Effect of analgesia nociception index monitor-based nociception control on perioperative stress responses during laparoscopic surgery in Trendelenburg position: a randomized controlled trial
- 1Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Republic of Korea
- 2Department of Obstetrics and Gynecology, Institute of Women’s Life Medical Science, Yonsei University College of Medicine, Seoul, Republic of Korea
- 3Department of Anesthesiology and Pain Medicine, Yonsei University College of Medicine, Seoul, Republic of Korea
Introduction: The analgesia nociception index (ANI) monitor is a nociception monitoring device based on heart rate variability. We aimed to determine the effect of ANI monitor-based intraoperative nociception control on the perioperative stress response during laparoscopic surgery in the Trendelenburg position.
Methods: Altogether, 72 female patients who underwent total laparoscopic hysterectomy were randomized to either the control or ANI group. Intraoperative nociception was controlled by remifentanil administration in a conventional manner (based on blood pressure and heart rate) in the control group and by ANI monitoring in the ANI group. Perioperative stress responses were estimated by measuring the levels of serum catecholamines and catabolic stress hormones at three timepoints: after loss of consciousness, at the end of surgery, and 1 h after the end of surgery.
Results: The serum cortisol level at the end of surgery was significantly higher in the ANI group than in the control group (p < 0.001), although more remifentanil was administered in the ANI group than in the control group (p < 0.001). Changes in the other estimators’ levels were comparable between groups during the perioperative period. The hemodynamic profiles during surgery were also significantly different between the two groups. Phenylephrine use to treat hypotension was more common in the ANI group than in the control group (p = 0.005). However, postoperative clinical outcomes such as pain and nausea/vomiting did not differ between groups.
Conclusion: ANI monitor-based nociception control in laparoscopic surgery in the Trendelenburg position did not improve perioperative stress responses, intraoperative opioid consumption, or postoperative clinical outcomes.
Clinical trial registration: ClinicalTrials.gov (NCT04343638).
1. Introduction
Adequate pain control is important for enhanced recovery after surgery. Although laparoscopy has improved postoperative clinical outcomes (1, 2), such as early mobilization and hospital discharge in gynecologic abdominal surgeries, patients undergoing total laparoscopic hysterectomy have been reported to experience severe pain after surgery (3).
Pain in conscious patients is assessed based on the patient’s self-reported pain scores such as numerical rating scale (NRS); however, the pain scores cannot be used to monitor nociception in unconscious patients. Conventionally, anesthesiologists control nociception according to indirect indicators of pain such as patient movement, high blood pressure, and tachycardia during surgery (4). However, these are not objective indicators of nociception, and nociception cannot be excluded even in the absence of these clinical symptoms.
Recently, several objective monitoring devices for nociception have been developed and applied in clinical practice (5). The analgesia nociception index (ANI) monitor (MetroDoloris, Lille, France) is a nociception monitoring device that non-invasively quantifies a patient’s nociception based on heart rate variability. Heart rate variability can be affected by various factors such as the autonomic system, body temperature, baroreflex, and neuroendocrine system. However, high-frequency heart rate variability >0.15 Hz specifically reflects parasympathetic nerves (6), and the ANI monitor calculates heart rate variability mediated by parasympathetic changes. Nociceptive stimulation reduces parasympathetic action and consequently decreases the ANI score. The ANI score ranges from 0–100, with 100 indicating maximum parasympathetic action and the lowest level of nociception and 0 indicating minimum parasympathetic action and the highest level of nociception. According to the manufacturer’s recommendation, the target level for adequate nociceptive control is between 50 and 70 of the average ANI value measured over the previous 240 s (ANIm) (7).
CO2 pneumoperitoneum induces hemodynamic changes, such as decreased cardiac output, increased systemic and pulmonary vascular resistance, increased arterial pressure, and activation of the sympathetic nervous system. Cardiac output decreases with increasing insufflation pressure (8), and vascular resistance increases immediately after pneumoperitoneum, which is related to neurohormonal factors (9). The steep Trendelenburg position, which is needed in laparoscopic pelvic surgeries, increases central blood volume and activates the baroreflex to decrease sympathetic nerve activity (10). Therefore, pneumoperitoneum and the steep Trendelenburg position in laparoscopic hysterectomy may disturb ANI monitoring because they can affect autonomic nervous system activity.
The previous studies on ANI monitor-based nociception control have reported inconsistent pain outcomes (4, 11–17), and this study aimed to evaluate the effectiveness of ANI monitoring-based nociception control in laparoscopic surgery in the steep Trendelenburg position. Although there is no definitive method to assess nociception during surgery, nociceptive stimulation during surgery can induce various neuroendocrine stress responses, resulting in increased catecholamines and catabolic hormones such as cortisol and adrenocorticotropic hormone (18–21). Therefore, we investigated whether ANI monitor-based nociception control can reduce the perioperative stress response compared with conventional nociception control by comparing serum catecholamines, stress hormones, and inflammatory cytokines in patients undergoing total laparoscopic hysterectomy.
2. Materials and methods
The Institutional Review Board of Yonsei University Health System, Seoul, South Korea, approved this trial (#4–2020-0130) on April 1, 2020. This study was registered before patient enrolment at ClinicalTrial.gov (NCT04343638, Principal investigator: Jae Hoon Lee, Date of registration: April 13, 2020) and conducted between May 2020 and October 2021. All participants provided written informed consent. Female patients aged 20–65 years who underwent elective total laparoscopic hysterectomy for uterine myoma or adenomyosis were recruited. We excluded patients with ASA physical status class ≥IV, endometriosis, anteroposterior diameter of uterus >12 cm, cognitive disorders, cardiac arrhythmia, implantable pacemaker, chronic opioid use, diseases affecting the autoimmune system (such as immune disease or diabetic neuropathy), use of medications affecting ANI monitoring (antimuscarinics, alpha-agonists, beta blockers), illiteracy, or foreigners. A computer-generated random code generator was used to randomly allocate participants to either the control group (conventional nociception control group) or ANI group (ANI monitor-guided nociception control group) in a 1:1 ratio. The patients and investigators in charge of data collection and outcome analyses were blinded, but the anesthesia providers were not blinded to randomization.
