- 1Department of Neurology, Central People's Hospital of Zhanjiang, Zhanjiang, China
- 2Zhanjiang Institute of Clinical Medicine, Central People's Hospital of Zhanjiang, Zhanjiang, China
- 3Department of Neurology, The Second Affiliated Hospital of Guangzhou Medical University, Zhanjiang, China
- 4Department of Histology and Embryology, Guangdong Medical University, Zhanjiang, China
- 5Jiangsu Key Laboratory of Neuropsychiatric Diseases and Institute of Neuroscience, Soochow University, Suzhou, China
Introduction: Patients with epilepsy are particularly vulnerable to the negative effects of anxiety disorders. In particular, temporal lobe epilepsy with anxiety disorders (TLEA) has attracted more attention in epilepsy research. The link between intestinal dysbiosis and TLEA has not been established yet. To gain deeper insight into the link between gut microbiota dysbiosis and factors affecting TLEA, the composition of the gut microbiome, including bacteria and fungi, has been examined.
Methods: The gut microbiota from 51 temporal lobe epilepsy patients has been subjected to sequencing targeting 16S rDNA (Illumina MiSeq) and from 45 temporal lobe epilepsy patients targeting the ITS-1 region (through pyrosequencing). A differential analysis has been conducted on the gut microbiota from the phylum to the genus level.
Results: TLEA patients' gut bacteria and fungal microbiota exhibited distinct characteristics and diversity as evidenced by high-throughput sequencing (HTS). TLEA patients showed higher abundances of Escherichia-Shigella (genus), Enterobacterales (order), Enterobacteriaceae (family), Proteobacteria (phylum), Gammaproteobacteria (class), and lower abundances of Clostridia (class), Firmicutes, Lachnospiraceae (family), Lachnospirales (order), and Ruminococcus (genus). Among fungi, Saccharomycetales fam. incertae sedis (family), Saccharomycetales (order), Saccharomycetes (class), and Ascomycota (phylum) were significantly more abundant in TLEA patients than in patients with temporal lobe epilepsy but without anxiety. Adoption and perception of seizure control significantly affected TLEA bacterial community structure, while yearly hospitalization frequency affected fungal community structures in TLEA patients.
Conclusion: Here, our study validated the gut microbiota dysbiosis of TLEA. Moreover, the pioneering study of bacterial and fungal microbiota profiles will help in understanding the course of TLEA and drive us toward preventing TLEA gut microbiota dysbiosis.
Introduction
Epilepsy is a chronic disease characterized by sudden, short-lived, and recurrent central nervous system dysfunction caused by abnormal discharge of brain neurons (Christensen et al., 2022). Temporal lobe epilepsy, one of the most common types of epilepsy, affects ~65 million people worldwide (Beghi, 2020; Buchin et al., 2022). Epilepsy patients frequently experience more psychological pressure and are more prone to mental illness than the general population (Tang et al., 2022). Up to 60% of epilepsy patients experience anxiety and/or depression (Seid et al., 2022).
Numerous studies have confirmed the alteration of enterobacterial structure in patients with neuropsychiatric and neurodegenerative disorders, whereas few studies have assessed the potential associations of epilepsy and intestinal fungi (Ding et al., 2021; Fusco et al., 2022; Iannone et al., 2022). Specifically, only a few population-based studies have confirmed gut flora dysbiosis in epilepsy patients. The sample size is relatively limited, so the results could be inconsistent (Ding et al., 2021). A study from Western China (Guizhou province) showed that Fusobacterium sp., Fusobacterium mortiferum, Ruminococcus gnavus, and Bacteroides fragilis were significantly positively correlated with the occurrence of epilepsy (r ≥ 0.5, P < 0.05) (Dong et al., 2022). Another study also from Western China (Sichuan Province) showed Actinomyces, Verrucomicrobia, Nitrospirae and Blautia, Bifidobacterium, Subdoligranulum, Dialister, and Anaerostipes were predominantly found in the intestines of patients with anti-seizure medications (Kruskal–Wallis test, P < 0.05) (Gong et al., 2020). Although most epilepsy patients are from Western China, their intestinal flora structure is different. We hypothesized that different types of epilepsy have different prognoses and effects on the intestinal flora. Therefore, it is necessary to determine the type of epilepsy before performing intestinal flora analysis.
As defined for epilepsy patients in the Diagnostic and Statistical Manual of Mental Disorders, anxiety is one of the most common psychiatric comorbidities as well as one of the most complex ones exhibiting a wide spectrum of manifestations from paroxysmal symptoms to epilepsy-specific anxiety and classic anxiety disorders (Munger Clary, 2022). However, the specific mechanisms underlying the comorbidity of epilepsy and anxiety are so far unclear. The neurotransmitters such as 5-hydroxytryptamine, gamma-aminobutyric acid (GABA), norepinephrine, and dopamine have been reported to play a crucial role in the pathogenesis of temporal lobe epilepsy with anxiety disorders (TLEA) (Nutt et al., 2022; Xu et al., 2022).
Given the role of gut microbiota in mental health, breakthroughs happened in the last decade. We note that animal experiments and clinical trials have shown the beneficial effects of probiotics such as Lactobacillus R0052 and Bifidobacterium longum R0175 on anxiety (Mitrea et al., 2022). This cumulative evidence points to the vital role of gut microbes in anxiety disorders. Regrettably, the gut microbiota structure and function in TLEA patients and how these differ from those in epilepsy individuals are still not fully explored.
In this study, the composition of the gut microbiota, including bacteria and fungi, has been assessed and thereby identified potent microecological biomarkers of the disease. Despite the small cohort size, this is the first study comparing the gut bacterial and fungal flora of individuals with TLEA and patients with temporal lobe epilepsy but without anxiety disorder (TLEW).
Materials and methods
The temporal lobe epilepsy patients at the Department of Neurology and the Neuroelectrophysiology Department of the Central People's Hospital of Zhanjiang (CPHZ) have been recruited for this study. Temporal lobe epilepsy was diagnosed by using an electroencephalogram (EEG) based on observed or reported seizures and interictal/seizure phases, epileptic focal localization, patients' clinical presentations, imaging, and EEG localizing the epileptogenic zone in the ipsilateral temporal lobe. The food intake of each patient was normal during the week before enrollment, and all patients included in the study had a balanced diet.
In the following instances, individuals were excluded: (1) if patients had received anti-seizure medication treatment, such as anti-anxiety and depression medications, antidiarrheal medications, laxatives, and antibiotics, or probiotic supplements 1 month before enrollment; (2) if they had undergone bowel resection or experienced acute gastric intestinal bleeding or intestinal tumors; (3) if they had undergone gastroscopy, colonoscopy, gastrointestinal barium meal examination, or other invasive digestive system examinations within 6 months and had a history of digestive system-related surgery; (4) if they were suffering from inflammatory disease enteropathy, Crohn's disease, or other digestive system diseases; (5) if they had diseases that may affect the stability of the intestinal flora due to coagulation dysfunction, severe cardiopulmonary disease, hypertension, diabetes, digestive system, immune system, and others; and (6) if they had a history of alcoholism, alcohol dependence, and smoking.
Clinical assessment
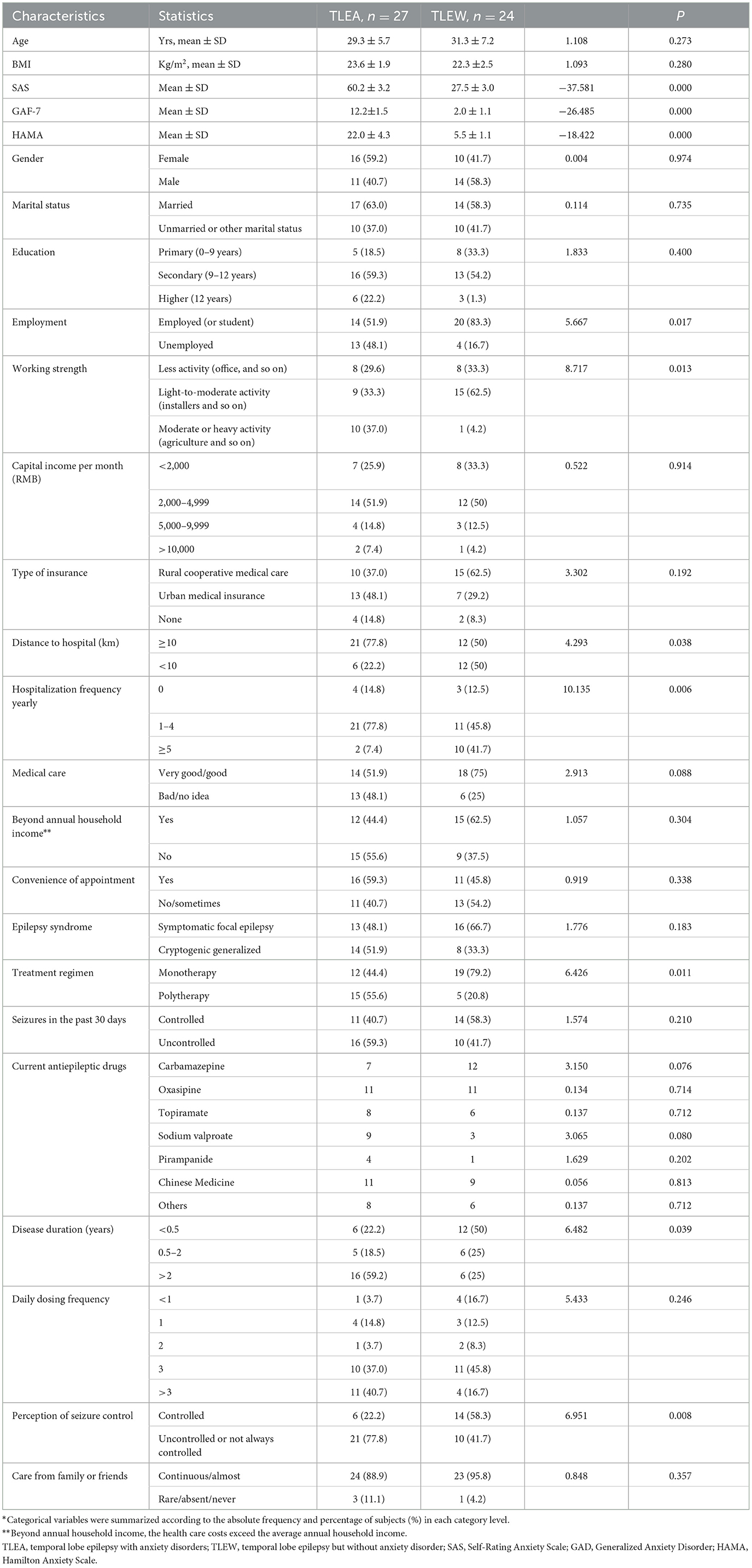
All temporal lobe epilepsy patients were recruited consecutively between December 2021 and December 2022. Patients were between the ages of 18 and 48 years and were of Han Chinese descent, residing in tropical China (Figure 1). The presence or absence of concomitant anxiety in patients with temporal lobe epilepsy served as the basis to establish TLEA and TLEW groups. Information on each participant's gender, epilepsy disease characteristics, and use of medication was recorded as demographic (age, gender, and education) and anxiety impact factors (AIFs, including the type of epilepsy, age at onset, duration of epilepsy, seizure frequency, and seizure type), to identify key factors influencing the presence of anxiety manifestations in patients with epilepsy. Questionnaires were produced using the Questionnaire Star tool (Figures 2A, B).
Figure 1. Geographic distribution of the study population. Only Han Chinese individuals residing in populations in tropical China were included, thus mitigating the effect of ethnic and geographic bias.
Figure 2. Anonymous questionnaire for collecting demographic characteristics, disease characteristics, and treatment characteristics. (A) QR code for logging in to the anonymous questionnaire; (B) the user interface of the anonymous questionnaire.
In this study, anxiety has been defined utilizing the Hamilton Anxiety Rating Scale (Ham-A), generalized anxiety disorder 7 (GAD-7), and self-rating anxiety scale (SAS) scores, with higher scores indicating higher levels of anxiety. The Ham-A score for TLEA was ≥14 and that for TLEW was <7. The GAD-7 TLEA score was >5. According to the Chinese normative results, the cutoff value of the SAS standard score was 50; thus, the TLEA score was >50.
