The Analysis of Two BDNF Polymorphisms G196A/C270T in Chinese Sporadic Amyotrophic Lateral Sclerosis
- Department of Neurology, Peking University Third Hospital, Beijing, China
Amyotrophic lateral sclerosis (ALS) is an ethnically heterogeneous motor neuron disease that results from the selective death of motor neurons in the brain and spinal cord. Brain-derived neurotrophic factor (BDNF) is widely distributed across the central and peripheral nervous systems and plays neurotrophic and other physiological roles in various brain regions. Alterations of neurotrophin availability have been proposed as a pathogenic mechanism underlying ALS neurodegeneration. Several genetic studies have shown a significant association between schizophrenia, Alzheimer's disease, and Parkinson's disease and certain BDNF polymorphisms, specifically G196A (rs6265) and C270T (rs56164415). However, the relationship between the G196A and C270T polymorphisms and ALS has never been investigated. We hypothesized that sporadic ALS (sALS) and disease susceptibility could arise due to BDNF polymorphisms and investigated the relationship between ALS and the BDNF polymorphisms G196A and C270T in a large Chinese cohort. We demonstrate that the frequency of the CT genotype and of the C270T T allele was significantly higher in the ALS group than in controls, although G196A was not associated with sALS. These data provide the first demonstration that the BDNF C270T polymorphism may be a candidate susceptibility locus for sALS, at least in Han Chinese.
Introduction
Amyotrophic lateral sclerosis (ALS) is an ethnically heterogeneous motor neuron disease that results from the selective death of motor neurons in the brain and spinal cord (Chen et al., 2015; Huynh and Kiernan, 2015; Shahrizaila et al., 2016). The crude annual incidence rate of ALS in the general European population was 2.16 per 100,000 person years (Logroscino et al., 2010). There are several phenotypes of ALS, including limb-onset ALS, bulbar-onset ALS, progressive muscular atrophy (PMA), primary lateral sclerosis (PLS), and several regional limb variants, such as flail-arm syndrome (FAS). The pathogenesis of ALS is unclear. Approximately 90% of ALS cases are sporadic (sALS), while approximately 10% of cases are familial (fALS). The heritability of ALS is high; twin studies have estimated the genetic component to be 0.61 and the unshared environmental component to be 0.39 (Al-Chalabi et al., 2010). Based on the hypothesis that sporadic disease may arise from complex interactions between genetic susceptibility and the environment, the scientific community began to explore sALS susceptible genes. Several previous studies have identified different susceptibility genes in different ethnic cohorts, including DPP6, ITPR2, UNC13A, FGGY, ELP3, KIFAP3, 9p21.2, ZNF512B, TIMA1, and SCNN1A (Van Es et al., 2007, 2008, 2009a; Cronin et al., 2008; Landers et al., 2009; Simpson et al., 2009; Van Es et al., 2009b; Iida et al., 2011; Chen et al., 2014). However, it is believed that there are more susceptible genes need to be identified to help better understand the pathophysiology of sALS.
Brain-derived neurotrophic factor (BDNF), as a member of the neurotrophin family of growth factors, is widely distributed across the central nervous system and plays neurotrophic and other physiological roles in various brain regions (Leibrock et al., 1989; He et al., 2013). Defective BDNF expression and/or function is thought to be associated with depression, schizophrenia, Alzheimer's disease (AD), Parkinson's disease (PD), and ALS (Hyman et al., 1991; Phillips et al., 1991; Holsinger et al., 2000; Castrén et al., 2007; Favalli et al., 2012; Libman-Sokołowska et al., 2015). Alterations of neurotrophic availability have been proposed as a pathogenic mechanism underlying ALS neurodegeneration (Duberley et al., 1997; Kawamoto et al., 1998; Nishio et al., 1998; Tremolizzo et al., 2016). Although therapeutic trials of BDNF infusion have failed to show significant clinical benefit (1999; Ochs et al., 2000). BDNF has been shown to impact the survival of motor neurons in both in vitro and in vivo models of neuron injury or death (Sendtner et al., 1992; Henderson et al., 1993; Koliatsos et al., 1993; Mitsumoto et al., 1994; Ikeda et al., 1995). Transplantation of a mixture of such MPC populations (BDNF, GDNF, VEGF, IGF-1) into the hind legs of SOD1 G93A transgenic mice (SOD1 mice), the commonly used model of ALS, delayed the onset of disease symptoms by 30 days and prolonged the average lifespan by 13 days (Dadon-Nachum, 2015). Treated mice also showed a decrease in the degeneration of neuromuscular junction and an increase in axonal survival (Deepa et al., 2011). These information indicated that BNDF may play an important role in pathophysiology of sALS. So we hypothesized that some polymorphisms of the BNDF gene resulted in deceased neurotrophic availability and/or changed physiological roles may increase susceptibility to sALS.
