- Key Laboratory of Agricultural Microbiology, College of Plant Protection, Shandong Agricultural University, Tai’an, China
Fusarium graminearum is one of the causal agents of Fusarium head blight and produces the trichothecene mycotoxin, deoxynivalenol (DON). Thioredoxin reductases (TRRs) play critical roles in the recycling of oxidized thioredoxin. However, their functions are not well known in plant pathogenic fungi. In this study, we characterized a TRR orthologue FgTRR in F. graminearum. The FgTRR-GFP fusion protein localized to the cytoplasm. FgTRR gene deletion demonstrated that FgTRR is involved in hyphal growth, conidiation, sexual reproduction, DON production, and virulence. The ΔTRR mutants also exhibited a defect in pigmentation, the expression level of aurofusarin biosynthesis-related genes was significantly decreased in the FgTRR mutant. Furthermore, the ΔTRR mutants were more sensitive to oxidative stress and aggravated apoptosis-like cell death compared with the wild type strain. Taken together, these results indicate that FgTRR is important in development and pathogenicity in F. graminearum.
Introduction
Fusarium head blight (FHB), caused mainly by Fusarium graminearum, is an importantly destructive disease of wheat and barley worldwide (Goswami and Kistler, 2004). The disease not only reduces wheat yield, but also endangers the health of humans and animals because of consumption of mycotoxin-contaminated grain (McMullen et al., 1997; Goswami and Kistler, 2004, 2005; Desjardins and Proctor, 2007). Deoxynivalenol (DON), one of trichothecene mycotoxins produced by F. graminearum, is recognized as an important virulence factor during plant infection (Desjardins et al., 1993, 1996; Proctor et al., 1995) and is a potent protein synthesis inhibitor (Pestka and Smolinski, 2005; Arunachalam and Doohan, 2013). The TRI gene cluster, including TRI5, TRI6, TRI10, and other TRI genes, are involved in DON synthesis in F. graminearum (Proctor et al., 1995; Brown et al., 2004; Alexander et al., 2009).
The thioredoxin system, comprised of thioredoxin reductase (TRR), thioredoxin (TRX), and NADPH, is involved in regulating methionine biosynthesis, cell growth, gene transcription, and apoptosis (Lindner et al., 2002; Koc et al., 2006; Bobba et al., 2014). TRR is a member of the pyridine nucleotide-disulfide oxidoreductase family and usually includes FAD-and NADPH-binding domains and an active site containing a redox-active disulfide (Williams, 1995; Mustacich and Powis, 2000; Arnér, 2009). Hallmarks of apoptosis include externalization of phosphatidylserine(PS), DNA fragmentation, and intracellular reactive oxygen species (ROS) accumulation. In microbial eukaryotes, ROS, including superoxide anion (O2-), hydrogen peroxide (H2O2), and hydroxyl radical (OH-), are involved in immunization, cell proliferation, signal transduction, and other biochemical reactions (Aguirre et al., 2005; Tudzynski et al., 2012). The cellular redox regulation is achieved mainly through the thioredoxin system (Powis et al., 2000; Bobba et al., 2014).
Yeast contains two genes encoding TRRs (cytoplasmic TRR1 and mitochondrial TRR2), and deletion of TRR1 increased sensitivity to oxidative and reductive stress, high temperature, and auxotrophic requirement for methionine (Pearson and Merril, 1998; Carmel-Harel et al., 2001; Trotter and Grant, 2002). In addition, TRR1 affects the transcription of cell cycle-regulated genes at the G1/S boundary and the activity of the p53 tumor suppresser gene in yeast (Machado et al., 1997; Pearson and Merril, 1998). In Acremonium chrysogenum, TrxR1 (TRR1) is required for the normal growth and related cephalosporin C production in methionine-supplemented medium (Liu et al., 2012). Characterization of the role of TRR in phytopathogenic fungi, Magnaporthe oryzae, and Botrytis cinerea, showed that TRR is involved in growth and virulence (Fernandez and Wilson, 2014; Viefhues et al., 2014).
In this study, we hypothesized that FgTRR may play important roles in development and virulence in F. graminearum. Therefore, we characterized the role of FgTRR in F. graminearum. Deletion of FgTRR led to defects in hyphal growth, asexual and sexual reproduction, pathogenicity, and DON production, indicating that FgTRR is involved in regulating development and virulence in F. graminearum.
Materials and Methods
Strains and Culture Conditions
Fusarium graminearum strain PH-1 was used as the wild-type (WT) strain in this study. The WT and its derivative mutants were routinely cultured on potato dextrose agar (PDA) and complete medium (CM) at 25°C for mycelial growth tests. Liquid carboxymethyl cellulose (CMC) medium was used to analyze induction of asexual reproduction (Hou et al., 2002). For determining sensitivity to various stresses, mycelial growth was assayed on CM plates supplemented with 0.7 M NaCl, 0.7 M KCl, 0.2 g/L Congo Red (CR), 1 M sorbitol, 0.05% SDS, 5 mM H2O2, and 15 μM menadion. Fungal mycelia were harvested from potato dextrose broth (PDB) and used for extraction of genomic DNA and RNA. Escherichia coli DH5α was used for routine transformations and subsequently cultured in Luria-Bertani broth at 37°C.
Generation of FgTRR Deletion Mutants
The split-marker approach (Catlett et al., 2003) was used to generate the FgTRR deletion mutants. Briefly, the 1422-bp upstream and 1061-bp downstream flanking sequences were amplified with primer pairs TRR-A1/TRR-A2 and TRR-B1/TRR-B2 (Supplementary Table S1), respectively. The amplicon containing hygromycin phosphotransferase (hph) was amplified from the pCB1003 with primer pairs HYG-F/HY-R and YG-F/HYG-R (Supplementary Table S1). FgTRR gene-replacement constructs were generated by overlapping PCR. Then, the resulting constructs were directly transformed into protoplasts of the WT strain with PEG-mediated transformation, and transformants were selected on TB3 medium with the final concentration of 200 μg/mL of Hygromycin B, and further identified by PCR and Southern blot analysis (Supplementary Table S1 and Supplementary Figure S2).
Generation of ΔTRR Complementation and Subcellular Localization
The yeast gap repair approach (Bruno et al., 2004) was used to generate the ΔTRR complementation (ΔTRR-C) strain. Briefly, a 3.3-kb DNA fragment from the FgTRR gene carrying its native promoter was amplified with primer TRR-CF/TRR-CR (Supplementary Table S1) and co-transformed with XhoI-digested pYF11 vector harboring GFP into S. cerevisiae XK1-25. The resulting construct was directly transformed into protoplasts of the FgTRR deletion mutant. Transformants were selected with 200 μg/mL G418 and identified by PCR with the primer pair TRR-CF/TRR-CR (Supplementary Table S1 and Supplementary Figure S2). GFP signals in hyphae were visualized with a laser confocal microscope (LSM880NLO, ZEISS).
Asexual and Sexual Reproduction Assays
Conidiation was examined in CMC medium after 5 days of incubation at 25°C (Hou et al., 2002). The number of conidia were counted for each strain using a haemocytometer. For sexual reproduction assays, aerial hyphae were gently removed on carrot agar (CA) plates after adding with 0.1% Tween 20 solution (Bowden and Leslie, 1999; Cavinder et al., 2012). Perithecia and ascospores were examined after 2–3 weeks of incubation at 25°C. The experiments were repeated three times with three replicates each time.
Plant Infection and DON Production Assays
Virulence assays were performed on wheat cv. Jimai 22 during flowering. A 10-μL suspension of conidia (4.0 × 105 conidia/mL) was injected into a floret in the central section of wheat ear (Gale et al., 2002; Han et al., 2004). Disease index was assayed 14 days post-inoculation. For infection of corn silks, mycelial plugs (5 mm in diameter) taken from PDA plates were inoculated on a group of silks and kept for 5 days at 25°C as described previously (Seong et al., 2005). For assays of DON production, 5 g of rice grains in per flask were autoclaved and inoculated with three mycelial plugs per flask. After 20 days of incubation at 25°C, DON concentrations were determined by a liquid chromatography-mass spectrometer/mass spectrometer (HPLC–MS/MS) system (AB Sciex, 5500) (Soleimany et al., 2012; Zheng et al., 2012). Ergosterol levels were used to normalize DON production per fungal mass. The experiments were repeated three times.
DNA Isolation and Southern Blot
Genomic DNA was extracted from mycelia as described previously (Leslie and Summerell, 2006) and digested with NdeI. Southern blot analysis was performed with the DIG-High Prime DNA Labeling and Detection Starter kit I according to the manufacturer’s protocol (Roche Diagnostics, Mannheim, Germany). The probe was amplified by PCR using the primer pair tTRR-F/tTRR-R (Supplementary Table S1).
RNA Extraction and qRT-PCR Analysis
Total RNAs were extracted from mycelia grown in PDB or DON-inducing medium (Gardiner et al., 2009) for 3 days using the RNAiso Plus reagent (TaKaRa, TaKaRa Biotechnology Co., Dalian, China). cDNA was synthesized by PrimeScriptTM RT reagent as instructed by the manufacturer (TaKaRa Biotechnology). The expression level of genes related to aurofusarin and DON synthesis were detected by qRT-PCR with the primers listed in Supplementary Table S1. Relative quantification of each transcript was calculated by the 2-ΔΔCT method (Livak and Schmittgen, 2001).