All scores were independently evaluated by clinicians who were blind concerning the patients' clinical conditions and who had attended a training course on how to administer the tests before the study. All patients considered to suffer from a psychiatric disorder or to be in clinical need of psychiatric help were offered assistance by a psychiatrist or psychotherapist. The Clinical Trials Ethics Committee CPHZ (approval number: PJI117-2022-027-013) approved the study protocols, and all methods followed the principles outlined in the 1975 Declaration of Helsinki. All participants participated voluntarily and provided written informed consent. The personal privacy of each participant was protected.
Sample collection and other: DNA extraction and sequencing
Sterile plastic cups were provided to each participant to collect a fresh fecal sample in the morning. All samples were transported to the Institute of Clinical Medicine affiliated with the CPHZ in a transfer box containing ice packs, and samples were kept at −80°C for 1 h after collection. These samples have been transported to Major-Bio (Shanghai, China) for DNA extraction and sequencing.
The microbial DNA from 51 samples has been extracted utilizing the E.Z.N.A.® Soil DNA Kit (Omega Bio-Tek, Norcross, GA, United States), following the manufacturer's instructions. A final DNA concentration, as well as purity, was assessed using a NanoDrop 2000 UV-VIS spectrophotometer (Thermo Scientific, Wilmington, DE, United States), and the quality of DNA was determined through 1% agarose gel electrophoresis. For amplifying the bacterial 16S rRNA in the V3–V4 region, the polymerase chain reaction (PCR) has been performed using 338F (ACTCCTACGGGAGGCAGCAG) and 806R (ACTCCTACGGGAGGCAGCAG) primers with Trans Start Fast pfu DNA polymerase (Trans Gen, Beijing, China) in an ABI Gene Amp 9700 device (Applied Biosystems, CA, United States). For amplifying the ITS-1 fragment of fungi, ITS1F (CTTGGTCATTTAGAGGAAGTAA) and ITS2R (GCTGCGTTCTTCATCGATGC) primers were utilized. PCR products were excised from 2% agarose gels, further purified using an AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, United States), and quantified using QuantiFluor™-ST (Promega, Madison, WI, United States).
Sequencing and data analyses
Purified amplicons were pooled in equimolar amounts, following the standard protocol of Bio-Pharm Technology Co. Ltd. at Majorbio (Shanghai, China), by demultiplexing, quality-filtering [using Trimmomatic software (V0.36, http://www.usadellab.org/cms/?page=trimmomatic)], and merging [with Flash software (V 1.2.11, https://ccb.jhu.edu/software/FLASH/index.shtml)], and raw FastQ files were further processed. The datasets presented in this study can be found in online repositories (Bio Project ID: PRJNA934740).
Abundances of operational taxonomic units (OTUs) were subjected to normalization according to the minimum sequence of the sample using standard sequence numbers and thereby clustered at 97% similarity using UPARSE (V 11, http://drive5.com/uparse). UCHIME was used to identify and remove chimeric sequences. Rare taxa were <10 OTUs (Wasserstrom et al., 2017). A classification analysis has been conducted utilizing the 16S rRNA SILVA database (SSU132) and a fungal database (Unite 8.0) with a 70% confidence threshold.
The data analyses were conducted utilizing an open online platform of the Majorbio cloud platform (https://cloud.majorbio.com/) (Ren et al., 2022). QIIME (V1.9.1, http://qiime.org/install/index.html) has been applied for determining α- as well as β-diversities and also for both principal component analysis (PCA) and principal coordinate analysis (PCoA). Bar diagrams of the microbial community were used to show the community structure composed of different groups at various taxonomic levels (Li et al., 2018). To search for statistically different biomarkers of TLEA, linear discriminant analysis (LDA) and effect size measurements (LEfSe) were performed using the LEfSe tool. The similarity test (ANOSIM) of the PRIMER 6 software package (PRIMER-E Ltd., Luton, UK) has been utilized to assess the fecal flora variations between the TLEA and TLEW groups. Identifying the distinct functional groups and relating corresponding abundances among bacterial as well as fungal communities have been evaluated utilizing PICRUSt 2 software (V2.2.0, https://github.com/picrust/picrust2/) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (bacteria) and an open annotation tool, FunGuild (V1, http://www.funguild.org/), Fungi Functional Guild (fungi). The composition of TLEA bacterial and fungi communities and their potential correlations with AIFs have been determined by redundancy analysis (RDA) using R software (V3.3.1, https://www.r-project.org/).
Statistical analyses
Bacteria, as well as fungi, have been evaluated at phylum, class, order, family, and genus levels, while the data series were analyzed using several different scales. Subgroup analyses were performed based on demographic and treatment regimens. The SPSS statistical package (V20.0) (IBM, Armonk, NY, United States) was applied to analyze the baseline data. The results of the measurement data were analyzed to identify differences between groups using t-tests and Wilcoxon rank-sum tests, whereas differences between groups in count data were analyzed using chi-square tests. Statistical significance is reported at the criterion of P < 0.05, and the P-value was adjusted by the false discovery rate (FDR) on the Majorbio cloud platform.
Results
Demographic characteristics of TLEA and TLEW patients
A total of 51 and 45 temporal lobe epilepsy patients provided stool samples for gut bacteria and gut fungi analyses, respectively. All participants were from a tropical Chinese population (Figure 1) and were of Han ethnicity. The demographic and epileptic disease features of the subjects are presented in Table 1 and Supplementary Table 2. The lifestyle and clinical data of all participants were recorded. TLEA and TLEW patients did not vary considerably in terms of age, gender, and body mass index (Table 1). However, TLEA patients showed significantly higher Ham-A (P < 0.05), SAS (P < 0.05), and GAD-7 (P < 0.05) scores. Analysis of the AIFs that may affect the occurrence of anxiety among patients with epilepsy revealed significant differences in the TLEA group regarding employment, working strength, distance to hospital, annual hospitalization frequency, treatment regimen, disease duration, and perception of seizure control.
Lower gut bacteria diversity in TLEA patients
16S sRNA gene sequencing has been applied for analyzing the bacteria fractions in the samples as well as for assessing the degree of bacterial flora dysbiosis associated with TLEA. Coverage in all samples was estimated at >99.9%. After removing rare OTUs, 11 phyla, 16 classes, 38 orders, 75 families, 208 genera, 404 species, and 598 OTUs have been retained to carry out further analyses.
The α-diversity of gut bacteria was first assessed. A decrease in microbial richness estimated by Chao and Ace indices was observed in the TLEA group, as compared with the TLEW group (Wilcoxon rank-sum test, P < 0.01; Figures 3A, B). The Simpson index showed that bacteria communities in the TLEA group were more abundant than those in the TLEA group (Figure 3C). However, according to the Shannon index, the microfloral diversity in the TLEW group was lower than in the TLEA group, which was, however, not statistically significant (P = 0.401; Figure 3D). Venn diagrams were produced to visualize unique and common taxa among the 51 samples (Figure 3E). There were 487 common OTUs in both groups, i.e., the two groups had a high similarity. We found 28 unique OTUs in the TLEA group and 83 unique OTUs in the TLEW group, suggesting a difference in the distribution of the two groups. Our results indicated that bacterial α-diversity was lower in TLEA patients.
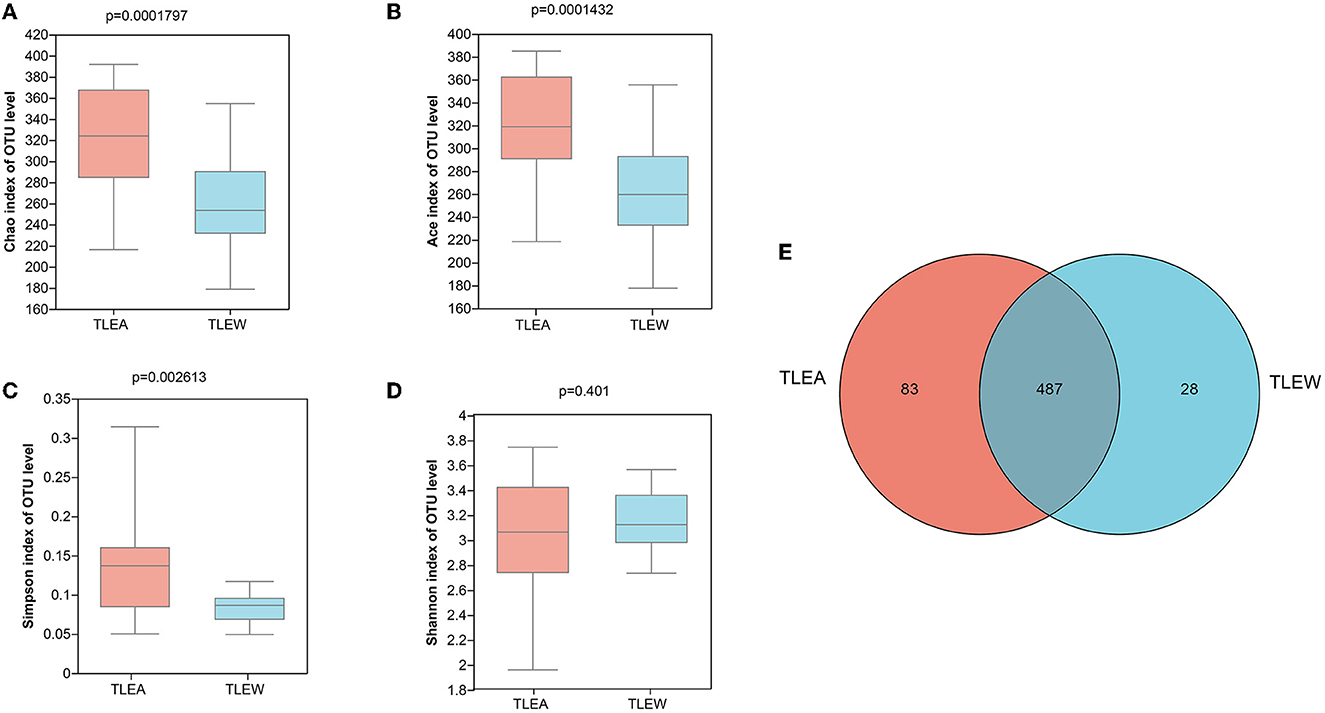
Figure 3. α-diversity of different groups of gut bacteria at the OTU level. (A–D) α-diversity of different groups of gut bacteria at the level of operational taxonomic units (OTUs) and index values represent species diversity. (A) Variations in Chao (A), Ace (B), Simpson (C), and Shannon (D) diversity indices between TLEA and TLEW. (E) Comparing the type and number of OTUs. Rare microbial OTUs were eliminated from the Venn diagram, and no subsampling was done. Wilcoxon rank-sum test. TLEA, temporal lobe epilepsy with anxiety disorders; TLEW, temporal lobe epilepsy but without anxiety disorder; OTU, operational taxonomic unit.
TLEA altered bacterial microbiome structures
The bacterial β-diversity has been examined utilizing PCA and PCoA, showing considerable variations across sample clusters as per the OTU level (Figures 4A, B). Samples clustered in the TLEA group, whereas inter-sample distances were larger in the TLEW group. The TLEA group has shown considerably varied bacterial β-diversity in comparison to the TLEW group.
