- 1College of Health Sciences, Noguchi Memorial Institute for Medical Research, University of Ghana, Accra, Ghana
- 2Ghana Health Service, Accra, Ghana
- 3The Institute of Medical Science, The University of Tokyo, Tokyo, Japan
- 4Graduate School of Medicine, Institute for Global Prominent Research, Chiba University, Chiba, Japan
- 5Joint Research Center for Human Retrovirus Infection, Kumamoto University, Kumamoto, Japan
- 6Department of Medicine, CU-UCSD Center for Mucosal Immunology, Allergy and Vaccines (cMAV), University of California San Diego, San Diego, CA, United States
Diarrheal disease remains a major global health problem particularly in children under 5 years and the emergence of antibiotic-resistant strains of causative pathogens could slow control efforts, particularly in settings where treatment options are limited. This surveillance study conducted in Ghana aimed to determine the prevalence and antimicrobial susceptibility profile of diarrhea-causing bacteria. This was a cross-sectional study carried out in five health facilities in the Ga West Municipality of Ghana between 2017 and 2021. Diarrheic stool samples from patients were collected and cultured on standard differential/selective media and isolates identified by standard biochemical tests, MALDI-TOF assay, and serological analysis. The antibiogram was determined using Kirby-Bauer disk diffusion and Microscan autoScan4 MIC panels which were used for extended-spectrum beta-lactamase (ESBL) detection. Bacteria were isolated from 97.5% (772/792) of stool samples, and 167 of the isolates were diarrheagenic and met our inclusion criteria for antimicrobial resistance (AMR) analysis. These included Escherichia coli (49.1%, 82/167), Salmonella species (23.9%, 40/167), Vibrio species (16.8%, 28/167), and Shigella species (10.2%, 17/167). Among 24 Vibrio species, we observed resistances to cefotaxime (21/24, 87.5%), ceftriaxone (20/24, 83.3%), and ciprofloxacin (6/24, 25%), including four multi-drug resistant isolates. All 13 Vibrio parahaemolyticus isolates were resistant to cefazolin. All 17 Shigella isolates were resistant to tetracycline with resistance to shigellosis drugs such as norfloxacin and ciprofloxacin. Salmonella isolates were highly susceptible to norfloxacin (40/40, 100%) and tetracycline (12/34, 35%). Two ESBL-producing E. coli were also identified with marked susceptibility to gentamicin (66/72, 91.7%) and amikacin (57/72, 79.2%) prescribed in the treatment of E. coli infections. This study showed the different bacteria implicated in diarrhea cases in Ghana and the need for differential diagnoses for better treatment outcomes. Escherichia coli, Shigella, Salmonella, and Vibrio have all been implicated in diarrhea cases in Ghana. The highest prevalence was E. coli and Salmonella with Shigella the least prevalent. Resistance to commonly used drugs found in these isolates may render bacteria infection treatment in the near future nearly impossible. Routine antimicrobial susceptibility testing, effective monitoring, and nationwide surveillance of AMR pathogens should be implemented to curb the increase of antimicrobial resistance in Ghana.
Introduction
Diarrhea disease, although preventable and highly treatable is the second leading cause of death among children under the age of five globally, killing about 525,000 children annually (World Health Organization, 2017). Diarrhea as defined by WHO is the excessive and frequent evacuation of watery stools usually indicating gastrointestinal disease or disorder of 3–7 days duration (World Health Organization, 2017). The etiology of diarrheal diseases mainly by a wide variety of viral, bacterial, and parasitic pathogens is variable depending on a range of conditions including but not limited to geographic and climate conditions, host factors, and socioeconomic situations (Hodges and Gill, 2010). Viral pathogens such as norovirus and rotavirus infection are known to be a leading cause of diarrhea among children under 2 years with the latter causing the highest fatality among children (Tate et al., 2016). Parasites such as Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum have also been recorded to cause morbid diarrhea episodes (Nkrumah and Nguah, 2011; Gilchrist et al., 2016; Yang et al., 2021).
Among the bacterial causes, Escherichia coli is the most common pathogen for childhood diarrhea in developing countries and an emerging antimicrobial-resistant entero-pathogen in developed countries (Zhou et al., 2018). Bacteria pathogens causing diarrhea-associated diseases have however been somewhat limited to Vibrio cholerae with some strains such as Ogawa capable of causing epidemics when not well managed (Danso et al., 2020). In Sub-Saharan Africa, diarrhea is known to cause high fatality among children in rural and low-income communities as compared to the Americas and Europe. This is mainly because of unsanitary conditions such as defecation into rivers and streams which double as drinking water for livestock and humans, lack of potable drinking water, and improper handling of household food can lead to increased diarrhea cases (Larbi et al., 2021; Takyi et al., 2021).
In Ghana, cholera outbreaks and epidemics were recorded in 1970, 2012, 2014, and 2015. The 2014–2015 epidemic affected all 10 regions of the country accounting for a total of 28,922 cases including 243 deaths (Government of Ghana, 2015; Noora et al., 2017) with observed clustering of isolates in some areas of southern Ghana (Danso et al., 2020). However, it has been established that diarrhea can be caused by different types of bacteria other than V. cholerae including Campylobacter spp., Salmonella spp., Shigella spp., and pathogenic Escherichia coli O15:H7 (although unresolved), and can be equally fatal when left untreated (William et al., 2020; Wang et al., 2021).
Infections with these bacteria are often easily treated with Oral Rehydration Solutions (ORS) and zinc as recommended by WHO; however, some patients require antimicrobial therapy in cases of bloody, severe, or persistent diarrhea (World Health Organization, 2005). With increasing reports of high antibiotic resistance among enterobacteria in Ghana, the treatment of diarrhea caused by bacteria will be a challenge (Kunadu et al., 2018; Obeng-Nkrumah et al., 2019). Reports of over 40% of non-bacterial diarrhea in children being treated with antibiotics, easy accessibility to antibiotics through unapproved means in Ghana, and wrongful prescription of drugs are of grave concern (Arhinful, 2009; Ahiabu et al., 2016). There are some studies implicating bacteria in diarrhea cases in Ghana (Akuffo et al., 2017; Mizutani et al., 2021), with a prevalence of rate of 3% and high resistance to commonly abused drugs such as tetracycline (Newman et al., 2011; Akuffo et al., 2017). This study aimed to isolate bacterial organisms associated with diarrhea in patients reporting to five health facilities in Ga West, Accra-Ghana, and determine the antimicrobial susceptibility profile of the isolates.
Materials and Methods
Ethical Considerations
This cross-sectional surveillance study conducted from August 2017 to May 2021 was approved by the Institutional Review Board (IRB) of Noguchi Memorial Institute for Medical Research (NMIMR; approval number: 096/16-1; dated May 3, 2017). It was exempted from additional approval by the Ghana Health Service Ethics Committee which considered the procedures as compliant with routine service for patients care and surveillance.