Analysis of Phosphatidylserine Externalization and Intracellular ROS Accumulation
Protoplasts were stained with the Annexin V-FITC Apop kit (Invitrogen, United States) to analyze PS externalization (Madeo et al., 1997). Briefly, protoplasts of WT and ΔTRR#1 were resuspended in 1.2 M KCl and treated with 5 mM H2O2 for 1 h. After washing protoplasts with 1.2 M KCl and resuspending in 200 μL binding buffer (1×), a 5-μL Annexin V-FITC was added into 195 μL of the protoplast suspension and the mixture was incubated for 10 min at room temperature. Then, after washing protoplasts with 200 μL of binding buffer (1×) and resuspending again in 190 μL of binding buffer (1×), 10-μL of propidium iodide (PI) (20 μg/mL) was added into the protoplast suspension and incubated for 15 min in the dark. Protoplasts were resuspended in 1.2 M KCl and analyzed by fluorescence microscopy. The experiments were repeated three times with three replicates each time.
Intracellular ROS accumulation was measured with a fluorescent probe, dihydrorhodamine (DHR) 123 (Sigma–Aldrich, United States) as previously described (Royall and Ischiropoulos, 1993). The mycelia cultured in liquid YEPD for 18 h were collected and stained with 5 μM DHR123 for 30 min, rinsed, and re-suspended in PBS. Fluorescence intensity was visualized by fluorescence microscopy.
Statistical Analysis
Data are presented as means ± standard deviations from three independent experiments. Different letters and asterisks indicate a significant difference (P < 0.05) by Duncan’s multiple range test or Student’s t-tests.
Results
Identification and Subcellular Localization of FgTRR in F. graminearum
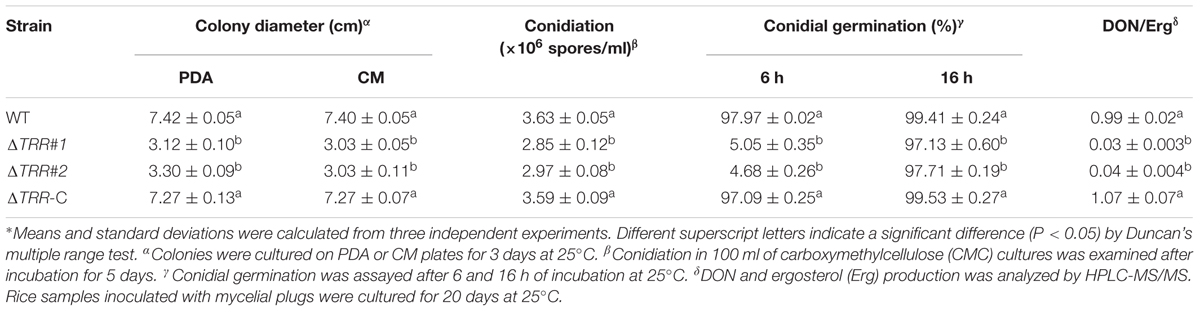
One putative TRR (FGSG_00871, designated as FgTRR) in F. graminearum was retrieved by BLAST search of the NCBI with the M. oryzae TRR1 (MGG_01284) as a query. The FgTRR gene is predicted to encode a 330-amino acid protein showing 46% identity to S. cerevisiae TRR1 and 79% to M. oryzae TRR1. The domain analysis showed that FgTRR was predicted to harbor all domains typical for the TRR family, including a FAD-binding domain I (GXGXX, where X is any amino acid), a FAD-binding domain II (GXFAXGDV), and a NADPH-binding domain (GGGXXA). Additionally, it contains a redox-active cysteine pair (CAVC) (Figure 1A). Phylogenetic analysis by the neighbor-joining method revealed that TRR is highly conserved among three Fusarium species (Supplementary Figure S1).
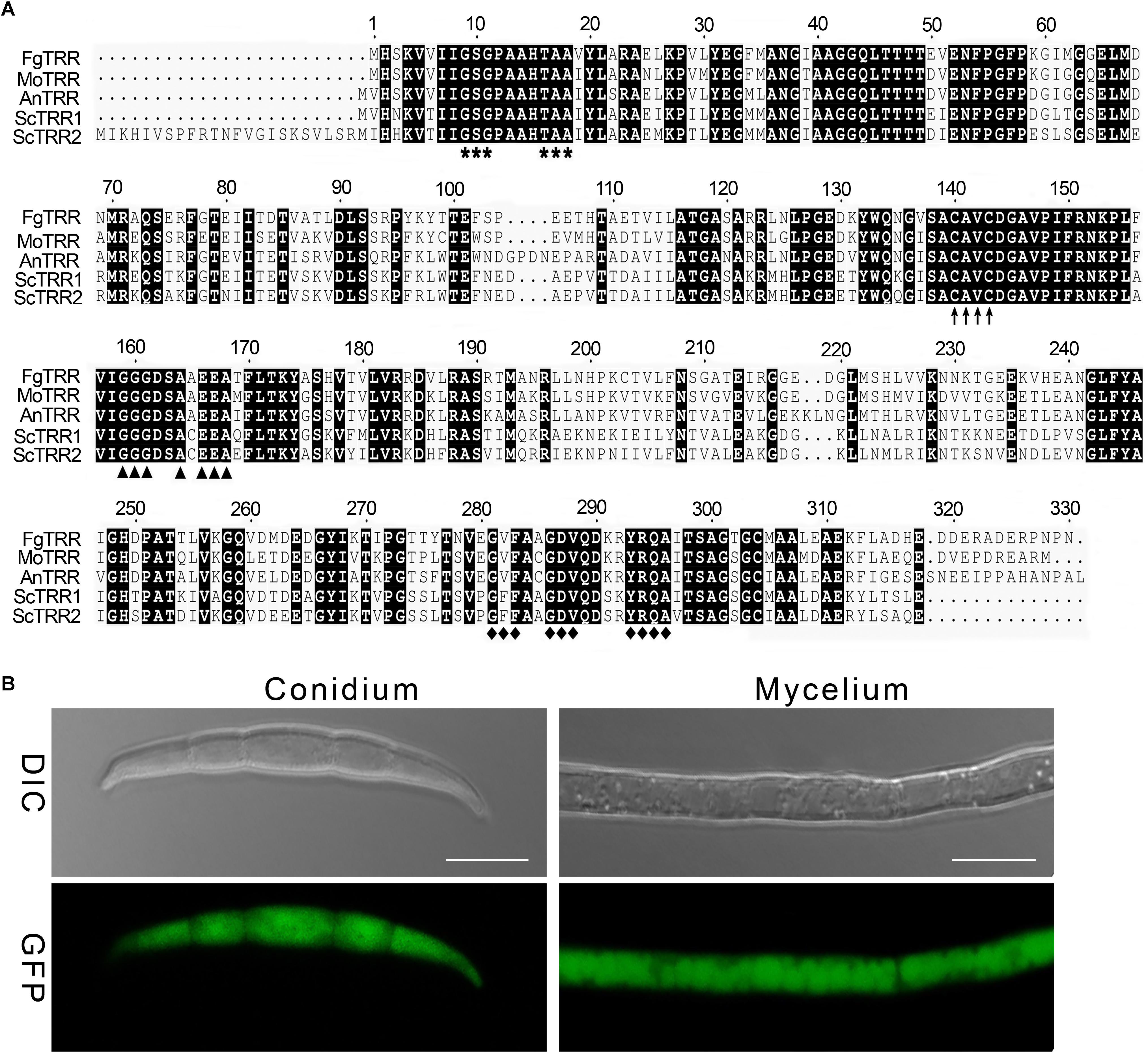
Figure 1. Sequence alignment and phylogenetic analysis of thioredoxin reductases (TRRs) in fungi. (A) Sequence alignment of FgTRR with other fungal TRRs. Fg, Fusarium graminearum; Mo, Magnaporthe oryzae; An, Aspergillus nidulans; Sc, Saccharomyces cerevisiae. Bold asterisks, arrows, triangles, and rhombuses denote a FAD-binding domain I (GXGXX), a redox-active cysteine pair (CAVC), a NADPH-binding domain (GGGXXA), and a FAD-binding domain II (GXFAXGDV), respectively. (B) Subcellular localization of the FgTRR-GFP fusion protein in F. graminearum. GFP signal observation in hyphae and conidia was determined using a laser confocal microscope. Bar = 10 μm.
To explore the subcellular localization of FgTRR, we generated the FgTRR-GFP fusion protein by using its native promoter. Transformants were validated by selection on G418-containing medium and PCR analysis (Supplementary Figure S2). Bright green fluorescence was observed and distributed uniformly throughout hyphae and conidia (Figure 1B). Therefore, we assumed that FgTRR localized to the cytoplasm.
