Case report: A pediatric case of repeated false-positive urea breath test for Helicobacter pylori without decreased gastric acid secretion
- 1Department of Pediatrics, Faculty of Medicine, Saga University, Saga, Japan
- 2Department of Gastroenterology, Hiramatsu Hospital, Ogi, Saga, Japan
The urea breath test (UBT) is often used to diagnose Helicobacter pylori infection and for its eradication. However, this text can give positive results even for other urease-active bacteria other than H. pylori. Even after the successful eradication of H. pylori, the presence of other urease-active bacteria in the gut and oral cavity can lead to positive UBT results in patients with decreased gastric acid secretion. Herein, a 15-year-old boy was diagnosed with H. pylori infection through the testing and treatment program for H. pylori for third-year junior high-school students in Saga Prefecture initiated in 2016. He underwent triple therapy comprising vonoprazan; however, UBT was found to be positive even after therapy. The results remained positive even after fourth-line eradication therapy. Stool antigen, PCR using gastric fluid, microscopy, culture, and rapid urease tests were all negative. Pepsinogen levels were normal, and none of the findings suggested autoimmune gastritis. Gastric microflora analysis revealed oral flora showing urease activity. UBT is considered useful for determining the successful eradication of H. pylori; however, it may give false-positive results for both H. pylori infection and eradication judgment. Although the patient did not have autoimmune gastritis or decreased gastric acid secretion, it is presumed that oral commensal bacteria showing urease activity inhabited the stomach, resulting in the persistently positive UBT results. In conclusion, repeated false-positive UBT results for H. pylori may occur even without gastric acid hyposecretion. If H. pylori eradication is unsuccessful based on UBT, additional test by stool H. pylori antigen tests should be considered.
1. Introduction
One of the major risk factors for gastric cancer is Helicobacter pylori infection (1). Prompt eradicating of H. pylori reduces the risk of gastric cancer (2). Accordingly, H. pylori screening and treatment (test and treatment) program for middle-and high-school students for the primary prevention of gastric cancer is now being take up by local governments in Japan (3).