Study Area
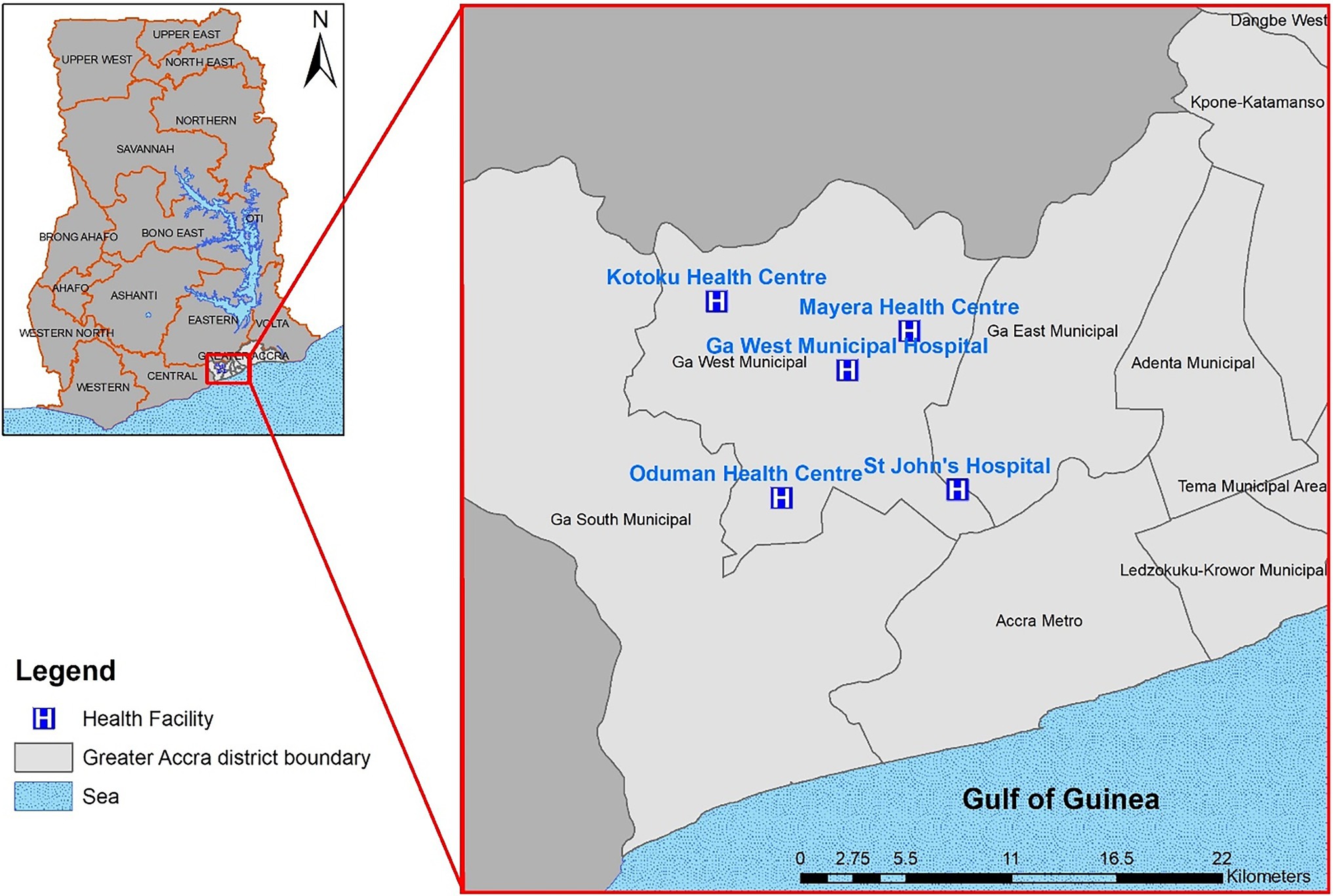
The study was conducted in the Ga West Municipality which lies between 5°35′North, 5°29′North, and longitude 0°10′West and 0°24′West. It occupies a total surface area of 299.578 square kilometers with about 412 communities. Five health facilities including a private facility within the municipality were chosen as surveillance sites (Figure 1). The main occupation or industry of inhabitants is trading and sales work of which females dominate. Public toilets and pit latrines are the main toilet facilities in the Municipality (Ghana Statistical Service, 2013).
Participants’ Data and Sample Collection
Individuals passing watery or loose stools three or more times within 24 h with/without mucus and or blood, vomiting, fever, and abdominal pain were eligible for this study.
Samples were taken between August 2017 and May 2021. Before stool sample collection into a container, patients’ clinical and demographic data including age, gender, height, weight, food, and water taken 3 days before the onset of diarrhea, temperature, number of stools per day, antibiotics taken, places visited among others were recorded using a structured questionnaire.
All collected stool samples were screened for V. cholerae using a rapid diagnostic test kit (Abbott Bioline, United States) at the various health facilities before transportation to the NMIMR laboratory for analysis. With the aid of a swab stick, a portion of the stool was inoculated into Cary Blair transport medium on-site and transported to NMIMR in a cold chain for culture and antimicrobial susceptibility testing.
Bacteria Isolation and Characterization
Each sample was inoculated on four media: MacConkey agar (ThermoScientific, United States) for isolation of non-lactose fermenters and E. coli, Thiosulphate Citrate Bile Salt agar, TCBS (Park Scientific, United Kingdom) for isolation of Vibrio spp., Alkaline Peptone Water, APW (Himedia, India), an enrichment medium for isolation of Vibrio spp. and Selenite Faecal Broth, SFB (Himedia, India) an enrichment medium for isolation of Salmonella and Shigella spp. and then incubated aerobically at 37°C for 18–24 h for isolation of enteric bacteria (Bolinches et al., 1988; Akuffo et al., 2017). A loopful of SFB and APW broth cultures were sub-cultured onto Salmonella Shigella Agar (SS) and TCBS respectively and incubated as previously. Suspected colonies of Salmonella spp., Shigella spp., E. coli, and Vibrio spp. were purified and identified by colonial characteristics, Gram staining reaction (Park Scientific, United Kingdom), standard biochemical methods-Analytical Profile Index (Biomerieux, United States), and Microscan Autoscan4 (Beckman Coulter, United States), and Matrix-Assisted Laser Desorption/Ionization-Time of Flight, MALDI-TOF (Bruker, United States). Shigella, E. coli, Salmonella, and Vibrio cholerae isolates were antigenically characterized using serological kits from Denka Seiken, Japan (Nath et al., 2013; Pun, 2014).
Antimicrobial Susceptibility Testing
Only isolates that were regarded as diarrheagenic strains were included in our analysis for antimicrobial susceptibility testing. Susceptibility to antimicrobial agents was done by Kirby Bauer disk diffusion technique per Clinical and Laboratory Standard Institute (CLSI) guidelines (CLSI, 2020). Isolates were tested against ceftriaxone (30 μg), norfloxacin (5 μg), cefotaxime (30 μg), amikacin (30 μg), gentamycin (30 μg), clotrimazole (50 μg), nalidixic acid (30 μg), tetracycline (30 μg), ceftazidime (30 μg), ciprofloxacin (5 μg), nitrofurantoin (300 μg), and cefazolin (30 μg; BD BBL, United Kingdom). Escherichia coli ATTC 25922 reference strain was used as a control. MicroScan autoScan4 MIC panels-Gram-Negative combo type 66 and Gram-Positive PB 44 (Beckman Coulter, United States) were also used to determine the susceptibility profiles of some isolates. Zones of Inhibition were measured on Mueller Hinton Agar plates in Kirby Bauer method and MIC used in MicroScan autoscan4. Resistance, intermediate susceptibility, and susceptibility were defined according to the CLSI breakpoints (CLSI, 2020).
ESBL Characterization/Multidrug Resistance
All extended spectrum-beta-lactamase (ESBLs) in this study were identified using a Gram-negative Combo type 66 panel on a MicroScan autoScan-4. This panel detects MIC of drugs and also virulent markers such as ESBL, and carbapenems. Multidrug resistance was defined as isolates being resistant to more than three antibiotic drug classes (Magiorakos et al., 2012).
Data Management and Analysis
Participants’ clinical, demographic, and laboratory data were entered into Epi Info version 2.0 Software (Centers for Disease Control and Prevention, CDC, Atlanta, GA, United States) and analyzed using Stata v14.2 (Stata Corporation, College Station, TX, United States). Descriptive statistics were carried out for both categorical and numerical variables. Cross-tabulations were further employed to explore the relationship between the different outcomes and selected variables using Chi-square and student t-test where applicable. Where appropriate, the Fisher’s exact or the chi-square tests were used to assess statistical significance. A p-value of less than 0.05 at a 95% confidence level was considered significant. The ArcMap tool employed in ArcGIS (Economic and Social Research Institute, version 10.1; ESRI, 2010) was used for constructing maps.
