- 1Department of Biosciences, Himalayan School of Biosciences, Swami Rama Himalayan University, Dehradun, India
- 2School of Agriculture, Graphic Era Hill University, Bhimtal Campus, Uttarakhand, India
- 3Amity Institute of Biotechnology, Amity University Jharkhand, Ranchi, Jharkhand, India
- 4Guru Nanak College of Pharmaceutical Sciences, Dehradun, Uttarakhand, India
- 5Laboratory of Animal Immunology and Biotechnology, Department of Animal Morphology, Physiology and Genetics, Faculty of AgriSciences, Mendel University in Brno, Brno, Czechia
Biochar is a carbonaceous by-product of lignocellulosic biomass developed by various thermochemical processes. Biochar can be transformed into “nano-biochar” by size reduction to nano-meters level. Nano-biochar presents remarkable physico-chemical behavior in comparison to macro-biochar including; higher stability, unique nanostructure, higher catalytic ability, larger specific surface area, higher porosity, improved surface functionality, and surface active sites. Nano-biochar efficiently regulates the transport and absorption of vital micro-and macro-nutrients, in addition to toxic contaminants (heavy metals, pesticides, antibiotics). However an extensive understanding of the recent nano-biochar studies is essential for large scale implementations, including development, physico-chemical properties and targeted use. Nano-biochar toxicity on different organisms and its in-direct effect on humans is an important issue of concern and needs to be extensively evaluated for large scale applications. This review provides a detailed insight on nanobiochar research for (1) development methodologies, (2) compositions and properties, (3) characterization methods, (4) potentiality as emerging sorbent, photocatalyst, enzyme carrier for environmental application, and (5) environmental concerns.
1. Introduction
Extensive industrialization, urbanization, and modern agricultural methods have resulted in accumulation of innumerous toxic compounds (pesticides, pharmaceutical and personal care products, antibiotics, hormones, organic compounds, nano-compounds, endocrine disruptors, steroids, surfactants and their metabolites, industrial additives, and heavy metals) in the different environmental matrices (Bhatt et al., 2022; Gangola et al., 2022). Anthropogenic activities such as healthcare, industries, power plants, oil refineries, mining, improper waste treatment, agriculture and household activities can lead to build-up of pollutants ranging from 1 μg/kg to 10 mg/kg in the different environments (Zhou et al., 2021). Furthermore, aquatic and soil sediments also function as potent sink for innumerous hydrophobic compounds (polychlorinated biphenyl, poly-and perfluoroalkyl compounds, and organochloride insecticides) (Bhatt et al., 2022). The immoderate enhancement in the amount of such contaminants in the environment has alarmed the scientific and regulatory bodies across the globe due to acute and chronic human health toxicities. With time, various physico-chemical and biological processes such as adsorption, advanced oxidation methods, sonocatalysis, nano-filtration/reverse osmosis and bioremediation have been developed for efficient treatment of contaminated environments (Amusat et al., 2021). However, majority of these advanced methods are energy and cost extensive and release more toxic secondary by-products in the environment. The sustainable, eco-friendly nature, easy operation, and low cost of bioremediation in comparison with traditional and advanced physico-chemical methods have resulted in the establishment of bioremediation technologies recently (Bhatt et al., 2020; Suresh et al., 2022). Nonetheless, limitations such as dynamic microbial habitat fluctuation, reproducibility, cross contamination with other contaminants, and interfacial physical and biogeochemical methods in the soil-aquatic shift may render biodegradation slow and inefficient (Mukherjee et al., 2022).
Several authors have recently concentrated on the utilization of nano-compounds for the development of better remediation methods (Mahmoud et al., 2022; Rajput et al., 2022). Due to distinct physical characters of nano-materials such as excellent surface-to-volume proportion, higher reactivity, ability modify surface chemistry, smaller intra-particle diffusion distance, higher contaminant removal efficiency, stable nature and reusable and recyclable capacity, nanobiotechnology has recently received great attention for environmental applications recently (Xia et al., 2022). Biochar (BC) is a carbon containing solid compound fabricated by pyrolytic degradation of biomass (agricultural, animal and solid waste) in the absolute vacuum conditions (Bolan et al., 2022). It is generally produced using different thermochemical methods; fast and slow pyrolysis, flash and hydrothermal carbonization, gasification and torrefaction (Bolan et al., 2022; Mukherjee et al., 2022). Biochar has shown a substantial ability to remediate pollutants since it is cheap accessibility of feedstock, economical, and desirable physicochemical surface properties (Xiao et al., 2021). Among these physicochemical properties, the biodegradable nature plays a crucial role especially in agricultural activities (Širić et al., 2022). The synthesis and applications of biochar have however also faced few hurdles due to low catalysis, inadequate pore size and surface area, deficiency of simple and chemical-free functionalization processes (Li et al., 2019a).
Recently, studies on the production of nano-biochar (nano-BC) for environmental and agriculture applications has been documented (Nath et al., 2019; Li et al., 2019b; Rajput et al., 2022). Carbonization results in fabrication of micro-sized BC with size 1 μm-1 nm referred to as “dissolved” or “nano-BC.” The elemental composition, aromatic/polar nature, cation exchange capacity, crystalline form, graphitic nature, pH, specific surface area, pore size, stability, temperature-dependent dispersibility and zeta potential of nano-BC vary in comparison with bulk-BC (Ramanayaka et al., 2020a). Colloidal and nano-BC possess features such as surface hydrophobicity, nano-scale size, significantly high specific surface area, micro-porous structure, diverse surface functionality (hydroxyl, carboxy, lactonyl) and thus significantly enhance the adsorption and immobilization capability of nano-BC for different pollutants, including heavy metals, pesticides, PCBs, PAHs, and others (Nath et al., 2019; Mahmoud et al., 2022). Nano-BC assisted adsorption for the removal of toxicants from water bodies have been developed recently, which also enable for both “C” sequestration in addition to remediation (Xia et al., 2022).
Furthermore, due to high porosity, surface functionality and larger surface-to-volume ratio, nano-BC functions as an excellent immobilization material for enzymes and can thus function as a nanocatalyst in bioremediation (Naghdi et al., 2018). The chemical and physical properties of nano-BC dictate its ability to remove various pollutants, which are dependent on feedstock material, production method, pyrolysis temperature, and other pre-or post-treatment methods (Xia et al., 2022). Thus, nano-BC, with its unique features and applications, opens up new avenues for a long-term, cost-effective, and sustainable solution to environmental pollution. Therefore, the present review provides updated information on the methodologies for fabrication and characterization of nano-BC and its application for managing hazardous contaminants in the environment. Furthermore, for future research, an extensive appraisal of the potentiality of nano-BC-assisted contaminant removal is presented.
2. Production of nanobiochar
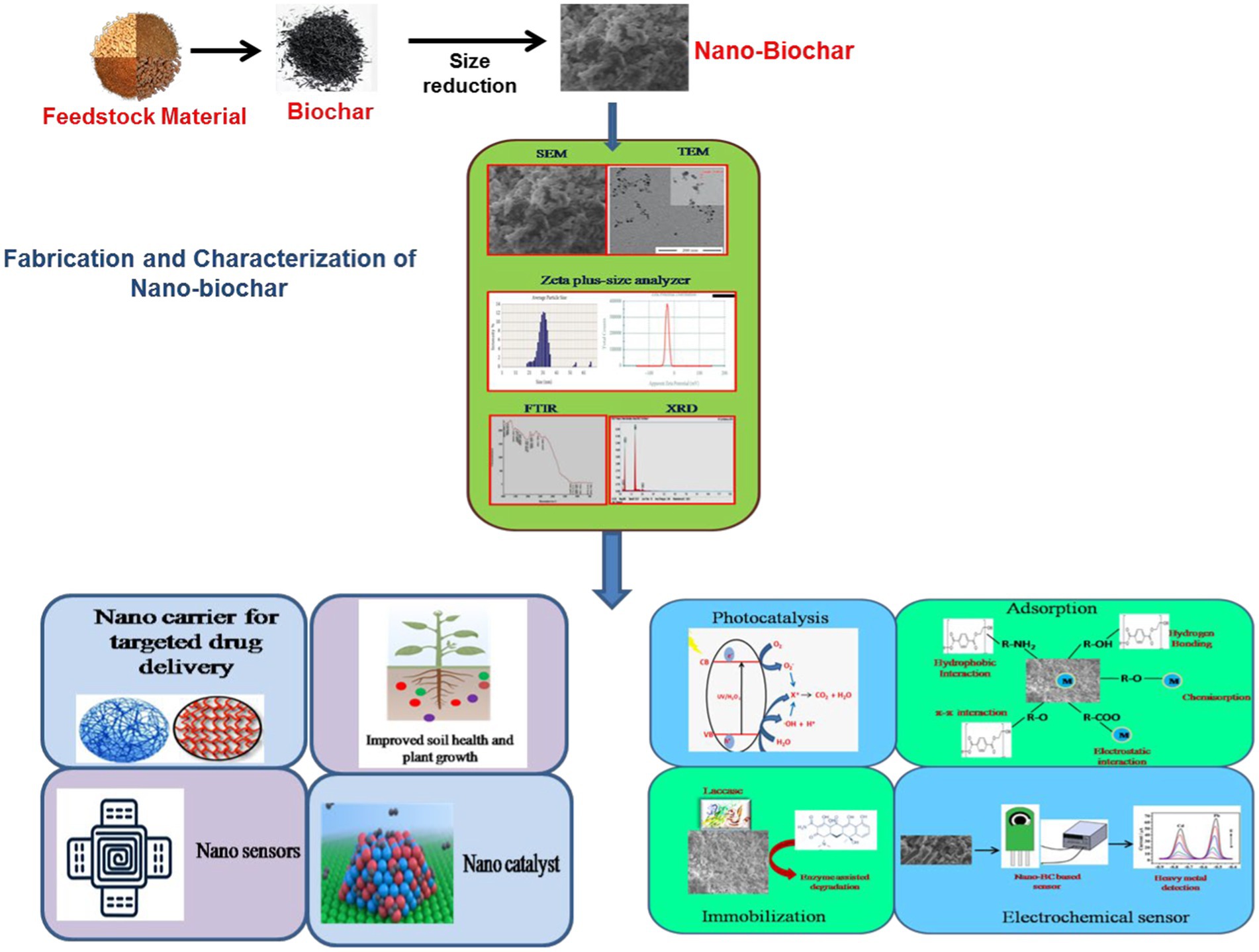
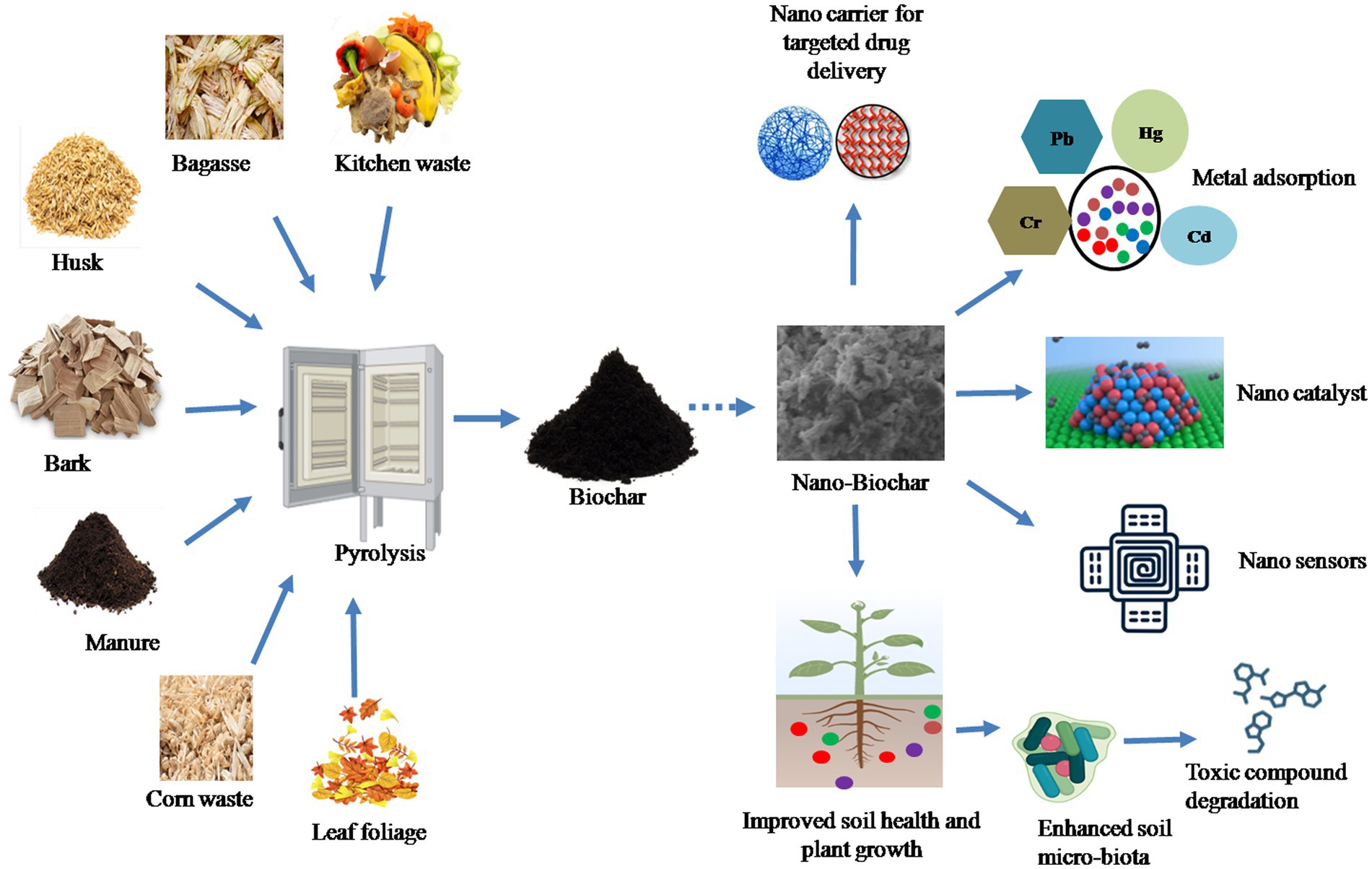
Nano-BC is a novel nano-sized carbonaceous material generally manufactured using green and energy-saving nanotechnology methods. Nano-BC differs from macrochar by of possessing higher specific surface area, higher porosity, lower hydrodynamic radius, stronger negative zeta potential, better oxygen-consisting surface functional groups, and lower carbon defects (Qin et al., 2018; Ramanayaka et al., 2020a). The most widely employed feedstocks for fabrication of nano-BC include animal wastes, municipal wastes, lignocellulosic agricultural wastes (grass, palm, peanut shell, rice husk and straw, sugar cane bagasse, bamboo, and soy bean stover), woody forest residues and sewage sludge. Initially the biomass is transformed into bulk-BC followed by size reduction through various fractionation approaches to produce nano-BC (Figure 1).
