- 1Department of Neurology, Osijek University Hospital Center, Osijek, Croatia
- 2Faculty of Medicine, Josip Juraj Strossmayer University of Osijek, Osijek, Croatia
- 3Department of Molecular Medicine and Biotechnology, Faculty of Medicine, University of Rijeka, Rijeka, Croatia
- 4Department of Radiology, Osijek University Hospital Center, Osijek, Croatia
- 5Institute of Nuclear Medicine and Radiation Protection, Osijek University Hospital Center, Osijek, Croatia
- 6Institute of Laboratory Diagnostics, Osijek University Hospital Center, Osijek, Croatia
- 7Department of Neurology, University Clinical Center of Serbia, Belgrade, Serbia
- 8Faculty of Medicine, University of Belgrade, Belgrade, Serbia
Neurological manifestations with basal ganglia involvement following Hymenoptera stings are rare and clinically ill-defined conditions. We present a patient with acute parkinsonism non-responsive to levodopa, who developed striatal lesions after a hornet sting. We report his response to immunomodulatory treatment and subsequent clinical and brain magnetic resonance imaging (MRI) follow-up. We also searched the literature for patients with acute extrapyramidal syndromes following an insect sting. Fourteen cases have been published; 12 of them are reviewed here. The majority of cases presented with symmetric akinetic syndrome with axial rigidity and/or gait impairment. Six patients were treated with levodopa and only two of these had a modest response to therapy. Brain MRI/computed tomography scan revealed lesions of the basal ganglia, which resulted in fatal outcome in four patients, whereas only one achieved complete recovery. Clinicians should be aware of this rare but devastating cause of acute-onset parkinsonism and specific clinical presentation of this condition, and should consider prompt and prolonged immunomodulatory treatment to prevent irreversible basal ganglia damage.
Introduction
Neurological manifestations of Hymenoptera stings are rare and mostly present with central or peripheral demyelination syndrome, intracranial hemorrhage and stroke (1–4). In addition, involvement of basal ganglia including pallidostriatal necrosis, coupled with different types of acute-onset parkinsonism, has been previously reported (5–17). Clinical presentation, treatment reaction and disease outcome are still unknown in this rare condition. We present a patient with acute parkinsonism non-responsive to levodopa and striatal lesions after a hornet sting, and document his response to immunomodulatory treatment with clinical and brain MRI follow-up. We also reviewed the literature for similar cases and discuss clinical presentation and prognosis of this rare condition.
Case report
A 70-year-old, right-handed Caucasian male was referred to our department for a second opinion 2 months after he had developed acute neurological symptoms following a hornet sting. On July 22, 2020, the patient had an anaphylactic reaction with hypotension and a brief loss of consciousness soon after a hornet had stung him in the nose. He was followed for the next 24 h in his local general hospital and discharged the next day after complete recovery following antihistamine therapy with desloratadine.
Over the next 3 days, he developed a feeling of malaise and noticed that his walk had become slower and with smaller steps. Desloratadine was discontinued after 8 days, but his gait became even worse with severe hesitation at gait initiation and sudden episodes during walking when he was unable to take a step. In addition, his family members noticed that he was more taciturn and slower to respond to questions. Up until the hornet incident, he had no major health problems except for well-controlled high blood pressure treated with lisinopril.
Diagnostic assessment
Non-contrast axial computed tomography (CT) scan of his brain performed 2 weeks after the hornet sting did not show any signs of acute stroke, tumor, or intracranial hemorrhage. Four days later, non-contrast head MRI showed a slight bilateral striatum hyperintensity signal (HIS) on T1-weighted images, mixed bilateral striatum signal intensity on T2-weighted images with HIS on fluid-attenuated inversion recovery (FLAIR) images, without restriction of diffusion, suggesting petechial blood products corresponding to a T2* susceptibility artifact on SWI sequence (Figure 1A). Levodopa/carbidopa was started and titrated slowly up to 1000 mg per day for the next 3 weeks, but no appreciable improvement was noted.