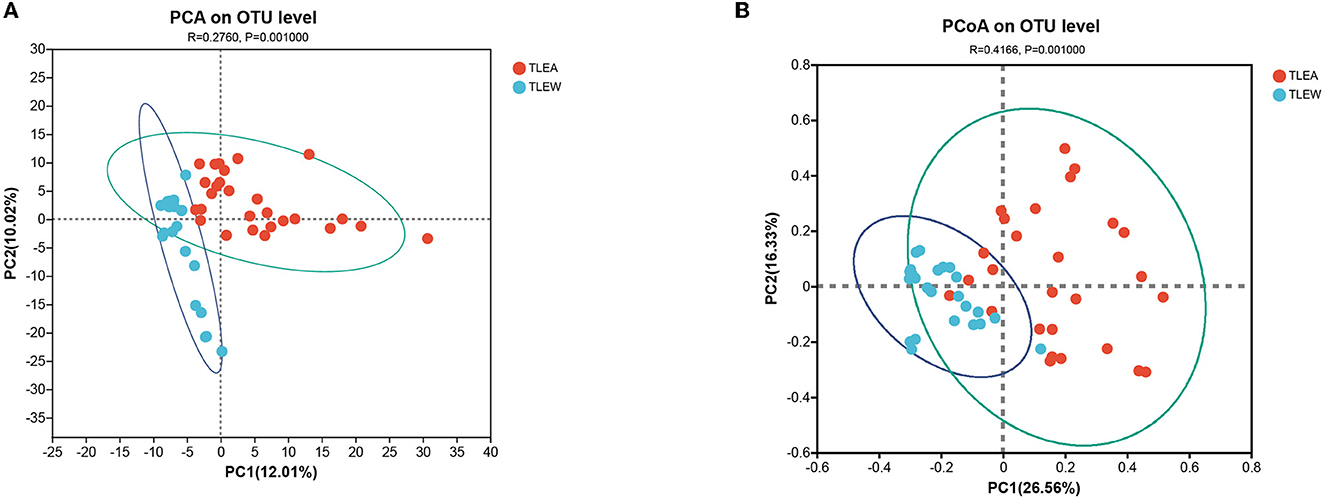
Figure 4. β-diversity of different groups of gut bacteria at the OTU level. Biodiversity of bacterial microbiota. (A) PCA of microbiota in TLEA and TLEW patients. (B) PCoA of microbiota in TLEA and TLEW patients. TLEA, temporal lobe epilepsy with anxiety disorders; TLEW, temporal lobe epilepsy but without anxiety disorder; OTU, operational taxonomic unit; PCA, principal component analysis; PCoA, principal coordinate analysis.
Bacterial abundance in TLEA
Operational taxonomic units (OTUs) were compared with the database, and bar graphs were created at the level of phylum, class, order, family, and genus. The results of the distribution of the bacterial community in both groups are shown in Figures 5A–E. In both groups, Firmicutes, Proteobacteria, Actinobacteriota, and Fusobacteriota were the predominant phyla (other phyla: <0.2%). Firmicutes in TLEA samples (67.10%) were significantly less abundant than in TLEW samples, whereas the abundances of Proteobacteria (18.08%), as well as Fusobacteriota (4.15%), were considerably elevated in TLEA samples (Figure 5A). At the class level, the TLEA group mainly showed Clostridia (52.44%), Gammaproteobacteria (18.08%), and Bacilli (13.09%). Gammaproteobacteria were significantly more abundant, and Clostridia were less abundant in the TLEA group compared with the TLEW group (Figure 5B). At the order level, the main bacteria in the TLEA group were found as Lachnospirales (42.17%), Enterobacterales (18.06%), Oscillospirales (6.38%), Lactobacillales (9.08%), Bifidobacteriales (5.55%), Erysipelotrichales (4.00%), and Coriobacteriales (3.88%). Lachnospirales and Oscillospirales were significantly less abundant in the TLEA than in the TLEW group, while Enterobacterales and Lactobacillales abundances were higher in the TLEA than in the TLEW group (Figure 5C). At the family level, the predominant bacteria in the TLEA samples were Lachnospiraceae (42.17%), Enterobacteriaceae (18.07%), Ruminococcaceae (4.92%), and Bifidobacteriaceae (5.55%). Lachnospiraceae and Ruminococcaceae were significantly less abundant in the TLEA group than in the other group. The abundances of Bifidobacteriaceae were significantly higher in the TLEA than in the TLEW group (Figure 5D).
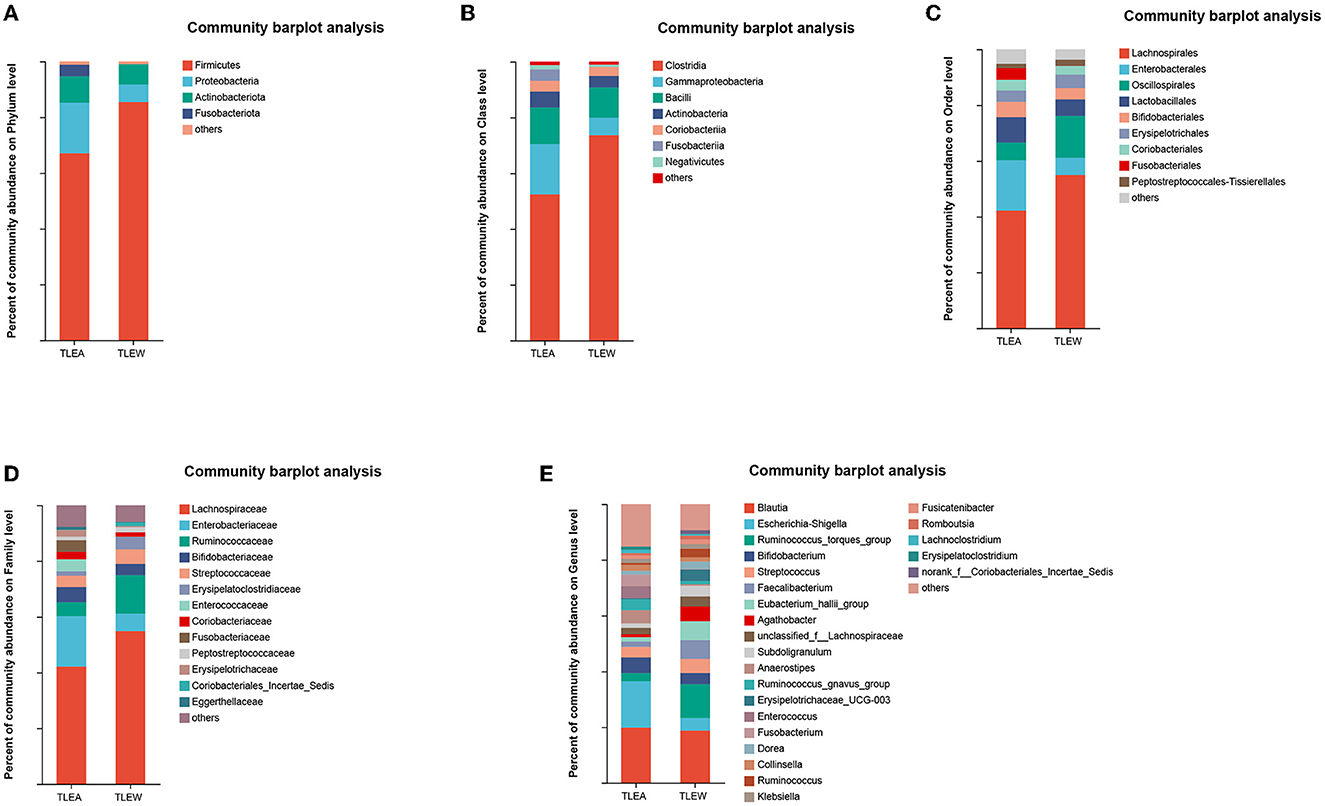
Figure 5. The abundance of gut bacteria in TLEA. The abundance of bacterial communities in TLEA patients at the phylum (A), class (B), order (C), family (D), and genus (E) levels. TLEA, temporal lobe epilepsy with anxiety disorders; TLEW, temporal lobe epilepsy but without anxiety disorders.
At the genus level, Escherichia–Shigella (12.14%) and Bifidobacterium (5.54%) were in considerable abundance in the TLEA group as compared with the TLEW group. Ruminococcus torques group (3.00%), Faecalibacterium (1.89%), Eubacterium hallii group (1.62%), and Agathobacter (1.01%) in the TLEA group were significantly less abundant than in the TLEW group (Figure 5E).
Biomarkers of gut bacteria in TLEA patients
The LEfSe has been utilized to determine variations in metagenomic biomarkers across the two groups. As shown in Figure 6, the biomarkers of TLEA were Proteobacteria and Fusobacteriota at the phylum level (Figures 6A, F, G); Gammaproteobacteria and Fusobacteriia at class level (Figures 6B, F, G); Enterobacterales and Fusobacteriales at the order level (Figures 6C, F, G); Enterobacteriaceae, Fusobacteriaceae Enterococcaceae at the family level (Figures 6D, F, G); Escherichia–Shigella, Fusobacterium, Anaerostipe and Enterococcus at the genus level (Figures 6E–G). In contrast, Clostridia at class level (Figures 6B, F, G); Lachnospirales and Oscillospirales at the order level (Figures 6C, F, G); Firmicutes and Lachnospiraceae Ruminococcaceae at the family level (Figures 6D, F, G); Eubacterium hallii group, Faecalibacterium, Agathobacter, Erysipelotrichaceae UCG-003, and Ruminococcus torques group at the genus level (Figures 6E–G), showed higher abundances in the TLEW group.
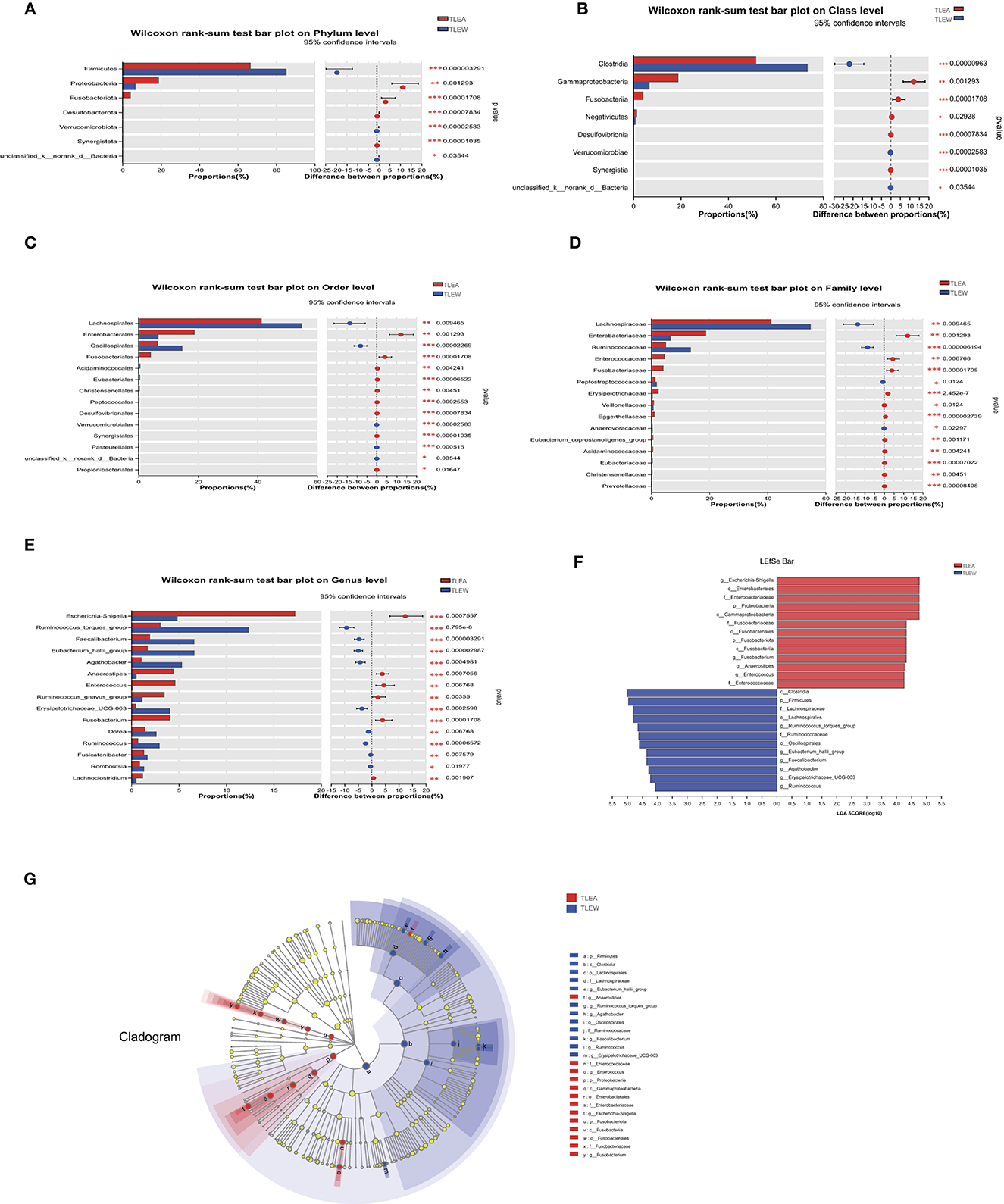
Figure 6. Different levels of bacterial biomarkers in TLEA patients. The abundance of bacterial communities at the phylum (A), class (B), order (C), family (D), and genus (E) levels for TLEA samples. (F) Macrogenomic biomarkers were analyzed utilizing LEfSe. Significant taxonomic variations in intestinal bacterial communities were identified between the three groups (LDA > 4, non-parametric factor Kruskal–Wallis rank-sum test, P < 0.05). (G) Branching map of bacterial compartments. Nodes of different colors indicate enriched microbiota and show significant differences between the groups. The size of the circles in the branching map is proportional to fungal abundance. From inside to outside, the circles represent the phylum, order, phylum, and family of fungi, respectively. *P < 0.05; **P < 0.01; and ***P < 0.001. LDA, linear discriminant analysis; LEfSe, LDA effect size; TLEA, temporal lobe epilepsy with anxiety disorders; TLEW, temporal lobe epilepsy but without anxiety disorders.