FgTRR Is Responsible for Hyphal Growth, Conidiation, and Conidial Germination
To study the function of the FgTRR gene in F. graminearum, we generated the FgTRR deletion mutants by replacing the targeted coding region with the hph gene. After hygromycin screening, seven independent putative FgTRR deletion mutants were verified by PCR and confirmed by Southern blot hybridization (Supplementary Table S1 and Supplementary Figure S2). For complementation, we performed FgTRR complementation (ΔTRR-C) by reintroducing the entire FgTRR gene under the control of its native promoter into the ΔTRR#1 mutant, one of FgTRR gene deletion mutants.
In comparison with the WT and ΔTRR-C strain, the ΔTRR mutants displayed reduced hyphal growth rates on PDA and CM plates. The mutants were reduced by approximately 56% on PDA and 60% on CM, respectively (Table 1 and Figure 2A). The FgTRR mutants produced fewer conidia but with normal morphology (Table 1 and Figure 2B). We then investigated the germination of conidia. After 6 h post-incubation (hpi) in YEPD medium, approximately 6% of the ΔTRR conidia germinated but the germination rate of the WT was over 97% under the same conditions. In addition, compared with the WT, the length of germ tube was obviously decreased in the ΔTRR mutants (Figure 2C). After 16 hpi, the germination rate of the ΔTRR mutants showed a slight decrease compared with the WT. Taken together, FgTRR is involved in vegetative growth, conidiation, and conidial germination.
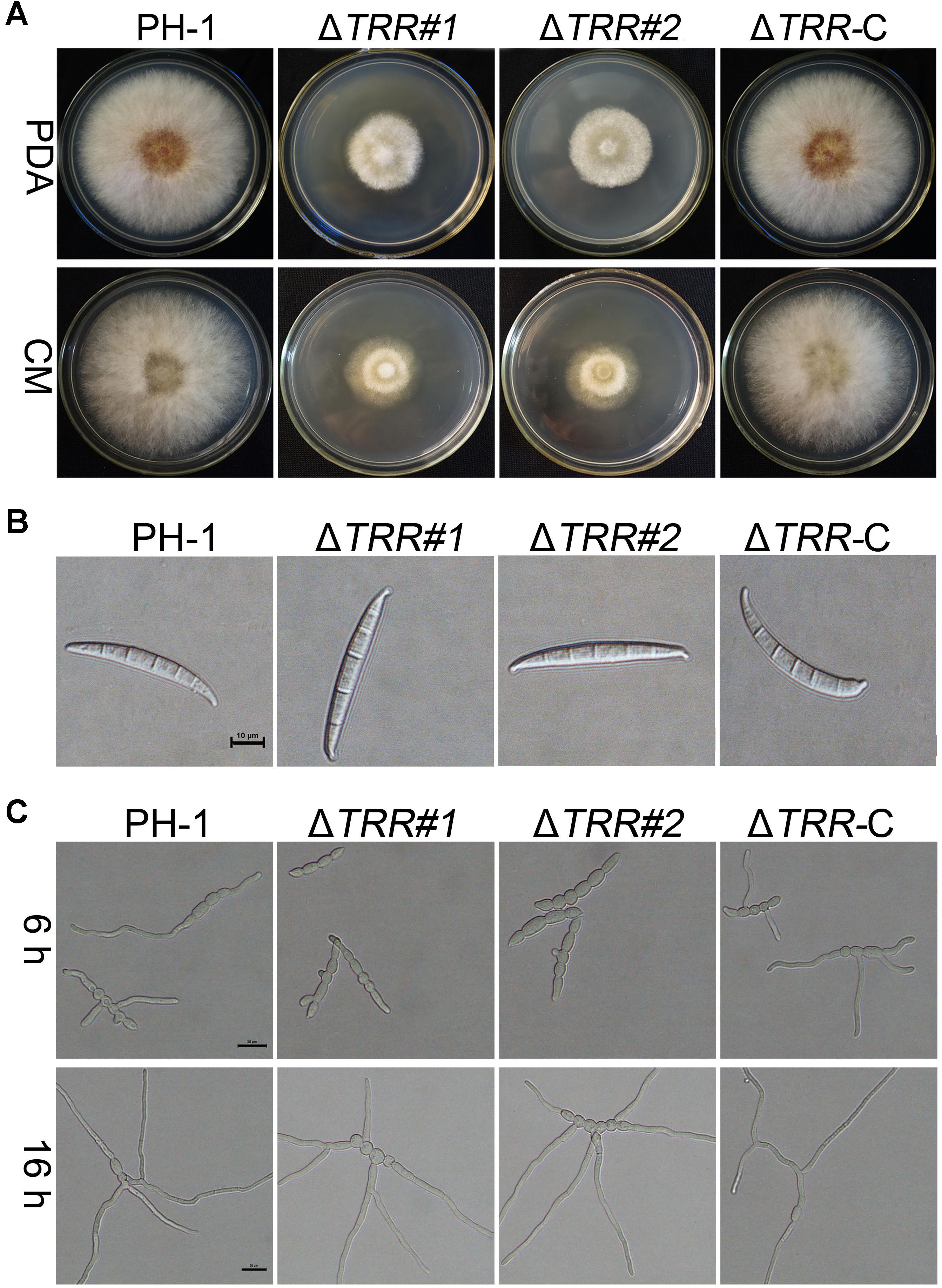
Figure 2. Hyphal growth, conidial morphology, and germination of the FgTRR deletion mutants. (A) Colonies of the wild type (PH-1), FgTRR deletion mutants, and ΔTRR-C strains were cultured on potato dextrose agar (PDA) and complete medium (CM) agar plates. Photos were recorded after growth for 3 days at 25°C. (B) Conidial morphology of the ΔTRR mutants. Conidia were examined by differential interference contrast microscopy. Bar = 10 μm. (C) Conidia of the ΔTRR mutants were incubated in liquid YEPD at 25°C and examined for germination. Bar = 20 μm.
FgTRR Is Involved in Ascus and Ascospore Development
In FHB development, ascospore formation is crucial for plant infection. Therefore, we further investigated the ability of the ΔTRR mutants in sexual reproduction. We found that the WT and ΔTRR-C strains produced many perithecia and normal ascospores on CA plates. Although perithecia of the ΔTRR mutants were observed, there were no asci and ascospores released in the ΔTRR mutants even after 30 days of induction (Figures 3A,B), indicating deletion of FgTRR blocks ascus development but has no effect on the formation of protoperithecia. In order to further determine whether the ΔTRR mutants are male fertile, the ΔTRR mutants were crossed as the male with the mat1-1-1 strain (Zheng et al., 2013). As shown in Figure 3C, asci and ascospores were normally produced in the ΔTRR mutants after mating with the mat1-1-1 strain, indicating that FgTRR mutants are male fertile in F. graminearum.
Figure 3. Assays for sexual reproduction in the FgTRR deletion mutants. (A) Perithecia produced by PH-1, the ΔTRR mutants, and ΔTRR-C at 14 days post fertilization. (B) Asci and ascospores observed under a microscope after squeezing perithecia. (C) Asci and ascospores from the ΔTRR mutants (male) × the mat1-1-1 strain (female). Bar = 50 μm.
FgTRR Is Essential for Pathogenicity and DON Production
Pathogenicity of the mutants was assayed by inoculation of wheat heads with 4.0 × 105 conidia/mL. As shown in Figure 4A, at 14 days post-inoculation (dpi), typical lesions spread from the inoculation site to other parts in wheat heads inoculated with the WT and ΔTRR-C strains. However, the ΔTRR mutants failed to develop, and the disease lesion was restricted to the inoculation site. Pathogenicity of the ΔTRR mutants was decreased by approximately 94% compared with the WT (Figure 4C). Similarly, the ΔTRR mutants caused only limited brown lesions on corn silks at 5 dpi (Figure 4B). These results confirm that FgTRR is important for virulence in F. graminearum.
