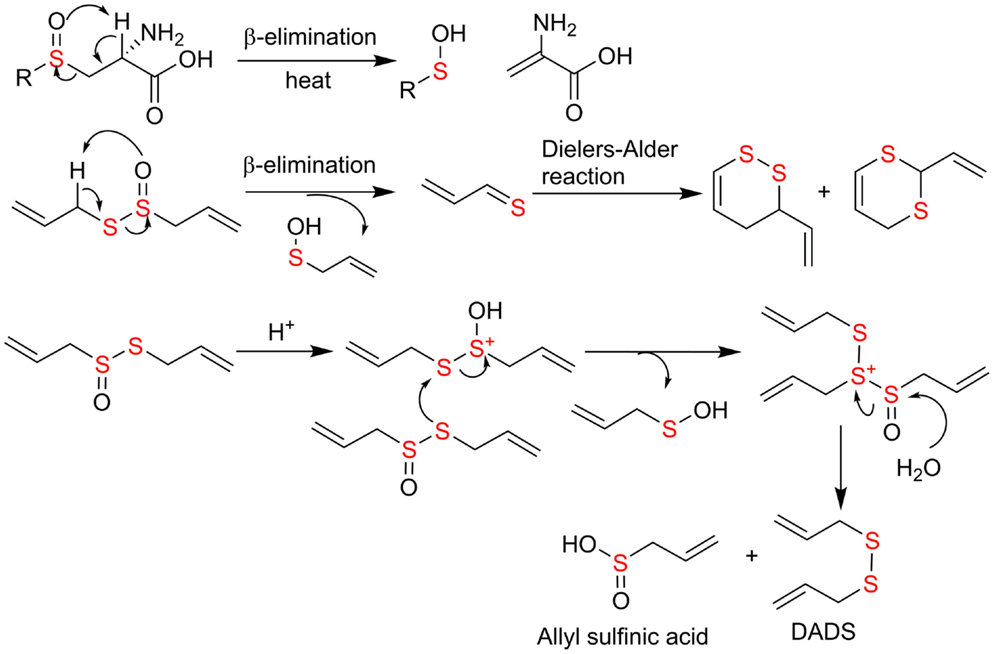
Figure 5. Proposed mechanism of chemical transformation from ACSO to organosulfides through elimination and acid-mediated nucleophilic substitution reactions.
In home cooking, cloves or whole onions are often cut into small pieces or crushed into pastes. Biotransformation of organosulfides in Alliums begins when cytoplasmic ACSOs are released and mixed with the vacuolar alliinase. When garlic cloves or onion are cooked as whole, deactivation of the alliinase would limit the transformation of ACSOs, whereas fresh macerates of garlic or onion would have high contents of thiosulfinates. Thiosulfinates were found to be present in Allium homogenate with stability lasting for 26 h under room temperature, except for considerable losses observed in those represented by MeCH = CHS(O)SR and MeS(O)SMe (60). Thiosulfinates can decompose to generate other form of organosulfides (i.e., linear polysulfides) (15). This decomposition is greatly affected by typical processing parameters such as temperature, pH, and storage time (77, 78). Commercial Allium products are available in the form of macerates (e.g., crushed garlic in oil) or homogenates, but the more commonly available ones are freeze-dried powder, essential oil, or cloves in fermented or acidified brine (11, 79). In garlic or onion essential oils, thermal decomposition of thiosulfinates generates various mono-, di- and trisulfides as a result of different distillation processes (11, 13). Indeed, GC-MS analysis of Allium essential oil has revealed predominance of mixed polysulfides (Table 1). Essential oils from Alliums are highly valued for their medical and biological functions because of their contents of organopolysulfides, including DADS, DATS, allyl methyl trisulfide, diallyl tetrasulfide in garlic distilled oil and dipropyl disulfide, dipropyl trisulfide, methyl propenyl disulfide, and methyl propyl trisulfide in distilled onion oil (11). An overview of the organosulfide transformations in Alliums as affected by various processing conditions is illustrated in Figure 6.
Aside from distilled oils, powdered form of garlic or onion is widely sold in the market. Freeze-drying is a major processing step employed in making this product. Since cloves or whole onions are typically used during freeze-drying, tissues remain intact; hence, hydrolysis of ACSOs by alliinase may not occur. Therefore, the powder basically has similar content of ACSO profile as the fresh ones. Freeze-drying of high-pressure processed (HPP) onion resulted in variable effects on the concentrations of major polysulfides (80). Significant decreases (P < 0.05) in dipropyl disulfide (59–82%), dipropyl trisulfide (62–85%), and trans-propenyl propyl disulfide (38–65%) were observed, retaining the levels of dimethyl trisulfide and methyl 1-propenyl disulfide. In our recently published work, we found that freeze-drying preserved most of the individual organosulfides in shallot, although total organosulfur compounds decreased due to reduction in the concentrations of only a few compounds (52). Moreover, freeze-drying showed retention of polysulfides comparable with other drying methods (air- and over-drying) (40). These observations indicate that freeze-drying is a good alternative method in producing dried Allium products with comparable polysulfide contents as those of the fresh.
Other drying methods have also been shown to have direct effects on the organosulfides of different Allium species. The effects of microwave drying and cabinet drying on the volatile components of hydrodistilled oil derived from garlic powder have been compared (81). DADS and diallyl tetrasulfide increased with both drying methods, however, DATS and allyl methyl trisulfide decreased, with microwave drying showing greater reduction. The observed reduction of polysulfides with microwave drying can be attributed to the effect of heat generated that might have driven off the volatiles and deactivated alliinase. It has been previously demonstrated that heating at 80°C for 10 min can partially deactivate alliinase (82).
The effect of long-term frozen storage (−20°C) of leek slices showed significant reduction of 14 sulfur compounds after 12 months (45). Some polysulfides including, propyl (E)-propenyl di- and trisulfides and dipropyl disulfide, increased after 2 weeks but compounds such as dimethyl disulfide and 2-propenyl disulfide were not detected after 4–6 months. The increase in concentration after 2 weeks of frozen storage implies that alliinase maintains its catalytic activity in sliced leeks even during 2 weeks of freezing.
Due to the sensitivity of alliinase to heat, boiled Alliums are expected to have no heat sensitive thiosulfinates, which have their purported bioactivity. For instance, the loss in the in vitro anti-aggregatory activity (IVAA) of garlic was suggested to be due to the sensitivity of thiosulfinates to heat after 10 min of boiling (100°C) (83). Similar results were observed in onion thiosulfinates. Boiling for 46 min completely suppressed IVAA, which the author correlates with the decrease in thiosulfinates (84). Minimal duration of boiling (<3 min) may preserve thiosulfinates as indicated by a maintained IVAA of both onion and garlic (83, 84). In another study, heat treatment by microwave (500 W) and convection oven (200°C) generally decreased thiosulfinate content as indicated by a reduction in IVAA (78). However, the extent to which these antiplatelet agents are destroyed varied depending on the method (whole, quarters, and crushed) of sample preparation with whole showing longer retention of IVAA (after 30 min) as compared to quarters (20 min) and crushed samples (10 min).
Among the Allium products in the market, there appears to be a fair acceptance of pickled blanched garlic, which comes as either fermented or unfermented. The main step in the process is blanching (90°C) of garlic cloves, which may deactivate alliinase and, therefore, have direct implication in the formation of other organosulfur compounds. The next step is brining whereby garlic cloves are immersed in an acidified brine containing lactic acid (85). Fermented pickled garlic involves subjecting the blanched garlic cloves to lactic acid bacteria fermentation, typically Lactobacillus plantarum or Lactobacillus pentosus (86). The final pH of the brine is around 3.9 with a titratable acidity of 1.2% (as lactic acid) (86). It was found that only SAC and GSAC slightly decreased after blanching (79). Therefore, blanching for 5 min is not that detrimental to the major OSC precursors in garlic such as alliin. However, after pickling and fermentation, the contents of alliin, isoalliin, and cycloalliin significantly decreased and became even much lower (23–28% loss) after 1 year of storage at refrigerated temperature (79). The blanching step is carried out mainly to deactivate alliinase. Consequently, the contents of oil-soluble polysulfides will be impacted by blanching. Pickling and fermenting garlic may eliminate the pungent flavor brought about by formation of thiosulfinates. Flavor improvement using these processing techniques could be useful as a marketing strategy. However, these processing methods may have critical implications on the formation of other bioactive organosulfur compounds in Alliums.
The sensitivity of organosulfides to processing methods used pose a challenge to processing Alliums so that the end products have optimal bioactive organosulfides. This would require research work that can delineate the structure and activity relationship through mechanistic studies.
Action Mechanisms of Polysulfides: As H2S Donors
Perhaps the most interesting and promising aspects of dietary organosulfur compounds are their potentials to be precursors of hydrogen sulfide (H2S), a colorless, flammable, and toxic gas with the characteristic foul odor of rotten eggs. For quite a long time, H2S attracted attention mainly because of its toxicity, however, in the past decades H2S has been found as a gaseous signaling molecule, which plays important roles in many physiological and pathological conditions (87). H2S is the third and newest member of gasotransmitter family, along with nitric oxide (NO) and carbon monoxide (CO) (88, 89).