2.1. Surgery, anesthesia, and nociception management
On arrival in the operating room, standard monitoring, including pulse oximetry, non-invasive blood pressure monitoring, electrocardiography, and bispectral index (BIS) monitoring, was performed in all patients. ANI monitoring was also applied to all participants. While nociception control was performed based on ANI monitoring in the ANI group, the ANI monitor was covered with black paper so that it could not be observed in the control group. For the induction of general anesthesia, 2 mg/kg of propofol was administered, and remifentanil was infused using an effect-site target-controlled infusion pump with an initial target effect-site concentration of 3 ng/mL according to the Minto model. After confirmation of loss of consciousness, 0.6 mg/kg of rocuronium was administered. After endotracheal intubation, the patients were mechanically ventilated with 6–8 mL/kg predicted body weight of tidal volume, and respiratory rates were adjusted to maintain the end-tidal CO2 between 35 and 40 mmHg. Anesthetic depth was maintained with sevoflurane to adjust the BIS value between 40 and 60 in all patients throughout the surgery. According to the attending anesthesiologist’s decision, an additional 10 mg of rocuronium was administered to maintain a train-of-four count of less than 2 throughout the surgery.
In the control group, the remifentanil concentration was adjusted to 0.5 or 1.0, according to the judgment of the attending anesthesiologist based on mean arterial pressure (MAP) and heart rate. In the ANI group, when the ANIm values deviated from the target value of 50–70 (7), the concentration of remifentanil was adjusted by 0.5 or 1.0. Remifentanil concentration lower than 1.5 ng/mL was not allowed in either group.
Total laparoscopic hysterectomies were performed with two ports in the 30° Trendelenburg position by four expert gynecologists. Pneumoperitoneum was maintained with an intra-abdominal pressure of 12 mmHg and a CO2 flow rate of 20 L/min during the surgery. The specimens were extracted transvaginally in specimen bag.
All patients received 8 mg ondansetron 30 min before the end of surgery. In addition, 1 g acetaminophen was administered to patients weighing >50 kg, and 15 mg/kg acetaminophen was administered to patients weighing <50 kg 30 min before the end of surgery.
2.2. Hemodynamic management
Phenylephrine was used to correct isolated hypotension (MAP <60 mmHg) without bradycardia. Combined hypotension and bradycardia (<45 bpm) was managed with ephedrine. Glycopyrrolate was administered to treat the isolated bradycardia. In the case of bradycardia refractory to glycopyrrolate, atropine was used.
2.3. Post-anesthetic care unit
On arrival at the post-anesthetic care unit (PACU), NRSs for pain and nausea were assessed. Oxycodone (0.05 mg/kg) was used when the NRS score for pain was higher than 4, and 10 mg metoclopramide was infused when the NRS score for nausea was higher than 4. Patients were discharged from the PACU after an NRS for pain lower than 4 was reported.
2.4. ANI value analysis
ANI data recorded at 1-s intervals were downloaded after discontinuation of the main anesthetic at the end of surgery. The mean ANIm values from the start of induction of general anesthesia to cessation of an anesthetics, percentage of time with adequate analgesia with an ANIm value of 50–70, and percentage of time with inadequate analgesia with an ANIm value <50 were assessed for each patient.
2.5. Outcome measures
The primary outcomes in the present study were perioperative levels of catecholamines, stress hormones, and inflammatory cytokines. Blood sampling was performed at three timepoints: after loss of consciousness, at the end of surgery, and 1 h after the end of surgery. A peripheral venous catheter for blood sampling was inserted immediately after loss of consciousness and maintained until the final sampling. Blood samples were centrifuged and stored at −80°C until analysis. Quantification of serum catecholamines (norepinephrine, epinephrine), stress hormones (cortisol, adrenocorticotropic hormone), and inflammatory cytokines (interleukin-6, interleukin-10, and high mobility group box 1) was performed using enzyme-linked immunosorbent assay kits.
Secondary outcomes included intraoperative MAP and heart rate, duration of surgery, total dose of remifentanil and phenylephrine usage, ephedrine and anticholinergic use, NRS for pain at the PACU at five timepoints (at admission and 10, 20, 30, and 40 min after admission), NRS score for nausea at PACU admission and 30 min after PACU admission, usage of oxycodone and metoclopramide in the PACU, and duration of PACU stay.
2.6. Statistical analyses
Previously, the mean (standard deviation) of serum norepinephrine levels in patients undergoing laparoscopic gynecologic surgery at the end of surgery was reported to be 750 (320) pg./mL (22). Assuming that a 30% reduction in norepinephrine level is clinically meaningful, and considering a 10% dropout rate, 72 patients were required to provide 80% power at a significance level of 0.05.
Descriptive data are presented as the mean (standard deviation) or median (interquartile range). For between-group comparisons, Student’s t-test or Mann–Whitney U-test was used for continuous variables, and the chi-squared test or Fisher’s exact test was used for categorical variables. We performed repeated analyses of variance with group, time, and the interaction between group and time for repeated variables (catecholamine and stress hormone levels, mean arterial pressure, heart rate, and postoperative pain scores). All statistical analyses were performed using SPSS Statistics for Windows version 23 (IBM, Armonk, NY).