The urea breath test (UBT) is often used to diagnose H. pylori (4). However, UBT is also positive for urease-active bacteria other than H. pylori. Even after the successful eradication of H. pylori, the presence of urease-active bacteria in the gut and oral cavity can lead to positive UBT results in patients with decreased gastric acid secretion, such as those with autoimmune gastritis (AIG). This is defined as “repeated false-positive UBT for H. pylori,” as advocated by Furuta et al. (5), which occurs when only UBT is used to determine H pylori eradication.
Herein, we report a pediatric case of repeated futile H. pylori eradication regimen because of false-positive UBT without decreased gastric acid secretion. This is the first pediatric case of this phenomenon.
2. Case report
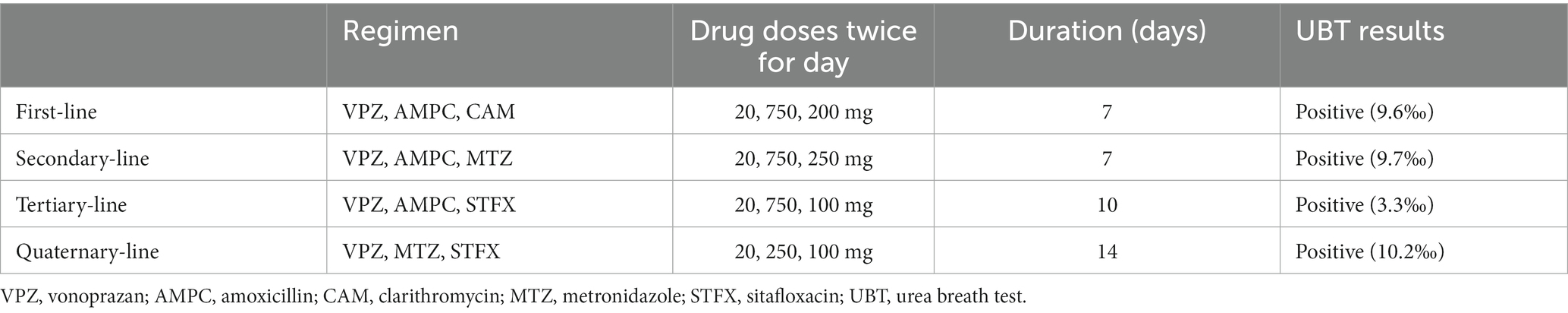
A 15-year-old boy was diagnosed with H. pylori infection at the test-and-treat program for H. pylori for third-year junior high-school students in Saga Prefecture in 2016 (6). Figure 1 presents the flowchart for the H. pylori test and treatment program in Saga Prefecture that is being conducted over the 8 years of the study period (2016–2023). We present the case of a patient diagnosed with H. pylori infection through both positive urine H. pylori antibody and stool antigen tests (SATs). This patient was included in the 2.0% (1,211/58, 281) H. pylori infection rate in our program. He exhibited no recent gastrointestinal symptoms and had no family history of H. pylori infection. He underwent eradication therapy for H. pylori at a medical institution participating in the Saga program. He was treated with a triple therapy of 20 mg vonoprazan (VPZ), 750 mg amoxicillin (AMPC), and 200 mg clarithromycin (CAM) twice daily for 7 days (Table 1). After 8 weeks, a UBT indicated that the eradication therapy was unsuccessful. He underwent second-line eradication therapy with 20 mg VPZ, 750 mg AMPC, and 250 mg metronidazole (MTZ) twice daily for 7 days. However, his UBT tests remained still positive. Third-line eradication therapy was performed with 20 mg VPZ, 750 mg AMPC, and 