- 1Department of Physical Therapy, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
- 2KITE, Toronto Rehabilitation Institute-University Health Network, Toronto, ON, Canada
- 3Rehabilitation Sciences Institute, Temerty Faculty of Medicine, University of Toronto, Toronto, ON, Canada
Introduction: Following central nervous system damage, the recovery of motor function is a priority. For some neurological populations, functional electrical stimulation (FES) is recommended in best practice guidelines for neurorehabilitation. However, limited resources exist to guide FES application, despite clinicians reporting that a lack of FES knowledge prevents use in clinical practice. The FES Clinical Decision Making Tool was developed to assist clinicians with FES application and translation into clinical practice. The purpose of this study was to evaluate the content validity of the Tool from the perspectives of Canadian physical and occupational therapists using FES in neurorehabilitation.
Methods: Thirteen participants (twelve women, one man), aged 40.5 ± 10.3 years, participated in individual semi-structured interviews to explore their clinical decision making experiences when applying FES and to evaluate the content validity (i.e., appropriateness, comprehensibility, and comprehensiveness) of the Tool. Interviews were analyzed using a qualitative conventional content analysis following the DEPICT model.
Results: Three themes were identified. 1) Clinician context influences FES usage. Participants' experiences with FES use varied and application was influenced by treatment goals. 2) Parameter selection in clinical practice. Participants identified decision-making strategies and the challenges of parameter selection. 3) With modifications, the Tool is a valid resource to inform FES applications. Participants discussed its strengths, limitations, and suggested changes. While the Tool is useful, a more extensive resource (e.g., appendix) for the Tool is warranted.
Discussion: A revised Tool was created to improve its comprehensiveness and comprehensibility. Thus, the Tool is a valid resource for applying FES in neurorehabilitation.
Introduction
Research supports the use of functional electrical stimulation (FES) as an adjunct therapy in neurorehabilitation (1, 2). FES produces sequenced and timed muscle contractions by delivering an electrical current through electrodes on the skin to activate motor neurons, with the purpose of executing functional tasks, such as walking, reaching and standing (1, 3). FES was initially developed in the 1960s as an assistive device (4), such that when stimulation was applied, an instantaneous change in movement or performance would be observed (commonly referred to as an orthotic effect) (5–7). For example, an FES application for foot drop resulted in immediate increases in gait speed in individuals living with the effects of stroke (8). When FES is used to practice a task repeatedly over time, therapeutic gains may be realized, such that improvements in motor performance persist when the electrical stimulation is removed (1, 7, 9–11). For example, FES has been used to achieve lasting improvements in the performance of grasping, reaching, and walking (1, 7–10).
FES is recommended as a therapeutic intervention in best practice guidelines for individuals with stroke and SCI. The Canadian Stroke Best Practice Guideline includes FES as a therapy recommendation to improve both upper and lower extremity function and reduce overall motor impairment in flaccid limbs (9, 12). Likewise, the literature supports the use of FES for individuals with SCI to help address common impairments and activity limitations, such as spasticity, gait impairments, and reduced ability to grasp and reach (10, 11, 13, 14). Despite FES being recommended in several clinical practice guidelines, its use in clinical practice by PTs remains low (3). Auchstaetter et al. (3) assessed how frequently Canadian PTs used FES in stroke rehabilitation and found that among the almost 300 PTs surveyed, most “never” or “rarely” used FES. The PTs reported their lack of knowledge, training, and expertise in FES, and the perceived lengthy set-up time as barriers to FES implementation in clinical practice (3). PTs acknowledged the importance of understanding FES parameters and the opportunity for hands-on training (3). Indeed, being “comfortable and confident in applying FES”(3) was one of the most commonly cited facilitators of FES use amongst PTs who use the modality in their practice.
To date there is no published guide on how to select electrical stimulation parameters (e.g., frequency, pulse duration/width, waveform, and amplitude) for the myriad of FES applications used with neurological populations. Summaries of the stimulation parameters used in previous research studies have been produced (15), yet there is considerable variety in the prescription of parameters across studies targeting the same orthotic or therapeutic goal. In an effort to contribute to the translation of FES into clinical practice and address the knowledge gap surrounding FES application, a FES Clinical Decision Making Tool (FES CDM Tool) (Figure 1) was developed by a research team member (KEM) through literature review, expert opinion, and clinical experience. More specifically, the electrical stimulation parameters that a clinician may need to set for any given FES application were identified as important to include in a decision-making tool. These parameters included stimulation amplitude, frequency, pulse duration and waveform. To provide recommendations on how to set these parameters, knowledge of the characteristics of electrical currents and their neurophysiological effects were translated into clinically relevant questions to guide a clinician's prescription of FES. This tool aims to help therapists determine the appropriate parameters for a given FES application. However, the content validity of this tool, or the extent to which it reflects the target construct, has yet to be examined. An evaluation of content validity typically considers the appropriateness, comprehensiveness, and comprehensibility of a tool with respect to the construct and population (16). Such an evaluation is a necessary step in the development of clinical decision-making tools, like the FES CDM Tool.