3. Results
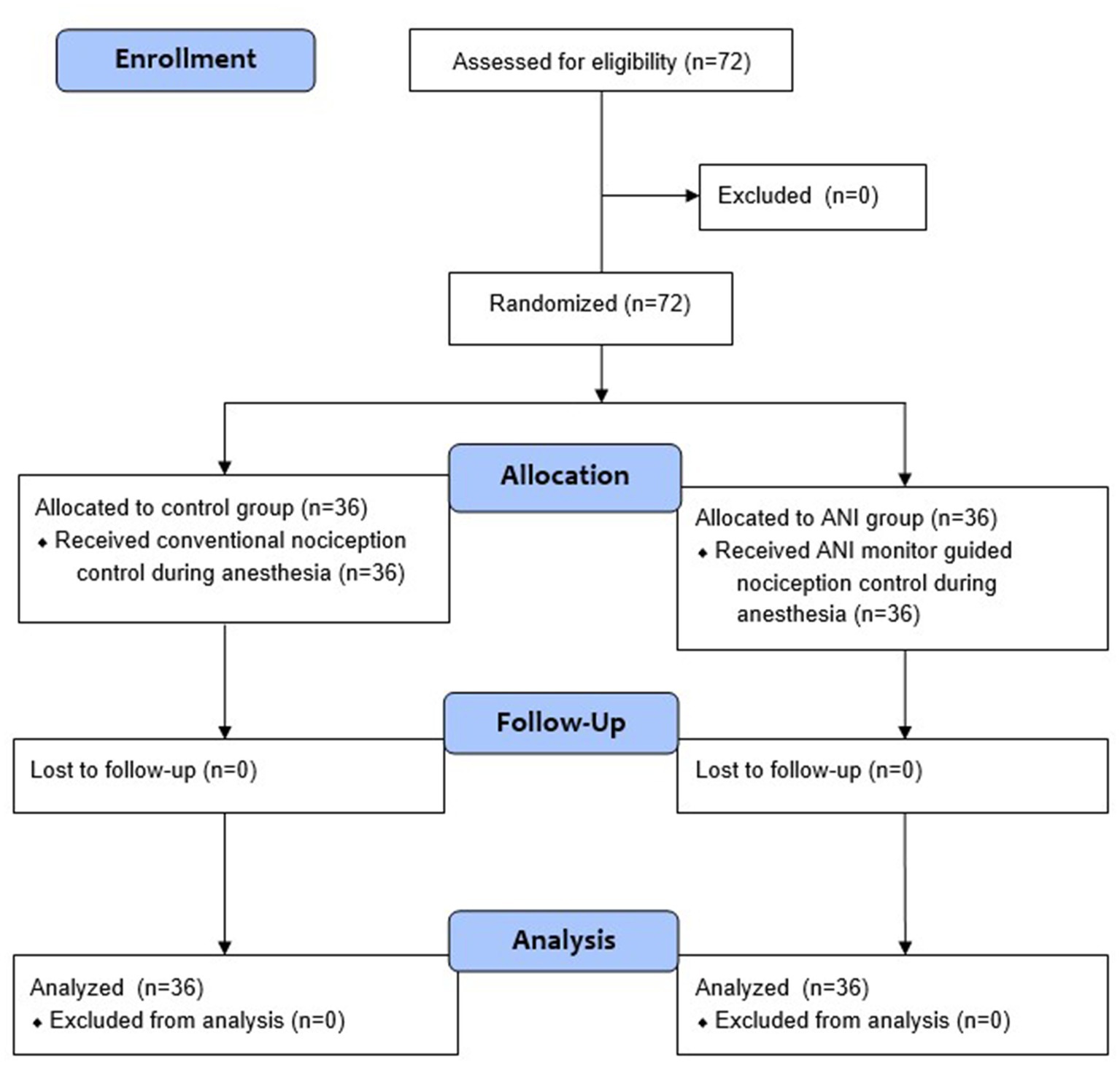
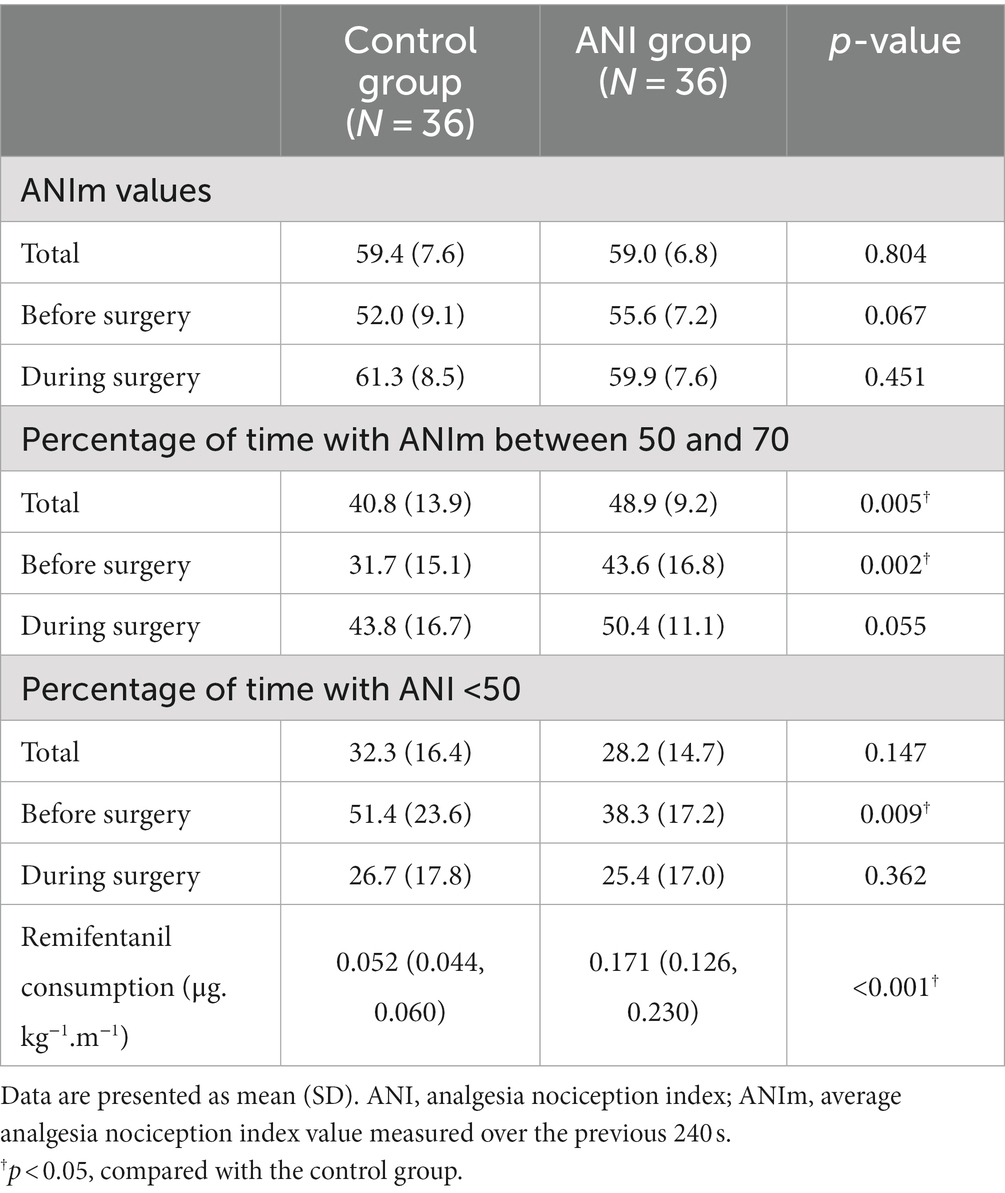
A total of 72 patients deemed eligible for this study were randomly allocated to either the control group or the ANI group (Figure 1). The baseline patient demographics are presented in Table 1. Table 2 shows the ANI values and remifentanil administration data. There was no significant difference in the mean ANIm values during anesthesia and surgery. The percentage of time spent with adequate ANIm values between 50 and 70 was higher in the ANI group than in the control group (48.9 [9.2] % vs. 40.8 [13.9] %, p = 0.005). However, during surgery, the percentage of time spent with adequate ANIm values did not differ between the two groups. Remifentanil consumption was significantly higher in the ANI group than in the control group.