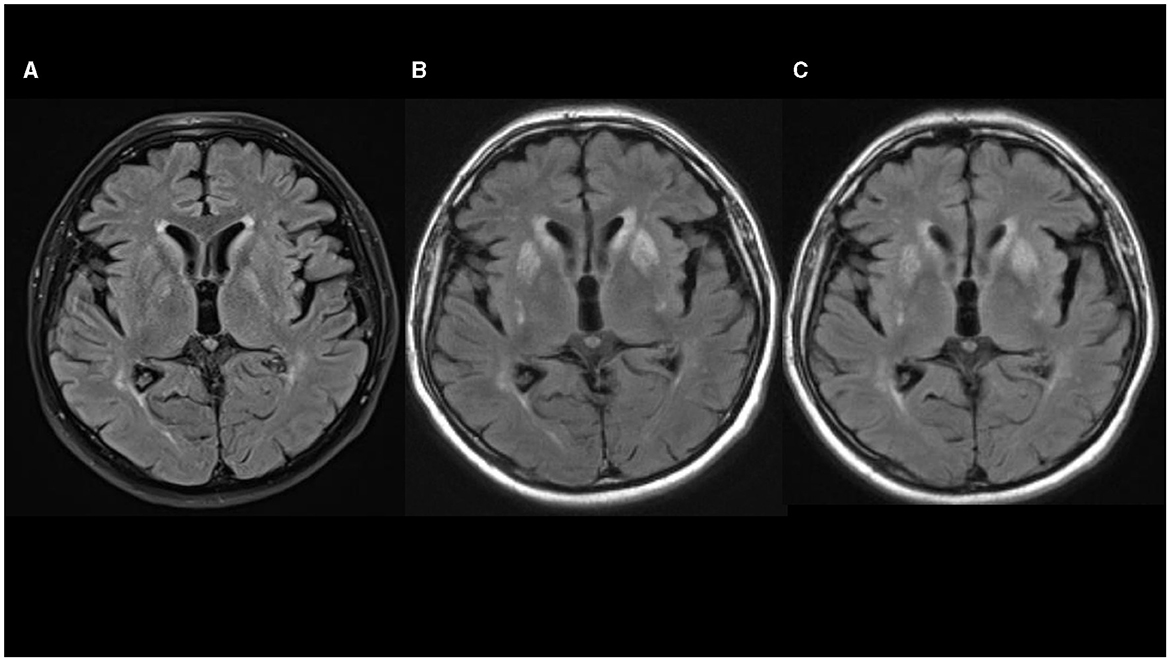
Figure 1. (A–C) Fluid-attenuated inversion recovery (FLAIR) sequences in axial plane show progression of hyperintensities in basal ganglia [(A) 08/2020; (B) 10/2020; (C) 02/2021].
Two months after the hornet incident, he came to our hospital. On examination, the patient had an impassive face, speech was hypophonic and he manifested palilalia. He had symmetric rigidity of his neck and limbs, and mild symmetric bradykinesia was present in all extremities without tremor. Reflexes were brisk but his plantar responses were normal. On postural reflex testing, he recovered with 2–3 steps. The main problem was his gait with start hesitation and freezing, especially on turning or passing through the door (Supplementary Video 1_Segment 1).
His Movement Disorder Society-Unified Parkinson's Disease Rating Scale (MDS-UPDRS) III score was 35 and he was 2.5. on the Hoehn and Yahr scale. Levodopa/carbidopa treatment was slowly down-titrated and stopped. Lumbar puncture showed a small increase in protein content (0.548 g/L; reference interval 0.170–0.370 g/L) with normal cell numbers. There were no oligoclonal bands or signs of immunoglobulin intrathecal synthesis. Autoimmune antibodies (anti-NMDA-R, anti-AMPA-R1, anti-AMPA-R2, anti-GABAB-R, anti-LGI 1 and anti-CASP-R 2) from cerebrospinal fluid were normal. DaTSPECT showed normal findings. Non-contrast head MRI showed mixed striatum signal intensity on T1-weighted images with hyperintensity in ventral putamen, hyperintensity on T2-weighted and FLAIR images, predominantly in bilateral putamen and caudate nuclei with a patchy restricted diffusion-weighted image signal suggesting necrosis (Figure 1B).