Furthermore, we found that compared with male patients, female patients with TLEA had lower bacterial abundances in unclassified_c__Clostridia (order), Eubacterium_coprostanoligenes_group (family) norank_f__Eubacterium_coprostanoligenes_group (genus), Lachnospiraceae_FCS020_group (genus), and Marvinbryantia (genus) but higher in Bacteroidaceae (family), Bacteroides (genus), Bacteroides_thetaiotaomicron (species), uncultured_bacterium_g__Sellimonas (species), Bacteroides_uniformis (species), Bacteroides_dorei (species), and Bacteroides_caccae (species) (Supplementary Figures 1A–D). Moreover, the abundance of TLEA bacteria varied with the different treatment regimens, including Micrococcales (order), Micrococcaceae (family), Morganellaceae (family), Morganella (genus), uncultured_organism_g__norank_f__Ruminococcaceae (species), Streptococcus_agalactiae (species), Streptococcus_sobrinus (species), and Morganella_morganii (species) (Supplementary Figures 2A–D).
Diversity of fungal microbiota in TLEA patients
The Sobs, Ace, and Chao indices on an OTU level have shown that fungal α-diversity was reduced for the TLEA group, as compared with the TLEW group (P < 0.05), whereas the Simpson index of fungal α-diversity displayed a non-significant increase for the TLEA than in the TLEW group (Figures 7A–D). Based on the β-diversity index, the PoCA showed significant differences between the fungal microbiota of the TLEA and TLEW groups (Figure 7E). Similarity analysis revealed that the distances derived at the OTU level for each sample varied considerably (ANOSIM/Adonis, Figure 7F; P < 0.05).
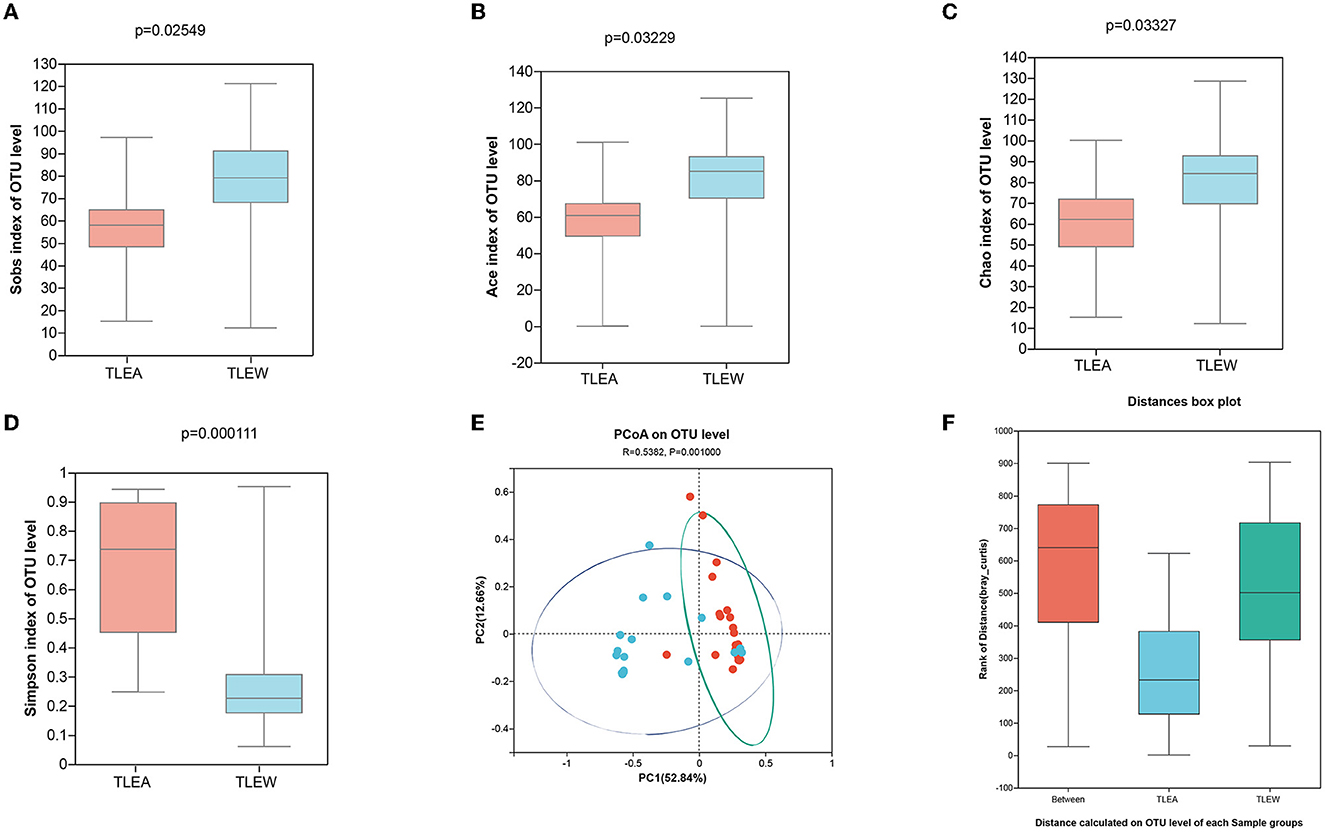
Figure 7. Diversity of gut fungi in TLEA patients. Sobs (A), Ace (B), Chao (C), and Simpson (D) differences in OTU levels of fungal flora in patients with TLEA and TLEW groups. Values represent the diversity of species. (E) Analysis of fungal β-diversity utilizing principal component analysis (PCA). There was no significant clustering among the groups of samples. (F) Analysis of similarity between fungi (ANOSIM/Adonis). The vertical coordinate indicates the distance rank calculated at the level of operational taxonomic units (OTUs) for each group. TLEA, temporal lobe epilepsy with anxiety disorders; TLEW, temporal lobe epilepsy but without anxiety disorder; OTU, operational taxonomic unit; ANOSIM, analysis of similarities; TLEA, temporal lobe epilepsy with anxiety disorders; TLEW, temporal lobe epilepsy but without anxiety disorders.
Composition and structure of the fungal microbiota in TLEA
Ascomycota, as well as Basidiomycota, were the most prevalent phyla found for the gut fungal microbiota. At the phylum level, the proportion of Ascomycota (95.51%) was higher in the TLEA than in the TLEW group, while that of Basidiomycota (3.87%) was significantly lower in the TLEA group (Figure 8A). At the class level, lowered levels of Eurotiomycetes (1.70%) and Tremellomycetes (2.54%), as well as elevated levels of Saccharomycetes (93.50%), have been found for the TLEA group (Figure 8B). At the order level, the TLEA group showed lower abundances of Eurotiales and Trichosporonales and higher abundances of Saccharomycetales (Figure 8C). At the family level, the abundances of Aspergillaceae, Saccharomycetaceae, Trichosporonaceae, Agaricostilbaceae, and Metschnikowiaceae were lower, and those of Saccharomycetales fam. incertae sedis were higher in the TLEA group (Figure 8D). At the genus level, Saccharomyces, Aspergillus, Cutaneotrichosporon, Sterigmatomyces, and Penicillium were less abundant in the TLEA group (Figure 8E).
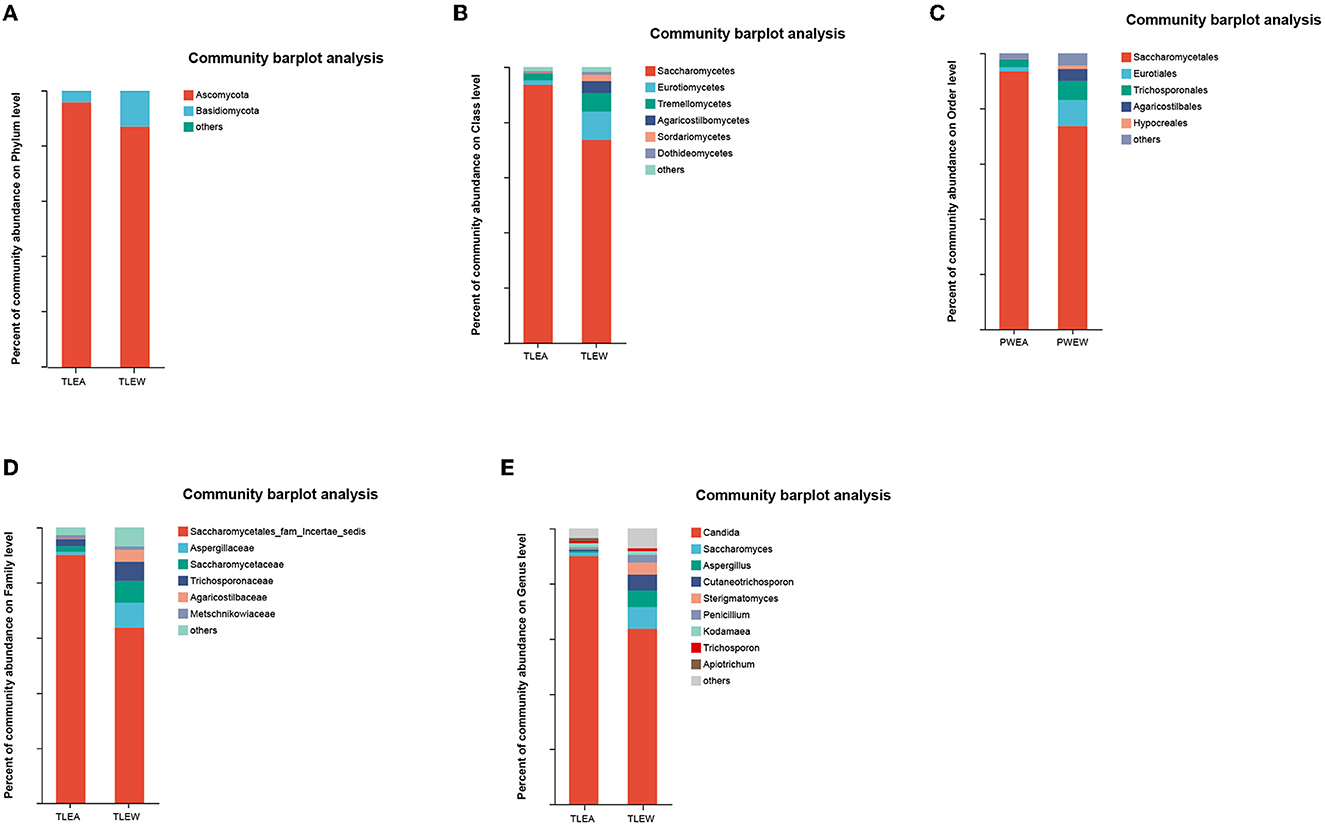
Figure 8. The abundance of fungal communities in TLEA patients at the phylum (A), class (B), order (C), family (D), and genus (E) levels. TLEA, temporal lobe epilepsy with anxiety disorders; TLEW, temporal lobe epilepsy but without anxiety disorders.