Since the discovery that endogenous H2S selectively enhance N-methyl-d-aspartate (NMDA) receptor (a glutamate receptor controlling synaptic plasticity and memory function) mediated response in 1996 (90), tremendous progress has been made in the understanding of H2S physiology. H2S is associated with a wide yet still expanding range of physiological events – their cardiovascular benefits attracting the most attention (87, 91). The first paper concerning the cardiovascular effect of H2S was published in 1997 (92), in which H2S was found to induce smooth muscle relaxation in vitro. Four years later it was reported that the H2S was a KATP channel activator, which was responsible for the vasorelaxation effect in vivo (93). Since then, the beneficial effects of H2S have been shown in many other studies (94). Hydrogen sulfide dilates blood vessel (95), protects against ischemia-reperfusion injury in myocardium (96), protects against heart failure by reducing oxidative stress, increasing myocardial vascular density, and preserving mitochondrial function (97). Furthermore, H2S prevents atherosclerosis by reducing smooth muscle cell proliferation and inhibiting adhesion molecule expression (98).
Hydrogen sulfide is produced endogenously in mammalian tissues mainly by enzymatic metabolism of l-cysteine. Currently, four enzymes, including cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), 3-mercaptopyruvate sulfur transferase (3-MST), and cysteine aminotransferase (CAT) have been found to be involved in its biological production (87, 99, 100). CSE and CBS are believed to be the major enzymes responsible for endogenous H2S synthesis. CSE has been reported in various organs including kidney, liver, uterus, and placenta but it is predominantly found in the vasculature and liver; while CBS is expressed in the liver and central nervous system.
Some non-enzymatic pathways that lead to H2S generation also exist, for example, the reaction between naturally occurring polysulfides and biological thiols, mainly through thiol-disulfide exchange reactions. The generation of H2S from the reaction between glutathione and calicheamicin γ1 (a natural antitumor agent with an allyl trisulfide group) was reported in 1994, when Myers et al. were trying to elucidate how calicheamicin γ1 initiated the DNA cleavage process (101). H2S was also generated upon the reaction between glutathione and 7-methylbenzopentathiepin, an analog of naturally occurring antibiotics varacin (102), however the H2S generation in these studies attracted little attention.
In 2007, Benavides et al. reported that DATS and DADS can be converted to H2S by human red blood cells or rat aorta ring, and the H2S produced exerts a vasorelaxant effect on rat aorta (103). Hence, they suggested that H2S mediates the vasoactivity of garlic. They also demonstrated that H2S production was through the reaction between DADS/DATS and glutathione. Since the conventional thiol-disulfide exchange between DADS and GSH does not produce H2S, they proposed that DADS undergo nucleophilic substitution at α carbon, producing the key intermediate allyl perthiol, which subsequently reacts with GSH to release H2S (103). They also found that the H2S releasing activity of organosulfurs from garlic is higher for those with allyl substituents and increased with increasing number of tethering sulfur atoms (103). Another study found that DATS treatment can significantly increase the H2S level, reduce the infarct size, and preserve cardiac function in mice after myocardial ischemia-reperfusion. This study substantiated the notion that DATS may be cardioprotective via H2S-related pathway (104).
Besides these polysulfide H2S donors, another group of organosulfur compounds that exert cardioprotective effects through H2S mediated pathways are cysteine derivatives, including SAC and its synthetic analog S-propargyl cysteine (R)-2-amino-3-(2-propynylthio) propanoic acid, SPRC. Instead of releasing H2S by themselves or by reacting with other compounds, they are believed to function by mediating endogenous H2S production. Increased CSE gene expression, elevated plasma H2S level and decreased mortality, infarct size, and ventricular hypertrophy were observed in acute myocardial infarction mice treated with SAC (105) or SPPC (106). The abolishment of these beneficial effects by a CSE inhibitor propargylglycine substantiated that their cardioprotective functions are H2S dependent.
Although many organosulfur compounds have been found from dietary source, only a few of them have been studied for H2S releasing activity. From a chemical point of view, polysulfides with more than two tethering sulfur atoms should be able to release H2S through thiol-disulfide exchange with GSH. Research on the H2S releasing activity by these compounds might provide new explanations for their purported cardiovascular benefits, and, therefore, warrants in-depth investigation.
Organopolysulfides as Reactive Oxygen Species Scavengers
The implication of reactive oxygen species (ROS) in the development of CVDs has been shown in numerous studies in the past decades. The most important ROS in cardiovascular system are superoxide anion radical () is produced in vascular cells by a wide variety of oxidases, including the predominant NADPH oxidases, as well as lipoxygenases, xanthine oxidase, cytochrome P450, uncoupled mitochondrial electron transfer chain, and uncoupled endothelial nitric oxide synthase (eNOS) (107). undergoes dismutation by superoxide dismutase (SOD) to generate H2O2. Superoxide anion rapidly would react with NO to generate peroxynitrite (ONOO−). This reaction consumes NO and reduces it activity in maintaining healthy cardiovascular functions. On the contrary, the resulting product, ONOO−, is an important lipid oxidation mediator that will lead to the oxidation of low-density lipoprotein (LDL), forming strong proatherogenic oxidized LDL (ox-LDL) (108). Besides, ROS contributes to cardiovascular injury through the modulation of multiple cellular responses, including monocyte adhesion, platelet aggregation, vascular smooth muscle cell apoptosis, migration and proliferation, inflammatory gene expression, and dysfunction of endothelium dependent relaxation (109).
Sulfur atoms in dietary organosulfides are electron rich and ready to donate electrons in a redox reaction; therefore, they are supposed to be good oxidant scavengers. AGE and garlic oil containing high amounts of organosulfurs have been shown to scavenge ROS and prevent damage caused by oxidative stress (110–113). However, very limited reports studied the scavenging activities of individual organosulfur compounds to certain types of free radicals. Those reported works are inconsistent and incomparable likely because different evaluating methods and sample concentrations were employed. In addition, nearly all of the studies were in vitro tests carried out in simple chemical systems because of the lack of analytical methods that can selectively target a specific free radical in vivo. But the general trend is, SAC, the water soluble, major organosulfur compound in AGE, possess strong scavenging activities against peroxynitrite (114, 115) and hydroxyl radical (114–116), but has negligible effects toward or H2O2 (114, 116). However, the major lipophilic polysulfides in garlic oil DADS and DATS can inhibit as much as SOD/ascorbic acid can (114) and their activity increases with the number of sulfur atoms; besides, they are good ONOO− scavengers but have very limited effects on hydroxyl radical.or H2O2 (114). The potent hypochlorite acid scavenging effect of SAC, DADS, and DATS as well as some other thioallyl compounds such as allyl mercaptan, allyl methyl sulfide, dipropyl sulfide, and allicin has been reported in by several studies (115, 117, 118). Allicin is a good scavenger against , OH⋅, and ONOO− (116, 117), but their activity seems to be attributed to the sulfenic acid formed from the copper elimination of allicin (119).
The cardioprotective effects of organosulfur compounds against oxidative damage in cell lines or in animals have been shown in several studies. SAC, which was able to inhibit LDL oxidation, was also reported to dose-dependently inhibit the H2O2 formation in ox-LDL challenged human umbilical vein endothelial cells (HUVEC) (120). Similar protective effect was also found in bovine pulmonary artery endothelial cells (121). DATS and DADS are reported to decrease the cellular peroxide level in ox-LDL-treated HUVEC cells by as much as 50 and 43%, respectively (122). DATS was shown to decrease the ROS and levels in H9c2 cells induced by high-glucose treatment, and protect cardiac myocytes from apoptotic cells death in culture medium as well as in diabetic rats (123) through ROS related pathways. Similar effects were also found in rats treated with DADS and garlic oil (124). However, it needs to be pointed out that these protective effects against ROS damage observed in cell lines or in experimental animals might come from the combination of a wide range of physiological pathways such as enhancement of antioxidant enzyme expression or inhibition of peroxidation enzymes production activity, instead of direct free radical scavenging.
