Virally mediated gene manipulation in the adult CNS
- Department of Neurobiology and Ethology, Center for Gene Manipulation in the Brain (CGMB), University of Haifa, Haifa, Israel
Understanding how the CNS functions poses one of the greatest challenges in modern life science and medicine. Studying the brain is especially challenging because of its complexity, the heterogeneity of its cellular composition, and the substantial changes it undergoes throughout its life-span. The complexity of adult brain neural networks results also from the diversity of properties and functions of neuronal cells, governed, inter alia, by temporally and spatially differential expression of proteins in mammalian brain cell populations. Hence, research into the biology of CNS activity and its implications to human and animal behavior must use novel scientific tools. One source of such tools is the field of molecular genetics—recently utilized more and more frequently in neuroscience research. Transgenic approaches in general, and gene targeting in rodents have become fundamental tools for elucidating gene function in the CNS. Although spectacular progress has been achieved over recent decades by using these approaches, it is important to note that they face a number of restrictions. One of the main challenges is presented by the temporal and spatial regulation of introduced genetic manipulations. Viral vectors provide an alternative approach to temporally regulated, localized delivery of genetic modifications into neurons. In this review we describe available technologies for gene transfer into the adult mammalian CNS that use both viral and non-viral tools. We discuss viral vectors frequently used in neuroscience, with emphasis on lentiviral vector (LV) systems. We consider adverse effects of LVs, and the use of LVs for temporally and spatially controllable manipulations. Especially, we highlight the significance of viral vector-mediated genetic manipulations in studying learning and memory processes, and how they may be effectively used to separate out the various phases of learning: acquisition, consolidation, retrieval, and maintenance.
Introduction
Our understanding of the molecular mechanisms underlying cellular functions represents one of the major revolutions of our era. The associated advances in genetics, molecular biology, and biochemistry enable both conceptual breakthroughs and the development of new technologies to study various cell types, including neurons, in culture and in vivo. However, the nature of mature post-mitotic neurons, and the complexity, vulnerability, and inaccessibility of the brain impose additional constraints on gene manipulation and molecular analysis of brain function, both in health and in sickness. These constraints include the difficulty of exposing and manipulating the brain, the specific difficulty of introducing DNA or RNA into neurons without harming the cells, the challenging task of manipulating a specific neuronal population among the thousands of neuronal types present, and the limitations of genetic approaches to manipulation of gene expression while maintaining the desired temporal and spatial resolution.
A major way to overcome some of these limitations and complexities is to use the natural ability of viruses to introduce genes into host-cells, including neurons, and during the last decade viral vector technology has been developed as a safe and reasonably easy tool to use. Several features of viral vectors are specifically attractive with regard to manipulation of the mature brain: (a) the ability to manipulate gene expression, and protein level and activity in specific cells, e.g., in neurons rather than in glia, by directing the viral transduction to the desired cell type; (b) the capability to identify the manipulated cells by co-expressing reporter genes, e.g., Enhanced Green Fluorescent Protein (EGFP), together with the gene of interest, a capability that is especially useful for phenotypic examination of the morphological and electrophysiological properties of the transduced cells; (c) co-expression of optogenetic tools, e.g., halorhodopsin, in manipulating neuronal activity; (d) cell-specific expression of viral DNA vectors, by using specific promoters; and (e) localization of the genetic manipulation induced by local injection of viral vectors into a specific brain region.
In this review we discuss the advantages and disadvantages of using viral vectors to enhance or to reduce expression of specific genes in the mature CNS, from the perspectives of research into neuroscience in general, and into the molecular and cellular mechanisms underlying learning and memory processes, in particular. We first discuss the methodology of gene transfer by using viral vectors, with specific attention to the differences between adult and non-adult tissues. We briefly describe the viral vectors most frequently used in neuroscience research: herpes simplex virus type-1 (HSV-1), adeno virus (Ad), adeno-associated virus (AAV), and lentivirus (LV), with emphasis on LV. We then briefly review several studies that aimed to elucidate molecular and cellular mechanisms of the various phases of learning and memory by using viral vectors. This review does not address the great potential of viral vectors in gene therapy of neurodegenerative or psychiatric diseases; these topics have been reviewed elsewhere (Verma and Weitzman, 2005; Lundberg et al., 2008; Nanou and Azzouz, 2009; Dreyer, 2011).
Gene Transfer Technologies
The mammalian brain undergoes significant changes in structural architecture and functional organization in the course of its life-span. Even though the brain has already grown to about 90% of its adult total size by late childhood, it continues to undergo dynamic modifications throughout adolescence and into young adulthood. Developmental changes include proliferation and migration of cells, which occur mostly during fetal development, regional changes in synaptic density that take place during postnatal development (Huttenlocher, 1979; 1990; Bourgeois et al., 1994), and prolonged development of myelination in adulthood. Therefore, brain maturation may be considered as progress toward ever-growing complexity. One aspect of this complexity is exhibited in the brain's thousands of intercalated but distinct neuronal populations, which are interlinked by huge numbers of synaptic connections.
To gain a better understanding of brain function at the cellular and molecular levels, a technology is needed that facilitates manipulation of gene expression in vivo. Within the brain, region-restricted and neuronal-subpopulation-restricted genetic manipulations serve as a powerful means for elucidation of gene function in the adult animal. Furthermore, one would like to be able to monitor neurobiological functions, in order to replace, modify, induce, or block expression of target genes in a temporally and spatially controlled manner. Over the past decade, application of gene manipulation technologies has gained momentum in neuroscience research in the adult brain. The ideal vector for gene delivery should efficiently package and protect the transgene, target specific neuronal subpopulations in the body, and express the transgene within a desired time window. In furthering this aim, research efforts have focused on evaluating existing vectors and developing novel vectors for efficient neuronal gene modifications.
At present, gene transfer technologies include viral and non-viral approaches, and non-viral nucleic acid carriers have been evaluated extensively, for neuronal gene delivery. These carriers include cationic polymers, lipids, engineered polypeptides, gene carriers based on synthetic nanoparticles, and, more recently, in vivo electroporation-based techniques (Pere et al., 2008). The majority of studies that use non-viral in vivo gene manipulation in the brain employ these techniques for manipulating the injured or the immature (neonatal and postnatal) brain (Petros et al., 2009; De Vry et al., 2010; Molotkov et al., 2010; Wong et al., 2010).
Viral vectors form another efficient tool for genetic manipulation in the adult mammalian brain. Viruses have evolved to infect host-cells and to regulate their genome expression, in order to replicate within these cells, so that the generic virus life cycle comprises two phases: infection and replication. The infection step involves attachment of virions to target cells, and subsequent introduction of the viral genome into the cell. The replication step involves expression of the viral structural and regulatory proteins; this step is essential for viral genome replication and for assembly of viral particles, a process termed packaging. Viral vector technology exploits the natural features of viruses to develop, through genetic engineering, a highly efficient tool for gene transfer: the genes mediating viral pathogenesis and replication are deleted and replaced with an expression cassette of exogenous transgenes. The outcome of such changes is a recombinant viral vector that effectively infects target cells and, introduces desired transgenes into the host-cell, but is incompetent with respect to replication. To achieve such a useful vector several technological obstacles need to be overcome: production of recombinant viral vectors requires replication-associated genes for packaging, but these genes will have been deleted from the viral vector itself. To overcome this issue, replication-associated genes are supplied to the producer cell line as trans-acting elements, so that the resulting recombinant viral vectors can be considered relatively safe with regard to transduction capabilities, because cell transduction can occur only once.
