Gender Differences in Elderly With Subjective Cognitive Decline
- 1Institutes of Brain Science, Fudan University, Shanghai, China
- 2Department of Neurology, Zhongshan Hospital, Fudan University, Shanghai, China
- 3State Key Laboratory of Medical Neurobiology, Fudan University, Shanghai, China
- 4Department of Neurology, The Second Hospital, Lanzhou University, Lanzhou, China
Objective: Subjective cognitive decline (SCD), also known as significant memory concern (SMC), has been suggested as a manifestation of Alzheimer’s Disease (AD) preceding mild cognitive impairment (MCI). This study assessed the impact of gender on cognition, amyloid accumulation, the volumes of hippocampus, entorhinal cortex (EC), fusiform and medial temporal lobe (MTA) and cerebrospinal fluid (CSF) pathology biomarkers in patients reporting SMC.
Methods: Twenty-nine males (mean age ± SD: 72.3 ± 5.7 years) and 40 females (mean age ± SD: 71.0 ± 5.1 years) with SMC from the AD Neuroimaging Initiative (ADNI) were included in the study. We explored the gender discrepancies in cognition, [18F] AV45 amyloid positivity, volumes of hippocampus, EC, fusiform and MTA and CSF biomarkers.
Results: Compared with females, males showed significantly worse performance in Assessment Scale-cognitive subscale 13 (ADAS-13; P = 0.004) and lower amyloid deposition (P < 0.001). However, females showed greater advantage on the task of Rey Auditory Verbal Learning Test-5 (RAVLT-5) sum (P = 0.021), RAVLT-immediate recall (P = 0.010) and reduced volumes of the hippocampus, EC, fusiform and MTA (P = 0.001, P < 0.001, P < 0.001, P = 0.007) than males. No gender differences were found in CSF Aβ42, CSF Tau and CSF P-tau (P = 0.264, P = 0.454, P = 0.353).
Conclusions: These findings highlight that gender discrepancies should be considered in the interpretation of cognitive measures when evaluating SMC.
Introduction
Significant memory concern (SMC; also known as subjective cognitive decline (SCD) or subjective memory impairment), is defined as a self-reported cognitive complaints in the absence of objective cognitive deficits, which is common in older adults (Jessen et al., 2014; Jenkins et al., 2015). Recent mounting evidences indicated that SMC is a risk factor for future accelerated cognitive decline and progression to preclinical or clinical state of Alzheimer’s disease (AD), with AD-type changes in amyloid deposition, neuroimaging and cerebrospinal fluid (CSF) biomarkers (Petersen, 2000; Visser et al., 2009; Reisberg et al., 2010; Perrotin et al., 2012; Scheef et al., 2012; Wang et al., 2012; Mitchell et al., 2014). Taken together, these results suggested that SMC might be an initial symptomatic indicator of preclinical AD (Jessen et al., 2014).
Gender-specific discrepancies in mild cognitive impairment (MCI) and AD have been observed (Roberts et al., 2012; Lin et al., 2015). Thus, sex-specific research in SMC is crucial to ensure early correct detection and pre-clinical intervention. However, to date, few studies have focused on the role of gender in SMC across a comprehensive profile of the cognitive assessment, neuroimaging and CSF AD biomarkers.
Therefore, the purpose of the current study was to go further to analyze whether the gender discrepancies are related to neuropsychological performance, CSF and positron emission tomography (PET) and magnetic resonance imaging (MRI) biomarkers of AD pathology in older adults reporting SMC.