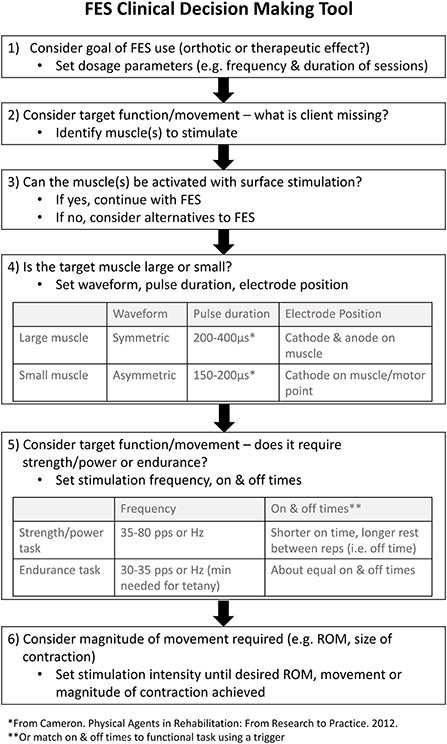
Figure 1. The FES CDM Tool used during semi-structured interviews. The Functional Electrical Stimulation (FES) Clinical Decision Making (CDM) Tool was developed to assist clinicians in the selection of electrical stimulation parameters when applying FES. ROM, range of motion.
Due to the inclusion of FES in best practice guidelines for neurological populations and the increasing interest in its use in neurorehabilitation (3), creating FES-related resources for clinicians, such as the FES CDM Tool, is a priority. The purpose of this study was to evaluate the content validity of the FES CDM Tool from the perspectives of Canadian PTs and OTs that use FES in neurorehabilitation. Specifically, the study objectives were to (1) understand the factors that PTs and OTs consider when selecting electrical stimulation parameters for applications of FES, and (2) evaluate the content validity of the FES CDM Tool.
Methods
Study design
This cross-sectional qualitative descriptive study used individual semi-structured interviews to explore the perspectives of Canadian PTs' and OTs' clinical decision making experiences when applying FES in clinical practice, and evaluated the content validity of the proposed FES CDM Tool. This study received approval from and was conducted in accordance with the established ethical standards of the Health Sciences Research Ethics Board (REB) at the University of Toronto.
Recruitment
Recruitment notices were circulated through the Neurosciences Division of the Canadian Physiotherapy Association (CPA), the Canadian Association of Occupational Therapists (CAOT), the Canadian Activity-Based Therapy Community of Practice, and KEM's professional network. Prospective participants contacted the research team via email and Zoom or telephone interviews were scheduled after informed consent was provided and study eligibility was confirmed.
Participants
Participants who met the following inclusion criteria were eligible to participate:
1. PTs and OTs who were licensed to practice in Canada.
2. Practiced in the area of neurorehabilitation with >50% of their caseload involving adults or children with upper motor neuron damage.
3. Practiced in any clinical setting (including but not limited to: acute care, rehabilitation hospital, private practice, home care, community care, etc.).
4. Used FES in their clinical practice, at least occasionally (i.e., with a minimum of 21–40% of their neurological clients/patients).
5. Able to participate in an interview conducted in English.
The study aimed to recruit 12–15 participants with at least four participants being OTs. While in Canada the majority of PTs are exposed to FES in the entry-to-practice PT curriculum, most OTs are not and thus, it was anticipated that fewer OTs were using FES in their practice compared to PTs. The target sample size of 12–15 participants aligned with a study by Guest et al. (17), which found that data saturation was largely achieved by the time 12 interviews were analyzed. Despite the research team receiving training for conducting interviews, a lack of previous interviewing experience may have affected the quality of the dialogue, thus a sample size slightly greater than 12 was targeted. Consecutive sampling was used, such that PTs and OTs meeting the eligibility criteria were enrolled in the order in which they self-referred to the study until the target sample size, including at least four OTs, was reached.
Data collection
Data were collected via Zoom or telephone using a demographic questionnaire and semi-structured interview guide. The demographic questionnaire queried participant age, gender, employment status, job title, work setting, province of practice, years of experience as a clinician, years of FES use in practice, and percentage of neurological clients for which FES is used. The guide consisted of 12 open-ended interview questions (Table 1) created by the research team, which included six PT students and a clinician-scientist with FES expertise. The questions were developed based on recommendations of Brod et al. for qualitative research to examine content validity, as well as the interview guide of a similar qualitative study evaluating the content validity of a decision-making tool (16, 18). The first part of the interview queried how participants selected electrical stimulation parameters for FES applications, and explored the factors considered when making clinical decisions. In the second part of the interview, participants were shown the FES CDM Tool (Figure 1). Cognitive debriefing interviewing with a verbal probing approach was used to query the appropriateness, comprehensiveness, and comprehensibility (i.e., content validity) of the FES CDM Tool (16). Participants were asked to explain their interpretation of each step, consider the relevance of each step to setting electrical stimulation parameters, and how easy or difficult it would be to apply each step (16). Interviews were conducted by two team members where one team member interviewed the participant (RF or NA), and the second (CT) reported observations in a reflective journal. Interviews lasted 36–76 min and were audio recorded and transcribed verbatim. Synthesized member checking (SMC) was used to allow participants to provide feedback as to whether the data accurately reflected their experiences (19).