Functional polymorphisms of the BDNF gene have been investigated in AD, PD, schizophrenia, depression patients, such as rs2030324, rs2049045, rs6265, rs2203877, rs7103411, rs988748, rs6265 [G196A], rs56164415[C270T], rs16917204 [G11757C], rs13306221 [G-712A] (Kunugi et al., 2001; Parsian et al., 2004; Bodner et al., 2005; Vepsäläinen, 2005; Dmitrzak-Weglarz et al., 2008; Borroni et al., 2009; Zdanys et al., 2009; Su et al., 2011; Zhang et al., 2011). Of these BDNF gene polymorphisms, the G196A and C270T SNPs were significantly associated with this diseases in some studies (Kunugi et al., 2001; Ventriglia et al., 2002; Parsian et al., 2004; Olin et al., 2005; Nagata et al., 2011; Dai et al., 2013; Watanabe et al., 2013; Lee and Song, 2014), especially C270T polymorphism was susceptibility to East Asian schizophrenia and AD (Watanabe et al., 2013). Then several researcher replicate this two loci analysis in different diseases, such as PD, schizophrenia, depression patients. ALS, AD, and PD are neurodegenerative diseases that share some common pathways in their onset and progression (Hetz and Mollereau, 2014). Numerous studies have described the overlap of clinical phenotype and gene variants between ALS and schizophrenia (Byrne et al., 2013; Fahey et al., 2014). So we hypothesized that the polymorphisms are susceptible to schizophrenia and neurodegenerative disease may be also susceptible to ALS. So we choose the two widely reported polymorphisms G196A/C270T to analyze its relationship with sALS in a Chinese cohort.
Subjects and Methods
Participants
This study consisted of 499 sALS patients and 488 healthy control subjects. All participants were of Han Chinese origin. The diagnosis of ALS was made in Peking University Third Hospital (PUTH) between January 2013 and September 2014 using the El Escorial Word Federation criteria for definite or probable ALS (Brooks et al., 2000). This study was approved by the institutional ethics committee of PUTH (IRB00006761), and all patients and controls gave written informed consent.
SNP Analysis
Genomic DNA was collected from peripheral blood leukocytes via standard phenol-chloroform procedures. Genotyping for G196A and C270T BDNF polymorphisms was performed by direct sequencing. The G196A polymorphism was genotyped using the following pair of primers: FW: 5′-ACTCTGGAGAGCGTGAAT-3′ and Rev: 5′-ATACTGTCACACACGCTC-3′. The C270T polymorphism was genotyped using the following pair of primers: FW: 5′-AATGAGACACCCACCGCTGCTG-3′ and Rev: 5′-CTCCTGCACCAAGCCCCATTC-3′. The PCR products were directly sequenced using an ABI3100 automated DNA sequencing system by Tsingke Biotechnology Co., Ltd. (Beijing, China).
Retrospective Observation
We conducted a retrospective review and evaluated both the outcomes and clinical manifestations of these patients. Survival time, which was an endpoint in this study, was defined as the number of months between symptom onset and death from any cause or between symptom onset and tracheostomy for the purpose of permanent mechanical ventilation. “Alcohol abuse” was defined as consuming an alcoholic drink more than twice a week for more than 1 year. “Use of riluzole” was defined as treatment with riluzole (50 mg) twice a day for longer than 2 weeks. The patient characteristics entered in the univariate analyses included age at onset, sex, site (bulbar/spinal) of symptom onset, disease progression ΔFS [progression rate of ALSFRS-R: (48-ALSFRS-R score at first visit)/(time in months between the first symptoms and the first examination)].
Statistical Analysis
Continuous variables were presented as means ± SDs or medians with interquartile ranges, whereas categorical variables were expressed as numbers and percentages. Chi-square test was used to compare group difference for categorical variables. The mean age at onset was compared using Student's t-test, while the Mann–Whitney U-test was applied to disease progression ΔFS. Univariate and multivariate logistic regressions were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between sALS and any of the confounds. In the multivariate logistic regressions, enter method was used with whether ALS or not serves as dependent variable, and gender, age, whether contains risk allele or not as independent variables. The effects of prognostic factors on survival were assessed using the Kaplan–Meier life-table method for all 499 patients according to their risk allele carrier status. The log-rank test was used to assess the equality of the outcome functions. A P <0.05 was considered significant after correcting for the number of tests (Bonferroni correction). All analyses were performed using the SPSS V.17.0 software package.
