Genetically-Driven Enhancement of Dopaminergic Transmission Affects Moral Acceptability in Females but Not in Males: A Pilot Study
- 1Department of Experimental and Clinical Medicine, University of Pisa, Pisa, Italy
- 2Department of Surgical, Medical, Molecular Pathology and Critical Care, University of Pisa, Pisa, Italy
- 3Department of Pharmacy, University of Pisa, Pisa, Italy
- 4Clinical Psychology Branch, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy
- 5Applied Research Division for Cognitive and Psychological Science, European Institute of Oncology, Milan, Italy
- 6Department of Developmental Psychology and Socialization and Center for Cognitive Neuroscience, University of Padova, Padova, Italy
- 7Department of General Psychology and Center for Cognitive Neuroscience, University of Padova, Padova, Italy
- 8IMT School for Advanced Studies, Lucca, Italy
Moral behavior has been a key topic of debate for philosophy and psychology for a long time. In recent years, thanks to the development of novel methodologies in cognitive sciences, the question of how we make moral choices has expanded to the study of neurobiological correlates that subtend the mental processes involved in moral behavior. For instance, in vivo brain imaging studies have shown that distinct patterns of brain neural activity, associated with emotional response and cognitive processes, are involved in moral judgment. Moreover, while it is well-known that responses to the same moral dilemmas differ across individuals, to what extent this variability may be rooted in genetics still remains to be understood. As dopamine is a key modulator of neural processes underlying executive functions, we questioned whether genetic polymorphisms associated with decision-making and dopaminergic neurotransmission modulation would contribute to the observed variability in moral judgment. To this aim, we genotyped five genetic variants of the dopaminergic pathway [rs1800955 in the dopamine receptor D4 (DRD4) gene, DRD4 48 bp variable number of tandem repeat (VNTR), solute carrier family 6 member 3 (SLC6A3) 40 bp VNTR, rs4680 in the catechol-O-methyl transferase (COMT) gene, and rs1800497 in the ankyrin repeat and kinase domain containing 1 (ANKK1) gene] in 200 subjects, who were requested to answer 56 moral dilemmas. As these variants are all located in genes belonging to the dopaminergic pathway, they were combined in multilocus genetic profiles for the association analysis. While no individual variant showed any significant effects on moral dilemma responses, the multilocus genetic profile analysis revealed a significant gender-specific influence on human moral acceptability. Specifically, those genotype combinations that improve dopaminergic signaling selectively increased moral acceptability in females, by making their responses to moral dilemmas more similar to those provided by males. As females usually give more emotionally-based answers and engage the “emotional brain” more than males, our results, though preliminary and therefore in need of replication in independent samples, suggest that this increase in dopamine availability enhances the cognitive and reduces the emotional components of moral decision-making in females, thus favoring a more rationally-driven decision process.
Introduction
Morality and moral judgments are crucial for human social interactions. Since the early days, moral behavior has been a matter of intense philosophical debate. Psychology has mostly focused on the study of the mental processes that subtend the complexity of moral behavior (Osman and Wiegmann, 2017). Over the last decades, the developments of novel methodologies for the in vivo study of the brain morphological and functional architecture in a non-invasive manner in humans (Pietrini, 2003; Poldrack, 2012; Poldrack and Yarkoni, 2016), along with the enormous acquisitions from molecular biology and genetics that led to the decoding of the human genome (Venter et al., 2001), have prompted cognitive sciences to venture into the study of the neurobiological mechanisms that subtend mental processes involved in moral behavior. In this perspective, a few brain-imaging studies have investigated brain neural activity in individuals who were asked to make moral choices in regard to distinct scenarios (Greene et al., 2001, 2004; Hutcherson et al., 2015). In their pioneer work, Greene and colleagues have proposed a “dual process theory” of moral decision-making, according to which both cognition and emotion are involved in moral judgments (Greene et al., 2001, 2004, 2008, 2009; Shenhav and Greene, 2014). These authors identified distinct neural patterns associated with emotion and cognition, and suggested that a conflict between these two components occurs during moral judgment formulation. The dual process theory has received additional support by independent studies (Schaich Borg et al., 2006; Valdesolo and DeSteno, 2006; Ciaramelli et al., 2007; Koenigs et al., 2007; Bartels, 2008; Fumagalli et al., 2010a). Moreover, some authors showed that pro-social emotions including aversive emotional reactions to harmful scenarios are highly variable among individuals (Moll and de Oliveira-Souza, 2007; Decety and Cowell, 2014a,b). Similarly, responses to moral dilemmas differ among individuals as well (Sarlo et al., 2014; Rota et al., 2016). The mechanisms that underlie this variability still remain to be understood. Distinct genetic profiles may likely be involved, as different polymorphisms have been associated with definite aspects of behavior including violent and antisocial behaviors (Rigoni et al., 2010; Sartori et al., 2011; Buades-Rotger and Gallardo-Pujol, 2014; Iofrida et al., 2014).
Dopamine is known to affect several aspects of social behavior that are fundamental for moral choices (i.e., motivation, reward, and reinforcing learning). The 7-repeat allele of a polymorphic region within the third exon of the Dopamine Receptor D4 gene (DRD4), for example, has been linked to impaired altruistic behavior (Bachner-Melman et al., 2005; Anacker et al., 2013) and decreased empathy (Uzefovsky et al., 2014), both powerful enhancers of pro-social behavior (Eisenberg, 2000, 2007).
These findings consistently suggest that gene variants in the dopaminergic pathway may affect moral decision-making, a crucial function in human sociality. To investigate this hypothesis, we combined a moral judgment paradigm with genetic testing, so to assess the potential role in moral choices of five genetic variants that affect dopaminergic neurotransmission: rs1800955 in the dopamine receptor D4 (DRD4) gene, the DRD4 48 bp variable number of tandem repeat (VNTR), the solute carrier family 6 member 3 (SLC6A3) 40 bp VNTR, rs4680 in the catechol-O-methyl transferase (COMT) gene, and rs1800497 in the ankyrin repeat and kinase domain containing 1 (ANKK1) gene (Table 1).
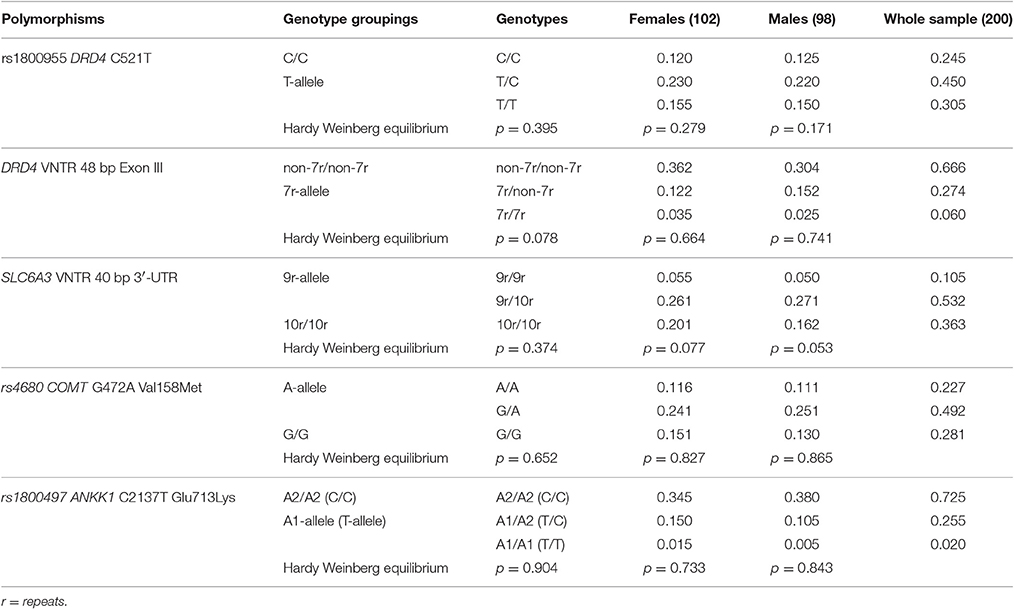
Table 1. Genotype frequencies and Hardy Weinberg equilibrium statistics for each genetic variant in the whole sample (males plus females) and in the two separate genders.
Each of these variants has been found individually associated with the modulation of personality traits and cognitive abilities linked to moral behavior.
Specifically, the C-allele of rs1800955 variant increases the DRD4 transcriptional efficiency (Okuyama et al., 1999) and has been associated with augmented extraversion (Bookman et al., 2002; Eichhammer et al., 2005; Golimbet et al., 2005) and novelty-seeking (Munafò et al., 2008), whereas the T-allele has been associated with attention deficits (Yang et al., 2008).
The DRD4 VNTR encodes the third intracellular loop of the receptor that interacts with a Gi protein with an inhibitory effect on cAMP formation (Van Tol et al., 1991). The 7-repeat allele of this polymorphism affects receptor function by inhibiting the ligand binding and DRD4 expression (Asghari et al., 1994, 1995; Grady et al., 2003; Borroto-Escuela et al., 2011; Knafo et al., 2011; González et al., 2012). It is known that, upon ligand binding, DRD4 forms a functional heterodimer with DRD2; interestingly, the 7-repeat allele of DRD4 interferes with this dimerization, thus causing a reduction of DRD2 activity as well (Borroto-Escuela et al., 2011; González et al., 2012). The same allele negatively influences altruistic traits (Bachner-Melman et al., 2005) and impairs prefrontal cortex activation and connectivity patterns linked to executive functions (Herrmann et al., 2007; Gilsbach et al., 2012). In particular, DRD4 plays a central role in the synchronization of glutamatergic and GABA-ergic activities and the 7-repeat allele impairs the balance between these two networks by causing a higher suppression of glutamatergic signaling (Zhong et al., 2016).