The Wilcoxon rank-sum tests also indicated considerable variations in fungal microbiota abundance among phylum, class, order, family, and genus, including Ascomycota (phylum), Saccharomycetes (class), Saccharomycetales (order), Saccharomycetales fam. incertae sedis (family), and Candida (genus) (Figures 9A–E).
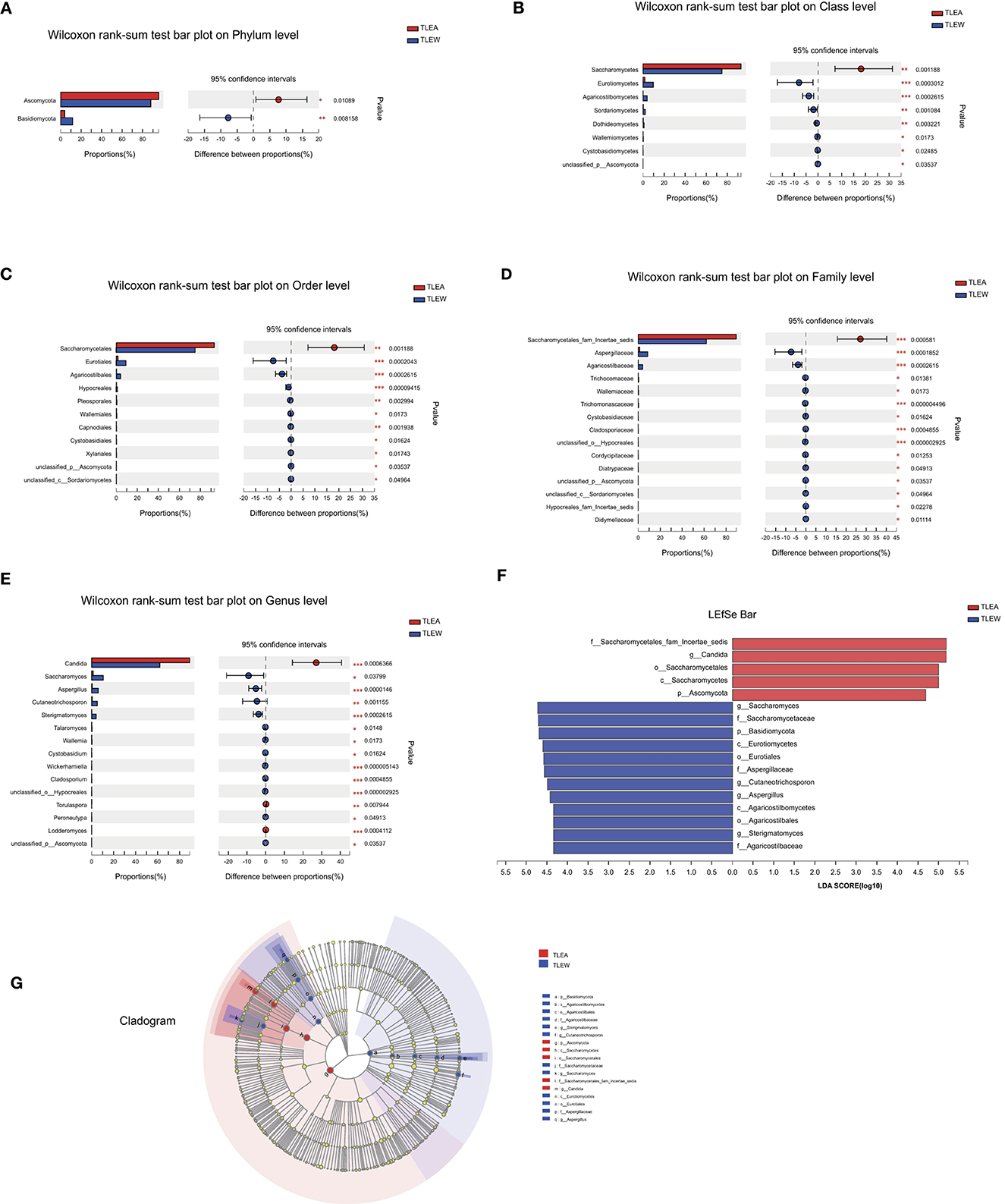
Figure 9. Different levels of fungal biomarkers in TLEA patients. The abundance of fungal communities at the phylum (A), class (B), order (C), family (D), and genus (E) levels for TLEA samples. (F, G) LEfSe bar plot of fungal communities. Linear discriminant analysis (LDA) has been performed to assess the influence of each component's abundance on differential effects. *P < 0.05; **P < 0.01; and ***P < 0.001. LDA, Linear discriminant analysis; LEfSe, LDA effect size; TLEA, temporal lobe epilepsy with anxiety disorders; TLEW, temporal lobe epilepsy but without anxiety disorders.
Furthermore, we found that, compared with male patients with TLEA, female patients had lower bacterial abundances in Malasseziomycetes (class), Agaricomycetes (class), Malasseziales (order), Filobasidiales (order), Malasseziaceae (family), and Malassezia (genus) (Supplementary Figures 3A–D). Moreover, the abundances of TLEA bacteria varied with different treatment regimens, including Sordariales (order), Chaetomiaceae (family), Morganellaceae (family), Pichia (genus), Choanephora (genus), Candida_orthopsilosis (species), Penicillium_allii (species), Cladosporium_delicatulum (species), and Choanephora_cucurbitarum (species) (Supplementary Figures 4A–D).
LEfSe plots, along with cladograms, illustrated the fungal microbiota to be the most pronounced variations in terms of relative abundance (Figures 9F, G). In the TLEA group, Saccharomycetales fam. incertae sedis (family), Saccharomycetales (order), Saccharomycetes (class), and Ascomycota (phylum) were significantly more abundant than in the TLEW group (LDA = 4.0).
Predicted microbial functions altered in TLEA patients
TLEA gut bacteria have been characterized utilizing PICRUSt's predictions of functional compositions from 16S rRNA sequencing data. Several KEGG (module level) categories such as uridine monophosphate biosynthesis (M00051), glycogen biosynthesis (M00854), glycogen degradation (M00855), C5 isoprenoid biosynthesis, non-mevalonate pathway (M0096), trehalose biosynthesis (M00565), cobalamin biosynthesis (M00122), tryptophan biosynthesis (M0023), and histidine biosynthesis (M00026) were lower in the TLEA group (Figure 10A). Ascorbate degradation (M00550), reductive pentose phosphate cycle (Calvin cycle) (M00165), reductive pentose phosphate cycle (M00167), KDO2-lipid A biosynthesis, Raetz pathway (M00866), citrate cycle, second carbon oxidation (M0011), KDO2-lipid A biosynthesis, LpxL-LpxM type (M00060), menaquinone biosynthesis (M00116), formaldehyde assimilation, xylulose monophosphate pathway (M00344), pyrimidine deoxyribonucleotide biosynthesis (M0053), and heme biosynthesis (M00121) were enriched in the TLEA group.
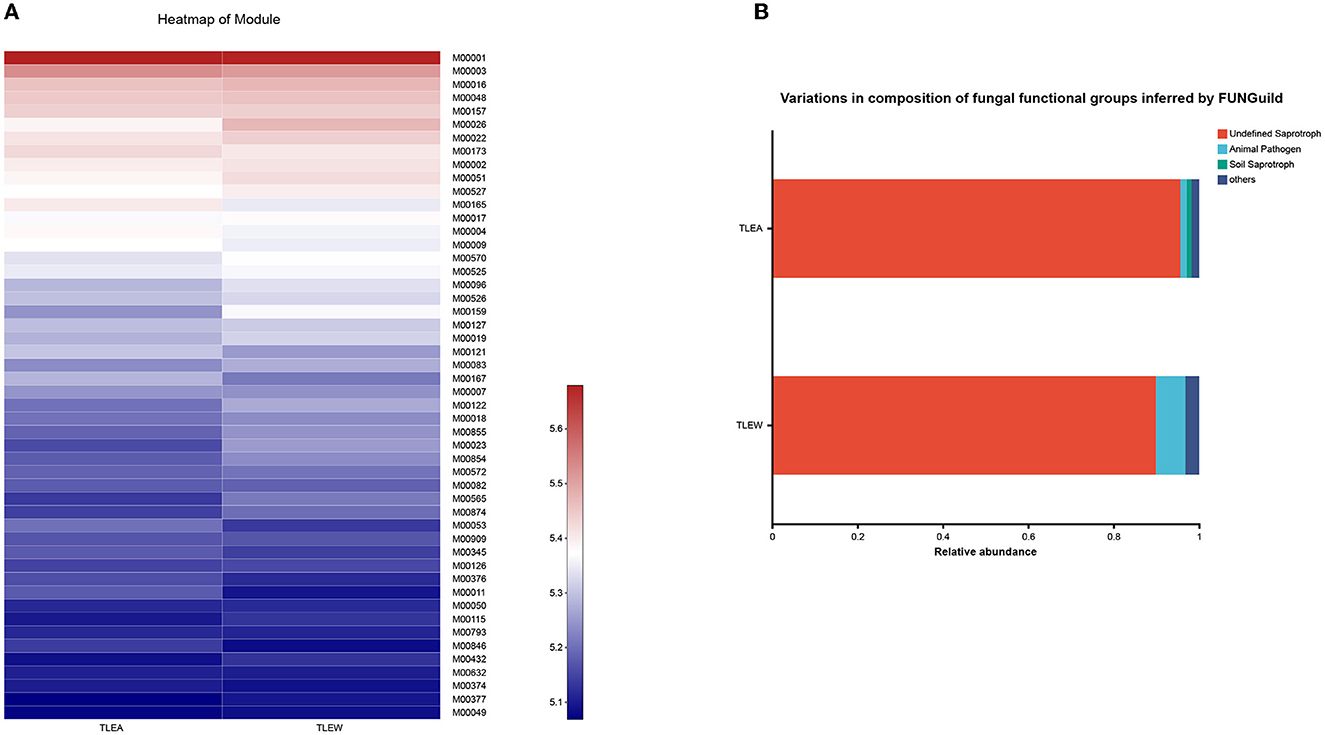
Figure 10. Predicted microbial functions altered in TLEA patients. (A) A heatmap of the module based on PICRUSt 1. Differences among TLEA and TLEW. (B) Variations in the composition of fungal functional groups inferred by FUNGuild. Differences in fungal functional groups among TLEA and TLEW patients. TLEA, temporal lobe epilepsy with anxiety disorders; TLEW, temporal lobe epilepsy but without anxiety disorders.
The FunGuild database was used to assign fungal OTUs to specific functional groups, and identified guilds are shown in Figure 10B. Undefined saprotroph accounted for ~90% of all detected fungal OTUs. TLEA had a higher relative abundance of saprotrophs of uncertain taxonomic classification as compared with TLEW, whereas TLEW possessed a higher relative abundance of animal pathogens.
Taken together, these results point to the possibility that changes in the composition of the host's microbial community may disrupt the host's physiological processes.
Relationship between microbial community structures and TLEA AIFs
Anxiety impact factors (AIFs) are involved in the progression of TLEA and may affect the structure of TLEA intestinal bacterial and fungal microbial communities to varying degrees (Table 1 and Supplementary Table 1). Therefore, an attempt is made to investigate whether AIF affects microbial community structures. AIF with statistically significant variations was included in the RDA. RDA of bacteria was conducted using six factors, i.e., employment, working strength, distance to distance, annual hospitalization frequency, treatment regimen, and perception of seizure control (Figure 11A). Fungal RDA identified three risk factors, namely, working strength, annual hospitalization frequency, and treatment regimen (Figure 11B). Employment (P = 0.009) and perception of seizure control (P = 0.019) significantly affected TLEA bacterial community structure, whereas annual hospitalization frequency (P = 0.014) significantly affected TLEA fungal community structures.
