Microglial polarization in TBI: Signaling pathways and influencing pharmaceuticals
- Department of Neurosurgery, The 904th Hospital of PLA, Medical School of Anhui Medical University, Wuxi, China
Traumatic brain injury (TBI) is a serious disease that threatens life and health of people. It poses a great economic burden on the healthcare system. Thus, seeking effective therapy to cure a patient with TBI is a matter of great urgency. Microglia are macrophages in the central nervous system (CNS) and play an important role in neuroinflammation. When TBI occurs, the human body environment changes dramatically and microglia polarize to one of two different phenotypes: M1 and M2. M1 microglia play a role in promoting the development of inflammation, while M2 microglia play a role in inhibiting inflammation. How to regulate the polarization direction of microglia is of great significance for the treatment of patients with TBI. The polarization of microglia involves many cellular signal transduction pathways, such as the TLR-4/NF-κB, JAK/STAT, HMGB1, MAPK, and PPAR-γ pathways. These provide a theoretical basis for us to seek therapeutic drugs for the patient with TBI. There are several drugs that target these pathways, including fingolimod, minocycline, Tak-242 and erythropoietin (EPO), and CSF-1. In this study, we will review signaling pathways involved in microglial polarization and medications that influence this process.
Introduction
Traumatic brain injury (TBI) occurs when the brain is hit by an external force, with a series of serious consequences. The global annual incidence of TBI exceeds 50 million individuals, and a study has said that half of the world’s population is likely to have one or more TBIs in their lifetime (Maas et al., 2017). The morbidity of TBI in China is estimated to be approximately 0.013%, which is similar to the rates reported in other countries (Jiang et al., 2019). China has the world’s largest population, which means that China would also have the most individuals who would encounter a TBI, making TBI a major public health concern in China. In another review, China was found to have approximately 770,060–890,990 new cases of TBI every year, and the average cost of patients ranges from ¥28,000 to 129,000 (Gao et al., 2020). Damage to neuronal tissues following TBI has two stages: the primary injury, which is directly caused by an external force when TBI occurs; and the secondary injury, which follows primary insult and causes tissue and cellular damage. The primary injury in TBI is largely irreversible, so the research focuses on changing the course of secondary injury. However, the pathophysiological mechanisms of the secondary injury are not well understood, including but not limited to excitotoxicity, mitochondrial dysfunction, oxidative stress, lipid peroxidation, neuroinflammation, axon degeneration, and apoptotic cell death (Ray et al., 2002). Neuroinflammation is the most intensively studied of these pathological mechanisms. In accordance with Bergold (2016) review, neuroinflammation plays a major role in traumatic brain damage, regulating the inflammatory process that can effectively treat TBI.
Microglia are cells present in the adult mammalian brain that account for 5–20% of all glial cells. They are derived from erythroid myeloid precursors in the embryonic yolk sac and are distributed within the embryonic mouse brain (Ginhoux et al., 2010). Microglia are vital to CNS homeostasis and are involved in the evolution of a variety of neurological pathophysiological states such as neuropsychiatric disorders, neurodegeneration, neuroinflammation, sterile injury responses, and infectious diseases (Nayak et al., 2014). The most important role of microglia is in neuroinflammation. Under normal conditions, microglia assume a neural-specific phenotype (Schmid et al., 2009) and retain a relative quiescent surveillance phenotype for constant monitoring of the brain parenchyma (Davalos et al., 2005). Modulation of neuroinflammation following TBI may require addressing both inflammatory pathways and facilitating repair (Corrigan et al., 2016). Within injured tissues, microglia exist in various states of activation and retain the capability to shift their functional phenotype during the inflammatory response (Stout et al., 2005). When an injury occurs, microglia activation can be divided into two processes. First, microglia polarize toward the pro-inflammatory (M1) phenotype that produces pro-inflammatory cytokines, such as TNF-α, interleukin (IL-1β, IL-12), present antigens, and express high levels of inducible NO synthase (iNOS) for NO production (Gordon and Taylor, 2005; Villalta et al., 2009). Then, microglia polarize to the M2 phenotype, expressing anti-inflammatory cytokines (IL-4, IL-10, IL-13, and TGF-β), arginase-1 (Arg-1), CD206, and chitinase-3-like-3 (Ym1 in rodents) (Colton, 2009; Henkel et al., 2009).
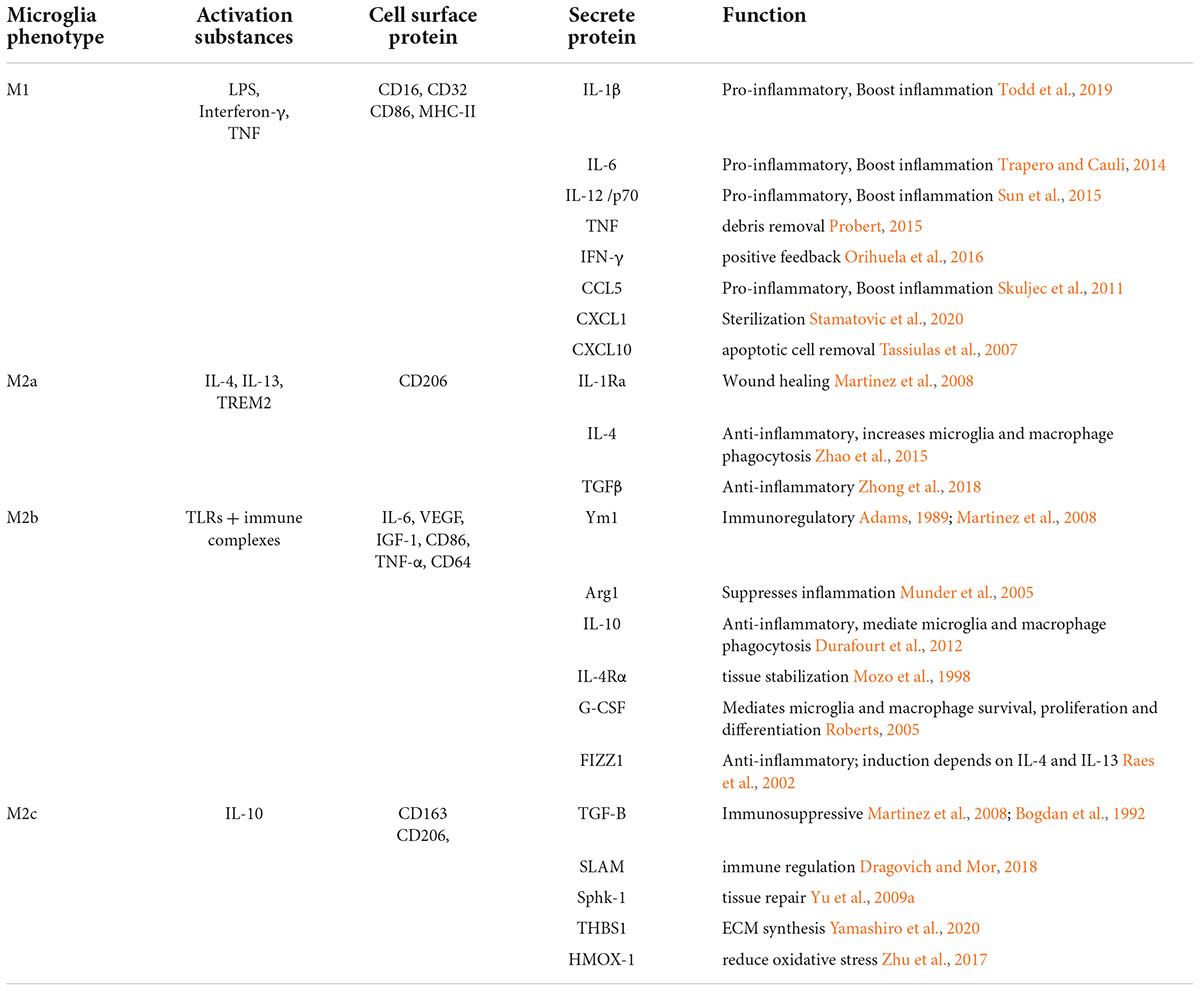
Classical activation (M1) is defined as the stimulation of microglia by external factors or elements, such as microorganisms and some cytokines, resulting in high expression of pro-inflammatory cytokines and an enhanced capacity for phagocytosis. Activating substances include two aspects, external matters, such as exogenous particles and bacteria, and endogenous matters, such as interferon-γ, TNF, and cell debris. The M1 phenotype is usually associated with the host defense against intracellular pathogens (Mackaness, 1977; Gordon and Taylor, 2005; Dale et al., 2008). The M2 phenotype is more complex (see Table 1). The M2 phenotype can be divided into three subtypes: M2a, M2b, and M2c. In the early 1990s, the concept of macrophage alternative activation was developed largely based on research showing a role for IL-4 in the induction of an alternative (M2) activation state (Stein et al., 1992) inducing the expression of anti-inflammatory cytokines IL-4, IL-10, IL-13, and TGF-β and arginase-1 (Arg-1), CD206, and chitinase-3-like-3 (Ym1 in rodents) (Colton, 2009; Henkel et al., 2009). M2 microglia play an important role in allergy response, parasite clearance, inflammatory dampening, tissue remodeling, angiogenesis, immunoregulation, and tumor promotion (Sica and Mantovani, 2012). Further studies have found that the M2a activation state is induced by parasitic products or associated signals (IL-4 and IL-13) with a longer term function for resolution and repair (Rutschman et al., 2001; Gordon and Taylor, 2005; Lawrence and Natoli, 2011; Wynn et al., 2013). In this case, the IL-4 receptor and its downstream molecules lead to the inhibition of nuclear factor kappa B (NF-κB) signaling and subsequent inhibition of M1 phenotype activation. M2b polarization is observed with the triggering of Fc-γ receptors, TLRs, and immune complexes (Murray et al., 2014). M2c polarization occurs in response to specific anti-inflammatory factors such as IL-10, TGF-β, and glucocorticoids (Vodovotz et al., 1993; Gordon and Taylor, 2005; Martinez et al., 2008). The alternative activation of microglia has been proposed for 40 years, but there is crosstalk among the subtypes of M2 phenotype, indicating that the polarization subtypes and functions of M2 microglia subtypes have not been fully understood so far. A recent study showed that energy metabolism plays an important role in the process of microglial polarization (Ghosh et al., 2018). Energy metabolism in microglia is mainly supplied by the tricarboxylic acid cycle (TCA). When microglia are induced to move toward the M1 phenotype by the external microenvironment, they will inhibit mitochondrial oxidative phosphorylation (OXPHOS) (Baseler et al., 2016). In contrast, “alternatively activated” microglia adopt a metabolic program dominated by fatty acid-fueled OXPHOS. More studies focus on how to facilitate the two processes to regulate microglial polarization. Compared with serum-cultured microglia, microglia cultured in a defined medium performed less well after the same stimulation, which was related to their metabolic state (Montilla et al., 2020). However, how metabolic states affect microglial polarization levels by regulating related cellular signaling pathways has not been clearly explained. Some researchers have focused on the mechanistic target of the rapamycin (mTOR) pathway. mTOR inhibition led to decreased production of LPS-induced pro-inflammatory cytokines by suppressing glycolysis (Hu et al., 2020). mTOR receptor inhibits the classical polarization of M1 microglia by enhancing anaerobic glycolysis in microglia and weakening mitochondrial function. Now, more and more studies have shown that microglia are not simply divided into M1 and M2 types, but also include other types, such as rod microglia. In addition to the classic M1/M2 classification of microglia, rod-shaped microglia, which were first introduced in 1899 (Au and Ma, 2017), are the current focus of research. Despite 100 years of study, little is known about the specific functions of rod-shaped microglia due to the lack of technology to cultivate rod-shaped microglia in vitro. Rod microglia, also called bipolar microglia, were observed in a single trajectory with their highly polarized processes seemingly connected (Ziebell et al., 2012). In a recent study, rod microglia were identified as an abundance of iba1-positive microglia with phagocytosis. With