Case report: Injected corticosteroids for treating leprosy isolated neuritis
- 1Leprosy Laboratory, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
- 2Post-Graduate Program in Neurology, Federal University of the State of Rio de Janeiro, Rio de Janeiro, Brazil
- 3Pedro Ernesto University Hospital, Rio de Janeiro State University, Rio de Janeiro, Brazil
- 4Department of Neurology, Antonio Pedro University Hospital, Fluminense Federal University, Niteroi, Brazil
- 5National Institute of Science and Technology on Neuroimmunomodulation, Oswaldo Cruz Institute, Oswaldo Cruz Foundation, Rio de Janeiro, Brazil
One of the main manifestations of leprosy is peripheral nerve impairment. Early diagnosis and treatment are important to reduce the impact of neurological impairment, which can cause deformities and physical disabilities. Leprosy neuropathy can be acute or chronic, and neural involvement can occur before, during, or after multidrug therapy, and especially during reactional episodes when neuritis occurs. Neuritis causes loss of function in the nerves and can be irreversible if left untreated. The recommended treatment is corticosteroids, usually through an oral regimen at an immunosuppressive dose. However, patients with clinical conditions that restrict corticosteroid use or that have focal neural involvement may benefit from the use of ultrasound-guided perineural injectable corticosteroids. In this study, we report two cases that demonstrate how the treatment and follow-up of patients with neuritis secondary to leprosy, using new techniques, can be provided in a more individualized way. Nerve conduction studies in association with neuromuscular ultrasound were used to monitor the response to treatment with injected steroids, focusing on neural inflammation. This study provides new perspectives and options for this profile of patients.
Introduction
Leprosy remains a public health problem despite efforts to eradicate it. The main manifestations are a result of the skin and peripheral nerve involvement. Neuritis may lead to nerve damage that can occur before, during, or after multidrug therapy (MDT), predominantly in multibacillary (MB) and borderline leprosy, and is commonly associated with type 1 reactions (1). It causes loss of function and can be irreversible if not properly treated. Neuritis has been defined as nerve inflammation, which presents as pain and nerve thickening in conjunction with sensory impairment, associated or not with signs of motor impairment (2–5). Leprosy was initially attributed to Mycobacterium leprae as the etiologic agent, but in 2008, Mycobacterium lepromatosis was described as a second causative species; however, there is still no consensus on its importance in the epidemiology of leprosy in humans (6). Although MDT targets the causative bacteria and promotes antigenic load reduction, corticosteroids play the most important role in the management of neuritis as an anti-inflammatory therapy (7). The optimal dose and duration of corticosteroid therapy to treat neuritis is still a matter of debate. The World Health Organization recommends a standard regimen of 12 weeks to treat acute neuritis, starting with 40 mg of prednisolone, the dose of which is reduced over the following 12 weeks. Some studies recommend longer courses for the treatment of type 1 reactions (8). Other studies have compared treatment with 40 and 60 mg of prednisone and both regimens were found to be effective; however, most of the recurrences occurred within a 6-month period after completion of the low-dose regimen (9). Van Brakel et al. (10) concluded that improvement following treatment was directly related to the severity of the nerve damage observed at the beginning of treatment. In patients who did not have neuropathy prior to acute neuritis, steroid treatment resulted in full recovery in 88% of nerves with neuropathy, but only 51% of those with chronic disease or recurrent neuropathy recovered nerve function (11).