The SLC6A3 VNTR modulates the dopamine transporter (DAT1) expression, as the 9-repeat allele decreases DAT-binding capacities and increases dopamine availability (Heinz et al., 2000; VannNess et al., 2005). This variant seems to support decision-making processes under risky situations, reward seeking behavior, and cognitive flexibility (Dreher et al., 2009; Zhong et al., 2009; Mata et al., 2012; Fagundo et al., 2014).
rs4680 affects the enzymatic activity of COMT, as the G/A base change leads to a Val/Met amino acidic change and to a less efficient degradation of dopamine (Chen et al., 2004). Brain imaging studies have shown that the A/A genotype increases prefrontal cortex activation related to cognitive performances, providing additional support to the hypothesis that rs4680 plays a role in moral choices (Egan et al., 2001; Malhotra et al., 2002; Bertolino et al., 2004, 2006; Winterer et al., 2006; Ettinger et al., 2008).
rs1800497, also known as Taq1A, is a tag SNP for some genetic variants located in the dopamine receptor D2 (DRD2) (Zhang et al., 2007). Imaging studies showed that, compared to A2/A2 carriers, the A1-carriers have a significant reduction in the number of DRD2-binding sites in the caudate nucleus (Noble et al., 1991; Ritchie and Noble, 2003) and in the striatum (Pohjalainen et al., 1998) and a decreased dopaminergic activity (Noble et al., 1991, 1997). This deficiency in dopaminergic system due to the A1-allele has been associated with substance dependency and abuse (Blum et al., 1996; Vereczkei et al., 2013), with lower performance in executive functions (Fossella et al., 2006; Klein et al., 2007), and with poor cognitive flexibility and decision-making abilities (Fagundo et al., 2014; Marinos et al., 2014).
Because these variants are all located in genes that belong to the same pathway, namely the dopaminergic pathway, they should not be considered as acting independently from each other, but rather synergistically. Therefore, we combined them in multilocus genetic profiles—following the example of Nikolova et al. (2011), Stice et al. (2012), Davis et al. (2013), Davis and Loxton (2013), and Kohno et al. (2016)—representative of the overall functional effect of these variants both on the dopaminergic neurotransmission on one hand and on the cognitive processes that underlie moral choices on the other.
Materials and Methods
Subjects
Two hundred unrelated Caucasian subjects (102 females) of Italian ancestry, aged 23.1 ± 6.6 SD (standard deviation) years (mean age: females 23.5 ± 7.9 SD; males 22.6 ± 4.8 SD; Table 2), were recruited among students at Pisa and Padua Universities. As the genetic variability of the Italian population is not discrete but continuous, and even more so among people from the Italian peninsula (Di Gaetano et al., 2012), the population stratification was considered of no relevant effect in this group of subjects.
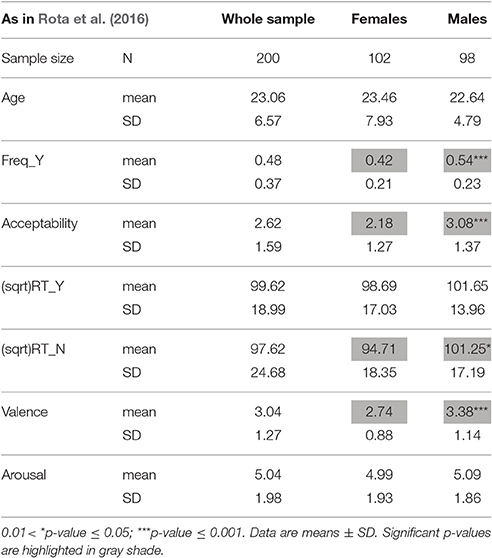
Table 2. Demographic and descriptive data of response variables to moral dilemmas, in the whole sample (males plus females) and in the two separate genders (as reported in Rota et al., 2016).
None of the subjects reported any history of neurological or psychiatric disorders, as assessed by anamnestic interview conducted by board-certified psychologists. The study was approved by the Local Ethic Committees at both Padua and Pisa Universities. Each participant signed an informed written consent to participate in the study and retained the right to drop out from the study at any moment.
Experimental Paradigm
Participants provided their saliva samples for DNA extraction and answered 56 written moral dilemmas characterized by different types of scenarios, modified from the standardized set of Lotto et al. (2014) (see Supplementary File 1). Each dilemma included a short story that ended by proposing an utilitarian resolution (i.e., the sacrifice of one person to save more people) to the portrayed situation, thus facing the reader with a moral dilemma. Participants read each dilemma at their own pace on a computer screen and indicated whether they would engage in the proposed action by pressing the YES/NO labeled buttons. Labeling of the right and left buttons was counterbalanced across participants. YES answers represented utilitarian responses; for each subject, the frequency of YES answers (Freq_Y) was calculated. Response times for YES (RT_Y) and NO (RT_N) were collected. Furthermore, subjects ranked the moral acceptability (Acceptability) of the proposed actions by using an 8-point Likert-type scale (0 = not at all acceptable, 7 = completely acceptable). The degree of pleasantness in engaging in the proposed actions (Valence) (1 = very unpleasant, 9 = very pleasant) and the extent of emotional activation (Arousal) (1 = not at all, 9 = very much) was evaluated by using the Self-Assessment Manikin (Bradley and Lang, 1994).
Genotyping
Saliva samples were collected by the ORAGENE•DNA Self-Collection kit OG-500 (DNA Genotek Inc., Kanata, Canada) and DNA was extracted by prepIT•L2P® kit (DNA Genotek Inc., Kanata, Canada), according to the manufacturer's instructions.
rs1800955 and rs1800497 were genotyped by Polymerase Chain Reaction (PCR)-Restriction Fragment Length Polymorphism (RFLP) by using the primers Forward-5′-TCAACTGTGCAACGGGTG-3′/Reverse-5′-GAGAAACCGACAAGGATGGA-3′ (Barr et al., 2001) and Forward-5′-CACGGCTGGCCAAGTTGTCTA-3′/Reverse-5′-CACCTTCCTGAGTGTCATCAA-3′ (Eisenberg et al., 2007), respectively. Digestions were performed with the FastDigest FspI (NsbI) enzyme (Thermo Fisher Scientific Inc., Waltham, MA, USA) and the TaqIα enzyme (New England Biolabs, Ipswich, USA).
DRD4 VNTR and SLC6A3 VNTR were genotyped by PCR-Fragment Length Analysis by using the primers Forward-5′-GCGACTACGTGGTCTACTCG-3′/Reverse-5′-AGGACCCTCATGGCCTTG-3′ (Serretti et al., 2006) and Forward-5′-TGTGGTGTAGGGAACGGCCTGAG-3′/Reverse-5′-CTTCCTGGAGGTCACGGCTCAAGG-3′ (Vandenbergh et al., 1992), respectively. PCR products were visualized on agarose gel.
rs4680 was genotyped by PCR-High Resolution Melting (HRM) by using the primers Forward-5′-CAGCGGATGGTGGATTTC-3′/Reverse-5′-TTCCAGGTCTGACAACGG-3′. The HRM analysis was performed with a temperature resolution of 0.2°C ranging from 75°C to 90°C. Data collection and genotype calls were obtained by the Rotor-Gene 6000 series software v1.7 (Qiagen, Venlo, Netherlands) using previously sequenced DNA samples as reference genotypes.
Statistical Analyses
The SPSS Advanced Statistics v21 (IBM Corporation, Armonk, NY, USA) was used to perform the statistical pre-processing and analysis of the collected data.
Deviations from normality of response variables and behavioral scores were evaluated by Kolmogorov-Smirnov and Shapiro-Wilks tests; outlier elimination (below the 5th and above the 95th percentiles) was applied to obtain normalized data. RT_Y and RT_N variables were square root (sqrt)-transformed to normalize their distribution.
In a previously reported behavioral study conducted in the same sample of individuals enrolled for the present research, we found significant associations between the responses to moral dilemmas and personality traits—including impulsivity, venturesomeness, and empathy—and mood states (Rota et al., 2016). Thus, the individual scores from the behavioral scales—the Impulsivity-Venturesomeness-Empathy Questionnaire (I7) (Russo et al., 2011), the Interpersonal Reactivity Index (IRI) (Davis, 1980), and the Profile of Mood State (POMS) (McNair et al., 1971)—were included as covariates in the subsequent genetic association analyses.
Concerning the age of subjects, as it did not correlate with the response variables (Supplementary Table 1), it was not included in the analysis as a covariate.
Deviation from the Hardy-Weinberg equilibrium was evaluated by using the HardyWeinberg (Graffelman and Camarena, 2008) and genetics (Warnes, 2003) packages in R (www.r-project.org).
The Fisher exact test was used to evaluate group differences in genotype distribution.
To investigate the association between response variables and genotypes in each gender, the Generalized Estimating Equations (GEEs) were used, as they provide an optimal framework to analyze the correlated data that also show different distributions like the adopted variables (Hardin and Hilbe, 2012). Loglinear Poisson or Tweedie with log link function distributions were used to analyze the Freq_Y variable, whereas Gaussian or Tweedie distributions with identity link function were chosen to analyze Acceptability, Valence, Arousal, and sqrt-transformed RT_Y and RT_N variables, as suggested by the goodness of fit values of Quasi Likelihood under Independence Model Criterion (QICC). An exchangeable working matrix appeared to be the most suitable method to model the within-subject dependency.