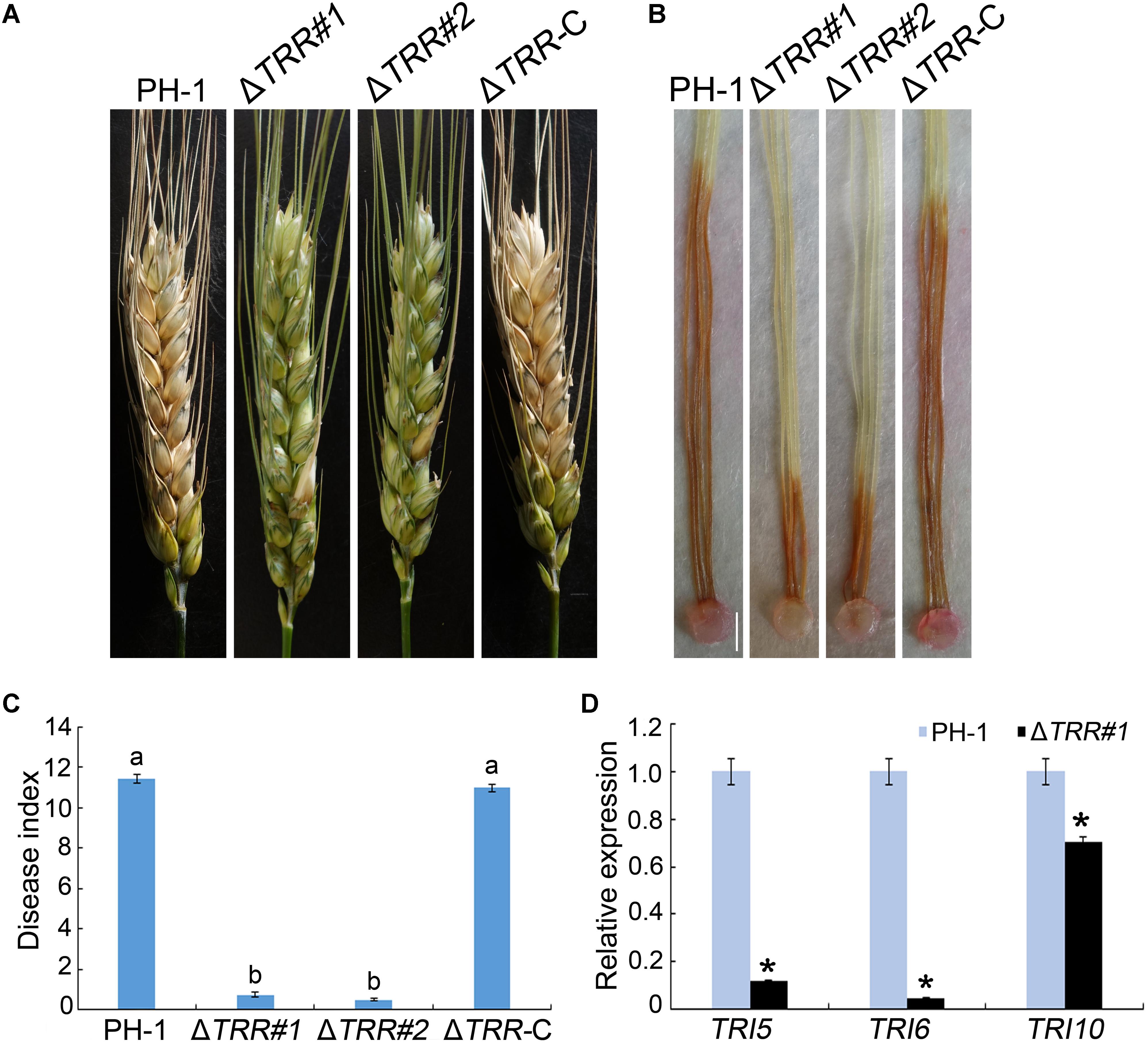
Figure 4. Pathogenicity and TRI expression assays of the FgTRR deletion mutants. (A) Flowering wheat heads were inoculated with conidial suspensions and examined 14 days post-inoculation (dpi). (B) Corn silks were inoculated with mycelial plugs and examined 5 dpi. Bar = 5 mm. (C) Disease index was examined from the number of symptomatic spikelets per wheat head 14 dpi. Different letters indicate statistically significant difference (P < 0.05) by Duncan’s multiple range test. (D) Relative expression levels of TRI5, TRI6, and TRI10 in PH-1 and the ΔTRR#1 mutant. The GAPDH gene was used as an internal control. Asterisks represent statistically significant difference (P < 0.05) by Student’s t-tests.
In view of pathogenicity defects, we assayed DON production in the WT and ΔTRR mutants on autoclaved rice grains in flasks. As shown in Table 1, DON/ergosterol ratio of the ΔTRR mutants was significantly reduced compared to the WT. Furthermore, the expression of TRI (TRI5, TRI6, and TRI10) genes, which are essential for the regulation of DON biosynthesis, were assayed by qRT-PCR. The expression levels of TRI5, TRI6, and TRI10 in the ΔTRR#1 mutant were reduced by approximately 9-, 21-, and 1.5-fold relative to the WT, respectively (Figure 4D).
Deletion of FgTRR Results in Defective Pigmentation
When all strains were incubated on PDA plates for 3 days, the WT formed pink colonies, whereas the FgTRR mutants formed slightly pale yellowish colonies visible on the bottom of plates (Figure 5A). In PDB flasks, the ΔTRR mutants, similarly, exhibited the yellowish pigment (Figure 5B). These results showed that FgTRR is involved in the pigment biosynthesis in F. graminearum. To further confirm this conclusion, we performed qRT-PCR to determine the expression levels of FgPKS12, Fggip1, Fggip2, FgAurJ, FgAurF, and FgAurO that are related the biosynthesis of pigment aurofusarin (Kim et al., 2005, 2006; Frandsen et al., 2006). As shown in Figure 5C, the expression levels of six genes in the WT were significantly higher than those of the ΔTRR#1 mutant.
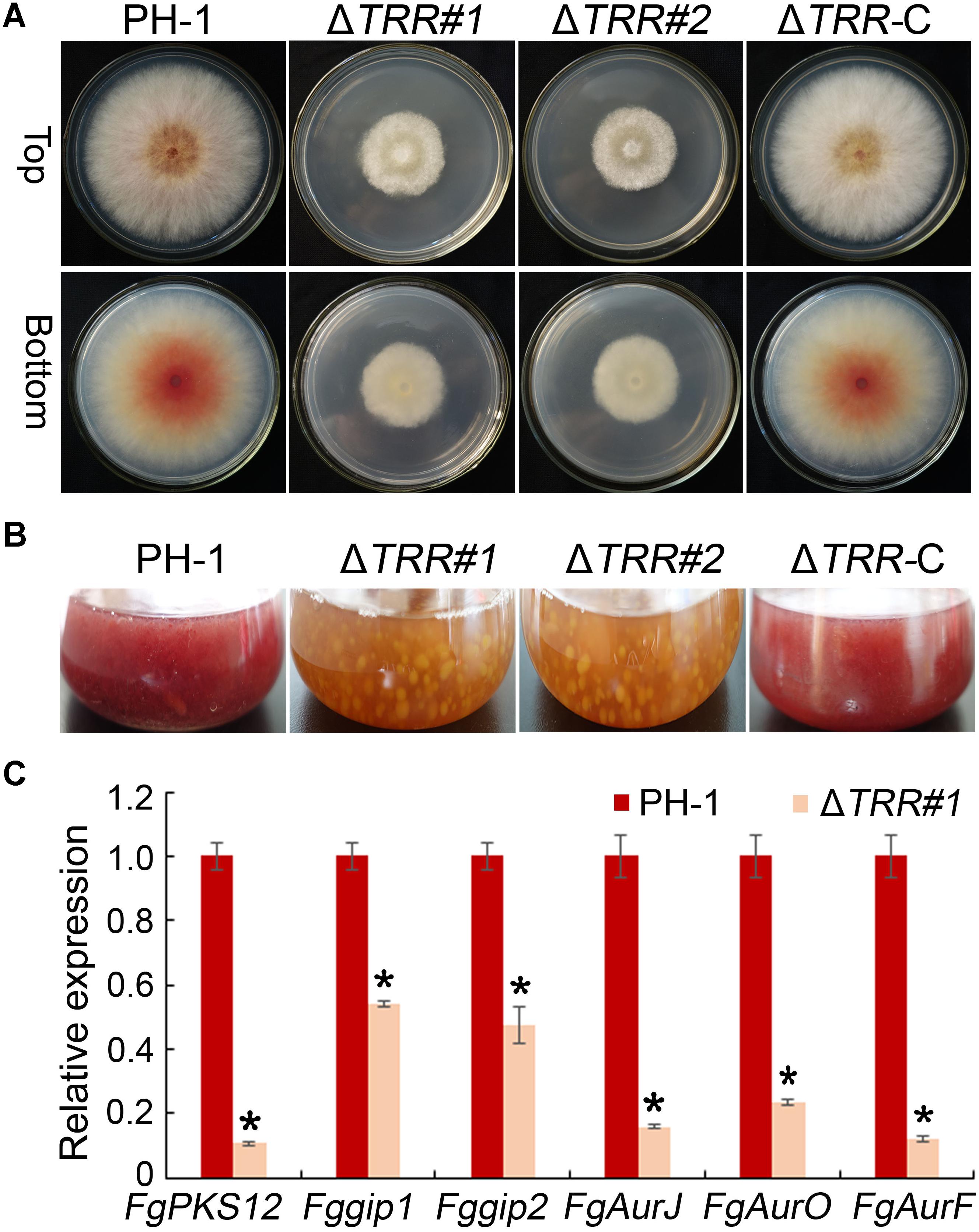
Figure 5. Defective pigmentation of the FgTRR deletion mutants. (A) Colony morphology on PDA plates 3 days post-incubation (dpi) at 25°C. (B) Liquid culture in flasks containing 50 mL of PDB medium 3 dpi at 25°C. (C) Relative expression levels of aurofusarin biosynthesis-related genes FgAurJ (FGSG_02326), FgAurO (FGSG_02321), FgAurF (FGSG_02327), FgPKS12 (FGSG_12040), Fggip1 (FGSG_02328), and Fggip2 (FGSG_02320). The GAPDH gene was used as an internal control. Asterisks indicate statistically significant difference (P < 0.05) by Student’s t-tests.
