Corrigendum: Unraveling the interplay between norovirus infection, gut microbiota, and novel antiviral approaches: a comprehensive review
- 1Department of Internal Medicine, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan
- 2Department of General Medicine, Taipei Medical University Hospital, Taipei, Taiwan
- 3Department of Pediatrics, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 4Department of Pediatrics, Division of Allergy, Asthma and Immunology, Shuang Ho Hospital, New Taipei, Taiwan
- 5Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA, United States
- 6Broad Institute of MIT and Harvard, Cambridge, MA, United States
- 7Department of Pediatrics, Division of Pediatric Gastroenterology and Hepatology, Shuang Ho Hospital, New Taipei, Taiwan
- 8TMU Research Center for Digestive Medicine, Taipei Medical University, Taipei, Taiwan
Norovirus infection is a leading cause of acute gastroenteritis worldwide and can also cause harmful chronic infections in individuals with weakened immune systems. The role of the gut microbiota in the interactions between the host and noroviruses has been extensively studied. While most past studies were conducted in vitro or focused on murine noroviruses, recent research has expanded to human noroviruses using in vivo or ex vivo human intestinal enteroids culture studies. The gut microbiota has been observed to have both promoting and inhibiting effects on human noroviruses. Understanding the interaction between noroviruses and the gut microbiota or probiotics is crucial for studying the pathogenesis of norovirus infection and its potential implications, including probiotics and vaccines for infection control. Recently, several clinical trials of probiotics and norovirus vaccines have also been published. Therefore, in this review, we discuss the current understanding and recent updates on the interactions between noroviruses and gut microbiota, including the impact of norovirus on the microbiota profile, pro-viral and antiviral effects of microbiota on norovirus infection, the use of probiotics for treating norovirus infections, and human norovirus vaccine development.
1. Introduction
Acute gastroenteritis is the second most burdensome infectious disease worldwide. Norovirus, a key pathogen associated with nearly one-fifth of all cases of acute gastroenteritis (Ahmed et al., 2014), is responsible for 18% of diarrheal disease globally, affecting both high-and low-income countries alike. Norovirus causes approximately 200,000 deaths annually worldwide, with over 70,000 of these deaths occurring among children in developing countries (Lopman, 2015; Centers for Disease Control and Prevention, 2023). Additionally, norovirus is responsible for 58% of foodborne illnesses in the United States, costing approximately $2 billion in lost productivity each year. Noroviruses belong to the Caliciviridae family and are non-enveloped, single-stranded RNA viruses. In 2019, the classification of noroviruses was updated, with 10 genogroups (GI-GX) and 48 genotypes [9 GI, 27 GII, 3 GIII, 2 GIV, 2 GV, 2 GVI, and 1 genotype each for GVII, GVIII, GIX (formerly GII.15), and GX] (Chhabra et al., 2019). Norovirus GII genotype 4 (GII.4) variants have been the predominant strains worldwide for decades (White, 2014), but recent studies have reported the emergence of GII.17 variants causing significantly increased outbreaks of acute gastroenteritis (Jin et al., 2016; Lu et al., 2016; Cheung et al., 2019). The presence of histo-blood group antigens (HBGAs) on the intestinal epithelium is essential for human norovirus infection, and GII.17 norovirus causing outbreak infections has been shown to have a wide spectrum of HBGAs susceptibility (Chan et al., 2015; Zhang et al., 2015). In addition, the gut microbiota has been found to play a role in norovirus infection with pro-viral and antiviral effects on both human and murine noroviruses (Sullender and Baldridge, 2018; Walker and Baldridge, 2019; Pena-Gil et al., 2021; Soorneedi and Moore, 2022). Recent years have seen an increase in in vitro trials using cell lines, in vivo clinical trials in animals and humans, and successful cultivation of human norovirus in ex vivo human intestinal enteroid (HIE) cultures (Ettayebi et al., 2016). Understanding the potential promotive or inhibitory role of the microbiota, the application of probiotics, and vaccine development in norovirus infection remains an area of active interest. Further understanding of the role of the microbiota in norovirus infection could provide insights into human norovirus pathogenesis and aid in the development of antiviral strategies. Therefore, the purpose of this review is to investigate and update the impact of norovirus on the gut microbiota profile, the role of the microbiota in norovirus infection, the use of probiotics for norovirus infections, human norovirus vaccine development.
2. Histo-blood group antigens and human milk oligosaccharides
Glycan interaction plays a crucial role in norovirus infection. Glycan epitopes have been found on histo-blood group antigens (HBGAs) and human milk oligosaccharides (HMOs) (Etzold and Bode, 2014). HBGAs are soluble antigens present in saliva and expressed on the mucosal epithelium of the digestive tract. HBGAs are catalyzed by a set of glycosyltransferases encoded by three major gene families: secretor, Lewis, and ABO. Oligosaccharides containing Lewis and ABH antigenic epitopes are involved in the variable binding activity with norovirus (Huang et al., 2003). The Secretor (Se) gene codes for an α-1,2 fucosyltransferase (FUT2), the Lewis (Le) gene codes for an α-1,3 or α-1,4 fucosyltransferase (FUT3), while the ABO family codes for two glycosyltransferases (A and B enzymes) (Tan and Jiang, 2014). Moreover, the FUT2 gene, which synthesizes the H-type 1 antigen in saliva and mucosa, is associated with susceptibility to norovirus infections. Individuals who express a functional FUT2 enzyme (known as secretor status) are more susceptible to norovirus infections (Nordgren and Svensson, 2019), while those who do not express a functional FUT2 enzyme (known as non-secretors) like the FUT2 non-secretor (se428se428) genotype are resistant to nosocomial and sporadic outbreaks of norovirus (Thorven et al., 2005). Clinical studies have shown that individuals with a homozygous recessive inactivating G428A mutation of the FUT2 gene, who do not express the H type-1 oligosaccharide ligand for norovirus binding, may be genetically resistant to norovirus infection (Lindesmith et al., 2003). Another clinical study showed that GII.4 strains of human norovirus exclusively infect secretors (Currier et al., 2015). Moreover, human noroviruses have a preference to certain types of HBGAs. Observational studies have also shown that individuals with blood group O (H antigen) are more likely to be infected with norovirus, while individuals with blood group B appear to have reduced susceptibility to symptomatic norovirus infection (Hutson et al., 2002; Hennessy et al., 2003).
Human milk oligosaccharides (HMOs) are complex carbohydrates synthesized in the breast gland and are abundant in human milk. The amount and composition of HMOs vary highly between women, and each structurally defined HMO may have distinct functionality. HMOs directly or indirectly modulate the infant’s physiological systems (Zhang S. et al., 2021). Firstly, since HMOs are not digested by the infant and serve as metabolic substrates for select microbes, they can shape the infant gut microbiome. Next, HMOs are soluble decoy receptors that block the attachment of viral, bacterial, or protozoan parasite pathogens to epithelial cell surface sugars, which may prevent infectious diseases in the gastrointestinal tract. HMOs are also antimicrobials that act as bacteriostatic or bacteriocidal agents. In addition, HMOs alter host epithelial and immune cell responses with potential benefits for the neonate (Bode, 2015). The presence and quantity of HMOs are also related to the secretor status and the Lewis group type. FUT2-dependent HMOs include 2′-fucosyllactose (2’FL), difucosyllactose (DFLac), and lactose-N-fucopentaose (LNFP) I. Non-secretor mothers have been found to produce significantly less HMOs compared to secretor mothers due to the absence of 2’FL, which accounts for a high proportion in secretor milk (Azad et al., 2018). Unlike HBGAs, HMOs provide protection against norovirus infection by competing with the HBGAs binding site on the norovirus capsid (Jiang et al., 2004; Shang et al., 2013). HMOs such as 2’FL and 3′-fucosyllactose (3’FL) could block norovirus from binding to HBGAs (Weichert et al., 2016), and 2′FL and 3’FL might function as broadly reactive antivirals against multiple norovirus genogroups (Koromyslova et al., 2017; Lalithamaheswari and Anu Radha, 2022).
3. Human intestinal enteroids
To address the challenges in evaluating the role of gut microbiota on norovirus infection, a recent advancement is the establishment of the human intestinal enteroids (HIEs) system. HIEs provide a valuable tool for studying norovirus replication (Cates et al., 2020). Estes et al. (2019) reviewed the applications of HIEs, which include studying virus-specific replication requirements, evaluating human host-pathogen interactions, and supporting pre-clinical assessment of methods to prevent and treat human norovirus infections. Initially, Ettayebi et al. (2016) demonstrated successful ex vivo human intestinal enteroid cultures that supported human norovirus replication. They found that replication was restricted to the small intestine based on viral replication in duodenal and ileal HIEs, but not colonoid HIEs. They further optimized new medium conditions to enhance human norovirus cultivation in HIEs (Ettayebi et al., 2021). Another study evaluated virus inactivation strategies using the HIEs model. Alcohols were found to slightly reduce but not completely inactivate human norovirus, regardless of concentration or exposure time. In contrast, complete inactivation of GII.4 viruses occurred at concentrations as low as 50 ppm of chlorine (Costantini et al., 2018). Moreover, another study showed that aged green tea extract could be an effective natural compound against human norovirus GII.4 through HIE cultures (Randazzo et al., 2020). Additionally, one study evaluated the correlation between viral load measured by real-time reverse transcription PCR and virus infectivity through HIE cultures. Norovirus inocula with a cycle threshold value <30 were found to robustly yield productive virus replication in HIEs, indicating the presence of infectious virus (Chan et al., 2019).
Recently, there have been more studies investigating the host-pathogen interaction of norovirus using human intestinal enteroids (HIEs) cultures. In one study, the role of FUT2, a gene involved in glycosylation, in human norovirus replication was evaluated. The results showed that FUT2 expression affected both the binding of human norovirus to the HIE cell surface and the susceptibility to viral infection, indicating that binding to a molecule glycosylated by FUT2 was critical for human norovirus infection (Haga et al., 2020). Another study identified a predominant type III interferon (IFN)-mediated innate response to human norovirus infection in HIE cultures. The study further revealed strain-specific sensitivities to IFN, showing that in signal transducer and activator of transcription 1 (STAT-1)-knockout HIEs compared to parental HIEs, there was enhanced replication and virus spread for GII.3, instead of the globally dominant GII.4 human norovirus. This indicated that IFN restricted GII.3 but not GII.4 replication, which might explain why GII.4 infections were more widespread and pandemic (Lin et al., 2020). Furthermore, another study showed that GII.3 replication in HIEs was dependent on bile acids. Glycochenodeoxycholic acid (GCDCA) induced multiple cellular responses that promoted GII.3 replication in HIEs, including endosomal uptake enhancement, endosomal acidification, and subsequent activation of endosomal/lysosomal enzyme acid sphingomyelinase (ASM). Inhibitors of endosomal acidification or ASM reduced GII.3 infection, but exogenous addition of ceramide alone permitted infection (Murakami et al., 2020). Therefore, HIE cultures provide an ex vivo model for studying human norovirus and have been valuable in uncovering important insights into host-pathogen interactions, and strain-specific sensitivities to host immune responses.
