- 1Student Research Committee, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 2Student Research Committee, School of Medicine, Shahroud University of Medical Sciences, Shahroud, Iran
- 3Department of Tissue Engineering, School of Advanced Technologies in Medicine, Fasa University of Medical Sciences, Fasa, Iran
- 4Fertility and Infertility Research Center, Health Technology Institute, Kermanshah University of Medical Sciences, Kermanshah, Iran
- 5Department of Tissue Engineering, School of Medicine, Kermanshah University of Medical Sciences, Kermanshah, Iran
In tissue engineering, the decellularization of organs and tissues as a biological scaffold plays a critical role in the repair of neurodegenerative diseases. Various protocols for cell removal can distinguish the effects of treatment ability, tissue structure, and extracellular matrix (ECM) ability. Despite considerable progress in nerve regeneration and functional recovery, the slow regeneration and recovery potential of the central nervous system (CNS) remains a challenge. The success of neural tissue engineering is primarily influenced by composition, microstructure, and mechanical properties. The primary objective of restorative techniques is to guide existing axons properly toward the distal end of the damaged nerve and the target organs. However, due to the limitations of nerve autografts, researchers are seeking alternative methods with high therapeutic efficiency and without the limitations of autograft transplantation. Decellularization scaffolds, due to their lack of immunogenicity and the preservation of essential factors in the ECM and high angiogenic ability, provide a suitable three-dimensional (3D) substrate for the adhesion and growth of axons being repaired toward the target organs. This study focuses on mentioning the types of scaffolds used in nerve regeneration, and the methods of tissue decellularization, and specifically explores the use of decellularized nerve tissues (DNT) for nerve transplantation.
Introduction
The central nervous system (CNS) includes the nervous tissue of the brain and spinal cord, and diseases such as multiple sclerosis, stroke, Alzheimer’s, Parkinson’s, and Huntington’s disease cause disorders in this system. Peripheral nervous system (PNS) refers to nerve bundles outside of the CNS and its disorders include Guillain-Barre syndrome, peripheral neuropathy, and radiculopathy (Borsook, 2012). Although no long-lasting treatments exist for neurological illnesses, especially in the CNS, cell therapy offers a potential way to repair degenerative tissue. Based on biomaterials, tissue engineering holds promise for neuro-regeneration and repair (Akhtar et al., 2022). This can be achieved using embryonic or fetal cells, but ethical concerns and a high likelihood of tissue rejection are associated with this approach. On the other hand, using adult cells like neural stem cells allows for autologous grafts with fewer ethical issues (Sivandzade and Cucullo, 2021; Sharifi et al., 2022).
In the field of neurological regeneration, the use of decellularized tissues through various physical and chemical protocols has shown significant promise in both animal and human clinical applications (Teebken et al., 2000). The tissues obtained from the normal nerve structure and extracellular matrix (ECM) can be transplanted as graft tissues (Gilbert et al., 2006; Nune et al., 2016). This review article specifically focuses on nerve regeneration using decellularized nerve tissues (DNT). In recent studies, the use of biological scaffolds has been the focus of researchers in nerve repair (Hsu et al., 2023; Yu et al., 2023). Therefore, we decided to mention this issue in this paper, and we hope that we have helped those interested in this field.
Figure 1 illustrates the history of nerve regeneration with and without DNT, along with the number of related articles and their research field. Nerve regeneration is a topic that has been receiving the attention of medical science for many years, and the process of nerve grafting using different materials has been researched over time. But recently, researchers’ attention has grown to repair damaged nerves using decellularized tissues, which has led to the publication of numerous articles and books and the holding of various scientific conferences in this field. The use of decellularized tissues as the new approaches in nerve transplantation, especially in different scientific branches, has been analyzed due to its unique features. This innovative technique can help repair and regenerate nerves over time. Therefore, all these efforts and developments can be significantly effective in advancing science in the field of nerve regeneration.

Figure 1. Scientific attention to nerve regeneration based on Scopus, PubMed, and Web of Science reports: (A) Number of published articles on nerve regeneration with and without DNT from 2000 to 2024, (B) type of published literature on nerve regeneration with and without DNT, (C) statistics of scientific attention to different branches of nerve regeneration with and without DNT.
CNS/PNS injury
CNS traumatic injuries, with their destructive and persistent complications, include traumatic brain injury (TBI) and traumatic spinal cord injury (SCI) (Roozenbeek et al., 2013; Walter and Zweckberger, 2018). TBI encompasses various neurological disorders such as tissue contusion, subdural and epidural hematoma, penetrating injuries, or diffuse damage, and can be classified by the Glasgow Coma Scale (GCS) into severe [3–8], moderate [9–12], and mild [13–15] (Maas et al., 2008). Although there is no precise analysis of physical function, determining the exact location and size of the lesion is significant for assessing the extent of functional recovery after the injury (Fouad et al., 2021).
Wallerian degeneration (WD) is a process that occurs in both CNS and PNS traumatic injuries, as well as in neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD), leading to degeneration of distal axons detached from their cell bodies (Rotshenker, 2011). Furthermore, there are contradictory findings regarding the effect of inflammation after CNS trauma on neurons; some studies show a detrimental effect, while others suggest it stimulates axon regeneration (Yin et al., 2006). The pathophysiology of SCI includes the death of neural cells and disruption of axonal connections. This degenerative process in distal long axons is called WD, but in the proximal stem, it can lead to the vulnerability of cell bodies (Ruff et al., 2008).
The PNS is composed of neuronal cells, glial cells, and stromal cells that conduct signals between the CNS and the body (Menorca et al., 2013). Challenges resulting from PNS injury include focal demyelination (neurapraxia), axon damage in addition to focal demyelination with normal connective tissue (axonotmesis), and complete dissection of axons and connective tissue (neurotmesis). Additionally, inflammation in the PNS plays a positive role in axon regeneration, but the precise mechanism of this immune reaction is not fully understood (Ankeny et al., 2009; Figure 2).

Figure 2. TBI (A) (Crupi et al., 2020) and SCI (B) (Feng et al., 2021) are serious neurological conditions, each with its pathophysiological processes following the injury. Adapted and reprinted.
Nerve repair with tissue engineering scaffolds
The treatment of PNS, particularly in the crucial gap of more than 3 cm, remains a significant clinical issue despite the tremendous advancements in medicine (Hussin et al., 2018). According to reports, many variables, such as the extent of the injury, the presence of neurotrophic factors, the existence of Schwann cells, etc., affect the restricted regeneration of peripheral nerves. Peripheral nerve damage is one of the most frequent causes of sensorimotor abnormalities and decreased productivity in adults, according to the literature (Moore et al., 2011). Since peripheral nerves have a limited capacity for self-regeneration, tissue engineering is employed as a potential method for nerve repair. The defect and formation of a gap in the peripheral nerve result in a severe loss of sensory or motor function in the affected animal or person. When the damage is minor, the nervous system may mend itself, which is a complicated biological event. It is challenging to restore the injured nerve in severe injuries that result in a large gap (Gontika et al., 2018).
Stem cells, natural and man-made materials, decellularized grafts, and other cell types are frequently utilized in regenerative medicine, a large branch of medical sciences, to restore tissues or damaged organs (Campbell, 2008). The creation and restoration of an organ’s normal function are the ultimate objectives of regenerative medicine. Tissue engineers have created innovative compounds and scaffolds of organic and synthetic materials with physical, chemical, and biological characteristics, targeting tissues. The creation of nerve conduits results from researchers’ efforts to address the drawbacks of nerve autograft transplantation (Yu et al., 2023).
To find a solution for the reconstruction and transplantation of organs, xenografts were proposed, but they were also rejected. The existing limitations for transplantation have led to the use of gene-edited pigs as an effective source for this purpose. The creation of pigs devoid of xenoantigens has been made possible via nuclease-based genome alteration techniques combined with somatic cell nuclear transfer (SCNT). These pigs’ organs prevent hyperacute rejection (HAR) and last longer after being transplanted into non-human primates (NHPs) (Navarro-Serna et al., 2022). A clinical xenotransplantation experiment using porcine skin grafts from pigs was launched in 2019 with FDA approval. At the end of the 30-day experimental period, preliminary findings indicated satisfactory graft acceptance and there was no indication of zoonotic disease transmission (Eyestone et al., 2020). These efforts have led to the development of several efficient xenotransplantation techniques.
Currently, both natural and artificial materials, including both autologous and non-autologous tissue transplants, and tissue, and cell transplants, are employed to regenerate neural tissues. End-to-end repair is the main therapy used to get rid of these lesions. Peripheral nerve grafts or nerve conduits will be employed if end-to-end repair is not possible (Jiang et al., 2023).
Neural conduits made of synthetic and natural polymers
Recent advancements in nerve conduit technology have utilized a combination of synthetic and natural polymers, such as Oligo-polyethylene glycol fumarate (OPF), Poly caprolactone (PCL), Poly-L-lactic acid (PLLA), Polycaprolactone fumarate (PCLF), Polylactic -co-glycolic acid (PLGA), collagen, chitosan, and fibrin, to facilitate nerve regeneration (Liu et al., 2017). Engineered nerve conduits, resembling nerve tissue with a collagen basis, encourage axon rebuilding, inhibit scar tissue invasion, and enhance the production of neurotrophic factors (Daly et al., 2013).
Bioactive properties in proteins (silk, collagen, etc.), polysaccharides (hyaluronic acid, chitosan, etc.), and polyesters (P3HB and PHBV) improve cell-scaffold interaction, facilitating tissue regeneration (Arslantunali et al., 2014; Ehterami et al., 2022). Biodegradable polymeric nerve conduits based on copolymers like poly-L lactide, polyurethane, and polyglycolic acid show promise as ideal scaffolds due to their bio-absorbability and mechanical characteristics (Cingolani et al., 2018; Ehterami et al., 2021).
These thermoplastic materials can be produced through various methods such as extruding, molding, printing, and dipping (Li et al., 2020). Natural polymers, when combined with synthetic polymers like PCL with collagen-based beads, exhibit enhanced strength and flexibility. Several neural conduits made from synthetic and natural polymers have received: food and drug administration (FDA) approval for clinical use (Deal et al., 2012, Kehoe et al., 2012). While artificial nerve conduits have some drawbacks, including inflammation and stiffness, biological conduits made of veins, arteries, and fibroblasts show promise (Hussin et al., 2018). Natural biological nerve conduits, including vessels, decellularized nerves, and muscle tissue, have been successfully used in animal models and human practice, offering a favorable environment for cell adhesion and proliferation, promoting axon regeneration and vascularization (Gontika et al., 2018; Table 1).
Extracellular matrix
ECM consists of proteoglycans, fibronectin, vitronectin, laminin, tenascin, thrombospondin, fibrillin, and collagen, which play a crucial role in adherence, duplication, and differentiation functions (Badylak et al., 2015). Any disruption in the regular arrangement of these biomolecules can lead to various diseases (Hinderer et al., 2016).
As we all know, tissue engineering efficiently regenerates damaged tissues using a mix of cells, scaffolds, and growth factors. Unfortunately, designed scaffolds of non-native materials frequently fail to establish adequate cell connections, which prevents essential functions from occurring during tissue healing. Using natural ECMs with various functional properties, such as structural support for cells, provision of a mechanical environment for cells, biodegradability to form functional microvascular networks, biocompatibility, and appropriate biological activities, can help overcome these difficulties (O'brien, 2011). Therefore, a scaffold matching the target tissue’s original ECM architecture would be optimal for regenerative medicine. However, given the present technology, effective natural ECM mimicking for synthetic tissues continues to be difficult (Kim et al., 2016).
Unfortunately, there are several obstacles in the way of tissue-derived ECM’s therapeutic use. ECM made from animal tissue is used as an alternate source due to the restricted availability of human cadaveric tissue. Immune rejection and disease spread are possible with incomplete tissue decellularization. Due to the difficulty in isolating some specialized tissues, including stem cell niches, some ECMs are not readily accessible. Due to these reasons, not all tissue regeneration procedures may employ ECM produced from decellularized tissues (Assunção et al., 2020). In particular, neural ECMs enable neural stem cell differentiation and promote axonal development in vitro, which have demonstrated positive outcomes for several neural tissue regeneration issues such as spinal cord injuries. The decellularized swine brain in mice with spinal cord injury (SCI) restored motor function up to two months after injury and increased the reparative macrophage phenotype. It’s interesting to note that optic nerve decellularization produced the best possible optic nerve performance by removing some axonal growth inhibitory molecules while leaving others intact (Ma et al., 2021; da Silva et al., 2023).