Enzyme Activation/Inactivation and Gene Regulation
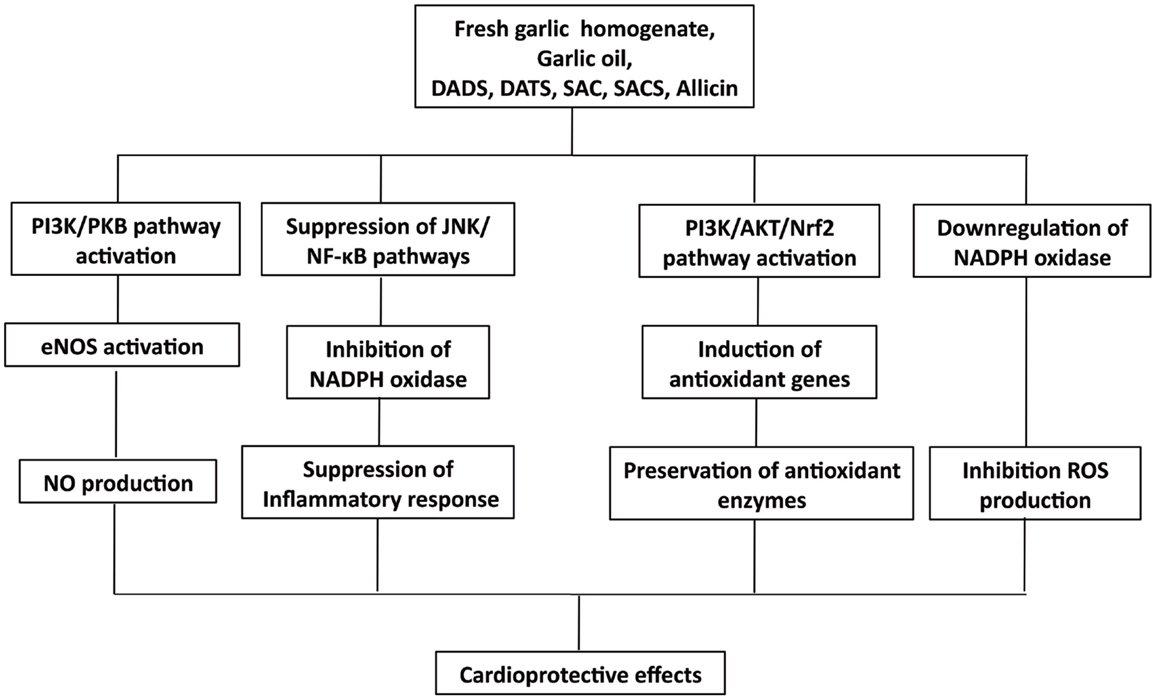
Recently, a number of studies have suggested that the beneficial effects of organosulfides in cardiovascular health are associated with their ability to modulate antioxidant genes and enzyme expression (Table 3). In this section, we discuss these mechanisms and present an overview of the important biochemical pathways associated in the cardioprotective effects of organosulfides (Figure 7).
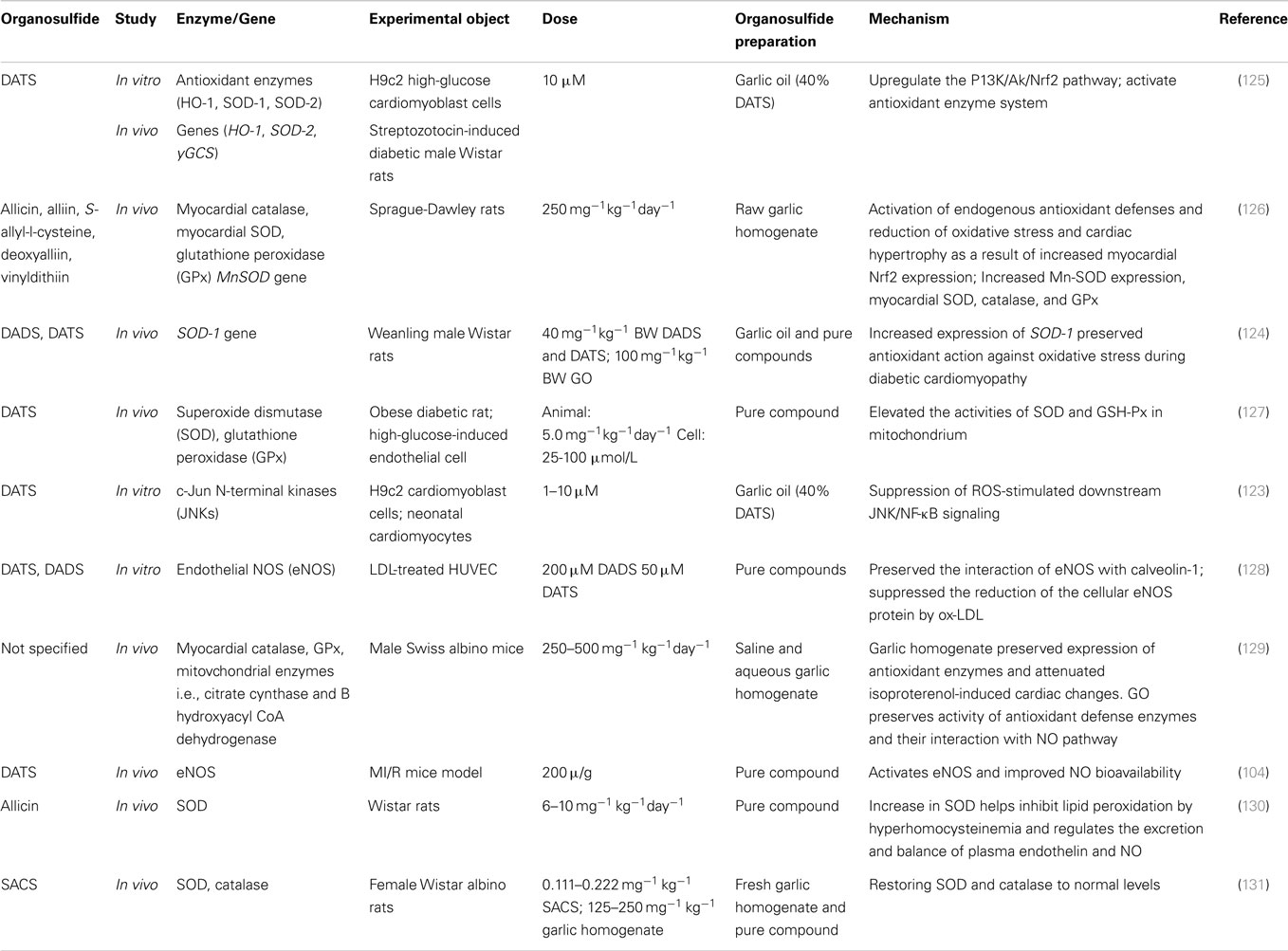
Table 3. In vivo and in vitro studies showing the effect of organosulfides on enzyme and gene regulation and the underlying mechanisms.
Nrf2 Activation and Antioxidant Gene Modulation
Oxidative stress, viewed at the molecular level, is linked to the activities of nuclear factor-E-2-related factor (Nrf2) in the nucleus, which upregulates genes that encode for the expression of antioxidant enzymes (132). It has been demonstrated that Nrf2 coordinates the expression and upregulation of various antioxidant enzymes, including heme oxygenase-1 (HO-1), glutathione S-transferase (GST), and SOD (133, 134). These enzymes collectively protect cardiomyocytes from oxidative stress (135, 136). For example, SOD-1, one of the three SOD, works together with catalase to detoxify superoxides and hydrogen peroxide (H2O2) (137). Induced upregulation or downregulation of SOD-1 genes and expression of the antioxidant enzyme SOD-1 are often evaluated as an indicator of ROS stress.
The induced Nrf2-mediated antioxidant gene activation by DATS is linked to several pathways, including MAPK, PKC, and PI3K/AKT (138). Tsai et al. showed that the cytoprotective effect of DATS against oxidative stress in high-glucose exposed neonatal cardiomyocytes and streptozotocin-induced diabetic rats is by activation of the P13K/Akt/Nrf2 pathway (125). Expression of Nrf2 proteins was upregulated by DATS in a time-dependent manner resulting in a dose-dependent improvement of the expression of antioxidant genes, including HO-1 and SOD2. Moreover, PI3K-specific SiRNA and Nrf2-specific SiRNA transfected cells showed normal levels of superoxide than those administered with DATS alone, indicating that the antioxidative effect of DATS is suppressed by Nrf2 and PI3K SiRNA, further suggesting that the cytoprotective effect of DATS against high-glucose-induced oxidative stress is by the activation of P13K/Akt/Nrf2 pathway (125). The ability of DATS to induce Nrf2 activation was further demonstrated in a study that specifically looked at Keap1 cysteine 288 residue, one of the cysteine residues that is essential in regulating Nrf2 activation (139). It was found that mono-allyl mono-sulfide may attach to the Keap1 peptide fragment containing Cys288, indicating that DATS specifically interacts with this cysteine residue accounting for its ability to induce Nrf2 activation (139). Recently, raw garlic homogenate administration to fructose fed-diabetic rats showed increased myocardial Nrf2 expression, along with increased Mn-superoxide dismutase (Mn-SOD) gene expression and, elevated myocardial SOD, catalase, and glutathione peroxidase (GPx) activities (126). In addition to enhanced antioxidant gene and enzyme expressions, this study also showed that garlic homogenate can increase the levels of phospho-PI3K and phospho-AKT, indicating that PI3K/AKT pathway plays a major role in cardiac hypertrophy and oxidative stress by activating Nrf2, which, in turn regulates the activation of antioxidant defense enzymes during cardiovascular complications (126). In another study, administration of garlic oil and pure compounds of DADS and DATS increased level of SOD-1 expression and enhanced the PI3K/AKT signaling in diabetic rats (124).