aging society as an aggravating factor, individuals with AD place considerable pressure on the healthcare systems in society. Due to its contribution to the development of AD, rod microglia are now the focus of several studies (Bachstetter et al., 2015). A cost-effective and highly reproducible method has been established to enrich bipolar/rod-shaped microglia in vitro (Tam and Ma, 2014; Tam et al., 2016), leading to accelerated research on these cells over the past few years. In the optic nerve transection (ONT) model, the appearance of rod microglia is not only closely related to injury time but is also related to the cortical position (Yuan et al., 2015). The distribution density of rod-shaped microglia differs in different cortices, which may be related to the different phenotypes of static microglia induced by specific microenvironment in different parts of the brain after injury. The process by primitive microglia are transformed into rod microglia is mediated by the granulocyte-macrophage colony stimulating factor (GM-CSF) and intracellular calcium concentration ([Ca2+]i) of microglial cells (Suzumura et al., 1990; Frei et al., 1992). In the ONT model, rod microglia showed strong phagocytosis, and rod-shaped microglia disappeared after tissue fragments were removed (Yuan et al., 2015). Therefore, cell debris may also be factors inducing the formation of rod microglia. The development of new therapeutic interventions by switching the microglial phenotype from amoeboid to rod microglia might shed new light on pathogenesis and identify targets for treating neurodegenerative diseases. As mentioned above, in addition to the classical and alternative pathways, there are other pathways involved in microglia activation. Therefore, the current international description of the polarization state of microglia focuses more on its function, following which they are classified into “pro-inflammatory” (or the M1) phenotype and the “anti-inflammatory” (or the M2) phenotype. Different microglial subtypes are dominant between 1 and 3 weeks after injury (Jin et al., 2012). Indeed, the M2 phenotype peaks at 1 week of TBI, decreases thereafter, and returns to normal level within 4 weeks. However, the M1 phenotype increases 4 weeks after injury. Different results were reported by Kumar et al. (2016) who found that M1 and M2 phenotypes were activated after TBI, but the M2 phenotype was replaced by the M1 phenotype at 7 day post-injury. This shift toward the M1 phenotype was associated with increased neurodegeneration. The focus of this study is to identify the process so that the transformation could be prevented or slowed down, as well as to prolong the expression of M2 microglia. Here, we organized a number of cellular signaling pathways that influence the transformation of microglial phenotypes.
Cell signaling pathways that influence microglial polarization
Microglia play a very important role in neuroinflammation, and many signaling pathways are involved in their polarization. Clarifying the interaction between these pathways and their influence on microglia is very important for regulating the polarization of microglia, while also providing a basis for the development of therapeutical drugs. Here, we summarize some of the classical and important signaling pathways involved in microglial polarization.
Toll-like receptor-4/NF-κB pathway
A toll-like receptor (TLR) is a pattern-recognition receptor that detects microbial components, and the TLR family consists of 13 members (Takeda and Akira, 2015). TLR-4 is expressed in microglia, astrocytes, and macrophages in the brain, and can be activated by LPS (Badshah et al., 2016). NF-κB is a key regulator of immune development, immune responses, and inflammation (Mitchell et al., 2016). The canonical pathway of the NF-κB pathway is triggered by TLR, and RelA is an activating substance between them. The pathway function is to regulate the expression of pro-inflammatory and cell survival genes (Karin et al., 2006; Lawrence, 2009).
In microglia, TLR-4/NF-κB is a traditional transcription factor that is activated by lipopolysaccharide (LPS) and regulates the expression of most M1-signature genes encoding pro-inflammatory cytokines (Taetzsch et al., 2015). Polarization of microglia in the direction of the M1 phenotype induced by the TLR-4 signaling pathway leads to damage to white matter tracts in the corpus callosum (Yang et al., 2018). In another study, the development of PD-associated neuroinflammation was reduced in TLR-4-deficient Parkinson’s disease (PD) mouse models compared with normal mice without PD (Campolo et al., 2019). TLR-4 could be considered an encouraging therapeutic target in neurodegenerative disease.
The diagnosis and treatment of patients with TBI now focus on inhibiting the TLR-4/NF-κB pathway. For instance, the viral inhibitory peptide of TLR-4 (VIPER) interacts with the myeloid differentiation factor 88 (MyD88) adaptor-like (Mal) and TRIF-related adaptor molecule (TRAM) to inhibit the TLR-4/NF-κB pathway and attenuate microglia activation (Lysakova-Devine et al., 2010; Masson et al., 2015). Vascular endothelial growth inhibitor (VEGI) could alleviate the post-traumatic excessive inflammatory response and remit the secondary brain damage by downregulating the expression of the TLR-4/NF-κB signaling pathway and inflammatory cytokines (Gao et al., 2015). Therefore, the TLR-4/NF-κB pathway can be said to promote M1-type polarization after TBI.
Janus kinase/signal transducers and activators of transcription pathway
The Janus Kinase/Signal Transducers and Activators of Transcription (JAK/STAT) signaling pathways have been recognized as one of the most important pathways in mediating innate and adaptive immunity (O’Shea and Plenge, 2012). The JAK family has four main members with over 1,000 amino acids each, and their molecular weights range from 120 to 140 kDa (Cai et al., 2015). The STAT family in the cytoplasm is a downstream target of JAKs, which consists of seven members with molecular weights ranging from 79 to 113 kDa (Darnell, 1997; Boengler et al., 2008; Yu et al., 2009b). When JAK is attached to the ligand, STAT is phosphorylated, dimerized, and transported into the nucleus to regulate the expression of related genes. The JAK/STAT pathway is well known to modulate various signals to maintain homeostasis in inflammatory conditions. It induces neuroinflammatory diseases such as PD and multiple sclerosis (MS) by modulating microglial polarization (Xin et al., 2020). The phenotype of microglia changes to M2 from M1 after the JAK/STAT signaling pathway phosphorylation process is inhibited, and the microglia then produce fewer inflammatory cytokines (Qu et al., 2019).
CNS homeostasis is disrupted for which microglia are overactivated, leading to an inflammatory storm after TBI. The severity of this inflammatory storm can be regulated via the JAK/STAT pathway to improve the prognosis of TBI. JAK2/STAT1 is a pro-apoptotic pathway that upregulates the expression of Fas, FasL, and IRF-1 and inhibits the anti-apoptotic NF-κB (Chin et al., 1997; Kumar et al., 1997; Wang et al., 2000). Additionally, this pathway promotes the polarization of microglia into the M1 phenotype (Porro et al., 2019). JAK2/STAT1 activation induces the expression of genes encoding IL-1β, TNF, and CXC motif chemokine 10 (CXCL10), indicating that the JAK2/STAT1 pathway promotes the polarization of macrophages into the M1 phenotype (Lawrence and Natoli, 2011). When the JAK2/STAT3 pathway was activated by paraquat, microglia were found to polarize into the M1 phenotype, consequently causing inflammatory damage to the hippocampus (Fan et al., 2022). Unlike this, the JAK2/STAT6 pathway promotes polarization of microglia to the M2 phenotype (Yang et al., 2017). The effect of the JAK/STAT family on microglia is not fully understood; thus, further studies are needed to clarify the different JAK/STAT pathways that could influence the polarization of microglia and the therapeutic needs of TBI.
HMGB1
High mobility group box 1 (HMGB1) is a non-histone nuclear protein with high electrophoretic mobility of 215 amino acids. HMGB1 was first described by Goodwin and Johns (1977). It is estimated that each nucleus contains approximately 1 × 106 HMGB1 molecules, which is only just lower than the core histone (Romani et al., 1979). The function of HMGB1 in the nucleus is DNA-binding activity, DNA chaperone, and DNA-bending activity (Kang et al., 2014). Extracellular HMGB1 functions as an immune adjuvant to trigger a robust response to activation or suppression of cells including T cells, macrophagocytes, and dendritic cells.
HMGB1 plays an important role in regulating the systemic inflammatory response in infectious diseases, and the serum level of HMGB1 in patients with sepsis is elevated (Wang et al., 1999). Current studies show that the regulation of HMGB1 on local inflammation can effectively change the occurrence, development, and prognosis of diseases such as stroke, TBI, PD, epilepsy, and Alzheimer’s disease (AD) via the regulation of microglial polarization (Nishibori et al., 2019). Paudel et al’s paper discussed the contribution of the HMGB1/TLR4/RAGE signaling pathway in TBI and other neuroinflammatory diseases, arguing the possibilities of HMGB1 as a common viable biomarker of TBI (Paudel et al., 2018).