Data analysis
Age, number of years as a PT/OT, and number of years using FES in clinical practice were reported as mean (± 1 standard deviation). Nominal demographic data (i.e., work setting, work location, patient populations) were reported as frequency counts. A qualitative conventional content analysis was conducted; a flexible method of analyzing text data where the objective is to describe a phenomenon (20). This method of analysis was deemed appropriate as limited research or theory on FES-related clinical decision making exists in the literature.
The six-step DEPICT model was followed to allow for collaboration of all team members: (1) dynamic reading, (2) codebook development, (3) participatory coding, (4) reviewing and summarizing categories, (5) collaborative analysis, and (6) translating findings (21). First, researchers worked in pairs to read an initial transcript and created marginal notes to highlight relevant sections. A preliminary codebook was developed from the marginal notes and the remaining transcripts were coded. New codes that emerged were discussed and transcripts were re-coded with the updated codebook. Through team discussion, generated codes were arranged into categories and sub-themes based on relatedness, from which overarching themes were explored. Conclusions on the FES CDM Tool's content validity were determined by the synthesized data based on its appropriateness, comprehensibility, and comprehensiveness. These findings were used to inform changes to the existing tool. Rigor was established through reflective journaling, SMC, and an audit trail.
Results
Participant demographics
Nine PTs and four OTs (one man, 12 women), aged 40.5 ± 10.3 years, participated. Participants had worked as a PT/OT for 14.9 ± 10.0 years, with seven currently working in Ontario, two in British Columbia, and the remaining four in Saskatchewan, Manitoba, Quebec, and Alberta. Participants had 9.5 ± 9.2 years of experience using FES in practice. Twelve participants worked in rehabilitation hospital settings and one participant worked in a private practice homecare setting. SCI was the most commonly reported patient population in which participants used FES (n = 12), followed by stroke (n = 8), multiple sclerosis (n = 3), brain injury (n = 3), Parkinson's disease (n = 1), and cerebral palsy (n = 1).
Three themes were identified: (1) Clinician Context Influences FES Usage and (2) Parameter Selection in Clinical Practice, which together informed the third theme (3) With Modifications, the FES CDM Tool is a Valid Resource to Inform FES Application. Several sub-themes were identified for each theme. Table 2 provides additional supporting quotes, with Q1, Q2, etc. linking each quote to the relevant text.
Theme 1: Clinician Context Influences FES Usage
Participants described their FES usage as dependent on their clinical context. The influences were grouped into two sub-themes. Participants explained their (a) Experience with FES Use is Variable, and dependent on their individual learning strategies and the devices they used. The (b) Treatment Goals Defined by Clinicians in their Clinical Practice included both therapeutic and orthotic goals and guided FES use.
Sub-theme 1a: Experience with FES Use is Variable
Across participants, experience with FES varied. Participants explained that FES use was influenced by access to FES education and devices. With respect to education, participants reported that Canadian entry-to-practice programs in PT and OT either did not include or did not adequately teach FES applications (Q1). Thereby, many participants reported using alternative learning methods such as reading literature to help inform parameter selection and appropriate clinical populations (n = 4). Additionally, many participants reported learning through post-graduate FES training (n = 9) (Q2), their clinical experience (n = 8), patient feedback (n = 8), and mentorship from colleagues (n = 3) (Q3).
Participants reported using different FES devices that varied in the number of channels and the ability to modulate each parameter. The device one was familiar with affected clinical practice (Q4). The differences in the devices can impact how FES is used. Depending on the device, the movements that can be practiced and the level of input from the PT/OT (e.g., parameter selection) can change (Q5). For example, a FES device with preset protocols requires minimal parameter selection.
Sub-theme 1b: Treatment Goals Defined by Clinicians in their Clinical Practice
Participants described using FES to address various treatment goals in their clinical practice, impacting FES usage. Participants primarily reported using FES for therapeutic rather than orthotic treatment goals. Eight participants reported using FES for orthotic use, at least occasionally, with the primary goal being to correct foot drop (n = 7) (Q6). Seven participants reported using FES to address spasticity. Therapeutic goals also included strength and endurance training (Q7) and functional goals (Q8–10).
Theme 2: Parameter Selection in Clinical Practice
Participants described their clinical decision making when selecting electrical stimulation parameters for FES applications. The Parameter Selection in Clinical Practice theme included two sub-themes: (a) Strategies Used for Decision Making (i.e., literature, patient feedback, matching to functional goals, using the same parameters, following a tool), and (b) Challenges of Selecting Stimulation Parameters.
Sub-theme 2a: Strategies Used for Decision Making
Participants described their clinical decision making strategies for setting up an FES application; this included referring to the literature and incorporating patient feedback to adjust parameters (Q11), including input on pain (Q12) and tolerance of the stimulation. Further, a patient's level of sensation affected parameter selection (Q13). Additionally, participants described considering patients' biopsychosocial factors (Q14) and functional goals (Q15).