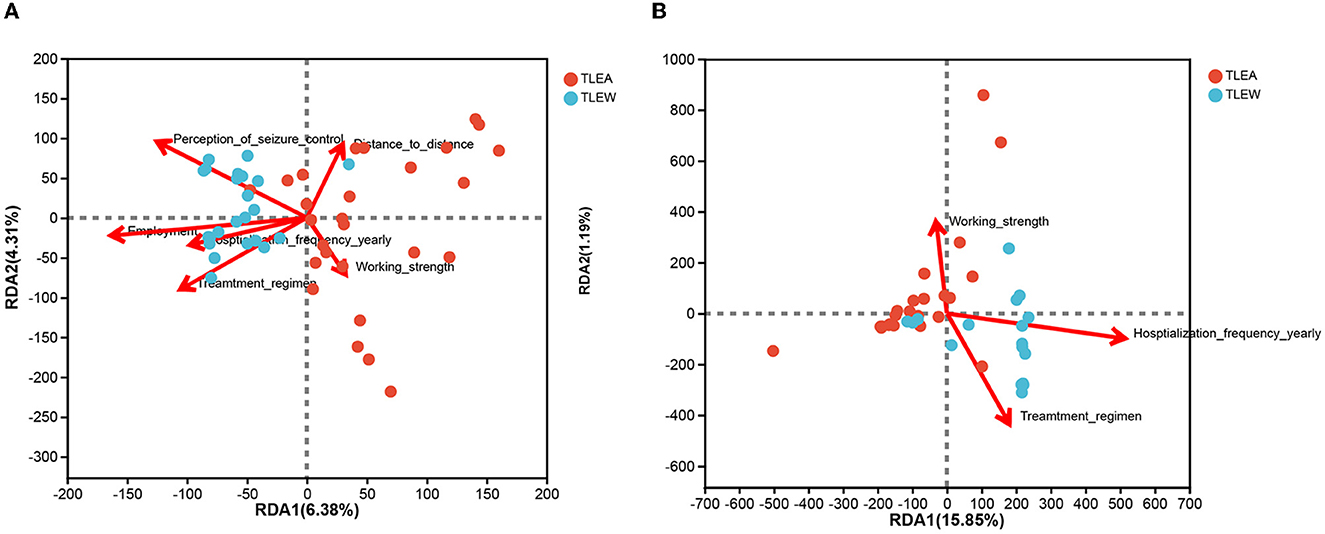
Figure 11. RDA of anxiety impact factors (arrows). Bacterial and fungal flora are shown in (A, B), respectively. The values of axis 1 and axis 2 are the percentages explained by the corresponding factor. RDA, redundancy analysis; TLEA, temporal lobe epilepsy with anxiety disorders; TLEW, temporal lobe epilepsy but without anxiety disorders.
Discussion
Although research has linked altered gut microbiota to disease etiology in epileptic patients, the link between gut microbiology and TLEA remains obscure. Intestinal microbes and fungi were compared between TLEA and TLEW patients for the first time in this research. The results from this study illustrate significant variations in gut flora composition and functional pathways between TLEA and TLEW patients.
The gut microbiota can influence the central as well as enteric nervous systems through a variety of mechanisms, such as the production and expression of neurotransmitters and neurotrophic factors, regulation of intestinal sensory afferents, metabolite production, mucosal immune regulation, and maintenance of the integrity of the intestinal barrier and tight junctions (Młynarska et al., 2022). TLEA patients endure enormous stress. The stress decreases the specific proteins of intestinal epithelial tight junction, such as Recombinant Claudin 1 (Da Silva et al., 2014), and disrupts the integrity of the intestinal epithelium, modifying the intestinal motility, secretions, and mucin production (Wong et al., 2016; Rutsch et al., 2020). On the other hand, we (Wei et al., 2020) and previous studies (Keita and Söderholm, 2018; Serek and Oleksy-Wawrzyniak, 2021) also found that disturbance of intestinal flora would increase the permeability of intestinal mucosal membranes, leading to the brain inflammation. One of the most relevant is the translocation of outer membrane vesicles and allergens produced by gram-negative bacteria into the bloodstream, which will trigger brain inflammation signaling (Wong et al., 2016; Rutsch et al., 2020; Wei et al., 2020). Thus, we conclude that unbalanced fungal diversity and abundance may contribute to the intestinal inflammatory process or increase the development of TLEA.
Considerable alterations in gut bacterial composition were also identified in TLEA patients. This was corroborated by α-diversity analysis that revealed a reduced gut bacteria diversity in the TLEA group. Moreover, the β-diversity analyses indicated that the TLEA group had clusters of gut bacteria that differed from those of the TLEW group, which clustered together. At the phylum level, there was a significant drop in Firmicutes and an increase in Proteobacteria and Fusobacteriota in TLEA patients. Anxiety disorder patients have lower microbial richness than those without anxiety (Jiang et al., 2018). In a cross-sectional study, Jiang et al. reported a significant increase in the phylum Firmicutes (especially, Lachnospira and the anti-inflammatory symbiotic Faecalibacterium) and a significant decrease in Fusobacteria (Jiang et al., 2018). This is consistent with the finding for patients with TLEA. In particular, Fusobacteriaceae, an invasive and pro-inflammatory pathogen, increased from the family level to the genus level of intestinal microorganisms in TLEA. Previous microbiome investigations in individuals with epilepsy and Alzheimer's disease have also shown similar alterations and reductions in bacterial diversity (Safak et al., 2020; Lim et al., 2022).
At the family level, Lachnospiraceae and Ruminococcaceae were less abundant in the TLEA group. These symbiotic bacteria are associated with intestinal health. Lachnospiraceae are involved in human energy supply and immunomodulatory functions (Arpaia et al., 2013; Pascale et al., 2018) while maintaining health by producing short-chain fatty acids, converting primary bile acids into secondary bile acids, and averting colonization by intestinal pathogens (Byndloss et al., 2017; Lan et al., 2022). It has been hypothesized that the gut microbiota of TLEA patients may be more vulnerable than that of TLEW patients when concomitant anxiety occurs, which may be due to their prolonged medication use and lack of timely adjustment of medication regimens, among other reasons. However, this conclusion is limited by the sample size of this study and requires confirmation. In addition, Ruminococcus proliferate during symptomatic episodes in patients with Crohn's disease and produce inflammatory polysaccharides (Yan et al., 2022). A reduction of Ruminococcus gnavus was found in both anxious (Jiang et al., 2018) and TLEA patients. It is hypothesized that lower levels of Lachnospiraceae and Ruminococcaceae may contribute to the disruption of intestinal functioning and increased intestinal mucosal inflammation in TLEA patients.
At the genus level, we and another study of 36 anxiety disorder patients (Chen et al., 2019) demonstrated a greater enrichment in Escherichia–Shigella in patients with anxiety. It is noteworthy that Chen also observed a positive association between Escherichia–Shigella and the severity of anxiety. Other groups such as Enterobacteriaceae (Chen et al., 2019), Enterobacteriales (Chen et al., 2019), and Proteobacteria (Dong et al., 2021) not only increased in anxiety disorder patients but also TLEA patients, highlighting the relationship between the presence of pathogens in the gut and anxiety again. Nevertheless, the observation is inconsistent with data from children with autism and epilepsy (Dan et al., 2020; Safak et al., 2020). Escherichia-Shigella include bacteria with pro-inflammatory activity (Reinoso Webb et al., 2016), which causes intestinal inflammation by bacterial structural components (i.e., microbial-associated molecular patterns) such as lipopolysaccharides and bacterial metabolism (Ceccarani et al., 2020). With the exception of Escherichia-Shigella, TLEA patients showed higher abundances of Gram-negative bacteria such as Enterobacteriaceae, compared with TLEW patients. Lipopolysaccharides are a major cell wall component in Gram-negative bacteria that assists in binding to Toll-like receptor 4 (TLR4) while activating MyD88-dependent signaling pathways in the lamina propria, leading to secretion of pro-inflammatory mediators, which elicit and sustain local inflammation and promote seizures (Ceccarani et al., 2021). In addition, activation of the TLR4 signaling pathway promotes neuroinflammation (Paudel et al., 2020). GABA type A receptor α1 binding and negative regulation of TLR4 leads to epilepsy–migraine comorbidity, and TLR4 is a key intermediate in epilepsy–migraine comorbidity (Lin et al., 2022). These studies provide further evidence that epilepsy and anxiety synergistically contribute to gut dysfunction.
In short, Faecalibacterium, Fusobacteria, Lachnospiraceae, Ruminococcaceae, and Escherichia–Shigella were not only different in anxious and healthy people but also different in TLEW and TLEA. We infer that the bacteria mentioned are also different in TLEA and healthy individuals.
Interestingly, we also found a reduced abundance of probiotics such as the Ruminococcus torques group, Faecalibacterium, Eubacterium hallii group, and Agathobacter in TLEA patients. These probiotics are reduced in inflammation-associated microbiota (Bai et al., 2022; Bonnechère et al., 2022). This suggests that the TLEA intestinal microenvironment is detrimental to the growth of bacterial strains that exert anti-inflammatory effects, which indirectly promotes the development of anxiety disorders.
The biodiversity of fungal microbiota in TLEA patients is low. The fungal microbiota is often considered a relatively small part of the gut microbiota, representing ~0.1%. As fungal taxa are rare in existing genomic databases, the role of the fungal microbiota in the human gut remains a mystery (Wang et al., 2022). An aberrant growth of several taxa in the fungal microbiome that is linked to opportunistic infections and inflammation has been observed. Consistent with the altered bacterial diversity reported in the TLEA group, we found that fungal α-diversity was lower in the TLEA group. However, significant biodiversity indices are insufficient in TLEA. The composition differences of the fungal flora in the TLEA group were fewer than that of the bacterial flora, and the individual differences were greater.
Although there was little change in fungal abundance in TLEA compared with TLEW patients, a trend toward decreased fungal diversity occurred in the TLEA group. Thus, there may be an association between bacteria and fungi in the development of epileptic disorders. The change in fungal abundances was not significant compared with that of bacteria, probably because of the insufficient sample size, the small proportion of fungi, and the lack of comprehensive fungal databases. More research with larger sample sizes and sophisticated assay methods is required to identify the involvement of fungi and the link between bacteria and fungi in the onset and progression of TLEA.
We found an increased proportion of Ascomycota and Candida albicans in the TLEA group. Interestingly, Zheng et al. have also reported an elevated proportion of Candida albicans in fecal fungal microbiota in patients with intestinal diseases (Zeng et al., 2022). Candida albicans is thought to be an inducer of T helper 17 cells, which are involved in the immunity of the intestinal mucosal barrier (Zeng et al., 2022). Under pathological conditions, such as during inflammatory bowel disease, T helper 17 cells secrete pro-inflammatory cytokines that exacerbate intestinal inflammation (Zeng et al., 2022), suggesting that Candida albicans may promote anxiety in epilepsy.
The composition of the intestinal bacterial and fungal flora is highly influenced by gender, medication, geography, and food (Chowdhury and Fong, 2020; De and Dutta, 2022). Unfortunately, not all previous studies have examined the effects of medications on gut microbiota profiles. Only one study of eight female participants exclusively diagnosed with anxiety was performed in the Caucasian population (Mason et al., 2020). In Mason's study, neither α-diversity nor β-diversity was significantly associated with anxiety, contrary to us and other previous studies where anxiety was associated with a lower fecal bacterial α-diversity. Similarly, the abundance of Bacteroides was also inconsistent with previous studies.
The Han Chinese epilepsy population in tropical China recruited for this study had a regular diet and lifestyle and exhibited similar dietary habits and lifestyles. The biases of diet and geographic and ethnic factors are likely minimal. Furthermore, the effect of gender and treatment regimen on intestinal microbial composition between the groups was observed in the study. We investigated the effects of the treatment regimen on the profiles of intestinal microbiota. There was an increase in the abundance of Morganella (family), Morganella (genus), and Morganella morganii (species) in TLEA patients treated with polytherapy vs. monotherapy. Considered to be a significant opportunistic pathogen, Morganella morganii can cause various infections, such as septicemia, abscess, chorioamnionitis, cellulitis, and purple urine bag syndrome (Liu et al., 2016). Accumulated data have demonstrated that the virulence of evolution makes Morganella morganii a major pathogen (Liu et al., 2016). From these results, we can conclude that long-term polytherapy in epileptic patients will lead to the development of resistance to harmful bacteria and the disorder of intestinal flora. An increase in unusual opportunistic pathogens is a serious challenge in addressing clinical infections.
