Effectiveness of non-pharmacological therapies on cognitive function in patients with dementia—A network meta-analysis of randomized controlled trials
- 1School of Public Health, North China University of Science and Technology, Tangshan, China
- 2School of Clinical Medicine, North China University of Science and Technology, Tangshan, China
- 3Department of Psychology, The Fourth People’s Hospital of Wuhu, Wuhu, China
- 4Department of General Medicine, Affiliated Anqing First People’s Hospital of Anhui Medical University, Anqing, China
- 5Hebei Province Key Laboratory of Occupational Health and Safety for Coal Industry, School of Public Health, North China University of Science and Technology, Tangshan, China
Objective: Non-pharmacological therapies (NPTs) have received increasing attention from researchers as a category of treatment to improve cognitive impairment in patients with dementia because of their fewer side effects. In this study, photobiomodulation (PBM), enriched environment (EE), exercise therapy (ET), computerized cognitive training (CCT), and cognitive stimulation therapy (CST) were selected to compare the effects of NPTs that improve dementia by quantifying information from randomized controlled trials (RCTs).
Methods: We did a systematic review and network meta-analysis. We searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), China National Knowledge Infrastructure Database, Wan Fang Database, Chinese Biomedical Literature Database, Web of Science, and VIP Database from the time of database creation to 1 August 2022. Two investigators independently screened the literature, extracted information, and assessed the RCTs’ quality with the Cochrane Collaboration Network Risk of Bias 2.0. Network meta-analysis was performed using R language (X64 version 4.1.3) and STATA 17.0.
Results: We identified 1,268 citations and of these included 38 trials comprising 3,412 participants. For improving dementia, the results of the network meta-analysis showed that compared with the control group (CON), PBM (SMD = 0.90, 95% CI: 0.43–1.37), EE (SMD = 0.71, 95% CI: 0.02–1.41), ET (SMD = 0.42, 95% CI: 0.16–0.68), and CST (SMD = 0.36, 95% CI: 0.11–0.62) were significantly different (P < 0.05); There was no significant difference in CCT (SMD = 0.41, 95% CI: −0.07–0.88) (P > 0.05). The ranked results showed that PBM has more potential to be the best intervention (P = 0.90). In addition, there was a significant difference between PBM and CST in improving cognitive function (SMD = 0.54, 95% CI: 0.00; 1.08, P < 0.05).
Conclusion: In this study, NPTs have excellent potential to improve cognition in people with dementia, and PBM may have more significant benefits in improving cognition than the other four NPTs.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42022363746.
1. Introduction
Dementia, a common neurodegenerative disease, was getting more and more attention with the progress of the global population aging. According to the World Health Organization (WHO), there were approximately 55 million cases of dementia patients worldwide, and the number of dementia patients will continue to rise as the world population ages. It is estimated that by 2,050, the number of dementia patients will increase to 139 million (Serge Gauthier et al., 2022). In addition, the total estimated cost of dementia was $1.3 trillion by 2020, which was set to rise with dementia patients in 2030 (World Health Organization, 2021). These ever-increasing patients have brought a double burden on society and economy, while the increase of the estimated prevalence and incidence of dementia emphasized the necessity of effective treatment.
At present, there was a controversy about the pathogenesis of dementia, which had led to the failure to make breakthroughs in drug research on the etiological treatment at this stage. The current drug treatment mainly included two types of cholinesterase inhibitors and ionotropic glutamate receptor antagonists (Tisher and Salardini, 2019), which may cause side effects such as gastrointestinal discomfort, constipation, syncope, falls, arrhythmias, and extrapyramidal symptoms (Cummings et al., 2019), although they had specific improvement effects on patients’ clinical manifestations. In contrast, non-pharmacological therapies (NPTs), which aimed at improving dementia in the elderly, had attracted considerable attention due to their safe, relatively inexpensive, and scalable intervention. At present, the routine NPTS research on dementia patients showed that exercise therapy (ET), cognitive stimulation therapy (CST), and computerized cognitive training (CCT) indicate better treatment effects (Liang et al., 2019). And enriched environment (EE) and photobiomodulation (PBM), as new treatment modalities, have shown sound therapeutic effects in recent studies (Bourdon and Belmin, 2021; Salehpour et al., 2021). However, the efficacy of NPTs were controversial, because the results of individual studies vary widely depending on the training contents. Meanwhile, most of the above NPTs in previous studies have focused on comparing the effectiveness of single non-pharmacological intervention with conventional care in reducing cognitive impairment in patients with dementia. The lack of direct comparative studies of different NPTs leads to differences on which non-pharmacological interventions are most effective (Sikkes et al., 2021).
To tackle this problem, a network meta-analysis is well suited, because it facilitates comparisons of multiple pairs of interventions in one statistical model (Dias et al., 2018).
It’s considered that there were few systematic reviews or meta-analyses have pooled data of randomized controlled trials (RCTs) of dementia patients covering all above aspects, especially the novel non-pharmacological treatment approaches. There was no evidence in the literature to prove which interventions is the best for improving the cognitive function of dementia patients. Therefore, this study provided an optimal evidence-based basis for selecting non-pharmacological treatment options for dementia patients by comparing the magnitude effects of different non-pharmacological treatments on dementia cognition through the frequentist model of network meta-analysis (network meta-analysis).
2. Materials and methods
This systematic review was performed according to the Cochrane Handbook for the Systematic Review of Interventions (Deeks et al., 2019) and according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Liberati et al., 2009). The review protocol was registered with PROSPERO (CRD42022363746).
2.1. Literature search strategies
The computer searched PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), China National Knowledge Infrastructure Database, Wan Fang Database, Chinese Biomedical Literature Database, Web of Science, and VIP Database from their inception to 1 August 2022, without language restrictions. This article used the search terms “Dementia” OR “Senile Paranoid Dementia” OR “Alzheimer’s Disease” OR “Vascular Dementia” OR “Mixed Dementia” combined with a list of all included non-pharmacological therapies. In addition, this study supplemented the relevant literatures through manual search to obtain some of the relevant literature from the review literature and references in the Meta-analysis or reviews in our specialty, which could reduce to some extent the omission of literature that met the inclusion criteria of this study.
2.2. Inclusion and exclusion criteria
To include the central relevant published studies, the inclusion criteria for this study were as follows: Firstly, participants were over 65 years old and diagnosed with dementia by clinical examination tools such as National Institute on Aging and Alzheimer’s Association (NIA-AA) guidelines (Hyman et al., 2012), Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (Wakefield, 2016), Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) criteria (McKhann et al., 1984). All patients had no other primary or secondary disease; Secondly, interventions including EE, PBM, CCT, ET, and CST; Thirdly, comparisons were focused on core treatments (EE, PBM, CCT, ET, and CST) vs. other types of NPTs or control groups (CON). Fourthly, outcome indicators Mini-mental State Examination (MMSE), Montreal Cognitive Assessment (MOCA), and Alzheimer’s disease assessment scale (ADAS-cog) for symptom assessment of dementia patients; Fifthly, the type of study was a published RCT; Exclusion criteria were as follows: Firstly, studies whose research object was confounded with other cognitive impairment-related disorders, such as Parkinson’s, mild cognitive impairment, etc.; Secondly, studies in which various types of cognitive interventions were used in combination with each other; Thirdly, studies in which all regionalized versions of the MMSE scale, such as the Korean-MMSE (K-MMSE), Hong Kong-MMSE (H-MMSE); Fourthly, conference papers, or papers presented in abstract only; Fifthly, studies for which data could not be extracted because of missing or incomplete data (Middelstädt et al., 2016; Berman et al., 2017); Sixthly, duplicate publications.
2.3. Literature selection and data extraction
The literature was screened by reading the title and abstract for initial screening. After excluding irrelevant literature, the full text was further read to exclude the literature that can’t get the full text or can’t meet the inclusion criteria. A uniform data extraction form was used to extract data from the included literature, which including the first author, year of publication, country, study population type, age, sample size, male or female ratio, interventions, duration, frequency, and mean and standard deviation (SD) of outcome indicators. The screening process was independently performed by two investigators, which was screened the literature to extract information and cross-checking, and they will consult a third to assist in judgment in case of disagreement.
2.4. Quality assessment
The risk of bias 2 (ROB 2) in the included literature was evaluated by two researchers independently using the RCT risk of bias two assessment tool (Sterne et al., 2019) recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins, 2019), which was consist of five aspects of the randomization process, deviation from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported results. Each entry was evaluated by the “low risk of bias,” “high risk of bias,” or “unclear.” If there was a disagreement, it will be decided by the third party or agreed upon by mutual agreement.
2.5. Statistical analyses
This study began with a similarity hypothesis test to evaluate the clinical and methodological similarity of the included studies (Salanti et al., 2008). A frequency science perspective was used to calculate efficacy of each treatment modality. This article analyzed the pooled data and demographic characteristics of each study and quantitatively estimated the heterogeneity of studies with I2 statistics (Chen and Benedetti, 2017) (ranging from 0 to 100%, the higher the I2, the more significant the heterogeneity, of which 25, 50, and 75% were considered as mild, moderate and high heterogeneity, respectively). After the network meta-analysis was conducted, the funnel plots were used to evaluate obvious publication biases based on visual inspection. Notably, this review used a random-effects model rather than a fixed-effects model because it might be the most appropriate and conservative analysis of the between-study variance (Kanters, 2022).