100 mg sitafloxacin (STFX) twice for 10 days daily. The fourth-line eradication therapy included 20 mg VPZ, 250 mg MTZ, and 100 mg STFX twice daily for 14 days. However, the UBT results after both therapies (10.2%, normal range < 2.5%) were positive, indicating eradication therapy failure. The patient was 100% compliant with all four eradication therapies and did not experience any adverse effects during or immediately after each line of therapy.
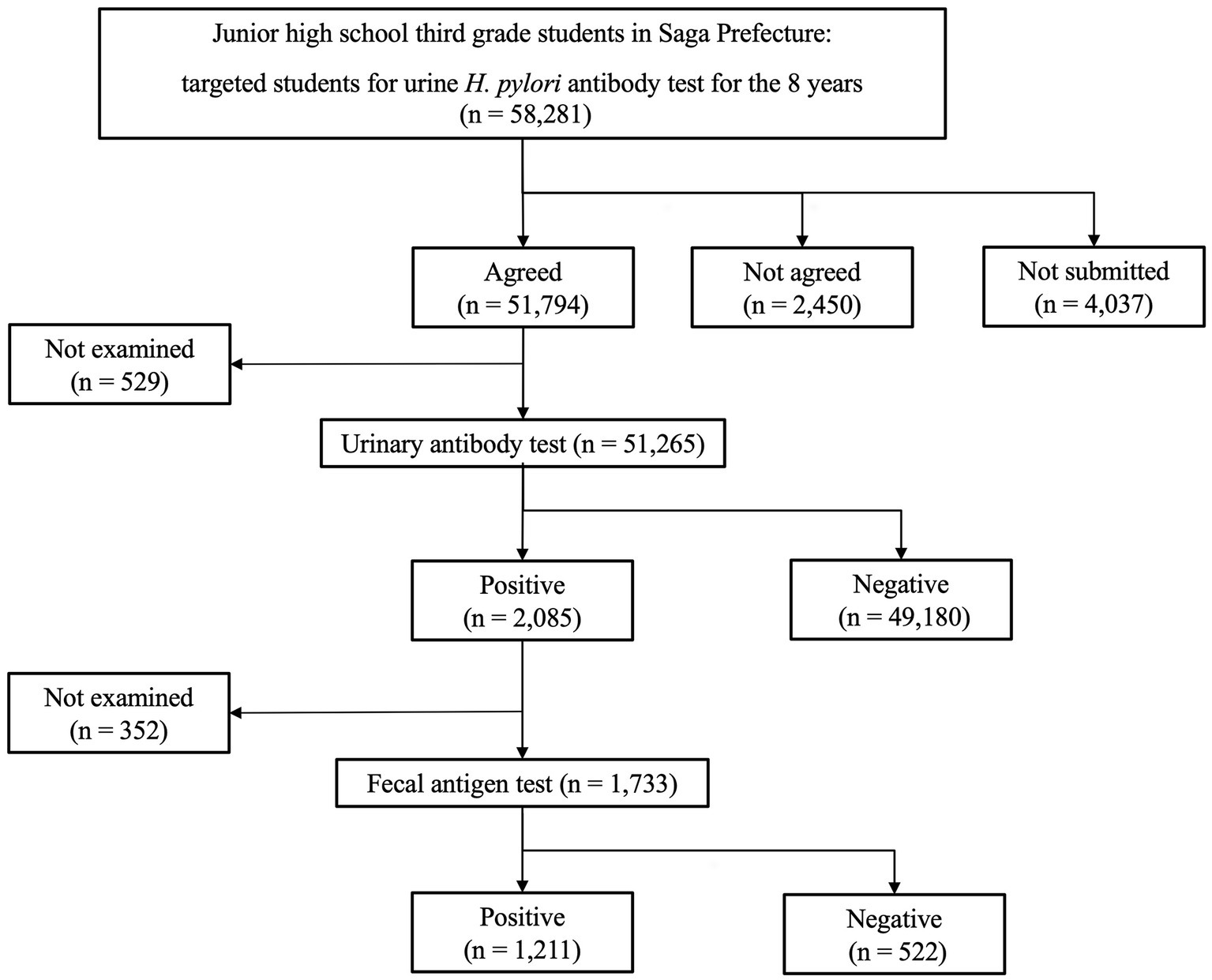
Figure 1. Summary for Helicobacter pylori screening and treatment in the Saga Prefecture for over 8 years of the study period (2016–2023). Of the total 58,281 patients, 51,265 underwent primary urinary antibody test for H. pylori and 1,733 underwent a secondary stool antigen test. Finally, 1,211 students had two positive tests and were diagnosed with H. pylori infection.
Considering the possibility of false-positive UBT results, we decided to conduct more tests. The serum H. pylori antibody titer was 13 U/mL (normal range, <10 U/mL), and the SATs was negative. Esophagogastroduodenoscopy (EGD) revealed mild antral atrophy (Kimura–Takemoto classification C-2; Figure 2A). Atrophy of the fundus and corpus was not observed (Figure 2B). There were no findings suggestive of H. pylori, such as diffuse redness, mucosal swelling, patchy redness, enlarged folds, foveolar-hyperplastic polyp, nodularity, or xanthoma. Negative results were obtained for both the rapid urease test as well as the point-of-care testing kit with intragastric fluid, a novel kit for detecting H. pylori and CAM resistance (7). H. pylori bacteria were not identified on microscopic examination with hematoxylin–eosin and Giemsa staining. A culture test for H. pylori using intragastric fluid was also negative. Mucosal pathology of the gastric antrum showed mild atrophy and intestinal metaplasia in a part of the epithelium. In the lamina propria, chronic inflammatory cell infiltration mainly comprising lymphocytes and plasma cells was observed, along with fibrosis (Figure 2C). Gastrin immunostaining revealed no evidence of positive G-cell hyperplasia (Figure 2D). Mucosal pathology of the gastric corpus indicated no atrophy or intestinal metaplasia of the epithelium (Figure 2E).