Materials and Methods
ADNI Study Design
Data used in the preparation of this article were obtained from the AD Neuroimaging Initiative (ADNI) database1 during January 2018. The data collectors were blind to participant information during the experiments. The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial MRI, PET, other biological markers and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. The Principal Investigator of this initiative is Michael W. Weiner, MD, VA Medical Center and University of California-San Francisco. ADNI is a global research effort that actively supports the investigation and development of treatments that slow or stop the progression of AD and subjects have been recruited from over 50 sites across the US and Canada. The overall goal of ADNI is to determine biomarkers for use in AD clinical treatment trials. To date, it has three phases: ADNI1, ADNI GO and ADNI2, consisting of cognitively normal (CN) individuals, early MCI (EMCI), to late MCI (LMCI), and dementia or AD. For more information, see www.adni-info.org. This study was carried out in accordance with the recommendations of each ADNI site. The protocol was approved by the ADNI. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Subjects
The current study sample consisted of 69 ADNI-2 participants, including 29 males and 40 females. Participants were selected if they were diagnosed as having SMC. Diagnosis was made using the standard criteria described in the ADNI-2 procedures manual2. Briefly, SMC participants had subjective memory concerns as evaluated using the Cognitive Change Index (CCI; total score from first 12 items >16), which was based on selected items from a larger compilation of measures analyzed in an independent sample (Saykin et al., 2006), but no informant-reported memory complaints, and normal cognitive performance on the Wechsler Logical Memory Delayed Recall (LM-Delayed) and the Mini-Mental State Exam (MMSE).
Neuropsychological Assessment
All participants underwent a standardized cognitive evaluation including the following items: (1) Global cognitive function: MMSE (Folstein et al., 1975), Montreal Cognitive Assessment (MoCA; Nasreddine et al., 2005), AD Assessment Scale-cognitive subscale 13 (ADAS-13; Mohs et al., 1997), Global Clinical Dementia Rating Scale (CDR-SB; Morris, 1993); (2) Memory: the Rey Auditory Verbal Learning Test (RAVLT), including trials 1–5 total recall (RAVLT-5 sum), 5-min delayed recall (RAVLT-immediate recall), 30-min delayed recall (RAVLT-delayed recall), yes-no recognition (RAVLT-recognition; Schmidt, 1996); (3) Attention/executive function: the Trail Making Test-A and B (TMT-A/B; Reitan, 1955); (4) Language: animal fluency, 30-item Boston Naming Task (BNT-30; Domoto-Reilly et al., 2012); (5) Visuospatial: clock Drawing Test (CDT; Brodaty and Moore, 1997); (6) 15-item Geriatric Depression Scale (GDS-15; Yesavage et al., 1982), Functional Assessment Questionnaire (FAQ; Pfeffer et al., 1982), Neuropsychiatric Inventory (NPI; Cummings et al., 1994).
Apolipoprotein E Genotyping
Apolipoprotein E (APOE; gene map locus 19q13.2) genotypes of the study subjects were obtained from the ADNI database1. All subjects were classified as APOE ε4 carriers with phenotypes ε2/ε4, ε3/ε4, and ε4/ε4, APOE ε4 non-carriers group with ε2/ε2, ε2/ε3 and ε3/ε3 genotypes.
Detection of CSF Aβ42, Tau and P-tau
The CSF Aβ42, Tau and P-tau immunoassays were used following a Roche Study Protocol at the University of Pennsylvania/ADNI Biomarker Laboratory, according to the preliminary kit manufacturer’s instructions, as described in previous studies (Bittner et al., 2016). Values are given in pg/mL for both tau and Aβ42.
[18F] AV 45 (Florbetapir) PET Scans
[18F] AV 45 (Florbetapir) PET data were processed and acquired as described previously (Landau et al., 2012, 2013b). Mean florbetapir standard uptake value ratios (SUVRs) were measured within four regions (frontal, anterior cingulate, precuneus, and parietal cortex) and normalized to the whole cerebellum reference region. Participants were classified as amyloid positivity when the SUVRs were >1.11, and amyloid negativity when the SUVRs were ≤1.11 based on a previously established threshold (Landau et al., 2013a). For more detailed information about PET protocols and data, see the ADNI website3.