Definition and methods of tissue decellularization
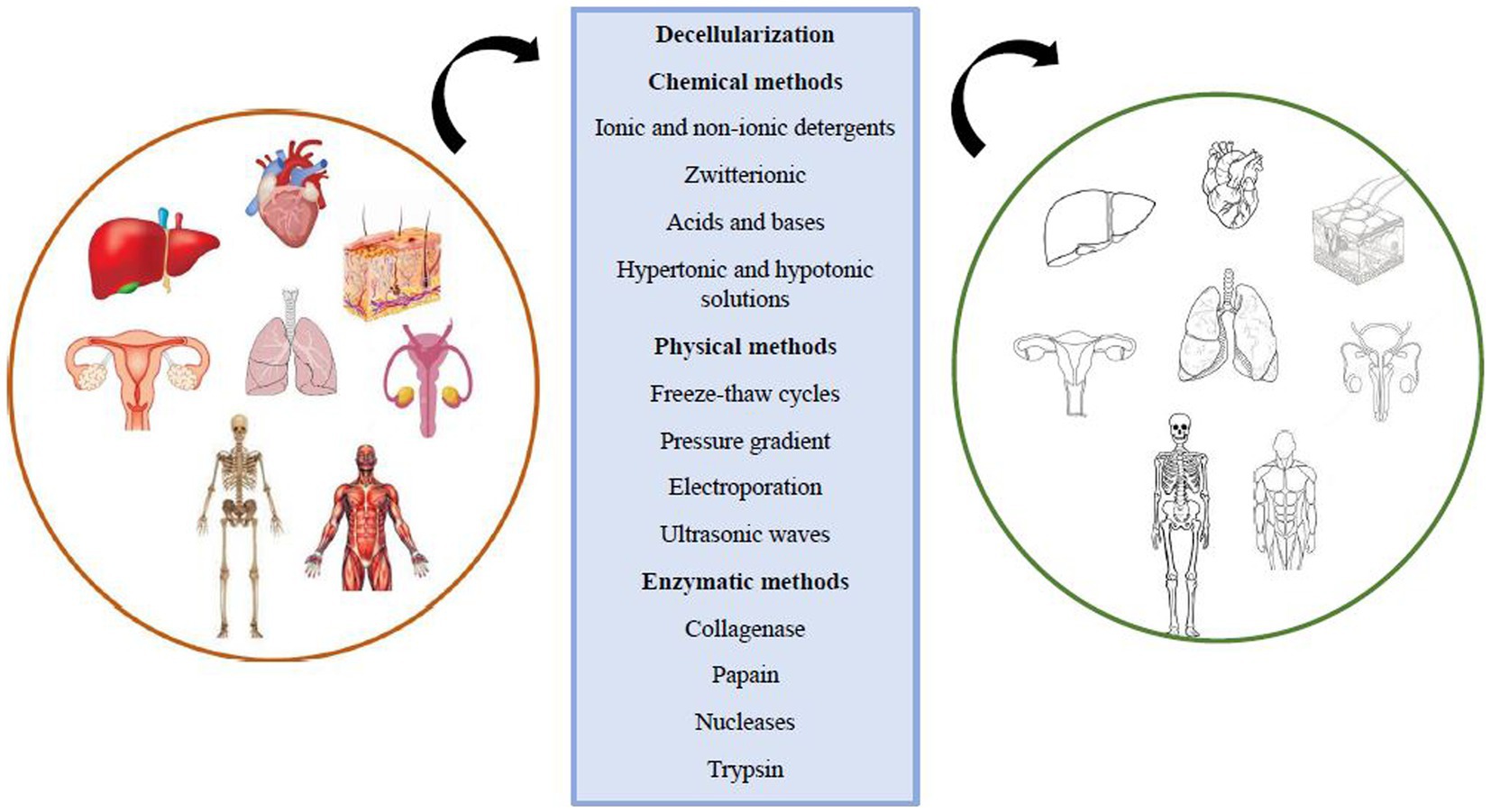
Scaffolds are crucial in tissue engineering as they facilitate the attachment and growth of foreign cells, providing mechanical and structural stability while distributing necessary growth factors for tissue regeneration. Scaffolds can be made from synthetic materials or natural tissues, with natural scaffolds offering biocompatibility and encouraging proper cellular interactions. Decellularization is a process that removes cellular components from the extracellular matrix (ECM) while preserving its structural and functional proteins and glycosaminoglycans. This creates a natural 3D form with regenerative properties (Mendibil et al., 2020). This comment is mentioned in the nerve repair with tissue engineering scaffolds section (Alizadeh et al., 2020). Decellularization eliminates the risk of inflammatory reactions and immunological rejection at the transplant site by removing cellular components and antigens. However, striking a balance between retaining the ECM structure and removing cellular waste products is essential to prevent inflammatory responses and support healthy regeneration. The ECM composition, surface topology, and 3D structure play crucial roles in host tissue regeneration responses, cell migration, proliferation, differentiation, mitogenesis, and chemotaxis. Decellularization can be applied to various tissues using physical, chemical, and enzymatic methods, or their combinations (Alizadeh et al., 2021; Khazaei et al., 2022) (Figure 3).
Chemical techniques
Chemical detergents, acids, bases, alcohols, and hypotonic and hypertonic solutions are used in decellularization processes. Detergents are polar and hydrophobic amphipathic molecules that can dissolve hydrophobic substances in water. Based on their polar head, detergents are categorized into three groups: ionic, non-ionic, and zwitterionic. Detergents that are ionic, non-ionic, or zwitterionic effectively remove biological components from the tissue by separating DNA from proteins and cell membranes. However, these elements disrupt the ECM’s protein structure (Zahmati et al., 2017; Khazaei et al., 2023).
Ionic detergents: Ionic detergents are potent cleaning agents that may fully denature proteins and obliterate cell membranes. Among the most used ionic decellularization agents are sodium hypochlorite, sodium deoxycholate (SDC), and sodium dodecyl sulfate (SDS). Cytoplasmic membranes, lipids, and DNA may all be successfully dissolved by these detergents. The process of decellularization frequently employs SDS (Montoya and McFetridge, 2009). SDS is more efficient than other detergents at removing cell waste products like nuclear and cytoplasmic substances from dense and thick tissues like the heart. As a result, it is the detergent that is employed in the decellularization process the most frequently. 1%SDS (w/v) is the most typical concentration in the decellularization procedure. SDS breaks down the covalent links that hold proteins together (Syazwani et al., 2015). Non-ionic detergents: less damaging to the ECM’s structural integrity. However, the decellularization process makes extensive use of these detergents. Protein–protein bindings cannot be broken by non-ionic detergents, however lipid-lipid and lipid-protein bonds can be (Alizadeh et al., 2022).
Decellularization uses Triton X-100 as a non-ionic detergent (El-Husseiny et al., 2023). When decellularizing thin tissues, zwitterionic or bipolar detergents—which have both ionic and nonionic properties—are frequently employed. Proteins are protected in their native condition throughout the decellularization process by zwitterionic detergents’ hydrophilic groups, which have zero net electric charges. These cleaners include: (3-cholamidopropyl) (dimethylammonio]-1-propane sulfonate (CHAPS), Sulfobetaine 16 (SB-16) and Sulfobetaine 16 (SB-16), for instance (Faulk et al., 2014).
Acids and bases: Acids and bases are catalysts or agents that hydrolyze biomolecules. Chromosomes and plasmid DNA are disrupted by bases. Ammonium hydroxide, sodium sulfide, sodium hydroxide, and calcium hydroxide are examples of bases (Reing et al., 2010). During the earliest steps of decellularization, the hair from dermis samples is typically removed using sufficiently coarse blades. Collagen fiber degradation and collagen cross-link disruption are the main mechanisms through which bases impair mechanical characteristics (Gupta et al., 2018). By dissolving cytoplasmic substances and rupturing nucleic acids, acids operate to remove DNA from ECM. They can also denature ECM proteins such as glycosaminoglycans (GAGs), collagen, and growth factors (Bourgine et al., 2013). Acetic acid, peracetic acid, hydrochloric acid, and sulfuric acid are among the acids employed in decellularization procedures. It should be mentioned that it’s crucial to optimize both the dose and the timing of the acids employed for decellularization (Gilbert, 2012).
Alcohols: By lysing and drying out cells, alcohols like glycerol aid in the decellularization process. According to reports, alcohol can be used to tackle the problem of phospholipids causing calcification and prosthesis failure in valves and conduits (Montoya and McFetridge, 2009). Alcohols like isopropanol, ethanol, and methanol may turn a tissue into one that is free of fat in a very short amount of time and is far more efficient than lipases in doing so. A crucial factor is the need for extreme caution while decellularizing tissues with alcohol. Because alcohols like ethanol and methanol may fix tissue, precipitate proteins, and harm the ECM’s structure. Additionally, as a last wash to get rid of any remaining nucleic acid in the tissue, ethanol or methanol might be utilized (Somuncu, 2020).
Solutions that are either hypertonic or too hypotonic lead to osmotic stress in the tissue and cell membrane breakdown in tissues or organs, which results in cell lysis. Proteins and DNA can be separated by hypertonic solutions. The washing of cellular debris is one of the purposes of utilizing hypertonic and hypotonic solutions, yet these solutions fall short of properly cleaning and removing the cellular debris from the tissues. Simple osmotic actions in hypotonic fluids can quickly lead to cell lysis with no alteration of the matrix molecules or their structural makeup. Tissues should be submerged alternately in hypertonic and hypotonic solutions to see the greatest osmotic effects. After the cells have been lysed, washing repeatedly with hypertonic and hypotonic liquids aids in washing the leftover cells from the tissue (Gilbert, 2012).
Chelators and Toxins: By binding to divalent cations, ethylene glycol, tetraacetic acid, and ethylene diamine tetraacetic acid functions as a chelating agent in the decellularization of organs. By separating ions like Ca2+ and Mg2+, the chemical components of these substances like ethylenediaminetetraacetic acid (EDTA) and ethylene glycol-bis (β-aminoethyl ether)-N, N,N′,N′-tetraacetic acid (EGTA) break the cell’s attachment to collagen and fibronectin. Chelating agents are typically employed in conjunction with other detergents or enzymes like trypsin since they are ineffective on their own (Goldstein and Thayer, 2014). Additionally, it has been mentioned that cytotoxic substances can be utilized in the decellularization of cells since toxins can harm cells (Keane et al., 2015).
Physical techniques
Cells in tissue and organs are extensively destroyed by thermal shock and freezing and thawing cycles. However, some of the membrane and cell material are still present and can be eliminated using further decellularization techniques. Due to the geometrical form of the ice crystals, this approach may disrupt the cell scaffold and has little impact on the mechanical capabilities of the ECM. It also partially destroys the ECM structure (Khazaei et al., 2021). In this process, ice crystals fill the inside of the cell, causing the cell membrane to burst. The use of freezing and thawing cycles might lessen immunological reactions that are unfavorable, including leukocyte infiltration in vascular ECM scaffolds. The melting temperature (37°C) and the freezing temperature can be unilaterally altered in this method’s temperature range. Varied authors have employed varied and arbitrary numbers of thermal shock cycles (Poornejad et al., 2015).
Mechanical and hydrostatic pressure: By physically scraping the tissue or organ with the use of sharp or abrasive instruments with enzymes or salt solution, the surface cells of the tissue or organ can be successfully removed. Better decellularization of tissues and organs is facilitated by the physical removal of the surface layers, but the amount of force used must be exact since the membrane connections and underlying structure are sensitive to any form of direct mechanical stress (Santoso et al., 2014). In the hydrostatic pressure approach, the target tissue is sprayed with pressured water, which works faster and more effectively than the procedure that uses detergents or enzymes. However, the ECM structure might be harmed by the ice crystals that develop when there is water present. Therefore, using this approach to raise the temperature during the decellularization process eliminates the chance of ice crystal formation. However, this temperature rise also raises entropy, which makes the ECM more prone to failure (Watanabe et al., 2019).
Pressure gradient: Along with the use of enzymes, pressure gradient induction is a technique to aid in improved decellularization. The use of a pressure gradient offers the chance for more appropriate penetration of the enzyme agent into the tissue, and as a result, the tissue decellularization process will be carried out with a higher percentage in the decellularization of hollow tissues like veins or thicker tissues like tendons (Sierad et al., 2015).