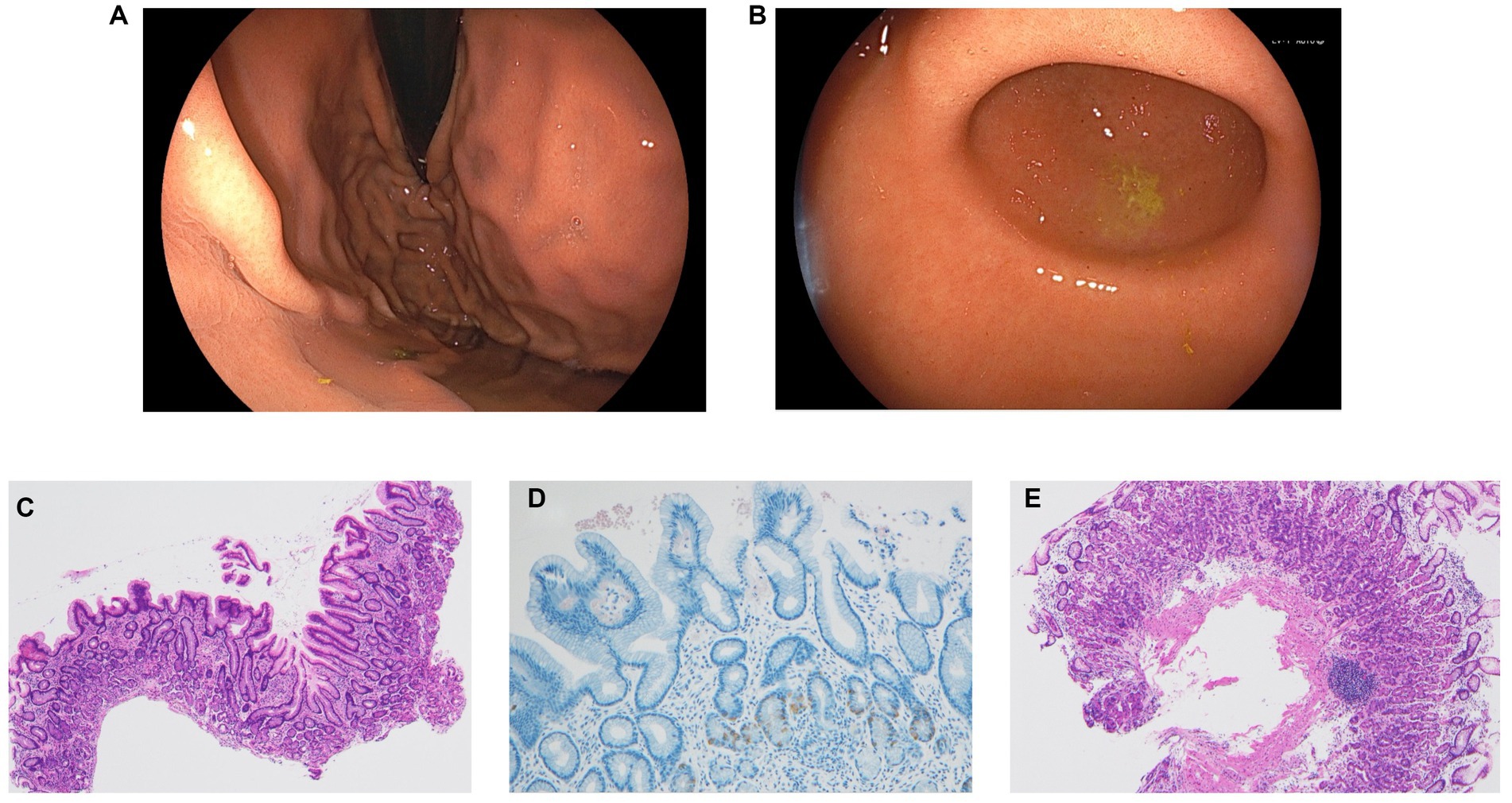
Figure 2. (A) Esophagogastroduodenoscopy revealed mild antral atrophy (Kimura–Takemoto classification C-2). (B) Atrophy of the fundus and corpus was not observed. None of the findings were suggestive of H. pylori, such as diffuse redness, mucosal swelling, patchy redness, enlarged fold, foveolar-hyperplastic polyp, nodularity, and xanthoma. (C) Mucosal pathological findings in the gastric antrum showed mild atrophy and intestinal metaplasia in part of the epithelium. Chronic inflammatory cell infiltration mainly comprising lymphocytes and plasma cells along with fibrosis was observed in the lamina propria (hematoxylin–eosin stain, ×40). (D) Gastrin immunostaining showed no evidence of positive G-cell hyperplasia (×100). (E) Mucosal pathological findings of the gastric corpus showed no atrophy or intestinal metaplasia of the epithelium (hematoxylin–eosin stain, ×40).
