- 1Department of Internal Medicine, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
- 2Department of Neurology, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States
- 3Department of Internal Medicine, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania
- 4Department of Cardiology, Jakaya Kikwete Cardiac Institute, Dar es Salaam, Tanzania
- 5Department of Internal Medicine, Ocean Road Cancer Institute, Dar es Salaam, Tanzania
- 6Department of Radiology, Muhimbili National Hospital, Dar es Salaam, Tanzania
- 7Department of Neurology, Texas Tech University Health Sciences Center, Paul L Foster School of Medicine El Paso, El Paso, TX, United States
Background: Large vessel ischemic strokes account for more than one-third of all strokes associated with substantial morbidity and mortality without early intervention. The incidence of large vessel occlusion (LVO) is not known in sub-Saharan Africa (SSA). Definitive vessel imaging is not routinely available in resource-limited settings.
Aims: We aimed to investigate the burden and outcomes of presumed LVO among patients with ischemic stroke admitted to a large tertiary academic hospital in Tanzania.
Methods: This cohort study recruited all consenting first-ever ischemic stroke participants admitted at a tertiary hospital in Tanzania. Demographic data were recorded, and participants were followed up to 1 year using the modified Rankin Scale (mRS). A diagnosis of presumed LVO was made by a diagnostic neuroradiologist and interventional neurologist based on contiguous ischemic changes in a pattern consistent with proximal LVO on a non-contrast computed tomography head. We examined factors associated with presumed LVO using logistic regression analysis. Inter-observer Kappa was calculated.
Results: We enrolled 158 first-ever ischemic strokes over 8 months with a mean age of 59.7 years. Presumed LVO accounted for 39.2% [95% confidence interval (CI) 31.6–47.3%] and an overall meantime from the onset of stroke symptoms to hospital arrival was 1.74 days. Participants with presumed LVO were more likely to involve the middle cerebral artery (MCA) territory (70.9%), p < 0.0001. Independent factors on multivariate analysis associated with presumed LVO were hypertension [adjusted odds ratio (aOR) 5.74 (95% CI: 1.74–18.9)] and increased waist-hip ratio [aOR 7.20 (95% CI: 1.83–28.2)]. One-year mortality in presumed LVO was 80% when compared with 73.1% in participants without presumed LVO. The Cohen's Kappa inter-observer reliability between the diagnostic neuroradiologist and interventional neurologist was 0.847.
Conclusion: There is a high burden of presumed LVO associated with high rates of 1-year morbidity and mortality at a tertiary academic hospital in Tanzania. Efforts are needed to confirm these findings with definitive vessel imaging, promoting cost-effective preventive strategies to reduce the burden of non-communicable diseases (NCDs), and a call for adopting endovascular therapies to reduce morbidity and mortality.
Introduction
Stroke is a leading cause of death and disability, particularly in low and middle-income countries (LMICs), contributing to 80% of all incident cases, 87% of all deaths, and 89% of stroke-related disability-adjusted life years (DALYs) (1, 2). According to the 2019 Global Burden of Disease (GBD) report, there were 12.2 million stroke incident cases, 6.55 million stroke fatal cases, and 143 million stroke DALYs (3). Globally, the proportions of ischemic strokes (62.4%) are higher as compared to intracerebral hemorrhages (27.9%) and subarachnoid hemorrhages (9.7%) (3). In LMIC, ischemic stroke accounts for approximately 7 million cases (63%) and 1.5 (57%) million deaths as a proportion of all strokes and deaths, respectively (4). A similar trend is observed in sub-Saharan Africa (SSA), where ischemic strokes account for 68% of all strokes as compared to 32% of hemorrhagic strokes (5).
Large vessel occlusions (LVOs) account for 20–40% of all ischemic strokes associated with substantial morbidity and mortality (6, 7). In high-income countries (HICs), the incidence of LVO is estimated at 24 per 100,000-person years, summing up to 80,000 cases annually (8). In the pre-endovascular era, mortality was two times (64% vs. 24%) among those with LVO as compared to those without, respectively (6). This eventually led to the adoption of endovascular interventions that have proven cost-effective in preventing mortality and disability from a stroke in HIC based on previous pivotal clinical trials (9, 10). Notably, such resources are limited in the vast majority of facilities sub-serving people living in SSA contributing to increased stroke mortality. In addition, it is unusual for patients to arrive in the early time window for stroke intervention. Little is known about the true burden and outcomes of LVO among patients with ischemic stroke in SSA. We therefore aimed to investigate the prevalence and outcomes of first-ever ischemic strokes, with a particular focus on presumed LVO in patients admitted to a tertiary academic hospital in Tanzania.
Materials and Methods
Study Design and Population
This cohort study was conducted at Muhimbili University of Health and Allied Sciences Academic Medical Center (MAMC), medical wards in Dar es Salaam, Tanzania. This is a tertiary academic hospital that receives referral patients from both public and private hospitals and offers specialized medical care for all medical subspecialties in Tanzania.
Consecutive participants aged ≥18 years admitted at MAMC with a diagnosis of first-ever ischemic stroke according to the World Health Organization definition (WHO) (11) between June 2018 and January 2019 were recruited. Written informed consent was obtained from either the participants or their next of kin if the participant was unable to consent before study enrollment.