Multivariate analysis methods based on GEEs are still under development (see Xu et al., 2014) for an example) and no optimal correction method exists to control for multiple comparisons and multiple testing in GEEs. Thus, a Bonferroni correction was applied, though it may be considered even too conservative for interconnected variables, like the selected genetic variants.
First, a single variant analysis was performed to test whether any genetic variant was individually associated to the response variables [Bonferroni correction: (a) analysis in the whole sample: p = number of genetic variants (5) × number of response variables (6) = 30; (b) sex by genotype interaction: p = number of genetic variants (5) × number of response variables (6) = 30; (c) post hoc: p = number of genetic variants (5) × number of response variables (6) × genders (2) = 60]. Then, after excluding any driving effect by any of these single variants, a genetic profile analysis was performed.
Multilocus genetic profiles were created by assigning a score to each homozygous genotype based on the functional effect of the two alleles on dopaminergic signaling (1 = high activity, 0 = low activity). Scores to the heterozygous genotypes were assigned based on scientific literature data describing their combination with one or the other homozygous genotype, in relation to cognitive processes and personality traits associated with moral behavior (see Table 3). Then, for each subject, a global score ranging from 0 to 5 was calculated by counting the number of high activity genotypes. None of the subjects showed an overall count equal to zero or to five.
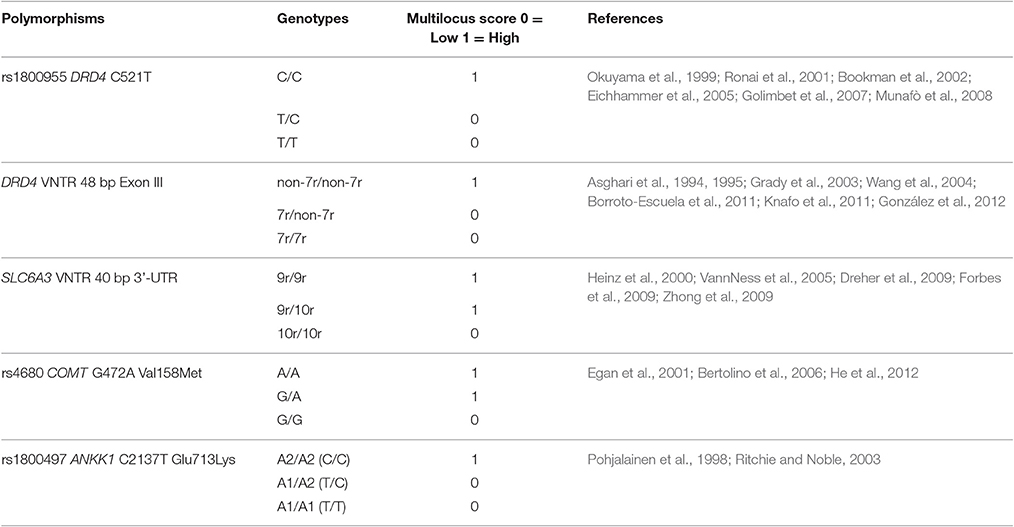
Table 3. Scores assigned to each variant genotype (1 = high activity; 0 = low activity), according to the indicated references, to create the multilocus genetic profiles.
The association analysis was performed both by considering the different multilocus genetic profiles as ordinal variables and by subdividing them into two groups, thus creating a dichotomic variable:
- Multilocus ordinal variable: 1 (18 females and 16 males), 2 (38 females and 44 males), 3 (36 females and 27 males), and 4 (10 females and 11 males) [Bonferroni correction: (a) analysis in the whole sample: p = number of response variables (6) = 6; (b) sex by genotype interaction: p = number of response variables (6) = 6; (c) post hoc: p = number of response variables (6) × genders (2) = 12).
- Multilocus dichotomic variable: Low (scores 1–2) (56 females and 60 males) and High (scores 3–4) (46 females and 38 males) [Bonferroni correction: (a) analysis in the whole sample: p = number of response variables (6) = 6; (b) sex by genotype interaction: p = number of response variables (6) = 6; (c) post hoc: p = number of response variables (6) × genders (2) = 12].
To give additional strength to the results of the multilocus analysis, a multivariate permutation test (10.000 permutations) followed by a Closed Testing procedure (Tippett Step-Down combining function) was run by using the dichotomic variable. The permutation analysis was performed by the Non Parametric Combination based “NPC Test R10” software (Pesarin and Salmaso, 2010).
Results
Allele and genotype frequencies in our sample were consistent with those reported by 1,000Genome (http://www.1000genomes.org/) and HapMap (http://hapmap.ncbi.nlm.nih.gov/) projects. None of the genotype frequencies deviated from the Hardy-Weinberg equilibrium (see Table 1) and they showed equal distribution in the two genders (Fisher's exact test: p = 0.87 for rs1800955; p = 0.40 for DRD4 VNTR; p = 0.56 for SLC6A3 VNTR; p = 0.64 for rs4680; p = 0.15 for rs1800497).
Descriptive data of response variables to moral dilemmas for each single variant genotype grouping, each multilocus genetic profile (ordinal variable), and each multilocus genetic profile group (dichotomic variable) are summarized in Supplementary Tables 2–4, respectively.
Single Variant Association Analysis with Response Variables to Dilemmas
No association was detected between the individually analyzed genetic variants and any of the response variables (Bonferroni adjusted p > 0.05) (see Supplementary Table 2 for descriptive data).
We observed only an interaction between DRD4 rs1800955 genotype and gender (Wald chi-square test = 6.785, df = 2, punadjusted = 0.034, pBonferroni adjusted = 1), as the C/C females, but not the males, rated these actions as more acceptable than the T-allele carriers (C/C females > T-allele females: punadjusted =0.025, pBonferroni adjusted = 0.75), and an interaction between gender and rs4680 (Wald chi-square test = 31.567, df = 2, punadjusted = 0.001, pBonferroni adjusted = 0.03), as female A-allele carriers rated utilitarian choices more acceptable than G/G females (A-allele females > G/G females: punadjusted = 0.0135, pBonferroni adjusted = 0.81). Neither one of these p-values, however, did survive the Bonferroni correction.
Multilocus Association Analysis with Response Variables to Dilemmas
Acceptability:
• GEE analysis by using the multilocus genetic profiles as an ordinal variable. No genotype effect was observed when considering the whole sample (males + females). However, an interaction between gender and multilocus genetic profiles was detected (Wald chi-square test = 11.766, df = 2, punadjusted = 0.003, pBonferroni adjusted = 0.018), as female carriers of High genetic profiles rated utilitarian choices as more acceptable than Low genetic profile females (High females > Low females: punadjusted = 0.001, pBonferroni adjusted = 0.012; Figure 1B).
• GEE analysis by using the multilocus genetic profile as a dichotomic variable. No genotype effect was observed when considering the whole sample (males + females). However, an interaction between gender and multilocus genetic profiles was detected (Wald chi-square test = 11.597, df = 2, punadjusted = 0.003, pBonferroni adjusted = 0.018), as female carriers of High genetic profiles rated utilitarian choices as more acceptable than Low genetic profile females (High females > Low females: punadjusted = 0.001, pBonferroni adjusted = 0.012; Figure 2B).
• Multivariate permutation analysis. The multivariate permutation analysis confirmed the data obtained by GEEs. Overall, a significant effect of multilocus genetic profiles was observed on response variables (Combining function: punadjusted = 0.007, pTippett adjusted = 0.019), which survived only in females (Combining function: punadjusted = 0.004, pTippett adjusted = 0.012). Specifically, female carriers of High genetic profiles rated utilitarian choices as more acceptable than Low genetic profile females (High females > Low females: punadjusted = 0.002, pTippett adjusted = 0.007; Figure 2B).
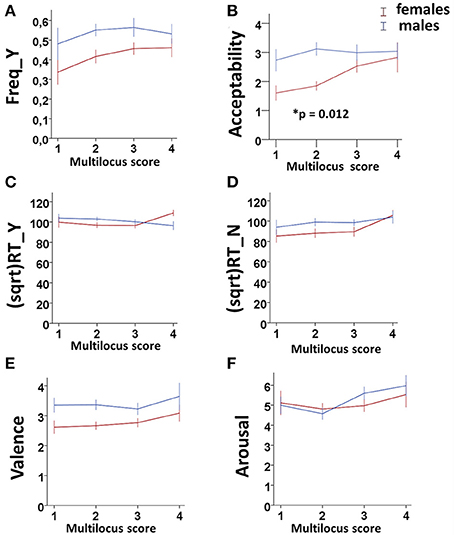
Figure 1. Association results between the dopaminergic Multilocus score and Freq_Y (A), Acceptability (B), (sqrt)RT_Y (C), (sqrt)RT_N (D), Valence (E), and Arousal (F) in the two genders. Bars represent mean ± SEM. 0.01 < *p-value ≤ 0.05.