Approaches for parameter setting ranged from structured approaches including preset parameters to unstructured approaches, such as using “trial-and-error” (P06 and P07). Some participants did not alter parameters at all either due to following literature guidelines, limitations of the FES device setting options, or they habitually used the same parameters (P01, P03, P09, P10, and P13). Other participants described a process where they would have guidelines in mind, either from the literature or frameworks that they learned about in previous courses, and then altered parameters based on their understanding of each parameter and patient comfort (P02, P04, P05, P08, P11, and P12). Lastly, P06 and P07 described a process for setting up FES parameters that was rooted in principles, but involved alterations and trial-and-error to get the desired outcome.
Sub-theme 2b: Challenges of Selecting Stimulation Parameters
Participants discussed the decision making challenges previously experienced when setting up FES, such as how “finicky” parameter setting was (P11 and P13) and the variability across and within patients day to day (Q16–17). Interestingly, the participants' level of confidence in FES application influenced their clinical decision making (Q18–19).
Theme 3: With Modifications, the FES CDM Tool is a Valid Resource to Inform FES Application
Overall, the participants reported the FES CDM Tool to be valid, but they suggested modifications to improve its clarity. Two sub-themes were identified within this theme. First, (a) Clinician Feedback on the FES CDM Tool included the strengths, limitations, and suggested additions and changes for each step of the tool. Second, most participants recommended the (b) Addition of an Appendix that would contain more FES resources for clinicians without cluttering the simplicity of the tool itself.
Sub-theme 3a: Clinician Feedback on the FES CDM Tool
Step 1
Step one of the FES CDM Tool asks clinicians to determine their goal when using FES (i.e., orthotic or therapeutic) in order to help set dosage parameters (i.e., frequency and duration of sessions) (Figure 1). Participants described step one of the FES CDM Tool as understandable and an appropriate step to include (n = 12) (Q20). In contrast, P06 stated they did not find step one to be understandable due to the limited use of FES as an orthosis in Canada. Participants reported that one limitation of this step is whether clinicians will understand the difference between therapeutic and orthotic goals (n = 4) (Q21–22). Three participants suggested adding a section on contraindications or patient appropriateness, specifically, within or prior to step one (Q23).
Step 2
Step two prompts the clinician to consider the target function or movement to then determine what muscle(s) they need to target with the FES (Figure 1). Twelve participants noted that this step was both understandable and appropriate (Q24).
In contrast, P10 noted that identifying the muscles to stimulate was appropriate, however, the prompt ‘what is the client missing?' was “extra, just not helpful.” Another suggested change to step two was to modify the wording to include a spasticity-related goal, since spasticity is not what a patient is missing but rather something that a patient has too much of (P13).
Step 3
Step three asks the clinician to consider if the muscle(s) being targeted can be activated with surface-level stimulation (Figure 1). While participants understood this step and felt that it was appropriate (n = 11), some required further prompting from the interviewer to help interpret it (Q25–26). For example, three participants highlighted how this step could be interpreted to be asking if FES is appropriate given the patient's diagnosis. P12 suggested that adding in examples may help clarify the step's intended purpose and meaning. In contrast, two participants did not think this was a necessary step (Q27).
Step 4
Step four asks the clinician to consider whether the target muscle is large or small in order to set the waveform, pulse duration, and electrode position (Figure 1). Many participants described that they understood this step and it was an appropriate step to include (n = 12). In contrast, P06 stated the table provided in this step is “not as simple as what it's listed as…because it's person specific to some degree.” Additionally, P12 suggested adding examples either into the step or an Appendix of how to differentiate muscle size for setting parameters (Q28).
Step 5
The fifth step is to consider if the target function or movement requires strength/power or endurance to set the stimulation frequency and on/off times (Figure 1). Many participants found this step understandable and appropriate (n = 12). Similar to step four, P06 stated they often selected frequencies outside of the given ranges recommended in step five depending on the patient's response to the simulation. The most common feedback received for this step was to add guidance around using FES for spasticity management (n = 6). However, some participants were not convinced spasticity management should be included in the FES CDM Tool (Q29).
Step 6
The final step of the FES CDM Tool is to consider the desired magnitude of movement in order to adjust the current intensity to achieve the desired range of motion, movement, or magnitude of muscle contraction (Figure 1). Ten participants understood the step and confirmed its appropriateness (Q30). One participant misinterpreted this step to suggest the stimulation should achieve full range of motion (Q31). Five participants noted the importance of taking into account patient factors when increasing the intensity such as skin sensation, fatigue, psychosocial considerations, and comfort (Q32). Four participants wanted the step to state that the patient should be actively performing the movement in conjunction with the muscle stimulation.
Sub-theme 3b: Addition of an Appendix
During the interviews, all participants expressed a need for FES resources in the form of an Appendix to the FES CDM Tool (n = 13) (Q33–35). The most frequent suggestion was to include more information about pad placement (n = 6). Another suggestion was to include contraindications to FES (n = 5) (Q36). Four participants suggested clarifying small vs. large muscles. Some less common suggestions were to include more information about: pad size (n = 2), commonly targeted muscles (n = 2), frequency and duration of sessions (n = 2), parameters for spasticity (n = 2), photos depicting different ways to use FES (n = 2), various FES devices (n = 2), case studies (n = 2), and definitions of common terms such as orthotic and therapeutic goals (n = 3).