A previous study showed that early treatment with anti-HMGB1 monoclonal antibody (m-Ab) might be a promising strategy for TBI (Okuma et al., 2012). One in vitro study demonstrated that HMGB1 induced the polarization of microglia toward the pro-inflammatory phenotype (Fan et al., 2020b). The study also demonstrated that inhibiting the HMGB1-RAGE axis prevented pro-inflammatory microglial polarization and afforded neuroprotection after SCI in rats. Li et al. (2020) found that the HMGB1 inhibitor BAI could improve acute neurocognitive impairment by HMGB1-mediated inhibition of neuroinflammation in LPS-induced mice.
Members of the mitogen-activated protein kinases signaling pathway
Members of the mitogen-activated protein kinases (MAPKs) family are typically activated by various mitogenic agents such as growth factors and hormones. This family plays an important role in regulating cell division and differentiation (Gustin et al., 1998). The MAPK family, including extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase, is a group of signaling molecules that plays an important role in the expression of pro-inflammatory cytokines (Liu et al., 2020). NF-κB is a downstream molecule of MAPK. MAPK/NF-κB pathway plays an important role in regulating the release of inflammatory mediators (Deng et al., 2018).
The MAPK/NF-κB pathway has been shown to be involved in the production of pro-inflammatory mediators in LPS-treated BV2 cells (Do et al., 2020). In spinal cord injury, when this pathway is activated, it induces the production of pro-inflammatory cytokines, including IL-6, TNF-α, or IL-1β, from microglial cells, which is indicative of microglial polarization toward the M1 phenotype (Liu et al., 2020). Methionine sulfoxide reductase A (MsrA) is an enzyme that plays a role in demyelination and has been shown to suppress inflammatory activation of microglia and oxidative stress via inhibition of the MAPK/NF-κB signaling pathway (Fan et al., 2020a).
In the CCI mice model, bazedoxifene was found to protect cerebral cognitive functions after TBI and attenuate impairments in blood-brain barrier (BBB) damage by blocking the MAPK/NF-κB signaling pathway (Lan et al., 2019). Research tends to focus almost entirely on using drugs to suppress one specific inflammatory signaling pathway, but one interesting phenomenon is that splenectomy in TBI mice can downregulate the MAPK/NF-κB signaling pathway, thereby inhibiting the polarization of microglia to the M1 phenotype (Chu et al., 2013). Together, these results support a potential role for the MAPK/NF-κB signaling pathway in the modulation of microglial polarization after TBI.
Peroxisome proliferation-activated receptors-gamma pathway
Peroxisome proliferation-activated receptors (PPARs) are nuclear hormone receptors that directly bind and respond to ligands such as steroids, thyroid hormone, retinoids, cholesterol by-products, and lipids (Chandra et al., 2008). PPARs are composed of three isoforms: PPAR-α, PPAR-β/δ, and PPAR-γ (Berger and Moller, 2002). These receptors contain poorly conserved A/B regions that, in some cases, act as potent transcriptional activators, provide sites of protein phosphorylation, or form direct interactions with other receptor domains or regulatory proteins (Bain et al., 2007). These three isotypes differ from each other in terms of their tissue distribution, ligand specificities, and physiological roles. PPAR-γ is highly expressed in white and brown adipose tissue, and it plays a key role in the regulation of adipogenesis, energy balance, and lipid biosynthesis (Lehrke and Lazar, 2005; Medina-Gomez et al., 2007).
It was previously thought that this receptor only participates in lipoprotein metabolism; however, a recent study showed that PPAR-γ also participates in the regulation of microglial polarization (Zhou et al., 2020). Why does a receptor that is primarily found in adipose tissue cells exert a regulatory effect on microglia? in an article by Fujisaka et al., The M1-to-M2 ratio was increased by a high-fat-diet and decreased by subsequent pioglitazone, PPAR-γ agonist, treatment (Fujisaka et al., 2009). This indicates that lipid metabolism can affect microglial polarization, but its internal molecular mechanisms need more research. It is confirmed that PPAR-γ agonists can be a promising therapy for PD because it suppresses the M1 phenotype and production of pro-inflammatory cytokines (Carta and Pisanu, 2013).
A research team at Zhejiang University, China, was among those who studied the role of PPAR-γ in TBI. They confirmed that axonal injury after TBI can be alleviated by PPAR-γ agonists, which induced microglia polarized to the M2 phenotype (Wen et al., 2018). In another study using a mouse model, Jiang et al., found that the Chinese herb phyllyrin inhibits inflammation of microglia via the PPAR-γ signaling pathway, protecting mice from TBI (Jiang et al., 2020). As discussed above, microglia are polarized to the M1 phenotype when anaerobic glycolysis increases within the cell; thus, the inhibition of microglia via the PPAR-γ signaling pathway may be related to the increase in aerobic glucose metabolism. Further investigations are needed to determine the validity of the PPAR-γ pathway in the polarization of microglia.
Specific inhibitors/agonists targeting specific signaling
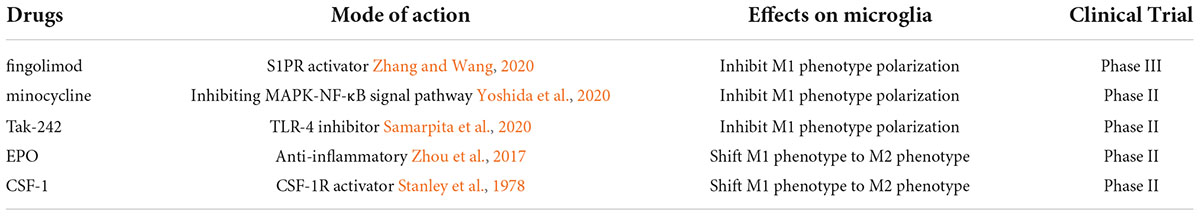
Currently, several promising anti-TBI drugs are undergoing clinical trials (Table 2). Most exert neuroprotective effects by inhibiting M1 phenotype microglia or enhancing M2 phenotype microglia. In this review, we emphasized the importance of the M2 phenotype in microglial responses following TBI, and treatment strategies with a focus on modulating or enhancing microglia with the M2 phenotype.
Fingolimod
Fingolimod (FTY720, R,2-amino-2[2-(4-octylphenyl) ethyl]-1,3-propanediol)), sold under the brand name Gilenya, was originally synthesized by the Japanese chemist Tetsuro Fujita (Huwiler and Zangemeister-Wittke, 2018). It is a high-affinity agonist of sphingosine-1-phosphate (SP1) receptor (Fujita et al., 1996). SP1 is well known to be involved in immune regulation in the body (Obinata and Hla, 2019). Therefore, FTY720 is involved in immunoregulation.
When FTY720 was synthesized, it was first applied to solid organ transplantation for its function as an agonist of SP1, which regulates inflammatory processes (Napoli, 2000). A growing body of evidence suggests that FTY720 is neuroprotective in CNS injuries due to its effects on the improvement of cognitive function, protection of BBB function, inhibition of apoptosis and inflammation, suppression of oxidative stress, and regulation of autophagy [87]. FTY720 has been used in various models of stroke and neurological disorders (Carreras et al., 2019; Wang et al., 2020; Rajabi et al., 2021).
Fingolimod is the first FDA-approved drug for the treatment of multiple sclerosis (MS) (Brunkhorst et al., 2014). Now, more and more scientists focus on its role in TBI. Consecutive administration of FTY720 for 3 days in a CCI mouse model was found to improve neurological functions and modulate multiple immune responses by attenuating the generally activated microglia and augmenting the M2/M1 ratio accompanied by decreased axonal damage (Gao et al., 2017). On the contrary, in a C57BL/6 mouse model of focal cortical cryo-lesion injury, FTY720 attenuated neuroinflammation but did not alter the lesion size or affect functional recovery (Mencl et al., 2014). These contradictory results may be because of the different types of brain injury in the above studies. There are also some contradictory results regarding the signaling pathway involved in FTY720. FTY720 has been found to significantly transform pro-inflammatory microglia into anti-inflammatory microglia by suppressing autophagy via STAT1 (Hu et al., 2021). Qin et al. found that FTY720 facilitated M1 to M2 switch of microglia via the STAT3 pathway (Qin et al., 2017). FTY720 does mitigate post-traumatic neuroinflammatory responses via a variety of signaling pathways.
Minocycline
Minocycline was first introduced in 1967 as a second-generation tetracycline derivative (Jonas and Cunha, 1982). It has a wide spectrum of activity against both gram-positive and gram-negative bacteria (Garrido-Mesa et al., 2013). Minocycline shares the basic four-ring structure of the other commonly used tetracyclines, having as its chemical characteristic the substitution of a dimethylamino group in the seventh position (Allen, 1976). It is usually prepared as dihydrated hydrochloride and, in that form, has a molecular weight of 530.
Obviously, minocycline is a broad-spectrum antibiotic, but how does it play an anti-inflammatory role? Some proposed mechanisms for its anti-inflammatory properties include the inhibitory effects on the activities of key enzymes, like iNOS and MMPs (Garrido-Mesa et al., 2013). In particular, minocycline has been shown capable of inhibiting the M1 polarization state of microglia in the CNS via inhibition of NF-κB and via interference of the MAPK pathways (Kobayashi et al., 2013). Minocycline also promotes M2 microglial polarization via upregulating the TrkB/BDNF pathway after intracerebral hemorrhage (ICH)(Miao et al., 2018). In addition, the minocycline could shift the activated M1 microglia phenotype into the M2 phenotype.