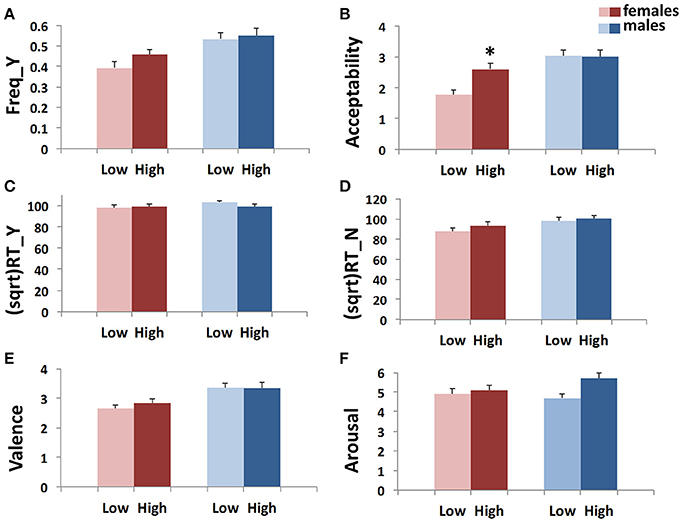
Figure 2. Association results between the dopaminergic dichotomic Multilocus variable and Freq_Y (A), Acceptability (B), (sqrt)RT_Y (C), (sqrt)RT_N (D), Valence (E), and Arousal (F) in the two genders. Bars represent mean ± SEM. 0.01 < *p-value ≤ 0.05.
Freq_Y, (sqrt)RT_Y, (sqrt)RT_N, Valence and Arousal
No associations were detected between multilocus genetic profiles and any of these response variables (Figures 1A,C–F, 2A,C–F).
Raw data are reported in Supplementary Data Sheet 1.
Discussion
In light of the well-known role of dopamine in modulating neural processes associated with executive functions and social cognition, including decision-making (van Schouwenburg et al., 2010; Logue and Gould, 2014; Arnold et al., 2016), reward (Everitt et al., 1999; Schott et al., 2008; Tunbridge et al., 2012), and altruism (Bachner-Melman et al., 2005), the present study tested the hypothesis that genetic variants modulating dopaminergic neurotransmission would affect moral decision-making in healthy individuals. Two hundred individuals were asked to respond to fifty-six moral dilemmas, each one proposing to adopt an utilitarian choice, that is, to sacrifice a person in order to save a larger group of people. Behavioral responses were analyzed in respect to five alleles of genes that regulate dopaminergic neurotransmission and that, taken individually, are known to affect behavioral and personality traits in humans (Balestri et al., 2014; Iofrida et al., 2014; Cherepkova et al., 2016; Heinrich et al., 2016). As the five selected genetic variants were all located in genes belonging to the same biological pathway, they were considered to act synergistically. Thus, after running a single variant analysis that showed no significant association between the single gene variants and the response variables to moral dilemmas, we performed a multilocus analysis following the methodology implemented by other authors (Nikolova et al., 2011; Stice et al., 2012; Davis et al., 2013; Kohno et al., 2016). To this aim, single variant genotypes were combined in multilocus genetic profiles, which are the representatives of the overall effect of different combinations of these alleles both on dopaminergic neurotransmission and on cognitive processes and behavioral traits associated with moral choices.
Interestingly, by applying the multilocus analysis, a gender effect was observed in females carrying genetic profiles that result in a more efficient dopamine signaling due to increased prefrontal dopamine availability (Heinz et al., 2000; Chen et al., 2004), enhanced expression of DRD2 and DRD4 (Pohjalainen et al., 1998; Okuyama et al., 1999; Ritchie and Noble, 2003), or augmentation of cognitive processes (Egan et al., 2001; Bertolino et al., 2006; Gilsbach et al., 2012; Fagundo et al., 2014). These females showed a higher acceptability than females with genetic profiles that impair the dopamine signaling (Figures 1B, 2B). Substantially, females carrying a genetic profile that potentiates the dopamine signaling judged moral dilemmas significantly more acceptable than the other females did, in a way that resembled male behavior.
That moral choices may differ between males and females is a well-known finding (Harenski et al., 2008; Fumagalli et al., 2010a,b; Youssef et al., 2012; Friesdorf et al., 2015). Males usually are more utilitarian than females (Friesdorf et al., 2015). Indeed, in our sample as well, males, as compared to females, opted for the utilitarian choice more frequently, took more time in responding when they opted for the NO answer, and judged the proposed actions more acceptable and less unpleasant (Table 2; Rota et al., 2016).
In addition, our findings are in agreement with results from a study that used a completely different experimental approach (Fumagalli et al., 2010a). These authors observed an increase in utilitarian responses to a moral judgment task in a group of females who underwent anodal transcranial Direct Current Stimulation (tDCS) over their ventral prefrontal cortex (VPC). As dopamine is an anionic catecholamine, the authors hypothesized that the anodal VPC-tDCS increased dopamine levels in the frontal lobe of these individuals, thus influencing their decisional processes. In contrast, anodal VPC-tDCS did not produce any significant effects in males.
Altogether, these findings raise the challenging question of why a further increase in dopamine signaling makes females more similar to males in moral judgment. Women, in fact, have higher levels of dopamine than men in the prefrontal cortex, independently from genotype, as estrogens down-regulate COMT gene expression (Xie et al., 1999) and function (Ball et al., 1972). They also have a higher D2-like receptor binding potential (Kaasinen et al., 2001), so that one would expect that a further increase in dopamine availability should amplify, rather than reduce, differences between genders. However, males and hyper-dopaminergic females may stand at the opposite ends of the inverted U-shaped curve that describes the relationship between dopamine levels and cortical function (Vijayraghavan et al., 2007; Cools and D'Esposito, 2011; Avery et al., 2013). As far as COMT is concerned, a gene by gender interaction has been reported also to modulate cortical thickness, neuronal density, and working memory performance differently in males and females, both in humans and in mice (Sannino et al., 2015). Specifically, a genetic reduction in COMT enzyme activity increased cortical thickness in the prefrontal and postero-parieto-temporal cortex in males but not in females, increased neuronal density in males whereas reducing it in females, and impaired working memory in females, but not in males (Sannino et al., 2015).
These findings reinforce our observation of a sexual dimorphism of dopaminergic genetic variants and are in line with the assumption of a gender-specific functional organization in the brain. Males and females, for example, are different as far as addiction behavior is concerned and these differences seem to be due to dissimilarities in the neural systems that mediate positive and negative reinforcement, probably modulated by hormones (Bobzean et al., 2014; Barth et al., 2015; Hammerslag and Gulley, 2016). Furthermore, a sexual dimorphism exists for cognition, as the gender differences in cognitive profiles seem to be associated with distinct multivariate patterns of resting-state functional connectivity detected by magnetic resonance imaging (Satterthwaite et al., 2015).
Males and females activate different cortical brain areas during moral tasks (Harenski et al., 2008; Juan Yang and Mingming, 2014). For example, in individuals rating the degree of moral violation in a series of unpleasant pictures, a stronger association between moral ratings and neural activity in posterior cingulate and insula was seen in females, and between moral ratings and neural activity in inferior parietal cortex was seen in males (Harenski et al., 2008). These results are in line with the hypothesis that female moral concerns may be mostly based on empathetic skills, whereas male moral assessment is mainly rational. Indeed, involvement of the posterior cingulate has been observed in response to social moral dilemmas (Robertson et al., 2007), whereas the involvement of the inferior parietal cortex may indicate that males used mostly cognitive resources to complete the moral tasks (Harenski et al., 2008).
On the basis of the results of the present study, we propose that the genetically driven increase in dopamine signaling may enhance specific executive functions in females, including attention and cognitive flexibility (Logue and Gould, 2014), making them more similar to males in approaching moral issues.
To date, only a very few studies have ventured in exploring the genetic correlates of moral choices. Three studies have identified associations between three different polymorphisms in the oxytocin receptor gene and moral judgment (Walter et al., 2012; Bernhard et al., 2016; Shang et al., 2017). Another work, published by Marsh et al. (2011), has shown that a genetic variation within the promoter region of the serotonin transporter gene (5-HTTLPR) has an impact on moral judgment as well. Our findings expand the current knowledge by providing a first indication in support of a gender-specific role for dopamine-related genes in human moral behavior.
Limitations of the Study
The use of a candidate gene approach, with a restrict number of a priori selected genetic alleles, may be considered a limitation of this study. The main concern about candidate gene studies, in fact, is the low rate of data reproducibility (Duncan and Keller, 2011; Dick et al., 2015). However, even Genome Wide Association Studies (GWAS), in which all the most common genetic variants are genotyped simultaneously without the need of making any a priori selection, are not able to overcome the risk of generating artifacts (Flint and Munafò, 2013). As a matter of fact, data from scientific literature suggest that these two approaches are complementary and they are both valid instruments to find genetic associations (Chang et al., 2014). Furthermore, the genetic variants for the present study were selected based on a strong a priori hypothesis.
Also, although our sample size—two hundred subjects—is comparable to that of the Study 1 described by Bernhard et al. (2016) or even larger than those in other published genetic association studies regarding moral dilemmas (Marsh et al., 2011; Walter et al., 2012), it is still relatively small, so that this may limit the statistical power.
However, to increase the statistical power of our sample, we performed a multilocus analysis by combining the different genotypes for each single variant in genetic profiles representative of the overall effect of these variants on dopaminergic transmission. This methodology has been successfully implemented by other authors (Nikolova et al., 2011; Stice et al., 2012; Davis and Loxton, 2013; Davis et al., 2013; Kohno et al., 2016). The main criticism to this approach is represented by the assumption that the effects of the single variants are considered additive rather than epistatic and with similar magnitude. However, compared to the single gene variant analysis, this strategy allows for a better representation of the effect of biological networks on complex phenotypes, as it is the case with human behavior (Saez et al., 2014).
Furthermore, in order to avoid type I errors, we applied a Bonferroni correction to GEE analysis to control for multiple comparisons and for multiple testing.
Finally, a multivariate permutation analysis was conducted in parallel.
Nonetheless, though our investigation was based on a strong a priori hypothesis and data were subjected to a conservative and rigorous statistical procedure, yet it should be considered as a pilot study with original preliminary findings that warrant replication in independent and larger samples.
