- 1College of Food Science, Southwest University, Chongqing, China
- 2Food Storage and Logistics Research Center, Southwest University, Chongqing, China
Utilizing antagonistic yeasts is a promising approach for managing postharvest decay of fruits. However, it is well established that various severe stresses encountered in the environment and production process cause the intracellular reactive oxygen species (ROS) accumulation in yeast cells, resulting in cell damage and loss of vitality. Here, proline has been shown to function as a cell protectant and inducer of biofilm formation able to increase the oxidative stress tolerance and the biocontrol ability of the antagonistic yeast Metschnikowia citriensis. Addition of proline to M. citriensis cells induced a significant rise in superoxide dismutase (SOD) and catalase (CAT) activity in the early and late stages of oxidative stress, respectively, and increased the maroon pigment production that directly reduced intracellular iron content and indirectly diminished intracellular ROS levels and thus inhibited ROS- and iron-induced apoptosis. Treating cells with iron chelator tropolone yielded similar results. Pigment production induced by proline also enhanced the capability of biofilm formation of M. citriensis. These results suggested an important role for pigment of M. citriensis in response to oxidative stress. The abilities of proline to scavenge intracellular ROS and inhibit apoptosis, increase pigment production, and promote biofilm formation contribute to the improvements in oxidative stress tolerance and biocontrol efficacy of M. citriensis.
Introduction
Postharvest fruits decay caused by fungal pathogens results in a large amount of economic losses and possible occurrence of mycotoxin contamination (Mahunu et al., 2016; Zhang et al., 2018). Utilizing various disease control agents to reduce postharvest losses is the most frugal ways to improve food security and nutritional value (Zhang et al., 2018). Based on the severe environmental pollution, human poisoning, and various diseases brought by the massive use of chemicals, biologicals (including biocontrol microbes) appear to be significant tools for the diseases control (Hazell and Wood, 2008; Aktar et al., 2009; Pandin et al., 2017; Yang et al., 2017; Ab Rahman et al., 2018; Yan et al., 2018).
Over the past 30 years, isolating wild species of antagonistic yeasts has become a research topic that receives considerable attention, since antagonistic yeasts have simple nutritional requirements and many of them have been proven to be harmless to potential consumers (Zhimo et al., 2014; Sui et al., 2015; Ocampo-Suarez et al., 2017). A growing body of new antagonists has been isolated from environment, and many biocontrol products have been developed and are mostly available in the agricultural markets in North America and Europe (Borriss, 2015). However, several of these antagonists have not achieved commercial success due to inconsistent results, and most of the biocontrol products have limited application on fruit crops (Droby et al., 2016). In contrast, Metschnikowia fructicola (Shemer, Bayer, Leverkusen, Germany) is a more successful biocontrol product for pre- and post-harvest applications on various fruits and vegetables, which registered in Israel and acquired by Bayer CropScience (Germany) and then delegated to Koppert (Netherlands) (Droby et al., 2016). In the genus Metschnikowia, not only M. fructicola, Metschnikowia pulcherrima and Metschnikowia andauensis have also been used as postharvest biocontrol agents (Kurtzman and Droby, 2001; Spadaro and Gullino, 2004; Manso and Nunes, 2011). Metschnikowia citriensis, an effective biocontrol agent for citrus postharvest green and blue molds, was identified in our previous study (Liu et al., 2017, 2018b, 2019). Iron depletion by forming maroon pigment has been known as an important action mechanism of Metschnikowia against Botrytis cinerea, Alternaria alternata, Penicillium digitatum, Penicillium expansum, Penicillium italicum, and Aspergillus oryzae (Saravanakumar et al., 2008; Türkel et al., 2014; Liu et al., 2019). Ferric ions and pulcherriminic acid non-enzymatically form pulcherrimin that is the maroon pigment produced by Metschnikowia (MacDonald, 1965; Sipiczki, 2006). In addition to iron depletion, the strong ability of M. citriensis to adhere tightly to the mycelia of pathogens and the surface of fruit as well as form biofilm was also hypothesized to play a key role in its biocontrol activity (Liu et al., 2019). Biofilms are spatially organized and dense communities of microorganisms, and the extracellular polymeric substances (EPS) produced by them consist mainly of water and extracellular biopolymers (polysaccharides, proteins, DNA, and lipids), making them have strong adhesion (Pandin et al., 2017). Biocontrol agent biofilms on the surface of the host tissue that prevent new growth of pathogens and avoid their invasion greatly improve the biocontrol efficacy of antagonistic yeasts against postharvest diseases (Liu et al., 2013).
Biocontrol yeasts do not show the same effect comparable to synthetic fungicides, because the biotic and abiotic stresses such as oxidative, pH, temperature, UV, and osmotic stresses impact the yeast viability and biocontrol activity (Liu et al., 2011, 2018a; Zhao et al., 2012; Sui et al., 2015). When yeast cells are subjected to these environmental stresses, large amounts of ROS accumulate in yeast cells, resulting in severe oxidative damage (Liu et al., 2013; Sui et al., 2015). There is some evidence that yeast antagonists produce relatively large amounts of superoxide anions ( ) and induce transient ROS production in the host (Macarisin et al., 2010). In addition, H2O2 accumulation in response to wounding and pathogen attack in apple and citrus fruit has been studied (Torres et al., 2003, 2011; Macarisin et al., 2007; Su et al., 2011; Buron-Moles et al., 2015). These ROS play a signaling role in the host by controlling the redox of transcription factors or by interacting with other signaling components (e.g., the mitogen-activated protein kinase cascade) to mediate defense gene activation (Zhang and Klessig, 2001; Macarisin et al., 2010; Hershkovitz et al., 2012). The oxidative burst in fruit wounds is due to the reactive oxygen species produced by the yeast and the host, and large amounts of H2O2 are detected in the yeast cells collected from fruit wounds (Droby et al., 2009; Piombo et al., 2018). Survival and reproduction in host wounds are essential for postharvest biocontrol yeasts, and thus finding effective approaches to improve their oxidative stress tolerance is necessary to improve their biocontrol ability.
Proline has showed an antioxidant feature, suggesting direct ROS scavenging feature, functioning as a molecular chaperone able to enhance the activities of ROS scavenging enzymes, and activating alternative detoxification pathways (Matysik et al., 2002; Hoque et al., 2008; Szabados and Savoure, 2010). Proline treatment can diminish ROS levels in fungi and thus inhibits ROS-induced apoptosis (Chen and Dickman, 2005). Under oxidative stress, apoptosis has been reported as a major cause of loss of cell viability for biocontrol yeasts (Chen et al., 2015; Zhang et al., 2017). In addition to its antioxidant feature, proline can also stabilize cellular homeostasis during stress through proline metabolism (Szabados and Savoure, 2010). Although several studies have reported on the strategies to improve oxidative stress resistance of antagonistic yeasts, the application of proline is remain rare. In addition, our preliminary unreported data showed that proline could induce the pigment production of M. citriensis that plays an important role in inhibition of pathogens by depleting iron, but we know little about its role in response to oxidative stress.
The aim of this study was to investigate the effect of proline on oxidative stress tolerance and biocontrol ability of M. citriensis, and the possible mechanisms involved. Moreover, the role of pigment production was elucidated. More specifically, we investigated (1) the survival of M. citriensis exposed to oxidative stress stimulated by different concentrations of H2O2; (2) the effect of proline on oxidative stress tolerance, ROS accumulation, membrane integrity, and apoptosis of M. citriensis cells; (3) the effect of proline on antioxidant enzymes, including CAT and SOD in M. citriensis; and (4) the effect of proline on population dynamics, biofilm formation, and biocontrol ability of M. citriensis against P. digitatum on citrus fruits.
Materials and Methods
Yeast and Pathogen
M. citriensis strain FL01T, an epiphytic yeast of citrus leaves (Liu et al., 2018b), was stored in a tube with nutrient yeast dextrose agar (NYDA) medium [5 g/L yeast extract, 8 g/L nutrient broth, 10 g/L dextrose, 20 g/L agar (Aobox, China)]. The yeast culture was incubated at 28°C at 200 rpm for 16 h to reach the mid-log phase from an initial concentration of 1 × 105 cells/ml. In addition, the P. digitatum strain was obtained from citrus fruits showing symptoms of disease, and its internal transcribed spacer (ITS) region was sequenced (unpublished data). The mold stock culture was incubated at 25°C for 7 days on potato dextrose agar (PDA) medium [200 g/L potatoes, 20 g/L dextrose, 20 g/L agar (Aobox, China)], and spores suspension was obtained by flooding the spores of the 7-day culture with sterile distilled water (SDW) and then filtering through four layers of sterile gauze.
Fruits
Citrus fruits [Citrus sinensis (L.) Osbeck cv. Olinda Valencia orange] were harvested at commercial harvest maturity from a conventional orchard (Zhongxian, Chongqing). The fruits were superficially disinfected with sodium hypochlorite (2% v/v) for 2 min, washed with tap water, and dried in the air prior to further use.
Evaluation of Survival of M. citriensis Under Oxidative Stress
The oxidative stress tolerance of M. citriensis was investigated according to the method of Zhang et al. (2017) with slight modification. The mid-log phase yeast cells were harvested by centrifugation (8,000 × g, 5 min) and then washed twice with SDW. The yeast cells were re-suspended in fresh nutrient yeast dextrose broth (NYDB), and the concentration was adjusted to 5 × 107 cells/ml and then exposed to 0, 20, 40, 60, and 80 mM H2O2 at 28°C, 150 rpm for 90 min. After that, yeast cells were collected by centrifugation and then washed twice. Serial 10-fold dilutions of the samples to 5 × 103 cells/ml were made, and 50 μl of diluted cultures was spread on NYDA medium. After 2 days of incubation at 28°C, the number of colony-forming units per milliliter (CFU/ml) was calculated. The viability of M. citriensis under moderately lethal oxidative stress was expressed as a percentage of the number of colonies with and without H2O2 exposure. For each treatment, there were three replicates and the experiment was repeated three times.
Tests for Pigment Production and Intracellular Iron Content
A loopful of the cell suspension (1 × 108 cells/ml) of M. citriensis was streaked onto the NYDA plate amended with or without 1 mM proline to test pigment production. Intracellular iron levels were measured according to the method of Xu et al. (2014) with slight modification, relying on the phenomenon that bathophenanthroline disulfonate (BPS) forms a colored complex with iron. The yeast cells (1 × 105 cells/ml) were incubated in NYDB medium (control) and NYDB medium supplemented with (1) 1 mM proline (the determination of concentration was based on preliminary unreported data), (2) 10 μM tropolone that has strong chelating ability for iron ions, and (3) 10 μM FeCl3. The mid-log phase yeast were harvested by centrifugation and washed twice with SDW and then re-suspended in 500 μl HNO3 (3%). When the cells are completely digested after boiled for 2 h, 400 μl of supernatant was transferred to a new tube and mixed with 160 μl ascorbic acid (34 mg/ml), 126 μl ammonium acetate (4 M), and 320 μl BPS (1.7 mg/ml). After incubation for 10 min at room temperature, the absorbance of the BPS-Fe complex at 535 nm and the nonspecific absorbance at 680 nm were measured by the Multiskan Spectrum microplate spectrophotometer (BioTek Instrument Inc., USA). The iron content was displayed in arbitrary units (A.U.) and calculated as follows: (OD535 − OD680)/the number of cells. For each treatment, there were three replicates and the experiment was repeated three times.