Results
Demographic and Clinical Characterization of Cases
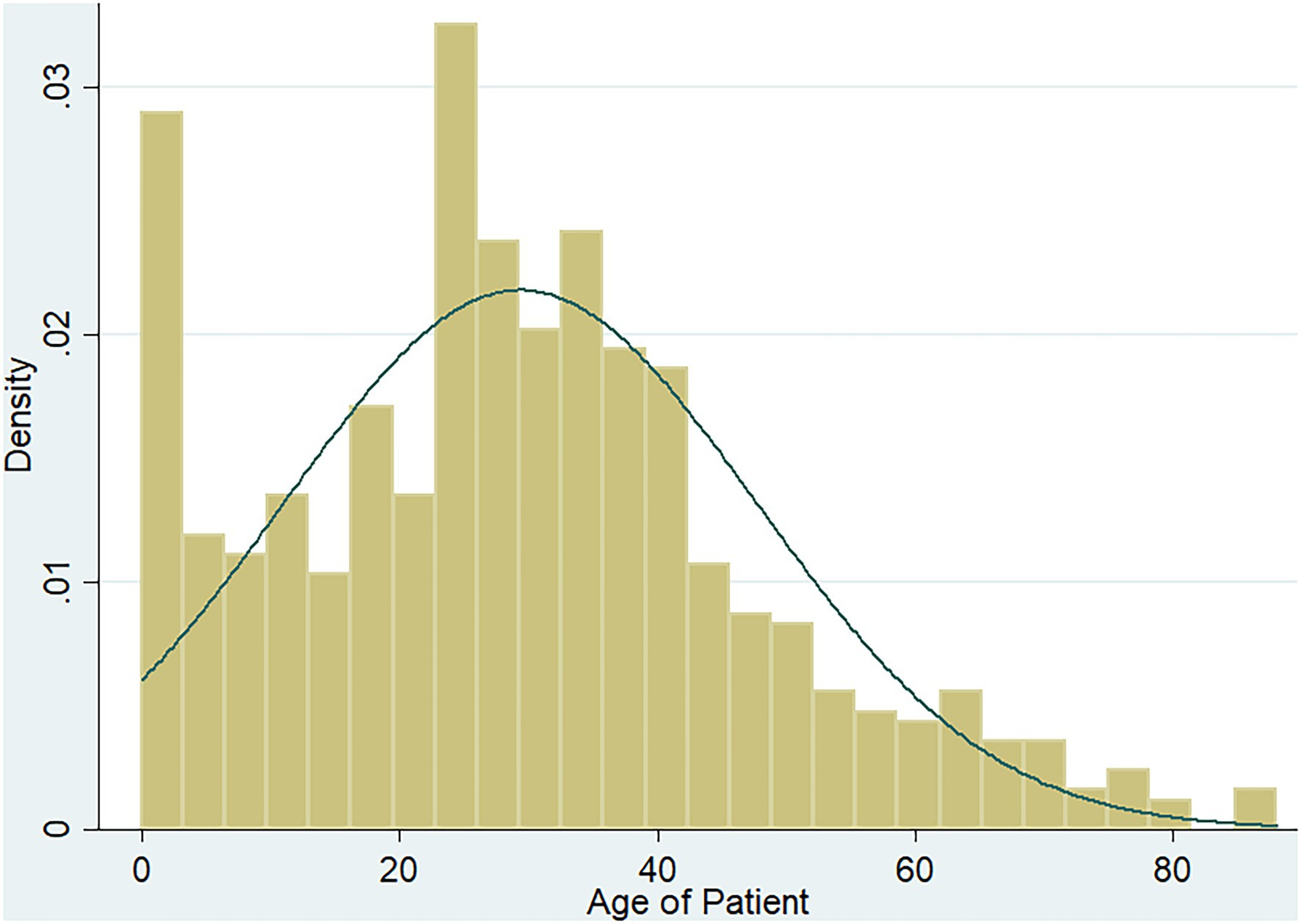
Seven hundred and ninety-two diarrheic stool samples were collected from the health facilities; 297 (37.5%) males and 495 (62.5%) females. The ages of participants ranged between 5 months and 88 years with the majority (589/792, 74.3%) being adults (>17 years) and a mean age of 29.3 years (Figure 2).
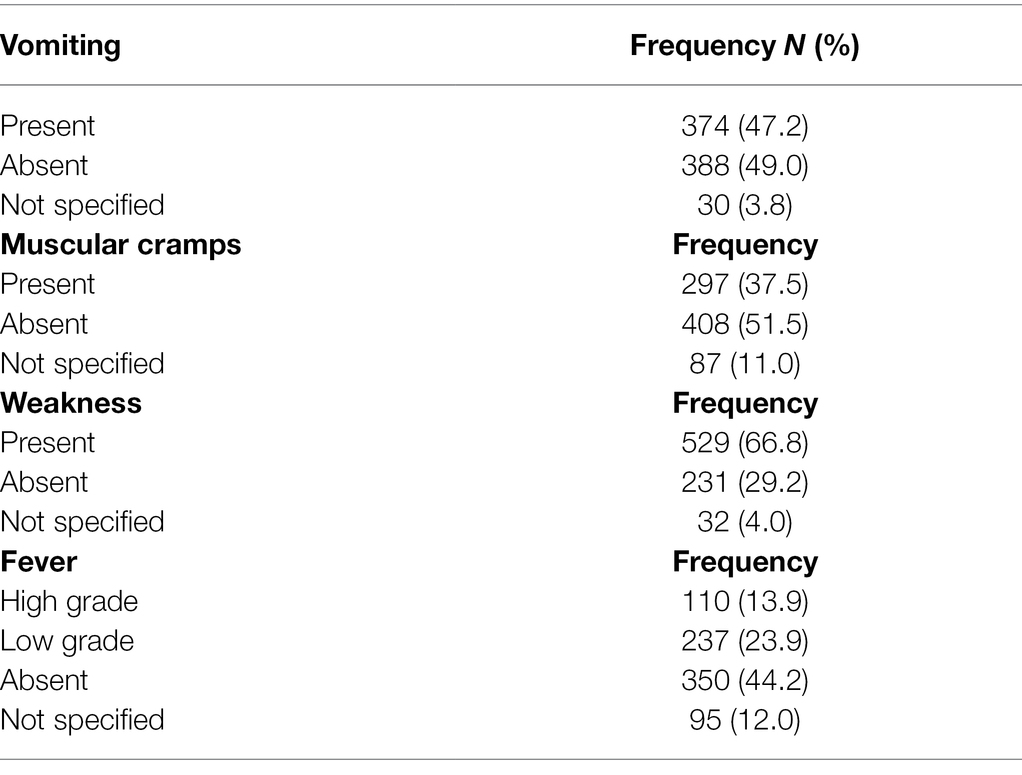
Clinical records indicated that 124 (15.7%) patients had taken antibiotics, 85 (10.7%) antimalarials, 40 (5.0%) anthelminthic, 37 (4.7%) both antibiotics and antimalarials, 17 (2.1%) all three drugs, and 497 (62.8%) had not taken any drug before visiting the health facilities. The highest temperature recorded among participants was 39.8°C and the lowest 30.1°C with a mean temperature of 36.3°C. Symptoms such as fever (347, 36.8%) vomiting (374, 47.2%), weakness (529, 66.8%), and muscle cramps (297, 37.5%) were recorded among the patients (Table 1).