Electroporation (permeabilizing the cell membrane): This technique might be cited among others for decellularizing tissues or organs. In this technique, the tissue is exposed to microsecond electrical pulses that cause the cell membrane to develop tiny holes. Cell homeostasis loss and eventual cell death are also possible outcomes of these holes. The relatively tiny electrodes used in this approach, which restrict tissue decellularization and make it unsuitable for vast tissues, are one of its drawbacks. More crucially, since the immune system will be responsible for the cell elimination mechanism, the decellularization process must be carried out inside the body. As a result, the applications of this technology are severely constrained (Sano et al., 2010).
Ultrasonic waves: Cells in a tissue or organ bath are separated using ultrasonic waves, which are frequently utilized at frequencies higher than 20 kHz. Cell membranes, internal components, and intermolecular connections can all be destroyed by these strong waves. The waves’ destructiveness increases with decreasing frequency (Azhim et al., 2011). Sonication refers to the use of ultrasonic waves for a variety of purposes. It’s crucial to keep bubbling under control while the procedure is going on. Due to the extreme fluid pressure fluctuations brought on by waves, this physical phenomenon is unavoidable, yet unchecked bubbling can seriously harm the tissue’s structure and mechanical capabilities. Additionally, the temperature, viscosity, and amount of gas dissolved in the various fluids all affect how much bubbling occurs (Forouzesh et al., 2019). Vacuum: The transmission of detergents to the target tissue for decellularization is accelerated and improved by the use of a vacuum (Butler et al., 2017).
Supercritical fluid: After initial decellularization using detergents like alcohol, supercritical fluid can be employed as a neutralizing agent to remove cellular debris from the tissue since it eliminates it as it travels through the tissue. Supercritical fluid has a fast passage rate and low viscosity, which allows for quick and easy decellularization procedures. The use of an inert material (such as carbon dioxide) to remove cells and little change in the mechanical characteristics of the ECM are two benefits of decellularization using the supercritical fluid approach. The ECM, however, might be obliterated by the pressure needed to introduce the supercritical fluid phase. Additionally, the tissues are dried following the decellularization procedure, doing away with the necessity for lyophilization, a typical method for facilitating long-term preservation (Boer et al., 2015).
Supercritical fluid is permeable like gasses and has the same density as liquids. SC-CO2 has a critical temperature and pressure of 31.1°C and 7.40 MPa, respectively. These values match the physiological circumstances of 37°C and 15 MPa. Additionally, because of the tissue’s great permeability, carbon dioxide gas is promptly expelled from it without the need for extra washing. The polar portion of the membrane, which is a phospholipid, may readily be removed by the addition of ethanol even though carbon dioxide gas is non-polar. Additionally, the tissue’s whole mechanical and structural characteristics are unaltered (Gilpin and Yang, 2017).
One of the useful methods of decellularization is immersion and the induction of turbulence, which allows chemicals to reach tissue cells more effectively and improves decellularization. This technique is used to decellularize tissues that lack a substantial circulatory network, making it impossible for decellularization agents to directly access every region of the tissue. The bladder, esophagus, trachea, skeletal muscles, tendons, heart valves, and cartilage are just a few examples of these tissues. In this technique, the target tissue or organ is submerged in the decellularization agent-filled chambers. The type of decellularization agent used, the thickness and density of the tissue, and the duration of immersion are all factors (Keane et al., 2015).
Perfusion: One way to decellularize organs is to create flow and perfusion in two opposing directions, fully separating the organ from its primary artery. Additionally, chemicals are pumped into its vascular system after washing with detergents. Controlling the flow rate of fluids is crucial because the pressure needed to push detergents and chemicals along the vascular system can lead to capillaries and tiny arteries rupturing (Crapo et al., 2011).
Enzymatic techniques
The most popular decellularization techniques involve the use of enzymes, which are frequently combined with chemical techniques to aid in eliminating cells and fully demolishing nuclear material from tissue. By stifling connections between cells and the ECM or by locating and eliminating undesirable proteins, enzymes are employed to decellularize cells. Enzymes including trypsin, protease, lipase, hyaluronidase, dispase, collagenase, papain, and nucleases are utilized in these procedures (Rieder et al., 2004; Pellegata et al., 2013).
One of the most used enzymatic decellularization agents is trypsin. ECM proteins do, however, exhibit some degree of resistance to trypsin, therefore use caution when exposed to it. Trypsin eliminates cells more gradually than detergents, damages elastin and collagen more severely, but better retains GAG content. Trypsin usage causes changes in the mechanical characteristics of ECM and decreases its strength by destroying collagen and breaking the connections that hold it together. To disrupt the tissue’s architecture and provide the potential of quick dissolving by detergents or enzymatic chelating agents, trypsin is an aggressive enzyme that particularly cuts peptide chains from the carboxyl side of the amino acids lysine or arginine. Rarely is trypsin utilized as the primary decellularization agent. Trypsin concentrations of 0.05–0.2% (w/v) are often only used during the first stage of therapy, before chemical decellularization. The biomechanics of the ECM can be harmed by trypsin at higher doses or after prolonged exposure (Olsen et al., 2004; Giraldo-Gomez et al., 2016).
Pepsin: Pepsin is one of the key components used to create hydrogels from decellularized tissues for the creation of therapeutic therapies and in vitro tissue ECM modeling. ECM of lyophilized tissue is dissolved in an acidic buffer using pepsin. This procedure involves dissolving Decellularized ECM (dECM) powder in a monomeric suspension at room temperature, neutralizing the pH, and then preparing a heat-sensitive pre-gel solution that will polymerize into ECM hydrogel when incubated at 37°C (Fruton, 1970).
Collagenase: Collagenase is a powerful enzyme that breaks down collagen II in cartilage and collagen I and III in other tissues during tissue decellularization. Because of the high abundance of collagen in these samples, collagenase is crucial for decellularization in studies intended to investigate ECM proteins by proteomic methods. Treatment with it can be used for the selective metabolism of ECM collagens, allowing the identification of proteins that control cell function in low-abundance decellularization scaffolds (Kuljanin et al., 2017).
Phospholipase: Phospholipase targets lipids and hydrolyzes ester bonds; it is useful for fatty tissues. But often, it is insufficient to eliminate all lipids when used alone. Phospholipase A2, an esterase, hydrolyzes the phospholipids in the cell but leaves intact the collagen and proteoglycan content. It also significantly reduces the amount of glycosaminoglycan while causing modest structural damage to the ECM. To produce an appropriate decellularized ECM, phospholipase is typically employed to remove cellular material from tissue in conjunction with chemical detergent or non-detergent techniques (Wu et al., 2009). Dispase is a neutral protease that swiftly divides fibronectin and collagen IV to release cells from tissue. This is therefore perfect for decellularizing tissues since collagen IV and laminin make up most of the basement membrane. Dispase can also be used to stop cellular aggregation (Prasertsung et al., 2008; Gonzalez-Andrades et al., 2011).
Nucleases are enzymes that can dissociate the phosphodiester linkages that hold single-stranded and double-stranded nucleic acids’ nucleotides together. In post-treatment procedures for chemical, physical, or biological decellularization, nucleases like RNase and DNase are frequently utilized. To effectively remove considerable amounts of cellular material from the matrix, these agents fracture the nucleus’ contents (Liao et al., 2007).
Following treatment with decellularization chemicals that cause cell lysis, DNase and RNase are very helpful for eliminating nucleotides from the ECM. According to studies, adding DNase treatment to various decellularization tactics based on chemical, enzymatic, and physical approaches can help preserve the biomechanical and glycosaminoglycan characteristics. ECM that has been exposed to DNase or RNase treatment has to go through several washing cycles since these enzymes generate immunogenicity that can thwart recellularization (Rieder et al., 2004).
Chondroitinase is excellent for decellularizing thick cartilage tissue and may aggressively break down proteoglycans. The dramatic decrease in glycosaminoglycan content, which changes the mechanical characteristics of the ECM and makes it stiffer, is directly connected to chondroitinase (Natoli et al., 2009; Bautista et al., 2016). The content of residual DNA in different neural tissues is different depending on the method of cell removal. It should be noted to choose a method that causes the least damage to the ECM structure and on the other hand, cell removal is done successfully. As can be seen in Figure 4, nerve tissues from different sources such as sciatic, spinal, and optic have been decellularized by different methods. The DNA content in all tissues is significantly reduced, but as can be seen in Figures 4B,C, where decellularization was performed with several different detergents, the DNA content is greatly reduced compared to the protocols that One or two substances that have been used in decellularization. A list of the key methods employed in the decellularization process is provided in Table 2.
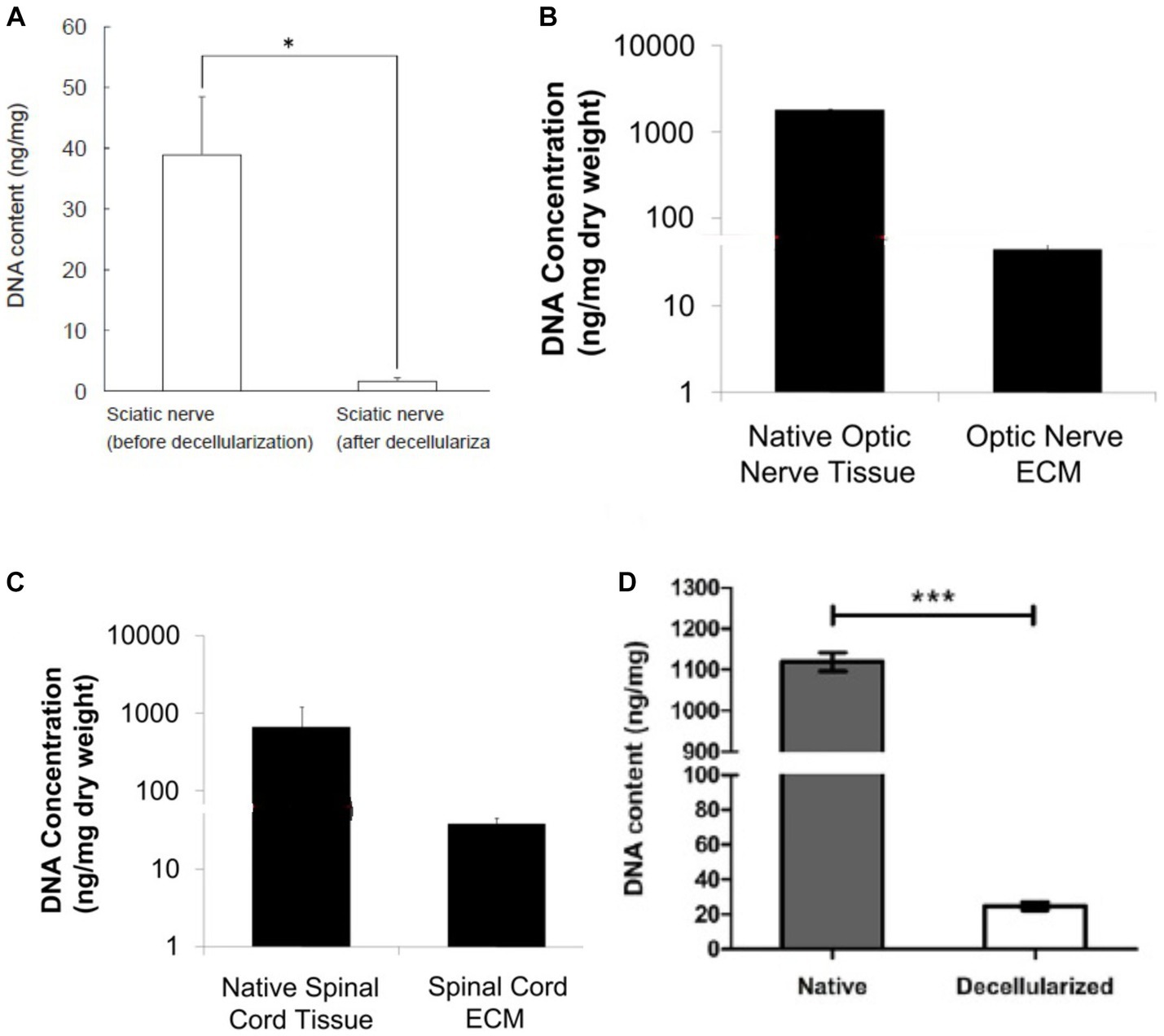
Figure 4. DNA content in DNT by different methods, (A) decellularization of pig sciatic nerve with ethanol and 0.5 NaOH solution (Choi et al., 2018), (B,C) decellularized porcine optic nerve and spinal cord using 0.02% trypsin/0.05% EDTA (w/v), 4.0% SDC (w/v) and 0.1% peracetic acid (vol) (Crapo et al., 2012), (D) decellularized porcine sciatic nerve using 4% Triton X-100 (w/v) (Kong et al., 2022b). In all the methods, the amount of DNA has been greatly reduced compared to the native tissue. Adapted and reprinted.