Data Collection
An interviewer-based structured questionnaire was administered to all study participants or their caregivers capturing the following: demographic information, date of onset of stroke symptoms, date of admission, contact details, and premorbid stroke risk factors (e.g., hypertension, diabetes mellitus (DM), and HIV infection). Medication history for hypertension, DM, HIV, and hormonal contraception for women was also obtained. We also inquired about smoking and alcohol consumption.
Clinical Measurements
Physical examination included measurement of blood pressure (BP) using a standard digital BP machine, AD Medical Inc. Three BP readings were collected spaced 5-min apart, while the participant was at rest, and an average BP was computed. Participants were regarded as hypertension when the average BP readings for systolic blood pressure (SBP) ≥140 mmHg or diastolic blood pressure (DBP) ≥90 mmHg or if the participant was on anti-hypertensive therapy according to the Joint National Committee 7 (JNC-7) definition (12). All participants had their waist and hip circumference measured using a tape measure and recorded in centimeters. The waist-hip ratio was interpreted according to the WHO guidelines; in men, the ratio of ≥0.90 and in women, the ratio of ≥0.85 were regarded as substantially increased (13). The examination also included precordial and neck carotid auscultation.
Laboratory Investigations
Capillary fingertip blood samples were collected to check for random blood glucose (RBG) levels and HIV rapid testing using a glucometer GLUCOPLUSTM and SD Bioline, respectively. A fasting blood glucose (FBG) sample was collected the following morning for participants with RBG levels of ≥11.1 mmol/l (equivalent to ≥200 mg/dl). DM diagnosis was defined as an RBG reading of ≥11.1 mmol/l (equivalent to ≥200 mg/dl) or an FBG reading of ≥7 mmol/l (equivalent to ≥126 mg/dl). HIV testing was performed using sequential rapid test SD Bioline, followed by Unigold Biotech – these are both rapid immune-chromatographic antibody assays for HIV 1/2 antibodies.
We aseptically collected 5 ml of venous blood from each study participant. We analyzed random total cholesterol, triglycerides (TGA), low-density lipoproteins (LDL), and high-density lipoproteins (HDL) using machine model A15 of BioSystems. Cutoffs for total cholesterol >240 mg/dl were regarded as hypercholesterolemia, TGA >200 mg/dl as hypertriglyceridemia, LDL >129 mg/dl as increased, and HDL <35 mg/dl as reduced levels.
Brain Imaging
A non-contrast brain computed tomography (NCCT) using GE Healthcare Optima was performed on all the study participants only at baseline, and images were independently interpreted by a diagnostic neuroradiologist (FL) and an interventional neurologist (FS). Ischemic stroke was defined based on a clinical stroke syndrome and objective evidence of focal cerebral ischemia in a vascular territory by an NCCT (14) (Figure 1). Presumed LVO was defined as occlusion of the proximal segments of the MCA (i.e., M1 or proximal M2), anterior cerebral arteries, posterior cerebral arteries, vertebral arteries, and basilar arteries that were determined radiographically based on contiguous hypo-density noted on NCCT that can be attributed to the involved vascular territory with or without the presence of additional findings, such as hyperdense MCA or basilar signs (15, 16). Hemorrhagic transformation was defined as per European Cooperative Acute Stroke Study (ECASS II) (17). Hemorrhagic infarction type 1 (HI1) was defined as petechial hemorrhages at the infarct margins. Hemorrhagic infarction type 2 (HI2) was defined as petechial hemorrhages throughout the infarct and no mass-effect was attributable to the hemorrhages. Parenchymal hematoma type 1 (PH1) was defined as ≤ 30% of the infarcted area and minor mass effect attributable to the hematoma. Parenchymal hematoma type 2 (PH2) was defined as >30% of the infarct zone and substantial mass effect attributable to the hematoma. Midline shift was defined as any measurable shift of midline cerebral structures seen on axial images of an NCCT, specifically the septum pellucidum and/or the pineal gland (18).
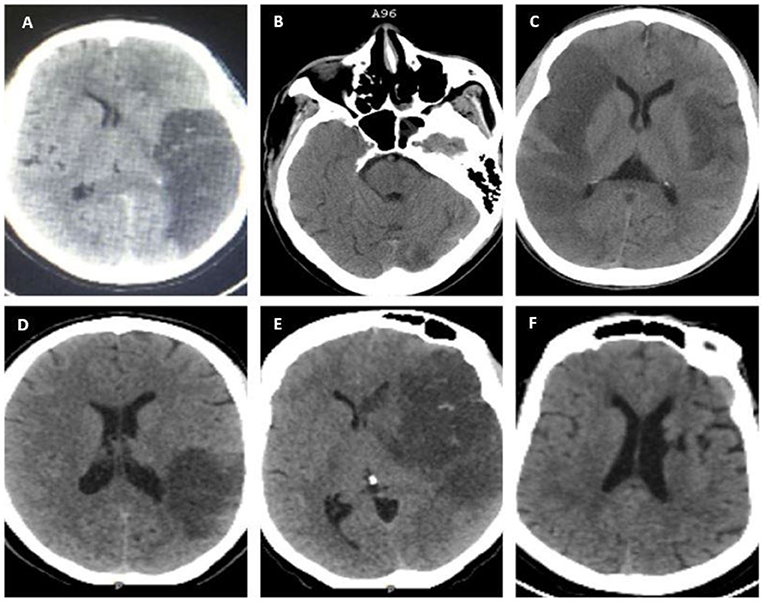
Figure 1. Non-contrast brain computed tomography (NCCT) scans showing evidence of ischemic infarcts in various vascular territories; (A) left distal middle cerebral artery (MCA) M1 with HI 1; (B) basilar and left posterior cerebral artery (PCA) infarction with a hyperdense basilar sign; (C) bilateral MCA: right M1 and left distal M1/ proximal M2; (D) inferior division M2 MCA with HI 1; (E) left proximal M1 with HI 1; and (F) left Lacunar infarct (non-LVO).