2.1. Preparation of bulk biochar
Biochar is fabricated from lignocellulosic biomass using thermo-chemical approaches such as pyrolysis (slow and fast), torrefaction, carbonization (hydrothermal or flash), and gasification (Amusat et al., 2021). The feedstock material is thermochemically or pyrolytically decomposed at 350–700°C in vacuum (<1% O2) for the generation of BC. Slow pyrolysis is an eco-friendly process commonly employed for BC fabrication and results in high yield of bio-oil and 35% yield of dry mass (Tomczyk et al., 2020). Zhang et al. (2017) used slow pyrolytically produced BC for soil remediation and sorption of different pollutants from wastewater. For biofuel production, fast pyrolysis is favored over other processes; gasification is primarily employed for the synthesis of syngas, which subsequently produces heat and energy. Additionally, lignocellulosic material caused higher BC yield than municipal solid waste (Ashiq et al., 2019). To expedite the nano-BC production the employment of BC produced by traditional thermochemical methods is advised, while optimizing the quality of biomass materials through transforming them to nano-particles. The traditional methods produce BC with different yields and elemental constitution (C, H, O). Biomass normally becomes more carbonized as treatment intensity increases, which corresponds to an elevation in C composition but a reduction in O and H composition. Additionally, BC is modified physically and chemically for a variety of purposes to enhance its functionality. Steam coating, chemical oxidation, acidic/basic treatment, CO2 activation, saturation with native and artificial nano-materials is used for chemically modifying BC (Song et al., 2022).
2.2. Conversion of bulk biochar to nanobiochar
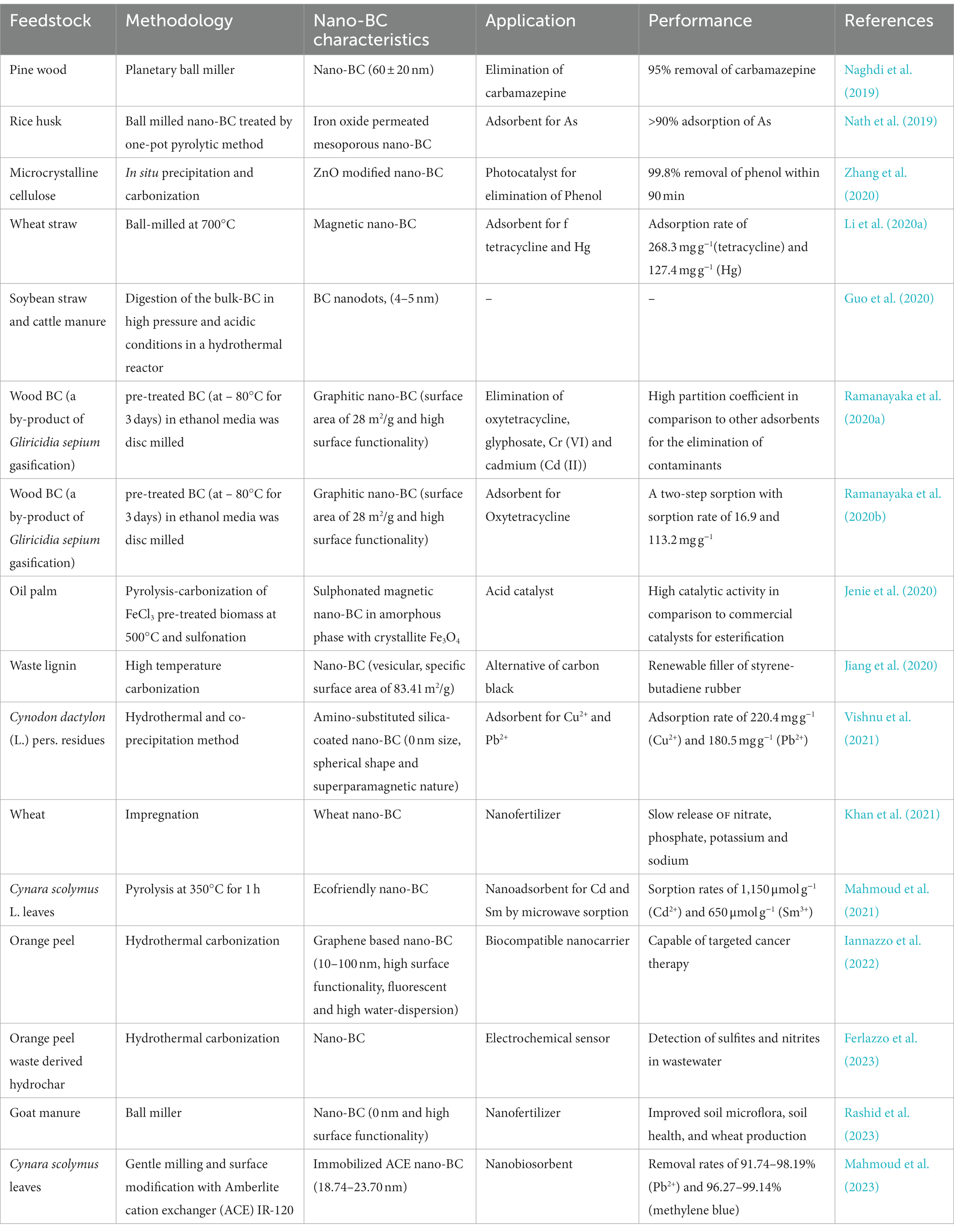
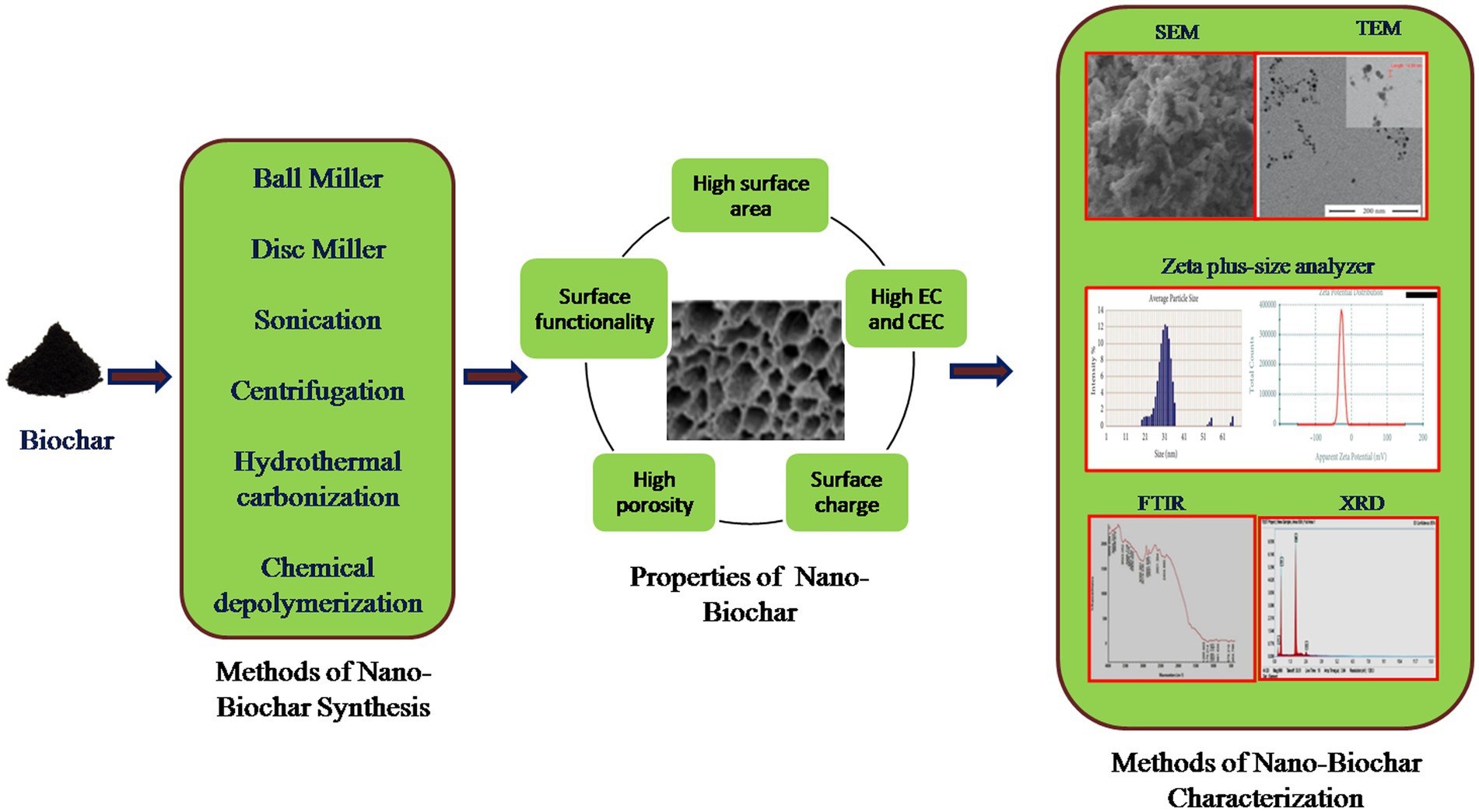
Nano-BC inherently forms while synthesizing macro-BC, however its output is limited (<2.0% from peanut shell-derived BC) (Liu et al., 2018). It is thus necessary to undergo a size reduction process in order to enhance the amount of nano-BC (Table 1). The production of nanomaterials can be performed by top-down or bottom-up processes. In the top-down process, the size of the macro BC is minimized to nanoscale; whereas in the bottom-up method, the nanomaterial is amassed from the atomic level. Top–down methods such as grinding, cutting, centrifugation and etching are used for the fabrication of nano-BC in an economical manner. Lonappan et al. (2016), Dong et al. (2018), Lian et al. (2020), and Ramanayaka et al. (2020a) have employed grinders for reducing the size of macro-BC to nanoscale. The bottom up method includes disintegration by ball milling or sonication and carbonization. Ball milling enables fabrication of nano-BC with improved properties without destroying its crystal structure (Amusat et al., 2021). Ball-milling has received great attention because of its low cost and energy demand during manufacturing, eco-friendly nature and wide range of application. The ball milling method disintegrates bulk-BC into nanoscale by the colliding it between metallic balls. The desired particle size may be attained by regulating the aggregation and modifying the ball sample ratio and milling time. There are two ways to ball mill BC at the nanoscale: wet and dry techniques. The wet approach is more preferred due to synthesis of nano-BC with superior dispersivity, higher surface functionality, eco-friendly and less labor intensive approach (Yuan et al., 2020). Ball milling method effectively tailors nano-BC characteristics by enhancing surface area, decreasing material size, improving surface oxygen functionality, and increasing sorption and photocatalytic efficacy (Lyu et al., 2017, 2018; Naghdi et al., 2017; Wang et al., 2018). Nano-BC with particle size smaller than 100 nm was fabricated within 30 h using planetary ball milling method (Richard et al., 2016). Naghdi et al. (2019) suggested a pre-treatment of BC at 80°C for 24 h before conversion to nano-BC using planetary ball milling within 100 min. The pre-conditioning subdued the agglomeration of nano-particles and reduced the size of BC from 212 to 60 nm. Double-disc milling is also an alternative method for nano-BC fabrication; however it demands high operational costs. Among the different ball mill methods, vibrating disc milling produces greater quantities of nano-BC with consistent size and shape due to attrition and shear stress (Karinkanta et al., 2018). Several studies have reported fabrication of nano-BC in a controlled environment using process parameters such as milling period of 120–1,200 min, number of balls from 25 to 800, ball weight from 0.5 to 100 g, and ball size from 3/4 to 15 mm. Ma et al. (2022) optimized the process parameters for synthesis of ball-milled nano-BC by regulating grinding time, rotating speed, and ball-to-powder mass ratio. The BC mixtures must be subsequently dispersed in different solvents post milling to improve particle distribution before separation (Song et al., 2022). The pre-treatment at 80°C however enables the reduction in size aggregation, but is a high-cost method thus restricting its scale-up. Iron oxides can be also added into BC for suppressing the agglomeration of particles and enhancing their distribution (Li et al., 2020a). Ball milling is a high atom economy method and generates nano-scale biodegradable products using renewable sources by limiting the usage of hazardous chemical-assisted procedures.
Among physical methods, sonication is an efficient method for production of nano-BC by employing high-energy ultrasonic radiations to disintegrate BC in suspension. The microporous region in BC increases due to shock waves resulting in opening of clogged pores and exfoliating the carbon structure. The small exfoliated particles then adhere to the surface or embed in the pores of BC resulting in nano-BC production (Liu et al., 2018). The uniformity in nano-BC surface and the development of porosity without obstruction are two prime benefits of sonication (Yang Y. et al., 2020). Few investigations also reported the generation of nano-BC from waste lignin carbonization as a post or pre-treatment with milling for enhancing the surface features and size of nano-BC with subsequent removal of impregnating salts (Jiang et al., 2020; Makshut et al., 2020). Guo et al. (2020) employed hydrothermal reaction for fabricating nano-BC from agricultural waste biomass. Soybean straw and animal wastes were employed as feedstock and digested with acids in a high-pressure hydrothermal reactor. Furthermore, multiple rounds of centrifugation are also employed for separating highly dispersed nano-BC particles (Ullmann et al., 2017). Different feedstock material and pyrolytic conditions, centrifugation period (2–30 min) and rotational speed (3,500–1,000 rpm) were used to prepare nano-BC (Anupama and Khare, 2021).