Protocols of nerve decellularization
The primary purpose of decellularization is to remove all cell components, such as myelin and Schwann cells, from the functional structure of the basal lamina and ECM. Aggressive decellularization protocols aim to reduce immunogenicity to utilize the tissue as a neural scaffold (Nakamoto et al., 2021). Various combined protocols involve enzymatic approaches to remove cellular parts from the ECM, followed by the dissolution of cytoplasmic and nuclear components using detergents (Gilbert et al., 2006).
Table 3 presents the most common protocols used in nerve tissue decellularization. One significant protocol is Sondell’s nerve, which involves soaking the nerves in distilled water for 7 h, followed by a shift to a mixture of distilled water and 3% Triton X-100 (w/v) or one night, and finally placing them in a mixture of 4% SDC (w/v) and distilled water for 24 h. After several repetitions at room temperature, the nerves are washed with distilled water and stored in phosphate-buffered saline (PBS) at 4°C (Philips et al., 2018).
CNS regeneration
Tissue engineering offers several approaches to traumatic brain injury (TBI), one of which is ECM decellularization, where the scaffold preserves growth factors and inflammatory factors, promotes neural stem cell transformation, and supports regeneration (Crapo et al., 2012). Another approach involves using brain patches made from decellularized brain tissue to treat injuries (Zhang et al., 2022a). In a specific in vivo study targeting Parkinson’s disease (PD) treatment, researchers explored the potential benefits of using bFGF (basic fibroblast growth factor) on rats after performing a complete decellularization protocol and analyzing the tissue through hematoxylin and eosin and DAPI staining. The evaluation of physical response was conducted by counting apomorphine-induced rotations in the rats, and the results showed an improvement in their behavior (Lin et al., 2017). However, despite these efforts, achieving a full recovery remains challenging.
PNS regeneration
In animal studies, the effectiveness of decellularized nerve transplantation has been demonstrated in preventing muscle atrophy and paresis after nerve transection. Recent studies have shown that Sondell-based protocols for grafts are effective and function well in humans for nerve injuries below 3 cm in length (Song et al., 2017). However, poor outcomes have been reported in animal studies for allografts with diameters longer than 14 mm (Whitlock et al., 2009; Moore et al., 2011).
The sciatic nerve is crucial in peripheral nerve injury (PNI) studies due to its length and easy accessibility for surgery (Geuna, 2015). Emerging from the lumbosacral plexus, it divides into the tibial nerve, the sural nerve, and the common peroneal nerve near the knee (Rupp et al., 2007). dECM can be sourced from nerve tissues like the sciatic nerve in rats and other mammals, as well as from the central nervous system in the context of PD and TBI (Choi et al., 2018; Qiu et al., 2020). These dECM applications aim to decrease immunogenicity, promote regeneration, and achieve functional recovery in tissue defects. In mammalian models like adult beagle dogs, dECM has shown enhancement in regeneration and recovery after autografts, particularly in PNS repair (Qiu et al., 2020) (Table 4).
Advantages of using decellularized tissues in nerve regeneration
The use of decellularized tissues, particularly decellularized nerve tissues, has been the focus of more research since the use of polymers in neural regeneration is sometimes hampered by complications. Decellularized tissues have several benefits, including their distinct structure, the presence of bioactive chemicals, relatively subdued immunological responses, excellent biodegradability, and a reduction in the difficulties associated with organ replacement (Liao et al., 2020). To rebuild peripheral nerve damage, the review of the study data suggests that designed nerve grafts are an appropriate substitute for autologous nerve grafting. The creation of peripheral nerves requires the use of an appropriate scaffold. Because it offers a favorable environment for cell adhesion and growth. Additionally, it can release growth hormones and quicken axon regeneration. Engineered scaffolds can improve regeneration conditions and hasten vascularization in seizures (Gontika et al., 2018; Asadi et al., 2020).
According to reports, the fundamental elements of decellularized matrices, such as collagen, elastin, and laminin, are what enable the aforementioned biological processes (Zheng et al., 2014; Liao et al., 2020). In studying the interactions of the ECM of the PNS, decellularized nerve grafts for the restoration of peripheral nerves can be utilized as a model. The environment for cell growth, reproduction, and migration is favorable when using clamps of this sort as a physical scaffold. In fact, by lowering antigenicity, these grafts can be a viable therapeutic approach (particularly when autologous tissues are not accessible) (Abbaszadeh et al., 2020). The immune system’s actions can be modulated by decellularized tissues, which have been shown to have limited immunogenicity due to their generally weak immunological responses (Brown et al., 2015). After decellularization, the majority of cells, antigen components, and other substances are typically eliminated, which reduces the immunogenicity of the decellularized tissues (Valentin et al., 2009; Yu et al., 2022). According to studies, the removal of cells and other antigen-containing elements from natural tissues can result in porous structures with the proper width, which can subsequently improve nutrition exchange while promoting adhesion and cell proliferation. Decellularized tissues are said to be capable of being crucial to the process of regenerating tissues and organs. They aid in tissue regeneration since they not only control the healing process but also when the repair is complete, are spontaneously eliminated (Liao et al., 2020). Decellularized scaffolds, a special structure made up of ECM structural proteins, growth factors, hormones, and other bioactive substances, can actually encourage repair and regeneration after nerve loss by creating a receptive environment (Buckenmeyer et al., 2020). The application of this method holds promise for the production of biologically active compounds that could promote the restoration of the nervous system’s functionality specifically (Maghsoudlou et al., 2013; Meng et al., 2014).
Studies show that the internal structural and molecular components of the extracellular matrix are preserved in decellularized nerve grafts, supporting neural regeneration (Szynkaruk et al., 2013). Many rat, mouse, and sheep models have been used to study the immune characteristics of cold-preserved allografts. These findings indicate a gradual loss of immunogenicity (Fox and Mackinnon, 2007). While the CNS has a limited capacity for regeneration, the spinal cord has shown more promise when using decellularized components. According to studies, using decellularized ECM scaffolds can influence host cell phenotypes, differentiation, migration, proliferation, and other aspects that can aid in the regeneration of CNS tissues. Additionally, it has been demonstrated that dECM-derived scaffolds can serve as a guiding substrate and a transporter of sequestered bioactive substances to support proper axonal sprouting (Guo et al., 2010; Xu et al., 2016). The evidence suggests that the decellularized allograft can improve motor and sensory function in relatively small nerve gaps (approximately 2.3 cm) clinically (Tang and Chauhan, 2015).
Challenges, limitations, and future prospects of using decellularized tissues in nerve regeneration
While some preclinical and clinical studies have shown encouraging results using decellularized matrices, there are several technical and grafting challenges in the clinical use of dECM scaffolds. For example, there have been reports of non-infectious edema, severe pain, and inflammatory response from decellularized scaffolds. Evidence also suggests that acellular scaffolds may not be suitable for injuries requiring long-term mechanical support. Furthermore, it has been reported that dECM scaffold implantation may lead to scar tissue deposition without net tissue regeneration (Elmashhady et al., 2017). The evidence indicates that the most common methods of decellularization of nerve tissues include perfusion, chemical, and biological agents, which are often used in combination with a physical method. The mentioned methods may change or destroy the important components of ECM, which in turn affects the structure, adhesion, and proliferation of future cells (Uriarte et al., 2018).
For example, treatment with acidic or alkaline solutions and ionic detergents may dissolve acidic components and disrupt the structure of nucleic acids. Non-ionic detergents may also leave protein–protein interactions intact and this is not desirable. The use of hypertonic and hypotonic solutions may also lead to osmotic shock and eventually cell lysis. The use of enzymes, in turn, may change the stability of the ECM due to the change in collagen content (Arslantunali et al., 2014).
Preservation of ECM microarchitecture and composition during decellularization requires optimal protocols that provide efficient removal of cells with minimal disruption. It has been reported that the balance between the effective removal of cells and preservation of structure and biochemical and biomechanical properties to obtain a dECM scaffold that minimizes immunogenicity after the implantation process and provides an optimal balance between cells and ECM is challenging. The evidence indicates that inefficient decellularization by the mentioned substances can cause immune system rejection after in vivo implantation. Another challenge is the effective and optimal cellularization of the nerve and determining the number of cells needed for the optimal cellularization process (Barbulescu et al., 2022).
The decellularization of tissues and organs to create ECM bioscaffolds, according to studies, necessitates a delicate balancing act between preserving the ECM’s structure and removing cellular components such as DNA, mitochondria, membrane lipids, and cytosolic proteins. These cellular remnants can cause an unfavorable inflammatory reaction and prevent the healthy regeneration of the tissue if they are not properly eliminated (Keane et al., 2015; Kubinova, 2017; Liao et al., 2020). Therefore, care should be taken to minimize the presence of normal cells that can trigger negative immunological reactions during the decellularization of an organ. According to studies, insufficient decellularization can hinder recellularization, which in turn causes graft rejection (Keane et al., 2015). The low mechanical stability of models based on decellularized ECM is one of their main drawbacks. The mechanical characteristics of decellularized tissues frequently differ significantly from those of their native equivalents, which may limit the scaffold’s capacity to hold its shape in vitro and modify cellular behavior (Lovati et al., 2018; Barbulescu et al., 2022). Utilizing natural and synthetic polymers, such as polycaprolactone, silk fibroin, polylactic-co-glycolic acid, chitosan, hyaluronic acid, alginate, and polyethylene glycol, is one way to solve this problem. Utilizing cross-linkers such as glutaraldehyde, riboflavin/ultraviolet light, genipin, and carbodiimide is another technique to enhance the mechanical characteristics (McCrary et al., 2020).
The re-endothelialization of decellularized tissue is the next difficult task. There should be more efforts to preserve ECM structure and endothelialization (e.g., physical, enzymatic, and chemical means), as research has shown that variations in the microstructure and quantity of ECM components (e.g., GAGs, elastin, and fibronectin) might affect re-endothelialization. The density of the target organ, its fat content and thickness, the type of decellularization agent, their concentration, solution pH, temperature, and decellularization duration should all be taken into account throughout the decellularization process (Mazloomnejad et al., 2023).
Artificial and biological bioreactors have produced endothelialized acellular vasculature in the field of recellularization, but full endothelialization has not yet been accomplished. The issues that arise from transplanting acellular structures and the lack of flow rate, pressure, and shear stress parameters for each organ in artificial bioreactors should be completely addressed in future studies. Another important factor in the endothelialization of acellular structures that was studied was surface alteration. To enhance ECM structure and endothelialization levels, adhesive molecules and GFs have been employed (Ye et al., 2013; Marinval et al., 2018).
The transition of these investigations into human clinical trials remains a top priority notwithstanding the encouraging results. Alternative strategies should also be investigated to enhance re-endothelialization outcomes. For comprehensive endothelium coverage, exosomes—extracellular vesicles carrying RNA, DNA, proteins, and lipids—might be useful. For their efficacy to be confirmed, more research is required (Nazerian et al., 2021). Another challenge is the effective and optimal cellularization of the nerve and determining the number of cells needed for the optimal cellularization process (Huang et al., 2017; Barbulescu et al., 2022). According to reports, even though the use of dECM may offer a different treatment to promote peripheral nerve regeneration, the antigenic material transmitted from allograft specimens during this process mandates the use of immunosuppressants and requires surgery (Buckenmeyer et al., 2020). Therefore, based on the information provided, it is highly advised to carry out additional research that will aid in resolving the issues raised. Combining decellularized nerve tissue with other methods, such as stem cell transplantation, provides a better perspective on the potential for nerve repair in the future. Also, the use of such decellularized tissues with polymers can be effective in creating appropriate porosity and preventing microbial contamination in the transplant. In summary, these methods have the potential to become a valuable choice for the regeneration of damaged central and peripheral nerves and can bring promising products to the market (Hopf et al., 2022; Kong et al., 2022a).
