- 1Faculty of Pharmacy, Siam University, Bangkok, Thailand
- 2Division of Clinical Pharmacy, Department of Pharmacy, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand
- 3Division of Pharmacy Practice, Faculty of Pharmaceutical Sciences, Ubon Ratchathani University, Ubon Ratchathani, Thailand
To the best of our knowledge, to date, no study has investigated the optimal dosage regimens of either colistin or sitafloxacin against drug-resistant Acinetobacter baumannii (A. baumannii) infections by using specific parameters. In the current study, we aimed to explore the optimal dosage regimens of colistin and sitafloxacin, either in monotherapy or in combination therapy, for the treatment of carbapenem-, multidrug-, and colistin-resistant A. baumannii infections. A Monte Carlo simulation was applied to determine the dosage regimen that could achieve the optimal probability of target attainment (PTA) and cumulative fraction of response (CFR) (≥90%) based on the specific parameters of each agent and the minimal inhibitory concentration (MIC) of the clinical isolates. This study explored the dosage regimen of 90, 50, 30, and 10 mL/min for patients with creatinine clearance (CrCL). We also explored the dosage regimen for each patient with CrCL using combination therapy because there is a higher possibility of reaching the desired PTA or CFR. Focusing on the MIC90 of each agent in combination therapy, the dosage regimen for colistin was a loading dose of 300 mg followed by a maintenance dose ranging from 50 mg every 48 h to 225 mg every 12 h and the dosage regimen for sitafloxacin was 325 mg every 48 h to 750 mg every 12 h. We concluded that a lower-than-usual dose of colistin based on specific pharmacokinetic data in combination with a higher-than-usual dose of sitafloxacin could be an option for the treatment of carbapenem-, multidrug-, and colistin-resistant. A. baumannii. The lower dose of colistin might show a low probability of adverse reaction, while the high dose of sitafloxacin should be considered. In the current study, we attempted to find if there is a strong possibility of drug selection against crucial drug-resistant pathogen infections in a situation where there is a lack of new antibiotics. However, further study is needed to confirm the results of this simulation study.
Introduction
Acinetobacter baumannii (A. baumannii) is one of the most important gram-negative pathogens that cause various nosocomial infections (García-Garmendia et al., 2001; Cisneros and Rodríguez-Baño, 2002; Werarak et al., 2012; Sieniawski et al., 2013; Almasaudi, 2018; Centers for Disease Control and Prevention, n.d.). Drug-resistant A. baumannii infections, including multidrug-resistant A. baumannii (MDR-AB), carbapenem-resistant A. baumannii (CRAB), and colistin-resistant A. baumannii (CoR-AB) infections, are a crucial problem because they cause prolonged hospitalization and a high mortality rate (Sunenshine et al., 2007; Kaye and Pogue, 2015). The study by Appaneal et al. showed that the inpatient mortality rate was higher in those with MDR-AB than those with non-MDR-AB infection (aOR 1.61) and in those with CRAB than non-CRAB infection (aOR 1.68). A hospitalization duration of more than 10 days was higher in those with MDR-AB compared to those with non-MDR-AB infection and in those with CRAB compared to those with non-CRAB infection (Appaneal et al., 2022). Moreover, the clinical outcomes were worse among patients with MDR-AB and/or CRAB infections (Appaneal et al., 2022). Similarly, the meta-analysis showed that the CRAB could increase the risk of high mortality rate in patients (Lemos et al., 2014). Compared to colistin-susceptible AB infection, patients with CoR-AB bloodstream infection had higher mortality (100% vs. 50%, respectively (p = 0.001)) and died sooner (p = 0.006) (Papathanakos et al., 2020).
Colistin has gained attention for its use in the treatment of drug-resistant A. baumannii infections (Falagas et al., 2010; Lim et al., 2011; Batirel et al., 2014; Kalin et al., 2014; López-Cortés et al., 2014; Yilmaz et al., 2015; Amat et al., 2018; Liang et al., 2018; Dickstein et al., 2019). The optimal dosage regimens for colistin have been investigated from the past to the present based on the population pharmacokinetic model and pharmacokinetic/pharmacodynamic (PK/PD) index of colistin (Garonzik et al., 2011; Nation et al., 2017). The usual PK/PD index of colistin is the average steady-state plasma colistin concentration (Css,avg). However, recent studies found that the most predictive PK/PD index of colistin against A. baumannii was the ratio of the area under the unbound concentration-time curve to the minimum inhibitory concentration (ƒAUC/MIC) (Dudhani et al., 2010; Cheah et al., 2015). A common adverse drug reaction from colistin is nephrotoxicity, which occurs in a dose- and time-dependent manner (Spapen et al., 2011; Dai et al., 2014). Therefore, the challenge of exploring a colistin dosage regimen is focused on both increasing efficacy and lowering toxicity.
Sitafloxacin is a fluoroquinolone that has shown excellent in vitro activity against drug-resistant A. baumannii (Dong et al., 2015; Huang et al., 2015; Rodjun et al., 2020). Its population pharmacokinetic model and PK/PD index have also been studied (Tanigawara et al., 2013). A key feature of sitafloxacin is its excellent penetration into the epithelial lining fluid (ELF) of critically ill patients with pneumonia; according to the data of Paiboonvong et al., the AUC0–8 h of ELF/unbound plasma ratio was 0.85 (Paiboonvong et al., 2019). Moreover, sitafloxacin in combination with colistin can decrease the MIC values of either colistin or sitafloxacin. This in vitro activity was observed when using this combination against extensively drug-resistant A. baumannii (XDR-AB) (Dong et al., 2015), MDR-AB, CRAB, and CoR-AB (Rodjun et al., 2020).
The World Health Organization (WHO) announced that there are declining private investments and a lack of innovation in the development of new antibiotics. Over 30 antibiotics are still in the clinical development pipeline (Kmietowicz, 2017; Butler et al., 2022). Because of the lack of novel antibiotics to treat drug-resistant bacterial infections, the standard guideline for treating drug-resistant pathogen infections still recommends using familiar antibiotics or some regimens that use the combination therapy (Tamma et al., 2023). The combination regimens of colistin with other antibiotics such as sulbactam, tigecycline, and carbapenems have been options for the management of drug-resistant A. baumannii infections (Garonzik et al., 2011; Lim et al., 2011; Batirel et al., 2014; Kalin et al., 2014; López-Cortés et al., 2014; Amat et al., 2018; Dickstein et al., 2019). From a previous in vitro study with sitafloxacin, the combination of colistin and sitafloxacin is one interesting possibility. This study aimed to explore the optimal dosage regimens of colistin and sitafloxacin, either in monotherapy or in combination therapy, for the treatment of MDR-AB, CRAB, and CoR-AB infections using a Monte Carlo simulation that was based on the specific population pharmacokinetics and PK/PD index of each agent.
Materials and methods
Microbiology
Data on A. baumannii were obtained from a prior study by Rodjun et al. (2020). Three hundred A. baumannii clinical isolates were comprised of MDR-AB–263 isolates (87.7%), CRAB–258 isolates (86%), and CoR-AB–43 isolates (14.3%). The MIC50/90 of colistin in MDR-AB and CRAB was 2/4 mg/L and that of CoR-AB was 8/8 mg/L. The MIC50/90 of sitafloxacin in MDR-AB and CRAB was 1/2 mg/L and that of CoR-AB was 0.5/1 mg/L. The MIC50/90 of colistin in combination regimens in MDR-AB and CRAB was 0.5/1 mg/L and that of CoR-AB was 1/2 mg/L. The MIC50/90 of sitafloxacin in combination regimens in MDR-AB and CRAB was 0.5/1 mg/L and that of CoR-AB was 0.25/1 mg/L.
Pharmacokinetic model
Colistin
The population pharmacokinetic models for colistimethate sodium (CMS) and colistin were two-compartment and one-compartment models, respectively. We used pharmacokinetic data from Nation et al. (2017), who studied the dosing guidance for colistin in critically ill patients with a CrCL of 0–236 mL/min. The parameters were randomly generated for each estimated mean, and the %IIV of the parameters are shown in Table 1. The equations below were modified according to the study of Garonzik et al. (2011) and represent the differential equations for the disposal of CMS and colistin. The unbound fraction of 0.49 +/− 0.11, determined by ultracentrifugation in samples collected from the patients in the prior study, was used (Nation et al., 2017).