Effect of Proline on Oxidative Stress Tolerance of M. citriensis
The effect of proline on cell viability of M. citriensis after exposure to H2O2 was determined according to the method of Zhang et al. (2017), with some modification. The yeast cells were incubated in NYDB medium and NYDB medium containing (1) 1 mM proline and (2) 10 μM tropolone, and the cell concentration was adjusted to 1 × 105 cells/ml. After overnight cultivation, cells were harvested by centrifugation. The cells were washed three times with SDW and then re-suspended in fresh NYDB medium, and the concentration was adjusted to 5 × 107 cells/ml. After that, the cells were exposed to 40 mM H2O2 for 90 min. Untreated yeast cells without exposure to H2O2 were used as controls. Survival rates were evaluated by the aforementioned method. Moreover, after exposure to oxidative stress, the concentration of yeast cells was adjusted to 1 × 106 cells/ml for the yeast spotting assay, and then a 5 μl yeast sample was seeded onto NYDA medium and cultured at 28°C. For each treatment, there were three replicates and the experiment was repeated three times.
Imaging of Intracellular ROS
The intracellular ROS production in M. citriensis was detected according to the method of Liu et al. (2011) with minor modification, using 2′, 7′-dichlorodihydrofluorescein diacetate (DCHF-DA, Molecular Probe) (Sigma, USA), a oxidant-sensitive probe. The mid-log phase yeast cells (untreated, proline-treated, tropolone-treated, and FeCl3-treated) were harvested as described above and then treated with 40 mM H2O2 for 90 min. After that, yeast cells were harvested by centrifugation and then washed twice with phosphate buffered saline (PBS, pH 7.0). The yeast cells were re-suspended with an equal volume of PBS with DCHF-DA at a final concentration of 25 μM and then incubated at 30°C in the dark. After an hour, yeast cells were washed twice with PBS and then detected under an Eclipse TS100 epifluorescence microscope (Nikon Instrument Inc., Japan) with excitation at 485 nm and emission at 530 nm. The percentage of stained cells was calculated, and each slide was randomly selected for six fields of view, each field containing at least 100 cells. Untreated yeast cells without exposure to H2O2 were used as controls. For each treatment, there were three replicates and the experiment was repeated three times.
Analysis of Membrane Integrity and Apoptosis of M. citriensis Cells Under Oxidative Stress
Membrane integrity and apoptosis of M. citriensis cells were analyzed according to the method of Chen et al. (2015), with minor modification. The mid-log phase yeast cells (untreated, proline-treated, tropolone-treated, and FeCl3-treated) were harvested as described above and then treated with 40 mM H2O2 for 90 min. Untreated yeast cells without exposure to H2O2 were used as controls. After that, yeast cells were harvested by centrifugation and then washed twice with PBS (pH 7.0). The yeast cells were re-suspended with an equal volume of PBS containing propidium iodide (PI, 10 μg/ml) and Hoechst 33342 (5 μg/ml) for 20 min at room temperature in the dark and then washed twice with PBS. After that, yeast cells were observed using an Eclipse TS100 epifluorescence microscope. PI-positive yeast cells indicated dead cells with damaged plasma membranes, including necrosis, necroptosis, and pyroptosis (Dixon and Stockwell, 2014; Nagata and Nakano, 2017). Hoechst 33342-positive and PI-negative cells indicated apoptotic cells (Hong et al., 2007). The percentage of apoptotic cells and plasma membrane integrity were calculated respectively, and each slide was randomly selected for six fields of view, each field containing at least 100 cells. For each treatment, there were three replicates and the experiment was repeated three times.
Assays of CAT and SOD Activities
Yeast cells (untreated and proline-treated) at mid-log phase were harvested by centrifugation as described above and then exposed to 40 mM H2O2. Extracts of enzymes of CAT and SOD were prepared according to the method of Zhang et al. (2017), with some modification. The yeast cells were collected by centrifugation (8,000 × g, 5 min) at specific intervals (0, 30, 60, and 90 min) after being treated with 40 mM H2O2, and then washed twice with SDW. The cells were suspended in cold 50 mM PBS (pH 7.0) containing 2 mM phenylmethanesulfonyl fluoride and 1 mM EDTA for CAT and SOD extraction by vortexing with glass beads to break the cell walls. After centrifugation at 10,000 × g for 20 min at 4°C, the supernatant was enzyme extract.
One hundred microliters of enzyme extract was mixed with 1.4 ml of 40 mM H2O2 to test CAT activity, and the absorbance at 240 nm was measured every 30 s for 5 min to determine the decomposition of H2O2. Inactivated enzyme extracts boiled for 5 min were used as controls. One unit of CAT activity was the amount of enzyme required to decompose 1 μM H2O2 per min. The reaction mixtures with SOD consisted of 50 μl enzyme extract and 2.95 ml 50 mM PBS containing 2 μM riboflavin, 13 mM methionine, 10 μM EDTA, and 75 μM nitroblue tetrazolium. After being illuminated by light (4,000 lx) for 20 min, the absorbance at 560 nm was determined. Non-illuminated solutions were used as controls. One unit of SOD activity was the amount of enzyme, which causes 50% of NBT reduction. The activities of both enzymes were expressed as U/mg protein. The determination of protein was based on the method of Bradford (1976). For each treatment, there were three replicates and the experiment was repeated three times.
Population Growth of M. citriensis in Wounds
The mid-log phase yeast cells (untreated and proline-treated) were harvested and then treated with 40 mM H2O2 for 90 min as described above. Untreated yeast cells without exposure to H2O2 were used as a control. The wounds of fruit samples (approximately 3 mm wide × 2 mm deep) were made with a sterile needle on superficially sanitized citrus fruits (three wounds per fruit). Twenty microliters of yeast suspension (1 × 108 cells/ml) was individually pipetted to the wounds, and then fruits were placed in plastic bags either incubated at 25 or 4°C. Tissue samples containing the whole wound of fruits were extracted after 0, 2, 4, 6, and 8 days of incubation at 25°C (after a 1-h incubation as the time 0), and the samples of fruits were extracted after 0, 5, 10, 15, and 20 days of incubation at 4°C, using a sterile cork borer. Each sample was ground in 10 ml PBS using a pestle and mortar, and 50 μl of diluted cultures was spread on NYDA plates after making serial 10-fold dilutions. Colonies were counted after 48 h of incubation at 28°C, and the population density was expressed as the Log10 CFU/wound. For each treatment, there were three replicates with five fruits per replicate and the experiment was repeated twice.
Biocontrol Assay of M. citriensis
Biocontrol ability of M. citriensis was evaluated on citrus fruits according to the method of Liu et al. (2017), with slight modification. The untreated (Treatment II) and proline-treated (Treatment III) yeast cells were harvested as described above and then treated with 40 mM H2O2 for 90 min. Untreated yeast cells without exposure to H2O2 were cells of Treatment I. Two wounds of fruit samples (approximately 3 mm wide × 2 mm deep) were made at the equator, using a sterile needle. Twenty microliters of yeast suspension (1 × 108 cells/ml) of each treatment group was inoculated into each wound, and the wounds inoculated with SDW were used as controls. After 4 h, 10 μl of P. digitatum spores suspension (1 × 105 spores/ml) was pipetted to each wound. The treated fruits were then stored in enclosed plastic trays at 25 and 4°C, putting wet paper towels in the trays to increase the humidity. The disease incidence (DI) and lesion diameter (ID) of the fruits were recorded daily and every 3 days, respectively, after ID of the controls grew to measurable sizes. The DI of treated fruits was calculated as a percentage of the number of decayed wounds and total wounds, and the LD was computed as mean value of long and short diameters of the damaged area. Fruits with LD more than 3mm (the size of wound) were considered to be decayed. For each treatment, there were three replicates with five fruits per replicate and the experiment was repeated twice.
Biofilm Formation of M. citriensis
Biofilm formation ability of M. citriensis on citrus fruits was analyzed using scanning electron microscopy (SEM). The fruits were treated and maintained as described above. After incubation for 26 days at 4°C, tissue samples were taken with a sterile knife and washed three times with 0.1 mM PBS (pH 6.8). Samples were then fixed in 0.1 mM PBS containing 2.5% (v/v) glutaraldehyde for 12–24 h at 4°C and dehydrated in ethanol series (30, 50, 70, 80, 90, and 100% twice). After that, the ethanol was replaced by tertiary butyl alcohol series (50, 70, 90, 95, and 100% twice). The samples were then dried at 65°C for 2 h with a DZF-6051 vacuum drying oven (Shanghai Jinghong Experimental Equipment Co., Ltd., China), and the dried samples were coated with a thin layer of gold before viewing in a JEOL JSM-6510LV SEM (JEOL Ltd., Japan).
Biofilm formation ability of M. citriensis in vitro was investigated according to the method of Parafati et al. (2015) with slight modification, by measuring the cell adherence to the surface of a polystyrene plate. The yeast cells with an initial concentration of 1 × 105 cells/ml were incubated in NYDB medium amended with or without 1 mM proline and YNB medium supplemented with different concentrations of iron (0, 1, 10, 100 μM). After overnight cultivation, the cells were harvested and washed twice with PBS (pH 7.2). The cell density was adjusted to 1 × 107 cells/ml with yeast nitrogen base (YNB) containing 100 mM glucose, and 100 μl of the cell culture was inoculated into wells of a 96-well polystyrene plate. The wells without yeast suspensions served as controls. After incubation for specific intervals (0, 30, 60, 120 min) at 28°C on a rotary shaker at 75 rpm, the wells were washed twice with PBS. One hundred microliters of 0.4% crystal violet solution was then added to the wells. After being stained for 45 min, the wells were washed four times with SDW. Subsequently, the wells were destained with 200 μl of 95% ethanol for 45 min, and then 100 μl of this solution was transferred to a new 96-well plate. The biofilm formation ability was expressed as crystal violet content and calculated as follows: OD590 of the test wells were subtracted that of the controls. For each treatment, there were three replicates and the experiment was performed twice.
Mutagenesis and mutant characterization were according to the method of Sipiczki (2006), with slight modification. Yeast cells in overnight NYDB culture (28°C, 100 rpm for 12 h) were collected by centrifugation (8,000 × g, 5 min, 10°C). The cells were re-suspended in fresh NYDB medium containing 300 μg/ml 1-methyl-3-nitro-1-nitrosoguanidine (Sigma-Aldrich, USA), and the cell concentration was adjusted to 1 × 107 cells/ml and then incubated at 28°C, 100 rpm for 30 min. After that, diluted cultures were spread on PDA plates supplemented with 10 μg/ml FeCl3 and incubated at 28°C for 3 days without light. The colonies that have narrower pigmented halos and are lighter in color than wild-type M. citriensis were isolated, and the mutagenized isolates whose growth ability that evaluated by OD600 significantly weaker than that of wild-type were discarded. The remaining candidate mutagenized isolates were then purified by single cell separation. To test the stability of their low-pigment phenotype, the mutagenized isolates were cultured at least 5 times. A loopful of the cell suspension (1 × 108 cells/ml) was streaked onto the PDA plates to test pigmentation, and the biofilm formation in vitro of the mutants was tested as described above.