Figure 2 depicts the changes in biochemical markers reflecting the stress response during surgery or perioperative inflammation and tissue damage. Although the changes in cortisol levels in the perioperative period were not significantly different between the two groups (Figure 2C pgroup × time = 0.062), the cortisol level at the end of surgery (T2) was significantly higher in the ANI group than in the control group in the post-hoc analysis (p < 0.001). The changes in other markers were comparable between the two groups.
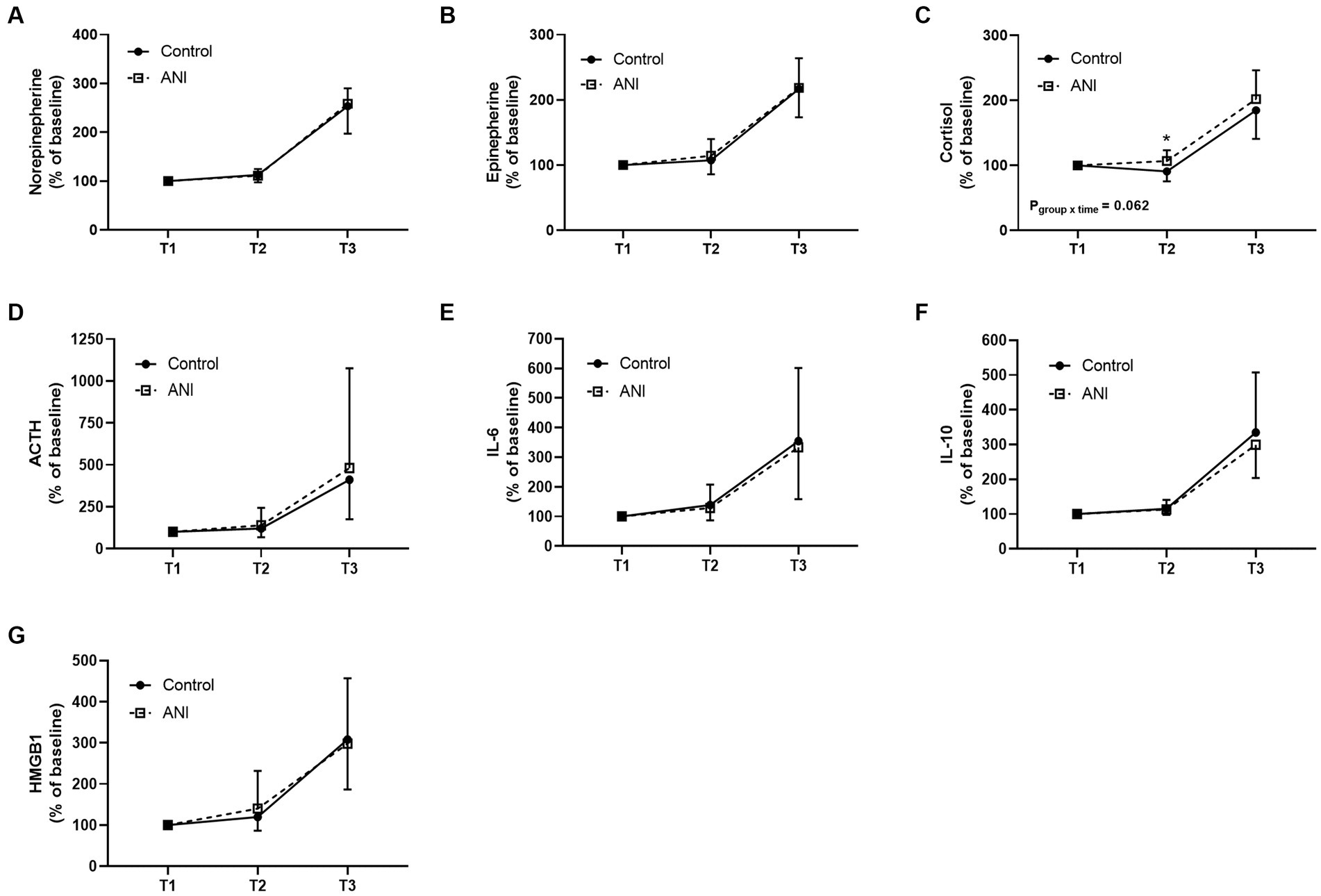
Figure 2. Perioperative changes in serum (A) norepinephrine, (B) epinephrine, (C) cortisol, (D) adrenocorticotropic hormone, (E) interleukin-6, (F) interleukin-10, and (G) high mobility group box 1 of the control and ANI group at three timepoints. T1, after the loss of consciousness; T2, at the end of surgery; T3, 1 h after the end of surgery; ANI, analgesia nociception index.