Conclusion
Decellularized tissues have the innate capacity to promote tissue regeneration, making them valuable in various disease models and therapies. Decellularization can be achieved through mechanical, chemical, or enzyme exposure techniques. The resulting acellular scaffolds contain growth factors, hormones, bioactive compounds, and essential proteins from the ECM, creating a favorable environment for repair and regeneration after injury. This method holds the potential to create physiologically active compounds that aid in the functional recovery of the nervous system. Previous attempts to promote axonal elongation using growth factors and biomaterial implants have shown limitations, resulting in random and abnormal axonal development, or limited functional outcomes. Decellularized tissue-derived scaffolds act as guiding substrates and delivery systems for isolated bioactive substances, encouraging healthy axon sprouting. Utilizing decellularization to create cell-free neural transplants eliminates donor-site complications associated with using neural autografts and reduces the need for post-surgical immunosuppression by removing antigenic material from allografts. The selection of appropriate decellularization techniques for tissues is crucial in facilitating nerve defect healing, as emphasized by this research.
Author contributions
MM: Writing – review & editing. TST: Writing – review & editing. ZA: Writing – review & editing. LR: Supervision, Writing – review & editing. MK: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was granted by the Kermanshah University of Medical Sciences, Kermanshah, Iran (IR.KUMS.REC.1402.160).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbaszadeh, S., Asadi, A., Zahri, S., Abdolmaleki, A., and Mahmoudi, F. (2020). Does phenytoin have neuroprotective role and affect biocompatibility of Decellularized sciatic nerve scaffold? Gene Cell Tissue 8:8726. doi: 10.5812/gct.108726
Ahmed, Z., and Brown, R. A. (1999). Adhesion, alignment, and migration of cultured Schwann cells on ultrathin fibronectin fibres. Cell Motil. Cytoskeleton 42, 331–343. doi: 10.1002/(SICI)1097-0169(1999)42:4<331::AID-CM6>3.0.CO;2-7
Akhtar, A., Farzamrad, V., Moradi, A.-R., Yar, M., and Bazzar, M. (2022). Emerging polymeric biomaterials and manufacturing-based tissue engineering approaches for neuro regeneration-a critical review on recent effective approaches. Smart Materials Med., 337–355. doi: 10.1016/j.smaim.2022.11.007
Alhosseini, S. N., Moztarzadeh, F., Mozafari, M., Asgari, S., Dodel, M., Samadikuchaksaraei, A., et al. (2012). Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 7, 25–34. doi: 10.2147/IJN.S25376
Alizadeh, M., Rezakhani, L., Khodaei, M., Soleimannejad, M., and Alizadeh, A. (2021). Evaluating the effects of vacuum on the microstructure and biocompatibility of bovine decellularized pericardium. J. Tissue Eng. Regen. Med. 15, 116–128. doi: 10.1002/term.3150
Alizadeh, A., Rezakhani, L., Shoa, M. A., and Ghasemi, S. (2020). Frequency of CD44 positive cells in MKN45 cell line after treatment with docetaxel in two and three-dimensional cell cultures. Tissue Cell 63:101324. doi: 10.1016/j.tice.2019.101324
Alizadeh, M., Rezakhani, L., Taghdiri Nooshabadi, V., and Alizadeh, A. (2022). The effect of Scrophularia striata on cell attachment and biocompatibility of decellularized bovine pericardia. Cell Tissue Bank. 23, 261–269. doi: 10.1007/s10561-021-09939-3
Amini, S., Salehi, H., Setayeshmehr, M., and Ghorbani, M. (2021). Natural and synthetic polymeric scaffolds used in peripheral nerve tissue engineering: advantages and disadvantages. Polym. Adv. Technol. 32, 2267–2289. doi: 10.1002/pat.5263
Ankeny, D. P., Guan, Z., and Popovich, P. G. (2009). B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest 119, 2990–2999. doi: 10.1172/JCI39780
Arslantunali, D., Dursun, T., Yucel, D., Hasirci, N., and Hasirci, V. (2014). Peripheral nerve conduits: technology update. Med. Dev. 7, 405–424. doi: 10.2147/MDER.S59124
Asadi, A., Zahri, S., and Abdolmaleki, A. (2020). Biosynthesis, characterization and evaluation of the supportive properties and biocompatibility of DBM nanoparticles on a tissue-engineered nerve conduit from decellularized sciatic nerve. Regener. Ther. 14, 315–321. doi: 10.1016/j.reth.2020.03.004
Assunção, M., Dehghan-Baniani, D., Yiu, C. H. K., Später, T., Beyer, S., and Blocki, A. (2020). Cell-derived extracellular matrix for tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol. 8:602009. doi: 10.3389/fbioe.2020.602009
Azhim, A., Yamagami, K., Muramatsu, K., Morimoto, Y., and Tanaka, M. (2011). The use of sonication treatment to completely decellularize blood arteries: a pilot study. 2011 annual international conference of the IEEE engineering in medicine and biology society, IEEE.
Bachtiar, E. O., Ritter, V. C., and Gall, K. (2021). Structure-property relationships in 3D-printed poly (l-lactide-co-ε-caprolactone) degradable polymer. J. Mech. Behav. Biomed. Mater. 121:104650. doi: 10.1016/j.jmbbm.2021.104650
Badylak, S. F., Freytes, D. O., and Gilbert, T. W. (2015). Reprint of: extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 23, S17–S26. doi: 10.1016/j.actbio.2015.07.016
Bae, J.-Y., Park, S. Y., Shin, Y. H., Choi, S. W., and Kim, J. K. (2021). Preparation of human decellularized peripheral nerve allograft using amphoteric detergent and nuclease. Neural Regen. Res. 16:1890. doi: 10.4103/1673-5374.306091
Barbulescu, G. I., Bojin, F. M., Ordodi, V. L., Goje, I. D., Barbulescu, A. S., and Paunescu, V. (2022). Decellularized extracellular matrix scaffolds for cardiovascular tissue engineering: current techniques and challenges. Int. J. Mol. Sci. 23:13040. doi: 10.3390/ijms232113040
Bautista, C. A., Park, H. J., Mazur, C. M., Aaron, R. K., and Bilgen, B. (2016). Effects of chondroitinase ABC-mediated proteoglycan digestion on decellularization and recellularization of articular cartilage. PLoS One 11:e0158976. doi: 10.1371/journal.pone.0158976
Biazar, E., and Heidari Keshel, S. (2013). A nanofibrous PHBV tube with Schwann cell as artificial nerve graft contributing to rat sciatic nerve regeneration across a 30-mm defect bridge. Cell Commun. Adhes. 20, 41–49. doi: 10.3109/15419061.2013.774378
Boer, E., Geldof, F., Gerritse, T., and Winkelhorst, E. (2015). Supercritical CO2 decellularized porcine pericardium is a promising material for an ascending aortic prosthesis. Enschede: University of Twente.
Bolaina-Lorenzo, E., Martínez-Ramos, C., Monleón-Pradas, M., Herrera-Kao, W., Cauich-Rodríguez, J. V., and Cervantes-Uc, J. M. (2016). Electrospun polycaprolactone/chitosan scaffolds for nerve tissue engineering: physicochemical characterization and Schwann cell biocompatibility. Biomed. Mater. 12:015008. doi: 10.1088/1748-605X/12/1/015008
Bourgine, P. E., Pippenger, B. E., Todorov, A. Jr., Tchang, L., and Martin, I. (2013). Tissue decellularization by activation of programmed cell death. Biomaterials 34, 6099–6108. doi: 10.1016/j.biomaterials.2013.04.058
Brown, B. N., Mani, D., Nolfi, A. L., Liang, R., Abramowitch, S. D., and Moalli, P. A. (2015). Characterization of the host inflammatory response following implantation of prolapse mesh in rhesus macaque. Am. J. Obstet. Gynecol. 213, 668.e1–668.e610. doi: 10.1016/j.ajog.2015.08.002
Buckenmeyer, M. J., Meder, T. J., Prest, T. A., and Brown, B. N. (2020). Decellularization techniques and their applications for the repair and regeneration of the nervous system. Methods 171, 41–61. doi: 10.1016/j.ymeth.2019.07.023
Burdick, J. A., and Prestwich, G. D. (2011). Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 23, H41–H56. doi: 10.1002/adma.201003963
Butler, C. R., Hynds, R. E., Crowley, C., Gowers, K. H., Partington, L., Hamilton, N. J., et al. (2017). Vacuum-assisted decellularization: an accelerated protocol to generate tissue-engineered human tracheal scaffolds. Biomaterials 124, 95–105. doi: 10.1016/j.biomaterials.2017.02.001
Campbell, W. W. (2008). Evaluation and management of peripheral nerve injury. Clin. Neurophysiol. 119, 1951–1965. doi: 10.1016/j.clinph.2008.03.018
Chato-Astrain, J., Philips, C., Campos, F., Durand-Herrera, D., García-García, O. D., Roosens, A., et al. (2020). Detergent-based decellularized peripheral nerve allografts: an in vivo preclinical study in the rat sciatic nerve injury model. J. Tissue Eng. Regen. Med. 14, 789–806. doi: 10.1002/term.3043
Chen, Y.-S., Chang, J.-Y., Cheng, C.-Y., Tsai, F.-J., Yao, C.-H., and Liu, B.-S. (2005). An in vivo evaluation of a biodegradable genipin-cross-linked gelatin peripheral nerve guide conduit material. Biomaterials 26, 3911–3918. doi: 10.1016/j.biomaterials.2004.09.060
Choi, J., Kim, J. H., Jang, J. W., Kim, H. J., Choi, S. H., and Kwon, S. W. (2018). Decellularized sciatic nerve matrix as a biodegradable conduit for peripheral nerve regeneration. Neural Regen. Res. 13:1796. doi: 10.4103/1673-5374.237126
Cingolani, A., Casalini, T., Caimi, S., Klaue, A., Sponchioni, M., Rossi, F., et al. (2018). A methodologic approach for the selection of bio-resorbable polymers in the development of medical devices: the case of poly (L-lactide-co-ε-caprolactone). Polymers 10:851. doi: 10.3390/polym10080851
Crapo, P. M., Gilbert, T. W., and Badylak, S. F. (2011). An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243. doi: 10.1016/j.biomaterials.2011.01.057
Crapo, P. M., Medberry, C. J., Reing, J. E., Tottey, S., van der Merwe, Y., Jones, K. E., et al. (2012). Biologic scaffolds composed of central nervous system extracellular matrix. Biomaterials 33, 3539–3547. doi: 10.1016/j.biomaterials.2012.01.044
Crupi, R., Cordaro, M., Cuzzocrea, S., and Impellizzeri, D. (2020). Management of traumatic brain injury: from present to future. Antioxidants 9:297. doi: 10.3390/antiox9040297
da Silva, D. M., Barroca, N., Pinto, S. C., Semitela, Â., de Sousa, B. M., Martins, P. A., et al. (2023). Decellularized extracellular matrix-based 3D nanofibrous scaffolds functionalized with polydopamine-reduced graphene oxide for neural tissue engineering. Chem. Eng. J. 472:144980. doi: 10.1016/j.cej.2023.144980
Daly, W. T., Knight, A. M., Wang, H., de Boer, R., Giusti, G., Dadsetan, M., et al. (2013). Comparison and characterization of multiple biomaterial conduits for peripheral nerve repair. Biomaterials 34, 8630–8639. doi: 10.1016/j.biomaterials.2013.07.086
Deal, N. D., Griffin, J. W., and Hogan, M. V. (2012). Nerve conduits for nerve repair or reconstruction. JAAOS 20, 63–68. doi: 10.5435/00124635-201202000-00001
Ehterami, A., Masoomikarimi, M., Bastami, F., Jafarisani, M., Alizadeh, M., Mehrabi, M., et al. (2021). Fabrication and characterization of Nanofibrous poly (L-lactic acid)/chitosan-based scaffold by liquid–liquid phase separation technique for nerve tissue engineering. Mol. Biotechnol. 63, 818–827. doi: 10.1007/s12033-021-00346-3
Ehterami, A., Rezaei Kolarijani, N., Nazarnezhad, S., Alizadeh, M., Masoudi, A., and Salehi, M. (2022). Peripheral nerve regeneration by thiolated chitosan hydrogel containing taurine: in vitro and in vivo study. J. Bioact. Compat. Polym. 37, 85–97. doi: 10.1177/08839115221085736
El-Husseiny, H. M., Mady, E. A., Kaneda, M., Shimada, K., Nakazawa, Y., Usui, T., et al. (2023). Comparison of bovine-and porcine-derived Decellularized biomaterials: promising platforms for tissue engineering applications. Pharmaceutics 15:1906. doi: 10.3390/pharmaceutics15071906
Elmashhady, H. H., Kraemer, B. A., Patel, K. H., Sell, S. A., and Garg, K. (2017). Decellularized extracellular matrices for tissue engineering applications. Electrospinning 1, 87–99. doi: 10.1515/esp-2017-0005
Eschbach, L. (2000). Nonresorbable polymers in bone surgery. Injury 31, D22–D27. doi: 10.1016/S0020-1383(00)80019-4
Evans, G., Brandt, K., Widmer, M., Lu, L., Meszlenyi, R., Gupta, P., et al. (1999). In vivo evaluation of poly (L-lactic acid) porous conduits for peripheral nerve regeneration. Biomaterials 20, 1109–1115. doi: 10.1016/S0142-9612(99)00010-1
Eyestone, W., Adams, K., Ball, S., Bianchi, J., Butler, S., Dandro, A., et al. (2020). Gene-edited pigs for xenotransplantation. Clinical xenotransplantation: Pathways and progress in the transplantation of organs and tissues between species. Springer: Berlin. 121–140.