Discussion
This study evaluated the content validity of the FES CDM Tool from the perspectives of Canadian PTs and OTs using FES in neurorehabilitation. Participants described previous academic and clinical experiences that influenced their use of FES. Although they had varying experiences, participants reported the FES CDM Tool to be appropriate and suggested modifications to increase its comprehensiveness and comprehensibility. Participants emphasized the importance of a FES CDM Tool being accessible for clinicians working in neurorehabilitation and encouraged future development of resources to support its use in clinical practice.
Clinical decision making when applying FES appeared to be challenging for study participants due to the lack of FES education and resources. The strategies used to select and manipulate parameters varied, with most participants using preset programs or trial-and-error when applying FES. This may have resulted from a lack of confidence in altering parameters and/or device selection. Four participants utilized devices with preset parameters, meaning they had little opportunity to customize the parameters for each patient. However, few participants articulated an evidence- or knowledge-informed approach to parameter selection; for example, participants did not discuss the relationship between stimulation frequency and muscle fatigue or the effect of increasing pulse duration/width on motor unit recruitment.
The FES content provided in Canadian PT and OT entry-to-practice programs was perceived as insufficient to prepare clinicians to apply FES. Similarly, Australian PTs and OTs reported gaining skills in FES application through post-graduate education and mentoring rather than entry-level education (22). A lack of knowledge, training, and expertise in FES is known to be the main barriers PTs and OTs face when applying FES (3, 23). In the present study, some participants attributed their increased confidence in parameter setting and manipulation to attending post-graduate courses. This aligns with prior research suggesting that one of the main facilitators to enabling FES use in clinical practice are increases in access to continuing education and the implementation of programs to increase awareness of FES application (23).
The findings from this study identified several suggestions to improve the FES CDM Tool that have been implemented to create a revised version (Figure 2). Wording was revised to increase clarity; for example, asking clinicians to consider whether the target muscle or peripheral nerve can be accessed, rather than activated, by surface-level stimulation, as step 3 in the original FES CDM Tool proved difficult for some participants to understand (Figure 1). An explanation of small vs. large target areas for the stimulation was added to step five (Figure 2), along with a statement about the placement of the anode electrode when using an asymmetric waveform. For step seven (Figure 2), text was added to remind clinicians to consider the patient's comfort and tolerance as the stimulation intensity is increased.
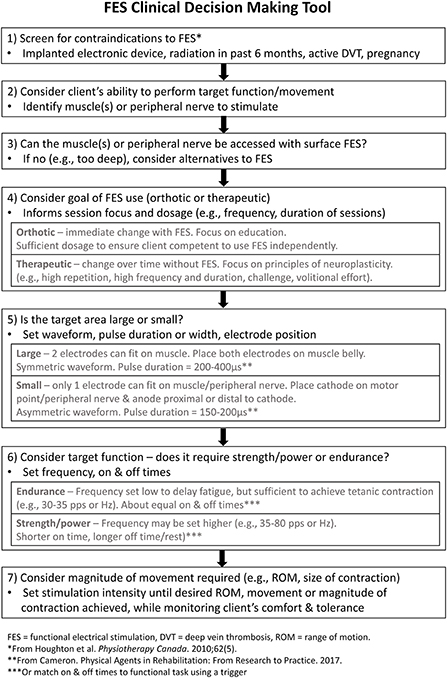
Figure 2. The revised FES CDM Tool. The revised Functional Electrical Stimulation (FES) Clinical Decisions Making (CDM) Tool, which incorporates the revisions and additions suggested by study participants.
In addition to editing the FES CDM Tool's wording, some conceptual changes were made. First, a step was added to prompt screening for potential contraindications to electrical stimulation [(step 1), Figure 2] (24, 25). This step is followed by identifying the target muscle(s) or peripheral nerve [(step 2), Figure 2] and determining whether the target muscle(s) or nerve can be accessed with surface-level stimulation [(step 3), Figure 2]. This order reflects the logical order of considerations when determining whether a specific FES application is safe and appropriate for an individual living with neurological injury or disease.
Second, definitions of therapeutic and orthotic goals were added to increase clarity of these terms [(step 4), Figure 2], since they are commonly used in FES literature and practice (26). When FES is being used as an orthotic assist, an assessment is typically completed to determine the optimal parameter settings for the FES application (27). In contrast, when FES is being used to achieve a therapeutic effect, electrical stimulation is incorporated into motor training that is characterized by principles of neuroplasticity, such as a high number of movement repetitions, sufficient motor challenge, volitional effort, and frequent practice (28). Hence, several dosage parameters, such as the frequency and duration of sessions, are dependent on the FES goal selected. Defining the terms orthotic and therapeutic may allow for increased understanding of this step, especially by clinicians not familiar with the FES literature. Interestingly, participants of the present study described using FES for primarily therapeutic goals. While using FES as an orthotic assist may not be common in Canada, it is frequently used for this purpose in other geographical regions, such as the United Kingdom (29). One possible reason for these regional differences is the availability of funding for FES devices. In Canada, both clinicians (3) and recipients of FES (30) have reported a lack of funding for patients to acquire FES devices, another barrier to using FES.