Immunohistochemistry was performed using the patient's serum to evaluate antibodies in brain tissue (for procedure, see additional material). As shown in Figure 2, rat brains reacted with the patient's serum in several regions (nucleus arcuatus, cornu ammonis 1 region of the hippocampus, cerebellum, dentate gyrus of the hippocampus, putamen, reticular formation of the pons and substantia nigra).
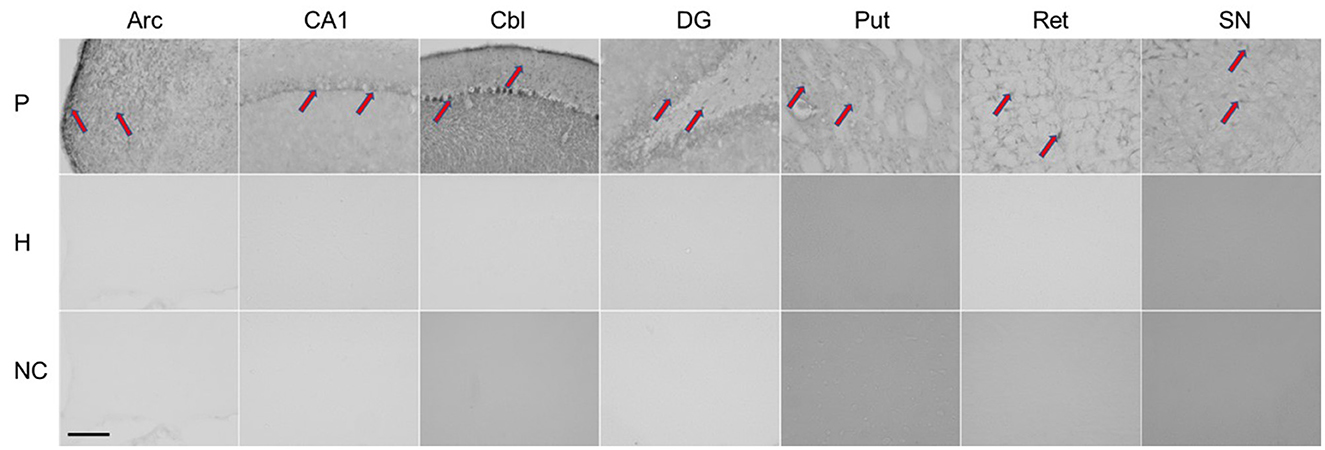
Figure 2. Immunohistochemical staining of rat brain slices using human serum as source of the primary antibodies. Red arrows indicate positive staining reaction in different regions of the rat brain. P, patient; H, healthy control; NC, negative controle; Arc, arcuatus; CA1, cornu ammonis 1 region of hippocampus; Cbl, cerebellum; DG, dentate gyrus of hippocampus; Put, putamen; Ret, reticular formation of pons; SN, substantia nigra. Total magnification of individual image 200x, scale 200 micrometers.
With intravenous corticosteroid treatment (methylprednisolone 250 mg/day for 5 days slowly tapered down over the subsequent 2 weeks), marked improvement of gait was observed (Supplementary Video 1_Segment 2). At discharge, the patient still had hesitation while turning, but freezing of gait had ceased, his MDS-UPDRS III score was 18 and he was 2 on the Hoehn and Yahr scale. Continuation of oral pronisone therapy with gradual dose tapering was recommended. Over the next 6 weeks (during which the patient stopped corticosteroid therapy on his own), gradual deterioration of gait with reappearance of freezing occurred. On that time his MDS- UPDRS III score was 30. Five courses of plasma exchange followed by rituximab were administered but without any significant gait improvement.