The STATA 16.0 was used to construct a network plot and provide all existing relationships, with different treatments represented by other nodes. The direct comparisons of results represented by lines connecting the appropriate nodes. The overall inconsistency and node split analysis were used to determine the inconsistency between direct and indirect evidence estimates for each intervention comparison, which was usually shown as p. If the p exceeds 0.05, the consistency model was used, indicating that there is no significant inconsistency (Higgins et al., 2012). The above analyses were performed using the “net-meta” package and the “Rjags” R language (X64 version 4.1.3). The rank probabilities of each treatment were calculated using the p-score, which values ranged from 0 to 1, where larger values indicated better treatment efficacy (Rücker and Schwarzer, 2015).
3. Results
3.1. Search process
The literature screening process was shown in Figure 1. The search for this study yielded a total of 1,268 articles. After removing 344 duplicates and 766 irrelevant articles, the remaining 158 articles were all read. Finally, 38 articles were included in our network meta-analysis by passing the strict eligibility criteria described above. All authors involved in this study agreed on the selection and evaluation method.
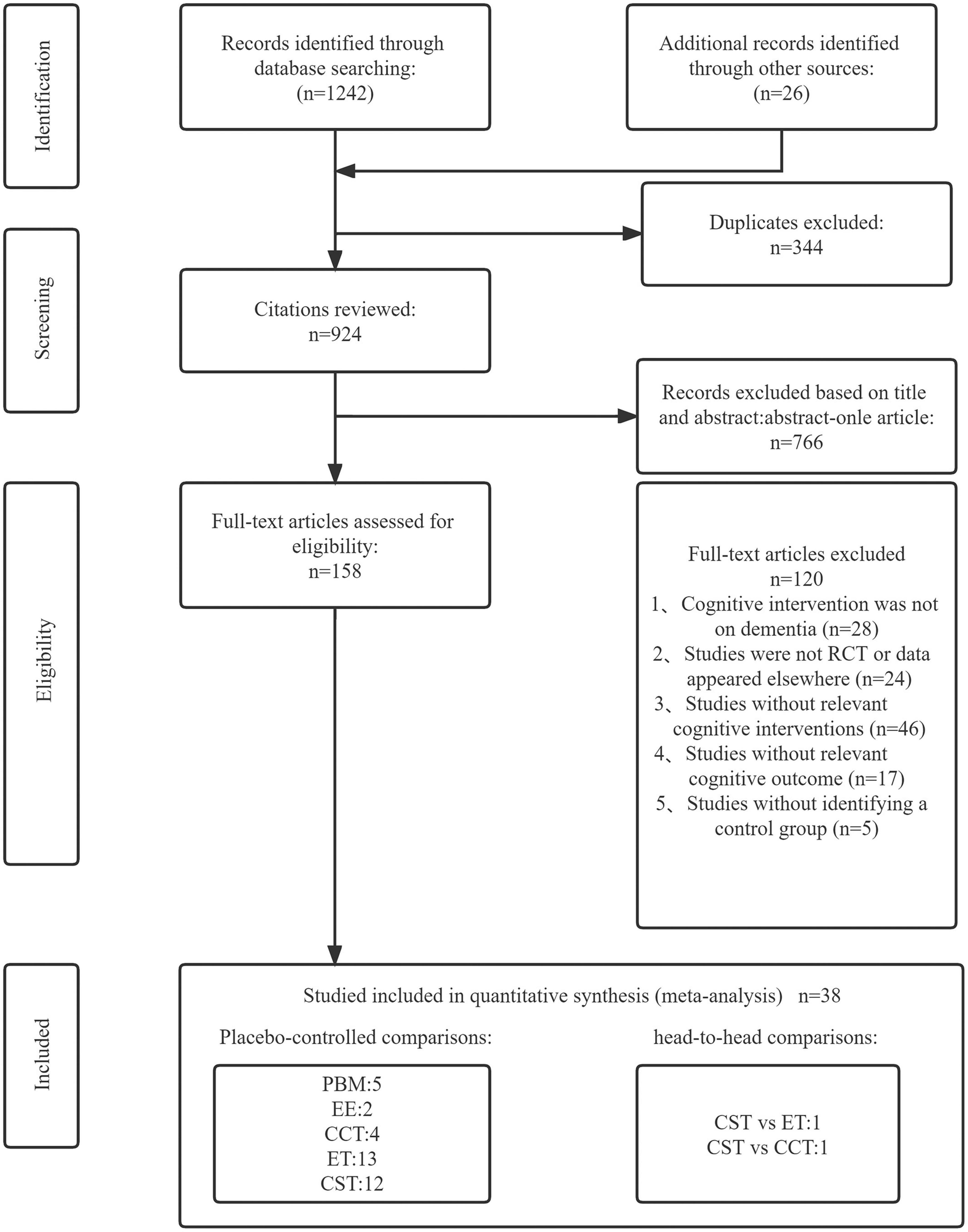
Figure 1. Literature review flowchart. EE, enriched environment; ET, exercise therapy; PBM, photobiomodulation; CCT, computerized cognitive training; CST, cognitive stimulation therapy; CON, control group.
3.2. Baseline characteristics and ROB 2 quality assessment
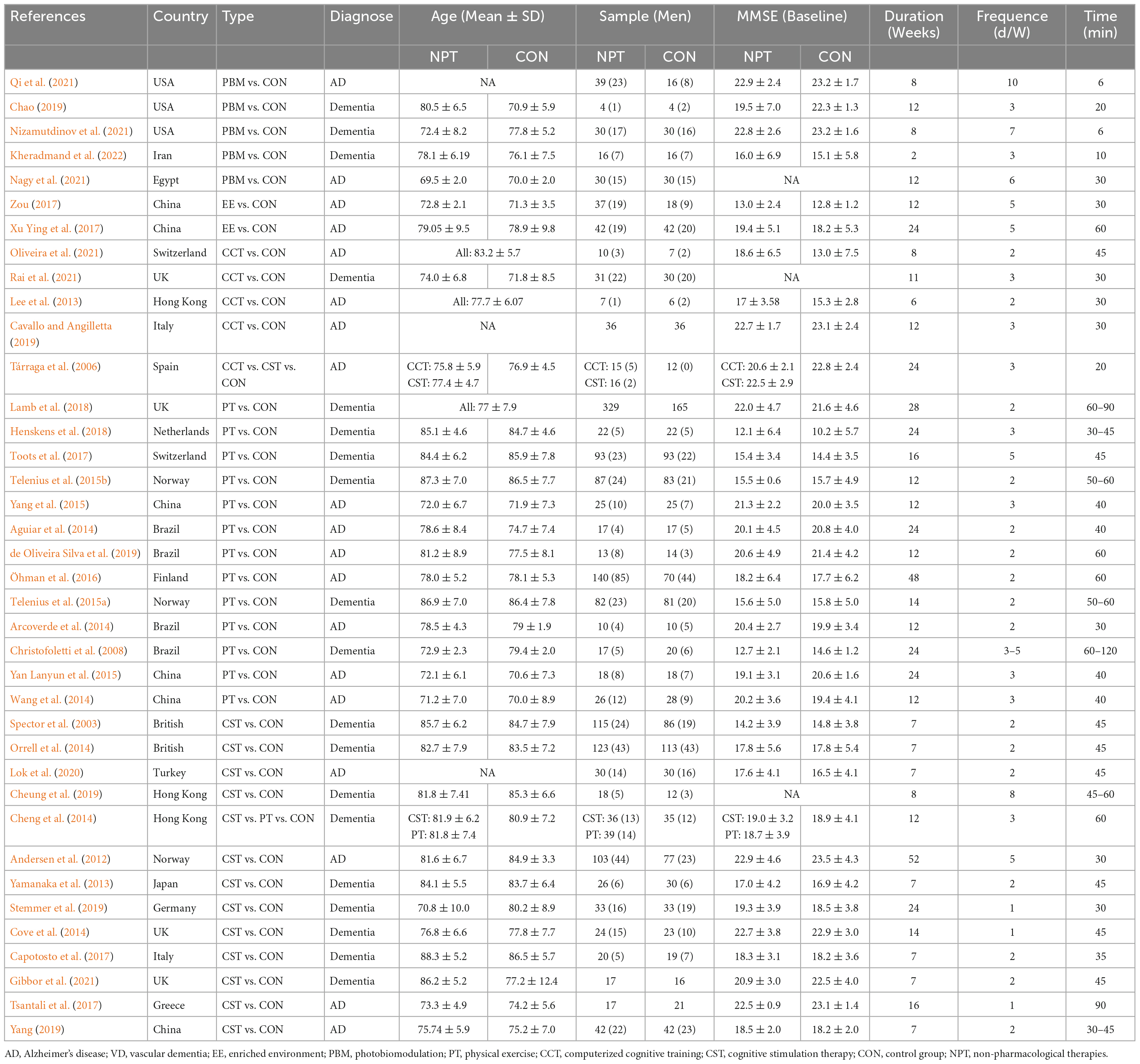
Table 1 showed that baseline data on the demographic characteristics of the 38 included trials, which included 3,721 participants at baseline and 309 participants who did not complete the entire intervention, and leaved a total of 3,412 participants (experimental: 1,920, control: 1,942). The mean age of the subjects ranged from 70.04 ± 8.90 to 88.25 ± 5.15, and the duration ranged from 2 to 52 weeks, with a mean of 14 weeks. In addition, the mean MMSE scores of all selected studies ranged from 5.1 to 23.5. Meanwhile, among the 38 baseline data on demographic characteristics, three studies did not record baseline values of cognitive function in subjects (Cheung et al., 2019; Nagy et al., 2021; Rai et al., 2021), and three studies did not report exact baseline age data (Cavallo and Angilletta, 2019; Lok et al., 2020; Qi et al., 2021), but all conform to our inclusion criteria.