2.3. Functionalization of nano biochar
The intrinsic characters of nano-BC can be readily modified thus providing a platform for the easy modification for wide applicability in different sectors. Surface fictionalization using amination, sulfonation and oxidation improves the performance of BC-based nanomaterials (Nath et al., 2019). It has been observed that employment of different combination of pure and acid mixtures (H2SO4, HNO3, and HCl) for surface functionalization increased carboxylic group formation with concurrent laccase adsorption (Naghdi et al., 2017). Similarly, Fe3O4 engineered nano-BC has a greater surface area and adsorption site owing to mesoporous structure (Nath et al., 2019). Cellulosic nanocrystal derived nano-BC was modified with ZnO and it displayed greater active sites and functioned as potent photo-catalysts for phenol removal (Zhang et al., 2021). Nano-BC obtained from artichoke leaves was base-modified with NaOH and employed for removal of metformin hydrochloride. The results revealed the existence of COOH, OH, and C=C groups and higher elimination rates of metformin by modified nano-BC in comparison with pristine nano-BC (Mahmoud et al., 2020). Ethylenediamine functionalized nano-BC was employed as an effective nano-sorbent for removing prednisolone and Cr(VI) (Mahmoud et al., 2022). The significance of magnetic nano-BC for treating tetracycline and Hg(II) polluted wastewater was assessed by Li et al. (2019b). The modified nano-BC exhibited high removal rates (>99%) for both tetracycline and Hg(II) (Li et al., 2019b). The employment of pristine BC imposes some limitations on the adsorption efficacy for various contaminants due to low surface functionality and pore size. The surface modification of BC by different approaches improves the surface area, furnishes additional surface functional groups and adsorption sites. Thus, functionalized BC is a promising potential substitute for treating wide range of contaminants.
3. Inherent properties of nano-BC and their characterization
The intrinsic properties of nano-BC are significant in their selection for wide applications. Plant derived nano-BC have large aromatic cluster size and high oxygen surface functionality resulting in higher affinity and coordinate binding of organic pollutants and heavy metals (Figure 2). Nano-BC fabricated from municipal wastes have abundant carbonate, sulfate and aluminosilicate groups, which enable heavy metal complexation and co-precipitation (Song et al., 2019). Likewise, the degree and type of functional groups and porosity influence nano-BC efficacy as a nano-adsorbent and nano-catalyst. The graphitic and amorphous character of BC (hardness and abrasion resistance) can influence the fabrication, characters and morphological and physiological diversity of nano-BC (Anupama and Khare, 2021). Nano-BC synthesized by bulk-BC fabricated at high-temperature has a higher carbon amount, bulk density, and extractable cations such as Ca, Fe, K, Mg, Mn, P, and Zn (Nath et al., 2019). The carbon amount of nano-BC derived from coconut fibers (90–94%) was greater than that of nano-BC from sewage sludge (4%). Generally, the nano-BC has comparatively greater ash content and lower aromatic and carbonized carbon content than the macro-BC (Wang et al., 2013).
The duration and operating temperature of pyrolysis affect the properties of fabricated nano-BC. An increase in pyrolysis temperature increases nano-BC size owing to improved solid density of micro-BC, resulting in the synthesis of large particle (Zhou et al., 2017). Likewise, increasing pyrolysis duration facilitates the transformation of less dense disordered carbon to small particles that form denser mass fractal architectures (Nath et al., 2019). Nano-BC synthesized at lower temperatures (300–400°C) have smaller surface areas (5.6–47.2 m2g−1), but nano-BC synthesized at higher temperatures (450–600°C), possess higher surface area (342–430 m2g−1) due to devolatilization of biomass and generation of surface porosity (Ramanayaka et al., 2020a). The surface area of nano-BC produced by ball milling, sonication, carbonization and centrifugation were in ranges of 3.67–1736, 0.76–264, 9.08–173, and 21.7–253 m2g−1, respectively (Anupama and Khare, 2021). The zeta potential describes the charge on nano-BC surface and stabilizes the efficiency of nano-BC colloidal solution. The higher zeta potential exhibits lesser particle aggregation and increased dispersion. Nano-BC display greater zeta potential (19.4 to 87 mv) as compared to bulk-BC indicating that nano-BC possesses higher degree of dispersity and colloidal stability.
4. Application of nano-BC in environmental remediation
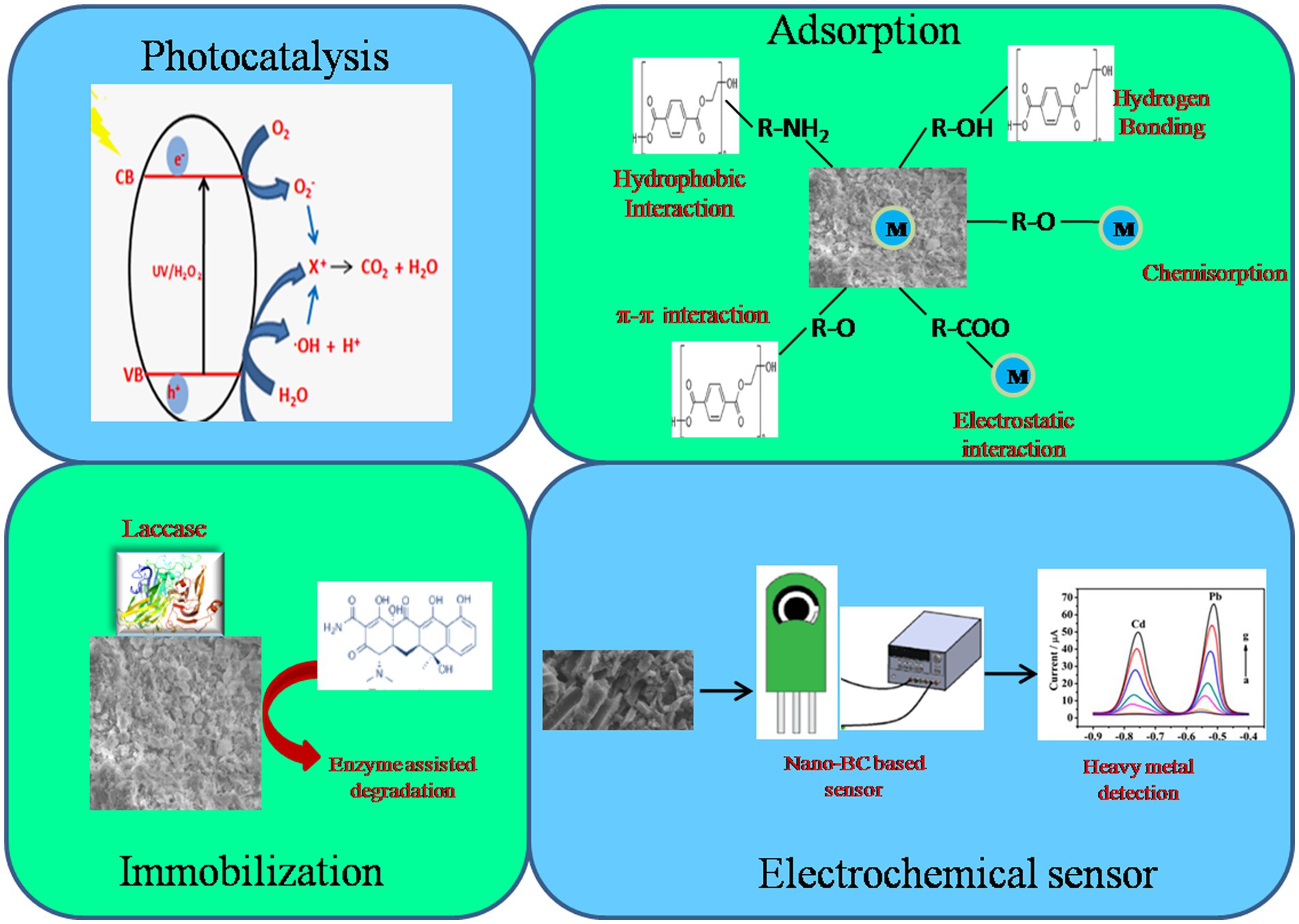
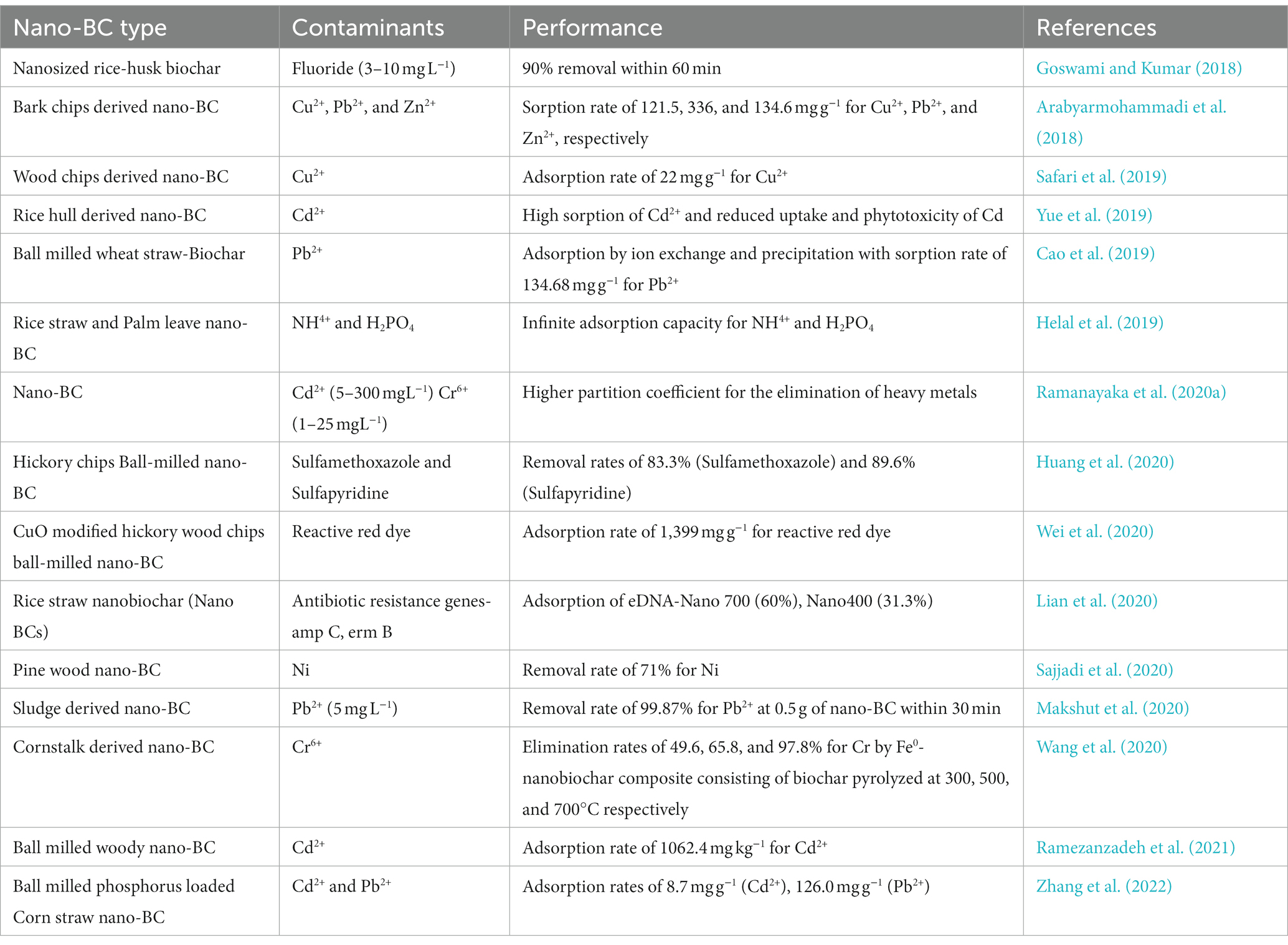
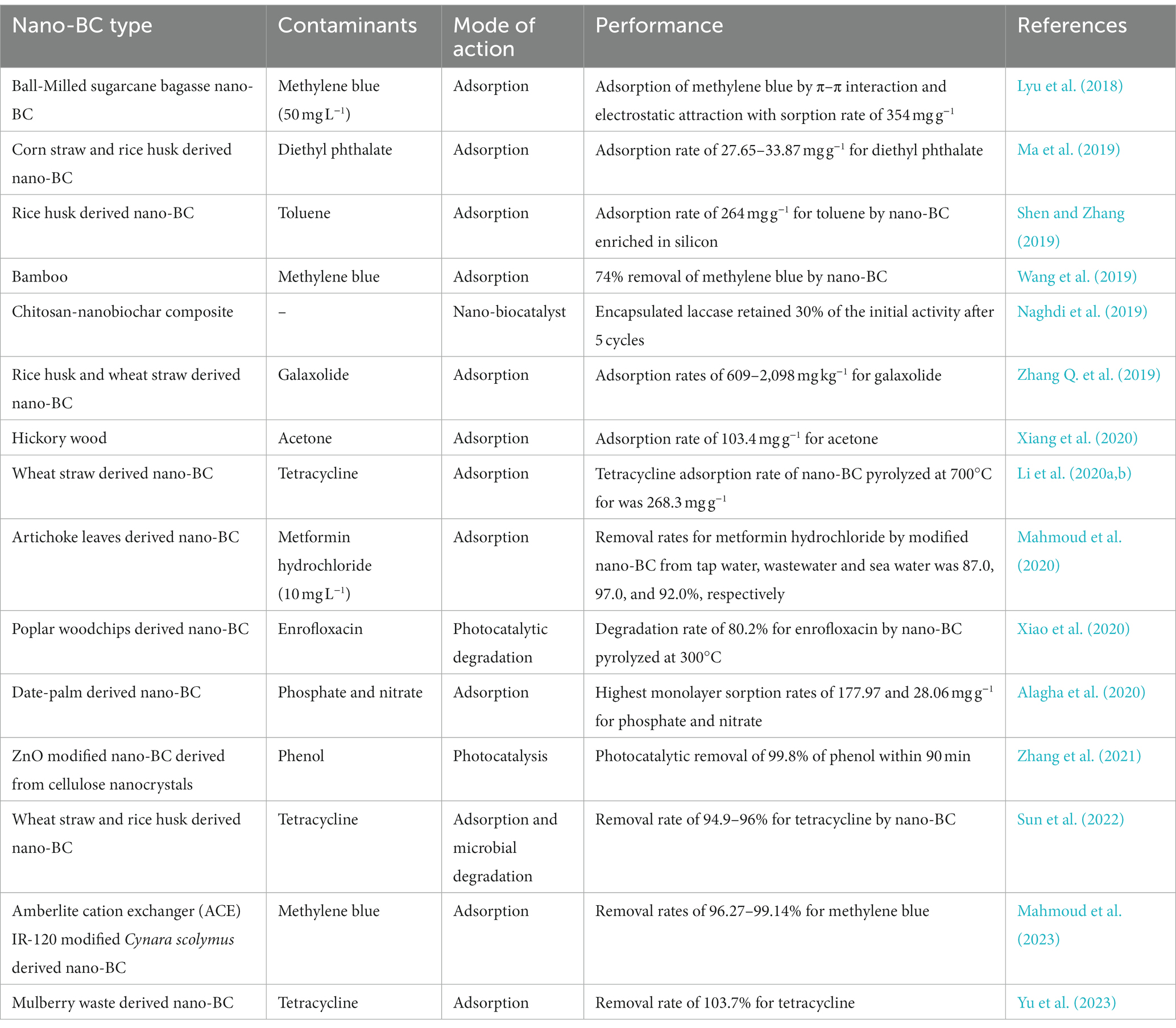
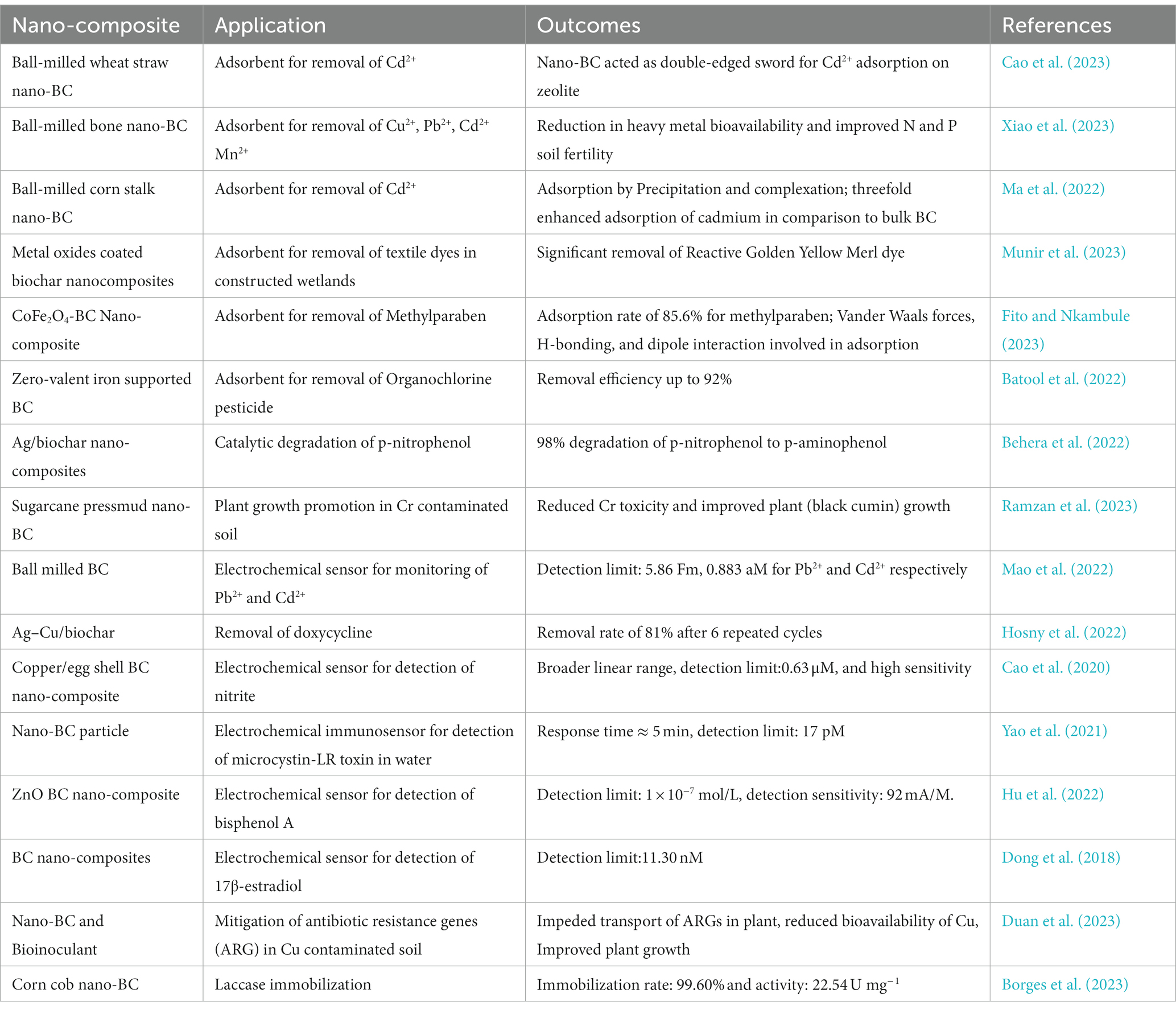
Biochar is recognized as a carbon-negative source since it produces energy while sequestering carbon and has emerged as a potential technology for dealing with several environmental challenges (Jiang et al., 2023). Moreover, the generation of eco-friendly energy and electrodes having enhanced properties using nano-BC is also being explored. Recently, nano-BC is being explored for diverse environmental applications including carbon sequestration, energy generation and treatment of emerging contaminants (agrochemicals, pharmaceuticals, inorganic and organic compounds) from contaminated sites (Figure 3). Nano-BC functions as an excellent adsorbent and thus displays remarkable adsorption capacity for wide range of contaminants (Tables 2, 3). Furthermore, nano-BC also accelerates the breakdown of organic compounds through catalytic electronic shifts like a biocatalyst (Yang et al., 2017).
4.1. Nano-biochar as an adsorbent
Nano-BC has demonstrated exceptional adsorption capability of hazardous organic and inorganic compounds, personal care products, pharmaceutically active compounds, insecticides, and heavy metals from various environmental matrices (Ma et al., 2022; Jiang et al., 2023; Mahmoud et al., 2023). The excellent sorption ability of nano-BC is due to the generation of a stable colloidal solution, greater surface areas, porosity, and surface charge. Chemical and physical adsorption, precipitation, and ion-exchange are the three primary process described for the adsorption of inorganic pollutants on nano-BC (Amusat et al., 2021). The carboxyl, phenol and hydroxyl groups present on nano-BC surface assist in chemi-sorption of contaminants by exchanging anionic ions with cationic contaminants. Physi-sorption occurs due to electrostatic and Van der Waals interactions among the freely mobile electrons of surface aromatic functional groups of the derived nano-BC, ultimately resulting in non-covalent attraction with C=C bonds (Amusat et al., 2021). Precipitation is also considered as one of the primary processes of sorption of inorganic contaminants. It generally involves heavy metal ion precipitation onto the nano-BC surface either in solid form or in the solvent during the adsorption process. The adsorption of organic contaminants by nano-BC consists of different sorption mechanisms including; electrostatic and hydrophobic interaction, ion exchange and pore–filling (Rajput et al., 2022). Moreover physical sorption is regarded as an initial removal mechanism indicating that nano-BC may transport and subsequently desorb the toxic contaminants from aquatic environment. High temperature pyrolysis enhances the specific surface area and void structure richness of nano-BC and reduces hydrophilic surface functional groups and thus physic-sorption is the prominent mechanism for adsorption. However, the surface area of low-temperature pyrolyzed nano-BC is comparatively low and hydrophilic surface functional groups are high, suggesting chemi-sorption to be the dominant mechanism of adsorption (Jiang et al., 2023).