At that time, head MRI scan (February 28, 2021) showed mixed striatum signal intensity and hyperintensity in the ventral putamen on T1-weighted, hyperintensity on T2-weighted and FLAIR MRI images, suggesting atrophy and gliosis of the striatum, without restriction of diffusion and without contrast enhancement on T1-weighted postcontrast images (Figure 1C). Symptoms remained unchanged and no new symptoms appeared over the next 6 months of follow-up.
Discussion
Basal ganglia necrosis associated with parkinsonism after an insect sting is a rare and ill-defined condition. In our case and the 12 previous cases from the literature (Table 1), presenting features consisted mainly of symmetric akinetic-rigid syndrome which developed within several days of the incident. Resting tremor was rare and mild, and in the majority of cases levodopa response was poor. Instead, the clinical picture consisted of gait impairment including freezing of gait, postural instabilities, and speech disturbances (including palilalia), all of which have been previously recognized as signs of pallidal damage (18). In addition, profound micrographia and akinetic mutism, which were presenting symptoms in one patient each, further support pallidal involvement (19). Interestingly, pyramidal signs including clonus and Babinski sign were noted in five out of eight patients (for the remaining cases, data are lacking). The combination of pyramidal signs coupled with specific, predominantly axial parkinsonism and gait disturbances after an insect sting more closely resemble distinctive, mainly genetically-determined pallido-pyramidal syndrome than Parkinson's disease (20).
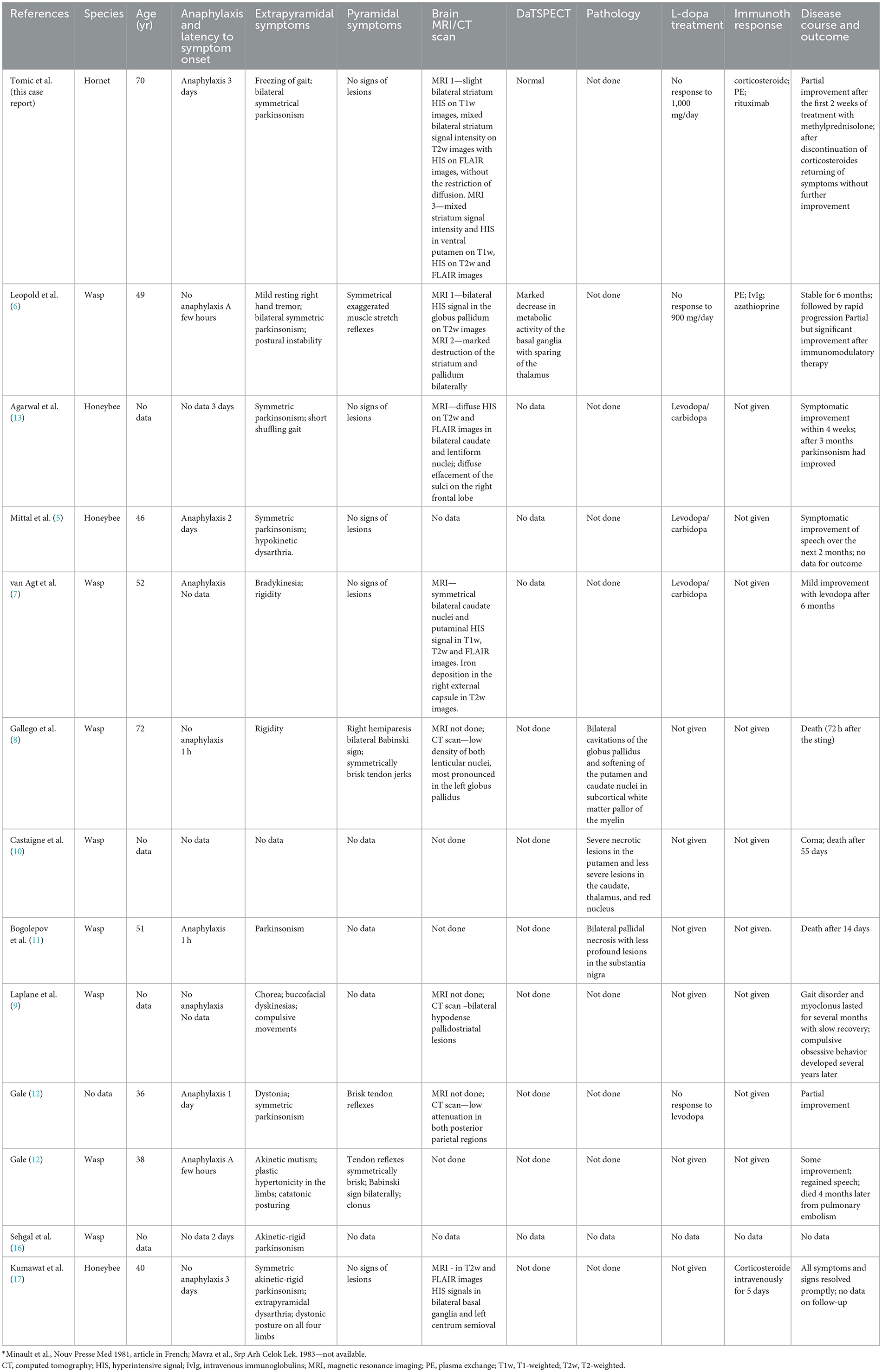
Table 1. Clinical characteristics, MRI findings, treatment response and outcomes of patients who developed parkinsonism following insect stings.
MRI was performed in six out of 12 patients, and HIS on T2-weighted and FLAIR images in the region of basal ganglia (striatum, mainly pallidum), were noted in all of them. In three additional cases, CT scans also demonstrated mainly symmetric hypodensity in lenticular nuclei. In our patient, non-contrast brain CT scan and brain MRI performed 2–3 weeks after the hornet sting showed bilateral petechial blood products in the striatum. Non-contrast brain MRI performed 3 months after hornet sting showed signs of bilateral striatal necrosis, and follow-up brain MRI performed after 7 months showed atrophy and gliosis of the striatum. Combining those data with normal DaTSPECT in our case suggest a postsynaptic cause of parkinsonism predominantly due to striatal damage with sparing of the nigrostriatal dopamine bundle.