Cardiovascular Assessment
Transthoracic echocardiography (ECHO) using GE Medical Systems was performed by a trained cardiologist, and interpretation was based on European Society of Cardiology/American Society of Echocardiography guidelines for evidence of any structural heart abnormalities and other cardiac risk factors for stroke, such as mitral stenosis, presence of vegetations and thrombus (19). A 12-lead electrocardiogram (ECG) using a machine of Bionet was performed on the study participants to look for evidence of atrial fibrillation.
Stroke Outcomes
Stroke severity was assessed using the National Institute of Health Stroke Scale (NIHSS) on admission (11). Stroke outcomes were categorized using the modified Rankin Scale (mRS) (11) at 24 h, 30 days, and 1 year from admission, with scores ranging from 0 (no symptoms) to 6 (death).
Study Variables
The dependent variable was presumed LVO. The independent variables included demographic characteristics, risk factors (hypertension, DM, smoking, cardiac disease, alcohol consumption, increased waist-hip ratio, hypercholesterolemia, and increased LDL), vascular territories, and stroke outcomes (death or survival with/without disabilities).
Data Analysis
Data were analyzed using SPSS version 20.0. Continuous variables were summarized and presented as means and standard deviation (SD) or medians with interquartile range [IQR]. Categorical variables were summarized as frequencies and proportions. Comparisons between proportions were done using Pearson's Chi-square test or Fisher's exact test. The logistic regression technique was used to determine independent factors associated with presumed LVO. All covariates with a p-value of <0.2 in the bivariable analysis were included in the multivariable analysis model. Unadjusted and adjusted odds ratios (OR), 95% confidence intervals (CI), and corresponding p-values were obtained from the models. A two-tailed significance level was set as a p-value of ≤ 0.05. Inter-observer Kappa between the diagnostic neuroradiologist (FL) and interventional neurologist (FS) was also calculated.
Results
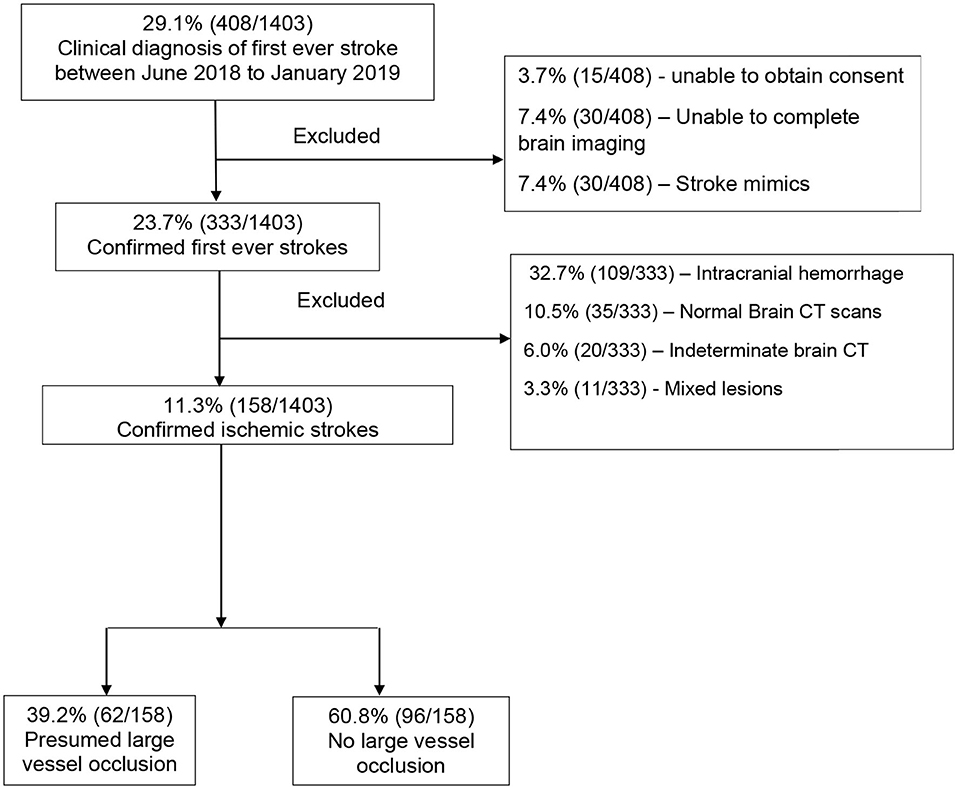
Between June 2018 to January 2019, there were 1,403 medical admissions, out of which 408 (29.1%) participants met the WHO clinical definition of first-ever ischemic stroke. We excluded 250 (15.9%) participants for the following reasons: inability to consent, those who did not complete brain imaging, those with stroke mimics based on brain imaging, intracranial hemorrhage, normal brain CT scans, indeterminate CT scans (those with artifacts or poor imaging quality), and those with mixed lesions. We recruited the remaining 158 (38.7%) participants with a confirmed diagnosis of ischemic stroke, as shown in Figure 2. The proportion of ischemic strokes over total admissions was 158 of 1,403 [(11.3%) (95% CI 9.7–13.0%)]. Of these, 62 of 158 [(39.2%) (95% CI 31.6–47.3%)] had presumed LVO.