Distribution of Diarrheic Bacteria Characterized
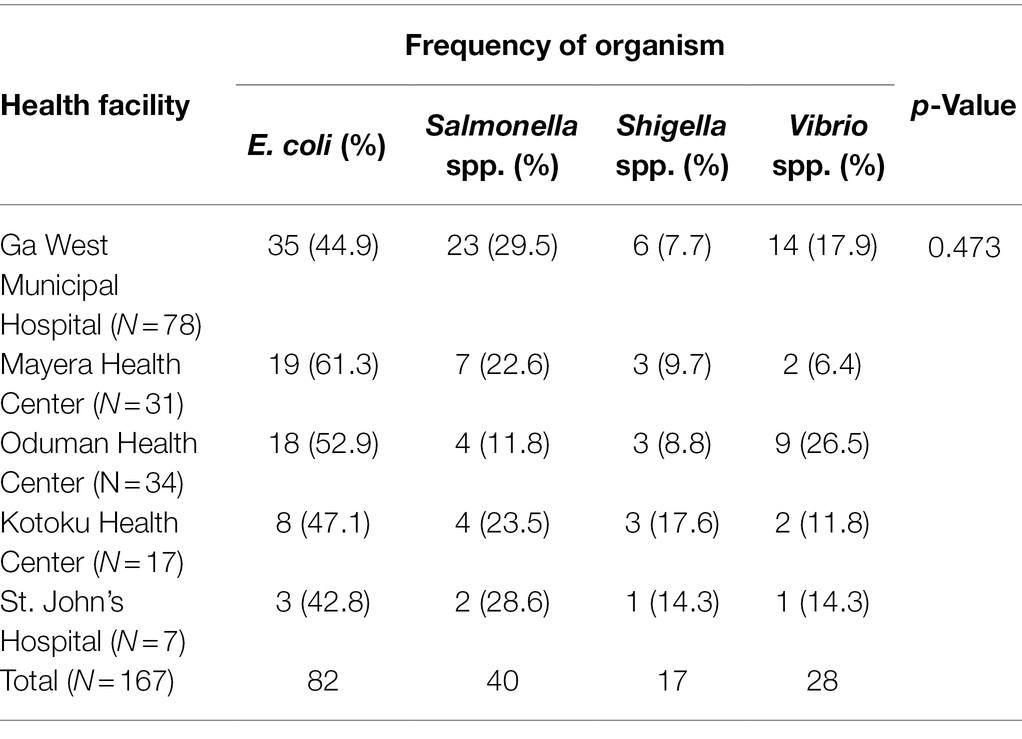
Bacterial isolates were obtained from 772/792 stool samples collected; 21.6% (167/772) were diarrheagenic bacteria including E. coli (from bloody stool as well as stool from children under five), and three other bacteria genera namely: Vibrio, Salmonella, and Shigella. There were 5.2% (40/772) Salmonella spp., 2.2% (17/772) Shigella spp., 3.6% (28/772) Vibrio sp., and 10.6% (82/772) E. coli, from bloody or infant stool (infant <5 years; Table 2). There was however no significant difference (p > 0.05) between the different bacteria etiology of diarrhea isolated from all five health facilities (Table 3).
Other species of bacteria including but not limited to Klebsiella (N = 50), Raoultella (N = 16), and Enterobacter (N = 6) were isolated but not further analyzed for this work.
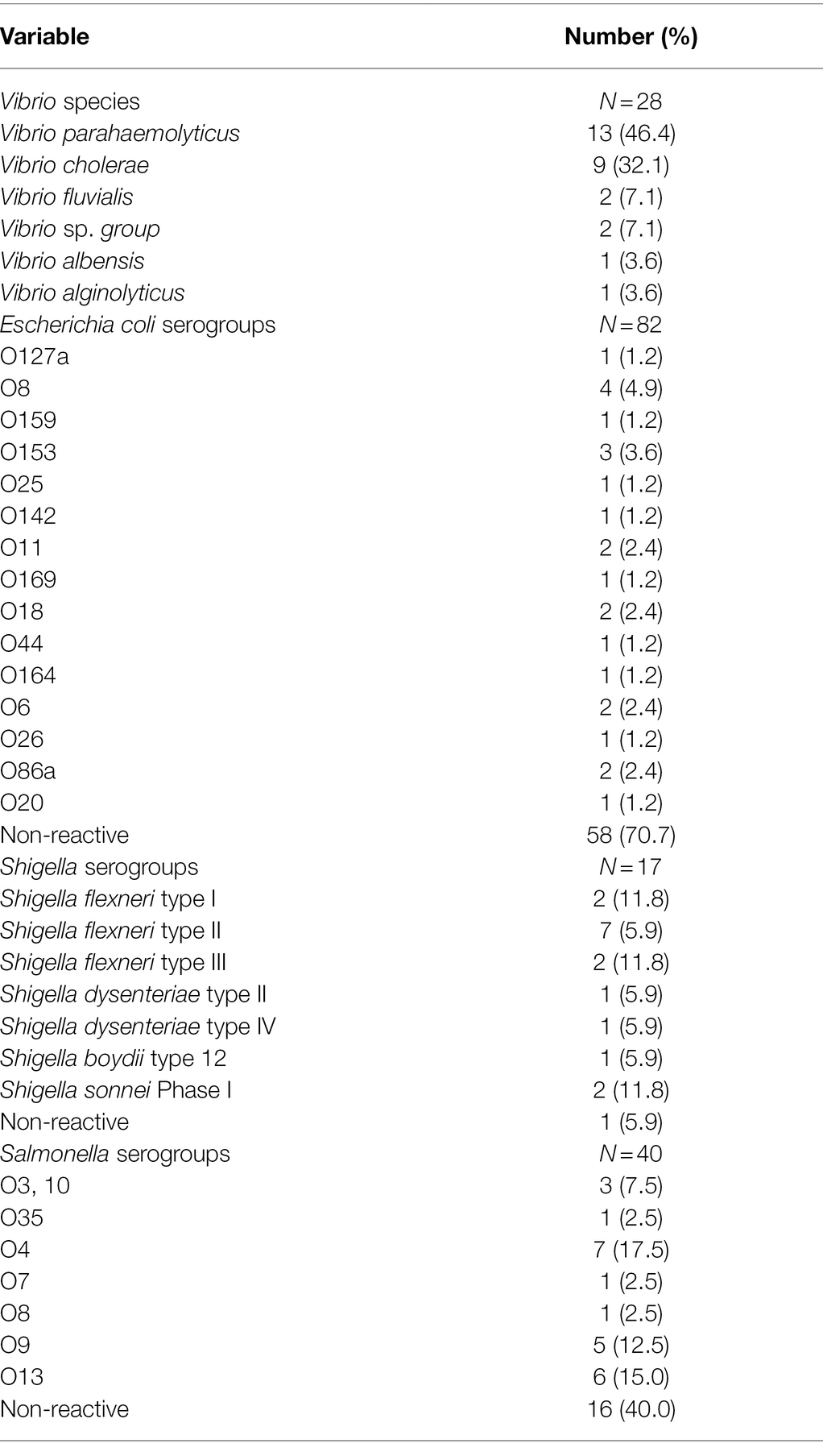
The Vibrio spp. (N = 28) were further characterized into six species namely: Vibrio parahaemolyticus (13/28, 46.4%), V. cholerae (9/28, 32.1%), Vibrio fluvialis (2/28, 7.1%), Vibrio sp. group (2/28, 7.1%), Vibrio albensis (1/28, 3.6%), and Vibrio alginolyticus (1/28, 3.6%). Sixteen out of the 17 (94.1%) Shigella species were identified as Shigella flexneri (11, 64.7%) Shigella dysenteriae (2, 11.8%), Shigella sonnei (2,11.8%), and Shigella boydii (1, 5.9%).
Upon serological analysis, E. coli strains were grouped, with the most prominent being serogroups O8 (4, 4.9%) and O153 (3, 3.7%), known to be enterotoxigenic (Escribano et al., 1987; Table 2). Among Salmonella isolates, serogroups O13 (15.0%) and O4 (17.5%) were the most dominant. More than one diarrhoeagenic bacteria were isolated from some of the patients; Salmonella and E. coli (4/792, 0.5%), Shigella, and E. coli (3/792, 0.4%) were isolated from mainly bloody stools.