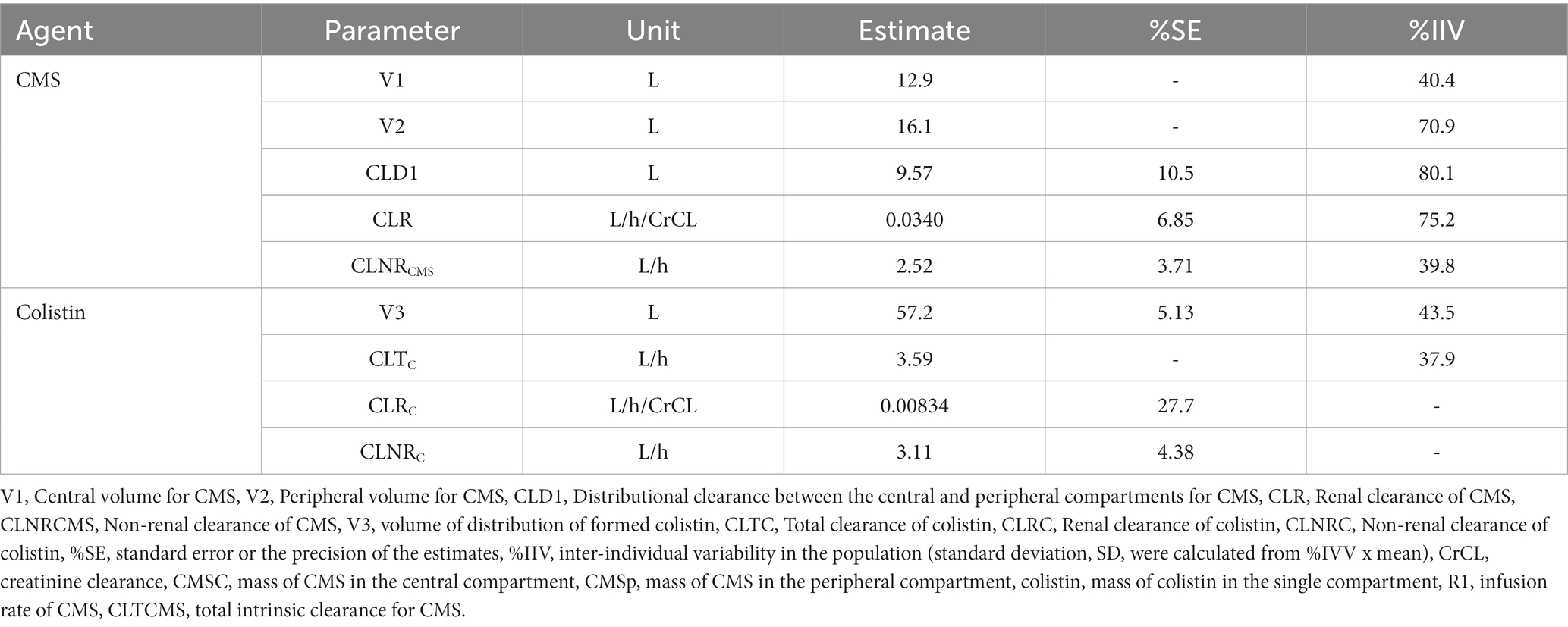
Table 1. Population pharmacokinetic parameters of colistin (Nation et al., 2017).
Sitafloxacin
The population pharmacokinetic model for oral sitafloxacin was assumed to follow the one-compartment model with first-order absorption (Tanigawara et al., 2013). We used pharmacokinetic data from Tanigawara et al. (2013), who used clinical data from clinical pharmacology studies, including a study on healthy, elderly but renally impaired patients (Nakashima et al., 1995; Nakashima, 2008; Nakashima and Kawada, 2008; Sekino, 2008), and the clinical PK/PD study on patients with respiratory tract infections (Saito et al., 2008). The parameters are shown in Table 2. The equation below was used to calculate the plasma sitafloxacin concentration (Jambhekar and Breen, 2012). An unbound fraction of sitafloxacin of 0.388 was used (Tanigawara et al., 2013).
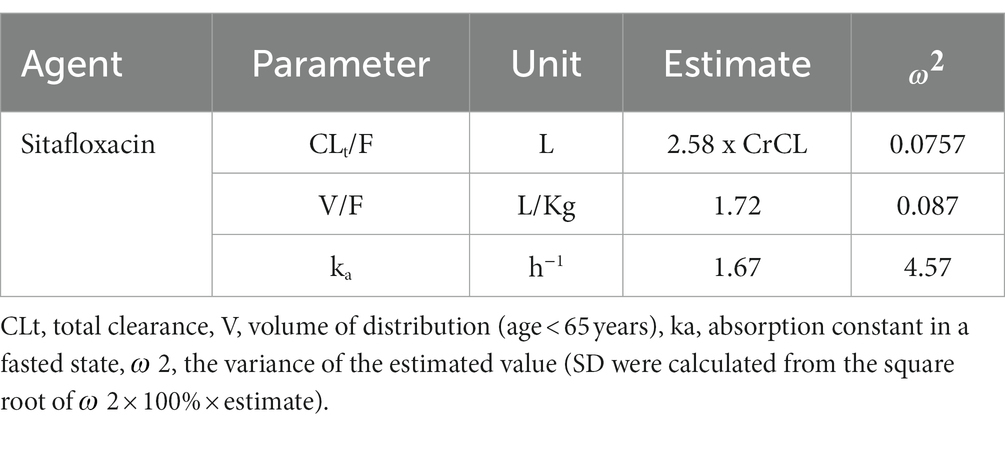
Table 2. Population pharmacokinetic parameters of sitafloxacin (Tanigawara et al., 2013).
where dX/dt = the rate of change of the amount of drug in the plasma, X = the mass of drug in the plasma at time t, Xa = the mass of absorbable drug at time t, Ka and K = the first-order of absorption and elimination rate constants, respectively, KaXa = the first-order rate of absorption, and KX = the first-order rate of elimination.
Pharmacokinetic or pharmacodynamic index
Colistin
The pharmacokinetic/pharmacodynamic index (PK/PD) index of colistin is characterized by ƒAUC/MIC ≥7.4, which showed a 2-log10 reduction of MDR A. baumannii clinical isolate strain 248-01-C.248 (MIC of colistin is 1 mg/L) in a mouse model with a thigh infection. This value resulted in bacterial burdens in mouse thighs determined at 2 h after inoculation (untreated controls) and 24 h later (untreated controls and colistin-treated subjects) (Cheah et al., 2015). The model with the thigh infection has been used in most in vivo studies (Craig, 1998) and is considered to be the gold standard for evaluating the efficacy of antimicrobials since its high degree of translation to human patients (Yedle et al., 2023). Moreover, the thigh infection model is considered to be an adequate simulator because the model allows for the dissemination of the offending pathogen in blood and viscera to occur as in the clinical status of bacteremia (Pantopoulou et al., 2007).
Sitafloxacin
The PK/PD index of sitafloxain is characterized by ƒAUC/MIC >30, which showed an eradication effect of 96.4% on respiratory tract infection (RTIs) isolates. This value was determined for individual PK parameters with MIC in 91 RTI isolates, and the attainment rates of the ƒAUC/MIC were calculated (Tanigawara et al., 2013).
Simulated dosage regimens
Colistin
The dosage regimens were chosen according to the study of Nation et al. (2017), the guidelines of the European Medicine Agency (EMA) (Nation et al., 2016) and the United States Food and Drug Administration (FDA) (Nation et al., 2016), and the recommended dosage regimens by Siriraj Hospital, Thailand, and our study’s dosage regimens. The creatinine clearance (CrCL) values used for the simulation were 90, 50, 30, and 10 mL/min. Each dose was infused for 30 min, and each dosage regimen starts with the loading dose (LD) of 450 mg or 300 mg. The maintenance doses vary from 50 mg every 48 h to 450 mg every 12 h according to the CrCL value.
Sitafloxacin
The dosage regimens were chosen according to the manufacturer’s recommendations (Pharmaceuticals and Medical Devices Agency, n.d.) and our study’s dosage regimens. The regimens were administered orally to an inpatient in a fasted state who weighed 60 kg and was under 65 years of age. The CrCL values used in this study were 90, 50, 30, and 10 mL/min. The doses vary from 50 mg every 48 h to 1,500 mg every 12 h according to the CrCL value.
Monte Carlo simulation
A Monte Carlo Simulation (Crystal Ball version 2017; Decisioneering Inc., Denver, CO United States) was applied to generate 10,000 subjects for each regimen. Log-normal distributions were studied for between-patient variability of each parameter except the unbound fraction of colistin, which was studied by the uniform distribution. The probability of target attainment (PTA) was determined as the percentage of all 10,000 estimates that achieved or exceeded the pharmacodynamic surrogate indices of each agent. Both colistin and sitafloxacin use ƒAUC/MIC. The AUC was determined using the linear trapezoidal rule, while ƒ was the unbound fraction of each agent. The MIC values were calculated from a prior study (Rodjun et al., 2020). The cumulative fraction of response (CFR) was calculated as the proportion of %PTA of each MIC according to the MIC distribution. The PTA and CFR (Asuphon et al., 2016; Jitaree et al., 2019; Leelawattanachai et al., 2020), which we calculated at the steady state, were considered optimal at ≥90%. This study was approved by the Ethics Committee of the Faculty of Dentistry/Faculty of Pharmacy, Mahidol University, Phutthamonthon District, Nakhon Pathom, Thailand (COE.No.MU-DT/PY-IRB 2020/001.1501).