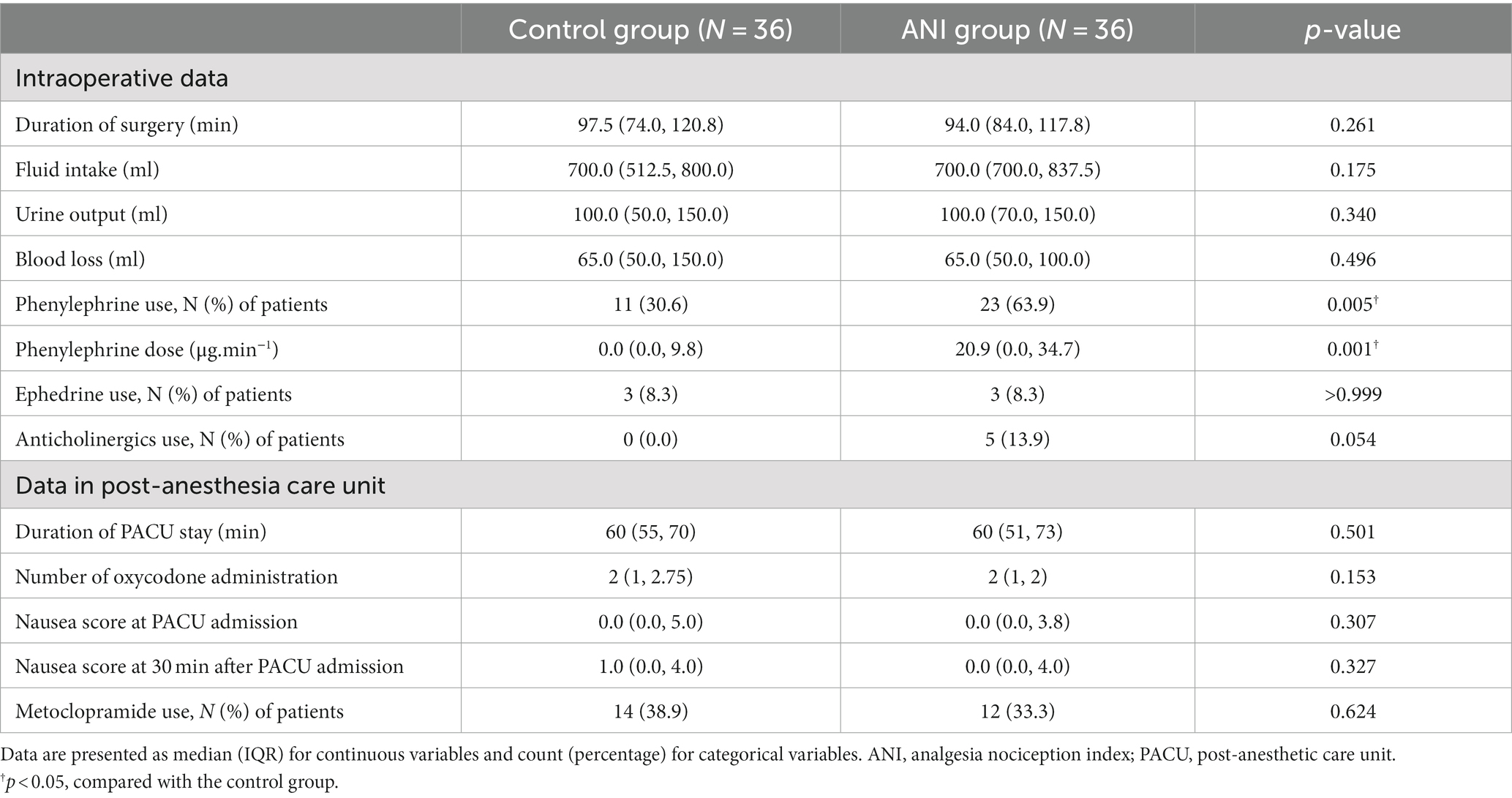
Other intraoperative and postoperative data are summarized in Table 3. Although the duration of surgery was similar between groups, a larger amount of phenylephrine was used in the ANI group than in the control group. Anticholinergics were used only in the ANI group. The change in MAP was different between the two groups (pgroup × time = 0.008, Figure 3A), and MAP immediately after anesthetic induction was significantly lower in the ANI group than in the control group. Heart rate was also lower in the ANI group than in the control group from anesthetic induction until the end of surgery (pgroup × time < 0.001, Figure 3B).
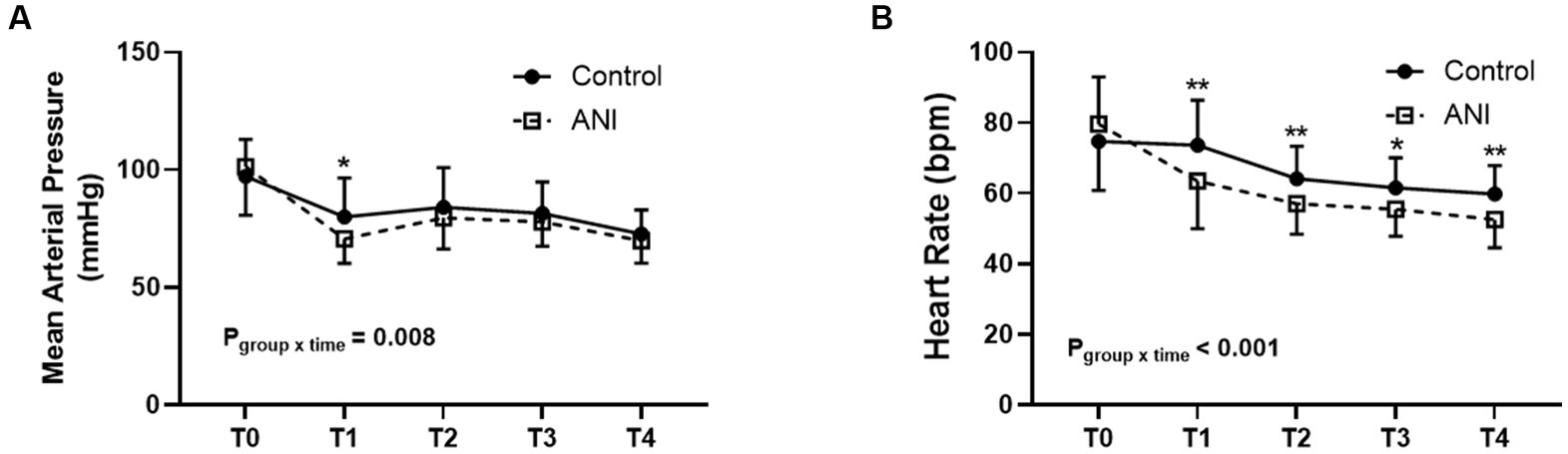
Figure 3. Intraoperative changes of mean arterial pressure (A) and heart rate (B) between the control and ANI group. T0, before induction of anesthesia; T1, after induction of anesthesia; T2, 10 min after the start of surgery; T3, 30 min after the start of surgery; T4, at the end of surgery. *p < 0.05; **p < 0.01. ANI, analgesia nociception index.
Pain scores during PACU stay did not differ between the two groups (pgroup × time = 0.255; Supplementary Figure 1). As shown in Table 3, oxycodone use did not differ between groups. Other parameters related to nausea and vomiting did not significantly differ between groups.