4.2. Nano-biochar as an adsorbent for removal of inorganic compounds
Elbehiry et al. (2022) studied the mono-and multi-sorption of metals (Cd, Cr, and Ni) on water hyacinths and black tea derived BC and nano-BC as a potent, economical and environmentally acceptable absorbents. The nano-BC eliminated >98.8% of Cr and Cd in mono-and competitive systems and the Freundlich isotherm model fitted most appropriately in the sorption kinetics (Elbehiry et al., 2022). The elimination of Cd(II) from an contaminated systems by nano-BC embedded in Ca-alginate beads and fabricated using ball-miller were reported (Wang et al., 2018). The improved surface functionality (oxygen-consisting groups) worked as effective sorption sites, promoting Cd(II)-calcium(II) ion exchange. The pH-dependent variations in Cd(II) sorption revealed the significance of oxygen-consisting surface groups (carboxylic, lactonic, and hydroxyl). The adsorption potential of nano-BC is determined by the surface area, humic acid, functional groups, and graphitic nature. Magnetic nano-BCs (nano zero-valent iron, iron sulfide, and iron oxide BC) display better functionality and magnetic properties that permit nano-BC recovery for recurrent usage. Improved chemical reduction, chemical precipitation, electrostatic interaction, surface complexation, ion exchange and radical activation due to synergistic effect of iron and nano-BC composites, better removal rate for wide range of pollutants are reported (Lyu et al., 2020a; Li et al., 2020b). Sisay et al. (2023) reported that Mg/Zr modified nano-BC derived from spent coffee grounds is an efficient sorbent for phosphate recovery and phosphorous release fertilizer. Furthermore, thiol-modified ball-milled BC demonstrated an improved Hg(II) elimination efficiency, with a sorption rate of 320.1 mg g−1, as compared to unmilled BC (105.7 mg g−1) (Lyu et al., 2020b). The amino-functional silica-coated magnetic nano-BC derived from Cynodon dactylon exhibited improved adsorption rates of 220.4 and 185.4 mg g−1 for Cu and Pb, respectively. The nano-composites also demonstrated a 15-fold reuse capability and highest elimination rates for Cu and Pb (Dhanya et al., 2022). The efficacy of ethylenediamine modified nano-BC in the elimination of Cr (VI) and prednisolone was investigated (Mahmoud et al., 2022). Electrostatic, hydrophobic and π-π interaction, ion exchange and complexation are the reported processes for Cr (VI) and prednisolone sorption onto the modified nano-BC. Similarly electrostatic interaction was involved in the elimination of anionic inorganic contaminants from aqueous systems by CuO modified nano-BC. CuO provides cationic sorption sites on surface of nano-BC for the contaminant binding and its subsequent elimination (Wei et al., 2020).
4.3. Nano-biochar as an adsorbent for treating organic compounds
Bulk-BC is the most commonly employed sorbent for treating a wide range of toxic contaminants (Yang F. et al., 2020). Nonetheless, nano-BC has an advantage above macro-BC due to larger specific surface area, higher negative Zeta-potential and greater surface functionality (Lian et al., 2018). Ball-milled BC displayed adsorption capabilities of 100.3 and 57.9 mg g−1 for removing antibiotics sulfamethoxazole and sulfapyridine, respectively (Huang et al., 2020). According to Shen et al. (2020), any changes in mechanical, physico-chemical, and morphological characters of nano-BC may improve its adsorption efficacy. Luong et al. (2020) described that the change in pH from acid to alkaline using a detergent (tween 80) can increase the sorption capacities of pinewood nano-BC by up to 63%. Xiao et al. (2020) reported that goethite modified peanut shell nano-BC exhibited intercalated hetero-structures and improved hetero-aggregation, which resulted in better adsorption rates. Few reports have employed nano-BC for efficiently removing oxytetracycline from aqueous systems (Li et al., 2020b; Ramanayaka et al., 2020b). The larger surface areas and higher oxygenic groups on nano-BC surface enabled remarkable removal of trichloroethylene with a degradation rate of 99.4% within 5 min, where nZVI-enhanced SO4• synthesis improved the degradation rate (Yan et al., 2015). The removal rate for dimethyl phthalate, diethyl phthalate, and dibutyl phthalate by BC-graphene nanosheets was substantially greater than that of bulk-BC. The aromatic groups on BC graphene nanosheets displayed π–π EDA linkages with the aromatic ring of dimethyl phthalate while hydrophobic groups are involved in dibutyl phthalate binding (Abdul et al., 2017).
The sorption of Cu(II), tylosin and sulfamethoxazole on nano-hydroxyapatite modified BC occurred due to electrostatic and π–π interaction, and hydrogen bonds (Li et al., 2020b). The occurrence of tylosin and/or sulfamethoxazole increased adsorption of Cu(II) significantly (Li et al., 2020b). Nano-BC obtained from hickory wood with specific surface area of 305 m2 g−1 efficiently adsorbed various compounds (acetone, cyclohexane, chloroform, ethanol, and toluene) with an adsorption rate in the range of 23.4–103.4 mg/g (Xiang et al., 2020). The volatile organic compounds easily diffuse through the pores of nano-BC to reach the interior during sorption, reaching equilibrium after about 1 h. The physico-chemical, morphological and mechanical characters of nano-BC such as pore size, specific surface area, BC composition and contaminant characters are the most important factors in surface sorption (Xiang et al., 2020). The volatile polar organic compounds (acetone, ethanol, and chloroform) are sorbed onto nano-BC by dipole–dipole interaction and hydrogen bonds. The sorption of weakly polar volatile organic compounds exhibited more heterogeneity than the sorption of polar compounds. Furthermore, due to stronger intermolecular forces, organic contaminants with high boiling points (ethanol, cyclohexane, and toluene) were efficiently sorbed by nano-BC. Thus, the sorption of volatile contaminants by ball-milled nano-BC is a highly potent alternative for removal of air pollutants and warranting detailed research into the mechanisms and critical factors (Anupama and Khare, 2021).