Modulation of Endothelial Nitric Oxide Synthase
Oxidized LDL is an important factor in the pathogenesis of atherosclerosis (108). Ox-LDL promotes vascular dysfunction in various mechanisms, one of which is its inhibition of eNOS activity resulting in the alteration of the NO-regulated responses in the endothelial cells (140, 141). Organosulfur compounds from garlic are involved in the modulation of eNOS activity and are responsible for garlic’s anti-atherogenic effect. Lei et al. studied garlic’s role against ox-LDL in HUVEC and found that the protective role of DADS and DATS in eNOS activation and NO production is associated with the PI3K/PKB pathway (128). Ox-LDL was found to decrease PKB and eNOS phosphorylation but this effect was abolished by DADS and DATS pretreatment along with restored production of NO. Moreover, treatment with PI3K inhibitor wortmanin attenuated the protective effect of DADS and DATS in eNOS activation and recovery of NO production (128). Serine phosphorylation and blocking of eNOS activation may result from the inhibition of PI3K/PKB pathway or from PKB site mutation on the eNOS protein (at serine 1177) signifying the importance of PI3K/PKB pathway in regulating eNOS activity (142). In another study, garlic homogenate (250 mg kg−1 day−1 for 30 days) treatment on isoproterenol-induced myocardial infarction mice model relieved oxidative stress by significantly increasing release of GPx and catalase activities (129). This study also demonstrated the positive association of NOS activation, NO production, and antioxidant enzyme activity protection with garlic treatment suggesting that the maintenance of redox balance through protecting the activation of NOS and, hence, production of NO, explain garlic’s protective role against myocardial damage. Moreover, eNOS activation was demonstrated by Predmore et al., showing that DATS treated rat myocardial tissue had an increased eNOS phosphorylation at Ser1177 and elevated levels of NO metabolites, including nitrite and nitrate (104).
Modulation of Antioxidant Enzymes and Inhibition of NADPH Oxidase Activity
The protective effect of DATS against hyperglycemia-induced oxidative stress was demonstrated in an in vivo model of obese diabetic rats and in high-glucose-treated endothelial cells (127). The reduction of mitochondrial oxidative stress was suggested to be the action mechanism because attenuation of endothelial cell impairment was observed along with enhanced activities of SOD and GPx in the mitochondria upon administration of DATS. SOD and GPx are antioxidative enzymes with detoxifying actions against mitochondrial ROS (143). DATS attenuated the hyperglycemia-induced NADPH oxidase and its related ROS production in the mitochondria mainly by preserving but not upregulating the activities of SOD and GPx (127). In another study, the effect of low (6 mg kg−1 day−1) and high (10 mg kg−1 day−1) allicin doses was demonstrated in hyperhomocysteinemia-induced vascular endothelial dysfunction animal model (130). Hyperhomocysteinemia is another cause of oxidative stress that may lead to vascular endothelial dysfunction and injury (144, 145). Formation of H2O2 may result from the self-oxidation of homocysteine at the active free sulfhydryl group. Homocysteine level in rats with hyperhomocysteinemia was reduced by treatment with allicin along with an increase in SOD activity (130). Similar results were demonstrated in another study employing fructose-induced hypertensive rat models (131). SOD and catalase were restored to normal levels after treatment with S-allyl cysteine sulfoxide (SACS) isolated from fresh garlic homogenate.
In a recent paper, expression of p22phox and gp91phox, the subunits comprising the membrane-bound component cytochrome b558, and the subunits responsible for the activity of NADPH oxidase increased along with the production of superoxide free radicals () in high-glucose-treated H9c2 cells but was attenuated by treatment with DATS (1–10 μM). Production of ROS by NADPH oxidase is related to the activation of c-Jun N-terminal kinases (JNK) signaling and the transcriptional factor NF-κB (146). Activation of JNK leads to their translocation into the nucleus where they phosphorylate transcription factors including c-Jun and p53 that are involved in the regulation of apoptosis (147, 148). Treatment with DATS (1–10 μM) dose-dependently inhibited the high-glucose activation of JNK, which was suggested to be associated with the inhibition of NADPH oxidase-regulated ROS generation in H9c2 cells and neonatal primary cardiomyocyte (123). Treatment with DATS (1–10 μM) inhibited the nuclear translocation of NF-κB in H9c2 cells and reduced the protein levels of NF-κB in streptozotocin-induced diabetic rats (40 mg/kg BW DATS) (123). Hyperglycemia is not only known to induce ROS generation but also inflammation, which could activate transcription regulators, including NF-κB, which, in turn, regulates intracellular apoptosis (149, 150). Treatment with DATS protects high-glucose-treated neonatal cardiomyocytes and H9c2 cells from ROS damage by inhibiting the activation of JNK/NF-κB pathways (123).
Conclusion
There are significant scientific research papers suggesting that dietary organosulfurs have broad range of bioactivity including cancer chemoprevention and promotion of cardiovascular health. While there is strong evidence to suggest that isothiocyanates may be the active form in cruciferous vegetables for their cancer chemopreventive property, the daily intake of such compounds could be very low because cooking of these vegetables will prevent their formation. For other organosulfides, it remains a challenge to pinpoint the compounds responsible for their purported health promoting effects. Allicin was considered as the active components of garlic and has been used as a marker compound in garlic supplement standardization. The null results from clinical trials make us reconsider other potential compounds. The discovery of DATS and DADS as donors of hydrogen sulfide opens up a new avenue for establishing evidence of organosulfide action mechanisms on promoting cardiovascular health. It remains to be seen whether H2S donating activity can be the unifying mechanism for dietary organopolysulfides to exert their health benefits. H2S is a “double-edged sword” as it is toxic at high concentrations. Rapid burst of H2S from organosulfides may lead toxic effects in vivo but it may be needed for their anti-microbial activity. For cardiovascular health, slow (or controlled) release of H2S from dietary polysulfides would be desired. There are many other organosulfides in our diet that have not been investigated yet. The outstanding ones include cyclic polysulfides founds in stinky beans and mushrooms. While these polysulfides are important flavoring molecules (sometime smelly!), little is known on their health promoting activity. The high sulfur loading of them (i.e., compounds shown in Figure 3) would make them ideal reservoirs of H2S, if they can be biotransformed to release H2S in human body. Research work in the future shall be focused on establishing the structure and H2S releasing potentials and rates of individual dietary polysulfides in cell line and animal model systems. Since processing conditions can greatly alter the polysulfide profiles in foods, an H2S releasing activity guided optimization of processing conditions would lead to optimal effectiveness of supplements or functional foods based on Alliums and stinky beans for their cardioprotective effects (Figure 8). The research is just at the beginning on taming the pesky dietary organosulfides for human health promoting and disease prevention.
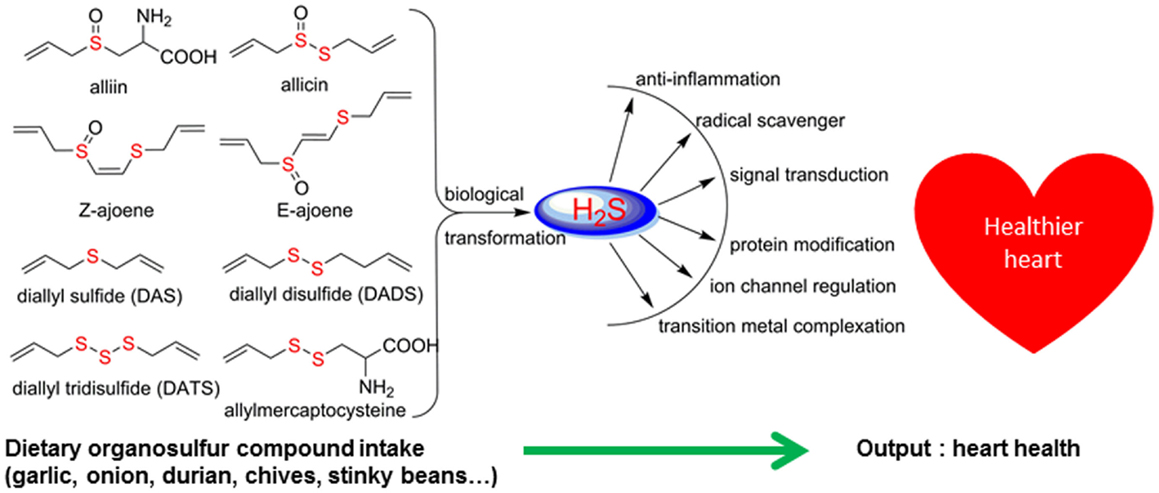
Figure 8. Hydrogen sulfide as the common denominator for bioactivity of dietary organosulfur compounds.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Agency of Science, Technology and Research (A*Star) of Singapore for financial support (Grant Number: 112 177 0036) and the support of a Jiangsu Province Grant to NUSRI for Food Science and Technology (platform2).