An additional issue to consider in viral vector technology is tropism. Natural viruses are most efficient at infecting specific host-cell populations; attachment to and infection of a susceptible cell are mediated by proteins expressed in the viral capsid or envelope, and in several viral species cell recognition and specificity can be modified by replacing viral capsid/envelope proteins. This process, termed pseudotyping, either restricts or extends the range of cells susceptible to transduction.
Viral Vectors Frequently Used in Neuroscience
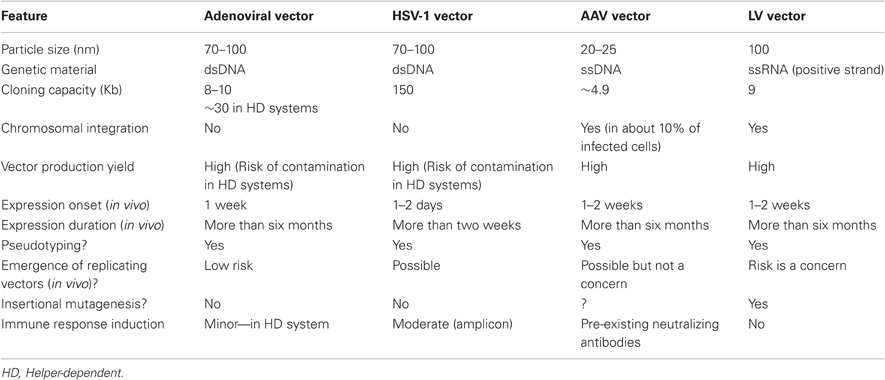
Several viral vectors derived from Ad, HSV-1, AAV, and human immunodeficiency virus type 1 (HIV-1) were found able to serve as powerful tools for adult brain transduction. Notably, each of these distinct vector types has its own individual features that should be considered before use; they include cloning capacity, target-cell specificity, targeted-cell viability, transgene expression duration, possible immune responses, controlled vector production, and biosafety (summarized in Table 1 and in Papale et al., 2009).
Adeno-Viral Vectors (Ad)
Ad is a non-enveloped virus that consists of a protein capsid surrounding a DNA/protein core. The capsid is composed of three major proteins—hexon (II), penton base (III), and a knobbed fibre (IV)—along with several minor proteins (Stewart et al., 1993). The viral genome is a linear double-stranded DNA with an average size of 36 kb. The Ad genome is segmented into 100 map units (mu) flanked by 100–150 bp repetitive DNA sequence elements termed inverted terminal repeats (ITRs). In addition, the 5′ terminus of the viral genome contains a ∼190 bp packaging sequence (Russell, 2000). Both of the ITRs and the packaging sequence are cis-acting elements, essential for viral DNA replication and packaging. The Ad genome can be divided into early (E) and late (L) transcriptional units, according to the time of onset of their expression during the viral life cycle. Among the early genes, E1a and E1b proteins encode trans-activating factors that help regulate the host-cell cycle. Furthermore, E2 is required for viral replication, E3 for host-immune modulation, and E4 to inhibit host-cell programmed cell death (Dharmapuri et al., 2009).
To date, more than 100 Ad serotypes, including 51 human Ad (hAd) serotypes, have been identified, and the hAd serotypes have been classified into six distinct subgroups (A–F), according to their characteristics such as genome size, organization, and nucleotide composition. The best-characterized serotype is group C, which consists of Ad serotypes 1, 2, 5, and 6, among which serotypes 2 and 5 (Ad2 and Ad5) are the most frequently used as gene transfer vectors (Dharmapuri et al., 2009). Ad-derived transfer vectors harbor broad tropism properties, can transduce both dividing and non-dividing cells without genome integration, and can be produced at very high titers.
In first-generation Ad systems, the E1 region was deleted by Xiang et al., (1996), which resulted in replication-incompetent viral vectors; however, first-generation vectors induced innate as well as adaptive immune responses (Hartman et al., 2008). In order to reduce this toxicity, second-generation vectors were further modified by deletion of the E2A, E3, and E4 genes, and these improved vectors resulted in transient transgene expression, because of induction of adaptive immune responses (Amalfitano and Chamberlain, 1997; Amalfitano et al., 1998). Third-generation, helper-dependent (HD) Ad vectors were produced by additional modifications; these vectors were able to facilitate high transgene expression levels with minor toxicity. In HD Ad vectors the gene transfer system consists of transgenic DNA of length up to 36 kb that can be delivered. However, there are production problems, such as obtaining high titers in large-scale production, and helper-virus contamination (Mitani et al., 1995; Kochanek et al., 1996) however, intensive research is being conducted in order to develop an Ad-helper-cell line that will overcome these difficulties.
Herpes Simplex Virus Type-1 Viral Vector (HSV-1)
HSV-1 is an important viral vector with natural neuron-infectivity properties. This neurotropic virus is enveloped with a double-stranded DNA genome of about 150 kb. HSV-1 is a non-integrating virus; in neurons, it forms a latent infection, characterized by lack of detectable viral protein production but continued transcriptional activity (Berges et al., 2007). Moreover, HSV-1 is able to spread in the nervous system through retrograde axonal transport (Cook and Stevens, 1973).
Brain infection with wild-type HSV-1 may result in encephalitis, therefore, a fundamental requirement for gene-transfer vectors derived from this virus is reduced pathogenicity. To achieve this, replication-deficient HSV-1-based vectors were generated by deleting the relevant genes. Furthermore, an advanced vector system, termed an amplicon was generated by deletion of all portions of the viral genome, except for the origin of replication and DNA packaging sequences, leaving about 150 kb available for transgene insertion. Production of amplicon HSV-1-based viral particles in packaging cells involves utilization of either a helper-virus or use of several plasmids that provide the necessary trans-acting elements (Epstein, 2005; Epstein et al., 2005; Neve et al., 2005).
Each of these two options has advantages and limitations that should be taken into account. Using a helper-virus will promote high titer but, with such an approach it is not possible to completely eliminate helper-virus contaminants. Alternatively, one can use the co-transfection method, in which the amplicon and a bacterial artificial chromosome (BAC) harboring the entire viral genome except for the packaging sequence are introduced into the producer cell line (Saeki et al., 2001). It is important to note that sensory neurons are the natural reservoir of latency (Kristensson et al., 1971). Nevertheless, gene transfer experiments have shown that HSV-1-based vectors can establish latency also in other neurons within the brain (Scarpini et al., 2001; Berges et al., 2005). However, there is indicative evidence that latency is not equivalent in all neuron subsets (Labetoulle et al., 2003), therefore, it is important to elucidate whether long-term transgene expression is achievable in a variety of neuronal populations.