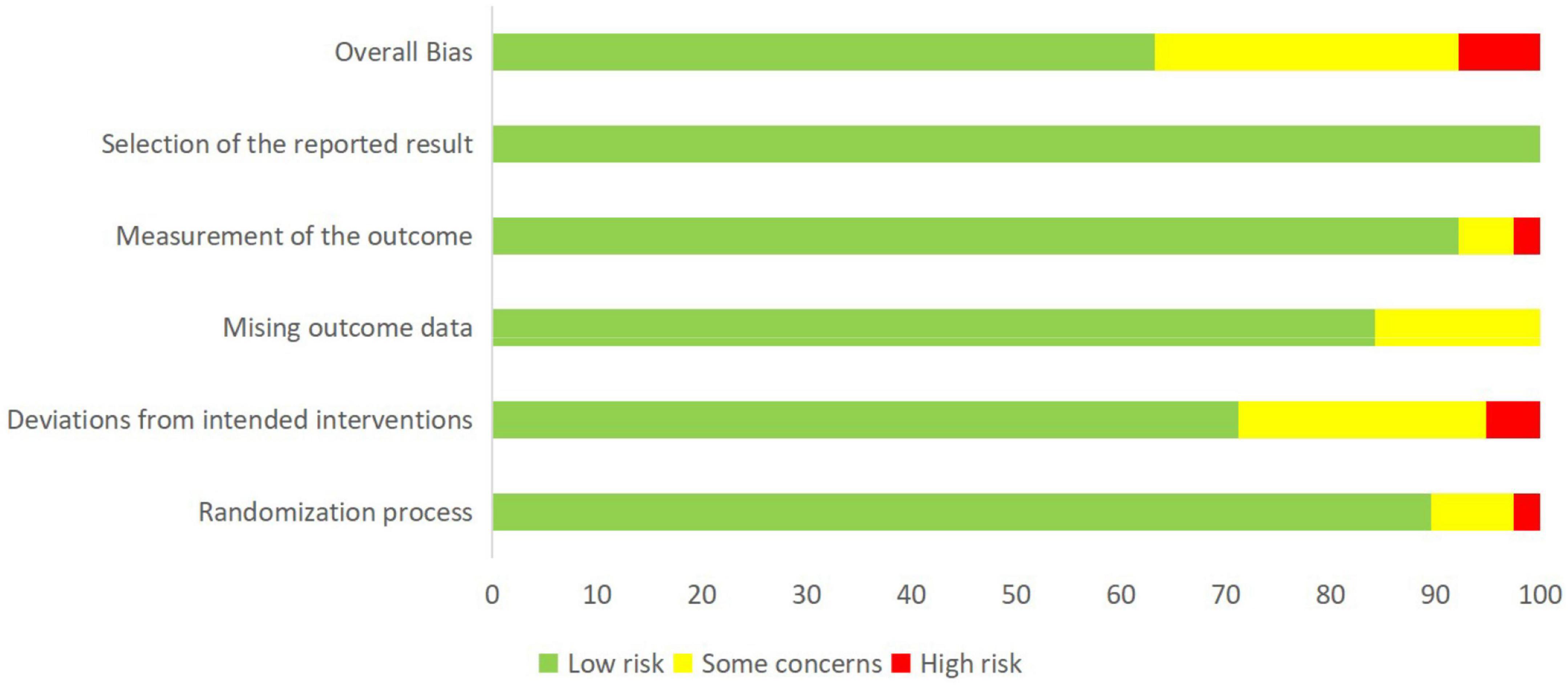
We evaluated the quality of included studies based on the Cochrane Collaboration Tool. Figure 2 and Supplementary Figure 1 summarized the risk of bias assessment for all data included in the network meta-analysis and the bias assessment risk of network meta-analysis at each outcome level in each study, respectively. We considered 63.2% as “low risk,” 7.9% as “high risk,” and 28.9% as “some concerns” about the articles of NPTs on cognitive function.
3.3. Network meta-analysis
The preliminary meta-analysis of the included studies showed mild heterogeneity (I2 = 64.3%). The symmetrical distribution of funnel plot indicated that there was no significant publication bias (p > 0.05) for Egger’s test, which showed that there was no significant bias in this study (Supplementary Figure 2).
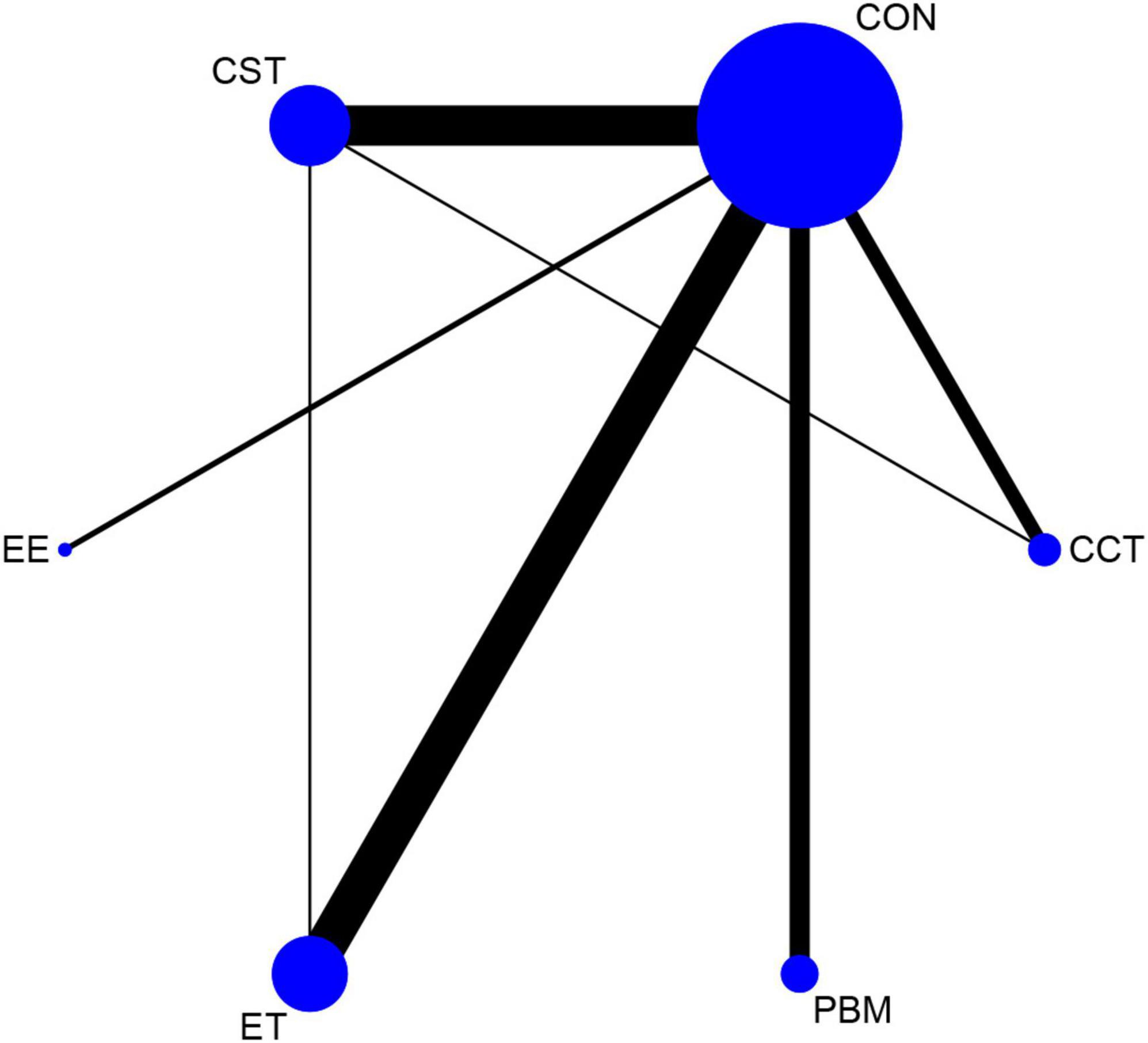
The net evidence of different interventions was shown in Figure 3. A total of five interventions were included: EE, PBM, CCT, ET, and CST. According to the network plot, ET had the most studies, and CST had more studies, and EE had the least. CST and CCT formed a closed loop, as well as CST and ET also created a closed loop, which indicated both direct and indirect comparisons. There was no evidence of direct comparisons for the other interventions.