The etiology of basal ganglia damage in this rare condition is unknown. Hypoxic-ischaemic mechanism due to an exaggerated anaphylaxis, associated hypotension, and eventual prolonged cerebral hypoxia is one possible mechanism. This sequence of events might explain cases with severe hypotension, prolonged loss of consciousness and respiratory failure. Nevertheless, in case presented by Gallego et al. patient presented with stupor 1 h after incident, soon progressed to coma but without significant cardiovascular, electrolytic or respiratory failure (8). In addition, authors stated that histological pattern on necropsy did not suggest a hypoxic-ischaemic nature of basal ganglia damage. In case we presented, as well as in other cases collected here the anaphylactic reaction led to hypotension without or only with short-term loss of consciousness that did not require cardiopulmonary resuscitation, and there was no need for respiratory support suggesting the hypoxic-ischemic mechanism of brain damage unlikely (6, 17).
Given the acute onset of parkinsonism and neuroimaging findings, an immune-mediated early hypersensitivity reaction is most likely. In support of this are evidence for autoimmunity obtained from immunohistochemistry and the excellent treatment response observed in one patient who received corticosteroids in the acute phase, as well as the beneficial but limited clinical response to corticosteroid treatment observed in our case and those reported by Leopold et al. (6). In the latter two cases, immunomodulatory treatment was applied with significant delay. Although we found that antibody reacted with different brain regions (nucleus arcuatus, cornu ammonis 1 region of hippocampus, cerebellum, dentate gyrus of hippocampus, putamen, reticular formation of pons and substantia nigra), only the striatum was damaged by necrosis. Susceptibility of the basal ganglia to toxic and hypoxic lesions has already been described in human and animal studies, and mitochondrial failure with energy deprivation is considered as the main underlying mechanism (21).