Conclusions
Our findings represent the first indication that genetic factors that modulate dopaminergic neurotransmission may exert a gender selective effect on human moral behavior. For the first time to our knowledge, in fact, we showed that genetics affects male and female moral judgment in a different manner. Specifically, we demonstrated that a genetic profile that improves dopaminergic signaling selectively influences moral judgment in females, making their responses to moral dilemmas more similar to those given by males. As females usually provide more emotionally based answers and engage more the ‘emotional brain’ than males do (e.g., Fumagalli et al., 2010a,b), the enhancement in dopamine availability may improve the cognitive and reduce the emotional counterparts of moral reasoning in females, thus favoring more rational choices.
Our findings, though obtained in a relatively small population and therefore in need of replication in independent samples, prompt additional research, including brain imaging studies designed to investigate patterns of brain activity in response to emotional and rational processing associated with moral judgment tasks (Hutcherson et al., 2015), in male and female carriers of the above reported genotype variants.
Ethics Statement
This study was approved by the Local Ethic Committees at both Padua and Pisa Universities. All subjects gave written informed consent in accordance with the Declaration of Helsinki and retained the right to drop out from the study at any moment.
Author Contributions
SPel and SPal equally contributed to the work: specifically, SPel conceived the work and the experimental design; contributed to data analysis; interpreted and discussed the data, and wrote the manuscript; SPal contributed to the experimental design, to genotyping, data interpretation and manuscript writing and performed most of the statistical analysis; CI performed genotyping and contributed to data interpretation; EM contributed to statistical analysis; GR recruited the subjects, administered moral dilemmas and psychometric scales and collected saliva samples at Pisa University; critically reviewed the manuscript; VM contributed to genotyping and data interpretation; critically reviewed the manuscript; TA performed the permutation tests; AM, LL, and MS enrolled subjects, administered moral dilemmas and psychometric scales and collected saliva samples at Padua University; critically reviewed the manuscript; RR contributed to the experimental design and critically reviewed the manuscript; PP conceived the work, contributed to data interpretation and manuscript writing and critically reviewed the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by a Grant from Fondazione Gio.I.A, Pisa (Italy), by PRIN 2010-2011 (Italian Ministry of Education, University and Research) and by Fondazione Cassa di Risparmio di Lucca (Grant 2016-2017).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/article/10.3389/fnbeh.2017.00156/full#supplementary-material
Supplementary Data Sheet 1. Examples of moral dilemmas administered to the subjects enrolled in the study.
References
Anacker, K., Enge, S., Reif, A., Lesch, K. P., and Strobel, A. (2013). Dopamine D4 receptor gene variation impacts self-reported altruism. Mol. Psychiatry 18, 402–403. doi: 10.1038/mp.2012.49
Arnold, C., Gispert, S., Bonig, H., von Wegner, F., Somasundaram, S., and Kell, C. A. (2016). Dopaminergic modulation of cognitive preparation for overt reading: evidence from the study of genetic polymorphisms. Cereb. Cortex 26, 1539–1557. doi: 10.1093/cercor/bhu330
Asghari, V., Sanyal, S., Buchwaldt, S., Paterson, A., Jovanovic, V., and Van Tol, H. H. (1995). Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J. Neurochem. 65, 1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x
Asghari, V., Schoots, O., van Kats, S., Ohara, K., Jovanovic, V., Guan, H. C., et al. (1994). Dopamine D4 receptor repeat: analysis of different native and mutant forms of the human and rat genes. Mol. Pharmacol. 46, 364–373.
Avery, M. C., Dutt, N., and Krichmar, J. L. (2013). A large-scale neural network model of the influence of neuromodulatory levels on working memory and behavior. Front. Comput. Neurosci. 7:133. doi: 10.3389/fncom.2013.00133
Bachner-Melman, R., Gritsenko, I., Nemanov, L., Zohar, A. H., Dina, C., and Ebstein, R. P. (2005). Dopaminergic polymorphisms associated with self-report measures of human altruism: a fresh phenotype for the dopamine D4 receptor. Mol. Psychiatry 10, 333–335. doi: 10.1038/sj.mp.4001635
Balestri, M., Calati, R., Serretti, A., and De Ronchi, D. (2014). Genetic modulation of personality traits: a systematic review of the literature. Int. Clin. Psychopharmacol. 29, 1–15. doi: 10.1097/YIC.0b013e328364590b
Ball, P., Knuppen, R., Haupt, M., and Breuer, H. (1972). Interactions between estrogens and catechol amines. 3. Studies on the methylation of catechol estrogens, catechol amines and other catechols by the ctechol-O-methyltransferases of human liver. J. Clin. Endocrinol. Metab. 34, 736–746. doi: 10.1210/jcem-34-4-736
Barr, C. L., Feng, Y., Wigg, K. G., Schachar, R., Tannock, R., Roberts, W., et al. (2001). 5′-untranslated region of the dopamine D4 receptor gene and attention-deficit hyperactivity disorder. Am. J. Med. Genet. 105, 84–90. doi: 10.1002/1096-8628(20010108)105:1<84::AID-AJMG1068>3.0.CO;2-Q
Bartels, D. M. (2008). Principled moral sentiment and the flexibility of moral judgment and decision making. Cognition 108, 381–417. doi: 10.1016/j.cognition.2008.03.001
Barth, C., Villringer, A., and Sacher, J. (2015). Sex hormones affect neurotransmitters and shape the adult female brain during hormonal transition periods. Front. Neurosci. 9:37. doi: 10.3389/fnins.2015.00037
Bernhard, R. M., Chaponis, J., Siburian, R., Gallagher, P., Ransohoff, K., Wikler, D., et al. (2016). Variation in the oxytocin receptor gene (OXTR) is associated with differences in moral judgment. Soc. Cogn. Affect. Neurosci. 11, 1872–1881. doi: 10.1093/scan/nsw103
Bertolino, A., Caforio, G., Blasi, G., De Candia, M., Latorre, V., Petruzzella, V., et al. (2004). Interaction of COMT (Val(108/158)Met) genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am. J. Psychiatry 161, 1798–1805. doi: 10.1176/ajp.161.10.1798
Bertolino, A., Rubino, V., Sambataro, F., Blasi, G., Latorre, V., Fazio, L., et al. (2006). Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biol. Psychiatry 60, 1250–1258. doi: 10.1016/j.biopsych.2006.03.078
Blum, K., Braverman, E. R., Wood, R. C., Gill, J., Li, C., Chen, T. J., et al. (1996). Increased prevalence of the Taq I A1 allele of the dopamine receptor gene (DRD2) in obesity with comorbid substance use disorder: a preliminary report. Pharmacogenetics 6, 297–305. doi: 10.1097/00008571-199608000-00003
Bobzean, S. A., DeNobrega, A. K., and Perrotti, L. I. (2014). Sex differences in the neurobiology of drug addiction. Exp. Neurol. 259, 64–74. doi: 10.1016/j.expneurol.2014.01.022
Bookman, E. B., Taylor, R. E., Adams-Campbell, L., and Kittles, R. A. (2002). DRD4 promoter SNPs and gender effects on Extraversion in African Americans. Mol. Psychiatry 7, 786–789. doi: 10.1038/sj.mp.4001075
Borroto-Escuela, D. O., Van Craenenbroek, K., Romero-Fernandez, W., Guidolin, D., Woods, A. S., Rivera, G., et al. (2011). Dopamine D2 and D4 receptor heteromerization and its allosteric receptor-receptor interactions. Biochem. Biophys. Res. Commun. 404, 928–934. doi: 10.1016/j.bbrc.2010.12.083
Bradley, M. M., and Lang, P. J. (1994). Measuring emotion: The self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 25, 49–59. doi: 10.1016/0005-7916(94)90063-9
Buades-Rotger, M., and Gallardo-Pujol, D. (2014). The role of the monoamine oxidase A gene in moderating the response to adversity and associated antisocial behavior: a review. Psychol. Res. Behav. Manag. 7, 185–200. doi: 10.2147/PRBM.S40458
Chang, C. Q., Yesupriya, A., Rowell, J. L., Pimentel, C. B., Clyne, M., Gwinn, M., et al. (2014). A systematic review of cancer GWAS and candidate gene meta-analyses reveals limited overlap but similar effect sizes. Eur. J. Hum. Genet. 22, 402–408. doi: 10.1038/ejhg.2013.161
Chen, J., Lipska, B. K., Halim, N., Ma, Q. D., Matsumoto, M., Melhem, S., et al. (2004). Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 75, 807–821. doi: 10.1086/425589
Cherepkova, E. V., Aftanas, L. I., Maksimov, N., and Menshanov, P. N. (2016). Frequency of 3′ VNTR polymorphism in the dopamine transporter gene SLC6A3 in humans predisposed to antisocial behavior. Bull. Exp. Biol. Med. 162, 82–85. doi: 10.1007/s10517-016-3551-7
Ciaramelli, E., Muccioli, M., Làdavas, E., and di Pellegrino, G. (2007). Selective deficit in personal moral judgment following damage to ventromedial prefrontal cortex. Soc. Cogn. Affect. Neurosci. 2, 84–92. doi: 10.1093/scan/nsm001
Cools, R., and D'Esposito, M. (2011). Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 69, e113–125. doi: 10.1016/j.biopsych.2011.03.028
Davis, C., and Loxton, N. J. (2013). Addictive behaviors and addiction-prone personality traits: associations with a dopamine multilocus genetic profile. Addict. Behav. 38, 2306–2312. doi: 10.1016/j.addbeh.2013.02.012
Davis, C., Loxton, N. J., Levitan, R. D., Kaplan, A. S., Carter, J. C., and Kennedy, J. L. (2013). ‘Food addiction’ and its association with a dopaminergic multilocus genetic profile. Physiol. Behav. 118, 63–69. doi: 10.1016/j.physbeh.2013.05.014
Davis, M. H. (1980). A Multidimensional Approach to Individual Differences in Empathy. JSAS Catalog of Selected Documents in Psychology, 10, 85.