Third, when discussing the final step in the original FES CDM Tool, four participants highlighted the importance of the patient voluntarily moving along with the electrical stimulation. This is an important concept to convey in a FES CDM Tool as FES combined with voluntary movement results in greater brain activation than FES alone (31, 32). Hence, encouraging patients to actively participate in the movement while receiving electrical stimulation may promote neuroplasticity and therapeutic goals. The importance of volitional effort is now mentioned in step four in the revised FES CDM Tool (Figure 2). These revisions were made to improve the Tool's comprehensiveness and comprehensibility, both of which contribute to a tool's content validity (16). We expect the FES CDM Tool may require additional modification in the future in response to new knowledge, new technology or changing practice patterns. Hence, the FES CDM Tool should be viewed as a living document that will be re-evaluated and revised as needed.
Participants suggested including FES applications for spasticity in the FES CDM Tool. Both sensory-level (i.e., transcutaneous electrical nerve stimulation (TENS)) and motor-level electrical stimulation (i.e., FES, neuromuscular electrical stimulation (NMES)) may be used to reduce spasticity in neurological populations. Current clinical practice guidelines for stroke rehabilitation indicates mixed evidence for the effects of NMES and FES on spasticity (9). In contrast, the guidelines indicate that TENS significantly reduces spasticity (9). Hence, if the primary goal of using electrical stimulation in post-stroke rehabilitation is to reduce spasticity, TENS would likely be more effective than FES. In SCI rehabilitation, however, TENS and FES have been shown to have similar anti-spasticity effects after a single session of electrical stimulation (33). Since FES is not more effective than TENS for spasticity management, we have not edited the FES CDM Tool to include a focus on spasticity. Moreover, the steps of the FES CDM Tool could be followed to set up an FES application to reduce spasticity; the evidence-based principles of motor-level electrical stimulation reflected in the Tool still apply.
All participants identified a need for additional resources pertaining to FES applications. While participants identified the FES CDM Tool as a useful resource to help guide clinicians in their clinical reasoning for FES application, they indicated that a more extensive resource, such as an Appendix for the FES CDM Tool, is warranted. These findings are consistent with the findings of Auchstaetter et al. (3), who reported a lack of readily available resources for FES as a barrier for FES application in stroke rehabilitation. Improving access to resources may promote confidence with FES application and facilitate its implementation into clinical practice. While the FES CDM Tool and an accompanying Appendix may support implementation, these resources are not expected to replace the need for formal education (i.e., FES education in entry-to-practice programs, continuing education courses) or informal training (e.g., mentoring by colleagues).
While a lack of educational resources and training on FES is a commonly reported barrier to FES use (3, 23), there are numerous additional barriers to consider. For example, a lack of time with patients, the perceived lengthy set-up time of FES, therapist preference for other treatment options and cost for the user are additional barriers that have been reported by health care providers working in stroke (3) and SCI (23) rehabilitation. According to the Knowledge-to-Action Framework, clinicians interested in using FES in their clinical practice should identify barriers in their local clinical context and then select the implementation strategies best suited to address these barriers (34). On its own, an educational resource like the FES CDM Tool will not address the majority of barriers to FES implementation. The Tool may provide clinicians with knowledge and greater confidence in applying FES, as well as reduce the time spent on setting up and changing FES parameters; however, these potential effects will need to be evaluated in future research.
This study had potential for bias as Canada consists of a small community of clinicians who utilize FES within their clinical practice. Some participants were already familiar with the FES CDM Tool since they took KEM's post-graduate FES courses. To mitigate this potential bias, KEM was not involved in the data collection and analysis for this study.
In conclusion, Canadian PTs and OTs using FES in their clinical practice indicated that the FES CDM Tool is appropriate in guiding clinical decision making for FES applications, supporting the tool's content validity. A revised version of the FES CDM Tool was created to improve the tool's comprehensiveness and comprehensibility. The FES CDM Tool may help clinicians working with neurological populations to confidently apply FES during neurorehabilitation sessions in a safe, effective, and patient-centered manner.
Data availability statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to Daniel Gyewu (Research Ethics Manager, Health Sciences), d.gyewu@utoronto.ca.
Ethics statement
The studies involving human participants were reviewed and approved by the Health Sciences Research Ethics Board (REB) at the University of Toronto. The patients/participants provided their written informed consent to participate in this study.
Author contributions
NA, SC, and RF contributed to design of study, data collection and analysis, and wrote the first draft of the manuscript. CT, KC, and SD contributed to design of study, data collection and analysis, and manuscript revisions. KM contributed to conception and design of study and manuscript revisions. All authors approved the submitted version.
Funding
Funding for this study was provided through the Canada Research Chairs Program (CRC-2020-00193) and the Coriat Family Research Fund to KM.