Statistical Analysis
Data from experiments were analyzed by using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). The arithmetic means were calculated and analyzed by using one-way analysis of variance (ANOVA) in all repeated experiments. The difference between the means were analyzed by Duncan’s multiple range test and considered significant at p < 0.05.
Results
Evaluation of Survival of M. citriensis Under Oxidative Stress
Under the oxidative stress of H2O2 (ranging from 0 to 80 mM) for 90 min, the cell viability decreased gradually with the increasing concentration of H2O2. The cells exposed to 20 mM H2O2 exhibited 62% viability and 47% at 40 mM H2O2. Therefore, the oxidative stress imposed by 40 mM H2O2 for 90 min was semi-lethal to M. citriensis cells, and this oxidative stress condition was chosen for subsequent experiments (Figure 1).
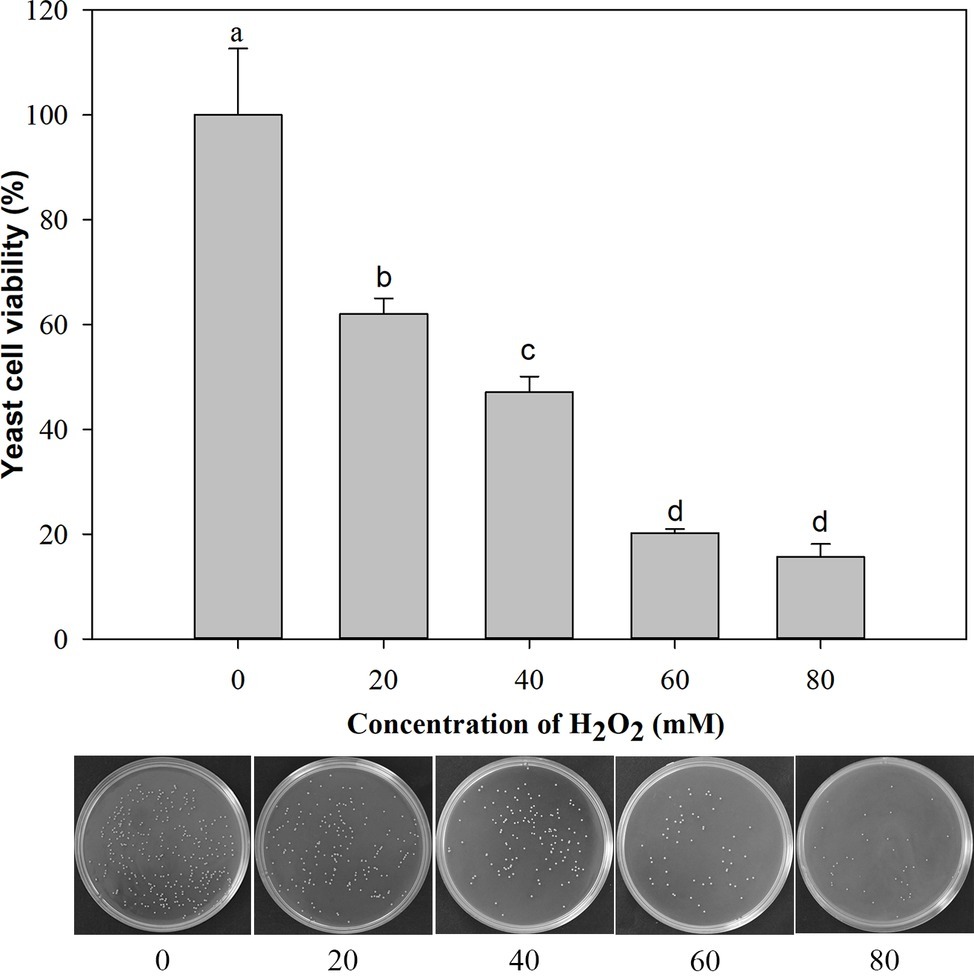
Figure 1. Viability of Metschnikowia citriensis exposed to oxidative stress (a series of concentrations of H2O2) for 90 min. The image of petri dishes shows the number of colony-forming units on NYDA medium after treatment with a series of concentrations of H2O2. Vertical bars represent standard errors of the mean. Data in columns with different letters are significantly different according to Duncan’s multiple range test (p < 0.05).
Tests for Pigment Production and Intracellular Iron Content
Proline treatment increased the pigment production of M. citriensis (Figure 2A), since proline-treated cells showed wider pigmented halo on NYDA plates compared with untreated yeast cells (control). In addition, the intracellular iron content of M. citriensis incubated in the NYDB medium containing proline or iron chelator tropolone was shown to be significantly lower, while that of FeCl3-treated cells were significantly higher than the control (Figure 2B). Therefore, maroon pigment formed by chelated iron decreased the intracellular iron content of M. citriensis.

Figure 2. The effect of proline treatment on pigment production and intracellular iron content of Metschnikowia citriensis. (A) Pigment production of M. citriensis on NYDA plates amended with or without proline. (B) Metal iron contents in the untreated (control) and proline-treated cells were quantified by the BPS-based colorimetric method. The tropolone-treated cells were used as a positive control, and FeCl3-treated cells were used as a negative control. Vertical bars represent standard errors of the mean. Data in columns with different letters are significantly different according to Duncan’s multiple range test at a 5% level.
Effect of Proline on Oxidative Stress Tolerance of M. citriensis
The viability of both proline-treated and untreated (control) yeast cells exposed to 40 mM H2O2 for 90 min was measured. Oxidative stress imposed by H2O2 significantly reduced the survival rate of M. citriensis. As indicated in Figure 3, the viability of M. citriensis could be greatly increased by proline (77%), compared with the control. Though the cell viability of M. citriensis incubated in NYDB amended with tropolone (60%) was not as high as that incubated in the NYDB amended with proline, tropolone-treated cells also showed a modest increase in cell viability compared with the control (55%).
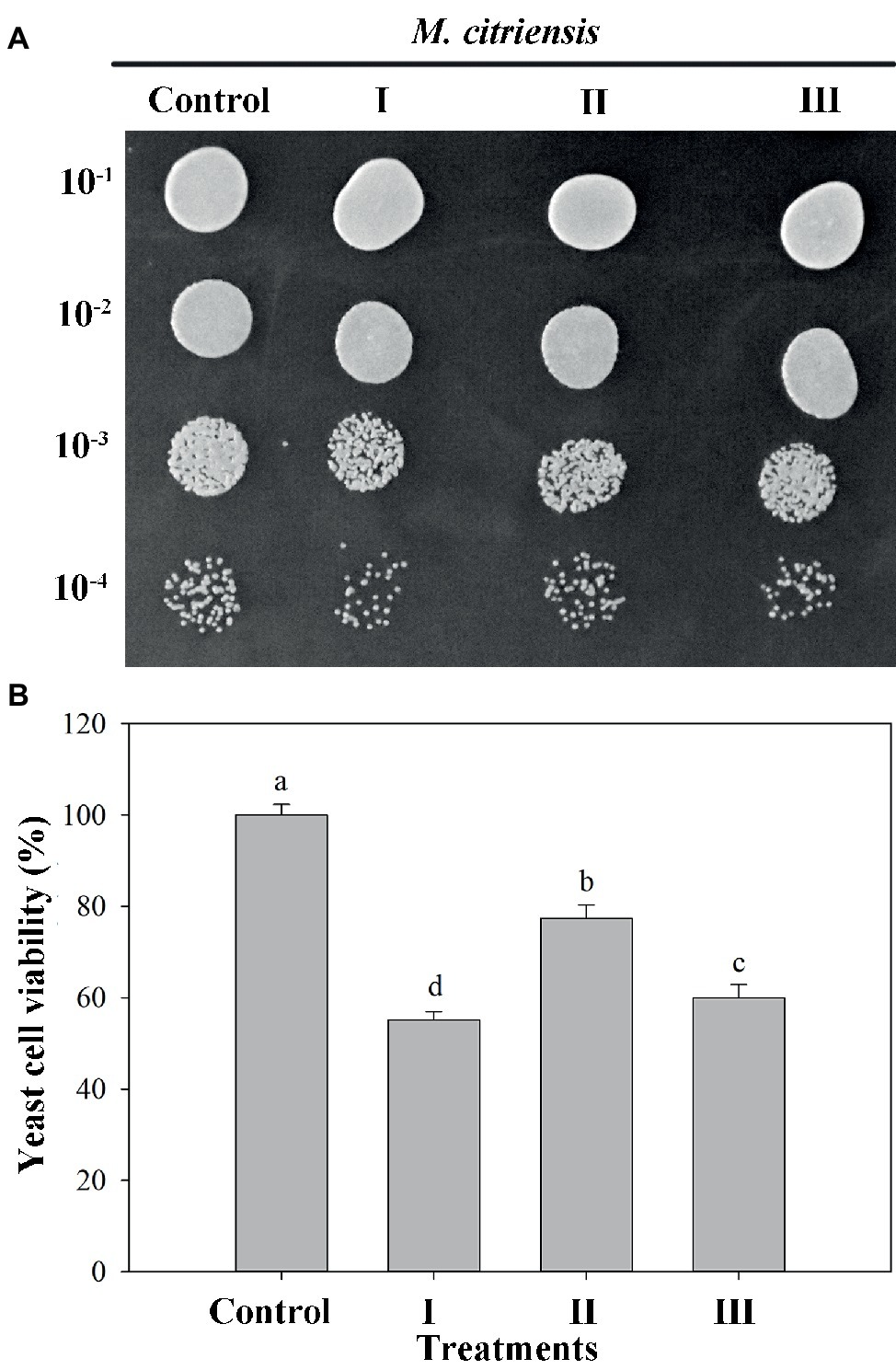
Figure 3. Viability of Metschnikowia citriensis under moderately lethal oxidative stress. (A) Image of the yeast spotting assay of viability of M. citriensis following exposure to 40 mM H2O2. (B) Viability of yeast cells following exposure to 40 mM H2O2. (I) Cells harvested from NYDB medium, (II) cells harvested from NYDB medium containing 1 mM proline, and (III) cells harvested from NYDB medium containing 10 μM tropolone were exposed to 40 mM H2O2 for 90 min. The cells harvested from NYDB medium without exposure to H2O2 were used as controls. The vertical bars represent standard errors of three replicates. Data in columns with different letters are significantly different according to the Duncan’s multiple range test (p < 0.05).
Imaging of Intracellular ROS
Intracellular ROS production was determined using the fluorescent dye DCHF-DA (Figure 4). Prior to H2O2 treatment (control), the percentage of ROS-positive cells was 2.3%. However, after H2O2 treatment for 90 min, the percentages of ROS of proline-treated and untreated cells exhibiting a visible ROS level were 18.0 and 33.5%, respectively. These results showed that oxidative stress could cause large amount accumulation of intracellular ROS in M. citriensis, but proline treatment decreased intracellular ROS level. In addition, tropolone-treated cells showed a significantly lower ROS level, compared with untreated cells.