4. Effect of norovirus on microbiota profiles
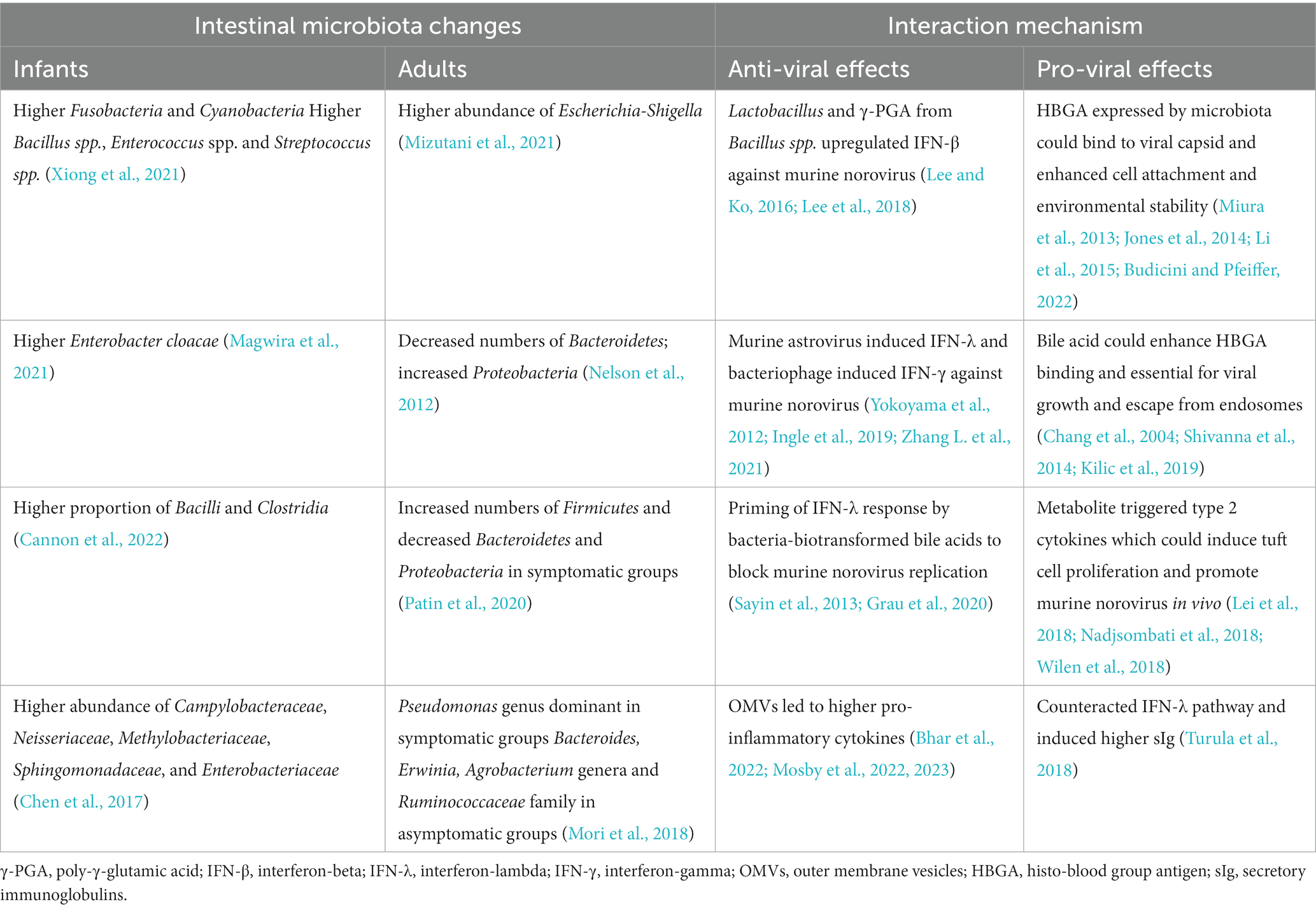
There have been several clinical studies that have reported changes in microbiota profiles following norovirus infections in infants and adults (Table 1). Infants infected with human norovirus were found to have higher abundance of Fusobacteria and Cyanobacteria at the phylum level, and higher abundance of Bacillus spp., Enterococcus spp., and Streptococcus spp. at the genus level compared to healthy infants. The Chao1 index, which indicates microbial diversity, was significantly higher in the human norovirus group, and there were significant differences in potentially pathogenic bacteria (Xiong et al., 2021). Infants with positive norovirus test results for diarrhea had significantly higher counts of Enterobacter cloacae compared to those who tested negative for norovirus (Magwira et al., 2021). Another study analyzed stool samples from infants and found that the microbiome was dominated by Actinobacteria before infection, with no change during norovirus infection episodes. However, there was a shift with a higher proportion of Bacilli and Clostridia observed several weeks after the first episode of acute gastroenteritis ended (Cannon et al., 2022). Fecal microbiome analysis from pediatric patients with severe norovirus or rotavirus infection revealed a decreased Shannon diversity index of the intestinal microbiota, and a greater abundance of Campylobacteraceae, Neisseriaceae, Methylobacteriaceae, Sphingomonadaceae, and Enterobacteriaceae compared to healthy children (Chen et al., 2017). Furthermore, the abundance of Bifidobacterium spp. and Lactobacillus spp. was significantly reduced in children with norovirus or rotavirus infection compared to healthy children (Solano-Aguilar et al., 2013).
Statistical differences were observed in the microbiota profiles of healthy adults and adult patients with diarrhea, with Firmicutes, Actinobacteria, Bacteroides, Cyanobacteria, and Proteobacteria showing significant differences. In healthy adults, Faecalibacterium was the dominant genus, while in patients with diarrhea, Escherichia-Shigella was the most abundant (Mizutani et al., 2021). Some norovirus-infected patients showed significant alterations in their microbiota compared to uninfected individuals, characterized by reduced relative numbers of Bacteroidetes and an increase in Proteobacteria due to a single operational taxonomic unit (OTU) of Escherichia coli. Factors such as gender, age, lactoferrin levels, and sampling time did not explain these changes in the microbiota (Nelson et al., 2012). Symptomatic individuals infected with norovirus exhibited a marked shift in their microbiome compared to asymptomatic individuals, with an increase in Firmicutes immediately after infection and a decrease in Bacteroidetes and Proteobacteria over the same time period. Genes enriched in the microbiomes of symptomatic subjects, such as the adenylyltransferase glgC, were associated with glycan metabolism and cell–cell signaling (Patin et al., 2020). Another study comparing symptomatic individuals with asymptomatic carriers found that the Pseudomonas genus was dominant in symptomatic groups, while Bacteroides, Erwinia, Agrobacterium genera, and Ruminococcaceae family were dominant in asymptomatic carriers (Mori et al., 2018). Studies on murine norovirus showed that malnutrition and murine norovirus infection could heavily influence the gut microbial composition, especially a decreased Bacteroidetes/Firmicutes ratio was observed (Hickman et al., 2014). However, another study found no major differences in intestinal bacterial communities between murine norovirus-infected mice and uninfected controls, both in tissue-associated samples and feces (Nelson et al., 2013).
5. Effects of microbiota on norovirus
5.1. Antiviral effects
Several studies have reported certain commensal bacteria play an important role in host susceptibility to norovirus infection. A higher abundance of Ruminococcaceae and Faecalibacterium bacteria was correlated with lower anti-viral immunoglobulin A (IgA) titers, suggesting that individuals with these taxa may have lower susceptibility to human norovirus infection (Rodriguez-Diaz et al., 2017). In a mouse model, human norovirus genotype GII.4 efficiently replicated in antibiotic-treated mice with depleted microbiota. Genera such as Adlercreutzia, Ruminococcus, and Dorea were depleted after antibiotic treatment, resulting in low microbial diversity, which was linked to increased permissiveness of norovirus replication. The expression of interleukin-4 (IL-4) and IL-13 was upregulated in all antibiotic-treated groups, but the expression levels of toll-like receptor 2 (TLR2) and tumor necrosis factor alpha (TNF-α) were diminished, resulting in efficient replication of norovirus (Santiso-Bellón et al., 2022).
Most of the following studies explored antiviral effects including interferon (IFN) modulation and increased production of smaller outer membrane vesicles (OMVs) from gut microbiome to block murine norovirus infection (Table 1). Firstly, both commensal bacteria-derived ligands and metabolites can signal and regulate IFN signaling pathways (Wirusanti et al., 2022). In an immunocompetent mouse model, STAT-1-dependent IFN responses restricted murine norovirus infection by inhibiting viral replication in the intestine, preventing virus-induced apoptosis of intestinal cells and splenocytes, and limiting viral dissemination to peripheral tissues (Mumphrey et al., 2007). Poly-γ-glutamic acid (γ-PGA), an extracellular biopolymer produced by Bacillus spp., was identified as a non-canonical toll-like receptor 4 (TLR4) agonist with anti-viral effects against norovirus infection. In ex-vivo culture of mouse ileum, oral administration of γ-PGA increased serum IFN-β levels, reduced murine norovirus loads in the ileal Peyer’s patches (PPs), and mesenteric lymph nodes in mice (Lee et al., 2018). Another in vivo and in vitro model showed that the Lactobacillaceae families were remarkably increased by murine norovirus inoculation and retinoic acid administration. Upregulated IFN-β by the abundance of Lactobacillus showed anti-viral effects against murine norovirus (Lee and Ko, 2016). A clinical study conducted with a similar concept showed the effect of retinoic acid supplementation on human norovirus infection among young children. Retinoic acid administration reduced the prevalence of human norovirus GII infections, increased the length of viral shedding, and decreased the prevalence of diarrhea (Long et al., 2007).
Next, research has shown that the virome, including bacteriophages, can have anti-viral effects. In immunodeficient hosts, the virome can protect against enteric pathogens. For instance, murine astrovirus has been observed in immunodeficient mice such as Rag1(−/−) mice, which are deficient in B and T cells (Yokoyama et al., 2012). The presence of murine astrovirus (STL5) in the gut has been found to protect primary immunodeficient mice from murine norovirus infection through IFN-λ signaling in gut epithelial cells. This protection can also be horizontally transferred between immunocompromised mice through co-housing and fecal transplantation (Ingle et al., 2019). IFN-λ has been identified as a key cytokine for curing virus infections. While cytokines such as IFN-α and IFN-β can prevent the systemic spread of murine norovirus, only IFN-λ can effectively control persistent enteric infections. The induction of IFN-λ is regulated by the murine norovirus capsid protein and is correlated with diminished enteric persistence (Nice et al., 2015). In addition, another study investigated the role of bacteriophages in murine norovirus replication. The results demonstrated that bacteriophages increased cellular response to IFN-γ and IFN-inducible GTPases, which exerted an antiviral effect in vitro (Zhang L. et al., 2021).