Quantification of Volumes of Hippocampus, Entorhinal Cortex, Fusiform and Medial Temporal Lobe
The ADNI neuroimaging standardized procedure has been described in great detail elsewhere (Jack et al., 2008). ADNI-2 MRI data were acquired on a 3 Tesla MRI scanners using T1-weighted sagittal 3D magnetization-prepared rapid gradient-echo (MPRAGE) sequences. Cortical reconstruction and volumetric segmentation were obtained using FreeSurfer version 5.1 image analysis suite in ADNI 24 (McDonald et al., 2009), as described in previous reports (Fischl et al., 2001, 2002; Fleisher et al., 2005; Han et al., 2006). In this study, hippocampus, EC, fusiform and medial temporal lobe (MTA) volumes were evaluated. Further details on ADNI imaging protocols can be found at http://adni.loni.usc.edu/methods/documents/mri-protocols/.
Statistical Analysis
Demographic and clinical variables were compared between genders in the overall sample using Student’s t-test by mean ± standard deviation (SD) according to the distribution, Mann–Whitney test for skewed distribution variables by median (M) and interquartile range (IQR), Chi-square test for categorical variables. All statistics were performed using SPSS software (version 23.0; IBM SPSS). All calculated tests were two-sided and statistical significance was set at P < 0.05. Figures were produced using GraphPad Prism 6.
Results
Demographic Characteristics by Gender
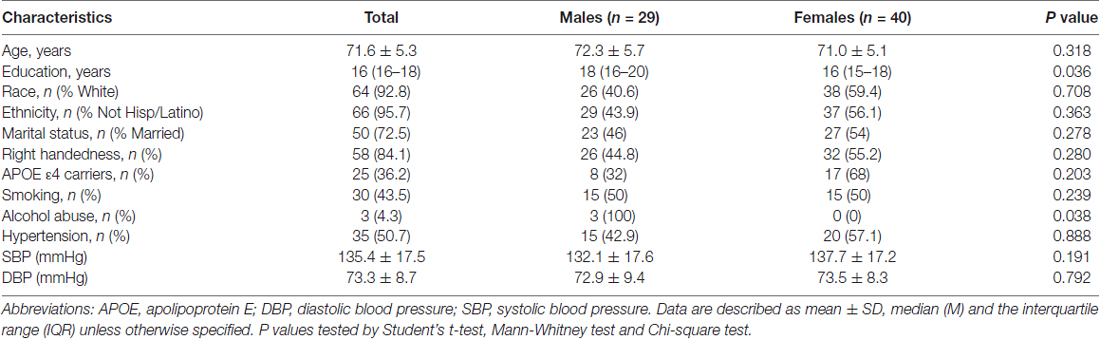
The overall sample was comprised of 69 participants including 29 males and 40 females were downloaded from the ADNI website. Socio-demographics and clinical characteristics of the study sample are presented in Table 1. In brief, females were less educated compared to males (P = 0.036) and males were reported with more alcohol drinking (P = 0.038). No gender differences were found in other variables (all P > 0.05).