Adeno-Associated Viral Vectors (AAV)
AAV is a non-enveloped virus harboring an outer protein capsid and a single-stranded DNA genome, approximately 4.7 kb long. The AAV genome consists of two open reading frames (ORFs) flanked by ITRs. The first ORF—termed rep—encodes four proteins that are involved in replication of the viral genome. The second ORF (cap) encodes three structural proteins (VP1, VP2, and VP3) (Srivastava et al., 1983). The ITRs are the minimal required cis-acting elements for viral genome integration, replication, and packaging into the capsid shell. In recombinant AAV-based vectors, rep, and cap are replaced with a gene of interest, and are supplied as trans-acting elements to facilitate transgene packaging inside the capsid. The AAV life cycle includes viral attachment to the cell surface, viral uptake, genome translocation into the nucleus and, finally, viral gene expression (Kwon and Schaffer, 2008). After transduction, the viral genome remains mainly episomal and, in some cases, integrates randomly into the host genome (Smith, 2008).
AAV is a naturally defective virus, and upon arrival into the nucleus, its replication requires functions supplied by a helper-virus such as an Ad, or it establishes a latent infection. It is important to clarify that recombinant AAV lacks both rep and cap genes, therefore, it is replication-deficient even in the presence of a helper-virus (Richardson and Westphal, 1984; Weindler and Heilbronn, 1991). Since AAV is inherently replication-defective and lacks any known pathogenicity, it is an attractive gene transfer vector for use in gene therapy. AAV has several available serotypes, of which the most studied is AAV-2, currently used in clinical trials for treatment of numerous diseases.
AAV-2 has a broad tropism and has been shown to deliver genes to a wide range of cell types, including muscle, brain, retina, liver, and lung. Nevertheless, a broad tropism can be a drawback for targeted gene delivery to specific types of cells, therefore, efforts have focused on modifying capsid proteins in order to alter the tropism and enhance the efficiency of transduction into specific cell types (Kwon and Schaffer, 2008). An additional consideration that should be taken into account is that gene transfer into the CNS could be compromised by immune responses against virion components; when AAV is used as a vector pre-existing circulating antibodies might inhibit gene transfer, thus limiting its effectiveness.
Lentiviral Vectors (LVs)
The best-characterized lentiviral vectors (LVs) are derived from the HIV-1, which is a subclass of retroviruses. However, in contrast to most retroviruses, which infect only actively replicating cells, LVs are capable of infecting both dividing and non-dividing cells, including fully differentiated neurons (Galimi and Verma, 2002). The natural virus consists of a glycoprotein envelope and a 9 kb single-stranded-RNA genome. Accordingly, the HIV-1 life cycle includes a step of reverse-transcription of the RNA genome into a double-stranded DNA, and a subsequent step of stable integration into the host-cell genome. This feature is maintained in HIV-1-based vectors, thereby enabling long-term expression of the gene of interest.
The HIV-1 genome contains nine ORFs, including the retroviral genes gag, pol, and env, which encode the structural proteins polymerase and reverse transcriptase, and envelope glycoproteins, respectively. Additionally, the LV genome encodes accessory proteins that function in infection maintenance. These accessory proteins were removed when the LV was developed into a gene transfer vector. Moreover, a number of cis-acting elements are required at various stages of the viral life cycle; these include the long terminal repeats (LTRs), the TAT activation region (TAR), splice donor and acceptor sites, packaging and dimerization signal (Ψ), Rev-responsive element (RRE), and the central and terminal polypurine tracts (PPT) (Ramezani and Hawley, 2002). Major research efforts have been dedicated to increasing the vector biosafety: the general strategy used to produce vector particles that are replication-defective has been to eliminate all dispensable genes from the HIV-1 genome and, as far as possible, to separate the cis-acting sequences from trans-acting factors that are absolutely required for viral particle production, infection, and integration. This approach resulted in the establishment of the third-generation self-inactivating (SIN) LV system (Naldini et al., 1996b; Dull et al., 1998; Zufferey et al., 1998).
Wild-type LVs have a natural tropism to T lymphocytes, but this tropism can be altered by pseudotyping. The most widely used LV pseudotypes are those that incorporate the attachment glycoprotein of the vesicular stomatitis virus (VSV-G), which both enables the production of high-titer vector stocks, and confers a broad host range (Jakobsson et al., 2003). Nevertheless, other pseudotyping options are available, and one can utilize this strategy to obtain cell-specific transduction properties (Waehler et al., 2007).
Efficient transduction of neurons in vivo was described in the very first report of a vector system based on HIV-1 (Naldini et al., 1996a). Since then, hundreds of reports have described the use of LVs in the CNS, for both therapeutic and experimental gene transfer purpose. It is important to mention that neurons transduced by LVs are both morphologically and physiologically normal, and remain healthy for many months after infection. Thus, LVs serve as an efficient tool to study the CNS, because infection per se has no apparent deleterious effect (Baekelandt et al., 2002).
Adverse Effects of LV-Mediated Manipulation
Several potential adverse effects of LV injection should be taken into account when using LVs as a vector (Pauwels et al., 2009). (1) Replication-competent lentiviruses (RCL) could be produced during the generation and propagation of LV production; generation of RCLs might arise through homologous recombination between overlapping sequences, and to prevent this a four-plasmid vector system is used in the third-generation LV, so that RCL may occur only after a number of recombination events (Dull et al., 1998). Although RCL events have not been detected so far, because of the SIN vector design, the possibility of such events should still be taken into account. (2) Because the LV produced DNA is inserted into the host-cell genome, the insertion might be associated with increased risk of insertional mutagenesis and transactivation of genome sequences. For example, high incidence of oncogenesis was detected following gene transfer with EIAV (equine infectious anaemia virus)-derived LVs in neonatal mice, and it was thought to be associated with insertional mutagenesis or transactivation (Themis et al., 2005). Studies have shown that LVs preferentially integrate into transcriptionally active genes, therefore, LV integration into such genes might be considered to pose a risk of tumorogenesis because of, for instance, the loss of normal tumor-suppressor gene functions. Furthermore, transactivation of neighbouring genome sequences is a risk, in the light of studies indicating that the LV enhancer/promoter elements have a role in cell transformation.
In order to address these major biosafety concerns, research is being conducted to develop integration-deficient lentiviral vectors (IDLVs). In contrast to integrating lentivectors, IDLVs remain as episomal DNA, and pose a much smaller risk of causing insertional mutagenesis and a lower risk of generating RCL. Currently, IDLV systems can mediate transient gene expression in proliferating cells, and stable expression in non-dividing cells in vitro and in vivo (Wanisch and Yanez-Munoz, 2009). In the CNS, stereotactic injections of IDLVs into mouse or rat brain established efficient Green Fluorescent Protein (GFP) expression which was stable for up to one month (Fabes et al., 2006; Markowitz et al., 2007); in all cases no differences were observed between integrating and non-integrating LVs, in their proficiency of titer matching in quiescent tissues (Markowitz et al., 2007).
Temporal and Spatial Control of Lentiviral-Mediated Genetic Manipulations
A major challenge in neuroscience is to define the roles of specific molecules in brain function, within neuronal circuits and in synaptic plasticity processes. To advance this type of research, genetic modifications need to induce refined manipulations, i.e., changes specific to a time and/or place such as a particular brain area or neuron population. Improved control of gene manipulation has been accomplished by utilization of genetically engineered mice harboring regulatory elements in addition to the gene of interest.