Antimicrobial Susceptibility Profiles of Isolates
Antimicrobial susceptibility testing (AST) was done for 24 out of the 28 isolated Vibrio spp. (Figure 3) of which all were susceptible to gentamicin, an aminoglycoside but moderately susceptible to amikacin (14/24, 58.3%). The Vibrio isolates showed resistance to cefazolin (18/24, 75%) among which all 13 V. parahaemolyticus isolates were resistant to cefazolin. Our Vibrio isolates showed 25% (6/24) resistance to ciprofloxacin, used to treat non-cholera Vibrio infections. Isolates tested against cefotaxime (21/24, 87.5%) and ceftriaxone (20/24, 83.3%) also used in Vibrio infection treatment were highly susceptible. A total of four multi-drug resistant Vibrio strains were identified with resistances to fluoroquinolones, cephalosporins, and aminoglycosides.
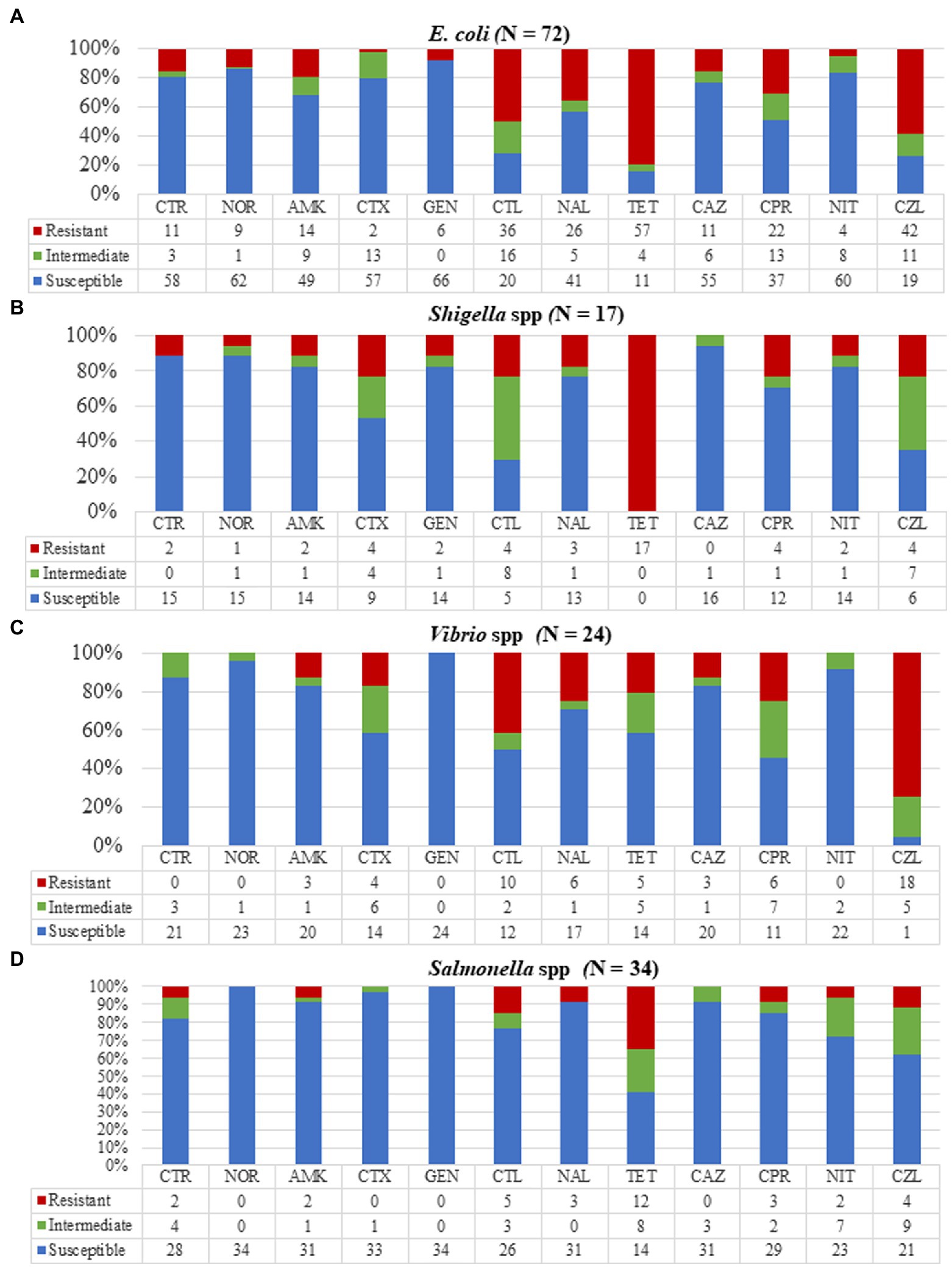
Figure 3. Resistance patterns of the various bacterial strains to 12 antibiotics. (A) Escherichia coli showing high resistance to tetracycline, cephalothin, and cefazolin. (B) Shigella isolates were totally resistant to tetracycline with no resistance to ceftazidime. (C) Vibrio isolates showed high resistance to cefazolin and complete susceptibility to gentamycin. (D) All Salmonella isolates were susceptible to norfloxacin, cefotaxime, and gentamycin with 35% of isolates resistant to tetracycline. CTR, ceftriaxone; NOR, norfloxacin; AMK, amikacin; CTX, cefotaxime; GEN, gentamycin; CTL, cephalothin; NAL, nalidixic acid; TET, tetracycline; CAZ, ceftazidime; CPR, ciprofloxacin; NIT, nitrofurantoin; and CZL, cefazolin.
Out of the 40 Salmonella isolates, AST was done for 34 isolates (Figure 3). We detected high resistance of Salmonella isolates to tetracycline (12/34, 35.3%) but relatively low resistance to ciprofloxacin (3/34, 8.8%) and ceftriaxone (2/34, 5.9%). The Salmonella isolates showed total susceptibility when tested against gentamycin and norfloxacin with no multi-drug resistant Salmonella strains however found.
All 17 Shigella isolates were tested against our selected antimicrobials (Figure 3) and were found to be resistant to tetracycline. Three multidrug-resistant Shigella isolates were identified. One (5.9%) isolate was resistant to three drugs (norfloxacin, ciprofloxacin, and tetracycline), and two (11.8%) were resistant to both norfloxacin and ciprofloxacin.
Antimicrobial susceptibility testing was done on 72/82 E. coli isolates and when tested against all our selected antimicrobials, at least one antimicrobial resistance for each isolate was observed (Figure 3). Escherichia coli isolates showed the highest resistance to tetracycline (57/72, 79.1%) and cefazolin (43/72, 58.3%). The isolates were highly susceptible to aminoglycosides, amikacin (57/72, 79.2%), and gentamicin (66/72, 91.7%), and nitrofurantoin (60/72, 83.3%) which is prescribed for treating E. coli infections. We detected high resistance to one fluoroquinolone, ciprofloxacin (22/72, 30.6%) with two extended-spectrum beta-lactamases-producing E. coli identified.
Discussion
Our routine surveillance study of diarrhea from Ga West Municipality in Ghana from 2017 to 2021 has revealed several important findings. The diversity and frequency of bacteria isolated from this study concur with similar reports from China, Kenya, and India (Mbuthia et al., 2018; William et al., 2020; Wang et al., 2021). The prevalence of Shigella, Salmonella, Vibrio, and diarrheagenic E. coli is consistent with previous studies in Ghana and other African countries (Akuffo et al., 2017; Kalule et al., 2019). However, reports from Lompo et al. (2021) did not isolate Salmonella from diarrheic stool samples. In our study, we isolated diarrheagenic bacteria from 21% (167/779) of stool samples received which is markedly high compared to previous studies in Ghana and elsewhere (4%; Akuffo et al., 2017; William et al., 2020). This high prevalence may be due to our sampling site, Ga West an area without potable drinking water and thus unsanitary living conditions. As previously reported, diarrheagenic bacteria have been isolated from street food samples in Ghana, which may result in more bacteria diarrhea cases in areas with unsanitary living conditions (Mensah et al., 2002). In a country where most diarrheal cases are thought to only be caused by V. cholerae, this revelation is very important. The frequency of Shigella, Salmonella, Vibrio, and enterotoxigenic E. coli emphasizes the need to scale up laboratory-based surveillance and appropriate determination of disease etiology for effective treatment of diarrheal diseases. The observable emerging resistance to potent drugs such as ciprofloxacin, norfloxacin, and nalidixic acid used in treating Vibrio and Shigella is of critical concern.