Cognitive Profiles by Gender
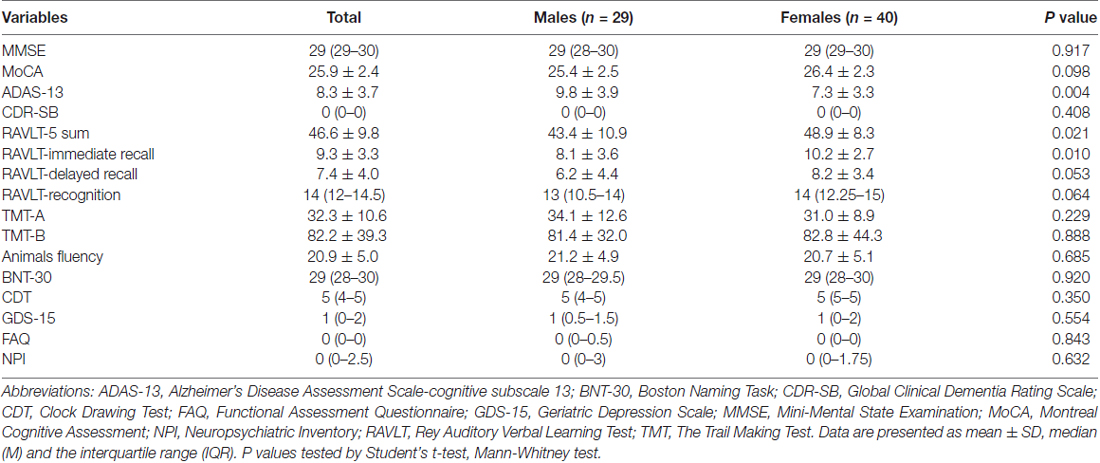
Gender differences in neuropsychological performances in the study sample are demonstrated in Table 2 and Figure 1. Men performed worse on ADAS-13 (P = 0.004), while women had statistically significant better cognitive function on RAVLT-5 sum (P = 0.021) and RAVLT-immediate recall (P = 0.010). However, there were no gender differences in performance on MMSE, MoCA, CDR-SB, RAVLT-delayed recall, RAVLT-recognition, TMT-A, TMT-B, Animals fluency, BNT-30, CDT, GDS-15, FAQ, NPI (P = 0.917, P = 0.098, P = 0.408, P = 0.053, P = 0.064, P = 0.229, P = 0.888, P = 0.685, P = 0.920, P = 0.350, P = 0.554, P = 0.843, P = 0.632).
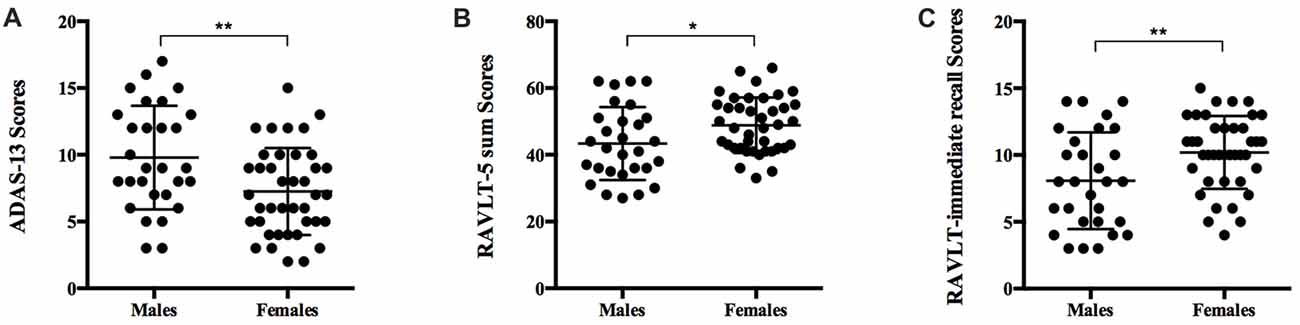
Figure 1. Comparison of neuropsychological measures in men and women with significant memory concern (SMC). Scatter plots displaying cognitive function in males and females. (A) Men had significantly worse cognitive function as measured by ADAS-13 (P = 0.004). (B) Women showed greater advantages on RAVLT-5 sum (P = 0.021). (C) Women excelled at RAVLT-immediate recall (P = 0.010). ADAS-13, Alzheimer’s Disease Assessment Scale-cognitive subscale 13; RAVLT, Rey Auditory Verbal Learning Test. P values tested by Student’s t-test. *P < 0.05, **P < 0.01.
CSF Biomarkers
There were no significant differences in CSF Aβ42, Tau and P-tau levels between males and females (P = 0.264, P = 0.454, P = 0.353; see Table 3).