Minocycline significantly reduced impairments of spatial learning and memory in the water maze test after TBI in mouse models (Lam et al., 2013). The effects of minocycline treatment in an animal model of TBI revealed that the protective effects could be detected in a short term after injury (3 days after injury) but not in long-term therapy (7 days after injury) (Hanlon et al., 2016). Further experiments confirmed that minocycline is ineffective in reducing microglial activation and ameliorating injury-induced deficits following repetitive neonatal traumatic brain injury. However, in mild blast-induced TBI (mb-TBI) models, acute minocycline treatment appears to prevent the development of neurobehavioral abnormalities likely through normalizing damage markers like NSE, NF-H, Tau, S100β, and glial markers of the injury (Kovesdi et al., 2012). In clinical trials, minocycline is beneficial in patients with moderate-to-severe TBI, but the therapeutic effect did not increase with the dose of minocycline administered (Meythaler et al., 2019). As it is an old pharmaceutical agent, further research on the newer applications, time and method of administration of minocycline is required.
TAK-242
TAK-242 (ethyl (6R)-6-[N-(2-chloro-4-fluorophenyl) sulfamoyl] cyclohex-1-ene-1-carboxylate) is a small molecule that selectively binds to TLR-4, thereby inhibiting TLR-4 signal transduction (Sha et al., 2007). TAK-242 binds selectively to Cys747, which is the intracellular domain of TLR-4 (Matsunaga et al., 2011). It is well known that TLR-4 plays an important role in inflammation, so its antagonist TAK-242 is first applied in anti-inflammation. TAK-242 has a lower molecular weight and liposoluble capacity, which allows it to cross the BBB (Hua et al., 2015).
TAK-242 has become a focus drug for neuroinflammatory disease due to its special molecular structure and anti-inflammatory effects. In AD mice models, TAK-242 administration significantly improved neurological function and increased the expression levels of the M2 phenotype of microglia. The mechanism of the phenomenon may be that TAK-242 modulates MyD88/NF-κB/NLRP3, which is the downstream signaling pathway of TLR-4 (Cui et al., 2020). Neuroinflammation contributes to the progression of amyotrophic lateral sclerosis (ALS). The microglial reaction is attenuated in TAK-242-treated mice (Fellner et al., 2017). Further research showed that TAK-242 also reduces spinal motor neuron loss in ALS; however, this effect did not result in an increased survival rate.
There is evidence indicating that TAK-242 is beneficial to TBI. Pre-injury treatment of mice with TAK-242 significantly enhances cognitive functional recovery after TBI by inhibiting autophagy and neuroinflammation (Feng et al., 2017). Further experiments confirmed that TAK-242 mainly inhibited the TLR4-MyD88/TRIF-NF-κB signaling pathway to exert anti-neuroinflammatory activity. Ischemia/reperfusion (I/R) injury is a mechanism of brain injury after TBI (Hua et al., 2015). TAK-242 mitigates I/R injury by inhibiting the TLR-4 pathway, which is associated with microglial polarization to the M1 phenotype (Bell et al., 2013). The effects of TAK-242 on patients with TBI have a wide therapeutic time window which is from 4 hours to 5 days after injury (Zhang et al., 2014). However, all experimental results are from animal experiments; therefore, more clinical trials are needed to validate these findings.
Erythropoietin
Erythropoietin is a well-known plasma factor that stimulates erythrocyte production and was first purified in 1977 (Witts, 1961; Miyake et al., 1977). EPO is mainly produced by interstitial cells in the adult kidney in response to hypoxia (Miyake et al., 1977; Kobayashi et al., 2017). In the late 1980s, recombinant human EPO (rh-EPO) became available for clinical use, revolutionizing the management of renal anemia. Now, more and more functions of EPO have been discovered, especially in neuroprotection (Hemani et al., 2021).
Erythropoietin significantly reduced brain tissue loss volume, ameliorated white matter injury, and improved neurobehavioral outcomes after ischemic stroke (Wang et al., 2017). The authors also demonstrated a shift from the M1 phenotype to the M2 phenotype at the infarct border after EPO treatment could attenuate brain injury. In a randomized, prospective clinical trial, it was confirmed that repeated, low-dose, rh-EPO treatment reduced the risk of disability for infants with moderate hypoxic-ischemic encephalopathy (HIE) without apparent side effects (Zhu et al., 2009).
The EPO receptor was shown to be highly expressed on the surface of microglia after brain injury, suggesting its role in the brain after an injury (Spandou et al., 2004). Reactive microglia are particularly effective at producing and releasing ROS/RNS (Block et al., 2007). Treatment with EPO activates the Akt/mTOR/NF-κB pathway, which is implicated in shifting macrophage activation state polarization from M1 to M2 (Xu et al., 2013). There is also evidence that EPO modulates neuroinflammation by decreasing levels of ROS/RNS, limiting microglial infiltration by preserving the health of the microvascular endothelial cells at the BBB (Bond and Rex, 2014). However, current clinical trial studies focus too much on the effectiveness and safety of EPO, but there is no corresponding clinical trial evidence on the method and time of administration.
Colony-stimulating factor 1
CSF-1 (Colony-stimulating factor 1, namely Macrophage colony-stimulating factor) is the primary growth factor required for the control of monocyte and macrophage differentiation (Sehgal et al., 2021). The CSF-1 was initially purified from human urine in Stanley et al. (1975). Similar to rh-EPO, rh-CSF-1 was synthesized from the c-DNA of CSF-1 in the 1980s. CSF-1 has not been found substantial clinical application, but CSF-1 administration promotes microglia infiltration, differentiation, clearance of damaged cells and repair (Stanley et al., 1978). Therefore now scientists are interested in its use in neurological diseases.
The specific role of CSF-1 in microglia polarization is unclear, but it is known that CSF-1 significantly promotes proliferation and differentiation of microglia (M1, M2, or other types) (Stanley and Chitu, 2014). CSF-1 usually interacts with other molecules on microglia to enhance its role in controlling the direction of microglia polarization. This effect is similar to that of glucocorticoids that enhance the vasoconstriction of catecholamines by permissive action. CSF-1 upregulates TLR-4 and CD14 expression in microglia through ERK1/2 and p38, and thus promotes the LPS-induced microglia polarizing to proinflammatory phenotype (Parajuli et al., 2012). When CSF-1 is inhibited, this “permissiveness” effect is mitigated, reducing the inflammatory response. The inhibition of colony-stimulating factor 1 receptor (CSF-1R) exerted neuroprotection in ischemia cerebral stroke mice model through inhibiting microglia M1 polarization and NLRP3 pathway and increasing the balance function of injured mice (Liu et al., 2020).
Colony-stimulating factor 1 also plays an important role in TBI. An experiment by Li et al. (2020) showed that both immediate administration (within 24 h after injury) and the sequelae stage (3 months after injury) could effectively improve the recovery of cognitive function in m-TBI mice (Rajabi et al., 2021). Further studies showed that microglia activity was inhibited after drug administration, but the changes in microglia function were not elaborated. A previously published paper showed that eliminating chronically activated microglia by inhibitors of the CSF-1R could improve long-term neurological function after TBI (Henry et al., 2020). This experiment further indicated that if the inflammatory response of microglia after TBI could be precisely controlled, the therapeutic window of TBI will be greatly increased. As a cytokine targeting microglia, CSF-1 needs more basic and clinical studies on how to better apply it in clinical practice.
Discussion
Traumatic brain injury has become a major health and socioeconomic problem worldwide. Primary injuries in TBI are largely irreversible, so the secondary damage stage becomes the only way to administer therapeutic measures. Neuroinflammation is the most important mechanism in secondary injury, and microglia play an important role in neuroinflammation. After microglial polarization, the cell phenotype changes, and its functions also change significantly. In this review, we have summarized that polarization of microglia to the M1 phenotype contributes to secondary damage after TBI, and M2 phenotype microglia aid in recovery from TBI. How to control the directions of polarization of microglia after TBI is an important consideration in the treatment of patients with TBI. We further summarized the cell-signaling pathways that were involved in microglial polarization after TBI, which provides a theoretical basis for further research and development of drugs targeting microglial polarization. In this paper, we also summarized some newly developed drugs and some new usages of old drugs that mainly inhibit the polarization of M1 microglia in the treatment of clinical patients. However, it is well known that M1 microglia are beneficial for a short period of time after TBI. Therefore, how to use existing clinical measures to detect when to use drugs to inhibit M1 microglia has become a current hotly discussed and difficult consideration, and more experiments are needed to put forward feasible measures in this aspect.