Acknowledgments
This research was completed in partial fulfillment of the requirements for a MScPT degree at the University of Toronto. We would like to thank the University of Toronto Research Unit faculty for their guidance throughout the project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vafadar AK, Côté JN, Archambault PS. Effectiveness of functional electrical stimulation in improving clinical outcomes in the upper arm following stroke: a systematic review and meta-analysis. Biomed Res Int. (2015) 2015:729768. doi: 10.1155/2015/729768
2. Eraifej J, Clark W, France B, Desando S, Moore D. Effectiveness of upper limb functional electrical stimulation after stroke for the improvement of activities of daily living and motor function: a systematic review and meta-analysis. Syst Rev. (2017) 6:40. doi: 10.1186/s13643-017-0435-5
3. Auchstaetter N, Luc J, Lukye S, Lynd K, Schemenauer S, Whittaker M, et al. Physical therapists' use of functional electrical stimulation for clients with stroke: frequency, barriers, and facilitators. Phys Ther. (2016) 96:995–1005. doi: 10.2522/ptj.20150464
4. Marquez-Chin C, Popovic MR. Functional electrical stimulation therapy for restoration of motor function after spinal cord injury and stroke: a review. Biomed Eng Online. (2020) 19:34. doi: 10.1186/s12938-020-00773-4
5. Miller L, McFadyen A, Lord AC, Hunter R, Paul L, Rafferty D, et al. Functional electrical stimulation for foot drop in multiple sclerosis: a systematic review and meta-analysis of the effect on gait speed. Arch Phys Med Rehabil. (2017) 98:1435–52. doi: 10.1016/j.apmr.2016.12.007
6. Springer S, Khamis S. Effects of functional electrical stimulation on gait in people with multiple sclerosis—a systematic review. Mult Scler Relat Disord. (2017) 13:4–12. doi: 10.1016/j.msard.2017.01.010
7. Pool D, Valentine J, Bear N, Donnelly CJ, Elliott C, Stannage K. The orthotic and therapeutic effects following daily community applied functional electrical stimulation in children with unilateral spastic cerebral palsy: a randomised controlled trial. BMC Pediatr. (2015) 15:154. doi: 10.1186/s12887-015-0472-y
8. Kottink AIR, Oostendorp LJM, Buurke JH, Nene AV, Hermens HJ, IJzerman MJ. The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: a systematic review. Artif Organs. (2004) 28:577–86. doi: 10.1111/j.1525-1594.2004.07310.x
9. Teasell R, Iruthayarajah J, Saikaley M, Longval M. Executive Summary: Evidence Based Review of Stroke Rehabilitation. (2018). Available online at: http://www.ebrsr.com/sites/default/files/documents/Executive%20Summary%20Total%202020_JI.pdf (accessed Jun 20, 2022).
10. Rehabilitation Evidence—Spinal Cord Injury Research Evidence. Spinal Cord Injury Research Evidence. Available online at: https://scireproject.com/evidence/rehabilitation-evidence/ (accessed September 15, 2021).
11. Popovic MR, Kapadia N, Zivanovic V, Furlan JC, Craven BC, McGillivray C. Functional electrical stimulation therapy of voluntary grasping vs. only conventional rehabilitation for patients with subacute incomplete tetraplegia: a randomized clinical trial. Neurorehabil Neural Repair. (2011) 25:433–42. doi: 10.1177/1545968310392924
12. Teasell R, Salbach NM, Foley N, Mountain A, Cameron JI, de Jong A, et al. Canadian stroke best practice recommendations: rehabilitation, recovery, and community participation following stroke. part one: rehabilitation and recovery following stroke. Int J Stroke. (2020) 15:763–88. doi: 10.1177/1747493019897843
13. Howlett OA, Lannin NA Ada L, McKinstry C. Functional electrical stimulation improves activity after stroke: a systematic review with meta-analysis. Arch Phys Med Rehabil. (2015) 96:934–43. doi: 10.1016/j.apmr.2015.01.013
14. Rayegani SM, Shojaee H, Sedighipour L, Soroush MR, Baghbani M, Amirani OB. The effect of electrical passive cycling on spasticity in war veterans with spinal cord injury. Front Neurol. (2011) 2:39. doi: 10.3389/fneur.2011.00039
15. Nussbaum EL, Houghton P, Anthony J, Rennie S, Shay BL, Hoens AM. Neuromuscular electrical stimulation for treatment of muscle impairment: critical review and recommendations for clinical practice. Physiother Can. (2017) 69:1–76. doi: 10.3138/ptc.2015-88
16. Brod M, Tesler LE, Christensen TL. Qualitative research and content validity: developing best practices based on science and experience. Qual Life Res. (2009) 18:1263–78. doi: 10.1007/s11136-009-9540-9
17. Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. (2006) 18:59–82. doi: 10.1177/1525822X05279903
18. Musselman KE, Lemay J-F, Walden K, Harris A, Gagnon DH, Verrier MC. The standing and walking assessment tool for individuals with spinal cord injury: a qualitative study of validity and clinical use. J Spinal Cord Med. (2019) 42:108–18. doi: 10.1080/10790268.2019.1616148
19. Birt L, Scott S, Cavers D, Campbell C, Walter F. Member checking: a tool to enhance trustworthiness or merely a nod to validation? Qual Health Res. (2016) 22:1802–11. doi: 10.1177/1049732316654870
20. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. (2005) 15:1277–88. doi: 10.1177/1049732305276687
21. Flicker S, Nixon SA. The DEPICT model for participatory qualitative health promotion research analysis piloted in Canada, Zambia and South Africa. Health Promot Int. (2015) 30:616–24. doi: 10.1093/heapro/dat093
22. Howlett O, McKinstry C, Lannin NA. Using functional electrical stimulation with stroke survivors: a survey of Victorian occupational therapists and physiotherapists. Aust Occup Ther J. (2018) 65:306–13. doi: 10.1111/1440-1630.12482
23. Tedesco Triccas L, Donovan-Hall M, Dibb B, Burridge JH. A nation-wide survey exploring the views of current and future use of functional electrical stimulation in spinal cord injury. Disabil Rehabil Assist Technol. (2021) 1–11. doi: 10.1080/17483107.2021.1916631. [Epub ahead of print].