This article used global inconsistency and nodal splitting to test the inconsistency between direct and indirect evidence from the included studies, with p = 0.2859 for the former and results for the latter p = 0.7339 (CCT vs. CON), p = 0.3197 (CCT vs. CST), p = 0.1603 (CST vs. CON), p = 0.2735 (ET vs. CON), and p = 0.7978 (ET vs. CST) showed that none of the inconsistencies in evidence between direct comparisons for each cognitive intervention were statistically significant, indicating a good fit for consistency (Supplementary Figure 3).
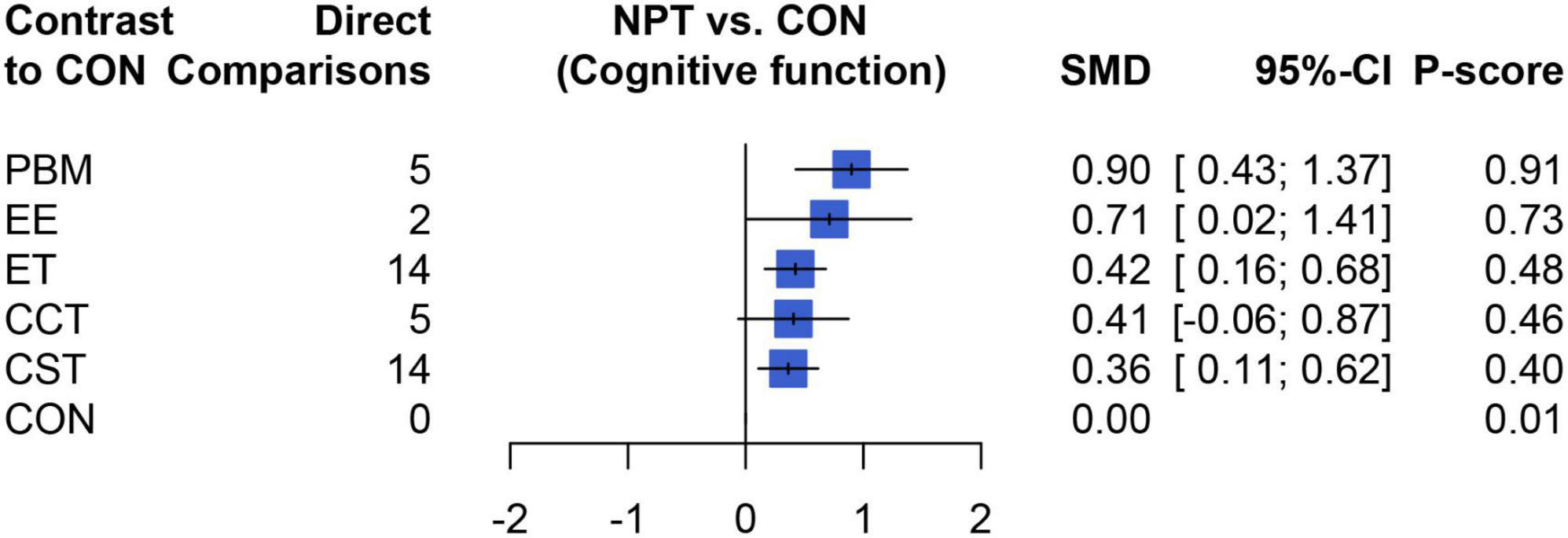
Table 2 showed the results of the network meta-analysis of the primary outcomes, In terms of curative effect, most of the included NPTS were statistically significantly superior to the CON group, including PBM (SMD = 0.90, 95% CI: 0.43–1.37), EE (SMD = 0.71, 95% CI: 0.02–1.41), ET (SMD = 0.42, 95% CI: 0.16–0.68) and CST (SMD = 0.36, 95% CI: 0.11–0.62). Compared with the control group, the results of the CCT group (SMD = 0.41, 95% CI: −0.07–0.88) failed to show significant efficacy compared to the control group (P > 0.05). In addition, the comparison between different NPTs showed that PBM had a better treatment effect than CST, but there was no significant difference among other NPTs. Figure 4 showed the therapeutic effect ranking of each NPTS that PBM (P-score = 0.90) ranked the highest in improving cognitive function of dementia patients, which were followed by EE (P-score = 0.73), ET (P-score = 0.48), CCT (P-score = 0.46), and CST (P-score = 0.40) ranked the lowest.
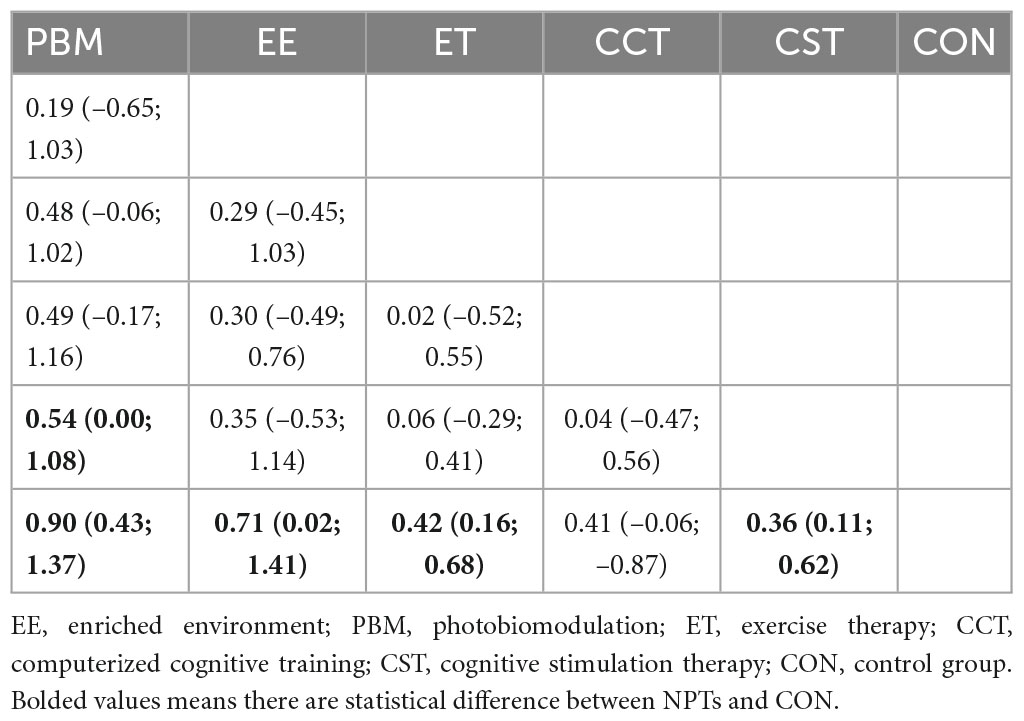
Table 2. The effect of each non-pharmacological therapy on cognition based on cognition examination.
4. Discussion
As far as we know, from the science, from the perspective of frequency science, especially when combining traditional and more recent NPTs, previous studies have not proposed which intervention is the best way to treat cognitive function of dementia patients. Therefore, direct and indirect evidence were used to evaluate the relative effectiveness of different NPTs in cognitive interventions of dementia patients. According to the available data summarized in this study, the efficacy of the five NPTs were ranked from good to bad were: PBM, EE, ET, CCT, and CST. PBM, EE, ET, and CST show significant differences compared to CON (p < 0.05).
This study analyzed the improvement of cognitive function in patients with dementia by non-pharmacological treatments, which was consistent with the findings of a previous network meta-analysis (Wang et al., 2020). All NPTs had great potential to improve cognitive performance in patients with dementia, while CST and ET were shown to be the most beneficial interventions. Based on this, this study included additional new non-pharmacological treatment modalities and further evaluated their therapeutic effects compared with traditional non-pharmacological treatments such as CCT, CST, and ET. It was found that CST and ET had better therapeutic effects in improving cognitive function in patients. Notably, we found that PBM performed best in treating cognitive dysfunction in dementia patients by adding the latest non-pharmacological treatment modalities through frequency science perspective.
Photobiomodulation refers to a type of light therapy that utilizes that visible or near-infrared (NIR) light (600–1,100 nm) from lasers or light-emitting diodes (LEDs) stimulates or modulates various cellular and biological processes (Salehpour et al., 2018; Glass, 2021). Although most of the studies included in this article used red light or NIR at wavelengths from 650 to 1,080 nm for intracranial and intranasal irradiation methods, the treatment regimen for PBM was still largely dependent on patient-physician preference. Our findings were consistent with those reported in a previous systematic review (Zhu et al., 2022) that PBM was effective as a novel therapeutic approach to improve the level of cognitive function in dementia patients. This positive effect of PBM relied on four potential mechanisms (Hamblin, 2016), the basic ones involving photon absorption in the mitochondria (cytochrome c oxidase) (Hennessy and Hamblin, 2017), terminal enzymes in the electron transport chain, triggering downstream molecular and biochemical pathways in the mitochondrial respiratory chain, and exerting therapeutic effects (Lane, 2006; Karu, 2008; Hamblin, 2018); and adjust regional cerebral blood flow to increase perfusion levels (Chao, 2019; Salehpour et al., 2020; Baik et al., 2021); and open the light-mediated of calcium channels (Jung et al., 2019; Kim et al., 2020) and promote the activation of signaling mediators and transcription factors (Wu et al., 2022). In addition, PBM can reduce Aβ production and plaque formation by shifting amyloid precursor protein (APP) to non-amyloidogenic pathways (Zhang et al., 2020). These specific mechanisms of PBM are effective in improving mitochondrial function and increasing oxygen activity and ATP production, inhibiting aspects such as the downregulation of inflammation through inhibition of the NF-κB pathway. These aspects have a more significant role in improving cognitive function in patients with dementia. Moreover, the results of this study show that PBM has better efficacy in improving cognitive function methods in dementia patients compared to the other four non-pharmacological treatments. PBM therapy was a safe, non-invasive, non-thermal, and economical approach to improving cognitive function in patients with dementia while significantly reducing the pain of treatment, the adverse effects of treatment, and the financial burden on the family. In conclusion, PBM was a promising non-pharmacological option associated with cognitive improvement in patients with dementia. However, the optimal treatment regimen for different dementia severity and other modifying factors needs to be clarified to provide more precise individualized treatment plans in the future.