In addition, intrinsic factor and gastric parietal cell antibody tests were negative. The level of pepsinogen 1 was within normal at 23.4 ng/mL (normal range, 15–100), and the ratio of pepsinogen 1:2 was 4.9 (normal range, >3). Gastric microbiota analysis using 16S rRNA was positive for Neisseria spp., Corynebacterium spp., Haemophilus spp., and Actinomyces spp. at the genus level (Figure 3).
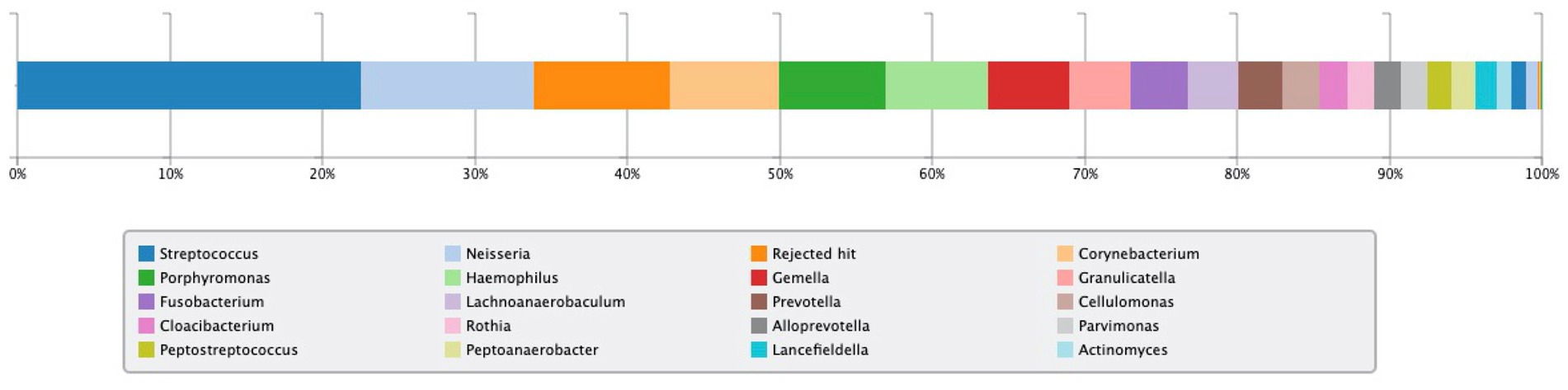
Figure 3. Result of 16S rRNA gastric microbiota analysis. Neisseria spp., Corynebacterium spp., Haemophilus spp., and Actinomyces spp. were detected at the genus level and have been evaluated as having urease activity.
3. Discussion
The clinical course in the present case highlights two important issues. First, repeated false-positive UBT results for H. pylori can also occur in children; therefore, physicians must be aware of these false-positive results while determining H. pylori eradication in this population. Second, paying attention to such false-positive UBT results is necessary, even in cases without gastric acid hyposecretion, such as AIG.
The current patient was diagnosed with H. pylori infection based on positive urine and serum antibody tests and SATs before eradication along with mild atrophy in the antrum through endoscopic and pathological findings. Other examination methods other than UBT after four eradication treatments did not indicate infection with H. pylori, indicating that eradication was successful at some stage during treatment. In Japan, triple therapy with VPZ is largely successful up to secondary eradication (8). This is true in both adults and children (3). In the test-and-treat program for H. pylori in the Saga Prefecture, the success rates of the first-and second-line eradication treatment were 83.3% (777/933) and 95.5% (128/134) over 8 years, respectively. UBT can only be used to determine the presence of urease activity in the stomach; it is not a test that directly confirms the presence of H. pylori. UBT is also considered useful for determining the eradication of H. pylori in the guidelines (9); however, as shown in this case, a positive result may be obtained even if H. pylori infection has been eradicated. Depending on the drug used for eradication therapy and whether a previous H. pylori drug resistance test was performed, if UBT reveals secondary eradication failure, it may be important to consider eradication confirmation tests other than UBT. Pediatric H. pylori guidelines recommend UBT or SATs to determine the success or failure of H. pylori eradication (9, 10). However, as mentioned in the guidelines, performing both tests is recommended. To that end, the frequency of UBT false-positive cases, like the present case, among patients with H. pylori eradicated must be examined because this indicates the cost effectiveness of treatment. Furuta et al. (5) described repeated false-positive UBT results for H. pylori in adult patients as continued positive UBT results despite the eradication of H. pylori. Our report indicates that such a phenomenon could also occur in children.