4. Discussion
ANI monitoring has been approved and adopted in clinical practice in many countries; however, the effect of ANI monitor-based nociception control during surgery on clinical outcomes has not been fully determined. The current study demonstrated that the application of ANI in laparoscopic hysterectomy did not reduce perioperative catecholamine levels when compared with conventional nociception control using hemodynamic variables. Furthermore, the serum cortisol level at the end of surgery was higher in the ANI group than in the control group, despite the higher dose of intraoperative remifentanil used. Therefore, the conventional application of ANI monitoring cannot improve the perioperative stress response in laparoscopic hysterectomy. Other clinical outcomes, including postoperative pain and nausea/vomiting, did not differ between the two groups.
Since the ANI monitor calculates heart rate variability mediated by parasympathetic changes, factors that can disturb the autonomic nervous system, such as pneumoperitoneum and Trendelenburg position in our study, may affect the values of ANI monitoring. CO2 pneumoperitoneum activates the sympathetic nervous system, which increases plasma catecholamine concentrations (23, 24). In previous studies based on heart rate variability (25–27), the ratio of low-frequency to high-frequency (LF/HF) band power increased after CO2 insufflation, which suggests sympathetic activation. While the baroreceptor reflex due to decreased cardiac output stimulates the sympathetic nervous system during pneumoperitoneum, increased venous return in the Trendelenburg position can reduce sympathetic overstimulation (27). In laparoscopic surgery in the Trendelenburg position, increased plasma levels of catecholamines, adrenocorticotropic hormone, and cortisol have been reported (28); however, the results of heart rate variability are conflicting (29–32). A previous study based on laparoscopic appendectomy or cholecystectomy demonstrated a decrease in ANI values after pneumoperitoneum inflation (33). Therefore, CO2 pneumoperitoneum and steep (30°) Trendelenburg position during surgery in our study can affect ANI values in addition to surgical nociception. In fact, we had more trouble maintaining adequate ANI values after the induction of pneumoperitoneum and Trendelenburg positioning than before surgery. Considering that the total duration of adequate ANIm values during surgery was not significantly different between groups despite the higher remifentanil administration in the ANI group, decreased parasympathetic activity (or increased sympathetic activity) by pneumoperitoneum may not be controlled well by opioid administration or may not be reflected by the ANI monitor.
Previous studies have reported that adequate nociception control guided by ANI monitoring or other nociception monitoring based on heart rate variability during surgery can reduce intraoperative opioid use and perioperative stress responses (34, 35). However, this study showed that ANI monitor-based nociception control during surgery did not reduce perioperative stress responses when compared with conventional nociception control. Furthermore, a higher dose of remifentanil was administered to the ANI group than to the control group to achieve adequate ANI values during surgery. In addition, heart rate and arterial blood pressure during anesthesia were significantly lower in the ANI group than in the control group, although more vasopressors were administered to avoid hypotension in the ANI group than in the control group. The low hemodynamic profiles in the ANI group may be due to the larger amount of remifentanil administered, which may be reflected in the profiles of perioperative stress responses between the two groups. Increased levels of catecholamines and glucocorticoid hormones have been reported in hypotensive situations (36, 37). Increased stress responses due to hypotension may mask the reduced stress responses following opioid administration.
The association between the autonomic nervous system and the immune system has been revealed. The vagus nerve, one of the main nerves of the parasympathetic nervous system, monitors and regulates peripheral inflammation (38). Therefore, we hypothesized that maintaining parasympathetic dominance during surgery by ANI monitoring could affect intra- and postoperative inflammatory responses, which may result in differences in tissue damage and inflammatory response during surgery between the two groups. However, there were no differences in inflammatory cytokines or markers of tissue damage (high mobility group box 1) between groups.
Postoperative pain outcomes after intraoperative ANI monitor-based nociception control can be affected by various factors, such as the type of surgery, anesthetic methods, or pain management strategy. However, previous studies have reported conflicting results. In contrast to favorable pain outcomes in spine and vascular surgeries (4, 11), ANI monitoring guidance did not improve postoperative pain in a variety of surgical patients (12–15) including laparoscopic surgery (16) and gynecologic surgery (17) (which included both laparoscopic and open abdominal surgeries). In the current study, although a higher dose of remifentanil was used in the ANI group than in the control group, the duration of the effect of remifentanil was short, and the dose of remifentanil in both groups was not high enough to induce hyperalgesia (39), postoperative pain outcomes were not different between the two groups. In addition, there was no difference between the two groups in the perioperative stress response or inflammatory response, which is consistent with the postoperative pain outcomes.
This study had some limitations. Firstly, the investigator in charge of anesthesia was not blinded to the randomization. However, the investigator in charge of laboratory measurements and postoperative outcome assessments was blinded to group allocation. Secondly, this study was conducted based on special clinical settings, such as laparoscopy in the steep Trendelenburg position in relatively young female patients, and the results of this study cannot be generalized to other patient populations. Thirdly, anticholinergic and ephedrine were used to treat bradycardia and hypotension, which can influence ANI monitoring. However, this medication can represent a clinical picture in this surgical setting.