Result
Colistin monotherapy
The dosage regimens of colistin that achieved a PTA of ≥90% for the MIC50 of MDR-AB and CRAB (2 mg/L) were the maintenance doses of 225 mg every 12 h, 150 mg every 24 h, 75 mg every 24 h, and 50 mg every 24 h for CrCL values of 90, 50, 30, and 10 mL/min, respectively. The dosage regimens that achieved a PTA of ≥90% for the MIC90 of MDR-AB and CRAB (4 mg/L) were maintenance doses of 300 mg every 12 h, 150 mg every 24 h, and 75 mg every 24 h for CrCL values of 50, 30, 10 mL/min, respectively. The dosage regimens that achieved a PTA of ≥90% for the MIC50/90 of CoR-AB (8 mg/L) were maintenance doses of 450 mg every 12 h, 300 mg every 12 h, and 150 mg every 24 h for CrCL values 50, 30, and 10 mL/min, respectively. No dosage regimen was recommended for patients with CrCL 90 mL/min at MIC of 2 and 4 mg/L.
The dosage regimens of colistin that achieved a CFR of ≥90% for MDR-AB and CRAB were maintenance doses of 300 mg every 12 h, 150 mg every 12 h, 150 mg every 24 h, and 50 mg every 24 h for CrCL values of 90, 50, 30, and 10 mL/min, respectively. The maintenance doses of 450 mg every 12 h, 300 mg every 12 h, and 150 mg every 24 h were recommended for patients with CrCL values 50, 30, and 10 mL/min, respectively. No dosage regimen was recommended for patients with CrCL values of 90 mL/min.
As can be observed from the results, a lower CrCL value (50, 30, and 10 mL/min) can achieve the target by the usual dosage regimen including the USFDA recommended. Meanwhile, a CrCL value of 90 mL/min should be used in our regimen, which is higher than the usual dose. The results table of colistin monotherapy is shown in the Supplementary Table S1.
Sitafloxacin monotherapy
The dosage regimens that achieved a PTA of ≥90% for the MIC50 of CoR-AB (0.5 mg/L) were doses of 375 mg every 12 h, 225 mg every 12 h, 250 mg every 24 h, and 175 mg every 48 h for CrCL values of 90, 50, 30, and 10 mL/min, respectively. The dosage regimen that achieved a PTA of ≥90% for the MIC50 of MDR-AB and CRAB, and the MIC90 of CoR-AB (1 mg/L) were doses of 750 mg every 12 h, 425 mg every 12 h, 500 mg every 24 h, and 325 mg every 48 h for CrCL values of 90, 50, 30, and 10 mL/min, respectively. The dosage regimens that achieved a PTA of ≥90% for the MIC90 of MDR-AB and CRAB (2 mg/L) were doses of 1,500 mg every 12 h, 750 mg every 12 h, 1,000 mg every 24 h, and 675 mg every 48 h for CrCL values of 90, 50, 30, and 10 mL/min, respectively.
The dosage regimens of sitafloxacin that achieved a CFR of ≥90% of MDR-AB and CRAB were doses of 1,500 mg every 12 h, 750 mg every 12 h, and 500 mg every 48 h for CrCL 90, 50, and 10 mL/min, respectively. For CrCL 30 mL/min, these doses were 800 mg every 24 h and 750 mg every 24 h of MDR-AB and CRAB, respectively. The dosage regimens for CoR-AB were 1,000 mg every 12 h, 500 mg every 12 h, 750 mg every 24 h, and 500 mg every 48 h for CrCL values of 90, 50, 30, and 10 mL/min, respectively.
As can be observed from the results, the manufacturer’s regimen cannot reach the target in any CrCL values. All the recommended dosage regimens were generated in this study. The lowest dose to achieve the target was 500 mg every 48 h. The results table of sitafloxacin monotherapy is shown in the Supplementary Table S2.
Colistin in combinations
The dosage regimens of colistin in combinations that achieved a PTA of ≥90% for the MIC50 of MDR-AB and CRAB (0.5 mg/L) were maintenance doses of 100 mg every 24 h for CrCL values of 90 mL/min and 50 mg every 48 h for 50, 30, and 10 mL/min. The dosage regimens that achieved a PTA of ≥90% for the MIC90 of MDR-AB, CRAB, and the MIC50 of CoR-AB (1 mg/L) were maintenance doses of 150 mg every 12 h for CrCL 90 mL/min, 75 mg every 24 h for CrCL 50 mL/min, and 50 mg every 48 h for CrCL 30 and 10 mL/min. The dosage regimens for the MIC90 of CoR-AB were the same as those for the MIC50 of MDR-AB and CRAB in monotherapy.
The dosage regimens that achieved a CFR of ≥90% for MDR-AB and CRAB were maintenance doses of 100 mg every 12 h for CrCL 90 mL/min and 50 mg every 48 h for CrCL values of 50, 30, and 10 mL/min. The maintenance doses for CoR-AB were 180 mg every 12 h, 150 mg every 24 h, 75 mg every 24 h, and 50 mg every 48 h for CrCL values of 50, 30, and 10 mL/min, respectively.
Overall, the doses of colistin in combination were lower than in monotherapy. The lower CrCL values (50, 30, and 10 mL/min) can achieve the target by the usual regimen while the CrCL values of 90 mL/min should use a higher dose, especially at MIC 2 mg/L.
Sitafloxacin in combinations
The dosage regimens of sitafloxacin in combinations that achieved a PTA of ≥90% for the MIC50 of CoR-AB (0.25 mg/L) were doses of 200 mg every 12 h, 125 mg every 12 h, 125 mg every 24 h, and 50 mg every 24 h for CrCL values of 90, 50, 30, and 10 mL/min, respectively. The dosage regimens that achieved a PTA of ≥90% for the MIC50 of MDR-AB and CRAB (0.5 mg/L) were the same as those for the MIC50 of CoR-AB in monotherapy. The dosage regimens that achieved a PTA of ≥90% for the MIC90 of MDR-AB and CRAB (1 mg/L) were the same as those for the MIC50 of MDR-AB and CRAB (1 mg/L) in monotherapy.
The optimal doses of sitafloxacin in combinations that achieved a CFR of ≥90% for MDR-AB and CRAB were doses of 750 mg every 12 h, 400 mg every 12 h, 500 mg every 24 h, and 300 mg every 48 h for CrCL values of 90, 50, 30, and 10 mL/min, respectively. The dosage regimens that achieved a CFR of ≥90% for CoR-AB were doses of 750 mg every 12 h, 400 mg every 12 h, 300 mg every 24 h, and 200 mg every 48 h for CrCL values of 90, 50, 30, and 10 mL/min, respectively.
The dosage regimens in combinations were lower than those in monotherapy. However, the manufacturer’s regimen cannot reach the target in any CrCL values.
The PTA analyses of various colistin and sitafloxacin regimens and CrCL are shown in Figures 1, 2, respectively. The PTA and CFR of each dosage regimen are shown in Tables 3–5 and in Supplementary material.