Author contributions
Y-FL: literature collection and manuscript writing. XR: literature collection and manuscript writing. LZ: literature collection and manuscript writing. TC: validation, writing – review and editing, and supervision. Y-HW: Supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82072168 and 81871589), the Natural Science Foundation of Jiangsu Province (No. BK20211044), the Major Scientific Research Project of Wuxi Health Commission (No. Z202001), the top talent support program for young and middle-aged people of Wuxi health committee (BJ2020118), the Translational Medicine Research Major Project of Wuxi Health Commission (No. ZH201901), the China Postdoctoral Science Foundation funded project (No. 2019M651803), the Key Scientific Research Project of Jiangsu Health Commission (No. K2019018), the Social Development Science and Technology Demonstration Project of Wuxi (N20201008), and the Logistics Scientific Research Project of PLA (No. CLB20J027).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, D. O. (1989). Molecular interactions in macrophage activation. Immunol. Today 10, 33–35. doi: 10.1016/0167-5699(89)90298-3
Au, N. P. B., and Ma, C. H. E. (2017). Recent advances in the study of bipolar/rod-shaped microglia and their roles in neurodegeneration. Front. Aging Neurosci. 9:128. doi: 10.3389/fnagi.2017.00128
Bachstetter, A. D., Van Eldik, L. J., Schmitt, F. A., Neltner, J. H., Ighodaro, E. T., Webster, S. J., et al. (2015). Disease-related microglia heterogeneity in the hippocampus of Alzheimer’s disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol. Commun. 3:32. doi: 10.1186/s40478-015-0209-z
Badshah, H., Ali, T., and Kim, M. O. (2016). Osmotin attenuates LPS-induced neuroinflammation and memory impairments via the TLR4/NFkappaB signaling pathway. Sci. Rep. 6:24493. doi: 10.1038/srep24493
Bain, D. L., Heneghan, A. F., Connaghan-Jones, K. D., and Miura, M. T. (2007). Nuclear receptor structure: implications for function. Annu. Rev. Physiol. 69, 201–220. doi: 10.1146/annurev.physiol.69.031905.160308
Baseler, W. A., Davies, L. C., Quigley, L., Ridnour, L. A., Weiss, J. M., Hussain, S. P., et al. (2016). Autocrine IL-10 functions as a rheostat for M1 macrophage glycolytic commitment by tuning nitric oxide production. Redox Biol. 10, 12–23. doi: 10.1016/j.redox.2016.09.005
Bell, M. T., Puskas, F., Agoston, V. A., Cleveland, J. C., Freeman, K. A., Gamboni, F., et al. (2013). Toll-Like receptor 4-Dependent microglial activation mediates spinal cord ischemia-reperfusion injury. Circulation 128, S152–S156. doi: 10.1161/CIRCULATIONAHA.112.000024
Berger, J., and Moller, D. E. (2002). The mechanisms of action of PPARs. Annu. Rev. Med. 53, 409–435. doi: 10.1146/annurev.med.53.082901.104018
Bergold, P. J. (2016). Treatment of traumatic brain injury with anti-inflammatory drugs. Exp. Neurol. 275(Pt 3), 367–380. doi: 10.1016/j.expneurol.2015.05.024
Block, M. L., Zecca, L., and Hong, J. S. (2007). Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57–69. doi: 10.1038/nrn2038
Boengler, K., Hilfiker-Kleiner, D., Drexler, H., Heusch, G., and Schulz, R. (2008). The myocardial JAK/STAT pathway: from protection to failure. Pharmacol. Ther. 120, 172–185. doi: 10.1016/j.pharmthera.2008.08.002
Bogdan, C., Paik, J., Vodovotz, Y., and Nathan, C. (1992). Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J. Biol. Chem. 267, 23301–23308. doi: 10.1016/S0021-9258(18)50091-0
Bond, W. S., and Rex, T. S. (2014). Evidence that erythropoietin modulates neuroinflammation through differential action on neurons, astrocytes, and microglia. Front. Immunol. 5:523. doi: 10.3389/fimmu.2014.00523
Brunkhorst, R., Vutukuri, R., and Pfeilschifter, W. (2014). Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front. Cell Neurosci. 8:283. doi: 10.3389/fncel.2014.00283
Cai, B., Cai, J. P., Luo, Y. L., Chen, C., and Zhang, S. (2015). The specific roles of JAK/STAT signaling pathway in sepsis. Inflammation 38, 1599–1608. doi: 10.1007/s10753-015-0135-z
Campolo, M., Paterniti, I., Siracusa, R., Filippone, A., Esposito, E., and Cuzzocrea, S. (2019). TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson’s diseases in vivo model. Brain Behav. Immun. 76, 236–247. doi: 10.1016/j.bbi.2018.12.003
Carreras, I., Aytan, N., Choi, J. K., Tognoni, C. M., Kowall, N. W., Jenkins, B. G., et al. (2019). Dual dose-dependent effects of fingolimod in a mouse model of Alzheimer’s disease. Sci. Rep. 9:10972. doi: 10.1038/s41598-019-47287-1
Carta, A. R., and Pisanu, A. (2013). Modulating microglia activity with PPAR-gamma agonists: a promising therapy for Parkinson’s disease? Neurotox Res. 23, 112–123. doi: 10.1007/s12640-012-9342-7
Chandra, V., Huang, P., Hamuro, Y., Raghuram, S., Wang, Y., Burris, T. P., et al. (2008). Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature 456, 350–356. doi: 10.1038/nature07413
Chin, Y. E., Kitagawa, M., Kuida, K., Flavell, R. A., and Fu, X. Y. (1997). Activation of the STAT signaling pathway can cause expression of caspase 1 and apoptosis. Mol. Cell. Biol. 17, 5328–5337. doi: 10.1128/MCB.17.9.5328
Chu, W., Li, M., Li, F., Hu, R., Chen, Z., Lin, J., et al. (2013). Immediate splenectomy down-regulates the MAPK-NF-kappaB signaling pathway in rat brain after severe traumatic brain injury. J. Trauma Acute Care Surg. 74, 1446–1453. doi: 10.1097/TA.0b013e31829246ad
Colton, C. A. (2009). Heterogeneity of microglial activation in the innate immune response in the brain. J. Neuroimmune Pharmacol. 4, 399–418. doi: 10.1007/s11481-009-9164-4
Corrigan, F., Mander, K. A., Leonard, A. V., and Vink, R. (2016). Neurogenic inflammation after traumatic brain injury and its potentiation of classical inflammation. J. Neuroinflamm. 13:264. doi: 10.1186/s12974-016-0738-9
Cui, W., Sun, C., Ma, Y., Wang, S., Wang, X., and Zhang, Y. (2020). Inhibition of TLR4 induces M2 microglial polarization and provides neuroprotection via the NLRP3 inflammasome in Alzheimer’s disease. Front. Neurosci. 14:444. doi: 10.3389/fnins.2020.00444
Dale, D. C., Boxer, L., and Liles, W. C. (2008). The phagocytes: neutrophils and monocytes. Blood 112, 935–945. doi: 10.1182/blood-2007-12-077917
Darnell, J. E. Jr. (1997). STATs and gene regulation. Science 277, 1630–1635. doi: 10.1126/science.277.5332.1630
Davalos, D., Grutzendler, J., Yang, G., Kim, J. V., Zuo, Y., Jung, S., et al. (2005). ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758. doi: 10.1038/nn1472
Deng, Y., Tang, K., Chen, R., Liu, Y., Nie, H., Wang, H., et al. (2018). Effects of shugan-jianpi recipe on the expression of the p38 MAPK/NF-kappaB signaling pathway in the hepatocytes of NAFLD rats. Medicines (Basel) 5:106. doi: 10.3390/medicines5030106
Do, H. T. T., Bui, B. P., Sim, S., Jung, J. K., Lee, H., and Cho, J. (2020). Anti-Inflammatory and anti-migratory activities of isoquinoline-1-carboxamide derivatives in LPS-Treated BV2 microglial cells via inhibition of MAPKs/NF-kappaB pathway. Int. J. Mol. Sci. 21:2319. doi: 10.3390/ijms21072319
Dragovich, M. A., and Mor, A. (2018). The SLAM family receptors: potential therapeutic targets for inflammatory and autoimmune diseases. Autoimmun. Rev. 17, 674–682. doi: 10.1016/j.autrev.2018.01.018
Durafourt, B. A., Moore, C. S., Zammit, D. A., Johnson, T. A., Zaguia, F., Guiot, M. C., et al. (2012). Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 60, 717–727. doi: 10.1002/glia.22298
Einck, L., and Bustin, M. (1985). The intracellular distribution and function of the high mobility group chromosomal proteins. Exp. Cell Res. 156, 295–310. doi: 10.1016/0014-4827(85)90539-7
Fan, H., Li, D., Guan, X., Yang, Y., Yan, J., Shi, J., et al. (2020a). MsrA suppresses inflammatory activation of microglia and oxidative stress to prevent demyelination via inhibition of the NOX2-MAPKs/NF-kappaB signaling pathway. Drug Des. Devel. Ther. 14, 1377–1389. doi: 10.2147/DDDT.S223218
Fan, H., Tang, H. B., Chen, Z., Wang, H. Q., Zhang, L., Jiang, Y., et al. (2020b). Inhibiting HMGB1-RAGE axis prevents pro-inflammatory macrophages/microglia polarization and affords neuroprotection after spinal cord injury. J. Neuroinflamm. 17:295. doi: 10.1186/s12974-020-01973-4
Fan, Z., Zhang, W., Cao, Q., Zou, L., Fan, X., Qi, C., et al. (2022). JAK2/STAT3 pathway regulates microglia polarization involved in hippocampal inflammatory damage due to acute paraquat exposure. Ecotoxicol. Environ. Saf. 234:113372. doi: 10.1016/j.ecoenv.2022.113372
Fellner, A., Barhum, Y., Angel, A., Perets, N., Steiner, I., Offen, D., et al. (2017). Toll-Like Receptor-4 inhibitor TAK-242 attenuates motor dysfunction and spinal cord pathology in an amyotrophic lateral sclerosis mouse model. Int. J. Mol. Sci. 18:1666. doi: 10.3390/ijms18081666
Feng, Y., Gao, J., Cui, Y., Li, M., Li, R., Cui, C., et al. (2017). Neuroprotective effects of resatorvid against traumatic brain injury in rat: involvement of neuronal autophagy and TLR4 signaling pathway. Cell Mol. Neurobiol. 37, 155–168. doi: 10.1007/s10571-016-0356-1
Frei, K., Nohava, K., Malipiero, U. V., Schwerdel, C., and Fontana, A. (1992). Production of macrophage colony-stimulating factor by astrocytes and brain macrophages. J. Neuroimmunol. 40, 189–195. doi: 10.1016/0165-5728(92)90133-6
Fujisaka, S., Usui, I., Bukhari, A., Ikutani, M., Oya, T., Kanatani, Y., et al. (2009). Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diab. Metab. Res. Rev. 58, 2574–2582. doi: 10.2337/db08-1475
Fujita, T., Hirose, R., Yoneta, M., Sasaki, S., Inoue, K., Kiuchi, M., et al. (1996). Potent immunosuppressants, 2-alkyl-2-aminopropane-1,3-diols. J. Med. Chem. 39, 4451–4459. doi: 10.1021/jm960391l
Gao, C., Qian, Y., Huang, J., Wang, D., Su, W., Wang, P., et al. (2017). A three-day consecutive fingolimod administration improves neurological functions and modulates multiple immune responses of CCI mice. Mol. Neurobiol. 54, 8348–8360. doi: 10.1007/s12035-016-0318-0
Gao, G., Wu, X., Feng, J., Hui, J., Mao, Q., Lecky, F., et al. (2020). Clinical characteristics and outcomes in patients with traumatic brain injury in China: a prospective, multicentre, longitudinal, observational study. Lancet Neurol. 19, 670–677. doi: 10.1016/S1474-4422(20)30182-4
Gao, W., Zhao, Z., Yu, G., Zhou, Z., Zhou, Y., Hu, T., et al. (2015). VEGI attenuates the inflammatory injury and disruption of blood-brain barrier partly by suppressing the TLR4/NF-kappaB signaling pathway in experimental traumatic brain injury. Brain Res. 1622, 230–239. doi: 10.1016/j.brainres.2015.04.035
Garrido-Mesa, N., Zarzuelo, A., and Galvez, J. (2013). Minocycline: far beyond an antibiotic. Br. J. Pharmacol. 169, 337–352. doi: 10.1111/bph.12139
Ghosh, S., Castillo, E., Frias, E. S., and Swanson, R. A. (2018). Bioenergetic regulation of microglia. Glia 66, 1200–1212. doi: 10.1002/glia.23271
Ginhoux, F., Greter, M., Leboeuf, M., Nandi, S., See, P., Gokhan, S., et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845. doi: 10.1126/science.1194637
Goodwin, G. H., and Johns, E. W. (1977). The isolation and purification of the high mobility group (HMG) nonhistone chromosomal proteins. Methods. Cell. Biol. 16:257–267.