Patient perspective
In conclusion, clinicians should be aware of this rare but devastating cause of acute-onset parkinsonism, it‘s specific clinical presentation and clinical course. Although limited, current knowledge suggest that prompt and prolonged immunomodulatory therapy could be a rational therapeutic choice in attempt to prevent irreversible basal ganglia damage.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ST: Conceptualization, Funding acquisition, Investigation, Writing—original draft, Writing—review & editing. MZ: Investigation, Writing—original draft, Writing—review & editing. ZP: Investigation, Writing—original draft, Writing—review & editing. ZK: Data curation, Investigation, Writing—original draft, Writing—review & editing. MH: Data curation, Writing—original draft, Writing—review & editing. DS: Data curation, Investigation, Writing—original draft, Writing—review & editing. DM: Data curation, Investigation, Writing—original draft, Writing—review & editing. SG: Data curation, Investigation, Writing—original draft, Writing—review & editing. IP: Conceptualization, Formal analysis, Methodology, Project administration, Resources, Validation, Writing—original draft, Writing—review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1365199/full#supplementary-material
References
1. Riggs JE, Ketonen LM, Bodensteiner JB, Benesch CG. Wasp sting-associated cerebral infarction: a role for cerebrovascular sympathetic innervation. Clin Neuropharmacol. (1993) 16:362–5. doi: 10.1097/00002826-199308000-00009
2. Romano JT, Riggs JE, Bodensteiner JB, Gutmann L. Wasp sting associated occlusion of the supraclinoid internal carotid artery: implications regarding the pathogenesis of moyamoya syndrome. Arch Neurol. (1989) 46:607–8. doi: 10.1001/archneur.1989.00520420025018
3. Schoene WC, Carpenter SS, Behan PO, Geschwind N. ‘Onion bulb' formation in the central and peripheral nervous system in association with multiple sclerosis and hypertrophic polyneuropathy. Brain. (1977) 100:755–73. doi: 10.1093/brain/100.4.755
4. Keller M. Optic neuritis after wasp sting. Klin Monatsbl Augenheilkd. (1995) 206:367–8. doi: 10.1055/s-2008-1035464
5. Mittal R, Munjal S, Khurana D, Gupta A. Parkinsonism following bee sting: a case report. Case Rep Neurol Med. (2012) 47, 6523. doi: 10.1155/2012/476523
6. Leopold NA, Bara-Jimenez W, Hallett M. Parkinsonism after a wasp sting. Mov Disord. (1999) 14:122–7.
7. van Agt TFA, Oliveira KLS, Bezerra MER, Pedroso JL, Franco CMR, Melo ES. Acute parkinsonism and basal ganglia lesions after wasp sting. Arq Neuropsiquiatr. (2022) 80:869–70. doi: 10.1055/s-0042-1755215
8. Gállego J, Tuñón T, Soriano G, Delgado G, Lacruz F, Villanueva JA. Bilateral pallidostriatal necrosis caused by a wasp sting: a clinical and pathological study. J Neurol Neurosurg Psychiatry. (1995) 58:474–6. doi: 10.1136/jnnp.58.4.474
9. Laplane D, Widlocher D, Pillon B, Baulac M, Binoux F. Comportement compulsif d'allure obsessionnelle par nécrose circonscrite bilatérale pallido-striatale. Encéphalopathie par piqûre de guêpe [Obsessional-type compulsive behavior caused by bilateral circumscribed pallidostriatal necrosis Encephalopathy caused by a wasp sting]. Rev Neurol (Paris). (1981) 137:269–76.
10. Castaigne P, Bertrand I, Buge A, Godet Guillain J, Escourolle R, Martin M. Coma prolonge secondaire a une piqfure d'insecte necrose symetrique des putamens ramollissement laminaire cortical etendu. Etude anatomo-clinique. Rev Neurol (Paris). (1962) 107:401–16.