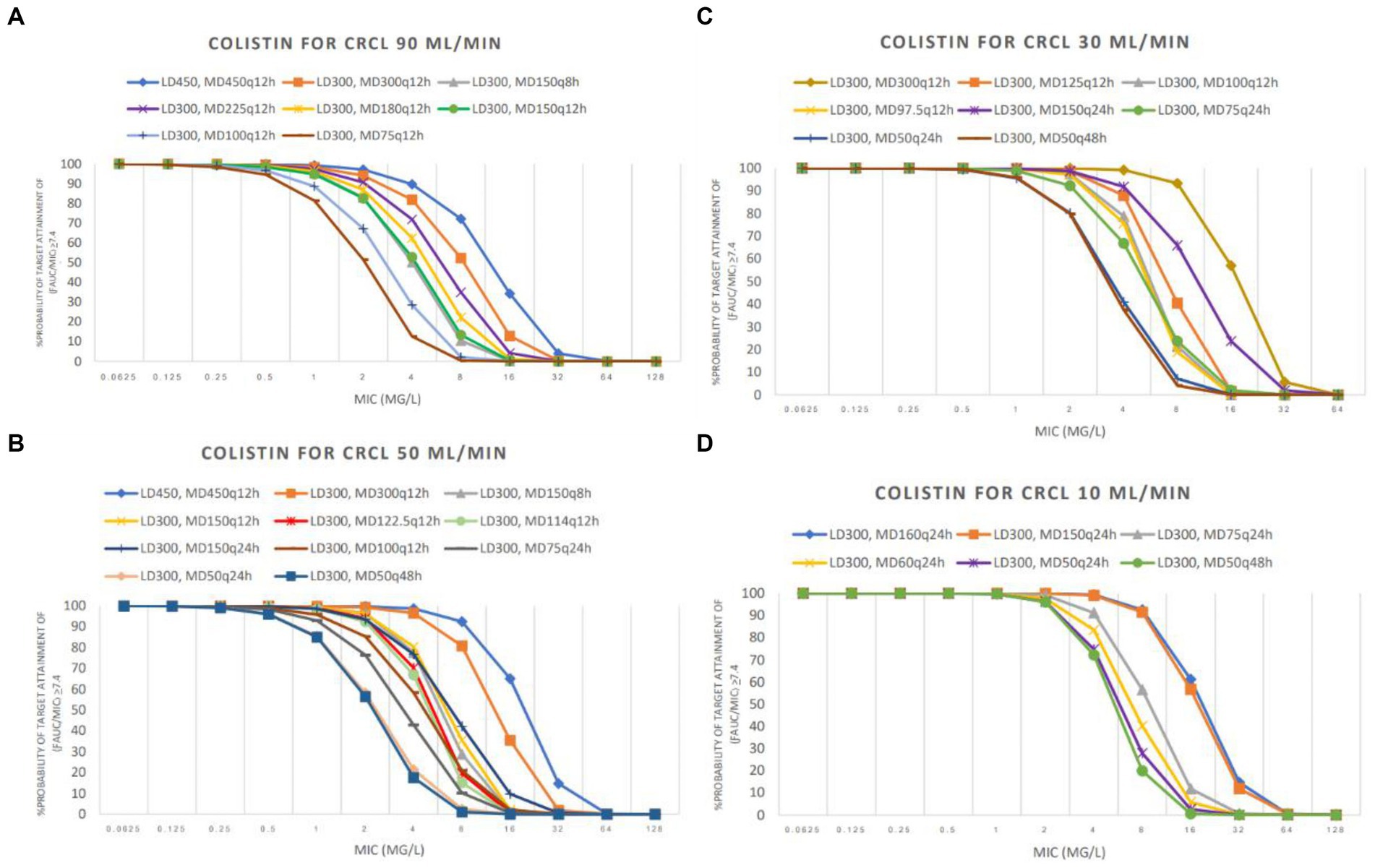
Figure 1. Probability of target attainment (PTA) achieved with colistin used in groups CrCL 90 mL/min (A), CrCL 50 mL/min (B), CrCL 30 mL/min (C), and CrCL 10 mL/min (D). LD: loading dose (in mg colistin base activity), MD: maintenance doses (in mg colistin base activity).
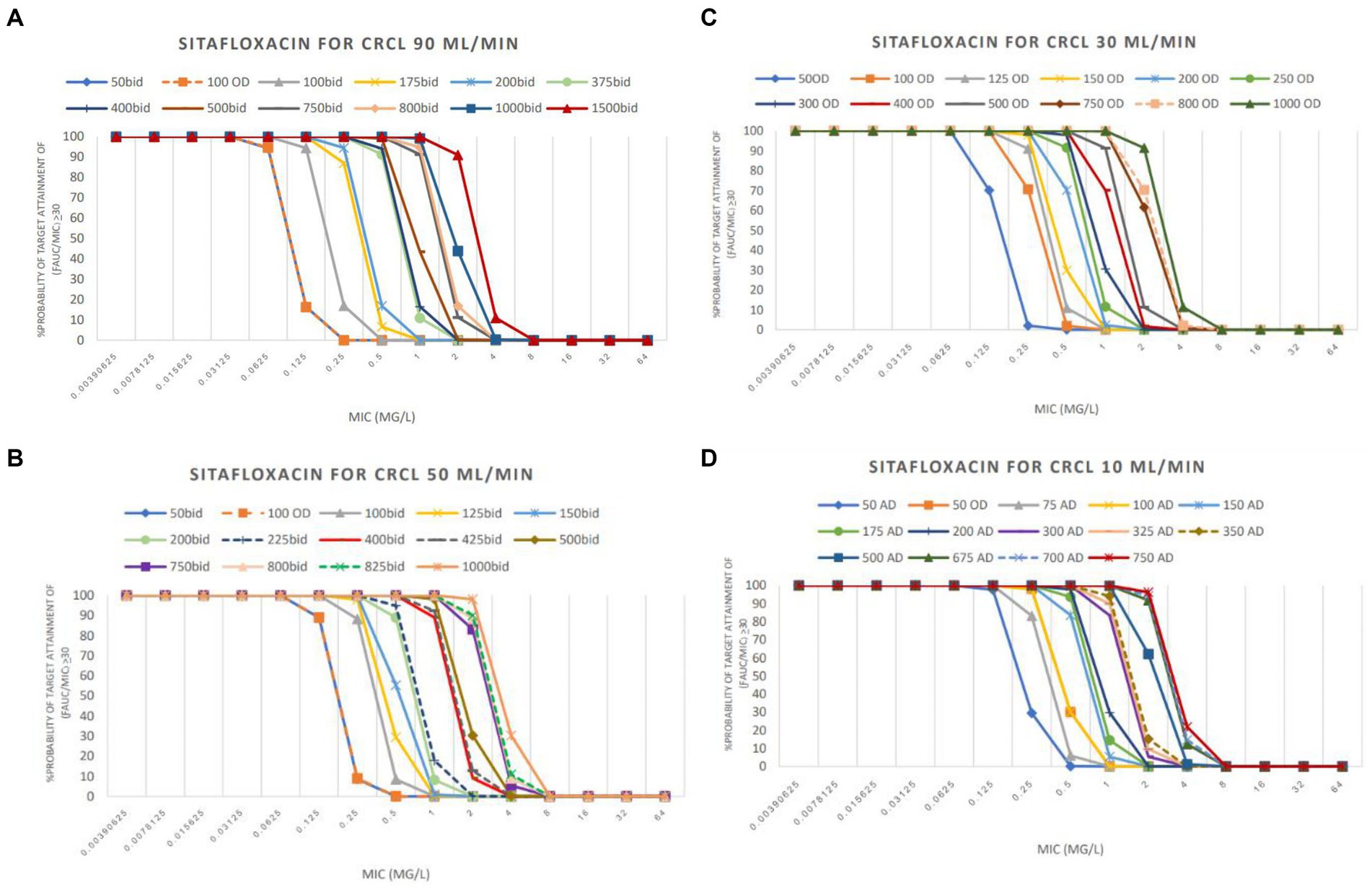
Figure 2. PTA achieved with sitafloxacin used in groups CrCL 90 mL/min (A), CrCL 50 mL/min (B), CrCL 30 mL/min (C), and CrCL 10 mL/min (D).
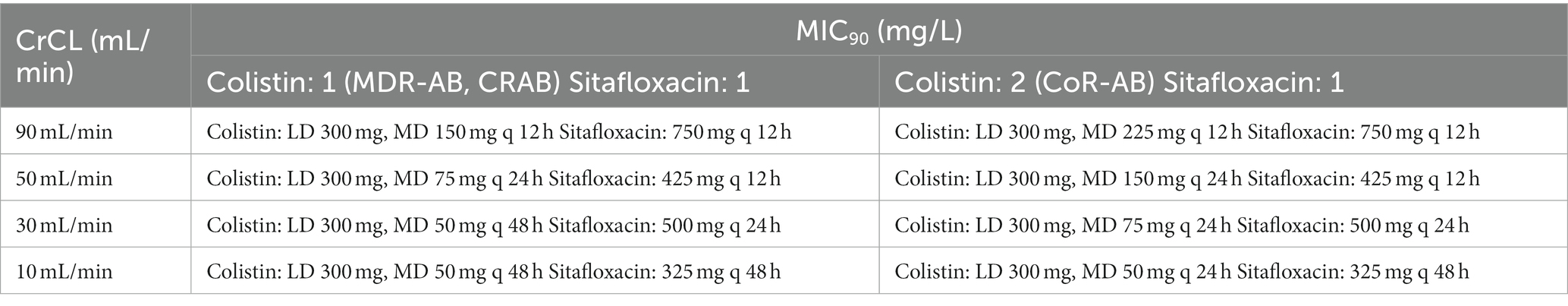
Table 3. The lowest recommended dose of colistin-sitafloxacin: focus on the MIC90 of each agent in combination.