Decety, J., and Cowell, J. M. (2014a). The complex relation between morality and empathy. Trends Cogn. Sci. 18, 337–339. doi: 10.1016/j.tics.2014.04.008
Decety, J., and Cowell, J. M. (2014b). Friends or foes: is empathy necessary for moral behavior? Perspect. Psychol. Sci. 9, 525–537. doi: 10.1177/1745691614545130
Dick, D. M., Agrawal, A., Keller, M. C., Adkins, A., Aliev, F., Monroe, S., et al. (2015). Candidate gene-environment interaction research: reflections and recommendations. Perspect. Psychol. Sci. 10, 37–59. doi: 10.1177/1745691614556682
Di Gaetano, C., Voglino, F., Guarrera, S., Fiorito, G., Rosa, F., Di Blasio, A. M., et al. (2012). An overview of the genetic structure within the Italian population from genome-wide data. PLoS ONE 7:e43759. doi: 10.1371/journal.pone.0043759
Dreher, J. C., Kohn, P., Kolachana, B., Weinberger, D. R., and Berman, K. F. (2009). Variation in dopamine genes influences responsivity of the human reward system. Proc. Natl. Acad. Sci. U.S.A. 106, 617–622. doi: 10.1073/pnas.0805517106
Duncan, L. E., and Keller, M. C. (2011). A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am. J. Psychiatry 168, 1041–1049. doi: 10.1176/appi.ajp.2011.11020191
Egan, M. F., Goldberg, T. E., Kolachana, B. S., Callicott, J. H., Mazzanti, C. M., Straub, R. E., et al. (2001). Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 98, 6917–6922. doi: 10.1073/pnas.111134598
Eichhammer, P., Sand, P. G., Stoertebecker, P., Langguth, B., Zowe, M., and Hajak, G. (2005). Variation at the DRD4 promoter modulates extraversion in Caucasians. Mol. Psychiatry 10, 520–522. doi: 10.1038/sj.mp.4001658
Eisenberg, D. T., Mackillop, J., Modi, M., Beauchemin, J., Dang, D., Lisman, S. A., et al. (2007). Examining impulsivity as an endophenotype using a behavioral approach: a DRD2 TaqI A and DRD4 48-bp VNTR association study. Behav. Brain Funct. 3:2. doi: 10.1186/1744-9081-3-2
Eisenberg, N. (2000). Emotion, regulation, and moral development. Annu. Rev. Psychol. 51, 665–697. doi: 10.1146/annurev.psych.51.1.665
Eisenberg, N. (2007). Empathy-Related Responding and Prosocial Behaviour. Novartis Found Symp. 278, 71–80; discussion 80–96, 216–221.
Ettinger, U., Kumari, V., Collier, D. A., Powell, J., Luzi, S., Michel, T. M., et al. (2008). Catechol-O-methyltransferase (COMT) val158met genotype is associated with BOLD response as a function of task characteristic. Neuropsychopharmacology 33, 3046–3057. doi: 10.1038/sj.npp.1301658
Everitt, B. J., Parkinson, J. A., Olmstead, M. C., Arroyo, M., Robledo, P., and Robbins, T. W. (1999). Associative processes in addiction and reward. The role of amygdala-ventral striatal subsystems. Ann. N. Y. Acad. Sci. 877, 412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x
Fagundo, A. B., Fernández-Aranda, F., de la Torre, R., Verdejo-García, A., Granero, R., Penelo, E., et al. (2014). Dopamine DRD2/ANKK1 Taq1A and DAT1 VNTR polymorphisms are associated with a cognitive flexibility profile in pathological gamblers. J. Psychopharmacol. 28, 1170–1177. doi: 10.1177/0269881114551079
Flint, J., and Munafò, M. R. (2013). Candidate and non-candidate genes in behavior genetics. Curr. Opin. Neurobiol. 23, 57–61. doi: 10.1016/j.conb.2012.07.005
Forbes, E. E., Brown, S. M., Kimak, M., Ferrell, R. E., Manuck, S. B., and Hariri, A. R. (2009). Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Mol. Psychiatry 14, 60–70. doi: 10.1038/sj.mp.4002086
Fossella, J., Green, A. E., and Fan, J. (2006). Evaluation of a structural polymorphism in the ankyrin repeat and kinase domain containing 1 (ANKK1) gene and the activation of executive attention networks. Cogn. Affect. Behav. Neurosci. 6, 71–78. doi: 10.3758/CABN.6.1.71
Friesdorf, R., Conway, P., and Gawronski, B. (2015). Gender differences in responses to moral dilemmas: a process dissociation analysis. Pers. Soc. Psychol. Bull. 41, 696–713. doi: 10.1177/0146167215575731
Fumagalli, M., Vergari, M., Pasqualetti, P., Marceglia, S., Mameli, F., Ferrucci, R., et al. (2010a). Brain switches utilitarian behavior: does gender make the difference? PLoS ONE 5:e8865. doi: 10.1371/journal.pone.0008865
Fumagalli, M., Ferrucci, R., Mameli, F., Marceglia, S., Mrakic-Sposta, S., Zago, S., et al. (2010b). Gender-related differences in moral judgments. Cogn. Process. 11, 219–226. doi: 10.1007/s10339-009-0335-2
Gilsbach, S., Neufang, S., Scherag, S., Vloet, T. D., Fink, G. R., Herpertz-Dahlmann, B., et al. (2012). Effects of the DRD4 genotype on neural networks associated with executive functions in children and adolescents. Dev. Cogn. Neurosci. 2, 417–427. doi: 10.1016/j.dcn.2012.05.001
Golimbet, V. E., Alfimova, M. V., Gritsenko, I. K., and Ebstein, R. P. (2007). Relationship between dopamine system genes and extraversion and novelty seeking. Neurosci. Behav. Physiol. 37, 601–606. doi: 10.1007/s11055-007-0058-8
Golimbet, V. E., Gritsenko, I. K., Alfimova, M. V., and Ebstein, R. P. (2005). [Polymorphic markers of the dopamine D4 receptor gene promoter region and personality traits in mentally healthy individuals from the Russian population]. Genetika 41, 966–972. doi: 10.1007/s11177-005-0161-2
González, S., Rangel-Barajas, C., Peper, M., Lorenzo, R., Moreno, E., Ciruela, F., et al. (2012). Dopamine D4 receptor, but not the ADHD-associated D4.7 variant, forms functional heteromers with the dopamine D2S receptor in the brain. Mol. Psychiatry 17, 650–662. doi: 10.1038/mp.2011.93
Grady, D. L., Chi, H. C., Ding, Y. C., Smith, M., Wang, E., Schuck, S., et al. (2003). High prevalence of rare dopamine receptor D4 alleles in children diagnosed with attention-deficit hyperactivity disorder. Mol. Psychiatry 8, 536–545. doi: 10.1038/sj.mp.4001350
Graffelman, J., and Camarena, J. M. (2008). Graphical tests for Hardy-Weinberg equilibrium based on the ternary plot. Hum. Hered. 65, 77–84. doi: 10.1159/000108939
Greene, J. D., Cushman, F. A., Stewart, L. E., Lowenberg, K., Nystrom, L. E., and Cohen, J. D. (2009). Pushing moral buttons: the interaction between personal force and intention in moral judgment. Cognition 111, 364–371. doi: 10.1016/j.cognition.2009.02.001
Greene, J. D., Morelli, S. A., Lowenberg, K., Nystrom, L. E., and Cohen, J. D. (2008). Cognitive load selectively interferes with utilitarian moral judgment. Cognition 107, 1144–1154. doi: 10.1016/j.cognition.2007.11.004
Greene, J. D., Nystrom, L. E., Engell, A. D., Darley, J. M., and Cohen, J. D. (2004). The neural bases of cognitive conflict and control in moral judgment. Neuron 44, 389–400. doi: 10.1016/j.neuron.2004.09.027
Greene, J. D., Sommerville, R. B., Nystrom, L. E., Darley, J. M., and Cohen, J. D. (2001). An fMRI investigation of emotional engagement in moral judgment. Science 293, 2105–2108. doi: 10.1126/science.1062872
Hammerslag, L. R., and Gulley, J. M. (2016). Sex differences in behavior and neural development and their role in adolescent vulnerability to substance abuse. Behav. Brain Res. 298, 15–26. doi: 10.1016/j.bbr.2015.04.008
Hardin, J. W., and Hilbe, J. M. (2012). Generalized Estimating Equations. Boca Raton, FL: CRC Press; Chapman and Hall/CRC.