Results
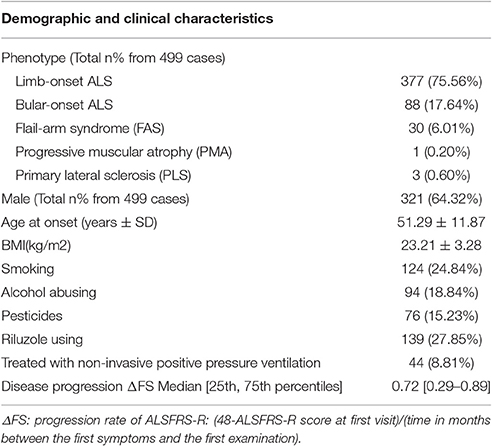
Of the 499 patients with sALS, the male: female ratio was 1.8:1, and the mean age at onset was 51.29 ± 11.87 years. Other demographic and clinical characteristics were shown in Table 1. Of the 488 healthy control subjects, the male: female ratio was 1.4:1, and the mean age was 52.71 ± 11.81 years in control group. There were no difference between the patients and control subjects in age and gender.
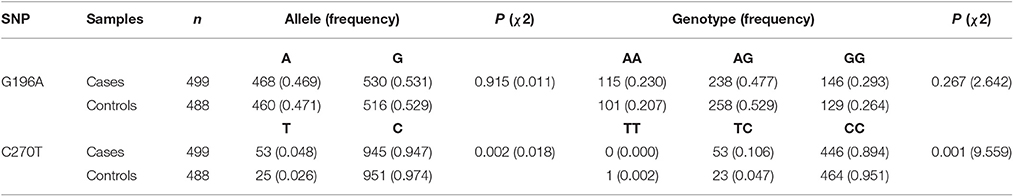
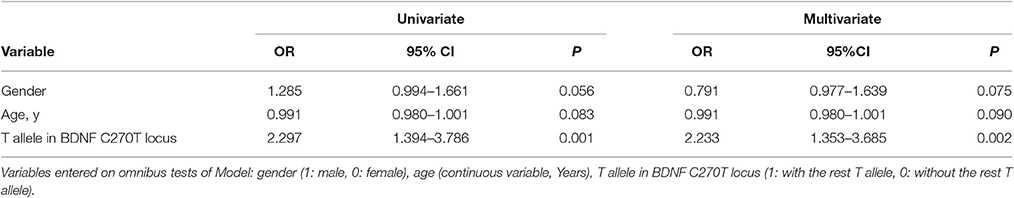
The two polymorphisms (G196A and C270T) were in Hardy-Weinberg equilibrium in both patients and controls. G196A was not associated with sALS. For C270T, the frequency of the CT genotype and of the T allele was significantly higher in the ALS group than in the control group (Table 2). This difference was significant after Bonferroni correction. The association with T270 was still significant even after adjusting for age and gender P = 0.002, OR95%CI = 2.233 [1.353–3.685] (Table 3).
This locus was included in 1000 Genomes project. We can learn that the T allele frequency in Beijing Han Chinese is 2.43% (5/206) from the 1,000 Genomes project database. So we add this data to our control group in order to slightly enlarge our size in the control group. After pooling this data with our control data, the T allele frequency in the new control group is 2.53% (30/1182). The frequency difference between the ALS group and control group was still significant (P = 0.0008, OR [95%CI] = 2.154 [1.365–3.398]).