In conclusion, the present study showed that nociception control with ANI monitoring according to the manufacturer’s recommendations did not improve perioperative stress response in terms of catecholamine and stress hormones in laparoscopic gynecologic surgery performed in the steep Trendelenburg position. In this clinical setting, despite the larger amount of opioid required to achieve adequate ANI values in the ANI group compared to the control group, ANI guidance could not improve postoperative clinical outcomes. When ANI monitoring is applied in laparoscopic surgeries in the Trendelenburg position, strategies other than maintaining the target range of ANIm may be required.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Yonsei University Health System, Seoul, South Korea, approved this trial (#4-2020-0130) on April 1, 2020. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SK: acquisition, analysis, and interpretation of data, drafting and critical revision of the manuscript. CC, J-RL, SS, and YK: acquisition, analysis, interpretation of data, and critical revision of the manuscript. JL: study concept and design, acquisition, analysis, interpretation of data, drafting and critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the Basic Science Research Program to JL through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2019R1C1C1005201).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1196153/full#supplementary-material
References
1. Orhan, A, Ozerkan, K, Kasapoglu, I, Ocakoglu, G, Cetinkaya Demir, B, Gunaydin, T, et al. Laparoscopic hysterectomy trends in challenging cases (1995-2018). J Gynecol Obstet Hum Reprod. (2019) 48:791–8. doi: 10.1016/j.jogoh.2019.06.007
2. Bartels, HC, Rogers, AC, Janda, M, Obermair, A, and Brennan, DJ. Quality of life following minimally invasive hysterectomy compared to abdominal hysterectomy: a metanalysis. Eur J Obstet Gynecol Reprod Biol. (2020) 252:206–2. doi: 10.1016/j.ejogrb.2020.06.055
3. Choi, JB, Kang, K, Song, MK, Seok, S, Kim, YH, and Kim, JE. Pain characteristics after Total laparoscopic hysterectomy. Int J Med Sci. (2016) 13:562–8. doi: 10.7150/ijms.15875
4. Upton, HD, Ludbrook, GL, Wing, A, and Sleigh, JW. Intraoperative "analgesia nociception index"-guided fentanyl administration during sevoflurane anesthesia in lumbar discectomy and laminectomy: a randomized clinical trial. Anesth Analg. (2017) 125:81–90. doi: 10.1213/ane.0000000000001984
5. Ledowski, T. Objective monitoring of nociception: a review of current commercial solutions. Br J Anaesth. (2019) 123:e312–21. doi: 10.1016/j.bja.2019.03.024
6. Task force of the European Society of Cardiology and the north American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. (1996) 93:1043–65. doi: 10.1161/01.CIR.93.5.1043
8. Ivankovich, AD, Miletich, DJ, Albrecht, RF, Heyman, HJ, and Bonnet, RF. Cardiovascular effects of intraperitoneal insufflation with carbon dioxide and nitrous oxide in the dog. Anesthesiology. (1975) 42:281–7. doi: 10.1097/00000542-197503000-00008
9. Wahba, RW, Béïque, F, and Kleiman, SJ. Cardiopulmonary function and laparoscopic cholecystectomy. Can J Anaesth. (1995) 42:51–63. doi: 10.1007/bf03010572
10. London, GM, Levenson, JA, Safar, ME, Simon, AC, Guerin, AP, and Payen, D. Hemodynamic effects of head-down tilt in normal subjects and sustained hypertensive patients. Am J Phys. (1983) 245:H194–202. doi: 10.1152/ajpheart.1983.245.2.H194
11. Daccache, G, Caspersen, E, Pegoix, M, Monthé-Sagan, K, Berger, L, Fletcher, D, et al. A targeted remifentanil administration protocol based on the analgesia nociception index during vascular surgery. Anaesth Crit Care Pain Med. (2017) 36:229–2. doi: 10.1016/j.accpm.2016.08.006
12. Dundar, N, Kus, A, Gurkan, Y, Toker, K, and Solak, M. Analgesia nociception index (ani) monitoring in patients with thoracic paravertebral block: a randomized controlled study. J Clin Monit Comput. (2018) 32:481–6. doi: 10.1007/s10877-017-0036-9
13. Le Gall, L, David, A, Carles, P, Leuillet, S, Chastel, B, Fleureau, C, et al. Benefits of intraoperative analgesia guided by the analgesia nociception index (ANI) in bariatric surgery: an unmatched case-control study. Anaesth Crit Care Pain Med. (2019) 38:35–9. doi: 10.1016/j.accpm.2017.09.004
14. Dostalova, V, Schreiberova, J, Bartos, M, Kukralova, L, and Dostal, P. Surgical Pleth index and analgesia nociception index for intraoperative analgesia in patients undergoing neurosurgical spinal procedures: a comparative randomized study. Minerva Anestesiol. (2019) 85:1265–72. doi: 10.23736/s0375-9393.19.13765-0
15. Tribuddharat, S, Sathitkarnmanee, T, Sukhong, P, Thananun, M, Promkhote, P, and Nonlhaopol, D. Comparative study of analgesia nociception index (ANI) vs. standard pharmacokinetic pattern for guiding intraoperative fentanyl administration among mastectomy patients. BMC Anesthesiol. (2021) 21:50. doi: 10.1186/s12871-021-01272-2
16. Szental, JA, Webb, A, Weeraratne, C, Campbell, A, Sivakumar, H, and Leong, S. Postoperative pain after laparoscopic cholecystectomy is not reduced by intraoperative analgesia guided by analgesia nociception index (ANI®) monitoring: a randomized clinical trial. Br J Anaesth. (2015) 114:640–5. doi: 10.1093/bja/aeu411
17. Sabourdin, N, Burey, J, Tuffet, S, Thomin, A, Rousseau, A, Al-Hawari, M, et al. Analgesia nociception index-guided remifentanil versus standard care during Propofol anesthesia: a randomized controlled trial. J Clin Med. (2022) 11:333. doi: 10.3390/jcm11020333
18. Price, HL, Linde, HW, Jones, RE, Black, GW, and Price, ML. Sympatho-adrenal responses to general anesthesia in man and their relation to hemodynamics. Anesthesiology. (1959) 20:563–5. doi: 10.1097/00000542-195909000-00001
19. Parker, SD, Breslow, MJ, Frank, SM, Rosenfeld, BA, Norris, EJ, Christopherson, R, et al. Catecholamine and cortisol responses to lower extremity revascularization: correlation with outcome variables. Perioperative ischemia randomized anesthesia trial study group. Crit Care Med. (1995) 23:1954–61. doi: 10.1097/00003246-199512000-00003