The rank probability of efficacy indicated that EE ranked second in effectiveness among the five different non-pharmacological interventions. This finding was similar to previous reports that EE was effective in improving cognitive function in patients with dementia (Bourdon and Belmin, 2021; Cutuli et al., 2022). EE was a non-invasive treatment that provides plasticity to the brain by combining cognitive training, such as memory and thinking, with dynamic stimulation, such as color, sound, and light, in an enriched environment (Figuracion and Lewis, 2021). A large number of animal studies have demonstrated the superiority and effectiveness of “Enriched environments” in improving cognitive functions in the brain (Nakano et al., 2020; Cordier et al., 2021). However, the network meta-analysis showed that there was no significant difference between EE and other non-pharmacological treatments, which may be due to the different study methods in the data pool. In the network meta-analyses, various interventions had slight interactions in the adjusted pooling and data comparison. Currently, there were few studies in this area of EE, and it’s a need to use more rigorous designs, more standard protocols, and more extensive studies to evaluate the effects of EE on improving cognitive function in patients with dementia.
The results of this study suggested that exercise therapy significantly improved the cognitive function of patients, which was consistent with previous meta-analyses that exploring the effects of exercise on dementia patients (Jia et al., 2019; López-Ortiz et al., 2021; Huang et al., 2022). In recent years, ET had been widely used as a low-risk and low-cost non-pharmacological treatment for patients with dementia. A large number of RCTs had reported the positive effects of exercise on cognitive function in patients with dementia (Henskens et al., 2018; Lamb et al., 2018; de Oliveira Silva et al., 2019). Exercise therapy improved cognitive performance mechanisms, such as increasing growth factors (Ruiz-González et al., 2021; Xue et al., 2022), modulating inflammatory cytokines (Hashiguchi et al., 2020; de Farias et al., 2021), alleviating oxidative stress (Hu et al., 2022), increasing cerebral blood flow (Lu et al., 2019; Tomoto et al., 2021), decreasing antibody concentrations (Giménez-Llort et al., 2013), and inhibiting tau phosphorylation from slowing the progression of dementia (Wang et al., 2021; Xu et al., 2022). However, these potential mechanisms have been proved to exist only in animal models, and some studies examining these mechanisms have not yet to prove their applicability to humans. This research included medium and long-term aerobic exercise, resistance training, physical and mental exercise, Tai Chi exercise and multi-component exercise. There was moderate heterogeneity among all RCTs on ET, which indicated that ET may have some variability in cognitive improvement due to the differences in exercise modality, intensity, frequency, and duration of study design. It was worth noting that recent studies had some differences in exploring the effects of different exercise modalities on cognitive improvement. Some studies have found that multi-component exercise could better improve cognitive dysfunction in patients (McDermott et al., 2019; Yang et al., 2021). Recent studies showed that resistance training appears to have the best therapeutic effect on improving cognitive function in patients (Huang et al., 2022). Therefore, future research on the process of exercise therapy for cognitive improvement needs to describe more specific exercise modalities and find more accurate ways to mitigate the process of dementia.
The results showed that there was no significant difference in the effect of CCT on the cognitive performance of patients with dementia, which was consistent with the findings of previous meta-analysis studies (Gates et al., 2019). Several studies found that cognitive training improves cognitive function in multiple cognitive domains in patients with mild cognitive impairment and dementia (Kanaan et al., 2014; Trebbastoni et al., 2018). CCT, a new cognitive training system that presents cognitive training tasks in a computer program, had a better effect on cognitive function (Bauer and Andringa, 2020). Despite the vital role of CCT in improving cognitive function, the current findings were not optimistic, which may be due to fewer included studies or the lower sensitivity of MMSE to cognitive function changes than other scales (Fu et al., 2017). All RCTs in this study used the MMSE as an assessment tool, which can’t accurately evaluates subtle changes in cognitive function (Weuve et al., 2006).
Cognitive stimulation therapy can improve cognition more effectively than controls, which was consistent with the previous meta-analysis that reported a more significant effect on cognitive function (Saragih et al., 2022). Using repetitive activities, especially tasks and games can help improve brain connectivity and generate new synapses and myelinated neural circuits, which was contributed to restoring or reorganizing neuronal structures behind cognitive function (Bryck and Fisher, 2012). Meanwhile, studies showed that CST has better clinical efficacy than drug therapy (Liang, 2019; Devita et al., 2021). It was noteworthy that CST did not show better efficacy compared to other non-pharmacological therapies in the present study.
Our study also had some limitations. First, the quality of the included studies was moderately heterogeneous due to the significant differences in treatment frequency, and treatment modality between different NPTs. Secondly, EE in our network only included a few studies, and there was less evidence-based evidence from studies of EE as a new non-pharmacological treatment modality, which may make the results biased. Thirdly, most of the included studies in this network meta-analysis compared non-pharmacological treatments with controls, while the number of actual head-to-head trials was relatively small, so comparative efficacy between interventions was often based on indirect comparisons. Fourthly, although we assessed the three assumptions of the network meta-analysis (homogeneity assumption, transferability assumption, and consistency assumption) to ensure their plausibility, there was moderate heterogeneity. Finally, our study did not analyze the safety of cognitive interventions because only four included studies described their adverse effects.
5. Conclusion
In conclusion, our network meta-analysis concluded that the best non-pharmacological treatment modality for patients with dementia was PBM, followed by EE, ET, and CST. However, the results should be interpreted with caution, considering the limitations of our meta-analysis described above and the insufficient number of studies in the existing literature. In the future, more multi-arm randomized controlled trials should be conducted to provide more direct evidence for the relative effectiveness of various non-pharmacological treatment.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
FG played a guiding role in the manuscript writing and revision and data analysis. FW contributed to the conception or design of the work, the writing and revision of the manuscript, and the processing of the data. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Innovation and Entrepreneurship Training Programmed for University Students Funding (X2022308).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1131744/full#supplementary-material
Abbreviations
PBM, photobiomodulation; EE, enriched environment; CCT, computerized cognitive training; CST, cognitive stimulation therapy; ET, exercise therapy; CON, control group; NPTs, non-pharmacological therapies; RCTs, randomized controlled trials; ROB 2, risk of bias 2; SMD, standardized mean difference; MMSE, mini-mental state examination; MOCA, montreal cognitive assessment; ADAS-cog, Alzheimer’s disease assessment scale.
References
Aguiar, P., Monteiro, L., Feres, A., Gomes, I., and Melo, A. (2014). Rivastigmine transdermal patch and physical exercises for Alzheimer’s disease: A randomized clinical trial. Curr. Alzheimer Res. 11, 532–537. doi: 10.2174/1567205011666140618102224
Andersen, F., Viitanen, M., Halvorsen, D. S., Straume, B., Wilsgaard, T., and Engstad, T. A. (2012). The effect of stimulation therapy and donepezil on cognitive function in Alzheimer’s disease. A community based RCT with a two-by-two factorial design. BMC Neurol. 12:59. doi: 10.1186/1471-2377-12-59
Arcoverde, C., Deslandes, A., Moraes, H., Almeida, C., Araujo, N. B., Vasques, P. E., et al. (2014). Treadmill training as an augmentation treatment for Alzheimer’s disease: A pilot randomized controlled study. Arq. NeuroPsiquiatr. 72, 190–196.