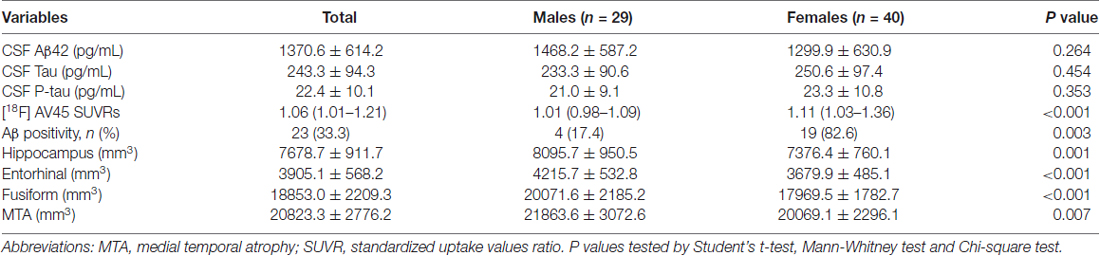
Table 3. Measurements of cerebrospinal fluid (CSF) biomarkers, positron emission tomography (PET) and magnetic resonance imaging (MRI) in the subjects.
Aβ Deposition in Different Groups
Significant decreases in [18F] AV45 SUVRs were found in males compared with females (P < 0.001). Aβ positivity was detected in 17.4% of males, 82.6% of females respectively. The percentage of Aβ positive subjects was significantly higher in females vs. males (P = 0.003; Table 3).
Volumes of Hippocampus, Entorhinal Cortex, Fusiform and Medial Temporal Lobe by Gender
The volumes of hippocampus, EC, fusiform, medial temporal lobe (MTA) are illustrated in Table 3. There were significantly larger volumes of hippocampus, EC, fusiform and MTA in men compared to women (P = 0.001, P < 0.001, P < 0.001, P = 0.007).
Discussion
In the present study, we explored a statistically significant gender discrepancy in cognition function, Aβ deposition and brain volume in older adults with SMC. The results confirmed that women with SMC outperformed men with SMC on the tasks of RAVLT-5 sum and RAVLT-immediate recall, while the advantage was eliminated on the task of RAVLT-delayed recall and a floor effect might limit interpretation. However, we found that men with SMC were associated with worse performance on ADAS-13. No significant differences were observed between males and females on other cognitive domains. Our results are in concordance with previous studies showing that the female advantage in verbal memory task was more apparent than men (Herlitz et al., 1997; Sundermann et al., 2016a,b). These observations suggest that gender discrepancies among SMC subjects might be appropriate to a specific cognitive domain. If so, then implementing sex-adjusted norms in clinical memory tests might ameliorate the diagnostic accuracy in women.
Females with SMC participants showed increased Aβ deposition relative to males. Elevated risk of AD was in women compared to men, although the underlying mechanism remains elusive as previously reported (Seshadri et al., 2006). However, we did not discover differences in CSF Aβ42, Tau and P-tau between males and females with subjective cognitive impairment. It is noteworthy that we observed significant reduction in volumetric measurements of hippocampus, EC, fusiform gyrus and MTA in females with SMC subjects. Consistent with the cognitive reserve theory (Klonoff and Landrine, 1992; Stern et al., 1994, 2003; Stern, 2002), better premorbid performance of women on verbal episodic memory tests might confer an advantage in the ability to maintain normal verbal memory performance despite reduced volumes of hippocampal, EC, fusiform gyrus, MTA and accumulating AD pathology (Sundermann et al., 2016a,b, 2017). However, women may have more accelerated decline once neuropathology reached a threshold level (Klonoff and Landrine, 1992; Stern et al., 1994).
Several limitations should be mentioned in the current study. First, a simple cross-sectional design used in the study does not definitively permit the theory that female advantage in verbal memory may act as a specific form of cognitive reserve, further longitudinal researches would be more necessary to closely confirm the conclusions. Second, The ADNI cohort included a potential selection bias based on the fact that ADNI participants had a high education level (Petersen et al., 2010; Grill et al., 2013), which may affect the generalizability to a greater population sections. Third, the relatively small group size could limit the interpretation of our results, larger sample of numbers need to be collected in future studies.