References
1. WHO. Cardiovascular Disease. World Health Organization (2014). Available from: <http://www.who.int/cardiovascular_diseases/en/>
2. Davison K, Coates AM, Buckley JD, Howe PRC. Effect of cocoa flavanols and exercise on cardiometabolic risk factors in overweight and obese subjects. Int J Obes (2008) 32:1289–96. doi: 10.1038/ijo.2008.66
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
3. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on the modification of the authorisation of a health claim related to cocoa flavanols and maintenance of normal endothelium-dependent vasodilation pursuant to Article 13(5) of Regulation (EC) No 1924/2006 following a request in accordance with Article 19 of Regulation (EC) No 1924/2006. EFSA J (2014) 12:3654. doi:10.2903/j.efsa.2014.3654
4. Tsai CW, Chen HW, Sheen LY, Lii CK. Garlic: health benefits and actions. BioMedicine (2012) 2:17–29. doi:10.1016/j.biomed.2011.12.002
5. Moriarty RM, Naithani R, Surve B. Organosulfur compounds in cancer chemoprevention. Mini Rev Med Chem (2007) 7:827–38. doi:10.2174/138955707781387939
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
6. Alvarez-Jubete L, Smyth TJ, Valverde J, Rai DK, Barry-Ryan C. Simultaneous determination of sulphoraphane and sulphoraphane nitrile in Brassica vegetables using ultra-performance liquid chromatography with tandem mass spectrometry. Phytochem Anal (2014) 25:141–6. doi:10.1002/pca.2480
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
7. Kassie F, Rabot S, Uhl M, Huber W, Qin HM, Helma C, et al. Chemoprotective effects of garden cress (Lepidium sativum) and its constituents towards 2-amino-3-methyl-imidazo[4,5-f]quinoline (IQ)-induced genotoxic effects and colonic preneoplastic lesions. Carcinogenesis (2002) 23:1155–61. doi:10.1093/carcin/23.7.1155
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
8. Rose P, Yen KW, Choon NO, Whiteman M. β-Phenylethyl and 8-methylsulphinyloctyl isothiocyanates, constituents of watercress, suppress LPS induced production of nitric oxide and prostaglandin E2 in RAW 264.7 macrophages. Nitric Oxide (2005) 12:237–43. doi:10.1016/j.niox.2005.03.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
9. Houghton CA, Fassett RG, Coombes JS. Sulforaphane: translational research from laboratory bench to clinic. Nutr Rev (2013) 71:709–26. doi:10.1111/nure.12060
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
10. Cui J, Li S. Inhibitors and prodrugs targeting CYP1: a novel approach in cancer prevention and therapy. Curr Med Chem (2014) 21:519–52. doi:10.2174/09298673113206660277
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
11. Corzo-Martínez M, Corzo N, Villamiel M. Biological properties of onions and garlic. Trends Food Sci Technol (2007) 18:609–25. doi:10.1016/j.tifs.2007.07.011
12. Gardner CD, Lawson LD, Block E, Chatterjee LM, Kiazand A, Balise RR, et al. Effect of raw garlic vs commercial garlic supplements on plasma lipid concentrations in adults with moderate hypercholesterolemia: a randomized clinical trial. Arch Intern Med (2007) 167:346–53. doi:10.1001/archinte.167.4.346
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
13. Rose P, Whiteman M, Moore PK, Yi ZZ. Bioactive S-alk(en)yl cysteine sulfoxide metabolites in the genus Allium: the chemistry of potential therapeutic agents. Nat Prod Rep (2005) 22:351–68. doi:10.1039/b417639c
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
14. Benkeblia N, Lanzotti V. Allium thiosulfinates: chemistry, biological properties and their potential utilization in food preservation. Food (2007) 1:193–201.
15. Block E. The organosulfur chemistry of the genus Allium – Implications for the organic chemistry of sulfur. Angew Chem Int Ed Engl (1992) 31:1135–78. doi:10.1002/anie.199211351
16. Wafler U, Shaw ML, Lancaster JE. Effect of freezing upon alliinase activity in onion extracts and pure enzyme preparations. J Sci Food Agric (1994) 64:315–8. doi:10.1002/jsfa.2740640311
17. Manchali S, Chidambara Murthy KN, Patil BS. Crucial facts about health benefits of popular cruciferous vegetables. J Funct Foods (2012) 4:94–106. doi:10.1016/j.jff.2011.08.004
18. Kubec R, Drhová V, Velíšek J. Thermal degradation of S-methylcysteine and its sulfoxide – important flavor precursors of Brassica and Allium vegetables. J Agric Food Chem (1998) 46:4334–40. doi:10.1021/jf980379x
19. Kubec R, Svobodová M, Velíšek J. Gas-chromatographic determination of S-methylcysteine sulfoxide in cruciferous vegetables. Eur Food Res Technol (2001) 213:386–8. doi:10.1007/s002170100384
20. Hamamoto A, Mazelis M. The C-S lyases of higher plants: isolation and properties of homogeneous cystine lyase from broccoli (Brassica oleracea var botrytis) buds. Plant Physiol (1986) 80:702–6. doi:10.1104/pp.80.3.702
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
21. Marks HS, Hilson JA, Leichtweis HC, Stoewsand GS. S-methylcysteine sulfoxide in Brassica vegetables and formation of methyl methanethiosulfinate from brussels sprouts. J Agric Food Chem (1992) 40:2098–101. doi:10.1021/jf00023a012
22. Kyung KH, Lee YC. Antimicrobial activities of sulfur compounds derived from S-Alk(en)yl-l-cysteine sulfoxides in Allium and Brassica. Food Rev Int (2001) 17:183–98. doi:10.1081/FRI-100000268
23. Edmands WMB, Gooderham NJ, Holmes E, Mitchell SC. S-methyl-l-cysteine sulphoxide: the Cinderella phytochemical? Toxicol Res (2013) 2:11–22. doi:10.1039/c2tx20030a
25. Griffiths DW, Macfarlanesmith WH, Boag B. The effect of cultivar, sample date and grazing on the concentration of S-methylcysteine sulfoxide in oilseed and forage. J Sci Food Agric (1994) 64:283–8. doi:10.1002/jsfa.2740640307
26. Kopsell DE, Randle WM, Eiteman MA. Changes in the S-alk(en)yl cysteine sulfoxides and their biosynthetic intermediates during onion storage. J Am Soc Hortic Sci (1999) 124:177–83.
27. Montaño A, Beato VM, Mansilla F, Orgaz F. Effect of genetic characteristics and environmental factors on organosulfur compounds in garlic (Allium sativum L.) grown in Andalusia, Spain. J Agri Food Chem (2011) 59:1301–7. doi:10.1021/jf104494j
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
28. Fujiwara M, Itokawa Y, Uchino H, Inoue K. Anti-hypercholesterolemic effect of a sulfur containing amino acid, S-methyl-l-cysteine sulfoxide, isolated from cabbage. Experientia (1972) 28:254–5. doi:10.1007/BF01928671
29. Komatsu W, Miura Y, Yagasaki K. Suppression of hypercholesterolemia in hepatoma-bearing rats by cabbage extract and its component, S-methyl-l-cysteine sulfoxide. Lipids (1998) 33:499–503.
30. Delaha EC, Garagusi VF. Inhibition of mycobacteria by garlic extract (Allium sativum). Antimicrob Agents Chemother (1985) 27:485–6. doi:10.1128/AAC.27.4.485
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
31. Arora DS, Kaur J. Antimicrobial activity of spices. Int J Antimicrob Agents (1999) 12:257–62. doi:10.1016/S0924-8579(99)00074-6
32. Briggs WH, Xiao H, Parkin KL, Shen C, Goldman IL. Differential inhibition of human platelet aggregation by selected Allium thiosulfinates. J Agric Food Chem (2000) 48:5731–5. doi:10.1021/jf0004412
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
33. Iciek M, Kwiecien I, Włodek L. Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen (2009) 50:247–65. doi:10.1002/em.20474
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
34. Lawson LD, Hughes BG. Characterization of the formation of allicin and other thiosulfinates from garlic. Planta Med (1992) 58:345–50. doi:10.1055/s-2006-961482
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
35. Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr (2001) 131:955S–62S.