Two main systems were applied to introduce controllable genetic modifications: the Cre-LoxP system and the tetracycline-dependent transcription system. Cre recombinase is an enzyme isolated from bacteriophage P1, which specifically recombines two 34-bp loxP recognition sites. This characteristic of Cre recombinase is utilized to target recombination events in the mouse genome, endogenously devoid of loxP sites (Hoess et al., 1982; 1986; Sauer and Henderson, 1988). The Cre-LoxP approach was effectively applied in the field of neuroscience initially in creating mice lacking functional N-methyl D-aspartate (NMDA) receptors, specifically in the CA1 region of the hippocampus (Tsien et al., 1996a, b). This cell-type-specific manipulation required crossing of two distinct mouse lines. In one of these mouse lines, established by standard transgenic techniques, Cre recombinase is expressed under regulation of the brain-region-specific αCaMKII promoter; the second was generated by homologous recombination in Embryonic Stem (ES) cells, in which loxP sites were inserted into intronic and downstream sequences surrounding the NMDA receptor 1 gene. Cross-breeding of these two mouse lines resulted in deletion of the NMDAR1 gene only in Cre-recombinase-expressing neurons, thus providing a brain-region-specific genetic manipulation. Temporal fine tuning of the Cre-LoxP system was further, achieved by generation of ligand-dependent Cre recombinase (CreERT2), which is active only in the presence of specific ligands. CreERT2 is a fusion protein in which Cre enzyme has been fused to a mutant estrogen receptor fragment. This fusion protein is not activated by endogenous estrogen, but is sensitive to a synthetic ligand, tamoxifen. CreERT2 is localized in the cell cytoplasm and upon binding to tamoxifen enters the nucleus to mediate recombination of loxP sites (Metzger and Chambon, 2001). Therefore, in CreERT2 expressing cells, temporal control is accomplished by tamoxifem administration.
The tetracycline-dependent Tet/tetO system was developed by Hermann Bujard and colleagues, and is based on the tetracycline resistance of bacteria (Gossen and Bujard, 1992; Furth et al., 1994). The basic principal involves introduction of an exogenous molecule with unknown mammalian targets, which is able to regulate transcription in an inducible and reversible manner. The Tet/tetO system consists of two elements: a transcription repressor (TetR), and a DNA motif specific for TetR binding and named tetO sequence. The tTA is a fusion protein composed of the TetR repressor and the VP16 transcription activation domain from the HSV. The tTA binds to the tetO element and activates the transcription of the nearby gene. Binding of tTA to the antibiotic tetracycline derivative, doxycycline, lowers its affinity to tetO thereby preventing transactivation of target genes (Tet-off). In a modified version of this system a four-amino-acid change in the tetR DNA binding domain alters the tTA binding properties, thereby, creating the rtTA protein that, in contrast to the tTA, can recognize only the tetO sequences and activate transgene expression in the presence of the tetracycline analog doxycycline (Tet-on). Thus, by controlling the doxycycline supply to the genetically engineered mouse we can turn the expression of a gene downstream of the tetO sequence on or off, depending on the Tet system used.
Mouse models harboring the regulatory systems described above can serve as a valuable tool for elucidating brain function in health and under pathological conditions. However, these techniques suffer from several limitations. First, some promoters may facilitate a cell-type-specific expression in one mouse strain, but will fail to do so when transferred to a different strain (Gupta et al., 2001; Everett et al., 2004). Second, several examples indicated that different mouse strains behaved differently, a phenomenon that can complicate behavioral analysis of the impact of the genetic manipulation (Miller et al., 2010; Lederle et al., 2011). Moreover, in transgenic approaches, the manipulation is performed at the germline level, which limits development of potential therapeutic agents. Stereotactic delivery of viral vectors to relevant brain regions provides an alternative to traditional mouse genetics and enables relatively high spatio-temporal control over the genetic manipulation, and a short time from experimental design to data collection and analysis. Currently, lenti- and adeno-associated viral vectors are regarded as best-choice tools for gene manipulation in the CNS. The performance of LVs is superior to that of AAVs with regard to packaging capacity, stability of transgene expression, and absence of an inflammatory response.
In order to achieve temporal and spatial control of LV-mediated gene manipulation, two main strategies are available. The first involves combination of the LV gene transfer approach and a Cre mouse model, in which the Cre recombinase is expressed under the regulation of a cell-type-specific promoter, and the LV contains a trangene that is regulated via loxP sites e.g., by using a loxP-flanked stop cassette between the transgene and its promoter. Another possibility is to apply a vector that carries a doubly floxed inverted gene. Upon transduction into Cre recombinase-expressing cells, the gene will be irreversibly inverted and enable cell-specific gene expression (Cardin et al., 2010; Zhang et al., 2010). The identification of cell-type-specific promoters is crucial for the success of cell-specific gene targeting. Initially, the neuronal population as a whole was targeted by using expression of neuron-specific enolase (NSE) minimal promoter-derived Cre. Since then a significant number of brain-region and cell-type-specific, Cre-dependent regulated systems have been established in mice and are expanding rapidly (Gavériaux-Ruff and Kieffer, 2007). Websites of Nagy (www.mshri.on.ca/nagy/) and Jackson (www.jax.org/) laboratories are recommended for finding available Cre mice. In addition, transgenic BAC-Cre recombinase driver lines can be found in the GENSAT database (www.gensat.org/cre.jsp).
Similarly, one could employ the Tet system (Gascon et al., 2008; Hioki et al., 2009; Konopka et al., 2009): a single LV vector can contain both the rtTA transactivator that is regulated by a cell-type-specific promoter, and a transgene expression cassette that is regulated by a promoter containing tetO sequences. In such a case the transgene expression is regulated by the presence of Dox in the brain. Thus, temporal control is achieved by Dox administration or removal, and spatial regulation is induced by cell-type-specific promoters.
A different approach for targeted LV-mediated gene transfer is to use envelope proteins that bind to specific receptors found only on the desired cell type; a process known as pseudotyping. As mentioned above, LV vectors are most frequently pseudotyped with the VSV-G glycoprotein, which has broad tropistic properties. Moreover, VSV-G-pseudotyped LV particles are resistant to freeze-thaw cycles as well as to ultracentrifugation during production, which are major aspects for gene-delivery vectors. Nevertheless, an optimal in vivo gene manipulation in the adult brain should facilitate cell-type-specific targeting. Selective tropism can be achieved by using the natural tropism of glycoproteins from other membrane-enveloped viruses. For instance, Mazarakis and colleagues demonstrated that pseudotyping of LVs with rabies-G glycoprotein enhanced gene transfer into neurons by facilitating retrograde axonal transport and transduction of neurons at distally connected sites within the nervous system (Wong et al., 2004). Another option is to apply ligand-receptor bridge proteins. Recently published findings demonstrated a successful application of bridge protein targeting of a LV to selected cells within the brain in vivo: cortical ErbB4-expressing neurons were targeted in vivo by using an EnvB-pseudotyped LV and the TVB–NRG1 bridge protein (Choi et al., 2010). Alternatively, single-chain antibodies that recognize cell-surface antigens can also be utilized to direct LV particles into specific neuronal populations (Anliker et al., 2010).