Nevertheless, it is known that prolonged therapy and/or high doses of corticosteroids result in a high frequency of side effects, such as arterial hypertension, dysglycemia, skin rashes, and Cushing's syndrome (12, 13). Another treatment option in this patient profile is the use of high-dose intravenous methylprednisolone in the pulse therapy regimen, which can reduce the occurrence of side effects (14). Local corticosteroid injection is a common non-surgical treatment for carpal tunnel syndrome (CTS). Several studies have shown that local corticosteroid injection provides significantly greater clinical improvement of CTS than oral steroids up to 3 months after treatment, as well as an improvement in symptoms compared with a single systemic injection at 1 month follow-up (15, 16). Dammers et al. (16) used a short-acting injectable corticosteroid, 40 mg of methylprednisolone, with 10 mg of lidocaine, for the treatment of CTS. In this study, we report two cases attended at the Ambulatory Souza Araújo (ASA) Leprosy Outpatient Clinic (Oswaldo Cruz Institute—IOC, Fiocruz), a Leprosy Reference Center, in Rio de Janeiro, Brazil, in which corticosteroid injections were used for the management of focal leprosy neuritis.
Case description
The two cases of the present study were diagnosed with leprosy according to the criteria of Ridley and Jopling (17) and were subsequently treated with MDT. They were evaluated by a dermatologist and a neurologist throughout the treatment.
Case 1
A 24-year-old male resident of the metropolitan region of Rio de Janeiro, Brazil, was referred to the Leprosy Outpatient Clinic by the primary care service on account of diffuse skin lesions over the body, which began 10 months previously. Past medical history: yellow fever 4 years previously, was a non-drinker and non-smoker, and said he did not use medications regularly. He said there was no history of leprosy in his family. A dermatological evaluation identified >20 diffuse lesions over the body (face, upper limbs, lower limbs, and trunk) in the form of papules, nodules, and tubercles. A Mitsuda test and bacilloscopy were requested, which were positive (5 mm) and 5.25, respectively, and MDT for MB leprosy was started after classification of the borderline lepromatous form (BL/LL). The patient was assessed by a neurologist at the beginning of treatment despite not having neurological symptoms, and a neurological examination did not show nerve thickening or sensory or motor changes.
After 4 months of MDT, the patient returned to the neurologist claiming he had been suffering from paresthesia in the fourth and fifth fingers of the left hand for ~1 month. On examination, the patient had pain upon palpation in the region above the left elbow and thickening of the ulnar nerve, associated with tactile hypoesthesia, thermal and pain insensitivity, and grade 4 muscle weakness according to the Medical Research Council (MRC) Scale (18) in the hypothenar region (little finger abductor and first dorsal interosseous muscles). A nerve conduction study (NCS) showed an absence of sensory nerve action potentials (SNAPs) in the left ulnar nerve and a reduced amplitude of compound motor action potentials (CMAPs) with a conduction block and reduced motor speed in the elbow segment (Table 1). Other nerves did not show alterations upon clinical examination and an NCS. Acute neuritis of the left ulnar nerve type 1 reaction was diagnosed.
The neuromuscular ultrasound (NMUS) evaluation showed marked ulnar thickening, measuring 24 mm2 at the epicondylar level and 92 mm2 at the supraepicondylar level [reference value (RV): 8 mm2], as well as homogeneous fascicular hypoechogenicity and vascular flow on the Power Doppler (Note: Power Doppler is more sensitive than the Color Doppler for detecting blood flow, but it does not provide information on the direction of flow). Ultrasound-guided local injection of 40 mg methylprednisolone with lidocaine was performed every 2 weeks above the elbow groove where the ulnar nerve was most damaged.