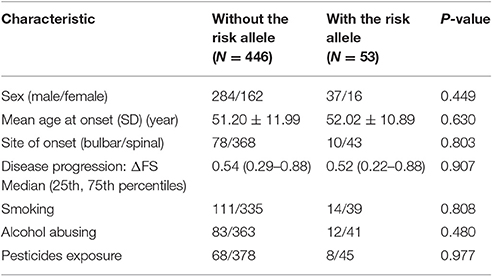
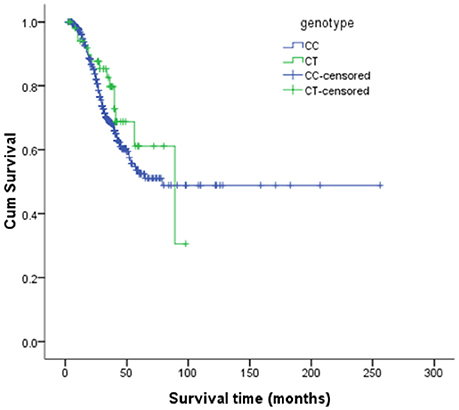
The risk allele (T allele; CT genotype) of SNP C270T was detected in 53 of the 499 patients with ALS. We divided the sALS patients into two groups: with and without the T risk allele group. Subgroup analysis was conducted to uncover the association between T risk allele with modifiable exposure and clinical phenotype. There were no difference between the two groups in smoking, alcohol and pesticide exposure, p-value respectively was 0.808, 0.480, and 0.977. We could not identify a significant difference in the mean age at onset, sex, site (bulbar/spinal) of onset, disease progression ΔFS, or survival time between the two groups (Table 4). The mean age at onset of ALS was 51.20 (±11.99) years in the patients without the risk allele and 52.02 (±10.89) years in those with the risk allele; this difference was not significant (t-test, P = 0.630). The sex ratio (male/female) was 284/162 in the patients without the risk allele and 37/16 in those with the risk allele. The site (bulbar/spinal) of symptom onset was 78/368 in the patients without the risk allele and 10/43 in those with the risk allele; again, this difference was not significant (Chi-square test, P = 0.803). The disease progression ΔFS also did not significantly differ between the two groups (Mann–Whitney U-test, P = 0.907). The Kaplan–Meier survival curves of the patients did not depend on C270T T allele carrier status; the curves of the patients with versus without the risk allele did not significantly differ (log-rank test, P = 0.316) (Figure 1).
Discussion
Here, we provide the first evidence that the BDNF C270T polymorphism might be associated with sporadic ALS in China. Our study suggests that the CT genotype and T allele of C270T in the BDNF gene might increase the risk of sALS in Han Chinese.
Our result is consistent with previous post-mortem and animal studies that have suggested that BDNF plays a role in the pathophysiology of ALS. If the T270 allele itself has a risk-increasing effect, it may result from changes in translation efficacy. The C270T SNP is located in a non-coding region of the 5′-untranslated region (UTR) of the BDNF gene and acts as a functional promoter polymorphism. We searched for potential modifications of transcription factor binding sites in the region of variant using Matinspector prediction software (Cartharius et al., 2005). The analyses indicated that the substitution of allele C with allele T could lend to the loss of transcription factors HINFP and ZIC3 binding sites. As a result, it may alter or control the efficacy of BDNF translation in the somatic, dendritic, or axonal regions of neurons, leading to region-specific quantitative BDNF imbalances in the cortex in patients. Another possibility is that other, currently unknown polymorphisms that are in linkage disequilibrium with the C270T polymorphism confer susceptibility to sALS.
However, we did not find strong evidence of a relationship between the different genotypes and the clinical symptoms of sALS. Altered levels of this neurotrophin have been linked to both cognitive and mood dysfunction (Teixeira et al., 2010). Accordingly, decreased BDNF levels have been reported in patients with depression, and a recent meta-analysis showed that altered peripheral BDNF levels are associated with ongoing depressive disorders (Molendijk et al., 2014). Depressive traits might contribute to major comorbidities and common and overlooked complications that often arise during the course of ALS (Kurt et al., 2007; Thakore and Pioro, 2016; Ye et al., 2016). We did not analyze depression disorders or cognitive function in the patients in this study. The T risk allele may be associated with depression disorders and cognitive function in ALS patients. Clinical trials that evaluate the relationship between genotype and depression disorders and cognitive function in ALS patients will be needed to test this hypothesis.
Our study had some limitations. First, our study just doing SNPs for two loci have some limitations, additional BDNF gene polymorphisms should be included in further study. Second, the sample size of this study was relative small and may be not enough to give a more confirmed conclusion in view of the statistic. However, as ALS is a rare disease, this sample size is relative large for a single center. Further studies with larger sample size especially multiple center studies with different ethnicities should be performed to verify the relationship between BNDF polymorphisms and sALS. Third, the T allele within the patients does not seem to be associated with the clinical phenotype and any modifiable exposure. Our explanation to this phenomena is that the number of the patients with T allele is too small to find any association.
In conclusion, this is the first report about the relationship between BDNF C270T/G196A loci and sporadic ALS. The result of this study indicate that the polymorphism C270T in BDNF gene might be associated with sporadic ALS in China and this relationship should be verified in future studies with larger and more homogeneous samples of different ethnicities.
Author Contributions
DF conceived this study and provided financial support. DF and LX designed the study. LX, DT, and JL took part in the design of the study and in sample collection. DF, LC, and LT conducted data management. LC and LT undertook data checking. LX and DF undertook statistical analysis. DF was responsible for project management. LX and DF were responsible for preparing and revising the manuscript. DF and LX had key roles in the study.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the National Natural Sciences Foundation of China [81030019].