24. Houghton PE, Nussbaum EL, Hoens AM, Electrophysical agents. Contraindications and precautions: an evidence-based approach to clinical decision making in physical therapy. Physiother Can. (2010) 62:1–80. doi: 10.3138/ptc.62.5
25. Badger J, Taylor P, Swain I. The safety of electrical stimulation in patients with pacemakers and implantable cardioverter defibrillators: a systematic review. J Rehabil Assist Technol Eng. (2017) 4:2055668317745498. doi: 10.1177/2055668317745498
26. Johnston TE, Keller S, Denzer-Weiler C, Brown L. A clinical practice guideline for the use of ankle-foot orthoses and functional electrical stimulation post-stroke. J Neurol Phys Ther. (2021) 45:112–96. doi: 10.1097/NPT.0000000000000347
27. Miller Renfrew L, Lord AC, McFadyen AK, Rafferty D, Hunter R, Bowers R, et al. A comparison of the initial orthotic effects of functional electrical stimulation and ankle-foot orthoses on the speed and oxygen cost of gait in multiple sclerosis. J. Rehabil. Assist. Technol. Eng. (2018) 5:2055668318755071. doi: 10.1177/2055668318755071
28. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. (2008) 51:S225–39. doi: 10.1044/1092-4388(2008/018)
29. Taylor P, Street T. The Case for the Odstock Dropped Foot Stimulator (ODFS®). Salisbury, MD: National Clinical FES Centre (2021). Available online at: https://odstockmedical.com/wp-content/uploads/the_case_for_the_odstock_dropped_foot_stimulator_28th_june_2021.pdf
30. Swaffield E, Yang JF, Manns P, Chan K, Musselman KE. Parents' perceptions of functional electrical stimulation as an upper limb intervention for young children with hemiparesis: qualitative interviews with mothers. BMC Pediatr. (2022) 22:346. doi: 10.1186/s12887-022-03403-1
31. Iftime-Nielsen SD, Christensen MS, Vingborg RJ, Sinkjaer T, Roepstorff A, Grey MJ. Interaction of electrical stimulation and voluntary hand movement in SII and the cerebellum during simulated therapeutic functional electrical stimulation in healthy adults. Hum Brain Mapp. (2012) 33:40–9. doi: 10.1002/hbm.21191
32. Joa K-L, Han Y-H, Mun C-W, Son B-K, Lee C-H, Shin Y-B, et al. Evaluation of the brain activation induced by functional electrical stimulation and voluntary contraction using functional magnetic resonance imaging. J Neuroeng Rehabil. (2012) 9:48. doi: 10.1186/1743-0003-9-48
33. Sivaramakrishnan A, Solomon JM, Manikandan N. Comparison of transcutaneous electrical nerve stimulation (TENS) and functional electrical stimulation (FES) for spasticity in spinal cord injury—A pilot randomized cross-over trial. J Spinal Cord Med. (2018) 41:397–406. doi: 10.1080/10790268.2017.1390930
Keywords: physical therapist, occupational therapist, functional electrical stimulation, qualitative research, evidence-based practice, neurorehabilitation
Citation: Abouzakhm N, Choy S, Feld R, Taylor C, Carter K, Degroot S and Musselman KE (2022) Evaluating the validity of a functional electrical stimulation clinical decision making tool: A qualitative study. Front. Neurol. 13:1001123. doi: 10.3389/fneur.2022.1001123
Received: 22 July 2022; Accepted: 31 October 2022;
Published: 15 November 2022.
Edited by:
Michael J. Grey, University of East Anglia, United KingdomReviewed by:
Gad Alon, University of Maryland, United StatesFrancesca Cecchi, University of Florence, Italy
Copyright © 2022 Abouzakhm, Choy, Feld, Taylor, Carter, Degroot and Musselman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kristin E. Musselman, kristin.musselman@utoronto.ca
†These authors have contributed equally to this work and share first authorship
 Nathalie Abouzakhm1†
Nathalie Abouzakhm1† Samantha Choy
Samantha Choy Spencer Degroot
Spencer Degroot Kristin E. Musselman
Kristin E. Musselman