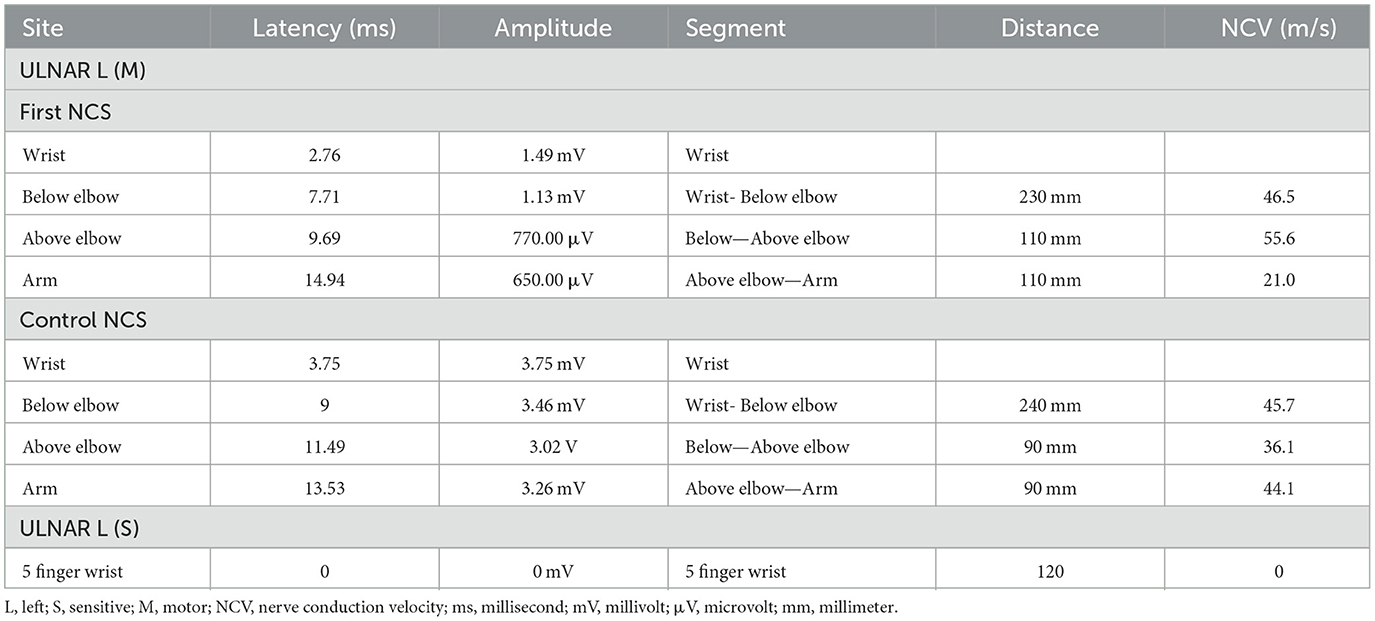
The patient was followed up in neurological appointments every 2 weeks, and after 2 months, the patient was reassessed and there was no complaint of paresthesia or pain upon neurological examination, an MRC grade 5 was determined in all muscles, and a subjective reduction in neural thickening was noted. An NCS was repeated within 3 months after the start of corticosteroid therapy and an improvement in electrophysiological values was observed (Table 1). NMUS showed an improvement in echogenicity and fascicular disarray, a 50% reduction in the cross-sectional diameter at the two levels described above, and no flow in the Power Doppler (Figure 1).
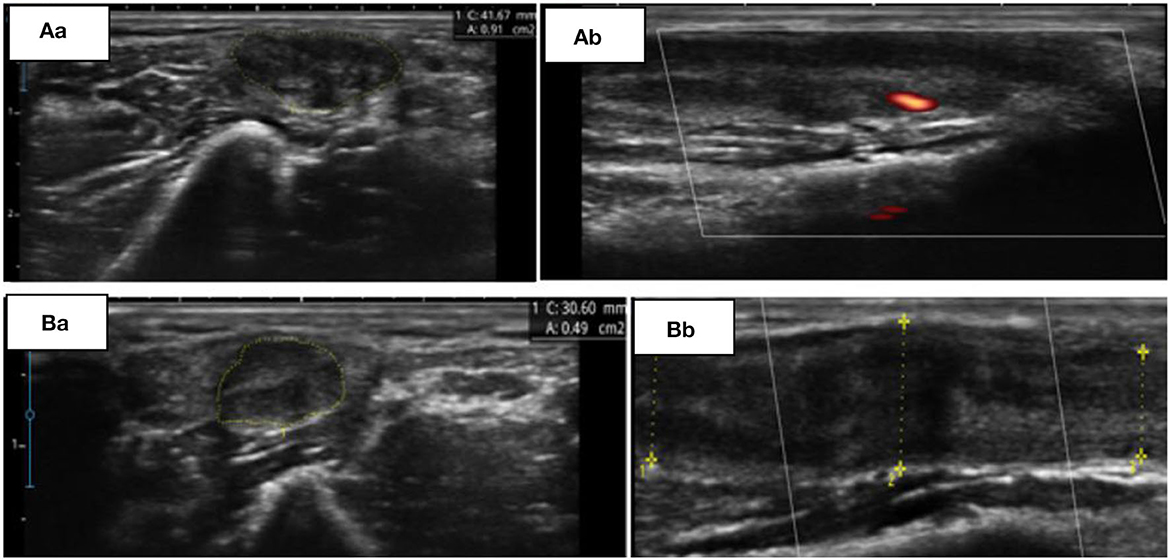
Figure 1. (A) Initial findings from ultrasonography (USG). Transverse (Aa) and longitudinal (Ab) view of the left ulnar nerve at the supraepicondylar region. (B) Control USG, 60 days later. Transverse (Ba) and longitudinal (Bb) view of the same nerve at the supraepicondylar region.
Case 2
A 52-year-old female resident of the metropolitan region of Rio de Janeiro, Brazil, was referred to the Leprosy Outpatient Clinic by the primary care service because of skin lesions and neuropathic pain in her hands for the last year. The patient was under endocrinological follow-up because of insulin-dependent diabetes, for which she was using neutral protamine Hagedorn (NPH) and regular insulin (according to blood glucose measurements) with good control. The patient was dyslipidemic, a smoker (35 packs/year), a non-drinker, and had previous contact with a brother that had leprosy. She said there was no history of leprosy in her family. Physical and dermatological examination showed 11–20 well-defined, erythematous, and hypochromic lesions, with a hypoesthetic lesion in the left upper limb. Bacilloscopy