11. Bogolepov NK, Luzhetskaia TA, Fedin AI, Artomasova AV, Vasil'ev PN. Allergicheskii éntsefalomielopoliradikulonevrit ot uzhaleniia osy (kliniko-patomorfologicheskoe soobshchenie) [Allergic encephalomyelopolyradiculoneuritis from a wasp sting (clinico-pathologic report)]. Zh Nevropatol Psikhiatr Im S S Korsakova. (1978) 78:187–91.
12. Gale AN. Insect-sting encephalopathy. Br Med J (Clin Res Ed). (1982) 284:20–1. doi: 10.1136/bmj.284.6308.20
13. Agarwal V, Singh R, Chauhan S, D'Cruz S, Thakur R. Parkinsonism following a honeybee sting. Indian J Med Sci. (2006) 60:24–5. doi: 10.4103/0019-5359.19673
14. Mavra M, Nikolić M, Pavlović D, Kovacević MS. Pojava parkinsonizma posle uboda ose [Occurrence of parkinsonism after a wasp sting]. Srp Arh Celok Lek. (1983) 111:501–4.
15. Minault P, Madigand M, Sabouraud O. Nécrose pallido-striatale après piqûre d'hyménoptère. Syndrome parkinsonien [Pallidostriatal necrosis after Hymenoptera sting Parkinsonian syndrome]. Nouv Presse Med. (1981) 10:3725–6.
16. Sehgal B, Malhotra M, Dhanoa J, Loomba V. Multiple wasp stings – an unusual cause of reversible akinetic parkinsonism. Indian Acad Clin Med. (2014) 15:1.
17. Kumawat BL, Sharma CM, Satija V, Tripathi G, Kumar S, Dixit S. A case of acute akinetic rigid parkinsonism due to honeybee sting. J Neurol Sci. (2009) 26:256–8.
18. Bhatia KP, Marsden CD. The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain. (1994) 117:859–76. doi: 10.1093/brain/117.4.859
19. Kuoppamäki M, Rothwell JC, Brown RG, Quinn N, Bhatia KP, Jahanshahi M. Parkinsonism following bilateral lesions of the globus pallidus: performance on a variety of motor tasks shows similarities with Parkinson's disease. J Neurol Neurosurg Psychiatry. (2005) 76:482–90. doi: 10.1136/jnnp.2003.020800
20. Paisán-Ruiz C, Guevara R, Federoff M, Hanagasi H, Sina F, Elahi E, et al. Early-onset L-dopa-responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Mov Disord. (2010) 25:1791–800. doi: 10.1002/mds.23221
Keywords: rapid onset, parkinsonism, basal ganglia damage, sting, insect
Citation: Tomic S, Zjalic M, Popovic Z, Krivdic Dupan Z, Heffer M, Snajder Mujkic D, Mandic D, Guljas S and Petrovic IN (2024) Case report: Rapid-onset parkinsonism after a hornet sting. Front. Neurol. 15:1365199. doi: 10.3389/fneur.2024.1365199
Received: 03 January 2024; Accepted: 18 March 2024;
Published: 03 April 2024.
Edited by:
Alberto Albanese, Catholic University of the Sacred Heart, ItalyReviewed by:
Pratibha Surathi, Rutgers New Jersey Medical School, United StatesRuth Schneider, University of Rochester, United States
Copyright © 2024 Tomic, Zjalic, Popovic, Krivdic Dupan, Heffer, Snajder Mujkic, Mandic, Guljas and Petrovic. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Svetlana Tomic, svetlana.tomic.osijek@gmail.com
†ORCID: Svetlana Tomic orcid.org/0000-0002-1613-3831
 Svetlana Tomic
Svetlana Tomic Milorad Zjalic
Milorad Zjalic Zvonimir Popovic
Zvonimir Popovic Zdravka Krivdic Dupan
Zdravka Krivdic Dupan Marija Heffer
Marija Heffer Darija Snajder Mujkic2,5
Darija Snajder Mujkic2,5 Dario Mandic
Dario Mandic Igor N. Petrovic
Igor N. Petrovic