References
(1999). A controlled trial of recombinant methionyl human BDNF in ALS: the BDNF study group (Phase III). Neurology 52, 1427#1433. doi: 10.1212/WNL.52.7.1427
Al-Chalabi, A., Fang, F., Hanby, M. F., Leigh, P. N., Shaw, C. E., Ye, W., et al. (2010). An estimate of amyotrophic lateral sclerosis heritability using twin data. J. Neurol. Neurosurg. Psychiatry 81, 1324–1326. doi: 10.1136/jnnp.2010.207464
Bodner, S. M., Berrettini, W., van Deerlin, V., Bennett, D. A., Wilson, R. S., Trojanowski, J. Q., et al. (2005). Genetic variation in the brain derived neurotrophic factor gene in Alzheimer's disease. Am. J. Med. Genet. B Neuropsychiatr. Genet. 134B, 1–5. doi: 10.1002/ajmg.b.30154
Borroni, B., Grassi, M., Archetti, S., Costanzi, C., Bianchi, M., Caimi, L., et al. (2009). BDNF genetic variations increase the risk of Alzheimer's disease-related depression. J. Alzheimers Dis. 18, 867–875. doi: 10.3233/JAD-2009-1191
Brooks, B. R., Miller, R. G., Swash, M., and Munsat, T. L. (2000). El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Mot. Neuron Disord. 1, 293–299. doi: 10.1080/146608200300079536
Byrne, S., Heverin, M., Elamin, M., Bede, P., Lynch, C., Kenna, K., et al. (2013). Aggregation of neurologic and neuropsychiatric disease in amyotrophic lateral sclerosis kindreds: a population-based case-control cohort study of familial and sporadic amyotrophic lateral sclerosis. Ann. Neurol. 74, 699–708. doi: 10.1002/ana.23969
Cartharius, K., Frech, K., Grote, K., Klocke, B., Haltmeier, M., Klingenhoff, A., et al. (2005). MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21, 2933–2942. doi: 10.1093/bioinformatics/bti473
Castrén, E., Võikar, V., and Rantamäki, T. (2007). Role of neurotrophic factors in depression. Curr. Opin. Pharmacol. 7, 18–21. doi: 10.1016/j.coph.2006.08.009
Chen, L., Zhang, B., Chen, R., Tang, L., Liu, R., Yang, Y., et al. (2015). Natural history and clinical features of sporadic amyotrophic lateral sclerosis in China. J. Neurol. Neurosurg. Psychiatry 86, 1075–1081. doi: 10.1136/jnnp-2015-310471
Chen, X., Huang, R., Chen, Y., Zheng, Z., Chen, K., Song, W., et al. (2014). Association analysis of four candidate genetic variants with sporadic amyotrophic lateral sclerosis in a Chinese population. Neurol. Sci. 35, 1089–1095. doi: 10.1007/s10072-014-1656-1
Cronin, S., Berger, S., Ding, J., Schymick, J. C., Washecka, N., Hernandez, D. G., et al. (2008). A genome-wide association study of sporadic ALS in a homogenous Irish population. Hum. Mol. Genet. 17, 768–774. doi: 10.1093/hmg/ddm361
Dadon-Nachum, M., Ben-Yaacov, K., Ben-Zur, T., Barhum, Y., Yaffe, D., Perlson, E., et al. (2015). Transplanted modified muscle progenitor cells expressing a mixture of neurotrophic factors delay disease onset and enhance survival in the SOD1 mouse model of ALS. J. Mol. Neurosci. 55, 788–797. doi: 10.1007/s12031-014-0426-0
Dai, L., Wang, D., Meng, H., Zhang, K., Fu, L., Wu, Y., et al. (2013). Association between the BDNF G196A and C270T polymorphisms and Parkinson's disease: a meta-analysis. Int. J. Neurosci. 123, 675–683. doi: 10.3109/00207454.2013.798784
Deepa, P., Shahani, N., Alladi, P. A., Vijayalakshmi, K., Sathyaprabha, T. N., Nalini, A., et al. (2011). Down regulation of trophic factors in neonatal rat spinal cord after administration of cerebrospinal fluid from sporadic amyotrophic lateral sclerosis patients. J. Neural Transm. (Vienna). 118, 531–538. doi: 10.1007/s00702-010-0520-6
Dmitrzak-Weglarz, M., Rybakowski, J. K., Suwalska, A., Skibinska, M., Leszczynska-Rodziewicz, A., Szczepankiewicz, A., et al. (2008). Association studies of the BDNF and the NTRK2 gene polymorphisms with prophylactic lithium response in bipolar patients. Pharmacogenomics 9, 1595–1603. doi: 10.2217/14622416.9.11.1595