FgTRR Is Involved in Sensitivity to Various Stresses
In order to test the role of FgTRR in various stresses, we examined the sensitivity related to osmotic stress, oxidative stress, and cell wall stress. On CM plates containing sorbitol, the growth rate of the ΔTRR mutants was obviously inhibited compared to the WT. Similar trends in relative growth rates were observed on media supplemented with KCl or NaCl, indicating that the ΔTRR mutants are more sensitive to osmotic stress. The mutants exhibited sensitivity to the cell wall inhibitor CR and tolerance to SDS which causes cell membrane damage (Figures 6A,B). These results indicate that FgTRR is involved in stress response and cell wall integrity.
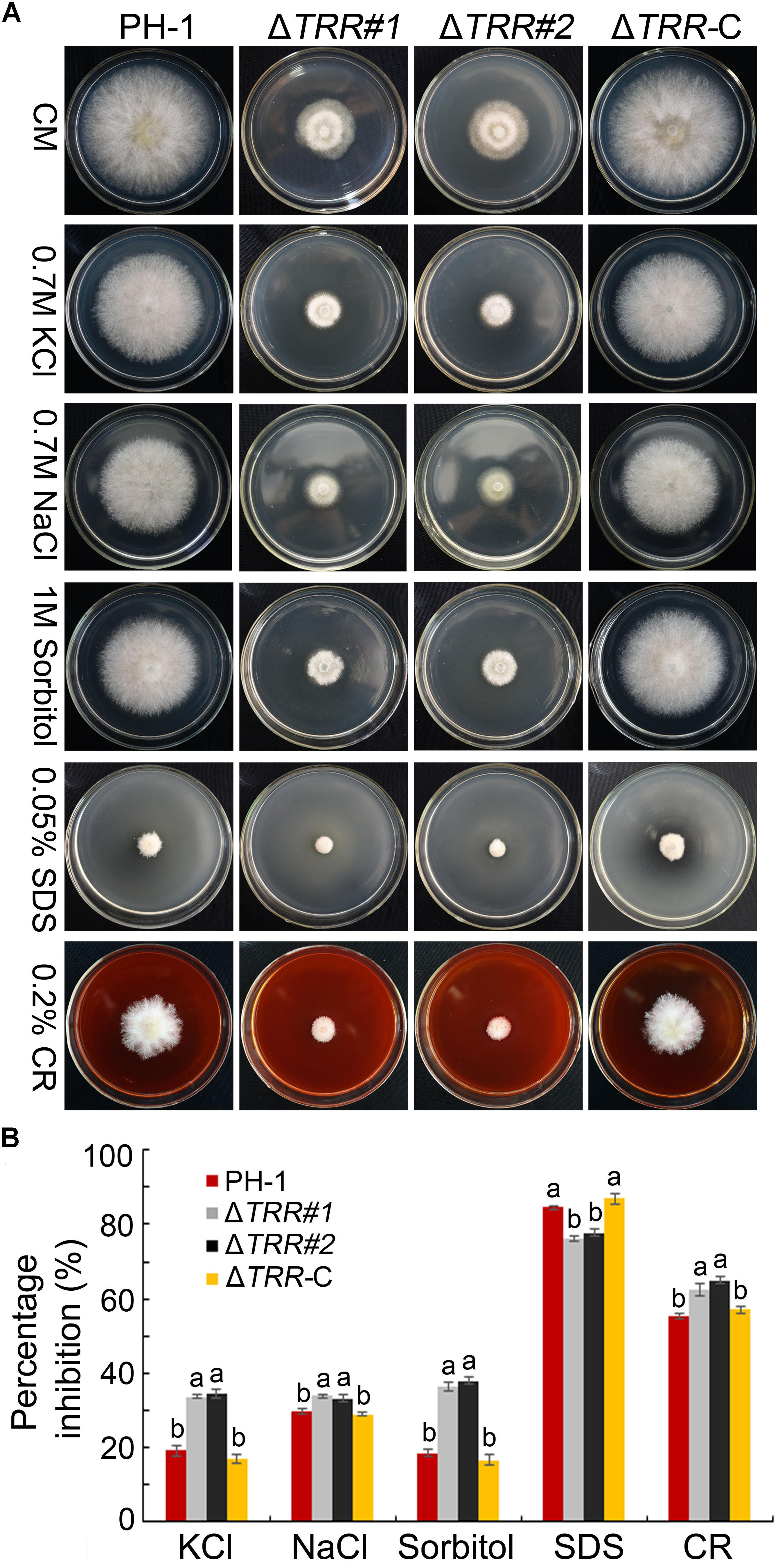
Figure 6. Sensitivity of the FgTRR deletion mutants to osmotic and cell wall-damaged stresses. (A) CM plates were supplemented with various stress agents at indicated concentrations. Photographs were taken after incubation for 3 days at 25°C. (B) Percentage inhibition was examined after incubation for 3 days. Means and standard deviations were calculated from three replicates. Different letters denote statistically significant differences (P < 0.05) by Duncan’s multiple range test.
The ΔTRR mutants were incubated on CM plates supplemented with H2O2 and menadion to confirm the sensitivity to oxidative stress. As shown in Figures 7A,B, the inhibition rate was significantly increased in mutants compared with the WT, indicating that FgTRR deletion mutants are more sensitive to oxidative stress.
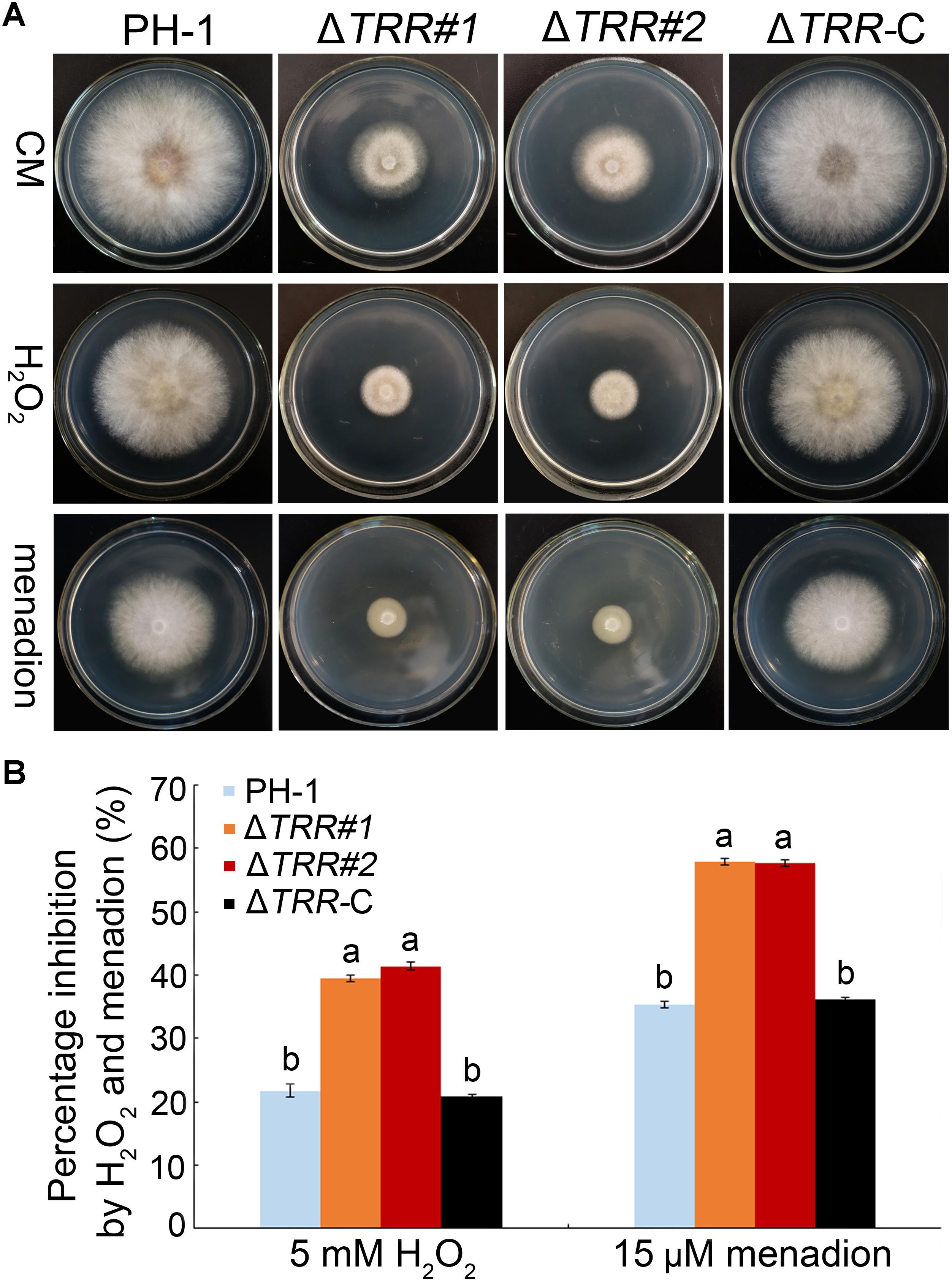
Figure 7. Sensitivity of the FgTRR deletion mutants to oxidative stress. (A) PH-1, ΔTRR mutants, and ΔTRR-C strains were cultured on CM plates supplemented with 5 mM H2O2 and 15 μM menadion. Photographs were taken after incubation for 3 days at 25°C. (B) Percentage inhibition was examined after incubation for 3 days. Means and standard deviations were calculated from three replicates. Different letters denote statistically significant differences (P < 0.05) by Duncan’s multiple range test.