4.4. Nano-biochar as an immobilization material for enzymes/biocatalysts
Nano-BC can be utilized as an enzyme/microbe/biocatalyst carrier material for achieving continued breakdown of contaminants due to its high mobility and tunable surface chemistry (Table 4). Enzymatic catalysis is a sustainable process for degradation of pollutants and thus laccases immobilized nano-BC is being extensively employed for biodegradation of different contaminants. Oxygen functionalized nano-BC supported Lacasse was utilized for treating carbamazepine contamination (Naghdi et al., 2017). The acidic treatment introduced hydroxyl groups, with its subsequent oxidation to carboxyl groups. The degradation rates of >80% for carbamazepine with recyclability for 3 cycles at a comparative removal efficacy were reported (Naghdi et al., 2017). The immobilization process can be improved by addition of cross-linking compounds such as carbodiimide hydrochloride or glutaraldehyde (Naghdi et al., 2018). Naghdi et al. (2019) recently described a hydrogel technique for encapsulating laccase on nano-BC and chitosan. They reported that adding laccase onto nano-BC significantly improved its thermostability (4–70°C) during storage. Lonappan et al. (2018) used laccase immobilized nano-BC to completely remove (100%) diclofenac (2.5 mg L−1) in 2 h, which was faster than laccase immobilized carbon nanotube (6 h), and 40% of the efficacy was retained post 5 cycles. More recently, laccase immobilized onto magnetic nano-BC was fabricated and employed for elimination of bisphenol A from aqueous environment (Zhang et al., 2020). The complete elimination of bisphenol A (25 mg L−1) was reported within 75 min and 85% efficacy of the composite remained after 7 cycles. Cold-active toluene/o-xylene monooxygenase and catechol 1,2-dioxygenase were immobilized onto micro/nano-BC or chitosan and employed for petroleum hydrocarbon degradation (Miri et al., 2021). The results suggested that immobilization improved the storage stability of the enzymes (>50% recyclability after 1 month at 4°C) and degradative ability (>80% degradation of BTEX).
4.5. Nanobiochar as photocatalyst
In recent times nano-BC supported photocatalysts have been fabricated through different methods to photo-catalytically breakdown water pollutants (phenolics, dyes, pharmaceutically active components, antibiotics). The photocatalysis is dependent on the methods of nano-BC synthesis. BC is an excellent support for photocatalysts owing to its tunable functional groups, chemo-stability, and electrical conductance. BC as a photocatalytic support reduces e−/h + recombination and thus displays increased catalytic efficiency (Ahmaruzzaman, 2021). Recently, the nano-BC/ZnO photocatalyst derived from carbon/ZnO nanocomposite were employed for photocatalytic breakdown of phenol (Zhang et al., 2020). In-situ precipitation and carbonization were used to create the photocatalyst, with carbon nano-composites serving as both the template and the carbon source. These composites demonstrated remarkable stability and durability with photodegradation rate of 95% suggesting that the integration of carbon nano-composites could efficiently reduce the photo-corrosion of ZnO (Zhang et al., 2020). The BC caused a reduction in the band gap of ZnO through continuous electron/hole separation and transport, increasing phenol degradation rates. A unique core-shell P-laden BC/ZnO/g-C3N4 nano-composite was recently reported as an excellent catalyst for atrazine breakdown and a potential regulated-release nanofertilizer for enhancing P utilization rates (An et al., 2022). The results revealed the formation of a Z-shaped heterojunction between ZnO and g-C3N4 in Pbi-ZnO-g-C3N4. The authors suggested that BC functions as an electron-transfer agent promoting the disjunction of electron–hole pairs. Highest atrazine photodegradation rates of 85.3% within 260 min were reported (An et al., 2022).
4.6. Nano-biochar used in electrochemical biosensor
The electrochemical characteristics of nano-BC have recently received attention for its potential application as an alternative to carbon electrodes. The high adsorption capacity of nano-BC enables selective entrapment of chemicals to improve their concentration on electrode surface thus increasing the sensitiveness of electrochemical biosensors for detection (Table 4). The water-dispersible nature of nano-BC allows its use in film-forming methods for creating film electrodes with potent electrochemical applications (Plácido et al., 2019). In supercapacitors also, the electrode materials have recently been substituted with nano-BC due to its both meso and microporous structures having higher specific surface area resulting in improved performance. Biochar is also being used to replace cathode materials in batteries. Water dispersible nano-BC has been successfully used as an electrode material for Pb(II) and Cd(II) voltammetric sensors (Liu et al., 2016; Li et al., 2017). Li et al. (2017) investigated the impact of loading a hybrid of both bulk and nano-BC on the electrodes of a voltammetric sensor for detection of Pb2+, and increased sensitivity and electric current (3.24–4.0 and 4.5 μA) were reported. Moreover, fluorescence assay confirmed that BC releases dissolved organic matter containing fluorescent humic compounds (Hernandez-Soriano et al., 2016). The fluorescent characters of nano-BC have also been exploited for development of fluorescent detectors for metals (Plácido et al., 2019). Nano-BC fabricated from sorghum and rice straw and dairy manure was utilized as a probe for heavy metal detection and the results revealed a 100, 66, 66, and 33% accuracy for Pb2+, Ni2+, Cu2+, and Hg2+ detection, respectively, (Plácido et al., 2019). This was the initial report to suggest the application of nano-BC quenching data as a simple and accurate method for detection of toxic metal ions.
Furthermore, functionalized magnetic baggase nano-BC was fabricated by combining carboxyl groups and enzymes for bisphenol A monitoring in aqueous systems, demonstrating high sensitiveness as well as excellent electrochemical activity. Dong et al. (2018) studied the possibility of loading nano-BC on glass carbon electrodes for 17-estradiol monitoring and the electric current increased from 0 to 1.5 μA at the 17-estradiol amount of 3 M. The transfer resistance was lowered from 495 to 325 Ω on loading electrodes with nano-BC fabricated at 800°C in comparison with pristine BC resulting in increased electrode conductance. Ball-milled BC modified carbon electrodes displayed remarkable electrochemical characters and electrocatalysis as indicated by conductance, peak-to-peak disjunction, resistivity, and charge transfer resistance (Lyu et al., 2019). He et al. (2020) employed tyrosinase immobilized magnetic nano-BC for detection of bisphenol A. The developed electrochemical biosensor demonstrated a monitoring range of 2.78 nM with a linear range of 0.01–1.01 M, and the sensitivity remained consistent after 8 cycles without signal reduction. The use of nano-BC in electrochemistry can be expanded into new fields such as biomass electrocatalysis, fuel cells, and CO2 reduction. A novel electrochemical biosensor for detection of lead and cadmium was synthesized by fabricating a high conductance and contaminant specific electrode. Ball milled BC was employed as the conductive material having large conductance, oxygen rich functionality and pores of ion-imprinted polymer functioned as target interacting sites (Mao et al., 2022). The ion imprinted bulk-BC electrode was created by in situ electro-polymerization of L-Cysteine and template metal ions on glassy carbon-modified bulk-BC, followed by template removal. The electrode detected very low concentrations of lead and cadmium using anodic dissolved differential pulse voltammetry. The monitoring range of 5.86 fM and 0.883 aM, and linear ranges of 25 fM ~ 1 μM and 0.1 fM ~ 1 μM, respectively were reported. The electrodes displayed no interaction with other ionic and organic molecules, and could be recycled for 7 cycles without losing detection sensitivity (Mao et al., 2022). Ferlazzo et al. (2023) described an electrochemical biosensor fabricated by nano-BC for the detecting nitrites and sulfites in contaminated water bodies. The nano-BC was placed on a commercial screen-printed carbon electrode (SPCE). The fabricated sensor outperformed the normal SPCE sensor in terms of detection limits and electrochemical oxidization of sulfites and nitrites in water (Ferlazzo et al., 2023).
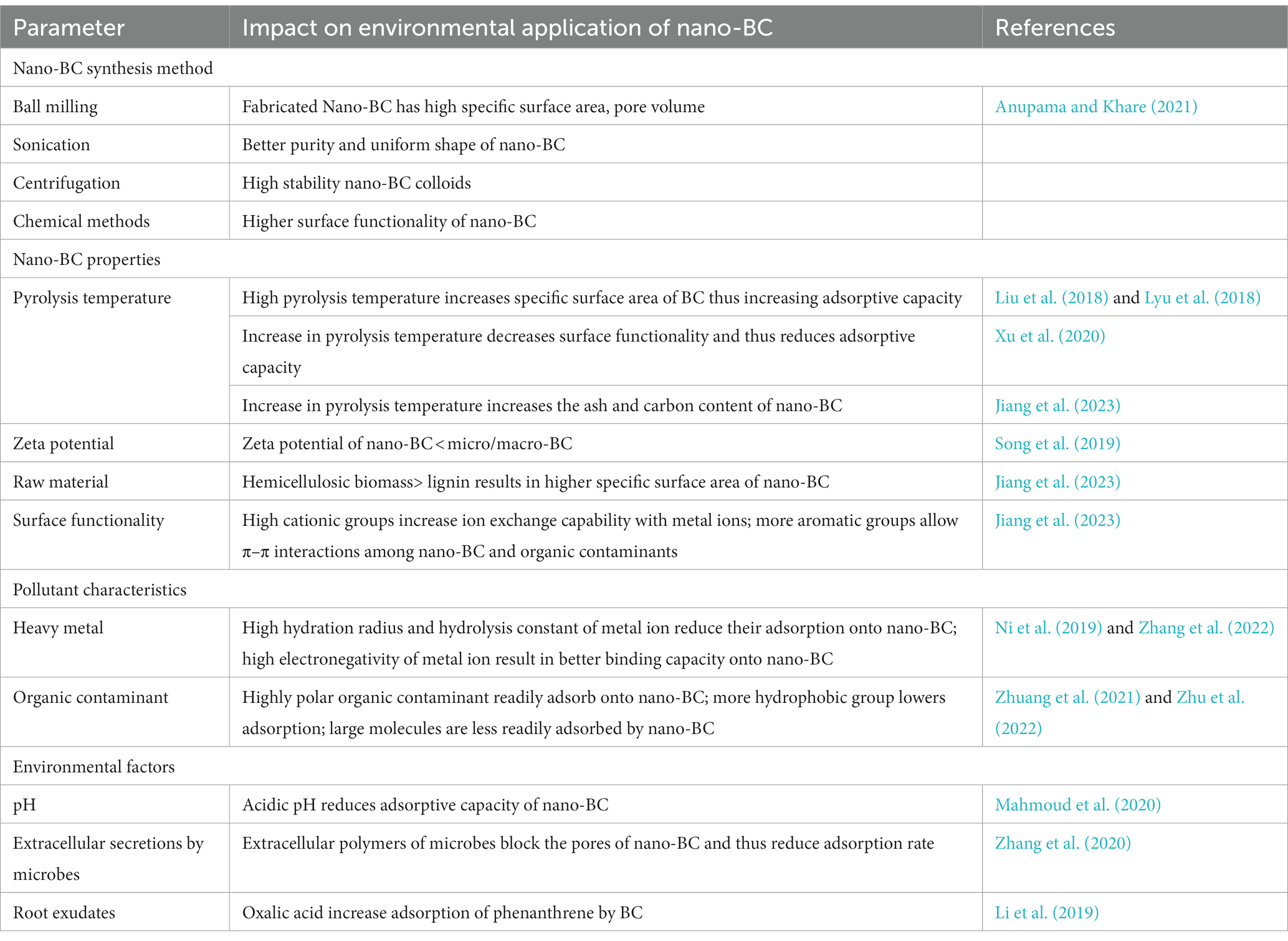
5. Factors affecting performance of nano-BC for environmental remediation
The biogeochemical nature of nano-BC during contaminant elimination is influenced by a variety of physico-chemical parameters (Table 5). Nano-BC possessing high cationic surface groups allows improved ion exchange capacity of nano-BC with toxic metal ions. A higher concentration of aromatic groups on surface of nano-BC results in better π-π-interactions with organic contaminants (Jiang et al., 2023). The aggregation capability, suspension stability, and electrokinetic characters of nano-BC impact the sorption of contaminants that may be determined by the zeta potential (Filipinas et al., 2021). The surface functionality is also dependent on pyrolysis temperature used for BC synthesis and low pyrolytic temperature was found to have abundant surface functional groups, higher zeta potential and stronger colloidal stability (Xu et al., 2020). In case of metal ions, the valency, hydration area, electronegative nature and hydrolytic constant are the dominant parameters that influence the metal ion removal by nano-BC. Zhang et al. (2022) reported that the sorption rate of nano-BC for Pb2+ was significantly higher than that for Cd2+ in same treatment conditions. The authors suggested that variation in metal characteristics (hydration area, hydrolytic constant) and their affinity for binding sites are responsible for different sorption rates. For organic contaminants, groups such as polar, hydrophobic, aromatic, and molecular weight of the contaminant affect their interaction with nano-BC. In general, the sorption of highly hydrophobic compounds by carbonaceous compounds is slow (Choi et al., 2014). Galaxolide is a highly hydrophobic contaminant and therefore shows high sorption to ball milled nano-BC (Zhang Q. et al., 2019). Moreover, high molecular weight contaminants are hardly sorbed by nano-BC due to size exclusion and pore filling effect resulting in restriction of these compounds from entering small pores on nano-BC (Zhu et al., 2022). Nano-BC assisted remediation of contaminants is also influenced by environmental parameters including: pH, soil microbes, dissolved organic matter, root exudates and coexisting contaminants (Jiang et al., 2023). In lower pH environments, the surface functional groups of nano-BC get protonated to generate H+. This causes competition between H+ and cationic contaminants for sorption sites thus affecting nano-BC’s sorption capability (Mahmoud et al., 2023). Wang et al. (2017) reported that co-existence of lead and p-nitrophenol improved the sorption of p-nitrophenol on nano-BC. In soil systems, carbon of nano-BC may functions as a source of nutrition to soil microbes, thus improving their metabolism and contaminant degradation efficacy (Mukherjee et al., 2022). The root exudates released from plant in contaminated soils can also impact the physical and chemical characteristics of nano-BC thus influencing its contaminant sorption ability (Li et al., 2019).