Finally, the interactions between norovirus and bacteria also had an impact on the bacteria themselves. These interactions induced stress responses in the bacteria and increased the production of bacterial extracellular vesicles. Specifically, when norovirus interacted with commensal bacteria such as Enterobacter cloacae, Bacteroides thetaiotaomicron, and Lactobacillus acidophilus, it led to an increased production of smaller OMVs. The stool samples collected from mice infected with the virus showed a significant increase in vesicle production compared to mock-infected controls (Mosby et al., 2022). Norovirus infection also resulted in changes in the DNA, protein, and lipid content of the bacteria. The increased accumulation of phospholipids was associated with increased blebbing, but there was no change in the lipid content of the bacterial outer membrane or the metabolite content of the bacterial cell (Mosby et al., 2023). Additionally, another study showed that murine norovirus could attach to the OMVs, facilitating co-inoculation of target cells with both the virus and vesicles. When murine noroviruses and OMVs were co-inoculated into macrophages, viral infection was reduced. Co-inoculation of murine norovirus with OMVs resulted in higher production and release of pro-inflammatory cytokines (IL-1β, TNF-α, and IFN-β) in response to viral infection compared to murine norovirus alone (Bhar et al., 2022).
5.2. Pro-viral effects
Gut microbiota may facilitate norovirus replication through various mechanisms, such as binding of the virus to HBGAs expressed by both the host and certain bacteria, bile acid-associated immune response, and tuft cells induced by type 2 cytokine (Table 1). Some human microbiota, such as Enterobacter cloacae, Escherichia coli, and Helicobacter pylori, have been shown to express HBGAs (Rasko et al., 2000; Yi et al., 2005; Jones et al., 2014), and human norovirus can bind to these bacteria. Both probiotic and non-probiotic bacteria have been found to possess the ability to bind to norovirus genotypes GI.1 and GII.4 through the protruding (P)-domain of the norovirus VP1 capsid protein (Rubio-del-Campo et al., 2014). Studies have shown that the extracellular polymeric substances (EPS) of Enterobacter cloacae containing HBGA-like substances play a key role in binding with human norovirus (Miura et al., 2013). Moreover, human norovirus has been found to enhance its infectivity to host cells in the presence of HBGA-positive bacteria by binding viral particles to these bacteria, allowing uptake of the virus into host cells (Jones et al., 2014). HBGA-positive bacteria are likely to affect the transmission and infection process of human norovirus. For instance, HBGA-positive Escherichia coli has been shown to maintain higher mucin-binding ability of norovirus-like particles even after heat treatment (Li et al., 2015). Additionally, both gram-positive and gram-negative bacteria have been found to bind to murine norovirus, and virion stability was enhanced in the presence of several gram-positive bacterial strains due to small heat-stable molecules (Budicini and Pfeiffer, 2022). Interestingly, human norovirus-bacteria binding has been observed around the outer cell surfaces and pili structures of gut microbiota, even if they do not produce HBGA (Almand et al., 2017). Other carbohydrate moieties, such as terminal sialic acids, glycoprotein, glycolipid, or glycosaminoglycans, have also been reported as receptors for both murine (Taube et al., 2009, 2012) and human norovirus (Tamura et al., 2004; Rydell et al., 2009). In terms of environmental stability, enteroviral particles, including norovirus, were packaged within vesicles enriched with phosphatidylserine (PS) lipids. These vesicles were released from cells without causing lysis and facilitated greater infection efficiency through bacterially-mediated viral clustering (Chen et al., 2015). These vesicles, which enveloped virus clusters, remained intact during fecal-oral transmission, allowing for the transport of multiple viral particles to the next hosts and enhancing viral tolerance to extreme temperatures, pH levels, and salt concentrations (Santiana et al., 2018). The influence of these vesicles on viral interaction with bacteria has yet to be explored, but it presents an interesting topic for future research.
Furthermore, it has been found that bile acid is required for certain genotypes of human norovirus (Ettayebi et al., 2016). Bile acid enhances the binding of HBGA for the HBGA binder genotype GII.10, and the non-HBGA binder genotype GII.1 can be converted to a HBGA binder after bile acid binding. Human norovirus genotypes GII.4 and GII.17, which do not bind bile acid, are responsible for large epidemics, whereas genotypes GII.1 and GII.10, which do bind bile acid, are not as widespread, indicating that these viruses have modified their bile acid requirements on the capsid (Kilic et al., 2019). In studies on porcine enteric calicivirus, bile acid was found to be critical for viral escape from the endosomes, enabling entry into the cell cytoplasm for viral replication (Shivanna et al., 2014). Bile acid was also found to be essential for viral growth and was associated with the down-regulation of IFN-mediated STAT-1 phosphorylation (Chang et al., 2004). Bile acids are synthesized from cholesterol in the liver and further metabolized by gut microbiota into secondary bile acids. Intestinal anaerobic bacteria also carry out bile salt hydrolysis, hydroxy group dehydrogenation reactions, and other biotransformation processes (Ridlon et al., 2006). The bile acid receptor farnesoid X receptor (FXR) plays a role in regulating both pro-viral and antiviral capacities. Bile acid synthesis is under negative feedback control through activation of the nuclear receptor FXR in the ileum and liver. High levels of FXR suppress bile acid-mediated enhancement of IFN-λ expression. One in vitro study showed that gut microbiota may suppress cholesterol 7α-hydroxylase (CYP7A1) and bile acid synthesis by reducing the levels of Tauro-beta-muricholic Acid (a natural FXR antagonist) and promoting FXR-dependent fibroblast growth factor 15 (FGF15) expression in the ileum (Sayin et al., 2013). Another in vitro study revealed that gut microbiota has opposing regional effects on murine norovirus infection, inhibiting viral infection of the proximal gut while promoting viral infection of the distal gut. The inhibition of proximal gut infection was due to the priming of IFN-λ response by bacteria-biotransformed bile acids, which in turn blocked viral replication. However, the promotion of distal gut infection was due to the more abundant FXR expression in the distal gut (Grau et al., 2020). Therefore, bile acid is necessary for certain genotypes of human norovirus, and the immune response associated with bile acid may be controlled by gut microbiota.
Furthermore, CD300lf, a proteinaceous receptor, was found to be essential for the binding and replication of murine norovirus. CD300lf-deficient mice [Cd300lf (−/−)] showed resistance to murine norovirus infection, indicating that CD300lf was the primary determinant of murine norovirus species tropism (Orchard et al., 2016). The dimeric protruding (P) domain of murine norovirus VP1 was found to form a complex with its cellular receptor CD300lf. The P2 subdomain, which has a cleft between the AB and DE loops, overlaps with the epitopes of neutralizing antibodies (Nelson et al., 2018). The structure of CD300lf-P domain complexed with glycochenodeoxycholic acid (GCDCA) and lithocholic acid (LCA) revealed that GCDCA induced conformational changes in the P domain, resulting in the elimination of P domain recognition by neutralizing antibodies as a viral immune escape mechanism (Creutznacher et al., 2021). Tuft cells, a rare type of intestinal epithelial cell, were found to express CD300lf and serve as the target cell for murine norovirus in the mouse intestine. Type 2 cytokines, including IL-4, which induce tuft cell proliferation, were found to promote murine norovirus infection in vivo (Wilen et al., 2018). Moreover, both succinate receptor 1 (Sucnr1) expressed on tuft cells and the microbial metabolite succinate, as an activating ligand, may trigger type 2 immunity (Lei et al., 2018; Nadjsombati et al., 2018).
Moreover, several studies have demonstrated that the bacterial microbiome can play a role in fostering enteric viral persistence by mediating the IFN-λ immune pathway. Antibiotics were found to prevent persistent murine norovirus infection by targeting IFN-λ receptor 1 (IFNLR1), the receptor for the antiviral cytokine IFN-λ, as well as the transcription factors STAT-1 and IFN regulatory factor 3 (IRF3). This prevention was reversed by replenishment of the bacterial microbiota (Baldridge et al., 2015). Heme-oxidized IRP2 ubiquitin ligase 1 (HOIL1) was shown to be critical for type I and III IFN induction during murine norovirus infection, as Hoil1(−/−) mice exhibited defective control of murine norovirus infection. Similarly, defective regulation of infection by the microbiome was observed in mice deficient in IFNLR1, STAT-1, and IRF3 (MacDuff et al., 2018). The normal microbiome was found to induce higher levels of secretory immunoglobulins (sIg) and promote murine norovirus infection. In mice lacking the polymeric immunoglobulin receptor (pIgR) relative to control mice, increased levels of IFN-γ and inducible nitric oxide synthase (iNOS) were observed, accompanied by reduced murine norovirus titers (Turula et al., 2018).