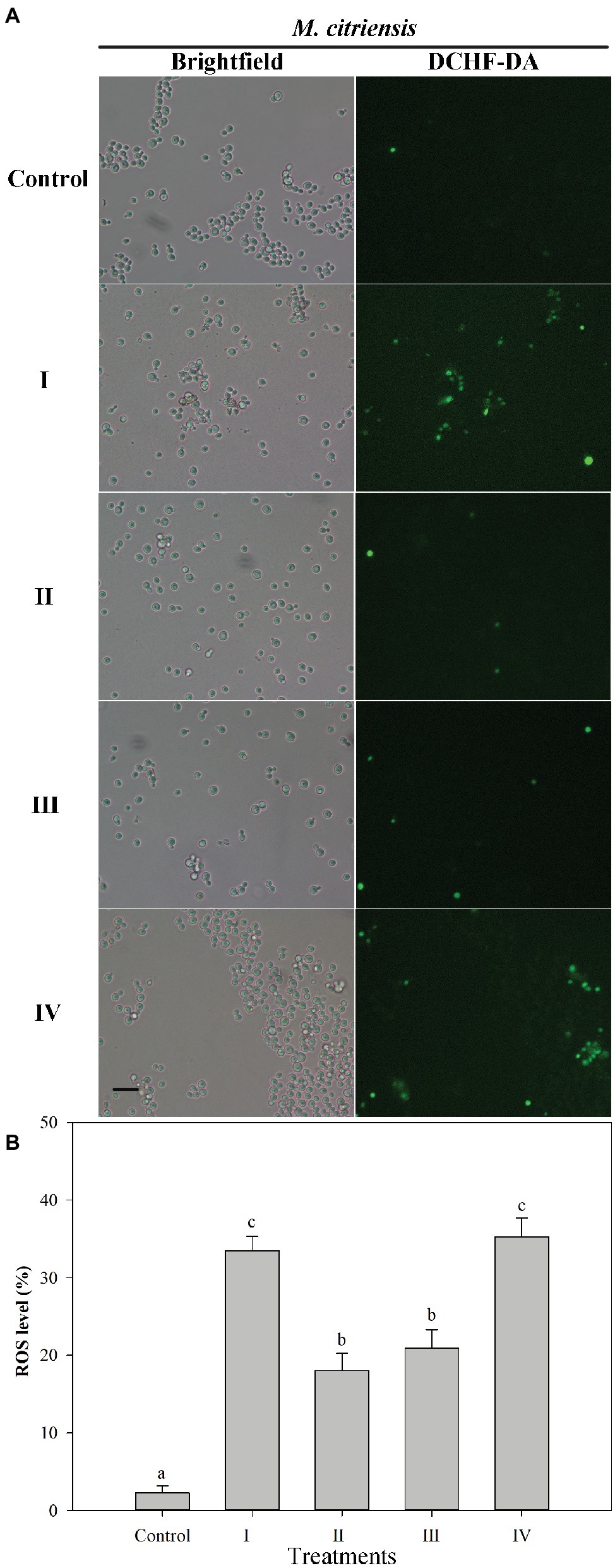
Figure 4. Intracellular ROS level in Metschnikowia citriensis under oxidative stress. (A) Fluorescence microscopic images of M. citriensis cells stained with the fluoroprobe DCHF-DA. (B) Percentage of M. citriensis cells exhibiting visible ROS accumulation. (I) Cells harvested from NYDB medium, (II) cells harvested from NYDB medium containing 1 mM proline, (III) cells harvested from NYDB medium containing 10 μM tropolone, and (IV) cells harvested from NYDB medium containing 10 μM FeCl3 were exposed to 40 mM H2O2 for 90 min. The cells harvested from NYDB medium without exposure to H2O2 were used as controls. The vertical bars represent standard errors of three replicates. Data in columns with different letters are significantly different according to the Duncan’s multiple range test (p < 0.05). Bar = 20 μm.
Analysis of Membrane Integrity and Apoptosis of M. citriensis Under Oxidative Stress
Membrane integrity and apoptosis of M. citriensis were demonstrated using Hoechst 33342 and PI staining (Figure 5). The dead yeast cells with damage of plasma membranes were detectable, since they were stained by PI and hence appeared red fluorescence (Figure 5A). Following exposure to 40 mM H2O2 for 90 min, the PI-negative/Hoechst33342-positive apoptotic cells (Figure 5C) and the dead yeast cells with damaged plasma membranes with PI-positive/Hoechst33342-positive (Figure 5B) increased to 32 and 6%, respectively. Proline treatment decreased the apoptosis to 20% and increased the plasma membrane integrity (97%) to the level comparable to untreated cells without exposure to H2O2 (control). Similarly, tropolone treatment also decreased the apoptosis to 21%. In addition, the dead yeast cells with damaged plasma membranes (7%) increased through cultivation in the NYDB media amended with FeCl3. These results indicated that exposure of M. citriensis cells to exogenous H2O2 can trigger iron-dependent apoptosis, which can be inhibited by an iron chelator, and increased iron concentration can promote plasma membrane damage under oxidative stress.
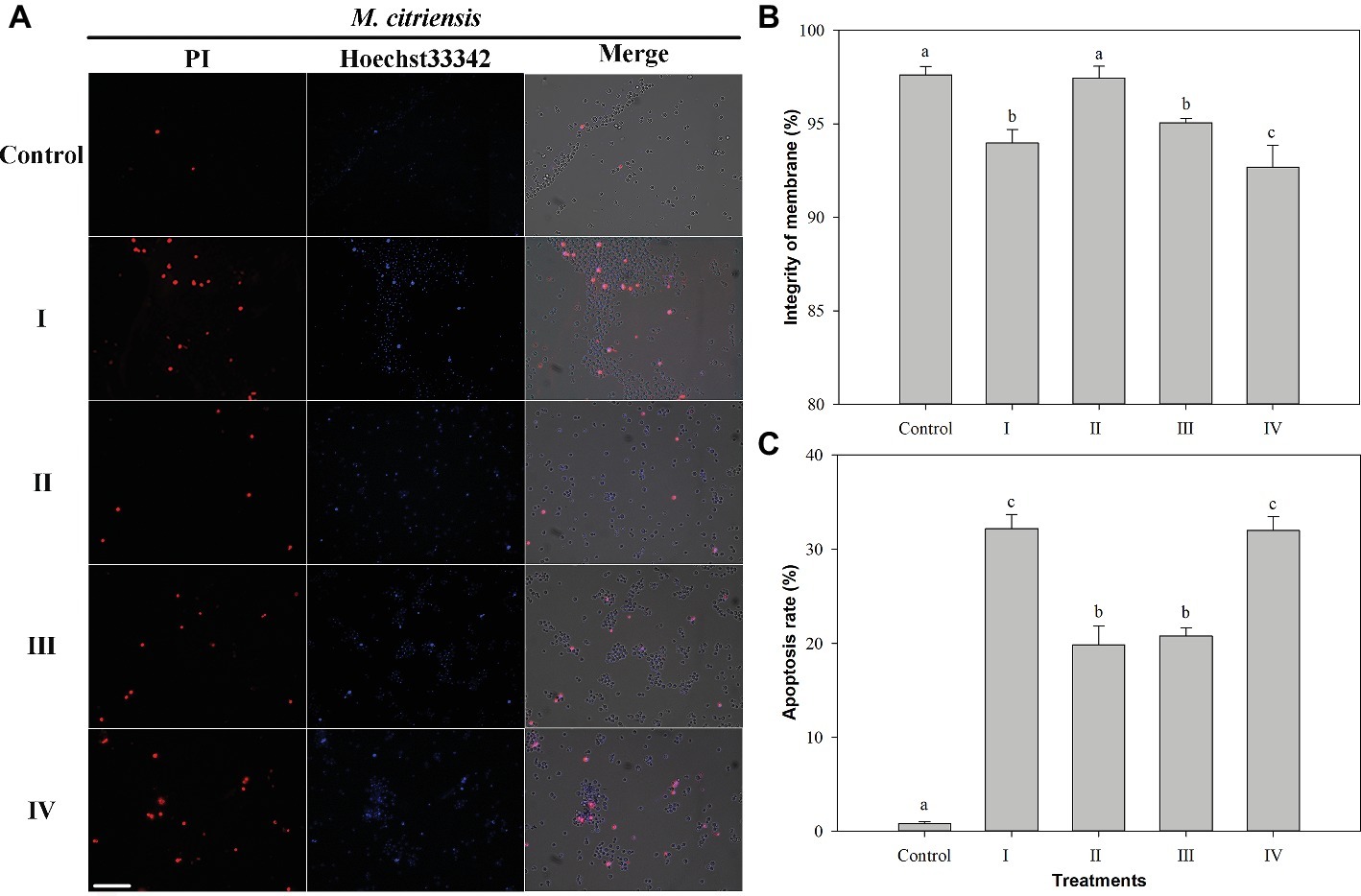
Figure 5. Membrane integrity and apoptosis analysis of Metschnikowia citriensis under oxidative stress. (A) Fluorescence microscopic images of M. citriensis cells double stained with PI/Hoechst 33342. (B) Percentage of plasma membrane integrity of M. citriensis cells. (C) Apoptosis rate of M. citriensis cells. (I) Cells harvested from NYDB medium, (II) cells harvested from NYDB medium containing 1 mM proline, (III) cells harvested from NYDB medium containing 10 μM tropolone, and (IV) cells harvested from NYDB medium containing 10 μM FeCl3 were exposed to 40 mM H2O2 for 90 min. The cells harvested from NYDB medium without exposure to H2O2 were used as controls. The vertical bars represent standard errors of three replicates. Data in columns with different letters within each parameter are significantly different according to the Duncan’s multiple range test (p < 0.05). Bar = 20 μm.
Assays of CAT and SOD Activities
Given that antioxidants, including CAT and SOD, are critical to protecting cells against oxidative stress by maintaining and H2O2 at low levels (Chen and Dickman, 2005), the status of the activities of these scavenging enzymes in M. citriensis under moderately lethal oxidative stress was evaluated. Following exposure to 40 mM H2O2 for 30 min, the untreated cells only showed a slight increase in CAT activity. Interestingly, proline-treated cells caused a nearly eightfold increase in CAT activity compared with untreated cells after 60 min of exposure to H2O2, and CAT activity continued to rise up to 90 min (Figure 6A). Moreover, addition of proline to the medium of M. citriensis increased SOD activity (time 0), and SOD activity of the proline-treated cells maintained a higher level than that of the untreated cells after 0–30 min of exposure to H2O2 (Figure 6B). In contrast, exogenous oxidative stress did not increase SOD activity of the untreated cells until 30 min of incubation while that in the proline-treated cells started to decrease. These data suggested that proline treatment induced a significant increase in SOD activity of M. citriensis in the early stage and increased CAT activity in the later stage, during oxidative stress.