We also found that Bacteroides increased significantly in women. Specifically, the abundance of Bacteroides is responsible for the deconjugation of conjugated bile acids synthesized in the liver (Siddiqui et al., 2022). Bacteroides vulgatus was significantly higher in women with polycystic ovary syndrome compared with controls (Qi et al., 2019; Siddiqui et al., 2022). We inferred that the TLEA female patients had disordered intestinal flora structures and increased Bacteroides compensatively. Due to the bias in the limited sample size, the value is limited, and these conclusions should be analyzed with caution. Overall, TLEA has similarities with the microbiome found in anxious patients, suggesting that microbiome modulation may be a preventative and therapeutic tool for TLEA.
At the functional level, a unique microbial metabolic pathway profile was present in TLEA's gut, such as ascorbic acid synthesis. Vitamin C(ascorbic acid) is a well-known antioxidant that is said to be involved in treating anxiety in humans (Oliveira et al., 2015; Pratiwi et al., 2019). Vitamin C supplementation at 3,000 mg daily lowered subjective stress against acute psychological stressors (Brody et al., 2002), and a relief effect on anxious mood was observed after vitamin C administration in healthy individuals (Oliveira et al., 2015; Moritz et al., 2017). Further studies will be needed to monitor changes in neurotransmitters, neurohormones, and neurotrophins in TLEA patients to understand the underlying mechanisms by which vitamin C affects TLEA brain function. Uridine, a precursor to cytidine diphosphate-choline, is involved in phospholipid synthesis, being evaluated as a potential medication for bipolar depression (Agarwal et al., 2011). Notably, nearly half of the patients with bipolar disorder have anxiety disorders during their lifetime (Qs et al., 2021). Our study found reduced uridine monophosphate biosynthesis in the intestinal microbiota of TLEA patients. This suggests that uridine supplementation might also modulate the imbalance of intestinal flora of TLEA. Further investigations are required to clarify the effects of uridine on anxiety disorder.
As a potent immunoreactive factor, Lipid A can be recognized by animal cells and triggers defense-related responses, causing gram-negative sepsis and endotoxic shock (Opiyo et al., 2010). We observed the KDO2-lipid A biosynthesis enriched in the TLEA group, indicating an increased risk of intestinal inflammation in TLEA patients.
In addition, we also found that several metabolic pathways in TLEA patients' gut microbiota are directly or indirectly related to anxiety, including tryptophan biosynthesis (M0023) (Songtachalert et al., 2018; Evrensel et al., 2020), reductive pentose phosphate cycle (Calvin cycle) (Peng et al., 2022), Raetz pathway (Nguyen et al., 2020), and menaquinone biosynthesis (Johnston and Bulloch, 2020). Together, these findings enrich TLEA's brain–gut axis studies.
Moreover, work-related factors such as employment and working strength increased anxiety in people with epilepsy. This is because of the psychological burden that can be caused by long periods of unemployment and working strength. Patients frequently develop guilt and fear of unemployment due to the disease. To better interpret the pathophysiology of TLEA and design individualized methods to change the gut microecology in TLEA patients, it is important to have a better understanding of the regulatory activities of bacteria in the gut.
Limitations
This study has some limitations. First, owing to the short sample size, a subgroup analysis of TLEA anxiety levels was not conducted. Possible mechanisms for the effect of gut flora on epilepsy remain to be elucidated, which requires further characterization of the mechanisms of gut microbiota involvement in epileptogenesis using more extensive sample-size studies based on macrogenomics, proteomics, and metabolomics. Second, data specific to the gut inflammation and permeabilities of the patients with TLEA are also essential for future research. Gut inflammation and permeabilities of TLEA will need to be compared with TLEW, including whether specific taxa associated with the gut inflammation and permeabilities can influence the development of TLEA. In addition, the microbiota at the species level was not investigated due to the unknown functions of many species. Current techniques and databases are not sufficiently comprehensive to allow a complete understanding of the functions of gut microbes. Moreover, the role of selecting specific gut microbiota in the development and progression of TLEA requires further investigation. Probiotics have been utilized to regulate the composition of the gut microbiota in patients with a variety of diseases in recent years. Transplanting probiotics or enterobacteria other than specific bacteria or fungi into the gut of animal models of mice would be interesting to see if the disease progression is successfully slowed, and if new therapeutic approaches for TLEA are offered.
Finally, the lack of control groups in the current study (i.e., groups of healthy controls and patients with anxiety disorders but without epilepsy) represents the main limitation. Therefore, future studies should be planned to include the use of a group of healthy controls, for instance, patients' relatives could reduce some bias related to environmental stressors, lifestyle, and diet, which are likely to contribute to gut microbiota dysbiosis. Many of the physical and mental health conditions are correlated with the gut microbiota. Recruiting individuals with anxiety disorder without epilepsy will be crucial in the next phase of the investigation, which could help identify the influence of this psychiatric disorder on gut microbiota in this specific geographical location.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving human participants were reviewed and approved by the Clinical Trials Ethics Committee Central People's Hospital of Zhanjiang (approval number: PJI117-2022-027-013) approved the study protocols. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SW, JW, and YC conceived and designed the study. RZ, YM, DZ, and LH carried out the statistical analysis and interpretation of data. All authors contributed toward drafting and revising the manuscript and agree to be accountable for all aspects of the manuscript.
Funding
The study was funded by the Doctoral Cooperation Project of Central People's Hospital of Zhanjiang (No. 2021H03), the GuangDong Basic and Applied Basic Research Foundation (2021A151515010827), and the Project of Traditional Chinese Medicine Bureau of Guangdong Province (No. 20221444).
Acknowledgments
We would like to thank Kun Li for the excellent technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1165787/full#supplementary-material
Supplementary Figure 1. TLEA subgroup analysis. The abundance of bacterial community at order (A), family (B), genus (C), and species (D) levels in different gender. TLEA, temporal lobe epilepsy with anxiety disorders.
Supplementary Figure 2. TLEA subgroup analysis. The abundance of bacterial community order (A), family (B), genus (C), and species (D) levels in the different treatment regimens. TLEA, temporal lobe epilepsy with anxiety disorders.
Supplementary Figure 3. TLEA subgroup analysis: the abundance of gender fungal community at class (A), order (B), family (C), and genus (D) levels. TLEA, temporal lobe epilepsy with anxiety disorders.
Supplementary Figure 4. TLEA subgroup analysis: the abundance of treatment regimen fungal community at order (A), family (B), genus (C), and species (D) levels. TLEA, temporal lobe epilepsy with anxiety disorders.
Supplementary Table 1. Redundancy analysis (RDA) of anxiety impact factors.
Supplementary Table 2. Characteristics of 45 temporal lobe epilepsy patients*.
Supplementary Table 3. Redundancy analysis (RDA) of anxiety impact factors.
References
Agarwal, N., Sung, Y. H., Jensen, J. E., Dacunha, G., Harper, D., Olson, D., et al. (2011). Short-term administration of uridine increases brain membrane phospholipid precursors in healthy adults: a 31-phosphorus magnetic resonance spectroscopy study at 4T. Bipolar Disord. 12, 825–833. doi: 10.1111/j.1399-5618.2010.00884.x
Arpaia, N., Campbell, C., Fan, X., Dikiy, S., van der Veeken, J., deRoos, P., et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. doi: 10.1038/nature12726
Bai, Y., Ma, K., Li, J., Ren, Z., Zhang, J., and Shan, A. (2022). Lactobacillus rhamnosus GG ameliorates DON-induced intestinal damage depending on the enrichment of beneficial bacteria in weaned piglets. J. Anim. Sci. Biotechnol. 13, 90. doi: 10.1186/s40104-022-00737-9
Beghi, E. (2020). The epidemiology of epilepsy. Neuroepidemiology 54, 185–191. doi: 10.1159/000503831
Bonnechère, B., Amin, N., and van Duijn, C. (2022). What are the key gut microbiota involved in neurological diseases? A systematic review. Int. J. Mol. Sci. 23, 13665. doi: 10.3390/ijms232213665
Brody, S., Preut, R., Schommer, K., and Schürmeyer, T. H. (2002). A randomized controlled trial of high dose ascorbic acid for reduction of blood pressure, cortisol, and subjective responses to psychological stress. Psychopharmacology 159, 319–324. doi: 10.1007/s00213-001-0929-6
Buchin, A., de Frates, R., Nandi, A., Mann, R., Chong, P., Ng, L., et al. (2022). Multi-modal characterization and simulation of human epileptic circuitry. Cell Rep. 41, 111873. doi: 10.1016/j.celrep.2022.111873
Byndloss, M. X., Olsan, E. E., Rivera-Chávez, F., Tiffany, C. R., Cevallos, S. A., Lokken, K. L., et al. (2017). Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575. doi: 10.1126/science.aam9949
Ceccarani, C., Bassanini, G., Montanari, C., Casiraghi, M. C., Ottaviano, E., Morace, G., et al. (2020). Proteobacteria overgrowth and butyrate-producing taxa depletion in the gut microbiota of glycogen storage disease type 1 patients. Metabolites 10, 133. doi: 10.3390/metabo10040133
Ceccarani, C., Viganò, I., Ottaviano, E., Redaelli, M. G., Severgnini, M., Vignoli, A., et al. (2021). Is Gut microbiota a key player in epilepsy onset? A longitudinal study in drug-naive children. Front. Cell. Infect. Microbiol. 11, 749509. doi: 10.3389/fcimb.2021.749509
Chen, Y.-h., Bai, J., Wu, D., Yu, S.-f., Qiang, X.-l., Bai, H., et al. (2019). Association between fecal microbiota and generalized anxiety disorder: severity and early treatment response. J. Affect. Disord. 259, 56–66. doi: 10.1016/j.jad.2019.08.014
Chowdhury, S., and Fong, S. S. (2020). Computational modeling of the human microbiome. Microorganisms 8, 197. doi: 10.3390/microorganisms8020197
Christensen, J., Dreier, J. W., Sun, Y., Linehan, C., Tomson, T., Marson, A., et al. (2022). Estimates of epilepsy prevalence, psychiatric co-morbidity and cost. Seizure. 107, 162–171. doi: 10.1016/j.seizure.2022.06.010
Da Silva, S., Robbe-Masselot, C., Ait-Belgnaoui, A., Mancuso, A., Mercade-Loubière, M., Salvador-Cartier, C., et al. (2014). Stress disrupts intestinal mucus barrier in rats via mucin O-glycosylation shift: prevention by a probiotic treatment. Am J Physiol. Gastrointest. Liver Physiol. 307, G420–G429. doi: 10.1152/ajpgi.00290.2013