6. Effects of probiotics on norovirus
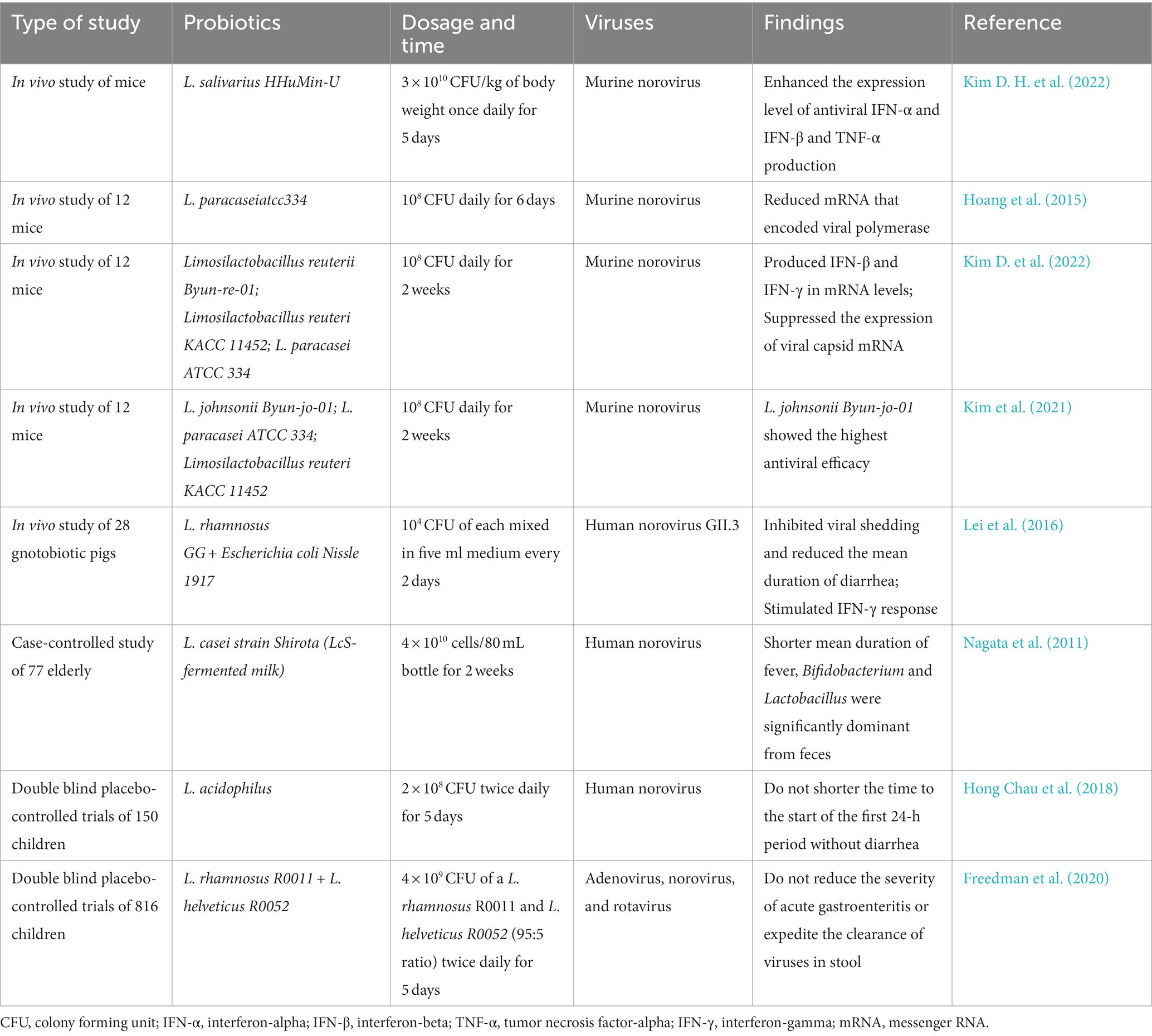
Probiotics have been used to prevent and reduce symptoms of common viruses, including digestive infections such as rotavirus, coronavirus, and norovirus. Various strains of Lactobacillus and Bifidobacterium have been frequently studied for their potential anti-viral effects (Lopez-Santamarina et al., 2021). Probiotics can modulate the immune response by promoting maturation of immune cells such as intestinal macrophages and dendritic cells. Additionally, probiotics can exert anti-viral activities through mechanisms such as competitive exclusion, bacteriocin production, and stimulation of antimicrobial defenses (Steyer et al., 2022). Probiotics can also protect the gut by increasing mucosal secretion, improving intestinal motility, and enhancing production of short-chain fatty acids (Vitetta et al., 2018). In vitro studies have shown that bacterial metabolic products from commercial probiotics containing strains such as Lactobacillus acidophilus, Lactobacillus rhamnosus, Bifidobacterium bifidum, Lactobacillus salivarius, and Streptococcus thermophilus have inhibitory effects on murine norovirus propagation (Adrienne et al., 2014). Other studies have demonstrated that strains such as Weissella cibaria, Pediococcus pentosaceus, Lactobacillus curvatus, and Lactobacillus sakei can significantly reduce murine norovirus (Seo et al., 2020), while Bifidobacterium adolescentis can inhibit the multiplication of murine norovirus (Li et al., 2016). Furthermore, a centenarian gut-derived strain, Limosilactobacillus fermentum, has been shown to exhibit the strongest antagonism against murine norovirus (Li et al., 2022).
Firstly, Lactobacillus salivarius HHuMin-U (HHuMin-U) effectively suppressed the replication of murine norovirus and decreased viral RNA levels in macrophages in vitro. HHuMin-U activated specific signaling pathways, including nuclear factor κB and TANK-binding kinase 1 (TBK1) - interferon regulatory factor 3 (IRF3), which led to increased production of IFN-α, IFN-β, and TNF-α in mouse macrophages (Kim D. H. et al., 2022). Next, a single-chain variable fragment (scFv) called 3D8, which is secreted by genetically engineered Lactobacillus paracasei, prevented apoptosis induced by murine norovirus infection and decreased the expression of viral capsid protein messenger RNA (Hoang et al., 2015). In vivo studies, both Lactobacillus salivarius and scFv 3D8 from Lactobacillus paracasei revealed antiviral effects (Table 2). In another live animal study, Limosilactobacillus reuteri strain Byun-re-01 was found to produce anti-inflammatory cytokines, mainly IFN-β and IFN-γ, at the mRNA level. It also suppressed the expression of viral capsid mRNA of murine norovirus (Kim D. et al., 2022). Furthermore, oral administration of Lactobacillus johnsonii strain Byun-jo-01 showed the highest antiviral efficacy against murine norovirus compared to feeding with other probiotic strains (Kim et al., 2021). In a study using a gnotobiotic pig model, colonization with Lactobacillus rhamnosus strain GG (LGG) and Escherichia coli strain Nissle 1917 (EcN) inhibited human norovirus shedding and significantly reduced the incidence and duration of diarrhea. The protective efficacy of these probiotic regimens was attributed to the stimulation of IFN-γ + T cell responses, increased production of intestinal IgA and IgG, and maintenance of healthy intestinal morphology (Lei et al., 2016).
In a clinical trial, a case-controlled study examined the effects of probiotic-fermented milk containing Lactobacillus casei strain Shirota in elderly individuals with norovirus gastroenteritis. The results showed that the mean duration of fever was significantly reduced in the group that received the probiotic. Analysis of fecal samples also revealed a significant increase in Bifidobacterium and Lactobacillus, while Enterobacteriaceae decreased in the probiotic group (Nagata et al., 2011). However, a double-blind, placebo-controlled trial investigating the effects of probiotic treatment containing Lactobacillus acidophilus on children with acute norovirus or rotavirus infection found no significant difference in the time to the start of the first 24-h period without diarrhea, indicating no benefits of probiotics in treating acute watery diarrhea in children (Hong Chau et al., 2018). Another double-blind, placebo-controlled trial involving 816 children with acute gastroenteritis also showed no virus-specific beneficial effects of the probiotic in reducing viral nucleic acid clearance from stool specimens (Freedman et al., 2020). Furthermore, there were no differences in diarrhea duration or the total number of diarrheal stools between the treatment and placebo groups across various common acute gastroenteritis pathogens, including adenovirus, norovirus, rotavirus, and bacteria (Freedman et al., 2021).
7. Norovirus vaccine development
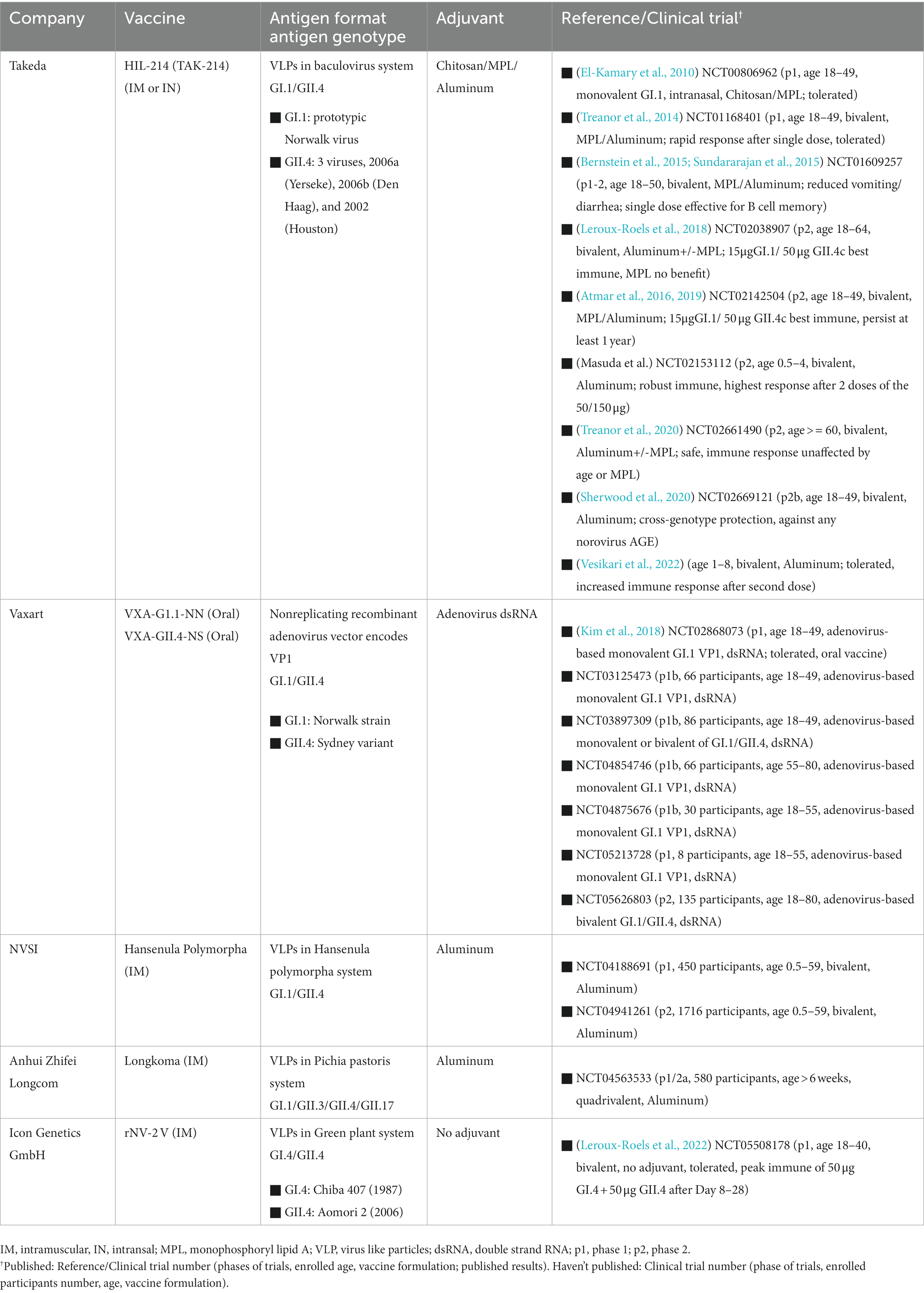
The development of a norovirus vaccine has been challenging due to the wide genetic and antigenic diversity of circulating norovirus strains. Additionally, there have been limitations in culture systems, animal models, and immune markers for vaccine evaluation (Tan, 2021). In recent years, several approaches have been explored for developing a norovirus vaccine, as summarized by Lucero et al. (2021). These vaccine platforms include virus-like particles (VLPs), P-particles, and adenovirus vector-based vaccines. Norovirus VLPs have been expressed in Escherichia coli (Tan et al., 2004; Huo et al., 2018), yeast (Tome-Amat et al., 2014; Parker et al., 2016) and plants (Mathew et al., 2014). Immune responses induced by these vaccines tend to be genotype-specific, requiring multiple genotypes in the vaccine formulation. Most of the vaccine candidates are in the pre-clinical stage, with some already reported in clinical trials. Clinical trial registrations were searched from https://clinicaltrials.gov/. Five vaccine candidates from Takeda, Vaxart, National Vaccine and Serum Institute China (NVSI), Anhui Zhifei Longcom Biologic Pharmacy, and Icon Genetics GmbH have been studied in either phase 1 or phase 2 clinical trials, with most of the studies complete and some reporting safety and efficacy results. The vaccine from Takeda, which contains two types of virus-like particles from GI.1 and GII.4 strains, is the most extensively studied. Several phase 1 clinical trials (El-Kamary et al., 2010; Treanor et al., 2014; Bernstein et al., 2015; Sundararajan et al., 2015) have demonstrated that the vaccine is well tolerated and induces robust immune responses in healthy adults. Subsequent phase 2 clinical trials (Atmar et al., 2016, 2019; Leroux-Roels et al., 2018; Masuda et al., 2018; Treanor et al., 2020) further confirmed the efficacy of the vaccine in participants ranging from 6 months old to older than 60 years old, with one study (Sherwood et al., 2020) even showing significant efficacy against all types of norovirus acute gastroenteritis, indicating cross-genotype protection. Table 3 provides information on the antigen genotypes and adjuvants used in the five vaccine candidates, as well as clinical trial information such as participant age, vaccine formulations, and published results.