Faulk, D. M., Carruthers, C. A., Warner, H. J., Kramer, C. R., Reing, J. E., Zhang, L., et al. (2014). The effect of detergents on the basement membrane complex of a biologic scaffold material. Acta Biomater. 10, 183–193. doi: 10.1016/j.actbio.2013.09.006
Feng, J., Zhang, Y., Zhu, Z., Gu, C., Waqas, A., and Chen, L. (2021). Emerging exosomes and exosomal MiRNAs in spinal cord injury. Front. Cell Dev. Biol. 9:703989. doi: 10.3389/fcell.2021.703989
Fertala, J., Rivlin, M., Wang, M. L., Beredjiklian, P. K., Steplewski, A., and Fertala, A. (2020). Collagen-rich deposit formation in the sciatic nerve after injury and surgical repair: a study of collagen-producing cells in a rabbit model. Brain Behav. 10:e01802. doi: 10.1002/brb3.1802
Forouzesh, F., Rabbani, M., and Bonakdar, S. (2019). A comparison between ultrasonic bath and direct sonicator on osteochondral tissue decellularization. J. Med. Signals Sensors 9, 227–233. doi: 10.4103/jmss.JMSS_64_18
Fouad, K., Popovich, P. G., Kopp, M. A., and Schwab, J. M. J. N. R. N. (2021). The neuroanatomical–functional paradox in spinal cord injury. Nat Rev Neurol 17, 53–62. doi: 10.1038/s41582-020-00436-x
Fox, I. K., and Mackinnon, S. E. (2007). Experience with nerve allograft transplantation. Seminars in plastic surgery. New York: Thieme Medical Publishers.
Fruton, J. S. (1970). Specificity and mechanism of pepsin action. Struct. Funct. Relationsh. Proteolytic Enzymes, 222–236.
Gaaz, T. S., Sulong, A. B., Akhtar, M. N., Kadhum, A. A. H., Mohamad, A. B., and Al-Amiery, A. A. (2015). Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 20, 22833–22847. doi: 10.3390/molecules201219884
Gautam, R., Bassi, A. S., and Yanful, E. K. (2007). Candida rugosa lipase-catalyzed polyurethane degradation in aqueous medium. Biotechnol. Lett. 29, 1081–1086. doi: 10.1007/s10529-007-9354-1
Geuna, S. (2015). The sciatic nerve injury model in pre-clinical research. J. Neurosci. Methods 243, 39–46. doi: 10.1016/j.jneumeth.2015.01.021
Gilbert, T. W. (2012). Strategies for tissue and organ decellularization. J. Cell. Biochem. 113, 2217–2222. doi: 10.1002/jcb.24130
Gilbert, T. W., Sellaro, T. L., and Badylak, S. F. (2006). Decellularization of tissues and organs. Biomaterials 27, 3675–3683. doi: 10.1016/j.biomaterials.2006.02.014
Gilpin, A., and Yang, Y. (2017). Decellularization strategies for regenerative medicine: from processing techniques to applications. Biomed. Res. Int. 2017:9831534. doi: 10.1155/2017/9831534
Giraldo-Gomez, D., Leon-Mancilla, B., Del Prado-Audelo, M., Sotres-Vega, A., Villalba-Caloca, J., Garciadiego-Cazares, D., et al. (2016). Trypsin as enhancement in cyclical tracheal decellularization: morphological and biophysical characterization. Mater. Sci. Eng. C 59, 930–937. doi: 10.1016/j.msec.2015.10.094
Goldstein, A. S., and Thayer, P. (2014). Bioreactor strategies for in vitro conditioning of engineered tissues. Scaffolds for tissue engineering: biological design, materials, and fabrication, Dubai: Jenny Stanford Publishing: 555–590.
Gontika, I., Katsimpoulas, M., Antoniou, E., Kostakis, A., Stavropoulos-Giokas, C., and Michalopoulos, E. (2018). Decellularized human umbilical artery used as nerve conduit. Bioengineering 5:100. doi: 10.3390/bioengineering5040100
Gonzalez-Andrades, M., de la Cruz Cardona, J., Ionescu, A. M., Campos, A., del Mar Perez, M., and Alaminos, M. (2011). Generation of bioengineered corneas with decellularized xenografts and human keratocytes. Invest. Ophthalmol. Vis. Sci. 52, 215–222. doi: 10.1167/iovs.09-4773
Guo, S., Ren, X., Wu, B., and Jiang, T. (2010). Preparation of the acellular scaffold of the spinal cord and the study of biocompatibility. Spinal Cord 48, 576–581. doi: 10.1038/sc.2009.170
Gupta, S. K., Mishra, N. C., and Dhasmana, A. (2018). “Decellularization methods for scaffold fabrication” in Decellularized Scaffolds and Organogenesis: Methods and Protocols (New York: Springer), 1–10.
Hazari, A., Johansson-Ruden, G., Junemo-Bostrom, K., Ljungberg, C., Terenghi, G., Green, C., et al. (1999). A new resorbable wrap-around implant as an alternative nerve repair technique. J. Hand Surg. 24, 291–295. doi: 10.1054/JHSB.1998.0001
Hinderer, S., Layland, S. L., and Schenke-Layland, K. (2016). ECM and ECM-like materials — biomaterials for applications in regenerative medicine and cancer therapy. Adv. Drug Deliv. Rev. 97, 260–269. doi: 10.1016/j.addr.2015.11.019
Hopf, A., Al-Bayati, L., Schaefer, D. J., Kalbermatten, D. F., Guzman, R., and Madduri, S. (2022). Optimized Decellularization protocol for large peripheral nerve segments: towards personalized nerve bioengineering. Bioengineering 9:412. doi: 10.3390/bioengineering9090412
Hsu, M.-W., Chen, S.-H., Tseng, W.-L., Hung, K.-S., Chung, T.-C., Lin, S.-C., et al. (2023). Physical processing for decellularized nerve xenograft in peripheral nerve regeneration. Front. Bioeng. Biotechnol. 11:1217067. doi: 10.3389/fbioe.2023.1217067
Huang, Z., Godkin, O., and Schulze-Tanzil, G. (2017). The challenge in using mesenchymal stromal cells for recellularization of decellularized cartilage. Stem Cell Rev. Rep. 13, 50–67. doi: 10.1007/s12015-016-9699-8
Huang, J., Hu, X., Lu, L., Ye, Z., Zhang, Q., and Luo, Z. (2010). Electrical regulation of Schwann cells using conductive polypyrrole/chitosan polymers. J. Biomed. Materials Res. Part A 93, 164–174. doi: 10.1002/jbm.a.32511
Hudson, T. W., Liu, S. Y., and Schmidt, C. E. (2004a). Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 10, 1346–1358. doi: 10.1089/ten.2004.10.1346
Hudson, T. W., Zawko, S., Deister, C., Lundy, S., Hu, C. Y., Lee, K., et al. (2004b). Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 10, 1641–1651. doi: 10.1089/ten.2004.10.1641
Hussin, H. M., Idrus, R. H., and Lokanathan, Y. (2018). Development of nerve conduit using decellularized human umbilical cord artery seeded with Centella asiatica induced-neurodifferentiated human mesenchymal stem cell. Sains Malays 47, 2789–2798. doi: 10.17576/jsm-2018-4711-22
Ishida, Y., Sakakibara, S., Terashi, H., Hashikawa, K., and Yamaoka, T. (2014). Development of a novel method for decellularizing a nerve graft using a hypertonic sodium chloride solution. Int. J. Artif. Organs 37, 854–860. doi: 10.5301/ijao.5000365
Jia, H., Wang, Y., Tong, X. J., Liu, G. B., Li, Q., Zhang, L. X., et al. (2012). Sciatic nerve repair by acellular nerve xenografts implanted with BMSCs in rats xenograft combined with BMSCs. Synapse 66, 256–269. doi: 10.1002/syn.21508
Jiang, Z., Zhang, Y., Wang, Y., Wang, S., Chang, J., Liu, W., et al. (2023). Multichannel nerve conduit based on chitosan derivates for peripheral nerve regeneration and Schwann cell survival. Carbohydr. Polym. 301:120327. doi: 10.1016/j.carbpol.2022.120327
Keane, T. J., Swinehart, I. T., and Badylak, S. F. (2015). Methods of tissue decellularization used for preparation of biologic scaffolds and in vivo relevance. Methods 84, 25–34. doi: 10.1016/j.ymeth.2015.03.005
Kehoe, S., Zhang, X., and Boyd, D. (2012). FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury 43, 553–572. doi: 10.1016/j.injury.2010.12.030
Khazaei, M. R., Ami, Z., Mozafar, K., and Rezakhani, L. (2023). The Decellularized calf testis: Introducing suitable scaffolds for spermatogenesis studies. Int. J. Fertil. Steril.