6. Challenges and environmental concern of N-BC
Nano-BC and colloidal BC possess high surface area, pore size, and surface functionality thus demonstrating remarkable contaminant removal efficiency as compared to pristine BC (Ramanayaka et al., 2020b). However there are several constraints to using native nano-BC in environmental applications including low yield and stability, easy mobilization, high agglomeration, uptake, accumulation, toxic nature and limitations in recovery (Liu et al., 2018). Functionalizing nano-BC with appropriate redox functional groups promotes its stability/suitability for contaminant removal in different environmental matrices but these studies are still in infancy and broad understanding is still necessary for on-site application. The inter-linkages among BC structure, oxygenic surface functional groups, feedstock types and pyrolytic parameters must be assessed for postulating molecular mechanisms of contaminant removal by electrochemical reaction pathways (Amusat et al., 2021). The technologies for large-scale fabrication of nano-BC need to be developed for achieving high yield of nano-BC for wide applications. The green and biogenic methods for fabricating nano-BC need to be investigated for reducing the risk of cross-contamination of chemicals used for synthesis during wastewater treatment. Nano-BC showed improved results when compared to the bulk-BC, however, the comparative performance to other nanomaterials and carbon based nanocompounds should be investigated further (Rajput et al., 2022). The economical assessment of the expenses is critical in evaluating the manufacture and deployment of the nano-BC for wide application. The lack of data on large level synthesis and employment of nano-BC makes estimating the economic aspect of nano-BC difficult. Furthermore, the utilization of wide range of raw feedstock material and different factors and processes for the fabrication of bulk-BC and the lack of standard procedures limit the cost analysis of nano-BC synthesis. The higher adsorption of contaminants and transportability of nano-BC, can cause the risk of cross-contamination of different ecosystems. High dispersion of nano–BC in natural aquatic systems can further expose different organisms to nanoparticle-associated risks (Freixa et al., 2018). The examination of toxic impact on respiratory system indicated a lower risk to human health (Dong et al., 2019). A very limited number of reports suggested the toxic impact of nano-BC and nanocarbon compounds on plant, mammals and soil microflora, thus in spite of their numerous benefits, the eco-toxicity must be investigated (Zhang K. et al., 2019; Rajput et al., 2022).
7. Conclusion
Nano-BC is an emerging and potential alternative to carbon-based nanomaterials for wide applicability in comparison to the pristine and bulk-BC. Nano-BC exhibits exceptional physicochemical characters such as high surface functionality and ease of surface modification. It is generally manufactured using ball miller, ultra-sonication, carbonization, centrifugation, and manual grinders. Ball milling is an economical, sustainable and green method, while ultrasonication is more energy-intensive and non-eco-friendly. Nonetheless, process optimization, eco-toxicity, and life cycle evaluation of nano-BC fabrication is necessary for individual process prior to its selection for commercial-scale synthesis. The distinctly minute size of nano-BC offers improved surface areas imparting it with the potential of applicability in environmental remediation. Nano-BC efficiently reduced toxic organic and inorganic pollutants from different environmental matrices as compared to bulk-BC. Nano-BC functions as detoxicant, playing vital part in waste management, reduction of soil erosion, and preventing nutrient loss from soil. The surface features of nano-BC allow it to function as carrier for the immobilizing enzymes, biocatalysts and microbes. Nano-BC is also a potential alternative to chemical electrode and thus functions as biosensor for detection and monitoring of toxic contaminants. Additionally the high surface area also provides habitat for microorganisms on nano-BC, thus understanding their interactions at molecular and genetic level can unfold new areas for hybrid remediation strategies, which however warrants future research. However, there is a considerable knowledge gap about the parameters of the nano-BC synthesis by different methods and their physicochemical characters. The optimization of process parameter for desirable properties (porosity, surface area and functionality and binding sites) and yield is required. Methods for rapid valorization of nano-BC and their transport and distribution into different ecosystems need to be studied for restricting the detrimental impact.
Author contributions
GB, AD, SGa, and VR: conceptualization and written the original draft. SGu, SM, and PS edited and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor PC declared a shared affiliation with the author SG at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdul, G., Zhu, X., and Chen, B. (2017). Structural characteristics of biochar– graphene nanosheet composites and their adsorption performance for phthalic acid esters. Chem. Eng. J. 319, 9–20. doi: 10.1016/j.cej.2017.02.074
Ahmaruzzaman, M. (2021). Biochar based nanocomposites for photocatalytic degradation of emerging organic pollutants from water and wastewater. Mater. Res. Bull. 140:111262. doi: 10.1016/j.materresbull.2021.111262
Alagha, O., Manzar, M. S., Zubair, M., Anil, I., and Muazu Qureshi, N. D. A. (2020). Comparative adsorptive removal of phosphate and nitrate from wastewater using biochar-MgAl LDH nanocomposites: coexisting anions effect and mechanistic studies. Nano 10:336. doi: 10.3390/nano10020336
Amusat, S. O., Kebede, T. G., Dube, S., and Nindi, M. M. (2021). Ball-milling synthesis of biochar and biochar–based nanocomposites and prospects for removal of emerging contaminants: a review. J. Water Process Eng. 41:101993. doi: 10.1016/j.jwpe.2021.101993
An, X., Wang, H., Dong, C., Jiang, P., Wu, Z., and Yu, B. (2022). Core-shell P-laden biochar/ZnO/g-C3N4 composite for enhanced photocatalytic degradation of atrazine and improved P slow-release performance. J. Colloid Interface Sci. 608, 2539–2548. doi: 10.1016/j.jcis.2021.10.166
Anupama,, and Khare, P. (2021). A comprehensive evaluation of inherent properties and applications of nano-biochar prepared from different methods and feedstocks. J. Clean. Prod. 320:128759. doi: 10.1016/j.jclepro.2021.128759
Arabyarmohammadi, H., Darban, A. K., Abdollahy, M., Yong, R., Ayati, B., Zirakjou, A., et al. (2018). Utilization of a novel chitosan/clay/biochar nanobiocomposite for immobilization of heavy metals in acid soil environment. J. Polym. Environ. 26, 2107–2119. doi: 10.1007/s10924-017-1102-6
Ashiq, A., Adassooriya, N. M., Sarkar, B., Rajapaksha, A. U., Ok, Y. S., and Vithanage, M. (2019). Municipal solid waste biochar-bentonite composite for the removal of antibiotic ciprofloxacin from aqueous media. J. Environ. Manag. 236, 428–435. doi: 10.1016/j.jenvman.2019.02.006
Batool, S., Shah, A. A., Bakar, A. F. A., Maah, M. J., and Bakar, N. K. A. (2022). Removal of organochlorine pesticides using zerovalent iron supported on biochar nanocomposite from Nephelium lappaceum (Rambutan) fruit peel waste. Chemosphere 289:133011. doi: 10.1016/j.chemosphere.2021.133011
Behera, M., Tiwari, N., Banerjee, S., Sheik, A. R., Kumar, M., Pal, M., et al. (2022). Ag/biochar nanocomposites demonstrate remarkable catalytic activity towards reduction of p-nitrophenol via restricted agglomeration and leaching characteristics. Colloids Surf A Physicochem. Eng. Aspects. 642:128616. doi: 10.1016/j.colsurfa.2022.128616
Bhatt, P., Bhandari, G., and Bilal, M. (2022). Occurrence, toxicity impacts and mitigation of emerging micropollutants in the aquatic environments: recent tendencies and perspectives. J. Environ. Chem. Eng. 10:107598. doi: 10.1016/j.jece.2022.107598
Bhatt, P., Gangola, S., Bhandari, G., Zhang, W., Maithani, D., Mishra, S., et al. (2020). New insights into degradation of synthetic pollutants in contaminated environment. Chemosphere 268:128827. doi: 10.1016/j.chemosphere.2020.128827
Bolan, N., Hoang, S. A., Beiyuan, J., Gupta, S., Hou, D., Karakoti, A., et al. (2022). Multifunctional applications of biochar beyond carbon storage. Int. Matter. Rev. 67, 150–200. doi: 10.1080/09506608.2021.1922047
Borges, J. F., Nascimento, P. A., Alves, A. N., Santos, M. P. F., Brito, M. J. P., Bonomo, R. C. F., et al. (2023). Laccase immobilization on activated carbon from hydrothermal carbonization of corn cob. Waste Biomass Valor. doi: 10.1007/s12649-023-02160-1
Cao, L., Kang, Z. W., Ding, Q., Zhang, X., Lin, H., Lin, M., et al. (2020). Rapid pyrolysis of cu2+−polluted eggshell membrane into a functional Cu2+-Cu+/biochar for ultrasensitive electrochemical detection of nitrite in water. Sci. Total Environ. 723:138008. doi: 10.1016/j.scitotenv.2020.138008
Cao, X., Meng, Z., Sheng, L., Hu, X., Wang, T., Sun, X., et al. (2023). Double-edged sword effect of nano-biochar for Cd2+ adsorption on zeolite. J. Environ. Chem. Eng. 11:109901. doi: 10.1016/j.jece.2023.109901
Cao, Y., Xiao, W., Shen, G., Ji, G., Zhang, Y., Gao, C., et al. (2019). Carbonization and ball milling on the enhancement of Pb(II) adsorption by wheat straw: competitive effects of ion exchange and precipitation. Bioresour. Technol. 273, 70–76. doi: 10.1016/j.biortech.2018.10.065
Choi, Y. J., Cho, Y. M., and Luthy, R. G. (2014). In situ sequestration of hydrophobic organic contaminants in sediments under stagnant contact with activated carbon. 1. Column studies. Environ. Sci. Technol. 48, 1835–1842. doi: 10.1021/es403335g
Dhanya, V., Dhandapani, B., Vaishnavi, G., and Preethi, V. (2022). Synthesis of tri-metallic surface engineered nanobiochar from Cynodon dactylon residues in a single step - batch and column studies for the removal of copper and lead ions. Chemosphere 28:131572. doi: 10.1016/j.chemosphere.2021.131572
Dong, X., He, L., Liu, Y., and Piao, Y. (2018). Preparation of highly conductive biochar nanoparticles for rapid and sensitive detection of 17β-estradiol in water. Electrochim. Acta 292, 55–62. doi: 10.1016/j.electacta.2018.09.129
Dong, C., Lung, S., Chen, C., Lee, J., Chen, Y., Wang, W., et al. (2019). Assessment of the pulmonary toxic potential of nano-tobacco stem-pyrolyzed biochars. Environ. Sci. Nano 6, 1527–1535. doi: 10.1039/C8EN00968F
Duan, M., Li, Z., Yan, R., Zhou, B., Su, L., Li, M., et al. (2023). Mechanism for combined application of biochar and Bacillus cereus to reduce antibiotic resistance genes in copper contaminated soil and lettuce. Sci. Total Environ. 884:163422. doi: 10.1016/j.scitotenv.2023.163422
Elbehiry, F., Darweesh, M., Al-Anany, F. S., Khalifa, A. M., Almashad, A. A., El-Ramady, H., et al. (2022). Using biochar and Nanobiochar of water hyacinth and black tea waste in metals removal from aqueous solutions. Sustainability 14:10118. doi: 10.3390/su141610118
Ferlazzo, A., Bressi, V., Espro, C., Iannazzo, D., Piperopoulos, E., and Neri, G. (2023). Electrochemical determination of nitrites and sulfites by using waste-derived nanobiochar. J. Electroana. Chem. 928:117071. doi: 10.1016/j.jelechem.2022.117071
Filipinas, J. Q., Rivera, K. K. P., Ong, D. C., Pingul-Ong, S. M. B., Abarca, R. R. M., and de Luna, M. D. G. (2021). Removal of sodium diclofenac from aqueous solutions by rice hull biochar. Biochar 3, 189–200. doi: 10.1007/s42773-020-00079-7
Fito, J., and Nkambule, T. T. I. (2023). Synthesis of biochar-CoFe2O4 nanocomposite for adsorption of methylparaben from wastewater under full factorial experimental design. Environ. Monit. Assess. 195:241. doi: 10.1007/s10661-022-10819-w
Freixa, A., Acuña, V., Sanchís, J., Farré, M., Barceló, D., and Sabater, S. (2018). Ecotoxicological effects of carbon based nanomaterials in aquatic organisms. Sci. Total Environ. 619–620, 328–337. doi: 10.1016/j.scitotenv.2017.11.095
Gangola, S., Bhatt, P., Kumar, A. J., Bhandari, G., Joshi, S., Punetha, A., et al. (2022). Biotechnological tools to elucidate the mechanism of pesticide degradation in the environment. Chemosphere 296:133916. doi: 10.1016/j.chemosphere.2022.133916
Goswami, R., and Kumar, M. (2018). Removal of fluoride from aqueous solution using nanoscale rice husk biochar. Groundw. Sustain. Dev. 7, 446–451. doi: 10.1016/j.gsd.2017.12.010