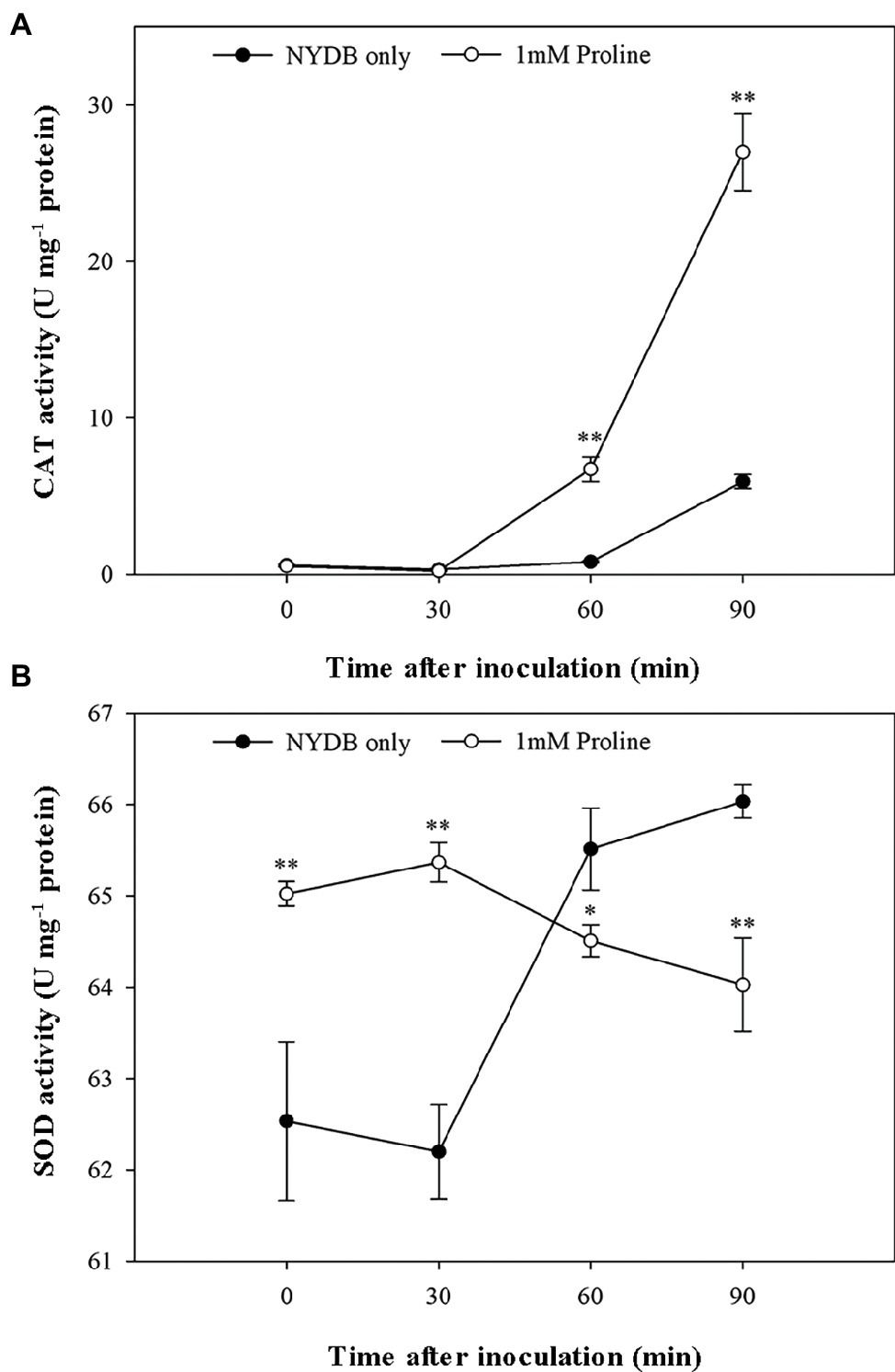
Figure 6. CAT and SOD activity of Metschnikowia citriensis following treatment with moderately lethal concentrations of H2O2. (A) CAT activity of M. citriensis harvested from NYDB medium with or without proline. (B) SOD activity of M. citriensis harvested from NYDB medium with or without proline. The vertical bars represent standard errors of three replicates. “*” indicates a significant difference according to the Duncan’s multiple range test (*p < 0.05, **p < 0.01).
Population Growth of M. citriensis in Wounds
The population growth of M. citriensis harvested from citrus wounds incubated at 25 and 4°C is shown in Figure 7. The untreated cells without exposure to H2O2 (control) multiplied quickly in the wounds and reached a higher level of population density at 25°C (log10 CFU/wound = 7.9). Moreover, the population of control cells reached a maximum (log10 CFU/ wound = 7.9) earlier than the untreated cells with exposure to H2O2 at 4°C. The untreated cells with exposure to H2O2 showed significantly lower populations than the control at almost all time points at 25 and 4°C (Figures 7A,B). These results indicated exogenous oxidative stress significantly inhibited the growth of M. citriensis cells. However, proline increased the population density of M. citriensis in wounds late in the storage (Figures 7A,B). After 8 days of incubation at 25°C, M. citriensis treated with 1 mM proline and 40 mM H2O2 showed a marked higher population than the untreated cells with exposure to H2O2 and the control (Figure 7A). Moreover, the population density of proline-treated cells with exposure to H2O2 reached a higher level at 4°C (log10 CFU/wound = 8.0) than the untreated cells with exposure to H2O2 and the control, though the cell growth rate was not as quick as that of the control (Figure 7B).

Figure 7. Population dynamics of Metschnikowia citriensis in wounds of citrus fruits stored at 25°C (A) and 4°C (B). The M. citriensis cells harvested from NYDB medium with or without 1 mM proline were exposed to 40 mM H2O2 for 90 min and then seeded to the wounds. The cells harvested from NYDB medium without exposure to H2O2 were used as controls. The vertical bars represent standard errors of three replicates. “*” indicates a significant difference from the control according to the Duncan’s multiple range test (p < 0.05).
Biocontrol Assay of M. citriensis
As shown in Figure 8, M. citriensis effectively inhibited the growth of P. digitatum on citrus fruits at 25 and 4°C. The exogenous oxidative stress significantly reduced the biocontrol ability of M. citriensis at 25°C, but not at 4°C. On the 6th day after inoculation at 25°C, the DI value of citrus fruits treated with M. citriensis cells without exogenous oxidative stress (Treatment I) was 23%, whereas DI value of fruits treated with H2O2-exposure cells (Treatment II) was as high as 33%. However, there was no significant difference of the DI or the LD value of the fruits between Treatment I and Treatment II. Obviously, the biocontrol performance of proline-treated cells showed a significant increase. The DI value (10%) and the LD value (7 mm) of citrus fruits treated with proline-treated yeast cells (Treatment III) were significantly lower than those of citrus fruits in Treatment I and Treatment II stored at 25°C (Figures 8A–C). Moreover, compared with the fruits in Treatment I (43%), DI value of the fruits seed with proline-treated cells distinctly lower (30%) after 26 days of incubation at 4°C, while DI value of the control (inoculated with SDW instead of M. citriensis cells) reached 100% (Figure 8D).
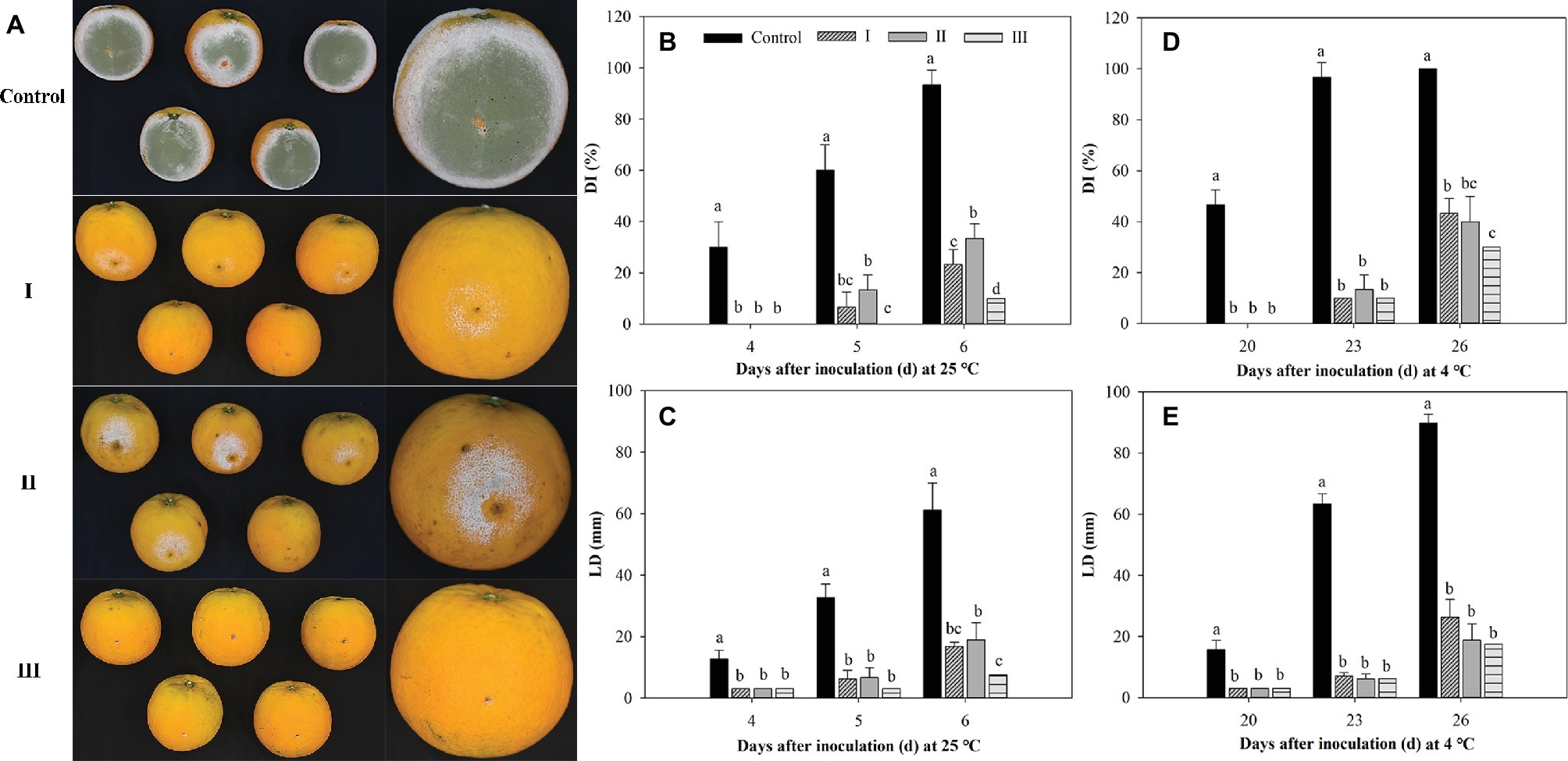
Figure 8. Effect of oxidative stress and proline treatment on the efficacy of Metschnikowia citriensis against Penicillium digitatum on citrus fruits. (A) Biocontrol performance of M. citriensis cells against P. digitatum on the 5th day after inoculation at 25°C. Statistical analysis of (B) decay incidence (DI) and (C) lesion diameters (LD) on citrus fruits after 4, 5, and 6 days of inoculation at 25°C. Statistical analysis of (D) DI and (E) LD on citrus fruits after 20, 23, and 26 days of inoculation at 4°C. The cells harvested from NYDB medium without exposure to H2O2 (I) and cells harvested from NYDB medium amended without (II) or with 1 mM proline (III) were exposed to 40 mM H2O2 for 90 min and then seeded to the wounds. Wounds inoculated with SDW followed by the pathogen were used as controls. Vertical bars represent standard errors of the mean. Data in columns with different letters within each parameter are significantly different according to Duncan’s multiple range test (p < 0.05).