The overall mean age ± SD of the recruited participants was 59.7 ± 16.6 years, and the majority resided in Dar-es-Salaam (72.8%), a former capital city. A quarter possessed health insurance (25.9%). The overall meantime from the onset of stroke symptoms to hospital arrival was 1.74 days with no statistically significant difference in the meantime from the onset of stroke symptoms to hospital arrival in the presumed LVO vs. non-LVO groups (1.87 days vs. 1.83, respectively, p < 0.32.). Overall, 29 (18.3%) participants were observed to arrive at the hospital within 24 h from stroke symptom onset with no participant arriving in <4.5-h and <8-h windows.
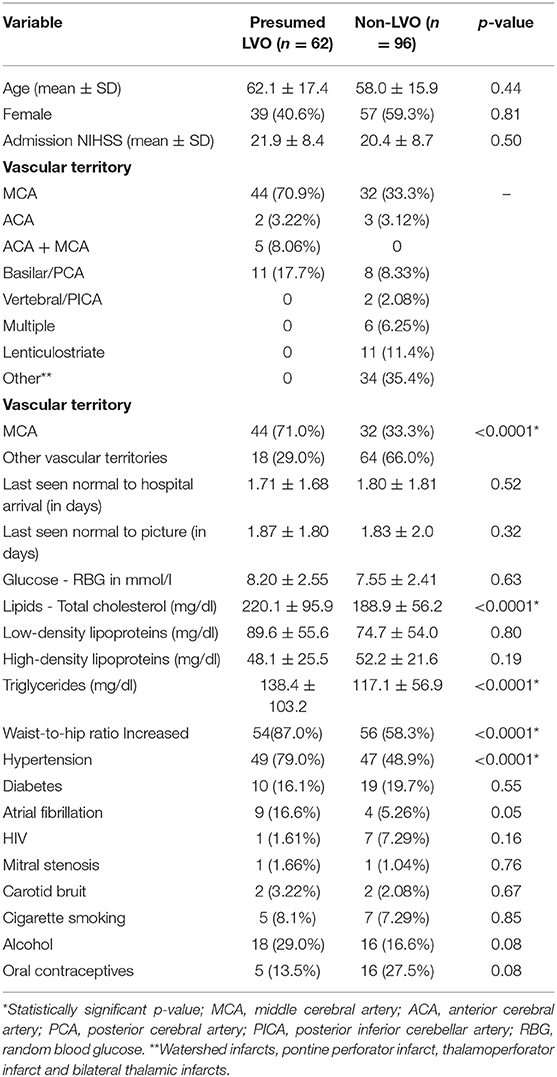
The MCA was the predominant major vascular territory involved. It was statistically seen more frequently in the presumed LVO group as compared to those with non-LVO, i.e., 44 (71.0%) vs. 32 (33.3%), p < 0.0001, respectively. Similarly, participants with presumed LVO were statistically more likely to have the following cardiovascular risk factors as compared to those with non-LVO: increased waist-hip ratio 54 (87%) vs. 56 (58.3%), p < 0.001, hypertension 49 (79.0%) vs. 47 (48.9%), p < 0.001, mean total cholesterol of 220.1 ± 95.9 mg/dl vs. 188.9 ± 56.2 mg/dl, p < 0.001, and mean TGA 138.4 ± 103.2 mg/dl vs. 117.1 ± 56.9 mg/dl, p < 0.001, respectively (Table 1).
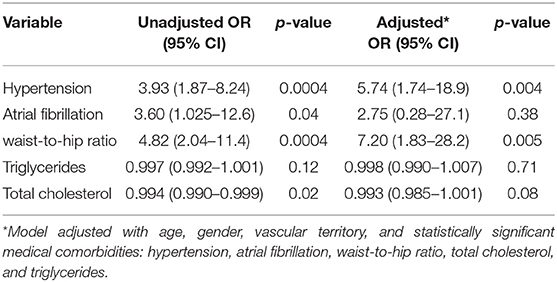
Predictors of presumed LVO are summarized in Table 2. In univariate analysis, factors that were significantly associated with presumed LVO were medical co-morbidities, such as hypertension, atrial fibrillation, increased waist-to-hip ratio, total cholesterol, and TGA. In multivariate analysis, presumed LVO was independently associated with hypertension [adjusted OR (aOR) 5.74 (95% confidence interval (CI): 1.74–18.9)] and increased waist-hip ratio [adjusted OR 7.20 (95% CI: 1.83–28.2)].