20. Sametz, W, Metzler, H, Gries, M, Porta, S, Sadjak, A, Supanz, S, et al. Perioperative catecholamine changes in cardiac risk patients. Eur J Clin Investig. (1999) 29:582–7. doi: 10.1046/j.1365-2362.1999.00509.x
21. Taschner, A, Kabon, B, Falkner von Sonnenburg, M, Graf, A, Adamowitsch, N, Fraunschiel, M, et al. Perioperative supplemental oxygen and plasma catecholamine concentrations after major abdominal surgery-secondary analysis of a randomized clinical trial. J Clin Med. (2022) 11:1767. doi: 10.3390/jcm11071767
22. Marana, E, Annetta, MG, Meo, F, Parpaglioni, R, Galeone, M, Maussier, ML, et al. Sevoflurane improves the neuroendocrine stress response during laparoscopic pelvic surgery. Can J Anaesth. (2003) 50:348–4. doi: 10.1007/bf03021031
23. Mann, C, Boccara, G, Pouzeratte, Y, Eliet, J, Serradel-Le Gal, C, Vergnes, C, et al. The relationship among carbon dioxide pneumoperitoneum, vasopressin release, and hemodynamic changes. Anesth Analg. (1999) 89:278–3. doi: 10.1097/00000539-199908000-00003
24. Mikami, O, Fujise, K, Matsumoto, S, Shingu, K, Ashida, M, and Matsuda, T. High intra-abdominal pressure increases plasma catecholamine concentrations during pneumoperitoneum for laparoscopic procedures. Arch Surg. (1998) 133:39–43. doi: 10.1001/archsurg.133.1.39
25. Sato, N, Kawamoto, M, Yuge, O, Suyama, H, Sanuki, M, Matsumoto, C, et al. Effects of pneumoperitoneum on cardiac autonomic nervous activity evaluated by heart rate variability analysis during sevoflurane, isoflurane, or propofol anesthesia. Surg Endosc. (2000) 14:362–6. doi: 10.1007/s004640000155
26. Bickel, A, Yahalom, M, Roguin, N, Frankel, R, Breslava, J, Ivry, S, et al. Power spectral analysis of heart rate variability during positive pressure pneumoperitoneum: the significance of increased cardiac sympathetic expression. Surg Endosc. (2002) 16:1341–4. doi: 10.1007/s00464-001-9211-6
27. Bickel, A, Kukuev, E, Popov, O, Ivry, S, Roguin, N, Yahalom, M, et al. Power spectral analysis of heart rate variability during helium pneumoperitoneum: the mechanism of increased cardiac sympathetic activity and its clinical significance. Surg Endosc. (2005) 19:71–6. doi: 10.1007/s00464-003-9304-5
28. Marana, E, Colicci, S, Meo, F, Marana, R, and Proietti, R. Neuroendocrine stress response in gynecological laparoscopy: TIVA with propofol versus sevoflurane anesthesia. J Clin Anesth. (2010) 22:250–5. doi: 10.1016/j.jclinane.2009.07.011
29. Shin, S, Bai, SJ, Rha, KH, So, Y, and Oh, YJ. The effects of combined epidural and general anesthesia on the autonomic nervous system and bioavailability of nitric oxide in patients undergoing laparoscopic pelvic surgery. Surg Endosc. (2013) 27:918–6. doi: 10.1007/s00464-012-2536-5
30. Lowenstein, L, Mustafa, M, Burke, YZ, Mustafa, S, Segal, D, and Weissman, A. Steep Trendelenburg position during robotic sacrocolpopexy and heart rate variability. Eur J Obstet Gynecol Reprod Biol. (2014) 178:66–9. doi: 10.1016/j.ejogrb.2014.03.046
31. Matanes, E, Weissman, A, Rivlin, A, Lauterbach, R, Amit, A, Wiener, Z, et al. Effects of pneumoperitoneum and the steep Trendelenburg position on heart rate variability and cerebral oxygenation during robotic Sacrocolpopexy. J Minim Invasive Gynecol. (2018) 25:70–5. doi: 10.1016/j.jmig.2017.07.009
32. Raimondi, F, Colombo, R, Costantini, E, Marchi, A, Corona, A, Fossali, T, et al. Effects of laparoscopic radical prostatectomy on intraoperative autonomic nervous system control of hemodynamics. Minerva Anestesiol. (2017) 83:1265–73. doi: 10.23736/s0375-9393.17.12024-9
33. Jeanne, M, Clément, C, De Jonckheere, J, Logier, R, and Tavernier, B. Variations of the analgesia nociception index during general anaesthesia for laparoscopic abdominal surgery. J Clin Monit Comput. (2012) 26:289–4. doi: 10.1007/s10877-012-9354-0
34. Funcke, S, Pinnschmidt, HO, Wesseler, S, Brinkmann, C, Beyer, B, Jazbutyte, V, et al. Guiding opioid administration by 3 different analgesia nociception monitoring indices during general anesthesia alters intraoperative Sufentanil consumption and stress hormone release: a randomized controlled pilot study. Anesth Analg. (2020) 130:1264–73. doi: 10.1213/ane.0000000000004388
35. Meijer, F, Honing, M, Roor, T, Toet, S, Calis, P, Olofsen, E, et al. Reduced postoperative pain using nociception level-guided fentanyl dosing during sevoflurane anaesthesia: a randomised controlled trial. Br J Anaesth. (2020) 125:1070–8. doi: 10.1016/j.bja.2020.07.057
36. Wood, CE. ACTH, cortisol, and renin responses to arterial hypotension in sheep. Am J Phys. (1986) 251:R18–22. doi: 10.1152/ajpregu.1986.251.1.R18
37. Rawlinson, WA, Loach, AB, and Benedict, CR. Changes in plasma concentration of adrenaline and noradrenaline in anaesthetized patients during sodium nitroprusside-induced hypotension. Br J Anaesth. (1978) 50:937–3. doi: 10.1093/bja/50.9.937
38. Steinberg, BE, Sundman, E, Terrando, N, Eriksson, LI, and Olofsson, PS. Neural control of inflammation: implications for perioperative and critical care. Anesthesiology. (2016) 124:1174–89. doi: 10.1097/aln.0000000000001083
Keywords: analgesia, catecholamine, glucocorticoid, laparoscopy, nociception
Citation: Kim SH, Chang CH, Lee J-R, Seo SK, Kwon YI and Lee JH (2023) Effect of analgesia nociception index monitor-based nociception control on perioperative stress responses during laparoscopic surgery in Trendelenburg position: a randomized controlled trial. Front. Med. 10:1196153. doi: 10.3389/fmed.2023.1196153
Edited by:
Pavel Michalek, General University Hospital in Prague, CzechiaReviewed by:
Sang Hun Kim, Chosun University, Republic of KoreaLei Zhao, Capital Medical University, China
Alessandro Gonfiotti, University of Florence, Italy
Sangseok Lee, Inje University Sanggye Paik Hospital, Republic of Korea
Copyright © 2023 Kim, Chang, Lee, Seo, Kwon and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jae Hoon Lee, neogens@yuhs.ac
 Seung Hyun Kim
Seung Hyun Kim Chul Ho Chang1
Chul Ho Chang1  Seok Kyo Seo
Seok Kyo Seo Young In Kwon
Young In Kwon Jae Hoon Lee
Jae Hoon Lee