Duberley, R. M., Johnson, I. P., Anand, P., Leigh, P. N., and Cairns, N. J. (1997). Immunocytochemical studies of neurotrophins in cerebral motor cortex in amyotrophic lateral sclerosis. Brain Res. 763, 259–263. doi: 10.1016/S0006-8993(97)00465-4
Fahey, C., Byrne, S., McLaughlin, R., Kenna, K., Shatunov, A., Donohoe, G., et al. (2014). Analysis of the hexanucleotide repeat expansion and founder haplotype at C9ORF72 in an Irish psychosis case-control sample. Neurobiol. Aging 35:1510.e1. doi: 10.1016/j.neurobiolaging.2013.12.003
Favalli, G., Li, J., Belmonte-de-Abreu, P., Wong, A. H., and Daskalakis, Z. J. (2012). The role of BDNF in the pathophysiology and treatment of schizophrenia. J. Psychiatr. Res. 46, 1–11. doi: 10.1016/j.jpsychires.2011.09.022
He, Y. Y., Zhang, X. Y., Yung, W. H., Zhu, J. N., and Wang, J. J. (2013). Role of BDNF in central motor structures and motor diseases. Mol. Neurobiol. 48, 783–793. doi: 10.1007/s12035-013-8466-y
Henderson, C. E., Camu, W., Mettling, C., Gouin, A., Poulsen, K., Karihaloo, M., et al. (1993). Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature 363, 266–270. doi: 10.1038/363266a0
Hetz, C., and Mollereau, B. (2014). Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 15, 233–249. doi: 10.1038/nrn3689
Holsinger, R. M., Schnarr, J., Henry, P., Castelo, V. T., and Fahnestock, M. (2000). Quantitation of BDNF mRNA in human parietal cortex by competitive reverse transcription-polymerase chain reaction: decreased levels in Alzheimer's disease. Brain Res. Mol. Brain Res. 76, 347–354. doi: 10.1016/S0169-328X(00)00023-1
Huynh, W., and Kiernan, M. C. (2015). A unique account of ALS in China: exploring ethnic heterogeneity. J. Neurol. Neurosurg. Psychiatry 86, 1051–1052. doi: 10.1136/jnnp-2015-311293
Hyman, C., Hofer, M., Barde, Y. A., Juhasz, M., Yancopoulos, G. D., Squinto, S. P., et al. (1991). BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350, 230–232. doi: 10.1038/350230a0
Iida, A., Takahashi, A., Kubo, M., Saito, S., Hosono, N., Ohnishi, Y., et al. (2011). A functional variant in ZNF512B is associated with susceptibility to amyotrophic lateral sclerosis in Japanese. Hum. Mol. Genet. 20, 3684–3692. doi: 10.1093/hmg/ddr268
Ikeda, K., Klinkosz, B., Greene, T., Cedarbaum, J. M., Wong, V., Lindsay, R. M., et al. (1995). Effects of brain-derived neurotrophic factor on motor dysfunction in wobbler mouse motor neuron disease. Ann. Neurol. 37, 505–511. doi: 10.1002/ana.410370413
Kawamoto, Y., Nakamura, S., Akiguchi, I., and Kimura, J. (1998). Immunohistochemical localization of brain-derived neurotrophic factor in the spinal cords of amyotrophic lateral sclerosis and non-amyotrophic lateral sclerosis patients. J. Neuropathol. Exp. Neurol. 57, 822–830. doi: 10.1097/00005072-199809000-00003
Koliatsos, V. E., Clatterbuck, R. E., Winslow, J. W., Cayouette, M. H., and Price, D. L. (1993). Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron 10, 359–367. doi: 10.1016/0896-6273(93)90326-M
Kunugi, H., Ueki, A., Otsuka, M., Isse, K., Hirasawa, H., Kato, N., et al. (2001). A novel polymorphism of the brain-derived neurotrophic factor (BDNF) gene associated with late-onset Alzheimer's disease. Mol. Psychiatry 6, 83–86. doi: 10.1038/sj.mp.4000792
Kurt, A., Nijboer, F., Matuz, T., and Kübler, A. (2007). Depression and anxiety in individuals with amyotrophic lateral sclerosis: epidemiology and management. CNS Drugs 21, 279–291. doi: 10.2165/00023210-200721040-00003
Landers, J. E., Melki, J., Meininger, V., Glass, J. D., van den Berg, L. H., van Es, M. A., et al. (2009). Reduced expression of the Kinesin-Associated Protein 3 (KIFAP3) gene increases survival in sporadic amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. U.S.A. 106, 9004–9009. doi: 10.1073/pnas.0812937106