Although the current patient did not have AIG or exhibit decreased gastric acid secretion, oral commensal bacteria with urease activity may have inhabited the stomach, resulting in the unnecessary continuation of H. pylori eradication therapy. Figure 2 shows the results of gastric microflora analysis using gastric fluid at the time of UBT positivity after four rounds of eradication. In the present case, four bacteria genera (Neisseria, Corynebacterium, Haemophilus, and Actinomyces), which may have urease activity as reported by Furuta et al. (5), were detected. The problem with the present case was that it was no possible to biochemically prove whether the bacterial genera detected in the gastric flora analysis actually showed urease activity.
Gastric microbiota comprises bacteria ingested primarily through the ororespiratory tract and secondarily from the intestines by transpyloric biliary reflux (11). Persistent H. pylori infection decreases gastric acid secretion, which might affect the gastric microbiota in adults (12) and children (13, 14). Andersson et al. (15) revealed that H. pylori was the dominant bacterium whenever isolated, although its absence was associated with a diverse microbiota. This information indicates that H. pylori can have inhibitory effects on the colonization of other bacteria harboring a significantly lower diversity in the stomach (16). On the contrary, even without H. pylori infection, previous reports have suggested that the predominant phyla in the gastric mucosa include Streptococcus, Rothia, Lactobacillus, Veillonella, Prevotella, Neisseria, and Hemophilus, counting more than one hundred sorts (11, 17). The predominance of any of these bacteria with urease activity in the stomach could result in a false-positive UBT. Further studies investigating the prevalence of oral commensal bacteria in cases of persistent positive UBT results following H. pylori eradication are warranted (Figure 3).
In conclusion, repeated false-positive UBT for H. pylori can occur even without gastric acid hyposecretion. If UBT results are persistently positive even after H. pylori is eradicated multiple times, the presence of urease-activating bacteria other than H. pylori must be considered. Specifically, eradication must be determined by testing with both UBT and SATs.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
MN: Conceptualization, Data curation, Writing – Original draft. TK: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Supervision, Writing – Review & editing. KF: Conceptualization, Data curation, Writing – Review & editing. MY: Conceptualization, Data curation, Supervision, Writing – Review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the patient’s family for providing consent and granting permission to draft and publish this case report. We acknowledge the medical staff of Saga University Hospital who were associated with this patient in the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Uemura, N, Okamoto, S, Yamamoto, S, Matsumura, N, Yamaguchi, S, Yamakido, M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. (2001) 345:784–9. doi: 10.1056/NEJMoa001999
2. Take, S, Mizuno, M, Ishiki, K, Nagahara, Y, Yoshida, T, Yokota, K, et al. Baseline gastric mucosal atrophy is a risk factor associated with the development of gastric cancer after Helicobacter pylori eradication therapy in patients with peptic ulcer diseases. J Gastroenterol. (2007) 42:21–7. doi: 10.1007/s00535-006-1924-9
3. Kakiuchi, T, Matsuo, M, Endo, H, Sakata, Y, Esaki, M, Noda, T, et al. Efficacy and safety of vonoprazan-based regimen for Helicobacter pylori eradication therapy in Japanese adolescents: a prospective multicenter study. J Gastroenterol. (2023) 58:196–204. doi: 10.1007/s00535-022-01942-z
4. Best, LM, Takwoingi, Y, Siddique, S, Selladurai, A, Gandhi, A, Low, B, et al. Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev. (2018) 2018:Cd012080. doi: 10.1002/14651858.CD012080.pub2