Harenski, C. L., Antonenko, O., Shane, M. S., and Kiehl, K. A. (2008). Gender differences in neural mechanisms underlying moral sensitivity. Soc. Cogn. Affect. Neurosci. 3, 313–321. doi: 10.1093/scan/nsn026
He, Q., Xue, G., Chen, C., Lu, Z., Chen, C., Lei, X., et al. (2012). COMT Val158Met polymorphism interacts with stressful life events and parental warmth to influence decision making. Sci. Rep. 2:667. doi: 10.1038/srep00677
Heinrich, A., Müller, K. U., Banaschewski, T., Barker, G. J., Bokde, A. L., Bromberg, U., et al. (2016). Prediction of alcohol drinking in adolescents: personality-traits, behavior, brain responses, and genetic variations in the context of reward sensitivity. Biol. Psychol. 118, 79–87. doi: 10.1016/j.biopsycho.2016.05.002
Heinz, A., Goldman, D., Jones, D. W., Palmour, R., Hommer, D., Gorey, J. G., et al. (2000). Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychophamracology 22, 133–139. doi: 10.1016/S0893-133X(99)00099-8
Herrmann, M. J., Walter, A., Schreppel, T., Ehlis, A. C., Pauli, P., Lesch, K. P., et al. (2007). D4 receptor gene variation modulates activation of prefrontal cortex during working memory. Eur. J. Neurosci. 26, 2713–2718. doi: 10.1111/j.1460-9568.2007.05921.x
Hutcherson, C. A., Montaser-Kouhsari, L., Woodward, J., and Rangel, A. (2015). Emotional and utilitarian appraisals of moral dilemmas are encoded in separate areas and integrated in ventromedial prefrontal cortex. J. Neurosci. 35, 12593–12605. doi: 10.1523/JNEUROSCI.3402-14.2015
Iofrida, C., Palumbo, S., and Pellegrini, S. (2014). Molecular genetics and antisocial behavior: where do we stand? Exp. Biol. Med. 239, 1514–1523. doi: 10.1177/1535370214529508
Juan Yang, L. G., and Mingming, Q. (2014). Gender differences in neural mechanisms underlying moral judgment of disgust: a functional MRI study. J. Behav. Brain Sci. 4, 214–222. doi: 10.4236/jbbs.2014.45023
Kaasinen, V., Någren, K., Hietala, J., Farde, L., and Rinne, J. O. (2001). Sex differences in extrastriatal dopamine d-like receptors in the human brain. Am. J. Psychiatry 158, 308–311. doi: 10.1176/appi.ajp.158.2.308
Klein, T. A., Neumann, J., Reuter, M., Hennig, J., von Cramon, D. Y., and Ullsperger, M. (2007). Genetically determined differences in learning from errors. Science 318, 1642–1645. doi: 10.1126/science.1145044
Knafo, A., Israel, S., and Ebstein, R. P. (2011). Heritability of children's prosocial behavior and differential susceptibility to parenting by variation in the dopamine receptor D4 gene. Dev. Psychopathol. 23, 53–67. doi: 10.1017/S0954579410000647
Koenigs, M., Young, L., Adolphs, R., Tranel, D., Cushman, F., Hauser, M., et al. (2007). Damage to the prefrontal cortex increases utilitarian moral judgements. Nature 446, 908–911. doi: 10.1038/nature05631
Kohno, M., Nurmi, E. L., Laughlin, C. P., Morales, A. M., Gail, E. H., Hellemann, G. S., et al. (2016). Functional genetic variation in dopamine signaling moderates prefrontal cortical activity during risky decision making. Neuropsychopharmacology 41, 695–703. doi: 10.1038/npp.2015.192
Logue, S. F., and Gould, T. J. (2014). The neural and genetic basis of executive function: attention, cognitive flexibility, and response inhibition. Pharmacol. Biochem. Behav. 123, 45–54. doi: 10.1016/j.pbb.2013.08.007
Lotto, L., Manfrinati, A., and Sarlo, M. (2014). A new set of moral dilemmas: norms for moral acceptability, decision times, and emotional salience. J. Behav. Decis. Mak. 27, 57–65. doi: 10.1002/bdm.1782
Malhotra, A. K., Kestler, L. J., Mazzanti, C., Bates, J. A., Goldberg, T., and Goldman, D. (2002). A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am. J. Psychiatry 159, 652–654. doi: 10.1176/appi.ajp.159.4.652
Marinos, G., Naziris, N., Limnaios, S. A., and Drakoulis, N. (2014). Genes and personality characteristics: possible association of the genetic background with intelligence and decision making in 830 Caucasian Greek subjects. Meta Gene 2, 844–853. doi: 10.1016/j.mgene.2014.10.006
Marsh, A. A., Crowe, S. L., Yu, H. H., Gorodetsky, E. K., Goldman, D., and Blair, R. J. (2011). Serotonin transporter genotype (5-HTTLPR) predicts utilitarian moral judgments. PLoS ONE 6:e25148. doi: 10.1371/journal.pone.0025148
Mata, R., Hau, R., Papassotiropoulos, A., and Hertwig, R. (2012). DAT1 polymorphism is associated with risk taking in the Balloon Analogue Risk Task (BART). PLoS ONE 7:e39135. doi: 10.1371/journal.pone.0039135
McNair, D. M., Lorr, M., and Droppleman, L. F. (1971). Manual for the Profile of Mood States. San Diego, CA: Educational and Industrial Testing Services.
Moll, J., and de Oliveira-Souza, R. (2007). Moral judgments, emotions and the utilitarian brain. Trends Cogn. Sci. 11, 319–321. doi: 10.1016/j.tics.2007.06.001
Munafò, M. R., Yalcin, B., Willis-Owen, S. A., and Flint, J. (2008). Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: meta-analysis and new data. Biol. Psychiatry 63, 197–206. doi: 10.1016/j.biopsych.2007.04.006
Nikolova, Y. S., Ferrel, R. E., Manuck, S. B., and Hariri, A. R. (2011). Multilocus genetic profile for dopamine signaling predicts ventral striatum reactivity. Neuropsychopharmacology 36, 1940–1947. doi: 10.1038/npp.2011.82
Noble, E. P., Blum, K., Ritchie, T., Montgomery, A., and Sheridan, P. J. (1991). Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch. Gne. Psychiatry 48, 648–654. doi: 10.1001/archpsyc.1991.01810310066012
Noble, E. P., Gottschalk, L. A., Follon, J. H., Ritchie, T. L., and Wu, J. C. (1997). D2 dopamine receptor polymorphism and brain regional glucose metabolism. Am. J. Med. Genet. 74, 162–166. doi: 10.1002/(SICI)1096-8628(19970418)74:2<162::AID-AJMG9>3.0.CO;2-W
Okuyama, Y., Ishiguro, H., Toru, M., and Arinami, T. (1999). A genetic polymorphism in the promoter region of DRD4 associated with expression and schizophrenia. Biochem. Biophys. Res. Commun. 258, 292–295. doi: 10.1006/bbrc.1999.0630
Osman, M., and Wiegmann, A. (2017). Explaining moral behavior. Exp. Psychol. 64, 68–81. doi: 10.1027/1618-3169/a000336
Pesarin, F., and Salmaso, L. (2010). Permutation Tests for Complex Data: Theory, Applications and Software. Chichester: Wiley series in probability and statistics. John Wiley & Sons, Ltd.