Khazaei, M., Khazaei, M. R., Alizadeh, M., Rahmati, S., and Rezakhani, L. (2021). Functional survey of decellularized tissues transplantation for infertile females. Cell Tissue Bank. 23, 407–415. doi: 10.1007/s10561-021-09979-9
Khazaei, F., Rezakhani, L., Alizadeh, M., Mahdavian, E., and Khazaei, M. (2022). Exosomes and exosome-loaded scaffolds: characterization and application in modern regenerative medicine. Tissue Cell 80:102007. doi: 10.1016/j.tice.2022.102007
Kim, Y., Ko, H., Kwon, I. K., and Shin, K. (2016). Extracellular matrix revisited: roles in tissue engineering. Int. Neurourol. J. 20:S23. doi: 10.5213/inj.1632600.318
Kim, J. K., Koh, Y.-D., Kim, J. O., and Seo, D. H. (2016). Development of a decellularization method to produce nerve allografts using less invasive detergents and hyper/hypotonic solutions. J. Plast. Reconstr. Aesthet. Surg. 69, 1690–1696. doi: 10.1016/j.bjps.2016.08.016
Kong, Y., Wang, D., Wei, Q., and Yang, Y. (2022a). Nerve decellularized matrix composite scaffold with high antibacterial activity for nerve regeneration. Front. Bioeng. Biotechnol. 9:840421. doi: 10.3389/fbioe.2021.840421
Kong, Y., Xu, J., Han, Q., Zheng, T., Wu, L., Li, G., et al. (2022b). Electrospinning porcine decellularized nerve matrix scaffold for peripheral nerve regeneration. Int. J. Biol. Macromol. 209, 1867–1881. doi: 10.1016/j.ijbiomac.2022.04.161
Krekoski, C. A., Neubauer, D., Zuo, J., and Muir, D. (2001). Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J. Neurosci. 21, 6206–6213. doi: 10.1523/JNEUROSCI.21-16-06206.2001
Kubinova, S. (2017). Extracellular matrix based biomaterials for central nervous system tissue repair: the benefits and drawbacks. Neural Regen. Res. 12:1430. doi: 10.4103/1673-5374.215249
Kuljanin, M., Brown, C. F., Raleigh, M. J., Lajoie, G. A., and Flynn, L. E. (2017). Collagenase treatment enhances proteomic coverage of low-abundance proteins in decellularized matrix bioscaffolds. Biomaterials 144, 130–143. doi: 10.1016/j.biomaterials.2017.08.012
Lee, S., Hongo, C., and Nishino, T. (2017). Crystal modulus of poly (glycolic acid) and its temperature dependence. Macromolecules 50, 5074–5079. doi: 10.1021/acs.macromol.7b00753
Li, F., Li, X., He, R., Cheng, J., Ni, Z., and Zhao, G. (2020). Preparation and evaluation of poly (D, L-lactic acid)/poly (L-lactide-co-ε-caprolactone) blends for tunable sirolimus release. Colloids Surf. A Physicochem. Eng. Asp. 590:124518. doi: 10.1016/j.colsurfa.2020.124518
Li, Y., Takanari, K., Nakamura, R., Kambe, M., Ebisawa, K., Oishi, M., et al. (2023). Artificial PGA/collagen-based bilayer conduit in short gap interposition setting provides comparable regenerative potential to direct suture. Plast. Reconstr. Surg. Glob. Open 11:4875. doi: 10.1097/GOX.0000000000004875
Liao, J., Xu, B., Zhang, R., Fan, Y., Xie, H., and Li, X. (2020). Applications of decellularized materials in tissue engineering: advantages, drawbacks and current improvements, and future perspectives. J. Mater. Chem. B 8, 10023–10049. doi: 10.1039/D0TB01534B
Liao, J., Yang, L., Grashow, J., and Sacks, M. S. (2007). The relation between collagen fibril kinematics and mechanical properties in the mitral valve anterior leaflet. J Biomech Eng 129, 78–87. doi: 10.1115/1.2401186
Lin, Q., Wong, H. L., Tian, F.-R., Huang, Y.-D., Xu, J., Yang, J.-J., et al. (2017). Enhanced neuroprotection with decellularized brain extracellular matrix containing bFGF after intracerebral transplantation in Parkinson’s disease rat model. Int. J. Pharm. 517, 383–394. doi: 10.1016/j.ijpharm.2016.12.028
Liu, C., Huang, Y., Pang, M., Yang, Y., Li, S., Liu, L., et al. (2015). Tissue-engineered regeneration of completely transected spinal cord using induced neural stem cells and gelatin-electrospun poly (lactide-co-glycolide)/polyethylene glycol scaffolds. PLoS One 10:e0117709. doi: 10.1371/journal.pone.0117709
Liu, X., Miller, A. L., Park, S., Waletzki, B. E., Zhou, Z., Terzic, A., et al. (2017). Functionalized carbon nanotube and graphene oxide embedded electrically conductive hydrogel synergistically stimulates nerve cell differentiation. ACS Appl. Mater. Interfaces 9, 14677–14690. doi: 10.1021/acsami.7b02072
Lovati, A. B., D’Arrigo, D., Odella, S., Tos, P., Geuna, S., and Raimondo, S. (2018). Nerve repair using decellularized nerve grafts in rat models. A review of the literature. Front. Cell. Neurosci. 12:427. doi: 10.3389/fncel.2018.00427
Lu, N., Wang, X., Li, X., Shi, W., Wang, X., Zou, Y., et al. (2023). EMSCs-seeded Micro-stripe patterned Polycaprolactone promoting sciatic nerve regeneration. Adv. Mater. Interfaces 10:2201929. doi: 10.1002/admi.202201929
Ma, Y.-H., Shi, H.-J., Wei, Q.-S., Deng, Q.-W., Sun, J.-H., Liu, Z., et al. (2021). Developing a mechanically matched decellularized spinal cord scaffold for the in situ matrix-based neural repair of spinal cord injury. Biomaterials 279:121192:121216. doi: 10.1016/j.biomaterials.2021.121216
Maas, A. I., Stocchetti, N., and Bullock, R. J. T. L. N. (2008). Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741. doi: 10.1016/S1474-4422(08)70164-9
Maghsoudlou, P., Totonelli, G., Loukogeorgakis, S. P., Eaton, S., and De Coppi, P. (2013). A decellularization methodology for the production of a natural acellular intestinal matrix. JoVE 80:e50658. doi: 10.3791/50658
Mao, X., Li, T., Cheng, J., Tao, M., Li, Z., Ma, Y., et al. (2023). Nerve ECM and PLA-PCL based electrospun bilayer nerve conduit for nerve regeneration. Front. Bioeng. Biotechnol. 11:1103435. doi: 10.3389/fbioe.2023.1103435
Marinval, N., Morenc, M., Labour, M., Samotus, A., Mzyk, A., Ollivier, V., et al. (2018). Fucoidan/VEGF-based surface modification of decellularized pulmonary heart valve improves the antithrombotic and re-endothelialization potential of bioprostheses. Biomaterials 172, 14–29. doi: 10.1016/j.biomaterials.2018.01.054
Mazloomnejad, R., Babajani, A., Kasravi, M., Ahmadi, A., Shariatzadeh, S., Bahrami, S., et al. (2023). Angiogenesis and re-endothelialization in decellularized scaffolds: recent advances and current challenges in tissue engineering. Front. Bioeng. Biotechnol. 11:1103727. doi: 10.3389/fbioe.2023.1103727
McCrary, M. W., Bousalis, D., Mobini, S., Song, Y. H., and Schmidt, C. E. (2020). Decellularized tissues as platforms for in vitro modeling of healthy and diseased tissues. Acta Biomater. 111, 1–19. doi: 10.1016/j.actbio.2020.05.031
Mendibil, U., Ruiz-Hernandez, R., Retegi-Carrion, S., Garcia-Urquia, N., Olalde-Graells, B., and Abarrategi, A. (2020). Tissue-specific decellularization methods: rationale and strategies to achieve regenerative compounds. Int. J. Mol. Sci. 21:5447. doi: 10.3390/ijms21155447
Meng, F., Modo, M., and Badylak, S. F. (2014). Biologic scaffold for CNS repair. Regen. Med. 9, 367–383. doi: 10.2217/rme.14.9
Menorca, R. M., Fussell, T. S., and Elfar, J. C. J. H. C. (2013). Nerve physiology: mechanisms of injury and recovery. Hand Clin. 29, 317–330. doi: 10.1016/j.hcl.2013.04.002
Mir, M., Ahmed, N., and Rehman, A. U. (2017). Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf. B: Biointerfaces 159, 217–231. doi: 10.1016/j.colsurfb.2017.07.038
Montoya, C. V., and McFetridge, P. S. (2009). Preparation of ex vivo–based biomaterials using convective flow decellularization. Tissue Eng. Part C Methods 15, 191–200. doi: 10.1089/ten.tec.2008.0372
Moore, A. M., MacEwan, M., Santosa, K. B., Chenard, K. E., Ray, W. Z., Hunter, D. A., et al. (2011). Acellular nerve allografts in peripheral nerve regeneration: a comparative study. Muscle Nerve 44, 221–234. doi: 10.1002/mus.22033
Nakamoto, J. C., Wataya, E. Y., Nakamoto, H. A., Santos, G. B., Ribaric, I., Herrera, A. K., et al. (2021). Evaluation of the use of nerve allograft preserved in glycerol. Plast. Reconstr. Surg. Glob. Open 9:3514. doi: 10.1097/GOX.0000000000003514
Natoli, R. M., Revell, C. M., and Athanasiou, K. A. (2009). Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue Eng. A 15, 3119–3128. doi: 10.1089/ten.tea.2008.0478
Navarro-Serna, S., Piñeiro-Silva, C., Romar, R., Parrington, J., and Gadea, J. (2022). Generation of gene edited pigs. Sustainable agriculture reviews 57: animal biotechnology for livestock production 2, Berlin: Springer: 71–130.
Nazerian, Y., Vakili, K., Ebrahimi, A., and Niknejad, H. (2021). Developing cytokine storm-sensitive therapeutic strategy in COVID-19 using 8P9R chimeric peptide and soluble ACE2. Front. Cell Dev. Biol. 9:717587. doi: 10.3389/fcell.2021.717587
Nune, M., Krishnan, U. M., and Sethuraman, S. (2016). PLGA nanofibers blended with designer self-assembling peptides for peripheral neural regeneration. Mater. Sci. Eng. C 62, 329–337. doi: 10.1016/j.msec.2016.01.057
O'brien, F. J. (2011). Biomaterials & scaffolds for tissue engineering. Mater. Today 14, 88–95. doi: 10.1016/S1369-7021(11)70058-X
Olsen, J. V., Ong, S.-E., and Mann, M. (2004). Trypsin cleaves exclusively C-terminal to arginine and lysine residues. Mol. Cell. Proteomics 3, 608–614. doi: 10.1074/mcp.T400003-MCP200
Ozdil, D., and Aydin, H. M. (2014). Polymers for medical and tissue engineering applications. J. Chem. Technol. Biotechnol. 89, 1793–1810. doi: 10.1002/jctb.4505
Pellegata, A. F., Asnaghi, M., Stefani, I., Maestroni, A., Maestroni, S., Dominioni, T., et al. (2013). Detergent-enzymatic decellularization of swine blood vessels: insight on mechanical properties for vascular tissue engineering. Biomed. Res. Int. 2013:918753. doi: 10.1155/2013/918753
Philips, C., Campos, F., Roosens, A., Sánchez-Quevedo, M. D. C., Declercq, H., and Carriel, V. (2018). Qualitative and quantitative evaluation of a novel detergent-based method for decellularization of peripheral nerves. Ann. Biomed. Eng. 46, 1921–1937. doi: 10.1007/s10439-018-2082-y
Pi, W., Zhou, L., Zhang, W., Liu, S., Li, C., Zhang, M., et al. (2022). Three-dimensional conductive polycaprolactone/carbon nanotubes scaffolds for peripheral nerve regeneration. J. Mater. Sci. 57, 11289–11299. doi: 10.1007/s10853-022-07336-z
Poornejad, N., Frost, T. S., Scott, D. R., Elton, B. B., Reynolds, P. R., Roeder, B. L., et al. (2015). Freezing/thawing without cryoprotectant damages native but not decellularized porcine renal tissue. Organogenesis 11, 30–45. doi: 10.1080/15476278.2015.1022009
Prasertsung, I., Kanokpanont, S., Bunaprasert, T., Thanakit, V., and Damrongsakkul, S. (2008). Development of acellular dermis from porcine skin using periodic pressurized technique. J. Biomed. Materials Res. Part B 85, 210–219. doi: 10.1002/jbm.b.30938
Qiu, S., Rao, Z., He, F., Wang, T., Xu, Y., Du, Z., et al. (2020). Decellularized nerve matrix hydrogel and glial-derived neurotrophic factor modifications assisted nerve repair with decellularized nerve matrix scaffolds. J. Tissue Eng. Regen. Med. 14, 931–943. doi: 10.1002/term.3050
Rao, Z., Lin, T., Qiu, S., Zhou, J., Liu, S., Chen, S., et al. (2021). Decellularized nerve matrix hydrogel scaffolds with longitudinally oriented and size-tunable microchannels for peripheral nerve regeneration. Mater. Sci. Eng. C 120:111791. doi: 10.1016/j.msec.2020.111791