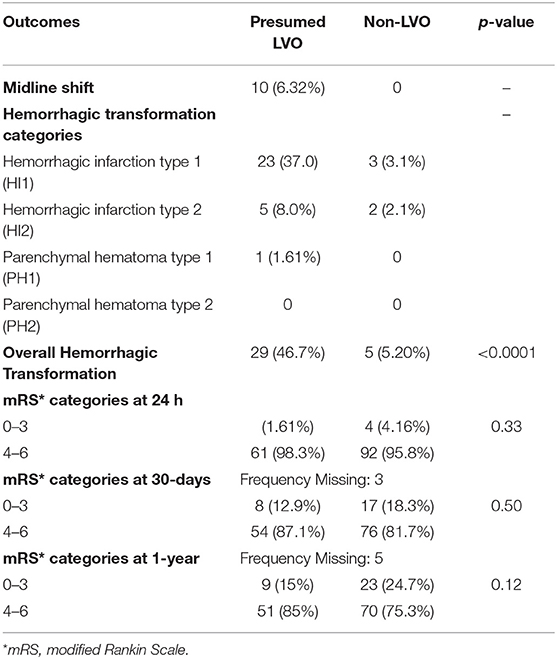
Table 3 describes the outcomes of the study participants. At 30 days, there were 3 participants who were lost to follow-up and were excluded from the analysis. Overall, the 30-day mortality was 89/155 (57.4%), with no statistically significant difference in mortality in the presumed LVO and non-LVO groups, 36/62 (58.1%) vs. 53/93 (57%), p = 0.51, respectively. At 1 year, there were 5 participants who were lost to follow-up and were excluded from the analysis. The overall 1-year mortality was 116/153 (75.8%), 48/60 (80%) in the presumed LVO group vs. 68/93 (73.1%) in the non-LVO group, p = 0.25. Those with presumed LVO were statistically more likely to have a hemorrhagic transformation on the NCCT head as compared to those with non-LVO, 29 (46.7%) vs. 5 (5.20%), p < 0.001, respectively. The Cohen's Kappa inter-observer reliability between the diagnostic neuroradiologist and interventional neurologist was calculated at 0.847.
Discussion
This is the first study looking at the burden and outcomes of presumed LVO among all presenting ischemic strokes in Tanzania, an East African nation representative of many LMICs located in SSA. The present study was conducted at a tertiary specialized academic hospital and found a proportion of 39.2% of presumed LVO. This is a large proportion of all presenting ischemic strokes and is comparable to a previously reported global prevalence of 31% in a large meta-analysis (20). Our findings are of particular importance given the high burden of ischemic strokes in Tanzania (21) and the need for good epidemiological data before advocating for evidence-based acute stroke interventions in resource-limited settings. There is currently no data on the prevalence of LVO ischemic strokes in SSA.
The mechanisms for LVO include intracranial artery atherosclerosis, cardioembolism, artery to artery embolism (from extracranial atherosclerosis or dissection), and unknown/cryptogenic causes (22). Our study found that risk factors for presumed LVO on the univariate analysis included hypertension, increased waist-to-hip ratio (a surrogate for obesity), increased total cholesterol, triglycerides, and atrial fibrillation. On multivariate analysis, only hypertension and increased waist-to-hip ratio remained statistically significant, which are known risk factors for both intracranial and extracranial vessel atherosclerosis (23). It is notable that our study showed a relatively lower prevalence of atrial fibrillation of 16.6% in the presumed LVO group and 5.3% in the non-LVO group when compared to a meta-analysis of 5 randomized trials that looked at the efficacy of endovascular thrombectomy over standard medical care where the prevalence of atrial fibrillation was noted to be 33% (24). A similar high prevalence of atrial fibrillation was noted in the DAWN trial with 40% in the mechanical thrombectomy arm and 24% in the medical therapy arm (25). Several factors might explain the lower prevalence of atrial fibrillation in this setting that included genetic predisposition, under surveillance resulting in lower detection of occult or paroxysmal atrial fibrillation using conventional resting ECG that was utilized as a part of routine clinical care and poor access to healthcare (26, 27). Stroke is preventable in SSA by ensuring early detection and control of modifiable risk factors, related to urbanization and lifestyle changes. Therefore, efforts need to be centered on promoting low-cost interventions to reduce this looming epidemic of non-communicable diseases (NCDs) since a stroke in Tanzania is associated with substantial morbidity and mortality, impacting the country's economy (28). The involvement of the MCA as the most common site for presumed LVO is in concordance with global trends (29).
The mainstay for the management of LVO is through endovascular interventions, which have been associated with improved outcomes; however, the majority of these clinical trials on the efficacy of thrombectomy have been done in HIC (9, 10). The barriers in LMIC that lead to inequity in endovascular care are mainly due to the high cost of the procedure, lack of endovascular specialists, and late times to presentation, among others. This was shown in the Mexican Endovascular Reperfusion Registry (MERR), which supported the effectiveness of thrombectomy but noted that treatment was mainly feasible in private hospitals (30). Similarly, the RESILIENT trial conducted in Brazil was the first clinical trial that evaluated the benefits of thrombectomy in patients with LVO in LMIC presenting within 8 h from stroke symptoms (31). Their results showed a better functional outcome at 90 days among the LVO group receiving endovascular therapy. Muhimbili National Hospital (MNH) and MAMC, where this study was conducted, can administer IV thrombolysis to patients presenting with an acute coronary syndrome, which will potentially translate to IV thrombolysis for acute ischemic stroke; however, we currently do not have mechanical thrombectomy capability in Tanzania. The poor functional outcomes in this cohort could be attributed to the severity of the stroke. It is notable that both groups had similar NIHSS scores (mean NIHSS score >20), which is a matter of concern, especially in the non-LVO group. One possible explanation for the severe symptoms in the non-LVO group is the anatomical location of the infarcts, which included watershed infarcts, pontine perforator infarcts, thalamoperforator infarcts, and bilateral thalamic infarcts. Further, reports have indicated that NIHSS scores can predict the presence of LVO among patients with ischemic stroke arriving at an early time window (within the first hours) but predicts less in late presenting patients (32). The latter was the case for this study and given the fact that our study did not use angiographic techniques to confirm the diagnosis of LVO; this could be another possible explanation for this discrepancy. Nonetheless, the severity of stroke also has multifactorial explanations that include lack of stroke readiness in the healthcare infrastructure and referral networks and lack of community awareness regarding stroke symptoms and signs translating into delays and progression of stroke syndromes. In our cohort, the time from stroke onset to presentation was suboptimal in both groups, with a mean of 1.87 days in the presumed LVO group vs. 1.83 days in the non-LVO group, p < 0.32 and no patients arriving within the first 8 h. This delay represents a critical barrier to adopting endovascular interventions and IV thrombolysis in our setting. Therefore, it is a call for prehospital referral strengthening approaches to facilitate transferring potential strokes to a stroke-capable facility.