Conclusion
In summary, the present results highlighted the urgent need to consider the sex differences in cognition evaluation, which contributes to clinical diagnosis even in preclinical stages, such as SMC.
Author Contributions
LW: conceived and designed the studies; wrote the article; is the corresponding author. LW and TT: performed the research; analyzed the data. All authors approved the final version of the manuscript for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI; National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81 X WH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Limited and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research and Development, LLC.; Johnson and Johnson Pharmaceutical Research and Development LLC.; Lumosity; Lundbeck; Merck and Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health5. The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Footnotes
- ^ adni.loni.usc.edu
- ^ http://www.adni-info.org
- ^ http://adni.loni.usc.edu/methods/
- ^ http://surfer.nmr.mgh.harvard.edu/
- ^ www.fnih.org
References
Bittner, T., Zetterberg, H., Teunissen, C. E., Ostlund, R. E. Jr., Militello, M., Andreasson, U., et al. (2016). Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of β-amyloid (1–42) in human cerebrospinal fluid. Alzheimers Dement. 12, 517–526. doi: 10.1016/j.jalz.2015.09.009
Brodaty, H., and Moore, C. M. (1997). The clock drawing test for dementia of the Alzheimer’s type: a comparison of three scoring methods in a memory disorders clinic. Int. J. Geriatr. Psychiatry 12, 619–627. doi: 10.1002/(sici)1099-1166(199706)12:6<619::aid-gps554>3.3.co;2-8
Cummings, J. L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., and Gornbein, J. (1994). The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. doi: 10.1212/wnl.44.12.2308
Domoto-Reilly, K., Sapolsky, D., Brickhouse, M., Dickerson, B. C., and Alzheimer’s Disease Neuroimaging Initiative. (2012). Naming impairment in Alzheimer’s disease is associated with left anterior temporal lobe atrophy. Neuroimage 63, 348–355. doi: 10.1016/j.neuroimage.2012.06.018
Fischl, B., Liu, A., and Dale, A. M. (2001). Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging 20, 70–80. doi: 10.1109/42.906426
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. doi: 10.1016/S0896-6273(02)00569-X
Fleisher, A., Grundman, M., Jack, C. R. Jr., Petersen, R. C., Taylor, C., Kim, H. T., et al. (2005). Sex, apolipoprotein E epsilon 4 status and hippocampal volume in mild cognitive impairment. Arch. Neurol. 62, 953–957. doi: 10.1001/archneur.62.6.953
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Grill, J. D., Di, L., Lu, P. H., Lee, C., Ringman, J., Apostolova, L. G., et al. (2013). Estimating sample sizes for predementia Alzheimer’s trials based on the Alzheimer’s disease neuroimaging initiative. Neurobiol. Aging 34, 62–72. doi: 10.1016/j.neurobiolaging.2012.03.006
Han, X., Jovicich, J., Salat, D., van der Kouwe, A., Quinn, B., Czanner, S., et al. (2006). Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage 32, 180–194. doi: 10.1016/j.neuroimage.2006.02.051
Herlitz, A., Nilsson, L. G., and Bäckman, L. (1997). Gender differences in episodic memory. Mem. Cognit. 25, 801–811. doi: 10.3758/bf03211324
Jack, C. R. Jr., Bernstein, M. A., Fox, N. C., Thompson, P., Alexander, G., Harvey, D., et al. (2008). The Alzheimer’s disease neuroimaging initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 27, 685–691. doi: 10.1002/jmri.21049
Jenkins, A., Bayer, A., Tree, J., and Tales, A. (2015). Self-reported memory complaints: implications from a longitudinal cohort with autopsies. Neurology 84:2384. doi: 10.1212/wnl.0000000000001677
Jessen, F., Wolfsgruber, S., Wiese, B., Bickel, H., Mosch, E., Kaduszkiewicz, H., et al. (2014). AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 10, 76–83. doi: 10.1016/j.jalz.2012.09.017