Guo, F., Bao, L., Wang, H., Larson, S. L., Ballard, J. H., Knotek-Smith, H. M., et al. (2020). A simple method for the synthesis of biochar nanodots using hydrothermal reactor. Methods X 7:101022. doi: 10.1016/j.mex.2020.101022
He, L., Yang, Y., Kim, J., Yao, L., Dong, X., Li, T., et al. (2020). Multi-layered enzyme coating on highly conductive magnetic biochar nanoparticles for bisphenol a sensing in water. Chem. Eng. J. 384:123276. doi: 10.1016/j.cej.2019.123276
Helal, M. I. D., Husein, M. E., Walaa, G., and Mostafa, E. D. (2019). Characterization of agricultural residues-based nano biochar and its efficiency in adsorption/desorption of nutrients. Int. J. Environ. 8, 130–141,
Hernandez-Soriano, M. C., Kerré, B., Kopittke, P. M., Horemans, B., and Smolders, E. (2016). Biochar affects carbon composition and stability in soil: a combined spectroscopymicroscopy study. Sci. Rep. 6:25127. doi: 10.1038/srep25127
Hosny, M., Fawzy, M., and Eltaweil, A. S. (2022). Phytofabrication of bimetallic silver-copper/biochar nanocomposite for environmental and medical applications. J. Environ. Manag. 316:115238. doi: 10.1016/j.jenvman.2022.115238
Hu, J., Mao, D., Duan, P., Li, K., Lin, Y., Wang, X., et al. (2022). Green synthesis of ZnO/BC nanohybrid for fast and sensitive detection of bisphenol a in water. Chemosensors 10:163. doi: 10.3390/chemosensors10050163
Huang, J., Zimmerman, A. R., Chen, H., and Gao, B. (2020). Ball milled biochar effectively removes sulfamethoxazole and sulfapyridine antibiotics from water and wastewater. Environ. Pollut. 258:113809. doi: 10.1016/j.envpol.2019.113809
Iannazzo, D., Celesti, C., Espro, C., Ferlazzo, A., Giofrè, S. V., Scuderi, M., et al. (2022). Orange-peel-derived nanobiochar for targeted cancer therapy. Pharmaceutics 14:2249. doi: 10.3390/pharmaceutics14102249
Jenie, S. N. A., Kristiani, A., Khaerudini, D. S., and Takeishi, K. (2020). Sulfonated magnetic nanobiochar as heterogeneous acid catalyst for esterification reaction. J. Environ. Chem. Eng. 8:103912. doi: 10.1016/j.jece.2020.103912
Jiang, C., Bo, J., Xiao, X., Zhang, S., Wang, Z., Yan, G., et al. (2020). Converting waste lignin into nano-biochar as a renewable substitute of carbon black for reinforcing styrene-butadiene rubber. Waste Manag. 102, 732–742. doi: 10.1016/j.wasman.2019.11.019
Jiang, M., He, L., Niazi, N. K., Wang, H., Gustave, H., Vithanage, M., et al. (2023). Nanobiochar for the remediation of contaminated soil and water: challenges and opportunities. Biochar 5:2. doi: 10.1007/s42773-022-00201-x
Karinkanta, P., Ammala, A., Illikainen, M., and Niinimaki, J. (2018). Fine grinding of wood overview from wood breakage to applications. Biomass Bioenergy 113, 31–44. doi: 10.1016/j.biombioe.2018.03.007
Khan, H. A., Naqvi, S. R., Mehran, M. T., Khoja, A. H., Khan Niazi, M. B., Juchelková, D., et al. (2021). A performance evaluation study of nano-biochar as a potential slow-release nano-fertilizer from wheat straw residue for sustainable agriculture. Chemosphere 285:131382. doi: 10.1016/j.chemosphere.2021.131382
Li, R., Deng, H., Zhang, X., Wang, J. J., Awasthi, M. K., Wang, Q., et al. (2019a). High-efficiency removal of Pb (II) and humate by a CeO2-MoS2 hybrid magnetic biochar. Bioresour. Technol. 273, 335–340. doi: 10.1016/j.biortech.2018.10.053
Li, X., Song, Y., Bian, Y., Wang, F., Gu, C., Yang, X., et al. (2019). Effects of root exudates on the sorption of polycyclic aromatic hydrocarbons onto biochar. Environ. Pollut. Bioavail. 31, 156–165. doi: 10.1080/26395940.2019.1593054
Li, Z., Sun, Y., Yang, Y., Han, Y., Wang, T., Chen, J., et al. (2020a). Biochar supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 383:121240. doi: 10.1016/j.jhazmat.2019.121240
Li, Z., Wang, Z., Wu, X., Li, M., and Liu, X. (2020b). Competitive adsorption of tylosin, sulfamethoxazole and cu(II) on nano-hydrocyapatite modifed biochar in water. Chemosphere 240:124884. doi: 10.1016/j.chemosphere.2019.124884
Li, L., Zhang, L., Chen, Z., Huang, G., Liu, M., and Li, Y. (2017). Mass preparation of micro/nano-powders of biochar with water dispersibility and their potential application. New J. Chem. 41, 9649–9657. doi: 10.1039/C7NJ00742F
Li, R., Zhang, Y., Deng, H., Zhang, Z., Wang, J. J., and Shaheen, S. M. (2019b). Removing tetracycline and hg(II) with ball-milled magnetic nanobiochar and its potential on polluted irrigation water reclamation. J. Haz. Mater. 384:121095. doi: 10.1016/j.jhazmat.2019.121095
Lian, F., Yu, W., Wang, Z., and Xing, B. (2018). New insights into black carbon nanoparticle-induced dispersibility of goethite colloids and configuration-dependent sorption for phenanthrene. Environ. Sci. Technol. 53, 661–670. doi: 10.1021/acs.est.8b05066
Lian, F., Yu, W., Zhou, Q., Gu, S., Wang, Z., and Xing, B. (2020). Size matters: nano-biochar triggers decomposition and transformation inhibition of antibiotic resistance genes in aqueous environments. Environ. Sci. Technol. 54, 8821–8829. doi: 10.1021/acs.est.0c02227
Liu, G., Li, L., Zhang, K., Wang, X., Chang, J., Sheng, Y., et al. (2016). Facile preparation of water-processable biochar based on pitch pine and its electrochemical application for cadmium ion sensing. Int. J. Electrochem. Sci. 11, 1041–1054. doi: 10.1016/S1452-3981(23)15903-7
Liu, G., Zheng, H., Jiang, Z., Zhao, J., Wang, Z., Pan, B., et al. (2018). Formation and physicochemical characteristics of nano biochar: insight into chemical and colloidal stability. Environ. Sci. Technol. 52, 10369–10379. doi: 10.1021/acs.est.8b01481
Lonappan, L., Liu, Y., Rouissi, T., Pourcel, F., Brar, S. K., Verma, M., et al. (2018). Covalent immobilization of laccase on citric acid functionalized micro-biochars derived from different feedstock and removal of diclofenac. Chem. Eng. J. 351, 985–994. doi: 10.1016/j.cej.2018.06.157
Lonappan, L., Rouissi, T., Das, R. K., Brar, S. K., Ramirez, A. A., Verma, M., et al. (2016). Adsorption of methylene blue on biochar microparticles derived from different waste materials. Waste Manag. 49, 537–544. doi: 10.1016/j.wasman.2016.01.015
Luong, D. X., Bets, K. V., Algozeed, W. A., Stanford, M. G., Kittrell, C., Chen, W., et al. (2020). Gram-scale bottom-up flash graphene synthesis. Nature 577, 647–651. doi: 10.1038/s41586-020-1938-0
Lyu, H., Gao, B., He, F., Ding, C., Tang, J., and Crittenden, J. C. (2017). Ball-milled carbon nanomaterials for energy and environmental applications. ACS Sustain. Chem. Eng. 5, 9568–9585. doi: 10.1021/acssuschemeng.7b02170
Lyu, H., Gao, B., He, F., Zimmerman, A. R., Ding, C., Huang, H., et al. (2018). Effects of ball milling on the physicochemical and sorptive properties of biochar: experimental observations and governing mechanisms. Environ. Pollut. 233, 54–63. doi: 10.1016/j.envpol.2017.10.037
Lyu, H., Tang, J., Cui, M., Gao, B., and Shen, B. (2020b). Biochar/iron (BC/Fe) composites for soil and groundwater remediation: synthesis, applications, and mechanisms. Chemosphere 246:125609. doi: 10.1016/j.chemosphere.2019.125609
Lyu, H., Xia, S., Tang, J., Zhang, Y., Gao, B., and Shen, B. (2020a). Thiol-modified biochar synthesized by a facile ball-milling method for enhanced sorption of inorganic Hg2+ and organic CH3Hg+. J. Hazard. Mater. 384:121357. doi: 10.1016/j.jhazmat.2019.121357
Lyu, H., Yu, Z., Gao, B., He, F., Huang, J., Tang, J., et al. (2019). Ball-milled biochar for alternative carbon electrode. Environ. Sci. Pollut. Res. 26, 14693–14702. doi: 10.1007/s11356-019-04899-4
Ma, S., Jing, F., Sohi, S. P., and Chen, J. (2019). New insights into contrasting mechanisms for PAE adsorption on millimeter, micron-and nano-scale biochar. Environ. Sci. Pollut. Res. 26, 18636–18650. doi: 10.1007/s11356-019-05181-3
Ma, W., Xu, Y., Zhou, D., Wang, L., Liang, X., and Sun, Y. (2022). Development and optimization of high–performance nano–biochar for efficient removal cd in aqueous: absorption performance and interaction mechanisms. Chem. Eng. Res. Des. 189, 516–529. doi: 10.1016/j.cherd.2022.11.051
Mahmoud, M. E., Abou-Ali, S. A. A., and Elweshahy, S. M. T. (2021). Efficient and ultrafast removal of cd (II) and Sm (III) from water by leaves of Cynara scolymus derived biochar. Mater. Res. Bull. 141:111334. doi: 10.1016/j.materresbull.2021.111334
Mahmoud, M. E., El-Ghanam, A. M., and Saad, S. R. (2022). Sequential removal of chromium (VI) and prednisolone by nanobiochar-enriched-diamine derivative. Biomass Conv. Bioref. doi: 10.1007/s13399-022-02888-1
Mahmoud, M. E., El-Ghanam, A. M., Saad, S. R., and Mohamed, R. A. H. (2020). Promoted removal of metformin hydrochloride anti-diabetic drug from water by fabricated and modified nanobiochar from artichoke leaves. Sus. Chem. Pharmacy 18:100336. doi: 10.1016/j.scp.2020.100336
Mahmoud, S. E. M. E., Ursueguia, D., Mahmoud, M. E., Fatteh, T. M. A., and Diaz, E. (2023). Functional surface homogenization of nanobiochar with cation exchanger for improved removal performance of methylene blue and lead pollutants. Biomass Conv. Bioref. doi: 10.1007/s13399-023-04098-9
Makshut, N. A., Ngaini, Z., Wahi, R., Hussain, H., Mahmut, N. I., and Bahrin, N. Q. (2020). Nano-sized adsorbent from pyrolysed sago activated sludge for removal of Pb (II) from aqueous solution. Pertanika J. Sci. Technol. 28, 893–916,
Mao, D., Hu, J., Duan, P., Qin, C., and Piao, Y. (2022). Ultrasensitive and highly reusable electrochemical sensor with ion imprinted nanobiochar. Sens. Actuat. B Chem. 371:132490. doi: 10.1016/j.snb.2022.132490
Miri, S., Perez, J. A. E., Brar, S. K., Rouissi, T., and Martel, R. (2021). Sustainable production and co-immobilization of cold-active enzymes from Pseudomonas sp. for BTEX biodegradation. Environ. Poll. 285:117678. doi: 10.1016/j.envpol.2021.117678
Mukherjee, S., Sarkar, B., Aralappanavar, V. K., Mukhopadhyay, R., Basak, B. B., Srivastava, P., et al. (2022). Biochar-microorganism interactions for organic pollutant remediation: challenges and perspectives. Environ. Poll. 308:119609. doi: 10.1016/j.envpol.2022.119609
Munir, R., Ali, K., Naqvi, S. A. Z., Muneer, A., Bashir, M. Z., Maqsood, M. A., et al. (2023). Green metal oxides coated biochar nanocomposites preparation and its utilization in vertical flow constructed wetlands for reactive dye removal: performance and kinetics studies. J. Contaminant Hydrol. 256:104167. doi: 10.1016/j.jconhyd.2023.104167
Naghdi, M., Taheran, M., Brar, S. K., Kermanshahi-Pour, A., Verma, M., and Surampalli, R. Y. (2017). Immobilized laccase on oxygen functionalized nanobiochars through mineral acids treatment for removal of carbamazepine. Sci. Total Environ. 584–585, 393–401. doi: 10.1016/j.scitotenv.2017.01.021
Naghdi, M., Taheran, M., Brar, S. K., Kermanshahi-Pour, A., Verma, M., and Surampalli, R. Y. (2018). Pinewood nanobiochar: a unique carrier for the immobilization of crude laccase by covalent bonding. Int. J. Biol. Macromol. 115, 563–571. doi: 10.1016/j.ijbiomac.2018.04.105
Naghdi, M., Taheran, M., Brar, S. K., Kermanshahi-Pour, A., Verma, M., and Surampalli, R. Y. (2019). Fabrication of nanobiocatalyst using encapsulated laccase onto chitosan-nanobiochar composite. Int. J. Biol. Macromol. 124, 530–536. doi: 10.1016/j.ijbiomac.2018.11.234
Nath, B. K., Chaliha, C., and Kalita, E. (2019). Iron oxide permeated mesoporous rice-husk nanobiochar (IPMN) mediatedremoval of dissolved arsenic (as): chemometric modelling and adsorption dynamics. J. Environ. Manag. 246, 397–409. doi: 10.1016/j.jenvman.2019.06.008
Ni, B. J., Huan, Q. S., Wang, C., Ni, T. Y., Sun, J., and Wei, W. (2019). Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 219, 351–357. doi: 10.1016/j.chemosphere.2018.12.053
Plácido, J., Bustamante López, S., Meissner, K. E., Kelly, D. E., and Kelly, S. L. (2019). Multivariate analysis of biochar-derived carbonaceous nanomaterials for detection of heavy metal ions in aqueous systems. Sci. Total Environ. 688, 751–761. doi: 10.1016/j.scitotenv.2019.06.342
Qin, Y., Li, G., Gao, Y., Zhang, L., Ok, Y. S., and An, T. (2018). Persistent free radicals in carbon-based materials on transformation of refractory organic contaminants (ROCs) in water: a critical review. Water Res. 137, 130–143. doi: 10.1016/j.watres.2018.03.012
Rajput, V. D., Minkina, T., Ahmed, B., Singh, V. K., Mandzhieva, S., Sushkova, S., et al. (2022). Nano-biochar: a novel solution for sustainable agriculture and environmental remediation. Environ. Res. 210:112891. doi: 10.1016/j.envres.2022.112891