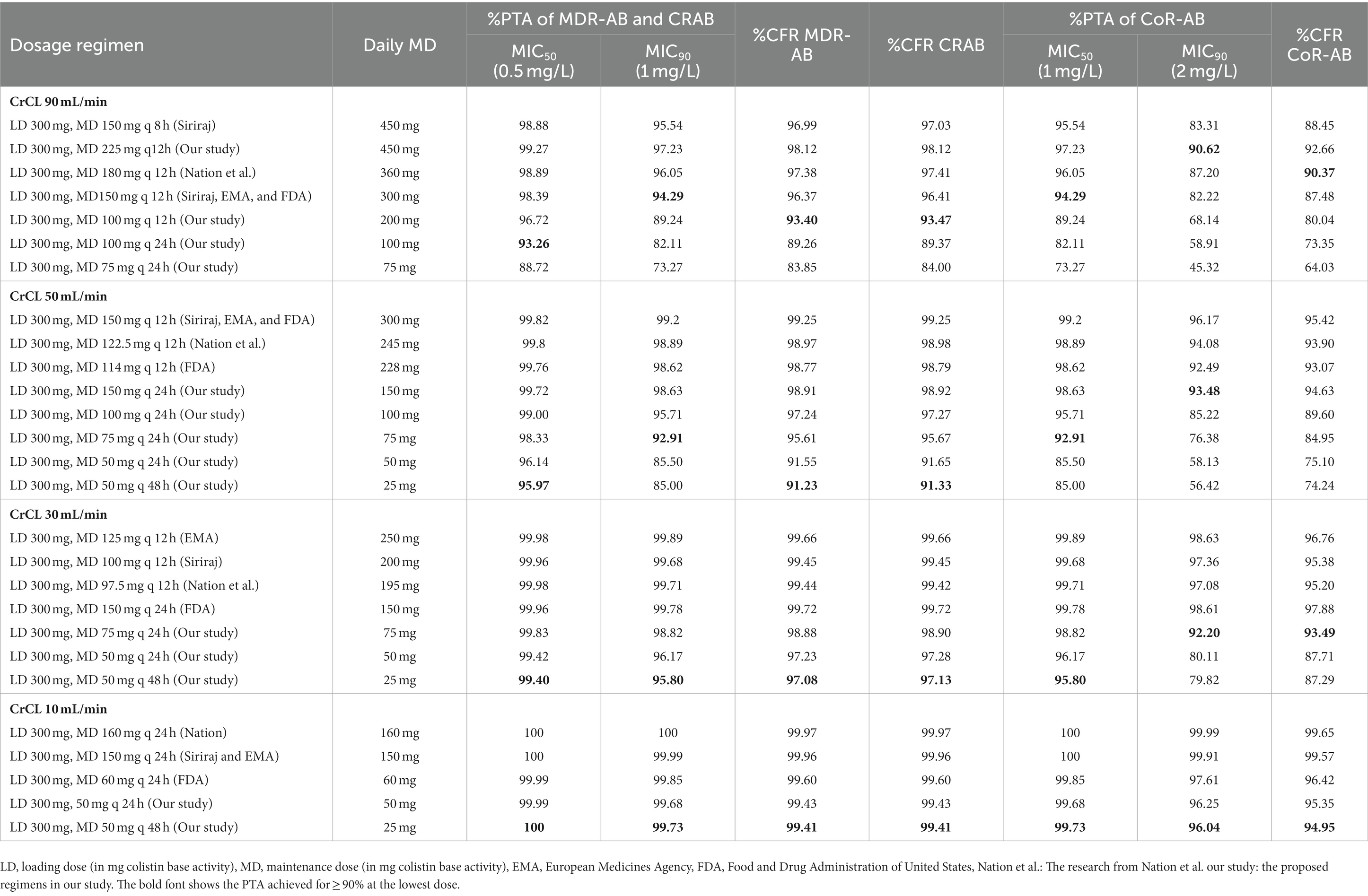
Table 4. %Probability of target attainment (PTA) and %Cumulative fraction of response (CFR) of the studied colistin regimens for each type of isolate in combinations.
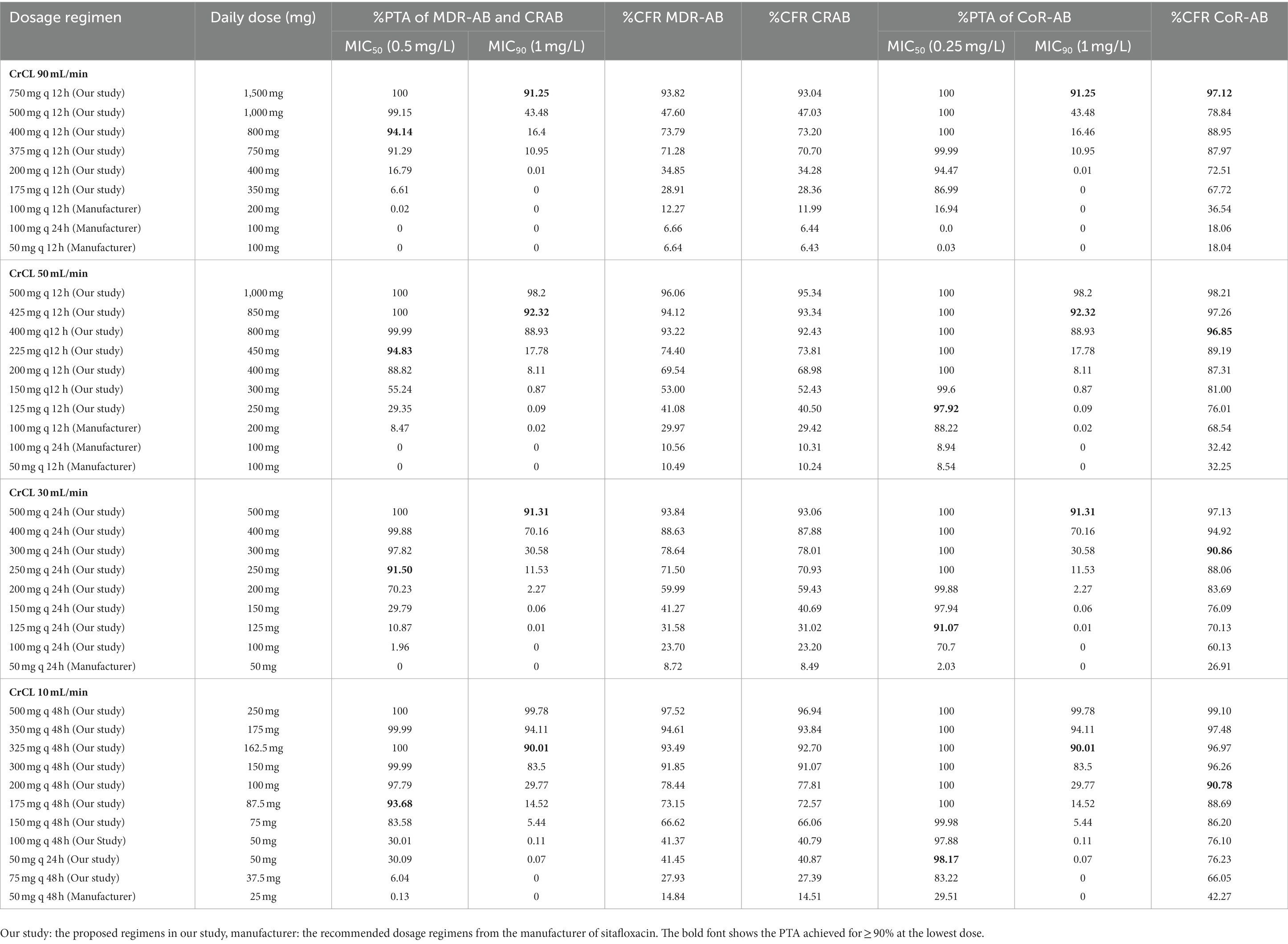
Table 5. %PTA and %CFR of the studied sitafloxacin regimens for each type of isolate in combinations.
Discussion
The infection caused by drug-resistant A. baumannii is a serious problem, especially MDR-AB and CRAB. Because of the various types of antibiotics that the pathogen resists, the choice of drug is limited. This study focuses on the use of colistin, which is known as the last resort for gram-negative bacteria, especially the drug-resistant pathogen. Moreover, sitafloxacin was chosen based on the good activity from the previous study (Rodjun et al., 2020). This is the first study to explore colistin dosage regimens based on the new pharmacokinetic/pharmacodynamic (PK/PD) index of A. baumannii. To date, the recommended colistin dosage regimen aims to achieve the desired Css,avg, especially at 2 mg/L (Garonzik et al., 2011; Nation et al., 2017), formerly the susceptibility breakpoint of gram-negative isolates, including Acinetobacter spp. This study used ƒAUC/MIC ≥7.4 as the desired index (Cheah et al., 2015). We ran simulations starting with a loading dose of 300 mg (the same as in the reference dosage regimens) and 450 mg, expecting them to rapidly reach the steady state of colistin. The reference dosage regimens recommended by FDA, EMA, Siriraj, and Nation et al. for the simulation in patients with a CrCL value of ≥90 mL/min cannot achieve the specific index at a PTA of ≥90%; however, our study’s dosage regimens can achieve it with the maintenance dose of 225 mg every 12 h at breakpoint MIC. The results of Jitaree et al., who studied the optimal dosage of colistin against carbapenem-resistant Klebsiella pneumonia and carbapenem-resistant Escherichia coli (Jitaree et al., 2019), were consistent with ours. In a patient who has a normal renal function (≥80 mL/min), one cannot use the reference dosage regimen to achieve the specific index (ƒAUC/MIC ≥25) when using the breakpoint as a desired MIC. Therefore, the usual colistin dosage regimens cannot achieve the specific index of each important gram-negative isolate in a patient who has normal renal function. However, the reference dosage regimen can achieve the specific index in a patient who has renal impairment (≤50 mL/min). Interestingly, our new dosage regimens (150 mg, 75 mg, and 50 mg every 24 h for CrCL value of 50, 30, and 10 mL/min, respectively), which are lower than the lowest reference dose (114 mg every 12 h, 150 mg every 24 h, and 60 mg every 24 h for CrCL value of 50, 30, and 10 mL/min, respectively), can achieve the target. To the best of our knowledge, the most common adverse event observed with colistin is dose-dependent nephrotoxicity (Nation et al., 2014; Eljaaly et al., 2021). A lower dose should be considered to reduce the risk of nephrotoxicity in a patient who has renal function impairment. However, patients with good renal function seem to be using the higher than usual. The other adverse reaction is neurotoxicity (Spapen et al., 2011). Though colistin is the last resort for the treatment of infection by a drug-resistant organism, the rate of colistin resistance is currently a problem (Cai et al., 2012). The reference dosage regimen for colistin cannot be used against the colistin-resistant A. baumannii in this study (MIC ≥2 mg/L), especially in a patient who has CrCL ≥50 mL/min. Therefore, combination regimens should be considered. However, the international consensus guidelines (Tsuji et al., 2019) for the optimal use of polymyxins recommended the use of a Css,avg of 2 mg/L instead of the new index because of the differences in the protein binding of mice and humans. However, our study used a protein binding profile from Nation et al. (2017), who ran simulations in a critically ill patient. The PK/PD index at a Css,avg of 2 mg/L did not depend on the variation in MIC values, which might complicate the choice of optimal doses. The new PK/PD index used in this study can be applied to any MIC value. Moreover, our PK/PD index is specific for A. baumannii (Cheah et al., 2015).