8. Discussion and future direction
The microbiota profile changes and the interaction between microbiota and norovirus infection were recently observed and highlighted in this review. In addition, this review summarizes the updated in vivo and clinical studies of probiotics on norovirus infection and investigates the clinical trials and published results of five vaccine candidates. This review deepens our understanding of the relationship between microbiota and norovirus infection, providing a powerful tool for the treatment of norovirus infections and the development of antiviral strategies, including probiotics or norovirus vaccines in the future.
There is a lot of ongoing research on host glycobiology which could potentially play a role in the outcome of norovirus infection. However, the inherent complexity of host glycobiology and microbiota complicates drawing definitive conclusions. Future studies investigating the interaction between host and pathogen using human intestinal enteroids system that mimic human intestinal epithelium may overcome this challenge. For instance, enteroids system generated from individuals with different secretor status may provide information about the relationship between host glycobiology and norovirus infection. The recent discovery of noroviruses potentially being packed within extracellular vesicles and the effect of gut microbiota further complicates the study of viral pathogenesis and inactivation. The role of outer membrane vesicles and bile acid-associated immune response controlled by bacteria also remains an area of interest for further study. Moreover, the virome, the viral fraction of the microbiome in the intestinal microbiome, requires further investigation of its interaction with norovirus infection.
Regarding antiviral strategies, probiotics have been studied for their antiviral effects in recent years; however, the specific immune mechanisms remain unclear. In addition to in vivo studies, clinical trials studying the antiviral effects of probiotics on individuals with norovirus infection are needed. Furthermore, challenges need to be addressed for norovirus vaccine development. Although young children experience the highest incidence of disease and severe disease outcomes are most common among young children and the elderly, most vaccine clinical studies have focused on adults. Separate clinical development for adults and children is required to determine the timing and number of doses, antigen concentration, and the need for adjuvants. Phase III clinical studies are necessary to demonstrate efficacy against natural infection in the field. Moreover, a multivalent vaccine that targets several pathogens in a single dose may be more attractive than introducing additional single-target vaccines; however, the role of these different groups in transmission and the transmission-blocking potential of a vaccine should be better understood. Lastly, testing the efficacy of antiviral strategies, including HBGAs and gut microbiota, in relation to host glycobiology and microbiota will be necessary due to their important role in norovirus infection.
Author contributions
G-HB and M-CT wrote the draft manuscript. S-CL, Y-HH, and S-YC reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by grants from NSTC 112-2622-E-038-004, Taiwan.
Acknowledgments
The authors sincerely thank all the authors who generously shared their experiences and took time to complete the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adrienne, E., Shearer, H., Dallas, G. H., and Kniel, K. E. (2014). Effect of bacterial cell-free supernatants on infectivity of norovirus surrogates. J. Food Prot. 77, 145–149. doi: 10.4315/0362-028X.JFP-13-204
Ahmed, S. M., Hall, A. J., Robinson, A. E., Verhoef, L., Premkumar, P., Parashar, U. D., et al. (2014). Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect. Dis. 14, 725–730. doi: 10.1016/S1473-3099(14)70767-4
Almand, E. A., Moore, M. D., Outlaw, J., and Jaykus, L. A. (2017). Human norovirus binding to select bacteria representative of the human gut microbiota. PLoS One 12:e0173124. doi: 10.1371/journal.pone.0173124
Atmar, R. L., Baehner, F., Cramer, J. P., Lloyd, E., Sherwood, J., Borkowski, A., et al. (2019). Persistence of antibodies to 2 virus-like particle norovirus vaccine candidate formulations in healthy adults: 1-year follow-up with memory probe vaccination. J. Infect. Dis. 220, 603–614. doi: 10.1093/infdis/jiz170
Atmar, R. L., Baehner, F., Cramer, J. P., Song, E., Borkowski, A., Mendelman, P. M., et al. (2016). Rapid responses to 2 virus-like particle norovirus vaccine candidate formulations in healthy adults: a randomized controlled trial. J. Infect. Dis. 214, 845–853. doi: 10.1093/infdis/jiw259
Azad, M. B., Robertson, B., Atakora, F., Becker, A. B., Subbarao, P., Moraes, T. J., et al. (2018). Human Milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J. Nutr. 148, 1733–1742. doi: 10.1093/jn/nxy175
Baldridge, M. T., Nice, T. J., Mccune, B. T., Yokoyama, C. C., Kambal, A., Wheadon, M., et al. (2015). Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 347, 266–269. doi: 10.1126/science.1258025
Bernstein, D. I., Atmar, R. L., Lyon, G. M., Treanor, J. J., Chen, W. H., Jiang, X., et al. (2015). Norovirus vaccine against experimental human GII.4 virus illness: a challenge study in healthy adults. J. Infect. Dis. 211, 870–878. doi: 10.1093/infdis/jiu497
Bhar, S., Zhao, G., Bartel, J. D., Sterchele, H., Del Mazo, A., Emerson, L. E., et al. (2022). Bacterial extracellular vesicles control murine norovirus infection through modulation of antiviral immune responses. Front. Immunol. 13:909949. doi: 10.3389/fimmu.2022.909949
Bode, L. (2015). The functional biology of human milk oligosaccharides. Early Hum. Dev. 91, 619–622. doi: 10.1016/j.earlhumdev.2015.09.001
Budicini, M. R., and Pfeiffer, J. K. (2022). Bacteria-mediated stabilization of murine norovirus. BioRxiv 2022:477311. doi: 10.1101/2022.01.21.477311
Cannon, J. L., Seabolt, M. H., Xu, R., Montmayeur, A., Suh, S. H., Diez-Valcarce, M., et al. (2022). Gut microbiome changes occurring with norovirus infection and recovery in infants enrolled in a longitudinal birth cohort in Leon, Nicaragua. Viruses 14:14. doi: 10.3390/v14071395
Cates, J. E., Vinjé, J., Parashar, U., and Hall, A. J. (2020). Recent advances in human norovirus research and implications for candidate vaccines. Expert Rev. Vaccines 19, 539–548. doi: 10.1080/14760584.2020.1777860
Centers for Disease Control and Prevention. (2023). Burden of Norovirus Illness in the U.S. Atlanta, [Online]. Georgia: Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/norovirus/trends-outbreaks/burden-US.html (Accessed April 14, 2023).