Biofilm Formation of M. citriensis
To investigate whether proline could promote the biofilm formation of M. citriensis, the yeast adherence on the polystyrene surface and the biofilm formation in citrus wounds by SEM were examined. Biofilms are microorganisms communities fixed to a surface and protected by EPS (Chmielewski and Frank, 2003).
SEM documented biofilm formation in the surface of wounds (Figure 9). Exogenous oxidative stress showed little effect on the adhesion capability of M. citriensis, since the number of cells adhered onto each wound was similar to that of the control (the untreated cells without exposure to H2O2). However, after 26 days of storage at 4°C, untreated cells with exposure to H2O2 showed a greater number of depressed and even broken cells compared with the control (Figures 9A1,B1). In vitro, proline-treated cells had a high film-forming capacity after a 30-min incubation, since its OD value was significantly higher than that of the cells cultured in NYDB without proline (Figure 10A). Moreover, the proline-treated cells (Figures 9C1,D1) displayed even heavier cell clusters adhering to wounds after repeated washes, compared with the control. Proline increased EPS production of M. citriensis, and the biofilms of proline-treated cells displayed varied phenotypes, including somewhat flat biofilm structures with large pieces of mucus (Figures 9C1–C3) and more robust and thick biofilms with crystalline EPS (Figures 9D1–D3). These results suggested that exogenous oxidative stress contributed to harmful changes of the cell morphology at low temperature, but proline treatment could protect cells and promote the biofilm formation.

Figure 9. Scanning electron micrographs of biofilm formation of Metschnikowia citriensis in citrus wounds on the 26th day after inoculation at 4°C. The cells harvested from NYDB medium (B1–B3) and cells harvested from NYDB medium containing 1 mM proline (C1–C3,D1–D3) were exposed to 40 mM H2O2 for 90 min and then seeded to the wounds. Arrows show the cell depression (B3), cell rupture (B2), and extracellular matrix of biofilm (C1–C3,D1–D3). The cells harvested from NYDB medium without exposure to H2O2 were used as controls (A1–A3). Magnification of 750× (A1–D1), 2000× (A2–D2), and 3,000× (A3–D3).
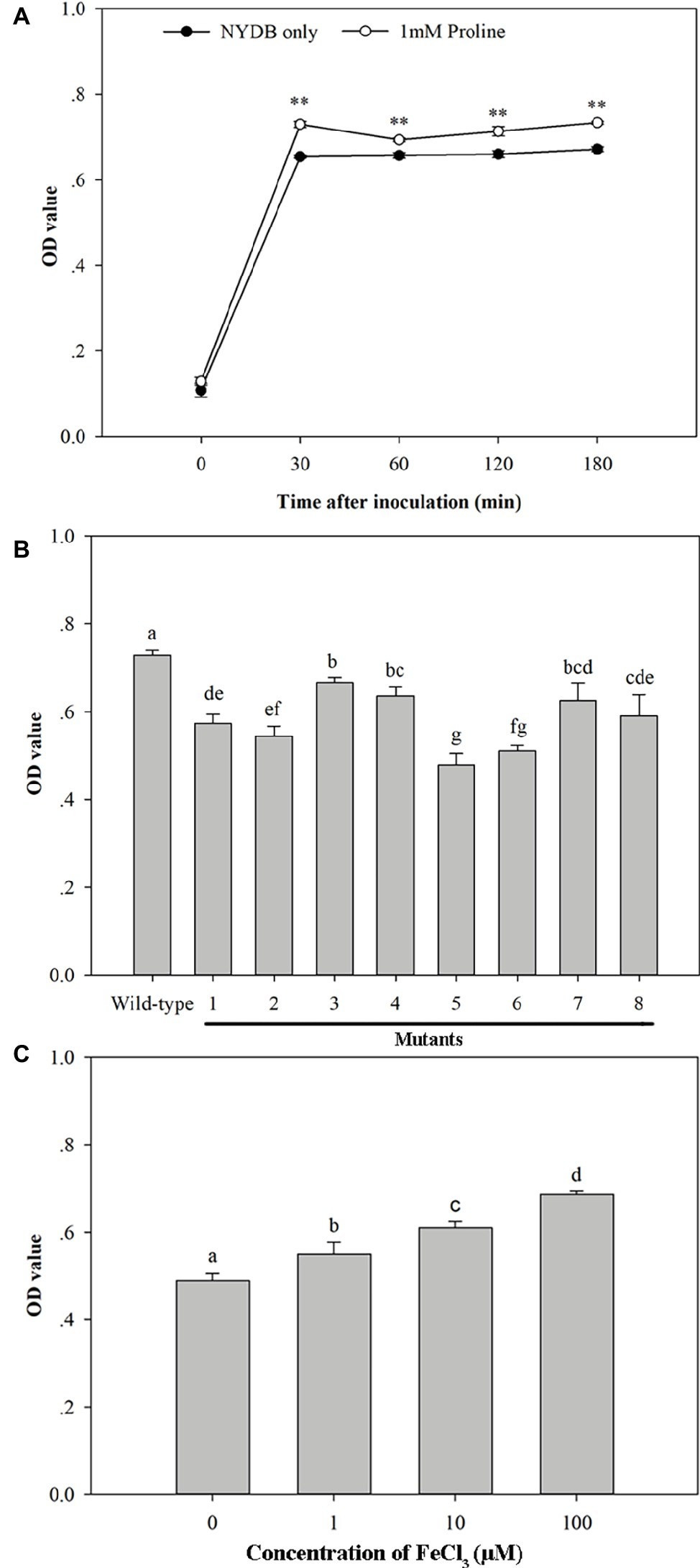
Figure 10. Biofilm formation ability of Metschnikowia citriensis in vitro. (A) Biofilm formation ability of wild-type M. citriensis FL01T harvested from NYDB medium with or without proline. (B) Biofilm formation ability of wild-type and the low-pigment mutants of M. citriensis FL01T harvested from NYDB medium. (C) Biofilm formation ability of wild-type M. citriensis FL01T grew in YNB medium supplemented with different iron concentrations. The vertical bars represent standard errors of three replicates. Data with “**” and different letters within each parameter are significantly different according to the Duncan’s multiple range test (p < 0.01).
Similar to the effect of proline treatment, the pigment production (i.e., the pigmentation of the yeast cultures) and biofilm formation ability of M. citriensis that incubated in YNB supplemented with different concentrations of iron (0, 1, 10, 100 μM) increased with increasing iron concentration (Figure 10C). In contrast, the iron chelator tropolone reduced biofilm formation ability of M. citriensis (data not show). To investigate whether the pigment production of M. citriensis is related to its biofilm formation ability, cells of M. citriensis FL01T were mutagenized with nitrosoguanidine. Eight pink mutants (with various degrees of pigmentation) that had less pigment production than the wild-type colonies (maroon) were selected, and the low-pigment mutants all showed lower capability of biofilm formation (Figure 10B), indicating that the pigment production promotes biofilm formation of M. citriensis.
Discussion
In this study, the effect of proline on oxidative stress tolerance and biocontrol ability of M. citriensis and its possible mechanisms were investigated. We found that inducing pigment production with exogenous substances is a promising approach to enhance the biocontrol ability of M. citriensis.
Metschnikowia strains have been reported to release a diffusible colorless precursor and then the precursor immobilized iron to form a pigment, playing an important role in their antimicrobial activity (Sipiczki, 2006; Parafati et al., 2015). But it is different from the low molecular iron chelators released by other microorganisms to absorb iron in the environment, since this pigment is not soluble in water and observed to cover the outside of the cell, instead of accumulating in the cell (Kluyver et al., 1953). Moreover, the intracellular iron content of M. citriensis decreased with the increase of pigment production, suggesting that the proposed mechanism of releasing this pigment precursor is to prevent excessive accumulation of cellular iron.
Iron is relevant to intracellular ROS production and cell death. Iron and iron derivatives such as [Fe-S] clusters and heme are not only incorporated into ROS-producing enzymes but are also essential for their function, while superoxide and H2O2 can damage [4Fe-4S] clusters of proteins that lead to the release of Fe2+ (Dixon and Stockwell, 2014). The small pools (<20 μM) of Fe2+, which can directly catalyze the formation of destructive free radicals by Fenton chemistry [Fe2+ react with peroxides to form soluble hydroxyl (HO•) or lipid alkoxy (RO•)] and then result in damage to various biomolecules, are redox-active and reside in the mitochondrial matrix and the cytosol of eukaryotic cells (Petrat et al., 2002; Kell, 2009; Dixon and Stockwell, 2014). In addition to reducing intracellular iron levels by pigment formation, proline treatment induced a significant increase in SOD activity in the early stage and increased CAT activity in the later stage of oxidative stress to reduce ROS accumulation in M. citriensis. Similar to proline treatment, the tropolone-treated cells showed an early rise in SOD activity under oxidative stress compared with the control, although their SOD activity was elevated later than the proline-treated cells; tropolone treatment also increased CAT activity of M. citriensis, although their CAT activity was lower than that of proline-treated cells (data not shown). So, tropolone also reduced ROS levels of M. citriensis as a result of reduced iron content and regulation of antioxidant enzyme activity. SOD acts as the first line of defense against ROS in the cells, and it may be the core of the defense mechanism since its activity determines the concentrations of both and H2O2 (Bowler et al., 1992; Blokhina et al., 2003). Although the reactivity of H2O2 is lower than , HO• formation can occur in the Fenton reaction in biological systems when reduced transition metals such as Fe2+ are present (Blokhina et al., 2003). SOD and other enzymes, such as CAT and antioxidants, may have a highly optimized balance that works together to reduce the risk of HO• formation; therefore, discussing the role of SOD requires the entire oxidant stress defense system to be considered as a whole (Bowler et al., 1992). Early rise of SOD activity of M. citriensis could accelerate the conversion of to H2O2, and then the increase in CAT activity could promote the conversion of H2O2 to water.
Apoptosis is a major contributor to the cell viability loss in M. citriensis under oxidative stress, which is consistent with the results of other antagonistic yeasts in previous reports (Chen et al., 2015; Zhang et al., 2017). Proline opposed the ROS- and iron-induced apoptosis of M. citriensis, and the pigment induced by proline played an important role in inhibiting iron-dependent apoptotic cell death. Moreover, proline also prevented M. citriensis from other types of cell death associated with damage of plasma membranes, and proline suppressed which might because of the capability to stabilize cellular homeostasis during stress conditions (Szabados and Savoure, 2010). Therefore, the viability of M. citriensis was greatly increased by proline under oxidative stress.