Gordon, S., and Taylor, P. R. (2005). Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964. doi: 10.1038/nri1733
Gustin, M. C., Albertyn, J., Alexander, M., and Davenport, K. (1998). MAP kinase pathways in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 62, 1264–1300. doi: 10.1128/MMBR.62.4.1264-1300.1998
Hanlon, L. A., Huh, J. W., and Raghupathi, R. (2016). Minocycline transiently reduces microglia/macrophage activation but exacerbates cognitive deficits following repetitive traumatic brain injury in the neonatal rat. J. Neuropathol. Exp. Neurol. 75, 214–226. doi: 10.1093/jnen/nlv021
Hemani, S., Lane, O., Agarwal, S., Yu, S. P., and Woodbury, A. (2021). Systematic Review of Erythropoietin (EPO) for neuroprotection in human studies. Neurochem. Res. 46, 732–739. doi: 10.1007/s11064-021-03242-z
Henkel, J. S., Beers, D. R., Zhao, W., and Appel, S. H. (2009). Microglia in ALS: the good, the bad, and the resting. J. Neuroimmune Pharmacol. 4, 389–398. doi: 10.1007/s11481-009-9171-5
Henry, R. J., Ritzel, R. M., Barrett, J. P., Doran, S. J., Jiao, Y., Leach, J. B., et al. (2020). Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J. Neurosci. 40, 2960–2974. doi: 10.1523/JNEUROSCI.2402-19.2020
Hu, Y., Mai, W., Chen, L., Cao, K., Zhang, B., Zhang, Z., et al. (2020). mTOR-mediated metabolic reprogramming shapes distinct microglia functions in response to lipopolysaccharide and ATP. Glia 68, 1031–1045. doi: 10.1002/glia.23760
Hu, Z. W., Zhou, L. Q., Yang, S., Chen, M., Yu, H. H., Tao, R., et al. (2021). FTY720 modulates microglia toward anti-inflammatory phenotype by suppressing autophagy via STAT1 pathway. Cell Mol. Neurobiol. 41, 353–364. doi: 10.1007/s10571-020-00856-9
Hua, F., Tang, H., Wang, J., Prunty, M. C., Hua, X., Sayeed, I., et al. (2015). TAK-242, an antagonist for Toll-like receptor 4, protects against acute cerebral ischemia/reperfusion injury in mice. J. Cereb. Blood Flow Metab. 35, 536–542. doi: 10.1038/jcbfm.2014.240
Huwiler, A., and Zangemeister-Wittke, U. (2018). The sphingosine 1-phosphate receptor modulator fingolimod as a therapeutic agent: recent findings and new perspectives. Pharmacol. Ther. 185, 34–49. doi: 10.1016/j.pharmthera.2017.11.001
Javaherian, K., Liu, J. F., and Wang, J. C. (1978). Nonhistone proteins HMG1 and HMG2 change the DNA helical structure. Science 199, 1345–1346. doi: 10.1126/science.628842
Jiang, J.-Y., Gao, G.-Y., Feng, J.-F., Mao, Q., Chen, L.-G., Yang, X.-F., et al. (2019). Traumatic brain injury in China. Lancet Neurol. 18, 286–295. doi: 10.1016/S1474-4422(18)30469-1
Jiang, Q., Chen, J., Long, X., Yao, X., Zou, X., Yang, Y., et al. (2020). Phillyrin protects mice from traumatic brain injury by inhibiting the inflammation of microglia via PPARgamma signaling pathway. Int. Immunopharmacol. 79:106083. doi: 10.1016/j.intimp.2019.106083
Jin, X., Ishii, H., Bai, Z., Itokazu, T., and Yamashita, T. (2012). Temporal changes in cell marker expression and cellular infiltration in a controlled cortical impact model in adult male C57BL/6 mice. PLoS One 7:e41892. doi: 10.1371/journal.pone.0041892
Jonas, M., and Cunha, B. A. (1982). Minocycline. Ther. Drug Monit. 4, 137–145. doi: 10.1097/00007691-198206000-00002
Kang, R., Chen, R., Zhang, Q., Hou, W., Wu, S., Cao, L., et al. (2014). HMGB1 in health and disease. Mol. Aspects Med. 40, 1–116. doi: 10.1016/j.mam.2014.05.001
Karin, M., Lawrence, T., and Nizet, V. (2006). Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124, 823–835. doi: 10.1016/j.cell.2006.02.016
Kobayashi, H., Liu, J., Urrutia, A. A., Burmakin, M., Ishii, K., Rajan, M., et al. (2017). Hypoxia-inducible factor prolyl-4-hydroxylation in FOXD1 lineage cells is essential for normal kidney development. Kidney Int. 92, 1370–1383. doi: 10.1016/j.kint.2017.06.015
Kobayashi, K., Imagama, S., Ohgomori, T., Hirano, K., Uchimura, K., Sakamoto, K., et al. (2013). Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 4:e525. doi: 10.1038/cddis.2013.54
Kovesdi, E., Kamnaksh, A., Wingo, D., Ahmed, F., Grunberg, N. E., Long, J. B., et al. (2012). Acute minocycline treatment mitigates the symptoms of mild blast-induced traumatic brain injury. Front. Neurol. 3:111. doi: 10.3389/fneur.2012.00111
Kumar, A., Alvarez-Croda, D. M., Stoica, B. A., Faden, A. I., and Loane, D. J. (2016). Microglial/Macrophage polarization dynamics following traumatic brain injury. J. Neurotrauma 33, 1732–1750. doi: 10.1089/neu.2015.4268
Kumar, A., Commane, M., Flickinger, T. W., Horvath, C. M., and Stark, G. R. (1997). Defective TNF-alpha-induced apoptosis in STAT1-null cells due to low constitutive levels of caspases. Science 278, 1630–1632. doi: 10.1126/science.278.5343.1630
Lam, T. I., Bingham, D., Chang, T. J., Lee, C. C., Shi, J., Wang, D., et al. (2013). Beneficial effects of minocycline and botulinum toxin-induced constraint physical therapy following experimental traumatic brain injury. Neurorehabil. Neural Repair. 27, 889–899. doi: 10.1177/1545968313491003
Lan, Y. L., Wang, X., Zou, Y. J., Xing, J. S., Lou, J. C., Zou, S., et al. (2019). Bazedoxifene protects cerebral autoregulation after traumatic brain injury and attenuates impairments in blood-brain barrier damage: involvement of anti-inflammatory pathways by blocking MAPK signaling. Inflamm. Res. 68, 311–323. doi: 10.1007/s00011-019-01217-z
Lawrence, T. (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1:a001651. doi: 10.1101/cshperspect.a001651
Lawrence, T., and Natoli, G. (2011). Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11, 750–761. doi: 10.1038/nri3088
Lehrke, M., and Lazar, M. A. (2005). The many faces of PPARgamma. Cell 123, 993–999. doi: 10.1016/j.cell.2005.11.026
Li, Y., Liu, T., Li, Y., Han, D., Hong, J., Yang, N., et al. (2020). Baicalin ameliorates cognitive impairment and protects microglia from LPS-Induced neuroinflammation via the SIRT1/HMGB1 pathway. Oxid. Med. Cell Longev. 2020:4751349. doi: 10.1155/2020/4751349
Liu, Z., Yao, X., Jiang, W., Li, W., Zhu, S., Liao, C., et al. (2020). Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-kappaB signaling pathway and pyroptosis after secondary spinal cord injury. J. Neuroinflamm. 17:90. doi: 10.1186/s12974-020-01751-2
Lysakova-Devine, T., Keogh, B., Harrington, B., Nagpal, K., Halle, A., Golenbock, D. T., et al. (2010). Viral inhibitory peptide of TLR4, a peptide derived from vaccinia protein A46, specifically inhibits TLR4 by directly targeting MyD88 adaptor-like and TRIF-related adaptor molecule. J. Immunol. 185, 4261–4271. doi: 10.4049/jimmunol.1002013
Maas, A. I. R., Menon, D. K., Adelson, P. D., Andelic, N., Bell, M. J., Belli, A., et al. (2017). Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 16, 987–1048. doi: 10.1016/S1474-4422(17)30371-X
Mackaness, G. B. (1977). Cellular immunity and the parasite. Adv. Exp. Med. Biol. 93, 65–73. doi: 10.1007/978-1-4615-8855-9_5
Martinez, F. O., Sica, A., Mantovani, A., and Locati, M. (2008). Macrophage activation and polarization. Front. Biosci. 13:453–461. doi: 10.2741/2692
Masson, G. S., Nair, A. R., Dange, R. B., Silva-Soares, P. P., Michelini, L. C., and Francis, J. (2015). Toll-like receptor 4 promotes autonomic dysfunction, inflammation and microglia activation in the hypothalamic paraventricular nucleus: role of endoplasmic reticulum stress. PLoS One 10:e0122850. doi: 10.1371/journal.pone.0122850
Matsunaga, N., Tsuchimori, N., Matsumoto, T., and Ii, M. (2011). TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharmacol. 79, 34–41. doi: 10.1124/mol.110.068064
Medina-Gomez, G., Gray, S., and Vidal-Puig, A. (2007). Adipogenesis and lipotoxicity: role of peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgammacoactivator-1 (PGC1). Public Health Nutr. 10, 1132–1137. doi: 10.1017/S1368980007000614