Reing, J. E., Brown, B. N., Daly, K. A., Freund, J. M., Gilbert, T. W., Hsiong, S. X., et al. (2010). The effects of processing methods upon mechanical and biologic properties of porcine dermal extracellular matrix scaffolds. Biomaterials 31, 8626–8633. doi: 10.1016/j.biomaterials.2010.07.083
Rieder, E., Kasimir, M.-T., Silberhumer, G., Seebacher, G., Wolner, E., Simon, P., et al. (2004). Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J. Thorac. Cardiovasc. Surg. 127, 399–405. doi: 10.1016/j.jtcvs.2003.06.017
Roozenbeek, B., Maas, A. I., and Menon, D. K. (2013). Changing patterns in the epidemiology of traumatic brain injury. Nat. Rev. Neurol. 9, 231–236. doi: 10.1038/nrneurol.2013.22
Rotshenker, S. (2011). Wallerian degeneration: the innate-immune response to traumatic nerve injury. J. Neuroinflammation 8, 1–14. doi: 10.1186/1742-2094-8-109
Ruff, R. L., McKerracher, L., and Selzer, M. E. (2008). Repair and neurorehabilitation strategies for spinal cord injury. Ann. N. Y. Acad. Sci. 1142, 1–20. doi: 10.1196/annals.1444.004
Rupp, A., Dornseifer, U., Fischer, A., Schmahl, W., Rodenacker, K., Jütting, U., et al. (2007). Electrophysiologic assessment of sciatic nerve regeneration in the rat: surrounding limb muscles feature strongly in recordings from the gastrocnemius muscle. J. Neurosci. Methods 166, 266–277. doi: 10.1016/j.jneumeth.2007.07.015
Sano, M. B., Neal, R. E., Garcia, P. A., Gerber, D., Robertson, J., and Davalos, R. V. (2010). Towards the creation of decellularized organ constructs using irreversible electroporation and active mechanical perfusion. Biomed. Eng. Online 9, 1–16. doi: 10.1186/1475-925X-9-83
Santoso, E. G., Yoshida, K., Hirota, Y., Aizawa, M., Yoshino, O., Kishida, A., et al. (2014). Application of detergents or high hydrostatic pressure as decellularization processes in uterine tissues and their subsequent effects on in vivo uterine regeneration in murine models. PLoS One 9:e103201. doi: 10.1371/journal.pone.0103201
Schacht, K., and Scheibel, T. (2014). Processing of recombinant spider silk proteins into tailor-made materials for biomaterials applications. Curr. Opin. Biotechnol. 29, 62–69. doi: 10.1016/j.copbio.2014.02.015
Sharifi, M., Farahani, M. K., Salehi, M., Atashi, A., Alizadeh, M., Kheradmandi, R., et al. (2022). Exploring the physicochemical, electroactive, and biodelivery properties of metal nanoparticles on peripheral nerve regeneration. ACS Biomater Sci. Eng. 9, 106–138. doi: 10.1021/acsbiomaterials.2c01216
Sierad, L. N., Shaw, E. L., Bina, A., Brazile, B., Rierson, N., Patnaik, S. S., et al. (2015). Functional heart valve scaffolds obtained by complete decellularization of porcine aortic roots in a novel differential pressure gradient perfusion system. Tissue Eng. Part C Methods 21, 1284–1296. doi: 10.1089/ten.tec.2015.0170
Sierpinski, P., Garrett, J., Ma, J., Apel, P., Klorig, D., Smith, T., et al. (2008). The use of keratin biomaterials derived from human hair for the promotion of rapid regeneration of peripheral nerves. Biomaterials 29, 118–128. doi: 10.1016/j.biomaterials.2007.08.023
Sivandzade, F., and Cucullo, L. (2021). Regenerative stem cell therapy for neurodegenerative diseases: an overview. Int. J. Mol. Sci. 22:2153. doi: 10.3390/ijms22042153
Somuncu, Ö. S. (2020). Decellularization concept in regenerative medicine. Adv. Exp. Med. Biol. 1212, 71–85. doi: 10.1007/5584_2019_338
Sondell, M., Lundborg, G., and Kanje, M. (1998). Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res. 795, 44–54. doi: 10.1016/S0006-8993(98)00251-0
Song, J., Sun, B., Liu, S., Chen, W., Zhang, Y., Wang, C., et al. (2016). Polymerizing pyrrole coated poly (l-lactic acid-co-ε-caprolactone)(PLCL) conductive nanofibrous conduit combined with electric stimulation for long-range peripheral nerve regeneration. Front. Mol. Neurosci. 9:117. doi: 10.3389/fnmol.2016.00117
Song, M., Wang, W., Ye, Q., Bu, S., Shen, Z., and Zhu, Y. (2017). The repairing of full-thickness skin deficiency and its biological mechanism using decellularized human amniotic membrane as the wound dressing. Mater. Sci. Eng. C 77, 739–747. doi: 10.1016/j.msec.2017.03.232
Syazwani, N., Azhim, A., Morimoto, Y., Furukawa, K., and Ushida, T. (2015). Decellularization of aorta tissue using sonication treatment as potential scaffold for vascular tissue engineering. J. Med. Biol. Eng. 35, 258–269. doi: 10.1007/s40846-015-0028-5
Szynkaruk, M., Kemp, S. W., Wood, M. D., Gordon, T., and Borschel, G. H. (2013). Experimental and clinical evidence for use of decellularized nerve allografts in peripheral nerve gap reconstruction. Tissue Eng. Part B Rev. 19, 83–96. doi: 10.1089/ten.teb.2012.0275
Tang, P., and Chauhan, A. (2015). Decellular nerve allografts. JAAOS 23, 641–647. doi: 10.5435/JAAOS-D-14-00373
Teebken, O., Bader, A., Steinhoff, G., and Haverich, A. (2000). Tissue engineering of vascular grafts: human cell seeding of decellularised porcine matrix. Eur. J. Vasc. Endovasc. Surg. 19, 381–386. doi: 10.1053/ejvs.1999.1004
Uriarte, J. J., Uhl, F. E., Enes, S. E. R., Pouliot, R. A., and Weiss, D. J. (2018). Lung bioengineering: advances and challenges in lung decellularization and recellularization. Curr. Opin. Organ Transplant. 23:673. doi: 10.1097/MOT.0000000000000584
Valentin, J. E., Stewart-Akers, A. M., Gilbert, T. W., and Badylak, S. F. (2009). Macrophage participation in the degradation and remodeling of extracellular matrix scaffolds. Tissue Eng. A 15, 1687–1694. doi: 10.1089/ten.tea.2008.0419
Wachs, R. A., Wellman, S. M., Porvasnik, S. L., Lakes, E. H., Cornelison, R. C., Song, Y. H., et al. (2022). Apoptosis-Decellularized peripheral nerve scaffold allows regeneration across nerve gap. Cells Tissues Organs. 211, 1–11. doi: 10.1159/000525704
Walter, J., and Zweckberger, K. (2018). Traumatic injuries of the central nervous system. Anasthesiol. Intensivmed Notfallmed Schmerzther 53, 668–681. doi: 10.1055/s-0043-118969
Wang, Q., Chen, J., Niu, Q., Fu, X., Sun, X., and Tong, X. (2017). The application of graphene oxidized combining with decellularized scaffold to repair of sciatic nerve injury in rats. Saudi Pharmaceut. J. 25, 469–476. doi: 10.1016/j.jsps.2017.04.008
Wang, X., He, J., Wang, Y., and Cui, F.-Z. (2012). Hyaluronic acid-based scaffold for central neural tissue engineering. Interface Focus 2, 278–291. doi: 10.1098/rsfs.2012.0016
Wang, X., Hu, W., Cao, Y., Yao, J., Wu, J., and Gu, X. (2005). Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain 128, 1897–1910. doi: 10.1093/brain/awh517
Watanabe, N., Mizuno, M., Matsuda, J., Nakamura, N., Otabe, K., Katano, H., et al. (2019). Comparison of high-hydrostatic-pressure decellularized versus freeze-thawed porcine menisci. J. Orthop. Res. 37, 2466–2475. doi: 10.1002/jor.24350
Whitlock, E. L., Tuffaha, S. H., Luciano, J. P., Yan, Y., Hunter, D. A., Magill, C. K., et al. (2009). Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 39, 787–799. doi: 10.1002/mus.21220
Williams, S. F., Martin, D. P., Horowitz, D. M., and Peoples, O. P. (1999). PHA applications: addressing the price performance issue: I. Tissue engineering. Int. J. Biol. Macromol. 25, 111–121. doi: 10.1016/S0141-8130(99)00022-7
Wu, Z., Zhou, Y., Li, N., Huang, M., Duan, H., Ge, J., et al. (2009). The use of phospholipase A2 to prepare acellular porcine corneal stroma as a tissue engineering scaffold. Biomaterials 30, 3513–3522. doi: 10.1016/j.biomaterials.2009.03.003
Xie, J., MacEwan, M. R., Willerth, S. M., Li, X., Moran, D. W., Sakiyama-Elbert, S. E., et al. (2009). Conductive core–sheath nanofibers and their potential application in neural tissue engineering. Adv. Funct. Mater. 19, 2312–2318. doi: 10.1002/adfm.200801904
Xu, H.-L., Tian, F.-R., Lu, C.-T., Xu, J., Fan, Z.-L., Yang, J.-J., et al. (2016). Thermo-sensitive hydrogels combined with decellularised matrix deliver bFGF for the functional recovery of rats after a spinal cord injury. Sci. Rep. 6:38332. doi: 10.1038/srep38332
Ye, X., Wang, H., Zhou, J., Li, H., Liu, J., Wang, Z., et al. (2013). The effect of heparin-VEGF multilayer on the biocompatibility of decellularized aortic valve with platelet and endothelial progenitor cells. PLoS One 8:e54622. doi: 10.1371/journal.pone.0054622
Yin, Y., Henzl, M. T., Lorber, B., Nakazawa, T., Thomas, T. T., Jiang, F., et al. (2006). Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells 9, 843–852. doi: 10.1038/nn1701
Yu, E., Chen, Z., Huang, Y., Wu, Y., Wang, Z., Wang, F., et al. (2023). A grooved conduit combined with decellularized tissues for peripheral nerve regeneration. J. Mater. Sci. Mater. Med. 34:35. doi: 10.1007/s10856-023-06737-z
Yu, Y., Zhang, W., Liu, X., Wang, H., Shen, J., Xiao, H., et al. (2022). Extracellular matrix scaffold-immune microenvironment modulates tissue regeneration. Compos. Part B 230:109524. doi: 10.1016/j.compositesb.2021.109524
Yu, W., Zhao, W., Zhu, C., Zhang, X., Ye, D., Zhang, W., et al. (2011). Sciatic nerve regeneration in rats by a promising electrospun collagen/poly (ε-caprolactone) nerve conduit with tailored degradation rate. BMC Neurosci. 12, 1–14. doi: 10.1186/1471-2202-12-68
Yuan, B., Zheng, X., Wu, M.-L., Yang, Y., Chen, J.-w., Gao, H.-C., et al. (2023). Platelet-rich plasma gel-loaded collagen/chitosan composite film accelerated rat sciatic nerve injury repair. ACS Omega 8, 2931–2941. doi: 10.1021/acsomega.2c05351
Zahmati, A. H. A., Alipoor, R., Shahmirzadi, A. R., Khori, V., and Abolhasani, M. M. (2017). Chemical decellularization methods and its effects on extracellular matrix. Intern. Med. Med. Investigat. J. 2, 76–83. doi: 10.24200/imminv.v2i3.63
Zeng, J.-B., Li, K.-A., and Du, A.-K. (2015). Compatibilization strategies in poly (lactic acid)-based blends. RSC Adv. 5, 32546–32565. doi: 10.1039/C5RA01655J
Zhang, X., Chen, X., Hong, H., Hu, R., Liu, J., and Liu, C. (2022a). Decellularized extracellular matrix scaffolds: recent trends and emerging strategies in tissue engineering. Bioactive Materials 10, 15–31. doi: 10.1016/j.bioactmat.2021.09.014
Zhang, Y., Jiang, Z., Wang, Y., Xia, L., Yu, S., Li, H., et al. (2022b). Nerve regeneration effect of a composite bioactive Carboxymethyl chitosan-based nerve conduit with a radial texture. Molecules 27:9039. doi: 10.3390/molecules27249039
Zheng, C., Zhu, Q., Liu, X., Huang, X., He, C., Jiang, L., et al. (2014). Improved peripheral nerve regeneration using acellular nerve allografts loaded with platelet-rich plasma. Tissue Eng. A 20, 3228–3240. doi: 10.1089/ten.tea.2013.0729
Glossary
Keywords: nerve regeneration, decellularized tissue, PNS, CNS, extracellular matrix
Citation: Mahdian M, Tabatabai TS, Abpeikar Z, Rezakhani L and Khazaei M (2023) Nerve regeneration using decellularized tissues: challenges and opportunities. Front. Neurosci. 17:1295563. doi: 10.3389/fnins.2023.1295563
Edited by:
Jose R. Pineda, University of the Basque Country, SpainReviewed by:
Sara Martín Colomo, University of the Basque Country, SpainNerea Garcia, Tecnalia Research & Innovation, Spain
Copyright © 2023 Mahdian, Tabatabai, Abpeikar, Rezakhani and Khazaei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leila Rezakhani, Leila_rezakhani@yahoo.com; Leila.rezakhani@kums.ac.ir; Mozafar Khazaei, mkhazaei@kums.ac.ir
 Maryam Mahdian1
Maryam Mahdian1 Leila Rezakhani
Leila Rezakhani