5. Furuta, T, Baba, S, Yamade, M, Uotani, T, Kagami, T, Suzuki, T, et al. High incidence of autoimmune gastritis in patients misdiagnosed with two or more failures of H. pylori eradication. Aliment Pharmacol Ther. (2018) 48:370–7. doi: 10.1111/apt.14849
6. Kakiuchi, T, Matsuo, M, Endo, H, Nakayama, A, Sato, K, Takamori, A, et al. A Helicobacter pylori screening and treatment program to eliminate gastric cancer among junior high school students in Saga prefecture: a preliminary report. J Gastroenterol. (2019) 54:699–707. doi: 10.1007/s00535-019-01559-9
7. Tsuda, M, Watanabe, Y, Oikawa, R, Watanabe, R, Higashino, M, Kubo, K, et al. Clinical evaluation of a novel molecular diagnosis kit for detecting helicobacter pylori and clarithromycin-resistant using intragastric fluid. Helicobacter. (2022) 27:e12933. doi: 10.1111/hel.12933
8. Kiyotoki, S, Nishikawa, J, and Sakaida, I. Efficacy of Vonoprazan for Helicobacter pylori eradication. Intern Med. (2020) 59:153–61. doi: 10.2169/internalmedicine.2521-18
9. Kato, S, Shimizu, T, Toyoda, S, Gold, BD, Ida, S, Ishige, T, et al. The updated JSPGHAN guidelines for the management of Helicobacter pylori infection in childhood. Pediatr Int. (2020) 62:1315–31. doi: 10.1111/ped.14388
10. Jones, NL, Koletzko, S, Goodman, K, Bontems, P, Cadranel, S, Casswall, T, et al. Joint ESPGHAN/NASPGHAN guidelines for the Management of Helicobacter pylori in children and adolescents (update 2016). J Pediatr Gastroenterol Nutr. (2017) 64:991–1003. doi: 10.1097/mpg.0000000000001594
11. Yu, G, Torres, J, Hu, N, Medrano-Guzman, R, Herrera-Goepfert, R, Humphrys, MS, et al. Molecular characterization of the human stomach microbiota in gastric cancer patients. Front Cell Infect Microbiol. (2017) 7:302. doi: 10.3389/fcimb.2017.00302
12. Oh, B, Kim, BS, Kim, JW, Kim, JS, Koh, SJ, Kim, BG, et al. The effect of probiotics on gut microbiota during the Helicobacter pylori eradication: randomized controlled trial. Helicobacter. (2016) 21:165–74. doi: 10.1111/hel.12270
13. Llorca, L, Pérez-Pérez, G, Urruzuno, P, Martinez, MJ, Iizumi, T, Gao, Z, et al. Characterization of the gastric microbiota in a Pediatric population according to Helicobacter pylori status. Pediatr Infect Dis J. (2017) 36:173–8. doi: 10.1097/inf.0000000000001383
14. Brawner, KM, Kumar, R, Serrano, CA, Ptacek, T, Lefkowitz, E, Morrow, CD, et al. Helicobacter pylori infection is associated with an altered gastric microbiota in children. Mucosal Immunol. (2017) 10:1169–77. doi: 10.1038/mi.2016.131
15. Andersson, AF, Lindberg, M, Jakobsson, H, Bäckhed, F, Nyrén, P, and Engstrand, L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One. (2008) 3:e2836. doi: 10.1371/journal.pone.0002836
16. Liatsos, C, Papaefthymiou, A, Kyriakos, N, Galanopoulos, M, Doulberis, M, Giakoumis, M, et al. Helicobacter pylori, gastric microbiota and gastric cancer relationship: unrolling the tangle. World J Gastrointest Oncol. (2022) 14:959–72. doi: 10.4251/wjgo.v14.i5.959
Keywords: Helicobacter pylori, child, autoimmune gastritis, urea breath test, urease activity
Citation: Nishino M, Kakiuchi T, Fukuda K and Yoshiura M (2023) Case report: A pediatric case of repeated false-positive urea breath test for Helicobacter pylori without decreased gastric acid secretion. Front. Med. 10:1267180. doi: 10.3389/fmed.2023.1267180
Edited by:
Raffaele Pellegrino, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Akira Umemura, Iwate Medical University, JapanJobin Philipose, Mountain View Regional Medical Center, United States
Jonathan Soldera, University of Caxias do Sul, Brazil
Copyright © 2023 Nishino, Kakiuchi, Fukuda and Yoshiura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshihiko Kakiuchi, kakiucht@cc.saga-u.ac.jp
 Masafumi Nishino1
Masafumi Nishino1  Toshihiko Kakiuchi
Toshihiko Kakiuchi