36. Calvo-Gómez O, Morales-López J, López MG. Solid-phase microextraction-gas chromatographic-mass spectrometric analysis of garlic oil obtained by hydrodistillation. J Chromatogr A (2004) 1036:91–3. doi:10.1016/j.chroma.2004.02.072
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
37. Kimbaris AC, Siatis NG, Daferera DJ, Tarantilis PA, Pappas CS, Polissiou MG. Comparison of distillation and ultrasound-assisted extraction methods for the isolation of sensitive aroma compounds from garlic (Allium sativum). Ultrason Sonochem (2006) 13:54–60. doi:10.1016/j.ultsonch.2004.12.003
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
38. Sowbhagya HB, Purnima KT, Florence SP, Appu Rao AG, Srinivas P. Evaluation of enzyme-assisted extraction on quality of garlic volatile oil. Food Chem (2009) 113:1234–8. doi:10.1016/j.foodchem.2008.08.011
39. Li R, Chen WC, Wang WP, Tian WY, Zhang XG. Extraction of essential oils from garlic (Allium sativum) using ligarine as solvent and its immunity activity in gastric cancer rat. Med Chem Res (2010) 19:1092–105. doi:10.1007/s00044-009-9255-z
40. Dziri S, Casabianca H, Hanchi B, Hosni K. Composition of garlic essential oil (Allium sativum L.) as influenced by drying method. J Essent Oil Res (2014) 26:91–6. doi:10.1080/10412905.2013.868329
41. Lawson LD, Wang ZYJ, Hughes BG. Identification and HPLC quantitation of the sulfides and dialk(en)yl thiosulfinates in commercial garlic products. Planta Med (1991) 57:363–70. doi:10.1055/s-2006-960119
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
42. Wu JL, Chou CC, Chen MH, Wu CM. Volatile flavor compounds from shallots. J Food Sci (1982) 47:606–8. doi:10.1111/j.1365-2621.1982.tb10133.x
43. Hanum T, Sinha NK, Guyer DE, Cash JN. Pyruvate and flavor development in macerated onions (Allium cepa L.) by γ-glutamyl transpeptidase and exogenous C-S lyase. Food Chem (1995) 54:183–8. doi:10.1016/0308-8146(95)00027-G
44. Pino JA, Fuentes V, Correa MT. Volatile constituents of Chinese chive (Allium tuberosum Rottl. ex Sprengel) and rakkyo (Allium chinense G. Don). J Agri Food Chem (2001) 49:1328–30. doi:10.1021/jf9907034
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
45. Studsgaard Nielsen G, Larsen LM, Poll L. Formation of aroma compounds and lipoxygenase (EC 1.13.11.12) activity in unblanched leek (Allium ampeloprasum Var. Bulga) slices during long-term frozen storage. J Agric Food Chem (2003) 51:1970–6. doi:10.1021/jf020921o
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
46. Yabuki Y, Mukaida Y, Saito Y, Oshima K, Takahashi T, Muroi E, et al. Characterisation of volatile sulphur-containing compounds generated in crushed leaves of Chinese chive (Allium tuberosum Rottler). Food Chem (2010) 120:343–8. doi:10.1016/j.foodchem.2009.11.028
47. Kim NY, Park MH, Jang EY, Lee J. Volatile distribution in garlic (Allium sativum L.) by solid phase microextraction (SPME) with different processing conditions. Food Sci Biotechnol (2011) 20:775–82. doi:10.1007/s10068-011-0108-4
48. Casella S, Leonardi M, Melai B, Fratini F, Pistelli L. The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytother Res (2013) 27:380–3. doi:10.1002/ptr.4725
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
49. Kallio H, Salorinne L. Comparison of onion varieties by headspace gas chromatography-mass spectrometry. J Agric Food Chem (1990) 38:1560–4. doi:10.1021/jf00097a029
50. Kuo MC, Ho CT. Volatile constituents of the distilled oils of welsh onions (Allium fistulosum L. variety Maichuon) and scallions (Allium fistulosum L. variety Caespitosum). J Agric Food Chem (1992) 40:111–7. doi:10.1021/jf00022a036
51. Mochizuki E, Yamamoto T, Komiyama Y, Nakazawa H. Identification of Allium products using flame photometric detection gas chromatography and distribution patterns of volatile sulfur compounds. J Agric Food Chem (1998) 46:5170–6. doi:10.1021/jf9803076
52. Tocmo R, Lin Y, Huang D. Effect of processing conditions on the organosulfides of shallot (Allium cepa L. Aggregatum Group). J Agric Food Chem (2014) 62:5296–304. doi:10.1021/jf500739n
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
53. Yu TH, Wu CM, Chen SY. Effects of pH adjustment and heat treatment on the stability and the formation of volatile compounds of garlic. J Agric Food Chem (1989) 37:730–4. doi:10.1021/jf00087a033
54. Wong KC, Tie DY. Volatile constituents of durian (Durio zibethinus Murr.). Flavour Fragr J (1995) 10:79–83. doi:10.1002/ffj.2730100205
55. Weenen H, Koolhaas WE, Apriyantono A. Sulfur-containing volatiles of durian fruits (Durio zibethinus Murr.). J Agric Food Chem (1996) 44:3291–3. doi:10.1021/jf960191i
56. Jiang J, Choo SY, Omar N, Ahamad N. GC-MS analysis of volatile compounds in durian (Durio zibethinus Murr.). Dev Food Sci (1998) 40:345–52. doi:10.1016/S0167-4501(98)80058-7
57. Voon YY, Abdul Hamid NS, Rusul G, Osman A, Quek SY. Characterisation of Malaysian durian (Durio zibethinus Murr.) cultivars: relationship of physicochemical and flavour properties with sensory properties. Food Chem (2007) 103:1217–27. doi:10.1016/j.foodchem.2006.10.038
58. Neti Y, Erlinda ID, Virgilio VG. The effect of spontaneous fermentation on the volatile flavor constituents of durian. Int Food Res J (2011) 18:635–41.
59. Lee PR, Toh M, Yu B, Curran P, Liu SQ. Manipulation of volatile compound transformation in durian wine by nitrogen supplementation. Int J Food Sci Technol (2013) 48:650–62. doi:10.1111/ijfs.12012
60. Block E, Naganathan S, Putman D, Zhao S-H. Allium chemistry: HPLC analysis of thiosulfinates from onion, garlic, wild garlic (ramsoms), leek, scallion, shallot, elephant (great-headed) garlic, chive, and Chinese chive. Uniquely high allyl to methyl ratios in some garlic samples. J Agric Food Chem (1992) 40:2418–30. doi:10.1021/jf00024a017
61. Sinha NK, Guyer DE, Gage DE, Lira CT. Supercritical carbon dioxide extraction of onion. Flavors and their analysis by gas chromatography-mass spectrometry. J Agric Food Chem (1992) 40(5):842–5. doi:10.1021/jf00017a027
62. Kuo MC, Ho CT. Volatile constituents of the solvent extracts of welsh onions (Allium fistulosum L. variety Maichuon) and scallions (Allium fistulosum L. variety Caespitosum). J Agric Food Chem (1992) 40:1906–10. doi:10.1021/jf00022a036
63. Frérot E, Velluz A, Bagnoud A, Delort E. Analysis of the volatile constituents of cooked petai beans (Parkia speciosa) using high-resolution GC/ToF-MS. Flavour Fragr J (2008) 23:434–40. doi:10.1002/ffj.1902
64. Miyazawa M, Osman F. Headspace constituents of Parkia speciosa seeds. Nat Prod Lett (2001) 15:171–6. doi:10.1080/10575630108041277
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
65. Gmelin R, Susilo R, Fenwick GR. Cyclic polysulphides from Parkia speciosa. Phytochemistry (1981) 20:2521–3. doi:10.1016/0031-9422(81)83085-3
66. Sakunpak A, Panichayupakaranant P. Antibacterial activity of Thai edible plants against gastrointestinal pathogenic bacteria and isolation of a new broad spectrum antibacterial polyisoprenylated benzophenone, chamuangone. Food Chem (2012) 130:826–31. doi:10.1016/j.foodchem.2011.07.088
67. Gan CY, Latiff AA. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem (2011) 124:1277–83. doi:10.1016/j.foodchem.2010.07.074
68. Aisha AFA, Abu-Salah KM, Alrokayan SA, Ismail Z, Abdul Majid AMS. Evaluation of antiangiogenic and antoxidant properties of Parkia speciosa Hassk extracts. Pak J Pharm Sci (2012) 25:7–14.
69. Siow HL, Gan CY. Extraction of antioxidative and antihypertensive bioactive peptides from Parkia speciosa seeds. Food Chem (2013) 141:3435–42. doi:10.1016/j.foodchem.2013.06.030
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
70. Jamaluddin F, Mohamed S, Lajis MN. Hypoglycaemic effect of Parkia speciosa seeds due to the synergistic action of β-sitosterol and stigmasterol. Food Chem (1994) 49:339–45. doi:10.1016/0308-8146(94)90002-7
71. Al Batran R, Al-Bayaty F, Jamil Al-Obaidi MM, Abdualkader AM, Hadi HA, Ali HM, et al. In vivo antioxidant and antiulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS One (2013) 8:e64751. doi:10.1371/journal.pone.0064751
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
72. Näf R, Velluz A. Sulphur compounds and some uncommon esters in durian (Durio zibethinus Murr.). Flavour Fragr J (1996) 11:295–303. doi:10.1002/(SICI)1099-1026(199609)11:5<295::AID-FFJ585>3.3.CO;2-W
73. Fenwick GR, Hanley AB. The genus Allium – Part 3. Crit Rev Food Sci Nutr (1985) 23:1–73. doi:10.1080/10408398509527419
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
74. Eustache J, Bisseret P, Van De Weghe P. 1.14 – One or more CC bond(s) by elimination of S, Se, Te, N, P, As, Sb, Bi, Si, Ge, B, or metal functions. In: Taylor ARKJK, editor. Comprehensive Organic Functional Group Transformations II. Oxford: Elsevier (2005). p. 601–68.
75. Pfister-Guillouzo G, Senio A, Gracian F, Khalid M, Ripoll J, Vallee Y. FVT-PES Study of reactivity of thionoacrylic compounds. New J Chem (1995) 19:1071–80.