was negative and skin biopsy revealed epithelioid granuloma in the dermis compatible with a reversal reaction (type 1). MDT for paucibacillary (PB) leprosy was started after it was classified as borderline tuberculoid (BT). At this time, the neurological assessment showed bilateral thickening of the ulnar nerves at the level of the elbow, thermal and painful hypoesthesia in the right ulnar nerve, and tactile hypoesthesia and thermal and pain insensitivity in the left ulnar nerve. Furthermore, muscle weakness (MRC grade 4) in the left hypothenar muscles (little finger abductor and first dorsal interosseous muscles) was observed. An NCS was performed, which demonstrated evidence of myelin lesions with secondary axonal involvement in both ulnar nerves. Ultrasonography (USG) showed ulnar nerves with thickening in the epicondylar and supraepincodylar regions and with homogeneous fascicular hypoechogenicity, as well as Power Doppler flow of the right and left ulnar nerves. There was no involvement of other neural territories observed in the initial clinical evaluation, NCS, or ultrasound. Bilateral ulnar neuritis was diagnosed and treatment with oral corticosteroids (1 mg/kg/day) was initiated, with a dose reduction of 0.1 mg/kg/day every 2 weeks until a dose of 0.5 mg/kg/day was reached, after which monthly weaning was performed until withdrawal. The total treatment time was 6 months. During this period, no significant side effects were observed, especially regarding dysglycemia, as the insulin adjustment and control were being monitored by a multidisciplinary team.
The patient was reevaluated with no signs of spontaneous pain or paresthesia, and objective sensory and motor findings were maintained. Electrophysiological examination and USG showed improvements in the ulnar parameters after 6 months.