Also importantly, there were high rates of hemorrhagic transformation on CT head statistically seen more in participants with presumed LVO (46.7% vs. 5.2%, p < 0.0001, respectively), even though most of these patients were not parenchymal hematomas (HI 1 and 2). This high rate is multifactorial and could represent the severity of reperfusion injury and uncontrolled hypertension; this highlights the need for specialized stroke units for peri-procedural management of these high-risk patients regardless of whether they undergo mechanical thrombectomy. This also highlights the need for a multidisciplinary approach with the involvement of neurosurgeons to facilitate decompressive hemicraniectomy to manage malignant cerebral edema and hemorrhagic transformation (33).
Finally, there was a higher rate of poor outcomes at 1 year (defined as mRS 4–6) in the presumed LVO group (85%) when compared with the non-LVO group (75.3%; p = 0.12), which is concerning but representative of global trends (1, 2). Similarly, 1-year mortality was higher in the presumed LVO group at 80% when compared with the non-LVO group at 73.1%. The overall high rate of poor outcomes in both groups represents a more significant problem with regard to post-stroke care and lack of rehabilitation in LMIC, particularly in SSA. Studies in SSA have previously demonstrated a low implementation of secondary preventive measures following stroke and limited access to rehabilitation services impacting the overall quality of life of these patients (34, 35).
Our study had the following strengths: it provides actionable information given the lack of similar data regarding the burden of presumed LVO in local and regional stroke demographics in LMIC in general and Tanzania as a representative country in SSA in particular. With the development of the MAMC, a state-of-the-art academic hospital sub-serving East and Central Africa, there is potential for more acute therapies for ischemic stroke reperfusion to be introduced into the local neurological landscape. Therefore, the results of this study show important challenges that need to be overcome, as highlighted above. Our study compared presumed large vessel and non-large vessel strokes enabling a unique analysis between these two entities in SSA, while previous data have only looked at overall ischemic strokes.
Finally, the high inter-rater Kappa boosts confidence in the reproducibility of these findings. Given previous infrastructural unavailability of CT/MR angiographic techniques to diagnose and manage LVOs, this study uses the next best method to provide preliminary data that will be the necessary stepping stone to generate discussions among different stakeholders to establish the mechanisms for introducing non-invasive and invasive angiography to diagnose and treat acute ischemic strokes.
This study is limited by the fact that the diagnosis of LVO was made without the use of angiographic techniques, which is the gold standard for this diagnosis. There are reliable data to suggest that the diagnosis of LVO can be inferred from non-angiographic CT studies based on contiguous hypo-density that can be attributed to the involved vascular territory and a compatible clinical syndrome (15, 16). In addition, given later times to presentation/imaging in our cohort, the reliability of NCCT findings increases with time (36, 37). However, it is entirely possible that there were patients in the non-LVO group that had not yet developed contiguous lobar hypodensities due to slow infarct progression; this technique, therefore, has the potential to underdiagnose LVO. Despite the late presentation of our patients (time from the onset of stroke symptoms to hospital arrival of (1.74 days), excluding the 35 patients with normal NCCT head might have underestimated the proportion of presumed LVO. Additionally, our study did not record specific neurological characteristics of these patients.
Conclusion
There is a high burden of presumed LVO associated with high rates of 1-year morbidity and mortality. Concerted efforts are required to promote cost-effective preventive strategies to combat the looming epidemic of non-communicable diseases and a call for adopting endovascular interventions to reduce morbidity and mortality.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Muhimbili University of Health and Allied Sciences Institutional Review Board approval number DA.287/298/01A/. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SM, RA, FL, MM, and FS conceptualized and designed the study and drafted the initial manuscript. MC carried out the data analysis and interpreted the results. SM, RA, MC, MM, FS, PM, KK, KT, FL, GR, VG, and AM critically reviewed and revised the final manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Catholic University of Health and Allied Sciences (Tanzania) and Texas Tech University Health Sciences Center, El Paso TX (USA). The funder has no role in the design, analysis, final write-up of the manuscript, and decision to submit the paper for publication.
Author Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Our sincere gratitude goes to the study participants for taking part in this study. SM reports support from the Fogarty International Center and National Institute of Neurological Disorders and Stroke D43TW009337.