FgTRR Deletion Mutant Aggravates Apoptosis-Like Cell Death
To test the role of FgTRR in apoptosis-like cell death in F. graminearum, we first examined the extent of PS externalization in WT and ΔTRR#1 by using Annexin V-FITC and PI staining. When treated with 5 mM H2O2 for 1 h, as shown in Figure 8A, protoplasts of the WT and the ΔTRR#1 mutant were frequently stained green, indicating that these protoplasts showed PS externalization, a typical marker of early apoptosis-like cell death. The proportion of Annexin V-FITC-positive protoplasts was approximately 18 and 33% in the WT and the ΔTRR#1 mutant treated with H2O2 for 1 h, respectively (Figure 8B). Similarly, in the absence of H2O2 treatment the proportion of Annexin V-FITC-positive protoplasts in the ΔTRR#1 mutant was higher than that of the WT (Figure 8B). We next measured the accumulation of intracellular ROS in hyphae of the ΔTRR#1 mutant. The results showed a stronger fluorescence in the ΔTRR#1 mutant compared with the WT (Figure 8C). These data indicate that FgTRR is negatively involved in apoptosis-like cell death in F. graminearum.
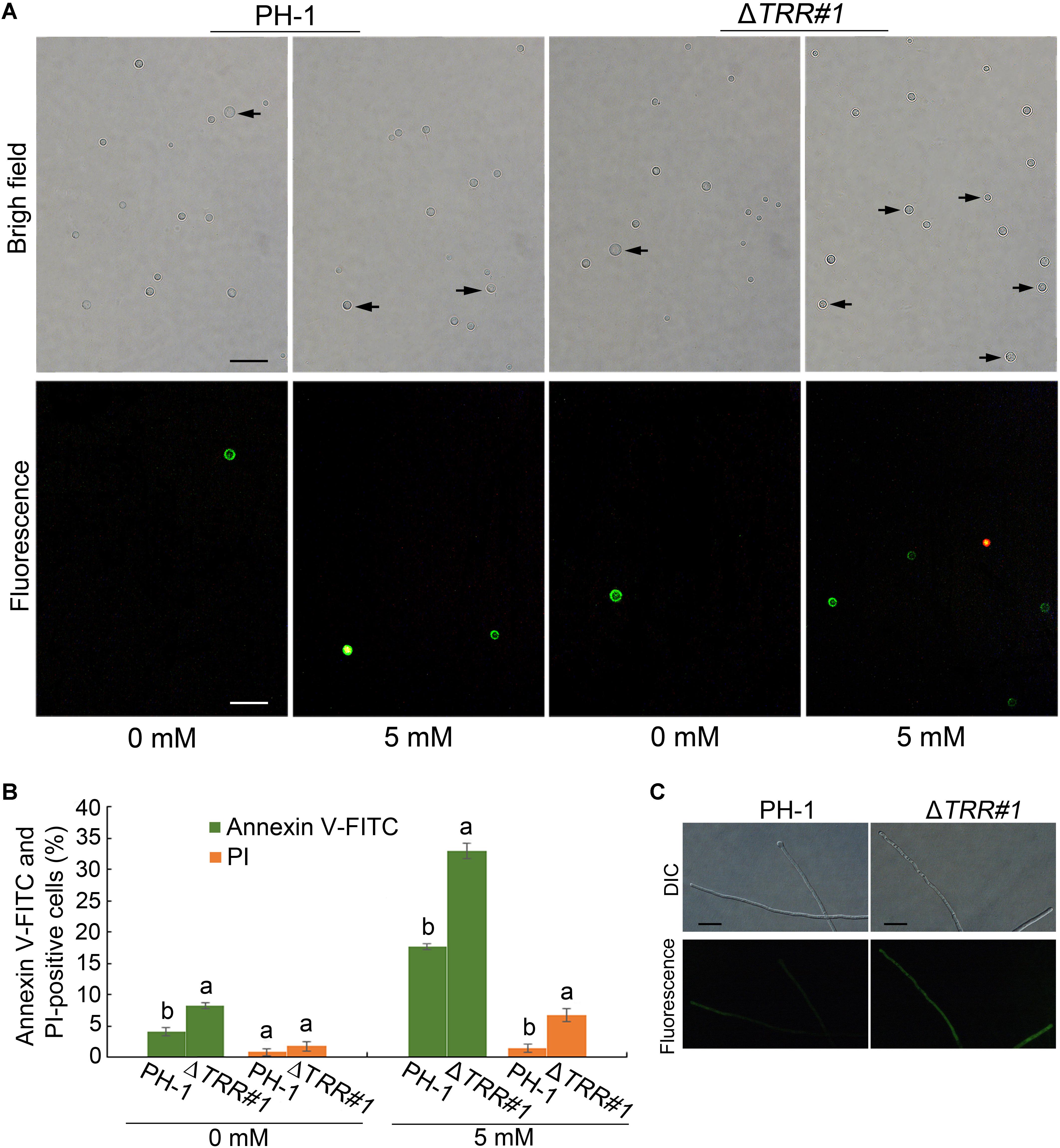
Figure 8. FgTRR is involved in apoptosis-like cell death in F. graminearum. (A) Protoplasts were treated with 5 mM H2O2 for 1 h and stained with Annexin V-FITC and propidium iodide (PI). Arrows indicate green and red cells showing Annexin V-FITC and PI-positive cells, respectively. Bar = 10 μm. (B) Percentage of Annexin V-FITC and PI-positive cells. At least 200 cells were counted in each repeat. Different letters denote statistically significant differences (P < 0.05) by Student’s t-tests. (C) The accumulation of intracellular ROS in hyphae of PH-1 and the ΔTRR#1 mutant. Hyphae cultured at 25°C for 18 h in liquid YEPD were collected and stained with 5 μM DHR123. Bar = 20 μm.
Discussion
The thioredoxin system consisting of TRR, TRX, and NADPH is ubiquitously present from yeast to filamentous fungi. In this study, we identified a TRR orthologue from F. graminearum. Sequence alignment revealed that FgTRR has features typical of TRRs from other fungi. The yeast S. cerevisiae contains two TRRs, TRR1 localizes in the cytoplasm and TRR2 in the mitochondrial matrix (Pedrajas et al., 1999; Carmel-Harel et al., 2001; Trotter and Grant, 2002), whereas only one TRR exists in some fungi (Fernandez and Wilson, 2014; Viefhues et al., 2014). Like yeast TRR1, FgTRR-GFP localizes to the cytoplasm, indicating that FgTRR is a cytosolic TRR.
The thioredoxin system is responsible for the redox regulation of DNA synthesis, development, gene transcription, and apoptosis (Lindner et al., 2002; Masutani et al., 2002; Koc et al., 2006). In this study, the FgTRR deletion mutants displayed a reduced hyphal growth rate, which is consistent with the TRR1 in B. cinerea (Viefhues et al., 2014). Cryptococcus neofoemans TRR1 is even essential for survival (Missall and Lodge, 2005). MoTrxs, a member of the thioredoxin system, play important roles in development, ROS signaling, and virulence in M. oryzae (Zhang S.J. et al., 2016). In contrast, M. oryzae TRR1 mutants exhibited normal radial growth (Fernandez and Wilson, 2014). Consistent with TRR in M. oryzae (Fernandez and Wilson, 2014), conidiation of the FgTRR mutants was also significantly reduced after a 5-day incubation in liquid CMC medium, conidial germination rates of the FgTRR mutants were also reduced compared with the WT. Therefore, the TRR may have a conserved role in normal vegetative growth and conidiation. Interestingly, the ΔTRR mutants were defective in ascus and ascospore development but produced protoperithecia on CA plates. Like TUB1 in F. graminearum (Chen et al., 2018), FgTRR is dispensable for initial stages of perithecium formation. Similarly, FgNoxR, a regulatory subunit of NADPH oxidases, is essential for perithecium formation in F. graminearum (Zhang C.K. et al., 2016). We propose that the thioredoxin system is required for sexual reproduction in F. graminearum.
Phytopathogenic species of Fusarium produce the red pigment aurofusarin, a secondary naphthoquinone metabolite (Medentsev and Akimenko, 1998; Frandsen et al., 2006). In this study, compared with the WT strain, the FgTRR mutants displayed reduced accumulation of red pigment, indicating that FgTRR deletion affects aurofusarin biosynthesis. The red coloration of CA plates was also reduced, which may be related to the downregulation of aurofusarin. Consistently, many genes including Gip, PKS, and Aur, which are responsible for aurofusarin production (Kim et al., 2005, 2006; Frandsen et al., 2006), were significantly decreased in the FgTRR mutant. The production of DON, an important virulence factor in F. graminearum (Desjardins et al., 1993, 1996; Proctor et al., 1995), was significantly decreased in the FgTRR mutants. Furthermore, qRT-PCR assays confirmed that FgTRR positively affected the expression of trichothecene biosynthesis genes TRI5, TRI6, and TRI10. TRR has been shown to be required for virulence in B. cinerea and M. oryzae (Fernandez and Wilson, 2014; Viefhues et al., 2014). We also found that deletion of FgTRR gene results in a defect in pathogenicity. Therefore, these results suggest that FgTRR is involved in secondary metabolism and pathogenicity.