Lee, Y. H., and Song, G. G. (2014). BDNF 196 G/A and 270 C/T polymorphisms and susceptibility to Parkinson's disease: a meta-analysis. J. Mot. Behav. 46, 59–66. doi: 10.1080/00222895.2013.862199
Leibrock, J., Lottspeich, F., Hohn, A., Hofer, M., Hengerer, B., Masiakowski, P., et al. (1989). Molecular cloning and expression of brain-derived neurotrophic factor. Nature 341, 149–152. doi: 10.1038/341149a0
Libman-Sokołowska, M., Drozdowicz, E., and Nasierowski, T. (2015). BDNF as a biomarker in the course and treatment of schizophrenia. Psychiatr. Pol. 49, 1149–1158. doi: 10.12740/PP/37705
Logroscino, G., Traynor, B. J., Hardiman, O., Chio, A., Mitchell, D., Swingler, R. J., et al. (2010). Incidence of amyotrophic lateral sclerosis in Europe. J. Neurol. Neurosurg. Psychiatry 81, 385–390. doi: 10.1136/jnnp.2009.183525
Mitsumoto, H., Ikeda, K., Klinkosz, B., Cedarbaum, J. M., Wong, V., and Lindsay, R. M. (1994). Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science 265, 1107–1110. doi: 10.1126/science.8066451
Molendijk, M. L., Spinhoven, P., Polak, M., Bus, B. A., Penninx, B. W., and Elzinga, B. M. (2014). Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol. Psychiatry 19, 791–800. doi: 10.1038/mp.2013.105
Nagata, T., Shinagawa, S., Nukariya, K., Ochiai, Y., Kawamura, S., Agawa-Ohta, M., et al. (2011). Association between brain-derived neurotrophic factor (BDNF) gene polymorphisms and executive function in Japanese patients with Alzheimer's disease. Psychogeriatrics 11, 141–149. doi: 10.1111/j.1479-8301.2011.00364.x
Nishio, T., Sunohara, N., and Furukawa, S. (1998). Neutrophin switching in spinal motoneurons of amyotrophic lateral sclerosis. Neuroreport 9, 1661–1665. doi: 10.1097/00001756-199805110-00073
Ochs, G., Penn, R. D., York, M., Giess, R., Beck, M., Tonn, J., et al. (2000). A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 1, 201–206. doi: 10.1080/14660820050515197
Olin, D., MacMurray, J., and Comings, D. E. (2005). Risk of late-onset Alzheimer's disease associated with BDNF C270T polymorphism. Neurosci. Lett. 381, 275–278. doi: 10.1016/j.neulet.2005.02.017
Parsian, A., Sinha, R., Racette, B., Zhao, J. H., and Perlmutter, J. S. (2004). Association of a variation in the promoter region of the brain-derived neurotrophic factor gene with familial Parkinson's disease. Parkinsonism Relat. Disord. 10, 213–219. doi: 10.1016/j.parkreldis.2003.12.003
Phillips, H. S., Hains, J. M., Armanini, M., Laramee, G. R., Johnson, S. A., and Winslow, J. W. (1991). BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer's disease. Neuron 7, 695–702. doi: 10.1016/0896-6273(91)90273-3
Sendtner, M., Holtmann, B., Kolbeck, R., Thoenen, H., and Barde, Y. A. (1992). Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature 360, 757–759. doi: 10.1038/360757a0
Shahrizaila, N., Sobue, G., Kuwabara, S., Kim, S. H., Birks, C., Fan, D. S., et al. (2016). Amyotrophic lateral sclerosis and motor neuron syndromes in Asia. J. Neurol. Neurosurg. Psychiatry 87, 821–830. doi: 10.1136/jnnp-2015-312751
Simpson, C. L., Lemmens, R., Miskiewicz, K., Broom, W. J., Hansen, V. K., van Vught, P. W., et al. (2009). Variants of the elongator protein 3 (ELP3) gene are associated with motor neuron degeneration. Hum. Mol. Genet. 18, 472–481. doi: 10.1093/hmg/ddn375
Su, N., Zhang, L., Fei, F., Hu, H., Wang, K., Hui, H., et al. (2011). The brain-derived neurotrophic factor is associated with alcohol dependence-related depression and antidepressant response. Brain Res. 1415, 119–126. doi: 10.1016/j.brainres.2011.08.005