Baik, J. S., Lee, T. Y., Kim, N. G., Pak, K., Ko, S. H., Min, J. H., et al. (2021). Effects of photobiomodulation on changes in cognitive function and regional cerebral blood flow in patients with mild cognitive impairment: A pilot uncontrolled trial. JAD 83, 1513–1519. doi: 10.3233/JAD-210386
Bauer, A., and Andringa, G. (2020). The potential of immersive virtual reality for cognitive training in elderly. Gerontology 66, 614–623. doi: 10.1159/000509830
Berman, M. H., Halper, J. P., Nichols, T. W., Jarrett, H., Lundy, A., and Huang, J. H. (2017). Photobiomodulation with near infrared light helmet in a pilot, placebo controlled clinical trial in dementia patients testing memory and cognition. J. Neurol. Neurosci. 8:176. doi: 10.21767/2171-6625.1000176
Bourdon, E., and Belmin, J. (2021). Enriched gardens improve cognition and independence of nursing home residents with dementia: A pilot controlled trial. Alzheimer Res. Ther. 13:116. doi: 10.1186/s13195-021-00849-w
Bryck, R. L., and Fisher, P. A. (2012). Training the brain: Practical applications of neural plasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. Am. Psychol. 67, 87–100. doi: 10.1037/a0024657
Capotosto, E., Belacchi, C., Gardini, S., Faggian, S., Piras, F., Mantoan, V., et al. (2017). Cognitive stimulation therapy in the Italian context: Its efficacy in cognitive and non-cognitive measures in older adults with dementia. Int. J. Geriatr. Psych. 32, 331–340. doi: 10.1002/gps.4521
Cavallo, M., and Angilletta, C. (2019). Long-lasting neuropsychological effects of a computerized cognitive training in patients affected by early stage Alzheimer’s disease: Are they stable over time. J. Appl. Gerontol. 38, 1035–1044. doi: 10.1177/0733464817750276
Chao, L. L. (2019). Effects of home photobiomodulation treatments on cognitive and behavioral function, cerebral perfusion, and resting-state functional connectivity in patients with dementia: A pilot trial. Photobiomodulation 37, 133–141. doi: 10.1089/photob.2018.4555
Chen, B., and Benedetti, A. (2017). Quantifying heterogeneity in individual participant data meta-analysis with binary outcomes. Syst. Rev. 6:243. doi: 10.1186/s13643-017-0630-4
Cheng, S. T., Chow, P. K., Song, Y. Q., Yu, E. C., Chan, A. C., Lee, T. M., et al. (2014). Mental and physical activities delay cognitive decline in older persons with dementia. Am. J. Geriatr. Psych. 22, 63–74. doi: 10.1016/j.jagp.2013.01.060
Cheung, D., Li, B., Lai, D., Leung, A., Yu, C., and Tsang, K. T. (2019). Cognitive stimulating play intervention for dementia: A feasibility randomized controlled trial. Am. J. AlzheimerDis. Dement. 34, 63–71. doi: 10.1177/1533317518808036
Christofoletti, G., Oliani, M. M., Gobbi, S., Stella, F., Bucken Gobbi, L. T., and Renato Canineu, P. (2008). A controlled clinical trial on the effects of motor intervention on balance and cognition in institutionalized elderly patients with dementia. Clin. Rehabil. 22, 618–626. doi: 10.1177/0269215507086239
Cordier, J. M., Aguggia, J. P., Danelon, V., Mir, F. R., Rivarola, M. A., and Mascó, D. (2021). Postweaning enriched environment enhances cognitive function and brain-derived neurotrophic factor signaling in the hippocampus in maternally separated rats. Neuroscience 453, 138–147. doi: 10.1016/j.neuroscience.2020.09.058
Cove, J., Jacobi, N., Donovan, H., Orrell, M., Stott, J., and Spector, A. (2014). Effectiveness of weekly cognitive stimulation therapy for people with dementia and the additional impact of enhancing cognitive stimulation therapy with a carer training program. Clin. Interv. Aging 9, 2143–2150. doi: 10.2147/CIA.S66232
Cummings, J. L., Tong, G., and Ballard, C. (2019). Treatment combinations for Alzheimer’s disease: Current and future pharmacotherapy options. JAD 67, 779–794. doi: 10.3233/JAD-180766
Cutuli, D., Landolfo, E., Petrosini, L., and Gelfo, F. (2022). Environmental enrichment effects on the brain-derived neurotrophic factor expression in healthy condition, Alzheimer’s disease, and other neurodegenerative disorders. JAD 85, 975–992. doi: 10.3233/JAD-215193
de Farias, J. M., Dos Santos Tramontin, N., Pereira, E. V., de Moraes, G. L., Furtado, B. G., Tietbohl, L., et al. (2021). Physical exercise training improves judgment and problem-solving and modulates serum biomarkers in patients with Alzheimer’s disease. Mol. Neurobiol. 58, 4217–4225. doi: 10.1007/s12035-021-02411-z
de Oliveira Silva, F., Ferreira, J. V., Plácido, J., Sant’Anna, P., Araújo, J., Marinho, V., et al. (2019). Three months of multimodal training contributes to mobility and executive function in elderly individuals with mild cognitive impairment, but not in those with Alzheimer’s disease: A randomized controlled trial. Maturitas 126, 28–33. doi: 10.1016/j.maturitas.2019.04.217
Deeks, J. J., Higgins, J. P. T., and Altman, D. G. (2019). “Analysing data and undertaking meta-analyses,” in Cochrane handbook for systematic reviews of interventions version 6.3, eds J. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. Page, et al. (Cochrane Library).
Devita, M., Masina, F., Mapelli, D., Anselmi, P., Sergi, G., and Coin, A. (2021). Acetylcholinesterase inhibitors and cognitive stimulation, combined and alone, in treating individuals with mild Alzheimer’s disease. Aging Clin. Exp. Res. 33, 3039–3045. doi: 10.1007/s40520-021-01837-8
Dias, S., Ades, A., Welton, N., Jansen, J., and Sutton, A. (2018). Network meta-analysis for decision making. United States, NY: Wiley.
Figuracion, K., and Lewis, F. M. (2021). Environmental enrichment: A concept analysis. Nurs. Forum. 56, 703–709. doi: 10.1111/nuf.12565
Fu, C., Jin, X., Chen, B., Xue, F., Niu, H., Guo, R., et al. (2017). Comparison of the Mini-Mental State Examination and Montreal Cognitive Assessment executive subtests in detecting post-stroke cognitive impairment. Geriatr. Gerontol. Int. 17, 2329–2335. doi: 10.1111/ggi.13069
Gates, N. J., Vernooij, R. W., Di Nisio, M., Karim, S., March, E., Martínez, G., et al. (2019). Computerised cognitive training for preventing dementia in people with mild cognitive impairment. Cochrane Datab. Systemat. Rev. 3:CD012279. doi: 10.1002/14651858.CD012279.pub2
Gibbor, L., Forde, L., Yates, L., Orfanos, S., Komodromos, C., Page, H., et al. (2021). A feasibility randomised control trial of individual cognitive stimulation therapy for dementia: Impact on cognition, quality of life and positive psychology. Aging Ment. Health 25, 999–1007. doi: 10.1080/13607863.2020.1747048
Giménez-Llort, L., Rivera-Hernández, G., Marin-Argany, M., Sánchez-Quesada, J. L., and Villegas, S. (2013). Early intervention in the 3xTg-AD mice with an amyloid β-antibody fragment ameliorates first hallmarks of Alzheimer disease. MAbs 5, 665–677. doi: 10.4161/mabs.25424
Glass, G. E. (2021). Photobiomodulation: A review of the molecular evidence for low level light therapy. JPRAS 74, 1050–1060. doi: 10.1016/j.bjps.2020.12.059
Hamblin, M. R. (2016). Shining light on the head: Photobiomodulation for brain disorders. BBA Clin. 6, 113–124. doi: 10.1016/j.bbacli.2016.09.002
Hamblin, M. R. (2018). Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem. Photobiol. 94, 199–212. doi: 10.1111/php.12864
Hashiguchi, D., Campos, H. C., Wuo-Silva, R., Faber, J., Gomes da Silva, S., Coppi, A. A., et al. (2020). Resistance exercise decreases amyloid load and modulates inflammatory responses in the APP/PS1 mouse model for Alzheimer’s disease. JAD 73, 1525–1539. doi: 10.3233/JAD-190729
Hennessy, M., and Hamblin, M. R. (2017). Photobiomodulation and the brain: A new paradigm. J. Optics 19:013003. doi: 10.1088/2040-8986/19/1/013003
Henskens, M., Nauta, I. M., van Eekeren, M., and Scherder, E. (2018). Effects of Physical Activity in Nursing Home Residents with Dementia: A Randomized Controlled Trial. Dement. Geriatr. Cogn. Disord. 46, 60–80. doi: 10.1159/000491818
Higgins, J. P., Jackson, D., Barrett, J. K., Lu, G., Ades, A. E., and White, I. R. (2012). Consistency and inconsistency in network meta-analysis: Concepts and models for multi-arm studies. Res. Synth. Methods 3, 98–110. doi: 10.1002/jrsm.1044
Higgins, J. P. T. (2019). Cochrane handbook for systematic reviews of interventions. Glasgow: Bell & Bain Ltd Press.