Recently, it has been shown that deletion of MoTRR resulted in increased sensitivity to CR in M. oryzae (Fernandez and Wilson, 2014). In this study, we also found that the FgTRR mutants were more sensitive to CR, indicating that deletion of FgTRR decreases cell wall integrity in F. graminearum. The FgTRR mutants showed increased sensitivity to osmotic and oxidative stresses, like in other fungi (Carmel-Harel et al., 2001; Fernandez and Wilson, 2014; Viefhues et al., 2014). The pigments produced by fungi may provide protection against excessive oxidative stress (Rangel et al., 2015), we propose that, due to reduced accumulation of pigment, deletion of FgTRR results in the decreased ability to remove excessive H2O2. Taken together, FgTRR is important for response to various stresses.
Fungal apoptosis-like cell death is essential for normal development and maintenance of multicellular organisms, and fungi undergo apoptosis-like cell death in response to antifungal drugs or stresses (Shlezinger et al., 2012; Gonçalves et al., 2017). Apoptosis-like cell death in filamentous fungi has been identified as a crucial component in the infection of plants. Overexpression of the anti-apoptotic gene BcBIR1 in B. cinerea confers enhanced pathogenicity and resistance to cell death (Shlezinger et al., 2011). Interestingly, deletion of FgTRR resulted in the accumulation of intracellular ROS, which ultimately aggravated apoptosis-like cell death. Therefore, these results indicate that FgTRR is responsible for apoptosis-like cell death in F. graminearum.
Conclusion
In summary, our results indicate that the FgTRR protein plays a pleiotropic role in F. graminearum, including the regulation of vegetative growth, asexual and sexual reproduction, stress responses, pathogenicity, and DON synthesis. Furthermore, FgTRR is involved in apoptosis-like cell death in F. graminearum.
Author Contributions
XF, FH, and MD conceived and designed the experiments. XF, FH, MD, CG, LC, and SZ performed the experiments. LC and SZ analyzed the data. XF, FH, and MD wrote the manuscript. YL, JY, and HD originated research leading up to this paper and provided guidance and review.
Funding
This work was supported by the Natural Science Foundation of Shandong Province (ZR2017MC020), State key R&D Program (2017YFD0201705), the wheat innovation team of Shandong province modern agricultural industry technology system (SDAIT-01-09), and Funds of Shandong “Double Tops” Program (SYL2017XTTD11).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2019.00393/full#supplementary-material
References
Aguirre, J., Rios-Momberg, M., Hewitt, D., and Hansberg, W. (2005). Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 13, 111–118. doi: 10.1016/j.tim.2005.01.007
Alexander, N. J., Proctor, R. H., and McCormick, S. P. (2009). Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 28, 198–215. doi: 10.1080/15569540903092142
Arnér, E. S. J. (2009). Focus on mammalian thioredoxin reductases-important selenoproteins with versatile functions. Biochim. Biophy. Acta. 1790, 495–526. doi: 10.1016/j.bbagen.2009.01.014
Arunachalam, C., and Doohan, F. M. (2013). Trichothecene toxicity in eukaryotes: cellular and molecular mechanisms in plants and animals. Toxicol. Lett. 217, 149–158. doi: 10.1016/j.toxlet.2012.12.003
Bobba, A., Casalino, E., Petragallo, V. A., and Atlante, A. (2014). Thioredoxin/thioredoxin reductase system involvement in cerebellar granule cell apoptosis. Apoptosis 19, 1497–1508. doi: 10.1007/s10495-014-1023-y
Bowden, R. L., and Leslie, J. F. (1999). Sexual recombination in Gibberella zeae. Phytopathology 89, 182–188. doi: 10.1094/PHYTO.1999.89.2.182
Brown, D. W., Dyer, R. B., McCormick, S. P., Kendra, D. F., and Plattner, R. D. (2004). Functional demarcation of the Fusarium core trichothecene gene cluster. Fungal. Genet. Biol. 41, 454–462. doi: 10.1016/j.fgb.2003.12.002
Bruno, K. S., Tenjo, F., Li, L., Hamer, J. E., and Xu, J. R. (2004). Cellular localization and role of kinase activity of PMK1 in Magnaporthe grisea. Eukaryot. Cell 3, 1525–1532. doi: 10.1128/EC.3.6.1525-1532.2004
Carmel-Harel, O., Stearman, R., Gasch, A. P., Botstein, D., Brown, P. O., and Storz, G. (2001). Role of thioredoxin reductase in the Yap1p-dependent response to oxidative stress in Saccharomyces cerevisiae. Mol. Microbiol. 39, 595–605. doi: 10.1046/j.1365-2958.2001.02255.x
Catlett, N. L., Lee, B. N., Yoder, O. C., and Turgeon, B. G. (2003). Split-marker recombination for efficient targeted deletion of fungal genes. Fungal Genet. Newsl. 50, 9–11. doi: 10.4148/1941-4765.1150
Cavinder, B., Sikhakolli, U., Fellows, K. M., and Trail, F. (2012). Sexual development and ascospore discharge in Fusarium graminearum. J. Vis. Exp. 61, 5407–5407. doi: 10.3791/3895
Chen, D. P., Wu, C. L., Hao, C. F., Huang, P. P., Liu, H. Q., Bian, Z. Y., et al. (2018). Sexual specific functions of tub1 beta-tubulins require stage-specific RNA processing and expression in Fusarium graminearum. Environ. Microbiol. 20, 4009–4021. doi: 10.1111/1462-2920.14441
Desjardins, A. E., Hohn, T. M., and McCormick, S. P. (1993). Trichothecene biosynthesis in Fusarium species: chemistry, genetics, and significance. Microbiol. Rev. 57, 595–604.
Desjardins, A. E., and Proctor, R. H. (2007). Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 119, 47–50. doi: 10.1016/j.ijfoodmicro.2007.07.024
Desjardins, A. E., Proctor, R. H., Bai, G. H., McCormick, S. P., Shaner, G., Buechley, G., et al. (1996). Reduced virulence of trichothecene-nonproducing mutants of Gibberella zeae in wheat field tests. Mol. Plant Microbe Interact. 9, 775–781. doi: 10.1094/MPMI-9-0775
Fernandez, J., and Wilson, R. A. (2014). Characterizing roles for the glutathione reductase, thioredoxin reductase and thioredoxin peroxidase-encoding genes of Magnaporthe oryzae during rice blast disease. PLoS One 9:e87300. doi: 10.1371/journal.pone.0087300
Frandsen, R. J. N., Nielsen, J., Maolanon, N., Sørensen, J. C., Olsson, S., Nielsen, J., et al. (2006). The biosynthetic pathway for aurofusarin in Fusarium graminearum reveals a close link between thenaphthoquinones and naphthopyrones. Mol. Microbiol. 61, 1069–1080. doi: 10.1111/j.1365-2958.2006.05295.x
Gale, L. R., Chen, L. F., Hernick, C. A., Takamura, K., and Kistler, H. C. (2002). Population analysis of Fusarium graminearum from wheat fields in eastern china. Phytopathology 92, 1315–1322. doi: 10.1094/PHYTO.2002.92.12.1315
Gardiner, D. M., Kazan, K., and Manners, J. M. (2009). Nutrient profiling reveals potent inducers of trichothecene biosynthesis in Fusarium graminearum. Fungal Genet. Biol. 46, 604–613. doi: 10.1016/j.fgb.2009.04.004
Gonçalves, A. P., Heller, J., Daskalov, A., Videira, A., and Glass, N. L. (2017). Regulated forms of cell death in fungi. Front. Microbiol. 8:1837. doi: 10.3389/fmicb.2017.01837
Goswami, R. S., and Kistler, H. C. (2004). Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 5, 515–525. doi: 10.1111/j.1364-3703.2004.00252.x
Goswami, R. S., and Kistler, H. C. (2005). Pathogenicity and in planta mycotoxin accumulation among members of the Fusarium graminearum species complex on wheat and rice. Phytopathology 95, 1397–1404. doi: 10.1094/PHYTO-95-1397
Han, Y. K., Lee, T., Han, K. H., Yun, S. H., and Lee, Y. W. (2004). Functional analysis of the homoserine O-acetyltransferase gene and its identification as a selectable marker in Gibberella zeae. Curr. Genet. 46, 205–212. doi: 10.1007/s00294-004-0528-2
Hou, Z., Xue, C., Peng, Y., Katan, T., Kistler, H. C., and Xu, J. R. (2002). A mitogen-activated protein kinase gene (MGV1) in Fusarium graminearum is required for female fertility, heterokaryon formation and plant infection. Mol. Plant Microbe Interact. 15, 1119–1127. doi: 10.1094/MPMI.2002.15.11.1119
Kim, J. E., Han, K. H., Jin, J. M., Kim, H., Kim, J. C., Yun, S. H., et al. (2005). Putative polyketide synthase and laccase genes for biosynthesis of aurofusarin in Gibberella zeae. Appl. Environ. Microbiol. 71, 1701–1708. doi: 10.1128/AEM.71.4.1701-1708.2005
Kim, J. E., Jin, J., Kim, H., Kim, J. C., Yun, S. H., and Lee, Y. W. (2006). GIP2, a putative transcription factor that regulates the aurofusarin biosynthetic gene cluster in Gibberella zeae. Appl. Environ. Microbiol. 72, 1645–1652. doi: 10.1128/AEM.72.2.1645-1652.2006
Koc, A., Mathews, C. K., Wheeler, L. J., Gross, M. K., and Merrill, G. F. (2006). Thioredoxin is required for deoxyribonucleotide pool maintenance during S phase. J. Biol. Chem. 281, 15058–15063. doi: 10.1074/jbc.M601968200
Leslie, J. F., and Summerell, B. A. (2006). The Fusarium laboratory manual. New Jersey, NJ: Blackwell Pub Professional. doi: 10.1002/9780470278376
Lindner, D. J., Ma, X., Hu, J., Karra, S., and Kalvakolanu, D. V. (2002). Thioredoxin reductase plays a critical role in IFN retinoid-mediated tumor-growth control in vivo. Clin. Cancer Res. 8, 3210–3218.