During the follow-up of ulnar neuritis, a neurological examination suggested the involvement of the right median nerve, which was confirmed by an NCS, wherein a conduction block in the right median nerve of the forearm was observed (Table 2). USG revealed thickening of the median nerve from the level of the middle third of the pronator quadratus muscle up to the main branch of the palmar terminal branches, with a cross-sectional area 2 cm from the carpal tunnel of 32 mm2 and 18 mm2 at the carpal tunnel (RV: 9 mm2), and Power Doppler flow detectable in the carpal region (Figure 2). An increased signal in those regions was detected by MR neurography of the median nerve with uptake by the intravenous contrast; there were no signs of nerve compression.
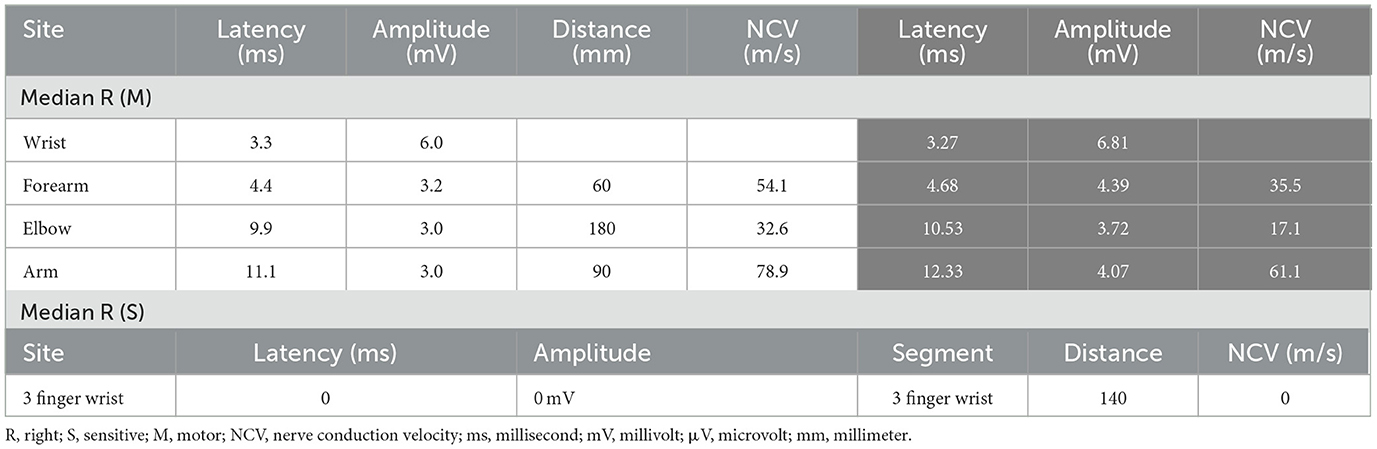
Table 2. Right median nerve conduction values at the first and control (gray column) NCS assessments.
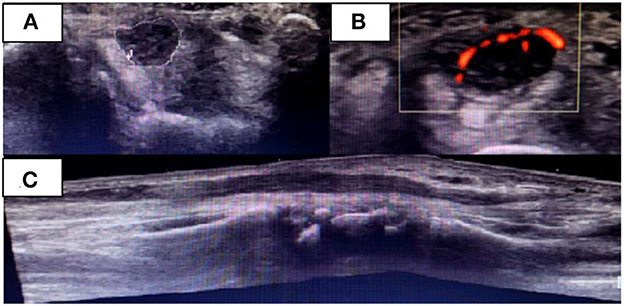
Figure 2. Right median nerve on the initial USG examination when median neuritis was diagnosed. Transverse (A, B) and (C) longitudinal nerve view in the carpal region. Presence of flow in the Power Doppler (B).
The diagnosis of right median neuritis was made when the patient was finishing the oral prednisone treatment for ulnar neuritis. Therefore, the patient was started on 40 mg methylprednisolone and lidocaine injected around the perineurium of the median nerve over 3 months. She underwent an NCS after this period, which showed improvement in the nerve conduction values (Table 2). Additionally, there was an improvement in the USG results in terms of the nerve thickening and absence of flow on the Power Doppler. The patient remains in control of the neuropathic pain through quarterly assessments by the neurology team.