Hu, S., Wan, X., Li, X., and Wang, X. (2022). Aerobic exercise alleviates pyroptosis-related diseases by regulating NLRP3 inflammasome. Front. Physiol. 13:965366. doi: 10.3389/fphys.2022.965366
Huang, X., Zhao, X., Li, B., Cai, Y., Zhang, S., Wan, Q., et al. (2022). Comparative efficacy of various exercise interventions on cognitive function in patients with mild cognitive impairment or dementia: A systematic review and network meta-analysis. J. Sport Health Sci. 11, 212–223. doi: 10.1016/j.jshs.2021.05.003
Hyman, B. T., Phelps, C. H., Beach, T. G., Bigio, E. H., Cairns, N. J., Carrillo, M. C., et al. (2012). National Institute on aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer Dement. 8, 1–13. doi: 10.1016/j.jalz.2011.10.007
Jia, R. X., Liang, J. H., Xu, Y., and Wang, Y. Q. (2019). Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr. 19:181. doi: 10.1186/s12877-019-1175-2
Jung, H., Kim, S. W., Kim, M., Hong, J., Yu, D., Kim, J. H., et al. (2019). Noninvasive optical activation of FLP recombinase for genetic manipulation in deep mouse brain regions. Nat. Commun. 10:314. doi: 10.1038/s41467-018-08282-8
Kanaan, S. F., McDowd, J. M., Colgrove, Y., Burns, J. M., Gajewski, B., and Pohl, P. S. (2014). Feasibility and efficacy of intensive cognitive training in early-stage Alzheimer’s disease. Am. J. Alzheimer’s Dis. Dement. 29, 150–158. doi: 10.1177/1533317513506775
Kanters, S. (2022). Fixed- and random-effects models. Methods Mole. Biol. 2345, 41–65. doi: 10.1007/978-1-0716-1566-9_3
Karu, T. I. (2008). Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochem. Photobiol. 84, 1091–1099. doi: 10.1111/j.1751-1097.2008.00394.x
Kheradmand, A., Donboli, S., Tanjani, P. T., Farhadinasab, A., Tabeie, F., Qutbi, M., et al. (2022). Therapeutic Effects of Low-Level Laser Therapy on Cognitive Symptoms of Patients with Dementia: A Double-Blinded Randomized Clinical Trial. Photobiomodul. 40, 632–638. doi: 10.1089/photob.2021.0135
Kim, S., Kyung, T., Chung, J. H., Kim, N., Keum, S., Lee, J., et al. (2020). Non-invasive optical control of endogenous Ca(2+) channels in awake mice. Nat. Commun. 11:210. doi: 10.1038/s41467-019-14005-4
Lamb, S. E., Sheehan, B., Atherton, N., Nichols, V., Collins, H., Mistry, D., et al. (2018). Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: Randomised controlled trial. BMJ 361:k1675. doi: 10.1136/bmj.k1675
Lee, G. Y., Yip, C. C., Yu, E. C., and Man, D. W. (2013). Evaluation of a computer-assisted errorless learning-based memory training program for patients with early Alzheimer’s disease in Hong Kong: A pilot study. Clin. Interv. Aging 8, 623–633. doi: 10.2147/CIA.S45726
Liang, J. (2019). Comparison of multiple cognitive interventions of elderly adults with dementia—A bayesian network meta-analysis. Medicine 97:e10744.
Liang, J. H., Lin, L., Wang, Y. Q., Jia, R. X., Qu, X. Y., Li, J., et al. (2019). Non-pharmacological therapeutic strategy options for patients with dementia based on cognitive function-A Bayesian network meta-analysis of randomized controlled trials. Ageing Res. Rev. 56:100965. doi: 10.1016/j.arr.2019.100965
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 151, W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136
Lok, N., Buldukoglu, K., and Barcin, E. (2020). Effects of the cognitive stimulation therapy based on Roy’s adaptation model on Alzheimer’s patients’ cognitive functions, coping-adaptation skills, and quality of life: A randomized controlled trial. Perspect. Psychiatr. Care 56, 581–592. doi: 10.1111/ppc.12472
López-Ortiz, S., Valenzuela, P. L., Seisdedos, M. M., Morales, J. S., Vega, T., Castillo-García, A., et al. (2021). Exercise interventions in Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Ageing Res. Rev. 72:101479. doi: 10.1016/j.arr.2021.101479
Lu, X., Moeini, M., Li, B., Lu, Y., Damseh, R., Pouliot, P., et al. (2019). A Pilot Study Investigating Changes in Capillary Hemodynamics and Its Modulation by Exercise in the APP-PS1 Alzheimer Mouse Model. Front. Neurosci. 13:1261. doi: 10.3389/fnins.2019.01261
McDermott, O., Charlesworth, G., Hogervorst, E., Stoner, C., Moniz-Cook, E., Spector, A., et al. (2019). Psychosocial interventions for people with dementia: A synthesis of systematic reviews. Aging Ment. Health 23, 393–403. doi: 10.1080/13607863.2017.1423031
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s Disease. Neurology 34, 939–944. doi: 10.1212/wnl.34.7.939
Middelstädt, J., Folkerts, A. K., Blawath, S., and Kalbe, E. (2016). Cognitive stimulation for people with dementia in long-term care facilities: Baseline cognitive level predicts cognitive gains, moderated by depression. JAD 54, 253–268. doi: 10.3233/JAD-160181
Nagy, E. N., Ali, A. Y., Behiry, M. E., Naguib, M. M., and Elsayed, M. M. (2021). Impact of combined photo-biomodulation and aerobic exercise on cognitive function and quality-of-life in elderly Alzheimer patients with anemia: A randomized clinical trial. Int. J. Gen. Med. 14, 141–152. doi: 10.2147/IJGM.S280559
Nakano, M., Kubota, K., Hashizume, S., Kobayashi, E., Chikenji, T. S., Saito, Y., et al. (2020). An enriched environment prevents cognitive impairment in an Alzheimer’s disease model by enhancing the secretion of exosomal microRNA-146a from the choroid plexus. Brain Behav. Immun. Health 9:100149. doi: 10.1016/j.bbih.2020.100149
Nizamutdinov, D., Qi, X., Berman, M. H., Dougal, G., Dayawansa, S., Wu, E., et al. (2021). Transcranial near infrared light stimulations improve cognition in patients with dementia. Aging Dis. 12, 954–963. doi: 10.14336/AD.2021.0229
Öhman, H., Savikko, N., Strandberg, T. E., Kautiainen, H., Raivio, M. M., Laakkonen, M. L., et al. (2016). Effects of exercise on cognition: The finnish Alzheimer disease exercise trial: A randomized, controlled trial. J. Am. Geriatr. Soc. 64, 731–738. doi: 10.1111/jgs.14059
Oliveira, J., Gamito, P., Souto, T., Conde, R., Ferreira, M., Corotnean, T., et al. (2021). Virtual reality-based cognitive stimulation on people with mild to moderate dementia due to Alzheimer’s Disease: A pilot randomized controlled trial. Int. J. Environ. Res. Public Health 18:5290. doi: 10.3390/ijerph18105290
Orrell, M., Aguirre, E., Spector, A., Hoare, Z., Woods, R. T., Streater, A., et al. (2014). Maintenance cognitive stimulation therapy for dementia: Single-blind, multicentre, pragmatic randomised controlled trial. Br. J. Psychiatry 204, 454–461. doi: 10.1192/bjp.bp.113.137414
Qi, X., Nizamutdinov, D., Berman, M. H., Dougal, G., Chazot, P. L., Wu, E., et al. (2021). Gender differences of dementia in response to intensive self-administered transcranial and intraocular near-infrared stimulation. Cureus 13:e16188. doi: 10.7759/cureus.16188
Rai, H. K., Schneider, J., and Orrell, M. (2021). An individual cognitive stimulation therapy app for people with dementia and carers: Results from a feasibility Randomized Controlled Trial (RCT). Clin Interv. Aging 16, 2079–2094. doi: 10.2147/CIA.S323994
Rücker, G., and Schwarzer, G. (2015). Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 15:58. doi: 10.1186/s12874-015-0060-8
Ruiz-González, D., Hernández-Martínez, A., Valenzuela, P. L., Morales, J. S., and Soriano-Maldonado, A. (2021). Effects of physical exercise on plasma brain-derived neurotrophic factor in neurodegenerative disorders: A systematic review and meta-analysis of randomized controlled trials. Neurosci. Biobehav. Rev. 128, 394–405. doi: 10.1016/j.neubiorev.2021.05.025
Salanti, G., Higgins, J. P., Ades, A. E., and Ioannidis, J. P. (2008). Evaluation of networks of randomized trials. Stat. Methods Med. Res. 17, 279–301. doi: 10.1177/0962280207080643
Salehpour, F., Gholipour-Khalili, S., Farajdokht, F., Kamari, F., Walski, T., Hamblin, M. R., et al. (2020). Therapeutic potential of intranasal photobiomodulation therapy for neurological and neuropsychiatric disorders: A narrative review. Rev. Neurosci. 31, 269–286. doi: 10.1515/revneuro-2019-0063
Salehpour, F., Khademi, M., and Hamblin, M. R. (2021). Photobiomodulation therapy for dementia: A systematic review of pre-clinical and clinical studies. JAD 83, 1431–1452. doi: 10.3233/JAD-210029
Salehpour, F., Mahmoudi, J., Kamari, F., Sadigh-Eteghad, S., Rasta, S. H., and Hamblin, M. R. (2018). Brain photobiomodulation therapy: A narrative review. Mol. Neurobiol. 55, 6601–6636. doi: 10.1007/s12035-017-0852-4
Saragih, I. D., Tonapa, S. I., Saragih, I. S., and Lee, B. O. (2022). Effects of cognitive stimulation therapy for people with dementia: A systematic review and meta-analysis of randomized controlled studies. Int. J. Nurs. Stud. 128:104181. doi: 10.1016/j.ijnurstu.2022.104181
Serge Gauthier, C. W., Stijn Servaes, J. A. M., and Rosa-Neto, P. (2022). World Alzheimer report 2022 life after diagnosis: Navigating treatment, care and support. Canada, QC: McGill University.