Ramanayaka, S., Kumar, M., Etampawala, T., and Vithanage, M. (2020b). Macro, colloidal and nanobiochar for oxytetracycline removal in synthetic hydrolyzed human urine. Environ. Poll. 267:115683. doi: 10.1016/j.envpol.2020.115683
Ramanayaka, S., Tsang, D. C. W., Hou, D., Ok, Y. S., and Vithanage, M. (2020a). Green synthesis of graphitic nanobiochar for the removal of emerging contaminants in aqueous media. Sci. Total Environ. 706:135725. doi: 10.1016/j.scitotenv.2019.135725
Ramezanzadeh, H., Reyhanitabar, A., Oustan, S., Mohammadi, M. H., and van der Zee, S. (2021). Enhanced sorption of cadmium by using biochar nanoparticles from ball milling in a sandy soil. Eurasian Soil Sci. 54, 201–211. doi: 10.1134/S1064229321020125
Ramzan, M., Zia, A., Naz, G., Shahid, M., Shah, A. A., and Farid, G. (2023). Effect of nanobiochar (nBC) on morpho-physio-biochemical responses of black cumin (Nigella sativa L.) in Cr-spiked soil. Plant Physiol. Biochem. 196, 859–867. doi: 10.1016/j.plaphy.2023.02.037
Rashid, M. I., Shah, G. A., Sadiq, M., Amin, N. U., Ali, A. M., Ondrasek, G., et al. (2023). Nanobiochar and copper oxide nanoparticles mixture synergistically increases soil nutrient availability and improves wheat production. Plan. Theory 12:1312. doi: 10.3390/plants12061312
Richard, S., Rajadurai, J. S., and Manikandan, V. (2016). Influence of particle size and particle loading on mechanical and dielectric properties of biochar particulate reinforced polymer nanocomposites. Int. J. Polym. Anal. Charact. 21, 462–477. doi: 10.1080/1023666X.2016.1168602
Safari, S., von Gunten, K., Alam, M. S., Hubmann, M., Blewett, T. A., Chi, Z., et al. (2019). Biochar colloids and their use in contaminants removal. Biochar 1, 151–162. doi: 10.1007/s42773-019-00014-5
Sajjadi, B., Chen, W. Y., Mattern, D. L., Hammer, N., and Dorris, A. (2020). Low-temperature acoustic-based activation of biochar for enhanced removal of heavy metals. J. Water Process Eng. 34:101166. doi: 10.1016/j.jwpe.2020.101166
Shen, Y., Shen, U., and Xiang, W. (2020). Role of nano-biochar in attenuating the allelopathic effect from Imperata cylindrica on rice seedlings. Environ. Sci. Nano 7, 116–126. doi: 10.1039/C9EN00828D
Shen, Y., and Zhang, N. (2019). Facile synthesis of porous carbons from silica-rich rice husk char for volatile organic compounds (VOCs) sorption. Bioresour. Technol. 282, 294–300. doi: 10.1016/j.biortech.2019.03.025
Širić, I., Eid, E. M., Taher, M. A., El-Morsy, M. H. E., Osman, H. E. M., Kumar, P., et al. (2022). Combined use of spent mushroom substrate biochar and PGPR improves growth, yield, and biochemical response of cauliflower (Brassica oleracea var. botrytis): a preliminary study on greenhouse cultivation. Horticulturae 8:830. doi: 10.3390/horticulturae8090830
Sisay, G. B., Atisme, T. B., Workie, Y. A., Negie, Z. W., and Mekonnen, M. L. (2023). Mg/Zr modified nanobiochar from spent coffee grounds for phosphate recovery and its application as a phosphorous release fertilizer. Environ. Nanotechnol. Monit. Manag. 19:100766. doi: 10.1016/j.enmm.2022.100766
Song, B., Cao, X., Gao, W., Aziz, S., Gao, S., Lam, C., et al. (2022). Preparation of nano-biochar from conventional biorefineries for high-value applications. Renewable Sus. Energy Rev. 157:112057. doi: 10.1016/j.rser.2021.112057
Song, B., Chen, M., Zhao, L., Qiu, H., and Cao, X. (2019). Physicochemical property and colloidal stability of micron-and nano-particle biochar derived from a variety of feedstock sources. Sci. Total Environ. 661, 685–695. doi: 10.1016/j.scitotenv.2019.01.193
Sun, Y., Lyu, H., Cheng, Z., Wang, Y., and Tang, J. (2022). Insight into the mechanisms of ball-milled biochar addition on soil tetracycline degradation enhancement: physicochemical properties and microbial community structure. Chemosphere 291:132691. doi: 10.1016/j.chemosphere.2021.132691
Suresh, R., Rajendran, S., Kumar, P. S., Dutta, K., and Vo, D. V. N. (2022). Current advances in microbial fuel cell technology toward removal of organic contaminants–a review. Chemosphere 287:132186. doi: 10.1016/j.chemosphere.2021.132186
Tomczyk, A., Sokołowska, Z., and Boguta, P. (2020). Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 19, 191–215. doi: 10.1007/s11157-020-09523-3
Ullmann, C., Babick, F., Koeber, R., and Stintz, M. (2017). Performance of analytical centrifugation for the particle size analysis of real-world materials. Powder Technol. 319, 261–270. doi: 10.1016/j.powtec.2017.06.057
Vishnu, D., Dhandapani, B., Vaishnavi, G., and Preethi, V. (2021). Synthesis of tri-metallic surface engineered nanobiochar from cynodon dactylon residues in a single step-batch and column studies for the removal of copper and lead ions. Chemosphere 286:131572. doi: 10.1016/j.chemosphere.2021.131572
Wang, H., Feng, M., Zhou, F., Huang, X., Tsang, D. C. W., and Zhang, W. (2017). Effects of atmospheric ageing under different temperatures on surface properties of sludge-derived biochar and metal/metalloid stabilization. Chemosphere 184, 176–184. doi: 10.1016/j.chemosphere.2017.05.175
Wang, B., Gao, B., and Wan, Y. (2018). Entrapment of ball-milled biochar in ca-alginate beads for the removal of aqueous cd (II). J. Ind. Eng. Chem. 61, 161–168. doi: 10.1016/j.jiec.2017.12.013
Wang, B., Gao, B., and Wan, Y. (2019). Comparative study of calcium alginate, ballmilled biochar, and their composites on aqueous methylene blue adsorption. Environ. Sci. Pollut. Res. 26, 11535–11541. doi: 10.1007/s11356-018-1497-1
Wang, K., Sun, Y., Tang, J., He, J., and Sun, H. (2020). Aqueous Cr(VI) removal by a novel ball milled Fe0-biochar composite: role of biochar electron transfer capacity under high pyrolysis temperature. Chemosphere 241:125044. doi: 10.1016/j.chemosphere.2019.125044
Wang, D., Zhang, W., Hao, X., and Zhou, D. (2013). Transport of biochar particles in saturated granular media: effects of pyrolysis temperature and particle size. Environ. Sci. Technol. 47, 821–828. doi: 10.1021/es303794d
Wei, X., Wang, X., Gao, B., Zou, W., and Dong, L. (2020). Facile ball-milling synthesis of CuO/biochar nanocomposites for efficient removal of reactive red 120. ACS Omega 5, 5748–5755. doi: 10.1021/acsomega.9b03787
Xia, C., Liang, Y., Li, X., Garalleh, H. A., Garaleh, M., Hill, J. M., et al. (2022). Remediation competence of nanoparticles amalgamated biochar (nanobiochar/nanocomposite) on pollutants: a review. Environ. Res. 218:114947. doi: 10.1016/j.envres.2022.114947
Xiang, W., Zhang, X., Chen, K., Fang, J., He, F., Hu, X., et al. (2020). Enhanced adsorption performance and governing mechanisms of ball-milled biochar for the removal of volatile organic compounds (VOCs). Chem. Eng. J. 385:123842. doi: 10.1016/j.cej.2019.123842
Xiao, J., Li, X., Cao, Y., and Chen, G. (2023). Does micro/nano biochar always good to phytoremediation? A case study from multiple metals contaminated acidic soil using Salix jiangsuensis '172′. Carbon Res. 2:21. doi: 10.1007/s44246-023-00053-5
Xiao, Y., Lyu, H., Tang, J., Wang, K., and Sun, H. (2020). Efects of ball milling on the photochemistry of biochar: enrofoxacin degradation and possible mechanisms. Chem. Eng. J. 384:123311. doi: 10.1016/j.cej.2019.123311
Xiao, Y., Raheem, A., Ding, L., Chen, W., Chen, X., Wang, F., et al. (2021). Pretreatment, modification and applications of sewage sludge-derived biochar for resource recovery-a review. Chemosphere 287:131969. doi: 10.1016/j.chemosphere.2021.131969
Xu, C. Y., Li, Q. R., Geng, Z. C., Hu, F. N., and Zhao, S. W. (2020). Surface properties and suspension stability of low-temperature pyrolyzed biochar nanoparticles: effects of solution chemistry and feedstock sources. Chemosphere 259:127510. doi: 10.1016/j.chemosphere.2020.127510
Yan, J., Han, L., Gao, W., Xue, S., and Chen, M. (2015). Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene. Bioresour. Technol. 175, 269–274. doi: 10.1016/j.biortech.2014.10.103
Yang, J., Pignatello, J. J., Pan, B., and Xing, B. (2017). Degradation of p-nitrophenol by lignin and cellulose chars: H2O2-mediated reaction and direct reaction with the char. Environ. Sci. Technol. 51, 8972–8980. doi: 10.1021/acs.est.7b01087
Yang, F., Zhang, Q., Jian, H., Wang, C., Xing, B., Sun, H., et al. (2020). Effect of biochar-derived dissolved organic matter on adsorption of sulfamethoxazole and chloramphenicol. J. Hazard. Mater. 396:122598. doi: 10.1016/j.jhazmat.2020.122598
Yang, Y., Zhou, B., Hu, Z., and Lin, H. (2020). The effects of nano-biochar on maize growth in northern Shaanxi province on the loess plateau. Appl. Ecol. Environ. Res. 18, 2863–2877. doi: 10.15666/aeer/1802_28632877
Yao, L., He, L., Yang, Y., Zhang, Y., Liu, Z., Liang, L., et al. (2021). Nanobiochar paper based electrochemical immunosensor for fast and ultrasensitive detection of microcystin-LR. Sci. Total Environ. 750:141692. doi: 10.1016/j.scitotenv.2020.141692
Yu, Z., Ji, L., Zuo, Y., Zhang, F., Wei, C., Jiang, F., et al. (2023). Removal of tetracycline hydrochloride by ball-milled mulberry biochar. Water Air Soil Pollut. 234:211. doi: 10.1007/s11270-023-06223-w
Yuan, Y., Zhang, N., and Hu, X. (2020). Effects of wet and dry ball milling on the physicochemical properties of sawdust derived-biochar. Instrum. Sci. Technol. 48, 287–300. doi: 10.1080/10739149.2019.1708751
Yue, L., Lian, F., Han, Y., Bao, Q., Wang, Z., and Xing, B. (2019). The efect of biochar nanoparticles on rice plant growth and the uptake of heavy metals: implications for agronomic benefts and potential risk. Sci. Total Environ. 656, 9–18. doi: 10.1016/j.scitotenv.2018.11.364
Zhang, H., Chen, C., Gray, E. M., and Boyd, S. E. (2017). Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass Bioenergy 105, 136–146. doi: 10.1016/j.biombioe.2017.06.024
Zhang, K., Mao, J., and Chen, B. (2019). Reconsideration of heterostructures of biochars: morphology, particle size, elemental composition, reactivity and toxicity. Environ. Pollut. 254:113017. doi: 10.1016/j.envpol.2019.113017
Zhang, Y., Piao, M., He, L., Yao, L., Piao, T., Liu, Z., et al. (2020). Immobilization of laccase on magnetically separable biochar for highly efficient removal of bisphenol a in water. RSC Adv. 10, 4795–4804. doi: 10.1039/C9RA08800H
Zhang, Q., Wang, J., Lyu, H., Zhao, Q., Jiang, L., and Liu, L. (2019). Ball-milled biochar for galaxolide removal: sorption performance and governing mechanisms. Sci. Total Environ. 659, 1537–1545. doi: 10.1016/j.scitotenv.2019.01.005
Zhang, P., Xue, B., Jiao, L., Meng, X., Zhang, L., Li, B., et al. (2022). Preparation of ballmilled phosphorus-loaded biochar and its highly efective remediation for cd-and Pb-contaminated alkaline soil. Sci. Total Environ. 813:152648. doi: 10.1016/j.scitotenv.2021.152648
Zhang, Y., Zhao, G., Xuan, Y., Gan, L., and Pan, M. (2021). Enhanced photocatalytic performance for phenol degradation using ZnO modified with nano-biochar derived from cellulose nanocrystals. Cellulose 28, 991–1009. doi: 10.1007/s10570-020-03581-0
Zhou, L., Huang, Y., Qiu, W., Sun, Z., Liu, Z., and Song, Z. (2017). Adsorption properties of nano-MnO2–biochar composites for copper in aqueous solution. Molecules 22:173. doi: 10.3390/molecules22010173
Zhou, Y., Qin, S., Verma, S., Sar, T., Sarsaiya, S., Ravindran, B., et al. (2021). Production and beneficial impact of biochar for environmental application: a comprehensive review. Bioresour. Technol. 337:125451. doi: 10.1016/j.biortech.2021.125451
Zhu, H. X., Liu, X. Y., Jiang, Y., Lin, H. D., and Yang, K. (2022). Sorption kinetics of 1,3,5-trini - trobenzene to biochars produced at various temperatures. Biochar. 4:32. doi: 10.1007/s42773-022-00157-y
Keywords: Nano-biochar, biochar, nanotechnology, environmental pollution, remediation
Citation: Bhandari G, Gangola S, Dhasmana A, Rajput V, Gupta S, Malik S and Slama P (2023) Nano-biochar: recent progress, challenges, and opportunities for sustainable environmental remediation. Front. Microbiol. 14:1214870. doi: 10.3389/fmicb.2023.1214870
Edited by:
Parul Chaudhary, Graphic Era Hill University, IndiaReviewed by:
Bartholomew Saanu Adeleke, Olusegun Agagu University of Science and Technology, NigeriaSami Abou Fayssal, University of Forestry, Sofia, Bulgaria
Copyright © 2023 Bhandari, Gangola, Dhasmana, Rajput, Gupta, Malik and Slama. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geeta Bhandari, geet33n@gmail.com; Sumira Malik, smalik@rnc.amity.edu; Petr Slama, petr.slama@mendelu.cz
 Geeta Bhandari
Geeta Bhandari Saurabh Gangola
Saurabh Gangola Archna Dhasmana
Archna Dhasmana Vishal Rajput1
Vishal Rajput1