Sitafloxacin, the fluoroquinolone antibiotic, demonstrates the concentration-dependent killing effect. The lower concentration showed less bacteriological efficacy (Tanigawara et al., 2013). The reference regimen for sitafloxacin monotherapy (50–100 mg every 12–48 h) cannot achieve the specific index in each CrCL at a PTA of ≥90%, while our study’s regimens can (≥175 mg every 48 h). We used the same PK/PD index as Tanigawara et al., who were the only authors to study the PK/PD index of sitafloxacin, ƒAUC/MIC ≥30 (Huang et al., 2015), but there were differences between their MIC values and ours. The lowest MIC from the study by Tanigawara et al. was ≤0.025 mg/L, which was less than the focus MIC value of this study, ≥0.5 mg/L, which led them to a lower recommended dose than the one in this study. In fact, the approved indications of sitafloxacin are likely to be a mild infection or community-acquired infection (Pharmaceuticals and Medical Devices Agency, n.d.), which is why the manufacturer-recommended dose seems to be low.
The colistin-sitafloxacin combination led to a greater opportunity to explore the optimal dosage regimen to achieve a PTA of 90% based on the specific PK/PD index. Because the MIC50/90 of CoR-AB (2 mg/L) was reduced to at least the intermediate breakpoint (Clinical and Laboratory Standards Institute, 2022) (≤2 mg/L), this study could determine the dosage regimen for all CrCL values. Although the MIC of sitafloxacin was also reduced, the dosage regimens that achieve a PTA of 90% are still higher than the reference dosage regimens. For example, 50 mg of sitafloxacin every 24 h was obtained for a patient with a CrCL value of 10 mL/min, while the recommended dose is 50 mg every 48 h. To our knowledge, fluoroquinolones are concentration-dependent antibiotics (Lode et al., 1998) and sitafloxacin is a fluoroquinolone antibiotic agent (Sun et al., 2021). Therefore, higher doses can have greater efficacy in isolate eradication. Saito et al. (2008) showed greater efficacy of sitafloxacin in a higher dose, which is consistent with our result that a higher dose achieved a higher PTA percentage. The point of concern is the possibility of a dose-dependent adverse drug reaction. Feldman et al. used sitafloxacin at a high dose (400 mg intravenously once daily) for the treatment of hospitalized patients with pneumonia and showed a 5% rate of drug-related adverse events but no severe reactions (Feldman et al., 2001). In a previous report, the dose-dependent prolongation of QTcF occurred after the administration of supratherapeutic dosages of sitafloxacin of 400, 600, or 800 mg twice daily to healthy volunteers (mean change in the QTcF interval of 0, 6, and 10 ms, respectively) (Keating, 2011). The highest recommended dose of sitafloxacin from this study for combination therapy in a patient with a CrCL value of 90 mL/min was 200–750 mg every 12 h, which might increase the QTcF. However, no reports of serious adverse reactions or adverse events based on recent data on sitafloxacin were observed.
This study explored all dosage regimens in a patient with various CrCL values, focusing on the one that achieved a PTA of 90% for the MIC90 of each agent in combination therapy. A colistin dose lower than the usual one seems to have led to less nephrotoxicity, while a dose of sitafloxacin appears to be higher than the usual regimen. A patient who is administered a high dose requires close monitoring.
An optimal CFR of ≥90% was used in this study. Some dosage regimens were different from the dosage regimen for a PTA of ≥90% because of the MIC distribution. The dose of sitafloxacin that achieved a CFR of ≥90% was lower than that which achieved a PTA of 90% in each CrCL value. The major MIC distribution of sitafloxacin in our study was lower than 0.5 mg/L (Rodjun et al., 2020), which increases the probability of achieving the desired CFR. The doses of colistin required to achieve a CFR of ≥90% in MDR-AB and CRAB for each CrCL were the same because most of the isolates in these two groups overlapped. Thus, the opportunity to achieve the desired CFR was dependent on the MIC distribution.
Our study has several limitations. First, this study used the PK/PD index from the thigh infection in the mouse model. For the treatment of other types of infections, another specific index should be considered. Drug-resistant A. baumannii, including MDR-AB, CRAB, and CoR-AB, could be treated by combination therapy because it increases the probability of achieving the specific target. Second, most of the recommended doses from this study were different from the reference dosage regimens. Therefore, close monitoring of clinical efficacy and toxicity is necessary. Third, our dosing recommendation used two population pharmacokinetics of two agents (colistin and sitafloxacin) to simulate. Therefore, our dosing recommendation for colistin and sitafloxacin could be used only in patients with similar characteristics to this study. According to the population pharmacokinetic model, for instance, there is a difference in the APACHE II score, the comorbid condition, the CrCL, the volume of distribution, and the drug clearance in critically ill patients compared to the healthy population. These parameters affect the plasma colistin level, which is important to reach the desired target in critically ill patients. Although the population pharmacokinetic analysis of sitafloxacin was based on the study on non-critically ill patients, the oral form of this drug should be considered for absorption in critically ill patients. However, we believe that sitafloxacin might have poor absorption as critically ill patients are hemodynamically unstable. The low plasma concentration might have occurred and cannot reach the target. Fourth, drug interaction should be considered because sitafloxacin (fluoroquinolone group) could reduce absorption when concomitant with antacids, ferrous sulfate, and other metallic cation-containing compounds (Deppermann and Lode, 1993) (this study simulates based on the fasted state of patient). Finally, this study used the PD index of efficacy. Thus, we cannot predict the resistant situation. However, the high dose of sitafloxacin should not develop pathogen resistance. The low dose of colistin in the combination therapy was against the lower MIC than usual. They should not develop pathogen resistance either. Currently, the problem with using colistin and sitafloxacin in combination is that there are no data that relate to the clinical outcome in terms of resistant genes. Although this study uses data from the most recent publications, further study is needed to confirm the results of this simulation study.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethics Committee of the Faculty of Dentistry/Faculty of Pharmacy, Mahidol University (COE.No.MU-DT/PY-IRB2020/001.1501).