Mencl, S., Hennig, N., Hopp, S., Schuhmann, M. K., Albert-Weissenberger, C., Siren, A. L., et al. (2014). FTY720 does not protect from traumatic brain injury in mice despite reducing posttraumatic inflammation. J. Neuroimmunol. 274, 125–131. doi: 10.1016/j.jneuroim.2014.07.010
Meythaler, J., Fath, J., Fuerst, D., Zokary, H., Freese, K., Martin, H. B., et al. (2019). Safety and feasibility of minocycline in treatment of acute traumatic brain injury. Brain Inj. 33, 679–689. doi: 10.1080/02699052.2019.1566968
Miao, H., Li, R., Han, C., Lu, X., and Zhang, H. (2018). Minocycline promotes posthemorrhagic neurogenesis via M2 microglia polarization via upregulation of the TrkB/BDNF pathway in rats. J. Neurophysiol. 120, 1307–1317. doi: 10.1152/jn.00234.2018
Mitchell, S., Vargas, J., and Hoffmann, A. (2016). Signaling via the NFkappaB system. Wiley Interdiscip Rev. Syst. Biol. Med. 8, 227–241. doi: 10.1002/wsbm.1331
Miyake, T., Kung, C. K., and Goldwasser, E. (1977). Purification of human erythropoietin. J. Biol. Chem. 252, 5558–5564. doi: 10.1016/S0021-9258(19)63387-9
Montilla, A., Zabala, A., Matute, C., and Domercq, M. (2020). Functional and metabolic characterization of microglia culture in a defined medium. Front. Cell Neurosci. 14:22. doi: 10.3389/fncel.2020.00022
Mozo, L., Gayo, A., Suarez, A., Rivas, D., Zamorano, J., and Gutierrez, C. (1998). Glucocorticoids inhibit IL-4 and mitogen-induced IL-4R alpha chain expression by different posttranscriptional mechanisms. J. Allergy Clin. Immunol. 102(6 Pt 1), 968–976. doi: 10.1016/S0091-6749(98)70335-5
Munder, M., Mollinedo, F., Calafat, J., Canchado, J., Gil-Lamaignere, C., Fuentes, J. M., et al. (2005). Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood 105, 2549–2556. doi: 10.1182/blood-2004-07-2521
Murray, P. J., Allen, J. E., Biswas, S. K., Fisher, E. A., Gilroy, D. W., Goerdt, S., et al. (2014). Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20. doi: 10.1016/j.immuni.2014.06.008
Napoli, K. L. (2000). The FTY720 story. Ther. Drug Monit. 22, 47–51. doi: 10.1097/00007691-200002000-00010
Nayak, D., Roth, T. L., and McGavern, D. B. (2014). Microglia development and function. Annu. Rev. Immunol. 32, 367–402. doi: 10.1146/annurev-immunol-032713-120240
Nishibori, M., Mori, S., and Takahashi, H. K. (2019). Anti-HMGB1 monoclonal antibody therapy for a wide range of CNS and PNS diseases. J. Pharmacol. Sci. 140, 94–101. doi: 10.1016/j.jphs.2019.04.006
Obinata, H., and Hla, T. (2019). Sphingosine 1-phosphate and inflammation. Int. Immunol. 31, 617–625. doi: 10.1093/intimm/dxz037
Okuma, Y., Liu, K., Wake, H., Zhang, J., Maruo, T., Date, I., et al. (2012). Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann. Neurol. 72, 373–384. doi: 10.1002/ana.23602
Orihuela, R., McPherson, C. A., and Harry, G. J. (2016). Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 173, 649–665. doi: 10.1111/bph.13139
O’Shea, J. J., and Plenge, R. (2012). JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity 36, 542–550. doi: 10.1016/j.immuni.2012.03.014
Parajuli, B., Sonobe, Y., Kawanokuchi, J., Doi, Y., Noda, M., Takeuchi, H., et al. (2012). GM-CSF increases LPS-induced production of proinflammatory mediators via upregulation of TLR4 and CD14 in murine microglia. J. Neuroinflamm. 9:268. doi: 10.1186/1742-2094-9-268
Paudel, Y. N., Shaikh, M. F., Chakraborti, A., Kumari, Y., Aledo-Serrano, Á, Aleksovska, K., et al. (2018). HMGB1: a common biomarker and potential target for TBI, neuroinflammation, epilepsy, and cognitive dysfunction. Front. Neurosci. 12:628. doi: 10.3389/fnins.2018.00628
Porro, C., Cianciulli, A., Trotta, T., Lofrumento, D. D., and Panaro, M. A. (2019). Curcumin regulates anti-inflammatory responses by JAK/STAT/SOCS signaling pathway in BV-2 microglial cells. Biology (Basel) 8:51. doi: 10.3390/biology8030051
Probert, L. (2015). TNF and its receptors in the CNS: the essential, the desirable and the deleterious effects. Neuroscience 302, 2–22. doi: 10.1016/j.neuroscience.2015.06.038
Qin, C., Fan, W.-H., Liu, Q., Shang, K., Murugan, M., Wu, L.-J., et al. (2017). Fingolimod protects against ischemic white matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke 48, 3336–3346. doi: 10.1161/STROKEAHA.117.018505
Qu, Z., Zheng, N., Wei, Y., Chen, Y., Zhang, Y., Zhang, M., et al. (2019). Effect of cornel iridoid glycoside on microglia activation through suppression of the JAK/STAT signalling pathway. J. Neuroimmunol. 330, 96–107. doi: 10.1016/j.jneuroim.2019.01.014
Raes, G., De Baetselier, P., Noel, W., Beschin, A., Brombacher, F., Hassanzadeh, et al. (2002). Differential expression of FIZZ1 and Ym1 in alternatively versus classically activated macrophages. J. Leukoc. Biol. 71, 597–602.
Rajabi, M., McConnell, M., Cabral, J., and Ali, M. A. (2021). Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 260:117768. doi: 10.1016/j.carbpol.2021.117768
Ray, S. K., Dixon, C. E., and Banik, N. L. (2002). Molecular mechanisms in the pathogenesis of traumatic brain injury. Histol. Histopathol. 17, 1137–1152.
Roberts, A. W. (2005). G-CSF: a key regulator of neutrophil production, but that’s not all! Growth Factors 23, 33–41. doi: 10.1080/08977190500055836
Romani, M., Rodman, T. C., Vidali, G., and Bustin, M. (1979). Serological analysis of species specificity in the high mobility group chromosomal proteins. J. Biol. Chem. 254, 2918–2922. doi: 10.1016/S0021-9258(17)30161-8
Rutschman, R., Lang, R., Hesse, M., Ihle, J. N., Wynn, T. A., and Murray, P. J. (2001). Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J. Immunol. 166, 2173–2177. doi: 10.4049/jimmunol.166.4.2173
Samarpita, S., Kim, J. Y., Rasool, M. K., and Kim, K. S. (2020). Investigation of toll-like receptor (TLR) 4 inhibitor TAK-242 as a new potential anti-rheumatoid arthritis drug. Arthritis Res. Ther. 22:16. doi: 10.1186/s13075-020-2097-2
Schmid, C. D., Melchior, B., Masek, K., Puntambekar, S. S., Danielson, P. E., Lo, D. D., et al. (2009). Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. J. Neurochem. 109, (Suppl. 1), 117–125. doi: 10.1111/j.1471-4159.2009.05984.x
Sehgal, A., Irvine, K. M., and Hume, D. A. (2021). Functions of macrophage colony-stimulating factor (CSF1) in development, homeostasis, and tissue repair. Semin. Immunol. 54:101509. doi: 10.1016/j.smim.2021.101509
Sha, T., Sunamoto, M., Kitazaki, T., Sato, J., Ii, M., and Iizawa, Y. (2007). Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur. J. Pharmacol. 571, 231–239. doi: 10.1016/j.ejphar.2007.06.027
Sica, A., and Mantovani, A. (2012). Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795. doi: 10.1172/JCI59643
Skuljec, J., Sun, H., Pul, R., Benardais, K., Ragancokova, D., Moharregh-Khiabani, D., et al. (2011). CCL5 induces a pro-inflammatory profile in microglia in vitro. Cell Immunol. 270, 164–171. doi: 10.1016/j.cellimm.2011.05.001
Spandou, E., Papoutsopoulou, S., Soubasi, V., Karkavelas, G., Simeonidou, C., Kremenopoulos, G., et al. (2004). Hypoxia-ischemia affects erythropoietin and erythropoietin receptor expression pattern in the neonatal rat brain. Brain Res. 1021, 167–172. doi: 10.1016/j.brainres.2004.06.057
Stamatovic, S. M., Phillips, C. M., Keep, R. F., and Andjelkovic, A. V. (2020). A novel approach to treatment of thromboembolic stroke in mice: redirecting neutrophils toward a peripherally implanted CXCL1-soaked sponge. Exp. Neurol. 330:113336. doi: 10.1016/j.expneurol.2020.113336
Stanley, E. R., Chen, D. M., and Lin, H. S. (1978). Induction of macrophage production and proliferation by a purified colony stimulating factor. Nature 274, 168–170. doi: 10.1038/274168a0
Stanley, E. R., and Chitu, V. (2014). CSF-1 receptor signaling in myeloid cells. Cold Spring Harb. Perspect. Biol. 6:a021857. doi: 10.1101/cshperspect.a021857
Stanley, E. R., Hansen, G., Woodcock, J., and Metcalf, D. (1975). Colony stimulating factor and the regulation of granulopoiesis and macrophage production. Fed Proc. 34, 2272–2278.