Dan, Z., Mao, X., Liu, Q., Guo, M., Zhuang, Y., Liu, Z., et al. (2020). Altered gut microbial profile is associated with abnormal metabolism activity of Autism Spectrum Disorder. Gut Microbes 11, 1246–1267. doi: 10.1080/19490976.2020.1747329
De, R., and Dutta, S. (2022). Role of the microbiome in the pathogenesis of COVID-19. Front. Cell. Infect. Microbiol. 12, 736397. doi: 10.3389/fcimb.2022.736397
Ding, M., Lang, Y., Shu, H., Shao, J., and Cui, L. (2021). Microbiota-gut-brain axis and epilepsy: a review on mechanisms and potential therapeutics. Front. Immunol. 12, 742449. doi: 10.3389/fimmu.2021.742449
Dong, L., Zheng, Q., Cheng, Y., Zhou, M., Wang, M., Xu, J., et al. (2022). Gut microbial characteristics of adult patients with epilepsy. Front. Neurosci. 16, 803538. doi: 10.3389/fnins.2022.803538
Dong, Z., Shen, X., Hao, Y., Li, J., Li, H., Xu, H., et al. (2021). Gut microbiome: a potential indicator for differential diagnosis of major depressive disorder and general anxiety disorder. Front. Psychiatry 12, 651536. doi: 10.3389/fpsyt.2021.651536
Evrensel, A., Nsalver, B. N., and Ceylan, M. E. (2020). Immune-kynurenine pathways and the gut microbiota-brain axis in anxiety disorders. Adv. Exp. Med. Biol. 1191, 155–167. doi: 10.1007/978-981-32-9705-0_10
Fusco, F., Perottoni, S., Giordano, C., Riva, A., Iannone, L. F., De Caro, C., et al. (2022). The microbiota-gut-brain axis and epilepsy from a multidisciplinary perspective: clinical evidence and technological solutions for improvement of in vitro preclinical models. Bioeng. Transl. Med. 7, e10296. doi: 10.1002/btm2.10296
Gong, X., Liu, X., Chen, C., Lin, J., Li, A., Guo, K., et al. (2020). Alteration of gut microbiota in patients with epilepsy and the potential index as a biomarker. Front. Microbiol. 11, 517797. doi: 10.3389/fmicb.2020.517797
Iannone, L. F., Gómez-Eguílaz, M., and De Caro, C. (2022). Gut microbiota manipulation as an epilepsy treatment. Neurobiol. Dis. 174, 105897. doi: 10.1016/j.nbd.2022.105897
Jiang, H.-y., Zhang, X., Yu, Z.-h., Zhang, Z., Deng, M., Zhao, J.-h., et al. (2018). Altered gut microbiota profile in patients with generalized anxiety disorder. J. Psychiatr. Res. 104, 130–136. doi: 10.1016/j.jpsychires.2018.07.007
Johnston, J. M., and Bulloch, E. M. (2020). Advances in menaquinone biosynthesis: sublocalisation and allosteric regulation. Curr. Opin. Struct. Biol. 65, 33–41. doi: 10.1016/j.sbi.2020.05.005
Keita, Å. V., and Söderholm, J. D. (2018). Mucosal permeability and mast cells as targets for functional gastrointestinal disorders. Curr. Opin. Pharmacol. 43, 66–71. doi: 10.1016/j.coph.2018.08.011
Lan, Y., Ning, K., Ma, Y., Zhao, J., Ci, C., Yang, X., et al. (2022). High-density lipoprotein cholesterol as a potential medium between depletion of lachnospiraceae genera and hypertension under a high-calorie diet. Microbiol. Spectrum 10, e0234922. doi: 10.1128/spectrum.02349-22
Li, Q., Longteng, Z., and Yongkang, L. (2018). Changes in microbial communities and quality attributes of white muscle and dark muscle from common carp (Cyprinus carpio) during chilled and freeze-chilled storage. Food Microbiol. 73, 237–244. doi: 10.1016/j.fm.2018.01.011
Lim, J.-M., Letchumanan, V., Tan, L.T.-H., Hong, K.-W., Wong, S.-H., Ab Mutalib, N.-S., et al. (2022). Ketogenic diet: a dietary intervention via gut microbiome modulation for the treatment of neurological and nutritional disorders (a narrative review). Nutrients 14, 3566. doi: 10.3390/nu14173566
Lin, Y., Ding, M., Gong, Q., and Xiao, Z. (2022). Downregulation of GABAARα1 aggravates comorbidity of epilepsy and migraine via the TLR4 signaling pathway. Brain Sci. 12, 1436. doi: 10.3390/brainsci12111436
Liu, H., Zhu, J., Hu, Q., and Rao, X. (2016). Morganella morganii, a non-negligent opportunistic pathogen. Int. J. Infect. Dis. 50, 10–17. doi: 10.1016/j.ijid.2016.07.006
Mason, B. L., Li, Q., Minhajuddin, A., Czysz, A. H., Coughlin, L. A., Hussain, S. K., et al. (2020). Reduced anti-inflammatory gut microbiota are associated with depression and anhedonia. J. Affect. Disord. 266, 394–401. doi: 10.1016/j.jad.2020.01.137
Mitrea, L., Nemeş, S.-A., Szabo, K., Teleky, B.-E., and Vodnar, D.-C. (2022). Guts imbalance imbalances the brain: a review of gut microbiota association with neurological and psychiatric disorders. Front. Medic. 9, 813204. doi: 10.3389/fmed.2022.813204
Młynarska, E., Gadzinowska, J., Tokarek, J., Forycka, J., Szuman, A., Franczyk, B., et al. (2022). The role of the microbiome-brain-gut axis in the pathogenesis of depressive disorder. Nutrients 14, 1921. doi: 10.3390/nu14091921
Moritz, B., Schwarzbold, M. L., Guarnieri, R., Diaz, A. P. S, Rodrigues, A. L., et al. (2017). Effects of ascorbic acid on anxiety state and affect in a non-clinical sample. Acta Neurobiol. Exp. 77, 362–372. doi: 10.21307/ane-2017-068
Munger Clary, H. M. (2022). Epilepsy-specific anxiety: a potential clue to the seizure onset zone. Epilepsy Curr. 22, 285–287. doi: 10.1177/15357597221109345
Nguyen, T. T., Kosciolek, T., Daly, R. E., Vázquez-Baeza, Y., and Jeste, D. V. (2020). Gut microbiome in Schizophrenia: altered functional pathways related to immune modulation and atherosclerotic risk. Brain Behav. Immunity 91, 245–256. doi: 10.1016/j.bbi.2020.10.003
Nutt, D. J., Tyacke, R. J., Spriggs, M., Jacoby, V., Borthwick, A. D., and Belelli, D. (2022). Functional alternatives to alcohol. Nutrients 14, 3761. doi: 10.3390/nu14183761
Oliveira, I., Souza, V., Motta, V., and Da-Silva, S. L. (2015). Effects of oral vitamin C supplementation on anxiety in students: a double-blind, randomized, placebo-controlled trial. Pak. J. Biol. Sci. 18, 11–18. doi: 10.3923/pjbs.2015.11.18
Opiyo, S. O., Pardy, R. L., Moriyama, H., and Moriyama, E. N. (2010). Evolution of the Kdo2-lipid A biosynthesis in bacteria. BMC Evol. Biol. 10, 362. doi: 10.1186/1471-2148-10-362
Pascale, A., Marchesi, N., Marelli, C., Coppola, A., Luzi, L., Govoni, S., et al. (2018). Microbiota and metabolic diseases. Endocrine 61, 357–371. doi: 10.1007/s12020-018-1605-5
Paudel, Y. N., Angelopoulou, E., Akyuz, E., Piperi, C., Othman, I., and Shaikh, M. F. (2020). Role of innate immune receptor TLR4 and its endogenous ligands in epileptogenesis. Pharmacol. Res. 160, 105172. doi: 10.1016/j.phrs.2020.105172
Peng, F., Zhang, Y.-H., Zhang, L., Yang, M., Chen, C., Yu, H., et al. (2022). Ketogenic diet attenuates post-cardiac arrest brain injury by upregulation of pentose phosphate pathway-mediated antioxidant defense in a mouse model of cardiac arrest. Nutrition 103–104, 111814. doi: 10.1016/j.nut.2022.111814
Pratiwi, A., Sukardi, S., Maliya, A., Sudiyanto, A., and Lestari, T. (2019). Effect of relaxation therapy and vitamin c supplementation on stress and CD4 levels of mentall illness patients. Biomed. Pharmacol. J. 11, 423–429. doi: 10.13005/bpj/1656
Qi, X., Yun, C., Sun, L., Xia, J., Wu, Q., Wang, Y., et al. (2019). Publisher correction: Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 25, 1459–1459. doi: 10.1038/s41591-019-0562-8
Qs, A., Sh, B., Mtc, D., Bpe, F., Sbc, D., and Olc, D. (2021). Properties of common anxiety scales among patients with bipolar disorder. J. Affect. Disord. 281, 972–979. doi: 10.1016/j.jad.2020.09.139
Reinoso Webb, C., Koboziev, I., Furr, K. L., and Grisham, M. B. (2016). Protective and pro-inflammatory roles of intestinal bacteria. Pathophysiology 23, 67–80. doi: 10.1016/j.pathophys.2016.02.002
Ren, Y., Yu, G., Shi, C., Liu, L., Guo, Q., Han, C., et al. (2022). Majorbio cloud: a one-stop, comprehensive bioinformatic platform for multiomics analyses. IMeta 1, e12. doi: 10.1002/imt2.12
Rutsch, A., Kantsj, J. B., and Ronchi, F. (2020). The gut-brain axis: how microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 11, 604179. doi: 10.3389/fimmu.2020.604179
Safak, B., Altunan, B., Topçu, B., and Eren Topkaya, A. (2020). The gut microbiome in epilepsy. Microb. Pathog. 139, 103853. doi: 10.1016/j.micpath.2019.103853
Seid, J., Mebrahtu, K., and Andualem, F. (2022). Prevalence and associated factors of anxiety disorder symptoms among people with epilepsy in Mekelle, Ethiopia, 2019: institutional-based cross-sectional study. Nursing open 9, 1731–1743. doi: 10.1002/nop2.1198
Serek, P., and Oleksy-Wawrzyniak, M. (2021). The effect of bacterial infections, probiotics and zonulin on intestinal barrier integrity. Int. J. Mol. Sci. 22, 11359. doi: 10.3390/ijms222111359
Siddiqui, R., Makhlouf, Z., Alharbi, A. M., Alfahemi, H., and Khan, N. A. (2022). The gut microbiome and female health. Biology 11, 1683. doi: 10.3390/biology11111683
Songtachalert, T., Roomruangwong, C., Carvalho, A. F., Bourin, M., and Maes, M. (2018). Anxiety disorders: sex differences in serotonin and tryptophan metabolism. Curr. Top. Med. Chem. 18, 1704–1715. doi: 10.2174/1568026618666181115093136
Tang, B., Fu, Y., Liu, B., and Yi, Q. (2022). Self-perceived burden and associated factors in Chinese adult epilepsy patients: a cross-sectional study. Front. Neurol. 13, 994664. doi: 10.3389/fneur.2022.994664
Wang, S., Huang, G., Wang, J., Tian, L., Zuo, X., Li, Y., et al. (2022). Altered gut microbiota in patients with peutz-jeghers syndrome. Front. Microbiol. 13, 881508. doi: 10.3389/fmicb.2022.881508
Wasserstrom, H., Ben-Ezra, V. E., Sherman, C., Unc, A., and Steinberger, Y. (2017). The influence of flint stones on a soil microbial community in the northern Negev Desert. AIMS Microbiol. 3, 580–595. doi: 10.3934/microbiol.2017.3.580
Wei, S., Peng, W., Mai, Y., Li, K., Wei, W., Hu, L., et al. (2020). Outer membrane vesicles enhance tau phosphorylation and contribute to cognitive impairment. J. Cell. Physiol. 235, 4843–4855. doi: 10.1002/jcp.29362
Wong, M. L., Inserra, A., Lewis, M., Mastronardi, C. A., Leong, L., Choo, J., et al. (2016). Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 21, 797. doi: 10.1038/mp.2016.46
Xu, Z., Tian, X., Wang, X., Chen, Y., Xu, Q., and Assenza, G. (2022). Editorial: the comorbid anxiety and depression disorder in patients with epilepsy: diagnosis, prevention and treatment. Front. Neurol. 13, 974462. doi: 10.3389/fneur.2022.974462
Yan, J., Wang, L., Gu, Y., Hou, H., Liu, T., Ding, Y., et al. (2022). Dietary patterns and gut microbiota changes in inflammatory bowel disease: current insights and future challenges. Nutrients 14, 4003. doi: 10.3390/nu14194003
Keywords: temporal lobe epilepsy patient, anxiety disorder, gut microbiota, bacteria, fungi
Citation: Wei S, Mai Y, Hu L, Zheng R, Zheng D, Chen W, Cai Y and Wang J (2023) Altered gut microbiota in temporal lobe epilepsy with anxiety disorders. Front. Microbiol. 14:1165787. doi: 10.3389/fmicb.2023.1165787
Received: 15 February 2023; Accepted: 12 April 2023;
Published: 22 May 2023.
Edited by:
Pasquale Striano, Giannina Gaslini Institute (IRCCS), ItalyReviewed by:
Greta Volpedo, The Ohio State University, United StatesEray Sahin, Acibadem University, Türkiye
Martina Fanella, Fabrizio Spaziani Hospital, Italy
Copyright © 2023 Wei, Mai, Hu, Zheng, Zheng, Chen, Cai and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Cai, caiyan@126.com; Junjun Wang, junjunwang@suda.edu.cn
 Shouchao Wei
Shouchao Wei Yingren Mai
Yingren Mai Li Hu4
Li Hu4