Author contributions
VR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. PM: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing. JH: Methodology, Resources, Writing – review & editing. KJ: Methodology, Writing – review & editing. WN: Methodology, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We thank Orarik Asuphon from Department of Pharmacy Practice, Faculty of Pharmaceutical Sciences, Naresuan University, Thailand, Pannee Leelawattanachai from Department of Pharmacy, Faculty of Medicine Vajira Hospital, Navamindradhiraj University, Thailand, and Taniya Paiboonvong from Department of Pharmacy Practice, College of Pharmacy, Rangsit University, Thailand for the valuable advice of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1275909/full#supplementary-material
References
Almasaudi, S. B. (2018). Acinetobacter spp. as nosocomial pathogens: epidemiology and resistance features. Saudi J. Biol. Sci. 25, 586–596. doi: 10.1016/j.sjbs.2016.02.009
Amat, T., Gutiérrez-Pizarraya, A., Machuca, I., Gracia-Ahufinger, I., Pérez-Nadales, E., Torre-Giménez, Á., et al. (2018). The combined use of tigecycline with high-dose colistin might not be associated with higher survival in critically ill patients with bacteraemia due to carbapenem-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 24, 630–634. doi: 10.1016/j.cmi.2017.09.016
Appaneal, H. J., Lopes, V. V., LaPlante, K. L., and Caffrey, A. R. (2022). Treatment, clinical outcomes, and predictors of mortality among a national cohort of admitted patients with Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 66, e01975–e01921. doi: 10.1128/aac.01975-21
Asuphon, O., Montakantikul, P., Houngsaitong, J., Kiratisin, P., and Sonthisombat, P. (2016). Optimizing intravenous fosfomycin dosing in combination with carbapenems for treatment of Pseudomonas aeruginosa infections in critically ill patients based on pharmacokinetic/pharmacodynamic (PK/PD) simulation. Int. J. Infect. Dis. 50, 23–29. doi: 10.1016/j.ijid.2016.06.017
Batirel, A., Balkan, I. I., Karabay, O., Agalar, C., Akalin, S., Alici, O., et al. (2014). Comparison of colistin-carbapenem, colistin-sulbactam, and colistin plus other antibacterial agents for the treatment of extremely drug-resistant Acinetobacter baumannii bloodstream infections. Eur. J. Clin. Microbiol. Infect. Dis. 33, 1311–1322. doi: 10.1007/s10096-014-2070-6
Butler, M. S., Henderson, I. R., Capon, R. J., and Blaskovich, M. A. (2022). Antibiotics in the clinical pipeline as of. J. Antibiot. 76, 431–473. doi: 10.1038/s41429-023-00629-8
Cai, Y., Chai, D., Wang, R., Liang, B., and Bai, N. (2012). Colistin resistance of Acinetobacter baumannii: clinical reports, mechanisms and antimicrobial strategies. J. Antimicrob. Chemother. 67, 1607–1615. doi: 10.1093/jac/dks084
Centers for Disease Control and Prevention. (n.d.) Available at: https://www.cdc.gov/hai/organisms/acinetobacter.html (Accessed March 21, 2022).
Cheah, S. E., Wang, J., Nguyen, V. T., Turnidge, J. D., Li, J., and Nation, R. L. (2015). New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J. Antimicrob. Chemother. 70, 3291–3297. doi: 10.1093/jac/dkv267
Cisneros, J. M., and Rodríguez-Baño, J. (2002). Nosocomial bacteremia due to Acinetobacter baumannii: epidemiology, clinical features and treatment. Clin. Microbiol. Infect. 8, 687–693. doi: 10.1046/j.1469-0691.2002.00487.x
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility Testing. Thirty-two informational supplement. CLSI document M100-Swayne; CLSI: Wayne, PA, USA, (2022).
Craig, W. A. (1998). Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26, 1–10. doi: 10.1086/516284
Dai, C., Li, J., Tang, S., Li, J., and Xiao, X. (2014). Colistin-induced nephrotoxicity in mice involves the mitochondrial, death receptor, and endoplasmic reticulum pathways. Antimicrob. Agents Chemother. 58, 4075–4085. doi: 10.1128/AAC.00070-14
Deppermann, K. M., and Lode, H. (1993). Fluoroquinolones: interaction profile during enteral absorption. Drugs 45, 65–72. doi: 10.2165/00003495-199300453-00013
Dickstein, Y., Lellouche, J., Ben Dalak Amar, M., Schwartz, D., Nutman, A., Daitch, V., et al. (2019). Ben Dalak Amar M, Schwartz D, Nutman a, Daitch V, Yahav D, Leibovici L, Skiada a, Antoniadou a, Daikos GL, Andini R, Zampino R, Durante-Mangoni E, Mouton JW, Friberg LE, Dishon Benattar Y, Bitterman R, Neuberger a, Carmeli Y, Paul M; AIDA study group. Treatment outcomes of colistin- and carbapenem-resistant Acinetobacter baumannii infections: an exploratory subgroup analysis of a randomized clinical trial. Clin. Infect. Dis. 69, 769–776. doi: 10.1093/cid/ciy988
Dong, X., Chen, F., Zhang, Y., Liu, H., Liu, Y., and Ma, L. (2015). In vitro activities of sitafloxacin tested alone and in combination with rifampin, colistin, sulbactam, and tigecycline against extensively drug-resistant Acinetobacter baumannii. Int. J. Clin. Exp. Med. 8, 8135–8140.
Dudhani, R. V., Turnidge, J. D., Nation, R. L., and Li, J. (2010). fAUC/MIC is the most predictive pharmacokinetic/pharmacodynamic index of colistin against Acinetobacter baumannii in murine thigh and lung infection models. J. Antimicrob. Chemother. 65, 1984–1990. doi: 10.1093/jac/dkq226
Eljaaly, K., Bidell, M. R., Gandhi, R. G., Alshehri, S., Enani, M. A., Al-Jedai, A., et al. (2021). Colistin nephrotoxicity: meta-analysis of randomized controlled trials. Open Forum Infect. Dis. 8:ofab026. doi: 10.1093/ofid/ofab026
Falagas, M. E., Rafailidis, P. I., Ioannidou, E., Alexiou, V. G., Matthaiou, D. K., Karageorgopoulos, D. E., et al. (2010). Colistin therapy for microbiologically documented multidrug-resistant gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int. J. Antimicrob. Agents 35, 194–199. doi: 10.1016/j.ijantimicag.2009.10.005
Feldman, C., White, H., O'Grady, J., Flitcroft, A., Briggs, A., and Richards, G. (2001). An open, randomised, multi-Centre study comparing the safety and efficacy of sitafloxacin and imipenem/cilastatin in the intravenous treatment of hospitalised patients with pneumonia. Int. J. Antimicrob. Agents 17, 177–188. doi: 10.1016/S0924-8579(00)00344-7
García-Garmendia, J. L., Ortiz-Leyba, C., Garnacho-Montero, J., Jiménez-Jiménez, F. J., Pérez-Paredes, C., Barrero-Almodóvar, A. E., et al. (2001). Risk factors for Acinetobacter baumannii nosocomial bacteremia in critically ill patients: a cohort study. Clin. Infect. Dis. 33, 939–946. doi: 10.1086/322584
Garonzik, S. M., Li, J., Thamlikitkul, V., Paterson, D. L., Shoham, S., Jacob, J., et al. (2011). Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55, 3284–3294. doi: 10.1128/AAC.01733-10
Huang, Y. S., Wang, J. T., Sheng, W. H., Chuang, Y. C., and Chang, S. C. (2015). Comparative in vitro activity of sitafloxacin against bacteremic isolates of carbapenem resistant Acinetobacter baumannii complex. J. Microbiol. Immunol. Infect. 48, 545–551. doi: 10.1016/j.jmii.2014.02.002
Jambhekar, S. S., and Breen, P. J. (Eds.) (2012). Basic Pharmacokinetics. Extravascular routes of drug administration.
Jitaree, K., Sathirakul, K., Houngsaitong, J., Asuphon, O., Saelim, W., Thamlikitkul, V., et al. (2019). Pharmacokinetic/pharmacodynamic (PK/PD) simulation for dosage optimization of colistin against carbapenem-resistant Klebsiella pneumoniae and carbapenem-resistant Escherichia coli. Antibiotics (Basel) 8:125. doi: 10.3390/antibiotics8030125
Kalin, G., Alp, E., Akin, A., Coskun, R., and Doganay, M. (2014). Comparison of colistin and colistin/sulbactam for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Infection 42, 37–42. doi: 10.1007/s15010-013-0495-y
Kaye, K. S., and Pogue, J. M. (2015). Infections caused by resistant gram-negative bacteria: epidemiology and management. Pharmacotherapy 35, 949–962. doi: 10.1002/phar.1636
Leelawattanachai, P., Wattanavijitkul, T., Paiboonvong, T., Plongla, R., Chatsuwan, T., Usayaporn, S., et al. (2020). Evaluation of intravenous Fosfomycin disodium dosing regimens in critically ill patients for treatment of carbapenem-resistant Enterobacterales infections using Monte Carlo simulation. Antibiotics (Basel) 9:615. doi: 10.3390/antibiotics9090615
Lemos, E. V., de La Hoz, F. P., Einarson, T. R., McGhan, W. F., Quevedo, E., Castañeda, C., et al. (2014). Carbapenem resistance and mortality in patients with Acinetobacter baumannii infection: systematic review and meta-analysis. Clin. Microbiol. Infect. 20, 416–423. doi: 10.1111/1469-0691.12363
Liang, C. A., Lin, Y. C., Lu, P. L., Chen, H. C., Chang, H. L., and Sheu, C. C. (2018). Antibiotic strategies and clinical outcomes in critically ill patients with pneumonia caused by carbapenem-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 24, 908.e1–908.e7. doi: 10.1016/j.cmi.2017.10.033
Lim, S. K., Lee, S. O., Choi, S. H., Choi, J. P., Kim, S. H., Jeong, J. Y., et al. (2011). The outcomes of using colistin for treating multidrug resistant Acinetobacter species bloodstream infections. J. Korean Med. Sci. 26, 325–331. doi: 10.3346/jkms.2011.26.3.325
Lode, H., Borner, K., and Koeppe, P. (1998). Pharmacodynamics of fluoroquinolones. Clin. Infect. Dis. 27, 33–39. doi: 10.1086/514623
López-Cortés, L. E., Cisneros, J. M., Fernández-Cuenca, F., Bou, G., Tomás, M., Garnacho-Montero, J., et al. (2014). Fernández-Cuenca F, Bou G, Tomás M, Garnacho-Montero J, Pascual a, Martínez-Martínez L, Vila J, Pachón J, Rodríguez Baño J; GEIH/REIPI-Ab2010 group. Monotherapy versus combination therapy for sepsis due to multidrug-resistant Acinetobacter baumannii: analysis of a multicentre prospective cohort. J. Antimicrob. Chemother. 69, 3119–3126. doi: 10.1093/jac/dku233
Nakashima, M. (2008). Pharmacokinetics of sitafloxacin (DU-6859a) in healthy volunteers (in Japanese). Jpn. J. Chemother. 56, 154–155.