Klonoff, E. A., and Landrine, H. (1992). Sex roles, occupational roles, and symptom-reporting: a test of competing hypotheses on sex differences. J. Behav. Med. 15, 355–364. doi: 10.1007/bf00844728
Landau, S. M., Breault, C., Joshi, A. D., Pontecorvo, M., Mathis, C. A., Jagust, W. J., et al. (2013a). Amyloid-β imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J. Nucl. Med. 54, 70–77. doi: 10.2967/jnumed.112.109009
Landau, S. M., Lu, M., Joshi, A. D., Pontecorvo, M., Mintun, M. A., Trojanowski, J. Q., et al. (2013b). Comparing positron emission tomography imaging and cerebrospinal fluid measurements of β-amyloid. Ann. Neurol. 74, 826–836. doi: 10.1002/ana.23908
Landau, S. M., Mintun, M. A., Joshi, A. D., Koeppe, R. A., Petersen, R. C., Aisen, P. S., et al. (2012). Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann. Neurol. 72, 578–586. doi: 10.1002/ana.23650
Lin, K. A., Choudhury, K. R., Rathakrishnan, B. G., Marks, D. M., Petrella, J. R., and Doraiswamy, P. M. (2015). Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement. 1, 103–110. doi: 10.1016/j.trci.2015.07.001
McDonald, C. R., McEvoy, L. K., Gharapetian, L., Fennema-Notestine, C., Hagler, D. J. Jr., Holland, D., et al. (2009). Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology 73, 457–465. doi: 10.1212/WNL.0b013e3181b16431
Mitchell, A. J., Beaumont, H., Ferguson, D., Yadegarfar, M., and Stubbs, B. (2014). Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr. Scand. 130, 439–451. doi: 10.1111/acps.12336
Mohs, R. C., Knopman, D., Petersen, R. C., Ferris, S. H., Ernesto, C., Grundman, M., et al. (1997). Development of cognitive instruments for use in clinical trials of antidementia drugs: additions to the Alzheimer’s Disease Assessment Scale that broaden its scope. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis. Assoc. Disord. 11, S13–S21. doi: 10.1097/00002093-199700112-00003
Morris, J. C. (1993). The clinical dementia rating (CDR): current version and scoring rules. Neurology 43, 2412–2414. doi: 10.1212/WNL.43.11.2412-a
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Perrotin, A., Mormino, E. C., Madison, C. M., Hayenga, A. O., and Jagust, W. J. (2012). Subjective cognition and amyloid deposition imaging: a Pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch. Neurol. 69, 223–229. doi: 10.1001/archneurol.2011.666
Petersen, R. C. (2000). Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia 15, 93–101. doi: 10.1002/0470846453.ch14
Petersen, R. C., Aisen, P. S., Beckett, L. A., Donohue, M. C., Gamst, A. C., Harvey, D. J., et al. (2010). Alzheimer’s disease neuroimaging initiative (ADNI): clinical characterization. Neurology 74, 201–209. doi: 10.1212/WNL.0b013e3181cb3e25
Pfeffer, R. I., Kurosaki, T. T., Harrah, C. H. Jr., Chance, J. M., and Filos, S. (1982). Measurement of functional activities in older adults in the community. J. Gerontol. 37, 323–329. doi: 10.1093/geronj/37.3.323
Reisberg, B., Shulman, M. B., Torossian, C., Leng, L., and Zhu, W. (2010). Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 6, 11–24. doi: 10.1016/j.jalz.2009.10.002
Reitan, R. M. (1955). The relation of the trail making test to organic brain damage. J. Consult. Psychol. 19, 393–394. doi: 10.1037/h0044509
Roberts, R. O., Geda, Y. E., Knopman, D. S., Cha, R. H., Pankratz, V. S., Boeve, B. F., et al. (2012). The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology 78, 342–351. doi: 10.1212/WNL.0b013e3182452862
Saykin, A. J., Wishart, H. A., Rabin, L. A., Santulli, R. B., Flashman, L. A., West, J. D., et al. (2006). Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 67, 834–842. doi: 10.1212/01.wnl.0000234032.77541.a2
Scheef, L., Spottke, A., Daerr, M., Joe, A., Striepens, N., Kölsch, H., et al. (2012). Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology 79, 1332–1339. doi: 10.1212/wnl.0b013e31826c1a8d
Schmidt, M. (1996). Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, CA: Psychological Services.