Discussion
The clinical form of leprosy depends on the host's immune response to M. leprae antigenic determinants (19, 20). The evolution of neurological manifestations in leprosy is related to the clinical forms and the leprosy reactions.
Acute neuritis in MB patients has been described; however, in general, there is little inflammatory response in MB and the symptoms evolve slowly, generating a progressive symmetrical polyneuropathy (19–21), like the neuritis that occurs by complement activation (22). Additionally, silent neuritis has been described and can occur in reactions without clinical manifestations. By contrast, in PB patients, the neural involvement is more limited, occurring as a mononeuropathy or mononeuritis multiplex; patients with borderline forms are the ones who most frequently have neurological damage and complications, as they have an unstable immune response (19–21). The patient of case 2 was PB, diagnosed as having borderline leprosy, and presented median neuritis with the additional involvement of the ulnar nerve, evidencing this immunological instability that generates edema and neural compression above the entrance to the carpal tunnel.
High-resolution USG has been used to evaluate peripheral nerves. Nerves are often enlarged in leprosy patients, especially those with type 1 reactions. Lugão et al. (23) noted that the greater the thickness of the nerve, the greater the flow on the Power Doppler. It is likely that increased blood flow is the first sign of neural injury (24). In the two reported cases, it was possible to see a clear loss of fascicular morphology and neural hypoechogenicity with significant thickening in the affected nerves. The presence of flow on the Power Doppler raises suspicion of the presence of nerve inflammation, and associated with the clinical and electrophysiological findings, neuritis can be diagnosed.
The median nerve of case 2 showed significant thickening proximal to the carpal tunnel (~55% greater than the thickening in CTS) with flow on the Power Doppler. These findings are similar to the USG changes of the median nerve reported in other studies of patients with leprosy. In these studies, morphological changes and fusiform neural thickening occurring 2–5 cm from the wrist were observed, which is different from what occurs in patients with CTS (25, 26).
Patients diagnosed with leprosy may have to receive high doses of corticosteroids, and perhaps even more than once in cases of recurrent neuritis (1). The use of systemic corticosteroids is limited in patients with comorbidities and other clinical conditions, such as diabetes mellitus, cataracts, hypertension, and immunosuppressed patients. Accordingly, steroids administered at the specific point of neuritis, guided by NCS and ultrasound, can be a safe and successful strategy in these patients. An anesthetic and corticosteroid solution injected around the nerve promotes the hydrodissection mechanism and release of the anti-inflammatory and perineural analgesic medication (27–29). This therapeutic modality may be a promising alternative in cases of leprosy-isolated neuritis.
The use of injectable medications has arisen and evolved with the improvement in clinical, NSC, and imaging parameters, and may be useful for leprosy-isolated neuritis in particular. It is expected that further studies will be carried out on this method to be able to offer these patients more treatment options with the aim of reducing the definitive neurological deficits.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
CS, MJ, and NV: conceived the study. CS, IP, LA, and AS: performed the review of medical records. CS and MJ: analyzed the data. CS: wrote the paper and performed radiology analysis. CS, MJ, IP, LA, and AS: performed clinical evaluation. CS, MJ, IP, and LA: performed neurological and electrophysiological testing. MJ: performed several edits of the draft manuscript and supervised neurological testing. NV: performed procedures. MJ and NV: contributed to the writing of the paper. ES and RP: technical and operational support. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are grateful to the people who agreed to participate in this study and the teams of the Leprosy Laboratory and the Center for Outpatient Care of the Oswaldo Cruz Institute that provided knowledgeable inputs regarding different areas of medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ministry of Health. Datasus. Leprosy Cases in TABNET: Expansion of the Database. (2015). Available online at: http://www.datasus.gov.br/DATASUS/index.php?acao=11&id=33270&tp=1 (accessed January 26, 2023).