Chan, M. C., Cheung, S. K. C., Mohammad, K. N., Chan, J. C. M., Estes, M. K., and Chan, P. K. S. (2019). Use of human intestinal Enteroids to detect human norovirus infectivity. Emerg. Infect. Dis. 25, 1730–1735. doi: 10.3201/eid2509.190205
Chan, M. C., Lee, N., Hung, T. N., Kwok, K., Cheung, K., Tin, E. K., et al. (2015). Rapid emergence and predominance of a broadly recognizing and fast-evolving norovirus GII.17 variant in late 2014. Nat. Commun. 6:10061. doi: 10.1038/ncomms10061
Chang, K. O., Sosnovtsev, S. V., Belliot, G., Kim, Y., Saif, L. J., and Green, K. Y. (2004). Bile acids are essential for porcine enteric calicivirus replication in association with down-regulation of signal transducer and activator of transcription 1. Proc. Natl. Acad. Sci. U. S. A. 101, 8733–8738. doi: 10.1073/pnas.0401126101
Chen, Y. H., Du, W., Hagemeijer, M. C., Takvorian, P. M., Pau, C., Cali, A., et al. (2015). Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cells 160, 619–630. doi: 10.1016/j.cell.2015.01.032
Chen, S. Y., Tsai, C. N., Lee, Y. S., Lin, C. Y., Huang, K. Y., Chao, H. C., et al. (2017). Intestinal microbiome in children with severe and complicated acute viral gastroenteritis. Sci. Rep. 7:46130. doi: 10.1038/srep46130
Cheung, S. K. C., Kwok, K., Zhang, L. Y., Mohammad, K. N., Lui, G. C. Y., Lee, N., et al. (2019). Higher viral load of emerging norovirus GII.P16-GII.2 than pandemic GII.4 and epidemic GII.17, Hong Kong China. Emerg. Infect. Dis. 25, 119–122. doi: 10.3201/eid2501.180395
Chhabra, P., De Graaf, M., Parra, G. I., Chan, M. C., Green, K., Martella, V., et al. (2019). Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 100, 1393–1406. doi: 10.1099/jgv.0.001318
Costantini, V., Morantz, 10.1080/14760584.2020.1777860 E. K., Browne, H., Ettayebi, K., Zeng, X. L., Atmar, R. L., et al. (2018). Human norovirus replication in human intestinal Enteroids as model to evaluate virus inactivation. Emerg. Infect. Dis. 24, 1453–1464. doi: 10.3201/eid2408.180126
Creutznacher, R., Maaß, T., Dülfer, J., Feldmann, C., Hartmann, V., Knickmann, J., et al. (2021). Murine norovirus capsid plasticity – Glycochenodeoxycholic acid stabilizes P-domain dimers and triggers escape from antibody recognition. BioRxiv 2021:433148. doi: 10.1101/2021.02.27.433148
Currier, R. L., Payne, D. C., Staat, M. A., Selvarangan, R., Shirley, S. H., Halasa, N., et al. (2015). Innate susceptibility to norovirus infections influenced by FUT2 genotype in a United States pediatric population. Clin. Infect. Dis. 60, 1631–1638. doi: 10.1093/cid/civ165
El-Kamary, S. S., Pasetti, M. F., Mendelman, P. M., Frey, S. E., Bernstein, D. I., Treanor, J. J., et al. (2010). Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 202, 1649–1658. doi: 10.1086/657087
Estes, M. K., Ettayebi, K., Tenge, V. R., Murakami, K., Karandikar, U., Lin, S.-C., et al. (2019). Human norovirus cultivation in nontransformed stem cell-derived human intestinal Enteroid cultures: success and challenges. Viruses 11:638. doi: 10.3390/v11070638
Ettayebi, K., Crawford, S. E., Murakami, K., Broughman, J. R., Karandikar, U., Tenge, V. R., et al. (2016). Replication of human noroviruses in stem cell-derived human enteroids. Science 353, 1387–1393. doi: 10.1126/science.aaf5211
Ettayebi, K., Tenge, V. R., Cortes-Penfield, N. W., Crawford, S. E., Neill, F. H., Zeng, X.-L., et al. (2021). New insights and enhanced human norovirus cultivation in human intestinal Enteroids. mSphere 6, e01136–e01120. doi: 10.1128/mSphere.01136-20
Etzold, S., and Bode, L. (2014). Glycan-dependent viral infection in infants and the role of human milk oligosaccharides. Curr. Opin. Virol. 7, 101–107. doi: 10.1016/j.coviro.2014.06.005
Freedman, S. B., Finkelstein, Y., Pang, X. L., Chui, L., Tarr, P. I., Vanburen, J. M., et al. (2021). Pathogen-specific effects of probiotics in children with acute gastroenteritis seeking emergency care: a randomized trial. Clin. Infect. Dis. 75, 55–64. doi: 10.1093/cid/ciab876
Freedman, S. B., Xie, J., Nettel-Aguirre, A., Pang, X. L., Chui, L., Williamson-Urquhart, S., et al. (2020). A randomized trial evaluating virus-specific effects of a combination probiotic in children with acute gastroenteritis. Nat. Commun. 11:2533. doi: 10.1038/s41467-020-16308-3
Grau, K. R., Zhu, S., Peterson, S. T., Helm, E. W., Philip, D., Phillips, M., et al. (2020). The intestinal regionalization of acute norovirus infection is regulated by the microbiota via bile acid-mediated priming of type III interferon. Nat. Microbiol. 5, 84–92. doi: 10.1038/s41564-019-0602-7
Haga, K., Ettayebi, K., Tenge, V. R., Karandikar, U. C., Lewis, M. A., Lin, S.-C., et al. (2020). Genetic manipulation of human intestinal Enteroids demonstrates the necessity of a functional Fucosyltransferase 2 gene for secretor-dependent human norovirus. Infection 11, e00251–e00220. doi: 10.1128/mBio.00251-20
Hennessy, E. P., Green, A. D., Connor, M. P., Darby, R., and Macdonald, P. (2003). Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 188, 176–177. doi: 10.1086/375829
Hickman, D., Jones, M. K., Zhu, S., Kirkpatrick, E., Ostrov, D. A., Wang, X., et al. (2014). The effect of malnutrition on norovirus infection. MBio 5, e01032–e01013. doi: 10.1128/mBio.01032-13
Hoang, P. M., Cho, S., Kim, K. E., Byun, S. J., Lee, T.-K., and Lee, S. (2015). Development of Lactobacillus paracasei harboring nucleic acid-hydrolyzing 3D8 scFv as a preventive probiotic against murine norovirus infection. Appl. Microbiol. Biotechnol. 99, 2793–2803. doi: 10.1007/s00253-014-6257-7
Hong Chau, T. T., Minh Chau, N. N., Hoang Le, N. T., Voong Vinh, P., Nguyen To, N. T., Ngoc, N. M., et al. (2018). A double-blind, randomized, placebo-controlled trial of Lactobacillus acidophilus for the treatment of acute watery diarrhea in Vietnamese children. Pediatr. Infect. Dis. J. 37, 35–42. doi: 10.1097/INF.0000000000001712
Huang, P., Farkas, T., Marionneau, S., Zhong, W., Ruvoen-Clouet, N., Morrow, A. L., et al. (2003). Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J. Infect. Dis. 188, 19–31. doi: 10.1086/375742
Huo, Y., Wan, X., Ling, T., Wu, J., Wang, W., and Shen, S. (2018). Expression and purification of norovirus virus like particles in Escherichia coli and their immunogenicity in mice. Mol. Immunol. 93, 278–284. doi: 10.1016/j.molimm.2017.07.014
Hutson, A. M., Atmar, R. L., Graham, D. Y., and Estes, M. K. (2002). Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 185, 1335–1337. doi: 10.1086/339883
Ingle, H., Lee, S., Ai, T., Orvedahl, A., Rodgers, R., Zhao, G., et al. (2019). Viral complementation of immunodeficiency confers protection against enteric pathogens via interferon-lambda. Nat. Microbiol. 4, 1120–1128. doi: 10.1038/s41564-019-0416-7
Jiang, X., Huang, P., Zhong, W., Tan, M., Farkas, T., Morrow, A. L., et al. (2004). Human milk contains elements that block binding of noroviruses to human histo-blood group antigens in saliva. J. Infect. Dis. 190, 1850–1859. doi: 10.1086/425159
Jin, M., Zhou, Y. K., Xie, H. P., Fu, J. G., He, Y. Q., Zhang, S., et al. (2016). Characterization of the new GII.17 norovirus variant that emerged recently as the predominant strain in China. J. Gen. Virol. 97, 2620–2632. doi: 10.1099/jgv.0.000582
Jones, M. K., Watanabe, M., Zhu, S., Graves, C. L., Keyes, L. R., Grau, K. R., et al. (2014). Enteric bacteria promote human and mouse norovirus infection of B cells. Science 346, 755–759. doi: 10.1126/science.1257147
Kilic, T., Koromyslova, A., and Hansman, G. S. (2019). Structural basis for human norovirus capsid binding to bile acids. J. Virol. 93. doi: 10.1128/JVI.01581-18
Kim, D., Cho, M.-J., Lee, Y., Kil, E.-J., Byun, S., and Lee, S.. (2021). In vivo characterization and genome-based approach to Lactobacillus Johnsonii Byun-jo-01 and its probiotic properties. Paper.
Kim, D. H., Jeong, M., Kim, J. H., Son, J. E., Lee, J. J., Park, S.-J., et al. (2022). Lactobacillus salivarius HHuMin-U activates innate immune defense against norovirus infection through TBK1-IRF3 and NF-κB signaling pathways. Research 2022:7. doi: 10.34133/research.0007
Kim, L., Liebowitz, D., Lin, K., Kasparek, K., Pasetti, M. F., Garg, S. J., et al. (2018). Safety and immunogenicity of an oral tablet norovirus vaccine, a phase I randomized, placebo-controlled trial. JCI Insight 3:e121077. doi: 10.1172/jci.insight.121077
Kim, D., Nurlaila, I., Sianipar, H., Cho, M., Lee, Y., and Cho, S. (2022). A novel Limosilactobacillus reuteri strain Byun-re-01 exhibits promising probiotic properties for further use as preservatives: An in vivo and in silico. J. Infect. Dis. Prevent. Med. 10, 1–10.
Koromyslova, A., Tripathi, S., Morozov, V., Schroten, H., and Hansman, G. S. (2017). Human norovirus inhibition by a human milk oligosaccharide. Virology 508, 81–89. doi: 10.1016/j.virol.2017.04.032
Lalithamaheswari, B., and Anu Radha, C. (2022). Structural and binding studies of 2′- and 3-fucosyllactose and its complexes with norovirus capsid protein by molecular dynamics simulations. J. Biomol. Struct. Dyn. 2022, 1–14. doi: 10.1080/07391102.2022.2153923
Lee, W., Kim, M., Lee, S. H., Jung, H. G., and Oh, J. W. (2018). Prophylactic efficacy of orally administered Bacillus poly-gamma-glutamic acid, a non-LPS TLR4 ligand, against norovirus infection in mice. Sci. Rep. 8:8667. doi: 10.1038/s41598-018-26935-y
Lee, H., and Ko, G. (2016). Antiviral effect of vitamin a on norovirus infection via modulation of the gut microbiome. Sci. Rep. 6:25835. doi: 10.1038/srep25835
Lei, S., Ramesh, A., Twitchell, E., Wen, K., Bui, T., Weiss, M., et al. (2016). High protective efficacy of probiotics and Rice bran against human norovirus infection and diarrhea in Gnotobiotic pigs. Front. Microbiol. 7:1699. doi: 10.3389/fmicb.2016.01699
Lei, W., Ren, W., Ohmoto, M., Urban, J. F. Jr., Matsumoto, I., Margolskee, R. F., et al. (2018). Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc. Natl. Acad. Sci. U. S. A. 115, 5552–5557. doi: 10.1073/pnas.1720758115
Leroux-Roels, G., Cramer, J. P., Mendelman, P. M., Sherwood, J., Clemens, R., Aerssens, A., et al. (2018). Safety and immunogenicity of different formulations of norovirus vaccine candidate in healthy adults: a randomized, controlled, double-blind clinical trial. J. Infect. Dis. 217, 597–607. doi: 10.1093/infdis/jix572
Leroux-Roels, I., Maes, C., Joye, J., Jacobs, B., Jarczowski, F., Diessner, A., et al. (2022). A randomized, double-blind, placebo-controlled, dose-escalating phase I trial to evaluate safety and immunogenicity of a plant-produced, bivalent, recombinant norovirus-like particle vaccine. Front. Immunol. 13:1021500. doi: 10.3389/fimmu.2022.1021500
Li, D., Breiman, A., Le Pendu, J., and Uyttendaele, M. (2015). Binding to histo-blood group antigen-expressing bacteria protects human norovirus from acute heat stress. Front. Microbiol. 6:659. doi: 10.3389/fmicb.2015.00659
Li, D., Breiman, A., Le Pendu, J., and Uyttendaele, M. (2016). Anti-viral effect of Bifidobacterium adolescentis against noroviruses. Front. Microbiol. 7:864. doi: 10.3389/fmicb.2016.00864
Li, Y., Gao, J., Xue, L., Shang, Y., Cai, W., Xie, X., et al. (2022). Determination of antiviral mechanism of centenarian gut-derived Limosilactobacillus fermentum against norovirus. Front. Nutr. 9:812623. doi: 10.3389/fnut.2022.812623
Lin, S.-C., Qu, L., Ettayebi, K., Crawford, S. E., Blutt, S. E., Robertson, M. J., et al. (2020). Human norovirus exhibits strain-specific sensitivity to host interferon pathways in human intestinal enteroids. Proc. Natl. Acad. Sci. 117, 23782–23793. doi: 10.1073/pnas.2010834117
Lindesmith, L., Moe, C., Marionneau, S., Ruvoen, N., Jiang, X., Lindblad, L., et al. (2003). Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9, 548–553. doi: 10.1038/nm860
Long, K. Z., Garcia, C., Santos, J. I., Rosado, J. L., Hertzmark, E., Dupont, H. L., et al. (2007). Vitamin a supplementation has divergent effects on norovirus infections and clinical symptoms among Mexican children. J. Infect. Dis. 196, 978–985. doi: 10.1086/521195
Lopez-Santamarina, A., Lamas, A., Del Carmen Mondragon, A., Cardelle-Cobas, A., Regal, P., Rodriguez-Avila, J. A., et al. (2021). Probiotic effects against virus infections: new weapons for an old war. Foods 10:130. doi: 10.3390/foods10010130
Lopman, B. A.. (2015). Global burden of norovirus and prospects for vaccine development. Atlanta, Georgia: Centers for Disease Control and Prevention.