76. Block E, John Dane A, Cody RB. Crushing garlic and slicing onions: detection of sulfenic acids and other reactive organosulfur intermediates from garlic and other Alliums using direct analysis in real-time mass spectrometry (DART-MS). Phosphorus Sulfur Silicon Relat Elem (2011) 186:1085–93. doi:10.1080/10426507.2010.507728
77. Shen C, Xiao H, Parkin KL. In vitro stability and chemical reactivity of thiosulfinates. J Agric Food Chem (2002) 50:2644–51. doi:10.1021/jf011013e
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
78. Cavagnaro PF, Galmarini CR. Effect of processing and cooking conditions on onion (Allium cepa L.) induced antiplatelet activity and thiosulfinate content. J Agric Food Chem (2012) 60:8731–7. doi:10.1021/jf301793b
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
79. Beato VM, Sánchez AH, De Castro A, Montaño A. Effect of processing and storage time on the contents of organosulfur compounds in pickled blanched garlic. J Agric Food Chem (2012) 60:3485–91. doi:10.1021/jf3002075
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
80. Colina-Coca C, González-Peña D, Vega E, De Ancos B, Sánchez-Moreno C. Novel approach for the determination of volatile compounds in processed onion by headspace gas chromatography-mass spectrometry (HS GC-MS). Talanta (2013) 103:137–44. doi:10.1016/j.talanta.2012.10.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
81. Rao PP, Nagender A, Rao LJ, Rao DG. Studies on the effects of microwave drying and cabinet tray drying on the chemical composition of volatile oils of garlic powders. Eur Food Res Technol (2007) 224:791–5. doi:10.1007/s00217-006-0364-3
82. Jansen H, Muller B, Knobloch K. Characterization of an alliin lyase preparation from garlic (Allium sativum). Planta Med (1989) 55:434–9. doi:10.1055/s-2006-962059
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
83. Cavagnaro PF, Camargo A, Galmarini CR, Simon PW. Effect of cooking on garlic (Allium sativum L.) antiplatelet activity and thiosulfinates content. J Agric Food Chem (2007) 55:1280–8. doi:10.1021/jf062587s
84. Galmarini CR, Cavagnaro PF, Sance MM. Effect of heating on onion (Allium cepa L.) antiplatelet activity and pungency sensory perception. Food Sci Technol Int (2007) 13:447–53. doi:10.1177/1082013207088108
85. Rejano L, Sanchez AH, De Castro A, Montano A. Chemical characteristics and storage stability of pickled garlic prepared using different processes. J Food Sci (1997) 62:1120–3. doi:10.1111/j.1365-2621.1997.tb12226.x
86. De Castro A, Montaño A, Sánchez AH, Rejano L. Lactic acid fermentation and storage of blanched garlic. Int J Food Microbiol (1998) 39:205–11. doi:10.1016/S0168-1605(98)00003-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
87. Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol (2011) 51:169–87. doi:10.1146/annurev-pharmtox-010510-100505
88. Mancardi D, Penna C, Merlino A, Del Soldato P, Wink DA, Pagliaro P. Physiological and pharmacological features of the novel gasotransmitter: hydrogen sulfide. Biochim Biophys Acta (2009) 1787:864–72. doi:10.1016/j.bbabio.2009.03.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
89. Gadalla MM, Snyder SH. Hydrogen sulfide as a gasotransmitter. J Neurochem (2010) 113:14–26. doi:10.1111/j.1471-4159.2010.06580.x
90. Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci (1996) 16:1066–71.
91. Polhemus DJ, Lefer DJ. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ Res (2014) 114:730–7. doi:10.1161/CIRCRESAHA.114.300505
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
92. Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun (1997) 237:527–31. doi:10.1006/bbrc.1997.6878
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
93. Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J (2001) 20:6008–16. doi:10.1093/emboj/20.21.6008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
94. Liu YH, Lu M, Hu LF, Wong PT, Webb GD, Bian JS. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid Redox Signal (2012) 17:141–85. doi:10.1089/ars.2011.4005
95. Yang D, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science (2008) 322:587–90. doi:10.1126/science.1162667
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
96. Johansen D, Ytrehus K, Baxter GF. Exogenous hydrogen sulfide (H2S) protects against regional myocardial ischemia-reperfusion injury – Evidence for a role of K ATP channels. Basic Res Cardiol (2006) 101:53–60. doi:10.1007/s00395-005-0569-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
97. Kondo K, Bhushan S, King AL, Prabhu SD, Hamid T, Koenig S, et al. H2S protects against pressure overload-induced heart failure via upregulation of endothelial nitric oxide synthase. Circulation (2013) 127:1116–27. doi:10.1161/CIRCULATIONAHA.112.000855
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
98. Mani S, Li H, Untereiner A, Wu L, Yang G, Austin RC, et al. Decreased endogenous production of hydrogen sulfide accelerates atherosclerosis. Circulation (2013) 127:2523–34. doi:10.1161/CIRCULATIONAHA.113.002208
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
99. Chan MV, Wallace JL. Hydrogen sulfide-based therapeutics and gastrointestinal diseases: translating physiology to treatments. Am J Physiol Gastrointest Liver Physiol (2013) 305:G467–73. doi:10.1152/ajpgi.00169.2013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
100. Song ZJ, Ng MY, Lee Z-W, Dai W, Hagen T, Moore PK, et al. Hydrogen sulfide donors in research and drug development. Med Chem Commun (2014) 5:557–70. doi:10.1039/c3md00362k
101. Myers AG, Cohen SB, Kwon BM. A study of the reaction of calicheamicin g1 with glutathionen in the presence of double-stranded DNA. J Am Chem Soc (1994) 116:1255–71. doi:10.1021/ja00083a012
102. Chatterji T, Gates KS. Reaction of thiols with 7-methylbenzopentathi epin. Bioorg Med Chem Lett (2003) 13:1349–52. doi:10.1016/s0960-894x(03)00103-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
103. Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, Patel RP, et al. Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci U S A (2007) 104:17977–82. doi:10.1073/pnas.0705710104
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
104. Predmore BL, Kondo K, Bhushan S, Zlatopolsky MA, King AL, Aragon JP, et al. The polysulfide diallyl trisulfide protects the ischemic myocardium by preservation of endogenous hydrogen sulfide and increasing nitric oxide bioavailability. Am J Physiol Heart Circ Physiol (2012) 302:H2410–8. doi:10.1152/ajpheart.00044.2012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
105. Chuah SC, Moore PK, Zhu YZ. S-allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway. Am J Physiol Heart Circ Physiol (2007) 293:H2693–701. doi:10.1152/ajpheart.00853.2007.-S-allylcysteine
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
106. Kan J, Guo W, Huang C, Bao G, Zhu Y, Zhu YZ. S-propargyl-cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxid Redox Signal (2014) 20:2303–16. doi:10.1089/ars.2013.5449
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
107. Cai H, Griendling KK, Harrison DG. The vascular NADPH oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci (2003) 24:471–8. doi:10.1016/S0165-6147(03)00233-5
108. Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest (1991) 88:1785–92. doi:10.1172/JCI115499
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
109. Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med (2007) 17:48–54. doi:10.1016/j.tcm.2006.11.005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
110. Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med (1994) 60:417–20. doi:10.1055/s-2006-959522
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
111. Ide N, Lau B. Aged garlic extract attenuates intracellular oxidative stress. Phytomedicine (1999) 6:125–31. doi:10.1016/S0944-7113(99)80047-6
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
113. Dillon SA, Burmi RS, Lowe GM, Billington D, Rahman K. Antioxidant properties of aged garlic extract: an in vitro study incorporating human low density lipoprotein. Life Sci (2003) 72:1583–94. doi:10.1016/S0024-3205(02)02475-X
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
114. Kim J-M, Chang HJ, Kim W-K, Chang N, Chun HS. Structure-activity relationship of neuroprotective and reactive oxygen species scavenging activities for Allium organosulfur compounds. J Agric Food Chem (2006) 54:6547–53. doi:10.1021/jf060412c
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
115. Medina-Campos ON, Barrera D, Segoviano-Murillo S, Rocha D, Maldonado PD, Mendoza-Patino N, et al. S-allylcysteine scavenges singlet oxygen and hypochlorous acid and protects LLC-PK(1) cells of potassium dichromate-induced toxicity. Food Chem Toxicol (2007) 45:2030–9. doi:10.1016/j.fct.2007.05.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
116. Chung LY. The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin, and allyl disulfide. J Med Food (2006) 9:205–13. doi:10.1089/jmf.2006.9.205
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
117. Arguello-Garcia R, Medina-Campos ON, Perez-Hernandez N, Pedraza-Chaverri J, Ortega-Pierres G. Hypochlorous acid scavenging activities of thioallyl compounds from garlic. J Agric Food Chem (2010) 58:11226–33. doi:10.1021/jf102423w
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
118. Wang H, Huang D. Dietary organosulfur compounds from garlic and cruciferous vegetables as potent hypochlorite scavengers. J Funct Foods (2014). doi:10.1016/j.jff.2014.07.001
119. Vaidya V, Ingold KU, Pratt DA. Garlic: source of the ultimate antioxidants – sulfenic acids. Angew Chem Int Ed (2009) 48:157–60. doi:10.1002/anie.200804560
120. Ho S, Ide N, Lau B. S-allyl cysteine reduces oxidant load in cells involved in the atherogenic process. Phytomedicine (2001) 8:39–46. doi:10.1078/0944-7113-00005
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
121. Ide N, Lau BH. Garlic compounds protect vascular endothelial cells from oxidized low density lipoprotein-induced Injury. J Pharm Pharmacol (1997) 49:908–11. doi:10.1111/j.2042-7158.1997.tb06134.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
122. Lei Y-P, Chen H-W, Sheen L-Y, Lii C-K. Diallyl disulfide and diallyl trisulfide suppress oxidized LDL–induced vascular cell adhesion molecule and E-selectin expression through protein kinase A–and B–dependent signaling pathways. J Nutr (2008) 138:996–1003.