2. Andrade PR, Jardim MR, da Silva AC, Manhaes PS, Antunes SLG, Vital R, et al. Inflammatory cytokines are involved in focal demyelination in leprosy neuritis. J Neuropathol Exp Neurol. (2016) 75:272–83. doi: 10.1093/jnen/nlv027
3. Garbino JA, Heise CO, Marques W Jr. Assessing nerves in leprosy. Clin Dermatol. (2016) 34:51–8. doi: 10.1016/j.clindermatol.2015.10.018
4. Andrade PR, Pinheiro RO, Sales AM, Illarramendi X, Barbosa MG, Moraes MO, et al. Type 1 reaction in leprosy: a model for a better understanding of tissue immunity under an immunopathological condition. Expert Rev Clin Immunol. (2015) 11:391–407. doi: 10.1586/1744666X.2015.1012501
5. Gelber RH. Leprosy (Hansen's Disease) In:Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, , editors. Harrison's Principles of Internal Medicine. 16th ed. New York, NY: McGraw-Hill (2005). p. 966–72.
6. Deps P, Collin SM. Mycobacterium lepromatosis as a second agent of Hansen's disease. Front Microbiol. (2021) 12:698588. doi: 10.3389/fmicb.2021.698588
7. Ebenezer GJ, Scollard DM. Treatment and evaluation advances in leprosy neuropathy. Neurotherapeutics. (2021) 18:2337–50. doi: 10.1007/s13311-021-01153-z
8. Naafs B. Treatment duration of reversal reaction: a reappraisal. Back to the past. Lepr Rev. (2003) 74:328–36. doi: 10.47276/lr.74.4.328
9. Pai VV, Tayshetye PU, Ganapati R. Ganapati A study of standardized regimens of steroid treatment in reactions in leprosy at a referral centre. Indian J Lepr. (2012) 84:9–15.
10. Van Brakel WH, Khawas IB, Lucas SB. Reactions in leprosy: an epidemiological study of 386 patients in West Nepal. Lepr Rev. (1994) 65:190–203. doi: 10.5935/0305-7518.19940036
11. Saunderson P, Gebre S, Desta K, Byass P, Lockwood DN. The pattern of leprosy-related neuropathy in the AMFES patients in Ethiopia: definitions, incidence, risk factors and outcome. Lepr Rev. (2000) 71:285–308. doi: 10.5935/0305-7518.20000033
12. Alves C, Moreira LA. Princípios para a retirada da corticoterapia. Resenha Médica Hosp São Rafael. (1992) 1:75–80.
13. Krasner AS. Glucocorticoid-induced adrenal insufficiency. JAMA. (1999) 282:671–6. doi: 10.1001/jama.282.7.671
14. Walker SL, Nicholls PG, Dhakal S, Hawksworth RA, Macdonald M, Mahat K, et al. A phase two randomised controlled double-blind trial of high dose intravenous methylprednisolone and oral prednisolone versus intravenous normal saline and oral prednisolone in individuals with leprosy type 1 reactions and/or nerve function impairment. PLoS Negl Trop Dis. (2011) 5:e1041. doi: 10.1371/journal.pntd.0001041
15. Hui AC, Wong SM, Wong KS Li E, Kay R, Yung P, et al. Oral steroid in the treatment of carpal tunnel syndrome. Ann Rheum Dis. (2001) 60:813–4. doi: 10.1136/ard.60.8.813
16. Dammers JW, Veering MM, Vermeulen M. Injection with methylprednisolone proximal to the carpal tunnel: randomised double blind trial. BMJ. (1999) 319:884–6. doi: 10.1136/bmj.319.7214.884
17. Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. (1966) 34:255–73.
18. Medical Research Council. Aids to Examination of the Peripheral Nervous System. Memorandum no. 45. London: Her Majesty's Stationary Office (1976).
19. Jardim MR. Pure Neural Form of Leprosy [Dissertation (Postgraduate)], Universidade Federal Fluminense, Niterói, Brazil (2004).