Abbreviations
AF, Atrial Fibrillation; DALYS, Disability Adjusted Life Years; DWI, Diffusion Weighted Imaging; FBG, Fasting Blood Glucose; HIV, Human Deficiency Virus; LVO, Large vessel occlusion; mRS, Modified Rankin Scale; NIHSS, National Institute of Health Stroke Scale; SSA, sub-Saharan Africa; WHO, World Health Organization.
References
1. Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Heal. (2013) 1:e259–81. doi: 10.1016/S2214-109X(13)70089-5
2. Krishnamurthi RV, Ikeda T, Feigin VL. Global, regional and country-specific burden of ischaemic stroke, intracerebral haemorrhage and subarachnoid haemorrhage: a systematic analysis of the global burden of disease study 2017. Neuroepidemiology. (2020) 54:171–9. doi: 10.1159/000506396
3. Feigin VL, Stark BA, Johnson CO, Roth GA, Bisignano C, Abady GG, et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
4. Bennett DA, Krishnamurthi RV, Barker-Collo S, Forouzanfar MH, Naghavi M, Connor M, et al. The global burden of ischemic stroke: findings of the GBD 2010 study. Glob Heart. (2014) 9:107–12. doi: 10.1016/j.gheart.2014.01.001
5. Owolabi MO, Sarfo F, Akinyemi R, Gebregziabher M, Akpa O, Akpalu A, et al. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Glob Heal. (2018) 6:e436–46. doi: 10.1016/S2214-109X(18)30002-0
6. Malhotra K, Gornbein J, Saver JL. Ischemic strokes due to large-vessel occlusions contribute disproportionately to stroke-related dependence and death: a review. Front Neurol. (2017) 8:651. doi: 10.3389/fneur.2017.00651
7. Dozois A, Hampton L, Kingston CW, Lambert G, Porcelli TJ, Sorenson D, et al. Plumber study (prevalence of large vessel occlusion strokes in mecklenburg county emergency response). Stroke. (2017) 48:3397–9. doi: 10.1161/STROKEAHA.117.018925
8. Rai AT, Seldon AE, Boo S, Link PS, Domico JR, Tarabishy AR, et al. A population-based incidence of acute large vessel occlusions and thrombectomy eligible patients indicates significant potential for growth of endovascular stroke therapy in the USA. J Neurointerv Surg. (2017) 9:722–6. doi: 10.1136/neurintsurg-2016-012515
9. Bannay A, Guillemin F, Soudant M, Bracard S, Epstein J, Achit H, et al. Cost-effectiveness of thrombectomy in patients with acute ischemic stroke. Stroke. (2017) 48:2843–7. doi: 10.1161/STROKEAHA.117.017856
10. Sweid A, Hammoud B, Ramesh S, Wong D, Alexander TD, Weinberg JH, et al. Acute ischaemic stroke interventions: large vessel occlusion and beyond. BMJ. (2020) 5:80–5. doi: 10.1136/svn-2019-000262
11. WHO Noncommunicable Diseases Mental Health. The WHO STEPwise Approach to Stroke Surveillance Report. (2005). Available online at: https://www.who.int/ncd_surveillance/en/steps_stroke_manual_v1.2.pdf (accessed July 01, 2022).
12. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. (2003) 42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2
13. World Health Organization. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. WHO (2011).
14. Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. (2013) 44:2064–89. doi: 10.1161/STR.0b013e318296aeca
15. Lim J, Magarik JA, Froehler MT. The CT-defined hyperdense arterial sign as a marker for acute intracerebral large vessel occlusion. J Neuroimaging. (2018) 28:212–6. doi: 10.1111/jon.12484
16. Truwit CL, Barkovich AJ, Gean-Marton A, Hibri N, Norman D. Loss of the insular ribbon: another early CT sign of acute middle cerebral artery infarction. Radiology. (1990) 176:801–6. doi: 10.1148/radiology.176.3.2389039
17. Boysen G, ECASS Study Group. European Cooperative Acute Stroke Study (ECASS): (rt-PA-Thrombolysis in acute stroke) study design and progress report. Eur J Neurol. (1995) 1:213–9. doi: 10.1111/j.1468-1331.1995.tb00074.x
18. Ropper AH. Lateral displacement of the brain and level of consciousness in patients with an acute hemispheral mass. N Engl J Med. (2009) 314:953–8. doi: 10.1056/NEJM198604103141504
19. Kim M, Kim HL, Park KT, Kim YN, Lim JS, Lim WH, et al. Echocardiographic parameters determining cardiovascular outcomes in patients after acute ischemic stroke. Int J Cardiovasc Imaging. (2020) 36:1445–54. doi: 10.1007/s10554-020-01841-5
20. Lakomkin N, Dhamoon M, Carroll K, Singh IP, Tuhrim S, Lee J, et al. Prevalence of large vessel occlusion in patients presenting with acute ischemic stroke: a 10-year systematic review of the literature. J Neurointerv Surg. (2019) 11:241–5. doi: 10.1136/neurintsurg-2018-014239
21. Walker R, Whiting D, Unwin N, Mugusi F, Swai M, Aris E, et al. Stroke incidence in rural and urban Tanzania: a prospective, community-based study. Lancet Neurol. (2010) 9:786–92. doi: 10.1016/S1474-4422(10)70144-7