We observed an emerging resistance of Vibrio isolates to ciprofloxacin, a potent cholera medication with 23.1% resistance and 30.8% intermediate resistance consistent with reports in Nigeria, Cameroon, and China (Quilici et al., 2010; de Melo et al., 2011). Our study also identified eight cases of V. cholerae which is a public health emergency when not properly managed. In our study, we isolated 13 V. parahaemolyticus from diarrhea stools. Two patients infected with V. parahaemolyticus presented bloody stool samples with 76.9% (10/13) of V. parahaemolyticus infected patients on admission. Bloody stool samples can be a result of V. parahaemolyticus damaging the epithelial layer of the small intestines through the creation of cavities (Wang et al., 2022). Classical V. parahaemolyticus infection symptoms such as high fever (53.8%), vomiting (53.8%), muscular cramps (46.2%), and general weakness (76.9%) were observed in all infected patients consistent with reports by Jung (2018).
Vibrio parahaemolyticus is highly prevalent in marine coastal areas and associated with seafood and endemic in South-Eastern Asia countries including Taiwan, Japan, and China causing many foodborne illnesses (Junhe et al., 2018; Zhao et al., 2020). These V. parahaemolyticus were isolated from stools in an area highly deprived of potable water hence depending on the river Densu for water. Residents living along the coast defecate, feed animals, wash and drink from this river, which is of huge public health concern. Vibrio parahaemolyticus is known to cause acute gastroenteritis characterized by diarrhea. Although self-limiting, strains of V. parahaemolyticus are virulent enough to cause outbreaks and can be fatal (Jung, 2018). With recent reports of antimicrobial-resistant strains (Tan et al., 2020; Jingjit et al., 2021), there is an urgent need to tackle it.
We found Shigella isolates to be totally (100%) resistant to tetracycline. This might be a result of the extensive use of tetracycline in Ghana over the years with several reports on antimicrobial resistance to these drugs (Newman et al., 2011; Yevutsey et al., 2017). There might also be some contribution from the irresponsible use in the animal farming industry as a result of easy access to the drug from pharmacies (Wilcox, 2009; Andoh et al., 2016). The Shigella spp. were additionally resistant to amikacin (4/17, 23.5%), ciprofloxacin (4/17, 23.5%), nalidixic acid (3/17, 17.6%), and norfloxacin (1/17, 5.9%) with the latter two formerly potent drugs used in Shigella dysentery infections in adults (Basnet et al., 2021). Observations of high susceptibility of Shigella sp. to ceftriaxone and high resistance to nalidixic acid, tetracycline, and amikacin is consistent with Pourakbari et al. (2010) and Basnet et al. (2021). Parenteral ceftriaxone is highly effective and recommended in the treatment of hospitalized children with severe shigellosis (Ashkenazi et al., 2003) so the emergence of high resistance to this drug is of great concern.
Serological analysis of isolated E. coli yielded a high prevalence of serotypes O8 (4.9%) and O153 (3.7%). The serotype O153, an enterotoxigenic Escherichia coli (ETEC) has been reported to harbor a large number of virulent genes capable of causing mild to severe infections (Díaz-Jiménez et al., 2020). There are reports of this E. coli strain being isolated from patients during an outbreak which may be indicative of its ability to cause an epidemic (Kennedy et al., 2018). Extended-spectrum beta-lactamase (ESBL)-producing E. coli were also detected in our study buttressing the high level of resistance occurring in our communities. The transmission and spread of these virulent E. coli strains in local communities can be of great danger to public health.
The identification of multi-drug resistant Vibrio species in this study continues to show the rising incidence of antimicrobial resistance in the communities. A major problem in Ghanaian communities is the indiscriminate use of antibiotics in animal husbandry and farming sectors as growth supplements for the prevention of infection and subsequently, increasing yield. This practice pre-exposes drugs to these pathogens hence hastening the rate at which antimicrobial resistance develops (Wilcox, 2009; Andoh et al., 2016). Multi-drug resistant Vibrio isolates may increase disease severity, morbidity, and mortality and increase constraints on our public health system.
Good hygiene practices will curb the spread of these pathogens since most of these infections are spread through person-to-person contact. The sentinel site chosen in this surveillance study is a community where inhabitants have limited access to pipe-borne water. Residents in some of the communities depend on streams, boreholes, and even rainwater as sources of water for drinking and household chores. Improper disposal of human waste is also another problem in the community. Open defecation into water bodies and bushes coupled with potable water scarcity could account for the high diarrhea cases (Larbi et al., 2021). Practices such as handwashing, proper cooking of food, as well as proper disposal of human and household waste, are known to curb the spread of bacterial pathogens causing diarrhea. Communities must be engaged regularly in the implementation of water, sanitation, and hygiene (WASH) practices. We identified a couple of limitations in the study. Molecular methods such as PCR would have been more sensitive and given a better understanding of the diarrheagenic E. coli and ESBLs isolated as compared to serological analysis only.
We did not have data on the total number of individuals presenting diarrhea to the health facilities as samples taken were from only patients who agreed to be in this study.
Conclusion
This study has shown the diverse bacteria etiology (Salmonella, Shigella, Vibrio, and some E. coli) implicated in diarrhea disease and the need for proper differential diagnosis for better treatment outcomes. Resistance observed in all isolates is of public health concern since drugs used in the treatment of Shigellosis, Salmonellosis, and other diarrhea diseases showed marked resistance. This can lead to pressure on our public health system should drugs not work against intended pathogens. A diarrheagenic prevalence rate of 21% further stresses the need for differential diagnosis in diarrhea cases for better treatment outcomes. The presence of multi-drug resistant Vibrio isolates and an increase in Vibrio parahaemolyticus which is highly associated with food poisoning as well as the high resistant rates of Shigella isolates and ESBLs detected is forewarning and raises the need for the implementation of preventive strategies to minimize transmission. Routine antimicrobial susceptibility testing, effective monitoring, and nationwide surveillance of AMR pathogens should be implemented to curb the increase of antimicrobial resistance in Ghana.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of Noguchi Memorial Institute for Medical Research (NMIMR; approval number: 096/16-1; dated on May 3, 2017). It was exempted from additional approval by the Ghana Health Service Ethics Committee which considered the procedures as compliant with routine service for patients care and surveillance. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
DY-M, KK, HK, KT, FA-B, AA-P, and GM: conceptualization, fund acquisition, and writing—review and editing. TA, DA, and AM: investigation. TA, DY-M, and PA: writing—original draft and formal analysis. DY-M, AA-P, GM, TA, DA, and AM: methodology. DY-M: resources, project administration, and supervision. DO, NG, TA, DA, KI, TMa, and TMi: writing—review and editing. AA: project administration. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by AMED-JICA [the Science and Technology Research Partnership for Sustainable Development (SATREPS); 19jm0110012].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors express our greatest gratitude to all laboratory staff at the various health facilities where samples were collected and in particular, Emmanuel Danso the project driver for his selflessness in his duties. We are sincerely grateful to all staff at the National Public Health reference laboratory and all participants of this study for their time and cooperation during this study.