Sikkes, S., Tang, Y., Jutten, R. J., Wesselman, L., Turkstra, L. S., Brodaty, H., et al. (2021). Toward a theory-based specification of non-pharmacological treatments in aging and dementia: Focused reviews and methodological recommendations. Alzheimer’s Dement. 17, 255–270. doi: 10.1002/alz.12188
Spector, A., Thorgrimsen, L., Woods, B., Royan, L., Davies, S., Butterworth, M., et al. (2003). Efficacy of an evidence-based cognitive stimulation therapy programme for people with dementia: Randomised controlled trial. Br. J. Psychiatry 183, 248–254. doi: 10.1192/bjp.183.3.248
Stemmer, R., Gräßel, E., and Schmid, M. (2019). [Individual activation of dementia sufferers in a home setting: A randomized controlled study]. Z. Gerontol. Geriatr. 52, 256–263. doi: 10.1007/s00391-018-1387-7
Sterne, J., Savoviæ, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366:l4898. doi: 10.1136/bmj.l4898
Tárraga, L., Boada, M., Modinos, G., Espinosa, A., Diego, S., Morera, A., et al. (2006). A randomised pilot study to assess the efficacy of an interactive, multimedia tool of cognitive stimulation in Alzheimer’s disease. J. Neurol. Neurosurg. Psych. 77, 1116–1121. doi: 10.1136/jnnp.2005.086074
Telenius, E. W., Engedal, K., and Bergland, A. (2015a). Effect of a high-intensity exercise program on physical function and mental health in nursing home residents with dementia: An assessor blinded randomized controlled trial. PLoS One 10:e0126102. doi: 10.1371/journal.pone.0126102
Telenius, E. W., Engedal, K., and Bergland, A. (2015b). Long-term effects of a 12 weeks high-intensity functional exercise program on physical function and mental health in nursing home residents with dementia: A single blinded randomized controlled trial. BMC Geriatr. 15:158. doi: 10.1186/s12877-015-0151-8
Tisher, A., and Salardini, A. (2019). A comprehensive update on treatment of dementia. Semin. Neurol. 39, 167–178. doi: 10.1055/s-0039-1683408
Tomoto, T., Liu, J., Tseng, B. Y., Pasha, E. P., Cardim, D., Tarumi, T., et al. (2021). One-year aerobic exercise reduced carotid arterial stiffness and increased cerebral blood flow in amnestic mild cognitive impairment. JAD 80, 841–853. doi: 10.3233/JAD-201456
Toots, A., Littbrand, H., Boström, G., Hörnsten, C., Holmberg, H., Lundin-Olsson, L., et al. (2017). Effects of exercise on cognitive function in older people with dementia: A randomized controlled trial. JAD 60, 323–332. doi: 10.3233/JAD-170014
Trebbastoni, A., Imbriano, L., Podda, L., Rendace, L., Sacchetti, M. L., Campanelli, A., et al. (2018). Cognitive training in patients with Alzheimer’s disease: Findings of a 12-month randomized controlled trial. Curr. Alzheimer Res. 15, 452–461. doi: 10.2174/1567205014666171113105044
Tsantali, E., Economidis, D., and Rigopoulou, S. (2017). Testing the benefits of cognitive training vs. cognitive stimulation in mild Alzheimer’s disease: A randomised controlled trial. Brain Impair. 18, 188–196. doi: 10.1017/BrImp.2017.6
Wakefield, J. C. (2016). Diagnostic issues and controversies in DSM-5: Return of the false positives problem. Annu. Rev. Clin. Psychol. 12, 105–132. doi: 10.1146/annurev-clinpsy-032814-112800
Wang, G., Zhou, H. H., Luo, L., Qin, L. Q., Yin, J., Yu, Z., et al. (2021). Voluntary wheel running is capable of improving cognitive function only in the young but not the middle-aged male APPSwe/PS1De9 mice. Neurochem. Int. 145:105010. doi: 10.1016/j.neuint.2021.105010
Wang, Y. Q., Jia, R. X., Liang, J. H., Li, J., Qian, S., Li, J. Y., et al. (2020). Effects of non-pharmacological therapies for people with mild cognitive impairment. A bayesian network meta-analysis. Int. J. Geriatr/Psychiatry 35, 591–600. doi: 10.1002/gps.5289
Wang, Z. Y., Yang, S. C. C., Wang Ying, L. I. H., Wang Qin, W. W., and Wang Tong, W. U. T. (2014). Effects of aerobic training on cognitive function and activities of daily living in patients with Alzheimer’s disease. Chin. J. Rehabil. Med. 29, 1151–1155.
Weuve, J., Kelsey, K. T., Schwartz, J., Bellinger, D., Wright, R. O., Rajan, P., et al. (2006). Delta-aminolevulinic acid dehydratase polymorphism and the relation between low level lead exposure and the mini-mental status examination in older men: The normative aging study. Occup. Environ. Med. 63, 746–753. doi: 10.1136/oem.2006.027417
World Health Organization (2021). Global statusreport on the public health response to dementia. Geneva: WHO.
Wu, X., Shen, Q., Chang, H., Li, J., and Xing, D. (2022). Promoted CD4(+) T cell-derived IFN-γ/IL-10 by photobiomodulation therapy modulates neurogenesis to ameliorate cognitive deficits in APP/PS1 and 3xTg-AD mice. J. Neuroinflamm. 19:253. doi: 10.1186/s12974-022-02617-5
Xu, L., Li, M., Wei, A., Yang, M., Li, C., Liu, R., et al. (2022). Treadmill exercise promotes E3 ubiquitin ligase to remove amyloid β and P-tau and improve cognitive ability in APP/PS1 transgenic mice. J. Neuroinflamm. 19:243. doi: 10.1186/s12974-022-02607-7
Xu Ying, C. J., Chen Mei, S. W., Cui Yanping, F. C., Shen Zhiqiang, W. J., and Jia Jie, W. Y. (2017). Effects of special environments on activities of daily living, cognitive function, and depressive status in patients with mild to moderate Alzheimer’s disease. Chin. J. Rehabil. Med. 32, 564–566.
Xue, B., Waseem, S., Zhu, Z., Alshahrani, M. A., Nazam, N., Anjum, F., et al. (2022). Brain-derived neurotrophic factor: A connecting link between nutrition, lifestyle, and Alzheimer’s Disease. Front. Neurosci. 16:925991. doi: 10.3389/fnins.2022.925991
Yamanaka, K., Kawano, Y., Noguchi, D., Nakaaki, S., Watanabe, N., Amano, T., et al. (2013). Effects of cognitive stimulation therapy Japanese version (CST-J) for people with dementia: A single-blind, controlled clinical trial. Aging Ment. Health 17, 579–586. doi: 10.1080/13607863.2013.777395
Yan Lanyun, W. W., Shen Feifei, Y. S., Wang Wei, W. Q., and Wang Tong, W. T. (2015). A clinical study of aerobic exercise with different training durations to intervene in mild to moderate Alzheimer’s disease. Chin. J. Rehabil. Med. 30, 771–776.
Yang, J. (2019). “Effects of cognitive stimulation therapy (CST) on cognitive function and quality of life in patients with Alzheimer’s disease (AD),” in School of nursing. ed. P. L. Meiqin (Guangzhou: Guangdong University of Pharmaceutical Sciences).
Yang, S. Y., Lee, H. C., Huang, C. M., and Chen, J. J. (2021). Efficacy of tai chi-style multi-component exercise on frontal-related cognition and physical health in elderly with amnestic mild cognitive impairment. Front. Aging 2:636390. doi: 10.3389/fragi.2021.636390
Yang, S. Y., Shan, C. L., Qing, H., Wang, W., Zhu, Y., Yin, M. M., et al. (2015). The effects of aerobic exercise on cognitive function of Alzheimer’s disease patients. CNS Neurol. Disor. Drug Targets 14, 1292–1297. doi: 10.2174/1871527315666151111123319
Zhang, Z., Shen, Q., Wu, X., Zhang, D., and Xing, D. (2020). Activation of PKA/SIRT1 signaling pathway by photobiomodulation therapy reduces Aβ levels in Alzheimer’s disease models. Aging Cell 19:e13054. doi: 10.1111/acel.13054
Zhu, G., Tong, Q., Ye, X., Li, J., Zhou, L., Sun, P., et al. (2022). Phototherapy for cognitive function in patients with dementia: A systematic review and meta-analysis. Front. Aging Neurosci. 14:936489. doi: 10.3389/fnagi.2022.936489
Keywords: dementia, cognitive, non-pharmacological therapy, network meta-analysis, randomized controlled trials
Citation: Luo G, Zhang J, Song Z, Wang Y, Wang X, Qu H, Wang F, Liu C and Gao F (2023) Effectiveness of non-pharmacological therapies on cognitive function in patients with dementia—A network meta-analysis of randomized controlled trials. Front. Aging Neurosci. 15:1131744. doi: 10.3389/fnagi.2023.1131744
Received: 30 December 2022; Accepted: 13 February 2023;
Published: 02 March 2023.
Edited by:
Ana Lloret, University of Valencia, SpainReviewed by:
Luodan Yang, South China Normal University, ChinaYing Shen, The First Affiliated Hospital of Nanjing Medical University, China
Copyright © 2023 Luo, Zhang, Song, Wang, Wang, Qu, Wang, Liu and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fujia Gao, gaofujia1983@163.com
 Guangxin Luo
Guangxin Luo Junqiu Zhang1
Junqiu Zhang1  Chengjiang Liu
Chengjiang Liu