22. Rennert RC, Wali AR, Steinberg JA, Santiago-Dieppa DR, Olson SE, Pannell JS, et al. Epidemiology, natural history, and clinical presentation of large vessel ischemic stroke. Neurosurgery. (2019) 85:S4–S8. doi: 10.1093/neuros/nyz042
23. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. (2013) 12:1106–14. doi: 10.1016/S1474-4422(13)70195-9
24. Goyal M, Menon BK, Van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
25. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
26. Hariri E, Hachem A, Sarkis G, Nasr S. Optimal duration of monitoring for atrial fibrillation in cryptogenic stroke: a nonsystematic review. Biomed Res Int. (2016) 2016:5704963. doi: 10.1155/2016/5704963
27. Rabinstein AA. Prolonged cardiac monitoring for detection of paroxysmal atrial fibrillation after cerebral ischemia. Stroke. (2014) 45:1208–14. doi: 10.1161/STROKEAHA.113.003389
28. Walker RW, Mclarty DG, Kitange HM, Whiting D, Masuki G, Mtasiwa DM. Stroke mortality in urban and rural Tanzania. Lancet. (2000) 355:1684–7. doi: 10.1016/S0140-6736(00)02240-6
29. Waqas M, Mokin M, Primiani CT, Gong AD, Rai HH, Chin F, et al. Large vessel occlusion in acute ischemic stroke patients: a dual-center estimate based on a broad definition of occlusion site. J Stroke Cerebrovasc Dis. (2020) 29:104504. doi: 10.1016/j.jstrokecerebrovasdis.2019.104504
30. Marquez-Romero JM, Góngora-Rivera F, Hernández-Curiel BC, Aburto-Murrieta Y, García-Cazares R, Delgado-Garzón P, et al. Endovascular treatment of ischemic stroke in a developing country. Vasc Endovascular Surg. (2020) 54:305–12. doi: 10.1177/1538574420906941
31. Martins SO, Mont'Alverne F, Rebello LC, Abud DG, Silva GS, Lima FO, et al. Thrombectomy for stroke in the public health care system of Brazil. N Engl J Med. (2020) 382:2316–26. doi: 10.1056/NEJMoa2000120
32. Heldner MR, Zubler C, Mattle HP, Schroth G, Weck A, Mono ML, et al. National Institutes of Health stroke scale score and vessel occlusion in 2152 patients with acute ischemic stroke. Stroke. (2013) 44:1153–7. doi: 10.1161/STROKEAHA.111.000604
33. Beez T, Munoz-Bendix C, Steiger HJ, Beseoglu K. Decompressive craniectomy for acute ischemic stroke. Crit Care. (2019) 23:209. doi: 10.1186/s13054-019-2490-x
34. Owolabi MO, Akarolo-Anthony S, Akinyemi R, Arnett D, Gebregziabher M, Jenkins C, et al. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc J Afr. (2015) 26:S27–38. doi: 10.5830/CVJA-2015-038
35. Tessua KK, Munseri P, Matuja SS. Outcomes within a year following first ever stroke in Tanzania. PLoS ONE. (2021) 16:1–13. doi: 10.1371/journal.pone.0246492
36. Nguyen TN, Abdalkader M, Nagel S, Qureshi MM, Ribo M, Caparros F, et al. Noncontrast computed tomography vs. computed tomography perfusion or magnetic resonance imaging selection in late presentation of stroke with large-vessel occlusion. JAMA Neurol. (2021) 79:22–31. doi: 10.1001/jamaneurol.2021.4082
37. Arsovska A. Can Non-Contrast Computed Tomography Alone Be Used as an Alternative to Advanced Imaging In Selecting Patients With Late-Presenting Large-Vessel Occlusion for Mechanical Thrombectomy? (2022). Available online at: https://www.world-stroke-academy.org/news/the-paper-of-the-month-january/ (accessed January 13, 2022).
Keywords: ischemic stroke, large vessel occlusion, thrombectomy, morbidity and mortality, Tanzania
Citation: Matuja SS, Ahmed RA, Munseri P, Khanbhai K, Tessua K, Lyimo F, Rodriguez GJ, Gupta V, Maud A, Chaudhury MR, Manji M and Sheriff F (2022) Ischemic Stroke at a Tertiary Academic Hospital in Tanzania: A Prospective Cohort Study With a Focus on Presumed Large Vessel Occlusion. Front. Neurol. 13:882928. doi: 10.3389/fneur.2022.882928
Received: 24 February 2022; Accepted: 17 June 2022;
Published: 14 July 2022.
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Davide Strambo, Center Hospitalier Universitaire Vaudois (CHUV), SwitzerlandRuiting Zhang, Zhejiang University, China
Copyright © 2022 Matuja, Ahmed, Munseri, Khanbhai, Tessua, Lyimo, Rodriguez, Gupta, Maud, Chaudhury, Manji and Sheriff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah Shali Matuja, dr.matujajunior@gmail.com
 Sarah Shali Matuja
Sarah Shali Matuja Rashid Ali Ahmed2
Rashid Ali Ahmed2 Khuzeima Khanbhai
Khuzeima Khanbhai Gustavo J. Rodriguez
Gustavo J. Rodriguez Vikas Gupta
Vikas Gupta Alberto Maud
Alberto Maud Mohammad Rauf Chaudhury
Mohammad Rauf Chaudhury Mohamed Manji
Mohamed Manji Faheem Sheriff
Faheem Sheriff