Lu, J., Fang, L., Zheng, H., Lao, J., Yang, F., Sun, L., et al. (2016). The evolution and transmission of epidemic GII.17 noroviruses. J. Infect. Dis. 214, 556–564. doi: 10.1093/infdis/jiw208
Lucero, Y., Matson, D. O., Ashkenazi, S., George, S., and O’ryan, M. (2021). Norovirus: facts and reflections from past, present, and future. Viruses 13:2399.
Macduff, D. A., Baldridge, M. T., Qaqish, A. M., Nice, T. J., Darbandi, A. D., Hartley, V. L., et al. (2018). HOIL1 is essential for the induction of type I and III interferons by MDA5 and regulates persistent murine norovirus infection. J. Virol. 92:e01368. doi: 10.1128/JVI.01368-18
Magwira, C. A., Steele, D., and Seheri, M. L. (2021). Norovirus diarrhea is significantly associated with higher counts of fecal histo-blood group antigen expressing Enterobacter cloacae among black south African infants. Gut Microbes 13:1979876. doi: 10.1080/19490976.2021.1979876
Masuda, T., Lefevre, I., Mendelman, P., Sherwood, J., Bizjajeva, S., and Borkowski, A. (2018). 2276. Immunogenicity of Takeda’s bivalent virus-like particle (VLP) norovirus vaccine (NoV) candidate in children from 6 months up to 4 years of age. Open Forum Infect. Dis. 5:S674. doi: 10.1093/ofid/ofy210.1929.eCollection, 2018 Nov.
Mathew, L. G., Herbst-Kralovetz, M. M., and Mason, H. S. (2014). Norovirus Narita 104 virus-like particles expressed in Nicotiana benthamiana induce serum and mucosal immune responses. Biomed. Res. Int. 2014:807539. doi: 10.1155/2014/807539
Miura, T., Sano, D., Suenaga, A., Yoshimura, T., Fuzawa, M., Nakagomi, T., et al. (2013). Histo-blood group antigen-like substances of human enteric bacteria as specific adsorbents for human noroviruses. J. Virol. 87, 9441–9451. doi: 10.1128/JVI.01060-13
Mizutani, T., Aboagye, S. Y., Ishizaka, A., Afum, T., Mensah, G. I., Asante-Poku, A., et al. (2021). Gut microbiota signature of pathogen-dependent dysbiosis in viral gastroenteritis. Sci. Rep. 11:13945. doi: 10.1038/s41598-021-93345-y
Mori, K., Konishi, N., Suzuki, Y., Harada, S., Maeda, M., Akase, S., et al. (2018). Comparison between patients with norovirus-related gastroenteritis and asymptomatic carriers with respect to distribution of antibody-complexed viral particles and intestinal flora. J. Med. Virol. 90, 1882–1887. doi: 10.1002/jmv.25079
Mosby, C. A., Bhar, S., Phillips, M. B., Edelmann, M. J., and Jones, M. K. (2022). Interaction with mammalian enteric viruses alters outer membrane vesicle production and content by commensal bacteria. J Extracell Vesicles 11:e12172. doi: 10.1002/jev2.12172
Mosby, C. A., Edelmann, M. J., and Jones, M. K. (2023). Murine norovirus interaction with Enterobacter cloacae leads to changes in membrane stability and packaging of lipid and metabolite vesicle content. Microbiology Spectrum 11, e04691–e04622. doi: 10.1128/spectrum.04691-22
Mumphrey, S. M., Changotra, H., Moore, T. N., Heimann-Nichols, E. R., Wobus, C. E., Reilly, M. J., et al. (2007). Murine norovirus 1 infection is associated with histopathological changes in immunocompetent hosts, but clinical disease is prevented by STAT1-dependent interferon responses. J. Virol. 81, 3251–3263. doi: 10.1128/JVI.02096-06
Murakami, K., Tenge, V. R., Karandikar, U. C., Lin, S.-C., Ramani, S., Ettayebi, K., et al. (2020). Bile acids and ceramide overcome the entry restriction for GII.3 human norovirus replication in human intestinal enteroids. Proc. Natl. Acad. Sci. 117, 1700–1710. doi: 10.1073/pnas.1910138117
Nadjsombati, M. S., Mcginty, J. W., Lyons-Cohen, M. R., Jaffe, J. B., Dipeso, L., Schneider, C., et al. (2018). Detection of succinate by intestinal tuft cells triggers a type 2 innate immune circuit. Immunity 49:e37. doi: 10.1016/j.immuni.2018.06.016
Nagata, S., Asahara, T., Ohta, T., Yamada, T., Kondo, S., Bian, L., et al. (2011). Effect of the continuous intake of probiotic-fermented milk containing Lactobacillus casei strain Shirota on fever in a mass outbreak of norovirus gastroenteritis and the faecal microflora in a health service facility for the aged. Br. J. Nutr. 106, 549–556. doi: 10.1017/S000711451100064X
Nelson, A. M., Elftman, M. D., Pinto, A. K., Baldridge, M., Hooper, P., Kuczynski, J., et al. (2013). Murine norovirus infection does not cause major disruptions in the murine intestinal microbiota. Microbiome 1:7. doi: 10.1186/2049-2618-1-7
Nelson, A. M., Walk, S. T., Taube, S., Taniuchi, M., Houpt, E. R., Wobus, C. E., et al. (2012). Disruption of the human gut microbiota following norovirus infection. PLoS One 7:e48224. doi: 10.1371/journal.pone.0048224
Nelson, C. A., Wilen, C. B., Dai, Y. N., Orchard, R. C., Kim, A. S., Stegeman, R. A., et al. (2018). Structural basis for murine norovirus engagement of bile acids and the CD300lf receptor. Proc. Natl. Acad. Sci. U. S. A. 115, E9201–E9210. doi: 10.1073/pnas.1805797115
Nice, T. J., Baldridge, M. T., Mccune, B. T., Norman, J. M., Lazear, H. M., Artyomov, M., et al. (2015). Interferon-lambda cures persistent murine norovirus infection in the absence of adaptive immunity. Science 347, 269–273. doi: 10.1126/science.1258100
Nordgren, J., and Svensson, L. (2019). Genetic susceptibility to human norovirus infection: an update. Viruses 11:226. doi: 10.3390/v11030226
Orchard, R. C., Wilen, C. B., Doench, J. G., Baldridge, M. T., Mccune, B. T., Lee, Y. C., et al. (2016). Discovery of a proteinaceous cellular receptor for a norovirus. Science 353, 933–936. doi: 10.1126/science.aaf1220
Parker, S. A., Maloy, M. H., Tome-Amat, J., Bardliving, C. L., Batt, C. A., Lanz, K. J., et al. (2016). Optimization of norovirus virus-like particle production in Pichia pastoris using a real-time near-infrared bioprocess monitor. Biotechnol. Prog. 32, 518–526. doi: 10.1002/btpr.2224
Patin, N. V., Pena-Gonzalez, A., Hatt, J. K., Moe, C., Kirby, A., and Konstantinidis, K. T. (2020). The role of the gut microbiome in resisting norovirus infection as revealed by a human challenge study. MBio 11:11. doi: 10.1128/mBio.02634-20
Pena-Gil, N., Santiso-Bellon, C., Gozalbo-Rovira, R., Buesa, J., Monedero, V., and Rodriguez-Diaz, J. (2021). The role of host Glycobiology and gut microbiota in rotavirus and norovirus infection, an update. Int. J. Mol. Sci. 22:13473. doi: 10.3390/ijms222413473
Randazzo, W., Costantini, V., Morantz, E. K., and Vinje, J. (2020). Human intestinal Enteroids to evaluate human norovirus GII.4 inactivation by aged-Green tea. Front. Microbiol. 11:1917. doi: 10.3389/fmicb.2020.01917
Rasko, D. A., Wang, G., Monteiro, M. A., Palcic, M. M., and Taylor, D. E. (2000). Synthesis of mono-and di-fucosylated type I Lewis blood group antigens by Helicobacter pylori. Eur. J. Biochem. 267, 6059–6066. doi: 10.1046/j.1432-1327.2000.01683.x
Ridlon, J. M., Kang, D. J., and Hylemon, P. B. (2006). Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259. doi: 10.1194/jlr.R500013-JLR200
Rodriguez-Diaz, J., Garcia-Mantrana, I., Vila-Vicent, S., Gozalbo-Rovira, R., Buesa, J., Monedero, V., et al. (2017). Relevance of secretor status genotype and microbiota composition in susceptibility to rotavirus and norovirus infections in humans. Sci. Rep. 7:45559. doi: 10.1038/srep45559
Rubio-Del-Campo, A., Coll-Marqués, J. M., Yebra, M. J., Buesa, J., Pérez-Martínez, G., Monedero, V., et al. (2014). Noroviral P-particles as an in vitro model to assess the interactions of noroviruses with probiotics. PLoS One 9:e89586. doi: 10.1371/journal.pone.0089586y
Rydell, G. E., Nilsson, J., Rodriguez-Diaz, J., Ruvoen-Clouet, N., Svensson, L., Le Pendu, J., et al. (2009). Human noroviruses recognize sialyl Lewis x neoglycoprotein. Glycobiology 19, 309–320. doi: 10.1093/glycob/cwn139
Santiana, M., Ghosh, S., Ho, B. A., Rajasekaran, V., Du, W. L., Mutsafi, Y., et al. (2018). Vesicle-cloaked virus clusters are optimal units for inter-organismal viral transmission. Cell Host Microbe 24:e208. doi: 10.1016/j.chom.2018.07.006
Santiso-Bellón, C., Gozalbo-Rovira, R., Buesa, J., Rubio-Del-Campo, A., Peña-Gil, N., Navarro-Lleó, N., et al. (2022). Replication of human norovirus in mice after antibiotic-mediated intestinal Bacteria depletion. Int. J. Mol. Sci. 23:10643. doi: 10.3390/ijms231810643
Sayin, S. I., Wahlstrom, A., Felin, J., Jantti, S., Marschall, H. U., Bamberg, K., et al. (2013). Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 17, 225–235. doi: 10.1016/j.cmet.2013.01.003