Nakashima, M., and Kawada, Y. (2008). Pharmacokinetic profiles of sitafloxacin in patients with renal dysfunction (in Japanese). Jpn. J. Chemother. 56, 21–24.
Nakashima, M., Uematsu, T., Kosuge, K., Umemura, K., Hakusui, H., and Tanaka, M. (1995). Pharmacokinetics and tolerance of DU-6859a, a new fluoroquinolone, after single and multiple oral doses in healthy volunteers. Antimicrob. Agents Chemother. 39, 170–174. doi: 10.1128/AAC.39.1.170
Nation, R. L., Garonzik, S. M., Li, J., Thamlikitkul, V., Giamarellos-Bourboulis, E. J., Paterson, D. L., et al. (2016). Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin. Infect. Dis. 62, 552–558. doi: 10.1093/cid/civ964
Nation, R. L., Garonzik, S. M., Thamlikitkul, V., Giamarellos-Bourboulis, E. J., Forrest, A., Paterson, D. L., et al. (2017). Dosing guidance for intravenous colistin in critically-ill patients. Clin. Infect. Dis. 64, 565–571. doi: 10.1093/cid/ciw839
Nation, R. L., Velkov, T., and Li, J. (2014). Colistin and polymyxin B: peas in a pod, or chalk and cheese? Clin. Infect. Dis. 59, 88–94. doi: 10.1093/cid/ciu213
Paiboonvong, T., Nosoongnoen, W., Sathirakul, K., Tangsujaritvijit, V., Kaemapairoj, J., Tragulpiankit, P., et al. (2019). Pharmacokinetics and penetration of sitafloxacin into alveolar epithelial lining fluid in critically ill Thai patients with pneumonia. Antimicrob. Agents Chemother. 63, e00800–e00819. doi: 10.1128/AAC.00800-19
Pantopoulou, A., Giamarellos-Bourboulis, E. J., Raftogannis, M., Tsaganos, T., Dontas, I., Koutoukas, P., et al. (2007). Colistin offers prolonged survival in experimental infection by multidrug-resistant Acinetobacter baumannii: the significance of co-administration of rifampicin. Int. J. Antimicrob. Agents 29, 51–55. doi: 10.1016/j.ijantimicag.2006.09.009
Papathanakos, G., Andrianopoulos, I., Papathanasiou, A., Priavali, E., Koulenti, D., and Koulouras, V. (2020). Colistin-resistant Acinetobacter baumannii bacteremia: a serious threat for critically ill patients. Microorganisms 8:287. doi: 10.3390/microorganisms8020287
Pharmaceuticals and Medical Devices Agency (n.d.). “Gracevit tablets, fine granules (sitafloxacin hydrate)” in Approval review information on ethical drugs [internet] (Tokyo: Daiichi Sankyo Co., Ltd) Japanesehttp://www.info.pmda.go.jp/shinyaku/P200800006/index.html
Rodjun, V., Houngsaitong, J., Montakantikul, P., Paiboonvong, T., Khuntayaporn, P., Yanyongchaikit, P., et al. (2020). In vitro activities of colistin and Sitafloxacin combinations against multidrug-, carbapenem-, and colistin-resistant Acinetobacter baumannii using the broth microdilution checkerboard and time-kill methods. Antibiotics (Basel) 9:516. doi: 10.3390/antibiotics9080516
Saito, A., Tanigawara, Y., Watanabe, A., Aoki, N., Niki, Y., Kohno, S., et al. (2008). Open study of sitafloxacin in patients with respiratory tract infections: PK-PD study (in Japanese). Jpn. J. Chemother 56, 63–80.
Sekino, H. (2008). Pharmacokinetic profiles of sitafloxacin in elderly volunteer (in Japanese). Jpn. J. Chemother. 56, 18–20.
Sieniawski, K., Kaczka, K., Rucińska, M., Gagis, L., and Pomorski, L. (2013). Acinetobacter baumannii nosocomial infections. Pol. Przegl. Chir. 85, 483–490. doi: 10.2478/pjs-2013-0075
Spapen, H., Jacobs, R., Van Gorp, V., Troubleyn, J., and Honoré, P. M. (2011). Renal and neurological side effects of colistin in critically ill patients. Ann. Intensive Care 1:14. doi: 10.1186/2110-5820-1-14
Sun, L. N., Sun, G. X., Yang, Y. Q., Shen, Y., Huang, F. R., Xie, L. J., et al. (2021). Effects of ABCB1, UGT1A1, and UGT1A9 genetic polymorphisms on the pharmacokinetics of Sitafloxacin granules in healthy subjects. Clin. Pharmacol. Drug Dev. 10, 57–67. doi: 10.1002/cpdd.848
Sunenshine, R. H., Wright, M. O., Maragakis, L. L., Harris, A. D., Song, X., Hebden, J., et al. (2007). Multidrug-resistant Acinetobacter infection mortality rate and length of hospitalization. Emerg. Infect. Dis. 13, 97–103. doi: 10.3201/eid1301.060716
Tamma, P. D., Aitken, S. L., Bonomo, R. A., Mathers, A. J., van Duin, D., and Clancy, C. J. (2023). Infectious Diseases Society of America 2023 guidance on the treatment of antimicrobial resistant gram-negative infections. Clin. Infect. Dis. :ciad428. doi: 10.1093/cid/ciad428
Tanigawara, Y., Kaku, M., Totsuka, K., Tsuge, H., and Saito, A. (2013). Population pharmacokinetics and pharmacodynamics of sitafloxacin in patients with community-acquired respiratory tract infections. J. Infect. Chemother. 19, 858–866. doi: 10.1007/s10156-013-0580-2
Tsuji, B. T., Pogue, J. M., Zavascki, A. P., Paul, M., Daikos, G. L., Forrest, A., et al. (2019). International consensus guidelines for the optimal use of the polymyxins: endorsed by the American college of clinical pharmacy (ACCP), European society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of America (IDSA), international society for anti-infective pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP). Pharmacotherapy 39, 10–39. doi: 10.1002/phar.2209
Werarak, P., Waiwarawut, J., Tharavichitkul, P., Pothirat, C., Rungruanghiranya, S., Geater, S. L., et al. (2012). Acinetobacter baumannii nosocomial pneumonia in tertiary care hospitals in Thailand. J. Med. Assoc. Thail. 95 Suppl 2, S23–S33.
Yedle, R., Reniguntla, M. K., Puttaswamy, R., Puttarangappa, P., Hiremath, S., Nanjundappa, M., et al. (2023). Neutropenic rat thigh infection model for evaluation of the pharmacokinetics/pharmacodynamics of anti-infectives. Microbiol. Spectr. 11, e00133–e00123. doi: 10.1128/spectrum.00133-23
Keywords: colistin, sitafloxacin, Acinetobacter baumannii, Monte Carlo simulation, multidrug-resistant Acinetobacter baumannii, carbapenem-resistant Acinetobacter baumannii, combination
Citation: Rodjun V, Montakantikul P, Houngsaitong J, Jitaree K and Nosoongnoen W (2023) Pharmacokinetic/pharmacodynamic (PK/PD) simulation for dosage optimization of colistin and sitafloxacin, alone and in combination, against carbapenem-, multidrug-, and colistin-resistant Acinetobacter baumannii. Front. Microbiol. 14:1275909. doi: 10.3389/fmicb.2023.1275909
Edited by:
Vijay Soni, NewYork-Presbyterian, United StatesReviewed by:
Tushar Dhanani, Florida Agricultural and Mechanical University, United StatesMadhavi Annamanedi, West Virginia University, United States
Arka Banerjee, NewYork-Presbyterian, United States
Copyright © 2023 Rodjun, Montakantikul, Houngsaitong, Jitaree and Nosoongnoen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Preecha Montakantikul, preecha.mon@mahidol.ac.th
 Vipavee Rodjun
Vipavee Rodjun Preecha Montakantikul2*
Preecha Montakantikul2* Wichit Nosoongnoen
Wichit Nosoongnoen