Direct LV mediated gene delivery into the adult brain represents a powerful tool in neuroscience research. Temporal and spatial control of LV-mediated genetic modifications can be facilitated by the strategies described above, because of the relatively high packaging capacity. It is worth mentioning that different gene expression regulation systems in LVs can be combined to induce fine tuning of time- and place-directed genetic modifications.
LV-Mediated Gene Manipulation in Different Temporal Phases of Learning/Memory Processes
Research into the molecular and cellular mechanisms underlying learning, memory, and synaptic plasticity processes is increasingly using various genetic tools, including viral vectors. One can divide the research on synaptic plasticity and memory into the following fields according to area of study and research tools. (1) Study of neuronal synaptic or intrinsic functions and properties by using electrophysiology and/or imaging techniques. (2) Elucidating neuronal functions and/or neuronal circuits within a given brain area by using imaging and optogenetic tools, and (3) Gaining understanding of the molecular and cellular mechanisms underlying learning and memory processes, with a specific aim of going beyond mere correlations, to prove the causality of molecular processes underlying learning.
Within the scope of the present review we focus mainly on studies that used viral gene manipulation in learning and memory processes—manipulation that aimed to dissect out the various phases of memory formation and storage: acquisition, consolidation, and maintenance. These studies pioneered the field and aimed to show causality between molecular and cellular events in specific brain regions, on the one hand, and specific phases of memory formation, on the other hand. We, therefore, put the spotlight specifically on use of the LV technique to investigate molecular mechanisms of memory formation in the gustatory cortex (GC), in taste memory formation (Elkobi et al., 2008; Shema et al., 2011).
One such type of learning paradigm comprises cortically dependent taste-learning paradigms in rodents. Taste memories, like any other sensory memory, can be formed and maintained without any associative external input, i.e., incidental learning (Merhav and Rosenblum, 2008), or can be associated with malaise to induce long-lasting negative memory, i.e., conditioned taste aversion (CTA) (Garcia et al., 1955). The functional integrity of the GC, which resides within the insular cortex (IC), is necessary both for creation of new positive or negative taste memories and also for maintaining these memories over time (Rosenblum, 2009). Rodents rely heavily on their chemical senses, which makes novel taste learning a natural and robust behavior to study at the anatomical, physiological, and molecular levels (Rosenblum et al., 1997; Jones et al., 1999; Berman et al., 2000; Merhav et al., 2006; Yefet et al., 2006).
At the biochemical level, novel taste learning is associated with changes in the phosphorylation state of several proteins, including extra-cellular regulated kinase (ERK1/2), Elk-1, and the 2B subunit of the NMDA receptor (Rosenblum et al., 1997; Berman et al., 2000; Barki-Harrington et al., 2009), expression levels of C/EBPβ (Yefet et al., 2006), and modulation of the protein synthesis machinery (Banko et al., 2006; Belelovsky et al., 2005; 2007; 2009). Inhibition of protein synthesis in the IC during novel taste learning disrupted long- but not short-term taste memory (Rosenblum et al., 1993; Houpt and Berlin, 1999; Merhav and Rosenblum, 2008). A proteomic study revealed induced expression of PSD-95 (post-synaptic protein 95/DLG1) in the GC, 3 h after novel taste learning (Elkobi et al., 2008). PSD-95 belongs to a family of synaptic scaffold proteins, PDZ-containing proteins, whose expression levels affect both depression, and potentiation of synaptic plasticity (Ehrlich and Malinow, 2004; Ehrlich et al., 2007).
In order to test the hypothesis that PSD-95 induction is necessary for memory formation, LVs containing RNAi to silence PSD-95 and EGFP as a fluorescent reporter were injected into the rat GC, before or after CTA training. Scrambled shRNA sequence or empty vectors were used as controls. In RNAi-injected rats there was no correlative induction of PSD-95 in the GC 3 h after taste learning, and CTA memory was diminished. However, the same manipulation of PSD-95 levels by using LVs expressing RNAi to silence PSD-95 in the GC after CTA training did not have any effect on memory retrieval or maintenance (Elkobi et al., 2008). These results indicated that transient increase in PSD-95 expression in the relevant cortical area is necessary for memory consolidation, but not for maintenance processes (Elkobi et al., 2008). The roles of other synaptic scaffold proteins such as PSD-93, SAP97, or SAP102 are yet to be studied.
In another set of experiments, researchers who were interested specifically in the processes underlying taste memory maintenance in the GC examined the role of PKMζ, which is an active form of PKM that peptide pharmacology has revealed to be relevant in Long-term Potentiation (LTP) and memory maintenance (Sacktor, 2008). By using an interfering peptide (ZIP sequence), it was shown that microinjection of PKMζ inhibitor into the GC long after CTA training diminished CTA memory but did not affect the ability to learn a new CTA association with a different taste (Shema et al., 2007). In a recent paper, the same authors used LVs expressing either RNAi to silence PKMζ or over-expression of PKMζ in the GC, weeks after CTA training. Indeed, by using LVs, the authors were able to manipulate PKMζ expression levels specifically in the GC, long after acquisition took place: reducing the expression of PKMζ attenuated CTA memory, whereas over-expression of PKMζ enhanced it (Shema et al., 2011).
Taken together, the above results demonstrate the potential for manipulating gene expression in the rodent brain by using LV vectors. However, one should take into account several questions, both technical and fundamental, that arise in this type of research. (1) Circuit questions, for example, what is the identity of cells responsible for such a strong behavioral phenotype? (2) Molecular questions regarding the integration site of the LV in these cells, and whether it plays a role in the phenotype. (3) Questions regarding the site of interference: does the phenotype relate directly to the target protein, or to a sequence of molecular events that were not addressed in the above studies? We believe that viral vector tools, together with well controlled physiological and behavioral analysis, will enable researchers to put the correlative molecular information into the context of a specific neuronal circuit, as was shown recently in the amygdala (Ciocchi et al., 2010).
Concluding Remarks
The field of viral-vector-mediated gene manipulation in the adult brain has achieved impressive progress during the last decade. In particular, LVs have now become a recognized experimental tool in neuroscience research; they offer interesting approaches for controlled, localized genetic manipulation in neurons, particularly in manipulating gene-targeted or transgenic mice. One major aspect that should be taken into account is the limited spread of LVs in the extracellular space (ECS) of the vertebrate brain, a feature that facilitates transduction of neurons located close to the injection site, but that makes these vectors inefficient in manipulating large populations of neurons. On the other hand, injection-based delivery provides modification that is highly specific in both time and location, which makes targeting of small neuronal populations with distinct cellular functions relatively easy. In addition, the fact that only a small number of neurons are transduced means that gene functions can be altered in a way that would not generate a lethal phenotype, or activate compensation mechanisms.
Vector systems may provide controllable therapy of the diseased brain. Knowledge regarding LV-mediated gene modification and its consequences is expanding continuously, and the technology is developing rapidly, therefore, this system is more attractive than other viral vectors. In viral-mediated human gene therapy a major cause for concern is possible induction of the host immune response; however, lack of established immunity to LVs in the majority of subjects represents an advantage over other vector systems, such as AAV and Ad.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by ISF (1305/08), Psychobiology and European Union Seventh Framework Program EUROSPIN (Contract HEALTH-F2-2009-241498) grants for Kobi Rosenblum.