Stein, M., Keshav, S., Harris, N., and Gordon, S. (1992). Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J. Exp. Med. 176, 287–292. doi: 10.1084/jem.176.1.287
Stout, R. D., Jiang, C., Matta, B., Tietzel, I., Watkins, S. K., and Suttles, J. (2005). Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 175, 342–349. doi: 10.4049/jimmunol.175.1.342
Sun, L., He, C., Nair, L., Yeung, J., and Egwuagu, C. E. (2015). Interleukin 12 (IL-12) family cytokines: role in immune pathogenesis and treatment of CNS autoimmune disease. Cytokine 75, 249–255. doi: 10.1016/j.cyto.2015.01.030
Suzumura, A., Sawada, M., Yamamoto, H., and Marunouchi, T. (1990). Effects of colony stimulating factors on isolated microglia in vitro. J. Neuroimmunol. 30, 111–120. doi: 10.1016/0165-5728(90)90094-4
Taetzsch, T., Levesque, S., McGraw, C., Brookins, S., Luqa, R., Bonini, M. G., et al. (2015). Redox regulation of NF-kappaB p50 and M1 polarization in microglia. Glia 63, 423–440. doi: 10.1002/glia.22762
Takeda, K., and Akira, S. (2015). Toll-like receptors. Curr. Protoc. Immunol. 109:14.12.1-14.12.10. doi: 10.1002/0471142735.im1412s109
Tam, W. Y., Au, N. P., and Ma, C. H. (2016). The association between laminin and microglial morphology in vitro. Sci. Rep. 6:28580. doi: 10.1038/srep28580
Tam, W. Y., and Ma, C. H. (2014). Bipolar/rod-shaped microglia are proliferating microglia with distinct M1/M2 phenotypes. Sci. Rep. 4:7279. doi: 10.1038/srep07279
Tassiulas, I., Park-Min, K. H., Hu, Y., Kellerman, L., Mevorach, D., and Ivashkiv, L. B. (2007). Apoptotic cells inhibit LPS-induced cytokine and chemokine production and IFN responses in macrophages. Hum. Immunol. 68, 156–164. doi: 10.1016/j.humimm.2006.12.008
Todd, L., Palazzo, I., Suarez, L., Liu, X., Volkov, L., Hoang, T. V., et al. (2019). Reactive microglia and IL1beta/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J. Neuroinflamm. 16:118. doi: 10.1186/s12974-019-1505-5
Trapero, I., and Cauli, O. (2014). Interleukin 6 and cognitive dysfunction. Metab. Brain Dis. 29, 593–608. doi: 10.1007/s11011-014-9551-2
Villalta, S. A., Nguyen, H. X., Deng, B., Gotoh, T., and Tidball, J. G. (2009). Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum. Mol. Genet. 18, 482–496. doi: 10.1093/hmg/ddn376
Vodovotz, Y., Bogdan, C., Paik, J., Xie, Q. W., and Nathan, C. (1993). Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J. Exp. Med. 178, 605–613. doi: 10.1084/jem.178.2.605
Wang, H., Bloom, O., Zhang, M., Vishnubhakat, J. M., Ombrellino, M., Che, J., et al. (1999). HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251. doi: 10.1126/science.285.5425.248
Wang, R., Li, J., Duan, Y., Tao, Z., Zhao, H., and Luo, Y. (2017). Effects of erythropoietin on gliogenesis during cerebral ischemic/reperfusion recovery in adult mice. Aging Dis. 8, 410–419. doi: 10.14336/AD.2016.1209
Wang, Y., Wu, T. R., Cai, S., Welte, T., and Chin, Y. E. (2000). Stat1 as a component of tumor necrosis factor alpha receptor 1-TRADD signaling complex to inhibit NF-kappaB activation. Mol. Cell. Biol. 20, 4505–4512. doi: 10.1128/MCB.20.13.4505-4512.2000
Wang, Z., Kawabori, M., and Houkin, K. (2020). FTY720 (Fingolimod) ameliorates brain injury through multiple mechanisms and is a strong candidate for stroke treatment. Curr. Med. Chem. 27, 2979–2993. doi: 10.2174/0929867326666190308133732
Wen, L., You, W. D., Wang, H., Meng, Y. Y., Feng, J. F., and Yang, X. F. (2018). Polarization of microglia to the M2 phenotype in a peroxisome proliferator-activated receptor gamma-dependent manner attenuates axonal injury induced by traumatic brain injury in mice. J. Neurotrauma 35, 2330–2340. doi: 10.1089/neu.2017.5540
Witts, L. J. (1961). Some aspects of the pathology of anaemia. i. theory of maturation arrest. Br. Med. J. 2, 325–328. doi: 10.1136/bmj.2.5248.325
Wynn, T. A., Chawla, A., and Pollard, J. W. (2013). Macrophage biology in development, homeostasis and disease. Nature 496, 445–455. doi: 10.1038/nature12034
Xin, P., Xu, X., Deng, C., Liu, S., Wang, Y., Zhou, X., et al. (2020). The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 80:106210. doi: 10.1016/j.intimp.2020.106210
Xu, F., Kang, Y., Zhang, H., Piao, Z., Yin, H., Diao, R., et al. (2013). Akt1-mediated regulation of macrophage polarization in a murine model of Staphylococcus aureus pulmonary infection. J. Infect. Dis. 208, 528–538. doi: 10.1093/infdis/jit177
Yamashiro, Y., Thang, B. Q., Ramirez, K., Shin, S. J., Kohata, T., Ohata, S., et al. (2020). Matrix mechanotransduction mediated by thrombospondin-1/integrin/YAP in the vascular remodeling. Proc. Natl. Acad. Sci. U S A. 117, 9896–9905. doi: 10.1073/pnas.1919702117
Yang, X., Xu, S., Qian, Y., and Xiao, Q. (2017). Resveratrol regulates microglia M1/M2 polarization via PGC-1α in conditions of neuroinflammatory injury. Brain Behav. Immun. 64, 162–172. doi: 10.1016/j.bbi.2017.03.003
Yang, X., Zhang, J. D., Duan, L., Xiong, H. G., Jiang, Y. P., and Liang, H. C. (2018). Microglia activation mediated by toll-like receptor-4 impairs brain white matter tracts in rats. J. Biomed. Res. 32, 136–144.
Yoshida, T., Das, N. A., Carpenter, A. J., Izadpanah, R., Kumar, S. A., Gautam, S., et al. (2020). Minocycline reverses IL-17A/TRAF3IP2-mediated p38 MAPK/NF-kappaB/iNOS/NO-dependent cardiomyocyte contractile depression and death. Cell. Signal. 73:109690. doi: 10.1016/j.cellsig.2020.109690
Yu, H., Okada, T., Kobayashi, M., Abo-Elmatty, D. M., Jahangeer, S., and Nakamura, S. (2009a). Roles of extracellular and intracellular sphingosine 1-phosphate in cell migration. Genes Cells 14, 597–605. doi: 10.1111/j.1365-2443.2009.01295.x
Yu, H., Pardoll, D., and Jove, R. (2009b). STATs in cancer inflammation and immunity: a leading role for STAT3. Nat. Rev. Cancer 9, 798–809. doi: 10.1038/nrc2734
Yuan, T. F., Liang, Y. X., Peng, B., Lin, B., and So, K. F. (2015). Local proliferation is the main source of rod microglia after optic nerve transection. Sci. Rep. 5:10788. doi: 10.1038/srep10788
Zhang, D., Li, H., Li, T., Zhou, M., Hao, S., Yan, H., et al. (2014). TLR4 inhibitor resatorvid provides neuroprotection in experimental traumatic brain injury: implication in the treatment of human brain injury. Neurochem. Int. 75, 11–18. doi: 10.1016/j.neuint.2014.05.003
Zhang, L., and Wang, H. (2020). FTY720 in CNS injuries: molecular mechanisms and therapeutic potential. Brain Res. Bull. 164, 75–82. doi: 10.1016/j.brainresbull.2020.08.013
Zhao, X., Wang, H., Sun, G., Zhang, J., Edwards, N. J., and Aronowski, J. (2015). Neuronal Interleukin-4 as a modulator of microglial pathways and ischemic brain damage. J. Neurosci. 35, 11281–11291. doi: 10.1523/JNEUROSCI.1685-15.2015
Zhong, C., Cao, G., Rong, K., Xia, Z., Peng, T., Chen, H., et al. (2018). Characterization of a microbial polysaccharide-based bioflocculant and its anti-inflammatory and pro-coagulant activity. Colloids Surf. B Biointerfaces 161, 636–644. doi: 10.1016/j.colsurfb.2017.11.042
Zhou, D., Ji, L., and Chen, Y. (2020). TSPO modulates IL-4-Induced Microglia/Macrophage M2 polarization via PPAR-gamma pathway. J. Mol. Neurosci. 70, 542–549. doi: 10.1007/s12031-019-01454-1
Zhou, Z. W., Li, F., Zheng, Z. T., Li, Y. D., Chen, T. H., Gao, W. W., et al. (2017). Erythropoietin regulates immune/inflammatory reaction and improves neurological function outcomes in traumatic brain injury. Brain Behav. 7:e00827. doi: 10.1002/brb3.827
Zhu, C., Kang, W., Xu, F., Cheng, X., Zhang, Z., Jia, L., et al. (2009). Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics 124, e218–e226. doi: 10.1542/peds.2008-3553
Zhu, X., Huang, S., Zeng, L., Ma, J., Sun, S., Zeng, F., et al. (2017). HMOX-1 inhibits TGF-beta-induced epithelial-mesenchymal transition in the MCF-7 breast cancer cell line. Int. J. Mol. Med. 40, 411–417. doi: 10.3892/ijmm.2017.3027
Keywords: TBI, microglial polarization, cytokine, signaling pathway, inhibitors/agonists
Citation: Li Y-F, Ren X, Zhang L, Wang Y-H and Chen T (2022) Microglial polarization in TBI: Signaling pathways and influencing pharmaceuticals. Front. Aging Neurosci. 14:901117. doi: 10.3389/fnagi.2022.901117
Received: 21 March 2022; Accepted: 28 June 2022;
Published: 01 August 2022.
Edited by:
Maria Jose Bellini, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Rebecca J. Henry, University of Maryland, Baltimore, United StatesMaria Grazia Giovannini, University of Florence, Italy
Huaqiu Zhang, Huazhong University of Science and Technology, China
Copyright © 2022 Li, Ren, Zhang, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Hai Wang, wangyuhai067@163.com; Tao Chen, fmmuchentao@163.com
 Yun-Fei Li
Yun-Fei Li Xu Ren
Xu Ren Liang Zhang
Liang Zhang  Tao Chen
Tao Chen