Seshadri, S., Beiser, A., Kelly-Hayes, M., Kase, C. S., Au, R., Kannel, W. B., et al. (2006). The lifetime risk of stroke: estimates from the Framingham Study. Stroke 37, 345–350. doi: 10.1161/01.STR.0000199613.38911.b2
Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. doi: 10.1017/s1355617702813248
Stern, Y., Gurland, B., Tatemichi, T. K., Tang, M. X., Wilder, D., and Mayeux, R. (1994). Influence of education and occupation on the incidence of Alzheimer’s disease. JAMA 271, 1004–1010. doi: 10.1001/jama.271.13.1004
Stern, Y., Zarahn, E., Hilton, H. J., Flynn, J., DeLaPaz, R., and Rakitin, B. (2003). Exploring the neural basis of cognitive reserve. J. Clin. Exp. Neuropsychol. 25, 691–701. doi: 10.1076/jcen.25.5.691.14573
Sundermann, E. E., Biegon, A., Rubin, L. H., Lipton, R. B., Landau, S., and Maki, P. M. (2017). Does the female advantage in verbal memory contribute to underestimating Alzheimer’s disease pathology in women versus men? J. Alzheimers Dis. 56, 947–957. doi: 10.3233/JAD-160716
Sundermann, E. E., Biegon, A., Rubin, L. H., Lipton, R. B., Mowrey, W., Landau, S., et al. (2016a). Better verbal memory in women than men in MCI despite similar levels of hippocampal atrophy. Neurology 86, 1368–1376. doi: 10.1212/WNL.0000000000002570
Sundermann, E. E., Maki, P. M., Rubin, L. H., Lipton, R. B., Landau, S., and Biegon, A. (2016b). Female advantage in verbal memory: evidence of sex-specific cognitive reserve. Neurology 87, 1916–1924. doi: 10.1212/WNL.0000000000003288
Visser, P. J., Verhey, F., Knol, D. L., Scheltens, P., Wahlund, L. O., Freund-Levi, Y., et al. (2009). Prevalence and prognostic value of CSF markers of Alzheimer’s disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 8, 619–627. doi: 10.1016/S1474-4422(09)70139-5
Wang, Y., West, J. D., Flashman, L. A., Wishart, H. A., Santulli, R. B., Rabin, L. A., et al. (2012). Selective changes in white matter integrity in MCI and older adults with cognitive complaints. Biochim. Biophys. Acta 1822, 423–430. doi: 10.1016/j.bbadis.2011.08.002
Keywords: gender, significant memory concern, cognitive function, hippocampus, entorhinal cortex, medial temporal lobe
Citation: Wang L and Tian T for the Alzheimer’s Disease Neuroimaging Initiative (2018) Gender Differences in Elderly With Subjective Cognitive Decline. Front. Aging Neurosci. 10:166. doi: 10.3389/fnagi.2018.00166
Received: 31 January 2018; Accepted: 16 May 2018;
Published: 04 June 2018.
Edited by:
Robert Perneczky, Ludwig-Maximilians-Universität München, GermanyReviewed by:
Baiying Lei, Shenzhen University, ChinaDeep R. Sharma, SUNY Downstate Medical Center, United States
Copyright © 2018 Wang and Tian for the Alzheimer’s Disease Neuroimaging Initiative. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijun Wang, lijunwang365@163.com
† Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
 Lijun Wang
Lijun Wang Ting Tian
Ting Tian