Liu, L., Long, L. K., An, Y., Yang, J., Xu, X. X., Hu, C. H., et al. (2012). The thioredoxin reductase-encoding gene ActrxR1 is involved in the cephalosporin C production of Acremonium chrysogenum in methionine-supplemented medium. Appl. Microbiol. Biotechnol. 97, 2551–2562. doi: 10.1007/s00253-012-4368-6
Livak, K. J., and Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Machado, A. K., Morgan, B. A., and Merril, G. F. (1997). Thioredoxin reductase-dependent inhibition of MCB cell cycle box activity in Saccharomyces cerevisiae. J. Biol. Chem. 272, 17045–17054. doi: 10.1074/jbc.272.27.17045
Madeo, F., Frohlich, E., and Frohlich, K. U. (1997). A yeast mutant showing diagnostic markers of early and late apoptosis. J. Cell. Biol. 139, 729–734. doi: 10.1083/jcb.139.3.729
Masutani, H., Ueda, S., and Yodoi, J. (2002). The thioredoxin system in retroviral infection and apoptosis. Cell Death Diff. 12, 991–998. doi: 10.1038/sj.cdd.4401625
McMullen, M., Jones, R., and Gallenberg, D. (1997). Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis. 81, 1340–1348. doi: 10.1094/PDIS-03-12-0291-FE
Medentsev, A. G., and Akimenko, V. K. (1998). Naphthoquinone metabolites of the fungi. Phytochemistry 47, 935–959. doi: 10.1016/S0031-9422(98)80053-8
Missall, T. A., and Lodge, J. K. (2005). Thioredoxin reductase is essential for viability in the fungal pathogen Cryptococcus neoformans. Eukaryot. Cell 4, 487–489. doi: 10.1128/EC.4.2.487-489.2005
Mustacich, D., and Powis, G. (2000). Thioredoxin reductase. Biochem. J. 346, 1–8. doi: 10.1042/bj3460001
Pearson, G. D., and Merril, G. F. (1998). Deletion of the Saccharomyces cerevisiae TRR1 gene encoding thioredoxin reductase inhibits p53-dependent reporter gene expression. J. Biol. Chem. 273, 5431–5434. doi: 10.1074/jbc.273.10.5431
Pedrajas, J. R., Kosmidou, E., Miranda-Vizuete, A., Gustafsson, J., Wright, A. P. H., and Spyrou, G. (1999). Identification and functional characterization of a novel mitochondrial thioredoxin system in Saccharomyces cerevisiae. J. Biol. Chem. 274, 6366–6373. doi: 10.1074/jbc.274.10.6366
Pestka, J. J., and Smolinski, A. T. (2005). Deoxynivalenol: toxicology and potential effects on humans. J. Toxicol. Environ. Health Part B 8, 39–69. doi: 10.1080/10937400590889458
Powis, G., Mustacich, D., and Coon, A. (2000). The role of the redox protein thioredoxin in cell growth and cancer. Free Radic. Biol. Med. 29, 312–322. doi: 10.1016/S0891-5849(00)00313-0
Proctor, R. H., Hohn, T. M., and McCormick, S. P. (1995). Reduced virulence of Gibberella zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant Microbe Interact. 8, 593–601. doi: 10.1094/MPMI-8-0593
Rangel, D. E. N., Alder-Rangel, A., Dadachova, E., Finlay, R. D., Kupiec, M., Dijksterhuis, J., et al. (2015). Fungal stress biology: a preface to the fungal stress responses special edition. Curr. Genet. 61, 231–238. doi: 10.1007/s00294-015-0500-3
Royall, J. A., and Ischiropoulos, H. (1993). Evaluation of dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch. Biochem. Biophys. 302, 348–355. doi: 10.1006/abbi.1993.1222
Seong, K., Hou, Z. M., Tracy, M., Kistler, H. C., and Xu, J. R. (2005). Random insertional mutagenesis identifies genes associated with virulence in the wheat scab fungus Fusarium graminearum. Phytopathology 95, 744–750. doi: 10.1094/PHYTO-95-0744
Shlezinger, N., Goldfinger, N., and Sharon, A. (2012). Apoptosis-like programmed cell death in fungi: the benefits in filamentous species. Front. Oncol. 2:97. doi: 10.3389/fonc.2012.00097
Shlezinger, N., Minz, A., Gur, Y., Hatam, I., Dagdas, Y. F., Talbot, N. J., et al. (2011). Anti-apoptotic machinery protects the necrotrophic fungus Botrytis cinerea from host-induced apoptotic-like cell death during plant infection. PLoS Pathog. 7:e1002185. doi: 10.1371/journal.ppat.1002185
Soleimany, F., Jinap, S., and Abas, F. (2012). Determination of mycotoxins in cereals by liquid chromatography tandem mass spectrometry. Food Chem. 130, 1055–1060. doi: 10.1016/j.foodchem.2011.07.131
Trotter, E. W., and Grant, C. M. (2002). Thioredoxins are required for protection against a reductive stress in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 46, 869–878. doi: 10.1046/j.1365-2958.2002.03216.x
Tudzynski, P., Heller, J., and Siegmund, U. (2012). Reactive oxygen species generation in fungal development and pathogenesis. Curr. Opin. Microbiol. 15, 653–659. doi: 10.1016/j.mib.2012.10.002
Viefhues, A., Heller, J., Temme, N., and Tudzynski, P. (2014). Redox systems in Botrytis cinerea: impact on development and virulence. Mol. Plant-Microbe Interact. 27, 858–874. doi: 10.1094/MPMI-01-14-0012-R
Williams, C. H. Jr. (1995). Mechanism and structure of thioredoxin reductase from Escherichia coli. FASEB J. 9, 1267–1276. doi: 10.1096/fasebj.9.13.7557016
Zhang, C. K., Lin, Y. H., Wang, J. Q., Wang, Y., Chen, M. P., Norvienyeku, J., et al. (2016). FgNoxR, a regulatory subunit of NADPH oxidases, is required for female fertility and pathogenicity in Fusarium graminearum. FEMS Microbiol. Lett. 363:fnv223. doi: 10.1093/femsle/fnv223
Zhang, S. J., Jiang, C., Zhang, Q., Qi, L. N., Li, C. H., and Xu, J. R. (2016). Thioredoxins are involved in the activation of the PMK1 MAP kinase pathway during appressorium penetration and invasive growth in Magnaporthe oryzae. Environ. Microbiol. 18, 3768–3784. doi: 10.1111/1462-2920.13315
Zheng, D., Zhang, S., Zhou, X., Wang, C., Xiang, P., Zheng, Q., et al. (2012). The FgHOG1 pathway regulates hyphal growth, stress responses, and plant infection in Fusarium graminearum. PLoS One 7:e49495. doi: 10.1371/journal.pone.0049495
Keywords: Fusarium graminearum, thioredoxin reductase, development, pathogenicity, apoptosis
Citation: Fan X, He F, Ding M, Geng C, Chen L, Zou S, Liang Y, Yu J and Dong H (2019) Thioredoxin Reductase Is Involved in Development and Pathogenicity in Fusarium graminearum. Front. Microbiol. 10:393. doi: 10.3389/fmicb.2019.00393
Received: 23 November 2018; Accepted: 14 February 2019;
Published: 07 March 2019.
Edited by:
Neil Andrew Brown, University of Bath, United KingdomReviewed by:
Joerg Bormann, University of Bremen, GermanyKemal Kazan, Commonwealth Scientific and Industrial Research Organisation (CSIRO), Australia
Copyright © 2019 Fan, He, Ding, Geng, Chen, Zou, Liang, Yu and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuancun Liang, liangyc@sdau.edu.cn
†These authors have contributed equally to this work
 Xinyue Fan†
Xinyue Fan† Shenshen Zou
Shenshen Zou Yuancun Liang
Yuancun Liang Hansong Dong
Hansong Dong