References
Ahiabu, M. A., Tersbøl, B. P., Biritwum, R., Bygbjerg, I. C., and Magnussen, P. (2016). A retrospective audit of antibiotic prescriptions in primary health-care facilities in eastern region, Ghana. Health Policy Plan. 31, 250–258. doi: 10.1093/heapol/czv048
Akuffo, R., Armah, G., Clemens, M., Kronmann, K., Jones, A., Agbenohevi, P., et al. (2017). Prevalence of enteric infections among hospitalized patients in two referral hospitals in Ghana. BMC Res. Notes 10, 1–7. doi: 10.1186/s13104-017-2621-x
Andoh, L., Dalsgaard, A., Obiri-Danso, K., Newman, M., Barco, L., and Olsen, J. (2016). Prevalence and antimicrobial resistance of Salmonella serovars isolated from poultry in Ghana. Epidemiol. Infect. 144, 3288–3299. doi: 10.1017/S0950268816001126
Arhinful, D. K. (2009). WHO pharmaceutical situation assessment: level II-health facilities survey in Ghana. Ghana: Ministry of Health.
Ashkenazi, S., Levy, I., Kazaronovski, V., and Samra, Z. (2003). Growing antimicrobial resistance of Shigella isolates. J. Antimicrob. Chemother. 51, 427–429. doi: 10.1093/jac/dkg080
Basnet, B., Niroula, D., Acharya, J., and Basnyat, S. (2021). Antibiotic susceptibility pattern of Shigella spp. isolated from patients suspected of acute gastroenteritis. J. Instr. Sci. Technol. 26, 1–7. doi: 10.3126/jist.v26i2.41261
Bolinches, J., Romalde, J. L., and Toranzo, A. E. (1988). Evaluation of selective media for isolation and enumeration of vibrios from estuarine waters. J. Microbiol. Methods 8, 151–160. doi: 10.1016/0167-7012(88)90016-4
CLSI (2020). Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute. [Internet]. Available at: https://clsi.org/media/3481/m100ed30_sample.pdf (Accessed April 21, 2022).
Danso, E. K., Asare, P., Otchere, I. D., Akyeh, L. M., Asante-Poku, A., Aboagye, S. Y., et al. (2020). A molecular and epidemiological study of Vibrio cholerae isolates from cholera outbreaks in southern Ghana. PLoS One 15:e0236016. doi: 10.1371/journal.pone.0236016
de Melo, L. M. R., Almeida, D., Hofer, E., dos Reis, C. M. F., Theophilo, G. N. D., Santos, A. F., et al. (2011). Antibiotic resistance of Vibrio parahaemolyticus isolated from pond-reared Litopenaeus vannamei marketed in Natal, Brazil. Braz. J. Microbiol. 42, 1463–1469. doi: 10.1590/S1517-83822011000400032
Díaz-Jiménez, D., García-Meniño, I., Herrera, A., García, V., López-Beceiro, A. M., Alonso, M. P., et al. (2020). Genomic characterization of Escherichia coli isolates belonging to a new hybrid aEPEC/ExPEC pathotype O153: H10-A-ST10 eae-beta1 occurred in meat, poultry, wildlife and human diarrheagenic samples. Antibiotics 9:192. doi: 10.3390/antibiotics9040192
Escribano, A., Ørskov, I., Ørskov, F., and Borras, R. (1987). Enterotoxigenic Escherichia coli O153: H45 from an outbreak of diarrhoea in Spain. Med. Microbiol. Immunol. 176, 241–244. doi: 10.1007/BF00190530
ESRI (2010). Introducing ArcGIS 10.1. Available at: https://www.esri.com/news/arcnews/spring12articles/introducing-arcgis-101.html (Accessed January 11, 2022).
Ghana Statistical Service (2013). 2010 Population and Housing Census. Regional Analytical Report. Greater Accra Region. [Internet]. Available at: https://www2.statsghana.gov.gh/docfiles/2010phc/2010_PHC_Regional_Analytical_Reports_Greater_Accra_Region.pdf (Accessed June 6, 2021).
Gilchrist, C. A., Petri, S. E., Schneider, B. N., Reichman, D. J., Jiang, N., Begum, S., et al. (2016). Role of the gut microbiota of children in diarrhea due to the protozoan parasite Entamoeba histolytica. J. Infect. Dis. 213, 1579–1585. doi: 10.1093/infdis/jiv772
Government of Ghana (2015). Ghana weekly epidemiological bulletin [Internet]. Available at: http://www.ghanahealthservice.org/downloads/Weekly_Epid_Bulletin_Week_1_2015.pdf (Accessed May 16, 2021).
Hodges, K., and Gill, R. (2010). Infectious diarrhea: cellular and molecular mechanisms. Gut Microbes 1, 4–21. doi: 10.4161/gmic.1.1.11036
Jingjit, N., Preeprem, S., Surachat, K., and Mittraparp-Arthorn, P. (2021). Characterization and analysis of clustered regularly interspaced short palindromic repeats (CRISPRs) in pandemic and non-pandemic Vibrio parahaemolyticus isolates from seafood sources. Microorganisms 9:1220. doi: 10.3390/microorganisms9061220
Jung, S. W. (2018). A foodborne outbreak of gastroenteritis caused by Vibrio parahaemolyticus associated with cross-contamination from squid in Korea. Epidemiol. Health 40:e2018056. doi: 10.4178/epih.e2018056
Junhe, L., Baisheng, L., Taohua, L., Zhaowen, P., Miaolin, H., and Jingwen, Z. (2018). Etiological characteristics of Vibrio parahaemolyticus strains isolated from a food poisoning event in Jiangmen, Guangdong. Dis. Surveill. 33, 387–390. doi: 10.3784/j.issn.1003-9961.2018.05.009
Kalule, J. B., Smith, A. M., Vulindhlu, M., Tau, N. P., Nicol, M. P., Keddy, K. H., et al. (2019). Prevalence and antibiotic susceptibility patterns of enteric bacterial pathogens in human and non-human sources in an urban informal settlement in cape town, South Africa. BMC Microbiol. 19, 1–11. doi: 10.1186/s12866-019-1620-6
Kennedy, C., Walsh, C., Karczmarczyk, M., O’Brien, S., Akasheh, N., Quirke, M., et al. (2018). Multi-drug resistant Escherichia coli in diarrhoeagenic foals: pulsotyping, phylotyping, serotyping, antibiotic resistance and virulence profiling. Vet. Microbiol. 223, 144–152. doi: 10.1016/j.vetmic.2018.08.009
Kunadu, A. P. H., Holmes, M., Miller, E. L., and Grant, A. J. (2018). Microbiological quality and antimicrobial resistance characterization of Salmonella spp. in fresh milk value chains in Ghana. Int. J. Food Microbiol. 277, 41–49. doi: 10.1016/j.ijfoodmicro.2018.04.025
Larbi, R. T., Atiglo, D. Y., Peterson, M. B., Biney, A. A., Dodoo, N. D., and Dodoo, F. N. A. (2021). Household food sources and diarrhoea incidence in poor urban communities, Accra Ghana. PLoS One 16:e0245466. doi: 10.1371/journal.pone.0245466
Lompo, P., Tahita, M. C., Sorgho, H., Kaboré, W., Kazienga, A., Nana, A. C. B., et al. (2021). Pathogens associated with acute diarrhea, and comorbidity with malaria among children under five years old in rural Burkina Faso. Pan. Afr. Med. J. 38:259. doi: 10.11604/pamj.2021.38.259.15864
Magiorakos, A. P., Srinivasan, A., Carey, R. B., Carmeli, Y., Falagas, M., Giske, C., et al. (2012). Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18, 268–281. doi: 10.1111/j.1469-0691.2011.03570.x
Mbuthia, O. W., Mathenge, S. G., Oyaro, M. O., and Ng’ayo, M. O. (2018). Etiology and pathogenicity of bacterial isolates: a cross sectional study among diarrheal children below five years in central regions of Kenya. Pan. Afr. Med. J. 31:88. doi: 10.11604/pamj.2018.31.88.15644
Mensah, P., Yeboah-Manu, D., Owusu-Darko, K., and Ablordey, A. (2002). Street foods in Accra, Ghana: how safe are they? Bull. World Health Organ. 80, 546–554.