Exposure of M. citriensis to exogenous H2O2 reduced its ability to grow and survive in wounds at 25 and 4°C and significantly reduced biocontrol efficacy of M. citriensis at 25°C, but not at 4°C, suggesting the effect of population density on its biocontrol efficacy at 4°C is less than that at 25°C. Although oxidative stress reduced the growth ability of M. citriensis, proline-treated cells with exogenous oxidative stress showed better biocontrol efficacy than the untreated cells with exogenous oxidative stress and the control (untreated cells without exogenous oxidative stress) not only at 25°C but also at 4°C, because proline increased pigment production and biofilm formation of M. citriensis. Biofilms generally exist in harsh environmental conditions (Fuqua et al., 1994; Rendueles and Ghigo, 2015), cells in which exhibit increased tolerance to harsh conditions (Whiteley et al., 2001; Harriott and Noverr, 2009; Bridier et al., 2011; Pu et al., 2014). As well as stress tolerance, biocontrol agent biofilms are frequently associated with spatial competition with pathogens, quickly forming layers on the surface of the nutrients and completely saturating the interface with the nutrients to limit nutrients into the pathogen (Habimana et al., 2011). Furthermore, since the presence of EPS in biofilms can locally concentrate the antimicrobial secondary metabolites produced by antagonists (Pandin et al., 2017), M. citriensis cells in biofilms can form higher concentrations of pigment relative to cells in solitary or planktonic forms. Cells in biofilms also exhibit an altered phenotype in growth rate (Harding et al., 2009). We found that the rate of cell growth of proline-treated cells with H2O2-exposure early in the storage was slower than that of the control and even slower than the untreated cells with H2O2-exposure when incubated at 4°C. Such behavior might imply that proline-treated yeast cells were preparing for the switch early in the storage, from growth to adaptation to environmental changes, because the physiological mechanisms of resistance to stress are likely to consume energy for its growth, including polymeric extracellular matrix production and biofilm formation. However, after cells are embedded in a self-secreted EPS, they can benefit from enhanced cell-to-cell communication and protect from stresses and efficient capture of nutrients, thereby promoting their growth (Harding et al., 2009). For this reason, proline enhanced the growth ability of M. citriensis late in the storage might be attributed to an increase in biofilm formation. It seems that the biofilm formation ability, which is one of the key biocontrol mechanisms of M. citriensis, is more important for its biocontrol efficacy, compared with the ability to grow rapidly in wounds.
Microorganisms in biofilms can use specific autoinducers that are signalling molecules allowing them to communicate with the surrounding populations, and these molecules are involved in the formation and development of biofilms (Fuqua et al., 1994; Rendueles and Ghigo, 2015). A vast number of natural signalling molecules regulate this quorum sensing (QS), especially the 2,5-diketopiperazines, and cyclodipeptides (CDPs) that belong to the non-ribosomal peptides and consist of two amino acids linked by peptide bonds (Bonnefond et al., 2011; Seguin et al., 2011; González et al., 2017). Because the intermediates cyclo-L-leucyl-L-leucyl (CDP) and pulcherriminic acid are produced during the formation of the pigment (pulcherrimin) of Metschnikowia from leucine (MacDonald, 1965), a hypothesis is that one of these intermediates might act as a diffusible signal factor to modulate QS and biofilm formation, but purified intermediates for this activity have not been tested. Since increased pigment production was accompanied by an increase in biofilm formation ability of M. citriensis and the low-pigment mutants of M. citriensis FL01T all showed lower capability of biofilm formation, pigment production could promote the biofilm formation of M. citriensis. The pigment production might be a mechanism by which M. citriensis promotes protective biofilm formation against stress conditions.
Conclusion
The results indicated that the application of proline was a useful approach to diminish the intracellular ROS level and enhance the oxidative stress tolerance of M. citriensis by regulating CAT and SOD enzyme activities and reducing intracellular iron content. Moreover, because of the increased pigment production and the robust biofilm formation in wounds, adding proline significantly improved biocontrol efficacy of M. citriensis against P. digitatum in citrus fruits. Pigment production contributed to the reduction of intracellular iron content and the biofilm formation of M. citriensis.
Data Availability
All datasets generated for this study are included in the manuscript.
Author Contributions
YL, CR, LD, and KZ designed the study. YL and SY conducted the experiments. YL, LY, and KZ wrote the manuscript. All authors have read and approved the submitted version.
Funding
This work was financially supported by grants from the National Natural Science Foundation of China (Grant NO. 31772027) and Chongqing Science and Technology Commission (Grant NO. cstc2017shms-xdny80058).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Ab Rahman, S. F. S., Singh, E., Pieterse, C. M., and Schenk, P. M. (2018). Emerging microbial biocontrol strategies for plant pathogens. Plant Sci. 267, 102–111. doi: 10.1016/j.plantsci.2017.11.012
Aktar, W., Sengupta, D., and Chowdhury, A. (2009). Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip. Toxicol. 2, 1–12. doi: 10.2478/v10102-009-0001-7
Blokhina, O., Virolainen, E., and Fagerstedt, K. V. (2003). Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann. Bot. 91, 179–194. doi: 10.1093/aob/mcf118
Bonnefond, L., Arai, T., Sakaguchi, Y., Suzuki, T., Ishitani, R., and Nureki, O. (2011). Structural basis for nonribosomal peptide synthesis by an aminoacyl-tRNA synthetase paralog. Proc. Natl. Acad. Sci. USA 108, 3912–3917. doi: 10.1073/pnas.1019480108
Borriss, R. (2015). “Bacillus, a plant-beneficial bacterium” in Princ plant-microbe interact. ed. B. Lugtenberg (Cham, Switzerland: Springer International Publishing), 379–391.
Bowler, C., Montagu, M. V., and Inze, D. (1992). Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. 43, 83–116. doi: 10.1146/annurev.pp.43.060192.000503
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. doi: 10.1016/0003-2697(76)90527-3
Bridier, A., Dubois-Brissonnet, F., Greub, G., Thomas, V., and Briandet, R. (2011). Dynamics of the action of biocides in Pseudomonas aeruginosa biofilms. Antimicrob Agents Chemother. 55, 2648–2654. doi: 10.1128/aac.01760-10
Buron-Moles, G., Torres, R., Teixidó, N., Usall, J., Vilanova, L., and Viñas, I. (2015). Characterisation of H2O2 production to study compatible and non-host pathogen interactions in orange and apple fruit at different maturity stages. Postharvest Biol. Technol. 99, 27–36. doi: 10.1016/j.postharvbio.2014.07.013
Chen, C. B., and Dickman, M. B. (2005). Proline suppresses apoptosis in the fungal pathogen Colletotrichum trifolii. Proc. Natl. Acad. Sci. USA 102, 3459–3464. doi: 10.1073/pnas.0407960102
Chen, J., Li, B. Q., Qin, G. Z., and Tian, S. P. (2015). Mechanism of H2O2-induced oxidative stress regulating viability and biocontrol ability of Rhodotorula glutinis. Int. J. Food Microbiol. 193, 152–158. doi: 10.1016/j.ijfoodmicro.2014.10.025
Chmielewski, R. A. N., and Frank, J. F. (2003). Biofilm formation and control in food processing facilities. Compr. Rev. Food Sci. F. 2, 22–32. doi: 10.1111/j.1541-4337.2003.tb00012.x
Dixon, S. J., and Stockwell, B. R. (2014). The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 10, 9–17. doi: 10.1038/nchembio.1416
Droby, S., Wisniewski, M., Macarisin, D., and Wilson, C. (2009). Twenty years of postharvest biocontrol research: is it time for a new paradigm? Postharvest Biol. Technol. 52, 137–145. doi: 10.1016/j.postharvbio.2008.11.009
Droby, S., Wisniewski, M., Teixidó, N., Spadaro, D., and Jijakli, M. H. (2016). The science, development, and commercialization of postharvest biocontrol products. Postharvest Biol. Technol. 122, 22–29. doi: 10.1016/j.postharvbio.2016.04.006
Fuqua, W. C., Winans, S. C., and Greenberg, E. P. (1994). Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 176, 269–275. doi: 10.1128/jb.176.2.269-275.1994
González, O., Ortíz-Castro, R., Díaz-Pérez, C., Díaz-Pérez, A. L., Magaña-Dueñas, V., López-Bucio, J., et al. (2017). Non-ribosomal peptide synthases from Pseudomonas aeruginosa play a role in cyclodipeptide biosynthesis, quorum-sensing regulation, and root development in a plant host. Microb. Ecol. 73, 616–629. doi: 10.1007/s00248-016-0896-4
Habimana, O., Guillier, L., Kulakauskas, S., and Briandet, R. (2011). Spatial competition with Lactococcus lactisin mixed-species continuous-flow biofilms inhibits Listeria monocytogenes growth. Biofouling 27, 1065–1072. doi: 10.1080/08927014.2011.626124
Harding, M. W., Marques, L. L., Howard, R. J., and Olson, M. E. (2009). Can filamentous fungi form biofilms? Trends Microbiol. 17, 475–480. doi: 10.1016/j.tim.2009.08.007
Harriott, M. M., and Noverr, M. C. (2009). Candida albicans and Staphylococcus aureus form polymicrobial biofilms: effects on antimicrobial resistance. Antimicrob. Agents Chemother. 53, 3914–3922. doi: 10.1128/AAC.00657-09
Hazell, P., and Wood, S. (2008). Drivers of change in global agriculture. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 363, 495–515. doi: 10.1098/rstb.2007.2166
Hershkovitz, V., Ben-Dayan, C., Raphael, G., Pasmanik-Chor, M., Liu, J., Belausov, E., et al. (2012). Global changes in gene expression of grapefruit peel tissue in response to the yeast biocontrol agent Metschnikowia fructicola. Mol. Plant Pathol. 13, 338–349. doi: 10.1111/j.1364-3703.2011.00750.x
Hong, S., Lee, J. E., Kim, C. Y., and Seong, G. J. (2007). Agmatine protects retinal ganglion cells from hypoxia-induced apoptosis in transformed rat retinal ganglion cell line. BMC Neurosci. 8:81. doi: 10.1186/1471-2202-8-81
Hoque, M. A., Banu, M. N. A., Nakamura, Y., Shimoishi, Y., and Murata, Y. (2008). Proline and glycine betaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J. Plant Physiol. 165, 813–824. doi: 10.1016/j.jplph.2007.07.013
Kell, D. B. (2009). Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genet. 2:2. doi: 10.1186/1755-8794-2-2
Kluyver, A. J., Van Der Walt, J. P., and Van Triet, A. J. (1953). Pulcherrimin, the pigment of Candida pulcherrima. Proc. Natl. Acad. Sci. USA 39, 583–593.