Seo, D. J., Jung, D., Jung, S., Yeo, D., and Choi, C. (2020). Inhibitory effect of lactic acid bacteria isolated from kimchi against murine norovirus. Food Control 109:106881. doi: 10.1016/j.foodcont.2019.106881
Shang, J., Piskarev, V. E., Xia, M., Huang, P., Jiang, X., Likhosherstov, L. M., et al. (2013). Identifying human milk glycans that inhibit norovirus binding using surface plasmon resonance. Glycobiology 23, 1491–1498. doi: 10.1093/glycob/cwt077
Sherwood, J., Mendelman, P. M., Lloyd, E., Liu, M., Boslego, J., Borkowski, A., et al. (2020). Efficacy of an intramuscular bivalent norovirus GI.1/GII.4 virus-like particle vaccine candidate in healthy US adults. Vaccine 38, 6442–6449. doi: 10.1016/j.vaccine.2020.07.069
Shivanna, V., Kim, Y., and Chang, K. O. (2014). The crucial role of bile acids in the entry of porcine enteric calicivirus. Virology 456-457, 268–278. doi: 10.1016/j.virol.2014.04.002
Solano-Aguilar, G., Fernandez, K. P., Ets, H., Molokin, A., Vinyard, B., Urban, J. F., et al. (2013). Characterization of fecal microbiota of children with diarrhea in 2 locations in Colombia. J. Pediatr. Gastroenterol. Nutr. 56, 503–511. doi: 10.1097/MPG.0b013e318282aa12
Soorneedi, A. R., and Moore, M. D. (2022). Recent developments in norovirus interactions with bacteria. Curr. Opin. Food Sci. 48:100926. doi: 10.1016/j.cofs.2022.100926
Steyer, A., Micetic-Turk, D., and Fijan, S. (2022). The efficacy of probiotics as antiviral agents for the treatment of rotavirus gastrointestinal infections in children: an updated overview of literature. Microorganisms 10:2392. doi: 10.3390/microorganisms10122392
Sullender, M. E., and Baldridge, M. T. (2018). Norovirus interactions with the commensal microbiota. PLoS Pathog. 14:e1007183. doi: 10.1371/journal.ppat.1007183
Sundararajan, A., Sangster, M. Y., Frey, S., Atmar, R. L., Chen, W. H., Ferreira, J., et al. (2015). Robust mucosal-homing antibody-secreting B cell responses induced by intramuscular administration of adjuvanted bivalent human norovirus-like particle vaccine. Vaccine 33, 568–576. doi: 10.1016/j.vaccine.2014.09.073
Tamura, M., Natori, K., Kobayashi, M., Miyamura, T., and Takeda, N. (2004). Genogroup II noroviruses efficiently bind to heparan sulfate proteoglycan associated with the cellular membrane. J. Virol. 78, 3817–3826. doi: 10.1128/jvi.78.8.3817-3826.2004
Tan, M. (2021). Norovirus vaccines: current clinical development and challenges. Pathogens 10:1641. doi: 10.3390/pathogens10121641
Tan, M., and Jiang, X. (2014). Histo-blood group antigens: a common niche for norovirus and rotavirus. Expert Rev. Mol. Med. 16:e5. doi: 10.1017/erm.2014.2
Tan, M., Zhong, W., Song, D., Thornton, S., and Jiang, X. (2004). E. coli-expressed recombinant norovirus capsid proteins maintain authentic antigenicity and receptor binding capability. J. Med. Virol. 74, 641–649. doi: 10.1002/jmv.20228
Taube, S., Perry, J. W., Mcgreevy, E., Yetming, K., Perkins, C., Henderson, K., et al. (2012). Murine noroviruses bind glycolipid and glycoprotein attachment receptors in a strain-dependent manner. J. Virol. 86, 5584–5593. doi: 10.1128/JVI.06854-11
Taube, S., Perry, J. W., Yetming, K., Patel, S. P., Auble, H., Shu, L., et al. (2009). Ganglioside-linked terminal sialic acid moieties on murine macrophages function as attachment receptors for murine noroviruses. J. Virol. 83, 4092–4101. doi: 10.1128/JVI.02245-08
Thorven, M., Grahn, A., Hedlund, K. O., Johansson, H., Wahlfrid, C., Larson, G., et al. (2005). A homozygous nonsense mutation (428G→a) in the human secretor (FUT2) gene provides resistance to symptomatic norovirus (GGII) infections. J. Virol. 79, 15351–15355. doi: 10.1128/JVI.79.24.15351-15355.2005
Tome-Amat, J., Fleischer, L., Parker, S. A., Bardliving, C. L., and Batt, C. A. (2014). Secreted production of assembled norovirus virus-like particles from Pichia pastoris. Microb. Cell Factories 13:134. doi: 10.1186/s12934-014-0134-z
Treanor, J. J., Atmar, R. L., Frey, S. E., Gormley, R., Chen, W. H., Ferreira, J., et al. (2014). A novel intramuscular bivalent norovirus virus-like particle vaccine candidate--reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J. Infect. Dis. 210, 1763–1771. doi: 10.1093/infdis/jiu337
Treanor, J., Sherwood, J., Cramer, J. P., Le Cam Bouveret, N., Lin, S., Baehner, F., et al. (2020). A phase 2 study of the bivalent VLP norovirus vaccine candidate in older adults; impact of MPL adjuvant or a second dose. Vaccine 38, 5842–5850. doi: 10.1016/j.vaccine.2020.06.011
Turula, H., Bragazzi Cunha, J., Mainou, B. A., Ramakrishnan, S. K., Wilke, C. A., Gonzalez-Hernandez, M. B., et al. (2018). Natural secretory immunoglobulins promote enteric viral infections. J. Virol. 92:e00826. doi: 10.1128/JVI.00826-18
Vesikari, T., Saez-Llorens, X., Blazevic, V., Lopez, P., Lopez, E., Masuda, T., et al. (2022). Immunogenicity of a bivalent virus-like particle norovirus vaccine in children from 1 to 8 years of age: a phase 2 randomized, double-blind study. Vaccine 40, 3588–3596. doi: 10.1016/j.vaccine.2022.04.089
Vitetta, L., Vitetta, G., and Hall, S. (2018). Immunological tolerance and function: associations between intestinal Bacteria, probiotics, prebiotics, and phages. Front. Immunol. 9:2240. doi: 10.3389/fimmu.2018.02240
Walker, F. C., and Baldridge, M. T. (2019). Interactions between noroviruses, the host, and the microbiota. Curr. Opin. Virol. 37, 1–9. doi: 10.1016/j.coviro.2019.04.001
Weichert, S., Koromyslova, A., Singh, B. K., Hansman, S., Jennewein, S., Schroten, H., et al. (2016). Structural basis for norovirus inhibition by human Milk oligosaccharides. J. Virol. 90, 4843–4848. doi: 10.1128/JVI.03223-15
White, P. A. (2014). Evolution of norovirus. Clin. Microbiol. Infect. 20, 741–745. doi: 10.1111/1469-0691.12746
Wilen, C. B., Lee, S., Hsieh, L. L., Orchard, R. C., Desai, C., et al. (2018). Tropism for tuft cells determines immune promotion of norovirus pathogenesis. Science 360, 204–208. doi: 10.1126/science.aar3799
Wirusanti, N. I., Baldridge, M. T., and Harris, V. C. (2022). Microbiota regulation of viral infections through interferon signaling. Trends Microbiol. 30, 778–792. doi: 10.1016/j.tim.2022.01.007
Xiong, L., Li, Y., Li, J., Yang, J., Shang, L., He, X., et al. (2021). Intestinal microbiota profiles in infants with acute gastroenteritis caused by rotavirus and norovirus infection: a prospective cohort study. Int. J. Infect. Dis. 111, 76–84. doi: 10.1016/j.ijid.2021.08.024
Yi, W., Shao, J., Zhu, L., Li, M., Singh, M., Lu, Y., et al. (2005). Escherichia coli O86 O-antigen biosynthetic gene cluster and stepwise enzymatic synthesis of human blood group B antigen tetrasaccharide. J. Am. Chem. Soc. 127, 2040–2041. doi: 10.1021/ja045021y
Yokoyama, C. C., Loh, J., Zhao, G., Stappenbeck, T. S., Wang, D., Huang, H. V., et al. (2012). Adaptive immunity restricts replication of novel murine astroviruses. J. Virol. 86, 12262–12270. doi: 10.1128/JVI.02018-12
Zhang, X. F., Huang, Q., Long, Y., Jiang, X., Zhang, T., Tan, M., et al. (2015). An outbreak caused by GII.17 norovirus with a wide spectrum of HBGA-associated susceptibility. Sci. Rep. 5:17687. doi: 10.1038/srep17687
Zhang, S., Li, T., Xie, J., Zhang, D., Pi, C., Zhou, L., et al. (2021). Gold standard for nutrition: a review of human milk oligosaccharide and its effects on infant gut microbiota. Microb. Cell Factories 20:108. doi: 10.1186/s12934-021-01599-y
Keywords: norovirus, microbiota, probiotics, norovirus vaccine, human intestinal enteroid
Citation: Bai G-H, Tsai M-C, Lin S-C, Hsu Y-H and Chen S-Y (2023) Unraveling the interplay between norovirus infection, gut microbiota, and novel antiviral approaches: a comprehensive review. Front. Microbiol. 14:1212582. doi: 10.3389/fmicb.2023.1212582
Edited by:
Bodo Linz, Friedrich-Alexander-Universität Erlangen-Nürnberg, GermanyReviewed by:
Yuta Kanai, Osaka University, JapanMaria Guadalupe Vizoso Pinto, CONICET Higher Institute of Biological Research (INSIBIO), Argentina
Copyright © 2023 Bai, Tsai, Lin, Hsu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shih-Yen Chen, 18159@s.tmu.edu.tw
†These authors have contributed equally to this work
 Geng-Hao Bai
Geng-Hao Bai Meng-Chen Tsai
Meng-Chen Tsai Sheng-Chieh Lin
Sheng-Chieh Lin Yi-Hsiang Hsu
Yi-Hsiang Hsu Shih-Yen Chen
Shih-Yen Chen