Teixeira, A. L., Barbosa, I. G., Diniz, B. S., and Kummer, A. (2010). Circulating levels of brain-derived neurotrophic factor: correlation with mood, cognition and motor function. Biomark Med. 4, 871–887. doi: 10.2217/bmm.10.111
Thakore, N. J., and Pioro, E. P. (2016). Depression in ALS in a large self-reporting cohort. Neurology 86, 1031–1038. doi: 10.1212/WNL.0000000000002465
Tremolizzo, L., Pellegrini, A., Conti, E., Arosio, A., Gerardi, F., Lunetta, C., et al. (2016). BDNF serum levels with respect to multidimensional assessment in amyotrophic lateral sclerosis. Neurodegener. Dis. 16, 192–198. doi: 10.1159/000441916
Van Es, M. A., Van Vught, P. W., Blauw, H. M., Franke, L., Saris, C. G., Andersen, P. M., et al. (2007). ITPR2 as a susceptibility gene in sporadic amyotrophic lateral sclerosis: a genome-wide association study. Lancet Neurol. 6, 869–877. doi: 10.1016/S1474-4422(07)70222-3
Van Es, M. A., van Vught, P. W., Blauw, H. M., Franke, L., Saris, C. G., Van den Bosch, L., et al. (2008). Genetic variation in DPP6 is associated with susceptibility to amyotrophic lateral sclerosis. Nat. Genet. 40, 29–31. doi: 10.1038/ng.2007.52
Van Es, M. A., Van Vught, P. W., Veldink, J. H., Andersen, P. M., Birve, A., Lemmens, R., et al. (2009b). Analysis of FGGY as a risk factor for sporadic amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 10, 441–447. doi: 10.3109/17482960802673042
Van Es, M. A., Veldink, J. H., Saris, C. G., Blauw, H. M., van Vught, P. W., Birve, A., et al. (2009a). Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat. Genet. 41, 1083–1087. doi: 10.1038/ng.442
Ventriglia, M., Bocchio, C. L., Benussi, L., Binetti, G., Zanetti, O., Riva, M. A., et al. (2002). Association between the BDNF 196 A/G polymorphism and sporadic Alzheimer's disease. Mol. Psychiatry 7, 136–137. doi: 10.1038/sj.mp.4000952
Vepsäläinen, S., Castren, E., Helisalmi, S., Iivonen, S., Mannermaa, A., Lehtovirta, M., et al. (2005). Genetic analysis of BDNF and TrkB gene polymorphisms in Alzheimer's disease. J. Neurol. 252, 423–428. doi: 10.1007/s00415-005-0667-5
Watanabe, Y., Nunokawa, A., and Someya, T. (2013). Association of the BDNF C270T polymorphism with schizophrenia: updated meta-analysis. Psychiatry Clin. Neurosci. 67, 123–125. doi: 10.1111/pcn.12018
Ye, S., Ji, Y., Li, C., He, J., Liu, X., and Fan, D. (2016). The Edinburgh cognitive and behavioural ALS screen in a Chinese amyotrophic lateral sclerosis population. PLoS ONE 11:e0155496. doi: 10.1371/journal.pone.0155496
Zdanys, K. F., Kleiman, T. G., Zhang, H., Ozbay, F., MacAvoy, M. G., Gelernter, J., et al. (2009). BDNF variants, premorbid educational attainment, and disease characteristics in Alzheimer's disease: an exploratory study. J. Alzheimers Dis. 17, 887–898. doi: 10.3233/JAD-2009-1106
Keywords: amyotrophic lateral sclerosis, brain-derived neurotrophic factor, Chinese cohort, polymorphisms, susceptibility
Citation: Xu L, Tian D, Li J, Chen L, Tang L and Fan D (2017) The Analysis of Two BDNF Polymorphisms G196A/C270T in Chinese Sporadic Amyotrophic Lateral Sclerosis. Front. Aging Neurosci. 9:135. doi: 10.3389/fnagi.2017.00135
Received: 25 December 2016; Accepted: 21 April 2017;
Published: 10 May 2017.
Edited by:
Oliver von Bohlen und Halbach, Universitätsmedizin Greifswald, GermanyReviewed by:
Neel Kamal Sharma, National Eye Institute, United StatesVassiliki Nikoletopoulou, Institute of Molecular Biology and Biotechnology, Greece
Copyright © 2017 Xu, Tian, Li, Chen, Tang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongsheng Fan, dsfan2010@aliyun.com
 Lianping Xu
Lianping Xu Danyang Tian
Danyang Tian Jiao Li
Jiao Li Lu Chen
Lu Chen Lu Tang
Lu Tang Dongsheng Fan
Dongsheng Fan