Pietrini, P. (2003). Toward a biochemistry of mind? Am. J. Psychiatry 160, 1907–1908. doi: 10.1176/appi.ajp.160.11.1907
Pohjalainen, T., Rinne, J. O., Någren, K., Lehikoinen, P., Anttila, K., Syvälahti, E. K., et al. (1998). The A1 allele of the human D2 dopamine receptor gene predicts low D2 receptor availability in healthy volunteers. Mol. Psychiatry 3, 256–260. doi: 10.1038/sj.mp.4000350
Poldrack, R. A. (2012). The future of fMRI in cognitive neuroscience. Neuroimage 62, 1216–2020. doi: 10.1016/j.neuroimage.2011.08.007
Poldrack, R. A., and Yarkoni, T. (2016). From brain maps to cognitive ontologies: informatics and the search for mental structure. Annu. Rev. Psychol. 67, 587–612. doi: 10.1146/annurev-psych-122414-033729
Rigoni, D., Pellegrini, S., Mariotti, V., Cozza, A., Mechelli, A., Ferrara, S. D., et al. (2010). How neuroscience and behavioral genetics improve psychiatric assessment: report on a violent murder case. Front. Behav. Neurosci. 4:160. doi: 10.3389/fnbeh.2010.00160
Ritchie, T., and Noble, E. P. (2003). Association of seven polymorphisms of the D2 dopamine receptor gene with brain receptor-binding characteristics. Neurochem. Res. 28, 73–82. doi: 10.1023/A:1021648128758
Robertson, D., Snarey, J., Ousley, O., Harenski, K., DuBois Bowman, F., Gilkey, R., et al. (2007). The neural processing of moral sensitivity to issues of justice and care. Neuropsychologia 45, 755–766. doi: 10.1016/j.neuropsychologia.2006.08.014
Ronai, Z., Barta, C., Guttman, A., Lakatos, K., Gervai, J., Staud, M., et al. (2001). Genotyping the−521C/T functional polymorphism in the promoter region of dopamine D4 receptor (DRD4) gene. Electrophoresis 22, 1102–1105. doi: 10.1002/1522-2683()22:6<1102::AID-ELPS1102>3.0.CO;2-3
Rota, G., Palumbo, S., Lattanzi, N., Manfrinati, A., Sarlo, M., Lotto, L., et al. (2016). Harm aversion explains utilitarian choices in moral decision-making in males but not in females. Arch. Ital. Biol. 154, 50–58. doi: 10.12871/00039829201622
Russo, P. M., Leone, L., and De Pascalis, V. (2011). Cross-cultural validity of the I7 impulsiveness-venturesomeness-empathy scales: evidence from the Italian I7. Compr. Psychiatry 52, 446–452. doi: 10.1016/j.comppsych.2010.07.008
Saez, I., Set, E., and Hsu, M. (2014). From genes to behavior: placing cognitive models in the contex of biological pathways. Front. Neurosci. 4:336. doi: 10.3389/fnins.2014.00336
Sannino, S., Gozzi, A., Cerasa, A., Piras, F., Scheggia, D., Managò, F., et al. (2015). COMT genetic reduction produces sexually divergent effects on cortical anatomy and working memory in mice and humans. Cereb. Cortex 25, 2529–2541. doi: 10.1093/cercor/bhu053
Sarlo, M., Lotto, L., Rumiati, R., and Palomba, D. (2014). If it makes you feel bad, don't do it! Egoistic rather than altruistic empathy modulates neural and behavioral responses in moral dilemmas. Physiol. Behav. 130, 127–134. doi: 10.1016/j.physbeh.2014.04.002
Sartori, G., Pellegrini, S., and Mechelli, A. (2011). Forensic neurosciences: from basic research to applications and pitfalls. Curr. Opin. Neurol. 24, 371–377. doi: 10.1097/WCO.0b013e3283489754
Satterthwaite, T. D., Wolf, D. H., Roalf, D. R., Ruparel, K., Erus, G., Vandekar, S., et al. (2015). Linked sex differences in cognition and functional connectivity in youth. Cereb. Cortex 25, 2383–2394. doi: 10.1093/cercor/bhu036
Schaich Borg, J., Hynes, C., Van Horn, J., Grafton, S., and Sinnott-Armstrong, W. (2006). Consequences, action, and intention as factors in moral judgments: an FMRI investigation. J. Cogn. Neurosci. 18, 803–817. doi: 10.1162/jocn.2006.18.5.803
Schott, B. H., Minuzzi, L., Krebs, R. M., Elmenhorst, D., Lang, M., Winz, O. H., et al. (2008). Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J. Neurosci. 28, 14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008
Serretti, A., Mandelli, L., Lorenzi, C., Landoni, S., Calati, R., Insacco, C., et al. (2006). Temperament and character in mood disorders: influence of DRD4, SERTPR, TPH and MAO-A polymorphisms. Neuropsychobiology 53, 9–16. doi: 10.1159/000089916
Shang, S., Wu, N., and Su, Y. (2017). How oxytocin receptor (OXTR) single nucleotide polymorphisms act on prosociality: the mediation role of moral evaluation. Front. Psychol. 8:396. doi: 10.3389/fpsyg.2017.00396
Shenhav, A., and Greene, J. D. (2014). Integrative moral judgment: dissociating the roles of the amydgdala and ventromedial prefrontal cortex. J. Neurosci. 34, 4741–4749. doi: 10.1523/JNEUROSCI.3390-13.2014
Stice, E., Yokum, S., Burger, K., Epstein, L., and Smolen, A. (2012). Multilocus genetic composite reflecting dopamine signaling capacity predicts reward circuitry responsivity. J. Neurosci. 32, 10093–10100. doi: 10.1523/JNEUROSCI.1506-12.2012
Tunbridge, E. M., Huber, A., Farrell, S. M., Stumpenhorst, K., Harrison, P. J., and Walton, M. E. (2012). The role of catechol-O-methyltransferase in reward processing and addiction. CNS Neurol. Disord. Drug Targets 11, 306–323. doi: 10.2174/187152712800672409
Uzefovsky, F., Shalev, I., Israel, S., Edelman, S., Raz, Y., Perach-Barzilay, N., et al. (2014). The dopamine D4 receptor gene shows a gender-sensitive association with cognitive empathy: evidence from two independent samples. Emotion 14, 712–721. doi: 10.1037/a0036555
Valdesolo, P., and DeSteno, D. (2006). Manipulations of emotional context shape moral judgment. Psychol. Sci. 17, 476–477. doi: 10.1111/j.1467-9280.2006.01731.x
Vandenbergh, D. J., Persico, A. M., Hawkins, A. L., Griffin, C. A., Li, X., Jabs, E. W., et al. (1992). Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics 14, 1104–1106. doi: 10.1016/S0888-7543(05)80138-7
VannNess, S. H., Owens, M. J., and Kilts, C. D. (2005). The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genet. 27, 6–55.
van Schouwenburg, M., Aarts, E., and Cools, R. (2010). Dopaminergic modulation of cognitive control: distinct roles for the prefrontal cortex and the basal ganglia. Curr. Pharm. Des. 16, 2026–2032. doi: 10.2174/138161210791293097
Van Tol, H. H., Bunzow, J. R., Guan, H. C., Sunahara, R. K., Seeman, P., Niznik, H. B., et al. (1991). Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350, 610–614. doi: 10.1038/350610a0
Venter, J. C., Adams, M. D., Myers, E. W., Li, P. W., Mural, R. J., Sutton, G. G., et al. (2001). The sequence of the human genome. Science 291, 1304–1351. doi: 10.1126/science.1058040
Vereczkei, A., Demetrovics, Z., Szekely, A., Sarkozy, P., Antal, P., Szilagyi, A., et al. (2013). Multivariate analysis of dopaminergic gene variants as risk factors of heroin dependence. PLoS ONE 8:e66592. doi: 10.1371/journal.pone.0066592
Vijayraghavan, S., Wang, M., Birnbaum, S. G., Williams, G. V., and Arnsten, A. F. (2007). Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory. Nat. Neurosci. 10, 376–384. doi: 10.1038/nn1846
Walter, N. T., Montag, C., Markett, S., Felten, A., Voigt, G., and Reuter, M. (2012). Ignorance is no excuse: moral judgments are influenced by a genetic variation on the oxytocin receptor gene. Brain Cogn. 78, 268–273. doi: 10.1016/j.bandc.2012.01.003
Wang, E., Ding, Y. C., Flodman, P., Kidd, J. R., Kidd, K. K., Grady, D. L., et al. (2004). The genetic architecture of selection at the human dopamine receptor D4 (DRD4) gene locus. Am. J. Hum. Genet. 74, 931–944. doi: 10.1086/420854
Winterer, G., Musso, F., Vucurevic, G., Stoeter, P., Konrad, A., Seker, B., et al. (2006). COMT genotype predicts BOLD signal and noise characteristics in prefrontal circuits. Neuroimage 32, 1722–1732. doi: 10.1016/j.neuroimage.2006.05.058
Xie, T., Ho, S. L., and Ramsden, D. (1999). Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol. Pharmacol. 56, 31–38.
Xu, H. M., Sun, X. W., Qi, T., Lin, W. Y., Liu, N., and Lou, X. Y. (2014). Multivariate dimensionality reduction approaches to identify gene-gene and gene-environment interactions underlying multiple complex traits. PLoS ONE 9:e108103. doi: 10.1371/journal.pone.0108103
Yang, J. W., Jang, W. S., Hong, S. D., Ji, Y. I., Kim, D. H., Park, J., et al. (2008). A case-control association study of the polymorphism at the promoter region of the DRD4 gene in Korean boys with attention deficit-hyperactivity disorder: evidence of association with the−521 C/T SNP. Prog. Neuropsychopharmacol. Biol. Psychiatry 32, 243–248. doi: 10.1016/j.pnpbp.2007.08.016
Youssef, F. F., Dookeeram, K., Basdeo, V., Francis, E., Doman, M., Mamed, D., et al. (2012). Stress alters personal moral decision making. Psychoneuroendocrinology 37, 491–498. doi: 10.1016/j.psyneuen.2011.07.017
Zhang, Y., Bertolino, A., Fazio, L., Blasi, G., Rampino, A., Romano, R., et al. (2007). Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc. Natl. Acad. Sci. U.S.A. 104, 20552–20557. doi: 10.1073/pnas.0707106104
Zhong, P., Liu, W., and Yan, Z. (2016). Aberrant regulation of synchronous network activity by the attention-deficit/hyperactivity disorder-associated human dopamine D4 receptor variant D4.7 in the prefrontal cortex. J. Physiol. 594, 135–147. doi: 10.1113/JP271317
Keywords: dopamine, genetic variant, moral behavior, decision-making, moral dilemma
Citation: Pellegrini S, Palumbo S, Iofrida C, Melissari E, Rota G, Mariotti V, Anastasio T, Manfrinati A, Rumiati R, Lotto L, Sarlo M and Pietrini P (2017) Genetically-Driven Enhancement of Dopaminergic Transmission Affects Moral Acceptability in Females but Not in Males: A Pilot Study. Front. Behav. Neurosci. 11:156. doi: 10.3389/fnbeh.2017.00156
Received: 28 February 2017; Accepted: 08 August 2017;
Published: 29 August 2017.
Edited by:
Giovanna Zamboni, University of Oxford, United KingdomReviewed by:
Andrea Lavazza, Centro Universitario Internazionale, ItalyHerta Flor, Central Institute of Mental Health, Germany
Copyright © 2017 Pellegrini, Palumbo, Iofrida, Melissari, Rota, Mariotti, Anastasio, Manfrinati, Rumiati, Lotto, Sarlo and Pietrini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Pellegrini, silvia.pellegrini@med.unipi.it
Pietro Pietrini, pietro.pietrini@imtlucca.it
†These authors have contributed equally to this work.
 Silvia Pellegrini
Silvia Pellegrini Sara Palumbo
Sara Palumbo Caterina Iofrida3
Caterina Iofrida3  Erika Melissari
Erika Melissari Giuseppina Rota
Giuseppina Rota Veronica Mariotti
Veronica Mariotti Teresa Anastasio
Teresa Anastasio Andrea Manfrinati
Andrea Manfrinati Rino Rumiati
Rino Rumiati Lorella Lotto
Lorella Lotto Michela Sarlo
Michela Sarlo Pietro Pietrini
Pietro Pietrini