References
Amalfitano, A., and Chamberlain, J. S. (1997). Isolation and characterization of packaging cell lines that coexpress the adenovirus E1, DNA polymerase, and preterminal proteins: implications for gene therapy. Gene Ther. 4, 258–263.
Amalfitano, A., Hauser, M. A., Hu, H., Serra, D., Begy, C. R., and Chamberlain, J. S. (1998). Production and characterization of improved adenovirus vectors with the E1, E2b, and E3 genes deleted. J. Virol. 72, 926–933.
Anliker, B., Abel, T., Kneissl, S., Hlavaty, J., Caputi, A., Brynza, J., Schneider, I. C., Munch, R. C., Petznek, H., Kontermann, R. E., Koehl, U., Johnston, I. C., Keinanen, K., Muller, U. C., Hohenadl, C., Monyer, H., Cichutek, K., and Buchholz, C. J. (2010). Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat. Methods 7, 929–935.
Baekelandt, V., Claeys, A., Eggermont, K., Lauwers, E., De Strooper, B., Nuttin, B., and Debyser, Z. (2002). Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Hum. Gene Ther. 13, 841–853.
Banko, J. L., Hou, L., Poulin, F., Sonenberg, N., and Klann, E. (2006). Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J. Neurosci. 26, 2167–2173.
Barki-Harrington, L., Elkobi, A., Tzabary, T., and Rosenblum, K. (2009). Tyrosine phosphorylation of the 2B subunit of the NMDA receptor is necessary for taste memory formation. J. Neurosci. 29, 9219–9226.
Belelovsky, K., Elkobi, A., Kaphzan, H., Nairn, A. C., and Rosenblum, K. (2005). A molecular switch for translational control in taste memory consolidation. Eur. J. Neurosci. 22, 2560–2568.
Belelovsky, K., Kaphzan, H., Elkobi, A., and Rosenblum, K. (2009). Biphasic activation of the mTOR pathway in the gustatory cortex is correlated with and necessary for taste learning. J. Neurosci. 29, 7424–7431.
Belelovsky, K., Maroun, M., and Rosenblum, K. (2007). MAPK activation in the hippocampus in vivo is correlated with experimental setting. Neurobiol. Learn. Mem. 88, 58–64.
Berges, B. K., Wolfe, J. H., and Fraser, N. W. (2005). Stable levels of long-term transgene expression driven by the latency-associated transcript promoter in a herpes simplex virus type 1 vector. Mol. Ther. 12, 1111–1119.
Berges, B. K., Wolfe, J. H., and Fraser, N. W. (2007). Transduction of brain by herpes simplex virus vectors. Mol. Ther. 15, 20–29.
Berman, D. E., Hazvi, S., Neduva, V., and Dudai, Y. (2000). The role of identified neurotransmitter systems in the response of insular cortex to unfamiliar taste: activation of ERK1-2 and formation of a memory trace. J. Neurosci. 20, 7017–7023.
Bourgeois, J. P., Goldman-Rakic, P. S., and Rakic, P. (1994). Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb. Cortex 4, 78–96.
Cardin, J. A., Carlén, M., Meletis, K., Knoblich, U., Zhang, F., Deisseroth, K., Tsai, L. H., and Moore, C. I. (2010). Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of Channelrhodopsin-2. Nat. Protoc. 5, 247–254.
Choi, J., Young, J. A., and Callaway, E. M. (2010). Selective viral vector transduction of ErbB4 expressing cortical interneurons in vivo with a viral receptor-ligand bridge protein. Proc. Natl. Acad. Sci. U.S.A. 107, 16703–16708.
Ciocchi, S., Herry, C., Grenier, F., Wolff, S. B., Letzkus, J. J., Vlachos, I., Ehrlich, I., Sprengel, R., Deisseroth, K., Stadler, M. B., Muller, C., and Luthi, A. (2010). Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282.
Cook, M. L., and Stevens, J. G. (1973). Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect. Immun. 7, 272–288.
De Vry, J., Martínez-Martínez, P., Losen, M., Temel, Y., Steckler, T., Steinbusch, H. W., De Baets, M. H., and Prickaerts, J. (2010). In vivo electroporation of the central nervous system: a non-viral approach for targeted gene delivery. Prog. Neurobiol. 92, 227–244.
Dharmapuri, S., Peruzzi, D., and Aurisicchio, L. (2009). Engineered adenovirus serotypes for overcoming anti-vector immunity. Expert Opin. Biol. Ther. 9, 1279–1287.
Dreyer, J. L. (2011). Lentiviral vector-mediated gene transfer and RNA silencing technology in neuronal dysfunctions. Methods Mol. Biol. 47, 169–187.
Dull, T., Zufferey, R., Kelly, M., Mandel, R. J., Nguyen, M., Trono, D., and Naldini, L. (1998). A third-generation lentivirus vector with a conditional packaging system. J. Virol. 72, 8463–8471.
Ehrlich, I., Klein, M., Rumpel, S., and Malinow, R. (2007). PSD-95 is required for activity-driven synapse stabilization. Proc. Natl. Acad. Sci. U.S.A. 104, 4176–4181.
Ehrlich, I., and Malinow, R. (2004). Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J. Neurosci. 24, 916–927.
Elkobi, A., Ehrlich, I., Belelovsky, K., Barki-Harrington, L., and Rosenblum, K. (2008). ERK-dependent PSD-95 induction in the gustatory cortex is necessary for taste learning, but not retrieval. Nat. Neurosci. 11, 1149–1151.
Epstein, A. L. (2005). HSV-1-based amplicon vectors: design and applications. Gene Ther. 12(Suppl. 1), S154–S158.
Epstein, A. L., Marconi, P., Argnani, R., and Manservigi, R. (2005). HSV-1-derived recombinant and amplicon vectors for gene transfer and gene therapy. Curr. Gene Ther. 5, 445–458.
Everett, R. S., Evans, H. K., Hodges, B. L., Ding, E. Y., Serra, D. M., and Amalfitano, A. (2004). Strain-specific rate of shutdown of CMV enhancer activity in murine liver confirmed by use of persistent [E1(-), E2b(-)] adenoviral vectors. Virology 325, 96–105.
Fabes, J., Anderson, P., Yanez-Munoz, R. J., Thrasher, A., Brennan, C., and Bolsover, S. (2006). Accumulation of the inhibitory receptor EphA4 may prevent regeneration of corticospinal tract axons following lesion. Eur. J. Neurosci. 23, 1721–1730.
Furth, P. A., St Onge, L., Boger, H., Gruss, P., Gossen, M., Kistner, A., Bujard, H., and Hennighausen, L. (1994). Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc. Natl. Acad. Sci. U.S.A. 91, 9302–9306.
Galimi, F., and Verma, I. M. (2002). Opportunities for the use of lentiviral vectors in human gene therapy. Curr. Top. Microbiol. Immunol. 261, 245–254.
Garcia, J., Kimfldorf, D. J., and Koelling, R. A. (1955). Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science 122, 157–158.
Gascon, S., Paez-Gomez, J. A., Diaz-Guerra, M., Scheiffele, P., and Scholl, F. G. (2008). Dual-promoter lentiviral vectors for constitutive and regulated gene expression in neurons. J. Neurosci. Methods 168, 104–112.