123. Kuo WW, Wang WJ, Tsai CY, Way CL, Hsu HH, Chen LM. Diallyl trisufide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFκB signaling via attenuating ROS generation. Int J Cardiol (2013) 168:270–80. doi:10.1016/j.ijcard.2012.09.080
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
124. Huang Y-T, Yao C-H, Way C-L, Lee K-W, Tsai C-Y, Ou H-C, et al. Diallyl trisulfide and diallyl disulfide ameliorate cardiac dysfunction by suppressing apoptotic and enhancing survival pathways in experimental diabetic rats. J Appl Physiol (2013) 114:402–10. doi:10.1152/japplphysiol.00672.2012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
125. Tsai CY, Wang CC, Lai TY, Tsu HN, Wang CH, Liang HY, et al. Antioxidant effects of diallyl trisulfide on high glucose-induced apoptosis are mediated by the PI3K/Akt-dependent activation of Nrf2 in cardiomyocytes. Int J Cardiol (2013) 168:1286–97. doi:10.1016/j.ijcard.2012.12.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
126. Padiya R, Chowdhury D, Borkar R, Srinivas R, Pal Bhadra M, Banerjee SK. Garlic attenuates cardiac oxidative stress via activation of PI3K/AKT/Nrf2-Keap1 pathway in fructose-fed diabetic rat. PLoS One (2014) 9:e94228. doi:10.1371/journal.pone.0094228
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
127. Liu LL, Yan L, Chen YH, Zeng GH, Zhou Y, Chen HP, et al. A role for diallyl trisulfide in mitochondrial antioxidative stress contributes to its protective effects against vascular endothelial impairment. Eur J Pharmacol (2014) 725:23–31. doi:10.1016/j.ejphar.2014.01.010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
128. Lei YP, Liu CT, Sheen LY, Chen HW, Lii CK. Diallyl disulfide and diallyl trisulfide protect endothelial nitric oxide synthase against damage by oxidized low density lipoprotein. Mol Nutr Food Res (2010) 54:S42–52. doi:10.1002/mnfr.200900278
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
129. Khatua TN, Padiya R, Karnewar S, Kuncha M, Agawane SB, Kotamraju S, et al. Garlic provides protection to mice heart against isoproterenol-induced oxidative damage: role of nitric oxide. Nitric Oxide (2012) 27:9–17. doi:10.1016/j.niox.2012.03.004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
130. Liu DS, Gao W, Liang ES, Wang SL, Lin WW, Zhang WD, et al. Effects of allicin on hyperhomocysteinemia-induced experimental vascular endothelial dysfunction. Eur J Pharmacol (2013) 714:163–9. doi:10.1016/j.ejphar.2013.05.038
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
131. Asdaq SM, Inamdar MN. Potential of garlic and its active constituent, S-allyl cysteine, as antihypertensive and cardioprotective in presence of captopril. Phytomedicine (2010) 17:1016–26. doi:10.1016/j.phymed.2010.07.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
132. Calvert JW, Jha S, Gundewar S, Elrod JW, Ramachandran A, Pattillo CB, et al. Hydrogen sulfide mediates cardioprotection through nrf2 signaling. Circ Res (2009) 105:365–74. doi:10.1161/CIRCRESAHA.109.199919
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
133. Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal (2005) 7:385–94. doi:10.1089/ars.2005.7.385
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
134. Kobayashi M, Li L, Iwamoto N, Nakajima-Takagi Y, Kaneko H, Nakayama Y, et al. The antioxidant defense system Keap1-Nrf2 comprises a multiple sensing mechanism for responding to a wide range of chemical compounds. Mol Cell Biol (2009) 29:493–502. doi:10.1128/MCB.01080-08
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
135. Zhu H, Jia Z, Misra BR, Zhang L, Cao Z, Yamamoto M, et al. Nuclear factor E2-related factor 2-dependent myocardiac cytoprotection against oxidative and electrophilic stress. Cardiovasc Toxicol (2008) 8:71–85. doi:10.1007/s12012-008-9016-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
136. Li J, Ichikawa T, Villacorta L, Janicki JS, Brower GL, Yamamoto M, et al. Nrf2 protects against maladaptive cardiac responses to hemodynamic stress. Arterioscler Thromb Vasc Biol (2009) 29:1843–50. doi:10.1161/ATVBAHA.109.189480
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
137. Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett (2005) 579:3029–36. doi:10.1016/j.febslet.2005.04.058
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
138. Lee JS, Surh YJ. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett (2005) 224:171–84. doi:10.1016/j.canlet.2004.09.042
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
139. Kim S, Lee HG, Park SA, Kundu JK, Keum YS, Cha YN, et al. Keap1 cysteine 288 as a potential target for diallyl trisulfide-induced Nrf2 activation. PLoS One (2014) 9:e85984. doi:10.1371/journal.pone.0085984
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
140. Blair A, Shaul PW, Yuhanna IS, Conrad PA, Smart EJ. Oxidized low density lipoprotein displaces endothelial nitric-oxide synthase (eNOS) from plasmalemmal caveolae and impairs eNOS activation. J Biol Chem (1999) 274:32512–9. doi:10.1074/jbc.274.45.32512
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
141. Ji Y, Diao J, Han Y, Huang Y, Bai H, Chen Q, et al. Pyridoxine prevents dysfunction of endothelial cell nitric oxide production in response to low-density lipoprotein. Atherosclerosis (2006) 188:84–94. doi:10.1016/j.atherosclerosis.2005.10.035
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
142. Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature (1999) 399:601–5. doi:10.1038/21224
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
143. Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med (2011) 51:1289–301. doi:10.1016/j.freeradbiomed.2011.06.033
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
144. Weiss N, Keller C, Hoffmann U, Loscalzo J. Endothelial dysfunction and atherothrombosis in mild hyperhomocysteinemia. Vasc Med (2002) 7:227–39. doi:10.1191/1358863x02vm428ra
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
145. Weiss N, Heydrick SJ, Postea O, Keller C, Keaney JF Jr, Loscalzo J. Influence of hyperhomocysteinemia on the cellular redox state – impact on homocysteine-induced endothelial dysfunction. Clin Chem Lab Med (2003) 41:1455–61. doi:10.1515/CCLM.2003.223
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
146. Tsai KH, Wang WJ, Lin CW, Pai P, Lai TY, Tsai CY, et al. NADPH oxidase-derived superoxide anion-induced apoptosis is mediated via the JNK-dependent activation of NF-κB in cardiomyocytes exposed to high glucose. J Cell Physiol (2012) 227:1347–57. doi:10.1002/jcp.22847
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
147. Huh JE, Kang KS, Chae C, Kim HM, Ahn KS, Kim SH. Roles of p38 and JNK mitogen-activated protein kinase pathways during cantharidin-induced apoptosis in U937 cells. Biochem Pharmacol (2004) 67:1811–8. doi:10.1016/j.bcp.2003.12.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
148. Ogino T, Ozaki M, Hosako M, Omori M, Okada S, Matsukawa A. Activation of c-Jun N-terminal kinase is essential for oxidative stress-induced Jurkat cell apoptosis by monochloramine. Leuk Res (2009) 33:151–8. doi:10.1016/j.leukres.2008.07.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
149. Shou Y, Li N, Li L, Borowitz JL, Isom GE. NF-κB-mediated up-regulation of Bcl-Xs and Bax contributes to cytochrome c release in cyanide-induced apoptosis. J Neurochem (2002) 81:842–52. doi:10.1046/j.1471-4159.2002.00880.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
150. Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-κB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J (2002) 367:729–40. doi:10.1042/BJ20020752
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: Alliums, stinky beans, dietary organopolysulfides, hydrogen sulfide, cardioprotection
Citation: Tocmo R, Liang D, Lin Y and Huang D (2015) Chemical and biochemical mechanisms underlying the cardioprotective roles of dietary organopolysulfides. Front. Nutr. 2:1. doi: 10.3389/fnut.2015.00001
Received: 15 October 2014; Accepted: 05 January 2015;
Published online: 02 February 2015.
Edited by:
Sascha Rohn, University of Hamburg, GermanyReviewed by:
Marcin Szymanski, Poznan University of Medical Sciences, PolandFranziska S. Hanschen, Leibniz-Institute of Vegetable and Ornamental Crops Großbeeren/Erfurt e.V., Germany
Copyright: © 2015 Tocmo, Liang, Lin and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dejian Huang, Food Science and Technology Programme, Department of Chemistry, National University of Singapore, Science Drive 2, 117543 Singapore e-mail: chmhdj@nus.edu.sg
 Restituto Tocmo
Restituto Tocmo Dong Liang
Dong Liang Yi Lin2
Yi Lin2  Dejian Huang
Dejian Huang