Kurtzman, C. P., and Droby, S. (2001). Metschnikowia fructicola, a new ascosporic yeast with potential for biocontrol of postharvest fruit rots. Syst. Appl. Microbiol. 24, 395–399. doi: 10.1078/0723-2020-00045
Liu, J., Sui, Y., Wisniewski, M., Droby, S., and Liu, Y. (2013). Review: utilization of antagonistic yeasts to manage postharvest fungal diseases of fruit. Int. J. Food Microbiol. 167, 153–160. doi: 10.1016/j.ijfoodmicro.2013.09.004
Liu, J., Sui, Y., Xie, Z., and Chi, M. (2018a). Transcriptome profiling reveals differential gene expression associated with changes in the morphology and stress tolerance of the biocontrol yeast, Pichia cecembensis. Biol. Control 120, 36–42. doi: 10.1016/j.biocontrol.2017.05.010
Liu, Y., Wang, W., Zhou, Y., Yao, S., Deng, L., and Zeng, K. (2017). Isolation, identification and in vitro screening of Chongqing orangery yeasts for the biocontrol of Penicillium digitatum on citrus fruit. Biol. Control 110, 18–24. doi: 10.1016/j.biocontrol.2017.04.002
Liu, J., Wisniewski, M., Droby, S., Vero, S., Tian, S., and Hershkovitz, V. (2011). Glycine betaine improves oxidative stress tolerance and biocontrol efficacy of the antagonistic yeast Cystofilobasidium infirmominiatum. Int. J. Food Microbiol. 146, 76–83. doi: 10.1016/j.ijfoodmicro.2011.02.007
Liu, Y., Yao, S., Deng, L., Ming, J., and Zeng, K. (2018b). Metschnikowia citriensis sp. nov., a novel yeast species isolated from leaves with potential for biocontrol of postharvest fruit rot. Biol. Control 125, 15–19. doi: 10.1016/j.biocontrol.2018.05.018
Liu, Y., Yao, S., Deng, L., Ming, J., and Zeng, K. (2019). Different mechanisms of action of isolated epiphytic yeasts against Penicillium digitatum and Penicillium italicum on citrus fruit. Postharvest Biol. Technol. 152, 100–110. doi: 10.1016/j.postharvbio.2019.03.002
Macarisin, D., Cohen, L., Eick, A., Rafael, G., Belausov, E., Wisniewski, M., et al. (2007). Penicillium digitatum suppresses production of hydrogen peroxide in host tissue during infection of citrus fruit. Phytopathology 97, 1491–1500. doi: 10.1094/PHYTO-97-11-1491
Macarisin, D., Droby, S., Bauchan, G., and Wisniewski, M. (2010). Superoxide anion and hydrogen peroxide in the yeast antagonist–fruit interaction: a new role for reactive oxygen species in postharvest biocontrol? Postharvest Biol. Technol. 58, 194–202. doi: 10.1016/j.postharvbio.2010.07.008
MacDonald, J. C. (1965). Biosynthesis of pulcherriminic acid. Biochem. J. 96, 533–538. doi: 10.1042/bj0960533
Mahunu, G. K., Zhang, H., Yang, Q., Li, C., and Zheng, X. (2016). Biological control of Patulin by antagonistic yeast: a case study and possible model. Crit. Rev. Microbiol. 42, 1–13. doi: 10.3109/1040841x.2015.1009823
Manso, T., and Nunes, C. (2011). Metschnikowia andauensis as a new biocontrol agent of fruit postharvest diseases. Postharvest Biol. Technol. 61, 64–71. doi: 10.1016/j.postharvbio.2011.02.004
Matysik, J., Alia, Bhalu, B., and Mohanty, P. (2002). Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 82, 525–532. https://www.jstor.org/stable/24105959
S. Nagata and H. Nakano (eds.) (2017). Apoptotic and non-apoptotic cell death. Vol. 403, (Switzerland: Springer International Publishing).
Ocampo-Suarez, I. B., López, Z., Calderón-Santoyo, M., Ragazzo-Sánchez, J. A., and Knauth, P. (2017). Are biological control agents, isolated from tropical fruits, harmless to potential consumers? Food Chem. Toxicol. 109, 1055–1062. doi: 10.1016/j.fct.2017.05.010
Pandin, C., Le Coq, D., Canette, A., Aymerich, S., and Briandet, R. (2017). Should the biofilm mode of life be taken into consideration for microbial biocontrol agents? Microb. Biotechnol. 10, 719–734. doi: 10.1111/1751-7915.12693
Parafati, L., Vitale, A., Restuccia, C., and Cirvilleri, G. (2015). Biocontrol ability and action mechanism of food-isolated yeast strains against Botrytis cinerea causing postharvest bunch rot of table grape. Food Microbiol. 47, 85–92. doi: 10.1016/j.fm.2014.11.013
Petrat, F., de Groot, H., Sustmann, R., and Rauen, U. (2002). The chelatable iron pool in living cells: a methodically defined quantity. Biol. Chem. 383, 489–502. doi: 10.1515/BC.2002.051
Piombo, E., Sela, N., Wisniewski, M., Hoffmann, M., Gullino, M. L., Allard, M. W., et al. (2018). Genome sequence, assembly and characterization of two Metschnikowia fructicola strains used as biocontrol agents of postharvest diseases. Front. Microbial. 9:593. doi: 10.3389/fmicb.2018.00593
Pu, L., Jingfan, F., Kai, C., Chao-an, L., and Yunjiang, C. (2014). Phenylethanol promotes adhesion and biofilm formation of the antagonistic yeast Kloeckera apiculata for the control of blue mold on citrus. FEMS Yeast Res. 14, 536–546. doi: 10.1111/1567-1364.12139
Rendueles, O., and Ghigo, J. M. (2015). Mechanisms of competition in biofilm communities. Microbiol. Spectr. 3, 1–18. doi: 10.1128/microbiolspec.MB-0009-2014
Saravanakumar, D., Ciavorella, A., Spadaro, D., Garibaldi, A., and Gullino, M. L. (2008). Metschnikowia pulcherrima strain MACH1 outcompetes Botrytis cinerea, Alternaria alternata and Penicillium expansum in apples through iron depletion. Postharvest Biol. Technol. 49, 121–128. doi: 10.1016/j.postharvbio.2007.11.006
Seguin, J., Moutiez, M., Li, Y., Belin, P., Lecoq, A., Fonvielle, M., et al. (2011). Nonribosomal peptide synthesis in animals: the cyclodipeptide synthase of Nematostella. Chem. Biol. 18, 1362–1368. doi: 10.1016/j.chembiol.2011.09.010
Sipiczki, M. (2006). Metschnikowia strains isolated from botrytized grapes antagonize fungal and bacterial growth by iron depletion. Appl. Environ. Microbiol. 72, 6716–6724. doi: 10.1128/AEM.01275-06
Spadaro, D., and Gullino, M. L. (2004). State of the art and future prospects of the biological control of postharvest fruit diseases. Int. J. Food Microbiol. 91, 185–194. doi: 10.1016/S0168-1605(03)00380-5
Su, J., Tu, K., Cheng, L., Tu, S. C., Wang, M., Xu, H. R., et al. (2011). Wound-induced H2O2 and resistance to Botrytis cinerea decline with the ripening of apple fruit. Postharvest Biol. Technol. 62, 64–70. doi: 10.1016/j.postharvbio.2011.05.001
Sui, Y., Wisniewski, M., Droby, S., and Liu, J. (2015). Responses of yeast biocontrol agents to environmental stress. Appl. Environ. Microbiol. 81, 2968–2975. doi: 10.1128/AEM.04203-14
Szabados, L., and Savoure, A. (2010). Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97. doi: 10.1016/j.tplants.2009.11.009
Torres, R., Teixidó, N., Usall, J., Abadias, M., Mir, N., Larrigaudiere, C., et al. (2011). Anti-oxidant activity of oranges after infection with the pathogen Penicillium digitatum or treatment with the biocontrol agent Pantoea agglomerans CPA-2. Biol. Control 57, 103–109. doi: 10.1016/j.biocontrol.2011.01.006
Torres, R., Valentines, M. C., Usall, J., Viñas, I., and Larrigaudiere, C. (2003). Possible involvement of hydrogen peroxide in the development of resistance mechanisms in ‘Golden delicious’ apple fruit. Postharvest Biol. Technol. 27, 235–242. doi: 10.1016/s0925-5214(02)00110-2
Türkel, S., Korukluoğlu, M., and Yavuz, M. (2014). Biocontrol activity of the local strain of Metschnikowia pulcherrima on different postharvest pathogens. Biotechnol. Res. Int. 2014, 1–6. doi: 10.1155/2014/397167
Whiteley, M., Bangera, M. G., Bumgarner, R. E., Parsek, M. R., Teitzel, G. M., Lory, S., et al. (2001). Gene expression in Pseudomonas aeruginosa biofilms. Nature 413, 860–864. doi: 10.1038/35101627
Xu, N., Dong, Y., Cheng, X., Yu, Q., Qian, K., Mao, J., et al. (2014). Cellular iron homeostasis mediated by the Mrs4–Ccc1–Smf3 pathway is essential for mitochondrial function, morphogenesis and virulence in Candida albicans. BBA-Mol. Cell. Res. 1843, 629–639. doi: 10.1016/j.bbamcr.2013.12.009
Yan, Y., Zhang, X., Zheng, X., Apaliya, M. T., Yang, Q., Zhao, L., et al. (2018). Control of postharvest blue mold decay in pears by Meyerozyma guilliermondii and it’s effects on the protein expression profile of pears. Postharvest Biol. Technol. 136, 124–131. doi: 10.1016/j.postharvbio.2017.10.016
Yang, Q., Wang, H., Zhang, H., Zhang, X., Apaliya, M. T., Zheng, X., et al. (2017). Effect of Yarrowia lipolytica on postharvest decay of grapes caused by Talaromyces rugulosus and the protein expression profile of T. rugulosus. Postharvest Biol. Technol. 126, 15–22. doi: 10.1016/j.postharvbio.2016.11.015
Zhang, Z., Chen, J., Li, B., He, C., Chen, Y., and Tian, S. (2017). Influence of oxidative stress on biocontrol activity of Cryptococcus laurentii against blue mold on peach fruit. Front. Microbial. 8:151. doi: 10.3389/fmicb.2017.00151
Zhang, S., and Klessig, D. (2001). MAPK cascades in plant defense signaling. Trends Plant Sci. 6, 520–527. doi: 10.1016/S1360-1385(01)02103-3
Zhang, H., Mahunu, G. K., Castoria, R., Yang, Q., and Apaliya, M. T. (2018). Recent developments in the enhancement of some postharvest biocontrol agents with unconventional chemicals compounds. Trends Food Sci. Technol. 78, 180–187. doi: 10.1016/j.tifs.2018.06.002
Zhao, L., Zhang, H., Li, J., Cui, J., Zhang, X., and Ren, X. (2012). Enhancement of biocontrol efficacy of Pichia carribbica to postharvest diseases of strawberries by addition of Trehalose to the growth medium. Int. J. Mol. Sci. 13, 3916–3932. doi: 10.3390/ijms13033916
Keywords: proline, Metschnikowia citriensis, maroon pigment, intracellular iron content, apoptosis, biofilm formation
Citation: Liu Y, Yi L, Ruan C, Yao S, Deng L and Zeng K (2019) Proline Increases Pigment Production to Improve Oxidative Stress Tolerance and Biocontrol Ability of Metschnikowia citriensis. Front. Microbiol. 10:1273. doi: 10.3389/fmicb.2019.01273
Edited by:
Haifeng Zhao, South China University of Technology, ChinaReviewed by:
Harminder Pal Singh, Panjab University, IndiaHongyin Zhang, Jiangsu University, China
Donald Becker, University of Nebraska-Lincoln, United States
Copyright © 2019 Liu, Yi, Ruan, Yao, Deng and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaifang Zeng, zengkaifang@hotmail.com
 Ye Liu
Ye Liu Lanhua Yi1,2
Lanhua Yi1,2 Kaifang Zeng
Kaifang Zeng