Gavériaux-Ruff, C., and Kieffer, B. L. (2007). Conditional gene targeting in the mouse nervous system: insights into brain function and diseases. Pharmacol. Ther. 113, 619–634.
Gossen, M., and Bujard, H. (1992). Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U.S.A. 89, 5547–5551.
Gupta, A. R., Dejneka, N. S., D'Amato, R. J., Yang, Z., Syed, N., Maguire, A. M., and Bennett, J. (2001). Strain-dependent anterior segment neovascularization following intravitreal gene transfer of basic fibroblast growth factor (bFGF). J. Gene Med. 3, 252–259.
Hartman, Z. C., Appledorn, D. M., and Amalfitano, A. (2008). Adenovirus vector induced innate immune responses: impact upon efficacy and toxicity in gene therapy and vaccine applications. Virus Res. 132, 1–14.
Hioki, H., Kuramoto, E., Konno, M., Kameda, H., Takahashi, Y., Nakano, T., Nakamura, K. C., and Kaneko, T. (2009). High-level transgene expression in neurons by lentivirus with Tet-Off system. Neurosci. Res. 63, 149–154.
Hoess, R. H., Wierzbicki, A., and Abremski, K. (1986). The role of the loxP spacer region in P1 site-specific recombination. Nucleic Acids Res. 14, 2287–2300.
Hoess, R. H., Ziese, M., and Sternberg, N. (1982). P1 site-specific recombination: nucleotide sequence of the recombining sites. Proc. Natl. Acad. Sci. U.S.A. 79, 3398–3402.
Houpt, T. A., and Berlin, R. (1999). Rapid, labile, and protein synthesis-independent short-term memory in conditioned taste aversion. Learn. Mem. 6, 37–46.
Huttenlocher, P. R. (1979). Synaptic density in human frontal cortex – developmental changes and effects of aging. Brain Res. 163, 195–205.
Huttenlocher, P. R. (1990). Morphometric study of human cerebral cortex development. Neuropsychologia 28, 517–527.
Jakobsson, J., Ericson, C., Jansson, M., Bjork, E., and Lundberg, C. (2003). Targeted transgene expression in rat brain using lentiviral vectors. J. Neurosci. Res. 73, 876–885.
Jones, M. W., French, P. J., Bliss, T. V., and Rosenblum, K. (1999). Molecular mechanisms of long-term potentiation in the insular cortex in vivo. J. Neurosci. 19, RC36.
Kochanek, S., Clemens, P. R., Mitani, K., Chen, H. H., Chan, S., and Caskey, C. T. (1996). A new adenoviral vector: replacement of all viral coding sequences with 28 kb of DNA independently expressing both full-length dystrophin and beta-galactosidase. Proc. Natl. Acad. Sci. U.S.A. 93, 5731–5736.
Konopka, W., Duniec, K., Klejman, A., Wawrzyniak, M., Owczarek, D., Gawrys, L., Maleszewski, M., Mallet, J., and Kaczmarek, L. (2009). Tet system in the brain: transgenic rats and lentiviral vectors approach. Genesis 47, 274–280.
Kristensson, K., Lycke, E., and Sjostrand, J. (1971). Spread of herpes simplex virus in peripheral nerves. Acta Neuropathol. 17, 44–53.
Kwon, I., and Schaffer, D. V. (2008). Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. Pharm. Res. 25, 489–499.
Labetoulle, M., Maillet, S., Efstathiou, S., Dezelee, S., Frau, E., and Lafay, F. (2003). HSV1 latency sites after inoculation in the lip: assessment of their localization and connections to the eye. Invest. Ophthalmol. Vis. Sci. 44, 217–225.
Lederle, L., Weber, S., Wright, T., Feyder, M., Brigman, J. L., Crombag, H. S., Saksida, L. M., Bussey, T. J., and Holmes, A. (2011). Reward-related behavioral paradigms for addiction research in the mouse: performance of common inbred strains. PLoS One 6, e15536. doi: 10.1371/journal.pone.0015536
Lundberg, C., Bjorklund, T., Carlsson, T., Jakobsson, J., Hantraye, P., Deglon, N., and Kirik, D. (2008). Applications of lentiviral vectors for biology and gene therapy of neurological disorders. Curr. Gene Ther. 8, 461–473.
Markowitz, M., Nguyen, B. Y., Gotuzzo, E., Mendo, F., Ratanasuwan, W., Kovacs, C., Prada, G., Morales-Ramirez, J. O., Crumpacker, C. S., Isaacs, R. D., Gilde, L. R., Wan, H., Miller, M. D., Wenning, L. A., and Teppler, H. (2007). Rapid and durable antiretroviral effect of the HIV-1 Integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J. Acquir. Immune Defic. Syndr. 46, 125–133.
Merhav, M., Kuulmann-Vander, S., Elkobi, A., Jacobson-Pick, S., Karni, A., and Rosenblum, K. (2006). Behavioral interference and C/EBPbeta expression in the insular-cortex reveal a prolonged time period for taste memory consolidation. Learn. Mem. 13, 571–574.
Merhav, M., and Rosenblum, K. (2008). Facilitation of taste memory acquisition by experiencing previous novel taste is protein-synthesis dependent. Learn. Mem. 15, 501–507.
Metzger, D., and Chambon, P. (2001). Site- and time-specific gene targeting in the mouse. Methods 24, 71–80.
Miller, B. H., Schultz, L. E., Gulati, A., Su, A. I., and Pletcher, M. T. (2010). Phenotypic characterization of a genetically diverse panel of mice for behavioral despair and anxiety. PLoS One 5, e14458. doi: 10.1371/journal.pone.0014458
Mitani, K., Graham, F. L., Caskey, C. T., and Kochanek, S. (1995). Rescue, propagation, and partial purification of a helper virus-dependent adenovirus vector. Proc. Natl. Acad. Sci. U.S.A. 92, 3854–3858.
Molotkov, D. A., Yukin, A. Y., Afzalov, R. A., and Khiroug, L. S. (2010). Gene delivery to postnatal rat brain by non-ventricular plasmid injection and electroporation. J. Vis. Exp. 43, e2244.
Naldini, L., Blomer, U., Gage, F. H., Trono, D., and Verma, I. M. (1996a). Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. U.S.A. 93, 11382–11388.
Naldini, L., Blomer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F. H., Verma, I. M., and Trono, D. (1996b). In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267.
Nanou, A., and Azzouz, M. (2009). Gene therapy for neurodegenerative diseases based on lentiviral vectors. Prog. Brain Res. 175, 187–200.
Neve, R. L., Neve, K. A., Nestler, E. J., and Carlezon, W. A. Jr. (2005). Use of herpes virus amplicon vectors to study brain disorders. Biotechniques 39, 381–391.
Papale, A., Cerovic, M., and Brambilla, R. (2009). Viral vector approaches to modify gene expression in the brain. J. Neurosci. Methods 185, 1–14.
Pauwels, K., Gijsbers, R., Toelen, J., Schambach, A., Willard-Gallo, K., Verheust, C., Debyser, Z., and Herman, P. (2009). State-of-the-art lentiviral vectors for research use: risk assessment and biosafety recommendations. Curr. Gene Ther. 9, 459–474.