Mizutani, T., Aboagye, S. Y., Ishizaka, A., Afum, T., Mensah, G. I., Asante-Poku, A., et al. (2021). Gut microbiota signature of pathogen-dependent dysbiosis in viral gastroenteritis. Sci. Rep. 11, 1–11. doi: 10.1038/s41598-021-93345-y
Nath, R., Saikia, L., Choudhury, G., and Sharma, D. (2013). Drug resistant Shigella flexneri in & around Dibrugarh, north-East India. Indian J. Med. Res. 137, 183–186.
Newman, M. J., Frimpong, E., Donkor, E. S., Opintan, J. A., and Asamoah-Adu, A. (2011). Resistance to antimicrobial drugs in Ghana. Infect. Drug Resist. 4, 215–220. doi: 10.2147/IDR.S21769
Nkrumah, B., and Nguah, S. B. (2011). Giardia lamblia: a major parasitic cause of childhood diarrhoea in patients attending a district hospital in Ghana. Parasit. Vectors 4, 1–7. doi: 10.1186/1756-3305-4-163
Noora, C. L., Issah, K., Kenu, E., Bachan, E. G., Nuoh, R. D., Nyarko, K. M., et al. (2017). Large cholera outbreak in Brong Ahafo region, Ghana. BMC Res. Notes 10:389. doi: 10.1186/s13104-017-2728-0
Obeng-Nkrumah, N., Labi, A. K., Blankson, H., Awuah-Mensah, G., Oduro-Mensah, D., Anum, J., et al. (2019). Household cockroaches carry CTX-M-15-, OXA-48-and NDM-1-producing enterobacteria, and share beta-lactam resistance determinants with humans. BMC Microbiol. 19, 1–11. doi: 10.1186/s12866-019-1629-x
Pourakbari, B., Mamishi, S., Mashoori, N., Mahboobi, N., Ashtiani, M. H., Afsharpaiman, S., et al. (2010). Frequency and antimicrobial susceptibility of Shigella species isolated in children medical center hospital, Tehran, Iran, 2001-2006. Braz. J. Infect. Dis. 14, 153–157. doi: 10.1016/S1413-8670(10)70029-5
Pun, S. B. (2014). The first appearance of classical-like phenotype Vibrio cholerae in Nepal. North Am. J. Med. Sci. 6, 183–184. doi: 10.4103/1947-2714.131248
Quilici, M. L., Massenet, D., Gake, B., Bwalki, B., and Olson, D. M. (2010). Vibrio cholerae O1 variant with reduced susceptibility to ciprofloxacin, Western Africa. Emerg. Infect. Dis. 16, 1804–1805. doi: 10.3201/eid1611.100568
Takyi, S. A., Amponsah, O., Yeboah, A. S., and Mantey, E. (2021). Locational analysis of slums and the effects of slum dweller’s activities on the social, economic and ecological facets of the city: insights from Kumasi in Ghana. GeoJournal 86, 2467–2481. doi: 10.1007/s10708-020-10196-2
Tan, C. W., Rukayadi, Y., Hasan, H., Thung, T. Y., Lee, E., Rollon, W. D., et al. (2020). Prevalence and antibiotic resistance patterns of Vibrio parahaemolyticus isolated from different types of seafood in Selangor, Malaysia. Saudi J. Biol. Sci. 27, 1602–1608. doi: 10.1016/j.sjbs.2020.01.002
Tate, J. E., Burton, A. H., Boschi-Pinto, C., Parashar, U. D., and World Health Organization–Coordinated Global Rotavirus Surveillance Network (2016). Global, regional, and national estimates of rotavirus mortality in children< 5 years of age, 2000–2013. Clin. Infect. Dis. 62, S96–S105. doi: 10.1093/cid/civ1013
Wang, J., Zhan, Y., Sun, H., Fu, X., Kong, Q., Zhu, C., et al. (2022). Regulation of virulence factors expression during the intestinal colonization of Vibrio parahaemolyticus. Foodborne Pathog. Dis. 19, 169–178. doi: 10.1089/fpd.2021.0057
Wang, L. P., Zhou, S. X., Wang, X., Lu, Q. B., Shi, L. S., Ren, X., et al. (2021). Etiological, epidemiological, and clinical features of acute diarrhea in China. Nat. Commun. 12, 1–12. doi: 10.1038/s41467-021-22551-z
Wilcox, M. H. (2009). The tide of antimicrobial resistance and selection. Int. J. Antimicrob. Agents 34, S6–S10. doi: 10.1016/S0924-8579(09)70550-3
William, A., Satija, S., and Kaur, R. (2020). Bacterial etiology of diarrhoea in a tertiary care hospital. Int. J. Recent Sci. Res. 11, 39022–39024. doi: 10.24327/ijrsr.2020.1106.5426
World Health Organization (2005). The treatment of diarrhoea: a manual for physicians and other senior health workers. World Health Organization. Report No: 9241593180.
World Health Organization (2017). WHO Diarrhoeal Disease Key Facts [Internet]. Available at: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (Accessed January 30, 2021).
Yang, X., Guo, Y., Xiao, L., and Feng, Y. (2021). Molecular epidemiology of human cryptosporidiosis in low-and middle-income countries. Clin. Microbiol. Rev. 34, e00087–e00119. doi: 10.1128/CMR.00087-19
Yevutsey, S. K., Buabeng, K. O., Aikins, M., Anto, B. P., Biritwum, R. B., Frimodt-Møller, N., et al. (2017). Situational analysis of antibiotic use and resistance in Ghana: policy and regulation. BMC Public Health 17, 1–7. doi: 10.1186/s12889-017-4910-7
Zhao, S., Zhao, Q., Ye, H., Yang, G., Li, J., and Huo, X. (2020). Investigation on a food poisoning caused by Vibrio parahaemolyticus and its etiology. J. Public Health Prev. Med. 113–117.
Keywords: diarrhea, bacteria, resistance, antimicrobial, susceptibility
Citation: Afum T, Asandem DA, Asare P, Asante-Poku A, Mensah GI, Musah AB, Opare D, Taniguchi K, Guinko NM, Aphour T, Arhin D, Ishikawa K, Matano T, Mizutani T, Asiedu-Bekoe F, Kiyono H, Anang AK, Koram KA and Yeboah-Manu D (2022) Diarrhea-Causing Bacteria and Their Antibiotic Resistance Patterns Among Diarrhea Patients From Ghana. Front. Microbiol. 13:894319. doi: 10.3389/fmicb.2022.894319
Edited by:
Steven Lee Foley, National Center for Toxicological Research (FDA), United StatesReviewed by:
Morteza Saki, Ahvaz Jundishapur University of Medical Sciences, IranMadubuike U. Anyanwu, University of Nigeria, Nsukka, Nigeria
Copyright © 2022 Afum, Asandem, Asare, Asante-Poku, Mensah, Musah, Opare, Taniguchi, Guinko, Aphour, Arhin, Ishikawa, Matano, Mizutani, Asiedu-Bekoe, Kiyono, Anang, Koram and Yeboah-Manu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dorothy Yeboah-Manu, dyeboah-manu@noguchi.ug.edu.gh
 Theophilus Afum
Theophilus Afum Diana Asema Asandem1
Diana Asema Asandem1 Prince Asare
Prince Asare Gloria Ivy Mensah
Gloria Ivy Mensah Abdul Basit Musah
Abdul Basit Musah Kiyosi Taniguchi
Kiyosi Taniguchi Thelma Aphour
Thelma Aphour Koichi Ishikawa
Koichi Ishikawa Tetsuro Matano
Tetsuro Matano Taketoshi Mizutani
Taketoshi Mizutani Hiroshi Kiyono
Hiroshi Kiyono Kwadwo Ansah Koram
Kwadwo Ansah Koram Dorothy Yeboah-Manu
Dorothy Yeboah-Manu