20. Jardim MR, Illarramendi X, Nascimento OJM, Nery JAC, Sales AM, Sampaio EP, et al. Pure neural leprosy: steroids prevent neuropathy progression. Arq Neuropsiquiatr. (2007) 65:969–73. doi: 10.1590/S0004-282X2007000600009
21. Garbino JAJardim, MR, Marques, JRW, Antunes, SL, Soarre, CT, Heise, CO, et al. Hanseníase neural primária. Projeto Diretrizes Associação Médica Brasileira e Conselho Federal de Medicina (2011). Available online at: https://amb.org.br/files
22. Bahia El Idrissi N, Das PK, Fluiter K, et al. M. leprae components induce nerve damage by complement activation: identification of lipoarabinomannan as the dominant complement activator. Acta Neuropathol. (2015) 129:653–67. doi: 10.1007/s00401-015-1404-5
23. Lugão HB, Frade MAC, Maques Jr W, Foss NT, Nogueira-Barbosa MH. Ultrasonography of leprosy neuropathy: a longitudinal prospective study. PLoS Negl Trop Dis. (2016) 10:e0005111. doi: 10.1371/journal.pntd.0005111
24. Jain S, Visser LH, Praveen TLN, Rao PN, Sureka T, et al. High-resolution sonography: a new technique to detect nerve damage in leprosy. PLoS Negl Trop Dis. (2009) 3:e498. doi: 10.1371/journal.pntd.0000498
25. Frade MAC, Nogueira-Barbosa MH, Lugão HB, Furini RB, Maques Jr W, Foss NT. New sonographic measures of peripheral nerves: a tool for the diagnosis of peripheral nerve involvement in leprosy. Mem Inst Oswaldo Cruz. (2013) 108:25–62. doi: 10.1590/S0074-02762013000300001
26. Nagappa M, Pujar GS, Keshavan AH, Bathala L, Jain RD, Das A, et al. Sonographic pattern of median nerve enlargement in Hansen's neuropathy. Acta Neurol Scand. (2021) 144:155–60. doi: 10.1111/ane.13432
27. Cass SP. Ultrasound-guided nerve hydrodissection: what is it? A review of the literature. Curr Sports Med Rep. (2016) 15:20–2. doi: 10.1249/JSR.0000000000000226
28. Bokey EL, Keating JP, Zelas P. Hydrodissection: an easy way to dissect anatomical planes and complex adhesions. Aust N Z J Surg. (1997) 67:643–4. doi: 10.1111/j.1445-2197.1997.tb04616.x
Keywords: leprosy, ultrasound, corticosteroid, case report, neuritis
Citation: Spitz CN, Pitta IJR, Andrade LR, Sales AM, Sarno EN, Villela NR, Pinheiro RO and Jardim MR (2023) Case report: Injected corticosteroids for treating leprosy isolated neuritis. Front. Med. 10:1202108. doi: 10.3389/fmed.2023.1202108
Received: 07 April 2023; Accepted: 26 May 2023;
Published: 15 June 2023.
Edited by:
Sebastian Vernal, University of São Paulo, BrazilReviewed by:
Pugazhenthan Thangaraju, All India Institute of Medical Sciences Raipur, IndiaBernard Naafs, Stichting Global Dermatology, Netherlands
Copyright © 2023 Spitz, Pitta, Andrade, Sales, Sarno, Villela, Pinheiro and Jardim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Clarissa Neves Spitz, spitzclarissa@hotmail.com
 Clarissa Neves Spitz
Clarissa Neves Spitz Izabela Jardim Rodrigues Pitta
Izabela Jardim Rodrigues Pitta Ligia Rocha Andrade1,2,3
Ligia Rocha Andrade1,2,3  Nivaldo Ribeiro Villela
Nivaldo Ribeiro Villela Roberta Olmo Pinheiro
Roberta Olmo Pinheiro Marcia Rodrigues Jardim
Marcia Rodrigues Jardim