Pere, D., Ignacio, S. L., Ramon, T., Fernando, L., Alberto, T., Pompeyo, V., Juan, G., Galindo, M. J., Paloma, G., Antonio, V., Jaime, C., Esteban, R., Bernardino, R., Garcia-Alcalde, M. L., Trinitario, S., Ferran, T., Juan Ramon, L., and Myriam, G. (2008). Dyslipidemia and cardiovascular disease risk factor management in HIV-1-infected subjects treated with HAART in the Spanish VACH cohort. Open AIDS J. 2, 26–38.
Petros, T. J., Rebsam, A., and Mason, C. A. (2009). In utero and ex vivo electroporation for gene expression in mouse retinal ganglion cells. J. Vis. Exp. 31, e1333.
Ramezani, A., and Hawley, R. G. (2002). Overview of the HIV-1 lentiviral vector system. Curr. Protoc. Mol. Biol. Chapter 16, Unit 16.21.
Richardson, W. D., and Westphal, H. (1984). Requirement for either early region 1a or early region 1b adenovirus gene products in the helper effect for adeno-associated virus. J. Virol. 51, 404–410.
Rosenblum, K. (2009). “Conditioned taste aversion and taste learning: molecular mechanisms,” in Concise Learning and Memory: The Editor's Selection, ed. John H Byrne (London: Academic Press), 465–482.
Rosenblum, K., Berman, D. E., Hazvi, S., Lamprecht, R., and Dudai, Y. (1997). NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J. Neurosci. 17, 5129–5135.
Rosenblum, K., Meiri, N., and Dudai, Y. (1993). Taste memory: the role of protein synthesis in gustatory cortex. Behav. Neural Biol. 59, 49–56.
Sacktor, T. C. (2008). PKMzeta, LTP maintenance, and the dynamic molecular biology of memory storage. Prog. Brain Res. 169, 27–40.
Saeki, Y., Fraefel, C., Ichikawa, T., Breakefield, X. O., and Chiocca, E. A. (2001). Improved helper virus-free packaging system for HSV amplicon vectors using an ICP27-deleted, oversized HSV-1 DNA in a bacterial artificial chromosome. Mol. Ther. 3, 591–601.
Sauer, B., and Henderson, N. (1988). Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl. Acad. Sci. U.S.A. 85, 5166–5170.
Scarpini, C. G., May, J., Lachmann, R. H., Preston, C. M., Dunnett, S. B., Torres, E. M., and Efstathiou, S. (2001). Latency associated promoter transgene expression in the central nervous system after stereotaxic delivery of replication-defective HSV-1-based vectors. Gene Ther. 8, 1057–1071.
Shema, R., Haramati, S., Ron, S., Hazvi, S., Chen, A., Sacktor, T. C., and Dudai, Y. (2011). Enhancement of consolidated long-term memory by overexpression of protein kinase Mzeta in the neocortex. Science 331, 1207–1210.
Shema, R., Sacktor, T. C., and Dudai, Y. (2007). Rapid erasure of long-term memory associations in the cortex by an inhibitor of PKM zeta. Science 317, 951–953.
Smith, R. H. (2008). Adeno-associated virus integration: virus versus vector. Gene Ther. 15, 817–822.
Srivastava, A., Lusby, E. W., and Berns, K. I. (1983). Nucleotide sequence and organization of the adeno-associated virus 2 genome. J. Virol. 45, 555–564.
Stewart, P. L., Fuller, S. D., and Burnett, R. M. (1993). Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 12, 2589–2599.
Themis, M., Waddington, S. N., Schmidt, M., von Kalle, C., Wang, Y., Al-Allaf, F., Gregory, L. G., Nivsarkar, M., Themis, M., Holder, M. V., Buckley, S. M., Dighe, N., Ruthe, A. T., Mistry, A., Bigger, B., Rahim, A., Nguyen, T. H., Trono, D., Thrasher, A. J., and Coutelle, C. (2005). Oncogenesis following delivery of a nonprimate lentiviral gene therapy vector to fetal and neonatal mice. Mol. Ther. 12, 763–771.
Tsien, J. Z., Chen, D. F., Gerber, D., Tom, C., Mercer, E. H., Anderson, D. J., Mayford, M., Kandel, E. R., and Tonegawa, S. (1996a). Subregion- and cell type-restricted gene knockout in mouse brain. Cell 87, 1317–1326.
Tsien, J. Z., Huerta, P. T., and Tonegawa, S. (1996b). The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell 87, 1327–1338.
Verma, I. M., and Weitzman, M. D. (2005). Gene therapy: twenty-first century medicine. Annu. Rev. Biochem. 74, 711–738.
Waehler, R., Russell, S. J., and Curiel, D. T. (2007). Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 8, 573–587.
Wanisch, K., and Yanez-Munoz, R. J. (2009). Integration-deficient lentiviral vectors: a slow coming of age. Mol. Ther. 17, 1316–1332.
Weindler, F. W., and Heilbronn, R. (1991). A subset of herpes simplex virus replication genes provides helper functions for productive adeno-associated virus replication. J. Virol. 65, 2476–2483.
Wong, L. F., Azzouz, M., Walmsley, L. E., Askham, Z., Wilkes, F. J., Mitrophanous, K. A., Kingsman, S. M., and Mazarakis, N. D. (2004). Transduction patterns of pseudotyped lentiviral vectors in the nervous system. Mol. Ther. 9, 101–111.
Wong, S. P., Argyros, O., Howe, S. J., and Harbottle, R. P. (2010). Systemic gene transfer of polyethylenimine (PEI)-plasmid DNA complexes to neonatal mice. J. Control Release 3, 298–306.
Xiang, Z. Q., Yang, Y., Wilson, J. M., and Ertl, H. C. (1996). A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219, 220–227.
Yefet, K., Merhav, M., Kuulmann-Vander, S., Elkobi, A., Belelovsky, K., Jacobson-Pick, S., Meiri, N., and Rosenblum, K. (2006). Different signal transduction cascades are activated simultaneously in the rat insular cortex and hippocampus following novel taste learning. Eur. J. Neurosci. 24, 1434–1442.
Zhang, F., Gradinaru, V., Adamantidis, A. R., Durand, R., Airan, R. D., de Lecea, L., and Deisseroth, K. (2010). Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 5, 439–456.
Keywords: viral vectors, lentivirus (LV), gene regulation, learning and memory
Citation: Edry E, Lamprecht R, Wagner S and Rosenblum K (2011) Virally mediated gene manipulation in the adult CNS. Front. Mol. Neurosci. 4:57. doi: 10.3389/fnmol.2011.00057
Received: 08 May 2011; Paper pending published: 05 October 2011;
Accepted: 15 December 2011; Published online: 26 December 2011.
Edited by:
Alistair N. Garratt, Max Delbrück Center for Molecular Medicine, GermanyReviewed by:
Josef Kittler, University College London, UKUrs Albrecht, University of Fribourg, Switzerland
Alistair N. Garratt, , Max Delbrück Center for Molecular Medicine, Germany
Copyright: © 2011 Edry, Lamprecht, Wagner and Rosenblum. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Efrat Edry, Department of Neurobiology and Ethology, Center for Gene Manipulation in the Brain (CGMB), University of Haifa, Mt Carmel, Haifa 31905, Israel. e-mail: eedry@univ.haifa.ac.il